Abstract
Background:
Immune checkpoint inhibitors (ICIs) have shown remarkable therapeutic outcomes among cancer patients. Durvalumab plus tremelimumab (DT) is under investigation as a new ICI combination therapy, and its efficacy has been reported in various types of cancer. However, the safety profile of DT remains unclear, especially considering rare adverse events (AEs).
Objective:
We aimed to assess the frequency of AEs associated with DT.
Design:
This study type is a systematic review and meta-analysis.
Data Sources and Methods:
Four databases were searched for articles. Randomized trials, single-arm trials, and prospective and retrospective observational studies were included. The type of cancer, previous treatment, and performance status were not questioned. Major AE indicators such as any AE and the pooled frequency of each specific AE were used as outcomes. As a subgroup analysis, we also compared cases in which DT was performed as first-line treatment with those in which it was performed as second-line or later treatment. The protocol for this systematic review was registered on the University Hospital Medical Information Network (UMIN) Center website (ID: UMIN000046751).
Results:
Forty-one populations including 3099 patients were selected from 30 articles. Pooled frequencies of key AE indicators are shown below: any AEs, 77.8% [95% confidence interval (CI): 67.9–87.6]; grade ⩾ 3 AEs, 29.3% (95% CI: 24.2–34.4); serious AEs, 34.9% (95% CI: 28.1–41.7); AE leading to discontinuation, 13.3% (95% CI: 9.3–17.4); treatment-related deaths, 0.98% (95% CI: 0.5–1.5). AEs with a frequency exceeding 15% are shown below: fatigue, 30.1% (95% CI: 23.8–36.3); diarrhea, 21.7% (95% CI: 17.8–25.6); pruritus 17.9% (95% CI: 14.4–21.3); decreased appetite, 17.7% (95% CI: 13.7–22.0); nausea, 15.6% (95% CI: 12.1–19.6). There were no significant differences in these pooled frequencies between subgroups.
Conclusions:
The incidence of any AE in DT therapy was approximately 78%, and the incidence of grade 3 or higher AEs was approximately 30%, which was independent of prior therapy.
Keywords: clinical trial, drug-related adverse events, immune checkpoint inhibitor, monoclonal antibodies, neoplasms
Introduction
It is well-established that immune checkpoint inhibitors (ICIs) block tumor cell signals that suppress T-cell activation. Importantly, the introduction of ICIs has drastically improved therapeutic outcomes among patients with cancer.
Tremelimumab, a monoclonal antibody targeting the cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) protein receptor, inactivates T-cell recognition and cancer cell proliferation, diversifies T-cell responses, and promotes T-cell infiltration into tumors.1,2 Durvalumab, another known ICI, can enhance the antitumor effect of T cells by inhibiting the binding of programmed death ligand 1 (PD-L1) to programmed cell death protein-1 (PD-1). 3
ICIs were initially used alone; however, clinical trials have revealed that combinations of cytotoxic agents or multiple ICIs can afford superior outcomes, resulting in the widespread application of nivolumab plus ipilimumab.
Currently, durvalumab plus tremelimumab (DT) is under investigation in clinical trials as a new ICI combination therapy, with efficacy reported in non-small cell lung cancer, 4 head and neck cancer, 5 and other types of cancers. However, the safety profile of DT remains unclear, especially considering rare adverse events (AEs). 6 DT has been associated with a greater number of grade ⩾ 3 AEs than monotherapy.7–10 Considering AEs from combined ICI therapy, Somekawa et al. 11 have systematically reviewed the combined use of nivolumab, an anti-PD-1 antibody, and ipilimumab, an anti-CTLA-4 antibody. The authors reported that approximately 40% of patients who received a combination of nivolumab and ipilimumab experienced grade ⩾ 3 AEs. It is estimated that treatment with DT can also result in grade ⩾ 3 AEs than monotherapy.
Although DT is gaining momentum as a standard treatment for various malignancies, detailed toxicity profiles need to be established. Therefore, in the present systematic review and meta-analysis, we aimed to assess the patient-level frequency of AEs associated with DT therapy.
Methods
Protocol registration
The protocol for this systematic review was established in accordance with meta-analyses of observational studies in epidemiology guidelines and was registered on the University Hospital Medical Information Network (UMIN) Center website (ID: UMIN000046751).12,13
Study search
The electronic database search formulas for PubMed, Web of Science Core Collection, Cochrane Advanced Search, and EMBASE are described in Supplemental Text 1. A database search was conducted on 15 February 2022. Two authors (HM and KS) independently performed additional searches manually.
The identified articles (HM and KS) were screened and thoroughly assessed. In the case of any disagreement, a third reviewer was consulted.
Publication type and trial design
In addition to randomized and single-arm trials, prospective and retrospective observational studies were also considered. However, case reports and case series were excluded owing to unsuitable study designs for estimating AE frequency. Eligible articles were limited to those published in English. Full articles and conference abstracts were also considered.
Patients
There was no restriction on the type of cancer, as it did not substantially impact the safety profile when the same regimen was selected. Patients who had undergone previous chemotherapy were considered. No restrictions on performance status or age were implemented.
Treatment
DT regimens combined with other anticancer medications, such as cytotoxic anticancer drugs, molecular-targeted drugs, and other ICI combinations, were excluded. The present analysis also excluded patients who received combined chemoradiotherapy. Furthermore, we excluded sequential combinations, such as three courses of durvalumab followed by three courses of tremelimumab. There were no restrictions on the dose, schedule, or frequency of DT combination therapy. However, clinical trials in which the study protocol required only one course of DT therapy were excluded. Perioperative treatments, such as adjuvant and neoadjuvant therapies, were permitted.
Quality assessment
The Newcastle-Ottawa quality assessment scale for cohort studies was used for quality assessment. 14
Outcomes
The binomial frequencies of major AE indicators (any AE, grade ⩾ 3 AEs, serious AEs, AE leading to discontinuation, and treatment-related death) were pooled. In addition, 22 specific AEs, including elevated alanine aminotransferase levels and skin rash, were reported.
Data extraction
Two review authors (HM and KS) extracted key study characteristics, including author name, year of publication, country of origin, study title, and the number of patients. If a study evaluated different doses of durvalumab and tremelimumab accompanied by AE profiles, these regimens were counted as independent populations.
Subgroup analysis
The subgroup analysis focused on patients who received DT therapy as first-line treatment, as well as on those who received DT therapy as second-line or later treatment.
Statistics
The frequency of each AE was pooled by random model meta-analysis using the generic inverse variance method (RevMan ver 5.4.1.; Cochrane Collaboration, London, UK). Standard errors were calculated using Agrestia’s method. 15 In addition to I2 statistics, between-subgroup differences were expressed using p-values for heterogeneity based on the RevMan random model analysis, with a significance level of p < 0.1.
Results
Study selection process
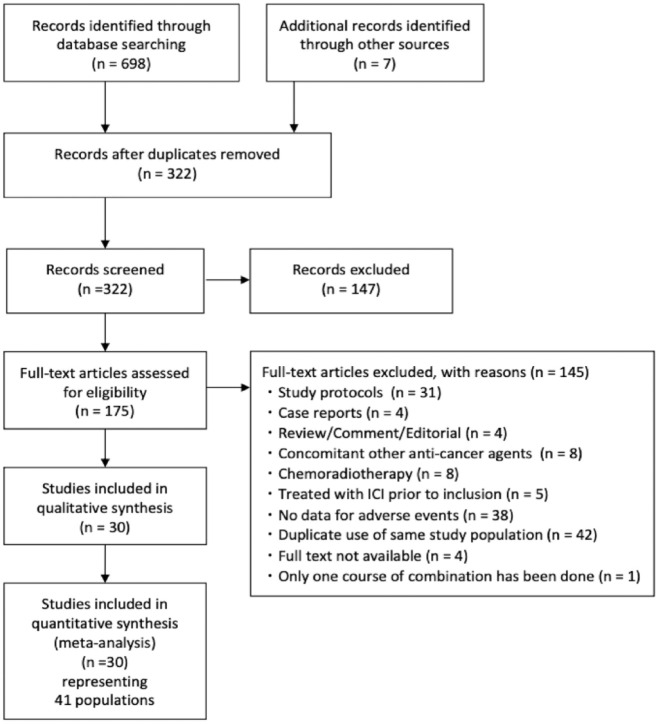
An electronic search of four major databases retrieved 698 articles, and a manual search identified seven additional articles (Figure 1). After deduplication (n = 147), screening (n = 322), and full-text scrutiny (n = 175), 41 populations from 30 studies were included in the quantitative analysis. (Figure 1 and Supplemental Table 3).
Figure 1.
The preferred reporting items for systematic reviews and meta-analyses flow chart.
Study characteristics
Among the 30 included papers, 17 were full articles and 13 were conference abstracts. More than 50% of included papers were from the U.S. (n = 18), followed by Canada (n = 5), France (n = 3), Italy, Korea, Spain, and the UK (n = 1 each). The studies included six phase I/IB studies, one phase Ib/II study, 14 phase II studies, six phase III studies, and one pilot study. However, some studies failed to describe the trial phase (Table 1).
Table 1.
Characteristics of included populations.
| Population | Country | Report | Design | Cancer | Stage | Regimen | Setting | n | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Antonia 2016 T1 | USA | FA | P1b | NSCLC | Locally advanced or metastatic | D10–20 q2w x26 or q4w x13 + T1 q4w x6 > q12w x3 | 1st or later | 56 | 4 |
| Antonia 2016 T10 | USA | FA | P1b | NSCLC | Locally advanced or metastatic | D15 q4w x13 + T10 q4w x6 > q12w x3 | 1st or later | 9 | 4 |
| Antonia 2016 T3 | USA | FA | P1b | NSCLC | Locally advanced or metastatic | D10–20 q2w x26 or q4w x13 + T3 q4w x6 > q12w x3 | 1st or later | 34 | 4 |
| Boilève 2021 | France | FA | P2 RCT | Biliary tract carcinoma | Recurrent or advanced | D1500 + T75 q4w x4 | 2nd or later | 10 | 4 |
| Brohawn 2018 P1b | USA | CA | P1b | Gastric cancer | Recurrent or metastatic | D20 + T1 q4w > D10 q2w | 2nd | 6 | 4 |
| Brohawn 2018 P2 ArmA | USA | CA | P2 RCT | Gastric cancer | Recurrent or metastatic | D20 + T1 q4w > D10 q2w | 2nd | 27 | 4 |
| Brohawn 2018 P2 ArmD | USA | CA | P2 RCT | Gastric cancer | Recurrent or metastatic | D20 + T1 q4w > D10 q2w | 3rd | 25 | 4 |
| Brohawn 2018 P2 ArmE | USA | CA | P2RCT | Gastric cancer | Recurrent or metastatic | D20 + T1 q4w > D10 q2w | 2nd or 3rd | 19 | 4 |
| Calabro 2018 | Italy | FA | Single arm P2 | Mesothelioma | Unresectable | D20 + T1 q4w x4 > D20 q4w x9 | 1st or 2nd | 40 | 5 |
| Capdevila 2020 | Spain | CA | Single arm P2 | Lung carcinoid, G1/2 gastric, G1/2 pancreatic, G3NEN | Advanced | D1500 q4x13 + T75 q4w x4 | 2nd or later | 123 | 4 |
| Chen 2019 | Canada | CA | P2 RCT | Colorectal carcinoma | Metastatic | D1500 + T75 q4w x4 | 2nd or later | 118 | 5 |
| Cho 2018 | USA | CA | P1 | SCLC | Extensive disease | D20 + T1 q4w | 2nd or later | 30 | 4 |
| Edenfield 2021 | USA | FA | Single arm P2 | Rare cancers | Advanced | D1500 q4w x13 + T75 q4w x7 > q12w x2 | 2nd or later | 44 | 4 |
| Ferrarotto 2020 | USA | FA | RCT | Oropharyngeal cancer | Stage 2–4A | D1500 + T75 day 1, 29 | Neoadj | 14 | 5 |
| Ferris 2020 | USA | FA | P3 | HNSCC | Recurrent or metastatic | D20 + T1 q4w > D10 mg q2w | 2nd-5th | 246 | 4 |
| Gao 2019 | USA | CA | Single arm | Bladder cancer | cT2-T4a | D1500 + T75 q4w x2 | Neoadj | 28 | 4 |
| Hotte 2019 | Canada | CA | P2 RCT | Prostate cancer | Metastatic | D1500 + T75 q4w x4 | 2nd or later | 39 | 4 |
| Karakunnel 2016 | USA | CA | P1 | NSCLC | Advanced | D20 + T1 q4w | Unknown | 102 | 4 |
| Kelley 2020 T300 + D | USA | CA | P3 | HCC | Unresectable | D1500 + T300 q4w | After sorafenib | 74 | 5 |
| Kelley 2020 T75 + D | USA | CA | P3 | HCC | Unresectable | D1500 + T75 q4w | After sorafenib | 82 | 4 |
| Kim 2020 | Korea | FA | Single arm P2 | Pulmonary sarcomatoid carcinoma | Recurrent or metastatic | D1500 + T75 q4w(x4) > D750 q2w(x18) | 1st or adj | 18 | 5 |
| Leighl 2021 | Canada | FA | P2 RCT | NSCLC | 4 | D1500 + T75 q4w x4 > D1500 q4w | Mainly chemo-naive | 149 | 5 |
| Nehra 2020 | Canada | FA | P1B | Solid tumor | Advanced, unresectable, recurrent or metastatic | D1500 + T75 q4w x4 | 1st or 2nd | 7’4 | |
| O’Reilly 2019 | USA | FA | P2 RCT | Pancreatic ductal adenocarcinoma | Recurrent or metastatic | D1500 q4w + T75 q4wx4 > D1500 q4w | 2nd | 32 | 4 |
| Ornstein 2020 cohort2 | USA | CA | P1b | RCC | High risk localized RCC (clinical stage T2b-4 and/or N1, M0 disease) | D + T- > D(x1dose), D + T- > D(x1 year), D + T- > D + T(x1dose) then D(x1 year), D1500 mgT75 mg | Neoadj, adj | 6 | 4 |
| Ornstein 2020 cohort2a | USA | CA | P1b | RCC | High risk localized RCC (clinical stage T2b-4 and/or N1, M0 disease) | D + T > D(x1dose), D + T > D(x1 year), D + T > D + T(x1dose) then D(x1 year), D1500 mgT75 mg | Neoadj, adj | 8 | 4 |
| Ornstein 2020 cohort3 | USA | CA | P1b | RCC | High risk localized RCC (clinical stage T2b-4 and/or N1, M0 disease) | D + T > D(x1dose), D + T > D(x1 year), D + T > D + T(x1dose) then D(x1 year), D1500 mgT75 mg | Neoadj, adj | 8 | 4 |
| Planchard 2020 | France | FA | P3 | NSCLC | Stage3B/4 | D20 mg/kg + T1 mg/kg q4w | 3rd or later | 173 | 4 |
| Powles 2020 | UK | FA | P3 | Urothelial carcinoma | Unresectable, locally advanced, metastatic | D1500 + T75 q4w x4 > D1500 m q4w | 1st | 340 | 5 |
| Ribrag 2021 | France | FA | P1b | DLBCL | Relapsed/refractory | D20 + T1 q4w | 2nd–5th | 3 | 4 |
| Rizvi 2020 | USA | FA | P3 | NSCLC | Stage4 | D20 + T1 q4w | 1st | 371 | 5 |
| Rubinstein 2019 | USA | CA | P2 RCT | Endometrial carcinoma, carcinosarcoma | Persistent or recurrent | D1500 + T75 q4wx4 > D1500 q4w | 2nd | 28 | 4 |
| Santa-Maria 2018 | USA | FA | Single arm (pilot) | Breast cancer | Metastatic | D1500 + T45 q4w x4 > D750 q2w 1 year | 2nd or later | 18 | 3 |
| Sarfaty 2021 | USA | FA | Single arm P2 | Non-urothelial carcinoma of the urinary tract | Unresectable or metastatic | D1500 + T75 q4w x4 > D1500 q4w | 1st–3rd | 13 | 4 |
| Seiwert 2016 | USA | CA | P3 | Head and neck cancer | Recurrent or metastatic | D1500 + T75 q4w > D1500 q4w | 1st | 408 | 4 |
| Siu 2019 | Canada | FA | P2 RCT | HNSCC | Recurrent or metastatic | D20 + T1 q4w x4 | After 1 platinum regimen | 133 | 4 |
| Somaiah 2020 | USA | CA | Single arm P2 | Sarcoma | Advanced or metastatic | D1500 + T75 q4w x4 > D1500 q4w | 1st or later | 57 | 4 |
| Song 2021 China Cohort | USA | CA | P2 RCT | HCC | Advanced | D20 + T1 q4w x4 | After sorafenib | 5 | 4 |
| Song 2021 T1 | USA | CA | P2 RCT | HCC | Advanced | D20 + T1 q4w x4 | After sorafenib | 40 | 4 |
| Song 2021 T300 | USA | CA | P2 RCT | HCC | Advanced | D1500 q4w + T300 x1 | After sorafenib | 74 | 4 |
| Song 2021 T75 | USA | CA | P2 RCT | HCC | Advanced | D1500 q4w + T75 q4w x4 | After sorafenib | 82 | 4 |
Adj, adjuvant therapy; CA, conference abstract; D10, durvalumab 10 mg/kg; D15, durvalumab 15 mg/kg; D1500, durvalumab 1500 mg/body; D20, durvalumab 20 mg/kg; FA, full article; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; 1L, first line; n, number of patients; NeoAdj, neo-adjuvant therapy; NOS, score of the Newcastle-Ottawa quality assessment scale for cohort studies wherein higher score means better quality; NS, not specified; NSCLC, non-small cell lung cancer; P1–4, phase 1–4; q2–6w, every 2–6 weeks; RCC, renal cell carcinoma; RCT, randomized controlled trial; T1, tremelimumab 1 mg/kg; T3, tremelimumab 3 mg/kg; T300, tremelimumab 300 mg/body; T45, tremelimumab 45 mg/body; T75, tremelimumab 75 mg/body; x1–26, administrated total 1–26 times; >, then.
Table 1 lists the target diseases identified in each study. The most frequently examined diseases were non-small cell lung cancer (n = 5), head and neck squamous cell cancer (n = 3), and hepatocellular carcinoma (n = 2). In addition, the present study included cancers such as small cell lung cancer and breast, colorectal, prostate, urinary tract, and rare cancers.
Approximately half (n = 16) of the included studies involved DT therapy as a second-line or later-line treatment. Three studies included only first-line therapy. In addition, three studies included adjuvant and neoadjuvant therapies, and one study failed to describe these therapies.
Based on the New-Ottawa Quality assessment scale, the median article quality was 4 (range 3–5). The analyzed population included 3099 patients (Table 1); five articles included multiple populations each, whereas others extracted one population each. Eventually, 41 independent populations were analyzed. The median population size was 34 patients (range 3–408).
Key AE indicators
In a random model meta-analysis with 19 populations and 1788 cases, the pooled frequency of all AEs was 77.8% [95% confidence interval (CI): 67.9–87.6, I2 = 97%, p for heterogeneity < 0.00001, Figure 2(a)]. Considering 21 populations (n = 1855) in which the presence or absence of grade ⩾ 3 AEs was recorded, 29.3 cases experienced grade ⩾ 3 AEs (95% CI: 24.2–34.4, I2 = 82%, p for heterogeneity < 0.00001, Figure 2(b)). The pooled frequency of serious AEs was 34.9% [24 populations, 95% CI: 28.1–41.7, I2 = 93%, p for heterogeneity < 0.00001, Figure 2(c)]. AE-related DT discontinuation occurred in 13.3% of patients [22 populations, 95% CI: 9.3–17.4, I2 = 84%, p for heterogeneity < 0.00001, Figure 2(d)]. Treatment-related deaths were documented in 0.98% of patients [28 populations, 95% CI: 0.5–1.5, I2 = 0%, p for heterogeneity = 1.00, Figure 2(e)].
Figure 2.
Forest plots to compare chemo-naive and pretreated for key adverse event indicators. (a) Any adverse event, (b) Grade 3 or higher adverse event, (c) Serious adverse event, (d) DT discontinuation due to adverse event and (e) Treatment-related deaths.
Specific AEs
The most frequently observed AE was fatigue (30.1%, 95% CI: 23.8–36.3). AEs with a frequency exceeding 15% included diarrhea (21.7%, 95% CI: 17.8–25.6), pruritus (17.9%, 95% CI: 14.4–21.3), decreased appetite (17.7%, 95% CI: 13.7–22.0), and nausea (15.6%, 95%CI: 12.1–19.6) (Table 2).
Table 2.
Estimated incidence of adverse events.
| Adverse event | N | n | Incidence (95% CI) |
|---|---|---|---|
| Key adverse event indicators | |||
| Any AE | 19 | 1788 | 77.8 (67.9–87.6) |
| Grade 3 or higher AE | 21 | 1865 | 29.3 (24.2–34.4) |
| Serious AE | 24 | 2536 | 34.9 (28.1–41.7) |
| AE leading to discontinuation | 22 | 1977 | 13.3 (9.3–17.4) |
| Treatment-related death | 28 | 2605 | 0.98 (0.45–1.5) |
| Gastrointestinal | |||
| Aspartate aminotransferase | 18 | 1332 | 8.3 (5.5–11.2) |
| Alanine aminotransferase | 18 | 1560 | 10.6 (6.8–14.4) |
| Amylase | 18 | 1349 | 7.0 (4.1–9.9) |
| Lipase | 20 | 1569 | 7.0 (4.3–9.7) |
| Diarrhea | 30 | 2720 | 21.7 (17.8–25.6) |
| Colitis | 18 | 1677 | 3.9 (2.1–5.7) |
| Decreased appetite | 20 | 2354 | 17.9 (13.7–22.0) |
| Nausea | 25 | 2383 | 15.9 (12.1–19.6) |
| Vomiting | 21 | 2118 | 10.8 (7.8–14.0) |
| Dermatological | |||
| Rash | 27 | 2357 | 14.8 (11.4–18.3) |
| Maculopapular rash | 9 | 326 | 9.9 (3.8–16.1) |
| Vitiligo | 4 | 201 | 0.5 (0–2.9) |
| Pruritus | 29 | 2669 | 17.9 (14.4–21.3) |
| Hormonal | |||
| Hypothyroidism | 22 | 1965 | 9.6 (7.6–11.6) |
| Hyperthyroidism | 14 | 1319 | 4.3 (2.9–5.7) |
| Adrenal insufficiency | 14 | 1510 | 0.7 (0.06–1.3) |
| Hypopituitarism | 7 | 1122 | 0.3 (0.2–0.8) |
| Other adverse events | |||
| Fatigue | 30 | 2740 | 30.1 (23.8–36.3) |
| Pyrexia | 18 | 1708 | 12.1 (9.1–15.2) |
| Headache | 14 | 916 | 5.7 (3.4–8.0) |
| Arthralgia | 18 | 1177 | 7.2 (3.7–11.0) |
| Pneumonitis | 23 | 1666 | 2.3 (1.5–3.2) |
Incidence (95% CI), pooled incidence using random model meta-analysis and its 95% confidence interval.
AE, adverse event; NA, not available; N, number of populations; n, number of patients.
The clinically important AEs included interstitial pneumonia, colitis, hyperthyroidism, hypothyroidism, and adrenal insufficiency. The pooled frequencies of these AEs were 2.3% for interstitial pneumonia (23 populations, 95% CI: 1.5–3.2), 3.9% for colitis (18 populations, 95% CI: 2.1–5.7), 4.3% for hyperthyroidism (14 populations, 95% CI: 2.9–5.7), 9.6% for hypothyroidism (22 populations, 95% CI: 7.6–11.6), and 0.67% for adrenal insufficiency (14 populations, 95% CI: 0.06–1.3).
Safety comparison of chemotherapy-naive and previously treated patients
A subgroup analysis of key AE indicators was conducted to compare the chemotherapy-naive and previously treated populations. There were no differences between subgroups for any AE (chemotherapy-naive 79.4% versus pretreated 70.8%, I2 = 0%, p = 0.39), grade ⩾ 3 AEs (21.4% versus 27.4%, I2 = 30.9%, p = 0.23), serious AEs (23.7% versus 34.8%, I2 = 54.4%, p = 0.14), treatment discontinuation due to AEs (13.8% versus 7.1%, I2 = 54.7%, p = 0.14), or treatment-related deaths (1.1% versus 0.6%, I2 = 0%, p = 0.38) (Figure 3).
Figure 3.
Forest plots to compare chemo-naive and pretreated for key adverse event indicators.
AE, adverse event; 95% CI, 95% confidence interval; IV, generic inverse variance.
Discussion
Based on the results of the present systematic review, more than three-quarters of patients who received DT experienced AEs, and approximately 30% of patients experienced grade ⩾ 3 AEs. Furthermore, AE-related treatment discontinuation was estimated to occur in 13% of patients, whereas treatment-related deaths occurred in less than 1% of patients. It is well-established that AEs are inevitable during cancer treatment, and combined therapy with two ICIs enhances toxicity.7–9 Therefore, we believe that our data provide information necessary for healthcare providers and patients to balance the benefits and risks of DT therapy.
To date, three systematic reviews on DT have been published. In 2020, Wang et al. 6 reported the first meta-analysis on DT combination therapy. The authors analyzed data from 587 patients extracted from five trials and found that double immunotherapy was superior to tremelimumab alone in head and neck squamous cell carcinoma. In addition, the authors reported that there was no difference in efficacy between double immunotherapy and monotherapy in pancreatic ductal adenocarcinoma and gastric/gastroesophageal junction cancer. The study also found no differences in treatment-related AEs between the two groups. In addition, a systematic review by Arru et al. 16 in 2021 found that dual immunotherapy was superior to monotherapy in certain tumor subsets, although it failed to exhibit a consistent advantage over single-agent durvalumab. In 2022, Fahmy et al. 17 published a study analyzing AEs, concluding that combination therapy resulted in greater treatment discontinuation and treatment-related deaths than durvalumab monotherapy.
All three systematic reviews included studies on regimens combining immunotherapy with cytotoxicity; therefore, it remains unclear whether the observed AEs could be solely attributed to immunotherapy. Furthermore, two of these studies focused on treatment efficacy and did not provide detailed data on AEs; therefore, data on AEs in DT-only regimens are required to establish the risks and benefits of DT therapy.
Standard dosing regimens and the optimal number of previous treatments for durvalumab and tremelimumab are yet to be established. Therefore, one of our main concerns was whether the safety profile was altered on administering the drug to patients who had never received chemotherapy when compared with those who had undergone prior therapy. The present systematic review did not reveal differences in the incidence of all AEs, grade ⩾ 3 AEs, serious AE, AEs leading to discontinuation, and treatment-related deaths between previously treated and untreated patients. Based on the findings of the present study, the DT regimen could be employed even in late-line treatment with the same safety profile as observed in first-line treatment.
One limitation of the present study was the inclusion of diverse tumor subtypes, therapeutic drug doses, dosing schedules, and lines of treatment. However, this may extend the external validity of the results.
In conclusion, this comprehensive systematic review summarized the AEs associated with DT therapy in ICI-naïve patients and incorporated 3099 cases from 41 populations. The data revealed the occurrence of AEs (77.8%), grade ⩾ 3 AEs (29.3%), serious AEs (34.9%), AEs resulting in treatment discontinuation (13.3%), treatment-related deaths (0.98%), documenting the occurrence of 22 specific AEs. Furthermore, no statistically significant differences in the safety profile were observed between chemotherapy-naive and chemotherapy-pretreated patients.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231198453 for Adverse events induced by durvalumab and tremelimumab combination regimens: a systematic review and meta-analysis by Hiromi Matsumoto, Kohei Somekawa, Nobuyuki Horita, Suguru Ueda, Megumi Kaneko, Ayami Kaneko, Nobuhiko Fukuda, Ami Izawa, Chisato Kamimaki, Katsushi Tanaka, Kota Murohashi, Hiroaki Fuji, Yoichi Tagami, Ayako Aoki, Keisuke Watanabe, Yu Hara, Nobuaki Kobayashi and Takeshi Kaneko in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
ORCID iDs: Hiromi Matsumoto  https://orcid.org/0000-0002-4819-1454
https://orcid.org/0000-0002-4819-1454
Nobuyuki Horita  https://orcid.org/0000-0002-8200-0340
https://orcid.org/0000-0002-8200-0340
Nobuhiko Fukuda  https://orcid.org/0000-0002-8498-2915
https://orcid.org/0000-0002-8498-2915
Nobuaki Kobayashi  https://orcid.org/0000-0002-7064-320X
https://orcid.org/0000-0002-7064-320X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hiromi Matsumoto, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Kohei Somekawa, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Nobuyuki Horita, Chemotherapy Center, Yokohama City University Hospital, 3-9 Fukuura, Kanazawa-ku, Yokohama 236-0004, Japan.
Suguru Ueda, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Megumi Kaneko, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Ayami Kaneko, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Nobuhiko Fukuda, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Ami Izawa, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Chisato Kamimaki, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Katsushi Tanaka, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Kota Murohashi, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Hiroaki Fuji, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Yoichi Tagami, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Ayako Aoki, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Keisuke Watanabe, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Yu Hara, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Nobuaki Kobayashi, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Takeshi Kaneko, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Hiromi Matsumoto: Data curation; Formal analysis; Investigation; Project administration; Visualization; Writing – original draft.
Kohei Somekawa: Formal analysis; Investigation.
Nobuyuki Horita: Conceptualization; Investigation; Supervision; Writing – review & editing.
Suguru Ueda: Writing – review & editing.
Megumi Kaneko: Writing – review & editing.
Ayami Kaneko: Writing – review & editing.
Nobuhiko Fukuda: Writing – review & editing.
Ami Izawa: Writing – review & editing.
Chisato Kamimaki: Writing – review & editing.
Katsushi Tanaka: Writing – review & editing.
Kota Murohashi: Writing – review & editing.
Hiroaki Fuji: Writing – review & editing.
Yoichi Tagami: Writing – review & editing.
Ayako Aoki: Writing – review & editing.
Keisuke Watanabe: Writing – review & editing.
Yu Hara: Writing – review & editing.
Nobuaki Kobayashi: Writing – review & editing.
Takeshi Kaneko: Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol 2022; 41: 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang RR, Jalil J, Economou JS, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res 2011; 17: 4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang H, Dai Z, Wu W, et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res 2021; 40: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab versus standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020; 6: 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019; 5: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu T, Jin B, Chen J, et al. Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: a network meta-analysis. Ther Adv Med Oncol 2020; 12: 175883592094092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou S, Khanal S, Zhang H. Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther Clin Risk Manag 2019; 15: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almutairi AR, McBride A, Slack M, et al. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol 2020; 10: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J, Li S, Yao Q, et al. The efficacy and safety of combined immune checkpoint inhibitors (nivolumab plus ipilimumab): a systematic review and meta-analysis. World J Surg Oncol 2020; 18: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Somekawa K, Horita N, Kaneko A, et al. Adverse events induced by nivolumab and ipilimumab combination regimens. Ther Adv Med Oncol 2022; 14: 175883592110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 13. UMIN Centor. UMIN crinical trial registry (UMIN-CTR), https://www.umin.ac.jp/ctr/ctr_regist.htm (accessed 24 November 2022).
- 14. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses, https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2000, accessed 24 November 2022).
- 15. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Statist 1998; 52: 119–126. [Google Scholar]
- 16. Arru C, De Miglio MR, Cossu A, et al. Durvalumab plus tremelimumab in solid tumors: a systematic review. Adv Ther 2021; 38: 3674–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fahmy O, Ahmed OAA, Khairul-Asri MG, et al. Adverse events and tolerability of combined durvalumab and tremelimumab versus durvalumab alone in solid cancers: a systematic review and meta-analysis. Biomedicines 2022; 10: 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231198453 for Adverse events induced by durvalumab and tremelimumab combination regimens: a systematic review and meta-analysis by Hiromi Matsumoto, Kohei Somekawa, Nobuyuki Horita, Suguru Ueda, Megumi Kaneko, Ayami Kaneko, Nobuhiko Fukuda, Ami Izawa, Chisato Kamimaki, Katsushi Tanaka, Kota Murohashi, Hiroaki Fuji, Yoichi Tagami, Ayako Aoki, Keisuke Watanabe, Yu Hara, Nobuaki Kobayashi and Takeshi Kaneko in Therapeutic Advances in Medical Oncology