Abstract
Objective
To identify the effects of metformin and kisspeptin structural polymorphism on the risk of polycystic ovary syndrome (PCOS) in Iraqi women.
Methods
Samples were collected at the family planning center of Al-Hassan Teaching Hospital (infertility clinic), Iraq. Hormonal and hematological parameters were measured. Kisspeptin structural polymorphisms were analyzed by polymerase chain reaction using a conventional thermal cycler and Phyre2 predictions. Kisspeptin concentrations were assessed by an enzyme-linked immunosorbent assay.
Results
Follicle-stimulating hormone (FSH) was the only sex hormone that changed in women with PCOS after metformin treatment. FSH concentrations were significantly increased after therapy compared with before therapy (9.39 ± 2.1 vs 5.13 ± 1.53 IU/L). We found that a single nucleotide polymorphism substituting G to C was related to PCOS. The kisspeptin structural polymorphism showed that the C allele was related to low FSH concentrations after treatment (6.92 ± 2.2 IU/L to 5.34 ± 1.58 IU/L). Kisspeptin concentrations were significantly lower after metformin treatment than before metformin treatment (395.44 ± 67.83 vs 273.18 ± 42.98 ng/mL).
Conclusion
A variation in the KISS1 gene or its protein structure may be involved in the development of PCOS. The response to metformin may be used as an indicator and could contribute to the early diagnosis and medical therapy of PCOS.
Keywords: Polycystic ovary syndrome, kisspeptin, polymorphism, metformin, follicle-stimulating hormone, KISS1 gene
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder that may be caused by a mixture of genetic, environmental, and intrauterine factors. There may be prenatal risk factors for the manifestation of PCOS characteristics in post-pubertal life. Overweight women can have female offspring with hirsutism and high androgen concentrations, while normal weight women can have offspring with high luteinizing hormone (LH) and normal androgen concentrations.1–4 PCOS can be due to hereditary causes. However, women with PCOS have two main genetic changes that affect how androgen and insulin are produced, as well as a higher incidence of several gene polymorphisms. 3
A neuropeptide called kisspeptin is the product of the KISS1 gene, which was first identified and discovered as a metastasis suppressor gene and added a new dimension to understanding the physiology of ovulation, reproduction, and fertility.5–7 Kisspeptin increases the release of gonadotropin-releasing hormone (GnRH) and is essential for the secretion and surge of LH and follicle-stimulating hormone (FSH) during the ovulation process. During an increase in estrogen levels and an LH surge, this negative feedback may change to a positive response. 8 Therefore, kisspeptin is a crucial component in triggering the LH surge and ovulation. Additionally, kisspeptin expression increases immediately before ovulation and the LH surge. 9 Kisspeptin neurons express estrogen receptor.10–13 Although the expression of ovarian kisspeptin in many animals and in humans has been confirmed, the precise mechanism by which kisspeptin is involved in ovulation is still unknown. PCOS is a neuroendocrine condition characterized by altered metabolism, hyperandrogenism, and ovulatory dysfunction. Because patients with PCOS have high LH and GnRH concentrations, kisspeptin concentrations may also be high. Numerous studies have examined kisspeptin function in the pathophysiology of PCOS and showed that patients with PCOS have higher kisspeptin concentrations than healthy women.14–18
Similar to most of the women worldwide, Iraqi women of reproductive age are also affected by PCOS. However, the etiology and indicators of PCOS are unclear. The strong relationship between PCOS and kisspeptin suggests that identifying kisspeptin concentrations and the mutations and polymorphisms in the kisspeptin gene including KISS1 is important. Genetic variations in the KISS1 gene are likely to be associated with PCOS in Iraqi women. Therefore, this study aimed to identify the genetic changes in single nucleotide polymorphisms (SNPs) (rs4889) and their relationships with physio-biochemical characteristics before and after metformin treatment. We also investigated the possible three-dimensional structural variations between allelic kisspeptins by using internet-based bioinformatics tools, and they are discussed with regard to the etiology of PCOS.
Materials and methods
Patients
This prospective cohort study was performed in the Karbala Governorate from January 2021 to March 2022. Samples were collected at Al-Hassan Teaching Hospital's family planning infertility clinic. The ethics committee of the College of Medicine at the University of Karbala approved (2123-5-2023) the study protocol. Written informed consent was obtained from each patient. All of the patients’ details were de-identified in this project. The reporting of this study conforms to the STROBE guidelines. 19
Infertile patients who were identified as having PCOS were diagnosed according to the Rotterdam Consensus Meeting on PCOS in 2003. Women with PCOS took oral treatment of metformin for 3 months. Women who had a regular menstrual cycle and were in good health were in the control group. They had a clinical evaluation to ensure that they were free from any symptoms of PCOS and had no history of infertility.
Measurement of parameters
To measure hormonal and hematological parameters, 5 mL of blood were drawn from each patient twice throughout the same menstrual cycle. Thyroid–stimulating hormone, LH, FSH, prolactin, testosterone, and progesterone were considered hormonal factors, and triglycerides (TGs), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), fasting blood sugar (FBS), glycated hemoglobin (HbA1c), and insulin were considered as hematological parameters. All of the parameters were measured by the Mini VIDS Cobas system (Siemens, Munich, Germany). Kisspeptin concentrations were assessed by an enzyme-linked immunosorbent assay.
Polymerase chain reaction analysis
A specific primer pair was designed for the amplification of the KISS1 gene using the Primer-Blast tool (Integrated DNA Technologies Inc., Coralville, Iowa, USA). The primers were created using the https://www.ncbi.nlm.nih.gov/ database. The sequences of the forward and reverse primers used to amplify the KISS1 gene of interest are shown in Table 1. Using a specific set of forward and reverse primers, polymerase chain reaction (PCR) was used to amplify the target gene (Macrogen Corp., Seoul, Korea). The total volume of the PCR reactions was 25 µL, which consisted of 3 µL of DNA, 1.5 µL of forward primer, 1.5 µL of reverse primer, and 12.5 µL of master mix (GoTaq® G2 Green Master Mix; Promega, Madison, Wisconsin, USA), Finally, the components of the reaction were added to PCR tubes, and under sterile conditions, the volume was increased to 25 µL by using deionized water. The following heat cycling profile was used for DNA amplification while using a conventional PCR thermal cycler (Biobase Co., Jinan, Shandong, China). An initial denaturation step was performed at 94°C for 5 minutes, which was then followed by 25 cycles of amplification consisting of the following: denaturation at 94°C for 30 s, annealing at 64°C for 30 s, and extension at 72°C for 46 s. A final extension at 72°C for 5 minutes was performed.
Table 1.
Nucleotide sequences of primers used for KISS1 gene amplification.
| Primers | Sequence | Length (bp) | Melting temperature (°C) | Product size |
|---|---|---|---|---|
| Forward primer | 5′AGTTCCAGTTGTAGTTCGGCAG3′ | 22 | 57.06 | 300 bp |
| Reverse primer | 5′CATCTTTCTGTGCCCTCTGTCCTA3′ | 24 | 58.95 |
A 300-bp amplicon was amplified using forward and reverse primers containing 22 and 24 bases, respectively.
bp, base pairs.
SNPs
To determine the sequence of the bases for the KISS1 gene polymorphisms, all PCR-purified products in the patient and control groups were sent to Macrogen Corporation in Korea. The sequences were read using the SnapGene Viewer (3.5) program (Dotmatics Scientific R&D Platform, Boston, MA, USA). The nucleotide sequences were then compared with the Genbank databases of the National Center for Biotechnology Information website (https://www.ncbi.nlm.nih.gov/) using the BLAST search engine, and SNPs were searched for. The tertiary structures of KISS1 gene polymorphisms were predicted by the Phyre2 internet-based online server.
Statistical analysis
The data were analyzed using PAST (Ver. 3) software (https://past.en.lo4d.com/windowsLO4D.com). Mean ± standard deviation values were compared between the groups by the independent t test. Values were considered significant at P ≤ 0.05. All of the data were examined in Excel (Microsoft Corporation India Ltd., Gurugram, India). The values of PCOS variables that were linearly related to other factors were found using correlation analysis.
Results
One hundred patients were included in the study before metformin treatment. The patients’ age ranged from 20 to 40 years (mean ± standard deviation: 27.64 ± 3.90 years). We evaluated the body mass index (BMI) in patients with PCOS. We found slight differences in BMI regarding the use of metformin treatment in female patients before and after treatment, but there were no significant differences between these times. A small number of women had a response to metformin treatment (Table 2). We also observed that most women with PCOS were overweight. A total of 54% of women with PCOS were overweight with a BMI value of ≥30 kg/m2.
Table 2.
BMI values in patients with polycystic ovary syndrome before and after metformin treatment.
| BMI (kg/m2) | Before metformin treatment |
After metformin treatment |
||
|---|---|---|---|---|
| Number (%) | Mean ± SD | Number (%) | Mean ± SD | |
| <18.5 | 0 | 0 | 0 | 0 |
| 18.5–24.9 | 13 (13) | 22.21 ± 1.8 | 11 (12.2) | 22.6 ± 1.8 |
| 25–29.9 | 33 (33) | 27.7 ± 1.5 | 29 (32.2) | 27.7 ± 1.4 |
| 30+ | 54 (54) | 33.7 ± 2.8 | 50 (55.5) | 33.8 ± 3. |
| Total | 100 | – | 90 | – |
| Statistical analysis | X2 = 0.05, P = 0.97, DF = 2 | |||
BMI, body mass index; SD, standard deviation; DF, degrees of freedom.
We also assessed some phenotypic features, such as hirsutism, alopecia, menstrual cycle regulation, and acne, in patients with PCOS. We found significant differences in these features with metformin treatment in patients with PCOS as follows (Table 3). We found a significantly lower number of women suffering from hirsutism and alopecia after metformin treatment than before metformin treatment (both P < 0.05) However, there were no significant differences in other factors, including a regular menstrual cycle and acne, between before and after metformin treatment.
Table 3.
Features associated with polycystic ovary syndrome before and after metformin treatment.
| Parameters | Before metformin treatment (n = 100) | After metformin treatment (n = 90) | P value |
|---|---|---|---|
| n (%) | n (%) | ||
| Hirsutism | |||
| Yes | 91 (91) | 66 (73) | X2 = 10.3P = 0.001 |
| No | 9 (9) | 24 (26) | |
| Alopecia | |||
| Yes | 84 (84) | 63 (70) | X2 = 25.3P = 0.02 |
| No | 16 | 27 | |
| Menstrual cycle regulation | |||
| Yes | 0 | 15 | X2 = 18.09P = 0.001 |
| No | 100 | 75 | |
| Acne | |||
| Yes | 64 | 48 | X2 = 2.22P = 0.13 |
| No | 36 | 42 |
We evaluated six hormones (i.e., LH, FSH, thyroid–stimulating hormone, prolactin, testosterone, and progesterone) in patients with PCOS. There were no significant differences in these hormones in women with PCOS between before and after 3 months of metformin treatment, except for FSH (Table 4). The mean FSH concentration was significantly higher after metformin treatment than before metformin treatment (P < 0.05). The mean kisspeptin concentration was significantly lower after metformin treatment than before metformin treatment (P = 0.011).
Table 4.
Hormonal concentrations in patients with polycystic ovary syndrome before and after metformin treatment.
| Hormones | Before metformin treatment (n = 100) | After metformin treatment (n = 90) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | P value | |
| LH (ng/mL) | 8.99 ± 2.9 | 9.39 ± 2.1 | 0.605 |
| FSH (IU/L) | 5.13 ± 1.53 | 9.39 ± 2.1 | 0.049 |
| TSH (µIU/mL) | 2.36 ± 0.23 | 2.36 ± 0.85 | 0.98 |
| Prolactin (ng/mL) | 26.29 ± 5.7 | 25.67 ± 6.22 | 0.77 |
| Testosterone (ng/mL) | 0.63 ± 0.15 | 0.71 ± 0.21 | 0.15 |
| Progesterone (ng/mL) | 0.62 ± 0.1 | 0.8 ± 0.13 | 0.51 |
| Kisspeptin (ng/L) | 395.44 ± 67.83 | 273.18 ± 42.98 | 0.011 |
SD, standard deviation; LH, luteinizing hormone; FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
We also assessed hematological parameters (i.e., TGs, FBS, LDL, HDL, TC, HbA1c, and insulin) in patients with PCOS before and after metformin treatment. The mean HbA1c concentration was significantly lower after metformin treatment than before metformin treatment (P = 0.04, Table 5). There were no significant differences in the rest of the other hematological parameters. There were no significant differences in hematological or lipid profiles between women with PCOS and healthy women.
Table 5.
Hematological parameters in women with polycystic ovary syndrome before and after metformin treatment.
| Hematological parameters | Before metformin treatment (n = 100) | After metformin treatment (n = 90) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | P value | |
| TGs (mmol/L) | 3.7 ± 0.064 | 0.09 ± 0.072 | 0.73 |
| FBS (mmol/L) | 2.56 ± 0.069 | 2.52 ± 0.011 | 0.38 |
| LDL (mmol/L) | 2.6 ± 0.035 | 2.22 ± 0.019 | 0.18 |
| HDL (mmol/L) | 1.22 ± 0.032 | 1.18 ± 0.011 | 0.22 |
| TC (mmol/L) | 6.85 ± 0.046 | 4.2 ± 0.027 | 0.50 |
| HbA1c (mg/L) | 48 ± 0.41 | 41 ± 0.68 | 0.04 |
| Insulin (mmol/L) | 1.411 ± 0.0086 | 1.406 ± 0.0034 | 0.96 |
SD, standard deviation; TGs, triglycerides; FBS, fasting blood sugar; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TC, total cholesterol; HbA1c, hemoglobin A1c.
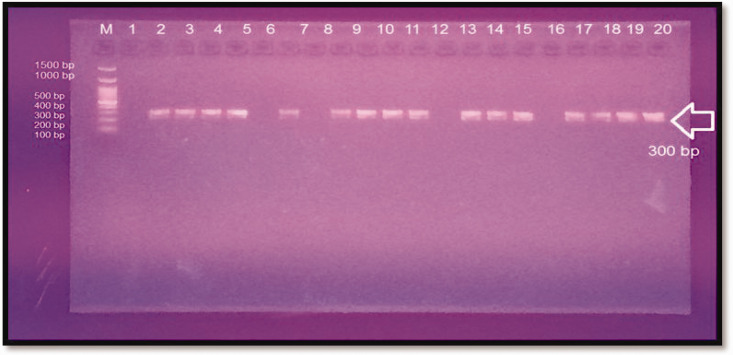
We then investigated the genetic variation in different patients with PCOS. The amplification result of the KISS1 gene showed an amplicon of 300 base pairs as observed by electrophoresis on a 2% agarose gel (Figure. 1).
Figure 1.
Electrophotography of the KISS1 gene (rs4889). Polymerase chain reaction product was loaded into each well on a 2% agarose gel (70 V, 120 minutes, and 5 μL in each well). Lanes 1 to 20: polymerase chain reaction product (300-base pair amplicon). Lanes 1, 6, 8, 12, and 16 are negative results, and lane M is the DNA ladder (100–1500 base pairs).
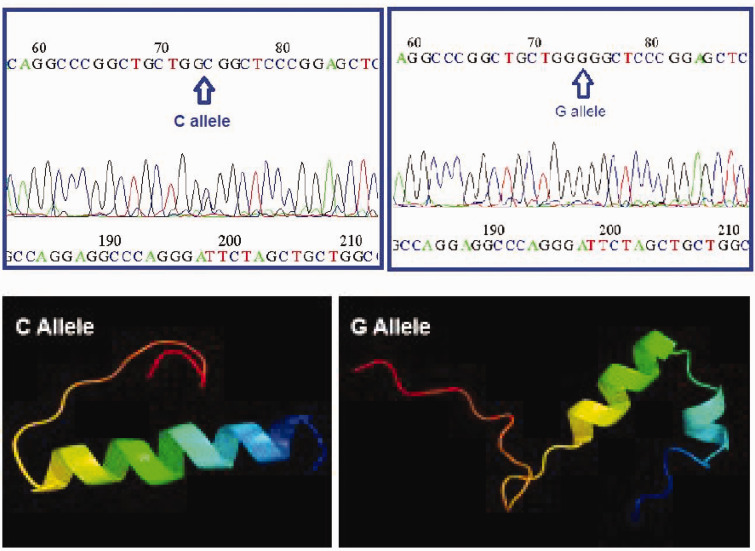
Most (75%) of the patients with PCOS had the G allele, which was a wild genotype, while 25% of the patients had the C allele, which was a mutated genotype with substitution of a proline residue to an arginine residue. The amino acid sequences of these two polymorphic kisspeptin coding regions were predicted and aligned by BLAST and CLASTALW similarity and alignment search tools, respectively (data not shown). The related three-dimensional structures were also predicted by the Phyre2 protein structure prediction online server. The three-dimensional structural patterns varied among C and G alleles related to kisspeptins, with substitution of the helix-turn-helix to a continuous helical structure (Figure 2). Because proline is responsible for the formation of bending structures, the replacement of a proline residue with an arginine residue in amino acid sequences might cause three-dimensional polymorphic structural variation among G and C alleles of kisspeptin.
Figure 2.
Sequence reading of KISS1 gene polymorphism and three-dimensional protein structure prediction. The upper right panel shows a G allele of the wild type in a patient with PCOS. The left panel shows a C allele of the mutated type in a patient with PCOS. The lower right panel shows the three-dimensional structure of the G allele of KISS1 with a continuous helical structure. The lower left panel shows the three-dimensional structure of the C allele of KISS1 with a helix-turn-helix structure.
The correlation between six different hormones and the allelic frequency of kisspeptin polymorphism is shown in Table 6. There were no significant differences in these hormones in relation to kisspeptin polymorphism before and after treatment with metformin, except for FSH. Blood FSH concentrations were significantly lower in women with PCOS carrying the C allele after metformin treatment than before metformin treatment (P = 0.03, Table 6). Kisspeptin concentrations were significantly lower in women with PCOS carrying the C allele after metformin treatment than before metformin treatment (P = 0.041).
Table 6.
Sex hormone and kisspeptin concentrations in relation to the allelic frequency of kisspeptin polymorphism before and after metformin treatment.
| Hormones | Alleles of kisspeptin polymorphism | Before metformin treatment | Before metformin treatment | P value |
|---|---|---|---|---|
| FSH (IU/L) | C | 6.92 ± 1.2 | 5.34 ± 1.28 | 0.03 |
| G | 5.8 ± 1.92 | 5.6 ± 1.22 | 0.47 | |
| LH (ng/mL) | C | 7.77 ± 2.6 | 7.58 ± 2.2 | 0.91 |
| G | 9.24 ± 2.95 | 9.75 ± 2.09 | 0.53 | |
| TSH (µIU/mL) | C | 2.13 ± 0.51 | 2.11 ± 0.53 | 0.95 |
| G | 2.39 ± 0.57 | 2.4 ± 0.65 | 0.92 | |
| Prolactin (ng/mL) | C | 9.83 ± 2.04 | 12.28 ± 2.95 | 0.42 |
| G | 17.17 ± 4.25 | 11.96 ± 2.83 | 0.56 | |
| Testosterone (ng/mL) | C | 0.54 ± 0.18 | 0.70 ± 0.10 | 0.16 |
| G | 0.65 ± 0.16 | 0.71 ± 0.20 | 0.3 | |
| Progesterone (ng/mL) | C | 0.17 ± 0.01 | 0.17 ± 0.08 | 0.16 |
| G | 1.32 ± 0.28 | 0.90 ± 0.12 | 0.52 | |
| Kisspeptin (ng/L) | C | 360.03 ± 58.02 | 244.82 ± 20.09 | 0.041 |
| G | 390.85 ± 77.46 | 301.45 ± 65.87 | 0.078 |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; TSH, thyroid-stimulating hormone.
Discussion
Hyperandrogenism, persistent anovulation, polycystic ovaries, insulin resistance, and overweight are the hallmarks of PCOS, which is a prevalent reproductive endocrine illness. 20 In this study, there was no significant difference in the BMI of women with PCOS between before they had metformin treatment and after metformin treatment for 3 months. Possible reasons for this finding may be an insufficient treatment period, the amount of the drug dose, the polymorphic status of the patient, and the patient’s lack of commitment to treatment. This finding is consistent with a study by Donnelly et al. who suggested that the glycemic response to metformin is similar in patients without obesity and in those with obesity. 21 Therefore, an individual’s BMI does not appear to affect the choice of the oral agent. However, most of the women with PCOS in this study had a BMI of more than 30 kg/m2. Gaining weight and being obese are two of the most common factors that lead to the clinical and biochemical manifestation of PCOS in women who are genetically prone to developing this condition. Therefore, there is a strong association between being overweight and having PCOS. The majority (from 38% to 88%) of women who have PCOS are either overweight or obese. 22
We found that there were significant differences in the frequency of hirsutism and alopecia in women with PCOS who used metformin treatment. Our finding is consistent with that by Spritzer et al. who was found that androgen hormones were related to the development of hirsutism. 23 Hirsutism can occur if the level of these hormones rises or if the body develops a greater sensitivity to them. PCOS is the most prevalent cause of hirsutism. Hirsutism is a disorder that affects the ovaries, and some of the symptoms it can cause include acne and erratic menstrual cycles, hair follicles, dihydrotestosterone binding to the androgen receptor, and the hormone–receptor complex stimulating the genes responsible for the progressive transition of big terminal follicles into smaller follicles. This process is known as a telogen effluvium. 24
A total of 15% women start menstruation when using metformin treatment, and this is due to the regularity of their androgen hormones. 25 When follicles in the ovary are too small to reach maturity, the follicle does not break and progesterone levels decrease. Menstruation often begins when progesterone levels decline after being elevated after ovulation. In most cases, menstruation will cease if ovulation does not occur. This explains why periods are so erratic or nonexistent in women with PCOS. 25
This study showed that there were no significant differences in the sex hormones that were measured in women with PCOS, except for FSH. FSH concentrations were significantly increased after metformin treatment. An imbalance in the release of LH and FSH occurs when GnRH secretion is disrupted. 26 Increased LH secretion stimulates estrogen production from the ovary, which through positive feedback, leads to the mid-cycle LH surge that causes ovulation. 23 Normally, the ratio of LH to FSH is between 1 and 2 in healthy women. This ratio is skewed in the opposite direction in women with PCOS, and may reach as high as 2 or 3. 27 This study is not consistent with that by Cho et al. who found that the median LH/FSH ratio was not significantly different between the PCOS group and the control group (1.6 versus 1.2, P = 0.14). 28 They found that, compared with 15.6% of samples from normally menstruating participants, only 7.6% of samples from patients with PCOS showed an LH/FSH ratio >3. Our study suggested that a pattern shift in FSH concentrations may be a suitable indicator for PCOS.
Although our study was a prospective cohort study during 3 months, there were no significant differences in hematological or lipid profiles between women with PCOS and healthy women. The reason for the absence of any significant differences may be due to the absence of a difference in the BMI between these two groups of women.
Agarose gel electrophoresis showed that there was a clear PCR product (300 base pairs) that could be used to identify genetic polymorphism of the KISS1 gene. This study showed that there were no significant differences in hormone concentrations in women with PCOS when they were sorted according to the allelic frequency of the KISS1 gene, except for FSH hormone. We found that the presence of the C allele was associated with significantly lower FSH concentrations in women with PCOS after metformin treatment. Kisspeptin controls the hypothalamic secretion of GnRH, and the pituitary gland secretes FSH when stimulated by GnRH. 29 Therefore, the low concentration of FSH patients with PCOS and the C allele may be due to low kisspeptin concentrations or its malfunction. Baranavan et al. suggested a correlation between high serum kisspeptin concentrations and PCOS. 30 Our kisspeptin assay results showed that its concentration was decreased in patients with PCOS when using metformin. This is an interesting result, which indicates that metformin may substitute the role of kisspeptin.
The response to GnRH in women with PCOS is comparable to that in ovulatory controls, suggesting that the C allele is related to low FSH concentrations or those within the lower follicular range in PCOS. 31 LH concentrations do not change in PCOS. The LH secretion pattern may shift for a number of reasons, including altered sex steroid production, metabolic malfunction, and obesity. 32
Based on the predicted three-dimensional structure, allelic polymorphism may affect the structure and function of kisspeptin in PCOS. This possibility needs to be studied in the future. In addition to the kisspeptin expression level, conformational changes in kisspeptin may also affect the etiology of PCOS.
There are some limitations to this study. These limitations include the difficulty in taking blood samples, as well as the difficulty of adhering to metformin treatment.
Conclusion and recommendation
The present findings suggest that a change in the KISS1 gene/protein structure plays a role in the onset of PCOS. Some physio-biochemical characteristics (especially including FSH and kisspeptin concentrations) in women with PCOS are changed after metformin treatment, and they might be useful as indicators of PCOS. These indicators may contribute to an early diagnosis and medical therapy in patients with PCOS. More large-scale, functional research is recommended to confirm the associations between kisspeptin expression levels, KISS1 gene and protein structural variants, and the clinical properties of PCOS.
Acknowledgements
The authors thank the University of Tabriz in Iran and Al-Hassan Teaching Hospital's family planning infertility clinic in Iraq for their assistance and supporting the present research work.
Footnotes
Author contributions: A.H. Kadhem prepared the data and wrote the manuscript. A. Gholizadeh supervised the study and revised the manuscript. M. Khalaj K was an advisor.
The authors declare that there are no conflicts of interest.
Funding: This research was financially supported by the Iraq Ministry of Science and Technology and the University of Tabriz, Iran.
ORCID iD: Ashraf Gholizadeh https://orcid.org/0000-0002-8730-6170
References
- 1.Yen SS. The polycystic ovary syndrome. Clin Endocrinol (Oxf) 1980; 12: 177–183. [DOI] [PubMed] [Google Scholar]
- 2.Mackenna TJ. Pathogenesis and treatment of polycystic ovary syndrome. N Engl J Med 1988; 318: 558–562. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Alexandraki K, Protogerou A, et al. Metformin administration improves endothelial function in women with polycystic ovary syndrome. Eur J Endocrinol 2005; 152: 749–756. [DOI] [PubMed] [Google Scholar]
- 4.Katsikis I, Karkanaki A, Misichronis G, et al. Phenotypic expression, body mass index and insulin resistance in relation to LH levels in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2011; 156: 181–185. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Miele ME, Hicks DJ, et al. Kiss1, a novel human malignant melanoma metastasis suppressor gene. J natl Cancer Inst 1996; 88: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 6.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. The N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 7.Hameed S, Jayasena CN, Dhillo WS. Kisspeptin and fertility. J Endocrinol 2010; 208: 97–105. [DOI] [PubMed] [Google Scholar]
- 8.Nejad SZ, Tehrani FR, Zadeh-Vakili A. The role of kisspeptin in female reproduction. Int J Endocrinol Metab 2017; 15: e44337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevisan CM, Montagna E, De Oliveira R, et al. Kisspeptin/GPR54 system: what do we know about its role in human reproduction? Cell Physiol Biochem 2018; 49: 1259–1276. [DOI] [PubMed] [Google Scholar]
- 10.Haung X, Harlen RE. Absence of androgen receptor in LHRH immunoreactive neuron. Brain Res 1993; 624: 309–311. [DOI] [PubMed] [Google Scholar]
- 11.Smith LA, Bukanov NO, Husson H, et al. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 2006; 17: 2821–2831. [DOI] [PubMed] [Google Scholar]
- 12.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 2006; 131: 623–630. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson J, D'Anglemont de Tassigny X, Moreno AS, et al. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008; 28: 8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon Y, Lee KE, Jung JA, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest 2013; 75: 268–274. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Mo Y, Li L, et al. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2010; 149: 72–76. [DOI] [PubMed] [Google Scholar]
- 16.Nyagolova PV, Mitkov MD, Orbetzova MM, et al. Kisspeptin and galanin-like peptide (galp) levels in women with polycystic ovary syndrome. Int J Pharmaceut Med Res 2016; 4: 6–12. [Google Scholar]
- 17.Yarmolinskaya MI, Ganbarli NF, Tkachenko NN, et al. Kisspeptin and polycystic ovary syndrome-is there any connection? J Obstet Women's Dis 2017; 66: 73–80. [Google Scholar]
- 18.Gorkem U, Togrul C, Arslan E, et al. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol 2018; 34: 157–160. [DOI] [PubMed] [Google Scholar]
- 19.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y, Lv J, Xu P, et al. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J Cell Biochem 2018; 119: 3913–3921. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly LA, Doney ASF, Hattersley AT, et al. The effect of obesity on glycaemic response to metformin or sulphonylureas in type 2 diabetes. Diabet Med 2006; 23: 128–133. [DOI] [PubMed] [Google Scholar]
- 22.Barber TM, Hanson P, Weickert MO, et al. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health 2019; 13: 1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spritzer PM, Marchesan LB, Santos BR, et al. Hirsutism, Normal Androgens and Diagnosis of PCOS. Diagnostics 2022; 12: 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torabi P, Behrangi E, Goodarzi A, et al. A systematic review of the effect of platelet‐rich plasma on androgenetic alopecia of women. Dermatol Ther 2020; 33: e13835. [DOI] [PubMed] [Google Scholar]
- 25.Jiao J, Sagnelli M, Shi B, et al. Genetic and epigenetic characteristics in ovarian tissues from polycystic ovary syndrome patients with irregular menstruation resemble those of ovarian cancer. BMC Endocr Disord 2019; 19: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadia Z. Follicle stimulating hormone (LH: FSH) ratio in polycystic ovary syndrome (PCOS)-obese vs. non-obese women. Med Arch 2020; 74: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malini NA, George KR. Evaluation of different ranges of LH: FSH ratios in polycystic ovarian syndrome (PCOS)–clinical based case control study. Gen Comp Endocrinol 2018; 260: 51–57. [DOI] [PubMed] [Google Scholar]
- 28.Cho LW, Jayagopal V, Kilpatrick ES, et al. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann Clinical Biochem 2006; 43: 217–219. [DOI] [PubMed] [Google Scholar]
- 29.Ruohonen ST, Poutanen M, Tena-Sempere M. Role of kisspeptins in the control of the hypothalamic-pituitary-ovarian axis: old dogmas and new challenges. Fertil Steril 2020; 114: 465–474. [DOI] [PubMed] [Google Scholar]
- 30.Branavan U, Muneeswaran K, Wijesundera WSS, et al. Association of Kiss1 and GPR54 gene polymorphisms with polycystic ovary syndrome among Sri Lankan women. Biomed Res Int 2019; 2019: 6235680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farsimadan M, Moammadzadeh GF, Takamoli S, et al. Association analysis of KISS1 polymorphisms and haplotypes with polycystic ovary syndrome. Br J Biomed Sci 2021; 78: 201–205. [DOI] [PubMed] [Google Scholar]
- 32.Daghestani MH, Daghestani MH, Daghistani M, et al. Influence of KISS1 gene polymorphisms on the risk of polycystic ovary syndrome and its associated variables, in Saudi women. BMC Endocr Disord 2020; 20: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]