Abstract
Introduction
The Florida-California Cancer Research, Education, and Engagement (CaRE2) Health Equity Center is a triad partnership committed to increasing institutional capacity for cancer disparity research, the diversity of the cancer workforce, and community empowerment. This article provides an overview of the structure, process innovations, and initial outcomes from the first 4 years of the CaRE2 triad partnership.
Methods
CaRE2 serves diverse populations in Florida and California using a “molecule to the community and back” model. We prioritize research on the complex intersection of biological, environmental, and social determinants health, working together with scientific and health disparities communities, sharing expertise across institutions, bidirectional training, and community outreach. Partnership progress and outcomes were assessed using mixed methods and four Program Steering Committee meetings.
Results
Research capacity was increased through development of a Living Repository of 81 cancer model systems from minority patients for novel cancer drug development. CaRE2 funded 15 scientific projects resulting in 38 publications. Workforce diversity entailed supporting 94 cancer trainees (92 URM) and 34 ESIs (32 URM) who coauthored 313 CaRE2-related publications and received 48 grants. Community empowerment was promoted via outreaching to more than 3000 individuals, training 145 community cancer advocates (including 28 Community Scientist Advocates), and publishing 10 community reports. CaRE2 members and trainees together have published 639 articles, received 61 grants, and 57 awards.
Conclusion
The CaRE2 partnership has achieved its initial aims. Infrastructure for translational cancer research was expanded at one partner institution, and cancer disparities research was expanded at the two cancer centers.
Keywords: lung cancer, outcomes, pancreatic cancer, prostate cancer, translation
Significance Statement
We aspire to eliminate cancer health disparities among Blacks and Latinos living in California and Florida, and contribute to paving the way to eliminate disparities in these populations across the US.
Introduction
Black or African American (B/AA) and Hispanic/Latino (H/L) individuals now comprise nearly 33% of the United States (U.S.) population, with a projected minority majority shift by 2045. 1 Unfortunately, significant disparities in cancer incidence, outcomes, research, and biomedical workforce representation exist within these underserved populations. 2 Despite these disparities, there is limited understanding of the complex intersection of biological, environmental, and social determinants of cancer health disparities, considering the genetic makeup, cultural diversity, and various ancestral origins across B/AA and H/L populations. Furthermore, another disparity persists relative to the dearth of highly trained underrepresented minority (URM) cancer researchers, particularly B/AA and H/L scientists.2,3 A well-trained and diverse workforce that leverages multiple perspectives to address the needs of underserved patient populations is vital to reducing these disparities.2-4 Finally, there is often limited engagement and involvement of minority populations and communities in cancer research. To address these needs, we established the Florida-California Cancer Research, Education, and Engagement (CaRE2) Health Equity Center, which was funded as one of the 16 Comprehensive Partnerships to Advance Cancer Health Equity (CPACHE) in September 2018. The purpose of this article is to disseminate to the cancer community key details about our partnership’s structure, process innovations, and initial outcomes of implementing the innovations over the first 4.5 years of the CaRE2 triad partnership.
Materials and Methods
Partners’ Main Scientific Focus
The CaRE2 minority cancer research and training triad partnership includes the Florida A&M University (FAMU), an institution serving underserved health disparity populations and underrepresented students (ISUPS); University of Florida (UF) Health Cancer Center (UFHCC); and University of Southern California Norris Comprehensive Cancer Center (USC NCCC). This triad partnership uses a “molecule to the community and back” approach, in partnership with the scientific and health disparities communities, sharing expertise and engaging in bidirectional training, education, and outreach. Engaging and serving the diverse populations of B/AA and H/L individuals in both Florida and California, the CaRE2 Center facilitates the study of cancer health disparities in the two U.S. states with the highest overall new cancer cases and deaths, 5 and institutions affiliated with incredibly heterogeneous catchment areas. The CaRE2 partnership combines advanced expertise in and resources for genomics and translational research, cancer research training and education, innovative community initiatives, and process and outcome evaluation to reduce disparities and improve outcomes for patients with cancer. The main scientific focus of the partnership is translational cancer disparities research in cancers of high burden and understudied in B/AA and H/L individuals, including pancreatic, prostate, and lung cancers. All research studies with human subjects or animals were approved by the FAMU, UFHCC, and USC NCCC Institutional Review Boards (Lung: HS-19-00642, 11/14/2019, HS-18-01049; Prostate: HS-21-00281, 3/25/2022; Pancreas pilot 1: IRB202200160, 3/22/2022; TMC Smart IRB: HS-21-00281, 8/10/2021; COC: IRB202000180, 5/8/2023, IRB202201133, 6/30/2023) or the FAMU Institutional Animal Care and Use Committee (IACUC: 022-07, October 6, 2022). Uniquely capturing the heterogeneity across these underserved groups and communities, the novel CaRE 2 Center’s work centers on four specific aims and nine benchmarks for success (Table 1).
Table 1.
CaRE2 Overall Specific Aims and Benchmarks for Success (2018-2023).
| Specific Aims | Focus |
|---|---|
| Aim 1 | Coalesce expertise, infrastructure, and shared resources for interdisciplinary and multi-institutional innovative translational research focused on understanding the biological basis of cancer disparities in B/AA and H/L populations capturing their broad heterogeneity |
| Aim 2 | Provide a continuum of cancer health disparities research training for URM trainees and early-stage investigators (ESIs) that fosters their individual career development toward conducting translational cancer research focused on the cancer disparities among B/AA and H/L populations |
| Aim 3 | Leverage existing community partnerships and infrastructure to: (i) Educate B/AA and H/L individuals about cancer prevention and control; and (ii) promote participation in biomedical research |
| Aim 4 | Enhance a systematic planning and evaluation plan to improve center effectiveness |
Key. URM: underrepresented minority; B/AA: Black/African American; H/L: Hispanic/Latino; URM: underrepresented minority.
Organizational Structure and Management Overview
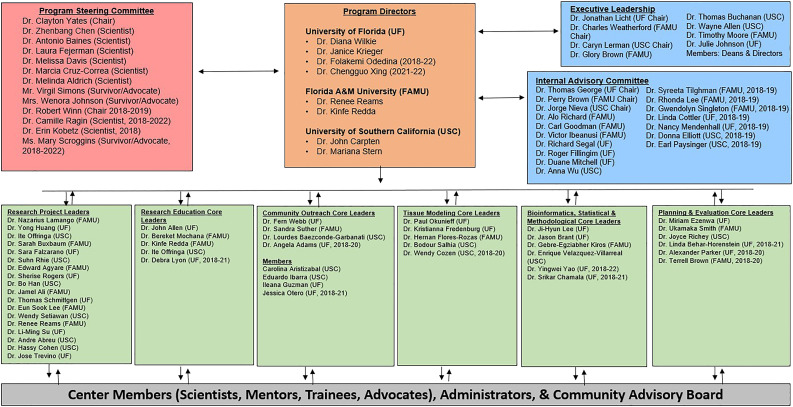
The CaRE2 partnership is strengthened by an investigative team with significant experience in cancer disparities research, translational sciences, research training, and community outreach. A team of six Multiple Principal Investigators (MPIs), two from each partner institution direct and administer the CaRE2 partnership (Figure 1). The MPIs form a leadership team of multidisciplinary scientists who are national leaders in cancer health disparities and are supported by an Executive Leadership Committee (ELC), an Internal Advisory Committee (IAC), and a Program Steering Committee (PSC). Top administrators at FAMU, UFHCC, and USC NCCC are committed to providing the Center with a strong foundation. Leadership and oversight for the CaRE2 Center is structured to maximize strong academic and community partnerships, effective communications among all groups, compliance with all policies and procedures, scientific excellence, training scholarship, research education, mentoring, community-centered outreach, effective monitoring, and continuous quality improvement (CQI). In addition, the leadership team collaborates with three CaRE2 administrators, one at each institution, who help to administer and coordinate networking activities at each institution.
Figure 1.
CaRE2 Health Equity Center: Structure and Initial Outcomes.
The MPIs established an innovative approach for sharing leadership responsibilities. The primary administrative responsibilities rotate on a 4-month schedule among the partners: UF leads Sept–Dec, USC leads Jan–April, and FAMU leads May–Aug. During the leadership trimester, the lead partner assumes responsibility for official Center-related communication with the NCI Program Officer, PSC, IAC, and ELC as well as organizing the meetings and functions occurring during the trimester (e.g., partnership meetings, PSC meeting, annual report). This delineation of responsibility minimizes the chance that key administrative tasks could be missed and threatens the success of the partnership.
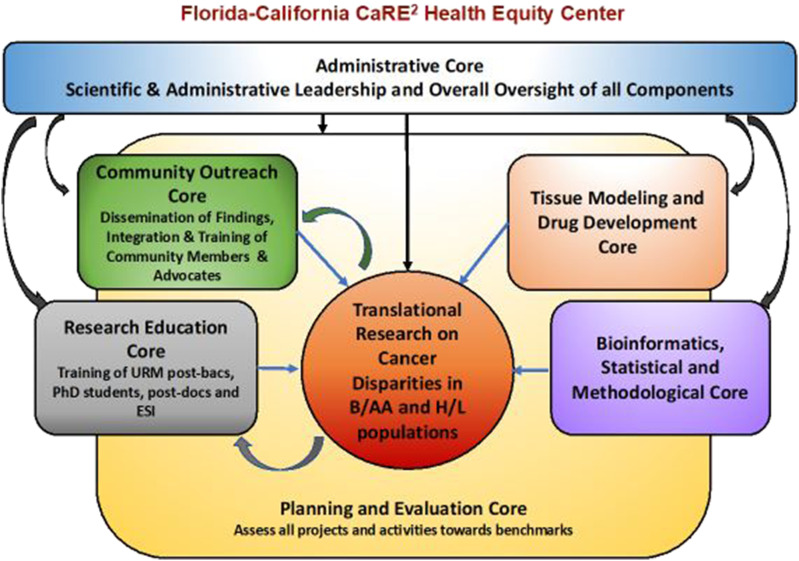
Figure 1 also displays the organizational structure of the Center’s four cores, two shared resources, and six research projects. A highly innovative feature of the CaRE2 organization structure is that each component includes principal investigators from each of the three partnering institutions, including all cores, shared resources, and projects, each with equal responsibility and voice. The CaRE2 partnership cores are highly integrated into the research platform to support the overall center mission in a synergistic manner (Figure 2). At the center of our partnership are highly collaborative and innovative translational cancer research projects supported by our cores and shared resources, whose overall structure is summarized in the following section.
Figure 2.
Interaction across the CaRE2 center components.
Description of Cores, Shared Resources, and Research Projects
The Administrative Core (AC) provides scientific leadership, centralized governance, and oversight; integrated coordination and management of center activities and monitoring for CQI. The AC meets twice per month and develops a strategy for effective management of communication across MPIs, with rotating responsibilities by center partners to ensure equity across the partnership. The MPIs facilitates and ensures oversight of all cores and projects through an MPI liaison system. The MPI liaison attends the core and project team meetings to support and promote an integrated center perspective. The MPIs are nationally and internationally recognized leaders and experts in cancer disparities research and training. The team’s diversity in race/ethnicity and gender has been shown to be a remarkable asset in the effective leadership of the Center to achieve its goals. The AC is primarily responsible for the Center’s strategic planning, including defining and upholding its mission and objectives, conducting environmental scans for strategy development, directing and managing strategy for implementation, and ensuring improvements are made based on program evaluation. The AC also coordinates and manages all relationships essential to the success of the Center, and maintains a web-based information-sharing, tracking, and monitoring system to facilitate communication across the Center.
The Research Education Core (REC) manages the training and educational efforts of the CaRE2 Center. The REC provides research education, academic career development, mentorship, and tailored research training opportunities to increase the competitive research capacity for URM trainees and ESIs in cancer health disparities. The REC focuses on (1) exposing URM undergraduate students at FAMU to translational cancer disparities research (Summer-CaRE2), 6 (2) training URM post-baccalaureate students across the partnership (CaRE2-Postbac), (3) and providing mentorship, and tailored cancer disparities research education opportunities that will increase the competitive research capacity of graduate students, postdoctoral fellows, and ESIs (CaRE2-Grad+) at all three partner universities. The REC implements a tracking system of the continued impact of the Center on career progression and growth of the next generation of URM translational cancer research scientists.
The Community Outreach Core (COC) leads community engagement and outreach events and training opportunities that promote the Center’s mission and goals while increasing community members’ knowledge, awareness, and engagement with cancer research and treatment in alignment with COC Strategic Plan. Specifically, the COC contributes to the Center’s plans to engage in bi-directional dialog with community members about the need for tissue/blood collection for biomedical research and participation in research, including clinical trials. With a tri-institutional Community Advisory Board, COC also designs, implements, and disseminates educational activities aimed to address disparities in pancreatic, lung, and prostate cancers for diverse populations of H/L and B/AA in our catchment areas, using innovative materials and approaches tailored to these communities. COC develops strategies for tracking the long-term impact of outreach activities in our communities.
The Planning and Evaluation Core (PEC) ensures the achievement of the Center’s overall goals and specific aims and for its cores and research projects. The main goal of the PEC is to implement a systematic planning and assessment plan for the CaRE2 Center that guides program improvement and documents accountability and effectiveness. PEC ensures that partnership meetings are effectively convened with information on action items captured for follow-up. The PEC establishes and facilitates the process for the selection and funding of new pilot studies during the current grant cycle and oversees the process for the selection of new research projects for the renewal proposal. PEC also provides formative evaluation and written feedback for CQI based on biannual core and project reports and the PSC annual report. PEC makes recommendations to AC for changes and plans for implementation over the years. PEC plans key partnership activities and events. PEC guides the CaRE2 Center toward increasing the pool of well-trained URM scientists who can conduct independent cancer disparity research efforts on the disproportionate burden of cancer. 7
The two Shared Resources Cores (SRC) collectively coordinate resources that support the core research mission of the CaRE2 Center. First, the Tissue Modeling & Drug Development Core (TMDDC) provides a virtual repository to access biospecimens from ethnically diverse individuals through a Center-provided, single-access point. These biospecimens are leveraged from existing resources and collaborations already available to the three institutions, including new sample collection when needed. Second, the Bioinformatics, Statistical, and Methodological Core (BSMC) provides bioinformatics and biostatistical support with expertise in bioinformatics including DNA, RNA, epigenomics, protein, microbiome, metabolomics, and correlation to human outcomes databases. This advanced bioinformatics and biostatistical expertise are being transferred to FAMU via the research projects and through their educational offerings. Our Shared Resources Core Leaders possess a unique combination of synergistic skills and experiences including highly technical pathological and research procedures, clinical pathology and therapeutics, bioinformatics, and “omics” technologies.
The Research Projects include two full research projects and one pilot research project funded in 2018 and three additional pilot research projects funded in 2021. Additionally, we supported other projects (supplements or developmental research program projects that were funded with institutional leverage funding). Two of the Administrative Supplements focused on COVID-19 research and outreach activities to improve our understanding of and to reduce the impact of the pandemic among B/AA and H/L communities. An innovative feature of the CaRE2 organizational structure is that we foster team science by requiring each full and pilot project to include a PI from each institution who has responsibility for specific aspects of the project specific aims. An important expectation of this team science model is that publications and future grants emanating from the project will include all three investigators. This organization model is specifically designed to reduce silos in translational cancer research to promote optimal sharing and translation of knowledge, skills, and technology to rapidly move cancer health disparities research forward.
Full Project 1 (funded 2018–2021) “Disparities in mitochondrial peptidomics and transcriptomics in prostate cancer.” Led by Drs. Reams (FAMU), Cohen (USC), and Su (UF), the central hypothesis of this project was that the expression of mitochondrial peptides and transcripts predicts prostate cancer (PCA) risk in a racial/ethnic-dependent manner. 8 Over the last few years, we have discovered a novel class of mitochondrial derived peptides (MDPs) that are transcribed from small open reading frames within the mtDNA, specifically the 16S rRNA and 12S rRNA genes in the mtDNA. These include humanin, SHLP-2, and MOTS-c, which are cytoprotective signaling peptides found in circulation and have both extracellular and intracellular activities. Decreased humanin and SHLP2 levels have been implicated in diseases of aging; we recently found that levels of these two MDPs are lower in Black than White individuals and thus may be involved in the racial differences in PCA risk and progression of Black men. The project goal was to test the potential of plasma MDPs and their prostate expression levels as biomarkers for PCA risk, both as pre-diagnostic biomarkers and as surrogate prognostic risk indicators in a group of White, Black, and Latino men with and without PCA. Also, we compared the prostate mito-transcriptome from men of various ethnicities for divergent expression of MDP transcripts and their relation to cancer severity and ethnic origin. Finally, we examined the contribution of mitochondrial DNA copy number in the circulation and/or in benign and malignant prostate tissues to PCA risk and severity.
Full Project 2 (funded 2018–2021) “Enhancing Efficacy of Gemcitabine (Gem) Nanoparticles in Pancreatic PDX Models.” Led by Drs. Agyare (FAMU), Trevino (UF), and Han (USC), the central hypothesis of this study was that molecular genomic and physiological features of PADC are different between B/AA, H/L, and NHW patients, 9 but could be overcome by the development of improved formulations of standard of care chemotherapies. 10 In this project, we sought to investigate the use of molecular profiles to predict the sensitivity of PADC to Gem, and improve on the delivery and stability of Gem. We proposed to expand on the health inequity in PADC and establish better efficacy for existing therapeutics. The rationale for our project was based on historical data where molecular profiling of lung, prostate, and breast cancer in Black patients has defined more effective therapeutic regimens. We hypothesized that genetic differences between tumors underlie the biological basis of differences in disease presentation and treatment response in PADC patients, and GemEnps represents a novel therapeutic alternative that can significantly inhibit PADC growth.
Pilot Project 1 (funded 2018–2021) “Contributions of racial disparity towards the early development of pancreatic cancer.” Led by Drs. Schmittgen (UF), Ali (FAMU), and Setiawan (USC), the central hypothesis of this study was that B/AA undergo acinar ductal metaplasia (ADM) to a greater degree than NHW and that genetic as well as epigenetic factors account for this disparity. 9 ADM is the earliest known precursor lesion for PDAC, making acinar to ductal transdifferentiation a key phase in the initiation of pancreatic cancer. Studying the early events in the development of human PDAC is challenging since the disease is often diagnosed at a stage often when the tumor has metastasized. Primary mouse pancreatic acini embedded into matrigel or collagen/TGFα will transdifferentiate into ductal epithelial cells with the accompanying changes in morphology and gene expression. Using this experimental system, the degree of ADM may be evaluated qualitatively (microscopic observation) as well as quantitatively (duct counts and gene expression). We hypothesize that Black individuals undergo ADM to a greater degree than White individuals and that genetic as well as epigenetic factors account for this disparity. Proposed in this project was a novel study to determine molecular and genetic factors that contribute to the early events in the development of PDAC by culturing and studying the ability of primary human pancreatic acini to undergo ADM in vitro.
Pilot Project 2 (funded 2021–2023) “Characterizing DNA Methylation Signatures in Prostate Tumors to Understand Health Disparities among Black and White Men.” Led by Drs. Rhie (USC), Buxbaum (FAMU), and Falzarano (UF), this ongoing project aims to measure global DNA methylation in PCA specimens from 100 B/AA men and 100 age-matched NHW men to identify differentially methylated CpG between groups. The study also aims to integrate additional multi-omic datasets to characterize gene regulation patterns in PCA from B/AA and NHW, including the use of publicly available datasets (i.e., TCGA) and other internal CaRE2 datasets. Successful completion of the proposed aims will identify key epigenetic alterations and molecular mechanisms impacting prostate health disparity. This finding would greatly assist researchers in developing novel biomarkers and therapeutic treatments.
Pilot Project 3 (funded 2021–2023) “An Organoid System Tailored to Studying Lung Cancer in Blacks.” Led by Drs. Lamango (FAMU), Huang (UF), and Offringa (USC), the ongoing study that aims to develop novel immortalized alveolar epithelium cell lines from B/AA individuals to determine the role of biology in lung cancer disparities. The investigative team will also develop novel isogenic lung adenocarcinoma (LUAD) cell lines with various driver mutations using CRISPR-Cas9 genome editing from specimens from B/AA subjects. The investigators are using novel 3D printing technologies to engineer an alveolar organoid for rapid drug screening and testing. These and other known LUAD cell lines are being used as models to determine the effectiveness of polyisoprenylated cysteinyl amide inhibitors (PCAIs), which target the commonly mutated RAS pathway in LUAD.
Pilot Project 4 (funded 2021–2023), “Target Pancreatic Cancer Mitochondrial Redox to Enhance Chemotherapy Efficacy in Blacks.” Led by Drs. Agyare (FAMU), Rogers (UF), and Han (USC), the foundational hypothesis for this ongoing study is that dense extracellular matrix (ECM) in the pancreatic cancer tumor microenvironment (TME), which could be more prevalent in B/AA, induces cancer cell metabolic adaptation and develops resistance to chemotherapy. The team proposes to characterize and identify correlations between dense ECM, GSTK genetic alterations, and drug sensitivity in B/AA and White pancreatic cancers using reagents developed in Full Project 2, including patient-derived models and whole exome sequencing data. They will also test how these physiological features modulate sensitivity to novel Gemcitabine analogs developed in Full Project 2 and new 5-FU analogs developed as part of this Pilot Project 4. The proposed studies will provide insights into mechanisms of treatment resistance in PDAC in different racial groups. Moreover, our observations will provide information to define the mechanistic correlation of intrinsic and acquired resistance that can be exploited therapeutically for effective therapeutics.
Innovations
The CaRE2 Center contributes to the CPACHE program by addressing gaps in knowledge and novel translational cancer disparities research in racially and ethnically diverse bicoastal populations of B/AA and H/L individuals in Florida and California. Our partnership provides a diverse academic platform to study and better understand cancer disparities in B/AA and H/L from coast to coast. Our multi-disciplinary program and aligned resources allow us to plan and conduct innovative research in cancer health disparities covering the spectrum from the molecular underpinnings of cancer health disparities to clinic-based and community-based interventions that will help improve outcomes among minority populations. In addition to the innovations previously described, four key innovative features of CaRE2 Center are:
1. Pioneering research focused on understudied heterogeneous and diverse Black populations such as American-born, African-born, and Caribbean-born Black individuals, as well as H/L diverse populations including Mexican, Central American, Caribbean, and US H/L populations of mixed ancestral origins, and individuals of mixed African and Caribbean/Latino ancestry. Examples include understanding the biological mechanisms of early pancreatic cancer development and developing and testing new drugs for pancreatic and lung cancer treatment using samples and models from minority individuals.
2. Novel translational research projects focusing on persistent and emerging disparities in prostate, lung, and pancreatic cancers, which utilize innovative approaches in omics and informatics, novel drug development, innovative diverse model systems (3D cultures, PDX models, 3D printed models), mitochondrial genetics, molecular epidemiology, and comprehensive genetic ancestry analysis.
3. Original training tools, structures, and approaches were validated and proven successful by early Year 5 in training URM scientists, trainees, and students in both Florida and California. This training model has led to cross-institutional collaborations, cross-training, and enhanced scholarship. Our early-pipeline and team-based focus provides an opportunity to facilitate URM scientists in launching successful cancer research careers.2,11 Through our U54 grant we were able to provide summer financial support for undergraduate trainees, year-long salary at the level of a predoctoral fellow for the postbac trainees, and research and travel support for graduate students, postdocs, and ESIs. Trainees were well-integrated into labs with productive scientists who were committed and generous in sharing with the trainees. As they launched their research and professional careers, ESIs worked closely with their CaRE2 mentoring committee to focus on productivity toward successful academic advancement. Additionally, we provided the trainee with tools for success, that is, mentorship from experts in their fields, funding opportunities, conferences, and access to NCI, AACR, CPACHE, and GMaP resources. These resources and opportunities allowed our trainees and ESIs to grow their research and publication skills and foster career development, which enhances and supports URMs to pursue additional education and advance their academic positions.
4. Novel, impactful, and culturally tailored community outreach approaches across B/AA and H/L communities in both Florida and California for: (1) bidirectional guidance of cancer disparities research, (2) development and best practices for dissemination of research results and cancer education, and (3) stimulation of bidirectional exchange of knowledge, build cancer advocacy capacity in the community, and determine the most appropriate approaches toward achieving health equity.
Results
Like other research teams around the world, the COVID-19 pandemic, which began approximately 6 months into Year 2 of our grant cycle, posed challenges and lead to innovative approaches. Restrictions imposed by the pandemic prevented face-to-face meetings, inter-institutional visits by trainees, and clinical activities such as collecting specimens for our partnership research projects. Restrictions also delayed laboratory-based translational research studies and prematurely ended important experiments with mandated euthanasia of animals as labs closed urgently. After the COVID-19 crisis period, the phenomenon of the great migration led to the transition of several key personnel to prestigious positions at other organizations. Although we were proud that our Center work facilitated our colleagues to obtain important career advancements, CaRE2 teams required reconfiguration and reformed relationships and work plans to achieve aims. Hiring into vacated positions required more time than usual as the bicoastal communities emerged from the pandemic and caused at times the workload to be unevenly distributed. Despite the hinderance to our work, the pandemic also challenged the teams to deploy technologies such as Zoom not only for collaboration but for offering training and community outreach. We learned that trainees and community members were able to engage in activities, socialize, and complete impressive projects via Zoom and other social media platforms. Despite these challenges, and as described in the results section shows, our partnership achieved our aims for 2018–2023.
Accomplishments: Scientific Projects and Cores
Scientific Projects
To date, our partnership has funded 15 translational cancer disparities research projects, which include 2 Full Projects, 4 Pilot Projects, 3 Administrative Supplements, 1 Diversity Supplement, and 5 Developmental Research Projects. Altogether, our Center has contributed to more than 639 publications, with 38 directly resulting from our funded projects, 313 from CaRE2 trainees and ESIs, and more than 288 from the CaRE2 members’ individual research programs that were relevant to cancer health disparities. CaRE2 members and trainees received 61 grants directly related to Center work. Table 1 shows a summary of the benchmark metrics.
Project 1
The study provided new discoveries of mitochondrial peptides overexpressed in PCA and in Black individuals. Major findings included the identification of novel Mitochondrial Derived Peptides, which are derived from ORFs that are differentially expressed in PCA from B/AA men and in response to androgens. The impact of these studies and discoveries is new knowledge for molecular determinants of PCA disparities. Four undergraduate students were trained as part of this research project, including students from FAMU who traveled to USC with Dr Reams for 2 weeks, and received a bioinformatics bootcamp and practical laboratory experiences in Drs. Cohen’s and Carpten’s laboratories. As a follow-up, Drs. Carpten, Salhia and Reams are collaborating on a new study using single-cell Multiome (RNA-seq and ATAC-seq) assays on frozen prostate specimens from B/AA and White patients.
Project 2
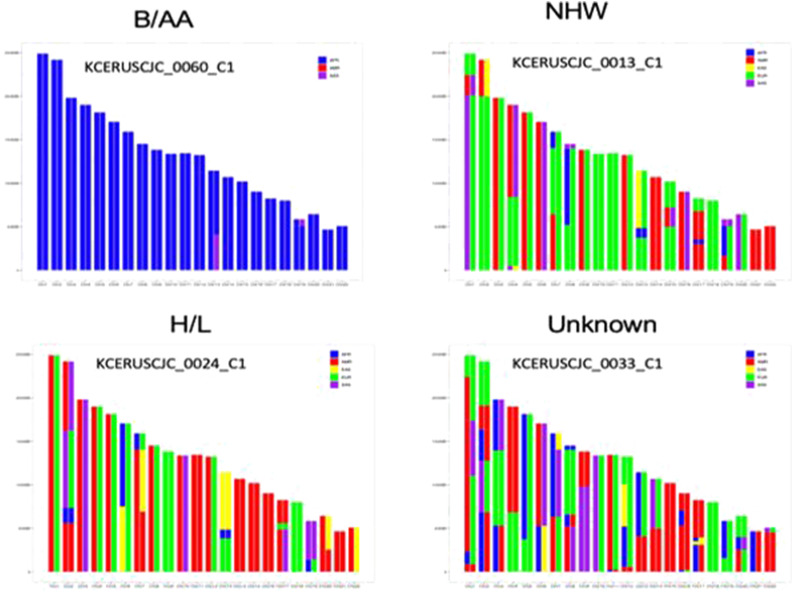
Whole exome sequencing of tumor and uninvolved tissue from a series of B/AA, H/L, and NHW PDAC patients has revealed local ancestry patterns across B/AA, H/L, and NHW patients (Figure 3) and somatic mutation frequencies for comparison with other datasets (i.e., TCGA). The study has also led to the development and synthesis of novel nanoparticle coated Gemcitabine analogs (GemEnps) by FAMU ESI Dr Agyare, which were tested on novel patient derived primary PDAC organoid cell models by Dr Han and xenografts (PDX) models derived from B/AA and NHW patients.12,13
Figure 3.
Self-identified race and ancestry of patients with pancreatic cancer.
Pilot Project 1
Using human pancreata of healthy donors obtained from pancreatic islet procurement centers, the team established the methodology to conduct sequencing of donor’s RNA from pancreatic cultures on matrigel using samples from B/AA, H/L, and NHW individuals. Using RNA sequencing they identified 10 transcriptional drivers of ADM and using genome-wide association data from the Multiethnic cohort study, they identified associations between Ptf1a and Rbpjl SNPs and pancreas cancer risk. Of significant impact is that two URM students, Alyssa Gosling (UF postbac) and Corey Perkins (UF graduate student) participated in this project, with one being a manuscript coauthor (Corey Perkins). 14 Finally, Dr Fredenburg is a URM ESI Pathologist at UF who obtained a diversity supplement related to this work with mentoring by Dr Schmittgen. The preliminary results of her study assessing the role of microRNAs in head and neck cancer disparities set the stage for her receiving a Department of Defense (DoD) Career Development Award in 2022. Impactfully, Dr Fredenburg mentored 3 CaRE2 postbacs who are coauthors on abstracts and manuscripts and received awards. Several publications emerged from this study that are published.14-18
Pilot Project 2
This team is collaborating with a DoD grant (Rhie, PI) to expand the assessment of epigenomic alterations in PCA, showing early synergy among this research team. Dr Falzarano, a pathologist at UF, has identified and reviewed the tissues and methylation assessment is underway with completion planned by mid-2023. Postbac and URM students involved in this project have presented at a national cancer conference. Several publications including research and review papers are in preparation.
Pilot Project 3
This team has successfully immortalized human alveolar epithelial cells and generated monoclonal cell lines; developed a gene editing strategy to replace KRAS G12 C in B/AA LUAD cell lines; designed a lung 3-D bioprinted model for drug testing; tested PCAIs and conducted functional analyses of these treated cells showing p-AKT levels are stimulated after treatment. Impactfully, several CaRE2 URM postbacs are working on this project in each of the three laboratories. All three postbacs submitted abstracts and attended national cancer conferences, and all three received Minority Scholar in Cancer Research travel awards. Several publications that include postbac trainees are in preparation.
Pilot Project 4
To date, the team has analyzed genomic data obtained from 20 B/AA and 20 H/L pancreatic cancer patients; established 8 pairs of 3D organoid models with defined ECM and mechanical properties to mimic stroma from B/AA and NHW patients; synthesized 6 chemo drug analogs; established toolbox for drug and drug combination test. Dr Rogers who is an URM ESI Medical Oncologist and received a career development award, provides critical translational and clinical insights about the research. Importantly, Dr Rogers trained a URM postbac, Guettchina Telisnor, who performed research and published a review manuscript on pancreatic cancer disparities. 19
Cores
Despite the challenges imposed by the COVID-19 pandemic, REC through its three programs has trained 128 trainees/ESIs (124 were URMs). Specifically, REC recruited and trained 35 Summer-CaRE2 students, surpassing the originally proposed 12. Thus far, of these 35 students, two were admitted to the CaRE2 postbac program, six were admitted to a PharmD program, and one was admitted to a PhD program. It has been more challenging than we anticipated to track the long-term outcomes of these Summer-CaRE2 students. The CaRE2 Postbac program has been incredibly successful and impactful with a total of 15 trained to date and seven currently being trained for a year at FAMU, UF, and USC. Thus far, one postbac has entered a PhD program, one enrolled in a PharmD program, four entered medical school, four entered masters programs, six are preparing applications to medical school, two are preparing applications to PhD programs, and one changed her area of focus to another field. We are following up to learn the plans of two postbacs who applied but were not yet accepted into medical school. To date, 21/22 postbacs have coauthored 19 unique abstracts and 17/22 postbacs have published 18 unique manuscripts. The Grad + -CaRE2 has also been very successful with three PhD students receiving T32 postdoc positions, one receiving a K99/R00 award, and one receiving a diversity supplement and then transitioning first to a cancer health disparities-specific postdoc position for additional training and then to a faculty position. Three ESIs transitioned to tenure track positions, four ESIs were each awarded a R01, and three ESIs received K awards. Altogether, the postbacs, Grad + trainees (29 PhD students, 8 postdocs, 34 ESIs) published 313 manuscripts to date and obtained 48 grants (e.g., R01, R13, R21, DOD, DOH, ACS, Pharma, and Air Force Office of Scientific Research). One ESI has a patent pending. REC has published one manuscript describing their Summer-CaRE2 approaches. 6
Following are a variety of comments from our trainees. One CaRE2 trainee reported that his mentor was “encouraging, resourceful, helpful and [showed] patience” while indicating that he would like “more community outreach days.” Another trainee commented about our health disparities training, “The graduate certificate was extremely vital to my experience as a post baccalaureate fellow. I think it coupled well with the research projects that I have been part of and will help in my matriculation as a medical student and clinician. One postbac stated “I want to thank the CaRE2 team for their help with my research, especially my mentor, Ite Offringa. Dr Offringa has been the most incredible mentor a student could ask for due to our shared enthusiasm for innovation. In addition, I want to thank my colleagues from the CaRE2 Health Equity Center for their unconditional support in my development as a scientist.” Another postbac stated “The CaRE2 program broadened my understanding regarding the importance of collaboration in cancer disparities research” and “I have grown in more ways than I can imagine in one year. I have improved as a writer, a collaborator, [and as] a leader from my experience in this program. I am also immensely grateful that I will be supported to attend AACR and present my projects.”
Guided by the Community Advisory Board’s feedback, the COC has trained 145 cancer advocates through the Cancer Advocacy and Community Scientist Research Advocate training programs, surpassing the original goal of 120. The Cancer Advocacy program was offered simultaneously and bilingually in Florida and California, with bilingual materials developed for H/L advocates. The flagship Community Scientist Research Advocate Program included 28 trainees, and its 2022 cohort was featured for its innovation in the Jacksonville Free Press. Among other highlights, COC:
• recruited 200 B/AA or H/L to a research contact registry facilitating research and clinical trial participation;
- • reached out to and educated
- o ∼ 750 people about prostate health care awareness, information on how to screen, treat, and live with prostate cancer through six ProTalk Facebook Live events and visiting more than 20 barbershops in person;
- o 225 individuals via virtual/in-person events about awareness, knowledge, and testing for PCA among B/AA men;
- o more than 3000 people through social media channels and our website,
• published and distributed 10 community reports, which are also available on our website (https://care2healthequitycenter.org/cancer-information-and-education-materials/),
• adapted a Toolkit and developed another, and
• presented at national conferences, with publications in preparation.
COC community members have commented about their experiences during several of the above activities:
Community Scientist Research Advocates commented, “Great program, I loved the diversity of backgrounds and education levels throughout the groups” “When I signed up for CaRE2 I wasn't quite sure what to expect, or if I would even get selected to participate, but now I am so grateful for the opportunity it presented my fellow group members, and myself to learn about how Community Advocacy works.” “I think the program is great and afforded me the opportunity to expand” Other community members stated that they signed up to be on the CaRE2 Contact Registry because “I wanted to be informed and educated on Cancer studies and how to best prevent or fight this disease.” “I like to stay informed and help others.” “My opinion matters.” “I want to help improve the health of my community, future generations, and self.” Finally, a community member who participated in the CaRE2 Connects with Our Community stated that it “Helped to continue connecting and highlighting the important and impactful work being done by CaRE2 researchers, physicians and administrators.”
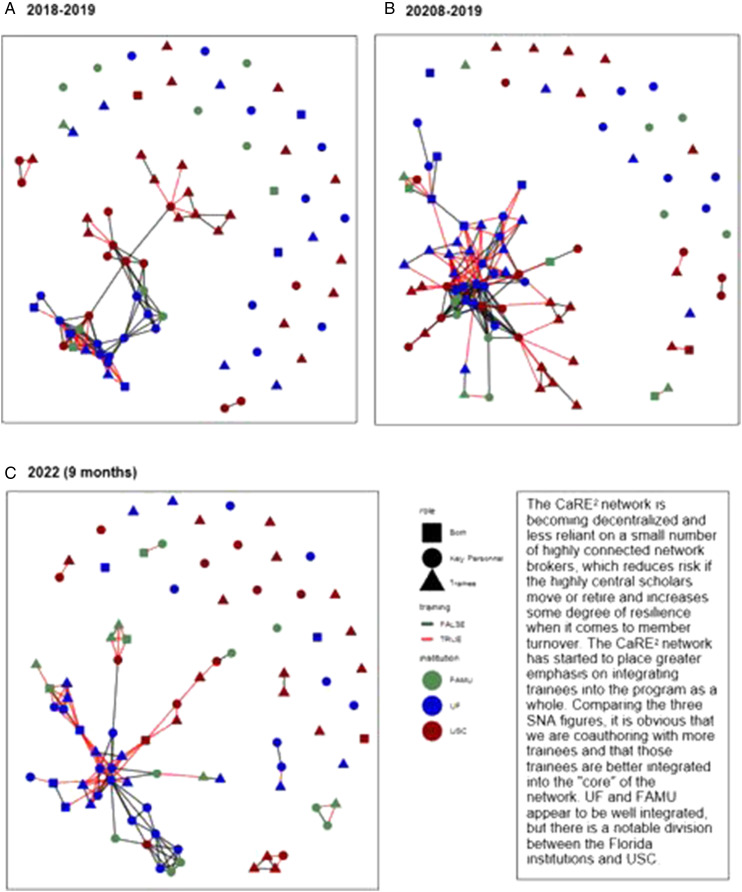
Guided by feedback the PEC obtained, we increased the number of partnership meetings from two to eight per year. With the National CPACHE Partnership Evaluators, 20 the PEC adopted standardized surveys for capturing partnership impact and effectiveness. Additionally, as an innovative evaluation approach, PEC conducted a social network analysis (SNA) in 2018 and again in 2022 to evaluate and characterize team science outcomes from a network science perspective. The SNA of publications authored by CaRE2 trainees, leaders, and investigators revealed that we had published more articles from 2018–2019 to 2020–2021 with similar trends so far for the first 9 months of 2022 (Figure 4). Center authors published with a larger number of new collaborators and less with their original collaborators, which would be expected as CaRE2 grows, and each individual expands their network within and between institutions from the work initiated through the partnership. We also progressed in cross-institutional scholarship, which tends to take a longer time to publish. Still these publications have been cited by other investigators at a faster rate than earlier publications. The findings indicated that we started off very strong with a tendency to collaborate within and between institutions at almost an equal rate. However, as CaRE2 has grown, there has been a greater tendency to collaborate within institutions, which likely reflects the growth in our group size. More extensive networks within institutions present more opportunities to collaborate. This information indicates that we should consider further incentives for cross-institutional publications, especially between Florida and California. Supplemental to the PEC evaluation process, the IAC’s and PSC’s feedback during quarterly and annual meetings, respectively, provided important opportunities to engage in CQI for strengthening the Center’s effectiveness. Therefore, evaluation was available from scientists across and external to the three institutions and the research projects/cores.
Figure 4.
CaRE2 coauthor network analysis (A) 2018-2019, (B) 20208-2019, (C) 2022 (9 months).
The TMDDC has been invaluable in providing access to biospecimens for partnership translational cancer disparities research projects. The TMDDC now has a regulatory-compliant access across the partnership to cancer cases. The cases include PDAC blocks (295 B/AA, 306 H/L), PDAC plasma/serum (136 B/AA, 53 H/L), PCA blocks (548 B/AA, 195 H/L), and PCA plasma/serum (595 B/AA, 92 H/L), and has facilitated the creation or identification of 4 new patient-derived xenografts (PDX) from B/AA patients with PDAC for studies at FAMU and USC.
The BSMC has been critical for CaRE2 research projects providing statistical and bioinformatics support to ensure rigor and reproducible scientific research, including analysis of whole exome sequencing and RNA sequencing data. BSMC developed a recorded Webinar Series with continuing education workshops on bioinformatics, statistics, and data science. The series sessions are available to the general community via our website. BSMC facilitates access to the UF supercomputing environment, HiPerGator, and USC Advanced Research Computing resources, for the data management and analysis of high-dimensional exome and RNA sequenced data.
Discussion
We have successfully managed the bicoastal distance and time difference between Florida and California with an effective model of face-to-face meetings during scientific national conferences and biweekly/monthly meetings via Zoom, which allowed us to communicate regularly and productively. We have fostered and facilitated innovative translational research that addresses cancer disparities from the molecular to the societal level and back. Our research has been conducted by a cadre of racially and ethnically diverse, well-trained URM scientists in partnership with consumer advocates (cancer survivors and advocates) and prioritized on cancers for which disparities have been documented or are understudied among B/AA and H/L populations. We have built novel and innovative research platforms and infrastructure at FAMU, the partnership ISUPS. The accomplishments related to these activities included the growth and expansion of a Living Biorepository with patient-derived cancer models from underrepresented patients for the development and testing of novel drugs created by FAMU investigators, as well as transfer of technology on cancer-model development through organoid and PDX training. We advanced, supported, and tracked the careers of URM trainees: namely postbac, graduate, postdoc, and ESIs. Of specific priority is fostering the acquisition of competitive independent research grants (R-type or equivalent) for URM ESIs, especially scientists at FAMU. We developed innovative approaches to deliver cancer research knowledge using culturally appropriate and multimedia approaches, build a workforce of trained cancer advocates, including community scientists, and train URM scientists who can engage in bi-directional community engagement.
We leveraged additional partnership opportunities within our triad partnership as well as with other CPACHE partnerships. An unexpected opportunity occurred due to the impact of the COVID-19 pandemic and was possible given the existing trust that we had built over the years with our community partners, which allowed us to quickly pivot to address the COVID-19 pandemic in our catchment communities. Specifically, we fostered their participation in COVID-19 vaccine clinical trials and the uptake of the COVID-19 vaccine among the B/AA and H/L populations with funding from an NIH CEAL Award led through UFHCC and an administrative supplement led through USC.
Moving forward, we will continue to expand collaborations to understand further the biological and environmental etiology of cancer disparities by expanding our focus to migration studies and global cancer health studies including B/AA and H/L populations outside the U.S. We have built strong collaborations with partners in Africa to explore the molecular features of prostate cancer in men from West Africa using transcriptome sequencing. This collaboration has led to supplemental funding from the NCI Center to Reduce Cancer Health Disparities (CRCHD) and NCI Center for Global Health.
We are also seeking novel collaborations with other entities, including other CPACHE partnerships, to synergize our efforts and effectively address cancer disparities nationally. For example, we will collaborate with Tempus, a company with molecular sequencing data from over 100,000 individuals, including many B/AA and H/L patients, to analyze the genomic features of cancers of interest for our partnership to validate our research findings. We will continue to seek other collaborative opportunities to expand the impact of our partnership.
Despite the many successes during the first funding cycle of the CaRE2 Health Equity Center, several limitations merit consideration. First, the laboratory-based research projects’ progress was slowed by the COVID-19 pandemic and publications for several aims are still in process. Second, there have been a large number of the Center’s investigators who transitioned to important positions at other institutions, which necessitated reconfiguration of teams and development and implementation of succession plans. The impact of these transitions would have been more disruptive to the Center’s work had our structure been less robust. Third, the Center’s initial focus was on integration across the three institutions with insufficient attention to integration within the center’s cores and projects as they worked to achieve their aims. Fourth, our initial trainee tracking processes were not as robust as needed for long-term tracking. Finally, with our laser focus on the big picture—our a priori established benchmarks of success—we missed opportunities to strengthen our evaluation of Center processes and other metrics that indicated successful achievement of all core and project aims. Identification of these limitations provides opportunities to strengthen the center as it moves toward sustainability.
Conclusion
Since its establishment in 2018, the CaRE2 Health Equity Center has established a structure and innovative processes enabling achievement of its foundational benchmarks. These initial outcomes are likely a function of the commitment of our investigators who embraced the team science model while they also encouraged and supported trainees to publish existing and new data and apply for grants to launch their scientific careers. Through our community outreach and bidirectional input, we have empowered our communities to understand, participate in, and advocate for cancer research. These efforts have created expanded infrastructure for translational cancer research at FAMU and cancer disparities research at the UFHCC and USC NCCC. Greater impact is expected in the future as the CaRE2 continues to mature and innovate.
Acknowledgements
We thank our NCI Program Officers, LeeAnn Bailey PhD, Branch Chief of the Integrated Networks Branch and Whitney (Barfield) Steward, Program Director for their thoughtful guidance regarding programmatic actions. We are grateful to our Community Advisory Board and community health educators for their tireless efforts to eliminate cancer health disparities and to Amelia Greenlee for her support of our work. For their constant advice and critical review of our program, we thank our Program Steering Committee members: Clayton Yates, PhD (Chair), Zhenbang Chen, PhD, Antonio Baines, PhD, Laura Fejerman, PhD, Virgil Simons, Robert Winn, MD, Camille Ragin, PhD, and Mary Scroggins.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was made possible by Grant Numbers U54CA233396, U54CA233444, U54CA233465 from the National Institutes of Health (NIH), National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
ORCID iDs
Brooke Hensel https://orcid.org/0000-0003-0854-4689
Thomas George https://orcid.org/0000-0002-6249-9180
Enrique I. Velazquez-Villarreal https://orcid.org/0000-0002-3603-6414
References
- 1.United States Census Bureau. https://www.census.gov/quickfacts/fact/table/US/PST045222#PST045222
- 2.American Association for Cancer Research (AACR) . Cancer Disparities Progress Report. Author. https://cancerprogressreport.aacr.org/. 2022.
- 3.Carpten JD, Fashoyin-Aje L, Garraway LA, Winn R. Making cancer research more inclusive. Nat Rev Cancer. 2021/10/012021;21(10):613-618. doi: 10.1038/s41568-021-00369-7 [DOI] [PubMed] [Google Scholar]
- 4.Odedina FT, Stern MC. Role of funders in addressing the continued lack of diversity in science and medicine. Nat Med. 2021;27(11):1859-1861. doi: 10.1038/s41591-021-01555-8 [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. Jan. 2023;73(1):17-48. doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 6.Mochona B, Lyon D, Offringa IA, et al. Developing a novel framework for an undergraduate cancer research education and engagement program for underrepresented minority students: the Florida-California CaRE(2) research education core (REC) training program. J Cancer Educ. Oct. 2021;36(5):914-919. doi: 10.1007/s13187-020-01762-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behar-Horenstein LS, Richey J, Parker A, Odedina FT, Askins N. Developmental evaluation model for the Florida-California CaRE2 health equity center. Cancer Epidem Biomar. Jun. 2020;29(6). doi: 10.1158/1538-7755.Disp19-D031 [DOI] [Google Scholar]
- 8.Xiao J, Cohen P, Stern MC, Odedina F, Carpten J, Reams R. Mitochondrial biology and prostate cancer ethnic disparity. Carcinogenesis. Dec 13 2018;39(11):1311-1319. doi: 10.1093/carcin/bgy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarton L, Yoon S, Oh S, et al. Pancreatic cancer related health disparities: A commentary. Cancers (Basel). Jul 18 2018;10(7). doi: 10.3390/cancers10070235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith T, Affram K, Nottingham EL, et al. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci Rep. Oct 12 2020;10(1):16989. doi: 10.1038/s41598-020-73218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norris KC, Agodoa LY. The need for health professionals and scientists from underrepresented minority and disadvantaged communities. Ethn Dis. Summer. 2005;15(3 Suppl 4):1-2. [PubMed] [Google Scholar]
- 12.Affram K, Udofot O, Singh M, et al. Smart thermosensitive liposomes for effective solid tumor therapy and in vivo imaging. PLoS One. 2017;12(9):e0185116. doi: 10.1371/journal.pone.0185116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inkoom A, Ndemazie N, Affram K, et al. Enhancing efficacy of gemcitabine in pancreatic patient-derived xenograft mouse models. Int J Pharm X. Dec 2020;2:100056. doi: 10.1016/j.ijpx.2020.100056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva L, Jiang J, Perkins C, et al. Pharmacological inhibition and reversal of pancreatic acinar ductal metaplasia. Cell Death Discov. Sep 2 2022;8(1):378. doi: 10.1038/s41420-022-01165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredenburg KM, Whitlock J, Morris C, et al. Lower disease control rates and survival outcomes among Blacks with pharyngeal squamous cell carcinomas compared with Whites: a retrospective analysis at the University of Florida. Cancer Causes Control. Nov. 2021;32(11):1269-1278. doi: 10.1007/s10552-021-01477-3 [DOI] [PubMed] [Google Scholar]
- 16.Fredenburg TB, Johnson TR, Cohen MD. The Fontan procedure: anatomy, complications, and manifestations of failure. Radiographics. Mar-Apr. 2011;31(2):453-463. doi: 10.1148/rg.312105027 [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Hakimjavadi H, Bray JK, et al. Transcriptional profile of human pancreatic acinar ductal metaplasia. Gastro Hep Advances. 2023;2(4):532-543. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutaria DS, Jiang J, Azevedo-Pouly AC, et al. Knockout of Acinar enriched microRNAs in mice promote duct formation but not pancreatic cancer. Sci Rep. Jul 31 2019;9(1):11147. doi: 10.1038/s41598-019-47566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telisnor G, DeRemer DL, Frimpong E, et al. Review of genetic and pharmacogenetic differences in cytotoxic and targeted therapies for pancreatic cancer in African Americans. J Natl Med Assoc. Apr. 2023;115(2):164-174. doi: 10.1016/j.jnma.2023.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behar-Horenstein LS, Suiter S, Snyder F, Laurila K. Consensus building to inform common evaluation metrics for the comprehensive partnerships to Advance cancer health equity (CPACHE) program. J Cancer Educ. 2021/11/06 2021;38(1):231-239. doi: 10.1007/s13187-021-02103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]