Abstract
Background and Objectives
The French Pompe disease registry was created in 2004 for study of the natural course of the disease in patients. It rapidly became a major tool for assessing the long-term efficacy of enzyme replacement therapy (ERT) after the market release of alglucosidase-alfa.
Methods
Approximately 10 years after publication of the baseline characteristics of the 126 initial patients of the French Late-Onset Pompe Disease registry, we provide here an update of the clinical and biological features of patients included in this registry.
Results
We describe 210 patients followed at 31 hospital-based French neuromuscular or metabolic centers. The median age at inclusion was 48.67 ± 14.91 years. The first symptom was progressive lower limb muscle weakness, either isolated (50%) or associated with respiratory symptoms (18%), at a median age of 38 ± 14.9 years. At inclusion, 64% of the patients were able to walk independently and 14% needed a wheelchair. Positive associations were found between motor function measure, manual motor test, and 6-minute walk test (6MWT) results, and these parameters were inversely associated with the time taken to sit up from a lying position at inclusion. Seventy-two patients had been followed for at least 10 years in the registry. Thirty-three patients remained untreated a median of 12 years after symptom onset. The standard ERT dose was administered for 177 patients.
Discussion
This update confirms previous findings for the adult population included in the French Pompe disease registry, but with a lower clinical severity at inclusion, suggesting that this rare disease is now diagnosed earlier; thanks to greater awareness among physicians. The 6MWT remains an important method for assessing motor performance and walking ability. The French Pompe disease registry provides an exhaustive, nationwide overview of Pompe disease and can be used to assess individual and global responses to future treatments.
Introduction
Pompe disease, glycogen storage disease type II (MIM #232300), is an autosomal recessive disorder characterized by impaired lysosomal acid α-glucosidase (GAA) activity with a very broad clinical spectrum. Severe forms with an onset in infancy can result in cardiorespiratory failure within a few months of birth, whereas other forms, with a much later onset, at ages of 30–50 years, affect the muscles, manifesting principally as diaphragm paralysis or limb-girdle weakness.1,2 Late-onset Pompe disease (LOPD) is the most frequent,3 accounting for more than 90% of Pompe disease diagnoses in France.
Pompe disease was one of the first neuromuscular disorders to benefit from specific treatment with intravenous enzyme replacement therapy (ERT) by alglucosidase-alfa. ERT has considerably improved the prognosis of infantile Pompe disease, by improving cardiac and motor function and prolonging survival in affected children.4,5 Several studies have reported improvements in the ability to walk in patients on ERT and a probable stabilization of respiratory function in adult patients with LOPD.6-9
A national Pompe disease registry was set up in France in 2004, through collaboration between the French reference centers for rare neuromuscular and metabolic diseases (the FILNEMUS and G2M networks), to provide insight into the natural course of Pompe disease in patients and the beneficial effects of treatments.10 This registry has prospectively collected data for most of the adults treated with alglucosidase-alfa in France, revealing a long-term effect of treatment on these clinical outcomes for LOPD.6 We present here an update of the data for all French patients with LOPD included in the French Pompe disease registry.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The registry is hosted on a secure server at Montpellier Hospital. It is covered by section IX of the French Data Protection Act, and it adheres to the Recommendations for Ethics and Good Practice in Epidemiology (French version, 2007). The study was authorized by the National Commission for Information Technology and Freedoms (no. 909139, April 23, 2009) and the Consultative Committee for the Treatment of Information in the Field of Health (reference 08.461, October 30, 2008). All patients for whom the diagnosis of Pompe disease was confirmed from whom a signed specific informed consent form was obtained were included in the registry.
Patient Inclusion
Thirty-one participating hospital-based centers from all over France enrolled the patients in the registry. Confirmation of the diagnosis was considered to have been obtained if an acid GAA enzyme deficiency was demonstrated or one of the 2 known allelic variant of the GAA gene was identified. Records were maintained with code numbers to protect patients' identity.
Data Collection
For each patient, demographic details, current symptoms, medical history, findings of muscle biopsies, and the results of genetic and biological analyses are recorded. All patients undergo examinations performed by a specialist in metabolic diseases or neuromuscular conditions, following national and international guidelines.11,12 Clinical data are collected for motor function, respiratory function, and comorbid conditions. The usual evaluations to assess motor performance include timed tests, manual muscle tests (MMTs), motor function measurements (MFMs),13,14 and 6-minute walk tests (6MWTs). For evaluations of respiratory function, forced vital capacity (FVC) is determined, with the patient in the sitting and supine positions. The difference between the 2 FVC values obtained is calculated to assess diaphragm function, together with maximal inspiratory/expiratory pressures (MIP/MEP). The results are expressed as a percentage of predicted normal values to minimize the contribution of confounding variables (age, height, weight, sex). Laboratory tests performed include serum creatinine kinase (CK) levels.
Statistical Analysis
Cross-sectional analysis was performed with R-4.0.3 software (the R-Foundation for Statistical Computing, Vienna, Austria). Data were expressed as number and percentage—n (%)—for qualitative variables and as mean and SD or median and interquartile range [Q1; Q3] for quantitative variables. The associations between clinical parameters were assessed by calculating the Pearson correlation coefficient. p Values of <0.05 were considered significant. Progression of the disease after the introduction of ERT was analyzed in survival analyses, in which the outcome variable of interest was time until the occurrence of an event and censored patients (those who had not yet changed status at the end of follow-up) were taken into account. The Kaplan-Meier method was used to estimate the percentage of patients with no change in status over time for walking ability or respiratory function, the major functions impaired in LOPD. Time until first progression in walking ability (independent, need for a walking stick, a walker or a manual or electric wheelchair) and respiratory function (no need for ventilatory assistance, noninvasive ventilation [NIV] and invasive ventilation [IV]) was considered in these analyses. We selected treated patients without missing data from the Pompe registry (N = 158) for comparison with the untreated patients (N = 24). Each treated patient was matched to an untreated patient for age at onset, sex, ventilation, and respiratory status (i.e., 1:1 matching). Pair-matching was optimized by minimizing the sum of absolute pairwise distances in the matched sample. Wilcoxon tests were used to compare quantitative variables between the 2 groups of patients, and McNemar tests were used to compare qualitative variables between the 2 groups.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Demographic Data
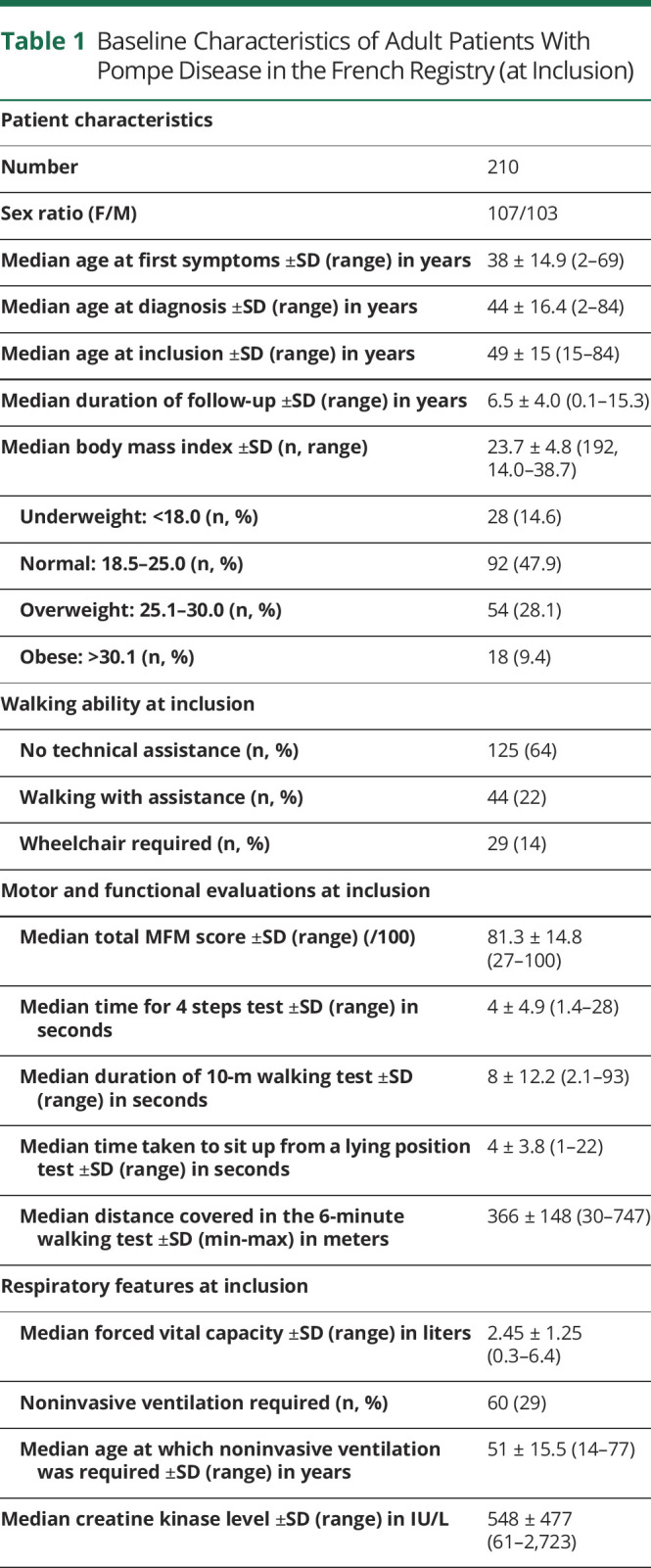
At the time of the most recent data extraction, 210 patients with LOPD had been included in the French Pompe disease registry: 107 women and 103 men. Parental consanguinity was reported for only 9 patients. The median age at inclusion was 48.7 ± 14.9 years (14.8–84.4), and age distribution was similar for men and women. The median body mass index at inclusion was 23.7 (14.0–38.7). Approximately half of the patients had a normal body mass index, 14% were underweight, 28% were overweight, and 9.4% were obese.
Diagnosis
Muscle Biopsy
In total, 157 muscle biopsies were performed in 141 patients. The quadriceps muscle was biopsied in 64 cases, deltoid in 61 cases, and another muscle in 32 cases. Vacuoles were observed in 66% of muscle biopsy specimens. Vacuoles were more frequent in the quadriceps (48%) than in the deltoid (35%) specimens or in specimens from other muscles (17%). Enhanced periodic acid–Schiff (PAS) staining was observed in 62% of the muscle biopsy specimens. Acid phosphatase activity was evaluated in 73% of the muscle biopsies. It was high in 82% of the specimens tested. The distribution of muscle biopsy assessments for Pompe disease diagnosis over time revealed a downward trend over the past 5 years.
Biochemical and Molecular Analysis
Blood analysis demonstrated the presence of GAA deficiency in 151 patients, and skin biopsy confirmed this deficiency in 41 patients. At inclusion, the mean total CK level was 583 IU/L (n = 137; 61–2,723). CK values were normal (<200 IU/L) in 26 patients (19%), between 200 and 954 IU/L in 92 patients (67%), and between 1,055 and 2,723 IU/L in 19 patients (14%). Genetic testing identified GAA gene mutations in 167 patients, with molecular analyses still underway for the remaining patients. The variants detected were bi-allelic in 155 patients (93%). Most patients were compound heterozygous (90%), and 3 patients were homozygous for the common intron-1 allelic variant (c.-32-13T>G) found in 145 patients studied here (87%). The “exon-18-deletion” c.2481+102-2646+31del was the second most frequent variant and was found to be associated with the c.-32-13T>G variant in 15 patients.
Characteristics of the Patients
The main characteristics of patients at inclusion are reported in Table 1.
Table 1.
Baseline Characteristics of Adult Patients With Pompe Disease in the French Registry (at Inclusion)
Initial Disease Course and Inaugural Symptoms
Progressive weakness of the lower limbs was the symptom most frequently reported initially, occurring either in isolation (50%) or together with respiratory symptoms (18%). Respiratory insufficiency was the symptom leading to the initial medical consultation in 15 cases (7%), despite preexisting muscle involvement warning signs in more than half of the patients, in the form of permanent muscle weakness (5/11, 45%) or high CK values (5/7, 71%).
Isolated hyper-CKemia was the sign leading to diagnosis in 14 patients. The other reasons given for the initial consultation included genetic counseling and episodes of muscle fatigue or lower back pain.
Patients frequently reported the presence of mild muscle symptoms since childhood. Twenty-one patients (10%) for whom the age at first steps was known had started walking late. The median age at which the patients learned to walk was 17 ± 2.9 (12–24) months for the entire study cohort. Walking difficulties were reported for 18 patients (8%) during childhood, whereas 56 (27%) found it difficult to run, and 23 (11%) were excused physical education activities at school. At inclusion, 13 patients (7%) presented with scapular winging and 67 (32%) had scoliosis detected at a median age of 15 ± 13.5 (7–60) years. Rigid-spine syndrome was observed in 2 patients,15 and 11 (5%) presented with ptosis at inclusion.
Involvement of the Limb Muscles at Inclusion
Progressive limb weakness began at a median age of 38 years. Almost all the patients found it difficult to stand from a squatting or sitting position at the initial visit for this study or to rise to a standing position from the supine position or to go upstairs. In total, 129 patients (64%) were able to walk without assistance, 44 (22%) were able to walk with assistance from a walking frame or cane, and 29 (14%) used a wheelchair. Fourteen of these patients had been confined to their wheelchair at all times since a median age of 42 years (14–73 years); another 11 patients used a wheelchair only some of the time. The median distance covered in the 6-minute walking test was 366 ± 148 (30–747) m.
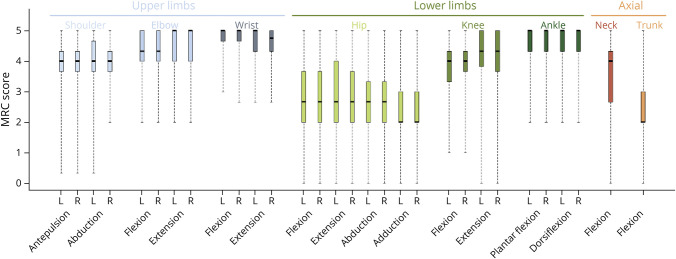
The distribution of muscle weakness was determined on the basis of median Medical Research Council score per muscle group (Figure 1). MMT results showed that muscle weakness was symmetric and predominantly affected the hip flexion, extension, adduction, abduction, and trunk muscles. This pattern of muscle weakness accounts for the waddling gait observed in most patients. Quadriceps, hamstrings, and shoulder girdle muscles were less severely affected, and distal muscles were spared.
Figure 1. Clinical Topography of Muscle Weakness.
Box plots are drawn for subscores of the MRC scale. The median is indicated by the line bisecting the box. The box represents the middle 50% of scores: the interquartile range. The upper and lower whiskers represent the minimum and maximum scores. The most severe weakness was that for hip muscles. The median score was below 3/5 for flexion, extension, abduction, and adduction of the hips, corresponding to an inability to move actively against gravity. Axial weakness predominated for trunk muscles, with a median score of 2/5, corresponding to active movement in the absence of gravity. Distal motor function was better preserved, with a median score of 4/5 or more for the wrist, ankle, elbow, and knee. L = left; MRC = Medical Research Council; R = right.
MFM scores were available for 117 patients as follows: 81.3% ± 14.8% (27–100) for the global score; 61.5% ± 26.9% (range: 0–100) for D1, exploring standing and positional transfers; 94.4% ± 12.7% (25–100) for D2, exploring the functioning of proximal and axial muscles; and 100% ± 5.2% (57–100) for D3, exploring the function of distal muscles. The low D1 scores indicated that the disease had a greater effect on standing and positional transfer activities than on limb-girdle function.
Respiratory Involvement
Seventy-seven patients (37%) required ventilatory assistance at inclusion. This ventilatory assistance took the form of NIV in 60 patients (29%) and a tracheostomy in 17 patients (8%). NIV was used only at night by 45 patients; 15 patients also required NIV during the day. Sixteen patients were on IV round the clock. The mean FVC in the sitting position was 2.61 L (71% the predicted normal value, n = 168 patients). The mean FVC was 1.95 L (53% the predicted normal value) for patients on NIV and 3.1 L (83.5% the predicted normal value) for patients without ventilatory assistance. Forty-six of the 60 patients requiring NIV were still able to walk (28 walked without aid, 15 with a stick, and 3 with a walker) and 12 used a wheelchair. Two of the 17 patients on IV were still able to walk.
Other Characteristics at Inclusion
Transthoracic cardiac ultrasound examinations were performed at inclusion in 135 patients (64.5%). The mean ventricular ejection fraction was 65.3% (range: 41%–81%). Only 2 patients had a dilated cardiomyopathy, and 9 patients had a hypertrophic cardiomyopathy. Other more frequent differential diagnoses may also account for cardiac involvement, particularly in older patients. Overall, 63% (7/11) of patients with cardiac disorders were older than 50 years at inclusion; 45% (5/11) were on antiplatelet aggregation treatment; and 44% (4/9) of the patients with hypertrophic cardiomyopathy were on antihypertensive treatment.
Forty-eight patients (22.8%) complained of digestive symptoms: 31 presented with diarrhea, 9 anal incontinence, 20 chronic constipation, and 16 (7.6%) with at least 2 of these symptoms. Thirty patients (15%) had symptoms of bladder-sphincter dysfunction. Twenty-three patients (11%) had dysphagia, which was associated with macroglossia in 4 cases.
Relationships Between the Principal Characteristics of the Patients
The associations between the various characteristics of the patients are demonstrated in the correlogram in Figure 2. Muscle function and strength parameters were strongly correlated with each other, with the exception of the MFM D3 score. Indeed, a positive association was found between MFM, lower limb MMT, and 6MWT results. Higher total or D1 MFM scores and lower limb MMT scores were associated with the coverage of a longer distance in the 6MWT. These parameters were found to be inversely associated with the time taken to sit up from a lying position. Weaker motor function was found to be associated with taking longer to sit up from a lying position. Total MFM score was also associated with respiratory parameters (MIP, MEP, and FVC). No association was found between muscle parameters and the MFM D3 score or between the CK level and muscle function or respiratory parameters.
Figure 2. Correlogram of All the Main Clinical Parameters of Patients at Inclusion.
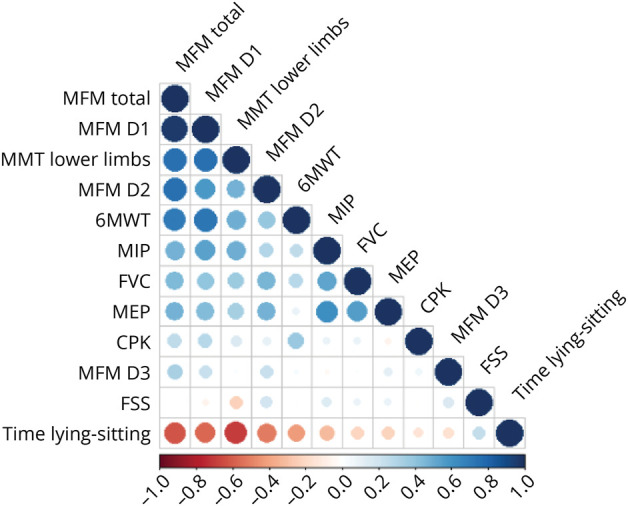
Blue indicates a positive correlation, and red indicates an inverse correlation. The intensity of the color indicates the strength of the correlation. 6MWT = 6-minute walk test; CPK = Creatine Phosphokinase; FSS = Fatigue Severity Scale; FVC = forced vital capacity; MEP = maximal expiratory pressure; MFM = motor function measurement; MIP = maximal inspiratory pressure; MMT = manual muscle test.
Disease Progression
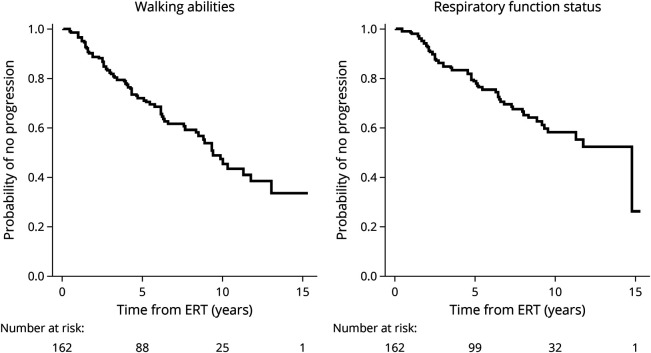
Kaplan-Meier curves for motor function and respiratory status (Figure 3) revealed a progression of muscle weakness and respiratory involvement, with a steady decline of both functions over time. Median survival was approximately 9.4 years for walking ability and 14.8 years for respiratory function status. This progressive worsening occurred despite most of the patients (84.3%) receiving ERT at the standard dose of 20 mg/kg every 2 weeks, confirming the results of previous studies and demonstrating the long-term limitations of current ERT with alglucosidase-alfa.6,7
Figure 3. Survival Curves for Walking Ability and Respiratory Function Status for Adult Patients With Pompe Disease in the French Registry.
Time in years from the start of ERT is plotted on the x-axis, whereas the probability of no progression or the proportion of individuals displaying stabilization is shown on the y-axis. The lines are the survival curves. Vertical drops in these curves correspond to events. The number of patients at risk is indicated below. At time 0, the probability of no progression is 1.0 (100% of the patients are in their baseline state). At 5 years, the probability of no progression is approximately 70% for walking ability and 80% for respiratory function status. Median survival is approximately 9.4 years for walking ability and 14.8 years for respiratory function status. ERT = enzyme replacement therapy.
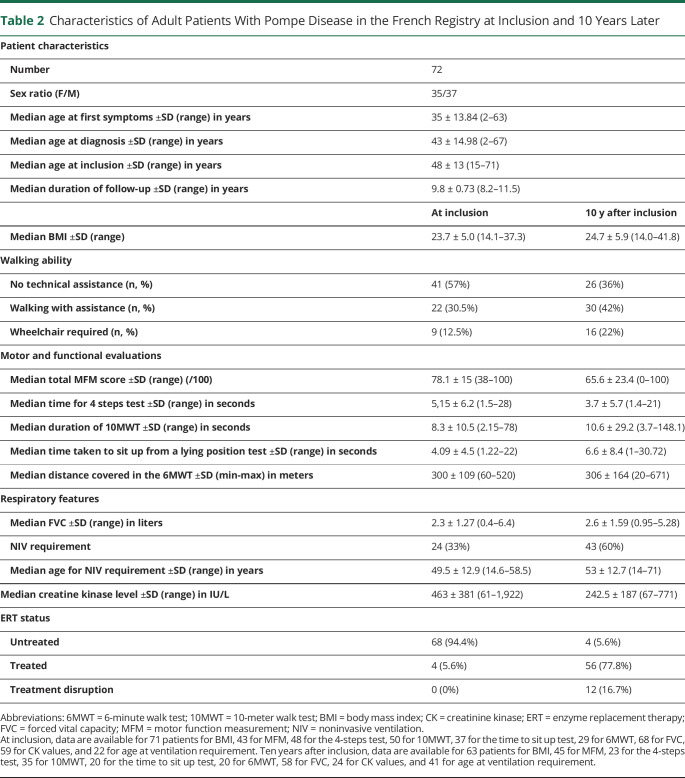
Seventy-two patients with LOPD had been followed for more than 10 years in the registry. Ten years after inclusion, there were fewer patients without technical assistance for walking (from 57% to 36%), twice as many patients required NIV (from 33% to 60%), and 77% of patients were treated, vs 5% at inclusion (Table 2).
Table 2.
Characteristics of Adult Patients With Pompe Disease in the French Registry at Inclusion and 10 Years Later
Focus on Specific Subgroups
Patients With Very LOPD
Disease onset occurred after the age of 60 years in 12 patients (5.7%), 6 men and 6 women. The median age at inclusion was 70 years (64–79 years), and the mean age at diagnosis was 67 ± 4.4 years (62–76 years). More than half of the patients (7/12, 58.3%) were heterozygous for the commonest mutation, and 25% (3/12) had normal CK values. Five patients (41.7%) walked with a stick, and 7 patients (58.3%) were able to walk without assistance.
Median FVC in the upright position at inclusion was 2.02 ± 0.96 L (1.1–3.4 L, 65% of the predicted value); 10 patients (83.3%) had dyspnea on exertion; and almost half of these patients (5/12, 41.7%) required NIV (exclusively nocturnal in 4 patients and both nocturnal and diurnal in 1 patient). Cardiac involvement was reported in only 1 patient, who had left ventricular hypertrophy.
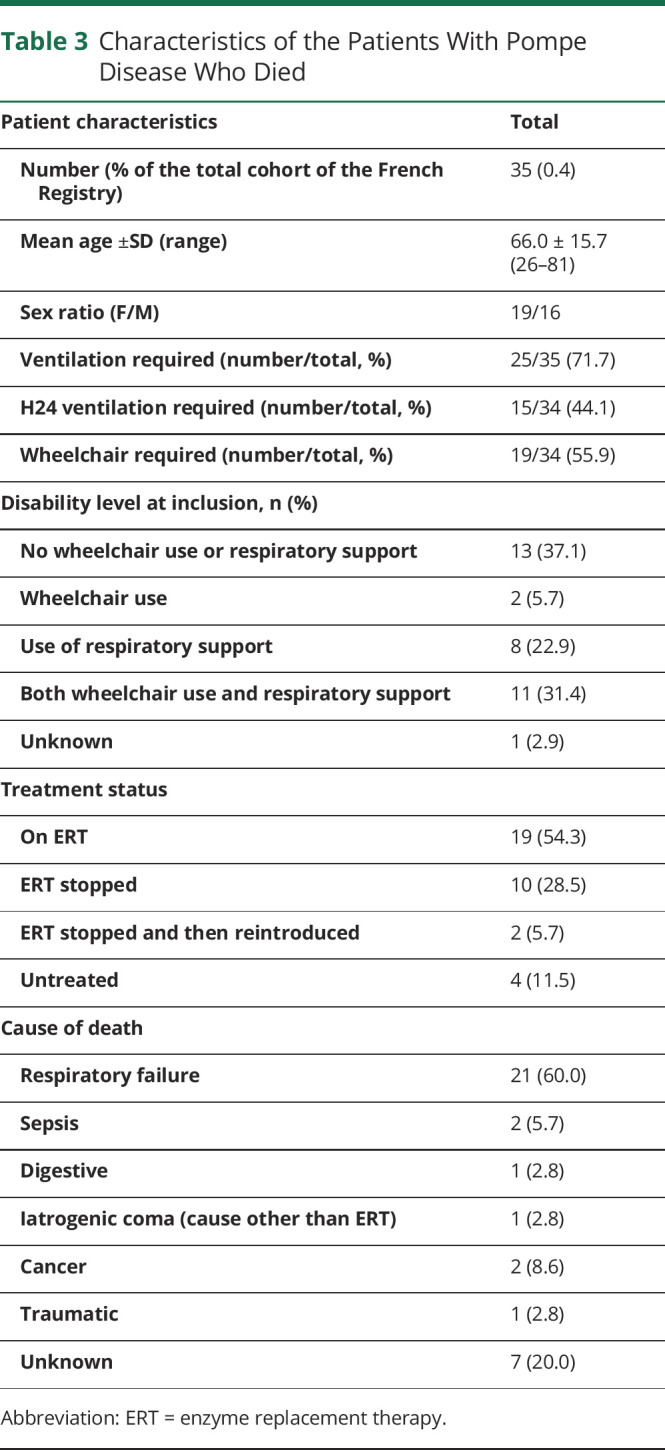
Deceased Patients
Thirty-five patients (16.7%) died during registry follow-up. The mean age at death was 66.0 ± 15.7 years (26–81 years). Most of the patients who died required ventilatory assistance (25/35, 71.5%), and 15 (44.1%) required round-the-clock ventilation. Half of the patients who died (19/34, 55.9%) required a wheelchair. Seventeen patients (8.1%) required both ventilatory assistance and a wheelchair. Half were still on ERT at the time of death (19/35, 54.3%); 10 stopped ERT a mean of 30 ± 31.7 months (2–104) before death; 2 stopped ERT and then restarted treatment (interruption of 15 ± 12.7 months [6–24] followed by 27.5 ± 12.0 months [19–36] of ERT); and 4 were untreated.
The cause of death was respiratory failure for 20 patients (60%); sepsis for 2 patients (6%); cancer for 2 patients (6%); digestive complications, iatrogenic coma, and traumatic complications in 1 patient each for 3 other patients (3%); and unknown for 7 patients (20%). These results are reported in Table 3.
Table 3.
Characteristics of the Patients With Pompe Disease Who Died
Course of the Disease in Untreated Patients and in Patients Receiving ERT
ERT was administered to 177 patients with the standard dose (84.3%): 90 women and 87 men. The median age at ERT initiation was 49.6 ± 15.0 years (13.3–87.5). The median duration of ERT was 103 ± 57.0 months (1–201).
Thirty-three patients (15.7%)—16 women and 17 men—with a confirmed diagnosis of LOPD remained untreated for a median duration of 12.1 ± 12.0 years (1.3–53.3) between symptom onset and the last follow-up visit. Their mean age at first symptoms was 37.5 ± 16.0 years (3–61), and their mean age at diagnosis was 44 ± 16.0 years (2–72). Two patients remained asymptomatic with isolated high CK levels. Most patients (27/33, 82%) required no technical assistance for walking at their last evaluation. Four patients (12%) required a stick for walking, and only 2 (6%) required a wheelchair. Only 8 patients (24%) required NIV, which was initiated at a mean age of 57 ± 10.9 years (44–74). The reason for treatment not being administered, particularly for the more severely affected patients, was not reported in the registry.
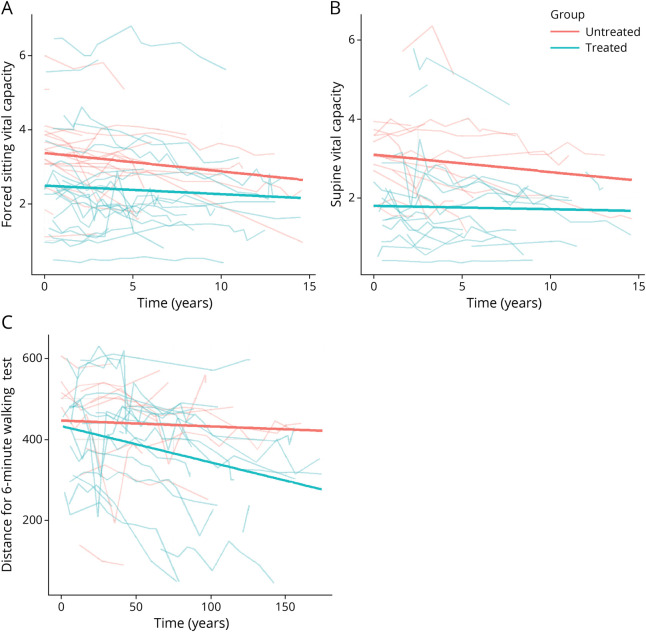
The comparison of 2 matched groups of 24 untreated and 158 treated patients with LOPD revealed a significant difference between the groups in changes over time in 6MWT and sitting FVC (p < 0.001 and p = 0.002, respectively). No significant difference was detected for supine FVC. These results are presented in Figure 4.
Figure 4. Disease Course in Untreated Patients vs Patients Treated by ERT: Walking and Respiration.
(A) Sitting FVC. At inclusion, the mean value for the untreated group was 3.36 ± 0.3 L; the mean value for the treated group was 0.75 ± 0.4 L lower, at 2.61 L (p = 0.056). Over time, the mean change in the untreated patients was −0.07 ± 0.009 (p < 0.001) and in the treated patients was 0.02 ± 0.01 L smaller, at −0.05 (p = 0.002). (B) Supine FVC. At inclusion, there was no significant difference between the 2 groups: the mean value for the untreated group was 2.95 ± 0.3 L and for the treated group was 0.82 ± 0.5 L lower, at 2.13 (p = 0.08). Over time, there was no significant difference in the changes observed between the 2 groups: the mean change for untreated patients was −0.07 ± 0.01 (p < 0.001) and for treated patients was 0.01 ± 0.01 L smaller, at −0.06 L (p = 0.40). (C) 6-MWT. At inclusion, there was no significant difference between the 2 groups: 444.6 ± 36.7 for the untreated group, 13.5 ± 46.6 lower, that is, 431.1 (p = 0.77) for the treated group. Over time, untreated patients remained stable with a coefficient of −0.15 ± 0.2; the slope for the treated patients was 0.85 ± 0.2, corresponding to a mean progression of −1.00 (p < 0.001). 6MWT = 6-minute walk test; ERT = enzyme replacement therapy; FVC = forced vital capacity.
Discussion
The French Pompe disease registry is an academic registry dedicated to the prospective collection of clinical and biological data for patients with Pompe disease at the nationwide level. This registry facilitates data collection and long-term standardized monitoring for one of the largest cohorts of patients with LOPD in the world, with financial support from both research grants and industry. Close collaboration between the French reference centers for rare neuromuscular (FILNEMUS) and metabolic (G2M) diseases provides an additional guarantee that the data collected are of high quality and exhaustive. The French Pompe disease registry, initially developed as an instrument for following the natural course of disease in patients, rapidly shifted focus to become a major tool for assessing the long-term efficacy of ERT after the market release of alglucosidase-alfa.6 However, a number of adult patients remain untreated, for various reasons, and follow-up data for these patients provide an important source of information. Approximately 10 years after publication of baseline characteristics for the first 126 patients included in the French LOPD cohort,10 we provide here an update of the clinical and biological features at inclusion in the French Pompe disease registry of a cohort that has now expanded to 210 patients with LOPD.
As we reported in the first description of the French Pompe disease registry, the range of age at onset of LOPD was broad, with occurrence peaking in patients aged between 20 and 30 years and onset delayed until 60 years and beyond in 12 patients. At inclusion, 64% of the patients were able to walk independently and 14% needed a wheelchair, whereas the corresponding figures 10 years ago were 55% of patients able to walk without assistance and 21% requiring a wheelchair.10 The median distance covered in the 6MWT by patients undergoing this test was 366 m, vs 340 m 10 years ago. These results probably reflect less severe lower limb muscle involvement at the time of diagnosis because of earlier diagnosis; thanks to an increase in physician awareness of LOPD. However, further efforts are still required to ensure that the disease is diagnosed before walking is severely impaired, a situation still observed in a large number of patients.
An analysis of MMT results for a large number of patients clearly showed that muscle weakness at inclusion predominantly affected the hip flexors and extensors, with a selective impairment of adductors. The muscles of the upper limbs were less severely affected than those of the lower limbs. The trunk muscles were also generally severely affected, and a weakness of these muscles was observable at early stages of the disease.16 We did not detect any additional patients with rigid-spine syndrome, which was the initial clinical presentation of only 2 patients, supporting the scarcity of this presentation.
Despite its limitations, the 6MWT remains an important method for assessing motor performance and walking ability. Vanherpe et al. provided evidence in favor of the use of the 6MWT rather than quantitative isometric strength measurement after 2 years of study for motor decline detection in treated patients with LOPD.11 They also confirmed that the 6MWT was more sensitive than ActivLim (“Activity Limitations”).17,18 The MFM scale has proved useful and is widely used to assess muscle function. It can be used to evaluate disease severity, regardless of disease stage, including, in particular, the D1 dimension, which evaluates standing activities and transfers, the score for which is well correlated with 6MWT and MMT results. We, therefore, propose the use of the MFM scale for patient assessments during follow-up and as an outcome measure for future clinical trials.
Respiratory involvement was severe in 37% of patients, who required either NIV (29%) or ventilation by tracheostomy (8%). These results contrast with the previous analysis of the French Pompe disease registry, in which 46% of patients were reported to require ventilatory assistance, providing further evidence in favor of the earlier diagnosis of LOPD over the past 10 years. Nevertheless, the proportion of patients with acute respiratory insufficiency as the inaugural manifestation of the disease (7%) remained identical to that 10 years previously, reflecting the difficulty establishing an early diagnosis of LOPD in patients with predominant respiratory muscle weakness.
This study also confirms that the severity of limb muscle weakness is not systematically linked to the severity of respiratory involvement, as shown by the absence of correlation between the results of respiratory assessments and those of the 6MWT, MFM32, and manual tests. Strikingly, sitting FVC was not correlated with MIP, despite both of these parameters being used to investigate the diaphragm weakness of patients with Pompe disease. This result may be due to the lower accuracy of sitting FVC than of supine FVC for assessing diaphragm involvement.
In addition to the typical clinical features of LOPD, we also detected gastrointestinal symptoms, bladder dysfunction, and dysphagia in 22%, 15%, and 11% of patients, respectively. These symptoms are often disabling, thus should be more systematically sought during patient follow-up. Further investigations are required to elucidate their relationship to Pompe disease. In 2021, Kishnani et al. reported that more than half of all patients with Pompe disease complain of gastrointestinal disorders, which are among the top 3 causes of a deterioration of quality of life.19 These symptoms are poorly understood and suggest possible smooth muscle involvement. We recently performed thorough investigations in patients complaining of dysphagia, which was found to be mostly related to macroglossia.20
Most patients (67%) present a moderate increase in the CK level to levels that remain below 1,000 IU/L, with only 14% of patients having higher CK values of up to 2,723 IU/L. It is of interest that normal CK values have also been reported in 19% of patients, regardless of disease severity. We found no correlation between CK level and the severity of either muscle weakness or pulmonary function deterioration. These findings could, therefore, make it possible to orientate diagnosis toward LOPD rather than other frequent causes of limb-girdle muscular dystrophy, which are generally associated with CK levels more than 1,000 IU/L.
As previously observed, muscle biopsies, when performed, only inconsistently revealed the typical pathologic features of lysosomal glycogenosis with vacuoles, excessive PAS staining, and an increase in acid phosphatase activity. These abnormalities were detected in less than two-thirds of patients, highlighting the limitations of muscle biopsy for establishing the correct diagnosis. However, we would like to stress the great relevance of acid phosphatase activity, which should be systematically analyzed, because we found that acid phosphatase activity was enhanced in 82% of the muscle biopsies for which this analysis was performed. This abnormality may even be the only pathologic abnormality in some cases of LOPD. The development of gene panels, and the parallel increase in the use of enzymatic assays on blood samples, probably account for the decrease in the number of muscle biopsies performed over the past 5 years. These results demonstrate the utility of biochemical analyses of acid GAA activity for Pompe disease diagnosis in adults with proximal and axial muscle weakness, including those with normal muscle biopsy findings and CK levels.
We identified a subgroup of 12 patients with a very late disease onset, older than 60 years. The clinical manifestations and severity of the disease do not seem to be different in this subgroup of patients, despite only half of these patients being heterozygous for the commonest mutation, vs approximately 90% of the total population studied. Dysphagia and speech impairment due to macroglossia are probably more frequent in this age range, as reported in a recent study,20 and it will be important to increase the awareness of this among physicians.
The 210 patients with LOPD included in the registry included 177 receiving ERT, the other 33 patients remaining untreated for various reasons (reluctance to agree to infusions, advanced age, concomitant serious disease, etc). The covariate analysis comparing untreated patients with a paired group of treated patients showed a significant difference in the changes in 6MWT and sitting FVC results over time between these 2 groups. Untreated patients had globally more stable 6MWT results but presented a higher aggravation of sitting FVC than treated patients over time. The decision to treat is not random, but depends on clinical progression. These results, therefore, suggest that there are several progression profiles in patients, with some remaining stable in the absence of treatment and others displaying a decline despite ERT. In 2018, Semplicini et al.21 reported that there was no straightforward relationship between genotype and age at disease onset. Further studies are required to determine the potential association with an evolutionary clinical profile. We recently published the results of an analysis of the long-term effects of ERT in the French LOPD cohort, providing further evidence that ERT improves walking ability and probably stabilizes respiratory function in LOPD, but with a ceiling effect for the 6MWT in the first 3 years of treatment.6 Despite these established benefits of ERT, we observed a treatment cessation rate of 20% and a mortality rate of 16% in treated patients. An analysis of the causes of these treatment cessations and deaths will be the subject of further studies.
In conclusion, we provide here a description of the characteristics of the LOPD population in France, which was made possible by the existence of the French Pompe disease registry and close collaboration between the French reference centers for rare neuromuscular and metabolic diseases. The number of patients with LOPD included in the registry has increased by 84 over the last 10 years, and the inclusion of patients with infantile Pompe disease is currently underway. Data collection for this registry is subject to several limitations, including, in particular, missing data because of the reluctance of some patients to perform clinical tests or differences in the frequency of assessments between reference centers. We, nevertheless, consider this registry to be a unique tool providing an exhaustive overview of Pompe disease at the nationwide level, together with industry-independent assessments of individual and global responses to current and future treatments.
Acknowledgment
We thank The Myology Institute (Mrs. Pr Bertrand Fontaine, Mrs. Christelle Gaultier, Mrs. Delphine Valleteau) for the administrative and legal support; and patient associations Association Francophone des glycogénoses (AFG), Association Française contre les Myopathies (AFM), and Vaincre les Maladies Lysosomales (VML) for their support; and Mrs. Julie Sappa for language editing.
Glossary
- 6MWT
6-minute walk test
- CK
creatinine kinase
- ERT
enzyme replacement therapy
- FVC
forced vital capacity
- GAA
α-glucosidase
- IV
invasive ventilation
- LOPD
late-onset Pompe disease
- MEP
maximal expiratory pressure
- MFM
motor function measurement
- MIP
maximal inspiratory pressure
- MMT
manual muscle test
- NIV
noninvasive ventilation
- PAS
periodic acid–Schiff
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
CME Course: NPub.org/cmelist
Study Funding
We thank Genzyme-Sanofi as a source of funding for the French Pompe Registry.
Disclosure
C. Lefeuvre received fees for consulting and sponsoring for scientific congress by Sanofi-Genzyme. F. Bouhour and E. Salort-Campana received fees for participation to scientific boards and sponsoring for scientific congress by Sanofi-Genzyme and Amicus Therapeutics. A. Behin, S. Attarian received fees for participation to scientific boards and sponsoring for scientific congress by Sanofi-Genzyme. A. Nadaj-Pakleza received fees for participation to scientific boards by Sanofi-Genzyme and Amicus Therapeutics. M. Spinazzi received fees for participation to scientific boards by Sanofi-Genzyme. C. Tard, M. Michaud, and A.-L. Bedat-Millet received sponsoring for scientific congress by Sanofi-Genzyme. J.-P. Noury received fees for participation to scientific boards by Amicus Therapeutics and sponsoring for scientific congress by Sanofi-Genzyme. P. Laforêt receive fees for participation to scientific boards by AMICUS Therapeutics, Sanofi Genzyme, Spark Therapeutics, consulting fees by Sanofi Genzyme, BioMarin, Sanofi Genzyme, Spark Therapeutics and sponsoring for scientific congress by Sanofi Genzyme, Amicus Therapeutics, Spark Therapeutics. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Engel AG. Acid maltase deficiency in adults: studies in four cases of a syndrome which may mimic muscular dystrophy or other myopathies. Brain. 1970;93(3):599-616. [DOI] [PubMed] [Google Scholar]
- 2.Laforêt P, Nicolino M, Eymard PB, et al. Juvenile and adult-onset acid maltase deficiency in France: genotype-phenotype correlation. Neurology. 2000;55(8):1122-1128. [DOI] [PubMed] [Google Scholar]
- 3.van der Ploeg AT, Reuser AJJ. Pompe's disease. Lancet. 2008;372(9646):1342-1353. [DOI] [PubMed] [Google Scholar]
- 4.Smith WE, Sullivan-Saarela JA, Li JS, et al. Sibling phenotype concordance in classical infantile Pompe disease. Am J Med Genet A. 2007;143A(21):2493-2501. [DOI] [PubMed] [Google Scholar]
- 5.Koeberl DD, Kishnani PS. Immunomodulatory gene therapy in lysosomal storage disorders. Curr Gene Ther. 2009;9(6):503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semplicini C, De Antonio M, Taouagh N, et al. Long-term benefit of enzyme replacement therapy with alglucosidase alfa in adults with Pompe disease: prospective analysis from the French Pompe Registry. J Inherit Metab Dis. 2020;43(6):1219-1231. [DOI] [PubMed] [Google Scholar]
- 7.Harlaar L, Hogrel JY, Perniconi B, et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology. 2019;93(19):e1756-e1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuperus E, Kruijshaar ME, Wens SCA, et al. Long-term benefit of enzyme replacement therapy in Pompe disease: a 5-year prospective study. Neurology. 2017;89(23):2365-2373. [DOI] [PubMed] [Google Scholar]
- 9.Stockton DW, Kishnani P, van der Ploeg A, et al. Respiratory function during enzyme replacement therapy in late-onset Pompe disease: longitudinal course, prognostic factors, and the impact of time from diagnosis to treatment start. J Neurol. 2020;267(10):3038-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laforêt P, Laloui K, Granger B, et al. The French Pompe registry. Baseline characteristics of a cohort of 126 patients with adult Pompe disease. Rev Neurol (Paris). 2013;169(8-9):595-602. [DOI] [PubMed] [Google Scholar]
- 11.Maladie de Pompe [Internet]. Haute Autorité de Santé. Accessed April 15, 2022. has-sante.fr/jcms/c_2659919/fr/maladie-de-pompe. [Google Scholar]
- 12.Schoser B, Laforêt P, Kruijshaar ME, et al. 208th ENMC International Workshop: formation of a European Network to develop a European data sharing model and treatment guidelines for Pompe disease Naarden, the Netherlands, 26-28 September 2014. Neuromuscul Disord. 2015;25(8):674-678. [DOI] [PubMed] [Google Scholar]
- 13.Bérard C, Payan C, Hodgkinson I, Fermanian J; MFM Collaborative Study Group. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. 2005;15(7):463-470. [DOI] [PubMed] [Google Scholar]
- 14.Benaïm C, Sacconi S, Fournier-Mehouas M, Tanant V, Desnuelle C. Validity of the motor function measurement scale when routinely used in the follow-up of adult outpatients in a neuromuscular center [in French]. Rev Neurol (Paris). 2010;166(1):49-53. [DOI] [PubMed] [Google Scholar]
- 15.Laforêt P, Doppler V, Caillaud C, et al. Rigid spine syndrome revealing late-onset Pompe disease. Neuromuscul Disord. 2010;20(2):128-130. [DOI] [PubMed] [Google Scholar]
- 16.Carlier PG, Azzabou N, de Sousa PL, et al. Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J Inherit Metab Dis. 2015;38(3):565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanherpe P, Fieuws S, D'Hondt A, et al. Late-onset Pompe disease (LOPD) in Belgium: clinical characteristics and outcome measures. Orphanet J Rare Dis. 2020;15(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tard C, Salort-Campana E, Michaud M, et al. Motor and respiratory decline in patients with late onset Pompe disease after cessation of enzyme replacement therapy during COVID-19 pandemic. Eur J Neurol. 2022;29(4):1181-1186. [DOI] [PubMed] [Google Scholar]
- 19.Korlimarla A, Lim JA, McIntosh P, Zimmerman K, Sun BD, Kishnani PS. New insights into gastrointestinal involvement in late-onset Pompe disease: lessons learned from bench and bedside. J Clin Med. 2021;10(15):3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupé C, Lefeuvre C, Solé G, et al. Macroglossia: a potentially severe complication of late-onset Pompe disease. Eur J Neurol. 2022;29(7):2121-2128. [DOI] [PubMed] [Google Scholar]
- 21.Semplicini C, Letard P, De Antonio M, et al. Late-onset Pompe disease in France: molecular features and epidemiology from a nationwide study. J Inherit Metab Dis. 2018;41(6):937-946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.