Abstract
Background and Objectives
Repeated impacts in high-contact sports such as American football can affect the brain's microstructure, which can be studied using diffusion MRI. Most imaging studies are cross-sectional, do not include low-contact players as controls, or lack advanced tract-specific microstructural metrics. We aimed to investigate longitudinal changes in high-contact collegiate athletes compared with low-contact controls using advanced diffusion MRI and automated fiber quantification.
Methods
We examined brain microstructure in high-contact (football) and low-contact (volleyball) collegiate athletes with up to 4 years of follow-up. Inclusion criteria included university and team enrollment. Exclusion criteria included history of neurosurgery, severe brain injury, and major neurologic or substance abuse disorder. We investigated diffusion metrics along the length of tracts using nested linear mixed-effects models to ascertain the acute and chronic effects of subconcussive and concussive impacts, and associations between diffusion changes with clinical, behavioral, and sports-related measures.
Results
Forty-nine football and 24 volleyball players (271 total scans) were included. Football players had significantly divergent trajectories in multiple microstructural metrics and tracts. Longitudinal increases in fractional anisotropy and axonal water fraction, and decreases in radial/mean diffusivity and orientation dispersion index, were present in volleyball but absent in football players (all findings |T-statistic|> 3.5, p value <0.0001). This pattern was present in the callosum forceps minor, superior longitudinal fasciculus, thalamic radiation, and cingulum hippocampus. Longitudinal differences were more prominent and observed in more tracts in concussed football players (n = 24, |T|> 3.6, p < 0.0001). An analysis of immediate postconcussion scans (n = 12) demonstrated a transient localized increase in axial diffusivity and mean/radial kurtosis in the uncinate and cingulum hippocampus (|T| > 3.7, p < 0.0001). Finally, within football players, those with high position-based impact risk demonstrated increased intracellular volume fraction longitudinally (T = 3.6, p < 0.0001).
Discussion
The observed longitudinal changes seen in football, and especially concussed athletes, could reveal diminished myelination, altered axonal calibers, or depressed pruning processes leading to a static, nondecreasing axonal dispersion. This prospective longitudinal study demonstrates divergent tract-specific trajectories of brain microstructure, possibly reflecting a concussive and repeated subconcussive impact-related alteration of white matter development in football athletes.
Introduction
Mild traumatic brain injury (mTBI) may affect the brains of players with possible long-term cognitive sequelae1,2 in high-contact sports, i.e., those that require physical contact between players leading to repeated subconcussive or concussive impacts, such as American football. Given the millions of people worldwide engaged in contact sports, it is imperative to understand the nature and time course of these brain changes. Diffusion MRI (dMRI) is an imaging modality sensitive to microstructural alterations3 and can noninvasively quantify subtle changes in brain microstructure along fiber tracts associated with sport impacts.4,5
Significant intersubject variability in brain microstructural anatomy complicates interpretation of cross-sectional studies of high-contact players vs controls. Identifying such changes is most specific when compared in reference with an individual subject's baseline imaging, which requires a longitudinal rather than cross-sectional study design. However, relatively few longitudinal studies have investigated these changes in sports. Even fewer studies incorporate baseline imaging, and studies with a duration greater than 1 year are even more scarce6,7 because of their logistical complexity.8
The brain's microstructural anatomy is dynamic and matures over the lifespan.7 Furthermore, it remains unknown which is a greater contributor to potential long-term brain injury: concussions, cumulative high-velocity subconcussive impacts, or both.9 Dissecting the evolution of these changes from normal development requires not only a mix of concussed and unconcussed high-contact players in a longitudinal study but also the inclusion of (unconcussed) low-contact players as a control group, which has been used sparsely in prior work.
dMRI provides a wealth of information but is a technique with myriad challenges and many possible interpretations.10 The most commonly studied metric, fractional anisotropy, can have multiple interpretations as a basis for microstructural changes.11,12 While the diffusion tensor imaging (DTI) model is generally used in clinical and research settings, advanced signal representation or multicompartment modeling techniques, such as diffusion kurtosis imaging (DKI), neurite orientation dispersion and density imaging (NODDI),13 and white matter tract integrity (WMTI), attempt to distinguish linear from nonlinear components of diffusion signals, which may correspond to intracellular and extracellular compartments. These more sophisticated techniques have the potential to disentangle such complex changes and better discern the underlying microstructural phenomena.3
Concussive and subconcussive impacts may differentially affect specific brain tracts or portions of tracts. Furthermore, these effects may interact with developmental and longitudinal neural processes, such as myelination and pruning.14 Thus, imaging studies need to pinpoint and investigate the location, magnitude, and mechanisms of (chronic and acute) tract-based microstructural alterations. However, most prior studies average diffusion changes over the entire length of fiber tracts, potentially obscuring local changes, and use voxel-wise statistics or skeletons that do not ensure voxel correspondence within the same tract across subjects.8,15
In this work, we aim to determine whether there are longitudinal differences between high-contact and low-contact players that reflect underlying brain changes associated with head impacts. Specifically, we study longitudinal alterations in brain microstructure in a high-contact college cohort (football), in comparison with a low-contact college cohort (volleyball) with baseline imaging and follow-up extending out to 4 years. We use a 2-shell dMRI acquisition, and in addition to traditional DTI measures, analyze advanced signal representation (DKI) or multicompartment modeling (NODDI and WMTI) metrics along fiber tracts. We further investigate the acute and chronic effects of both subconcussive and concussive impacts on tract-specific diffusion metrics. Finally, we study the associations between tract-specific diffusion changes with clinical, behavioral, and demographic measures including the Standardized Concussion Assessment Tool (SCAT) score, history of concussion, years of football experience, and player position-based impact risk. We hypothesize that the white matter (WM) will show a lower relative increase in measures of integrity in football when compared with volleyball and that these differences would be associated with SCAT scores and history of concussion.
Methods
Study Cohorts
Inclusion criteria included enrollment in the university and participation in either the university football or volleyball teams. Exclusion criteria included self-reported history of brain surgery, severe brain injury, or major neurologic, psychiatric, or substance abuse disorder. In conjunction with athletic trainers, the study procedures of annual and postconcussive MRI and the goals of studying brain injury were presented to the teams in conjunction with athletic trainers, and eligible (matching both inclusion/exclusion criteria) interested players underwent written informed consent. Almost all players enrolled at the beginning of the season, except for 1 football player who enrolled after a concussion. Players underwent MRI scans (1) annually at the beginning of every season, (2) within 24–96 hours after a concussion (when possible), and (3) at the end of their final athletic season.14 For the first year of this study, players had an extra end-of-season MRI scan.
Football players self-reported their history of prior concussions (number and date of concussion), their years of football involvement before enrolling in the study, and the current position played. One football athlete did not provide years of tackle football experience. Players underwent a pre-first season SCAT (either version 2 or 3) examination.16 The SCAT testing (maximum score 61) included the assessment of the concussion, balance, coordination, and cognitive assessment.14
Each player had 1 to 8 imaging time points, throughout a maximum of 4 playing years. During this study, incidences of concussion were identified by experienced trainers who proactively pulled subjects from the sport for testing after a perceived high-risk contact, loss of consciousness, if their helmet came off, or if there was any evidence on the field of cognitive dysfunction.14 A concussion was verified by team physicians, who documented the patient symptoms (headache, sensitivity to noise or light, nausea, balance problems, neck pain, etc). An experienced certificate of added qualification-certified and board-certified neuroradiologist (M.Z.) blindly reviewed all brain images for incidental findings.14 Three players (9 scans) were excluded due to incidental abnormalities.
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board at Stanford University (#5136 Panel 7) approved this study. All procedures were in accordance with the Health Insurance Portability and Accountability Act. All participants (players) provided written informed consent.
Image Acquisition
A 3-Tesla MRI (GE MR-750, Milwaukee, WI) protocol was acquired (eMethods, links.lww.com/WNL/C970). In brief, for the described analyses, the following sequences were acquired: (1) A 1-mm isotropic T1-weighted scan, (2) multishell whole-brain axial 2D pulsed gradient spin echo dMRI with 60/30 directions at b = 2,500/800 s/mm2 and voxel size=1.875 × 1.875 × 2.0 mm3, and (3) a 1-mm isotropic gradient echo field-map for distortion correction.
Diffusion Metrics
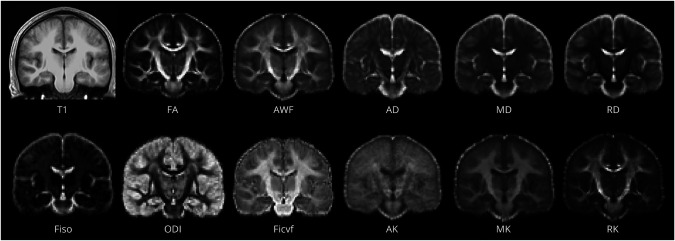
We focused on 9 metrics previously shown to be sensitive to changes after repeated subconcussive events.13,17 Diffusion preprocessing is described in eMethods, links.lww.com/WNL/C970 (diffusion preprocessing). Weighted-least squares diffusion tensor fitting yielded mean, axial, and radial diffusivity (MD, AD, RD) and fractional anisotropy (FA) using FMRIB software library's dtifit. NODDI13 metrics (orientation dispersion [ODI], intracellular volume fraction [FICVF], free water volume fraction) were derived using default parameters and a sigma parameter of 2.0. Kurtosis and WMTI metrics were calculated using DESIGNER software,17 which provided mean, axial, and radial kurtosis (MK, AK, RK), as well as axonal water fraction (AWF) (Figure 1).
Figure 1. Representative Images of All Diffusion-Based Imaging Metrics Used in the Study.
Average maps of first season volleyball players in T1 space through generation of a study-specific atlas (top left). AD = axial Diffusivity; AK = axial kurtosis; AWF = axonal water fraction; FA = fractional anisotropy; FICVF = intracellular volume fraction; FISO = free water (isotropic) volume fraction; MD = mean diffusivity; MK = mean kurtosis; ODI = orientation dispersion index; RD = radial diffusivity; RK = radial kurtosis.
Whole-Brain Tractography
Fiber tracts in each athlete's brain were generated using Mrtrix3 (eMethods, links.lww.com/WNL/C970, Tractography).
Automated Fiber Quantification
To quantify tissue properties along the major fiber tracts in each athlete's brain, we applied the automated fiber quantification (AFQ) method15 (eMethods, links.lww.com/WNL/C970). Briefly, AFQ samples metrics at equally spaced nodes along 20 major tracts. This enables comparisons of different metrics along the extent of fiber pathways. For our analyses, the nodes along each tract were linearly resampled to 10 nodes.
Statistics
Nested linear mixed-effects models were used for all statistical analyses. A linear mixed-effects model examined changes based on fixed effects of sports (football vs volleyball), age at the time of baseline scan, BMI, years after baseline scan (time), and interaction between time and sports. Random effects included subject ID and node number along tract (10 nodes per tract). To control for type 1 errors, we adjusted p values across all tracts and diffusion metrics using “hommel” family-wise error correction using R's p.adjust package (v1.6), a more conservative correction method than the typically used false discovery rate. Statistics were computed using R version 3.6.0 and additionally verified using Stata (v15.0). Normality of residuals was confirmed by plotting histograms and Q-Q plots. T-statistics and p values are reported for the nested mixed-effect models.
Football vs Volleyball Analysis
Applying the mixed-effects model to diffusion metrics, we examined: (1) baseline shifts between sports (effect of sport irrespective of time) and (2) differences between trajectories of football and volleyball players (interaction of time and sport). To visualize node effects (e.g., Figure 2B), a 3-way interaction model (time, sport, and node) was used. We excluded all immediate postconcussion time points in this analysis to focus on the possible chronic effects of injury.
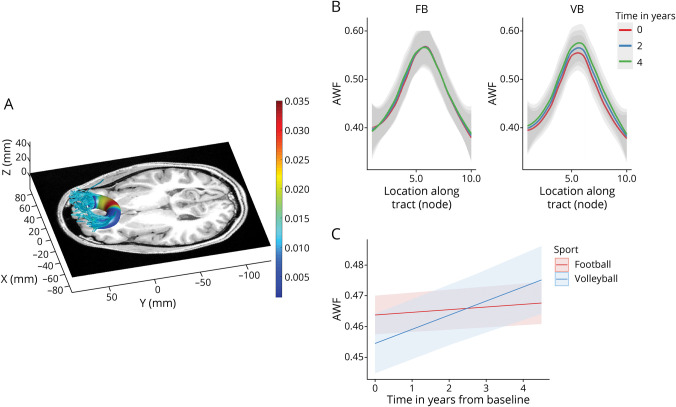
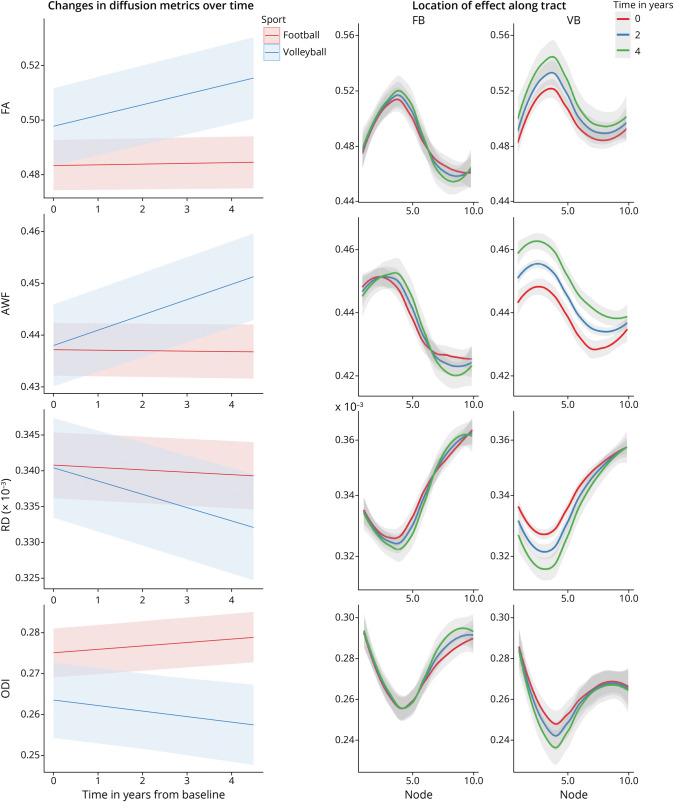
Figure 2. Divergent AWF Trajectory Between Football and Volleyball Players in the Callosum Forceps Minor (Highlighted From Table 2).
(A) Rendering of localized effects. Tractography was performed on a representative volleyball scan. Color coding indicates the node-wise predicted AWF difference between sports using a 3-way interaction model—red indicates larger and blue indicates smaller group differences/effects. (B) Plot of AWF values along the tract nodes (parameterized location 1–10) for football (FB) and volleyball (VB) players for different time points of the study (0 years—red, 2 years—blue, and 4 years—green). (C) The trajectories of AWF values over time across the whole tract, as estimated by the nested linear mixed-effects model, for football (red) and volleyball (blue) players. AWF = axonal water fraction.
Concussion Analyses
We sought to determine whether any prior or in-study concussion had chronic effects on tract microstructure across sports. To assess chronic effects, we excluded immediate postconcussive scans and used the above model, but separated our cohort into 3 groups: football players with prior (concussions sustained before study enrollment) and/or in-study concussion (sustained during the study), football players with no prior or in-study concussions, and volleyball players. We performed all 2-way comparisons of these 3 cohorts.
To examine acute effects of concussion, we analyzed football players scanned within 1 week of sustaining a concussion, comparing (1) baseline scans (preconcussion) with scans immediately following a player's concussion (concussion scans) and (2) concussion scans with follow-up non–post-concussive scans. Two previously excluded football players (because their only follow-up MRI was less than 6 months after baseline) were included in this analysis. For this analysis, we included 2 additional metrics putatively sensitive to postconcussive changes along the axons,18,19 AD and AK.
Within Football Analyses
To further interrogate the effect of subconcussive impacts, we investigated the association between localized microstructural changes in football players (at baseline and longitudinally) with the presence of prior concussions, prior football exposure, player position-based head impact risk, and baseline (global) SCAT.16 For the presence of prior concussions, a binary variable represented whether subjects had either a prior or an in-study concussion. To test whether position-based risk of impact was associated with microstructural changes, we relied on previously reported high position-based impact risk (HITsp) values per player position as an estimate of the severity of impacts.20 HITsp is a (composite) measure that quantifies a player's risk based on hit impact rotational and linear acceleration, quantity, duration, and location for other players of the same position.14 Nested linear mixed-effects models were again used. The results were corrected using “hommel’ adjustment separately for each behavior but across all microstructural metrics and nodes.
Data Availability
All data associated with this study are available in the main text or the supplementary materials. All the imaging data can be shared on request with a proposal and under a data transfer agreement.
Results
Cohort Demographics
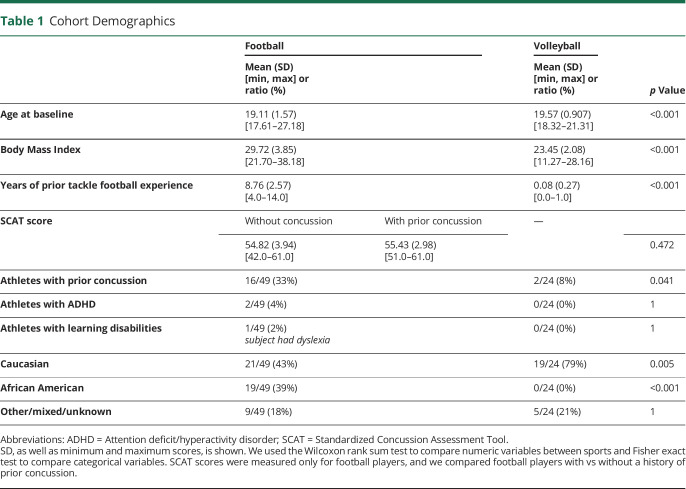
Sixty-three football (high-contact) and 34 volleyball (low-contact) college players were enrolled in our study prospectively (eFigure 1, links.lww.com/WNL/C970). There was a significant difference in age at baseline between football and volleyball players (football: 19.11  1.57 years, volleyball: 19.57
1.57 years, volleyball: 19.57  0.91 years, p < 0.001; Table 1) as well as in body mass index (BMI, football: 29.72
0.91 years, p < 0.001; Table 1) as well as in body mass index (BMI, football: 29.72  3.85, volleyball: 23.45
3.85, volleyball: 23.45  2.08, p < 0.001). A subset of volleyball players had prior tackle football experience; however, it was substantially shorter compared with football: 0.08 years (range: 0–1) vs 8.76 years (range: 4–14, p < 0.001). Sixteen of 49 football and 2 of 24 volleyball players experienced concussions before this study (p = 0.04). One football player had 3 prior concussions, 3 had 2 prior concussions, and the rest had 1 prior concussion. SCAT was only collected in football players, and there were no significant differences in baseline SCAT scores between players with vs without prior concussion. Football had very low instances of ADHD (2 players) and learning disabilities (1 player), whereas volleyball had none (no statistical difference). In football, there were significantly more African American players (19/49) compared with volleyball (0/24) (p < 0.001), and significantly fewer White players (21/49 vs 19/24 in volleyball, p = 0.005).
2.08, p < 0.001). A subset of volleyball players had prior tackle football experience; however, it was substantially shorter compared with football: 0.08 years (range: 0–1) vs 8.76 years (range: 4–14, p < 0.001). Sixteen of 49 football and 2 of 24 volleyball players experienced concussions before this study (p = 0.04). One football player had 3 prior concussions, 3 had 2 prior concussions, and the rest had 1 prior concussion. SCAT was only collected in football players, and there were no significant differences in baseline SCAT scores between players with vs without prior concussion. Football had very low instances of ADHD (2 players) and learning disabilities (1 player), whereas volleyball had none (no statistical difference). In football, there were significantly more African American players (19/49) compared with volleyball (0/24) (p < 0.001), and significantly fewer White players (21/49 vs 19/24 in volleyball, p = 0.005).
Table 1.
Cohort Demographics
The football cohort consisted of 25 players (100 total scans) without prior or in-study concussion and 24 players (80 scans) with a prior or in-study concussion. In total, 18 concussions occurred in 14 football players, 3 of whom had at least 2 concussions. One of these 14 was excluded from postconcussion analysis because their MRI was performed at a delay at 2 weeks postconcussion. Postconcussion MRI within 1 week was thus captured for 13 of these 18 concussions, among 12 football players (eFigure 1, links.lww.com/WNL/C970). For 1 of the 12 players with postconcussion MRIs, their first imaging time point was 2 days postconcussion, without prior baseline imaging, so their 2.5 months of follow-up MRI served as a baseline. One player had 2 concussions with postconcussion MRIs, but only the latter scan, which had a longer return-to-play time, was used in the concussion analysis. Of the 13 concussions, all were sports-related (with most being due to head-to-head contact), none involved loss of consciousness, and the mean return-to-play time was 10.3 days (excluding 1 athlete who did not return to play). For these postconcussion scans, the average time to scan from concussion was 1.83  1.26 days (range: 1–4 days) and the average time from concussion to follow-up scan was 165 ± 77 days (range 58–280). Four volleyball players suffered in-study concussions, and their subsequent scans were excluded from further analysis.
1.26 days (range: 1–4 days) and the average time from concussion to follow-up scan was 165 ± 77 days (range 58–280). Four volleyball players suffered in-study concussions, and their subsequent scans were excluded from further analysis.
Longitudinal Differences in Diffusion Metrics Between Sports
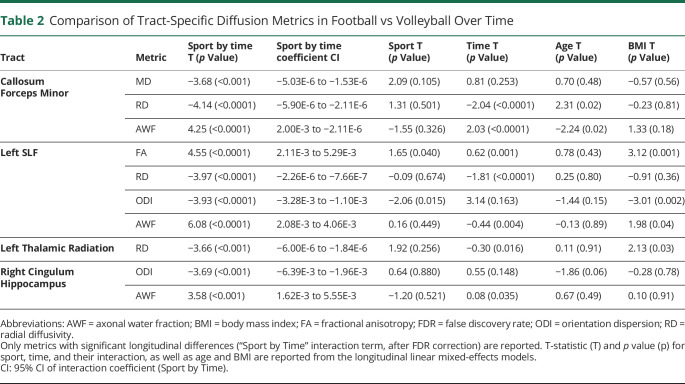
For the longitudinal analyses, we only included players with 2 or more scans that were at least 6 months apart, which resulted in 271 scans from 73 subjects, 193 scans from 49 football players and 78 scans from 24 volleyball players (eFigures 1 and 2, links.lww.com/WNL/C970). Football players had significantly divergent trajectories in multiple microstructural metrics and tracts compared with volleyball controls (Table 2), specifically in the forceps minor of corpus callosum, left superior longitudinal fasciculus [SLF], left thalamic radiation, and right cingulum hippocampus (absolute T-statistic “|T|” > 3.5, p < 0.0001). The temporal patterns were uniform across tracts: AWF and FA increased and MD/RD/ODI decreased in volleyball relative to football. In the callosum forceps minor, volleyball controls showed a decreasing MD and RD and increasing AWF over time, an effect substantially diminished in football players (Table 2, Sport by Time; eFigure 3). Figure 2 presents the longitudinal AWF differences between sports in the callosum forceps minor, both within locations (nodes) along the tract (Figure 2B) and across the longitudinal trajectory, based on the nested linear mixed-effects model (incorporating all the tract nodes) (Figure 2C).
Table 2.
Comparison of Tract-Specific Diffusion Metrics in Football vs Volleyball Over Time
The left SLF exhibited the largest interaction “Sport by Time” effects (for 4 diffusion metrics) (Table 2, Figure 3). As seen in the nested model over time (Figure 3, left), as well as in the distribution across the tract nodes (Figure 3, right), the low-contact (volleyball) players showed an increase in FA and AWF and decrease in RD and ODI with time. However, this longitudinal change was highly attenuated in high-contact (football) players. In these analyses, BMI and age were not significant (did not survive correction), except for 2 models where BMI was significant in the left SLF (with FA and ODI metrics), with negligible effects on diffusion findings. We relied on 2 layers of outlier detection to ensure only successful tractography, and quantification was included in the results (eMethods; eFigures 4-6, links.lww.com/WNL/C970). The results were unchanged when handedness was accounted for.
Figure 3. Longitudinal Diffusion Metrics in Left Superior Longitudinal Fasciculus (SLF) in Football (FB) vs Volleyball (VB) Players.
Left: The trajectories of diffusion metrics values over time across the whole SLF, as estimated by the nested linear mixed-effects model, for football (red) and volleyball (blue) players. Right: The same diffusion metrics for football (FB, left column) and volleyball (VB, right column), plotted along the tract nodes (parameterized location 1–10), for 3 distinct time points over the course of the study (0 years—red, 2 years—blue, and 4 years—green).
Effect of Concussions
Chronic Effects of Prior or In-Study Concussions
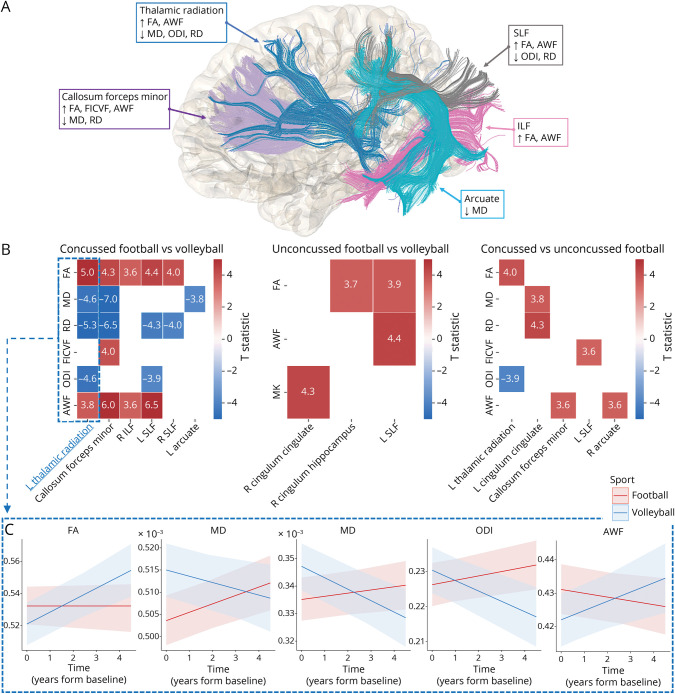
Of 49 football players, 25 did not report any concussions, 11 had prior concussions, 8 had in-study concussions, and 5 had both. The results of the nested linear mixed-effects models comparing volleyball (n = 24) and concussed football players (n = 24) are summarized graphically in Figure 4A, with model statistics presented in eTable 1, links.lww.com/WNL/C970. Football players with prior or in-study concussion exhibited widespread differences in multiple diffusion metrics and tracts when compared with volleyball controls (|T|> 3.6, p < 0.0001; Figure 4B; eTable 1, concussed football vs volleyball). Football players without a history of concussion (n = 25) similarly showed few differences over time against volleyball (Figure 4B; eTable 1, unconcussed football vs volleyball). A direct longitudinal comparison of concussed vs unconcussed football players (Figure 4B, eTable 1) showed that unconcussed players have relative increases in FA/AWF and decreases in RD/ODI over time compared with concussed players.
Figure 4. Longitudinal Comparison Between (1) Volleyball Players, (2) Football Players With Prior or In-Study Concussion (Concussed), or (3) Without Concussion (Unconcussed).
(A) Summary of tract-specific findings for volleyball vs concussed football players. Upward and downward arrows within each box represent significant longitudinal metric increases and decreases, respectively, in volleyball compared with concussed football players within respective tracts. (B) Heatmaps summarizing tract-specific findings across all comparisons between the 3 groups. Longitudinal changes were more prominent and observed in more tracts when volleyball was compared with concussed football players (left). Heatmap values represent the T-statistic of the “Group x Time” variable from the nested mixed-effect models. Nonsignificant findings are depicted as blank cells. (C) Trajectories of diffusion metric values over time across the left thalamic radiation, as estimated by the nested linear mixed-effects model, for concussed football (red) and volleyball (blue) players.
Acute Effects of Concussions
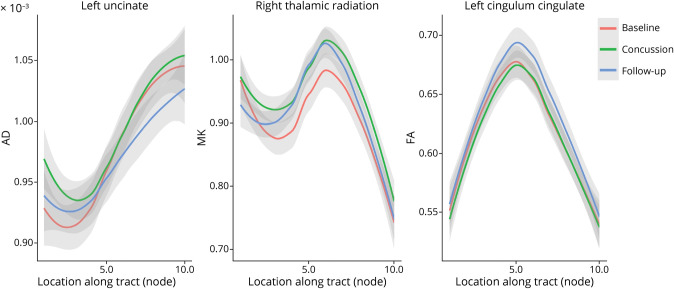
Figure 5 presents the examples of longitudinal metric changes within fiber tracts for 2 effects (baseline vs concussion and concussion vs follow-up). Acutely after concussion, we observed a localized increase in AD in both the left uncinate and right cingulum hippocampus. This significant increase was also observed in MK and RK within the same tracts. This increase in diffusion metrics was transient and decreased significantly at the follow-up scan (Figure 5A; eTable 2, links.lww.com/WNL/C970).
Figure 5. Acute Effects of Concussion Assessed by Comparing (1) Baseline to Immediately Postconcussion (Concussion) Scans and (2) Concussion to Follow-up Scans.
Examples of diffusion metric changes across nodes in baseline, concussion, and follow-up scans: Changes in AD within the left uncinate tract (left) represent a finding exhibiting both effects: baseline vs concussion (T-statistic: 3.9; p value: <0.001) and concussion vs follow-up (T-statistic: −4.2; p value: <0.001) (eTable 2, links.lww.com/WNL/C970). MK differences in the right thalamic radiation (middle) represent changes from baseline to concussion (T-statistic: 4.05; p value: <0.001), while FA differences in the left cingulum cingulate (right) represent changes from concussion to follow-up (T-statistic: 5.69; p value: <0.001). AD = axial diffusivity; FA = fractional anisotropy; MK = mean kurtosis.
Effect of Player Position
The left cingulum hippocampus showed a group difference (main effect) where football players with high position-based impact risk (HITsp) had higher ODI (T = 3.9, p = 0.0002, eFigure 7, left, links.lww.com/WNL/C970). The right SLF showed an interaction between time and position-based impact risk, where players with higher position-based impact risk had a greater increase in FICVF over time (T = 3.6, p = 0.0003, eFigure 7, right). No other position-based impact risk effects passed multiple comparison correction. Baseline SCAT score, prior football exposure, and prior concussion exposure did not show a statistical relationship to dMRI metrics.
Discussion
This study examined brain microstructure in high-contact (football) and low-contact (volleyball) college athletes across multiple seasons using advanced dMRI metrics and fiber quantification tools. In volleyball players, we found longitudinally decreasing RD, MD, and ODI, and increasing FA and AWF, in multiple tracts including callosum forceps minor, left SLF, left thalamic radiation, and right cingulum hippocampus, while in football players, these changes were relatively attenuated or absent. These effects were more prominent and observed in a larger number of tracts when volleyball players were compared with football players with a prior or in-study concussion, who showed a similar relative absence of FA/AWF increases and RD/ODI decreases over time. Football players who had a concussion during the study showed a transient localized increase in AD, MK, and RK in the left uncinate and right cingulum hippocampus. Finally, studying player position effect on microstructural changes in football revealed an increase in ODI over time in football players with high position-based impact risk. Overall, our results suggest that possible normative changes in diffusion metrics, which may reflect healthy brain developmental trajectories, may be significantly altered in football athletes.
Multicompartment longitudinal modeling demonstrated decreased tract-specific diffusivity and dispersion perpendicular to the primary pathway over time in low-contact athletes, whereas in high-contact athletes, these changes were attenuated. This finding is consistent with existing literature, suggesting decreasing tract diffusivity, including RD,21 MD,4 and ODI,22 in noncontact or low-contact sports compared with high-contact sports, particularly in the corpus callosum, where we additionally observed increasing AWF. In addition, we observed an increase in FA longitudinally in volleyball compared with football, consistent with findings in studies including noncontact sports vs a high-contact rugby cohort.21 WMTI and kurtosis metrics can be sensitive markers of WM injury after head impacts23,24 because they enable the quantification of non-Gaussian water diffusion and might be sensitive to effects such as inflammatory responses.25 Using these metrics in addition to DTI and NODDI enhance our ability to decipher underlying microstructural mechanisms and may be revealing in clinical studies of brain injury.
From a developmental perspective, myelination is thought to increase into adulthood and peak around 20–40 years,26-28 while pruning is generally considered complete by puberty/early adolescence.29 Although recent research suggests that pruning may continue to a lesser degree, into early adulthood,30 myelination is generally accepted to peak later in life. Myelination, as measured by DTI, has been mainly observed as FA increases, with MD and RD decreases, during childhood and adolescence. The relative attenuation of increasing AWF/FA and decreasing RD/ODI seen in football players could possibly reveal delayed myelination, altered axonal caliber, or depressed pruning processes leading to increased axonal dispersion.27 This potential delayed maturation in the WM parallels our prior finding of divergent trajectories in the GM (cortical thinning) in football vs volleyball players.14 These microstructural alterations could potentially render athletes more vulnerable to future injury, reduce cognitive reserve, and exacerbate cognitive sequalae of subsequent concussions. For clinical implications, the present multiseason results raise concerns about the cumulative effects of repeated impacts during adolescence and later in life. Inclusion of additional myelin or demyelination sensitive imaging sequences, such as magnetization transfer imaging, in future work may further enable distinguishing these cellular and developmental processes and their effects on WM maturation.
Players with a concussion (prior or in-study) had more extensive differences when compared with volleyball controls with larger divergences in multiple diffusion metrics and across several tracts (including callosum forceps minor, SLF and thalamic radiation), whereas those without prior or in-study concussions had smaller effect sizes and fewer group differences in metrics and tracts (eTable 1, links.lww.com/WNL/C970). These changes mirror the results where all football players were compared with volleyball players, with the same relative absence of FA/AWF increases and RD/ODI decreases over time. Most (but not all) “Sport by Time” interaction findings observed in the whole cohort analysis were also observed in the concussed football vs volleyball analysis, pointing to (prior or in-study) concussions being a strong determinant of chronic microstructural alterations. Nonetheless, some longitudinal alterations were only observed in the unconcussed football vs volleyball players, suggesting that microstructural alterations may be partially attributed to repetitive subconcussive impacts only. Similarly, some changes, such as FA and AWF in the left SLF, were observed in both analyses (albeit stronger in the concussed group), pointing toward a potential synergistic effect of concussive and subconcussive impacts. A direct longitudinal comparison of concussed vs unconcussed football players showed that concussed players have significantly divergent trajectories, with relative decreases in FA/AWF and increases MD, RD, and ODI over time (eTable 1). Taken together, this suggests that unconcussed football players may exhibit an intermediate phenotype between concussed football and volleyball. These results highlight that repetitive impacts experienced by football players affect multiparametric diffusion measures, even in the absence of concussion. Our findings support the hypothesis that concussive and subconcussive impacts may alter brain development and may drive divergent trajectories between low-contact and high-contact athletes.
These chronic effects are supported by several studies of WM abnormalities in concussed high-contact athletes in comparison with low-contact controls followed over a single season, where concussed players exhibited (1) decreased FA in tracts including the corpus callosum and cingulum hippocampus, associated with worsening impulse control31 and memory performance32 or (2) elevated MD and/or RD in the corpus callosum,33 the SLF, and corticospinal tract.34 The corpus callosum is a common site of microstructural injury resulting from concussive impacts.35 Finite element analyses demonstrate that the corpus callosum is sensitive to rotations and shear forces, leading to higher tract-oriented strains,36 potentially due to lateral motion of the stiff falx.37 Callosal injury is thought to affect perception, orientation, and reaction time.38 Other tracts we found that commonly exhibit trauma-related injury include the thalamic radiation, cingulum, and cortico-spinal tract.35
While several studies confirm our findings, some cross-sectional studies have reported opposing changes in diffusion metrics, i.e., elevated FA and lower MD and ODI in the concussed group.39,40 The discrepancies in diffusion metric directionality between dMRI studies may be due to the different investigated timelines and scan intervals of acute/subacute vs chronic effects postinjury (ranging from days to months or beyond) (see eDiscussion for detailed discussion). Other factors contributing to these differences may be the large heterogeneity across these athletes and concussed populations at baseline, the heterogeneity of microstructural changes in mTBI across different tracts, and the complexity of comparing across distinct image analysis techniques (voxel-wise, skeletons, regions of interests, tract-based) and imaging protocols. These discrepancies support the need for a longitudinal, multisite study design that accounts for baseline differences and more sophisticated diffusion metrics that have higher specificity to underlying intracellular and extracellular processes.
Football players who had a concussion during the study showed a transient localized increase in AD, MK, and RK in the left uncinate and right cingulum hippocampus after concussion, compared with their preconcussion scan. These effects seemed to only be transient and decreased significantly at follow-up. Previous work similarly found acutely elevated AD in the right corticospinal tract relative to unconcussed athletes at both 6 days and 6 months postconcussion.41 Another study found elevated AD and RD in the genu of the corpus callosum within 2 weeks of TBI.42 By contrast, reduced postconcussion AD has been reported over shorter periods,43,44 but without baseline scans and with different statistical techniques. Lancaster et al.44 also observed more widespread elevated AK, consistent with our detected increased kurtosis metrics. The dynamics of microstructural changes preinjury and postinjury are complex4 and depend on factors such as severity of injury, duration of follow-up, scan intervals, and assessed metrics. The transient increase in kurtosis metrics and AD we observed may reflect altered diffusion possibly due to diffuse axonal injury, axonal beading, and/or an acute neuroinflammatory response and microglial activation.19 These changes are subtle and best detected in a group longitudinal study rather than in individuals. Future work should use advanced modeling of microstructure in combination with neuroinflammatory biomarkers to investigate underlying acute physiologic mechanisms postconcussion.
We observed that players with HITsp had a greater increase in FICVF over time in the right SLF and an increased ODI in the left cingulum hippocampus at baseline. This effect mirrors some of our main longitudinal findings in football vs volleyball players, possibly reflecting the same underlying biological mechanism. The lack of other behavioral and clinical findings in our cohort may support the need for development of more sensitive clinical tools, e.g., novel cognitive tests and biomarkers, that target these subconcussive effects. These findings may suggest that caution is warranted in regard to clinical evaluation and return-to-play time when assessing impacts of athletes with higher position-based impact risk because these clinically silent microstructural changes may be potentially exacerbated by repeated injury.
This study should be interpreted considering some limitations. While we used advanced mapping techniques to localize changes along the length of tracts (avoiding biases of averaging and miscorrespondences of voxel/skeleton approaches), this technique averages effects per node across tracts and subjects. Future work should assess subject-specific changes across the whole brain. Although we followed the players for a period of up to 4 years, we only enrolled male athletes from one institution, and the number of in-study concussions was limited, arguing for larger studies of more sports across multiple institutions and including both sexes. Player concussion history was self-reported, allowing for underreported previous concussions. Similarly, participation in other high-contact sports should be formally quantified in future studies. We used the global SCAT score; future work can examine SCAT subscores and other more detailed neurocognitive testing.
Our study used advanced dMRI metrics based on signal representation (diffusion tensor and kurtosis) and multicompartment modeling (NODDI and WMTI) to assess chronic and acute microstructural changes in collegiate athletes of high-contact sports, with low-contact sport athletes as a control population. We found the callosum forceps minor and left SLF to be the most affected tracts, with microstructural changes in all our cohort comparisons. While the control, low-contact population showed changes that could signal higher myelination, such as lower RD, MD, and ODI and higher FA and AWF over time, these changes were attenuated in high-contact athletes. This study contributes to the body of literature examining the chronic effects of high-contact sports, leveraging multiyear study duration, advanced metrics, and tract specificity. The clinical impact is the consideration of possibly abnormal brain development measured at the group level associated with concussive impacts, with additional recognition of the presence of lesser albeit similar brain changes even in the group who did not have diagnosed concussions. Future longitudinal studies that include more sports will complement our understanding of brain changes due to head impacts, while combined prospective imaging and histopathology studies in animals or humans45,46 might elucidate the pathologic correlates of imaging changes.
Acknowledgment
The authors are grateful for assistance with player enrollment from Scott Anderson, Lexie Ross, Steven Bartlinsky, Anthony Pass Christopher Barrett, and Eitan Gelber. This article does not reflect official views of the Pac-12.
Glossary
- AD
axial diffusivity
- AFQ
automated fiber quantification
- AK
axial
- AWF
axonal water fraction
- BMI
body mass index
- DKI
diffusion kurtosis imaging
- dMRI
diffusion MRI
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FICVF
intracellular volume fraction
- HITsp
high position-based impact risk
- MD
mean diffusivity
- MK
mean kurtosis
- mTBI
mild traumatic brain injury
- NODDI
neurite orientation dispersion and density imaging
- ODI
orientation dispersion
- RD
radial diffusivity
- RK
radial kurtosis
- SCAT
Standardized Concussion Assessment Tool
- SLF
superior longitudinal fasciculus
- WM
white matter
- WM
white matter
- WMTI
white matter tract integrity
Appendix. Authors
Footnotes
Editorial, page 380
Study Funding
This research was conducted with funding from the Radiology Society of North America and American Society for Neuroradiology, the Center for Biological Imaging at Stanford, and General Electric Healthcare. This publication was also supported by the Pac-12 Conference's Student-Athlete Health and Well-Being Initiative.
Disclosure
M. Zeineh receives research funding from GE Healthcare. All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Manley G, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med. 2017;51(12):969-977. doi. 10.1136/bjsports-2017-097791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAllister T, McCrea M. Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J Athl Train. 2017;52(3):309-317. doi. 10.4085/1062-6050-52.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novikov DS, Fieremans E, Jespersen SN, Kiselev VG. Quantifying brain microstructure with diffusion MRI: theory and parameter estimation. NMR Biomed. 2019;32(4):e3998. doi. 10.1002/nbm.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAllister TW, Ford JC, Flashman LA, et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82(1):63-69. doi. 10.1212/01.wnl.0000438220.16190.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sollmann N, Echlin PS, Schultz V, et al. Sex differences in white matter alterations following repetitive subconcussive head impacts in collegiate ice hockey players. NeuroImage Clin. 2018;17:642-649. doi. 10.1016/j.nicl.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning KY, Brooks JS, Dickey JP, et al. Longitudinal changes of brain microstructure and function in nonconcussed female rugby players. Neurology. 2020;95(4):E402-E412. doi. 10.1212/WNL.0000000000009821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang YS, Owen JP, Pojman NJ, et al. White matter changes of neurite density and fiber orientation dispersion during human brain maturation. PLoS One. 2015;10(6):e0123656. doi. 10.1371/journal.pone.0123656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tayebi M, Holdsworth SJ, Champagne AA, et al. The role of diffusion tensor imaging in characterizing injury patterns on athletes with concussion and subconcussive injury: a systematic review. Brain Inj. 2021;35(6):621-644. doi. 10.1080/02699052.2021.1895313 [DOI] [PubMed] [Google Scholar]
- 9.Champagne AA, Coverdale NS, Germuska M, Bhogal AA, Cook DJ. Changes in volumetric and metabolic parameters relate to differences in exposure to sub-concussive head impacts. J Cereb Blood Flow Metab. 2020;40(7):1453-1467. doi. 10.1177/0271678X19862861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239-254. doi. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- 11.Alba-Ferrara LM, de Erausquin GA. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Neurosci. 2013;7:9. doi. 10.3389/fnint.2013.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douaud G, Jbabdi S, Behrens TEJ, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage. 2011;55(3):880-890. doi. 10.1016/j.neuroimage.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016. doi. 10.1016/j.neuroimage.2012.03.072 [DOI] [PubMed] [Google Scholar]
- 14.Mills BD, Goubran M, Parivash SN, et al. Longitudinal alteration of cortical thickness and volume in high-impact sports. Neuroimage. 2020;217:116864. doi. 10.1016/j.neuroimage.2020.116864 [DOI] [PubMed] [Google Scholar]
- 15.Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11):e49790. doi. 10.1371/journal.pone.0049790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. Br J Sports Med. 2009;43(suppl 1):i76-i84. doi. 10.1136/bjsm.2009.058248 [DOI] [PubMed] [Google Scholar]
- 17.Ades-Aron B, Veraart J, Kochunov P, et al. Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. NeuroImage. 2018;183:532-543. doi. 10.1016/j.neuroimage.2018.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: clues to axon and myelin repair capacity. Exp Neurol. 2016;275:328-333. doi. 10.1016/j.expneurol.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 19.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35-43. doi. 10.1016/j.expneurol.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crisco JJ, Wilcox BJ, Beckwith JG, et al. Head impact exposure in collegiate football players. J Biomech. 2011;44(15):2673-2678. doi. 10.1016/j.jbiomech.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefebvre G, Guay S, Chamard E, et al. Diffusion tensor imaging in contact and non-contact university-level sport athletes. J Neurotrauma. 2021;38(5):529-537. doi. 10.1089/neu.2020.7170 [DOI] [PubMed] [Google Scholar]
- 22.Mayer AR, Ling JM, Dodd AB, Meier TB, Hanlon FM, Klimaj SD. A prospective microstructure imaging study in mixed-martial artists using geometric measures and diffusion tensor imaging: methods and findings. Brain Imaging Behav. 2017;11(3):698-711. doi. 10.1007/s11682-016-9546-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davenport EM, Apkarian K, Whitlow CT, et al. Abnormalities in diffusional kurtosis metrics related to head impact exposure in a season of high school varsity football. J Neurotrauma. 2016;33(23):2133-2146. doi. 10.1089/neu.2015.4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung S, Wang X, Fieremans E, et al. Altered relationship between working memory and brain microstructure after mild traumatic brain injury. Am J Neuroradiol. 2019;40(9):1438-1444. doi. 10.3174/ajnr.A6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braeckman K, Descamps B, Pieters L, Vral A, Caeyenberghs K, Vanhove C. Dynamic changes in hippocampal diffusion and kurtosis metrics following experimental mTBI correlate with glial reactivity. NeuroImage Clin. 2019;21:101669. doi. 10.1016/j.nicl.2019.101669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan KM, Kamali A, Abid H, Kramer LA, Fletcher JM, Ewing-Cobbs L. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Struct Funct. 2010;214(4):361-373. doi. 10.1007/s00429-009-0238-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340-352. doi. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- 28.Westlye LT, Walhovd KB, Dale AM, et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055-2068. doi. 10.1093/cercor/bhp280 [DOI] [PubMed] [Google Scholar]
- 29.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31(19):7174-7177. doi. 10.1523/JNEUROSCI.0054-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petanjek Z, Judaš M, Šimić G, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108(32):13281-13286. doi. 10.1073/pnas.1105108108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazarian JJ, Zhu T, Zhong J, et al. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9(4):e94734. doi. 10.1371/journal.pone.0094734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuzminski SJ, Clark MD, Fraser MA, et al. White matter changes related to subconcussive impact frequency during a single season of high school football. Am J Neuroradiol. 2018;39(2):245-251. doi. 10.3174/ajnr.A5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YC, Harezlak J, Elsaid NMH, et al. Longitudinal white-matter abnormalities in sports-related concussion: a diffusion MRI study. Neurology. 2020;95(7):e781-e792. doi. 10.1212/WNL.0000000000009930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koerte IK, Kaufmann D, Hartl E, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus. 2012;33(6):E3. doi. 10.3171/2012.10.FOCUS12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gumus M, Santos A, Tartaglia MC. Diffusion and functional MRI findings and their relationship to behaviour in postconcussion syndrome: a scoping review. J Neurol Neurosurg Psychiatry. 2021;92(12):1259-1270. doi. 10.1136/jnnp-2021-326604 [DOI] [PubMed] [Google Scholar]
- 36.Viano DC, Casson IR, Pellman EJ, Zhang L, King AI, Yang KH. Concussion in professional football: brain responses by finite element analysis: part 9. Neurosurgery. 2005;57(5):891-916. doi. 10.1227/01.NEU.0000186950.54075.3B [DOI] [PubMed] [Google Scholar]
- 37.Hernandez F, Giordano C, Goubran M, et al. Lateral impacts correlate with falx cerebri displacement and corpus callosum trauma in sports-related concussions. Biomech Model Mechanobiol. 2019;18(3):631-649. doi. 10.1007/s10237-018-01106-0 [DOI] [PubMed] [Google Scholar]
- 38.Arenth PM, Russell KC, Scanlon JM, Kessler LJ, Ricker JH. Corpus callosum integrity and neuropsychological performance after traumatic brain injury: a diffusion tensor imaging study. J Head Trauma Rehabil. 2014;29(2):E1-E10. doi. 10.1097/HTR.0b013e318289ede5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Churchill NW, Caverzasi E, Graham SJ, Hutchison MG, Schweizer TA. White matter microstructure in athletes with a history of concussion: comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum Brain Mapp. 2017;38(8):4201-4211. doi. 10.1002/hbm.23658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lancaster MA, Meier TB, Olson DV, McCrea MA, Nelson LD, Muftuler LT. Chronic differences in white matter integrity following sport-related concussion as measured by diffusion MRI: 6-month follow-up. Hum Brain Mapp. 2018;39(11):4276-4289. doi. 10.1002/hbm.24245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry LC, Tremblay J, Tremblay S, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28(10):2049-2059. doi. 10.1089/neu.2011.1836 [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Gupta RK, Husain M, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23(7-8):675-685. doi. 10.1080/02699050903014915 [DOI] [PubMed] [Google Scholar]
- 43.Pasternak O, Koerte IK, Bouix S, et al. Hockey concussion education project, part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. J Neurosurg. 2014;120(4):873-881. doi. 10.3171/2013.12.JNS132090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lancaster MA, Olson DV, McCrea MA, Nelson LD, LaRoche AA, Muftuler LT. Acute white matter changes following sport-related concussion: a serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum Brain Mapp. 2016;37(11):3821-3834. doi. 10.1002/hbm.23278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uretsky M, Bouix S, Killiany RJ, et al. Association between antemortem FLAIR white matter hyperintensities and Neuropathology in brain donors exposed to repetitive head impacts. Neurology. 2022;98(1):e27-e39. doi. 10.1212/WNL.0000000000013012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360-370. doi. 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data associated with this study are available in the main text or the supplementary materials. All the imaging data can be shared on request with a proposal and under a data transfer agreement.