Abstract
Ictal asystole is a rare condition associated primarily with temporal lobe epilepsy that can cause syncope, falls, and head trauma. It is also associated with increased rates of sudden unexplained death in epilepsy. We present a case of a 33-year-old woman with a history of childhood epilepsy who presented with 3 years of recurrent syncope. Video-EEG revealed temporal lobe seizures with ictal asystole. EKG showed stepwise progression of bradycardia, asystole, and tachycardia. MRI showed focal cortical thickening at the right insular cortex with blurring of the gray-white matter interface, consistent with insular focal cortical dysplasia. The patient was transitioned from lacosamide to clobazam because of concern for PR interval prolongation and was referred to cardiology for pacemaker placement. Ictal asystole should be considered as a rare but serious cause of unexplained recurrent syncope, particularly in patients with a history of seizures. Management includes antiepileptic drug regimen optimization, consideration of epilepsy surgery, and referral for cardiac pacing when asystole lasts longer than 6 seconds.
Pearls
Ictal asystole is a cause of recurrent unexplained syncope.
Ictal asystole <6 seconds should be treated with antiseizure medication optimization and epilepsy surgery, when applicable.
Ictal asystole >6 seconds should be referred to cardiology for potential permanent pacemaker placement.
Oy-sters
The frequency of ictal asystole is likely underestimated as long-term video-EEG and associated EKG recording is required to make the diagnosis.
Arrhythmogenic antiepileptic drugs, such as sodium channel blockers, may increase risk of arrhythmias (ictal or postictal) and/or may prolong recovery.
Case Report
A 33-year-old woman with a history of childhood epilepsy was admitted to the epilepsy monitoring unit (EMU) for characterization of new-onset recurrent spells. Her seizures started at the age of 11 years and consisted of nocturnal convulsions and staring spells that were well-controlled with carbamazepine monotherapy. She was initially believed to have benign epilepsy with centrotemporal spikes, also known as benign rolandic epilepsy. She had a normal neurologic examination at admission.
Three years before EMU admission, she began experiencing new episodes characterized by slumping over and/or sudden collapse with occasional stiffening of upper extremities occurring up to once per week. She had no aura or incontinence and no postevent confusion. The episodes were refractory to medication trials, including lacosamide, cenobamate, and levetiracetam. Her home regimen at the time of EMU admission included levetiracetam 1500 mg twice a day, lacosamide 200 mg twice a day, and cenobamate 100 mg once a day. She had previously been on valproic acid, oxcarbazepine discontinued because of hyponatremia, and carbamazepine discontinued because of hyponatremia and pancytopenia. She had a normal neurologic examination.
She had prior EEG, MRI, PET, and ECG scans. An EEG performed in February 2018 showed a single sharp form over the left temporal region. A subsequent EEG in September 2021 was read as normal with no seizures or epileptiform discharges. MRI scans in January 2018 and December 2021 were both normal, as was her PET in December 2021. Prior EKG in 2019 showed HR 100, PR 140, QRS 90, and QTc 434 with nonspecific T-wave abnormalities in the inferior leads. Similar findings were seen on an EKG from 2021. The patient was admitted to the EMU in March 2022. At that time, video-EEG demonstrated right temporal lobe seizures with ictal asystole (Figure 1). On interictal EEG, there was intermittent paroxysmal fast activity over the right temporal region. Seizures electrographically began with right temporal focal fast activity, which would evolve bitemporally, followed by a diffuse generalized suppression. In sleep, there was no visible clinical change during the seizures, besides the ictal bradycardia and ictal asystole. In wakefulness, after the ictal onset of her seizure, there were associated heart rate changes. Her clinical symptoms on video correlated with those heart rate changes, such that ictal bradycardia correlated with pallor and ictal asystole correlated clinically with loss of muscle tone and electrographically with diffuse suppression on EEG. After her heart rate resumed, electrographically, there was emergence of diffuse delta slowing and clinically, intermittent myoclonic jerks occurred, likely as a result of cerebral reperfusion. She reported no aura. She had a total of 4 seizures during her EMU admission. The patient's baseline EKG showed HR 70, PR 148, QRS 92, and QTc 398 and redemonstrated the nonspecific T-wave abnormalities. She had no interictal EKG pathology.
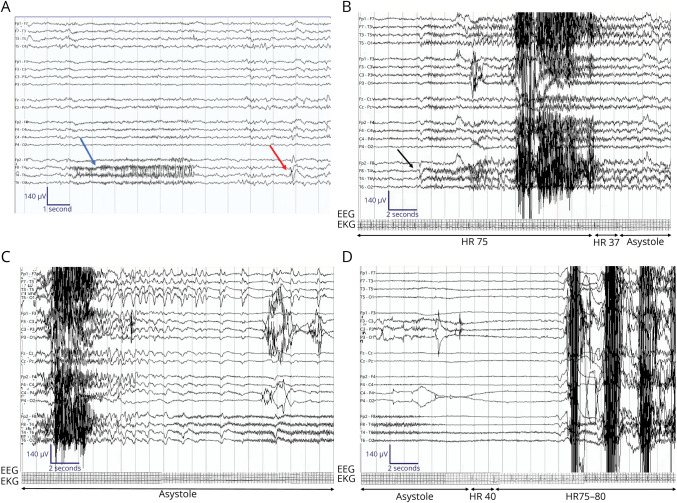
Figure 1. Representative EEG During Epilepsy Monitoring Unit Stay.
Sensitivity 7 μV/mm, low-frequency filter 1 Hz, high-frequency filter 70 Hz, bipolar montage. (A) A brief ictal rhythmic discharge consisting of paroxysmal fast activity in the right temporal region (blue arrow) and an example of a right temporal sharp form over T4 (red arrow). (B) Right temporal seizure onset with sentinel spike at T4 > F8 > F7. Clinically, the patient is sleeping. Eyes open 3 seconds after sentinel spike but continues to rest in bed. Onset of ictal asystole on EKG. (C) Termination of the seizure with ongoing ictal asystole. (D) Termination of the ictal asystole after 32 seconds. Postictal slowing seen on EEG after termination of the seizure.
Given the EEG focal fast activity, there was a high suspicion for focal cortical dysplasia (FCD). Because prior MRI 3 T seizure protocol studies were normal and a 7T MRI was not available within the state, a 3 T MRI with fast gray matter acquisition T1 inversion recovery (FGATIR) sequence was obtained. MRI with FGATIR sequence showed focal cortical thickening at the right insular cortex with blurring of the gray-white matter interface, consistent with FCD (Figure 2). The patient was transitioned from lacosamide to clobazam because of concern for PR interval prolongation, which may delay recovery. She had a pacemaker placed and is currently undergoing evaluation for epilepsy surgery with an upcoming stereo-EEG.
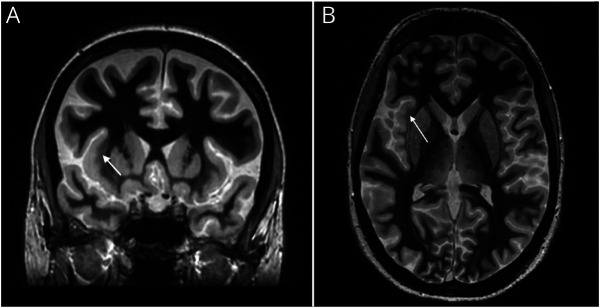
Figure 2. MRI With Fast Gray Matter Acquisition T1 Inversion Recovery Sequence Consistent With Focal Cortical Dysplasia.
Coronal (A) and axial (B) views of focal cortical thickening at the right insular cortex-external capsule margin with blurring of the gray-white matter interface.
Discussion
Ictal asystole is a rare event that occurs primarily in temporal lobe epilepsy. It is estimated to occur between 0.27% and 0.4% of patients with epilepsy undergoing monitoring.1 Ictal asystole is defined as epileptogenic activity accompanied by a cessation of ventricular complexes for >4 seconds.2 There is no clearly established underlying pathophysiology related to the heart or conduction system. Ictal asystole is believed to be due to activation of brain regions that influence cardiac function through efferent neural pathways causing a “vagal storm” that induces ictal asystole and ictal bradycardia.3 Cardiac dysfunction subsequently causes decreased cerebral perfusion, resulting in seizure termination and syncope. This hypothesis is based on the time delay between seizure onset and onset of asystole.3,4 A recent review of 103 cases of ictal asystole showed that asystole began on average 30 seconds after seizure onset and lasted for 20 seconds.5 This phenomenon has a higher prevalence in seizures with temporal lobe onset, as in this patient.3
Ictal asystole is most often associated with areas of the brain related to autonomic function, including the temporal lobe, insular cortex, and amygdala. While it was previously believed that ictal asystole was primarily related to the left hemisphere, both hemispheres have been found to contribute.3
The American Heart Association maintains that first-line treatment should be antiseizure medication optimization and epilepsy surgery for appropriate candidates. Cardiac pacing is reserved for cases in which anticonvulsant drug optimization has not prevented asystole episodes >6 seconds in length.3,6 Pauses greater than 6 seconds have been shown to contribute to syncope/loss of consciousness. Thus, pacing for asystole <6 seconds may not reduce risk of falls and syncopal injury. The second rationale behind the 6-second limit is that neuronal cells are believed to have a 6–9-second energy reserve.3,6
Additional factors to consider when determining whether a patient is a candidate for pacemaker placement include the lifelong need for pacemaker management and replacement. Drawbacks to pacing include the belief that asystole may contribute to seizure cessation through the resultant transient cerebral ischemia, and thus, eliminating asystole may paradoxically prolong the seizure duration.3
Permanent pacemaker implantation is recommended when epilepsy surgery is not an option and when AED regimen optimization has failed.6 However, in all cases of ictal asystole, if the asystole is severe and unremitting, pacemaker implantation should be considered.
FCDs are estimated to have a prevalence between 5% and 25% in focal epilepsy and are known to cause refractory epilepsy.7 There are several treatment modalities for FCDs, including surgical resection, stereotactic minimally invasive thermal ablation, subpial transection, or electrical stimulation implants.8 Treatment is tailored based on the FCD classification, location, and patient factors.
The frequency of sudden unexplained death in epilepsy (SUDEP) ranges from 0.4 to 9 deaths per 1,000 patient years.3 The most important risk factors include uncontrolled seizures, particularly generalized tonic-clonic seizures.3 There are conflicting opinions regarding the relationship between ictal bradycardia/asystole and SUDEP. Some authors claim that insufficient evidence exists to claim a relationship3 while other studies have shown that specifically postictal arrhythmias—rather than ictal arrhythmias—may have a higher risk of developing SUDEP.5,9
In this case, the clinical history, EEG, telemetry, and imaging findings were concordant with an insular focal cortical dysplasia as a cause of this patient's presentation. The addition of the FGATIR to a traditional 3 T MRI seizure protocol was instrumental in uncovering a lesional etiology for her previously nonlesional medically refractory epilepsy. Given the insula's deep location, interictal or ictal activity may be seen over the temporal leads on scalp EEG, so for this reason, the seizure's electrographic onset was referred to as temporal.10 However, based on her imaging that was suggestive of an insular focal cortical dysplasia, the insula is the likely true onset point of this patient's seizure. Medical optimization, pacemaker placement, and epilepsy surgery evaluation are all recommended management options for ictal asystole.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Ravat SH, Bhatti AA, Shah MV, Muzumdar DP, Ravat SH. Ictal asystole: a rare cardiac manifestation of temporal lobe epilepsy, treated with epilepsy surgery. Ann Indian Acad Neurol. 2017;20(1):55-57. doi: 10.4103/0972-2327.199916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moseley BD, Ghearing GR, Munger TM, Britton JW. The treatment of ictal asystole with cardiac pacing. Epilepsia. 2011;52(4):e16-e19. doi: 10.1111/j.1528-1167.2010.02972.x [DOI] [PubMed] [Google Scholar]
- 3.Benditt DG, van Dijk G, Thijs RD. Ictal asystole: life-threatening vagal storm or a benign seizure self-termination mechanism? Circ Arrhythm Electrophysiol. 2015;8(1):11-14. doi: 10.1161/CIRCEP.114.002546 [DOI] [PubMed] [Google Scholar]
- 4.Catenoix H, Mauguière F, Guénot M, Isnard J, Ryvlin P. Recording the insula during ictal asystole. Int J Cardiol. 2013;169(2):e28-e30. doi: 10.1016/j.ijcard.2013.08.100 [DOI] [PubMed] [Google Scholar]
- 5.van der Lende M, Surges R, Sander JW, Thijs RD. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry. 2016;87(1):69-74. doi: 10.1136/jnnp-2015-310559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bestawros M, Darbar D, Arain A, et al. Ictal asystole and ictal syncope: insights into clinical management. Circ Arrhythm Electrophysiol. 2015;8(1):159-164. doi: 10.1161/CIRCEP.114.001667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bast T, Ramantani G, Seitz A, Rating D. Focal cortical dysplasia: prevalence, clinical presentation and epilepsy in children and adults. Acta Neurol Scand. 2006;113(2):72-81. doi: 10.1111/j.1600-0404.2005.00555.x [DOI] [PubMed] [Google Scholar]
- 8.Guerrini R, Duchowny M, Jayakar P, et al. Diagnostic methods and treatment options for focal cortical dysplasia. Epilepsia. 2015;56(11):1669-1686. doi: 10.1111/epi.13200 [DOI] [PubMed] [Google Scholar]
- 9.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977. doi: 10.1016/S1474-4422(13)70214-X [DOI] [PubMed] [Google Scholar]
- 10.Levy A, Yen Tran TP, Boucher O, Bouthillier A, Nguyen DK. Operculo-insular epilepsy: scalp and intracranial electroencephalographic findings. J Clin Neurophysiol. 2017;34(5):438-447. doi: 10.1097/WNP.0000000000000391 [DOI] [PubMed] [Google Scholar]