Abstract
Background and Objectives
This study aimed to evaluate and predict the effects of interictal epileptiform discharges (IEDs) on driving ability using simple reaction tests and a driving simulator.
Methods
Patients with various epilepsies were evaluated with simultaneous EEGs during their response to visual stimuli in a single-flash test, a car-driving video game, and a realistic driving simulator. Reaction times (RTs) and missed reactions or crashes (miss/crash) during normal EEG and IEDs were measured. IEDs, as considered in this study, were a series of epileptiform potentials (>1 potential) and were classified as generalized typical, generalized atypical, or focal. RT and miss/crash in relation to IED type, duration, and test type were analyzed. RT prolongation, miss/crash probability, and odds ratio (OR) of miss/crash due to IEDs were calculated.
Results
Generalized typical IEDs prolonged RT by 164 ms, compared with generalized atypical IEDs (77.0 ms) and focal IEDs (48.0 ms) (p < 0.01). Generalized typical IEDs had a session miss/crash probability of 14.7% compared with a zero median for focal and generalized atypical IEDs (p < 0.01). Long repetitive bursts of focal IEDs lasting >2 seconds had a 2.6% miss/crash probabilityIED. Cumulated miss/crash probability could be predicted from RT prolongation: 90.3 ms yielded a 20% miss/crash probability. All tests were nonsuperior to each other in detecting miss/crash probabilitiesIED (zero median for all 3 tests) or RT prolongations (flash test: 56.4 ms, car-driving video game: 75.5 ms, simulator 86.6 ms). IEDs increased the OR of miss/crash in the simulator by 4.9-fold compared with normal EEG. A table of expected RT prolongations and miss/crash probabilities for IEDs of a given type and duration was created.
Discussion
IED-associated miss/crash probability and RT prolongation were comparably well detected by all tests. Long focal IED bursts carry a low risk, while generalized typical IEDs are the primary cause of miss/crash. We propose a cumulative 20% miss/crash risk at an RT prolongation of 90.3 ms as a clinically relevant IED effect. The IED-associated OR in the simulator approximates the effects of sleepiness or low blood alcohol level while driving on real roads. A decision aid for fitness-to-drive evaluation was created by providing the expected RT prolongations and misses/crashes when IEDs of a certain type and duration are detected in routine EEG.
Introduction
Driving a motor vehicle is an important societal skill, be it for adults who drive as a means of transportation or for professional mobility or for adolescents who often perceive it as a major step toward maturity.1 Evaluating the fitness to drive becomes relevant, particularly in the older individuals2 and for people with a medical disorder such as epilepsy. Numerous studies have analyzed the accident risk of persons with epilepsy.3-13 One consequence has been the development of national and international guidelines that define periods of seizure freedom required to obtain and maintain a driver's license. Opinions diverge on how to best evaluate fitness to drive and whether pathologic EEG findings should be considered. The focus of the EEG evaluation is on interictal epileptiform discharges (IEDs) that occur between seizures and are typically not perceived by patients nor recognizable by routine clinical observation. Nevertheless, IEDs can have serious consequences given their association with transitory cognitive impairment14-16 and their prevalence. IEDs can be up to 2,000 times more frequent than seizures in temporal lobe epilepsy.17 Their influence on cognition can be variable. Features that are responsible for the variability of IED effects include IED type, duration, configuration, and amplitude18,19 and IED location, particularly in intracranial EEGs.20,21 A recent survey of European epileptologists revealed varying opinions on the use of EEG and the consideration of IEDs for fitness-to-drive evaluation.22 However, there was consensus (>80%) on the need for more research to improve evaluation techniques and for coordination and regulation of best practices for evaluating fitness to drive.22 Existing evaluation techniques include a flash test that is used by several epilepsy centers to examine IED effects on responsiveness. A recent pilot study using a realistic driving simulator demonstrated the feasibility of a simulator to test the effects of generalized epileptiform discharges on driving performance.19
We previously characterized the effects of generalized IEDs on reaction time (RT) using a single flash test and a car-driving video game to support clinicians with objective data in their decision to use EEG outcomes for evaluating fitness to drive. We found that IED type and IED duration influenced RT. Specifically, RT prolongation was greater with increasing IED duration, and generalized typical (“well-organized”) IEDs prolonged RTs more than generalized atypical IEDs (“other type”) or generalized sharp theta activity.23 We then validated these IED effects on RTs.24 Focal IED bursts had the weakest effect on RT prolongation, while generalized typical IEDs showed RT prolongations between 105 and123 ms. Crashes in the car-driving video game and performance lapses in the flash test indicated test errors. IEDs increased the probability of crashes and lapses, but the higher test error rate could not be explained by a linear increase in RT during IEDs. We showed that the number of different antiseizure medications taken by the patient increasingly slowed responsiveness, but it was the IEDs that correlated with RT prolongation and test errors.24
Despite the wealth of knowledge about the effect of IEDs on cognition, it remains unclear how this knowledge can be applied in a standardized manner to the evaluation of fitness to drive and how the IED effects can be translated to real-world situations on the road.
Our study aimed to (1) consolidate the knowledge about IEDs that affect a person's fitness to drive; (2) clarify the applicability of simple tests and a realistic driving simulator to study missed reactions or crashes and RT prolongation; (3) determine the predictability of RT prolongation regarding the likelihood of a missed reaction or crash; (4) translate the IED-associated relative risk in the laboratory simulator to the real-road crash risk from the literature; and (5) provide clinicians with a decision aid that uses routine EEG to determine which patients are candidates for fitness-to-drive testing or, if tests are not available, to identify potentially clinically relevant IEDs based on expected RT prolongations and misses/crashes.
Methods
Reaction Tests
Effects of IEDs were studied using a flash test, a car-driving video game (hereafter abbreviated as car test), and a realistic driving simulator (simulator). During each test, a scalp EEG was recorded (Figure 1, eMethods, links.lww.com/WNL/C979).
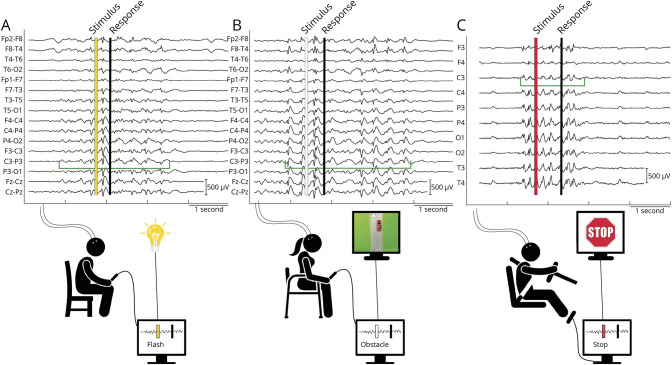
Figure 1. Reaction Tests.
(A) Patients with closed eyes reacted to a single light flash by button press while scalp EEG was recorded. Single flashes from a flickering light were manually triggered as soon as each IED was recognized. A yellow vertical bar indicates single flash appearance, IED onset and end are marked with vertical green lines, and duration is indicated by a horizontal green line. The trigger latency is the difference in time between the green horizontal bar indicating the onset of the IED and the yellow vertical bar indicating the appearance of the visual stimulus triggered by the experimentalist. Single flashes were triggered in random fashion during normal EEG (not shown). (B) Patients played a car-driving video game (car test) on a laptop. An obstacle (whitish cow on gray road, shown on the small monitor) appeared in the lane of a red car and the patient had to change lanes by pressing a button, otherwise the car crashed into the obstacle. Obstacles were triggered manually when an IED was detected and randomly during normal EEG. (C) Patients drove in a realistic driving simulator with an “empty highway at night” scenario projected in front of the driver. Red stop signs were triggered manually at random during normal EEG and by a real-time detection algorithm during IEDs. Patients responded by right foot brake. IED = interictal epileptiform discharge.
Interictal Epileptiform Discharges
EEG recordings were analyzed post hoc by neurologists (H.K., D.R.S., C.J.). IEDs (also termed IED bursts hereafter) were defined on a clinical level as “not recognizable” by regular observation and on an electrophysiologic level as a series of epileptiform potentials (>1 potential) without evolution in frequency (<1 Hz over the duration of the discharge) and amplitude. Each IED was identified according to its distribution as focal or generalized. A generalized IED was classified as “typical” if it consisted of classically configured spike-waves and/or polyspike-waves with reasonably constant organization and amplitude throughout (“well-organized”25). Generalized “atypical” IED consisted of more bluntly configured spike-waves and sharp theta activity and were less well organized often with variable amplitude over time (“other type”25). Focal IEDs were defined independently of their configuration of epileptiform potentials. Examples of the 3 IED types are shown in eFigure 1 (links.lww.com/WNL/C971). IEDs were examined both at the session level and at the "single" IED burst level (when sessions were resolved and all individual data pooled). Entire test sessions were labeled as focal, generalized atypical, or generalized typical according to the predominant type of IED in the EEG recording. Single IED bursts were labeled according to the test session from which they originated.
Study Parameters
Each test session consisted of a varying number of stimulus presentations during normal EEG and IEDs in variable order, as determined by IED appearance. For each patient, we recorded the following: (1) RT [ms], (2) trigger latency [ms], (3) IED duration [ms], (4) missed reactions in the flash test and simulator, and (5) crashes in the car test. A patient could complete 1 session or multiple sessions (eAppendix 1, links.lww.com/WNL/C979).
RT was measured during normal EEG (RTnormal EEG) and during IEDs (RTIED). In the flash test, RT was defined as the time between flash triggering and patient reaction and as time between on-screen obstacle appearance, as recorded by a photovoltaic sensor, and the patient's response in the car test and simulator, respectively. The trigger latency was defined as the time of appearance of the visual stimulus triggered by the experimentalist relative to IED onset (Figure 1A).
The definition of a missed reaction in the flash test and simulator during normal EEG and during IEDs was no response at any latency by the patient. As defined by the programming of the car test, the car would not swerve to avoid the obstacle but would crash into the obstacle unless the patient switched lanes by a button press within 1 second. A crash was defined as the collision of the car into the obstacle. For all 3 tests, the probability of IED-associated missed reactions or crashes (miss/crash probabilityIED) was the number of missed reactions or crashes during IEDs divided by the number of visual stimuli triggered during IEDs in that session. The mean RT prolongation of a session was mean RTIED minus mean RTnormal EEG of that test session. RT prolongation represented the IED-induced deficit on each participant's RT, controlled by their own normal/baseline RT. The reader is referred to eMethods (links.lww.com/WNL/C979) for calculation of the miss/crash probability during normal EEG, miss/crash probability and RT prolongation due to single IED bursts, and the cumulative distribution function that related miss/crash probability with RT prolongation. The total stopping distance in the realistic driving simulator was investigated as an additional behavioral measure (eMethods, eAppendix 1). Treatment of missing variables and control of confounders is described in eMethods.
Influence of Biological or Environmental Factors on Driving Performance in the Literature and Comparison With IEDs
First, we directly compared the increase in RT due to biological/environmental factors with IED-associated RT prolongation. Second, we compared the percent increase in RT or lane deviation of a vehicle (measured in cm) due to drowsiness, blood alcohol concentration, or medications/drugs with the percent increase in IED-associated RT prolongation. Third, we compared the reported odds ratios (ORs) and relative risk of collisions in a laboratory simulator due to blood alcohol or of fatal or nonfatal accidents in real-road studies due to drowsiness, or blood alcohol, with the OR and relative risk of IEDs in our simulator.
Statistical Analyses
Statistics were calculated with GraphPad Prism 9 and R. Recorded and calculated parameters were given as mean (SD) in eTable 1 (links.lww.com/WNL/C976) and presented as medians with 10%–90% interpercentile ranges (10%–90% IPRs) in the main text and as medians with 95% CIs in the Figures, eFigures (links.lww.com/WNL/C971, links.lww.com/WNL/C972, links.lww.com/WNL/C973, links.lww.com/WNL/C974, links.lww.com/WNL/C975) and eTable 2 (links.lww.com/WNL/C977). Group-wise comparisons were performed by paired or unpaired nonparametric tests, as indicated. Odds ratios and relative risk were calculated using contingency tables, tested with the χ2 test, and given as values with 95% CIs. eTable 3 (links.lww.com/WNL/C978) reports all analyses in terms of group size or number of values, whether the data were paired or unpaired, statistical test used, minimum and maximum values, range, median, 10th percentile, 90th percentile, and 10%–90% IPR. Results were significant at corrected p 2-tailed <0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
Our prospective cohort study was approved by the ethics committee of Bern University Hospital and University of Bern (KEK No. 165/10, Basec PB_2017_00574) and complies with the Declaration of Helsinki. All adults and all adolescents and their parents gave their written informed consent. The study was performed at the Departments of Pediatric Neurology and Neurology, Bern University Hospital, Switzerland. Patients were recruited by mail or in-person and examined during regular outpatient consultations or by appointment. Inclusion criteria were proven focal or generalized epilepsy (irrespective of medication intake) and age 14 years or older. Patients with early epileptic encephalopathy were excluded.
Data Availability
Data will be shared on request.
Results
Data from the following 2 cohorts were used: (1) 20 adolescent and 21 adult patients with epilepsy recruited between 2015 and 2018 and (2) 63 adults from our previous studies (23 individuals from the study in reference [23] and 40 individuals from the study in reference [24], eAppendix 1, links.lww.com/WNL/C979). Of the 104 patients tested, 95 met the data analysis criterion of stimulus appearance on average during the IEDs (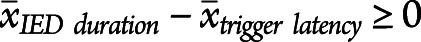 ): 18 adolescents and 20 adults were recruited during 2015–2018, and 57 adults were recruited from previous studies.23,24 These patients completed 155 test sessions in total (eAppendix 1). Female-to-male ratio was 48:47; the mean adolescent age was 15.7 years. (min. 14, max. 17), and the mean adult age was 37.8 years. (min. 18, max. 76). Codified patient information included epilepsy type/syndrome, antiseizure medication (including dose), patient age at testing, and sex (eTable 1, links.lww.com/WNL/C976). Patients had idiopathic generalized epilepsies, epilepsies of unknown origin, nonacquired focal epilepsies, or structural epilepsies.
): 18 adolescents and 20 adults were recruited during 2015–2018, and 57 adults were recruited from previous studies.23,24 These patients completed 155 test sessions in total (eAppendix 1). Female-to-male ratio was 48:47; the mean adolescent age was 15.7 years. (min. 14, max. 17), and the mean adult age was 37.8 years. (min. 18, max. 76). Codified patient information included epilepsy type/syndrome, antiseizure medication (including dose), patient age at testing, and sex (eTable 1, links.lww.com/WNL/C976). Patients had idiopathic generalized epilepsies, epilepsies of unknown origin, nonacquired focal epilepsies, or structural epilepsies.
RT and Miss/Crash Are Affected by IEDs and Vary With IED Type and IED Duration
IEDs increased median RT by 75.3 ms (p < 0.01, Wilcoxon test), but median miss/crash probability remained at zero (Table 1). Session miss/crash probabilities between normal EEG and IED nevertheless differed (p < 0.01, Wilcoxon test) because there were sessions with miss/crash during normal EEG and during IED, but their miss/crash probabilities were too few to be reflected in the median. Single IED bursts increased miss/crash probability from 0.1% during normal EEG to 4.6% (p < 0.01, χ2 test; Table 1).
Table 1.
Select Key Results of RTs and Misses/Crashes for All Patients and All Test Types, Grouped According to Test Type, IED Type, and Age Groups
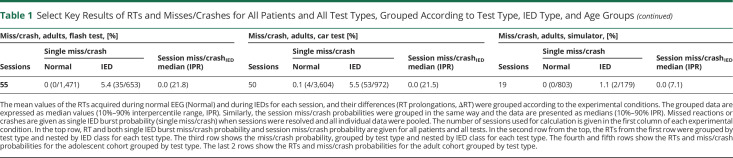
The median session RT prolongation increased from focal IED (48 ms, 10%–90% IPR 100.0 ms) to generalized atypical IED (77.0 ms, 10%–90% IPR 160.9 ms) and generalized typical IED (164.0 ms, 10%–90% IPR 498.0 ms) (p < 0.01 Kruskal-Wallis test; Figure 2B). The median session miss/crash probabilityIED was zero for focal IED (0%, 10%–90% IPR 5.6%) and generalized atypical IED (0%, 10%–90% IPR 8.9%) and increased to 14.7% (10%–90% IPR 38.6%) in generalized typical IED (p < 0.01 Kruskal-Wallis test; Figure 2D). Single IED burst miss/crash probability increased from focal IED (1.0%) to generalized atypical IED (2.5%) and generalized typical IED (22.4%) (p < 0.01 χ2 test; Figure 2E).
Figure 2. RT Prolongation and Miss/Crash Vary With IED Type and IED Duration.
(A) The number of IEDs from test sessions classified as having predominant focal, generalized atypical (gen atyp), or generalized typical (gen typ) IEDs was divided by the respective duration of the test session in minutes to get a metric of IED frequency. Each point of the scatter plot corresponds to the IED frequency of a session. The metric was presented as median with 95% CI in red (medians written above scatter plots). (B) IEDs without miss/crash generally resulted in RT prolongation. RT prolongation of each session (represented as 1 point) was grouped according to the IED type of the session. The appropriately grouped RT prolongations were presented as median with 95% CI. (C) The average duration of IEDs without miss/crash of each session (represented as 1 point) was grouped according to the IED type of the session. The median with 95% CI is shown for each group of IED durations. (D) At the session level, IED-associated missed reactions in the flash test and simulator and IED-associated crashes in the car test were divided by the total number of stimulus exposures during IED (each point corresponds to the miss/crash probabilityIED of a session). Miss/crash probabilitesIED were grouped by IED type and presented as median with 95% CI. (E) To calculate the single IED burst miss/crash probability separately for each IED type, the session level was resolved. Of all sessions classified as focal, generalized atypical, or generalized typical, the respective total number of IEDs with misses/crashes was divided by the respective total number of stimulus exposures during each IED. This plot is intended to complement the session miss/crash probabilityIED in (D) to show that miss/crash did occur in sessions that were classified as focal or generalized atypical. (F) The session average of IED duration for IEDs with miss/crash (shown as 1 point) was grouped according to the IED type of the session. The descriptive statistics used was the median with 95% CI (in red). IED = interictal epileptiform discharge.
On a session level, the median duration of IEDs without miss/crash was comparable between focal and generalized atypical IEDs (1,384.0 ms, 10%–90% IPR 1867.0 ms vs 1,359.0 ms, 10%–90% IPR 1324.6 ms) and were longer for generalized typical IEDs (2,374.0 ms, 10%–90% IPR 4435.5 ms) (p < 0.01 generalized atypical vs generalized typical IED, Kruskal-Wallis test; Figure 2C). A similar pattern could be observed for median session duration of IEDs with miss/crash with the longest duration of generalized typical IEDs (3,155.0 ms, 10%–90% IPR 9514.0 ms) (p = 0.03 for generalized atypical IED vs generalized typical IED, Kruskal-Wallis test; Figure 2F). No difference was found for median IED frequency per minute of test sessions according to IED type (p = 0.22, Kruskal-Wallis test; Figure 2A). Left hemispheric and right hemispheric focal IED bursts did not differ for RT prolongation (left: 32.7 ms, right: 56.8 ms; p = 0.22, Mann-Whitney test) and single IED burst miss/crash probability (left: 0.9%, right: 1.0%).
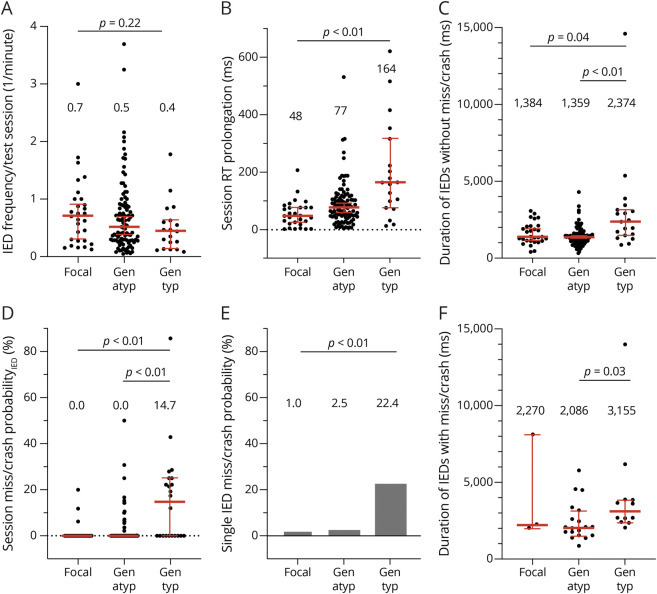
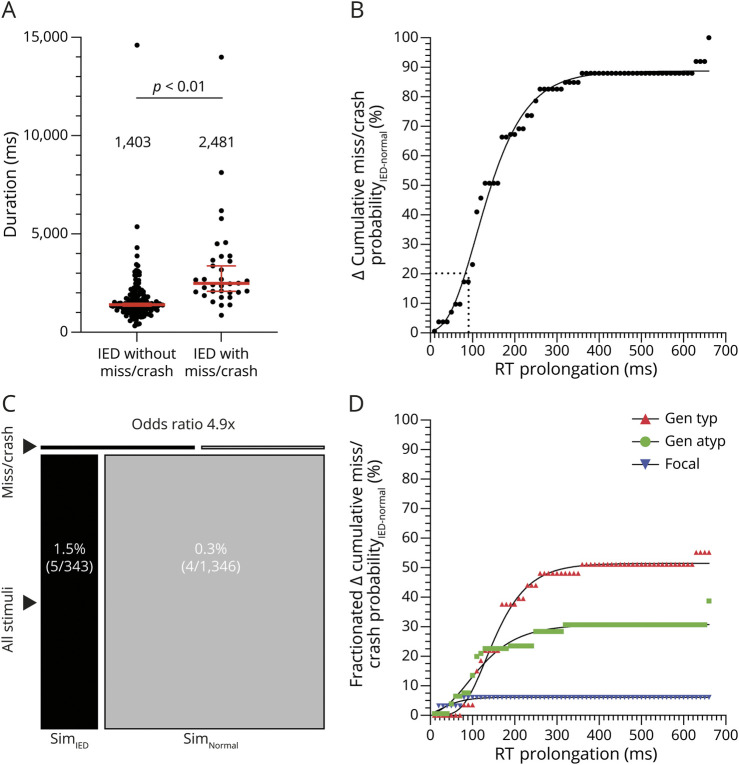
We performed a similar set of analyses when sessions were grouped according to test type. The median IED–associated RT prolongation was comparable between the flash test (56.4 ms, 10%–90% IPR 190.9 ms), car test (75.5 ms, 10%–90% IPR 146.1 ms), and simulator (86.6 ms, 10%–90% IPR 311.7 ms), indicating nonsuperior sensitivity to detect IED effects on this behavioral measure (p = 0.25, Kruskal-Wallis test; Figure 3B, Table 1). Similarly, the median miss/crash probabilityIED was zero for each of the 3 tests, indicating nonsuperiority in detecting IED-associated miss/crash when analyzed on a session level (p = 0.29, Kruskal-Wallis test; Figure 3D, Table 1). Conversely, when the session level was resolved and data were analyzed according to single IED burst miss/crash probability, a difference emerged in the percentage of miss/crash detected (car test 5.1% > flash test 4.8% > simulator 1.5%) (p = 0.02, χ2 test; Figure 3E). The duration of IEDs without miss/crash was lowest in the simulator (941.8 ms, 10–%90% IPR 700.1 ms) when compared with that in the flash test (1,469.0 ms, 10%–90% IPR 1726.6 ms) and car test (1,511.0 ms, 10%–90% IPR 1833.0 ms) (p < 0.01, Kruskal-Wallis test; Figure 3C). The duration of IEDs with miss/crash did not differ between the test types (p = 0.38, Kruskal-Wallis test; Figure 3F). A clear difference in duration was found when IEDs without miss/crash (1,403.0 ms, 10%–90% IPR 1869.9 ms) and IEDs with miss/crash (2,481.0 ms, 10%–90% IPR 4512.0 ms) were contrasted at the session level (p < 0.01, Mann-Whitney test; Figure 4A). In a subgroup analysis, we examined all the abovementioned parameters separately for adolescents and adults who were comparably affected by IEDs in terms of both session miss/crash probability and RT prolongation, independent of the test used (Table 1, eAppendix 1, links.lww.com/WNL/C979).
Figure 3. RT Prolongation and Miss/Crash on a Session Level are Not Influenced by Test Type.
(A) The session IED frequency was calculated as the number of IEDs divided by the duration of the test session in minutes and grouped according to session test type (flash test, car test, and simulator). Each point of the scatter plot corresponds to the IED frequency of a session. The descriptive statistics used for session IED frequencies was the median with 95% CI (in red, median values written about scatter plots). (B) Session RT prolongation (shown as 1 point) was grouped by test type. The correspondingly grouped session RT prolongations were described with the median and 95% CI. (C) The average duration of IEDs without miss/crash of each session (equivalent to 1 point) was grouped by test type. The median with 95% CI is shown for each group of IED durations. (D) Session miss/crash probabilityIED was grouped by test type and described using the median and 95% CI. (E) Single IED burst miss/crash probability is shown as a mosaic plot, grouped by test type, and generated from a contingency table. The areas of the rectangles reflect the ratio between the total number of IEDs with misses/crashes (miss/crashIED) and the total number of stimulus exposures during the IED (all IED) of each test type. For example, for the flash test, 42 divided by 861 gives 4.8%. The areas of the rectangles are in the (graphically vertical) ratio of 0.048 (miss/crashIED) to 1 (all IED) for the flash test. But they are also in the (graphically horizontal) ratio between the test types according to the calculated percentages to each other. (F) The average duration of IEDs with miss/crash of each session (equivalent to 1 point) was grouped by test type. The median with 95% CI is shown for each group of IED durations. IED = interictal epileptiform discharge.
Figure 4. Predictive Power of IEDs for Risk Assessment of Miss/Crash.
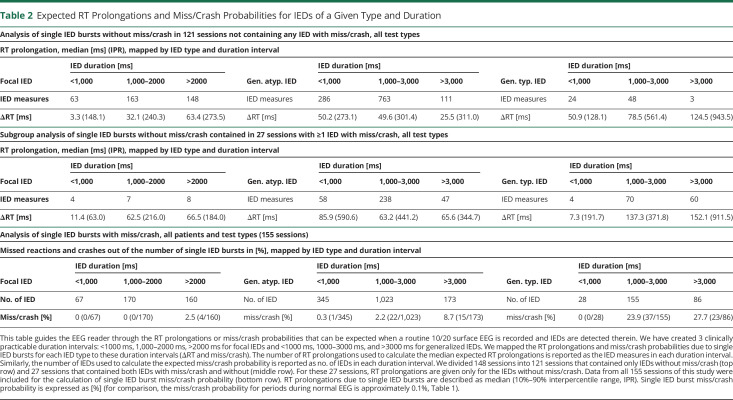
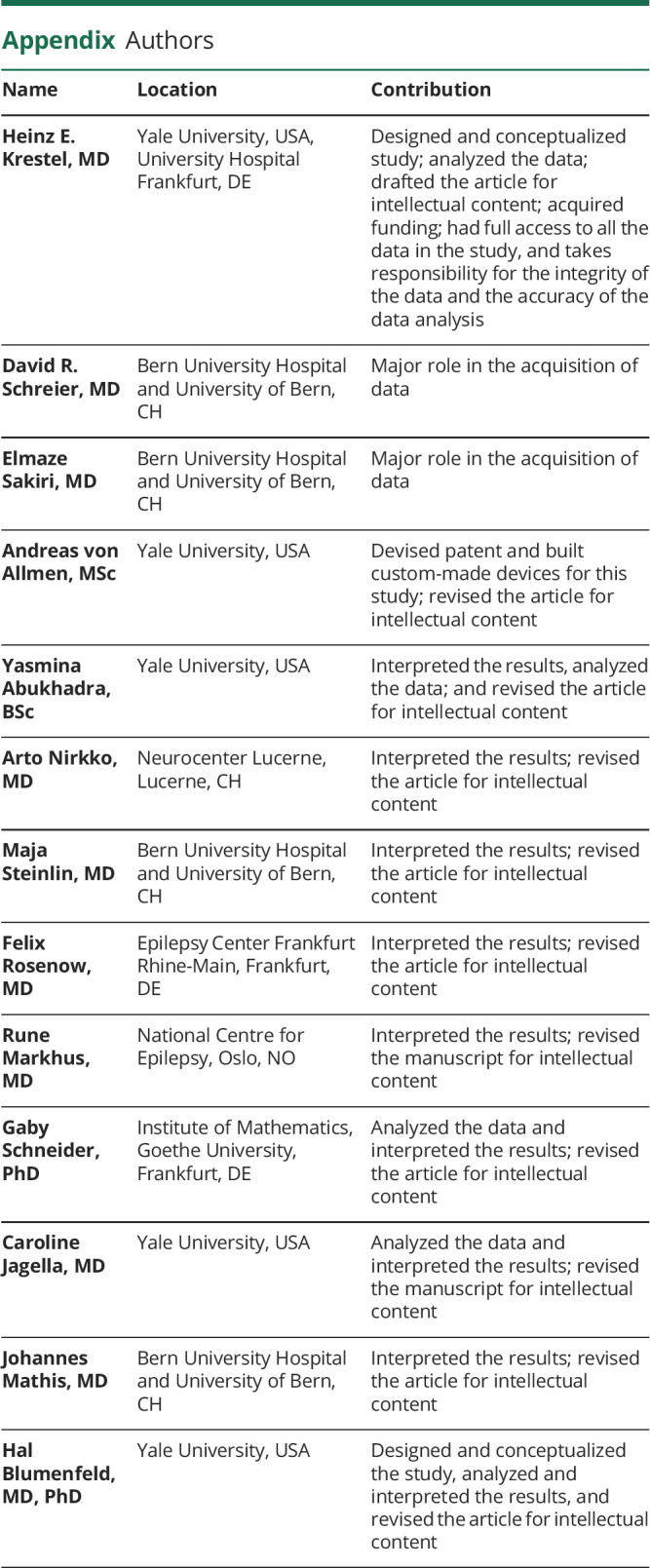
(A) The average duration of IEDs without miss/crash from 121 sessions plus the average duration of IEDs without miss/crash from 27 sessions that included both IEDs without miss/crash and IEDs with miss/crash is shown in this scatter plot above the label "IED without miss/crash" (1 dot represents 1 session). The average duration of IEDs with miss/crash from each session with at least 1 miss/crash (from 27 sessions that included both types of IEDs and from 7 sessions that included only IEDs with miss/crash) was included in the "IED with miss/crash" category. Grouped session data were described with the median and 95% CI (in red, median values written above scatter plots). (B) Empirical cumulative distribution function showing the accumulated difference in miss/crash probability between IED and normal EEG as a function of RT prolongation. Approximately 20% of misses/crashes (horizontal dotted line) were associated with RT prolongations ranging from 1 ms to X = 90.3 ms (vertical dotted line). (C) The single IED burst miss/crash probability in the simulator (SimIED) was 1.5% (visualized as the proportion of the thin black rectangle labeled "miss/crash," relative to the larger black rectangle below labeled "all stimuli"). The probability of suffering a miss/crash from a single visual stimulus during normal EEG in the simulator (SimNormal) was 0.3% (visualized as the proportion of the 2 gray rectangles). The OR of experiencing a miss/crash during an IED was increased 4.9-fold compared with experiencing a miss/crash during periods of normal EEG (the proportion of the black rectangles compared with that of the gray rectangles). The respective numerical ratios were visualized as corresponding area ratios in a mosaic plot for the simulator. (D) The differences in miss/crash probability between IED and normal EEG and RT prolongations were grouped by IED type, and the empirical cumulative distribution function was calculated for each subgroup. Compared with the curve in (B), it shows the relative accumulated difference in miss/crash probability between IED and normal EEG as a function of RT prolongation, grouped by sessions with predominantly generalized typical IED, generalized atypical IED, and focal IED. IED = interictal epileptiform discharge.
Predictive Power of IEDs for Risk Assessment of Miss/Crash
The empirical cumulative distribution function related the difference in cumulative miss/crash probability between IED and normal EEG to the RT prolongation (Figure 4B). The first inflection point was calculated to be 8.5% at a mean RT prolongation of 62.1 ms. The difference in cumulative miss/crash probability between IED and normal EEG was calculated to be 50% at a mean RT prolongation of 149.4 ms and 20% at a mean RT prolongation of 90.3 ms (Figure 4B). Twenty-two percent of 155 sessions contained at least 1 IED-associated miss/crash. Fifty-six percent of sessions had a mean RT prolongation of >62.1 ms, and 16% of sessions had a mean RT prolongation of >150.0 ms. The differences in miss/crash probability between IED and normal EEG and RT prolongations were grouped by IED type, and the empirical cumulative distribution function was calculated for each subgroup (Figure 4D). From this, the relative difference in cumulative miss/crash probability was almost twice as high for generalized typical IEDs as for generalized atypical IEDs and approximately 5 times higher than for focal IEDs.
The OR of a miss/crash during IED was increased 4.9-fold (relative risk 2.7-fold) compared with normal EEG in our simulator (Figure 4C, eTable 3, links.lww.com/WNL/C978 analysis 22). The rare case of miss/crash during normal EEG was most likely due to an attentional deficit ("daydreaming").
Influence of Biological or Environmental Factors on Driving Performance and Comparison With IEDs
In a computerized response speed test with RTs of 489–494 ms in rested and fasting patients, wakefulness of 18 hours prolonged RT by 45 ms, whereas a blood alcohol concentration of 0.05% or 0.1% prolonged RTs by 45 ms and 77 ms, respectively.26 In our car test, the median RT was 474 ms during normal EEG and was prolonged by a median 76 ms when all IEDs were combined.
More than 21 hours of wakefulness or 0.08% blood alcohol concentration caused a relative performance decline of 5%–10% in a computer tracking task.27 Standard doses of the sleeping aids, zolpidem or zopiclone, when taken 4.5 hours or 10.5 hours before a car trip, increased SD of lateral car position (on real road) by 3.4 cm (+18%) or 2.5 cm (+14%), respectively.28 An increase in SD of lateral car position by 2.4 cm could be caused by a blood alcohol concentration of 0.05% and was considered a benchmark.29 The IED-associated relative RT prolongation was +18% (502.6 ms during IED/423.5 ms during normal EEG = 1.185) for all patients with epilepsy and all test types combined. Relative RT prolongation ranged from +13% for focal IED bursts to +38% for generalized typical IED.
The alcohol-related relative risk of driver involvement in fatal and nonfatal crashes as a function of blood alcohol concentration was reexamined using recent real-road data.30 The relative risk was approximated by the OR. The relative driver involvement risk for nonfatal crashes was increased by a factor of 2.3–3.9 at a blood alcohol concentration of 0.035% (2.3 for road users older than 20 years.; 3.9 for road users aged 16–20 years.), and with a blood alcohol concentration of 0.065%, it increased by a factor of 4.8–12.6 (4.8 for road users older than 20 years.; 12.6 for road users aged 16–20 years). In our study, the OR to suffer a miss/crash in the simulator during IED was increased by a factor of 4.9 (95% CI 1.5–16.1) compared with that during normal EEG. The higher OR of 16- to 20-year-old road users could not be reproduced in our patients with epilepsy patients the simulator (data not shown). In a meta-analysis of 7,000 participants with self-reported drowsiness in real-road driving, the pooled OR of being involved in a traffic accident while driving drowsy was increased by a factor of 2.5 (95% CI 1.9–3.4).31
Expected RT Prolongations and Miss/Crash Probabilities for IEDs of a Given Type and Duration
We calculated RT prolongations and miss/crash probabilities for single IED bursts (eMethods, links.lww.com/WNL/C979). We grouped these values according to IED type and sorted the RT prolongations and miss/crashes by the duration of their IEDs from minimum to maximum in each group. We created 3 duration intervals: <1000 ms, 1,000–2000 ms, >2000 ms for focal IEDs and <1000 ms, 1000–3000 ms, and >3000 ms for generalized IEDs. We mapped the median RT prolongations and miss/crash probabilities due to single IED bursts for each IED type to these duration intervals (Table 2). We divided all 148 sessions that contained IEDs into 2 groups: 121 sessions that contained only IEDs without miss/crash and 27 sessions that contained both IEDs with miss/crash and without. The rationale behind this division was that the 2 groups were expected to behave differently regarding RT prolongation,24 which they did not when the corresponding duration intervals were compared (Mann-Whitney test not significant, data not shown). IED frequency in the 121 sessions (0.48/min, 95% CI 0.38–0.64) was comparable with that in the 27 sessions (0.63, 95% CI 0.39–0.86) (p = 0.23, Mann-Whitney test, data not shown). The situation was different for the duration of IEDs without miss/crash, which was shorter in the 121 sessions (1,427 ms, 95% CI 1398–1,477) than in the 27 sessions (1904 ms, 95% CI 1749–2052) (p < 0.01, Mann-Whitney test, data not shown).
Table 2.
Expected RT Prolongations and Miss/Crash Probabilities for IEDs of a Given Type and Duration
Discussion
In this study, we identified IEDs that are clinically relevant to the fitness to drive of individuals with epilepsy as determined in 3 different test types.
Our data confirmed that the IED type, that is focal or generalized distribution over the hemispheres, and configuration of epileptiform potentials (also called morphology in previous work14) were important determinants for IED effects. Longer duration increased the probability for an IED-induced deficit.14,32 Other determinants may include IED amplitude18,32 and increased EEG power during epileptiform discharges (called ramp configuration of EEG spectrum33).18 IEDs were, however, variable within EEG recordings. The subset of neurons involved in generating an IED in each patient varies from 1 spike to the next, that is, the neuronal ensembles that generate IEDs are probabilistic.34,35 Diurnal fluctuation in IED number also contributes to variability.36 The variability of IED effects (see, for example, SD of RT prolongations in eTable 1, links.lww.com/WNL/C976) can partly be explained by the coincidence of the probabilistic nature of IED generation with a probabilistic nature of information processing, that is, neuronal information is also not represented by a specific spatiotemporal pattern of electrical activity.34 Finally, the intraindividual and interindividual variability of IED effects may also be due to IED interaction with the test task. That is, tasks that engage a brain region with somatotopically arranged information processing, such as the primary visual cortex, may exhibit higher anatomical-functional specificity of IED effects than tasks that require networks for their processing, such as language, the prefrontal network for decision making, and probably the planning and execution of motor tasks.
We expanded our reaction tests and added a driving simulator. The main reason was to improve the transferability of laboratory results to the real road.37 We could have chosen lane deviation, and thus “SD lateral position”, as an indicator of IED effects, as in previous work.38,39 However, we decided to use the total stopping distance as a more descriptive parameter than lane deviation, but unfortunately, the use of total stopping distance failed due to the variation in the participants' driving speeds (eAppendix 1, links.lww.com/WNL/C979). Notably, session RT prolongation and session miss/crash probability were not significantly influenced by the test used, suggesting that the effectiveness of a simple driving video game or flash test is noninferior to a realistic driving simulator in assessing IED-related miss/crash and RT prolongation. We thus failed to show that increased task difficulty, which is associated with the simulator in comparison with the flash test and car test, provided greater sensitivity for IED-induced deficits, as was previously suggested.15 We confirmed for IEDs without miss/crash that increased task difficulty reduced IED number and shortened IED duration,14-16 using simulator data in comparison with data from the flash test and car test. We did, however, note that duration of IEDs with miss/crash was comparable across the 3 different tests, probably due to a stronger epileptogenic field of miss/crash IEDs. The laterality of focal IEDs did not affect the IED-induced deficit in our 3 tests. We had recorded too few single focal IEDs to test bifrontal location separately. In the only study that analyzed IED effects on real road, 2 of the 3 patients with the greatest increase in SD of lateral car position had focal discharges involving the frontal lobe (frontotemporal right and bifrontal more on the left).39 Future studies should focus on the location of focal IEDs rather than on their laterality to study the anatomical-functional specificity of IED-induced deficits in simulated driving.
A threshold of concern exists for epileptic seizures. A patient can drive if they have an annual seizure recurrence risk of up to 20%.7,8 A threshold of concern for a clinically relevant IED has not yet been formulated. In our previous work,24 we have theoretically proposed a clinically relevant IED as the 99th percentile of the SD of the mean RT of young healthy volunteers and have set it to 100 ms. We now propose a 20% miss/crash risk at an RT prolongation of 90.3 ms as a clinically relevant IED effect, based on the cumulative distribution curve we have calculated for the 2 variables. The predictiveness of IED-associated RT prolongation, which is usually measured much more frequently in a test than a missed reaction or crash, can be used to identify a clinically relevant IED. The essence of an IED with a missed reaction or crash is cognitive impairment, which is related to impaired awareness seizures that are known to cause the most accidents when they occur while driving.3
We limited our research to the effects of drowsiness, medication intake, and alcohol while driving. The idea of using blood alcohol–related impairment as a comparator across studies was proposed by others26,27 and is based on the substantial contribution of drinking and driving to real-road traffic accidents and the large number of studies on the subject. The IED-associated OR and relative risk for a missed reaction in our simulator is comparable with an estimated OR for a crash by self-reported drowsiness while driving,31 and with an estimated relative risk for collisions in the driving simulator from a blood alcohol concentration between 0.05% and 0.1% (eAppendix 1, links.lww.com/WNL/C979),40 and with an OR for a nonfatal crash on real roads from a blood alcohol concentration approximately 0.035%.30 It is important to carefully consider this translation of IED effects to the real road and identify the clinically relevant IEDs either by measuring their effect (see previous paragraph) or by analyzing the electrophysiologic features of IEDs in a routine EEG (next paragraph).
Our decision aid (in Table 2 and with practical examples in eAppendix 1, links.lww.com/WNL/C979) assists in determining who is eligible for a fitness-to-drive test by providing the EEG reader with the expected transitory deficits for IEDs of a given type and duration. We recommend testing whether EEGs show predominantly focal IED bursts with greater than 2-second duration or generalized IEDs with greater than 3-second duration. When EEGs contain generalized IEDs of 1,000–3,000 ms duration, it is recommended that the testing depends on the IED frequency per session (above 0.5/min) and the patient's contextual factors. From a clinical perspective, the IEDs that form the basis for assigning patients to behavioral testing are also the IEDs that we would classify as clinically relevant if there were no options for fitness-to-drive testing. The aim is not to test as many patients as possible but to individualize decisions about the fitness to drive and to standardize the decisions regarding practices at different epilepsy clinics.
Long trigger latencies, even with automated IED detection in the simulator, did not allow us to measure the effects of solitary epileptiform spikes. We are currently working on a real-time IED detection and stimulus-triggering algorithm. In classical literature and more recent work,14,15,39,41-44 the definition of an IED was variable, but so were the purposes of the studies. We believe that the search for the best possible IED criteria to study their effects on daily social functioning of the awake epilepsy patient is a work in progress.
Not only have IEDs recently become increasingly important in research focused on impaired consciousness, for example,11,18,19 dementia,45-47 and seizure prediction36 but are also a phenomenon with everyday social consequences. We have created a decision aid for fitness-to-drive evaluation and propose a clinically relevant IED effect. A flash test or a (freely available) car test can be used to screen for IED-induced deficits in regular clinical practice (for example, in 20 minutes). Future longitudinal studies should focus on determining which IEDs are treatable and, if so, whether the IEDs relevant to regaining fitness to drive can be suppressed and whether the therapeutic goals can be achieved to improve the patient’s quality of life. New machine learning approaches and automated neuropsychological bedside tests can assist in improved testing of IED-induced deficits.
Acknowledgment
The authors thank the patients for participating in this study and the technicians at the Inselspital Bern, headed by Janine Wettstein, for registering the RT-EEGs. The authors thank Philip Coish, PhD, and Robert K. Fulbright, MD, both from Yale University, for extensive proofreading and for their kindness and patience with the first author.
Glossary
- 10%–90% IPR
10%–90% interpercentile range
- Gen atyp
generalized atypical (IEDs)
- Gen typ
generalized typical (IEDs)
- IEDs
interictal epileptiform discharges
- miss/crash
missed reactions or crashes
- RTs
reaction times
Appendix. Authors
Footnotes
Editorial, page 377
CME Course: NPub.org/cmelist
Study Funding
Kernen Fonds, Switzerland; European Union's Framework Program for Research and Innovation Horizon 2020 (2014–2020) under the Marie Sklodowska‐Curie Grant Agreement No. 99791; LOEWE CMMS —Multiscale Modeling in the Life Sciences.
Disclosure
H. Krestel was supported by Kernen Fonds, Switzerland (to D. Schreier), and is supported by the European Union's Framework Program for Research and Innovation Horizon 2020 (2014–2020) under the Marie Sklodowska‐Curie Grant Agreement No. 99791; G. Schneider was supported by the LOEWE CMMS —Multiscale Modeling in the Life Sciences. Y. Abukhadra was supported by the Yale College DeanÂ’s Office Hahn Fellowship; A. von Allmen and H. Krestel hold a patent on the real-time IED detection and stimulus-triggering algorithm used in this study; A. Nirkko holds a patent concerning the synchronization of the EEG recordings with the driving simulator display; AvA, HK, and AN declare that none of these patents are involved in any actual commercial or other financial interest related to this manuscript; all other coauthors report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Nashef L, Capovilla G, Camfield C, Nabbout R. Transition: driving and exercise. Epilepsia. 2014;55(suppl 3):41-45. doi: 10.1111/epi.12717 [DOI] [PubMed] [Google Scholar]
- 2.Hill LJN, Pignolo RJ, Tung EE. Assessing and counseling the older driver: a concise review for the generalist clinician. Mayo Clin Proc. 2019;94(8):1582-1588. doi: 10.1016/j.mayocp.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 3.Gastaut H, Zifkin BG. The risk of automobile accidents with seizures occurring while driving: relation to seizure type. Neurology. 1987;37(10):1613-1616. doi: 10.1212/wnl.37.10.1613 [DOI] [PubMed] [Google Scholar]
- 4.Hansotia P, Broste SK. The effect of epilepsy or diabetes mellitus on the risk of automobile accidents. N Engl J Med. 1991;324(1):22-26. doi: 10.1056/NEJM199101033240105 [DOI] [PubMed] [Google Scholar]
- 5.Taylor J, Chadwick D, Johnson T. Risk of accidents in drivers with epilepsy. J Neurol Neurosurg Psychiatry. 1996;60(6):621-627. doi: 10.1136/jnnp.60.6.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lings S. Increased driving accident frequency in Danish patients with epilepsy. Neurology. 2001;57(3):435-439. doi: 10.1212/wnl.57.3.435 [DOI] [PubMed] [Google Scholar]
- 7.Kim LG, Johnson TL, Marson AG, Chadwick DW; MRC MESS Study Group. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurol. 2006;5:317-322. doi: 10.1016/S1474-4422(06)70383-0 [DOI] [PubMed] [Google Scholar]
- 8.Bonnett LJ, Tudur-Smith C, Williamson PR, Marson AG. Risk of recurrence after a first seizure and implications for driving: further analysis of the multicentre study of early epilepsy and single seizures. BMJ. 2010;341:c6477. doi: 10.1136/bmj.c6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal A, Carne R, Odell M, Ballek D, D'Souza WJ, Cook MJ. Characteristics of motor vehicle crashes associated with seizure: car crash semiology. Neurology. 2018;91(12):e1102-e1111. doi: 10.1212/WNL.0000000000006208 [DOI] [PubMed] [Google Scholar]
- 10.Sundelin HEK, Chang Z, Larsson H, et al. Epilepsy, antiepileptic drugs, and serious transport accidents: a nationwide cohort study. Neurology. 2018;90(13):e1111-e1118. doi: 10.1212/WNL.0000000000005210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antwi P, Atac E, Ryu JH, et al. Driving status of patients with generalized spike-wave on EEG but no clinical seizures. Epilepsy Behav. 2019;92:5-13. doi: 10.1016/j.yebeh.2018.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi CN, Vossler DG, Spanaki M, Draszowki JF, Towne AR; Members of the Treatments Committee of the American Epilepsy Society. "Chance takers are accident makers": are patients with epilepsy really taking a chance when they drive? Epilepsy Curr. 2019;19(4):221-226. doi: 10.1177/1535759719858647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skyving M, Forsman Å, Dukic Willstrand T, Laflamme L, Möller J. Medical impairment and road traffic crashes among older drivers in Sweden—a national, population-based, case-control study. Accid Anal Prev. 2021;163:106434. doi: 10.1016/j.aap.2021.106434 [DOI] [PubMed] [Google Scholar]
- 14.Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107(Pt 1):293-308. doi: 10.1093/brain/107.1.293 [DOI] [PubMed] [Google Scholar]
- 15.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2(12):725-730. doi: 10.1016/s1474-4422(03)00584-2 [DOI] [PubMed] [Google Scholar]
- 16.Kasteleijn-Nolst Trenité D, Vermeiren R. The impact of subclinical epileptiform discharges on complex tasks and cognition: relevance for aircrew and air traffic controllers. Epilepsy Behav. 2005;6(1):31-34. doi: 10.1016/j.yebeh.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Janszky J, Hoppe M, Clemens Z, et al. Spike frequency is dependent on epilepsy duration and seizure frequency in temporal lobe epilepsy. Epileptic Disord. 2005;7(4):355-359. [PubMed] [Google Scholar]
- 18.Guo JN, Kim R, Chen Y, et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15(13):1336-1345. doi: 10.1016/S1474-4422(16)30295-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen E, Antwi P, Banz BC, et al. Realistic driving simulation during generalized epileptiform discharges to identify electroencephalographic features related to motor vehicle safety: feasibility and pilot study. Epilepsia. 2020;61(1):19-28. doi: 10.1111/epi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horak PC, Meisenhelter S, Song Y, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia. 2017;58(3):373-380. doi: 10.1111/epi.13633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ung H, Cazares C, Nanivadekar A, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. 2017;140(8):2157-2168. doi: 10.1093/brain/awx143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markhus R, Henning O, Molteberg E, et al. EEG in fitness to drive evaluations in people with epilepsy—considerable variations across Europe. Seizure. 2020;79:56-60. doi: 10.1016/j.seizure.2020.04.013 [DOI] [PubMed] [Google Scholar]
- 23.Krestel H, Nirkko A, von Allmen A, et al. Spike-triggered reaction-time EEG as a possible assessment tool for driving ability. Epilepsia. 2011;52(10):e126-e129. doi: 10.1111/j.1528-1167.2011.03252.x [DOI] [PubMed] [Google Scholar]
- 24.Nirkko A, Bernasconi C, von Allmen A, Liechti C, Mathis J, Krestel H. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia. 2016;57(5):832-840. doi: 10.1111/epi.13361 [DOI] [PubMed] [Google Scholar]
- 25.Mirsky AF, Vanburen JM. On the nature of the “Absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic and autonomic factors. Electroencephalogr Clin Neurophysiol. 1965;18:334-348. doi: 10.1016/0013-4694(65)90053-2 [DOI] [PubMed] [Google Scholar]
- 26.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med. 2000;57(10):649-655. doi: 10.1136/oem.57.10.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388(6639):235. doi: 10.1038/40775 [DOI] [PubMed] [Google Scholar]
- 28.Leufkens TRM, Lund JS, Vermeeren A. Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J Sleep Res. 2009;18(4):387-396. doi: 10.1111/j.1365-2869.2009.00746.x [DOI] [PubMed] [Google Scholar]
- 29.Louwerens JW, Gloerich ABM, De Vries G, Brookhuis KA, O'Hanlon JF. The relationship between drivers' blood alcohol concentration (BAC) and actual driving performance during high speed travel. In: Noordzij PC, Roszbach R, eds. International Congress on Alcohol, Drugs and Traffic Safety, T86. Excerpta Medica; 1987:183-186. [Google Scholar]
- 30.Zador PL, Krawchuk SA, Voas RB. Relative Risk of Fatal Crash Involvement by BAC, Age, and Gender (Report HS-809-050). U.S. Department of Transportation, National Highway Traffic Safety Administration; 2000. [Google Scholar]
- 31.Bioulac S, Micoulaud-Franchi JA, Arnaud M, et al. Risk of motor vehicle accidents related to sleepiness at the wheel: a systematic review and meta-analysis. Sleep. 2017;40(10). doi: 10.1093/sleep/zsx134 [DOI] [PubMed] [Google Scholar]
- 32.Shewmon DA, Erwin RJ. Focal spike-induced cerebral dysfunction is related to the after-coming wave. Ann Neurol. 1988;23(2):131-137. doi: 10.1002/ana.410230205 [DOI] [PubMed] [Google Scholar]
- 33.Stevens JR, Lonsbury B, Goel S. Electroencephalographic spectra and reaction time in disorders of higher nervous function. Science. 1972;176:1346-1349. doi: 10.1126/science.176.4041.1346 [DOI] [PubMed] [Google Scholar]
- 34.Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time. II. Neuroanatomic specificity. Electroencephalogr Clin Neurophysiol. 1988;69(4):338-352. doi: 10.1016/0013-4694(88)90005-3 [DOI] [PubMed] [Google Scholar]
- 35.Conrad EC, Tomlinson SB, Wong JN, et al. Spatial distribution of interictal spikes fluctuates over time and localizes seizure onset. Brain. 2020;143(2):554-569. doi: 10.1093/brain/awz386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baud MO, Kleen JK, Mirro EA, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9(1):88. doi: 10.1038/s41467-017-02577-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreier D, Banks C, Mathis J. Driving simulators in the clinical assessment of fitness to drive in sleepy individuals: a systematic review. Sleep Med Rev. 2018;38:86-100. doi: 10.1016/j.smrv.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 38.O'Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol. 1984;18(suppl 1):121s-129s. doi: 10.1111/j.1365-2125.1984.tb02590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasteleijn-Nolst Trenité D, Riemersma JBJ, Binnie CD, Smit AM, Meinardi H. Influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalogr Clin Neurophysiol. 1987;67(2):167-170. doi: 10.1016/0013-4694(87)90040-x [DOI] [PubMed] [Google Scholar]
- 40.Moskowitz H, Burns M, Fiorentino D, Smiley A, Zador P. Driver Characteristics and Impairment at Various BACs, Report HS 809-075. National Highway Traffic Safety Administration; 2000. [Google Scholar]
- 41.Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time. I. General considerations. Electroencephalogr Clin Neurophysiol. 1988;69(4):319-337. doi: 10.1016/0013-4694(88)90004-1 [DOI] [PubMed] [Google Scholar]
- 42.Jing J, Sun H, Kim JA, et al. Development of expert-level automated detection of epileptiform discharges during electroencephalogram interpretation. JAMA Neurol. 2020;77(1):103-108. doi: 10.1001/jamaneurol.2019.3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatum WO, Shellhaas RA. Epileptiform discharges. Are we still defining them? Neurology. 2020;94(20):862-863. doi: 10.1212/WNL.0000000000009432 [DOI] [PubMed] [Google Scholar]
- 44.Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 version. J Clin Neurophysiol. 2021;38(1):1-29. doi: 10.1097/WNP.0000000000000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat Med. 2017;23(6):678-680. doi: 10.1038/nm.4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer's disease: causes and clinical relevance. Lancet Neurol. 2017;16(4):311-322. doi: 10.1016/S1474-4422(17)30044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tai XY, Koepp M, Duncan JS, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain. 2016;139(Pt. 9):2441-2455. doi: 10.1093/brain/aww187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared on request.