Abstract
Introduction
Choline participates in plant stress tolerance through glycine betaine (GB) and phospholipid metabolism. As a salt-sensitive turfgrass species, Kentucky bluegrass (Poa pratensis) is the main turfgrass species in cool-season areas.
Methods
To improve salinity tolerance and investigate the effects of choline on the physiological and lipidomic responses of turfgrass plants under salinity stress conditions, exogenous choline chloride was applied to Kentucky bluegrass exposed to salt stress.
Results
From physiological indicators, exogenous choline chloride could alleviate salt stress injury in Kentucky bluegrass. Lipid analysis showed that exogenous choline chloride under salt-stress conditions remodeled the content of phospholipids, glycolipids, and lysophospholipids. Monogalactosyl diacylglycerol, digalactosyl diacylglycerol, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and lysophosphatidylcholine content were increased and phosphatidic acid content were decreased in plants after exogenous choline chloride under salt treatment. Plant leaf choline content increased, but GB was not detected in exogenous choline chloride treatment plants under nonstress or salt-stress conditions.
Discussion
GB synthesis pathway related genes showed no clear change to choline chloride treatment, whereas cytidyldiphosphate‐choline (CDP‐choline) pathway genes were upregulated by choline chloride treatment. These results reveal that lipid remodeling through choline metabolism plays an important role in the salt tolerance mechanism of Kentucky bluegrass. Furthermore, the lipids selected in this study could serve as biomarkers for further improvement of salt-sensitive grass species.
Keywords: choline chloride, salt stress, phospholipids, glycolipids, turfgrass
1. Introduction
Salt stress, caused by soil salinization, is an important factor for plant distribution worldwide and greatly affects plant development and productivity (Deinlein et al., 2014). Salt stress primarily causes osmotic and ionic toxicity in plant cells, which induces secondary effects of oxidative stress that greatly damage cellular components, such as membrane lipids (Zhu, 2016). Halophytes and salt-tolerant glycophyte cultivars are tolerant to saline conditions and exhibit various mechanisms, including complex physiological traits, molecular or gene networks, and metabolic pathways (Annunziata et al., 2019). The research about plant salinity stress response, such as through osmotic adjustment (OA), which maintains cell membrane integrity and stability, could provide a combination of molecular tools for developing salt-tolerant varieties in saline environments. Therefore, it is an effective method to elevate plant salinity tolerance through increasing membrane stability (Lv et al., 2021).
Salinity enhances lipid peroxidation, which, in turn, impairs cellular membrane permeability. Phospholipids and glycolipids are two basic lipids in plant cell membranes and function as maintaining membrane structure and regulating plant salt stress tolerance (Mansour, 2013; Chalbi et al., 2015). Phospholipids, like phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), could work as signaling compounds in plants exposed to abiotic stress (Hou et al., 2016; Han and Yang, 2021). In structural phospholipids, PC and PE occupy a large proportion and the remainder consists of phosphatidylglycerol (PG), phosphoinositides (PI), phosphatidylserine (PS), and PA (Furt et al., 2011). The minor lipids PA and phosphatidylinositol bisphosphate (PIP2) act as salt stress-signaling lipids that rapidly accumulate in rice (Oryza sativa) leaves, as detected using 32P labeling (Darwish et al., 2009). Platre et al. (2018) showed that PS and PA are required to generate the electrostatic signature of the plant plasma membrane, so their function could be separated based on their varying surface charges. PC is induced by salt stress and might function in the maintenance of membrane structure and function. Liu et al. (2015) reported that PE might be indirectly converted to PC in Arabidopsis via a hypothetical methylation pathway involving the synthesis of lysophosphatidylethanolamine (LysoPE), its methylation to lysophosphatidylcholine (LysoPC), and conversion to PC. PC could be synthesized by choline from the cytidyldiphosphate‐choline (CDP‐choline) pathway (Annunziata et al., 2019). In maize roots, PC levels decreased significantly with the increased expression of some phospholipase genes under saline-alkaline stress, suggesting there was a PC mediated lipid reprogramming under stress condition (Xu et al., 2021).
Glycolipids, like monogalactosyl diacylglycerol (MGDG) and digalactosyl diacylglycerol (DGDG), are major chloroplast thylakoid membranes components and primarily affect the photosystem II (PSII) characteristic (Mizusawa and Wada, 2012). In addition, a higher ratio of DGDGs/MGDGs to PCs/PEs is an indicator of the maintenance of membrane fluidity in response to abiotic stress in different plant species. It has been reported that the DGDG/MGDG ratio is response to salt stress (Guo et al., 2019). In transgenic tobacco (Nicotiana tabacum) with OsMGD gene, plants had a high salt tolerance, increased MGD activity and higher DGDG/MGDG ratios, which revealed a more stable thylakoid membrane in transgenic plant (Wang et al., 2014). In addition, the variation of membrane lipids compositions and fatty acid desaturation under salt stress condition have been identified as a common strategy for plant via affecting membranes mobility and stress signal transduction. (Magdy et al., 1993; Mansour, 2013).
Glycine betaine (GB) could synthesized from choline with two key enzyme, choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH) (Annunziata et al., 2019). In halophyte Chenopodium quinoa, choline accumulation could help to increase GB to improve salt tolerance (Pottosin et al., 2014). For some species like kidney bean (Phaseolus vulgaris) and wheat (Triticum aestivum), GB is a highly abundant compatible solute during the regulation of osmotic stress (Kreslavski et al., 2001; Salama et al., 2011). In seashore paspalum, the application of choline induced salt tolerance both in different salt sensitivity cultivars with remodeling the lipid profile and increase the GB content (Gao et al., 2020). However, not all plant species has high levels of GB, such as Arabidopsis thaliana and some grass species (Sakamoto and Murata, 2002; Hu et al., 2012). The exogenous treatment of choline or GB has effectively enhanced plant tolerance to abiotic stress, particularly for these low GB level species. In the C3 Poa turfgrass, foliar application of GB alleviated the physiological injury caused by drought or salt stress by maintaining membrane stability and active SOD, POD, and APX activity (Yang et al., 2012). In rice, choline priming could mitigate the salt stress during seed germination (Huang et al., 2023). Therefore, other metabolic pathways, such as the phospholipid and glycolipid reprograming through CDP-choline pathway, might play important role in dealing with salt stress in these species.
In this study, we examined the physiological response of Kentucky bluegrass with exogenous choline chloride under salt stress. Characterizing the GB and lipids (mainly phospholipids and glycolipids) metabolism that may be involved in the choline regulation of salt tolerance could provide theoretical basis for the important role of lipid metabolism in CDP-choline pathway during plant under salt stress condition. The selected lipid species in our study could be molecular markers for further salt stress tolerance improvement in salt-sensitive grass species.
2. Materials and methods
2.1. Plant material and treatment
Kentucky bluegrass (‘Diva’) seeds were sown in plastic pots (length 10 cm, width 7 cm, height 8.5 cm) filled with fritted clay. After 2-month pre-cultivation, uniform-size seeding were transferred to hydroponic conditions (0.5×Hoagland’s nutrient solution) in a growth chamber. Seedlings were cultivated for 21 days to adapt to the environment before exposure to the experimental treatments.
Plants were foliar-sprayed with 1 mM choline chloride until it dripped from the plants based on the previous study (Gao et al., 2020). Choline chloride treatment was performed 7 days prior to the start of stress application and continued at 7-day intervals during the experimental processing. 100 mM sodium chloride (NaCl) was added for the salt stress treatment. The salt stress was starting with 30 mM NaCl, and then gradually increased to 100 mM in two days. Each treatment had four replicates. Physiological measurements were performed 7, 14, and 21 days after treatment with 100 mM NaCl. Leaf was sampled at 14 days after salt stress treatment, then washed with deionized water and stored in -80°C for GB and choline content measurements, gene analysis, and lipid analysis. The plant growth state was photographed after 21 days of salt stress treatment.
2.2. Physiological measurements
The leaf Fv/Fm, electrolyte leakage, relative water content, and chlorophyll content was measured according to the previous study (Zhang et al., 2019; Zhang et al., 2020). The leaf osmotic adjustment was determined following a previous study (Gao et al., 2020). Quantification of GB and choline was performed using HPLC–MS based on a method described previously with modifications (Koc et al., 2002).
2.3. Lipid extraction
Lipid extraction was performed according to previous studies (Zhang et al., 2019). 1 mL lipid extract (methyl alcohol:methylene dichloride=1:1) was added and vortexed for 30 min. Added 300 μL ultra-pure water and vortexed for 1 minute and incubated at 4°C for 10 minutes. After centrifugation at 12000 rpm and 4°C for 3 minutes, 400 μL supernatant evaporated with nitrogen gas and concentrated to dry. Then, the sample was redissolved in 200 μL isopropanol. After filtered with filter membrane, the solution was ready for LC–MS/MS analysis.
2.4. HPLC and ESI–MS/MS conditions
The lipid extracts were analyzed with an LC–ESI- QTRAP/MS system (UPLC, Exion LC AD; MS, QTRAP 6500+ System). The ESI source operation parameters were as follows: ion source, turbo spray; source temperature 500°C; ion spray voltage 5500 V(Positive), −4500 V (Negative); Ion source gas 1, gas 2, and curtain gas were set at 45, 55, and 35 psi, respectively. The lipid unsaturation index was calculated as previously described (Su et al., 2009).
2.5. qRT-PCR analysis
Leaf RNA was isolated with the RNA EASY Fast Kit (TIANGEN, China). cDNA synthesis and qRT-PCR analyses was performed with FastKing One Step RT-PCR Kit (TIANGEN, China) on QuantStudio 1 Plus (Applied biosystems, USA). The qRT-PCR primers are listed in Supplementary Table S1 . The PpGADPH gene was used as a reference control for qRT-PCR analysis according to a previous study (Niu et al., 2017). The gene expression was calculated using the 2-△△Ct method (Livak and Schmittgen, 2001). Each qRT-PCR analysis was performed in triplicate.
2.6. Statistical analysis
The data were analyzed with Spss 17.0 (SPSS Inc., Chicago, IL, USA). Fisher’s protected least significant difference (LSD) test was performed to calculate the significant differences (P < 0.05).
3. Results
3.1. The growth and physiological changes of Kentucky bluegrass
Kentucky bluegrass plants were cultured in nutrient solution were foliar-sprayed with choline chloride and subjected to a salt solution for 21 days ( Figure 1 ). Choline chloride treatment had improved the plant growth with more leaves compared to untreated plant. Under salt stress condition, greener and fewer wilted leaves were observed in the choline chloride treatment plants than untreated plant. Growth characteristics showed that exogenous choline chloride increased the salt tolerance of Kentucky bluegrass.
Figure 1.
Growth of Kentucky bluegrass treated with choline chloride and salt stress. The photographs were taken after 21 days of treatment. CK, control treatment; CC, choline chloride treatment; S, salt treatment; CS, choline chloride and salt treatment, the same as below.
In addition, physiological indicators were determined to illustrate the mechanism that increased salt tolerance in Kentucky bluegrass after the exogenous application of choline chloride. The Fv/Fm ratio decreased after salt stress treatments ( Figure 2A ). After 7 days of treatment, the difference of Fv/Fm ratio among the four treatments was not significant. After 14 days of treatment, the Fv/Fm ratio was significantly declined under stress conditions compared to the nonstress condition, whereas choline chloride did not affect the Fv/Fm ratio under stress and nonstress conditions. At 21 days of treatment, plants had a significantly higher Fv/Fm ratio in choline chloride treated plants, whereas salt treatment significantly decreased the Fv/Fm ratio compared to the nonstress plants. Electrolyte leakage were upregulated in both choline chloride untreated and untreated plants after salt stress ( Figure 2B ). At 7 and 14 days after salt stress, exogenous choline chloride resulted in lower EL (9.62% and 9.37%, respectively) than that of untreated plants. Chlorophyll content in all treatment were decreased during salt stress ( Figure 2C ) and it was significantly higher in choline chloride treated plants than that in untreated plants 7 (7.03%) and 21 days (10.00%) after salt treatment, whereas exogenous choline chloride significantly increased the chlorophyll content 14 and 21 days after the non-stress treatment. The relative water content in all treatment were decreased during salt stress ( Figure 2D ). Plants had significantly higher relative water content in choline chloride treated plants than that in untreated plants 7 (3.86%) and 14 days (5.37%) after salt treatment. Salt treatment significantly declined the relative water content compared to the nonstress treatment during days 7–21 of salt stress ( Figure 2E ). In addition, OA was detected in the leaves of the four treatments, and this value increased during the salt stress treatment. Significantly higher OA was found in choline chloride-treated plants from 7 to 21 days of stress treatment, with increases of 77.61%, 42.54%, and 13.55%, respectively, than in untreated plants.
Figure 2.
The physiological indicators of Kentucky bluegrass with choline chloride and salt stress treatments. (A) Fv/Fm. (B) Electrolyte leakage. (C) Chlorophyll content. (D) Relative water content. (E) Osmotic adjustment. All data are means ± SE for three biological replicates. The vertical bars indicate the values of LSD at P-value=0.05.
3.2. The leaf lipidomic changes of Kentucky bluegrass
After 14 days of salt stress, the leaf tissues of Kentucky bluegrass were collected for lipidomic analysis. The leaf lipidomic change profiles are shown in Figure S1 . In total, 150 lipid molecular species were tested, including DGDG, MGDG, PA, PC, PE, PG, PI, PS, and LysoPC in Kentucky bluegrass treated with choline chloride and salt stress.
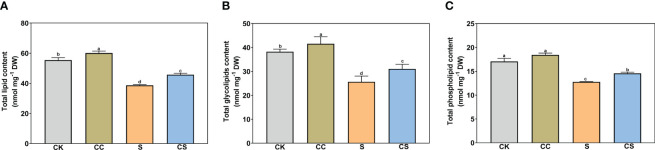
As shown in Figure 3 , the total lipid, total glycolipid, and total phospholipid contents significantly decreased after salt stress treatment. The total lipid content increased by 8.43% and 18.41% with exogenous choline chloride under nonstress and salt stress treatment ( Figure 3A ). The content of total glycolipid was significantly higher in choline chloride -treated plants than that in untreated nonstress control (8.67%) and salt-stressed plants (21.03%) ( Figure 3B ). For total phospholipids, an increase was found only in choline chloride-treated plants compared to non-treated plants under salt stress conditions ( Figure 3C ).
Figure 3.
The contents of total lipid, glycolipid, and phospholipid of Kentucky bluegrass with choline chloride and salt stress treatments. (A) Total lipid content. (B) Total glycolipid content. (C) Total phospholipid content. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05, the same as below.
In glycolipids, the total DGDG and MGDG contents were both significantly declined with or without choline chloride treatment under salt stress ( Figures 4A, B ). Exogenous choline chloride significantly increased the total DGDG content under nonstress and salt-stress conditions by 17.37% and 20.15%, respectively, and increased the total MGDG content by 19.76% under salt-stress conditions compared to the control. In phospholipids, the total PA, PC, and PE ( Figures 4C–E ) and total PG and PS ( Figures 5A, C ) contents significantly decreased after salt stress treatment with or without choline chloride treatment. Exogenous choline chloride significantly increased the total PC content by 9.73% under salt-stress conditions, decreased the total PA content by 17.33% and 38.70% under nonstress and salt-stress conditions, respectively, and increased the total PS content by 22.76% and 22.73% under nonstress and salt-stress conditions, respectively. The PI content had no significant changes under different treatments ( Figure 5B ). The lysophospholipid (LysoPC) content also had no significant difference with choline chloride treatment under nonstress conditions, but the difference was significant under salt-stress conditions (S, 0.11 nmol mg-1, CS 0.18 nmol mg-1) ( Figure 5D ). In addition, exogenous choline chloride significantly increased the DGDG:MGDG ratio compared with that in treated plants under nonstress conditions ( Figure 5E ). Under salt treatment, the PC:PE ratio was significantly higher (8.68%) in choline chloride -treated plants than in untreated plants ( Figure 5F ).
Figure 4.
The contents of total DGDG, MGDG, PA, PC, and PE of Kentucky bluegrass with choline chloride and salt stress treatments. (A) Total DGDG content. (B) Total MGDG content. (C) Total PA content. (D) Total PC content. (E) Total PE content. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05.
Figure 5.
The contents of total PG, PI, PS, and LysoPC, and the ratio of DGDG:MGDG and PC:PE of Kentucky bluegrass with choline chloride and salt stress treatments. (A) Total PG content. (B) Total PI content. (C) Total PS content. (D) Total LysoPC content. (E) DGDG:MGDG ratio. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05.
3.3. The variation of different lipid molecular species in Kentucky bluegrass
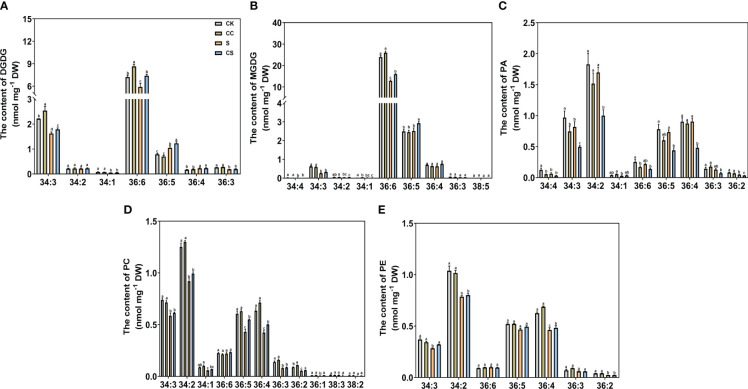
The different lipid specific molecular classes are shown in Figures 6 , 7 in Kentucky bluegrass leaves treated with choline chloride and salt stress.
Figure 6.
The specific molecular species of DGDG, MGDG, PA, PC, and PE of Kentucky bluegrass with choline chloride and salt stress treatments. (A) The content of DGDG content. (B) The content of MGDG content. (C) The content of PA content. (D) The content of PC content. (E) The content of PE content. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05.
Figure 7.
The specific molecular species of PG, PI, PS, and LysoPC of Kentucky bluegrass with choline chloride and salt stress treatments. (A) The content of PG content. (B) The content of PI content. (C) The content of PS content. (D) The content of LysoPC content. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05.
For glycolipid species, the 36:6 lipid species had the highest abundant in all treatments in MGDG and DGDG ( Figure 6A, B ). The content of 34:3 and 36:6 molecular classes in DGDG were decreased and the content of the 36:5 molecular class in MGDG was increased, with or without choline chloride treatment under salt stress condition. The DGDG (34:3, 36:6) content was significantly higher in choline chloride-treated plants under non stress conditions, whereas the DGDG (34:3, 36:6, 36:5) content was significantly higher in choline chloride -treated plants under salt and non-stress conditions. Compared to untreated plants, the MGDG (36:6, 36:5) content was significantly increased in choline chloride -treated plants in under salt-stress. For phospholipid species, under non stress conditions, exogenous choline chloride significantly decreased the PA (34:3, 36:6) content, whereas PA species (34:3, 34:2, 36:5, 36:4, and 36:2) content were lower in choline chloride-treated plants compared nontreated plants under salt stress ( Figure 6C ). The PC (34:3, 34:2, 36:5, 36:4, 36:3, and 36:2) content decreased significantly after salt stress treatment. Under salt stress, the content of PC (36:5 and 36:4) in choline chloride treated plant were significantly higher that of non-treated plants ( Figure 6D ). The PE (34:2, 36:4) content was significantly lower under salt stress than that under nonstress conditions, and PE (34:3, 36:4) was higher in choline chloride -treated plants ( Figure 6F ). For other phospholipid species, a significant decrease in PG (34:4, 34:3, 34:2) and PS (40:3, 40:2) was observed after salt stress and choline chloride treatment significantly increased the PG (34:4, 34:3), PI (34:3, 34:2), and PS (40:3, 40:2) contents under non stress conditions ( Figure 7A-C ). Under salt stress, PI (34:2) and PS (40:3, 40:2) contents were both greatly increased by choline chloride treatment. For lysophospholipids, the content of LysoPC (18:3, 18:2) significantly increased in choline chloride -treated plants under non stress conditions, whereas that of LysoPC (16:0, 18:3, 18:2) significantly increased in choline chloride -treated plants under salt and non-stress conditions ( Figure 7D ). The DGDG, PC, and PE unsaturation were significantly increased, whereas the PG unsaturation was significantly decreased with or without choline chloride treatment after salt stress treatment ( Table S2 ). In choline chloride -treated plants, a significantly lower unsaturation (3.02%) was observed in PC under salt stress.
3.4. The leaf GB and choline content determination
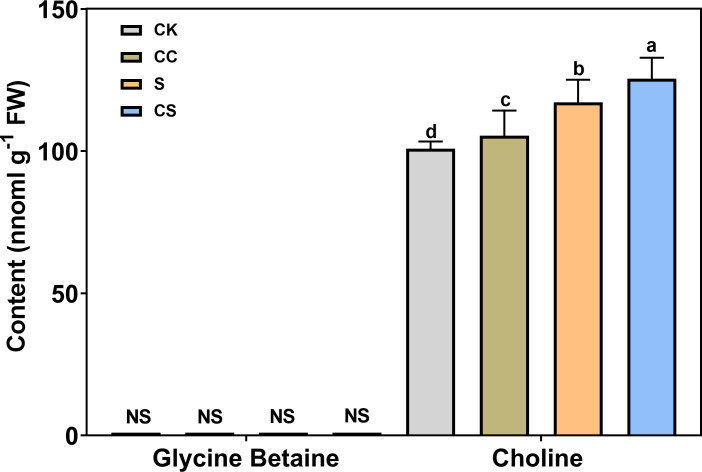
To determine the major pathways regulated by choline chloride, GB and choline contents were analyzed under different treatments ( Figure 8 ). The results showed that the GB content were not detected following either salt or choline chloride treatment. Salt stress significantly increased the choline content with or without choline chloride treatment. Exogenous choline chloride significantly increased choline content (4.52% and 7.13%) under nonstress and salt-stress conditions, respectively. These results revealed that exogenous choline chloride could affect endogenous choline content rather than the GB synthesis pathway under stress and nonstress conditions in Kentucky bluegrass.
Figure 8.
Leaf endogenous GB and choline content with choline chloride and salt stress treatments. NS means undetectable. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05.
3.5. The expression of GB synthesis and CPD-choline pathway genes
Furthermore, expression analysis of genes controlling lipid synthesis was performed to determine the molecular mechanisms affected by choline. For genes in the GB synthesis pathway, salt stress greatly increased the expression of CMO and BADH1 and decreased the expression of BADH2 ( Figures 9A-C ). Exogenous choline chloride upregulated the CMO (1.53-fold), BADH1 (2.97-fold), and BADH2 (1.74-fold) gene expressions under nonstress conditions. The expression of CMO and BADH1 had no significant changes to exogenous choline chloride after salt stress treatment, whereas BADH2 gene expression was downregulated by the choline chloride treatment under salt stress. For genes in the CPD-choline pathway, salt stress significantly induced the expression of Choline kinase (CK1), CTP-phosphocholine cytidylyltransferase (CCT1), and Choline/ethanolaminephosphotransferase (CEPT) ( Figures 9D-F ). Under salt stress, the expression of CK1 was significantly upregulated (73.19%) by choline chloride treatment. The expression of CCT1 and CEPT gene were significantly higher in choline chloride treated plants under nonstress and salt-stress conditions by 104.24% and 209.87% and 14.64% and 15.21%, respectively. These results revealed that choline chloride regulates salt stress in Kentucky bluegrass via choline metabolic pathways and not through the GB synthesis pathway.
Figure 9.
The gene expression of GB and CDP-choline metabolism pathway with choline chloride and salt stress treatments. (A) CMO. (B) BADH1. (C) BADH2. (D) CK1. (E) CCT1. (F) CEPT. All data are means ± SE for three biological replicates. Different letter indicated the significance at P < 0.05.
4. Discussion
Soil salinity is an important factor that affecting turfgrass growth and utilization. Kentucky bluegrass is sensitive to salt stress, and mitigation of the detrimental effects of salt stress and improvement in its tolerance is urgently required. Choline has been identified play role in plant salt stress tolerance (Incharoensakdi and Karnchanatat, 2003). In the present study, Fv/Fm, relative water content, chlorophyll content, and OA were increased, and EL were decreased in choline chloride -treated plants in salt treated plants. This illustrated that exogenous choline chloride could improve the salt tolerance of Kentucky bluegrass. Membrane remodeling is an important metabolic regulatory mechanism in plants exposed to salt stress (Guo et al., 2022). In soybeans, a short-term salt stress treatment could trigger a dynamic reprogramming in both galactolipids and phospholipids (Liu et al., 2021). Similar results have been reported in potato (Solanum tuberosum) (Yu et al., 2019), cotton (Gossypium herbaceum) (Liu et al., 2022), and Carex rigescens (Hu et al., 2021). As a precursor, choline is participated in two key pathways including GB metabolism pathway and lipid metabolism pathway in plant (Annunziata et al., 2019). In our study, endogenous GB metabolism pathway had not obviously response to choline application, while lipid (galactolipids and phospholipids) remodeling, were occurred with choline treatment, especially under salt stress. These results show that choline-mediated lipid metabolism is the central salt tolerance mechanism in Kentucky bluegrass.
Our data showed that the glycolipid and phospholipid contents were increased with choline chloride treatment. Choline alters the reorganization of lipid composition to protect membrane from the injury caused by salt stress, and similar results have been reported for the seashore paspalum (Gao et al., 2020). Plant glycolipids contribute to the formation of stacked thylakoid membranes and DGDG and MGDG content have been reported to decrease after salt stress in rice (Wang et al., 2014) and Suaeda salsa (Sui et al., 2010). In our study, an increase in the content of DGDG and MGDG was observed under salt stress with choline chloride treatment. DGDG and MDGD are likely to act as stabilizers in the organization of thylakoid membranes and are indispensable components of the light-harvesting complex II in plant photosynthesis (Guo et al., 2019). Furthermore, plants can alter the thylakoid membrane lipids component to resist abiotic stress. The content of MGDG 36:6, 36:5, and DGDG 34:3, 36:6, and 36:5 was significantly higher in choline chloride treated plants under salt stress, suggesting that these glycolipid species make a difference in choline mediated salt tolerance improvement. Similarly, the levels of 18:2-containing glycolipids (like MGDG-36:5 and DGDG-36:5) were upregulated by different abiotic stresses in tall fescue (Festuca arundinacea) (Zhang et al., 2019), C. rigescens (Hu et al., 2021), and rice (Wang et al., 2019). These results suggest that unsaturated 18:2-containing glycolipids might be significant in abiotic stress responses in plants.
Under salt stress condition, the PC content was significantly higher in choline chloride treated plants. PC participate in abiotic stress tolerance in plants (Tasseva et al., 2004). Studies have confirmed membrane PC has positive role in plant salt tolerance in Arabidopsis thaliana (Qiao et al., 2018), cordgrass (Spartina patens) (Wu et al., 2005) and tomato (Lycopersicon esculentum) (Kerkeb et al., 2001). In addition, in Catharanthus roseus and Mesembryanthemum crystallinum which had high salt tolerence (Elkahoui et al., 2004; Barkla et al., 2018), PC species (36:5 and 36:4) increased after salt stress compared to those in salt-sensitive plants, suggesting that they are involved in salt stress adaptation (Magdy et al., 1994). In the present study, the PC unsaturation index decreased with exogenous choline chloride. In PC-specific molecular species, the abundance of 16:0 acyl chains were higher than that of 18:3/18:2 acyl chains after choline chloride treatment. High lipid saturation contributes to plant cell membrane stability and integrity under abiotic stress (Li et al., 2020), indicating that these saturated lipid species may play an important role in choline-mediated salt stress tolerance in Kentucky bluegrass.
PE and PC are the major phospholipid components of extraplastidial membranes, and PE can regulate stress signal transduction under hyperosmotic stress via phospholipase C (Pokotylo et al., 2014; Singh et al., 2015). Under salt stress, the PE content was significantly increased in choline chloride treated plants. Studies have reported that PE increases in association with PC, resulting in abiotic stress due to increased membrane fluidity (Higashi and Saito, 2019). The choline group of PC is a bilayer-forming lipid, whereas the ethanolamine group of PE is a non-lamellar-forming lipid, which affects the formation of the bilayer phase and preserves membrane fluidity (Toumi et al., 2008; Narayanan et al., 2018). Therefore, the PC/PE ratio may reflect a cellular response to maintain membrane stability and balance between the membrane and storage lipids (Narayanan et al., 2018). In the present study, the PC/PE ratio increased in response to salt stress with exogenous choline chloride, illustrating that the elevated abundance of PC rather than PE with choline chloride treatment could help maintain the membrane integrity and fluidity in Kentucky bluegrass.
PA has been reported to crucial for salt stress responses by helping maintain ion homeostasis (Yao and Xue, 2018; Liu et al., 2019). In addition, PA is a major intermediate that guiding the PC and PE biosynthesis from the CDP-choline and CDP-ethanolamine pathways, respectively (Carman and Han, 2009). The PA content and specific molecular species decreased under salt stress in Kentucky bluegrass, and the result was the opposite of the PC and PE variation. Under abiotic stress conditions, phospholipase D could hydrolyze of PE and PC and produce PA in plants (Jiang et al., 2019). Therefore, these results suggest that choline can improve salt tolerance in Kentucky bluegrass by promoting PA downstream of PC and PE synthesis through the CDP-choline pathway.
The phosphatidylserine (PS) content can be regulated by abiotic stress in plants (Higashi and Saito, 2019). PS includes a large amount of lipid species, such as PS (40:3), which contains very-long-chain fatty acids and has been reported to increase in heat stress-susceptible wheat genotype plants (Narayanan et al., 2016). In the present study, choline chloride treatment increased the PS (40:3 and 40:2) content under salt stress conditions. However, the function of the increased PS content under salt stress is unknown. A previous study reported that PS decarboxylase (PSD) converts PS to PE through endoplasmic reticulum (Yamaoka et al., 2011). Therefore, the changes in PS in plants exposed to salinity stress with choline chloride treatment may rely on the PSD-mediated metabolic pathway and, together with PE, may play a role in membrane stability.
LysoPC is a lipid class with only one fatty acyl chain on the glycerol moiety of glycerolipids, which is surface-active, alters membrane fluidity, and affects membrane receptor functions (Zhang et al., 2018). In the present study, choline chloride treatment increased LysoPC content under salt stress conditions. In Schizochytrium sp., LysoPC lipid species were significantly enriched in the choline metabolism pathway, contributing to improved salt stress tolerance (Jiang et al., 2019). However, the role of LysoPC in stress tolerance in plants remains unclear. Plant Ca2+ plays a significant role in cell membrane integrity, and endogenous Ca2+ levels may decrease during salt stress (Hashemipetroudi et al., 2022). A previous study found that the content of LysoPC increased in response to Ca2+ deprivation (IsHizuKA et al., 2000), demonstrating that LysoPC might play an essential role in Ca2+ maintaining plasma membrane stability.
In summary, choline chloride increased the salt tolerance of Kentucky bluegrass by increasing Fv/Fm, relative water content, chlorophyll content, and OA and reducing leaf EL. Lipidomic results showed that the content of total MGDG, DGDG, PC, PE, PS, and LysoPC were increased, and PA content was decreased with exogenous choline chloride when exposed to salt stress. The leaf GB content and synthesis related genes had no response to exogenous choline chloride, indicating that choline triggered lipid metabolism was a primary mechanism in Kentucky bluegrass response to salt stress condition The specific lipid molecular species of glycolipids (MGDG 36:6, 36:5, and DGDG 34:3, 36:6, 36:5), phospholipids (PC 36:5, 36:4, PE 34:3, 34:2, 36:5, 36:4, PI 34:2, and PS 40:3, 40:2), and lysophospholipids (LysoPC 16:0, 18:3, 18:2) were increased exposed to exogenous choline chloride. Our results provide lipid biomarkers for further improvement of salt-sensitive grass species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZZ: Writing – original draft, Writing – review and editing, Software, Data curation, Formal Analysis, Validation. YL: Validation, Writing – review and editing, Data curation, Formal Analysis, Software. XM: Data curation, Software, Writing – review and editing, Formal Analysis. YL: Writing – review and editing, Investigation, Validation. CZ: Validation, Writing – review and editing, Data curation. GY: Writing – review and editing, Resources, Supervision. ZW: Supervision, Writing – review and editing, Funding acquisition. KZ: Funding acquisition, Supervision, Writing – review and editing, Software, Writing – original draft.
Funding Statement
The work was supported by the First-Class Grassland Science Discipline Program of Shandong Province, China and Start-Up Foundation for High Talents of Qingdao Agricultural Universtiy (no.665/1121011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1269286/full#supplementary-material
References
- Annunziata M. G., Ciarmiello L. F., Woodrow P., Dell'Aversana E., Carillo P. (2019). Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla B. J., Garibay-Hernández A., Melzer M., Rupasinghe T. W. T., Roessner U. (2018). Single cell-type analysis of cellular lipid remodelling in response to salinity in the epidermal bladder cells of the model halophyte Mesembryanthemum crystallinum . Plant Cell Environ. 41 (10), 2390–2403. doi: 10.1111/pce.13352 [DOI] [PubMed] [Google Scholar]
- Carman G. M., Han G. S. (2009). Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 284 (5), 2593–2597. doi: 10.1074/jbc.R800059200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalbi N., Martínez-Ballesta M. C., Youssef N. B., Carvajal M. (2015). Intrinsic stability of Brassicaceae plasma membrane in relation to changes in proteins and lipids as a response to salinity. J. Plant Physiol. 175, 148–156. doi: 10.1016/j.jplph.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Darwish E., Testerink C., Khalil M., El-Shihy O., Munnik T. (2009). Phospholipid signaling responses in salt-stressed rice leaves. Plant Cell Physiol. 50 (5), 986–997. doi: 10.1093/pcp/pcp051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinlein U., Stephan A. B., Horie T., Luo W., Xu G., Schroeder J. I. (2014). Plant salt-tolerance mechanisms. Trends Plant Sci. 19 (6), 371–379. doi: 10.1016/j.tplants.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahoui S., Smaoui A., Zarrouk M., Ghrir R., Limam F. (2004). Salt-induced lipid changes in Catharanthus roseus cultured cell suspensions. Phytochemistry 65 (13), 1911–1917. doi: 10.1016/j.phytochem.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Furt F., Simon-Plas F., Mongrand S. (2011). Lipids of the plant plasma membrane. Plant plasma membrane 19, 3–30. doi: 10.1007/978-3-642-13431-9_1 [DOI] [Google Scholar]
- Gao Y., Li M., Zhang X., Yang Q., Huang B. (2020). Up-regulation of lipid metabolism and glycine betaine synthesis are associated with choline-induced salt tolerance in halophytic seashore paspalum. Plant Cell Environ. 43 (1), 159–173. doi: 10.1111/pce.13657 [DOI] [PubMed] [Google Scholar]
- Guo Q., Liu L., Barkla B. J. (2019). Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 20 (17), 4264. doi: 10.3390/ijms20174264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Liu L., Rupasinghe T. W. T., Roessner U., Barkla B. J. (2022). Salt stress alters membrane lipid content and lipid biosynthesis pathways in the plasma membrane and tonoplast. Plant Physiol. 189 (2), 805–826. doi: 10.1093/plphys/kiac123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Yang Y. (2021). Phospholipids in salt stress response. Plants (Basel) 10 (10), 2024. doi: 10.3390/plants10102204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemipetroudi S. H., Ahmadian G., Fatemi F., Nematzadeh G., Yamchi A., Kuhlmann M. (2022). Ion content, antioxidant enzyme activity and transcriptional response under salt stress and recovery condition in the halophyte grass Aeluropus littoralis . BMC Res. Notes 15 (1), 201. doi: 10.1186/s13104-022-06090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Saito K. (2019). Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 75, 100990. doi: 10.1016/j.plipres.2019.100990 [DOI] [PubMed] [Google Scholar]
- Hou Q., Ufer G., Bartels D. (2016). Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 39 (5), 1029–1048. doi: 10.1111/pce.12666 [DOI] [PubMed] [Google Scholar]
- Hu Q., Cui H., Ma C., Li Y., Yang C., Wang K., et al. (2021). Lipidomic metabolism associated with acetic acid priming-induced salt tolerance in Carex rigescens . Plant Physiol. Biochem. 167, 665–677. doi: 10.1016/j.plaphy.2021.08.045 [DOI] [PubMed] [Google Scholar]
- Hu L., Hu T., Zhang X., Pang H., Fu J. (2012). Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hortic. Sci. 137 (1), 38–46. doi: 10.21273/JASHS.137.1.38 [DOI] [Google Scholar]
- Huang L., Yu M., Feng N., Di. Z., Jie Y., Li Y. (2023). Effects of seed initiators choline chloride and indole-3-butyric acid potassium on growth and physiological characteristics of rice seedlings under salt Stress. J. Nuc Agr Sci. 37 (1), 196–206. doi: 10.11869/j.issn.1000-8551.2023.01.0196 [DOI] [Google Scholar]
- Incharoensakdi A., Karnchanatat A. (2003). Salt stress enhances choline uptake in the halotolerant cyanobacterium Aphanothece halophytica . Biochim. Biophys. Acta 1621 (1), 102–109. doi: 10.1016/s0304-4165(03)00052-7 [DOI] [PubMed] [Google Scholar]
- IsHizuKA K., Kanayama A., Satsu H., Miyamoto Y., Furihata K., Shimizu M. (2000). Identification of a taurine transport inhibitory substance in sesame seeds. Bioscience biotechnology Biochem. 64 (6), 1166–1172. doi: 10.1271/bbb.64.1166 [DOI] [PubMed] [Google Scholar]
- Jiang J. Y., Zhu S., Zhang Y., Sun X., Hu X., Huang H., et al. (2019). Integration of lipidomic and transcriptomic profiles reveals novel genes and regulatory mechanisms of Schizochytrium sp. in response to salt stress. Bioresour Technol. 294, 122231. doi: 10.1016/j.biortech.2019.122231 [DOI] [PubMed] [Google Scholar]
- Kerkeb L., Donaire J. P., Venema K., Rodríguez-Rosales M. P. (2001). Tolerance to NaCl induces changes in plasma membrane lipid composition, fluidity and H+-ATPase activity of tomato calli. Physiol. Plant 113 (2), 217–224. doi: 10.1034/j.1399-3054.2001.1130209.x [DOI] [PubMed] [Google Scholar]
- Koc H., Mar M. H., Ranasinghe A., Swenberg J. A., Zeisel S. H. (2002). Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 74 (18), 4734–4740. doi: 10.1021/ac025624x [DOI] [PubMed] [Google Scholar]
- Kreslavski V., Balakhnina T., Khristin M., Bukhov N. (2001). Pre-treatment of bean seedlings with choline compounds increases the resistance of photosynthetic apparatus to UV-B radiation and elevated temperatures. Photosynthetica 39, 363–368. doi: 10.1023/A:1015174108937 [DOI] [Google Scholar]
- Li J., Liu L. N., Meng Q., Fan H., Sui N. (2020). The roles of chloroplast membrane lipids in abiotic stress responses. Plant Signal Behav. 15 (11), 1807152. doi: 10.1080/15592324.2020.1807152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ma D., Zhang Z., Wang S., Du S., Deng X., et al. (2019). Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 165, 174–184. doi: 10.1016/j.envexpbot.2019.06.005 [DOI] [Google Scholar]
- Liu Y., Wang L., Li X., Luo M. (2022). Detailed sphingolipid profile responded to salt stress in cotton root and the GhIPCS1 is involved in the regulation of plant salt tolerance. Plant Sci. 316, 111174. doi: 10.1016/j.plantsci.2021.111174 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang G., Wang X. (2015). Role of aminoalcoholphosphotransferases 1 and 2 in phospholipid homeostasis in Arabidopsis. Plant Cell 27 (5), 1512–1528. doi: 10.1105/tpc.15.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Xiao Z., Wang Z., Lam H. M., Chye M. L. (2021). Galactolipid and phospholipid profile and proteome alterations in soybean leaves at the onset of salt stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.644408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lv S., Tai F., Guo J., Jiang P., Lin K., Wang D., et al. (2021). Phosphatidylserine synthase from salicornia europaea is involved in plant salt tolerance by regulating plasma membrane stability. Plant Cell Physiol. 62 (1), 66–79. doi: 10.1093/pcp/pcaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdy M., Mansour F., Lee-Stadelmann O. Y., Stadelmann E. J. (1993). Salinity stress and cytoplasmic factors. A comparison of cell permeability and lipid partiality in salt sensitive and salt resistant cultivars and lines of Triticum aestivum and Hordeum vulgare . Physiologia Plantarum 88 (1), 141–148. doi: 10.1111/j.1399-3054.1993.tb01771.x [DOI] [Google Scholar]
- Magdy M., Mansour F., van Hasselt P. R., Kuiper P. J. (1994). Plasma membrane lipid alterations induced by NaCl in winter wheat roots. Physiologia Plantarum 92 (3), 473–478. doi: 10.1111/j.1399-3054.1994.tb08838.x [DOI] [Google Scholar]
- Mansour M. M. F. (2013). Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. plantarum 57, 1–10. doi: 10.1007/s10535-012-0144-9 [DOI] [Google Scholar]
- Mizusawa N., Wada H. (2012). The role of lipids in photosystem II. Biochim. Biophys. Acta 1817 (1), 194–208. doi: 10.1016/j.bbabio.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Narayanan S., Prasad P. V. V., Welti R. (2018). Alterations in wheat pollen lipidome during high day and night temperature stress. Plant Cell Environ. 41 (8), 1749–1761. doi: 10.1111/pce.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S., Tamura P. J., Roth M. R., Prasad P. V., Welti R. (2016). Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 39 (4), 787–803. doi: 10.1111/pce.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu K., Shi Y., Ma H. (2017). Selection of Candidate Reference Genes for Gene Expression Analysis in Kentucky Bluegrass (Poa pratensis L.) under Abiotic Stress. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platre M. P., Noack L. C., Doumane M., Bayle V., Simon M. L. A., Maneta-Peyret L., et al. (2018). A combinatorial lipid code shapes the electrostatic landscape of plant endomembranes. Dev. Cell 45 (4), 465–480.e411. doi: 10.1016/j.devcel.2018.04.011 [DOI] [PubMed] [Google Scholar]
- Pokotylo I., Kolesnikov Y., Kravets V., Zachowski A., Ruelland E. (2014). Plant phosphoinositide-dependent phospholipases C: variations around a canonical theme. Biochimie 96, 144–157. doi: 10.1016/j.biochi.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Pottosin I., Bonales-Alatorre E., Shabala S. (2014). Choline but not its derivative betaine blocks slow vacuolar channels in the halophyte Chenopodium quinoa: implications for salinity stress responses. FEBS Lett. 588 (21), 3918–3923. doi: 10.1016/j.febslet.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Qiao K., Wang M., Takano T., Liu S. (2018. 1772). Overexpression of acyl-coA-binding protein 1 (ChACBP1) from saline-alkali-tolerant chlorella sp. Enhances stress tolerance in arabidopsis . Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A., Murata N. (2002). The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 25 (2), 163–171. doi: 10.1046/j.0016-8025.2001.00790.x [DOI] [PubMed] [Google Scholar]
- Salama K. H., Mansour M. M., Hassan N. S. (2011). Choline priming improves salt tolerance in wheat (Triticum aestivum L.). Aust. J. Basic Appl. Sci. 5 (11), 126–132. doi: 10.13140/2.1.4228.9606 [DOI] [Google Scholar]
- Singh A., Bhatnagar N., Pandey A., Pandey G. K. (2015). Plant phospholipase C family: Regulation and functional role in lipid signaling. Cell Calcium 58 (2), 139–146. doi: 10.1016/j.ceca.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Su K., Bremer D. J., Jeannotte R., Welti R., Yang C. (2009). Membrane lipid composition and heat tolerance in cool-season turfgrasses, including a hybrid bluegrass. J. Am. Soc. Hortic. Sci. 134 (5), 511–520. doi: 10.21273/JASHS.134.5.511 [DOI] [Google Scholar]
- Sui N., Li M., Li K., Song J., Wang B.-S. (2010). Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica 48, 623–629. doi: 10.1007/s11099-010-0080-x [DOI] [Google Scholar]
- Tasseva G., Richard L., Zachowski A. (2004). Regulation of phosphatidylcholine biosynthesis under salt stress involves choline kinases in Arabidopsis thaliana. FEBS Lett. 566 (1-3), 115–120. doi: 10.1016/j.febslet.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Toumi I., Gargouri M., Nouairi I., Moschou P., Salem-Fnayou A. B., Mliki A., et al. (2008). Water stress induced changes in the leaf lipid composition of four grapevine genotypes with different drought tolerance. Biol. Plantarum 52, 161–164. doi: 10.1007/s10535-008-0035-2 [DOI] [Google Scholar]
- Wang Y., Jiang D., Hou J., Chen G. (2019). Physiological characterization and thylakoid ultrastructure analysis in super high-yield hybrid rice leaves under drought stress. Photosynthetica 57 (3), 890–896. doi: 10.32615/ps.2019.106 [DOI] [Google Scholar]
- Wang S., Uddin M. I., Tanaka K., Yin L., Shi Z., Qi Y., et al. (2014). Maintenance of chloroplast structure and function by overexpression of the rice MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE gene leads to enhanced salt tolerance in tobacco. Plant Physiol. 165 (3), 1144–1155. doi: 10.1104/pp.114.238899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Seliskar D. M., Gallagher J. L. (2005). The response of plasma membrane lipid composition in callus of the halophyte Spartina patens (Poaceae) to salinity stress. Am. J. Bot. 92 (5), 852–858. doi: 10.3732/ajb.92.5.852 [DOI] [PubMed] [Google Scholar]
- Xu X., Zhang J., Yan B., Wei Y., Ge S., Li J., et al. (2021). The adjustment of membrane lipid metabolism pathways in maize roots under saline-alkaline stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.635327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Yu Y., Mizoi J., Fujiki Y., Saito K., Nishijima M., et al. (2011). PHOSPHATIDYLSERINE SYNTHASE1 is required for microspore development in Arabidopsis thaliana . Plant J. 67 (4), 648–661. doi: 10.1111/j.1365-313X.2011.04624.x [DOI] [PubMed] [Google Scholar]
- Yang Z., Yu J., Merewitz E., Huang B. (2012). Differential effects of abscisic acid and glycine betaine on physiological responses to drought and salinity stress for two perennial grass species. J. Am. Soc. Hortic. Sci. 137 (2), 96–106. doi: 10.21273/JASHS.137.2.96 [DOI] [Google Scholar]
- Yao H. Y., Xue H. W. (2018). Phosphatidic acid plays key roles regulating plant development and stress responses. J. Integr. Plant Biol. 60 (9), 851–863. doi: 10.1111/jipb.12655 [DOI] [PubMed] [Google Scholar]
- Yu Y., Kou M., Gao Z., Liu Y., Xuan Y., Liu Y., et al. (2019). Involvement of phosphatidylserine and triacylglycerol in the response of sweet potato leaves to salt stress. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Cui H., Li M., Xu Y., Cao S., Long R., et al. (2020). Comparative time-course transcriptome analysis in contrasting Carex rigescens genotypes in response to high environmental salinity. Ecotoxicol Environ. Saf. 194, 110435. doi: 10.1016/j.ecoenv.2020.110435 [DOI] [PubMed] [Google Scholar]
- Zhang X., Xu Y., Huang B. (2019). Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant Cell Environ. 42 (3), 947–958. doi: 10.1111/pce.13405 [DOI] [PubMed] [Google Scholar]
- Zhang T., Yang J., Sun Y., Kang Y., Yang J., Qi Z. (2018). Calcium deprivation enhances non-selective fluid-phase endocytosis and modifies membrane lipid profiles in Arabidopsis roots. J. Plant Physiol. 226, 22–30. doi: 10.1016/j.jplph.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Zhu J. K. (2016). Abiotic stress signaling and responses in plants. Cell 167 (2), 313–324. doi: 10.1016/j.cell.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.