Abstract
BACKGROUND
Telomere shortening is a well-characterized cellular aging mechanism, and short telomere syndromes cause age-related disease. However, whether long telomere length is advantageous is poorly understood.
METHODS
We examined the clinical and molecular features of aging and cancer in persons carrying heterozygous loss-of-function mutations in the telomere-related gene POT1 and noncarrier relatives.
RESULTS
A total of 17 POT1 mutation carriers and 21 noncarrier relatives were initially included in the study, and a validation cohort of 6 additional mutation carriers was subsequently recruited. A majority of the POT1 mutation carriers with telomere length evaluated (9 of 13) had long telomeres (>99th percentile). POT1 mutation carriers had a range of benign and malignant neoplasms involving epithelial, mesenchymal, and neuronal tissues in addition to B- and T-cell lymphoma and myeloid cancers. Five of 18 POT1 mutation carriers (28%) had T-cell clonality, and 8 of 12 (67%) had clonal hematopoiesis of indeterminate potential. A predisposition to clonal hematopoiesis had an autosomal dominant pattern of inheritance, as well as penetrance that increased with age; somatic DNMT3A and JAK2 hotspot mutations were common. These and other somatic driver mutations probably arose in the first decades of life, and their lineages secondarily accumulated a higher mutation burden characterized by a clocklike signature. Successive generations showed genetic anticipation (i.e., an increasingly early onset of disease). In contrast to noncarrier relatives, who had the typical telomere shortening with age, POT1 mutation carriers maintained telomere length over the course of 2 years.
CONCLUSIONS
POT1 mutations associated with long telomere length conferred a predisposition to a familial clonal hematopoiesis syndrome that was associated with a range of benign and malignant solid neoplasms. The risk of these phenotypes was mediated by extended cellular longevity and by the capacity to maintain telomeres over time. (Funded by the National Institutes of Health and others.)
THE LENGTH OF TELOMERES, WHICH ARE made up of tandem (TTAGGG)n sequences at chromosome ends, predicts the onset of replicative senescence and functions as a mitotic clock.1 Telomeres shorten with cell division, and short dysfunctional telomeres signal DNA damage, which elicits a cellular response that leads to senescence or apoptosis.2 Germline loss-of-function mutations in genes that encode components of telomerase, the enzyme that synthesizes new telomere repeats, cause a short telomere progeria that is marked by pulmonary and hematopoietic disease.2 Its most common manifestation is idiopathic pulmonary fibrosis,2 which co-occurs with syndromic features such as premature hair graying,3 bone marrow failure,4 and B- and T-cell immunodeficiency.5,6 Long telomeres extend the replicative potential of cultured cells,1 which raises the possibility that mutations that prevent telomere shortening may influence specific aging phenotypes and disease risk.
The protein POT1 (protection of telomeres 1) binds the single-stranded 3′ end of the telomere.7 It is a conserved, essential protein and protects telomeres from exonuclease degradation.7 POT1 has also been implicated in the regulation of telomerase-dependent elongation of the telomere. Some studies have suggested that it increases the processivity of telomerase (i.e., its ability to continue to add telomere repeats),8 whereas others have identified a role of POT1 in the negative regulation of telomerase catalytic activity.9,10 Heterozygous somatic POT1 mutations were initially identified in chronic lymphocytic leukemia (CLL) cells,11 and germline mutations were later reported in families with isolated cancers, including melanoma,12,13 glioma,14 and CLL.15 Biallelic POT1 mutations have been reported in an infant with some short telomere features.16 However, the mechanisms underlying the risk of disease associated with heterozygosity for POT1 mutations are not understood; hypotheses have included telomere deprotection,17 genome instability,11 telomere elongation,18,19 and telomere shortening.20
METHODS
PARTICIPANTS AND OVERSIGHT
We recruited participants for a Johns Hopkins University research study dedicated to understanding the role of telomeres in disease. Persons who were heterozygous for POT1 mutations were identified in a clinical setting and, along with their relatives, were invited to participate in the study. An initial group of 17 mutation carriers from five families, along with 21 of their noncarrier relatives, were first included in the study; subsequently, 6 mutation carriers from three additional families were recruited and included in validation studies. The Johns Hopkins Medicine institutional review board approved the study, and the participants provided written informed consent. Two of the authors reviewed the primary clinical records. The authors vouch for the accuracy and completeness of the data.
TELOMERE LENGTH MEASUREMENT
Telomere length was measured with the use of flow cytometry and fluorescence in situ hybridization at Johns Hopkins Hospital Laboratories, and the data were plotted relative to a clinically validated nomogram.21 Additional details, including the methods used for germline and somatic bulk and single-cell colony sequencing, T-cell clonality studies, flow cytometry, and phylogenetic analyses, are provided in the Supplementary Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org. The human genome build and transcript identifiers used for all variant annotation are also provided in the Supplementary Methods section.
STATISTICAL ANALYSIS
The study was designed to be exploratory, and all analyses, including the analyses of the primary and exploratory end points, were assigned post hoc. The first primary objective was to compare the age-adjusted telomere length in POT1 mutation carriers with the expected length based on the validated nomogram of controls, as published previously.21 Specifically, we calculated the probability of observing the number of POT1 mutation carriers whose telomere length was above the 90th percentile and above the 99th percentile relative to the expected percentages of 10% and 1%, respectively, using the binomial test. We also evaluated the difference in telomere length among POT1 mutation carriers and related noncarriers as compared with the 50th percentile value at that age using the Mann–Whitney test. The second primary objective was to compare the incidence of clonal hematopoiesis of indeterminate potential (CHIP) among POT1 mutation carriers and related noncarriers with the use of Fisher’s exact test. A two-sided P value of less than 0.05 was considered to indicate statistical significance.
Exploratory analyses included a comparison of the proportions of POT1 mutation carriers and related noncarriers who had JAK2 p.V617F clones with variant-allele frequencies greater than 0.1%; this measure was also evaluated among GGCC haplotype carriers with or without POT1 mutations. In these analyses, odds ratios and 95% confidence intervals were adjusted for small sample sizes with the use of the epitab package in R software.22 The widths of the confidence intervals were not adjusted for multiplicity, and therefore the confidence intervals should not be used for hypothesis testing. P values are included only for the primary analyses. Data were analyzed with R software, version 4.2.1.
RESULTS
TELOMERE LENGTH AND FUNCTIONAL CONSEQUENCES OF POT1 MUTATIONS
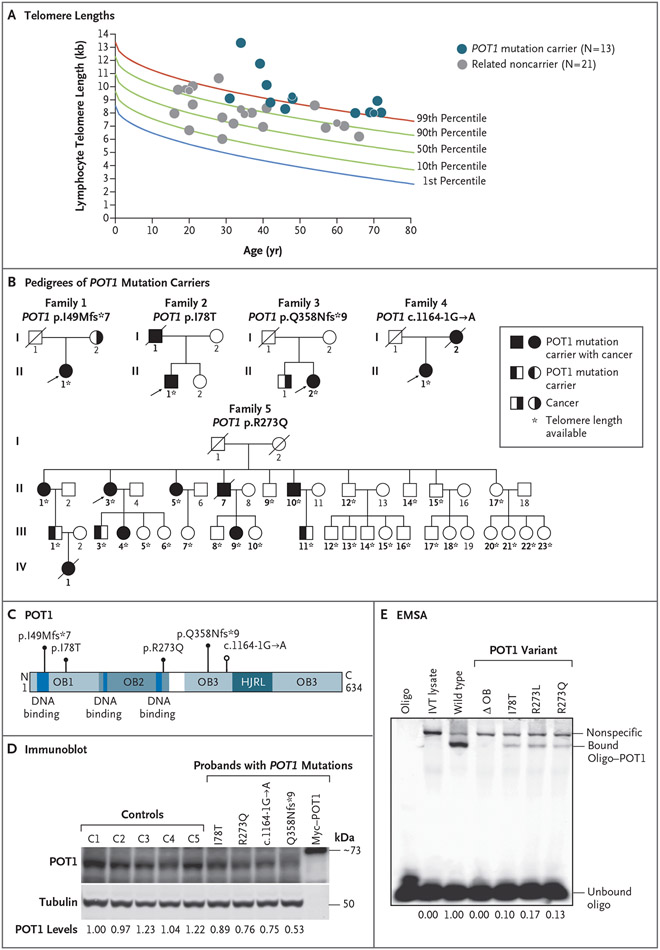
We consecutively included 17 persons who carried germline heterozygous mutations in POT1 from five unrelated families (Fig. 1A, 1B, and 1C). Of these persons, the 13 who were alive underwent evaluation for telomere length; in all 13, the mean telomere length was above the 90th percentile, and in 9 of the 13 the mean telomere length was above the 99th percentile (P<0.001 by binomial test for each comparison). Persons with a POT1 mutation had longer telomeres than did their noncarrier relatives (21 persons) (mean, 3.0 kb vs. 1.2 kb longer than the median for age; P<0.001 by Mann–Whitney test) (Fig. 1A).
Figure 1 (facing page). Telomere Length and Pedigrees of POT1 Mutation Carriers.
Panel A shows telomere lengths measured by flow cytometry and fluorescence in situ hybridization in 13 living POT1 mutation carriers and their noncarrier relatives. Data are plotted relative to a clinically validated nomogram derived from healthy controls.21 Some data points differ in size in order to make overlapping data points visible. Panel B shows pedigrees of POT1 mutation carriers. Probands are indicated with arrows. Bold identifiers indicate persons for whom data on the POT1 genotype were available. Circles denote female family members, and squares male family members; a line through a symbols indicates that the person is deceased. Panel C shows POT1 with mutations annotated relative to conserved domains. HJRL denotes holiday junction resolvase-like, and OB oligonucleotide-binding. Panel D shows an immunoblot of endogenous POT1 levels in lymphoblastoid cell lines with levels quantified below in arbitrary units relative to tubulin (replicate data in Fig. S1A). Panel E shows an electrophoretic mobility-shift assay (EMSA) for POT1 missense mutations. The ΔOB mutant is truncated for amino acids 127 through 635. POT1R273L, a variant reported in familial melanoma,12 was included as a positive control. DNA binding is quantified below as a proportion relative to wild type, and results were replicated twice. IVT denotes in vitro translated, and oligo oligonucleotide.
The POT1 mutations were absent from or rare in population databases (Table S1 in the Supplementary Appendix), although one mutation, p.I78T, is a known Ashkenazi founder mutation.23 The p.I78T and p.R273Q variant amino acids are located in conserved domains of POT1 that interact with telomere DNA. Assays of POT1 protein and mRNA in lymphoblastoid cell lines derived from carriers of POT1 p.I78T and p.R273Q showed decreased expression and defective binding to telomere DNA (Fig. 1D and 1E and Fig. S1A and S1B). The other mutations disrupted POT1 protein stability by altering the reading frame or altering splicing (Fig. 1D and Fig. S1C and S1D). These data support the hypothesis that POT1 haploinsufficiency is associated with long telomere length.
NEOPLASMS IN POT1 MUTATION CARRIERS WITH LONG TELOMERES
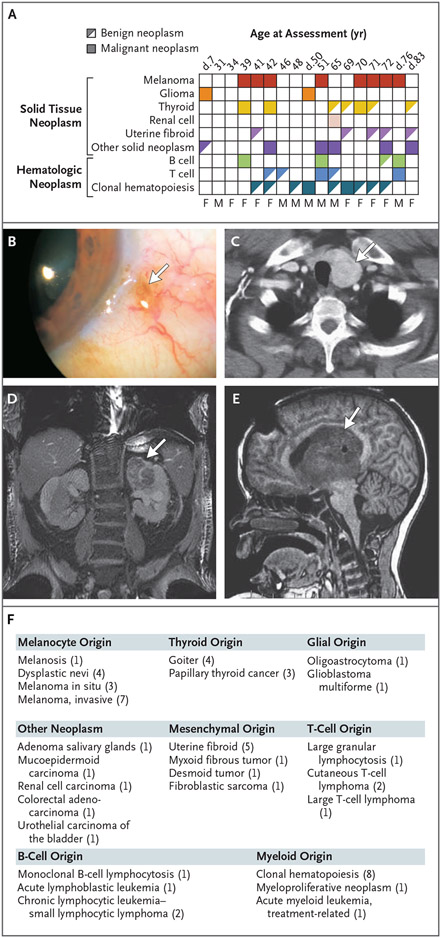
We found a spectrum of neoplasms involving solid and hematopoietic tissues that ranged from benign to malignant (Fig. 2). The spectrum of melanocyte neoplasms, for example, ranged from eye melanosis and dysplastic nevi to invasive and metastatic melanoma. Delayed hair graying was reported in all six participants 70 to 83 years of age who were queried (or whose relatives were queried, for those who were deceased), a finding consistent with a melanocyte spectrum phenotype (Table S2). Goiter and papillary thyroid cancer were also documented, with benign thyroid disease being more common than malignant disease (Fig. 2A and 2C). Mesenchymal neoplasms ranged from uterine fibroids to soft-tissue sarcomas and mesenteric desmoid tumors (Fig. 2A and 2F). Epithelial-derived cancers included renal cell, urothelial, and colorectal carcinoma (Fig. 2D and 2F). The most life-threatening neoplasm was malignant glioma, which affected two POT1 mutation carriers (12%) (Fig. 2A, 2E, and 2F). B-cell neoplasms included B-cell lymphocytosis of uncertain significance, pediatric acute lymphocytic leukemia, and adult small lymphocytic lymphoma (Fig. 2F). T-cell neoplasms included cutaneous T-cell lymphoma and large T-cell lymphoma (Fig. 2F), and two persons had both B-cell and T-cell neoplasms in addition to one or two solid tumors (Fig. 2A). One of the participants with metastatic melanoma was treated with an immune-checkpoint inhibitor; a large T-cell lymphoma subsequently developed and proved fatal (Fig. 3A). In another participant who received alkylating therapy for glioma, fatal acute myeloid leukemia developed 8 years later, and one participant had an evolving myeloproliferative neoplasm (Fig. 2A).
Figure 2. Benign and Malignant Neoplastic Manifestations among POT1 Mutation Carriers.
Panel A shows the diagnoses among 17 POT1 mutation carriers and the ages at last assessment. Age at death is indicated by “d.” The clonal hematopoiesis in all persons shown here met the threshold for clonal hematopoiesis of indeterminate potential (CHIP; i.e., variant-allele frequency of ≥2%); for four deceased persons and one person who was recruited at the end of the study, clonal hematopoiesis and T-cell clonality were not assessed. M denotes male and F female. Panel B shows a representative image of benign melanosis (arrow). Panel C shows a computed tomography (CT)–captured goiter (arrow). Panel D shows an axial CT image of a left kidney mass diagnosed as clear-cell carcinoma (arrow). Panel E shows a representative magnetic resonance image of pediatric-onset glioblastoma multiforme. Panel F shows the range of benign to malignant diagnoses grouped according to the affected tissue among 17 POT1 mutation carriers, with the number of affected persons in Panel A shown in parentheses.
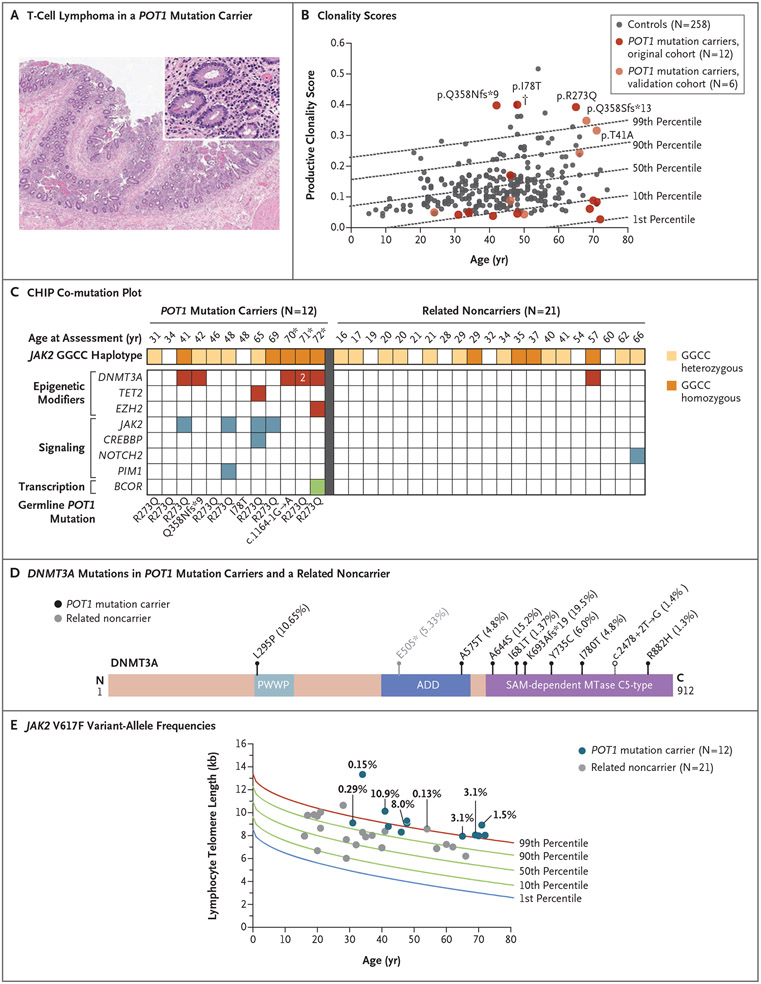
Figure 3 (facing page). Lymphoid and Myeloid Clonality among POT1 Mutation Carriers.
Panel A shows images (hematoxylin and eosin staining) of small bowel from an autopsy examination of a POT1 mutation carrier in whom large T-cell lymphoma developed after receipt of immune-checkpoint inhibitor therapy for metastatic melanoma. Lymphocyte infiltrates involving the lamina propria were positive for CD3. Panel B shows productive clonality scores plotted against age (calculated from T-cell receptor Vβ CDR3 sequencing as 1 minus the normalized Shannon’s entropy for all productive rearrangements). Scores range from 0 to 1, where 0 represents polyclonality and 1 indicates one rearrangement dominating the entire repertoire. The 18 POT1 mutation carriers are from seven unrelated families. Percentile lines are derived from 258 healthy bone marrow donors (controls) who were seropositive for cytomegalovirus, as derived from Emerson et al.24 One person with a history of cutaneous T-cell lymphoma but no known systemic disease is indicated with a dagger. Panel C shows a CHIP co-mutation plot including POT1 mutation carriers and their noncarrier relatives sorted according to increasing age with the germline mutations indicated below. JAK2 GGCC haplotype status is also color-coded in the key. The three persons indicated with an asterisk had additional DNMT3A mutations with variant-allele frequencies of 1% or higher, one of whom had two DNMT3A mutations with variant-allele frequencies of 2% or higher; all these mutations are shown in Panel D. Panel D shows DNMT3A mutations identified in POT1 mutation carriers and in one related noncarrier relative to the conserved domains of the protein and their allele frequency. Filled circles denote nonsynonymous mutations and the unfilled circle a splicing variant. Panel E shows JAK2 V617F variant-allele frequencies identified by targeted ultradeep sequencing in POT1 mutation carriers and their noncarrier relatives, shown in relation to lymphocyte telomere length.
LYMPHOID CLONALITY
The occurrence of both lymphoid and myeloid neoplasms in the carriers of POT1 mutations, together with an apparent absence of cellular hypersensitivity to DNA damage (Fig. S1E), support the hypothesis that long telomere length provides a selective advantage that sustains clonal evolution. To test this hypothesis, we assessed the diversity of peripheral T cells by sequencing TCRB (specifically, complementarity-determining region 3 of the T-cell receptor β-chain variable region) from 12 POT1 mutation carriers and found that 3 had productive clonality scores near the 99th percentile of scores of healthy age-matched controls24 (Fig. 3B), and the 10 most prevalent clones among these participants were overrepresented in the repertoire (Fig. S2A). In a second group of unrelated POT1 mutation carriers, 2 of 6 persons had a clonality score near the age-adjusted 99th percentile24 (Fig. 3B and Fig. S2B). Cytomegalovirus (CMV) exposure is the primary driver of T-cell clonality with aging,24 but the increased clonality was evident irrespective of whether we compared the clonality of peripheral T cells from POT1 mutation carriers with those from CMV-positive or CMV-negative controls (Fig. 3B and Fig. S2C and S2D). In total, 5 of 18 POT1 mutation carriers (28%) had evidence of increased clonality (>90th percentile) (probability by chance alone, 2.8%), including 4 persons with no history of hematologic cancer. Assessment by flow cytometry confirmed the presence of atypical or clonal T-cell populations in 4 of 5 of these persons, and all 5 were also incidentally identified as having clonal B-cell populations (Fig. S2E).
ASSESSMENT OF CLONAL HEMATOPOIESIS MUTATIONS
We next examined the prevalence of clonal variants, adopting standard clinical criteria for defining CHIP (i.e., mutations with a variant-allele frequency of ≥2%).25 Of the 12 POT1 mutation carriers included in this analysis, 8 (67%) had CHIP; in contrast, of 21 of their noncarrier relatives, 2 had CHIP (odds ratio, 10.1; 95% confidence interval [CI], 2.6 to 84.2; P = 0.001 by Fisher’s exact test) (Fig. 3C). The size of the clones was relatively large (of the 14 variants occurring in the 8 affected persons, 5 had a variant-allele frequency of >10%) as compared with those in noncarriers, in whom the clonal variants were small (variant-allele frequency, ≤5%), with two of three occurring in noncanonical CHIP genes (Table S3). In the clones in the carriers, DNMT3A was the most commonly mutated gene, followed by JAK2 (in which the p.V617F hotspot variant occurred). A second group of 3 POT1 mutation carriers older than 65 years of age from the validation cohort had CHIP (Fig. S3C and S3D), which supported a high penetrance among older carriers even relative to an older group of controls (in total [original cohort plus validation cohort], 8 of 8 POT1 mutation carriers >65 years of age [median age, 69.5 years] vs. 13 of 30 controls >70 years of age [median age, 74.5 years]) (Table S4) and despite a lower depth of coverage in the former group (600× for POT1 mutation carriers vs. 1300× for older controls) (see the Supplementary Methods section).
Ultradeep sequencing showed that 7 of 12 POT1 mutation carriers as compared with 1 of 23 related noncarriers had JAK2 V617F clones (odds ratio, 12.8; 95% CI, 2.8 to 148.9) (Fig. 3E). Genomewide association studies have identified an intronic GGCC haplotype within JAK2 that increases the risk of JAK2-associated disease by a factor of 4,26,27 and in two of the families, GGCC heterozygous or homozygous haplotypes were present (Fig. 3C). However, the GGCC genotype alone did not explain the difference in JAK2 variant clonal hematopoiesis between carriers and noncarriers; the GGCC genotype was similarly distributed among carriers and noncarriers (83% and 71%, respectively, had at least one GGCC allele) (Fig. 3C), but POT1 mutation carriers were more likely to have JAK2 V617F (6 of 10 GGCC-positive POT1 mutation carriers had JAK2 V617F vs. 0 of 15 GGCC-positive noncarriers; odds ratio, 18.0; 95% CI, 2.1 to 956.8) (Fig. S4A).
PHYLOGENETIC STUDIES
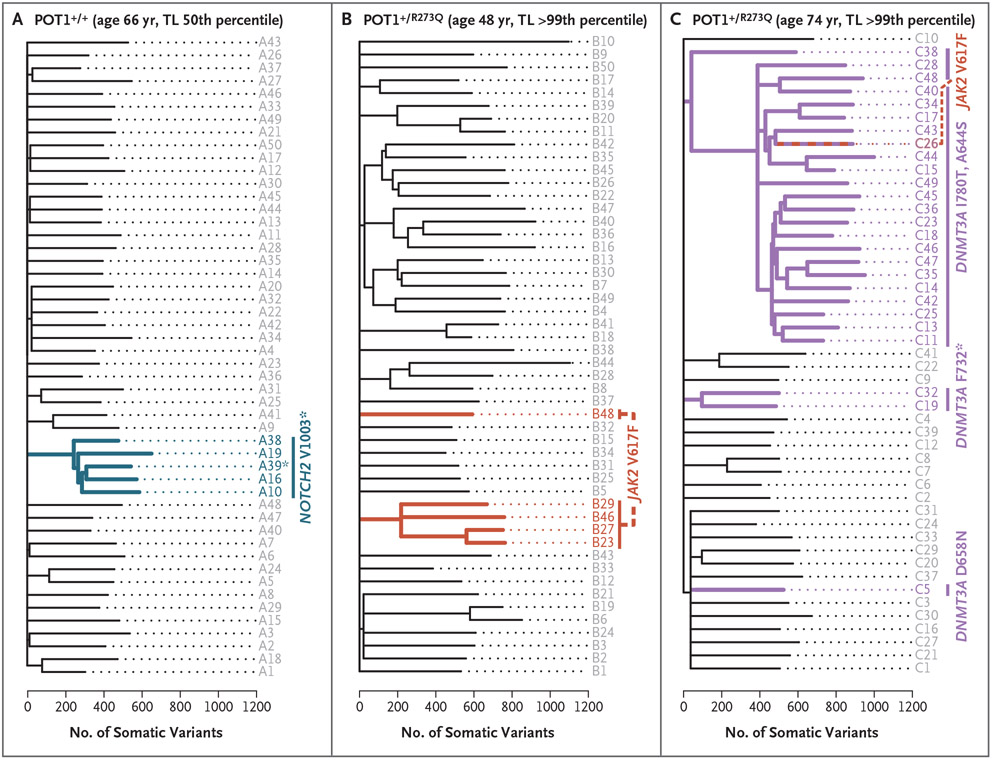
We queried the lineage of JAK2 V617F in leukocyte fractions and confirmed that they were myeloid-derived and that they expanded longitudinally with age (Fig. S4B and S4C). However, in some cases, DNMT3A mutations were shared across myeloid and lymphoid lineages, which supports their having arisen in a primitive progenitor (Fig. S4B). Phylogenetic inference of colonies derived from single-cell hematopoietic progenitors (with the use of whole-genome sequencing) in two related POT1 mutation carriers yielded results consistent with driver CHIP mutations arising early in life (e.g., before 4 years of age in one 74-year-old carrier), supporting our hypothesis that driver-carrying lineages are long-lived in POT1 mutation carriers (Fig. S5A-S5C). Moreover, progenitors from POT1 mutation carriers had higher somatic-mutation burdens than a related noncarrier (Fig. 4 and Fig. S5D), and the topologies of phylogenetic trees also showed evidence of oligoclonality relative to chronologic age. For example, the phylogenetic tree of a 48-year-old carrier showed two independent JAK2 V617F clonal events and phylogenetic evidence of oligoclonality that was greater than that in a relative who was 18 years older (Fig. 4) and similar to that in controls in their eighth decade of life.28 The other POT1 mutation carrier carried five identifiable DNMT3A somatic mutations (biallelic mutations defining a large clade, as well as three other mutations, including one identified by bulk sequencing) (Fig. 4 and Fig. S6). A dominance of C→T transitions among the single-base substitution signatures and clocklike, age-associated patterns are consistent with an extended replicative history for these clones (Fig. S7).29
Figure 4. Phylogenies of Hematopoietic Colonies.
Panels A, B, and C show phylogenies of single cell–derived hematopoietic colonies on a somatic variant scale (single-nucleotide variants and short insertions–deletions). The POT1 genotype, the person’s age, and the telomere length (TL) percentile are shown above each tree. In Panel B, two de novo JAK2 mutations are resolved in the tree, and the two cooccurring DNMT3A mutations in Panel C were found to be biallelic in phasing studies. The tree topology in the two POT1 mutation carriers shows the tips, which represent contemporary hematopoietic colonies that are descended from fewer ancestral progenitor lineages (internal branches), in contrast to the tree of the related noncarrier shown in Panel A. The number of somatic variants in POT1 mutation carriers was also higher than in the noncarrier, even in clades without detectable driver mutations.
MECHANISMS UNDERLYING CLONAL PREDISPOSITION
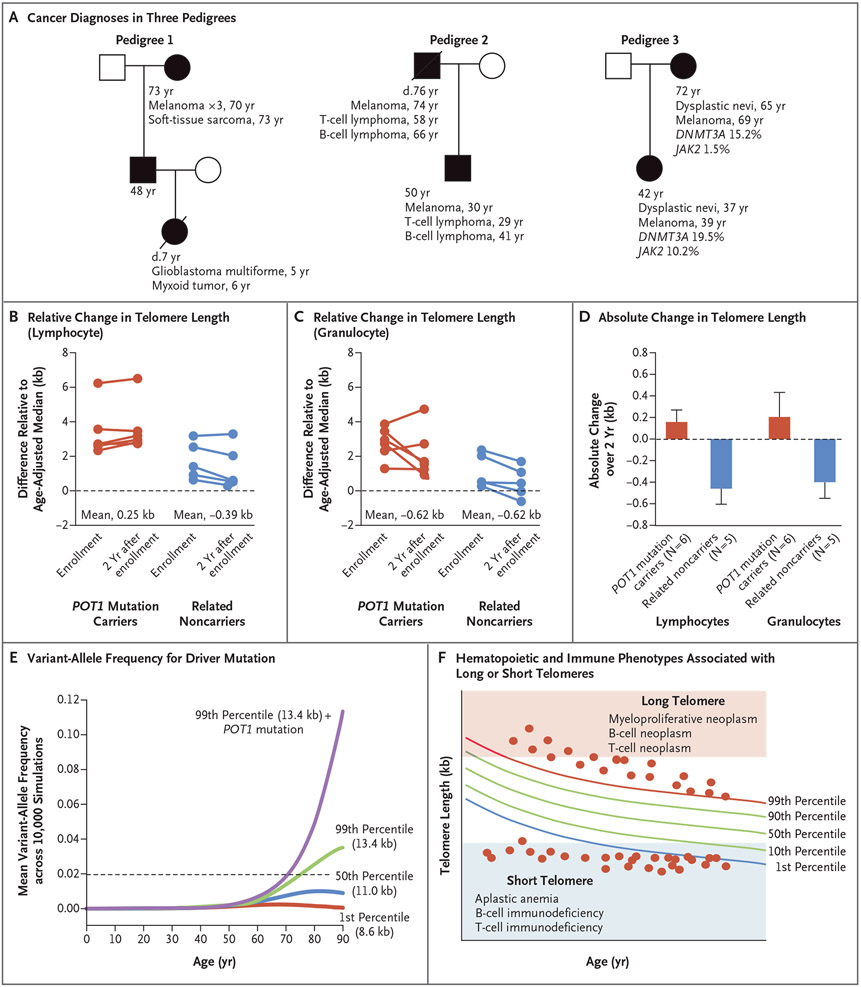
We asked whether the clonal predisposition was due to the long telomere length or a telomerelengthening advantage and found evidence supporting both mechanisms. A role for long telomere length was suggested by the appearance of genetic anticipation, wherein offspring of POT1 mutation carriers who carried the same germline mutation had cancer that developed several decades earlier than that in their parents (and in some cases, larger somatic clones developed at younger ages) (Fig. 5A). We propose that this genetic anticipation is due to inheritance of long telomeres, as has been documented for the short telomere syndromes,3 although we did not detect a difference in telomere length across consecutive generations in the families we studied here. The apparent genetic anticipation led us to hypothesize that POT1 mutations promote telomere lengthening with age, so we measured telomere length 2 years after study enrollment. Our data support a slower rate of shortening among POT1 mutation carriers than among controls, whereas their noncarrier relatives had a rate of shortening similar to that among controls (Fig. 5B and 5C). Telomeres of lymphocytes and granulocytes in POT1 mutation carriers showed an absolute gain in length as compared with that of their noncarrier children and siblings (mean change, 0.17 kb vs. −0.47 kb in lymphocytes and 0.22 kb vs. −0.41 kb in granulocytes) (Fig. 5D).
Figure 5 (facing page). Genetic Anticipation and Longitudinal Change in Telomere Length among POT1 Mutation Carriers.
Panel A shows the age at assessment and cancer diagnosis in three pedigrees. Mutation carriers (shaded) carry POT1 p.R273Q in Pedigrees 1 and 3 and carry p.I78T in Pedigree 2. One mutation carrier in Pedigree 1 had three separate melanoma diagnoses. Panels B and C show differences in lymphocyte and granulocyte telomere lengths relative to the median for age at study enrollment and at 2 years. Data are shown for POT1 mutation carriers and their siblings and children who are noncarriers. Panel D shows the mean absolute change in telomere length over a 2-year period in POT1 mutation carriers (red) and their noncarrier relatives (blue). Error bars indicate the standard error. Panel E shows the mean variant-allele frequency for a somatic heterozygous driver mutation acquired at birth over a lifetime in telomere length groups calculated from 10,000 simulations for each group. The dashed line indicates the 2% variant-allele frequency threshold defining CHIP. Panel F shows contrasting hematopoietic and immune phenotypes associated with POT1 mutations (long telomere length) and short telomere syndromes. Each dot represents a hypothetical person with a germline telomere maintenance defect plotted relative to the normal telomere length distribution in the human population. This panel was adapted from Armanios.38
To understand the mechanism that underlies the high incidence of CHIP among POT1 mutation carriers with aging, we simulated the effect of the inherited capacity to lengthen telomeres (such as with POT1 mutation carriers) and long telomere length (99th percentile) (such as with POT1 mutation carriers), both independently and together, on the clone size of a single somatic driver mutation (such as the JAK2 V617F hotspot variant) that arose in a hematopoietic progenitor at birth over a 90-year lifespan. We also simulated the effects of intermediate and short telomere length, at the 50th and 1st percentiles, respectively; percentiles were previously defined in clinically validated nomograms.21 On the basis of 10,000 replicate simulations per group, POT1 mutation carriers with long telomere length had the largest clone sizes (Fig. 5E; confidence intervals are shown in Fig. S8). In this simulation, long telomere length also supported clonal longevity with age, although to a lesser extent (Fig. 5E). By contrast, simulated clones with short telomeres initially expanded but then vanished because of dropout at the threshold of telomere-induced senescence (Fig. 5E and Fig. S9). These data support a model in which the proportion of persons with CHIP was the highest among POT1 mutation carriers with long telomere length, followed by persons with long telomere length alone (Fig. S10).
DISCUSSION
The risk of clonal hematopoiesis increases with age, but the germline drivers underlying its variable penetrance are not fully known. Here we found that excessively long telomeres, along with the inherited capacity to lengthen telomeres as a result of POT1 dysfunction, conferred a predisposition to lymphoid and myeloid clonal hematopoiesis in an autosomal dominant manner that showed increasing penetrance with age. The genetic data support the hypothesis that haploinsufficiency for POT1 facilitates telomerase-dependent telomere elongation, although the precise mechanisms by which telomerase repeat addition is enhanced in this context remains unclear. We determined that somatic driver mutations arise during the early decades of life and that their long-lived lineages sustain a high mutation burden, which shows clocklike signatures that are a hallmark of an extended replicative history. The loss of the tumor-suppressor mechanism of telomere shortening supports the expansion of clonal populations, which may explain the elevated risk of cancer. JAK2 driver mutations have been inferred to occur during the perinatal period or in the first or second decade of life in older patients with overt myeloproliferative neoplasms.30,31 Our data support the hypothesis that long telomere length provides an advantage for the “survival” of these variant clones into adulthood. They also support a model wherein telomere-independent germline factors, such as the GGCC haplotype, determine the risk of JAK2 mutagenesis, and replicative competence is necessary for sustaining the longevity of JAK2 variant clones. POT1 mutations were recently identified in 1% of patients with myeloproliferative neoplasms.32 Multiple genes regulate telomere length, and therefore variants in genes other than POT1 may account for the familial clustering of these disorders and their association with the risk of lymphoid neoplasms and solid tumors.33
An increased incidence of clonal hematopoiesis has also been documented among persons with mendelian short telomere syndromes, but the spectrum of mutations is distinct, with somatic-reversion mutations that offset the inherited defect being most common.18,34 These observations, together with our findings, suggest that the germline genetic background influences the spectrum of mutations under clonal selection in hematopoietic compartments. Moreover, our study provides a mendelian context for understanding the biology that underlies population-based observations linking common variants near TERT, the gene encoding telomerase reverse-transcriptase, which are associated with long telomere length, with the risk of CHIP and nearly all solid tumors.35-37
The neoplastic predisposition we describe presents a paradox at the intersection of aging and cancer biology. Long telomere length (obtained, in part, through an invulnerability to telomere shortening) and increasing telomere length with age manifests as delayed replicative senescence. Simultaneously, it sustains clonality, which is typically associated with older age. The paradox is evident in the observations that both short and long extremes of telomere length appear to mediate two distinct age-associated disease phenotypes. Their contrast is illustrated in the predisposition to B-cell and T-cell lymphoproliferative and myeloproliferative disease in POT1 mutation carriers and the predisposition to B-cell and T-cell immunodeficiency and aplastic anemia in persons with short telomere syndromes (see Fig. 5F, which depicts our hypothesis that the syndromes are diametric).
The syndrome we report is an archetype for a cellular pronearia (from the Greek “nearós,” meaning “youth”). Its phenotype would appear to affect several organ systems. We speculate that the relatively high burden of somatic variants in hematopoietic lineages may be similar to that of other tissues. We observed benign neoplasias, including cutaneous nevi, goiters, and uterine fibroids, in POT1 mutation carriers. The risk of these conditions has been linked to loci encompassing telomere genes39; perhaps longer telomere length confers a risk of these neoplasms in addition to clonal hematopoiesis. Overall, our data uncover an inherited cancer-predisposition mechanism that is distinct from that of mutations affecting tumor-suppressor proteins and oncoproteins: an extended cellular lifespan that supports clonal evolution with aging.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (NIH) (R01CA225027 and R01HL119476, to Dr. Armanios; and NIH R35133747, to Dr. McCoy), the S&R Foundation (to Dr. Armanios), the Commonwealth Foundation (to Dr. Armanios), and a gift from Godrej Industries in the name of Mrs. P. Godrej (to Dr. Armanios). Ms. DeBoy received support from NIH grant T32 GM136577 and the Turock Family Scholars Fund; Dr. Schratz was supported by NIH grant K08HL163468, an American Society of Hematology Scholar Award, and a grant from the Dresner Foundation; and Ms. Yan received support from NIH grant F31 HG012495.
We thank all the study participants and their health care providers; Ms. Anna M. Nicosia and Ms. Pamela Brock for helpful discussions; Ms. Gabrielle Scolaro for help with experiments; Dr. Ludmila Danilova for help with establishing the TCR-seq nomogram; Ms. Amanda Blackford for support with statistical analyses; the Johns Hopkins University Genetic Resources Core Facility, Single Cell and Transcriptomics Core, and Molecular Diagnostics Lab; the Bloomberg School of Public Health Flow Cytometry and Immunology Core; and the TCR Immunogenetics Core at the Sidney Kimmel Comprehensive Cancer Center.
Footnotes
Disclosure forms as provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Emily A. DeBoy, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Medical Scientist Training Program, Johns Hopkins University School of Medicine, Baltimore; Telomere Center, Johns Hopkins University School of Medicine, Baltimore
Michael G. Tassia, Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore
Kristen E. Schratz, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Telomere Center, Johns Hopkins University School of Medicine, Baltimore; Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
Stephanie M. Yan, Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore
Zoe L. Cosner, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Telomere Center, Johns Hopkins University School of Medicine, Baltimore
Emily J. McNally, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Telomere Center, Johns Hopkins University School of Medicine, Baltimore
Dustin L. Gable, Child Neurology Residency Program, Boston Children’s Hospital, Boston
Zhimin Xiang, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Telomere Center, Johns Hopkins University School of Medicine, Baltimore
David B. Lombard, Department of Pathology and Laboratory Medicine, Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, Miami
Emmanuel S. Antonarakis, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore; Division of Hematology, Oncology, and Transplantation, University of Minnesota Masonic Cancer Center, Minneapolis
Christopher D. Gocke, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore; Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
Rajiv C. McCoy, Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore
Mary Armanios, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore; Department of Genetic Medicine, Johns Hopkins University School of Medicine, Baltimore; Telomere Center, Johns Hopkins University School of Medicine, Baltimore; Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
REFERENCES
- 1.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990;345:458–60. [DOI] [PubMed] [Google Scholar]
- 2.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 2012;13: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A 2005;102: 15960–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood 2011;117:5607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner CL, Hanumanthu VS, Talbot CC Jr, et al. Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest 2018;128:5222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell 2013;12: 319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001;292: 1171–5. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Podell ER, Zaug AJ, et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 2007;445: 506–10. [DOI] [PubMed] [Google Scholar]
- 9.Ye JZ-S, Hockemeyer D, Krutchinsky AN, et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev 2004;18:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelleher C, Kurth I, Lingner J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol Cell Biol 2005;25:808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsay AJ, Quesada V, Foronda M, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet 2013;45:526–30. [DOI] [PubMed] [Google Scholar]
- 12.Robles-Espinoza CD, Harland M, Ramsay AJ, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet 2014;46:478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J, Yang XR, Ballew B, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet 2014;46:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bainbridge MN, Armstrong GN, Gramatges MM, et al. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst 2014;107:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speedy HE, Kinnersley B, Chubb D, et al. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 2016;128:2319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takai H, Jenkinson E, Kabir S, et al. A POT1 mutation implicates defective telomere end fill-in and telomere truncations in Coats plus. Genes Dev 2016;30:812–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinzaru AM, Hom RA, Beal A, et al. Telomere replication stress induced by POT1 inactivation accelerates tumorigenesis. Cell Rep 2016;15:2170–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schratz KE, Gaysinskaya V, Cosner ZL, et al. Somatic reversion impacts myelodysplastic syndromes and acute myeloid leukemia evolution in the short telomere disorders. J Clin Invest 2021;131(18): e147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim W-T, Hennick K, Johnson J, et al. Cancer-associated POT1 mutations lead to telomere elongation without induction of a DNA damage response. EMBO J 2021; 40(12):e107346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelich J, Aramburu T, van der Vis JJ, et al. Telomere dysfunction implicates POT1 in patients with idiopathic pulmonary fibrosis. J Exp Med 2022;219(5):e20211681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alder JK, Hanumanthu VS, Strong MA, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci U S A 2018;115(10): E2358–E2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R: a language and environment for statistical computing. Vienna: R Project for Statistical Computing; (http://www.R-project.org/). [Google Scholar]
- 23.Wong K, Robles-Espinoza CD, Rodriguez D, et al. Association of the POT1 germline missense variant p.I78T with familial melanoma. JAMA Dermatol 2019;155:604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emerson RO, DeWitt WS, Vignali M, et al. Immunosequencing identifies signatures of cytomegalovirus exposure history and HLA-mediated effects on the T cell repertoire. Nat Genet 2017;49:659–65. [DOI] [PubMed] [Google Scholar]
- 25.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones AV, Chase A, Silver RT, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet 2009;41:446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olcaydu D, Harutyunyan A, Jäger R, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet 2009;41:450–4. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell E, Spencer Chapman M, Williams N, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 2022;606:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer. Nature 2020;578: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams N, Lee J, Mitchell E, et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 2022;602:162–8. [DOI] [PubMed] [Google Scholar]
- 31.Van Egeren D, Escabi J, Nguyen M, et al. Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative neoplasms. Cell Stem Cell 2021;28(3):514–523.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim TL, Lieberman DB, Davis AR, et al. Germline POT1 variants can predispose to myeloid and lymphoid neoplasms. Leukemia 2022;36:283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brabrand M, Frederiksen H. Risks of solid and lymphoid malignancies in patients with myeloproliferative neoplasms: clinical implications. Cancers (Basel) 2020;12:3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schratz KE, Haley L, Danoff SK, et al. Cancer spectrum and outcomes in the Mendelian short telomere syndromes. Blood 2020;135:1946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bick AG, Weinstock JS, Nandakumar SK, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 2020;586:763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNally EJ, Luncsford PJ, Armanios M. Long telomeres and cancer risk: the price of cellular immortality. J Clin Invest 2019;129:3474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kar SP, Quiros PM, Gu M, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet 2022;54:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armanios M. The role of telomeres in human disease. Annu Rev Genomics Hum Genet 2022;23:363–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafnar T, Gunnarsson B, Stefansson OA, et al. Variants associating with uterine leiomyoma highlight genetic background shared by various cancers and hormone-related traits. Nat Commun 2018;9:3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.