Abstract
Purpose of review:
We review the key principles of kidney paired donation (KPD) and discuss the status and unique considerations for KPD in developing countries.
Recent findings:
Despite the advantages of KPD programs, they remain rare among developing nations, and the programs that exist have many differences with those of in developed countries. There is a paucity of literature and lack of published data on KPD from most of the developing nations. Expanding KPD programs may require the adoption of features and innovations of successful KPD programs. Cooperation with national and international societies should be encouraged to ensure endorsement and sharing of best practices.
Summary:
KPD is in the initial stages or has not yet started in the majority of the emerging nations. But the logistics and strategies required to implement KPD in developing nations differ from other parts of the world. By learning from the KPD experience in developing countries and adapting to their unique needs, it should be possible to expand access to KPD to allow more transplants to happen for patients in need world-wide.
Keywords: solid organ transplantation, deceased donor, living donor
Introduction
According to the global observatory on donation and transplantation data, a total of 151,299 solid organ transplants were performed across the world in 2021.1 Only a small amount of these data came from emerging nations, as some transplant programs in developing countries are still evolving and lack robust data reporting. Deceased donation requires a strong infrastructure and logistics, hence in the early phase of building a transplant program living donation programs are often established in many resource restricted regions of the world.2 However, there are some inherent barriers to living donation such as biological incompatibility between donor-recipient pairs (DRPs). ABO incompatible (ABOi) and HLA incompatible (HLAi) transplantation are feasible under careful a priori protocols, but these approaches add clinical complexity and financial barriers exist due to the need for greater levels of immunosuppression, increased risk of complications (e.g., infection, rejection and graft loss), and associated costs.3–5 Additionally, the logistics involved in crossing these barriers can be challenging to developing programs. Kidney paired donation (KPD) is a strategy to overcome incompatibility barriers by exchanging donors to create compatible combinations. In this article, we review the key principles of KPD and the status and unique considerations for KPD in developing countries.
Overview of kidney paired donation
Kidney paired donation was first proposed by Rapaport 6 in 1986 and first performed in South Korea in 1991 7 (Figure 1). Subsequent KPD program growth was slow, with KPD appearing in Europe in 1999 and in the United States in 2000. Since then, much progress has been made overcoming policy, legal, logistical, clinical and psychosocial barriers to KPD. The first national registry of paired donors was developed in 2001 through the Alliance for Paired Donation in the United States, which provided a central registry of potential donors and facilitated nationwide sharing of living donor kidneys. KPD is designed to surmount a number of barriers, classically including biological incompatibility (ABOi and/or HLAi). KPD programs can also help overcome chronological incompatibility allowing for various types of asynchronous donation (i.e. donors may donate before a recipient’s transplantation); geographic incompatibility by helping donors donate remotely in geographically distant locations to the transplant center; in addition to improve HLA, size and age mismatch. Figure 1 summarizes the main landmarks in the evolution of KPD over three decades.
Figure 1:

History of kidney exchange
Two types of donors are possible, directed (donation to known recipient) and non-directed (donation to unknown recipient). The use of the term “altruistic donor” was previously used for a non-directed donor but is no longer favoured, as all donors demonstrate altruism in their willingness to donate. Figure 2 summarizes the structures of KPD used in practice, the logistics of which can range from straightforward to quite elaborate. In the simplest form of KPD, two designated donors can “swap” recipients to provide a better match (Figure 2A). Multiple donor-recipient pairs can be added into this swap to facilitate multiple better-paired exchanges (Figure 2B).
Figure 2.
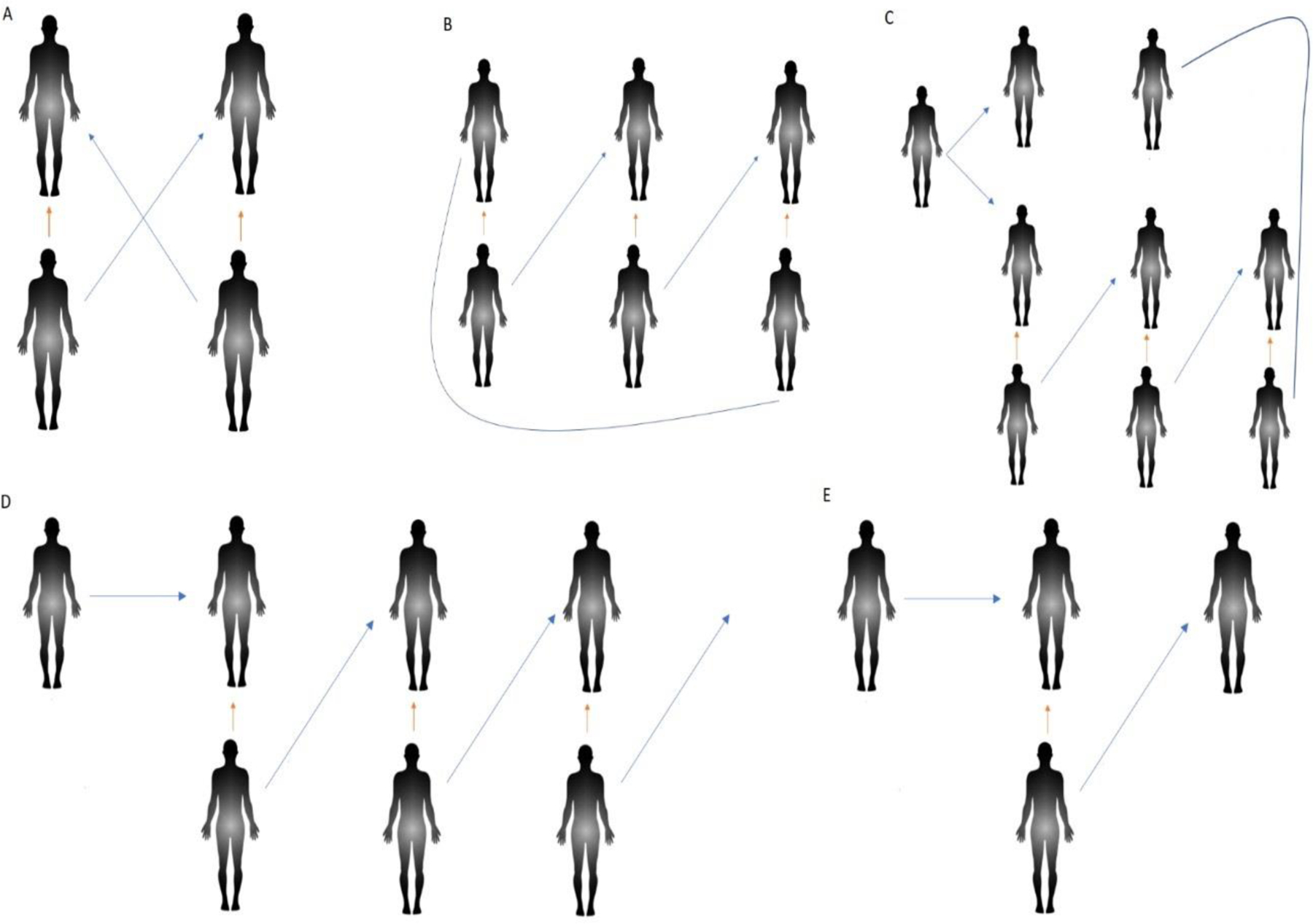
Overview of kidney exchange (KE): A = Two-way KE; B = Three-way KE; C = Deceased donor (DD) initiated KE where DD donates to first person on waitlist and to KE registry patient. This will create a chain of KE which will end at transplantation of second waitlist patient on DD from the donor of last donor-recipient pair in KE. D = Altruistic donor initiating an open-ended chain of KE which can extend to any numbers; E = Altruistic donor initiating a chain of KE which ends in transplantation of the recipient on waiting list for DD. Blue arrow represent KE and organ arrow represent the incompatibility of donor-recipient pair.
Non-directed donors can either donate directly to an unknown patient on a center’s waitlist or can start a chain in which multiple DRPs are involved, with the final donor donating to the waitlist (Figure 2D). KPD may also allow the incorporation of deceased donation into chains wherein a deceased donor kidney is transplanted to a patient listed on a KPD registry to start a chain of transplants (Figure 2C). Donor vouchers and advanced donation allow living donor candidate to donate a kidney temporally separated (by months to years) from the receipt of a kidney by their intended recipient; this is performed to overcome chronologic incompatibility. These strategies may allow donor candidates to donate at a time that is convenient for them, have time to recover and then serve as the recipient’s caretaker or provide a kidney while healthy, even if their recipient does not yet need renal replacement therapy. Specifically, voucher donors may choose a single recipient for a future transplant (directed donation), or name multiple recipients if one of them needs a kidney transplant in the future (semi-nondirected donation). The most developed of these programs is the Advanced Donation Program started by The National Kidney Registry (NKR).8,9 This innovative use of KPD can further be enhanced by the participation of blood-type O recipient and non-O donor pairs.
These three distinctive categories of KPD participation (non-directed, advanced, and voucher donors), 10 do not exist in India and they are not included in the The Transplantation of Human Organs Act in India and the developing world.
The status of KPD in developing nations
There is no published data of KPD from most of the developing nations. Table 1 shows transplant activity, including deceased donation, living donation, and kidney exchange among developing countries. The World Bank has categorized developing nations based on their income status into low-income countries (LICs) and low-middle income countries (LMICs). The four nations which report kidney exchanges are India, Iran, Nepal, and Pakistan, which all are categorized as LMIC and there have been no reports from LICs to date.
Table 1.
Developing nations with their transplantation activity and pattern
| Countries | Reporting year | Population for the year (million) | Total Actual DD | LD Kidney Tx | LD Liver Tx | KE program |
|---|---|---|---|---|---|---|
| Afghanistan | 2014 | 31.3 | - | |||
| Algeria | 2019 | 42.7 | 1 | 268 | 11 | - |
| Angola | 2010 | 19 | - | |||
| Bangladesh | 2019 | 168.1 | 0 | 205 | 0 | - |
| Belize | 2020 | 11.7 | 0 | 10 | 0 | - |
| Egypt, Arab Rep. | 2015 | 91.5 | 0 | 1417 | 450 | - |
| El Salvador | 2021 | 6.5 | 0 | 27 | 0 | - |
| Ethiopia | 2019 | 110.1 | 0 | 35 | 0 | - |
| Ghana | 2015 | 27.4 | 0 | 3 | 0 | - |
| India | 2019 | 1,368.70 | 715 | 8613 | 1991 | Yes |
| Indonesia | 2013 | 249.9 | 0 | 494 | 2 | |
| Iran, Islamic Rep | 2019 | 82.8 | 1078 | 747 | Yes | |
| Kenya | 2021 | 55 | 0 | 160 | 0 | - |
| Kyrgyz Republic | 2015 | 5.9 | 0 | 20 | 2 | - |
| Mongolia | 2019 | 3.2 | 9 | 18 | 31 | - |
| Morocco | 2019 | 36.6 | 4 | 36 | 1 | - |
| Myanmar | 2013 | 53.3 | 1 | 2 | 1 | - |
| Nepal | 2015 | 28.5 | 0 | 100 | 0 | Yes |
| Nicaragua | 2019 | 6.4 | 2 | 9 | 0 | - |
| Nigeria | 2019 | 201 | 0 | 165 | 0 | - |
| Pakistan | 2019 | 204.6 | 0 | 1306 | 285 | Yes |
| Philippines | 2019 | 108.1 | 283 | 0 | - | |
| Sri Lanka | 2020 | 21.4 | 98 | 210 | 0 | - |
| Sudan | 2019 | 42.5 | 0 | 313 | 0 | - |
| Syrian Arab Republic | 2019 | 18.5 | 0 | 275 | 1 | - |
| Tajikistan | 2015 | 8.5 | 0 | 25 | 0 | - |
| Tanzania | 2020 | 59.7 | 0 | 8 | 0 | - |
| Tunisia | 2015 | 11.3 | 9 | 10 | - | |
| Ukraine | 2015 | 44.8 | 3 | 120 | 10 | - |
| Vietnam | 2015 | 93.4 | 17 | 261 | 3 | - |
Countries with no transplantation activity reported: Benin, Bhutan, Bolivia, Burkina Faso, Burundi, Cabo Verde, Cambodia, Cameroon, Central African Republic, Chad, Comoros, Congo Dem. Rep, Congo Rep., Côte d’Ivoire, Djibouti, Eritrea, Eswatini, Gambia, Guinea, Guinea-Bissau, Haiti, Honduras, Kiribati, Korea, Dem. People’s Rep, Lao PDR, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Micronesia Fed. Sts., Mozambique, Niger, Papua New Guinea, Rwanda, Samoa, São Tomé and Principe, Senegal, Sierra Leone, Solomon Islands, Somalia, South Sudan, Timor-Leste, Togo, Uganda, Uzbekistan, Vanuatu, West Bank and Gaza, Yemen Rep., Zambia and Zimbabwe.
Abbreviations: DD: deceased donation; LD: living donation; Tx: transplant; KE: kidney exchange, Countries in italics represent low-income countries, and non-italics denotes low-middle income countries according to World bank classification. The data was retrieved from the official website of global observatory donation and transplantation. - represent no data reported to GODT in the last decade.
The Iranian model of kidney transplant established in 1988 is a unique example of a compensated and regulated living unrelated kidney donation. Through this model, a total of 436 paired kidney donors and recipients were benefited11. This model can increase transplantation, but careful attention must be paid to risks of coercion and protection of donor safety and well-being. In Pakistan, living donation practices are restricted to a few transplant centers, and there is only a single case report of KPD, where an exchange was performed to overcome the ABO incompatibility barrier.12 The majority of the KPD reports from developing nations belong to India, the seventh largest and the second most populous nation in the world. Interestingly, most reports from India are from a single center reporting 300 KPDs and performing non-simultaneous kidney exchanges without non-directed donation.13,14 This center recently performed their longest KPD chain of 10 DRPs and has become a champion for the development of KPD within India and the rest of the developing world.15,16 In 2015, the kidney exchange matching rate of Indian single center KPD programs was 62% which shows the percentage of pairs undergoing KPD within the registered pairs. This rate is significantly higher than the matching rates of national KPD programs in Australia, Canada, The Netherlands and the UK (49%, 44%, 37% and 27%, respectively).17
Global Kidney Exchange
In 2010, 5–7 million people died worldwide because they did not have access to renal replacement therapy.18 DRPs from LICs and LMICs participate in the Global Kidney Exchange (GKE) program to overcome financial incompatibility.19 Although the GKE has generated considerable support, it has also generated critics and rebuttals.20–32 International bodies such as the World Health Organization (WHO), The Transplantation Society (TTS), Council of Europe’s Committee on Organ Transplantation and the Declaration of Istanbul Custodian Group have expressed concerns over the absence of transparency in the selection of the compatible DRPs in the LICs and LMICs, stating GKE as transplant colonialism. GKE provide some donor supply, but the European Platform on Ethical, Legal and Psychosocial Aspects of Organ Transplantation has expressed concerns that GKE violates the non-payment principle, exploits donors in LICs and LMICs, and detracts from the aim of self-sufficiency and logistical simplicity (transcultural issues, travel visas). Some of the concerns surrounding GKE can be mitigated by allowing independent international committee review by organizations such as WHO and TTS. More research is required to establish the willingness of DRPs to participate in GKE, inclusion criteria for participating, management of escrow funds, and common global legislation. A few examples of Filipino and U.S. GKE pairs have been successful and remain well at 3 years follow up, making a strong case for this type of program expansion.33 Minerva et al. strongly advocates for the expansion of GKE based on the principle that all human lives have equal value, and opposes the concerns of organ trafficking, exploiting the poor, and involving coercion and commodification / exploitation of donors and suggest that GKE promotes global justice. So far 54 patients have been transplanted in GKE including 19 international patients and 35 Americans. 34
Challenges and opportunities for KPD in developing countries
Despite the advantages of KPD programs, they remain rare in the developing world, and the programs that exist have many differences with those of developed countries.
Program structure is one of these differences: multicenter, regional, and national KPD programs (Swiss, Australia, Canada, Dutch, UK, USA) are more common in the developed than the developing world, whereas single center programs are more common in Brazil, India, Pakistan, and Nepal.12,13,35–46 Bangladesh, Qatar, and many others have not started KPD programs.
System inefficiency often exists in developed countries due to bureaucracy and regulations: kidney exchanges frequently take weeks to months to obtain legal permission in India despite the fact that only closely-related family members (i.e. parents, spouse, siblings, children, and grandparents) are allowed to donate a kidney.47
Protecting the privacy of a donor, including maintaining anonymity when requested, is common practice among developed countries but uncommon in developing nations. Anonymous allocation during KPD is a standard practice in the Netherlands, Sweden, and other parts of Europe but, this is not the case in countries such as India, Korea, and Romania. 14,48,49 In areas where anonymity is not maintained, the intended donor/recipient pair must meet and share medical information once a potential exchange is identified, but before formal allocation of pairs occurs. The original donor/recipient pair may refuse the proposed exchange option for any reason and continue to be on the wait list. In India, non-anonymous KPD allocation is standard practice and has the goal of increasing trust and transparency between the transplant team and the administrative team.14,49 Countries differ in philosophical approaches to optimizing trust and transparency, and objective data on most effective practices would benefit the global community.
Donor age group matching is most commonly expected among all variables by DRP. Medical fitness of DRP should be established before allowing DRP to meet each other to avoid chain collapse. DRP meetings need to be arranged on virtual platforms given the diverse geographic regions. Transplant teams should solve any concerns surfacing after DRP’s meeting and allow DRP to reject offers and remain waitlisted for the next match run if desired.
Compared to developed nations, donor-recipient matching has been commonly performed manually in countries such as India. Recently, the Alliance for Paired Donation shared their computer software for KPD allocation for use in India. 14,49 With increasing automation in allocation, kidney exchange length should be gradually increased from two-way to three-ways and so on, depending on the logistical capacity of the program. Simultaneous transplant surgery should be encouraged over non-simultaneous surgery due to risk of donor renege, but this can limit the length of chains. Geographic differences of DRPs can make simultaneous surgery difficult. The geographic separation of recipient and matched donor may be addressed with transition from donor travel to the shipment of donor kidneys, as in Canada50 and the United States. 51,52 The logistical demands of donor versus travel must be reconciled regionally, based on resources and infrastructure.
Reimbursement for lost wages, travel costs, and lodging in the donation process are more frequently allowed in programs from developing nations. In a global perspective analysis, lost income was reimbursed in 17 countries, while travel, accommodation, meal and childcare costs were reimbursed in 19, 17, 14 and 12 countries, respectively.53 Ten countries had comprehensive programs where all major cost categories were reimbursed to some extent.53
The way forward
Expanding KPD in developing countries may benefit from adopting features of successful KPD programs (Table 2). Table 3 offers some potential solutions for developing nations. Increasing the engagement with national and international societies should be encouraged to ensure endorsement and sharing of best practices.
Table 2.
Key features of kidney exchange programs and approaches described in published literature37,63,70–73
| Country | Key features of kidney exchange programs |
|---|---|
| Australia | High transplant rate for highly sensitized, HLA-incompatible pairs due to accepting ABO-incompatible donor matching with ABO titer ≤1 : 6437 |
| Canada | Non-directed anonymous donors facilitate 62% of transplants38 |
| Korea | Favourable blood group distribution (non-O > O patients),less sensitized, more compatible pairs and non-directed anonymous donors |
| United Kingdom | Transplant rate on the rise due to use of altruistic donor chains, embedded 2 way in 3-way or 4 way exchange70 |
| UNOS Kidney Paired Donation, USA | Nondirected donation (NDD) of the kidneys increased significantly in the 20-year72 |
| National Kidney Registry USA | The largest US exchange network of 103 Member Centers spanning 35 states managed by a nonprofit organization and participate in voucher donation, remote donation, long chain and Donor Shield.73 NKR facilitates over 450 “ Kidney Paired Donation” or “Paired Exchange” transplants annually63 |
Table 3.
Proposed interventions for adapting KPD to developing nations
| 1. Limit length of KPD to 3-way in initial stages to limit complex logistics; after developing comfort with single-center KPD, shift gradually toward multi-center, regional, state, and national programs to expand the donor pool |
| 2. Include non-directed anonymous donors |
| 3. Include biologically compatible pairs |
| 4. Consider avoidance of anonymous donation in early stages to foster trust in the transplant system |
| 5. Employ computer allocation rather than manual allocation to increase match run frequency |
| 6. Start with simultaneous surgery and consider expanding to non-simultaneous surgeries as experience grows |
| 7. Implement robust protocols to protect recipients such as use of deceased donor allocation priority in the case of paired donors refusing to donate after their recipient has been transplanted |
| 8. Adapt strategies for organ shipping versus donor travelling to a transplant center based on regional feasibility |
| 9. Implement surveillance and monitoring from national and international regulatory bodies to prevent illegal organ trafficking during KPD |
| 10. once established, incorporate selected KPD innovations such as donor voucher programs and advanced and/or remote donation |
Ethical, legal, and policy considerations should respect donor autonomy during all phases of the evaluation and donation process and remain patient-centric. Nations desiring to implement a KPD program are expected to have a learning curve in managing complex logistics and would benefit from starting with simple single-center simultaneous two-way KPD and gradually expanding to more complex strategies such as advance donation. Graft survival, patient survival, and rejection rates with KPD have been shown to be similar to direct donation.8,13,27 Therefore, KPD should be encouraged and promoted in centers where cost is a major consideration since incompatible transplant is substantially more expensive before and after transplantation. Likewise, overcoming the incompatibility barrier of a DRP through KPD will be particular beneficial to countries with low rates of deceased donor transplantation.46
DRPs considering KPD, should be informed of expected time for matching, as waiting times are highly variable depending on donor-recipient blood type combination, recipient sensitization and donor pool size. Selected DRP blood type combinations may be more challenging to match than others (A/B patient and B/A donor, a blood type O donor). Recipients pursuing KPD should also enrolled in deceased donor kidney waiting lists when available. The cost and complications of maintenance dialysis increases with longer dialysis exposure. For easy to match incompatible DRPs, we prefer KPD due to the better long-term outcomes and costs-savings over desensitization therapy. Donor and recipient candidates should be evaluated according to uniform preoperative multidisciplinary team assessment as per local/national or KDIGO guidelines to minimize risk and prevent chain collapse.54,55 Non-standard and technically complex donors should be avoided in the early days of program development. Outcomes should be monitored and continuously reassessed as part of a program quality improvement.
Conclusion:
KPD is in initial stages or has not yet started in the majority of the developing nations. The logistics and innovations required to implement KPD vary across the world. By learning from the KPD experience in developing countries and adapting to their strategies to the unique needs in developing nations, it should be possible to expand access to KPD to allow more transplants happen for patients in need world-wide.
ACKNOWLEDGEMENTS
KLL is a Senior Scientist of the SRTR, receives research funding related to living donation from the National Institutes of Health (NIH R01DK120551), and is also supported by the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. KLL is chair of the AST LDCOP, member of the ASN Policy and Advocacy Committee, and a member of the National Kidney Foundation Transplant Advisory Committee. Unrelated to this work, KLL receives consulting fees from CareDx and speaker honoraria from Sanofi.
Footnotes
Conflict of Interest: None
Data availability statement: Not applicable
Informed consent: Not applicable
Ethics approval: Not required
Ethical statement: This article does not contain any studies with human and animal subjects performed by any of the authors. We abided with the rules and regulations declaration of Helsinki, and Istanbul. No institutional or any other ethical board approval was required as the data presented is retrospective, publicly available from the website. No informed consent was required as no individual patient level data was used.
References
- 1.Global Observatory on Donation and Transplantation. WHO-ONT Accessed 20 September, 2022. http://www.transplant-observatory.org/
- 2.Kute V, Ramesh V, Shroff S, Guleria S, Prakash J. Deceased-Donor Organ Transplantation in India: Current Status, Challenges, and Solutions. Exp Clin Transplant Jul 2020;18(Suppl 2):31–42. doi: 10.6002/ect.rlgnsymp2020.L6 [DOI] [PubMed] [Google Scholar]
- 3.Scurt FG, Ewert L, Mertens PR, Haller H, Schmidt BMW, Chatzikyrkou C. Clinical outcomes after ABO-incompatible renal transplantation: a systematic review and meta-analysis. Lancet May 18 2019;393(10185):2059–2072. doi: 10.1016/S0140-6736(18)32091-9 [DOI] [PubMed] [Google Scholar]
- 4.Held PJ, McCormick F. ABO-Incompatible Kidney Transplants: Twice as Expensive, Half as Good. Am J Transplant May 2016;16(5):1343–4. doi: 10.1111/ajt.13638 [DOI] [PubMed] [Google Scholar]
- 5.Pankhurst L, Hudson A, Mumford L, et al. The UK National Registry of ABO and HLA Antibody Incompatible Renal Transplantation: Pretransplant Factors Associated With Outcome in 879 Transplants. Transplant Direct Jul 2017;3(7):e181. doi: 10.1097/TXD.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapaport FT. The case for a living emotionally related international kidney donor exchange registry. Transplant Proc Jun 1986;18(3) Suppl. 2):5–9. [PubMed] [Google Scholar]
- 7.Kwak JY, Kwon OJ, Lee KS, Kang CM, Park HY, Kim JH. Exchange-donor program in renal transplantation: a single-center experience. Transplant Proc Feb-Mar 1999;31(1–2):344–5. doi: 10.1016/s0041-1345(98)01655-8 [DOI] [PubMed] [Google Scholar]
- 8.Cooper M, Leeser DB, Flechner SM, et al. Ensuring the need is met: A 50-year simulation study of the National Kidney Registry’s family voucher program. Am J Transplant Mar 2021;21(3):1128–1137. doi: 10.1111/ajt.16101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veale JL, Capron AM, Nassiri N, et al. Vouchers for Future Kidney Transplants to Overcome “Chronological Incompatibility” Between Living Donors and Recipients. Transplantation Sep 2017;101(9):2115–2119. doi: 10.1097/TP.0000000000001744 [DOI] [PubMed] [Google Scholar]
- 10.Matas AJ. Nondirected, Advanced, and Voucher-Based Donation-The Importance of Terminology. JAMA Surg Mar 1 2022;157(3):280. doi: 10.1001/jamasurg.2021.5767 [DOI] [PubMed] [Google Scholar]
- 11.Feizi M, Moeindarbari T. Characteristics of kidney donors and recipients in Iranian kidney market: Evidence from Mashhad. Clin Transplant Oct 2019;33(10):e13650. doi: 10.1111/ctr.13650 [DOI] [PubMed] [Google Scholar]
- 12.Nizam N, Mazhar F, Abbas K, et al. Kidney Swap in Pakistan: Experience at the Sindh Institute of Urology and Transplantation. Exp Clin Transplant Feb 2017;15(Suppl 1):76–78. doi: 10.6002/ect.mesot2016.O63 [DOI] [PubMed] [Google Scholar]
- 13.Kute VB, Patel HV, Shah PR, et al. Impact of single centre kidney paired donation transplantation to increase donor pool in India: a cohort study. Transpl Int Jul 2017;30(7):679–688. doi: 10.1111/tri.12956 [DOI] [PubMed] [Google Scholar]
- 14.Kute VB, Patel HV, Modi PR, et al. Non-simultaneous kidney exchange cycles in resource-restricted countries without non-directed donation - a prospective single-center cohort study. Transpl Int Apr 2021;34(4):669–680. doi: 10.1111/tri.13833 [DOI] [PubMed] [Google Scholar]
- 15.Kute VB, Patel HV, Modi PR, et al. Paired Kidney Exchange in India: Future Potential and Challenges Based on the Experience at a Single Center. Transplantation May 1 2021;105(5):929–932. doi: 10.1097/TP.0000000000003421 [DOI] [PubMed] [Google Scholar]
- 16.Ahmad I, Saxena S, Bansal R, et al. First Successful Three-Way Kidney Exchange Transplantation in North India. Indian J Nephrol Mar-Apr 2021;31(2):169–172. doi: 10.4103/ijn.IJN_116_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toews M, Giancaspro M, Richards B, Ferrari P. Kidney Paired Donation and the “Valuable Consideration” Problem: The Experiences of Australia, Canada, and the United States. Transplantation Sep 2017;101(9):1996–2002. doi: 10.1097/TP.0000000000001778 [DOI] [PubMed] [Google Scholar]
- 18.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet May 16 2015;385(9981):1975–82. doi: 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 19.Rees MA, Dunn TB, Kuhr CS, et al. Kidney Exchange to Overcome Financial Barriers to Kidney Transplantation. Am J Transplant Mar 2017;17(3):782–790. doi: 10.1111/ajt.14106 [DOI] [PubMed] [Google Scholar]
- 20.Minerva F, Savulescu J, Singer P. The ethics of the Global Kidney Exchange programme. Lancet Nov 9 2019;394(10210):1775–1778. doi: 10.1016/S0140-6736(19)32474-2 [DOI] [PubMed] [Google Scholar]
- 21.Singer P, Minerva F, Savulescu J. The Global Kidney Exchange programme - Authors’ reply. Lancet May 9 2020;395(10235):1485–1486. doi: 10.1016/S0140-6736(20)30619-X [DOI] [PubMed] [Google Scholar]
- 22.Ambagtsheer F, Haase-Kromwijk B, Dor F, et al. Global Kidney Exchange: opportunity or exploitation? An ELPAT/ESOT appraisal. Transpl Int Sep 2020;33(9):989–998. doi: 10.1111/tri.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rees MA, Paloyo SR, Roth AE, et al. Global kidney exchange: Financially incompatible pairs are not transplantable compatible pairs. Am J Transplant Oct 2017;17(10):2743–2744. doi: 10.1111/ajt.14451 [DOI] [PubMed] [Google Scholar]
- 24.Marino IRRA, Rees MA, Doria C. Open dialogue between professionals with different opinions builds the best policy. Am J Transplant 2017;17(10):2749. [DOI] [PubMed] [Google Scholar]
- 25.Roth AE, Krawiec KD, Paloyo S, et al. People should not be banned from transplantation only because of their country of origin. Am J Transplant Oct 2017;17(10):2747–2748. doi: 10.1111/ajt.14485 [DOI] [PubMed] [Google Scholar]
- 26.Roth AE, Marino IR, Ekwenna O, et al. Global kidney exchange should expand wisely. Transpl Int Sep 2020;33(9):985–988. doi: 10.1111/tri.13656 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Fraga M, Dominguez-Gil B. The Global Kidney Exchange programme. Lancet May 9 2020;395(10235):1484. doi: 10.1016/S0140-6736(20)30614-0 [DOI] [PubMed] [Google Scholar]
- 28.Valera LCM. The Global Kidney Exchange programme. Lancet 2020;395(10235):1484–1485. [DOI] [PubMed] [Google Scholar]
- 29.Danovitch GM. The Global Kidney Exchange programme. Lancet May 9 2020;395(10235):1485. doi: 10.1016/S0140-6736(20)30615-2 [DOI] [PubMed] [Google Scholar]
- 30.Roth AE, Wang SW. Popular repugnance contrasts with legal bans on controversial markets. Proc Natl Acad Sci U S A Aug 18 2020;117(33):19792–19798. doi: 10.1073/pnas.2005828117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baines LS, Jindal RM. Comment: Kidney Exchange to Overcome Financial Barriers to Kidney Transplantation. Am J Transplant Oct 2017;17(10):2742. doi: 10.1111/ajt.14325 [DOI] [PubMed] [Google Scholar]
- 32.Kute V, Jindal RM, Prasad N. Kidney Paired-Donation Program Versus Global Kidney Exchange in India. Am J Transplant Oct 2017;17(10):2740–2741. doi: 10.1111/ajt.14324 [DOI] [PubMed] [Google Scholar]
- 33.Bozek DN, Dunn TB, Kuhr CS, et al. Complete Chain of the First Global Kidney Exchange Transplant and 3-yr Follow-up. Eur Urol Focus Mar 2018;4(2):190–197. doi: 10.1016/j.euf.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 34.Rees M.
- 35.Stepkowski SM, Mierzejewska B, Fumo D, et al. The 6-year clinical outcomes for patients registered in a multiregional United States Kidney Paired Donation program - a retrospective study. Transpl Int Aug 2019;32(8):839–853. doi: 10.1111/tri.13423 [DOI] [PubMed] [Google Scholar]
- 36.Hadaya K, Fehr T, Rusi B, Ferrari-Lacraz S, Jean V, Ferrari P. Kidney paired donation: a plea for a Swiss National Programme. Swiss Med Wkly 2015;145:w14083. doi: 10.4414/smw.2015.14083 [DOI] [PubMed] [Google Scholar]
- 37.Cantwell L, Woodroffe C, Holdsworth R, Ferrari P. Four years of experience with the Australian kidney paired donation programme. Nephrology (Carlton) Mar 2015;20(3):124–31. doi: 10.1111/nep.12369 [DOI] [PubMed] [Google Scholar]
- 38.Cole EH, Nickerson P, Campbell P, et al. The Canadian kidney paired donation program: a national program to increase living donor transplantation. Transplantation May 2015;99(5):985–90. doi: 10.1097/TP.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 39.Malik S, Cole E. Foundations and principles of the Canadian living donor paired exchange program. Can J Kidney Health Dis 2014;1:6. doi: 10.1186/2054-3581-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Klerk M, Witvliet MD, Haase-Kromwijk BJ, Weimar W, Claas FH. A flexible national living donor kidney exchange program taking advantage of a central histocompatibility laboratory: the Dutch model. Clin Transpl 2008:69–73. [PubMed] [Google Scholar]
- 41.Johnson RJ, Allen JE, Fuggle SV, Bradley JA, Rudge C, Kidney Advisory Group UKTN. Early experience of paired living kidney donation in the United kingdom. Transplantation Dec 27 2008;86(12):1672–7. doi: 10.1097/TP.0b013e3181901a3d [DOI] [PubMed] [Google Scholar]
- 42.Osbun N, Thomas AG, Ronin M, et al. The benefit to waitlist patients in a national paired kidney exchange program: Exploring characteristics of chain end living donor transplants. Am J Transplant Jan 2022;22(1):113–121. doi: 10.1111/ajt.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mierzejewska B, Durlik M, Lisik W, et al. Current approaches in national kidney paired donation programs. Ann Transplant Mar 19 2013;18:112–24. doi: 10.12659/AOT.889096 [DOI] [PubMed] [Google Scholar]
- 44.Bastos J, Mankowski M, S EG, et al. Kidney paired donation in Brazil - a single center perspective. Transpl Int Aug 2021;34(8):1568–1570. doi: 10.1111/tri.13923 [DOI] [PubMed] [Google Scholar]
- 45.Kher V, Jha PK. Paired kidney exchange transplantation - pushing the boundaries. Transpl Int Sep 2020;33(9):975–984. doi: 10.1111/tri.13693 [DOI] [PubMed] [Google Scholar]
- 46.Chandra Shrestha P, Bhandari TR, Adhikari R, Baral H, Verma RK, Shrestha KK. Living donor kidney paired exchange: An observational study. Ann Med Surg (Lond) Jun 2022;78:103761. doi: 10.1016/j.amsu.2022.103761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahay M Transplantation of human organs and tissues Act-“Simplified”. Indian journal of transplantation 2018;12(2):84. [Google Scholar]
- 48.Lucan M Five years of single-center experience with paired kidney exchange transplantation. Transplant Proc Jun 2007;39(5):1371–5. doi: 10.1016/j.transproceed.2007.02.081 [DOI] [PubMed] [Google Scholar]
- 49.Huh KH, Kim MS, Ju MK, et al. Exchange living-donor kidney transplantation: merits and limitations. Transplantation Aug 15 2008;86(3):430–5. doi: 10.1097/TP.0b013e3181804a34 [DOI] [PubMed] [Google Scholar]
- 50.McGregor TB, Sener A, Yetzer K, Gillrie C, Paraskevas S. The impact of COVID-19 on the Canadian Kidney Paired Donation program: an opportunity for universal implementation of kidney shipping. Can J Surg Sep-Oct 2020;63(5):E451–E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treat E, Chow EKH, Peipert JD, et al. Shipping living donor kidneys and transplant recipient outcomes. Am J Transplant Mar 2018;18(3):632–641. doi: 10.1111/ajt.14597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Treat EG, Miller ET, Kwan L, et al. Outcomes of shipped live donor kidney transplants compared with traditional living donor kidney transplants. Transpl Int Nov 2014;27(11):1175–82. doi: 10.1111/tri.12405 [DOI] [PubMed] [Google Scholar]
- 53.Sickand M, Cuerden MS, Klarenbach SW, et al. Reimbursing live organ donors for incurred non-medical expenses: a global perspective on policies and programs. Am J Transplant Dec 2009;9(12):2825–36. doi: 10.1111/j.1600-6143.2009.02829.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation Aug 2017;101(8S Suppl 1):S1–S109. doi: 10.1097/TP.0000000000001769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lentine KL, Lam NN, Segev DL. Risks of Living Kidney Donation: Current State of Knowledge on Outcomes Important to Donors. Clin J Am Soc Nephrol Apr 5 2019;14(4):597–608. doi: 10.2215/CJN.11220918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallis CB, Samy KP, Roth AE, Rees MA. Kidney paired donation. Nephrol Dial Transplant Jul 2011;26(7):2091–9. doi: 10.1093/ndt/gfr155 [DOI] [PubMed] [Google Scholar]
- 57.Ellison B A Systematic Review of Kidney Paired Donation: Applying Lessons From Historic and Contemporary Case Studies to Improve the US Model Retrieved 23 September, 2016, from https://repository.upenn.edu/wharton_research_scholars/107/?utm_source=repository.upenn.edu%2Fwharton_research_scholars%2F107&utm_medium=PDF&utm_campaign=PDFCoverPages
- 58.The JHU Gazette. Hopkins Surgical Teams Perform Historic Quintuple Kidney Swap Retrieved 11 June, 2023, from https://pages.jh.edu/gazette/2006/27nov06/27swap.html.
- 59.de Klerk M, Keizer KM, Claas FH, Witvliet M, Haase-Kromwijk BJ, Weimar W. The Dutch national living donor kidney exchange program. Am J Transplant Sep 2005;5(9):2302–5. doi: 10.1111/j.1600-6143.2005.01024.x [DOI] [PubMed] [Google Scholar]
- 60.Malik S, Cole E et al. State of the Art Practices and Policies in Kidney Paired Donation. Curr Transpl Rep 1, 10–17 (2014). 10.1007/s40472-013-0002-5. [DOI] [Google Scholar]
- 61.Rees MA, Kopke JE, Pelletier RP, et al. A nonsimultaneous, extended, altruistic-donor chain. N Engl J Med Mar 12 2009;360(11):1096–101. doi: 10.1056/NEJMoa0803645 [DOI] [PubMed] [Google Scholar]
- 62.Congress.gov. H.R.710 – 110th Congress (2007–2008): Charlie W. Norwood Living Organ Donation Act (2007, December 21). Retrieved 11 June, 2023 from https://www.congress.gov/bill/110th-congress/house-bill/710.
- 63.Flechner SM, Thomas AG, Ronin M, et al. The first 9 years of kidney paired donation through the National Kidney Registry: Characteristics of donors and recipients compared with National Live Donor Transplant Registries. Am J Transplant Nov 2018;18(11):2730–2738. doi: 10.1111/ajt.14744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fortin MC, Williams-Jones B. Who should travel in kidney exchange programs: the donor, or the organ? Open Med 2011;5(1):e23–5. [PMC free article] [PubMed] [Google Scholar]
- 65.Johns Hopkins Medicine. Johns Hopkins Leads First 16-Patient, Multicenter “Domino Donor” Kidney Transplant Retrieved 11 June, 2023, from https://www.hopkinsmedicine.org/news/media/releases/johns_hopkins_leads_first_16_patient_multicenter_domino_donor_kidney_transplant.
- 66.New York Times. 60 Lives, 30 Kidneys, All Linked. New York Times Retrieved 11 June, 2023 from https://www.nytimes.com/2012/02/19/health/lives-forever-linked-through-kidney-transplant-chain-124.html.
- 67.ABC News. Donating a Kidney to a Complete Stranger in Order to Save a Loved One Retrieved 11 June, 2023 from https://abcnews.go.com/Health/donating-kidney-complete-stranger-order-save-loved/story?id=30288400.
- 68.Biro P, Haase-Kromwijk B, Andersson T, et al. Building Kidney Exchange Programmes in Europe-An Overview of Exchange Practice and Activities. Transplantation Jul 2019;103(7):1514–1522. doi: 10.1097/TP.0000000000002432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.European Cooperation in Science and Technology. European Network for Collaboration on Kidney Exchange Programmes (ENCKEP) Retreived 11 June, 2023. from https://www.enckep-cost.eu/.
- 70.Johnson RJ, Allen JE, Fuggle SV, Bradley JA, Rudge C. Early experience of paired living kidney donation in the United kingdom. Transplantation Dec 27 2008;86(12):1672–7. doi: 10.1097/TP.0b013e3181901a3d [DOI] [PubMed] [Google Scholar]
- 71.Bingaman AW, Wright FH Jr., Kapturczak M, Shen L, Vick S, Murphey CL. Single-center kidney paired donation: the Methodist San Antonio experience. Am J Transplant Aug 2012;12(8):2125–32. doi: 10.1111/j.1600-6143.2012.04070.x [DOI] [PubMed] [Google Scholar]
- 72.Jan MY, Yaqub MS, Adebiyi OO, et al. Nondirected Living Kidney Donation and Recipient Outcomes in the United States: A 20-Year Review. Kidney Int Rep Jun 2022;7(6):1289–1305. doi: 10.1016/j.ekir.2022.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leeser DB, Thomas AG, Shaffer AA, et al. Patient and Kidney Allograft Survival with National Kidney Paired Donation. Clin J Am Soc Nephrol Feb 7 2020;15(2):228–237. doi: 10.2215/cjn.06660619 [DOI] [PMC free article] [PubMed] [Google Scholar]


