Abstract
Magnetic Drug Targeting (MDT) is of particular interest to researchers because of its good loading efficiency, targeting accuracy, and versatile use in vivo. Cardiovascular Disease (CVD) is a global chronic disease with a high mortality rate, and the development of more precise and effective treatments is imminent. A growing number of studies have begun to explore the feasibility of MDT in CVD, but an up-to-date systematic summary is still lacking. This review discusses the current research status of MDT from guiding magnetic fields, magnetic nanocarriers, delivery channels, drug release control, and safety assessment. The current application status of MDT in CVD is also critically introduced. On this basis, new insights into the existing problems and future optimization directions of MDT are further highlighted.
Keywords: Magnetic field targeting, magnetic nanocarriers, drug release control, cardiovascular disease
GRAPHICAL ABSTRACT (BY FIGDRAW.)
Magnetic Drug Targeting (MDT) is a drug-targeted delivery method that has recently received much attention from researchers. The idea of guiding the accumulation of drugs at specific sites in the body through magnetic targeting was first proposed by Freeman in 1960 [1]. Compared with traditional drug delivery methods, MDT can achieve more drug aggregation at the target site, reduce free drug in circulation [2], achieve more precise and efficient drug delivery, and reduce the required drug dose as well as possible toxic side effects [3]. On the other hand, MDT also enables the targeted delivery of therapeutic "cargo" such as cells [4], genes [5], and exosomes [6] in the vasculature. For decades, magnetic targeting research has focused on oncology for imaging [7], thermal therapy [8], and drug delivery [9]. In the following, we present the current status of MDT research in five aspects: guiding magnetic fields, magnetic nanocarriers, delivery channels, drug release control, and safety assessment, and provide new insights into the existing problems and future directions of MDT optimization.
Part I. Main ideas of MDT and the current status of research
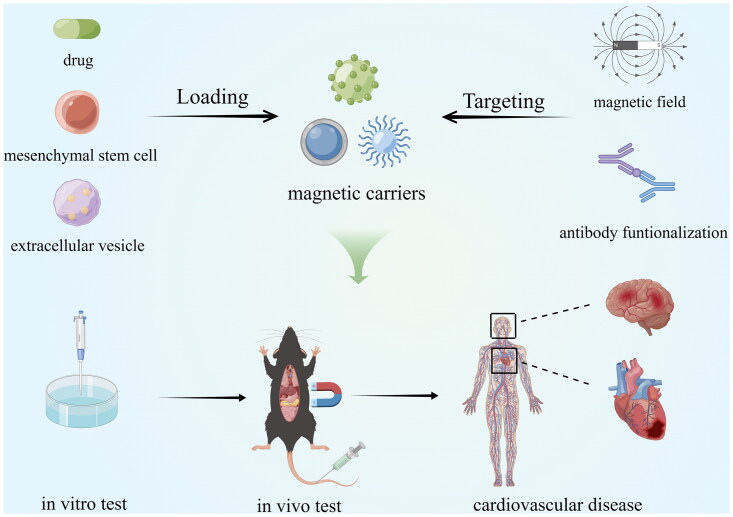
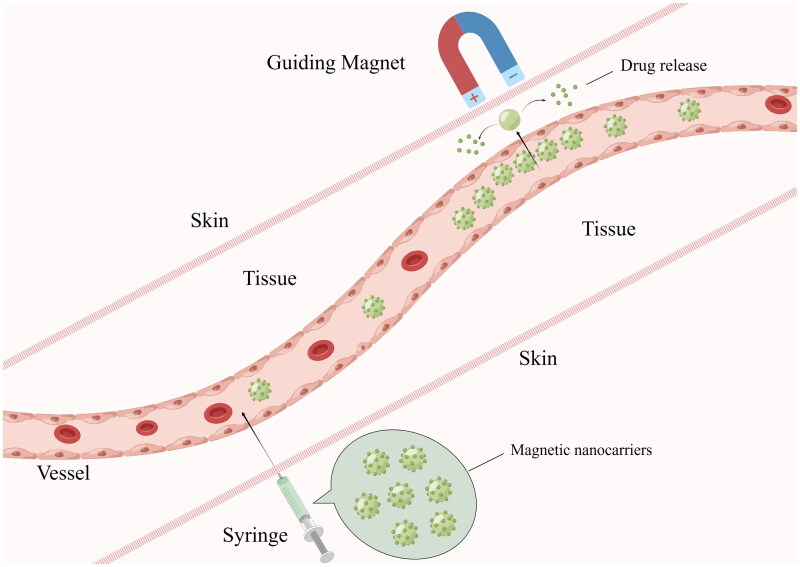
The main idea of MDT is to inject magnetic nanoparticles (MNPs) or other magnetic carriers bound to the drug/cargo into the body circulation, set up the magnetic field source at a specific location, use the magnetic force to make the magnetic carriers overcome the blood flow resistance, aggregate at the target site, and leak from the blood vessel into the tissue to complete the drug/cargo release at the specific site (Figure 1). Therefore, MDT focuses on guiding magnetic fields, magnetic nanocarriers, delivery channels, drug release control, and safety assessment.
Figure 1.
Design concept of MDT (by Figdraw.).
Guiding magnetic field
The magnetic field is the main driving force to guide the directional motion of magnetic carriers. Magnet systems for drug targeting are mainly divided into static magnetic field magnet systems, which are simple and easy to use for clinical purposes but have a single magnetic field force, and variable magnetic field magnet systems, which are complex to build but have a controlled magnetic field force and higher accuracy [10].
Assuming that the magnetic field is large enough to magnetize MNPs completely, the magnetic force on MNPs in the field can be expressed as follows [11]:
| Formula 1 |
In the above formulation, Msat is the magnetization strength, V is the volume of the material, and refers to the magnetic field gradient. The magnetic force is positively correlated with the magnetic field strength as well as the magnetic field gradient. The larger the magnetic field strength and gradient, the better the targeting effect of the same magnetic nanocarrier. The Halbach array is the best magnet arrangement that maximizes the magnetic field strength [12, 13]. The tip-top design can also increase the magnetic field strength and gradient [14]. Magnetic nanocarriers tend to be attracted toward the guiding magnet, which is often set on the skin surface, so how to target deep tissue has always been a research challenge.
Researchers have come up with new solutions that rely on the combination of two magnets to "push" particles into the deeper tissue [15, 16], and the magnetic field formed by two magnets seems to be more uniform than that of a single magnet [17]. Alternatively, the surgical construction of a magnet device at the site of the lesion is a solution, for example, in combination with endoscopic techniques [18]. MRI is a variable magnetic field magnet system based on superconducting coils that allow precise control of magnetic field gradients. Since it also has an imaging function, it has unique advantages in targeting and monitoring magnetic carriers, which helps implement precise targeting [19–21]. However, the weak magnetic fields and small magnetic field gradients generated by current commercial MRI instruments are not satisfactory for driving magnetic nanocarriers at the nanoscale [22]. Possible solutions include using specialized gradient inserts to obtain larger magnetic field gradients and better particle drive capabilities [23, 24]. It is important to note that the magnetic field strength is not the greater, the better. Shen et al. found that the 0.3 T group was the most effective in treating ischemic myocardium when comparing the effects of magnetically targeted mesenchymal stem cell transplantation in the 0.15 T, 0.3 T, and 0.6 T groups. In contrast, the 0.6 T group showed higher cell retention but may have had micro embolism, resulting in a less effective overall treatment than the 0.3 T group [25].
Currently, the magnets used in the clinical application of magnetic targeting technology are mainly permanent magnets [10]. The remaining various magnet systems have not been validated in clinical studies, and efforts should be made to promote more clinical studies of different types of magnet systems in the future [10]. In addition, optimizing the magnetic field source should consider the actual disease situation, taking into account safety, efficiency, convenience, and energy saving as much as possible.
Magnetic nanocarrier
Magnetic nanocarriers are nanoscale materials with magnetic properties that can be freely loaded/unloaded with other micro and nano substances. Commonly used magnetic nanocarriers are magnetic nanoparticles, magnetic nanowires, magnetic nanorings, magnetic nanotubes, and other shaped magnetic nanodevices, which can be used for loading drugs, magnetic targeting, magnetic thermotherapy, and magnetic resonance imaging [26–28]. The typical magnetic nanocarriers are composite nanoparticles formed by Fe3O4, γ-Fe2O3, or other substances with special treatment, which have properties such as superparamagnetism, large surface area to volume ratio, good biocompatibility and biodegradability, as well as high drug loading efficiency and strong dispersion ability [22]. Drugs/cargoes are usually physically or chemically loaded onto the particles. The widely used methods for synthesizing MNPs are co-precipitation and thermal decomposition, of which the former is suitable for large-scale synthesis, and the latter can control the distribution of nanocrystal sizes [26]. As Formula 1, the force on a magnetic particle in a magnetic field is positively related to its volume. Since the blood flow drag force is proportional to the diameter, and thus the magnetic force increases faster than the drag force as the particle size increases [27], researchers want to increase the particle size as much as possible to achieve the ideal magnetic navigation. However, large particles cause physical irritation and small vessel embolism and have a low circulating half-life, so the ideal size of magnetic carriers for application in the vasculature should usually not exceed 200 nm [22].
Suitable hydrophilic coatings allow MNPs to maintain a stable dispersion in aqueous solutions. Polyethylene glycol (PEG), which has the lowest immunogenicity and high biocompatibility, is currently the coating of choice [29]. It is worth mentioning that whether magnetic particles were delivered to the target tissue also depends on the interaction between the surface of the particles and the tissue, and the antibody functionalization strategy, which gives the particles dual targeting ability of antibody and magnetic field, is a promising direction to enhance the targeting effect of MNPs [30]. In addition, MNPs can gain some other advantages when combined with other carriers. For example, combining MNPs with liposomes to form magnetic liposomes can reduce drug toxicity [31, 32], and with microbubbles to form magnetic microbubbles can obtain ultrasound sensitivity [33].
Magnetic robots with good reconfigurability and programmability, including (quasi-)spherical robots, helical robots, flexible robots, wirelike robots, and biohybrid robots, are a class of magnetic carriers at the nanometer to micron level that have attracted much attention in recent years and have unique advantages over conventional MNPs [34]. Drive sources for magnetic robots include magnetic fields [35], light [36], and sound [37]. Different types of magnetic robots have different structures. The most suitable for complex human environments are soft robots, whose components include propulsion structures and feedback control systems [38]. In addition, some materials are stimulus-responsive, which can modulate the magnetic robot’s morphological motion and the targeted release of loads in response to specific stimuli, such as temperature [39]. Stimulus-responsive systems are discussed further in the "Drug release control" section below.
Magnetic robotic designs often mimic the unique functions of living organisms, such as the magnetic responsiveness of magnetotactic bacteria, the walking function of spiders, the balance of torpedo tubes, and the light-sensitive navigation ability of beetles, and have broad research potential [34]. Algae are also attractive to researchers due to their smaller size and good biodegradability. Gong et al. developed a Chlorella-based microrobot with low cytotoxicity, high drug delivery efficiency, and pH responsiveness [40]. Li et al. used Thalassiosira Weissflogii as a template to develop a micro-robot with high specific surface area, strong stability, and pH responsiveness to replace artificial mesoporous silica carriers with complicated preparation processes. By loading doxorubicin (DOX) and accomplishing magnetic targeting drug release, cancer cell viability was reduced by 11.16% [41]. Compared to hard robots, soft robots have greater deformability and higher spatial flexibility [42] but are less susceptible to cellular uptake [43]. Zhu et al. considered these issues and designed an ameboid nanorobot using polyphosphoester and PEG, and modified the tumor acidity-sensitive transactivator of transcription peptide on the surface of the robot to enhance its uptake by tumor cells uptake by tumor cells. The MNPs and DOX were encapsulated in the nucleus. Ultimately, the robot successfully targeted 92.3% of triple-negative breast cancer cells and achieved 96.1% tumor growth inhibition after drug release [44]. In addition, the "camouflage" of magnetic carriers with red blood cell membranes can extend their half-life and improve biocompatibility and biodegradability [45]. This micromotor has good cycling stability, magnetic targeting, and imaging capabilities.
The magnetic microwheel is a magnetic carrier for thrombolysis, which can greatly improve the efficiency of thrombus lysis and the speed of blood flow restoration [46, 47]. Disharoon et al. prepared magnetic mesoporous silica particles from iron oxide nanoparticles, immobilized them on the surface of colloidal particles to form studded beads, and modified the studded beads with tissue-type fibrinogen activator and fibrinogen. Under a rotating magnetic field, the studded beads also rotated, resulting in a microwheel structure. These microwheels can roll at high speed in the vasculature and target the thrombus site, lysing the thrombus beyond the biochemical speed limit [46]. In addition, this wheel-like structure is also used in drug-carrying robots to improve propulsion performance [40].
Delivery channel (vasculature)
For MDT, the movement of magnetic carriers in the delivery channel (vasculature) is crucial. By using theoretical modeling to analyze the intravascular motion of magnetic carriers, the parameters of the magnetic field and magnetic carriers can be accurately predicted and set according to the actual situation, thus saving resources for animal and clinical experiments. Three dimensionless values, Magnetic-Richardson number, Mass Pe’clet number, and the Renkin reduced diffusion coefficient, are introduced by Nacev et al. to improve the computational efficiency, and then the classical normalized equation is established [48]. By calculating the particle concentration profile in the cross-section, the model predicts the particle motion behavior in the blood vessel well, and the results match previously published results from in vitro and in vivo experiments and explain behaviors that were not once understood. However, the model assumes that blood is a Newtonian fluid while blood is a very complex non-Newtonian fluid. Moreover, the model is two-dimensional, ignoring the possible vortices and turbulence in the three-dimensional blood vessels.
The improved model at this stage uses the Carreau non-Newtonian fluid viscosity model, based on the analysis of specific shapes of 3D vessels to adapt to specific drug delivery contexts. Hewlin et al. developed the Willis arterial ring model to analyze the kinetic behavior of particles using the Euler-Lagrange technique [49]. Superparamagnetic particles also have good capture efficiency in weak fields with intensities less than 4 T, where 100 nm particles are significantly better than 10 nm particles. However, the viscosity predicted by the Carreau model is higher than the actual plasma viscosity, and the capture efficiency may be underestimated [49]. In addition, significant vortex phenomena were formed in the curved and bifurcated regions of the vessels, and nanoparticles in these regions were difficult to trap. Sodagar et al. developed a 90° curved vessel model to investigate the particle trapping efficiency in the presence of different degrees of atherosclerosis [50]. They used a turbulent flow model of blood as well as a discrete-phase model to study for the first time the effect of vascular transitive motion brought about by cardiac systole and diastole on magnetic particle trapping. The results showed that atherosclerosis and transitive motion might lead to blood flow perturbations that increase the probability of collision of magnetic particles with the vessel wall, thus increasing the capture rate of 1–5 μm magnetic particles. In addition, Manshadi et al. developed the first four-layer structural model of arterial tissue, making it possible to probe the interaction of particles with the arterial wall [51]. The results suggest that increasing the particle size and magnetic field strength helps to obtain more tissue retention. In conclusion, these model calculations further demonstrate the feasibility of applying MDT in CVD, and the delivery effects in specific contexts need to be further validated by in vivo experiments in conjunction with the theoretical modeling scheme.
Drug release control
Drug release is the last part of MDT. Designing a reasonable drug release method can help improve the accuracy of targeted drug release and reduce drug damage to healthy tissues. Controlling the rate of drug release can prolong the duration of drug action, thus reducing the drug dose as well as the associated side effects. Nanocarriers can achieve precise drug release by responding to external stimuli such as temperature, ultrasound, light, electric and magnetic fields, and internal stimuli such as pH, redox potential, and enzymes [52]. Magnetic nanocarriers are essentially nanocarriers, and their drug-release control modes are also focused on these aspects.
The principle of temperature-sensitive drug release systems is the rapid property change of thermosensitive materials in response to changes in temperature. The current applications of thermosensitive systems are mainly focused on magnetic liposomes. Due to hysteresis loss and Néel relaxation, MNPs generate heat when placed in an alternating magnetic field, which raises the temperature of the lipid membrane and increases its permeability [53]. Nitica et al. developed a simple and rapid method for the synthesis of thermosensitive magnetic liposomes, including two core steps of synthesizing the lipid gel and driving the drug incorporation [54]. The 300 nm magnetoliposomes prepared by this method had good physical properties, and DOX release resulted in the death of more than 90% of A549 cancer cells when placed in an alternating magnetic field of 20–30 kA/m, 355 kHz for 30 min.
Ultrasound can enable remote drug release through mechanical and thermal effects. Magnetic microbubbles have the unique advantage of being ultrasound-sensitive and magnetic field-sensitive simultaneously [33, 55]. Chertok et al. functionalized MNPs and microbubbles with heparin and protamine, respectively, and then complexed heparin and protamine to prepare an ideal magnetic microbubble that stabilizes circulation and reduces lung clearance in the tumor vasculature and remotely collapses at specific sites, thereby accomplishing drug release [33]. Beguin et al. constructed the magnetic-acoustic device, which is also essentially a magnetic microbubble. This delivery method obtained higher tumor retention and better therapeutic efficacy of the drug [55]. In addition, porous structures can also increase sensitivity to ultrasound. Park et al. developed a magnetically driven porous degradable microrobot loaded with 5-fluorouracil with good ultrasound responsiveness as well as cancer cell inhibition [56].
The photosensitive release system is a convenient, noninvasive stimulus-responsive drug release system. Near-infrared light is the most popular source replacing ultraviolet light for its excellent tissue penetration capability and higher safety. The main principles of photosensitive drug release are the photoisomerization of hydrogels and photochemical and photothermal reactions [57]. Song et al. prepared DOX-loaded three-bead azo micro-robots with good drug release, photothermal effect, and in vitro cancer cell-killing ability under NIR light irradiation [58]. Copper sulfide nanoparticles are capable of both photothermal and photodynamic therapy when excited by near-infrared light. Wang et al. constructed tumor-targeted photosensitive micromotors with biological and optical therapeutic efficacy by chemically loading the photosensitizer chlorin e6 and the magnetized photosensitizing bacterium AMB-1 [59]. Hollow mesoporous copper sulfide nanoparticles loaded with DOX and wrapped with superparamagnetic iron oxide nanoparticles (SPIONs) were prepared by Feng et al. [60]. This drug delivery vehicle achieves the synergy of controlled drug release and photochemotherapy and has demonstrated sound anti-tumor effects in both in vivo and in vitro experiments.
Electric field-based drug release control systems are mainly realized by electro-responsive materials such as carbon nanotubes and conductive polymer hydrogels [61, 62]. The presence of an electric current alters the magnetic field, and thus there are no examples of the use of electrical stimulation alone in magnetic carriers to release drugs. The magnetic field itself can also be used to "extrude" the drug from the carrier by mechanical force [52]. In addition, controlled drug release from magnetic carriers through alternating magnetic fields combined with thermal effects, as described above, is the main application of electromagnetic binding at present [54].
PH-regulated drug release is mainly achieved by designing polymers containing ionizable groups or acid-sensitive bonds to encapsulate the drug or by designing pH-sensitive shells to encapsulate specific ligands. This modulation type is suitable for targeting sites with abnormal pH, especially for acidic regions such as inflammation and tumors. With pH sensitivity, adhesion, biocompatibility, and degradability advantages, chitosan is widely used to prepare pH-sensitive magnetic nanocarriers [63]. Liu et al. prepared magnetic nanocarriers loaded with doxorubicin hydrochloride using carboxymethyl chitosan and aminated lignin sulfonate, which showed good responsiveness to the acidic environment and inhibitory effect on cancer cells [64].
Similar to pH-responsive magnetic carriers that require a specific pH at the target site, redox-responsive magnetic carriers require a specific redox potential change at the target site. For example, glutathione is a reducing agent that is found in higher levels within tumor cells than outside the cells in some malignant tumors. Therefore, polymeric structures containing disulfide bonds can be designed to allow magnetic carriers to respond to the reducing environment and break the disulfide bonds when targeted to certain tumor tissues, thereby releasing the drug. Based on this principle, Wang et al. designed redox-responsive polymeric magnetosomes loaded with DOX that can be monitored by MRI, and these magnetosomes have sound photothermal effects under near-infrared light irradiation, which can combine chemotherapy with tumor ablation therapy [65]. Notably, thione bond-containing nanoparticles are reactive oxygen responsive and are an ideal carrier for drug release at targets with inflammation [66–68].
Besides, increased levels of specific enzymes in the pathological environment can be used for the localized release of the drug. Xiong et al. designed a lipase-responsive nanocarrier to accomplish vancomycin release in the presence of Staphylococcus aureus only [69]. Notably, this approach requires an explicit enzyme expression profile characteristic of the target site to avoid drug release at other locations.
In summary, for the application of MDT in CVD, the most feasible and universal drug release system is the temperature-sensitive system. The thermal effect of MNPs can be stimulated by alternating magnetic fields, near-infrared light, and ultrasound, which in turn accomplishes drug release. Ultrasound-responsive magnetic microbubbles are another viable drug release system that requires constant attention for the stability of the drug-loaded magnetic microbubbles for transport in the vasculature. For pH, redox, and enzyme concentration responsive systems, which are susceptible to environmental conditions, can be used as adjunctive release means in specific diseases. In addition, it was possible to figure out the optimal drug release rate by setting the parameter gradient of the controlled release source. The advantages and disadvantages of these magnetic carrier drug release modalities are summarized in Table 1.
Table 1.
Drug release stimulation modalities and their characteristics.
| Modalities | Advantages | Disadvantages | Relevant references |
|---|---|---|---|
| Temperature sensitive system | State-of-the-art stimulus-response system for rapid, quantitative drug release | High requirements for the safety and sensitivity of materials | [53], [54] |
| Ultrasound System | Noninvasive, penetrating | High stability requirements for magnetic microbubbles | [33], [55], [56] |
| Photosensitive system | Noninvasive and convenient | High penetration requirements; safety to be specified | [57], [58], [59], [60] |
| Electrical and magnetic field systems | Sensitive and controllable; can realize the potential of magnetic carriers | Difficult to apply alone; high technical requirements of the device | [61], [62]; [52], [54] |
| PH, redox, and enzyme response systems | Responsive to subtle environmental changes | Can only target specific sites; challenging to apply in complex environments | [63], [64]; [65], [66], [67], [68]; [69] |
Part II. Safety assessment of MDT
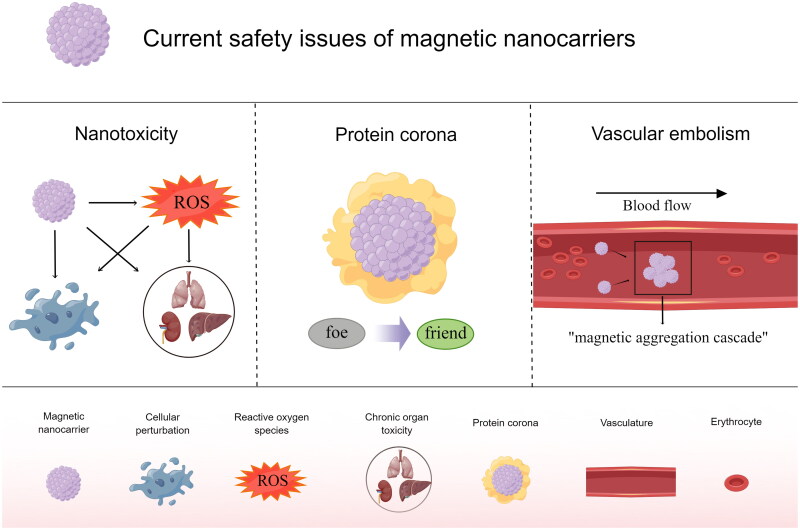
Safety assessment is a vital aspect of all therapeutic tools before their introduction into clinical applications, and the in vivo safety studies of magnetic carriers are crucial as they are in close contact with blood and tissues. The current safety issues of magnetic nanocarriers are mainly in nanotoxicity, protein corona, and vascular embolism (Figure 2).
Figure 2.
Current safety issues of magnetic nanocarriers (by Figdraw.).
An exhaustive summary of the toxicity profile of SPIONs was presented by Vakili-Ghartavol et al. [70]. Perhaps since living organisms have some ability to self-regulate, the in vivo experiments do not match the results of the cellular experiments [71]. In vivo tests in mice appear to show only mild toxicity, and this does not necessarily occur in humans, where the single injection dose is small [72]. The toxicity of MNPs is mainly manifested in terms of oxidative stress, cellular perturbation, and chronic organ toxicity, depending on factors such as size, surface modification, tissue concentration, and target cell type [70]. The formation of reactive oxygen species (ROS) is the most critical factor in the toxicity caused by MNPs. Metals can generate ROS through corrosion, catalysis, and photoexcitation, leading to oxidative stress events such as apoptosis, protein and lipid peroxidation, and DNA damage [73]. This case can be solved by treating the carrier surface to reduce ROS production and equipping it with ROS scavenging groups [74].
40- and 50-nm particles significantly affect cell signaling function [75]. Han et al. used proteomics for the first time to analyze cellular signaling potentially perturbed by toxicity and identified 197 up-regulated proteins and 75 down-regulated proteins, as well as the AKT/mTOR/TFEB pathway in splenic macrophages as the major activated signaling pathway [76]. Chrishtop et al. indicated that the nervous system, heart, lung, thyroid, mononuclear phagocytic system, and reproductive system are the main sites of chronic toxicity from MNPs [77], and they concluded that there is a need to further study the effects of nanotoxicity based on chronic disease models. In addition, positively charged surfaces and tissue concentrations above 100 g/ml result in higher toxicity, while surface functionalization decreases the toxicity of MNPs [70]. However, what exactly the nanotoxicity of MNPs will do to humans, and how to counteract it remains to be studied in further clinical trials.
Nanoparticles bind to serum proteins to form protein corona (PC). PC is thought to interfere with nanoparticle targeting ability and induce immune responses [78]. In fact, the effect of PC presence on the transport of nanoparticles is not limited to harm but has two sides, which are summarized and discussed in depth by Kim et al. and proposed to "turn enemies into friends" [79]. PC has a positive or negative effect on the colloidal stability, cellular uptake, cytotoxicity, and organ targeting of nanoparticles, the variability of which depends on the PC, the cell, the nanoparticle, the species, and the type of disease. For reducing PC formation, available tools include the use of "stealth polymers" such as PEG, poly(2-oxazoline)s, zwitterionic polymers, polyglycerols, hyaluronic acid, polyacrylamide-grafted guar gum, and chitosan, to modify the NP surface, or to wrap the NPs with cell membranes. Additionally, the disruptive effects of PC can be countered by antibody/ligand functionalization strategies or by direct modification of the coating with albumin, apolipoprotein, and CD47 protein. The main idea of "turning enemies into friends" is to form targeted PCs by attracting specific proteins, such as albumin, apolipoprotein, and vitreous binding proteins, to increase the accumulation of nanoparticles in specific organs [79]. It should be noted that the results of animal experiments may not be generalized to humans because of the different compositions of blood proteins, and we need some clinical trials to explore the optimal treatment of PC.
In general, embolism in blood vessels is formed due to the aggregation of larger magnetic units. Huang et al. reported that MNPs-labeled mesenchymal stem cells form cell clusters due to a "magnetic aggregation cascade", which in turn causes coronary embolism [80]. The "magnetic aggregation cascade" is because SPIONs may lose their superparamagnetism after aggregation and gain permanent magnetization, further attracting the surrounding SPIONs to agglomerate [81]. The embolic phenomenon brought about by magnetically targeted drug delivery is highly harmful to organisms, which requires strict control of the magnetic carrier and cargo/drug size in MDT design, setting up a precise and sensitive 3D magnetic field for navigation, and, more importantly, maintaining good intravascular colloid stability to avoid intravascular particle aggregation and embolism as much as possible [82–84]. Modifying polymer surface coatings to control the particle interactions is the foremost approach to solving this problem [82]. In addition, this "magnetic aggregation cascade" seems to have good aspects, such as increasing the volume, which allows the magnetic force to increase faster than the resistance [48]. This embolic phenomenon seems poorly reported in MDT studies so far, focusing on large-size cell targeting and with some contradictions [80]. Therefore, if the evidence at this stage is not sufficient to prove or solve this problem, perhaps efforts can be focused on real-time monitoring of the vasculature and probing the tolerance of the body’s vasculature to magnetic nanoparticles in preclinical and clinical trials to achieve the most direct safety assessment.
Part III. Application of MDT in CVD
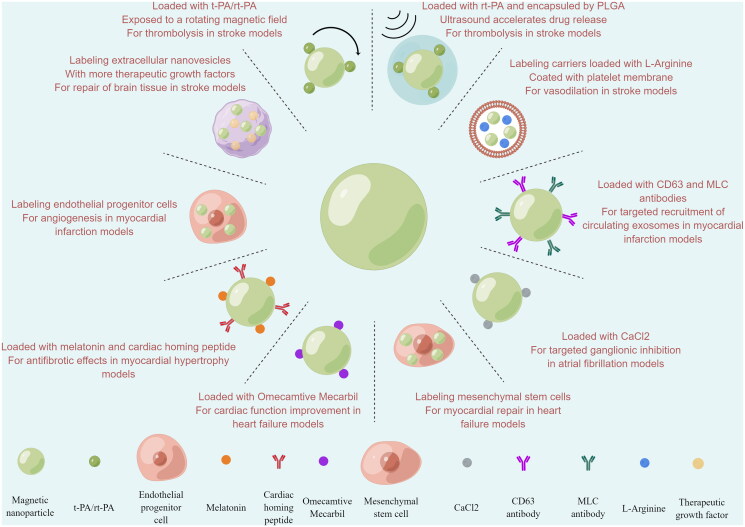
Cardiovascular Disease (CVD) is the leading cause of death worldwide [85], and there is an urgent need to improve the outcome of CVD treatment. The introduction of MDT into the clinical treatment and medication of CVD will enable drugs with short half-lives to obtain sufficient blood circulation time, achieve drug accumulation at the target site, avoid dose-dependent side effects and decreased patient compliance due to repeated drug administration, and will also enable the development and utilization of many restricted and innovative drugs, achieving twice the effect with half the effort. In addition, magnetic resonance imaging brought by magnetic nanocarriers into the cardiac system is expected to enable noninvasive real-time monitoring of the disease course. Thus, MDT may be an effective tool to improve CVD's efficacy. The intracellular magnetism of MNPs has been reported to disrupt the endothelial barrier at the target site and increase endothelial permeability, thereby enhancing site-specific drug delivery [86], making the application of MDT in CVD more promising. We will describe the application of MDT in CVD in the last five years in the following (Figure 3). Detailed data from relevant animal experiments are summarized in Table 2.
Figure 3.
Application of MDT in CVD in the last five years (by Figdraw.).
Table 2.
Detailed data from relevant animal experiments on MDT in CVD.
| Complex | MNP size (nm) | Complex size | Magnetic field strength (T) | Concentration | Function | Main Results | Model | Ref. |
|---|---|---|---|---|---|---|---|---|
| Magnetic microrods loaded with t-PA | 15 ± 3 | Length 1.3 ± 0.2 μm, Diameter 0.5 ± 0.1 μm | 0.24 | 1mg/kg | To enhance thrombolysis | Reduction in t-PA thrombolysis time by 2/3 | dMCAO/mouse | [87] |
| MNP loaded with rt-PA | \ | \ | \ | 1mg/ml | To enhance thrombolysis | Dramatic increase in thrombolytic efficiency | dMCAO/mouse | [88] |
| PLGA-coated disc-shaped particles loaded with rt-PA and SPION | 10.7 | Pitch 973.8 nm, Diameter 2.67 μm | 0.42 | 2mg/kg | To avoid hemorrhagic transformation |
Durable thrombolysis and avoidance of HT | Laser-induced thrombosis/mouse | [89] |
| Platelet membrane mimetic magnetic nanocarriers loaded with L-arginine | 27.94 ± 1.10 | Diameter 200 nm | 0.3 | (1.41 ± 0.16)×108/ml | To improve blood flow | Rapid targeting and successful reestablishment of blood flow | Cold light source-induced focal cerebral ischemia/mouse | [90] |
| Magnetic extracellular nano-vesicles | 12 | Diameter 194.2 ± 44.5 nm | 0.32 | 200μg in 300 μl of PBS | To repair infarcted tissue | Significant reduction in cerebral infarct volume | Transient MCAO/rat | [91] |
| Silica-coated MNP-labeled endothelial progenitor cells | 60 | \ | 0.39 | 1 × 106 in 100 μl of PBS | To enhance the targeting of EPCs | Enhanced aggregation of EPCs in the infarct border zone | LAD ligation/rat | [92] |
| Magnetic extracellular nano-vesicles | 25 | \ | \ | 150μg per rat | To repair infarcted tissue | Improvement in heart function | LAD ligation/rat | [93] |
| MNP loaded with CD63 and MLC antibodies | 200 | \ | 1.3 | 10mg/kg | To capture circulating exosomes | Successful capture and aggregation of exosomes | LAD ligation/rat and rabbit | [6] |
| Peptide functionalized SPION loaded with melatonin | 10 | Diameter 221 ± 13nm | 0.6 | \ | To improve cardiac hypertrophy | Prolonged melatonin half-life and increased utilization | TAC/rat | [94] |
| Fe3O4 nanoparticles loaded with OM | 45 | Diameter 45 nm | 0.3 | 4mg per rat | To reduce side effects | 118% cardiac efficacy achieved with 4% of previous dose | Heart failure/rat | [95] |
| SPION-labeled mesenchymal stem cell | 40 | \ | 0.3 | 2 × 106 in 0.5 ml of DMEM | To enhance tissue regeneration | Significant reduction in myocardial fibrosis | ISO subcutaneous injection/rat | [96] |
| PLGA-MNP loaded with CaCl2 | 213 ± 84 | Diameter 213 ± 84nm | 0.26 | 1mg/ml | To ablate the major atrial GP | Successful suppression of target GP function | RAP/dog | [97] |
Abbreviations: MNP: magnetic nanoparticle; PLGA: poly(lactic-co-glycolic acid); SPION: superparamagnetic iron oxide nanoparticle; OM: omecamtive mecarbil; DMEM: Dulbecco’s modified Eagle’s medium; EPCs: endothelial progenitor cells; GP: ganglionated plexi; HT: hemorrhagic transformation; (d)MCAO (distal): middle cerebral artery occlusion, LAD: left anterior descending artery; TAC: transverse aortic constriction; ISO: isoproterenol; RAP: rapid atrial pacing.
Ischemic stroke
Statistics for 2019 show that stroke is the second leading cause of death worldwide, with ischemic stroke being the leading type of stroke, accounting for 62.4% [98]. Currently available and being explored treatments for ischemic stroke include intravenous thrombolysis, thrombectomy, ultrasound-assisted thrombolysis, and neuroprotective agents [99]. Intravenous thrombolysis is the predominant treatment modality in clinical practice. Alteplase, a recombinant tissue-type fibrinogen activator (rt-PA), is the only thrombolytic drug currently approved by the US Food and Drug Administration (FDA) for acute ischemic stroke but has many well-known drawbacks, such as slow and incomplete thrombolysis, a therapeutic time window of only 4.5 h, short drug half-life, hemorrhagic transformation, and neurotoxicity [100]. Applying thrombectomy is strictly conditioned, and difficult to avoid tissue trauma. Ultrasonic thrombolysis and neuroprotective agents have not yet demonstrated verifiable clinical benefits. The introduction of magnetic targeting into stroke treatment may be a helpful attempt to improve the thrombolytic efficiency and safety of drugs, expand the treatment time window, and protect ischemic tissue.
In fact, the delivery of thrombolytic drugs is one of the current research hotspots in MDT, and a growing number of reports are highlighting the feasibility of magnetically targeted thrombolysis [101–106]. It is important to note that most current animal studies for thrombolysis use iliac or femoral vein thrombosis models [104, 107, 108]. This may be due to the complex structure of the brain and the fact that subtle trauma can produce significant dysfunction, making it challenging to advance the experiment. On the other hand, this demonstrates the potential of magnetically targeted thrombolysis for lower extremity deep vein thrombosis [109]. Nevertheless, we still collected three experiments based on stroke models within nearly five years. In 2018, Hu et al. prepared porous magnetic iron oxide microrods loaded with t-PA and induced CD1-IGS male mice with FeCl3 to establish distal middle cerebral artery occlusion (dMCAO) models [87]. Both the drug and control solutions were injected into the mice from the right external carotid artery. A 0.24 T magnet was placed perpendicular to the distal middle cerebral artery on the ischemic side to target the magnetic microrods to the thrombus site. After successful targeting, the magnet was removed, and a rotating magnetic field with a strength of 40 mT and a frequency of 20 Hz was placed near the infarcted area of the mouse head for thrombolysis. Aided by the mechanical effect of the rotating magnetic field, the time of t-PA thrombolysis was reduced by 2/3, and no re-embolization occurred within 24 hours. At the optimal dose (1 mg/kg), the magnetic microrods loaded with t-PA did not produce circulating embolism, cytotoxicity, or hepatorenal toxicity and were broken down into tiny particles that were excreted through the urine [87]. A similar study was reported in 2019. Huang et al. established dMCAO models by FeCl3 induction in male C57/BL6 mice and prepared polyacrylic acid-coated MNPs cross-linked with rt-PA [88]. At a dose of 1 mg/ml, magnetic field rotation resulted in a substantial increase in thrombus lysis efficiency and a significant reduction in cerebral infarct size. However, the strength of the magnetic field, the size of the particles, and the safety were not described. In 2022, a complete study was presented by Choi et al. [89]. They synthesized SPIONs by pyrolysis and assembled poly(lactic-co-glycolic acid) (PLGA), rtPA, and SPIONs into disc-shaped particles with a diameter of approximately 3 μm using a top-down approach. A 532 nm green dot laser was used to induce ischemic stroke models in male BALB/C mice. A saline solution of the particles at a dose of 2 mg/kg was injected into the mice from the tail vein, and a 0.42 T magnet was placed on the skull surface of the right hemisphere of the mouse brain. A 1.5 MHz focused transducer was used for in vivo magnetoacoustic thrombolysis, and near-infrared carbocyanine fluorescent was used to monitor the targeting of the particles. The results showed that the particles under the action of the magnetic field could reach the target within 20 minutes, and ultrasound could control the accelerated release of rt-PA from the particles. Even if the conventional treatment time window of rtPA is exceeded, the particles still have considerable thrombolytic efficiency. More importantly, they did not produce any hemorrhagic transformation after thrombolysis, the safety was good, and the mouse models obtained the recovery of motor function. However, the efficacy of this magnetoacoustic device did not differ much from that of rtPA alone, which may be its limitation [89]. Nevertheless, all three experiments provided encouraging results demonstrating the potential of magnetic targeting for thrombolytic therapy of ischemic stroke.
Nitric oxide (NO) is an important signaling molecule that can diastole blood vessels and reduce platelet (PLT) aggregation, with the potential to expand the therapeutic time window for ischemic stroke. However, NO is challenging to target the stroke region in an aerobic environment. Li et al. developed a PLT membrane and magnetic field dual-targeting L-Arginine delivery device [90]. L-Arginine is an NO donor that promotes NO production by endothelial cells. They used the membrane-extrusive method to assemble PLT membrane vesicles, L-Arginine, and γ-Fe2O3 MNPs into a mimetic particle with a diameter of about 200 nm and labeled with a near-infrared fluorescent dye. Male C57BL/6 mice were divided into three groups, the first group was injected with labeled particles via tail vein only, the second group was injected with labeled particles after stroke induction via a 4 mm aperture cold light source, and the third group had a 0.3 T magnet placed in the focal area of the head of the mice based on the second group. Near-infrared fluorescence in vivo imaging system showed significantly higher fluorescence intensity in the lesion area in the third group than in the other two groups with a rapid rise. Similar results were obtained with MRI, with the strongest carrier targeting efficiency for the group applying the magnetic field. Quantitative blood flow analysis showed that recanalization of the ischemic region occurred 0.5–1h after particle administration and application of an external magnetic field led to a further increase in the rate of blood flow reconstruction. Mice were executed six hours after particle injection and immunohistochemical analysis showed that brain tissue damage was significantly reduced in the experimental group, and no significant damage was seen in other organs [90].
Mesenchymal stem cells (MSCs) are investigated for treating tissue injury in ischemic stroke because of their multiple effects, such as regenerative vascularization, immune modulation, and neuroprotection [110]. However, direct implantation is invasive and has safety issues. Due to their large size, MSCs transplanted intravenously can be intercepted by pulmonary capillaries with poor results [111]. MSCs-derived exosomes can improve this situation, but their targeting still needs to be improved [112]. Extracellular nanovesicles (NVs) function similarly to exosomes and can be produced on a large scale. Kim et al. prepared magnetic NVs about 200 nm in size [91]. Male rats in the experimental group were treated with transient middle cerebral artery occlusion (i.e., transient MCAO) and wore magnetic helmets with an intensity of 0.32 T. Magnetic NVs were labeled with the lipophilic dye VivoTrack 680 and injected into the rats from the tail vein. Compared with the control group, where NVs were mainly absorbed by the liver and spleen, the magnetic field effect resulted in a significant increase in fluorescence signal in the focal area 24 h after injection. In addition, the volume of brain infarcts in the experimental group was significantly smaller than that in the control group three days after injection. Immunohistochemistry showed that MSCs-derived magnetic NVs had anti-inflammatory, anti-apoptotic, and pro-angiogenic effects [91].
Myocardial infarction
Acute myocardial infarction is a hazardous ischemic heart disease, and its treatment is divided into invasive interventional and surgical treatment and noninvasive drug treatment. Invasive treatments have a high application threshold, drugs have a short half-life and high side effects, and thus well-targeted MDT modalities need to be urgently developed.
Endothelial progenitor cells (EPCs) can differentiate into mature endothelial cells and proliferate, promoting angiogenesis and improving blood flow. However, the retention of EPCs in the infarct region is low, resulting in suboptimal clinical efficacy. In response, Zhang et al. used silica-coated MNPs to label EPCs (MEPCs) and enhanced the aggregation of EPCs in the marginal infarct zone in rats by magnetic targeting [92]. The results showed that MEPCs produced essentially no microcirculatory embolism and cytotoxicity, reduced cardiomyocyte apoptosis and fibrosis, and promoted angiogenesis in the infarct margins, with the smallest infarct size and best cardiac morphology in the magnetic targeting group [92]. For the same purpose, another idea was provided by Sun et al. [113]. To avoid the tumorigenic potential of exogenous EPCs, they recruited endogenous EPCs using SPIONs loaded with CD34 antibodies. These SPIONs were loaded into neutrophils (NEs) to complete the targeting and release process. Ultimately, EPCs were successfully recruited to the infarct site, with increased microvessel density and improved left ventricular ejection function [113]. Unfortunately, the SPIONs of this experiment were only used for imaging and recruitment, and targeting was provided by chemotaxis of NEs. Future exploration of the potential of this indirect treatment modality in combination with magnetic targeting is worthwhile.
MSCs-derived exosomes have been developed to treat ischemic stroke, as previously described. The same idea has also received attention in the treatment of heart attacks. Lee et al. modified the surface of cubic iron oxide nanoparticles (INOPs) of approximately 25 nm in size with PEG and fluorescent dyes and loaded them into MSCs-derived NVs [93]. The results showed that magnetically targeted INOP-NVs showed good retention at the infarct site and could promote myocardial repair in four ways: anti-inflammatory, anti-apoptotic, anti-fibrotic, and angiogenic. Interestingly, in vitro experiments showed that INOPs promote the expression of therapeutic molecules and miRNAs in MSCs, but the exact mechanism is unclear [93]. Similar to the previous idea of Sun et al. [113], Liu et al. used antibody-coupled MNPs to recruit endogenous circulating exosomes to treat heart attacks [6]. Differently, this study is a complete example of the magnetic targeting application. 200 nm Fe3O4 nanoparticles were encapsulated by SiO2 shells and subsequently modified with PEG, pH-sensitive hydrazone bonds, and two antibodies, CD63 and MLC. After female rats were ligated with the left anterior descending artery (LAD), 10 mg/kg of fluorescently labeled engineered MNPs were injected from the tail vein. A 1.3 T circular neodymium magnet was placed 5 mm from the heart within 10 min after injection. The results showed that the engineered MNPs could trap circulating exosomes, aggregate them to the infarct site, and complete their release in an acidic environment, thereby reducing infarct size, promoting angiogenesis, and improving left ventricular ejection fraction. Serum biochemical parameters and histological examination showed no significant evidence of toxicity. In addition, they injected the particles into a porcine vein and showed that the magnetic field continued to have a sufficient capture capacity at a flow rate of 50 ml/min. Notably, they also established a rabbit heart failure model and obtained the same results of improved cardiac function [6].
A recent interesting study from the team of Bao et al. suggests that magnetic stimulation itself has a therapeutic effect on myocardial infarction [114]. They loaded SPIONs into a hydrogel and wrapped it around the vagus nerve of infarcted rats. Under the stimulation of a pulsed magnetic field, the rats’ cardiac function was significantly improved. Future research on magnetic targeting in myocardial infarction treatment could try to combine drug delivery and magnetic neuromodulation.
Myocardial hypertrophy
Technically speaking, cardiac hypertrophy is a pathological change, but it can develop into severe heart disease, such as heart failure. Therefore, attention to myocardial hypertrophy is warranted. Currently, the means of remodeling for myocardial hypertrophy are unsatisfactory [115]. Myocardial fibrosis is the most crucial pathological factor leading to myocardial hypertrophy. Means to reverse myocardial fibrosis are still under investigation.
Melatonin, a hormone secreted by the pineal gland of the brain, has been shown to have anti-fibrotic effects on the myocardium [116]. However, conventional oral administration makes its bioavailability low. Accordingly, Zhao et al. combined poly (lactide) polycarboxybetaine, cardiac homing peptides, and 10 nm size SPIONs coupled with melatonin to prepare an approximately 200 nm large drug delivery vehicle with dual targeting capability [94]. Sprague-Dawley rats underwent transverse aortic constriction (TAC) surgery to induce myocardial hypertrophy. Fluorescent dye-labeled functionalized nanocarriers were injected into the rats from the tail vein, followed by a 0.6 T neodymium magnet placed outside the heart. The results showed that this carrier had good drug encapsulation ability, prolonged the circulating half-life of melatonin, and increased its accumulation in the heart. After treatment, parameters associated with myocardial hypertrophy and hydroxyproline concentrations in cardiac tissue were significantly reduced. RT-PCR showed a significant decrease in the expression of genes associated with myocardial hypertrophy and fibrosis [94].
Liu et al., on the other hand, focused on the TGF-β signaling pathway, which may be a key driver of myocardial hypertrophy and fibrosis. In 2019, they revealed that Foxp1 transcriptional repressors expressed in endothelial cells could reverse pathological myocardial fibrosis by regulating the TGF-β1-Endothelin-1 pathway [117]. RGD-peptide MNPs (RGD-MNPs) were used to deliver TGF-β1-siRNA to Foxp1-deficient endothelial cells, showing that TGF-β1 signaling was blocked and myocardial hypertrophy and fibrosis were reversed. In 2020, the team further found that simvastatin could modulate the Foxp1-TGF-β1 signaling pathway by activating Kruppel-like factor 2 (Klf2), an upstream factor of Foxp1, which in turn attenuates TAC-induced myocardial hypertrophy [118]. RGD-MNPs were used to block Klf2 signaling. Overall, the team’s study established a clear mechanistic pathway and implied the targeting potential of RGD-MNPs. Unfortunately, the specific magnetic targeting operation was not described in detail, and the RGD-MNPs were only used to block the signal.
Heart failure
Heart failure (HF) is one of the major public health problems today, and its mortality rate has been increasing in recent years due to a lack of effective management [119].
Omecamtive Mecarbil (OM) is a novel cardiac myosin activator for treating systolic heart failure. However, it has dose-dependent side effects, and repeated dosing decreases patient compliance. Accordingly, Kiaie et al. loaded it onto chitosan-coated SPIONs with a diameter of 45 nm to improve the targeting of OM and reduce the dose used [95]. Male Wistar rats were used to construct the HF model. A 0.3 T magnet was placed on the heart surface, and SPIONs loaded with OM were injected into the rats from the tail vein. The result showed a 2.5-fold increase in OM targeting, a 96% reduction in dose, and an 18% increase in left ventricular ejection fraction from 71.7 ± 1.41% to 89.6 ± 1.40% [95].
Mesenchymal stem cells (MSCs) can repair the myocardium but are difficult to be retained in the myocardium after exogenous injection. Repeated invasive injections to obtain sustained efficacy are impractical. Accordingly, Naseroleslami et al. used PEG-coated 40 nm diameter SPIONs to label MSCs to improve cell homing ability [96]. Isoproterenol (ISO) saline solution was injected subcutaneously into male Wistar rats for four consecutive days to induce heart failure. 2 × 106 SPION-labeled MSCs were injected into the heart, and a 0.3 T neodymium magnet, 8 mm long and 2 mm wide, was placed 1 mm above the heart and subsequently sutured incision. The results showed that the magnetic field improved the retention of MSCs, the ejection fraction was improved, and myocardial fibrosis was significantly reduced [96].
Cardiac arrest/ischemia-reperfusion injury
Cardiac arrest is a significant health problem with a high mortality rate, primarily due to reperfusion injury to the organism after resuscitation [120]. The potential of magnetically targeted drug delivery in cardiopulmonary resuscitation was discussed in 2012 [121]. Xanthos et al. suggested that disposable magnet pads could be added to the defibrillator to introduce well-targeted drug therapy without hindering CPR. Unfortunately, the last five years of research have not revived this idea. Nevertheless, there are still some examples of using MNPs as drug carriers.
Hydrogen sulfide (H2S) can reduce ischemia-reperfusion injury in the heart and brain. However, the lack of effective carriers to deliver it to target organs and control its release leads to its cytotoxicity causing damage to normal tissues. Wang et al. prepared mesoporous iron oxide nanoparticles (MIONs) loaded with diallyl trisulfide (DATS), an H2S donor, which were used in mouse models of myocardial ischemia with good biocompatibility and controlled release properties [122]. Sun et al. also prepared DATS-loaded MIONs. They modified them with PEG to obtain longer circulation times and introduced lactoferrin to help them cross the blood-brain barrier [123]. Huang et al., on the other hand, prepared DATS-loaded erythrocyte membrane-encapsulated MIONs, which showed long circulation and controlled release in the animal model of ischemia-reperfusion and improved cardiac function through anti-inflammatory, antioxidant, and anti-apoptotic pathways [124]. Unfortunately, none of these studies used magnetic fields to target MIONs, and it is entirely possible to introduce magnetic targeting systems into the study of H2S in the future.
Atrial fibrillation
Atrial fibrillation is the most common cardiac arrhythmia. The incidence has steadily increased in recent years, and the efficacy of therapeutic options has not been satisfactory [125]. Ablation is a common treatment, but it requires a specialized electrophysiologist to perform it, and the indications for its application are strict and costly. In this regard, Yu et al. proposed a way to intervene early in paroxysmal atrial fibrillation. They constructed approximately 200 nm large CaCl2-loaded PLGA-MNPs and subjected male mongrel dogs to 6 hours of rapid atrial pacing (RAP) after open-heart treatment to induce atrial fibrillation [97]. 1 mg/ml of Ca-MNPs were injected from the left circumflex artery into dogs, and 0.26 T of electromagnets were placed on the epicardial surface of the target atrial ganglionated plexi (GP). The results showed that Ca-MNPs were targeted to the target GP, and the neurotoxicity of calcium ions successfully inhibited GP function and prevented RAP-induced electrical remodeling of the atria [97].
Part IV. Conclusions and prospects
MDT has great potential for application in cardiovascular diseases. In this review, we present the current status of research on magnetically targeted drug/cargo delivery from five aspects: guiding magnetic fields, magnetic carriers, delivery channels, drug release control, and safety assessment, and summarize the evidence from animal experiments in the last five years. To improve the efficiency of targeted drug delivery, researchers have developed advanced magnetic carriers such as magnetic robots, adopted antibody functionalization strategies to achieve bio-magnetic targeting, and supplemented with various modalities, such as pH and ultrasound, to control drug release. In animal experiments on CVDs, MDT is mainly applied to ischemic stroke and cardiac diseases, and thrombolysis and stem cell therapy are repeatedly mentioned topics. In our opinion, new nano-delivery technologies can all be tried in combination with magnetic targeting. Moreover, researchers need to fully exploit the potential of magnetic carriers to move in magnetic fields, of which magnetic microwheels are an example. In addition, nanotoxicity, protein corona, and vascular embolism are three safety concerns of MDT that need to be further illustrated in clinical trials. In the future, MDT needs to be studied more extensively and in-depth in more CVDs to elucidate more details in specific situations.
Acknowledgments
The authors would like to acknowledge the convenience of Figdraw (https://www.figdraw.com/) for diagramming.
Funding Statement
This work was supported by grants from the Jiangxi Provincial Department of Education Science and Technology Research Project [GJJ2200176].
Ethical approval
Not applicable.
Authors’ contributions
XW was responsible for the conception, charting, and first draft of this paper. RB was responsible for the conception, critical revision, and final approval of this paper. All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Freeman MW, Arrott A, Watson JHL. (1960). Magnetism in medicine. J Appl Phys 31:1–15. doi: 10.1063/1.1984765. [DOI] [Google Scholar]
- 2.Lubbe AS, Bergemann C, Riess H, et al. (1996). Clinical experiences with magnetic drug targeting: a phase I study with 4’-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 56:4686–93. [PubMed] [Google Scholar]
- 3.Alexiou C, Arnold W, Klein RJ, et al. (2000). Locoregional cancer treatment with magnetic drug targeting. Cancer Res 60:6641–8. [PubMed] [Google Scholar]
- 4.Vandergriff AC, Hensley TM, Henry ET, et al. (2014). Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 35:8528–39. doi: 10.1016/j.biomaterials.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Schwerdt JI, Goya GF, Calatayud MP, et al. (2012). Magnetic field-assisted gene delivery: achievements and therapeutic potential. Curr Gene Ther 12:116–26. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Chen X, Bao L, et al. (2020). Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng 4:1063–75. doi: 10.1038/s41551-020-00637-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Chen S, Zhu Y, et al. (2018). Triple-modal imaging-guided chemo-photothermal synergistic therapy for breast cancer with magnetically targeted phase-shifted nanoparticles. ACS Appl Mater Interfaces 10:42102–14. doi: 10.1021/acsami.8b16323. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Bu W, Ren J, et al. (2018). Enhanced synergism of thermo-chemotherapy for liver cancer with magnetothermally responsive nanocarriers. Theranostics 8:693–709. doi: 10.7150/thno.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li WP, Liao PY, Su CH, Yeh CS. (2014). Formation of oligonucleotide-gated silica shell-coated Fe(3)O(4)-Au core-shell nanotrisoctahedra for magnetically targeted and near-infrared light-responsive theranostic platform. J Am Chem Soc 136:10062–75. doi: 10.1021/ja504118q. [DOI] [PubMed] [Google Scholar]
- 10.Liu YL, Chen D, Shang P, Yin DC. (2019). A review of magnet systems for targeted drug delivery. J Control Release 302:90–104. doi: 10.1016/j.jconrel.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Bietenbeck M, Florian A, Faber C, et al. (2016). Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: where are we now? Int J Nanomedicine 11:3191–203. doi: 10.2147/IJN.S110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riegler J, Lau KD, Garcia-Prieto A, et al. (2011). Magnetic cell delivery for peripheral arterial disease: a theoretical framework. Med Phys 38:3932–43. doi: 10.1118/1.3593363. [DOI] [PubMed] [Google Scholar]
- 13.Shen WB, Anastasiadis P, Nguyen B, et al. (2017). Magnetic enhancement of stem cell-targeted delivery into the brain following mr-guided focused ultrasound for opening the blood-brain barrier. Cell Transplant 26:1235–46. doi: 10.1177/0963689717715824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voronin DV, Sindeeva OA, Kurochkin MA, et al. (2017). In vitro and in vivo visualization and trapping of fluorescent magnetic microcapsules in a bloodstream. ACS Appl Mater Interfaces 9:6885–93. doi: 10.1021/acsami.6b15811. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro B, Dormer K, Rutel IB. (2010). A two-magnet system to push therapeutic nanoparticles. AIP Conf Proc 1311:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JF, Lan Z, Ferrari C, et al. (2020). Use of oppositely polarized external magnets to improve the accumulation and penetration of magnetic nanocarriers into solid tumors. ACS Nano 14:142–52. doi: 10.1021/acsnano.9b05660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Shen Z, Chen X. (2020). Tale of two magnets: an advanced magnetic targeting system. ACS Nano 14:7–11. doi: 10.1021/acsnano.9b06842. [DOI] [PubMed] [Google Scholar]
- 18.Roeth AA, Slabu I, Baumann M, et al. (2017). Establishment of a biophysical model to optimize endoscopic targeting of magnetic nanoparticles for cancer treatment. Int J Nanomedicine 12:5933–40. doi: 10.2147/IJN.S132162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riegler J, Wells JA, Kyrtatos PG, et al. (2010). Targeted magnetic delivery and tracking of cells using a magnetic resonance imaging system. Biomaterials 31:5366–71. doi: 10.1016/j.biomaterials.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Vartholomeos P, Fruchard M, Ferreira A, Mavroidis C. (2011). MRI-guided nanorobotic systems for therapeutic and diagnostic applications. Annu Rev Biomed Eng 13:157–84. doi: 10.1146/annurev-bioeng-071910-124724. [DOI] [PubMed] [Google Scholar]
- 21.Muthana M, Kennerley AJ, Hughes R, et al. (2015). Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat Commun 6:8009. doi: 10.1038/ncomms9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyak B, Friedman G. (2009). Magnetic targeting for site-specific drug delivery: applications and clinical potential. Expert Opin Drug Deliv 6:53–70. doi: 10.1517/17425240802662795. [DOI] [PubMed] [Google Scholar]
- 23.McNab JA, Edlow BL, Witzel T, et al. (2013). The human connectome project and beyond: initial applications of 300 mT/m gradients. Neuroimage 80:234–45. doi: 10.1016/j.neuroimage.2013.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker RR, Payne C, Yu Y, et al. (2022). Image-guided magnetic thermoseed navigation and tumor ablation using a magnetic resonance imaging system. Adv Sci 9:e2105333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Liu X, Huang Z, et al. (2015). Comparison of magnetic intensities for mesenchymal stem cell targeting therapy on ischemic myocardial repair: high magnetic intensity improves cell retention but has no additional functional benefit. Cell Transplant 24:1981–97. doi: 10.3727/096368914X685302. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Yan B, Li Y, et al. (2020). Graphene oxide-grafted magnetic nanorings mediated magnetothermodynamic therapy favoring reactive oxygen species-related immune response for enhanced antitumor efficacy. ACS Nano 14:1936–50. doi: 10.1021/acsnano.9b08320. [DOI] [PubMed] [Google Scholar]
- 27.Nana ABA, Marimuthu T, Kondiah PPD, et al. (2019). Multifunctional magnetic nanowires: design, fabrication, and future prospects as cancer therapeutics. Cancers 11:1956. doi: 10.3390/cancers11121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Son SJ, Reichel J, He B, et al. (2005). Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. J Am Chem Soc 127:7316–7. doi: 10.1021/ja0517365. [DOI] [PubMed] [Google Scholar]
- 29.Jiang K, Zhang L, Bao G. (2021). Magnetic iron oxide nanoparticles for biomedical applications. Curr Opin Biomed Eng 20:100330. doi: 10.1016/j.cobme.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng K, Shen D, Hensley MT, et al. (2014). Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat Commun 5:4880. doi: 10.1038/ncomms5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veloso SRS, Andrade RGD, Castanheira EMS. (2021). Magnetoliposomes: recent advances in the field of controlled drug delivery. Expert Opin Drug Deliv 18:1323–34. doi: 10.1080/17425247.2021.1915983. [DOI] [PubMed] [Google Scholar]
- 32.Allen TM, Cullis PR. (2013). Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Chertok B, Langer R. (2018). Circulating magnetic microbubbles for localized real-time control of drug delivery by ultrasonography-guided magnetic targeting and ultrasound. Theranostics 8:341–57. doi: 10.7150/thno.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou H, Mayorga-Martinez CC, Pane S, et al. (2021). Magnetically driven micro and nanorobots. Chem Rev 121:4999–5041. doi: 10.1021/acs.chemrev.0c01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chesnitskiy AV, Gayduk AE, Seleznev VA, Prinz VY. (2022). Bio-inspired micro- and nanorobotics driven by magnetic field. Materials 15:7781. doi: 10.3390/ma15217781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villa K, Pumera M. (2019). Fuel-free light-driven micro/nanomachines: artificial active matter mimicking nature. Chem Soc Rev 48:4966–78. doi: 10.1039/c9cs00090a. [DOI] [PubMed] [Google Scholar]
- 37.Aghakhani A, Yasa O, Wrede P, Sitti M. (2020). Acoustically powered surface-slipping mobile microrobots. Proc Natl Acad Sci U S A 117:3469–77. doi: 10.1073/pnas.1920099117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field RD, Anandakumaran PN, Sia SK. (2019). Soft medical microrobots: design components and system integration. Appl Phys Rev 6:041305. doi: 10.1063/1.5124007. [DOI] [Google Scholar]
- 39.Kobayashi K, Yoon C, Oh SH, et al. (2019). Biodegradable Thermomagnetically Responsive Soft Untethered Grippers. ACS Appl Mater Interfaces 11:151–9. doi: 10.1021/acsami.8b15646. [DOI] [PubMed] [Google Scholar]
- 40.Gong D, Celi N, Zhang D, Cai J. (2022). Magnetic biohybrid microrobot multimers based on chlorella cells for enhanced targeted drug delivery. ACS Appl Mater Interfaces 14:6320–30. doi: 10.1021/acsami.1c16859. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Wu J, Lin D, et al. (2022). A diatom-based biohybrid microrobot with a high drug-loading capacity and pH-sensitive drug release for target therapy. Acta Biomater 154:443–53. doi: 10.1016/j.actbio.2022.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Hines L, Petersen K, Lum GZ, Sitti M. (2017). Soft actuators for small-scale robotics. Adv Mater 29:1603483. doi: 10.1002/adma.201603483. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, Zhang L, Wang J, et al. (2015). Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv Mater 27:1402–7. doi: 10.1002/adma.201404788. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Song Y, Cao Z, et al. (2023). A magnetically driven amoeba-like nanorobot for whole-process active drug transport. Adv Sci 10:e2204793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou K, Zhang Y, Bao M, et al. (2022). A multifunctional magnetic red blood cell-mimetic micromotor for drug delivery and image-guided therapy. ACS Appl Mater Interfaces 14:3825–37. doi: 10.1021/acsami.1c21331. [DOI] [PubMed] [Google Scholar]
- 46.Disharoon D, Trewyn BG, Herson PS, et al. (2022). Breaking the fibrinolytic speed limit with microwheel co-delivery of tissue plasminogen activator and plasminogen. J Thromb Haemost 20:486–97. doi: 10.1111/jth.15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tasci TO, Disharoon D, Schoeman RM, et al. (2017). Enhanced fibrinolysis with magnetically powered colloidal microwheels. Small 13:1700954. doi: 10.1002/smll.201700954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nacev A, Beni C, Bruno O, Shapiro B. (2011). The behaviors of ferro-magnetic nano-particles in and around blood vessels under applied magnetic fields. J Magn Magn Mater 323:651–68. doi: 10.1016/j.jmmm.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hewlin RL Jr., Tindall JM. (2023). Computational assessment of magnetic nanoparticle targeting efficiency in a simplified circle of willis arterial model. Int J Mol Sci 24:2545. doi: 10.3390/ijms24032545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sodagar H, Shakiba A, Niazmand H. (2020). Numerical investigation of drug delivery by using magnetic field in a 90-degree bent vessel: a 3D simulation. Biomech Model Mechanobiol 19:2255–69. doi: 10.1007/s10237-020-01337-0. [DOI] [PubMed] [Google Scholar]
- 51.Manshadi MKD, Saadat M, Mohammadi M, et al. (2018). Delivery of magnetic micro/nanoparticles and magnetic-based drug/cargo into arterial flow for targeted therapy. Drug Deliv 25:1963–73. doi: 10.1080/10717544.2018.1497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mura S, Nicolas J, Couvreur P. (2013). Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 53.Amstad E, Kohlbrecher J, Muller E, et al. (2011). Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett 11:1664–70. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 54.Nitica S, Fizesan I, Dudric R, et al. (2022). Doxorubicin loaded thermosensitive magneto-liposomes obtained by a gel hydration technique: characterization and in vitro magneto-chemotherapeutic effect assessment. Pharmaceutics 14:2501. doi: 10.3390/pharmaceutics14112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beguin E, Gray MD, Logan KA, et al. (2020). Magnetic microbubble mediated chemo-sonodynamic therapy using a combined magnetic-acoustic device. J Control Release 317:23–33. doi: 10.1016/j.jconrel.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Park J, Kim JY, Pane S, et al. (2021). Acoustically mediated controlled drug release and targeted therapy with degradable 3D porous magnetic microrobots. Adv Healthc Mater 10:e2001096. [DOI] [PubMed] [Google Scholar]
- 57.Xing Y, Zeng B, Yang W. (2022). Light responsive hydrogels for controlled drug delivery. Front Bioeng Biotechnol 10:1075670. doi: 10.3389/fbioe.2022.1075670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song X, Chen Z, Zhang X, et al. (2021). Magnetic tri-bead microrobot assisted near-infrared triggered combined photothermal and chemotherapy of cancer cells. Sci Rep 11:7907. doi: 10.1038/s41598-021-87010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang B, Qin Y, Liu J, et al. (2023). Magnetotactic Bacteria-Based Drug-Loaded Micromotors for Highly Efficient Magnetic and Biological Double-Targeted Tumor Therapy. ACS Appl Mater Interfaces 15:2747–59. doi: 10.1021/acsami.2c19960. [DOI] [PubMed] [Google Scholar]
- 60.Feng Q, Zhang Y, Zhang W, et al. (2017). Programmed near-infrared light-responsive drug delivery system for combined magnetic tumor-targeting magnetic resonance imaging and chemo-phototherapy. Acta Biomater 49:402–13. doi: 10.1016/j.actbio.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 61.Servant A, Methven L, Williams RP, Kostarelos K. (2013). Electroresponsive polymer-carbon nanotube hydrogel hybrids for pulsatile drug delivery in vivo. Adv Healthc Mater 2:806–11. doi: 10.1002/adhm.201200193. [DOI] [PubMed] [Google Scholar]
- 62.Bansal M, Dravid A, Aqrawe Z, et al. (2020). Conducting polymer hydrogels for electrically responsive drug delivery. J Control Release 328:192–209. doi: 10.1016/j.jconrel.2020.08.051. [DOI] [PubMed] [Google Scholar]
- 63.Assa F, Jafarizadeh-Malmiri H, Ajamein H, et al. (2017). Chitosan magnetic nanoparticles for drug delivery systems. Crit Rev Biotechnol 37:492–509. doi: 10.1080/07388551.2016.1185389. [DOI] [PubMed] [Google Scholar]
- 64.Liu Q, Tan Z, Zheng D, Qiu X. (2023). pH-responsive magnetic Fe(3)O(4)/carboxymethyl chitosan/aminated lignosulfonate nanoparticles with uniform size for targeted drug loading. Int J Biol Macromol 225:1182–92. doi: 10.1016/j.ijbiomac.2022.11.179. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Xue X, He Y, et al. (2018). Novel redox-responsive polymeric magnetosomes with tunable magnetic resonance property for in vivo drug release visualization and dual-modal cancer therapy. Adv Funct Mater 28:1802159. doi: 10.1002/adfm.201802159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Z, Wang H, Zhang Z, et al. (2022). Cartilage targeting therapy with reactive oxygen species-responsive nanocarrier for osteoarthritis. J Nanobiotechnology 20:419. doi: 10.1186/s12951-022-01629-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhai Z, Ouyang W, Yao Y, et al. (2022). Dexamethasone-loaded ROS-responsive poly(thioketal) nanoparticles suppress inflammation and oxidative stress of acute lung injury. Bioact Mater 14:430–42. doi: 10.1016/j.bioactmat.2022.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinaldi A, Caraffi R, Grazioli MV, et al. (2022). Applications of the ROS-Responsive Thioketal Linker for the Production of Smart Nanomedicines. Polymers (Basel) 14:687. doi: 10.3390/polym14040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong MH, Bao Y, Yang XZ, et al. (2012). Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J Am Chem Soc 134:4355–62. doi: 10.1021/ja211279u. [DOI] [PubMed] [Google Scholar]
- 70.Vakili-Ghartavol R, Momtazi-Borojeni AA, Vakili-Ghartavol Z, et al. (2020). Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif Cells Nanomed Biotechnol 48:443–51. doi: 10.1080/21691401.2019.1709855. [DOI] [PubMed] [Google Scholar]
- 71.Moore A, Marecos E, Bogdanov A, Jr., Weissleder R. (2000). Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology 214:568–74. doi: 10.1148/radiology.214.2.r00fe19568. [DOI] [PubMed] [Google Scholar]
- 72.Jain TK, Reddy MK, Morales MA, et al. (2008). Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharmaceutics 5:316–27. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 73.Kessler A, Hedberg J, Blomberg E, Odnevall I. (2022). Reactive oxygen species formed by metal and metal oxide nanoparticles in physiological media-a review of reactions of importance to nanotoxicity and proposal for categorization. Nanomaterials 12:1922. doi: 10.3390/nano12111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Z, Li Q, Wang J, et al. (2020). Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett 15:115. doi: 10.1186/s11671-020-03344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang W, Kim BY, Rutka JT, Chan WC. (2008). Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3:145–50. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 76.Han J, Tian Y, Wang M, et al. (2022). Proteomics unite traditional toxicological assessment methods to evaluate the toxicity of iron oxide nanoparticles. Front Pharmacol 13:1011065. doi: 10.3389/fphar.2022.1011065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chrishtop VV, Mironov VA, Prilepskii AY, et al. (2021). Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 15:167–204. doi: 10.1080/17435390.2020.1842934. [DOI] [PubMed] [Google Scholar]
- 78.Panico S, Capolla S, Bozzer S, et al. (2022). Biological features of nanoparticles: protein corona formation and interaction with the immune system. Pharmaceutics 14:2605. doi: 10.3390/pharmaceutics14122605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim W, Ly NK, He Y, et al. (2023). Protein corona: friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv Drug Deliv Rev 192:114635. doi: 10.1016/j.addr.2022.114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang Z, Shen Y, Pei N, et al. (2013). The effect of nonuniform magnetic targeting of intracoronary-delivering mesenchymal stem cells on coronary embolisation. Biomaterials 34:9905–16. doi: 10.1016/j.biomaterials.2013.08.092. [DOI] [PubMed] [Google Scholar]
- 81.Morup S, Hansen MF, Frandsen C. (2010). Magnetic interactions between nanoparticles. Beilstein J Nanotechnol 1:182–90. doi: 10.3762/bjnano.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Champagne PO, Westwick H, Bouthillier A, Sawan M. (2018). Colloidal stability of superparamagnetic iron oxide nanoparticles in the central nervous system: a review. Nanomedicine 13:1385–400. doi: 10.2217/nnm-2018-0021. [DOI] [PubMed] [Google Scholar]
- 83.Guerrini L, Alvarez-Puebla RA, Pazos-Perez N. (2018). Surface modifications of nanoparticles for stability in biological fluids. Materials 11:1154. doi: 10.3390/ma11071154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Limongi T, Canta M, Racca L, et al. (2019). Improving dispersal of therapeutic nanoparticles in the human body. Nanomedicine (Lond) 14:797–801. doi: 10.2217/nnm-2019-0070. [DOI] [PubMed] [Google Scholar]
- 85.Zhao D, Liu J, Wang M, et al. (2019). Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 16:203–12. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 86.Qiu Y, Tong S, Zhang L, et al. (2017). Magnetic forces enable controlled drug delivery by disrupting endothelial cell-cell junctions. Nat Commun 8:15594. doi: 10.1038/ncomms15594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu J, Huang S, Zhu L, et al. (2018). Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl Mater Interfaces 10:32988–97. doi: 10.1021/acsami.8b09423. [DOI] [PubMed] [Google Scholar]
- 88.Huang L, Wang J, Huang S, et al. (2019). Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem Biophys Res Commun 516:565–70. doi: 10.1016/j.bbrc.2019.06.079. [DOI] [PubMed] [Google Scholar]
- 89.Choi W, Cho H, Kim G, et al. (2022). Targeted thrombolysis by magnetoacoustic particles in photothrombotic stroke model. Biomater Res 26:58. doi: 10.1186/s40824-022-00298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M, Li J, Chen J, et al. (2020). Platelet membrane biomimetic magnetic nanocarriers for targeted delivery and in situ generation of nitric oxide in early ischemic stroke. ACS Nano 14:2024–35. doi: 10.1021/acsnano.9b08587. [DOI] [PubMed] [Google Scholar]
- 91.Kim HY, Kim TJ, Kang L, et al. (2020). Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 243:119942. doi: 10.1016/j.biomaterials.2020.119942. [DOI] [PubMed] [Google Scholar]
- 92.Zhang BF, Jiang H, Chen J, et al. (2019). Silica-coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long-term cardiac function with magnetic field guidance in rats with myocardial infarction. J Cell Physiol 234:18544–59. doi: 10.1002/jcp.28492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JR, Park BW, Kim J, et al. (2020). Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv 6:eaaz0952. doi: 10.1126/sciadv.aaz0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao X, Wang X, Wang J, et al. (2020). A peptide-functionalized magnetic nanoplatform-loaded melatonin for targeted amelioration of fibrosis in pressure overload-induced cardiac hypertrophy. Int J Nanomedicine 15:1321–33. doi: 10.2147/IJN.S235518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiaie N, Emami SH, Rabbani S, et al. (2020). Targeted and controlled drug delivery to a rat model of heart failure through a magnetic nanocomposite. Ann Biomed Eng 48:709–21. doi: 10.1007/s10439-019-02394-y. [DOI] [PubMed] [Google Scholar]
- 96.Naseroleslami M, Aboutaleb N, Parivar K. (2018). The effects of superparamagnetic iron oxide nanoparticles-labeled mesenchymal stem cells in the presence of a magnetic field on attenuation of injury after heart failure. Drug Deliv Transl Res 8:1214–25. doi: 10.1007/s13346-018-0567-8. [DOI] [PubMed] [Google Scholar]
- 97.Yu L, Scherlag BS, Dormer K, et al. (2018). Targeted ganglionated plexi denervation using magnetic nanoparticles carrying calcium chloride payload. JACC Clin Electrophysiol 4:1347–58. doi: 10.1016/j.jacep.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feigin VL, Stark BA, Johnson CO, et al. (2021). Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsivgoulis G, Katsanos AH, Sandset EC, et al. (2023). Thrombolysis for acute ischaemic stroke: current status and future perspectives. Lancet Neurol 22:418–29. doi: 10.1016/S1474-4422(22)00519-1. [DOI] [PubMed] [Google Scholar]
- 100.Ma H, Jiang Z, Xu J, et al. (2021). Targeted nano-delivery strategies for facilitating thrombolysis treatment in ischemic stroke. Drug Deliv 28:357–71. doi: 10.1080/10717544.2021.1879315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grayston A, Zhang Y, Garcia-Gabilondo M, et al. (2022). Endovascular administration of magnetized nanocarriers targeting brain delivery after stroke. J Cereb Blood Flow Metab 42:237–52. doi: 10.1177/0271678X211028816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu X, Zhang Y, Wang L, et al. (2021). Development of L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv 28:380–9. doi: 10.1080/10717544.2021.1883158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Wang J, Hao J, et al. (2021). Guiding drug through interrupted bloodstream for potentiated thrombolysis by C-shaped magnetic actuation system in vivo. Adv Mater 33:e2105351. [DOI] [PubMed] [Google Scholar]
- 104.Chen HA, Ma YH, Hsu TY, Chen JP. (2020). Preparation of peptide and recombinant tissue plasminogen activator conjugated poly (Lactic-Co-Glycolic Acid) (PLGA) magnetic nanoparticles for dual targeted thrombolytic therapy. Int J Mol Sci 21:2690. doi: 10.3390/ijms21082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie M, Zhang W, Fan C, et al. (2020). Bioinspired soft microrobots with precise magneto-collective control for microvascular thrombolysis. Adv Mater 32:e2000366. doi: 10.1002/adma.202000366. [DOI] [PubMed] [Google Scholar]
- 106.Pernal SP, Willis AJ, Sabo ME, et al. (2020). An in vitro model system for evaluating remote magnetic nanoparticle movement and fibrinolysis. Int J Nanomedicine 15:1549–68. doi: 10.2147/IJN.S237395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang S, Guo X, Xiu W, et al. (2020). Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci Adv 6:eaaz8204. doi: 10.1126/sciadv.aaz8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu CH, Hsu HL, Chen JP, et al. (2019). Thrombolysis induced by intravenous administration of plasminogen activator in magnetoliposomes: dual targeting by magnetic and thermal manipulation. Nanomedicine 20:101992. doi: 10.1016/j.nano.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 109.Ouyang H, Zheng Z, Chen Y, et al. (2019). A magnetically modified black phosphorus nanosheet-based heparin delivery platform for preventing DVT accurately. J Mater Chem B 7:6099–108. doi: 10.1039/c9tb01459d. [DOI] [PubMed] [Google Scholar]
- 110.Li J, Zhang Q, Wang W, et al. (2021). Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential. J Neurol 268:4095–107. doi: 10.1007/s00415-020-10138-5. [DOI] [PubMed] [Google Scholar]
- 111.Fischer UM, Harting MT, Jimenez F, et al. (2009). Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18:683–92. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khan H, Pan JJ, Li Y, et al. (2021). Native and bioengineered exosomes for ischemic stroke therapy. Front Cell Dev Biol 9:619565. doi: 10.3389/fcell.2021.619565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun R, Wang X, Nie Y, et al. (2022). Targeted trapping of endogenous endothelial progenitor cells for myocardial ischemic injury repair through neutrophil-mediated SPIO nanoparticle-conjugated CD34 antibody delivery and imaging. Acta Biomater 146:421–33. doi: 10.1016/j.actbio.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 114.Bao S, Lu Y, Zhang J, et al. (2023). Rapid improvement of heart repair in rats after myocardial infarction by precise magnetic stimulation on the vagus nerve with an injectable magnetic hydrogel. Nanoscale 15:3532–41. doi: 10.1039/d2nr05073k. [DOI] [PubMed] [Google Scholar]
- 115.Martin TG, Juarros MA, Leinwand LA. (2023). Regression of cardiac hypertrophy in health and disease: mechanisms and therapeutic potential. Nat Rev Cardiol 20:347–63. doi: 10.1038/s41569-022-00806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Che H, Wang Y, Li H, et al. (2020). Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-beta1/Smads signaling in diabetic cardiomyopathy. Faseb J 34:5282–98. doi: 10.1096/fj.201902692R. [DOI] [PubMed] [Google Scholar]
- 117.Liu J, Zhuang T, Pi J, et al. (2019). Endothelial forkhead box transcription factor P1 regulates pathological cardiac remodeling through transforming growth factor-beta1-endothelin-1 signal pathway. Circulation 140:665–80. doi: 10.1161/CIRCULATIONAHA.119.039767. [DOI] [PubMed] [Google Scholar]
- 118.Li H, Wang Y, Liu J, et al. (2021). Endothelial Klf2-Foxp1-TGFbeta signal mediates the inhibitory effects of simvastatin on maladaptive cardiac remodeling. Theranostics 11:1609–25. doi: 10.7150/thno.48153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roger VL. (2021). Epidemiology of heart failure: a contemporary perspective. Circ Res 128:1421–34. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 120.Lazzarin T, Tonon CR, Martins D, et al. (2022). Post-cardiac arrest: mechanisms, management, and future perspectives. J Clin Med 12:259. doi: 10.3390/jcm12010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xanthos T, Chatzigeorgiou M, Johnson EO, Chalkias A. (2012). Magnetically targeted drug delivery during cardiopulmonary resuscitation and the post-resuscitation period. Resuscitation 83:803–5. doi: 10.1016/j.resuscitation.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, Liu H, Lu Y, et al. (2019). Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia-reperfusion injury. Int J Nanomedicine 14:875–88. doi: 10.2147/IJN.S186225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun X, Wang Y, Wen S, et al. (2021). Novel controlled and targeted releasing hydrogen sulfide system exerts combinational cerebral and myocardial protection after cardiac arrest. J Nanobiotechnology 19:40. doi: 10.1186/s12951-021-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang K, Wen S, Wang W, et al. (2021). Erythrocyte membrane coated nanoparticle-based control releasing hydrogen sulfide system protects ischemic myocardium. Nanomedicine (Lond) 16:465–80. doi: 10.2217/nnm-2020-0404. [DOI] [PubMed] [Google Scholar]
- 125.Brundel B, Ai X, Hills MT, et al. (2022). Atrial fibrillation. Nat Rev Dis Primers 8:21. doi: 10.1038/s41572-022-00347-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.