Abstract
The hypothalamic-pituitary-adrenal (HPA) axis activity has been demonstrated as one of the physiological mechanisms underlying the long-lasting effects of peer victimization on physical and mental health. However, the mechanisms linking peer victimization to dysregulations of HPA axis activity remain inadequately understood. The present study examined the potential mediating role of emotional regulation in the association between peer victimization and HPA axis activity in a large community-based sample of 645 children affected by parental HIV (, ranging from 8 to 15 years old). The three-level growth curve model revealed that higher peer victimization was associated with lower emotional regulation, which in turn was related to lower cortisol at awakening and more blunted diurnal slopes in girls, but not in boys. The findings highlight the protective effect of emotional regulation in relation to HPA axis activity in victimized children, particularly in girls.
Keywords: Peer victimization, Emotional regulation, Diurnal cortisol, Gender differences, Mediation
1. Introduction
Peer victimization is a wide-spread issue among school-aged children. Previous research estimated that approximately 70% of school children were targets of peer victimization (Finkelhor et al., 2009), with 10% experiencing frequent peer victimization (Reijntjes et al., 2010). Notably, peer victimization is a potent risk factor for poor physical and mental health during childhood and adolescence (Cluver et al., 2010; Hager and Leadbeater, 2016). The effect of childhood peer victimization on health may even persist through adulthood (Wolke et al., 2013). An underlying mechanism explaining the far-reaching effect of peer victimization on health is the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis activity (McEwen, 1998, 2008; Vaillancourt et al., 2013). However, there is a dearth of research on exploring potential psychological factors, such as emotional regulation, underlying the effect of peer victimization on HPA axis activity.
As a source of acute and chronic stress, peer victimization is linked to the activation of HPA axis with the production of cortisol (Vaillancourt et al., 2013). Cortisol concentration follows a circadian rhythm: it is high at awakening, increases to a peak about 30 min after awakening (cortisol awakening response, CAR), and then decreases throughout the day. In the short term, the activation of HPA axis is adaptive and increases cortisol secretion to mobilize energy and to facilitate the physiological responses to stress (Susman, 2006). In the long term, however, the repeated activation of HPA axis may be dysfunctional, exhibiting delayed shutting down after stress or inadequate cortisol responses to stress, and finally may lead to poor physical and mental health (McEwen, 1998; McEwen and Stellar, 1993). Evidence showed that blunted diurnal slopes (less decline throughout the day) were consistently linked to poor health (Adam et al., 2017), though less consistent associations were found between morning cortisol (e.g., cortisol at awakening, CAR) and health outcomes (Hartman et al., 2013).
Studies have shown that peer victimization is associated with dysregulations of HPA axis activity (Calhoun et al., 2014; Kliewer, 2006, 2016; Knack et al., 2011; Ouellet-Morin et al., 2011b; Vaillancourt et al., 2008). Low morning cortisol and a blunted CAR were observed in victimized children (Kliewer, 2006; Knack et al., 2011). Victimized children were also found to exhibit a blunted cortisol response to stress (Calhoun et al., 2014; Kliewer, 2016; Ouellet-Morin et al., 2011b). The association between peer victimization and the hypoactivity of HPA axis may reflect a down-regulation of HPA axis activity resulting from repeated HPA axis activation (Miller et al., 2007). A discordant monozygotic twin study showed that this down-regulation of HPA axis activity among victimized children might not be totally attributed to genetic factors (Ouellet-Morin et al., 2011a).
Identifying potential mechanisms for the effect of peer victimization on dysregulations of HPA axis activity is important to inform preventive interventions aiming at alleviating the disruptive effect of peer victimization on stress response system. One potential mechanism is emotional regulation proposed by Taylor et al. (2011) in the developmental model of stress-related health. Emotional regulation is defined as “the ability to manage and modify one’s emotional reactions to achieve goal-directed outcomes” (Matsumoto, 2006, p.421). As suggested by Taylor et al. (2011), emotional regulation may be a key psychological mechanism linking early adversity to stress response system and subsequent physical and mental health. Deficits in emotional regulation, which represent the lack of the ability to regulate the intensity of negative emotions and to shift emotional states associated with stress, have been found to mediate the association between peer victimization and mental health (McLaughlin et al., 2009).
Existing literature suggests that emotional regulation may mediate the association between peer victimization and dysregulations of HPA axis activity (Cărnuţă et al., 2015). Different forms of victimization, including peer victimization, abuse, and violence exposure, were found to be related to deficits in emotional regulation, both concurrently and longitudinally (Herts et al., 2012; Kim and Cicchetti, 2010; Maughan and Cicchetti, 2002; McLaughlin et al., 2009; Schwartz and Proctor, 2000). Deficits in emotional regulation, in turn, contribute to dysregulations in HPA axis activity (Cărnuţă et al., 2015; Kliewer et al., 2016). For example, Kliewer et al. (2016) found that emotional dysregulation among adolescents prospectively predicted blunted anticipatory cortisol over one year. In addition, Kliewer and colleagues found that such an association was only observed in girls. Similarly, associations between emotional dysregulation and mental health problems were found to be more evident in girls than in boys (Thayer et al., 2003). These results might suggest possible gender differences in the association between emotional regulation and health-related outcomes (Nolen-Hoeksema, 2012). However, another study found no gender differences in the association between emotional dysregulation and internalizing symptoms among adolescents (McLaughlin et al., 2009).
The current study aimed to examine the mediating role of emotional regulation in the associations between peer victimization and three parameters of diurnal cortisol rhythm including cortisol at awakening, CAR, and diurnal slopes, among a community-based sample of children affected by parental HIV. Children affected by parental HIV refer to children who lose one or both parents to AIDS or children who live with one or both HIV positive parents (Chi and Li, 2013). As of 2016, about 16.5 million children of 0–17 years old lost one or both parents to AIDS worldwide and millions more were living with HIV positive parents (UNICEF, 2016). These children are vulnerable to peer victimization, as they are likely to live in communities characterized by poverty and to be stigmatized due to parental HIV (Ainsworth and Filmer, 2006; Cluver et al., 2010). Furthermore, we examined whether there were gender differences in the mediating effect of emotional regulation in the association between peer victimization and diurnal cortisol rhythm. We hypothesized that peer victimization would be associated with a hypoactive diurnal cortisol pattern and such an association would be mediated by emotional regulation. We also hypothesized that gender would moderate the mediating effect of emotional regulation in the association between peer victimization and diurnal cortisol rhythm, such that an association between low emotional regulation and a hypoactive diurnal cortisol pattern would be more evident in girls than in boys.
2. Method
2.1. Participants
Data for this study were derived from the baseline assessment of a randomized controlled trial of psychological intervention among 790 children (6 to 17 years old) affected by parental HIV from a rural county in China, where many residents were infected with HIV through unhygienic blood-collection practices. A subsample of 746 children aged from 8 to 15 years were chosen to match the age range for which self-report measures used in present analyses were normed (Slatcher et al., 2015). Of 746 children, 645 (86.4%) children (335 boys, , ) provided valid saliva samples for cortisol analyses, therefore constituting the final sample for the current study. Among the study sample, approximately 12% lost at least one biological parent to AIDS and about 88% had a household income under 2000 Yuan (approximately USD 286) per month. About 94% of children’s caregivers had less than high school education.
2.2. Procedure
Participating children and their caregivers were randomly selected from a list of families caring for children affected by parental HIV in targeted communities. Children with known HIV infection were not included in the intervention trial. Children and their caregivers were instructed to complete the survey in Chinese, including several psychosocial scales and demographic information. For psychosocial measures that were available in English only, they were translated from English to Chinese by English-Chinese bilingual research team members. The translation was then back-translated into English to ensure the similarity in the meaning of items between this back-translation version and the original version. Children and caregivers completed the questionnaires individually or in a small group with the presence of two interviewers. For children who had reading difficulties, the interviewers read the items to them and recorded children’s oral responses on the survey. Also, children were instructed to collect their saliva samples four times a day for three consecutive days. They were also asked to complete a daily dairy during the period of saliva collection. At the completion of the survey, each child received an age-appropriate gift (toys or school supplies) for his/her participation. Appropriate informed consent from caregivers and children was obtained before participation, and the approval for the study protocol was obtained from the Institutional Review Boards at Wayne State University in the United States and Henan University in China.
2.3. Measures
2.3.1. Peer victimization
Peer victimization was measured using five items from a 14-item scale measuring children’s experience about victimization and harassment (Zhao et al., 2010). Children were asked to report how often each of the listed events, including “being beaten by other kids”, “being called names”, “being teased or picked on by other kids”, “peers do not play with me anymore”, and “my friends do not play with me anymore” happened to them during the previous six months on a 5-point scale (from 1 = never happened to 5 = always happened). The mean scores of all items were calculated, with higher scores reflecting higher levels of peer victimization. Cronbach alpha for this scale was 0.74. Construct validity was established by the significant correlation between peer victimization and depression (, ), with depression being measured by a short version of the Center for Epidemiologic Studies Depression Scale for children (Fendrich et al., 1990).
2.3.2. Emotional regulation
Emotional regulation was measured using a 6-item emotional regulation subscale from the Social Competence Scale (Corrigan, 2002). Children were asked to report how well each of the following statements described themselves on a 4-point scale (from 1 = not at all to 4 = very well): “I can accept things not going my way”, “I can cope well with failure”, “I think before acting”, “I can calm down when excited”, “I do what told to do”, and “I can control temper when disagreement”. The mean scores of all items were calculated, with higher scores reflecting greater emotional regulation ability. Cronbach alpha for this subscale was 0.63. Construct validity was established by significant correlations between emotional regulation and negative and positive affect (, ; , , respectively), with the latter being measured by the Positive and Negative Affect Schedule Scale (Watson et al., 1988).
2.3.3. Family socioeconomic status (SES)
Children’s family SES was estimated based on caregiver education and family income. Caregiver education was measured by caregivers’ reports of their education levels on a 5-point scale (from 1 = no schooling to 5 = college or above). Family income was measured by caregiver’ reports of their family monthly income on a 6-point scale (from = “0–999 Chinese Yuan” to 6 = “more than 5000 Chinese Yuan”). A family SES index was created by Z-scoring caregiver education and family income. The sum scores of the two z-scores were calculated, with higher scores reflecting higher family SES.
2.3.4. Daily sleep quality
Daily sleep quality was measured by asking children the quality of the prior nights’ sleep on a 4-point scale (from 1 = terrible to 4 = great) on each morning of saliva sample collection.
2.3.5. Perceived health status
Perceived health status was measured by children’s self-report of their overall health status on a 5-point scale (from 1 = very poor to 5 = very good).
2.3.6. Additional covariates
Additional covariates included age, gender (0 = boy, 1 = girl), wakeup time on the day of saliva collection, and day of the week for saliva collection (0 = weekday, 1 = weekend). Children were also asked whether they smoked (0 = no, 1 = yes) or did any sport exercise (0 = no, 1 = yes) prior to each time point of saliva sample collection.
2.3.7. Salivary cortisol
Children were instructed to self-collect saliva samples using Salivettes (Sarstedt, Rommelsdorft, Germany) four times a day from Thursday to Saturday based on the following schedule: immediately upon awakening (prior to any eating, drinking, or exercise), 30 min after awakening, 1 h before dinner-time, and immediately before bedtime. Prior to the start of saliva sample collection, the correct procedure of saliva sample collection using Salivettes was graphically shown to children, and specifically designed wristbands with a reminder were provided to remind children of saliva collection. The importance of the compliance with the schedule of sample collection was emphasized. Salivettes were stored at room temperature before being collected by researchers on the following Monday. Previous study has shown that cortisol concentrations are not adversely impacted when Salivettes are stored at room temperature for up to two weeks (Garde and Hansen, 2005). Salivettes then were refrigerated in the lab until being assayed. Cortisol concentrations were estimated in singles using chemiluminescent immunoassay (Assess cortisol kit YZB/USA 2802; Beckman Coulter, Inc, Fullerton, CA) with 8.4% and 11.4% of intra- and inter-assay coefficients of variation, respectively, reported by the manufacturer. Of the 645 children, 61.3% provided 12 saliva samples, and 96% provided at least 8 saliva samples across the 3 days. Of 1810 available CAR cortisol values, 525 deviated by 10 min or more from the requested 30-minute interval, and these cortisol values were dropped from the analysis. Cortisol values above or below 3 standardized deviations (SDs) were winsorized by replacing the value at 3 SDs within each time point (33 cortisol values were affected). Raw cortisol values were natural log-transformed to correct for positive skewness. In addition, we added a constant of 1 before the transformation to ensure that all transformed values were positive.
2.4. Data analyses
A three-level growth curve modeling approach was performed to test the hypotheses. This approach can model the nested structure of the data (cortisol data nested within days, days nested within individuals), as well as estimate the effect of predictors at Level 1 (cortisol-level), Level 2 (day-level), and Level 3 (person-level) on diurnal cortisol rhythm. At Level 1, the decline of diurnal cortisol level was modeled by regressing cortisol on time of saliva sample collection. Time was centered as hours since awakening (e.g., at awakening = 0), so that the intercept reflected the cortisol level at awakening. A quadratic time term (time since awakening squared) was also included in the model to assess the curvilinear effect of time since awakening (Adam and Kumari, 2009). To model the size of CAR, a dummy variable (30-minute sample = 1, other samples = 0) was added (see Eq. (1)). At Level 2, the effects of variables at the day-level (e.g., wakeup time and day of the week) on diurnal cortisol parameters were estimated (see Eqs. (2)–(4)). At Level 3, the effects of variables at the person-level (e.g., peer victimization and emotional regulation) on diurnal cortisol parameters were estimated (see Eqs. (5)–(7)). The intercept and linear slope of diurnal cortisol and the size of CAR were randomly estimated at Level 2 and Level 3. The curvilinear effect of time since awakening was fixed at Level 1 without predictors at Level 2 or Level 3. Continuous variables were mean-centered before calculating interaction terms. All continuous variables at Level 2 and Level 3 were grand-mean centered (Enders and Tofighi, 2007). All models were analyzed with Mplus 7.0 using maximum likelihood with robust standard errors, as well as dealing with missing data (Muthén and Muthén, 2012).
Level 1:
| (1) |
Level 2:
| (2) |
| (3) |
| (4) |
Level 3:
| (5) |
| (6) |
| (7) |
A sequence of five models was performed to test our hypotheses. First, an unconditional three-level growth curve model (Model 1) was analyzed to assess the average diurnal cortisol pattern across all participants. Second, some potential covariates identified in existing diurnal cortisol studies (Adam and Kumari, 2009), including sport exercise and smoking prior saliva sample collection, daily wakeup time, day of the week, daily sleep quality, gender, age, family SES, and perceived health status were added separately to Model 1 to examine whether each covariate had significant effects on diurnal cortisol (Model 2). To preserve model parsimony, covariates were dropped from the subsequent analyses if they had no significant effects on cortisol levels or diurnal cortisol parameters. Third, main effects of peer victimization were estimated by adding peer victimization as a predictor of diurnal cortisol parameters (Model 3). Fourth, the procedure recommended by Preacher et al. (2011) was used to examine the mediating effect of emotional regulation in the association between peer victimization and diurnal cortisol rhythm (Model 4). Sobel test was applied to test the significance of the mediation effect (Sobel, 1982). Finally, the procedure recommended by Preacher et al. (2007) was used to conduct the moderated mediation analysis (Model 5) in which we examined whether gender would moderate the hypothesized mediation model (see Fig. 1). Simple slope analyses were performed to probe significant interactions, and conditional indirect effects were estimated for boys and girls.
Fig. 1.
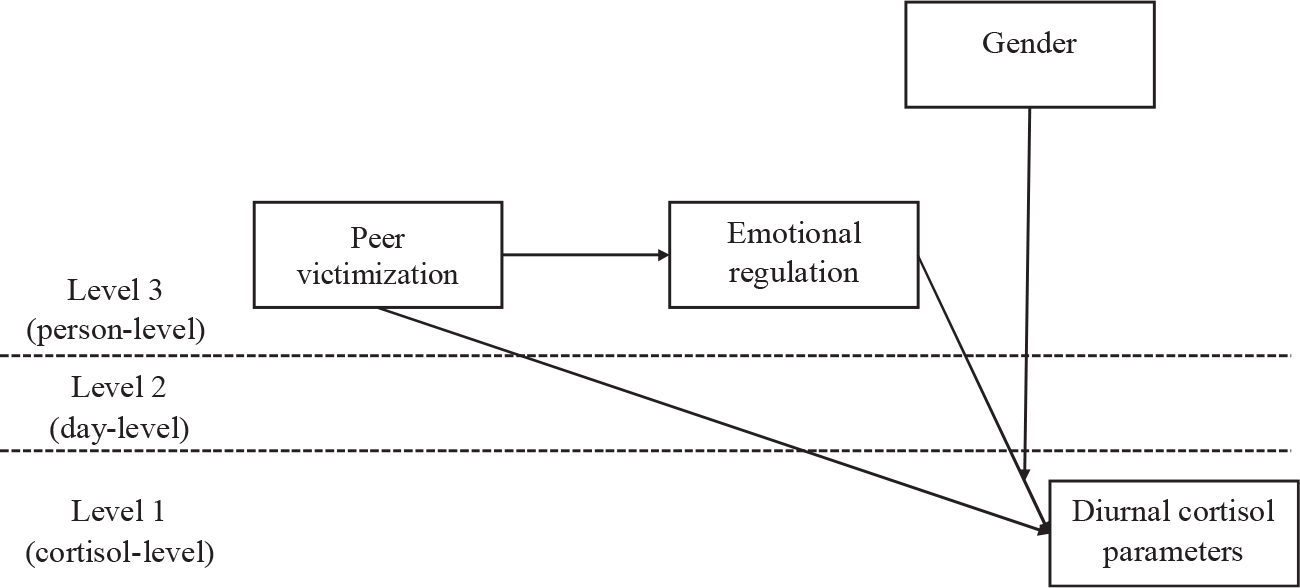
The hypothetical moderated mediation model (Model 5). Covariates were controlled but not displayed for model simplification. Gender was dummy coded with 0 = boy and 1 = girl.
To examine the robustness of the hypothesized mediation model, supplemental analyses were preformed to test two alternative multilevel mediation models. The first alternative model was to test whether diurnal cortisol would have an indirect effect on peer victimization via emotional regulation; the second alternative model was to test whether peer victimization would have an indirect effect on emotional regulation via diurnal cortisol.
3. Results
3.1. Preliminary analyses
Results from tests for continuous variables and chi-square tests for categorical variables showed that children who provided valid saliva samples did not significantly differ from those who were excluded from the current analyses because of lack of saliva samples in terms of peer victimization, emotional regulation, and person-level covariates (all ).
Table 1 displayed descriptive statistics of person-level variables by gender. Results showed that boys reported higher levels of peer victimization than girls , but reported lower levels of emotional regulation than girls . Table 2 displayed correlations between person-level variables by gender. For the entire sample, higher peer victimization was correlated with lower emotional regulation . Age was negatively correlated with peer victimization , indicating that younger children were more likely than older children to report peer victimization.
Table 1.
The mean and standard deviation of person-level variables by gender.
| Variables | Total | Skewness | Kurtosis | Boys | Girls | t | P |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age | 10.67(1.79) | 0.32 | −0.69 | 10.82(1.78) | 10.51(1.78) | 2.22 | .027 |
| Family SES | 0.01(1.54) | 1.06 | 2.22 | −0.04(1.43) | 0.06(1.65) | −0.75 | .452 |
| Perceived health status | 4.25(1.06) | −1.36 | 1.19 | 4.20(1.08) | 4.30(1.04) | −1.16 | .246 |
| Peer victimization | 1.82(0.73) | 1.01 | 0.92 | 1.90(0.72) | 1.73(0.73) | 2.85 | .005 |
| Emotional regulation | 2.74(0.56) | −0.21 | −0.01 | 2.68(0.57) | 2.80(0.55) | −2.73 | .007 |
Note. SES=socioeconomic status.
Table 2.
Correlations between person-level variables by gender.
| Variables | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
|
| |||||
| Age | - | −.07 | −.10 | −.14* | .09 |
| Family SES | .06 | - | −.02 | .02 | .01 |
| Perceived health status | −.11* | −.01 | - | −.12* | .11* |
| Peer victimization | −.06 | −.08 | .01 | - | −.13* |
| Emotional regulation | .04 | −.03 | .02 | −.08 | - |
Note: Data for boys were presented below the diagonal, and data for girls were presented above the diagonal. SES=socioeconomic status.
p < .05.
3.2. Average diurnal cortisol rhythm and covariates
Results from Model 1 indicated that children’s cortisol value was high at awakening , with a nonsignificant increase from awakening to 30 min later and a substantial decrease throughout the day . Results from Model 2 showed no significant effects of daily sleep quality, family SES, and perceived health status on cortisol at awakening, CAR, or diurnal slopes (all ). Therefore, these three variables were removed from the subsequent data analyses. Sport exercise and smoking were associated with higher cortisol levels ; respectively). Day of the week (weekday vs. weekend) was significantly related to three diurnal cortisol parameters for cortisol at awakening; for CAR; and for diurnal slopes). Wake-up time was associated with cortisol at awakening and ; respectively), but not with diurnal slopes . Gender and age were related to the size of CAR ; respectively). Neither gender nor age was associated with cortisol at awakening or diurnal slopes (all ). Therefore, sport exercise and smoking were included as covariates at Level 1; day of the week and wake-up time were included as covariates at Level 2; and gender and age were included as covariates at Level 3.
3.3. The effects of peer victimization and emotional regulation on diurnal cortisol parameters
Results from Model 3 showed that higher peer victimization was related to lower cortisol at awakening and more blunted diurnal respectively, see Table 3, Model 3). No significant relationship was found between peer victimization and CAR . The effect of peer victimization on diurnal cortisol rhythm was also graphically depicted in Fig. 2.
Table 3.
Results of multilevel modeling predicting diurnal cortisol rhythm.
| Fixed effects | Model 3 |
Model 4 |
Model 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | p | Coefficient | SE | p | Coefficient | SE | p | |
|
| |||||||||
| Morning cortisol, | |||||||||
| Average morning cortisol, , | 0.772 | 0.040 | < .001 | 0.764 | 0.040 | < .001 | 0.773 | 0.040 | < .001 |
| Gender, | −0.006 | 0.013 | .655 | −0.010 | 0.013 | .424 | −0.011 | 0.013 | .372 |
| Age, | 0.005 | 0.004 | .168 | 0.004 | 0.004 | .227 | 0.004 | 0.004 | .281 |
| Peer victimization, | −0.027 | 0.009 | .002 | −0.024 | 0.009 | .006 | −0.023 | 0.009 | .007 |
| Emotional regulation, | - | - | - | 0.038 | 0.011 | .001 | 0.007 | 0.014 | .596 |
| Emotional regulation × gender, | - | - | - | - | - | - | 0.067 | 0.022 | .002 |
| Day of the week, , | −0.101 | 0.010 | < .001 | −0.102 | 0.010 | < .001 | −0.101 | 0.010 | < .001 |
| Wake-up time, , | −0.012 | 0.006 | .054 | −0.011 | 0.006 | .093 | −0.012 | 0.006 | .059 |
| Cortisol awakening response (CAR), | |||||||||
| Average CAR, , | 0.060 | 0.046 | .193 | 0.062 | 0.046 | .183 | 0.060 | 0.047 | .199 |
| Gender, | −0.029 | 0.013 | .024 | −0.028 | 0.013 | .030 | −0.029 | 0.013 | .028 |
| Age, | 0.007 | 0.003 | .044 | 0.007 | 0.003 | .043 | 0.007 | 0.003 | .042 |
| Peer victimization, | 0.004 | 0.009 | .643 | 0.003 | 0.009 | .730 | 0.003 | 0.009 | .743 |
| Emotional regulation, | − | - | - | −0.009 | 0.010 | .411 | −0.010 | 0.014 | .477 |
| Emotional regulation × gender, | - | - | - | - | - | - | 0.003 | 0.021 | .904 |
| Day of the week, , | −0.028 | 0.013 | .030 | −0.028 | 0.013 | .033 | −0.028 | 0.013 | .028 |
| Wake-up time, , | −0.006 | 0.007 | .393 | −0.007 | 0.007 | .370 | −0.006 | 0.007 | .396 |
| Time since awakening, | |||||||||
| Average linear slope, , | −0.035 | 0.005 | < .001 | −0.034 | 0.004 | < .001 | −0.035 | 0.005 | < .001 |
| Gender, | −0.001 | 0.001 | .346 | −0.001 | 0.001 | .523 | −0.001 | 0.001 | .577 |
| Age, | 0.000 | 0.000 | .242 | 0.000 | 0.000 | .307 | 0.000 | 0.000 | .373 |
| Peer victimization, | 0.002 | 0.001 | .007 | 0.002 | 0.001 | .014 | 0.002 | 0.001 | .017 |
| Emotional regulation, | - | - | - | −0.003 | 0.001 | .006 | −0.001 | 0.001 | .496 |
| Emotional regulation × gender, | - | - | - | - | - | - | −0.004 | 0.002 | .041 |
| Day of the week, , | 0.007 | 0.001 | < .001 | 0.007 | 0.001 | < .001 | 0.007 | 0.001 | < .001 |
| Wake-up time, , | −0.001 | 0.001 | .244 | −0.001 | 0.001 | .168 | −0.001 | 0.001 | .284 |
| Time since awakening2, | |||||||||
| Average curvature, | 0.001 | 0.000 | < .001 | 0.001 | 0.000 | < .001 | 0.001 | 0.000 | < .001 |
| Sport Exercise, | |||||||||
| Intercept, | 0.017 | 0.008 | .030 | 0.017 | 0.008 | .021 | 0.017 | 0.008 | .023 |
| Smoking, | |||||||||
| Intercept, | 0.148 | 0.048 | .002 | 0.148 | 0.048 | .002 | 0.149 | 0.048 | .002 |
Note: The average morning cortisol indicates the average cortisol value at awakening; the average cortisol awakening response (CAR) indicates the change in cortisol during the 30 min after awakening; the average slopes of time since awakening indicate changes in cortisol values per 1-hour change in time; the average slopes of time since awakening2 indicate changes in cortisol values per 1-hour change in time2. Estimates were unstandardized regression coefficients. Gender was dummy coded with 0=boy and 1=girl; day of the week was dummy coded with 0=weekday and 1=weekend; Sport exercises was dummy coded with 0=no and 1=yes; smoking was dummy coded with 0=no and 1=yes.
Fig. 2.
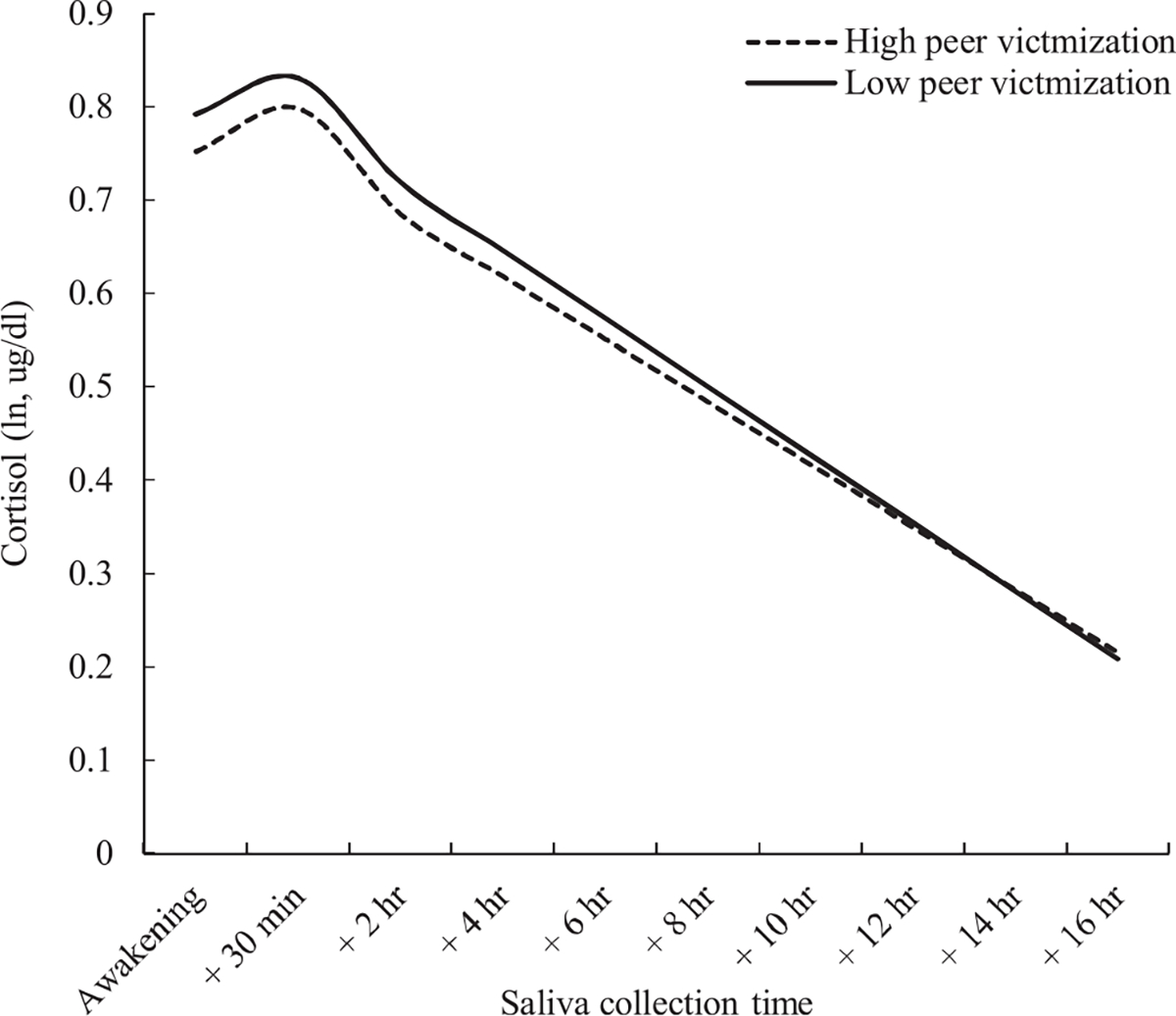
The effect of peer victimization on diurnal cortisol. Cortisol is depicted as a function of time since awakening at different levels of peer victimization.
Note. High and low levels of peer victimization represent on 1 standard deviation above and below the mean, respectively.
Results from Model 4 showed that higher peer victimization was related to lower emotional regulation . Lower emotional regulation, in turn, was associated with lower cortisol at awakening and more blunted diurnal slopes , respectively, see Table 3, Model 4), but not with CAR . A significant indirect effect was observed from peer victimization to cortisol at awakening through emotional regulation (unstandardized indirect effect = −0.00335, Sobel confidence interval (CI) = [−0.00648, −.00023]). However, the indirect effect from peer victimization to diurnal slopes through emotional regulation was not significant (unstandardized indirect effect = 0.00024, Sobel , 95%CI = [−0.00001, 0.00050]). Main effects of peer victimization on cortisol at awakening and diurnal slopes were reduced but remained significant.
Results from alternative mediation models did not show significant indirect effects from diurnal cortisol parameters to peer victimization via emotional regulation, or significant indirect effects from peer victimization to emotional regulations via diurnal cortisol parameters (all ), with an exception that there was a significant indirect effect from peer victimization to emotional regulation via cortisol at awakening (Sobel ).
3.4. Gender differences in the mediating effects of emotional regulation
Results from Model 5 showed significant interactive effects of emotional regulation and gender on cortisol at awakening and diurnal cortisol slopes , respectively, see, Table 3, Model 5), but not on CAR . Simple slope analyses were performed to interpret the interactive effects of emotional regulation and gender on cortisol at awakening and diurnal slopes. Cortisol at awakening and diurnal slopes were estimated by gender at one standard deviation (SD) above and one SD below the mean of emotional regulation (−0.56 and 0.56, respectively). Results showed that lower emotional regulation was related to lower cortisol awakening in girls (, see Fig. 3a), but not in boys . Similarly, lower emotional regulation was related to more blunted diurnal slopes in girls , see Fig. 3b), but not in boys . There was a significant conditional indirect effect of peer victimization on cortisol at awakening through emotional regulation in girls (unstandardized indirect effect = −0.00656, Sobel , , , but not in boys (unstandardized indirect effect = −0.00066, Sobel , , . A significant conditional indirect effect from peer victimization to diurnal slopes through emotional regulation was also found in girls (unstandardized indirect effect = 0.00044, Sobel , , , but not in boys (unstandardized indirect effect = 0.00008, Sobel , , .
Fig. 3.
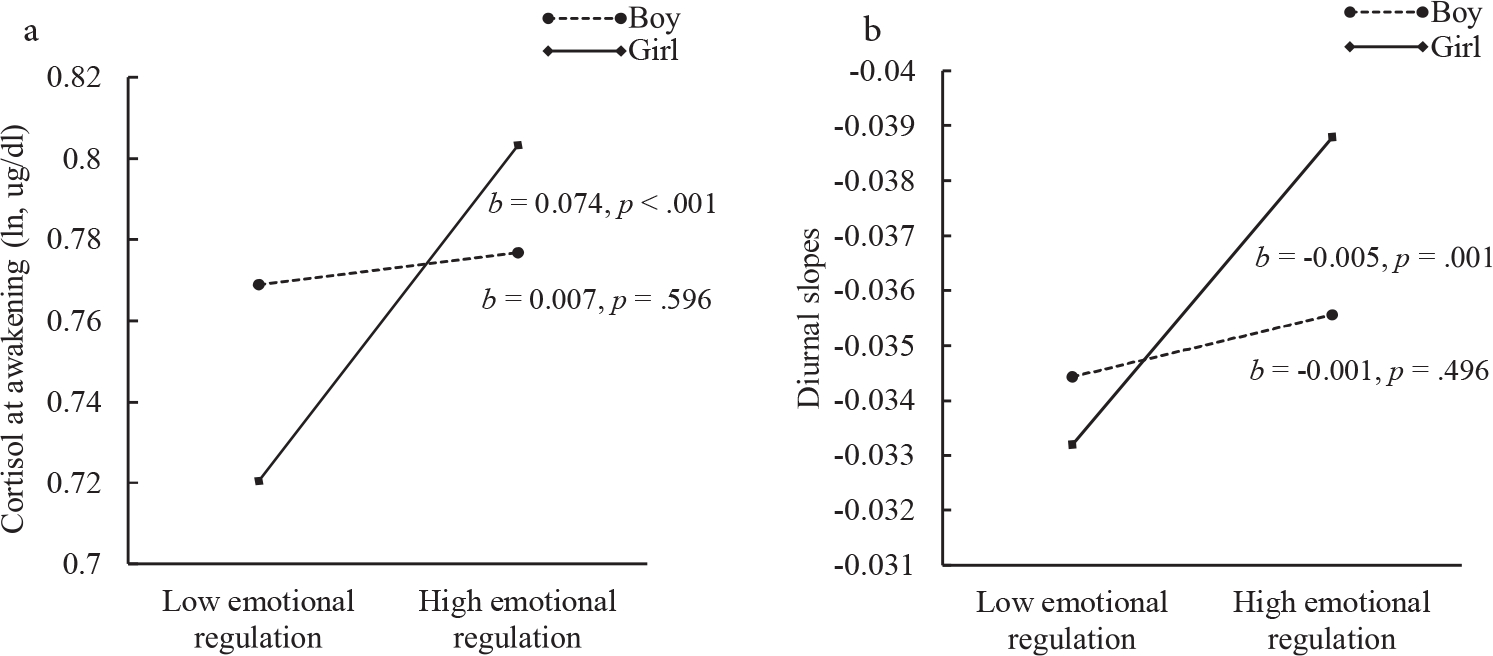
(a) represents simple slopes of cortisol at awakening at different levels of emotional regulation for boys and girls. (b) represents simple slopes of diurnal slopes at different levels of emotional regulation for boys and girl.
Note. High and low levels of emotional regulation represent on 1 standard deviation above and below the mean, respectively.
4. Discussion
The present study extends prior work by illustrating the potential mediating role of emotional regulation in the association between peer victimization and diurnal cortisol rhythm among children affected by parental HIV, an understudied population in the literature of peer victimization. Results showed that peer victimization was associated with low emotional regulation, which in turn was associated with a hypoactive diurnal cortisol pattern. Moreover, this study suggested gender differences underlying this process. A significant indirect effect from peer victimization to diurnal cortisol rhythm via emotional regulation was found in girls, but not in boys.
As hypothesized, our results showed that emotional regulation might be a potential psychological mechanism underlying the negative effect of peer victimization on the HPA axis activity. We found that higher peer victimization was associated with lower emotional regulation. Lower emotional regulation, in turn, was associated with lower cortisol at awakening and more blunted diurnal slopes. These findings are consistent with existing theoretical model linking early adversity to stress response system (Taylor et al., 2011), as well as previous research suggesting the mediating effect of emotional regulation on the association between stress and subsequent health outcomes (Hatzenbuehler et al., 2009; Kim and Cicchetti, 2010; McLaughlin et al., 2009). Other possible pathways may exist among peer victimization, HPA axis activity, emotional regulation. However, our results from alternative mediation models did not strongly support such possibilities. A hypoactive diurnal cortisol pattern among children with low emotional regulation found in our study is also consistent with recently published work indicating hypoactive cortisol reactivity among adults with emotional dysregulation (Cărnuţă et al., 2015; Kliewer et al., 2016). It is likely, as McLaughlin et al. (2009) suggested, that dealing with peer victimization and its associated emotional arousal may deplete children’s self-control and coping resources, which may eventually lead to the decrease in children’ emotional regulation ability. Deficits in emotional regulation ability, in turn, are associated with intensifying and ruminating negative affect aroused by stress, resulting in prolonged negative emotion and ultimately dysregulations in HPA axis activity (Gross and Jazaieri, 2014; Quirin et al., 2011; Taylor et al., 2011).
It should be noted, however, that direct effects of peer victimization on low cortisol at awakening and blunted diurnal slopes remained significant after controlling for the effect of emotional regulation. As hypothesized, the findings suggest that children, especially those vulnerable to peer victimization, may perceive peer victimization as a chronic stressor, which in turn contributes to down-regulation of HPA axis activity (Miller et al., 2007) and ultimately leads to poor physical and mental health (McEwen, 1998, 2008). Particularly, a significant association between peer victimization and blunted diurnal slopes observing in this study continuously underscores the importance of HPA axis in understanding the long-lasting effect of peer victimization on health (Vaillancourt et al., 2013). The findings also suggest that there might be other potential mechanisms linking peer victimization to HPA axis activity. One possible mechanism is self-esteem. Low self-esteem is common among victimized children (Overbeek et al., 2010), and low self-esteem has been documented to be related to dysregulations of HPA axis activity (Pruessner et al., 1999). Another possible mechanism is depressive symptoms. For example, a longitudinal study showed that peer victimization was associated with low morning and evening cortisol over 1-year period via increased depressive symptoms among children 12 years of age (Vaillancourt et al., 2011).
We found that lower emotional regulation was associated with a more hypoactive diurnal cortisol pattern in girls, and emotional regulation partially mediated the associations between peer victimization and diurnal cortisol. Conversely, no association between emotional regulation and diurnal cortisol rhythm was found in boys. The findings were not surprising, given the prior study observing an association between emotional dysregulation and blunted anticipatory cortisol in adolescent girls only (Kliewer et al., 2016), as well as the studies indicating a stronger effect of emotional regulation on mental health in girls than in boys (e.g., Bender et al., 2012; Thayer et al., 2003). One possible explanation for these findings might be the gender difference in the strategies involving in emotional regulation. It has been argued that females are more likely than males to report higher emotional awareness and rumination during stressful situations (Thayer et al., 2003). When emotional regulation deficits occur, girls might be more likely than boys to experience negative emotions but less likely than boys to use antirumination strategies, resulting in a greater effect on HPA axis activity in girls than in boys. However, an overall measure of emotional regulation ability rather than specific emotional regulation strategies in this study limits our ability to examine this potential explanation. Another explanation is that some unmeasured variables, such as sex hormones, may play a substantial role in HPA axis activity. It has been suggested that females might be more vulnerable to social instability stress than males (McCormick and Mathews, 2007).
This study did not confirm significant effects of peer victimization on CAR, which was inconsistent with prior study observing a smaller CAR in victimized children than in non-victimized children (Knack et al., 2011). While unexpected, our finding was not surprising, as the CAR literature is notoriously inconsistent with regard to associations with psychosocial factors (Chida and Steptoe, 2009; Clow et al., 2010). One potential explanation is that the current sample has different characteristics from the sample of Knack et al. (2011) which was composed of general school-aged children in the United States. It is likely that some other factors (e.g., parental HIV, chronic poverty), rather than peer victimization examined in this study, exerted a powerful influence on CAR. Another alternative explanation is the potential underestimation of CAR due to inaccurate reports of the timing of saliva collection in the current study. Although prior studies suggested that self-reports of time of awakening were reasonably accurate, inaccurate waketime reporting might occur and contribute to an underestimation of CAR, which makes the effect of peer victimization undetectable (DeSantis et al., 2010). However, both explanations need to be validated in future studies.
Identifying the adverse effect of peer victimization on HPA axis activity and the potential role of emotional regulation underlying such effect has important implications for developing health promotion interventions targeting children with victimization-related HPA axis dysregulations, especially children affected by parental HIV who are at high risk of peer victimization. Our findings suggest that targeting emotional regulation skills might be a promising strategy to promote HPA axis regulation among children affected by parental HIV. Indeed, previous studies have showed that emotional regulation and coping trainings are promising practices to promote neuroendocrine hormone regulation among people living with HIV (for a review, see Carrico and Antoni, 2008).
4.1. Limitations
The findings of the current study, however, should be cautiously interpreted because of the following limitations. First, the nature of cross-sectional data limits the interpretation of causal relationships among peer victimization, emotional regulation, and disruptions in diurnal cortisol rhythm. Future longitudinal data analyses are needed to examine how the link from peer victimization to diurnal cortisol through emotional regulation unfolds over time. Second, cortisol was assayed in singles in this study due to a large sample size and limited resources. The intra-and inter-assay precision for cortisol relied on the data provided by the manufacturer. Third, an overall measure of emotional regulation ability limits the understanding about the roles of different processes of emotional regulation in the association between peer victimization and diurnal cortisol rhythm. For example, Cărnuţă et al. (2015) found that difficulties in emotional response acceptance, but not difficulties in other processes of emotional regulation, such as emotional clarity, was a significant mediator underlying the effect of stressful life events on blunted cortisol reactivity. Future studies should examine whether different processes of emotional regulation may have different effects on HPA axis activity.
Fourth, the data on children’s pubertal stages were not available in this current study. Children in our sample might be at different stages of pubertal development because of the broad age range (8–15 years old). Some studies have indicated that pubertal stage may have an impact on CAR (Oskis et al., 2009). Fifth, the current sample was comprised of children that were affected by parental HIV, which might limit the generalizability of the findings to children with other sociodemographic backgrounds or adults. For example, a meta-analysis conducted by Bunea et al. (2017) suggested that the negative effect of early adversity on HPA axis might increase as children age and reach the peak in adulthood. It is possible to observe a stronger relationship between childhood peer victimization and dysregulations in HPA axis activity in adults than in children and adolescents.
4.2. Conclusions
In summary, the current study provided the empirical evidence supporting emotional regulation as a potential psychological mechanism underlying the effect of peer victimization on diurnal cortisol rhythm among children affected by parental HIV, although future longitudinal work is solely needed to support these findings over time. Peer victimization was associated with a hypoactive diurnal cortisol pattern, and such an association was partially mediated by emotional regulation in girls but not in boys. These findings illustrated the protective effect of emotional regulation on HPA axis activity and suggested enhancing emotional regulation as a promising strategy for health promotion interventions to mitigate the negative effect of peer victimization among children affected by parental HIV. The findings also highlight the needs of considering gender differences in future efforts of health promotion interventions among victimized children.
Acknowledgement
We thank Samuele Zilioli and Peilian Chi for their assistance on data cleaning.
Role of funding source
This work was supported by the National Institute of Nursing Research GrantR01-NR13466 and the National Natural Science Foundation of China [grant number 31470992].
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest with respect to the authorship or the publication of this manuscript.
References
- Adam EK, Kumari M, 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE, 2017. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology 83, 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth M, Filmer D, 2006. Inequalities in children’s schooling: AIDS, orphanhood, poverty, and gender. World Dev. 34, 1099–1128. [Google Scholar]
- Bender PK, Reinholdt-Dunne ML, Esbjørn BH, Pons F, 2012. Emotion dysregulation and anxiety in children and adolescents: gender differences. Pers. Individ. Differ. 53, 284–288. [Google Scholar]
- Bunea IM, Szentágotai-Tătar A, Miu AC, 2017. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl. Psychiatry 7, 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun CD, Helms SW, Heilbron N, Rudolph KD, Hastings PD, Prinstein MJ, 2014. Relational victimization, friendship, and adolescents’ hypothalamic–pituitary–adrenal axis responses to an in vivo social stressor. Dev. Psychopathol. 26, 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cărnuţă M, Crişan LG, Vulturar R, Opre A, Miu AC, 2015. Emotional non-acceptance links early life stress and blunted cortisol reactivity to social threat. Psychoneuroendocrinology 51, 176–187. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Antoni MH, 2008. The effects of psychological interventions on neuroendocrine hormone regulation and immune status in HIV-positive persons: a review of randomized controlled trials. Psychosom. Med. 70, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Li X, 2013. Impact of parental HIV/AIDS on children’s psychological well-being: a systematic review of global literature. AIDS Behav. 17, 2554–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, 2009. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol. 80, 265–278. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L, 2010. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 35, 97–103. [DOI] [PubMed] [Google Scholar]
- Cluver L, Bowes L, Gardner F, 2010. Risk and protective factors for bullying victimization among AIDS-affected and vulnerable children in South Africa. Child Abuse Neglect 34, 793–803. [DOI] [PubMed] [Google Scholar]
- Corrigan A, 2002. Social Competence Scale—Parent Version: Grade 1/Year 2 (Fast Track Project Technical Report). Retrieved October 12, 2007. [Google Scholar]
- DeSantis AS, Adam EK, Mendelsohn KA, Doane LD, 2010. Concordance between self-reported and objective wakeup times in ambulatory salivary cortisol research. Int. J. Behav. Med. 17, 74–78. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D, 2007. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol. Methods 12, 121–138. [DOI] [PubMed] [Google Scholar]
- Fendrich M, Weissman MM, Warner V, 1990. Screening for depressive disorder in children and adolescents: validating the center for epidemiologic studees depression scale for children. Am. J. Epidemiol. 131, 538–551. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Ormrod RK, Turner HA, 2009. The developmental epidemiology of childhood victimization. J. Interpers Violence 24, 711–731. [DOI] [PubMed] [Google Scholar]
- Garde A, Hansen ÅM, 2005. Long-term stability of salivary cortisol. Scand. J. Clin. Lab. Invest. 65, 433–436. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Jazaieri H, 2014. Emotion, emotion regulation, and psychopathology: an affective science perspective. Clin. Psychol. Sci. 2, 387–401. [Google Scholar]
- Hager AD, Leadbeater BJ, 2016. The longitudinal effects of peer victimization on physical health from adolescence to young adulthood. J. Adolesc. Health 58, 330–336. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, Ormel J, 2013. Self-or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: a systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypothesis. Biol. Psychol. 94, 175–184. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J, 2009. How does stigma “get under the skin”? The mediating role of emotion regulation. Psychol. Sci. 20, 1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herts KL, McLaughlin KA, Hatzenbuehler ML, 2012. Emotion dysregulation as a mechanism linking stress exposure to adolescent aggressive behavior. J. Abnormal Child Psychol. 40, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cicchetti D, 2010. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. J. Child Psychol. Psychiatry 51, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer W, 2006. Violence exposure and cortisol responses in urban youth. Int. J. Behav. Med. 13, 109–120. [DOI] [PubMed] [Google Scholar]
- Kliewer W, 2016. Victimization and biological stress responses in urban adolescents: emotion regulation as a moderator. J. Youth Adolesc. 45, 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer W, Riley T, Zaharakis N, Borre A, Drazdowski TK, Jäggi L, 2016. Emotion dysregulation, anticipatory cortisol, and substance use in urban adolescents. Pers. Individ. Differ. 99, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knack JM, Jensen-Campbell LA, Baum A, 2011. Worse than sticks and stones? Bullying is associated with altered HPA axis functioning and poorer health. Brain Cogn. 77, 183–190. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, 2006. Are cultural differences in emotion regulation mediated by personality traits? J. Cross Cult. Psychol. 37, 421–437. [Google Scholar]
- Maughan A, Cicchetti D, 2002. Impact of child maltreatment and interadult violence on children’s emotion regulation abilities and socioemotional adjustment. Child Dev. 73, 1525–1542. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, 2007. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 86, 220–233. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease: allostasis and allostatic load. Ann. N.Y. Acad. Sci. 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2008. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E, 1993. Stress and the individual: mechanisms leading to disease. Arch. Intern. Med. 153, 2093–2101. [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Hilt LM, 2009. Emotion dysregulation as a mechanism linking peer victimization to internalizing symptoms in adolescents. J. Consult. Clin. Psychol. 77, 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 133, 25–45. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 2012. Mplus Version 7 User’S Guide. Muthén &; Muthén, Los Angeles, CA. [Google Scholar]
- Nolen-Hoeksema S, 2012. Emotion regulation and psychopathology: the role of gender. Annual review of clinical psychology 8, 161–187. [DOI] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A, 2009. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology 34, 307–316. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Bowes L, Shakoor S, Ambler A, Pariante CM, Papadopoulos AS, Caspi A, Moffitt TE, Arseneault L, 2011a. A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. J. Amer Academy of Child & Adolescent Psychiatry 50, 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Caspi A, Moffitt TE, Arseneault L, 2011b. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological psychiatry 70, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek G, Zeevalkink H, Vermulst A, Scholte RH, 2010. Peer victimization, self-esteem, and ego resilience types in adolescents: a prospective analysis of person-context interactions. Soc. Dev. 19, 270–284. [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF, 2007. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivar. Behav. Res. 42, 185–227. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Zhang Z, Zyphur MJ, 2011. Alternative methods for assessing mediation in multilevel data: the advantages of multilevel SEM. Struct. Equ. Model. 18, 161–182. [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C, 1999. Low self-esteem, induced failure and the adrenocortical stress response. Pers. Individ. Differ. 27, 477–489. [Google Scholar]
- Quirin M, Kuhl J, Düsing R, 2011. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology 36, 898–904. [DOI] [PubMed] [Google Scholar]
- Reijntjes A, Kamphuis JH, Prinzie P, Telch MJ, 2010. Peer victimization and internalizing problems in children: a meta-analysis of longitudinal studies. Child Abuse Neglect 34, 244–252. [DOI] [PubMed] [Google Scholar]
- Schwartz D, Proctor LJ, 2000. Community violence exposure and children’s social adjustment in the school peer group: the mediating roles of emotion regulation and social cognition. J. Consult. Clin. Psychol. 68, 670–683. [PubMed] [Google Scholar]
- Slatcher RB, Chi P, Li X, Zhao J, Zhao G, Ren X, Zhu J, Stanton B, 2015. Associations between coping and diurnal cortisol among children affected by parental HIV/AIDS. Health Psychol. 34, 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME, 1982. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol. Methodol. 13, 290–312. [Google Scholar]
- Susman EJ, 2006. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci. Biobehav. Rev. 30, 376–389. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Seeman TE, 2011. Early adversity and adult health outcomes. Development and psychopathology 23, 939–954. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Rossy LA, Ruiz-Padial E, Johnsen BH, 2003. Gender differences in the relationship between emotional regulation and depressive symptoms. Cognit. Ther. Res. 27, 349–364. [Google Scholar]
- UNICEF, 2016. Statistics by area/HIV/AIDS UNICEF. http://www.childinfo.org/hiv_aids_global_trends.html (accessed 26 February 2018).
- Vaillancourt T, Duku E, Decatanzaro D, Macmillan H, Muir C, Schmidt LA, 2008. Variation in hypothalamic–pituitary–adrenal axis activity among bullied and non--bullied children. Aggress. Behav. 34, 294–305. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T, Duku E, Becker S, Schmidt LA, Nicol J, Muir C, MacMillan H, 2011. Peer victimization, depressive symptoms, and high salivary cortisol predict poorer memory in children. Brain Cogn. 77, 191–199. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T, Hymel S, McDougall P, 2013. The biological underpinnings of peer victimization: understanding why and how the effects of bullying can last a lifetime. Theory Pract. 52, 241–248. [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wolke D, Copeland WE, Angold A, Costello EJ, 2013. Impact of bullying in childhood on adult health, wealth, crime, and social outcomes. Psychol. Sci. 24, 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li X, Fang X, Hong Y, Zhao G, Lin X, Zhang L, Stanton B, 2010. Stigma against children affected by AIDS (SACAA): psychometric evaluation of a brief measurement scale. AIDS Behav. 14, 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]


