ABSTRACT
Apolipoprotein E (ApoE) is a lipid transport protein that is hypothesized to suppress proinflammatory cytokine production, particularly after stimulation with Toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS). Studies using transgenic ApoE human replacement mice (APOE) expressing one of three different allelic variants suggest that there is a hierarchy in terms of responsiveness to proinflammatory stimuli such as APOE4/E4 > APOE3/E3 > APOE2/E2. In this study, we test the hypothesis that APOE genotype can also predict susceptibility to infection with the facultative intracellular gram-positive bacterium Listeria monocytogenes. We found that bone-marrow-derived macrophages isolated from aged APOE4/E4 mice expressed elevated levels of nitric oxide synthase 2 and were highly resistant to in vitro infection with L. monocytogenes compared to APOE3/E3 and APOE2/E2 mice. However, we did not find statistically significant differences in cytokine or chemokine output from either macrophages or whole splenocytes isolated from APOE2/E2, APOE3/E3, or APOE4/E4 mice following L. monocytogenes infection. In vivo, overall susceptibility to foodborne listeriosis also did not differ by APOE genotype in either young (2 mo old) or aged (15 mo old) C57BL/6 mice. However, we observed a sex-dependent susceptibility to infection in aged APOE2/E2 male mice and a sex-dependent resistance to infection in aged APOE4/E4 male mice that was not present in female mice. Thus, these results suggest that APOE genotype does not play an important role in innate resistance to infection with L. monocytogenes but may be linked to sex-dependent changes that occur during immune senescence.
KEYWORDS: macrophages, lipid transport, foodborne listeriosis, TNFα
INTRODUCTION
Listeria monocytogenes are facultative intracellular bacteria that cause foodborne disease in humans and are widely used as a model pathogen to study immune function. The bacteria can infect a variety of different cell types, and when taken up by phagocytes, can escape into the cytosol where they replicate with a doubling time of about 45 min (1). From decades of published studies, we know that L. monocytogenes can activate many facets of the immune system, but sterilizing immunity requires the induction of a Th1-type proinflammatory response and antigen-specific cytotoxic T cells (2). Interferon gamma (IFNγ) is a key part of the proinflammatory cytokine response because it induces the expression of hundreds of genes that can restrict intracellular infection (3 - 5). Indeed, macrophages pretreated with IFNγ become activated and no longer support the intracellular growth of L. monocytogenes (6). This is thought to be due to increased expression of nitric oxide and reactive oxygen species which rapidly kill the bacteria in the phagocytic vacuole (7).
Apolipoprotein E (ApoE) is a lipid transport protein important for the metabolism of cholesterol that also has an established role in modulating neuroinflammation. ApoE-deficient mice have increased inflammatory responses in a variety of model systems which led to the hypothesis that ApoE functions to suppress proinflammatory cytokine production (8). In humans (but not mice), there are three different primary allelic variants that encode ApoE: APOE2, APOE3, and APOE4. APOE4 is the strongest genetic risk factor for developing late-onset Alzheimer’s disease, and people who carry this allele have earlier onset of disease symptoms (9, 10). In both humans and APOE-targeted replacement mice (APOE-TR) that express one of the human isoforms, proinflammatory stimuli induced differential expression of tumor necrosis factor alpha (TNFα) and release of interleukin (IL)-1β with APOE4/APOE4 (E4) mice having the highest response and APOE2/APOE2 (E2) mice having the lowest response (11, 12). Thus, current models of amyloid beta-induced neuroinflammation where ApoE has been studied the most suggest that the proteins encoded by each allele vary in their ability to serve as an anti-inflammatory protein with a hierarchy of E2 > E3 > E4.
Hepatocytes in the liver are the main source of peripheral ApoE and astrocytes in the brain produce most of the ApoE in the central nervous system (CNS), but other cell types such as activated macrophages and microglia can also express APOE during stress (13). Indeed, the E4 > E3 > E2 inflammatory phenotype has been recapitulated in vitro using both mixed glial cultures (14) and macrophages from APOE-TR mice (15). For example, in vitro stimulation of sodium periodate-induced peritoneal macrophages with either LPS alone or LPS plus IFNγ resulted in increased production of nitrite and TNFα in an APOE genotype-dependent manner (15).
Relatively, few studies have examined the role of ApoE in modulating inflammation outside the CNS or used inflammatory stimuli other than LPS, but the proinflammatory phenotype associated with E4 would be predicted to increase innate resistance to a variety of microbial pathogens. The only published study looking at the role of ApoE during L. monocytogenes infection found greater mortality in ApoE-deficient mice within 3 d compared with wildtype animals (16), indicating a role for the protein in mediating innate resistance to infection. ApoE-deficient mice were also significantly more susceptible to both Klebsiella pneumoniae and Borrelia hispanica, one of the species that causes relapsing fevers, and they had more inflammation and joint swelling following infection with Borrelia burgdorferi, the causative agent of Lyme disease (17, 18).
In this study, we used APOE-TR mice to test the hypothesis that animals with a homozygous E4/E4 genotype would be more resistant to L. monocytogenes infection and, conversely, that E2/E2 mice would be more susceptible relative to mice expressing E3, the most common human allele (19). We found that the E4 > E3 > E2 inflammatory response was readily recapitulated in vitro when using macrophages cultured from aged, but not young, bone marrow. However, the predicted resistance and susceptibility phenotypes were only observed in vivo during infection of aged male mice.
MATERIALS AND METHODS
Bacteria
All experiments in this study used L. monocytogenes SD2000 (20), a derivative of reference strain EGDe that expresses a mouse-adapted allele of internalin A (InlAm) to allow for efficient binding to murine E-cadherin, which promotes invasion of the intestinal epithelium (21, 22). Bacteria were grown shaking at either 30°C (for in vivo infection of mice) or 37°C (for in vitro infection of cells) in brain heart infusion (BHI) broth (Difco) to early stationary phase, and aliquots were prepared and frozen at −80°C until use (23).
Animals
Mice with targeted replacement of murine ApoE with human APOE alleles (24 - 26), backcrossed 10 + generations onto the C57BL/6 background (Taconic Labs), were originally obtained from Dr. Nobuyo Maeda (UNC Chapel Hill). After establishing breeding colonies at the University of Kentucky, the lines were rederived by backcrossing the APOE2/E2 and APOE4/E4 mice with APOE3/E3 mice (Fig. 1A) to mitigate any possible genetic drift that may have occurred in the long-existing UNC Chapel Hill colonies. With the exception of data shown in Fig. 1D, only homozygous mice were used for this study and are abbreviated as E2 (APOE2/APOE2), E3 (APOE3/APOE3), and E4 (APOE4/APOE4) throughout. Both male and female mice were used as indicated. APOE genotypes were confirmed by PCR analysis of tail DNA as described (27); mice were co-housed by genotype after weaning. Young mice were approximately 8 wk old (range 42–69 d) and aged mice were approximately 15 mo old (range 441–539 d) at the time of infection or harvest. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
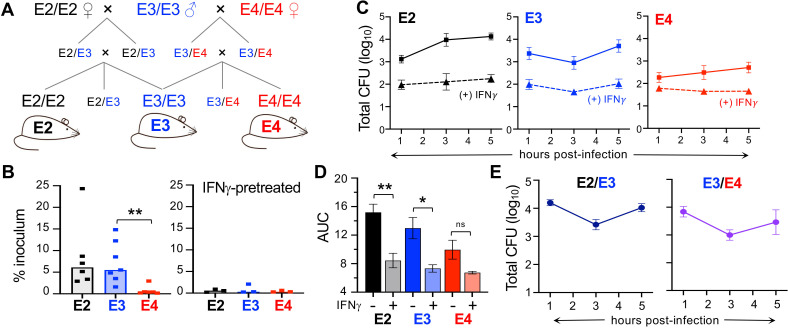
Fig 1.
Bone-marrow-derived macrophages from aged homozygous APOE4/APOE4 (E4) mice are more resistant to L. monocytogenes infection than cells from APOE3/APOE3 (E3) or APOE2/APOE2 (E2) mice. (A) Breeding scheme for the rederivation of humanized homozygous APOE mice. (B) Invasion assay for macrophages pretreated with phosphate-buffered saline (PBS; left) or IFNγ (right) for 24 h then infected with L. monocytogenes at MOI = 0.1 for 1 h. Each symbol represents the mean value of triplicate samples obtained from cells derived from a single aged mouse; median values (bars) for pooled data from multiple experiments were analyzed by Mann-Whitney U test: *P < 0.05, **P < 0.01. (C) Intracellular growth assay measuring gentamicin-resistant L. monocytogenes in macrophages ± IFNγ pretreatment. Symbols indicate colony forming unit values for cells derived from n = 6 different aged mice per genotype that were tested in triplicate (± SEM) in each assay; a representative growth curve for cells from one mouse per genotype. (D) Area under the curve (AUC) for the data points shown in panel C. Analyzed by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons. (E) Intracellular growth assay for macrophages derived from APOE heterozygous mice. Mean values ± SEM for triplicate samples from one of three independent experiments are shown. For panels (B) and (C), all but one animal in each group was female; for panel (E), there were n = 6 female and n = 3 male E2/E3 mice, and all the E3/E4 mice were male.
Cell culture
Bone marrow was extracted from the femurs and tibias of mice and cultured in BMM media consisting of Dulbecco’s modified Eagle’s medium ( Invitrogen cat. #11960) supplemented with 20% fetal bovine serum (FBS; Gemini Benchmark #100–106), 1X GlutaMax (Gibco), and 20% L929 cell supernatant as described (28). Cells were seeded in 24-well dishes on 12 mm round glass coverslips and maintained at 37°C in 7% CO2 with fresh media every 3–4 d for up to 4 wk. After day 7 of culture, the percentage of both FBS and L929 supernatant in the media was reduced to 10%. Macrophages were maintained in media containing penicillin and streptomycin; the day prior to infection, the cells were washed once in phosphate-buffered saline (PBS; Invitrogen cat. #14190) and then maintained in media lacking antibiotics.
Spleens harvested from APOE-TR mice were treated with collagenase D (Roche) as previously described (29) and then mashed through a sterile mesh screen (no. 80) to prepare a single-cell suspension. Red blood cells were lysed in a hypotonic solution and then splenocytes were seeded in 96-well round plates (5 × 105 cells/well) in 200 µL of antibiotic-free RP-10 medium consisting of RPMI 1640 (Invitrogen cat. # 11875) supplemented with 10% FBS, 1X GlutaMax, and 10 mM HEPES.
Invasion and intracellular growth assays
Macrophages were pretreated with mouse rIFNγ (final concentration of 100 U/mL; BD Biosciences) or media alone 24 h prior to infection. Bacterial aliquots were thawed and incubated shaking at 37°C for 1.5 h, then washed once in PBS and suspended in PBS at 1 × 106 colony forming unit (CFU)/mL. Fifty microliters was used to infect each well of confluent macrophages at a multiplicity of infection (MOI) of 0.1, the plates were centrifuged for 5 min at 700 × g to synchronize the infection, and incubated for 25 more minutes at 37°C in 7% CO2. Serial dilutions of the inoculum were prepared and plated on BHI agar to confirm the actual dose. At 30 min post-infection, cells were washed three times with 1 mL of prewarmed (37°C) PBS and then 1 mL of RP-10 media with gentamicin (final concentration of 10 µg/mL) was added. At 1 h post-infection (hpi), cells were washed once with PBS, the coverslips were transferred to a conical tube containing 5 mL of sterile water, the tubes were vortexed at a maximum speed for 1 min, and then serial dilutions were prepared in sterile water and plated on BHI agar. The percent invasion was calculated by dividing the total number of CFU recovered from each coverslip by the actual dose used to infect each well and multiplying by 100.
The ability of the bacteria to replicate in the cytosol of macrophages was monitored by assessing the number of gentamicin-protected CFU over time. Cells were infected as described above for the invasion assay, and coverslips were harvested at 1, 3, and 5 hpi. In each experiment, triplicate coverslips containing cells derived from a single APOE-TR mouse were used; the mean value of those technical triplicates was used as a biological replicate for statistical analysis of pooled data.
QRT-PCR
Total RNA was isolated from cultured macrophages using TRIzol reagent, according to the manufacturer’s instructions (Invitrogen, #15596026). cDNA was synthesized from total RNA using SuperScript III First-Strand Synthesis System (Invitrogen #18080). A StepOne Real-Time PCR system (Applied Biosystems) was used to detect products amplified from cDNA (10 ng) in the PowerUp SYBR Green Master Mix (Applied Biosystems, #100029284) with primers specific for NOS2 (forward, 5′-TCCTCACTGGGACAGCACAGAATG-3′; reverse, 5′-GTGTCATGCAAAATCTCTCCACTGCC-3′) (30). Glyceraldehyde 3-phosphate dehydrogenase primers (forward, 5′-AGGTCGGTGTGAACGGATTTG-3′; reverse, 5’- TGTAGACCATGTAGTTGAGGTCA-3′) were used as a control (31). Fold changes were determined using the 2–ΔΔC′T method, as previously described (32).
Cytokine and chemokine immunoassay
Supernatants were collected from the wells of L. monocytogenes infected or uninfected cultured bone-marrow-derived macrophages or from splenocytes harvested directly from APOE-TR mice at either 5 h or 24 hpi. The supernatants were centrifuged at 700 × g for 5 min to remove any cellular debris and frozen at −80°C. Samples from multiple infection experiments were batch tested in ProcartaPlex (Invitrogen) 26- or 36-Plex immunoassays, according to the manufacturer’s protocol. Cytokine and chemokine concentrations were determined using a Luminex 200 (UK Flow Cytometry and Immune Monitoring Core).
Foodborne infection of mice
Mice were denied food (but given water ad libitum) for 18–24 h prior to infection in BSL2-caging on raised wire flooring to prevent coprophagy. Frozen aliquots of Lm were thawed, incubated statically in BHI for 1.5 h at 30°C, and suspended in a small volume of PBS and then melted salted sweet cream butter (Kroger) was added (2:3 ratio of PBS/butter). A 2- to 3-cm piece of white bread (Kroger) saturated with 108 CFU Lm was fed to mice near the onset of their dark cycle as described previously (33, 34). Ileum (defined as the terminal third of the length of the small intestine) and colon contents were collected by squeezing with sterile forceps, and then each section was flushed with a total of 8–10 mL of PBS through a 25-g needle. To quantify the number of bacteria in the lumen, the pooled contents and flushes were centrifuged for 20 min at 12,000 × g. The bacterial pellet was suspended in 0.5–1.0 mL of sterile water and serial dilutions were plated on BHI agar supplemented with 15 g/L LiCl and 10 g/L glycine (BHI/L + G) to prevent the growth of most gut microbiota. Washed ileum and colon tissues were cut longitudinally with a sterile scalpel blade, placed in 2 mL of sterile water, and then homogenized for 1 min using a PowerGen 1000 homogenizer (Fisher) at 80% power. Serial dilutions were plated on BHI/L + G agar. Spleens and livers were harvested aseptically, homogenized in 5 mL of sterile water for 30 s, and then serial dilutions were plated on BHI agar to determine the total number of L. monocytogenes CFU present in each tissue.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 9; the specific test performed with each data set is indicated in the figure legends. P values < 0.05 were considered significant and are designated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
RESULTS
Macrophages from aged E4 mice were more resistant to L. monocytogenes infection
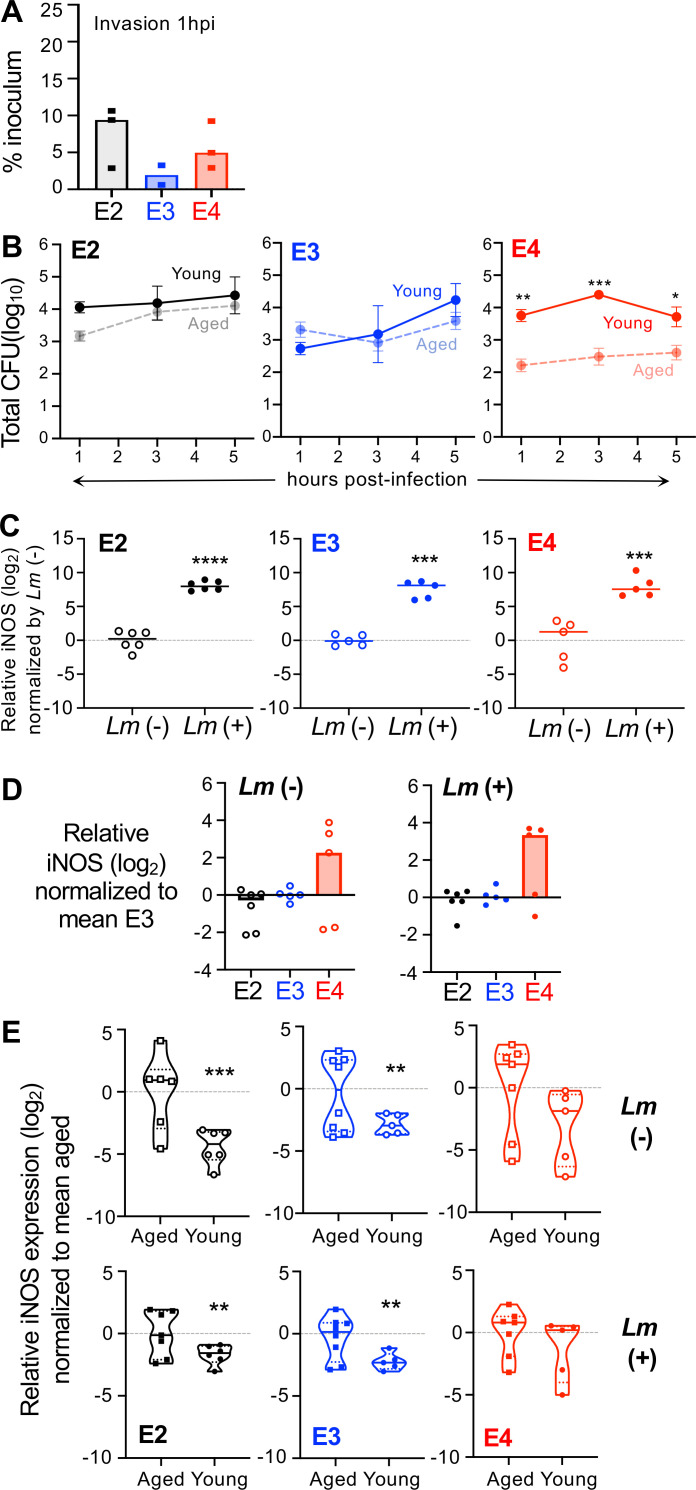
L. monocytogenes are readily internalized by macrophages, and the bacteria quickly escape from phagocytic vacuoles and localize to the host cell cytosol. However, if macrophages are pretreated with IFNγ, they become highly resistant to L. monocytogenes infection (6). To discern whether the hyperinflammatory state previously associated with the APOE4 allele rendered macrophages more resistant to L. monocytogenes infection, we infected bone-marrow-derived macrophages at low MOI and determined the number of intracellular (gentamicin protected) bacteria 1 h later. The bone marrow used to generate the macrophages was harvested from aged mice that were part of another study examining the role of ApoE in Alzheimer’s disease. As shown in Fig. 1B, the initial invasion rate for either E2 or E3 macrophages was approximately 5% of the inoculum, but cells from E4 mice were significantly more resistant to infection, with very few intracellular L. monocytogenes recovered. Pretreatment of the macrophages with IFNγ increased the resistance of both E2 and E3 cells such that there was no difference compared to E4 macrophages.
We next performed an intracellular growth assay to assess the ability of macrophages to support intracellular replication of L. monocytogenes over a 5-h period. As shown in Fig. 1C, the growth curves varied by APOE genotype. E2 cells were most susceptible to infection, with intracellular bacteria steadily increasing over time. The number of intracellular L. monocytogenes initially decreased in E3 cells, presumably due to killing of some bacteria in the phagocytic vacuole; however, the bacteria then increased exponentially (Fig. 1C). E4 cells supported only a modest two-fold increase in intracellular bacteria over the 5-h period. As expected, pretreatment of the macrophages with IFNγ decreased the initial invasion of L. monocytogenes and suppressed intracellular growth during the entire interval with the greatest difference observed for E2 cells and no significant difference for E4 cells (Fig. 1D).
Since homozygous E2/E2 and E4/E4 genotypes are rare in the human population, we next wanted to determine whether the presence of only a single copy of APOE2 or APOE4 would influence macrophage susceptibility to L. monocytogenes. To test this, we cultured bone-marrow-derived macrophages from heterozygous E2/E3 and E3/E4 mice and performed intracellular growth assays. As shown in Fig. 1E, the growth curves for these cells were very similar to the growth curve for homozygous E3/E3 mice in Fig. 1C. This suggests that the effects of the APOE3 allele are dominant and can counteract any hypoinflammatory (APOE2) or hyperinflammatory (APOE4) tendencies of the other alleles. Together, these results indicated that APOE genotype did affect the ability of aged macrophages to kill intracellular L. monocytogenes with a general pattern of E2 cells being more susceptible and E4 cells being more resistant than E3 macrophages.
Macrophages from aged E4 mice expressed increased inducible nitric oxide synthase
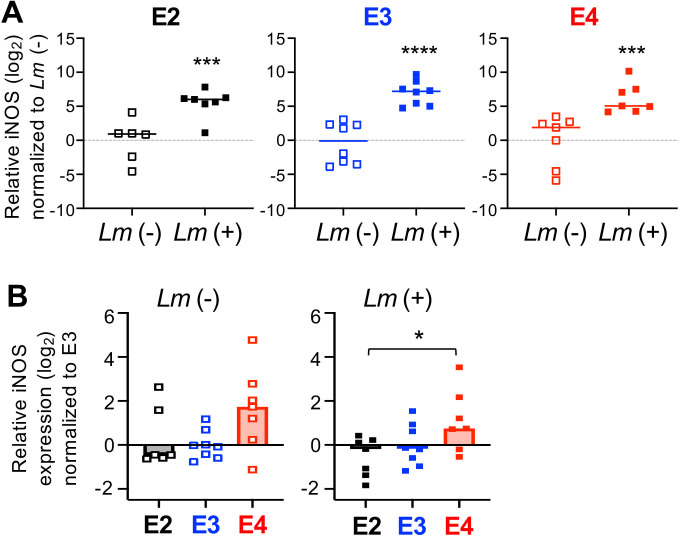
The increased resistance of IFNγ-pretreated macrophages to L. monocytogenes infection is known to be caused at least in part by the upregulation of inducible nitric oxide synthase (NOS2) (35). Vitek et al. previously showed that cells from APOE4/E4 mice stimulated in vitro with both IFNγ and LPS produced more NOS2 mRNA than cells from APOE3/E3 mice (15). In that study, they tested both microglia cells enriched from brain homogenates and inflammatory monocytic cells elicited to the peritoneal cavity by sodium periodate treatment. To find out whether increased iNOS could be responsible for the enhanced resistance we observed in E4 bone-marrow-derived macrophages, we quantified NOS2 mRNA levels with and without exposure to L. monocytogenes. As expected, L. monocytogenes infection increased NOS2 expression by more than 30-fold in cells of all three genotypes (Fig. 2A). However, we found that basal expression of NOS2 in uninfected, unstimulated macrophages was already higher in E4 cells compared to E3 cells (Fig. 2B). After infection, the induction of NOS2 was also slightly higher in E4 cells. Thus, E4 bone-marrow-derived macrophages may be more resistant to L. monocytogenes infection because they have a high steady state level of NOS2 which could kill most of the invading bacteria.
Fig 2.
Macrophages from aged homozygous APOE4 mice express higher levels of iNOS mRNA. Bone-marrow-derived macrophages were infected with L. monocytogenes (Lm+) at MOI = 0.1; RNA was harvested 5 hpi for qRT-PCR analysis. (A) iNOS induction following exposure to L. monocytogenes. Relative mRNA levels were normalized to mean value for uninfected (Lm−) samples. A one-sample t-test was performed, using a hypothetical mean value of 0. (B) iNOS expression in macrophages varies by APOE genotype. Relative mRNA levels were normalized to the mean value for uninfected E3 samples. Pooled data from three independent experiments are shown; each symbol represents expression levels in macrophages harvested from an individual female mouse. Bars indicate median values; significance was evaluated by Mann-Whitney U test: *P < 0.05, ***P < 0.01, ***P < 0.001, ****P < 0.0001.
Early cytokine and chemokine production following L. monocytogenes exposure did not vary by APOE genotype
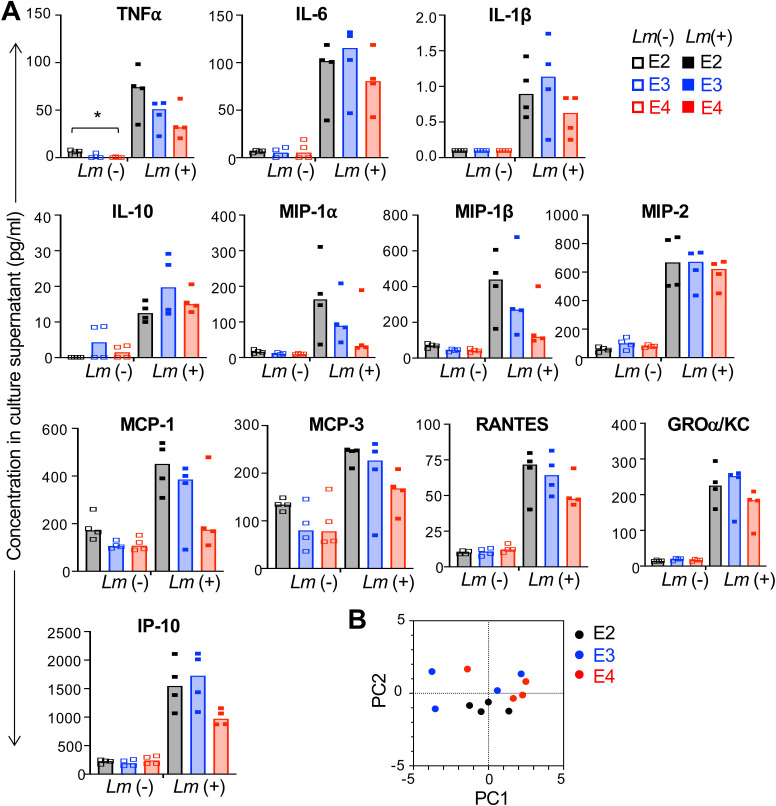
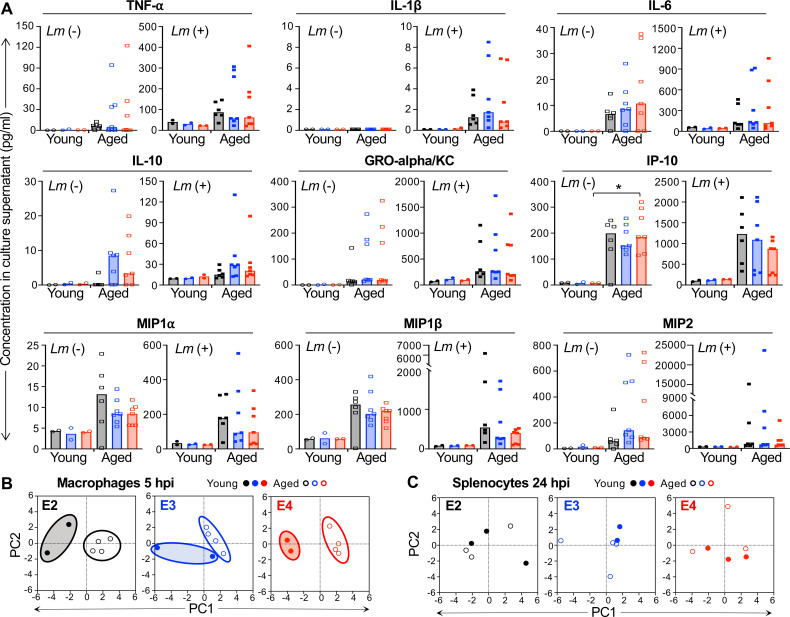
Macrophage secretion of proinflammatory cytokines such as TNFα can also influence the ability of surrounding cells to become activated and more resistant to L. monocytogenes infection. To test the role of APOE genotype on both the steady state and infection-induced cytokine and chemokine profiles of bone-marrow-derived macrophages, we infected the cells for 5 h and then collected culture supernatants for multiplex immunoassay. As shown in Fig. 3A, exposure to L. monocytogenes rapidly induced the expression of both cytokines and chemokines by all the macrophages. Although there was an E2 > E3 > E4 trend in Listeria-infected animals for TNFα and several chemokines (MIP-1α, MIP-1β, MCP-1, MCP-3, and RANTES), no significant differences were noted for cells of varying APOE genotype when the uninfected and infected groups were analyzed by two-way ANOVA. Furthermore, principal component analysis revealed that the samples did not cluster by APOE genotype for either component 1 or component 2, which accounted for 54.87% and 32.15% of the variance within the population, respectively (Fig. 3B). These results suggested that the early cytokine and chemokine response of aged macrophages was not a critical factor in determining the overall resistance to L. monocytogenes infection.
Fig 3.
Early cytokine and chemokine production in aged macrophages does not vary significantly by APOE genotype in response to L. monocytogenes infection. Bone-marrow-derived macrophages were infected with Lm SD2000 at MOI = 0.1 for 5 h and then supernatants were collected for subsequent 26-plex immunoassay. (A) Symbols represent values for samples collected from cells derived from individual aged female mice (n = 4 per APOE allele); bars indicate median values. Kruskal−Wallis tests (nonparametric one-way ANOVA) were performed for each group: *P < 0.05. (B) A principal component analysis using only the data presented in panel A was performed. Not included were the 13 cytokines (granulocyte-macrophage colony-stimulating factor, IFNg, IL-2, IL-4, IL-5, IL-9, IL-12p70, IL-13, IL-17a, IL-18, IL-22, IL-23, and IL-27) and one chemokine (eotaxin) which were either undetected or unchanged after L. monocytogenes infection.
Since macrophages are not the only cell type that secrete proinflammatory mediators during infection, we next determined whether the cytokine and chemokine output of splenocytes exposed to L. monocytogenes differed by APOE genotype. Single-cell suspensions of collagenase-digested spleens harvested from several different mice of each APOE genotype were infected with L. monocytogenes and supernatants were collected 24 h later. Once again, infection induced the expression of many cytokines and chemokines, but no significant differences could be attributed to a particular APOE allele (Fig. 4A). Similar trends were noted with lower overall values when supernatants collected just 6 hpi were assayed (data not shown). Principal component analysis showed that component 1 accounted for 48.66% and component 2 for 15.59% of the variance within the supernatant samples, but they did not cluster by APOE genotype (Fig. 4B).
Fig 4.
The cytokine and chemokine response of splenocytes following stimulation with L. monocytogenes is not dependent on APOE genotype. Collagenase-digested splenocytes were infected with L. monocytogenes at MOI = 1 and cell-free supernatants were collected at 24 hpi for subsequent 36-plex immunoassay. (A) Each symbol represents the concentration detected in a supernatant collected from cells harvested from an individual female mouse; bars indicate median values. A nonparametric one-way ANOVA (Kruskal–Wallis test) did not reveal any significant differences; P values for specific comparisons of median values by Mann-Whitney U test are indicated. (B) A principal component analysis using only the data presented in panel A was performed. Not included were the 15 cytokines (IFNa, IL-1a, IL-2, IL-3, IL-4, IL-5, IL-9, IL-13, IL-15, IL-23, IL-27, IL-28, IL-31, leukemia inhibitory factor, and macrophage colony-stimulating factor) and 3 chemokines (eotaxin, CXCL5, and MCP-3) which were either undetected or unchanged after L. monocytogenes infection.
APOE genotype did not affect susceptibility of young mice to foodborne infection with L. monocytogenes
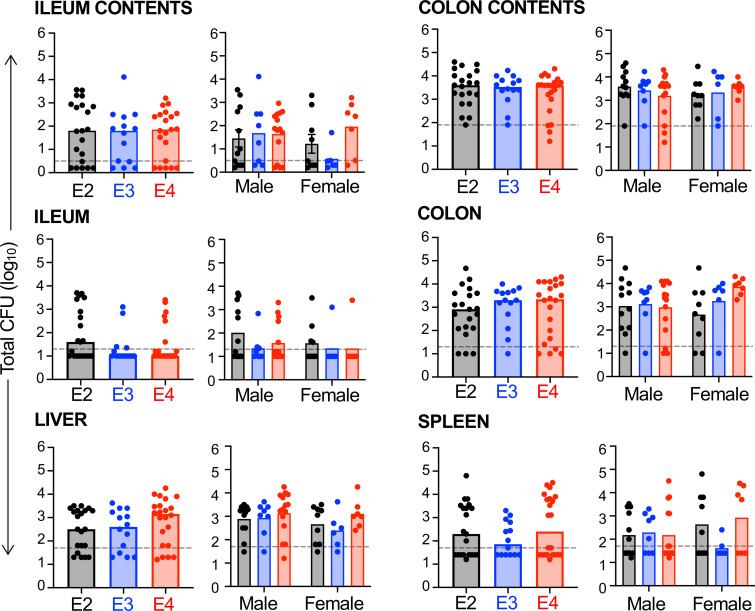
We next sought to determine whether the APOE-dependent susceptibility (E2) and resistance (E4) phenotypes observed in macrophages were also evident in vivo during infection of mice. Our standard mouse model of foodborne listeriosis involves feeding young (6–9-wk-old) female BALB/cByJ mice during their nocturnal phase and was carefully designed to avoid the large variances in CFU burdens that had previously been observed following oral challenge with L. monocytogenes (33, 34). Mice on a C57BL/6 background such as the APOE-TR used here can also be infected, but are more resistant, requiring a 10-fold higher dose to achieve the same initial bacterial burdens in the gut, which are then cleared more rapidly than in BALB/cByJ mice (20, 21). Regardless of APOE genotype, bacterial burdens were higher in the ileum and colon luminal content than in the flushed intestinal tissue (Fig. 5), consistent with previous studies which suggested that crossing the intestinal mucosal barrier is a relatively rare event for L. monocytogenes (33, 36). Also consistent with that earlier work, we found about 100-fold higher CFU in the colons of all mice compared with CFU counts in the ileum (Fig. 5). However, bacterial burdens did not differ by APOE genotype in the ileum, colon, spleen, or liver at 3 d post-infection (dpi) in either males or females (Fig. 5). Thus, in contrast to the results for aged macrophages, the presence of the E4 allele did not protect young mice from challenge with L. monocytogenes, and the E2 allele did not render mice more susceptible.
Fig 5.
APOE genotype does not affect susceptibility to foodborne infection with L. monocytogenes. Six- to eight-wk old male and female mice were fed L. monocytogenes-contaminated food and the total number of CFU was determined 3 dpi. For the ileum and colon, the luminal contents were collected and processed separately from the flushed tissues. Dashed lines indicate the limit of detection for each tissue. Pooled data from five independent infections are shown (inocula ranged from 108 to 109 CFU). Symbols represent CFU burdens in individual mice; bars indicate median values. For each tissue, pooled data of all mice are shown in the left graph and data sorted by sex are shown in the right graph. No significant differences were noted by either Mann-Whitney U test analysis for the pooled data (E2, n = 21; E3, n = 14; E4, n = 22) or two-way ANOVA for the male (E2, n = 12; E3, n = 8; E4, n = 15) vs female (E2, n = 9; E3, n = 6; E4, n = 7) data set.
Macrophages cultured from young E4 mice were more susceptible to L. monocytogenes infection than cells derived from aged E4 mice
Given the large age difference between the mice used to culture the bone marrow-derived macrophages used for experiments presented in Fig. 1 to 4 and the mice used for in vivo challenge studies, we next wanted to feed aged mice L. monocytogenes and determine whether increased resistance would develop in the older E4 animals. While we waited for a cohort of APOE-TR mice to age, we decided to examine bone-marrow-derived macrophages from young mice, as we postulated that they would not display APOE-dependent differences in susceptibility or resistance. As expected, macrophages from young E4 mice were not more resistant to invasion by L. monocytogenes (Fig. 6A), in contrast to what was observed with aged E4 mice (Fig. 1B). When we compared the intracellular growth over time in macrophages cultured from either young or aged mice, only cells from E4 mice displayed a significant difference (Fig. 6B), suggesting that the resistance phenotype was present only in older animals.
Fig 6.
Macrophages cultured from young humanized APOE mice are more susceptible to L. monocytogenes infection than those cells from aged mice. (A) Invasion efficiency for young macrophages infected with L. monocytogenes (MOI = 0.1) for 1 h. Each symbol represents the mean of triplicate values from a single experiment using cells derived from a single mouse; a mixture of male and female mice was used. No statistical significance by one-way ANOVA. (B) Growth of intracellular (gentamicin resistant) L. monocytogenes in young macrophages. Solid lines are representative growth curves for cells from one of n = 2–3 mice per genotype; each symbol represents the mean (±SEM) for triplicate samples in the assay. For comparison, the data of aged mice (from Fig. 1B) are represented by dashed lines. Two-way ANOVA analysis indicated that both genotype (**) and time of infection (*) varied but the only significant difference between young and aged cells was for APOE4 mice. (C) Young macrophages were infected with L. monocytogenes at MOI = 0.1 (Lm+) or left untreated (Lm−) and iNOS mRNA was measured 5 hpi. Each symbol represents expression levels in macrophages harvested from an individual mouse. Bars indicate median values; significance was evaluated by Mann-Whitney U test: *P < 0.05, ***P < 0.01, ***P < 0.001, ****P < 0.0001. (D) The same data shown in panel C were normalized by APOE genotype; no significant differences by Tukey’s multiple comparisons test. (E) Baseline values for iNOS mRNA (Lm−) and induced levels 5 hpi (Lm+) were compared in young and aged (data from Fig. 2A) macrophages. All values were normalized to the mean for aged mice of each genotype; solid horizontal lines in the violin plots indicate medians; short dashed lines indicate quartiles. In panels (C, D, E), each symbol is a biological replicate (RNA harvested from independent cell cultures derived from different mice) and one-sample t-tests using a hypothetical value of 0 were performed.
Macrophages cultured from young mice robustly induced the expression of iNOS upon infection with L. monocytogenes regardless of APOE genotype (Fig. 6C), similar to what was observed for cells from aged mice (Fig. 2A). Although there was a trend toward E4 macrophages producing higher levels of iNOS, it was not statistically significant (Fig. 6D). However, it was noted that macrophages cultured from young mice regardless of APOE genotype overall expressed less iNOS mRNA than macrophages from aged mice (Fig. 6E), consistent with the prevailing thought of “inflammaging” (37). Together, these results indicated that macrophages from young E4 mice were not more resistant to L. monocytogenes infection, perhaps explaining the lack of an APOE-dependent phenotype during foodborne infection of young mice.
Macrophages from young mice had diminished inflammatory responses to L. monocytogenes infection
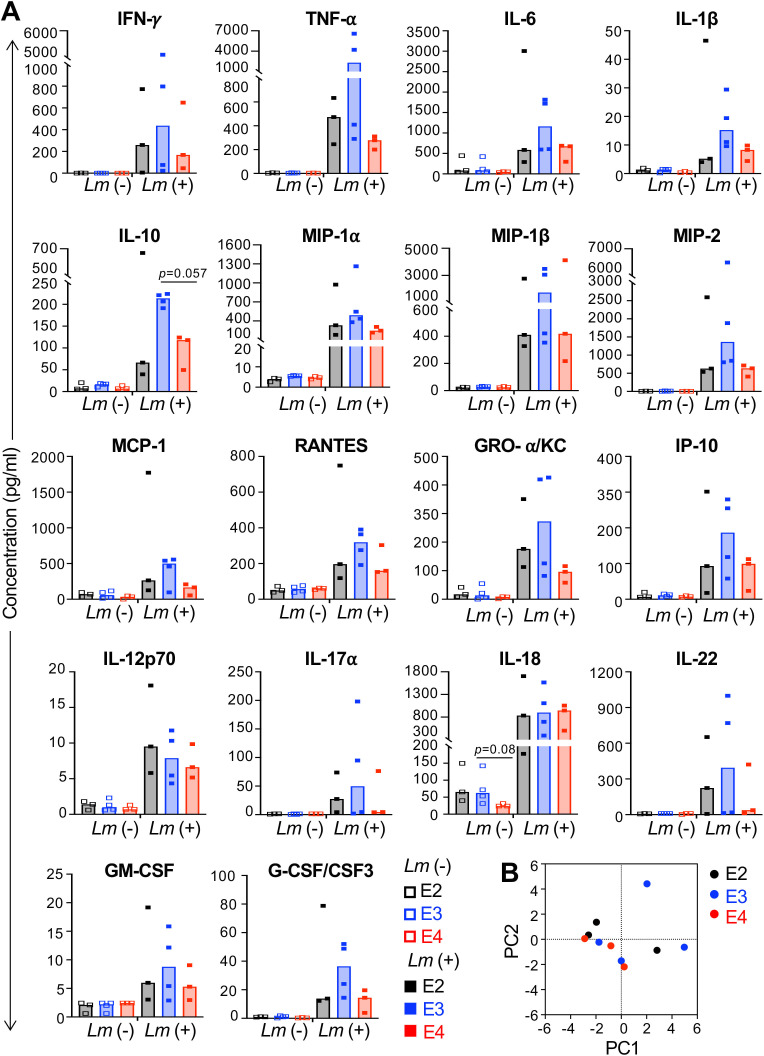
To determine whether the increased susceptibility of cells from young E4 mice was due to an altered cytokine or chemokine response, we infected either bone-marrow-derived macrophages or total splenocytes from E2, E3, and E4 young mice and collected supernatants at either 5 h (macrophages) or 24 hpi (splenocytes). Again, no significant differences in cytokine or chemokine output were noted for either the macrophages (Fig. 7) or the splenocytes (Fig. S1) from mice of varying APOE genotype. However, it was noted that macrophages cultured from young mice produced much lower levels of all cytokines and chemokines detected (Fig. 7A) and principal component analysis revealed that PC1 accounted for about 70% of the variance among the samples (Fig. 7B). In contrast, the cytokine and chemokine output of total splenocytes did not show the same pattern with young mice of all APOE genotypes producing more IFNγ and IL-18 (Fig. S1) and the samples did not separate into distinct groups by principal component analysis (Fig. 7C).
Fig 7.
Macrophages from young APOE mice have diminished inflammatory responses to L. monocytogenes infection compared to macrophages derived from aged mice. (A) Macrophages cultured from the bone marrow of young mice (males and females) were infected with L. monocytogenes at MOI = 0.1 for 5 hpi and then culture supernatants were collected for multiplex immunoassay. Only cytokines and chemokines that were upregulated following L. monocytogenes infection are shown. Symbols represent individual mice; bars indicate median values for data pooled from multiple experiments. Two-way ANOVA was performed. (B and C) Principal component analysis was performed using selected cytokine and chemokine immunoassay data (young data are shown in panel A; aged data are shown in Fig. 3) that were upregulated in young or old macrophage culture supernatant 5 hpi (B) or 24 hpi in splenocyte culture supernatants (C). Symbols are biological replicates (samples collected from cells isolated from individual mice).
Aged E2 and E4 mice displayed sex-dependent differences in bacterial burdens following foodborne L. monocytogenes infection
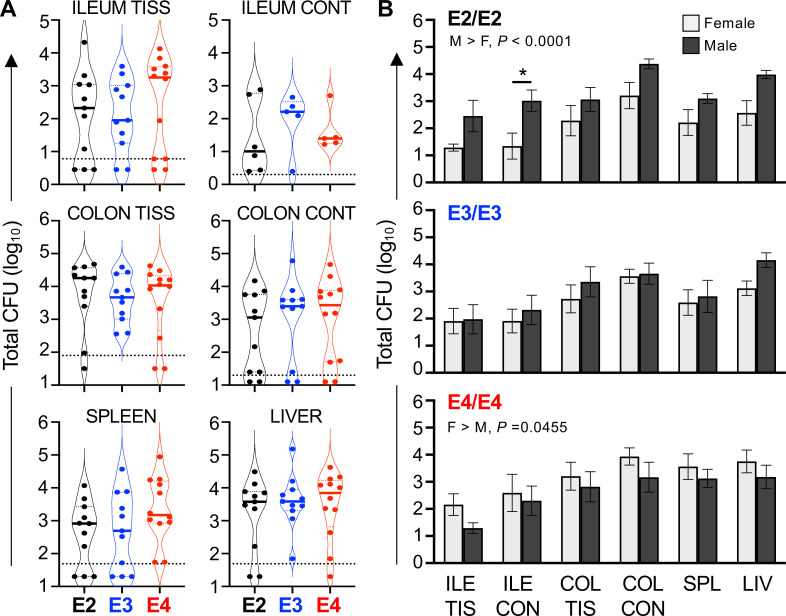
To assess the ability of the E4 genotype to protect aged mice from foodborne listeriosis, we used a cohort of mice approximately 15 mo old, similar in age to the mice used for the data shown in Fig. 1. Groups of mice were fed L. monocytogenes (the same dose given to the younger mice) and the total CFU found in the intestines, spleen, and liver was determined 3 dpi. As shown in Fig. 8A, there were no significant differences in the bacterial burdens in any tissue of E2 mice and E4 mice compared with E3 mice. The aged mice were not overall more susceptible to infection compared with young mice, with the median bacterial burdens being very similar in both sets of experiments (compare Fig. 8A with Fig. 5); however, the variability between individual animals of each genotype was much higher for the aged mice.
Fig 8.
Aged ApoE2 and ApoE4 mice display sex-dependent differences in bacterial burdens following foodborne Listeria monocytogenes infection. Aged mice were fed ~5 × 108 CFU L. monocytogenes and tissues were harvested 3 dpi. The total number of L. monocytogenes in the ileal (ILE CONT) or colon (COL CON) contents and the flushed ileum (ILE TIS) or colon (COL TIS) as well as the liver (LIV) and spleen (SPL) were determined for each mouse. (A) Pooled data from three separate in vivo challenge experiments are shown; symbols represent CFU from one mouse. No differences between mouse strains were noted by one-way ANOVA analysis of each tissue. (B) The same pooled data shown in panel A are separated by sex; two-way ANOVA revealed that there were opposing sex-dependent effects in E2 and E4 mice but not the E3 mice.
When we analyzed the data from male and female mice separately, we noted that E2 male mice were significantly more susceptible to L. monocytogenes than females, with 10- to 100-fold greater CFU in each tissue (Fig. 8B). In contrast, E4 males were much more resistant to infection with a small, but reproducible, reduction in bacterial burdens in each tissue compared with females. There were no sex-dependent differences in CFU counts for the E3 mice. Thus, the predicted resistance and susceptibility phenotypes based on APOE genotype were observed following foodborne challenge with L. monocytogenes but only in aged males.
DISCUSSION
Allelic variants of the lipid transport protein ApoE are known to differ in their ability to modulate inflammation and the established paradigm suggests that a homozygous E4 genotype is associated with a hyperinflammatory state while a homozygous E2 genotype is associated with a hypoinflammatory state relative to the most common variant E3 (38). However, this has been studied mainly in the context of neurodegenerative diseases studying elderly human populations or aged mouse models. In this study, we tested the hypothesis that the Th1-type inflammation associated with an E4 genotype would lead to greater resistance to infection with the facultative intracellular bacterial pathogen L. monocytogenes. We found that macrophages isolated from aged, but not young, E4 mice expressed high levels of iNOS and were significantly more resistant to in vitro infection with L. monocytogenes. However, using a foodborne model of in vivo infection, the predicted resistance in E4 mice and enhanced susceptibility in E2 mice was observed only in males.
Humans at both extremes of life (neonates and the elderly) carry the highest risk for developing severe life-threatening infections with L. monocytogenes. An early study showed that young mice become increasingly more resistant to intravenous infection as they age, acquiring maximum resistance by 8 mo; after that, there was a slow but progressive increase in susceptibility to high-dose infections (39). Smithey et al. showed that 18-mo-old C57BL/6 mice infected intravenously with a lethal dose of L. monocytogenes had a diminished CD8+ T-cell response with fewer antigen-specific cells overall and fewer cells making TNFα (40). T-cell expansion may have been limited by age-associated changes in CD8α+ dendritic cell functions including impaired uptake of Listeria and lower levels of costimulatory molecules (41). In contrast, when lower sublethal doses of bacteria were injected intravenously, both young and aged mice had similar bacterial burdens in the spleen and readily cleared the infection (39). It is much harder to achieve a high-dose lethal systemic infection when L. monocytogenes are administered by the natural foodborne route because the bacteria pass through many bottlenecks, and crossing the intestinal mucosa is a relatively rare event. Indeed, Khairallah et al. used the same foodborne model of infection we used here and they found no significant differences between young and aged mice when examining CFU counts in the spleen, liver, or mesenteric lymph nodes (42). They attributed this in part to the accumulation of a population of protective CD44hiCD27neg Vα4 mucosal T cells in the aged animals. Thus, the differences we observed in aged animals are likely to be linked to specific changes in ApoE variant protein function rather than a general feature of immune senescence.
Although females are typically thought to have enhanced Th1-type inflammatory responses when compared to males in many model systems, it has long been known that the opposite is true with regard to susceptibility to L. monocytogenes. Pasche et al. showed that female mice of several different strain backgrounds had higher mortality and increased bacterial loads in tissues which correlated with increased production of immune suppressive IL-10 early during infection (43). Other sex hormone-dependent mechanisms have also been proposed. For example, elevated serum progesterone in mice was shown to cause decreased bystander activation of memory CD8+ T cells resulting in lower IFNγ production and less protection against Listeria challenge (44). Rapid IFNγ production by CD44hi memory phenotype cells is known to be a critical component of innate resistance to infection of C57BL6 mice (45 - 47). Likewise, estrogen endogenously produced by females or administered to males also increased susceptibility to Listeria infection (44, 48). The female mice used in this study were not manipulated to be on synchronous estrous cycles, so it is conceivable that differences in systemic hormones among the young females caused greater spread in the CFU data. However, the aged mice we used would be expected to be well into reproductive senescence (49), and thus cycling hormones would no longer be an issue.
Most of the early work examining the effect of sex or age on susceptibility to listeriosis used the intravenous inoculation model. We chose to use the foodborne mouse model here not only because it is more physiologically relevant, but also because several studies have indicated a potential link between ApoE and the gut microbiome. For example, ApoE-deficient young mice fed a high-fat diet for 6 mo had some changes in gut microbiota composition relative to wildtype mice including a lower Firmicutes to Bacteroidetes ratio (50). More recently, Parikh et al. showed that beta diversity in 4- to 6-mo-old APOE-TR mice (human E2, E3, and E4) was strongly associated with APOE genotype (51); that study both confirmed and extended previous findings that had compared the microbiomes of only E3 and E4 mice (52). Given that susceptibility to low-dose foodborne listeriosis is highly dependent on an ability to compete with the resident gut microbiota (53, 54), we expected that the microbiome changes associated with APOE genotype might result in differences in bacterial burdens in the gut lumen. However, since that was not what we observed in this study, it suggests that the small changes in gut microbiota associated with APOE genotype are not sufficient to alter the ability of the L. monocytogenes to persist in the intestines. Consistent with this idea, we previously showed that fecal transplantation in young animals did not transfer the enhanced resistance of C57BL/6 mice to susceptible BALB/cByJ mice (55). Alam et al. showed that aged C57BL/6J mice had fewer Lactobacillaceae than young mice (56), and some strains of L. monocytogenes do secrete a bacteriocin called Listeriolysin S (LLS) that can specifically target other Firmicutes (57) to promote gut colonization, but the strain used in this study does not express LLS. Together, these data suggest that a drastic shift in the composition or density of the microbiota is needed to alter susceptibility to foodborne listeriosis.
Enhanced resistance to infection in E4 mice attributed to a hyperinflammatory state has been observed with other microbial pathogens. For example, Azevedo et al. showed that E4 mice were more protected from oral Cryptosporidium parvum challenge than E3 mice (58). In that study, the mice were fed a low-protein diet following infection to mimic the high incidence of this waterborne parasitic protozoan disease in malnourished children and they found elevated levels of IL-1β, IFNγ, IL-17, and TNFα in the ileums of the E4 mice. We only examined systemic cytokines in this study, but L. monocytogenes is more invasive than Cryptosporidium, which remains in the gut. Azevedo et al. further attributed the enhanced resistance to infection to an upregulation of L-arginine selective cationic protein transporter (CAT-1) which could enhance the barrier function of intestinal epithelial cells and increase the expression of NOS (58), and these are both protective functions that could alter susceptibility to systemic listeriosis. The cecal ligation and puncture model of peritonitis has also been examined in both E3 and E4 mice. As predicted, increased and more rapid mortality was observed in the E4 mice which had higher serum levels of multiple cytokines that presumably contributed to the “cytokine storm” associated with sepsis (59). However, Ostendorf et al. recently showed that both E2 and E4 mice had increased viral loads and higher mortality following infection with SARS-CoV-2 (60), which suggests that the established paradigm of E4 > E3 > E2 inflammatory responses does not always translate to a pattern of infection resistance, where the E4 genotype is the most protective.
We were surprised to find that neither macrophages nor splenocytes from E4 mice produced significantly more TNFα than either E3 or E2 cells in response to L. monocytogenes infection. ApoE-deficient mice had increased serum TNFα 3 dpi (16), and the E4 protein is predicted to be the least proficient at suppressing inflammation. Indeed, Vitek et al. compared the cytokine profiles of both microglia and peritoneal macrophages isolated from APOE-TR mice and found a modest increase in IL-6, IL-12, and TNFα expression by the E4 cells after in vitro stimulation with LPS plus IFNγ (15). Likewise, Gale et al. stimulated whole blood from healthy adult volunteers with a variety of TLR ligands and found elevated TNFα production from people carrying an E4 allele (11). They also found elevated serum TNFα levels associated with the E4 genotype when APOE-TR mice were injected directly with LPS. L. monocytogenes lacks LPS but have other cell wall components such as lipoteichoic acid that can be recognized by TLR2 to initiate the MyD88- and NFκB-dependent signaling pathways that lead to TNFα secretion. The bone marrow macrophages used here were plated at a relatively high density, and that could have affected the phagocytic capacity and overall level of cytokines produced (61), but would not be expected to affect the E4 cells disproportionately since bone marrow from all of the APOE-TR mice were plated at the same density. However, the baseline higher levels of iNOS expressed by E4 cells may preclude there being a high enough bacterial burden in the cells to fully induce cytokine expression.
Our study differs from the previous work mainly in that we used natural infection as the immune stimulus rather than purified TLR ligands. Thus, it is possible that E4-associated increases in cytokine production occur only when TLR stimulation occurs at a very high, consistent level as can be achieved with in vitro stimulation with perhaps super-physiological concentrations of bacterial cell wall components such as LPS. ApoE can directly bind free LPS and target it for degradation in the liver (62), and since both humans and rodents with an E4 genotype respond more robustly to injections of LPS (11), it is possible that the E4 variant does not bind LPS with the same efficiency and that is the basis for the hyperinflammatory state previously described. LPS has been linked to many of the neurodegenerative changes that occur in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, and an endotoxin hypothesis underlying all neurodegeneration has been proposed (63).
In summary, we found that resistance to foodborne listeriosis is a complex phenotype that was not completely linked to APOE genotype in all animals but instead had a direct effect only in aged males or macrophages derived from aged mice. It should be noted that all our experiments involving macrophages or splenocytes used biological replicates (cells that were derived from the bone marrow of different mice), rather than technical replicates (multiple wells of cells seeded from the same bone marrow). The somewhat unexpected amount of variation we observed for the cytokine responses of these cells following exposure to L. monocytogenes as well as the CFU burdens in the tissues of mice infected in vivo highlights the idea that the inflammatory phenotype associated with APOE genotype varies considerably among individuals and underscores the need for further study to isolate additional biological variables.
ACKNOWLEDGMENTS
We thank Dr. Don Cohen for assistance with the Luminex assays, Gabriela Hernandez for mouse husbandry, and Hiba Khan and Tanner Durst for assistance with processing tissue samples. The UK Flow Cytometry & Immune Monitoring core facility is supported in part by the Office of the Vice President for Research, the Markey Cancer Center, and an NCI Center Core Support Grant (P30 CA177558) to the University of Kentucky Markey Cancer Center.
This work was supported by Health and Human Services (HHS)/National Institutes of Health (NIH)/National Institute on Aging grant R56AG057589 to S.E. S.E.F.D. acknowledges the support of HHS/NIH/National Institute of Allergy and Infectious Disease grant R56AI132410. L.A.J. was supported by the National Institute on Aging (R01AG060056, R01AG062550, and R01AG080589) and Alzheimer’s Association.
L.A.J., S.E., and S.E.F.D participated in the conceptualization and design of the study. validation: J.C. formal analysis: J.C., K.A., and S.E.F.D.; investigation: J.C., J.F., K.A., and S.E.F.D. participated in the acquisition, analysis, and interpretation of data. L.A.J. provided the mice used in this study. J.C., K.A., and S.E.F.D. wrote the first draft of this manuscript and all authors participated in editing the manuscript.
The authors declare no conflict of interest.
Contributor Information
Sarah E. F. D’Orazio, Email: sarah.dorazio@uky.edu.
Andreas J. Bäumler, University of California, Davis, California, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00251-23.
Additional data to support results shown in Fig. 7.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Portnoy DA, Auerbuch V, Glomski IJ. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol 158:409–414. doi: 10.1083/jcb.200205009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Orazio SEF. 2019. Innate and adaptive immune responses during Listeria Monocytogenes infection. Microbiol Spectr 7:1–40. doi: 10.1128/microbiolspec.GPP3-0065-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eshleman EM, Delgado C, Kearney SJ, Friedman RS, Lenz LL. 2017. Down regulation of macrophage IFNGR1 exacerbates systemic L. monocytogenes infection. PLoS Pathog 13:e1006388. doi: 10.1371/journal.ppat.1006388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Radoshevich L, Impens F, Ribet D, Quereda JJ, Nam Tham T, Nahori M-A, Bierne H, Dussurget O, Pizarro-Cerdá J, Knobeloch K-P, Cossart P. 2015. ISG15 counteracts Listeria monocytogenes infection. Elife 4:e06848. doi: 10.7554/eLife.06848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoggins JW. 2019. Interferon-stimulated genes: what do they all do? Annu Rev Virol 6:567–584. doi: 10.1146/annurev-virology-092818-015756 [DOI] [PubMed] [Google Scholar]
- 6. Portnoy DA, Schreiber RD, Connelly P, Tilney LG. 1989. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med 170:2141–2146. doi: 10.1084/jem.170.6.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaughnessy LM, Swanson JA. 2007. The role of the activated macrophage in clearing Listeria monocytogenes infection. Front Biosci 12:2683–2692. doi: 10.2741/2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malik M, Parikh I, Vasquez JB, Smith C, Tai L, Bu G, LaDu MJ, Fardo DW, Rebeck GW, Estus S. 2015. Genetics ignite focus on microglial inflammation in Alzheimer's disease. Mol Neurodegener 10:52. doi: 10.1186/s13024-015-0048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261:921–923. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 10. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, Huentelman MJ, Beach TG, Caselli RJ, Chen Y, Su Y, Myers AJ, Hardy J, Paul Vonsattel J, Younkin SG, Bennett DA, De Jager PL, Larson EB, Crane PK, Keene CD, Kamboh MI, Kofler JK, Duque L, Gilbert JR, Gwirtsman HE, Buxbaum JD, Dickson DW, Frosch MP, Ghetti BF, Lunetta KL, Wang L-S, Hyman BT, Kukull WA, Foroud T, Haines JL, Mayeux RP, Pericak-Vance MA, Schneider JA, Trojanowski JQ, Farrer LA, Schellenberg GD, Beecham GW, Montine TJ, Jun GR, Alzheimer’s Disease Genetics Consortium . 2020. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun 11:667. doi: 10.1038/s41467-019-14279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T, Madenspacher JH, Draper DW, Ge W, Aloor JJ, Azzam KM, Lai L, Blackshear PJ, Calvano SE, Barnes KC, Lowry SF, Corbett S, Wurfel MM, Fessler MB. 2014. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol 134:127–134. doi: 10.1016/j.jaci.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ. 2012. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 60:559–569. doi: 10.1002/glia.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Y, Mahley RW. 2014. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis 72 Pt A:3–12. doi: 10.1016/j.nbd.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M, Lei AZ, Bahroos N, Green SJ, Hendrickson B, Van Eldik LJ, LaDu MJ. 2015. APOE-modulated Aβ-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective. J Neurochem 133:465–488. doi: 10.1111/jnc.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitek MP, Brown CM, Colton CA. 2009. APOE genotype-specific differences in the innate immune response. Neurobiol Aging 30:1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roselaar SE, Daugherty A. 1998. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res 39:1740–1743. doi: 10.1016/S0022-2275(20)32160-X [DOI] [PubMed] [Google Scholar]
- 17. de Bont N, Netea MG, Demacker PN, Kullberg BJ, van der Meer JW, Stalenhoef AF. 2000. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur J Clin Invest 30:818–822. doi: 10.1046/j.1365-2362.2000.00715.x [DOI] [PubMed] [Google Scholar]
- 18. Toledo A, Monzón JD, Coleman JL, Garcia-Monco JC, Benach JL. 2015. Hypercholesterolemia and APOE deficiency result in severe infection with Lyme disease and relapsing-fever Borrelia. Proc Natl Acad Sci U S A 112:5491–5496. doi: 10.1073/pnas.1502561112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egert S, Rimbach G, Huebbe P. 2012. Apoe genotype: from geographic distribution to function and responsiveness to dietary factors. Proc Nutr Soc 71:410–424. doi: 10.1017/S0029665112000249 [DOI] [PubMed] [Google Scholar]
- 20. Jones GS, Bussell KM, Myers-Morales T, Fieldhouse AM, Bou Ghanem EN, D’Orazio SEF. 2015. Intracellular Listeria monocytogenes comprises a minimal but vital fraction of the intestinal burden following foodborne infection. Infect Immun 83:3146–3156. doi: 10.1128/IAI.00503-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bou Ghanem EN, Jones GS, Myers-Morales T, Patil PD, Hidayatullah AN, D’Orazio SEF. 2012. InlA promotes dissemination of Listeria monocytogenes to the mesenteric lymph nodes during food borne infection of mice. PLoS Pathog 8:e1003015. doi: 10.1371/journal.ppat.1003015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wollert T, Pasche B, Rochon M, Deppenmeier S, van den Heuvel J, Gruber AD, Heinz DW, Lengeling A, Schubert W-D. 2007. Extending the host range of Listeria monocytogenes by rational protein design. Cell 129:891–902. doi: 10.1016/j.cell.2007.03.049 [DOI] [PubMed] [Google Scholar]
- 23. Jones GS, D’Orazio SEF. 2013. Listeria monocytogenes: cultivation and laboratory maintenance. Curr Protoc Microbiol 31:9B. doi: 10.1002/9780471729259.mc09b02s31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. 1997. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 272:17972–17980. doi: 10.1074/jbc.272.29.17972 [DOI] [PubMed] [Google Scholar]
- 25. Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. 1998. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse APOE with human APOE*2. J Clin Invest 102:130–135. doi: 10.1172/JCI2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. 1999. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest 103:1579–1586. doi: 10.1172/JCI6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farmer BC, Williams HC, Devanney NA, Piron MA, Nation GK, Carter DJ, Walsh AE, Khanal R, Young LEA, Kluemper JC, Hernandez G, Allenger EJ, Mooney R, Golden LR, Smith CT, Brandon JA, Gupta VA, Kern PA, Gentry MS, Morganti JM, Sun RC, Johnson LA. 2021. APOε4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis. Mol Neurodegener 16:62. doi: 10.1186/s13024-021-00483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92. doi: 10.1016/s0022-1759(98)00204-x [DOI] [PubMed] [Google Scholar]
- 29. Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. 2009. Isolation of dendritic cells. Curr Protoc Immunol 3:3. doi: 10.1002/0471142735.im0307s86 [DOI] [PubMed] [Google Scholar]
- 30. Li M, Wang J, Fang Y, Gong S, Li M, Wu M, Lai X, Zeng G, Wang Y, Yang K, Huang X. 2016. MicroRNA-146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci Rep 6:23351. doi: 10.1038/srep23351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suwanpradid J, Shih M, Pontius L, Yang B, Birukova A, Guttman-Yassky E, Corcoran DL, Que LG, Tighe RM, MacLeod AS. 2017. Arginase1 deficiency in monocytes/macrophages upregulates inducible nitric oxide synthase to promote cutaneous contact hypersensitivity. J Immunol 199:1827–1834. doi: 10.4049/jimmunol.1700739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 33. Bou Ghanem EN, Myers-Morales T, Jones GS, D’Orazio SEF. 2013. Oral transmission of Listeria Monocytogenes in mice via ingestion of contaminated food. J Vis Exp 75:e50381. doi: 10.3791/50381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bou Ghanem EN, Myers-Morales T, D’Orazio SEF. 2013. A mouse model of foodborne Listeria monocytogenes infection. Curr Protoc Microbiol 31:9B. doi: 10.1002/9780471729259.mc09b03s31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29–38. doi: 10.1016/s1074-7613(00)80004-7 [DOI] [PubMed] [Google Scholar]
- 36. Melton-Witt JA, Rafelski SM, Portnoy DA, Bakardjiev AI. 2012. Oral infection with signature-tagged Listeria monocytogenes reveals organ-specific growth and dissemination routes in guinea pigs. Infect Immun 80:720–732. doi: 10.1128/IAI.05958-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qu L, Matz AJ, Karlinsey K, Cao Z, Vella AT, Zhou B. 2022. Macrophages at the crossroad of meta-inflammation and inflammaging. Genes (Basel) 13:2074. doi: 10.3390/genes13112074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandez CG, Hamby ME, McReynolds ML, Ray WJ. 2019. The role of Apoe4 in disrupting the Homeostatic functions of Astrocytes and Microglia in aging and Alzheimer's disease. Front Aging Neurosci 11:14. doi: 10.3389/fnagi.2019.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel PJ. 1981. Aging and cellular defense mechanisms: age-related changes in resistance of mice to Listeria monocytogenes. Infect Immun 32:557–562. doi: 10.1128/iai.32.2.557-562.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smithey MJ, Renkema KR, Rudd BD, Nikolich-Žugich J. 2011. Increased apoptosis, curtailed expansion and incomplete differentiation of CD8+ T cells combine to decrease clearance of L. monocytogenes in old mice. Eur J Immunol 41:1352–1364. doi: 10.1002/eji.201041141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, Smithey MJ, Rudd BD, Nikolich-Žugich J. 2012. Age-associated alterations in CD8α+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium. Aging Cell 11:968–977. doi: 10.1111/j.1474-9726.2012.00867.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khairallah C, Chu TH, Qiu Z, Imperato JN, Yang D, Sheridan BS. 2022. The accumulation of Vγ4 T cells with aging is associated with an increased adaptive Vγ4 T cell response after foodborne Listeria monocytogenes infection of mice. Immun Ageing 19:19. doi: 10.1186/s12979-022-00275-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pasche B, Kalaydjiev S, Franz TJ, Kremmer E, Gailus-Durner V, Fuchs H, Hrabé de Angelis M, Lengeling A, Busch DH. 2005. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun 73:5952–5960. doi: 10.1128/IAI.73.9.5952-5960.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao Y, Li H, Ding J, Xia Y, Wang L. 2017. Progesterone impairs antigen-non-specific immune protection by CD8 T memory cells via interferon-γ gene hypermethylation. PLoS Pathog 13:e1006736. doi: 10.1371/journal.ppat.1006736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berg RE, Crossley E, Murray S, Forman J. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med 198:1583–1593. doi: 10.1084/jem.20031051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berg RE, Cordes CJ, Forman J. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol 32:2807–2816. doi: [DOI] [PubMed] [Google Scholar]
- 47. Bou Ghanem EN, Nelson CC, D’Orazio SEF. 2011. T cell-intrinsic factors contribute to the differential ability of CD8+ T cells to rapidly secrete IFN-γ in the absence of antigen. J Immunol 186:1703–1712. doi: 10.4049/jimmunol.1001960 [DOI] [PubMed] [Google Scholar]
- 48. Pung OJ, Luster MI, Hayes HT, Rader J. 1984. Influence of steroidal and nonsteroidal sex hormones on host resistance in mice: increased susceptibility to Listeria monocytogenes after exposure to estrogenic hormones. Infect Immun 46:301–307. doi: 10.1128/iai.46.2.301-307.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diaz Brinton R. 2012. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology 153:3571–3578. doi: 10.1210/en.2012-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saita D, Ferrarese R, Foglieni C, Esposito A, Canu T, Perani L, Ceresola ER, Visconti L, Burioni R, Clementi M, Canducci F. 2016. Adaptive immunity against gut microbiota enhances apoE-mediated immune regulation and reduces atherosclerosis and western-diet-related inflammation. Sci Rep 6:29353. doi: 10.1038/srep29353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parikh IJ, Estus JL, Zajac DJ, Malik M, Maldonado Weng J, Tai LM, Chlipala GE, LaDu MJ, Green SJ, Estus S. 2020. Murine gut microbiome association with APOE alleles. Front Immunol 11:200. doi: 10.3389/fimmu.2020.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tran TTT, Corsini S, Kellingray L, Hegarty C, Le Gall G, Narbad A, Müller M, Tejera N, O’Toole PW, Minihane A-M, Vauzour D. 2019. APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer's disease pathophysiology. FASEB J 33:8221–8231. doi: 10.1096/fj.201900071R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Becattini S, Littmann ER, Carter RA, Kim SG, Morjaria SM, Ling L, Gyaltshen Y, Fontana E, Taur Y, Leiner IM, Pamer EG. 2017. Commensal microbes provide first line defense against Listeria monocytogenes infection. J Exp Med 214:1973–1989. doi: 10.1084/jem.20170495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tucker JS, Cho J, Albrecht TM, Ferrell JL, D’Orazio SEF. 2023. Egress of Listeria monocytogenes from mesenteric lymph nodes depends on intracellular replication and cell-to-cell spread. Infect Immun 91:e0006423. doi: 10.1128/iai.00064-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Myers-Morales T, Bussell KM, D’Orazio SE. 2013. Fecal transplantation does not transfer either susceptibility or resistance to food borne listeriosis in C57BL/6 and BALB/c/by mice. F1000Res 2:177. doi: 10.12688/f1000research.2-177.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alam MS, Gangiredla J, Hasan NA, Barnaba T, Tartera C. 2021. Aging-induced dysbiosis of gut microbiota as a risk factor for increased Listeria monocytogenes infection. Front Immunol 12:672353. doi: 10.3389/fimmu.2021.672353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quereda JJ, Dussurget O, Nahori M-A, Ghozlane A, Volant S, Dillies M-A, Regnault B, Kennedy S, Mondot S, Villoing B, Cossart P, Pizarro-Cerda J. 2016. Bacteriocin from epidemic Listeria strains alters the host intestinal microbiota to favor infection. Proc Natl Acad Sci U S A 113:5706–5711. doi: 10.1073/pnas.1523899113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Azevedo OGR, Bolick DT, Roche JK, Pinkerton RF, Lima AAM, Vitek MP, Warren CA, Oriá RB, Guerrant RL. 2014. Apolipoprotein E plays a key role against cryptosporidial infection in transgenic undernourished mice. PLoS One 9:e89562. doi: 10.1371/journal.pone.0089562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang H, Christensen DJ, Vitek MP, Sullivan PM, Laskowitz DT. 2009. APOE genotype affects outcome in a murine model of sepsis: implications for a new treatment strategy. Anaesth Intensive Care 37:38–45. doi: 10.1177/0310057X0903700111 [DOI] [PubMed] [Google Scholar]
- 60. Ostendorf BN, Patel MA, Bilanovic J, Hoffmann H-H, Carrasco SE, Rice CM, Tavazoie SF. 2022. Common human genetic variants of APOE impact murine COVID-19 mortality. Nature 611:346–351. doi: 10.1038/s41586-022-05344-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee CM, Hu J. 2013. Cell density during differentiation can alter the phenotype of bone marrow-derived macrophages. Cell Biosci 3:30. doi: 10.1186/2045-3701-3-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Petruk G, Elvén M, Hartman E, Davoudi M, Schmidtchen A, Puthia M, Petrlova J. 2021. The role of full-length apoE in clearance of gram-negative bacteria and their endotoxins. J Lipid Res 62:100086. doi: 10.1016/j.jlr.2021.100086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown GC. 2019. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation 16:180. doi: 10.1186/s12974-019-1564-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional data to support results shown in Fig. 7.