ABSTRACT
Microbial species colonizing host ecosystems in health or disease rarely do so alone. Organisms conglomerate into dynamic heterotypic communities or biofilms in which interspecies and interkingdom interactions drive functional specialization of constituent species and shape community properties, including nososymbiocity or pathogenic potential. Cell-to-cell binding, exchange of signaling molecules, and nutritional codependencies can all contribute to the emergent properties of these communities. Spatial constraints defined by community architecture also determine overall community function. Multilayered interactions thus occur between individual pairs of organisms, and the relative impact can be determined by contextual cues. Host responses to heterotypic communities and impact on host surfaces are also driven by the collective action of the community. Additionally, the range of interspecies interactions can be extended by bacteria utilizing host cells or host diet to indirectly or directly influence the properties of other organisms and the community microenvironment. In contexts where communities transition to a dysbiotic state, their quasi-organismal nature imparts adaptability to nutritional availability and facilitates resistance to immune effectors and, moreover, exploits inflammatory and acidic microenvironments for their persistence.
KEYWORDS: polymicrobial community, dental caries, periodontal disease
INTRODUCTION
The strength of the pack is the wolf
And the strength of the wolf is the pack
—Rudyard Kipling from The Jungle Book
The polymicrobial communities that inhabit host surfaces and mucosal barriers are complex and dynamic, as well as compositionally and spatially heterotypic. Integration of spatially contextualized information from partner species and from the host microenvironment drives the emergence of community-specific properties, and the community can operate as a functionally cohesive, or quasi-organismal, unit (1). Underlying the development of a polymicrobial community is a regulatory system of interconnected switches and rheostats, which operate both transcriptionally and posttranscriptionally, and which calibrate the pathogenic potential, or nososymbiocity, of the community. Indeed, it is the community in toto that constitutes the etiological agent in many cases of disease at mucosal membranes and on host surfaces. Consequently, host responses should be considered with regard to the community in its entirety rather than to individual organisms.
While the principles of interbacterial communication are applicable in communities at any anatomical site, we shall focus here on the oral ecosystem in which many of the core concepts were originally established (2 - 5). The oral cavity comprises a diverse ecosystem containing an abundant microbiota and both hard and soft tissues; nonetheless, unlike other mucosal environments, for example, the gastrointestinal tract, the ecosystem of the oral cavity is readily accessible. Thus, the microbiome associated with tissue- and site-specific diseases, such as dental caries and periodontal disease, can be sampled and studied exclusively. An intricate network of microbe-microbe and microbe-host interconnectivity has been uncovered, which can receive input in the form of physical or chemical signals, and which allows organisms to sense and respond to neighboring cells to coordinate group behavior and adapt to the microenvironment (6). While the extent of interspecies communication is potentially limitless, with hundreds of species capable of colonizing the mouth, the in situ configuration suggests that individual organisms have a more restricted sphere of influence. Fluorescent image analysis of subgingival communities shows that any one organism has a limited range of binding partners within distinct spatial structures, while specific microbes in supragingival biofilms can cluster themselves to create a localized pathogenic niche (7 - 9) (Fig. 1A). This parsimony of association facilitates the development of tractable and scalable models for the study of community development. Interestingly, as we shall explore, responses of an organism to partners show a significant degree of specificity for each pairing (10, 11), which may facilitate rapid adaptation to emerging conditions and allow organisms to have differential influence of community properties.
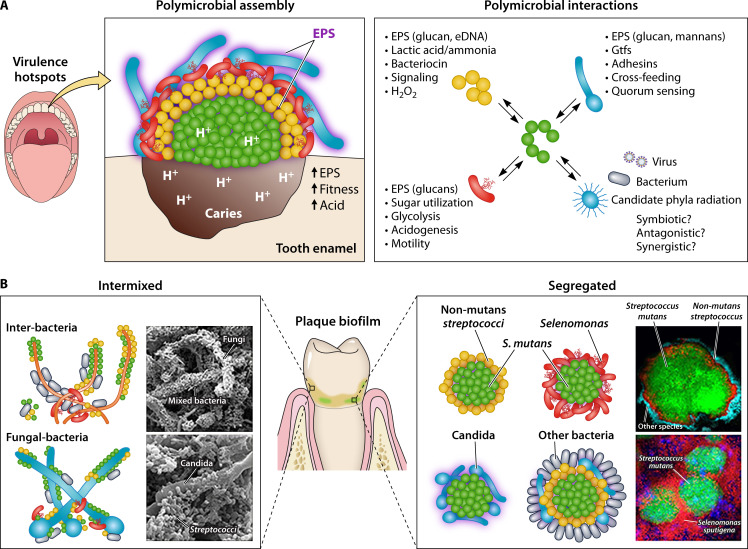
Fig 1.
Interbacterial and interkingdom interactions in oral polymicrobial communities. (A) The oral microbiota harbors a multitude of different microbes, including bacteria, fungi, viruses, and ultra-small organisms. These diverse microbial populations engage in complex interspecies or cross-kingdom interactions which drive cooperative, competitive, or both outcomes among community members. Certain species, such as Streptococcus mutans, form highly clustered communities with precise spatial structure at the infection site (supragingival) which promotes a disease-causing state (dental caries). (B) Complex physical and chemical interactions with different species promote a multilayered, corona-like spatial arrangement formed by an inner core composed almost exclusively of S. mutans and outer layers of other oral microbes, physically separated by extracellular polymeric substances. This spatial structure enhances bacterial fitness and protection, and creates a highly acidic microenvironment, leading to the localized onset of disease. Precise positioning and spatial arrangement combined with polymicrobial interactions can coordinate pathogenesis in situ to create virulence hotspots impacting the host tissues. [Adapted from reference (12) with permission from Elsevier.]
Interbacterial dialogue
The overall community developmental process of coadhesion and physiological integration belies an often multidimensional array of interbacterial interactions which can have differing outcomes depending on contextual cues. An overview of documented oral bacterial interactions is presented in Table 1, and these are extensively discussed in several excellent recent reviews (4, 13 - 16). In general, specialized adhesins mediate interbacterial binding which facilitates exchange of metabolites and other small molecules. Bacteria may utilize the information conveyed by attachment to a partner species to optimize fitness, resulting in new physiological functions that cannot be achieved by individual constituents alone. Although not yet resolved in oral bacteria, the outer membrane of other organisms, such as in Escherichia coli, can sense stressors including mechanical changes resulting from adhesion (17). In one such pathway, the lipoprotein NlpE senses surface adhesion and activates both the Cpx and BaeSR two-component systems, thus initiating an adhesion-dependent pattern of gene expression (18 - 20). Physiological integration can then involve cross-feeding or progressive metabolism of complex substrates (21). Increased fitness of community organisms has implications for pathogenicity, as in some instances virulence factors, for example, proteases, have dual functionality involving nutrient uptake in addition to tissue destruction. Indeed, current models of periodontal disease pathogenicity incorporate the role of interbacterial communication. In the polymicrobial synergy and dysbiosis (PSD) model, integration into subgingival communities of pathogens such as Porphyromonas gingivalis, even at low number, precipitates disruption of host homeostasis (22, 23). P. gingivalis thus fulfills the criteria for a keystone species; one whose supportive influence on its community is inordinately large relative to its abundance, thereby constituting the “keystone” of the community’s structure. A keystone pathogen, therefore, is a quantitatively minor but functionally critical component of a disease-provoking microbiota. For instance, at least in the mouse host, P. gingivalis fulfills both criteria (low relative abundance and disproportionately large impact) by manipulating host immunity and inflammation in ways that promote the nososymbiocity and persistence of its community, while present at <0.01% of the total bacterial load (22). The concept of keystone pathogen should be distinguished from the action of other important pathogenic species, such as dominant pathogens, which can have a large impact on their communities and the host simply by virtue of their outsize biomass. Interestingly, as we shall explore later, the role played by individual species can vary, and organisms that contribute to dysbiosis in one context can help maintain homeostasis in another.
TABLE 1.
Interspecies interactions among oral bacteria
| Organisms | Interactions | References |
|---|---|---|
| Interactions within communities | ||
| Streptococcus gordonii-Porphyromonas gingivalis | Context-dependent regulation of consortia pathogenic potential. P. gingivalis exhibits modulation of tyrosine phosphorylation-dependent signaling; regulation of genes encoding fimbrial adhesins; upregulation of genes associated with oxidative stress resistance; increased gingipain activity and hemin acquisition; differential flux through one-carbon metabolism (OCM) pathways. Streptococcal AI-2 regulates expression of genes involved in carbohydrate metabolism in P. gingivalis. Short-chain fatty acids from P. gingivalis inhibit the competence-stimulating peptide (CSP) quorum-sensing system of S. gordonii. | (24 - 30) |
| S. gordonii-Veillonella parvula | Consortia-dependent increased expression of streptococcal genes associated with carbohydrate metabolism and increased expression of oxidative stress-related processes in V. parvula. | (31) |
| S. gordonii-Fusobacterium nucleatum | S. gordonii genes involved in the biosynthesis and export of cell wall proteins and carbohydrate metabolism, and F. nucleatum genes associated with translation, protein export, and sialic acid metabolism are differentially regulated in consortia which have increased survival within macrophages. | (32, 33) |
| S. sanguinis-F. nucleatum | In a consortium, F. nucleatum masks the surface components recognized by H2O2 producing mouse oral microbiome constituents. | (34) |
| S. gordonii-Actinomyces oris | S. gordonii scavenges arginine from A. oris through the extracellular protease challisin which results in downregulation of streptococcal genes involved in arginine biosynthesis; actinomyces catalase can protect streptococci from oxidative damage. | (35, 36) |
| S. gordonii-Aggregatibacter actinomycetemcomitans (Aa) | Consortia are synergistically virulent, and Aa displays: a shift from fermentative to respiratory metabolism (cross-respiration); preferential utilization of lactate through carbon resource partitioning; increased production of catalase and complement resistance protein ApiA; regulation of iron uptake mechanisms which lead to modulation of dispersin B production and remodeling of the extracellular matrix to ensure optimal distance from S. gordonii. | (37 - 40) |
| S. parasanguinis-Aa | Aa promotes accumulation of S. parasanguinis through modulating the production of H2O2 by fine-tuning the expression of pyruvate oxidase. | (41) |
| S. gordonii-P. gingivalis, Prevotella intermedia, Tannerella forsythia | S. gordonii GAPDH binds heme and forms a reservoir that can be sequestered by HmuY of P. gingivalis, PinO of Pr. intermedia or Tfo of Ta. forsythia. | (42) |
| S. sanguinis, Aa-P. gingivalis | Increased catalase production by Aa in consortia protects P. gingivalis from H2O2 produced by S. sanguinis. | (43) |
| S. intermedius, S. cristatus-P. gingivalis | Streptococcal arginine deiminase (ArcA) represses expression of genes encoding fimbriae and gingipains in P. gingivalis. | (44, 45) |
| Mitis Group Streptococci (MGS)-Haemophilus parainfluenzae | MGS provide NAD and evoke distinct patterns of carbon utilization in H. parainfluenzae which is resistant to streptococcal H2O2. | (46) |
| V. parvula-P. gingivalis | V. parvula produces a cell-density-dependent soluble molecule which stimulates P. gingivalis growth at low cell density and enhances in vivo virulence. | (47) |
| V. parvula-F. nucleatum, S. gordonii | V. parvula catalase protects F. nucleatum in microaerophilic conditions and from streptococcal H2O2. | (48) |
|
V. parvula-F. nucleatum
S. gordonii-F. nucleatum |
V. parvula and S. gordonii increase amino acid availability for F. nucleatum, resulting in enhanced production of fermented and decarboxylated metabolites. | (49) |
| V. parvula, S. gordonii, F. nucleatum-P. gingivalis | Cross-feeding of F. nucleatum with V. parvula and S. gordonii increases polyamine production which accelerated accumulation with P. gingivalis and subsequent dispersal of planktonic cells. | (49) |
| Corynebacterium durum-S. sanguinis | Fatty acids produced by C. durum increase streptococcal chain length and promote resistance to phagocytosis. | (50) |
| P. gingivalis-S. mitis | P. gingivalis induces expression of transposases and cell death of S. mitis. | (51) |
| MGS-S. mutans | H2O2 from MGS inhibit S. mutans growth; however, this can be mitigated by pyruvate secretion which depletes H2O2; S. mutans lantibiotic and non-lantibiotic bacteriocins, tryglysin peptides, and tetramic acids are toxic to MGS; MGS block competence stimulating peptide and comX-inducing peptide (XIP) signaling and induce a common contact-dependent pattern of differential gene expression in S. mutans; a streptococcal protease (challisin) can degrade CSP and suppress mutacin gene expression; AI-2 from S. gordonii stimulates biofilm formation and regulates virulence gene expression in S. mutans; SsnA DNase of S. gordonii inhibits accumulation of S. mutans. | (52 - 58) |
| MGS, S. mutans-Veillonella | S. mutans produces lactate for Veillonella metabolism; Veillonella enhances expression of S. gordonii α-amylase to increase release of lactate. | (59, 60) |
| V. parvula-S. gordonii, S. mutans | V. parvula can protect S. mutans from H2O2 produced by S. gordonii and increases the expression of genes required for the uptake and metabolism of sugars in S. mutans. | (61) |
| S. parasanguinis-S. mutans, Candida albicans | S. parasanguinis accumulation in consortia is promoted by nitrite through upregulation of reactive nitrogen species (RNS) scavengers. Nitrite drives the metabolic signature of the consortia and restricts virulence factor production. | (62) |
| F. nucleatum-P. gingivalis | F. nucleatum can remove oxygen and promote the growth of P. gingivalis. | (63, 64) |
| F. nucleatum-P. gingivalis, T. denticola, Ta. forsythia | AI-2 from F. nucleatum induces expression of adhesins in P. gingivalis, T. denticola, and Ta. forsythia, and enhances consortia formation. | (65) |
| P. gingivalis-Pr. intermedia | Pr. intermedia interpain protease extracts heme from hemoglobin and converts to methemoglobin which is a substrate for the extraction of iron(III) protoporphyrin IX by HmuY of P. gingivalis. | (66, 67) |
| T. denticola-P. gingivalis | Glycine, isobutyrate, and thiamine produced by P. gingivalis stimulate the growth of T. denticola. Succinate and OCM metabolites produced by T. denticola stimulate glycine production and growth of P. gingivalis; T. denticola increases the expression of hemagglutinin adhesin domain proteins in P. gingivalis which enhances P. gingivalis adhesive capabilities. | (68 - 71) |
| Aa-P. gingivalis | AI-2 from Aa can regulate expression of genes involved in stress resistance and iron uptake in P. gingivalis. | (72) |
| T. denticola-P. gingivalis, F. nucleatum | Genes encoding T. denticola major antigens are suppressed by P. gingivalis and F. nucleatum. | (73) |
| Interactions involving host cells | ||
| S. gordonii-P. gingivalis | Streptococcal H2O2 incapacitates P. gingivalis gingipain and prevents Notch activation of epithelial cells; S. gordonii activates the TAK-NLK pathway and blocks P. gingivalis mobilization of FOXO1; S. gordonii induces a transcriptional profile which mitigates the impact of P. gingivalis. | (74 - 76) |
| S. gordonii-P. gingivalis | Spent culture supernatant of S. gordonii suppresses inflammatory responses of epithelial cells, fibroblasts, and macrophages to P. gingivalis lipopolysaccharide. | (77) |
| P. gingivalis-F. nucleatum | P. gingivalis suppresses endocytic pathway-mediated inflammasome activation in macrophages and prevents activation by F. nucleatum; in neutrophils P. gingivalis induces Toll-like receptor 2 (TLR2)-C5aR which activates PI3K and protects F. nucleatum from phagocytosis; P. gingivalis capsule-mediated association augments epithelial cell invasion by P. gingivalis. | (78 - 80) |
| S. gordonii-F. nucleatum | Coaggregation inhibits epithelial cell apoptosis and promotes secretion of tumor necrosis factor and interleukin (IL)-6. | (81) |
| S. gordonii, F. nucleatum-P. gingivalis | Consortium growth causes an increase in Mfa1 expression in P. gingivalis and elevated invasion of dendritic cells by P. gingivalis and F. nucleatum. | (82) |
| S. cristatus-F. nucleatum | S. cristatus stabilizes IκB-α in epithelial cells, blocking nuclear factor kappa B (NF-κB) activation and cytokine secretion induced by F. nucleatum. | (83) |
| P. gingivalis-F. nucleatum | The P. gingivalis serine phosphatase SerB dephosphorylates the p65 NF-κB subunit, blocking nuclear translocation and cytokine secretion induced by F. nucleatum. | (84) |
| T. denticola-F. nucleatum | T. denticola incapacitates the F. nucleatum-induced expression of human beta defensins and IL-8 in epithelial cells by interrupting endo-lysosomal maturation and reactive oxygen species-dependent TLR activation. | (85) |
In other instances, as exemplified by the cariogenic Streptococcus mutans, the bacterial cells conglomerate preferentially into homotypic structures. Analysis of intact supragingival communities formed on mineralized host tissue (i.e., the teeth) reveals microbial clusters comprised almost exclusively of S. mutans that are surrounded by outer layers of other microbial species forming a corona-like spatial arrangement (7, 86) (Fig. 1B). The inner core of S. mutans appears to be physically separated from the outer layer of other microbial species by extracellular polymeric substances, including glucans, adhesins, glycoproteins, and eDNA (87). Despite this isolation, the inner S. mutans cells interact with the outer members of this spatially ordered community. For example, the presence of S. oralis in the outer community induces the expression of S. mutans atpB, a key gene associated with acid tolerance and increased fitness at acidic pH, while helping to create a localized acidogenic state via metabolic interactions that contributes to dissolution of the tooth enamel (86). Furthermore, such spatial configuration of the cloistered S. mutans cells creates a protective microenvironment against antimicrobials such as chlorhexidine, thus establishing a retentive pathogenic niche (86). These findings highlight the importance of the spatial structure of the microbiome (termed biogeography) in mediating the function and outcome of host-microbe interactions (13)
The Porphyromonas gingivalis interactome
The full pathogenic potential of P. gingivalis in periodontal disease is only realized in the context of a microbial community (24, 88, 89), and thus networking with other organisms is of considerable importance in shaping the pathoecology of the gingival compartments. While the major component of the oral microbiota is acquired initially as an infant from caregivers and other family members (90), there is an order to the process based on fitness in the dynamic ecosystem (91). Facultative species such as the oral streptococci avidly adhere to the salivary pellicle on enamel surfaces and constitute an abundance of early colonizers (92 - 94). The reduction in oxygen tension as facultative accrete into densely packed communities facilitates successful colonization by anaerobes such as P. gingivalis. Further expansion of P. gingivalis is enhanced by inflammation-derived proteinaceous nutritional substrates such as will become available during gingivitis induced by an abundant early community (95). It has also been established that a propensity for slow growth at low cell density contributes to late colonization by P. gingivalis (47). This dependency on an autoinducer (AI) can be overcome by the early colonizing Veillonella parvula, which provides a soluble growth initiating cue (47) (Fig. 2A). Surfaces encountered by P. gingivalis during successful colonization and expansion are populated by a variety of organisms, and P. gingivalis is well equipped with adhesins mediating attachment to many of these, including fusobacteria, actinomyces, veillonellae, and streptococci (96 - 100). Once established in deeper subgingival areas, P. gingivalis also coadheres with spirochetes such as Treponema denticola (68). For binding to S. gordonii, P. gingivalis employs the FimA structural subunit fimbriae which engage GAPDH on the streptococcal surface, as well as the Mfa1 structural subunit fimbriae which engage SapA/SspB, members of the Ag I/II family of streptococcal surface proteins (24, 101). The functional domain on the SspB protein has been localized to a C-terminal region, designated BAR, spanning aa residues 1,167–1,193 (24, 102). Ag I/II members in other oral streptococcal species that possess a homologous BAR domain (such as S. mitis and S. oralis) support P. gingivalis adherence, whereas those lacking BAR homologs (such as S. cristatus and S. mutans) do not (25, 98). Of note, both S. cristatus and S. mutans lack physiological compatibility with P. gingivalis (Fig. 2A). Arginine deiminase produced by S. cristatus inhibits the expression of fimbrial- and protease-associated genes in P. gingivalis, consequently diminishing community formation and pathogenicity in vivo (103). Low pH values as are induced by S. mutans are antagonistic to P. gingivalis (104). Hence, P. gingivalis can be seen to have tailored its adhesive repertoire to favor attachment to non-antagonistic organisms. The situation is more nuanced, however, as the relationship between P. gingivalis and S. mitis can turn toxic through the induction of multiple transposases and cell death in the streptococci (105).
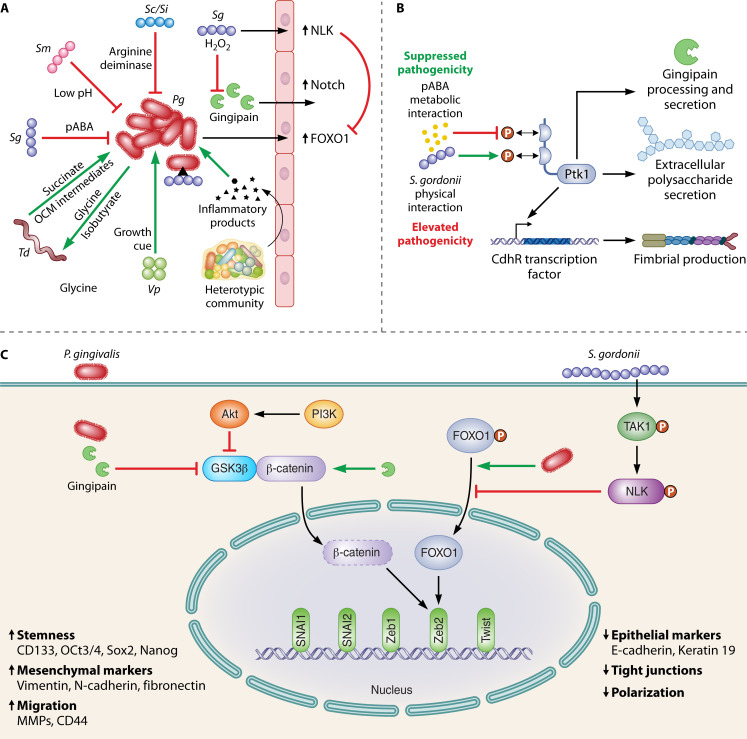
Fig 2.
The P. gingivalis interactome. (A) Overview (not to scale) of interactions of P. gingivalis with other bacteria, bacterial communities, and with gingival epithelial cells. Green arrows represent a synergistic relationship which increases the colonization, growth, or pathogenicity of P. gingivalis, or an increase in an epithelial cell signaling pathway. Red flat arrows represent an antagonistic relationship. (B) The streptococcal metabolite para-amino benzoic acid (pABA) and physical attachment between P. gingivalis and S. gordonii have opposing effects on pathogenicity, although both funnel through activation/inactivation of the Ptk1 tyrosine kinase signaling pathway. (C) Indirect communication between S. gordonii and P. gingivalis involving the epithelial cell as an intermediary. S. gordonii can activate the TAK1-NLK pathway, which mitigates P. gingivalis stimulation of FOXO1-Zeb2 signaling. P. gingivalis, however, can enhance Zeb2 activity through pathways that are insulated from S. gordonii.
In contrast, the relationship between P. gingivalis and S. gordonii remains harmonious and indeed begins prior to attachment (Fig. 2A). Oral streptococci such as S. gordonii produce para-amino benzoic acid (pABA) which diffuses freely in and out of bacterial cells and is an essential component of one-carbon metabolism (OCM) (26, 106, 107). Exogenous pABA acquired by P. gingivalis can interfere with tyrosine phosphorylation/dephosphorylation-based signaling in P. gingivalis, which funnels through the bacterial tyrosine (BY) kinase Ptk1. Ptk1 is a node in a regulatory network which controls the production of virulence factors, including the gingipain proteases, fimbriae, and extracellular polysaccharide (EPS) (27, 108) (Fig. 2B). In addition, Ptk1 is one of only two common fitness determinants of P. gingivalis identified in an abscess model with either S. gordonii or Fusobacterium nucleatum (10). Hence, in conditions of pABA excess, P. gingivalis virulence is dampened, both in abscess and alveolar bone loss models of disease (26). Through mechanisms that have yet to be unraveled, physical association between P. gingivalis and S. gordonii also impacts the phosphorylation/activation state of Ptk1 in a manner which reverses information flow through the circuitry. Consistently, physically integrated P. gingivalis-S. gordonii communities are more pathogenic in animal models of periodontal disease compared to either organism alone (89, 109) (Fig. 2B). In this context, we have proposed that S. gordonii does not function as a true commensal but rather as an accessory pathogen, an organism that, while not pathogenic in itself, can act synergistically to elevate the pathogenicity of another species or of a community (97).
The OCM pathway, of which pABA is an essential precursor, is an integral part of cellular intermediary metabolism, producing a number of one-carbon unit intermediates (formyl, methylene, methenyl, and methyl), which are required for the synthesis of various amino acids and other biomolecules, such as purines, thymidylate, folate, and redox regulators (110, 111). The participation of pABA in both OCM and virulence provides insight into coordination of physiologic and pathogenic properties by P. gingivalis. Tyrosine phosphorylation is required for processing and secretion of gingipains (108), and thus interference of Ptk1 activation by pABA will reduce the availability of amino acids that are also required as substrates to maintain OCM (110). Hence, accumulation of exogenous or endogenous pABA acts as a negative feedback loop to fine-tune OCM. While phosphorylation-mediated coupling of OCM and gingipain activities likely arose as a mechanism to ensure balanced flux through OCM, given the prominent pathological role of gingipains, this axis also drives pathogenicity. This landscape of metabolic pathogenicity may include other organisms, as T. denticola can provide OCM metabolites to P. gingivalis as a means to increase glycine availability for treponemal growth (69) (Fig. 2A).
The full nature of the trophic web involving P. gingivalis remains to be established, but all indications are that it is extensive and multicomponent. For example, in coculture with F. nucleatum, S. gordonii secretes ornithine via an arginine-ornithine antiporter (ArcD), which supports fusobacterial growth through an increase in amino acid availability. Higher levels of ornithine cause F. nucleatum to increase the production of putrescine, a polyamine derived from ornithine by decarboxylation (49). Similarly, coculture with V. parvula increases lysine availability, which promotes the production of the polyamine cadaverine by F. nucleatum. When P. gingivalis is present, both community coalescence and subsequent dispersal of planktonic cells are enhanced by polyamines (49).
The interplay between P. gingivalis and S. gordonii has relevance for host cell responses to the oral microbiome. P. gingivalis has emerged as a potential oncopathogen in oral and esophageal squamous cell carcinoma (112, 113). Certainly, as a monoinfection, P. gingivalis has a number of effects on gingival epithelial cells consistent with such a role. These include the suppression of apoptosis, acceleration through the cell cycle, and the induction of epithelial mesenchymal transition (EMT) as well as a dysbiotic inflammatory microenvironment (113, 114). However, S. gordonii can mitigate these effects through a variety of processes. For example, P. gingivalis upregulates genes encoding components of the Notch signaling pathway, including the downstream effector olfactomedin 4 (OLFM4), which is required for epithelial cell migratory, proliferative, and inflammatory responses to P. gingivalis (74). This regulation can be overridden by S. gordonii through the production of hydrogen peroxide, which inactivates the P. gingivalis gingipain proteases and prevents proteolytic cleavage and activation of the Notch1 extracellular domain (74) (Fig. 2A). In this context, we have proposed that S. gordonii functions as a homeostatic commensal, suppressing the impact of potential pathogens to help maintain eubiotic host responses. Production of hydrogen peroxide will be higher on oral mucosal membranes, compared to periodontal pockets, due to differences in oxygen availability, and this may be one reason that S. gordonii exhibits distinct “personalities” at these sites. Remarkably, S. gordonii can also influence the behavior of P. gingivalis indirectly by using the epithelial cell as an intermediary. S. gordonii can program gingival epithelial cells to resist FOXO1-Zeb2-dependent regulation of EMT markers initiated by P. gingivalis (75). Mechanistically, S. gordonii prevents serine-phosphorylation-mediated activation of FOXO1 by inducing the phosphorylation and activation of the TAK1-NLK-negative regulatory pathway, even in the presence of P. gingivalis (75) (Fig. 2C). Moreover, RNA-Seq of epithelial cells infected with P. gingivalis, S. gordonii, or both organisms in combination, shows that the dual organism challenge induces a pattern of gene expression which resembles that of S. gordonii more closely than that of P. gingivalis (74). It is likely, therefore, that S. gordonii possess additional mechanisms to diminish the influence of P. gingivalis on epithelial cell function. Related oral streptococcal species may perform similar roles, and S. sanguinis, for example, can override the effect of P. gingivalis on epithelial cells with regard to the production of inflammatory cytokines (115). Interestingly, however, P. gingivalis can impact epithelial cell circuitry in ways that are insulated from streptococci (Fig. 2C). This includes pathways that can promote Zeb2 activity and may allow P. gingivalis to manipulate host cell physiology to a sufficient degree even in the presence of organisms with opposing activity (116). In addition, P. gingivalis can suppress epithelial cell production of the neutrophil chemokine IL-8 and the T-cell chemokine IP-10 in a heterotypic infection with otherwise stimulatory organisms (117, 118). The immunosuppressive features of P. gingivalis can thus counteract the effects of community partners and may contribute to the keystone pathogen features of the organism.
Corynebacterium-streptococcal interactions
Studies of bacterial interplay in the oral microbiome have focused traditionally on organisms deemed important in terms of bulk presence or absence. More recent image analysis by fluorescent in situ hybridization of communities recovered in vivo has identified organisms playing functional roles in establishing biogeography (9). One such organism is Corynebacterium durum which assembles into structures known as “corncobs” with oral streptococci (119). C. durum has a dramatic effect on the morphology of S. sanguinis provoking an increase in chain length, a phenomenon effectuated by fatty acids likely delivered in corynebacterial membrane vesicles (50). Mixtures of palmitic, stearic, and/or oleic acids induce streptococcal chain length elongation; however, the process also involves metabolic coordination of fatty acid production in S. sanguinis. The streptococcal fab operon, which encodes fatty acid biosynthetic reactions, is downregulated in the presence of C. durum supernatants, and deletion of genes within the operon, such as fabH or acpP, phenocopies the chain length effect in the absence of exogenous fatty acids. C. durum also induces an increase in the expression of gldA, encoding an enzyme which converts glycerol into dihydroxyacetone (glycerone), and ablation of gldA expression in S. sanguinis prevents chain length regulation (50). Thus, there would appear to be regulatory connections between lipid metabolism and chain length in S. sanguinis, which can be intercepted by exogenous fatty acids provided by C. durum. The influence of C. durum on S. sanguinis extends beyond chain length, and interbacterial association increases streptococcal growth rate as well as resistance to phagocytosis and killing by macrophages (50). The association of S. sanguinis with C. durum in corncob structures will benefit the streptococci, therefore, by providing protection from innate immunity.
Streptococcal interaction with Candida
Oral streptococcal interactions with Candida albicans are associated with enhanced virulence of early childhood caries and oropharyngeal diseases (120 - 122). C. albicans interacts with mitis group streptococci (MGS) to enhance bacterial colonization and biofilm formation, while C. albicans becomes more invasive, thus promoting mucosal tissue infection and destruction (120). C. albicans physically interacts with MGS, including S. gordonii, S. sanguinis, and S. oralis, through cell surface proteins and receptors. Streptococcal cell surface adhesins, SspA and SspB, interact with the C. albicans surface, while Als and HWP adhesins on the fungal cell wall appear to mediate binding to MGS. Specifically, SspB and Als3 mediate intercellular binding through the N-terminal domain of Als3 (123). These interactions may also involve O-mannosyl residues in Als adhesins and other cell wall proteins, such as Sap9. In vivo studies show that coinfection of C. albicans and S. oralis results in increased mucosal tissue invasion and augmented inflammatory responses due to induction of neutrophil-activating cytokines (IL-17, CXCL1, MIP-2/CXCL2, TNF, IL1α, and IL-1β) and upregulation of Toll-like receptor 2-dependent proinflammatory signaling as well as increased epithelial μ-calpain activity (124). How the host orchestrates immune responses against C. albicans-streptococcal-mediated mucosal infection and the role of this cross-kingdom interaction in host immune evasion need further elucidation (120).
In contrast to MGS, the cariogenic S. mutans employs distinctive mechanisms to associate with C. albicans. In addition to antigen I/II adhesins (125), S. mutans secretes glucosyltransferase (Gtf) exoenzymes that bind avidly to the C. albicans cell surface and convert sucrose to large amounts of EPS α-glucans on the fungal surface. The EPS provides bacterial binding sites and promotes coassembly with C. albicans in saliva while promoting colonization of the tooth surface and interkingdom biofilm formation that exacerbates the severity of dental caries (126, 127). Mechanistically, S. mutans-derived GtfB binds with high affinity to N- or O-linked mannans located on the outermost layer of the C. albicans cell wall and maintains its catalytic activity to produce α-glucans in situ (128). The formation of glucan-rich matrix provides a scaffold for both surface adhesion and cell-to-cell cohesion while establishing chemical and nutrient gradients by modulating diffusion. It also provides an additional benefit to the fungi by creating a “drug-trapping matrix” that prevents uptake of the antifungal fluconazole, reducing C. albicans killing efficacy (129).
Complex signaling, cross-feeding, and metabolic interactions occur within the interkingdom MGS- or S. mutans-Candida biofilms. Signaling/quorum sensing (QS) and other biomolecules appear to facilitate these interactions, including AI-2, peptidoglycan fragments, exoenzymes, and hydrogen peroxide (H2O2) (130) (Fig. 1A). For example, nutrient byproducts as well as AI-2 signaling and H2O2 from S. gordonii stimulate C. albicans hyphal development within biofilm communities, while S. oralis activates expression of fungal aspartyl proteases. C. albicans can promote streptococcal proliferation by providing growth-stimulating factors and reducing oxygen tension. The impact of C. albicans and MGS synergism on the host-pathogen interaction has been demonstrated in vivo, whereby heterotypic C. albicans-S. oralis community growth enhances neutrophil infiltration, leading to increased severity of soft tissue lesions (120 - 122). C. albicans and S. mutans display interesting synergistic mechanisms, whereby S. mutans converts sucrose to glucose that can be more readily metabolized by C. albicans. C. albicans activates S. mutans competence, virulence gene expression, and GtfB production via QS molecules such as farnesol, while enhancing acidogenicity and aciduricity of the community (122, 131).
Notably, cross-kingdom interactions can also repress functions of the member species to modulate population growth, biofilm structure, and spatial organization. S. mutans-derived mutanobactin A and fatty acid signaling through trans-2-decenoic acid can inhibit C. albicans hyphal formation (132, 133). Furthermore, competence-stimulating peptides secreted by S. mutans (134) or S. gordonii (135) can inhibit C. albicans hyphal formation. Paradoxically, farnesol produced by C. albicans, which stimulates S. mutans growth and gtfB expression at low concentrations, disrupts bacterial growth at higher concentrations. Hence, similar to the situation described above for S. gordonii-P. gingivalis interactions, a tightly regulated cooperative and antagonistic balance through stimulus-inhibition mechanisms appears to mediate bacterial-fungal interactions, which can become synergistic when conditions are conducive for disease.
Host responses dependent on community properties
Since the early 20th century, different theories have been proposed for the microbial etiology of the inflammatory periodontal lesions, ranging from the “non-specific plaque hypothesis” (disease caused by mere increase in the quantity of subgingival plaque bacteria beyond a certain threshold, regardless of the species involved) to the implication of specific organisms (including an oral amoeba later named Entamoeba gingivalis) or a select few bacteria (such as the red complex of P. gingivalis, T. denticola, and T. forsythia) [reviewed in reference (23)]. As outlined above, it is now well established that periodontal disease is driven by mutually reinforcing interactions between a polymicrobial dysbiotic community and the host inflammatory response. The PSD model of periodontal disease pathogenesis was founded upon knowledge from modern metagenomic and metatranscriptomic studies with insights into the dynamic changes in the composition and structure of the periodontal microbiome, as well as from mechanistic studies in clinically relevant animal models on how bacteria synergize to maximize nososymbiocity [reviewed in reference (24)]. According to the PSD model, the emergence of dysbiosis (hence the potential for destructive inflammation) is determined not by specific individual organisms acting independently but by the combined output of community action; the latter is molded by interspecies interactions as well as host genetic and environmental variables that impact on both the microbial community and the immune response (24). In other words, what matters is not so much the identities of individual species but rather the presence of the appropriate complement of genes and their interaction with the host environment. This concept is consistent with published mechanistic studies, such as the ones briefly discussed below.
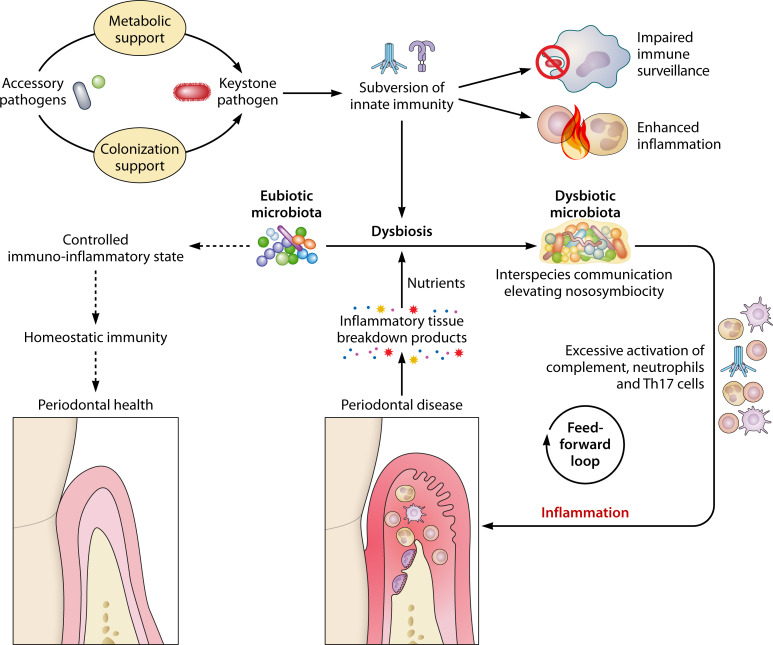
Independent studies in model organisms show that combinations of different oral pathogens cause increased periodontal inflammation and bone loss as compared to each pathogen alone (136 - 139). P. gingivalis is incapable of causing periodontitis by itself in germ-free mice, although it can colonize this host after repeated oral inoculations; however, the pathogenic potential, or keystone pathogen function, of P. gingivalis is readily expressed in the context of an oral microbial community, as occurs when the pathogen is introduced into the oral commensal microbiota of conventional mice (88). Mechanistically, P. gingivalis interacts with phagocytes in ways that disrupt immune surveillance (e.g., extracellular killing, phagocytosis) and stimulate inflammatory pathways, factors that favor the outgrowth of pathobionts, thus enhancing the community’s nososymbiocity (78, 88, 140). Reciprocally, P. gingivalis receives metabolic and colonization support from accessory pathogens (47, 89, 97, 141) (Fig. 3). Thus, in this give-and-take relationship, the capacity of P. gingivalis to provide keystone function to the community is dependent upon help from companion species.
Fig 3.
Interspecies and microbe-host interactions that promote dysbiosis and inflammatory disease. Whereas communities of predominantly eubiotic commensals induce balanced immune responses that contribute to homeostatic immunity and a healthy periodontal tissue, dysbiotic communities induce dysregulated inflammatory responses that are detrimental for the host and ineffective in controlling the bacteria. In polymicrobial communities associated with periodontitis, keystone pathogens are aided by accessory pathogens in terms of metabolic and/or colonization support and, once established, can subvert host immunity in a manner that contributes to the outgrowth of inflammophilic pathobionts. Community members engage in complex interspecies communication that elevates the expression of virulence factors and the pathogenicity of the entire community. A key environmental factor that aggravates dysbiosis and pathobiont expansion is destructive inflammation, which not only drives bone loss but also generates tissue breakdown products that can be used as nutrients by the dysbiotic community. These mutually reinforcing interactions between dysbiosis and inflammation represent a self-sustained feed-forward loop that constitutes the actual driver of periodontitis and can explain, in great part, its chronic nature.
The aptitude by which P. gingivalis manipulates the host immune response to promote dysbiosis depends heavily on its ability to exploit complement signaling pathways (78, 142, 143). The recent success of a complement-targeted clinical trial for the treatment of periodontal disease might thus not be attributable only to inhibition of inflammation but also to counteraction of complement-dependent immune subversion by P. gingivalis (144). In addition to subverting the host response, P. gingivalis may mediate keystone function via direct interactions with community members. For instance, introduction of this keystone pathogen into an otherwise health-compatible microbial community leads to major transcriptomic and proteomic alterations with potential for increased virulence expression (145, 146). P. gingivalis may also contribute to dysbiosis via elimination of health-associated species, such as by inducing cell death in S. mitis as mentioned above (105).
That inflammatory periodontal lesions are driven predominantly by dysbiotic, rather than multitudinous (as the non-specific plaque hypothesis would predict), communities is also supported by a study using the ligature-induced periodontitis model in mice. Ligature placement in posterior teeth of conventional mice results in rapidly increased bacterial biomass and structural changes in the microbial community of affected sites, as compared to unligated, hence, healthy, contralateral sites. These changes are associated with gingival inflammation and loss of alveolar bone (147). Administration—via the drinking water—of different antibiotics, either alone or in combinations, revealed that inflammatory bone loss was not necessarily associated with an increase in the total microbial load. Specifically, those antibiotic treatments which inhibited inflammation (as assessed by expansion of CD4+ T helper 17 cells) and bone loss also invariably caused compositional changes within the community, without—however—always decreasing the total microbial load (147). Therefore, although a dysbiotic community may be highly abundant relative to health-associated communities, it is mainly the qualitative differences (the collective gene pool as altered in dysbiosis) that dictate its increased pathogenic potential. Such dysbiotic communities are quite stable and can transmit disease both horizontally and vertically.
Specifically, a dysbiotic microbial community, which was established in the mouse oral cavity following inoculation with P. gingivalis, could be stably transferred to germ-free mice, which subsequently developed periodontal bone loss (148). The same study showed that the P. gingivalis-induced microbial community could also be transferred from parents to offspring, which developed significant bone loss relative to offspring of periodontally healthy control parents. Moreover, antibiotic treatment of mice with oral microbial dysbiosis could only transiently reverse dysbiosis, as dysbiosis was readily restored upon antibiotic termination (148). This implies a degree of resilience inherent in the interspecies communication within the dysbiotic community and perhaps also in the community’s interactions with a favorable host environment upon cessation of treatment. Inflammation is a major ecological factor that contributes to the dysbiotic shift in the microbial population structure associated with periodontitis. Through the availability of nutrients derived from inflammatory tissue breakdown, inflammation exerts selective pressure on the community organization, favoring the expansion of inflammophilic pathobionts at the expense of species that cannot endure or capitalize on an inflammatory environment (6, 149) (Fig. 3). This concept is further supported by observations in a controlled multispecies community environment. The addition of serum, hemoglobin, or hemin (which are by-products of destructive inflammation) to an in vitro generated oral multispecies community selectively favors the expansion of pathobiont species. These, moreover, upregulate the expression of genes that can facilitate increased exploitation of a nutritionally favorable inflammatory environment; indeed, the upregulated genes encode for proteases, hemolysins, and molecules required for the acquisition of hemin (150). Other host-related factors that contribute to the emergence of dysbiosis include inherited and acquired traits, such as immune deficiencies, smoking, unhealthy diet, obesity, diabetes, and systemic inflammatory disorders, as well as aging (1, 151, 152).
The effects of periodontal dysbiosis are not restricted locally since oral pathobionts and their proinflammatory products can spill into the circulation through the ulcerated and richly vascularized gingival epithelial barrier (153, 154). The resulting systemic inflammation can cause functional alterations in a variety of organs, including the bone marrow, where induction of maladaptive myelopoiesis exacerbates not only periodontitis but also systemic comorbidities, such as rheumatoid arthritis (155). Moreover, oral pathobionts may translocate to extraoral sites including, for example, the intestine, where they can aggravate colitis (156). It can readily be envisioned that ectopically colonizing oral microbes can cooperate or synergize with the resident dysbiotic microbiota (e.g., in the lungs or intestine), thereby further promoting disease at extraoral sites. This concept of an interconnected microbiome with enhanced virulence is supported, in principle, by certain observations. Orally aspirated P. gingivalis is detected together with Pseudomonas aeruginosa in tracheal aspirates of individuals with acute exacerbation of chronic obstructive pulmonary disease (157). Importantly, the ability of P. aeruginosa to invade respiratory epithelial cells, modulate host cell apoptosis, and ultimately cause host cell death is enhanced in the presence of P. gingivalis (158, 159).
Whereas periodontal dysbiotic communities induce immune and inflammatory responses that are ineffective, dysregulated, and detrimental for the host, both locally and systemically, communities of predominantly eubiotic commensals induce balanced immune responses that contribute to homeostatic immunity and maintain host-microbe equilibrium that characterizes a healthy periodontium (160). The mechanisms underlying homeostatic interactions between eubiotic communities and the host immune system have been extensively studied in the gut and might have parallels in the oral cavity. Different members of such communities contribute to diverse mechanisms that collectively contribute to homeostasis (e.g., by inducing antimicrobial proteins to resist pathogenic species, stimulating regenerative responses to promote tissue repair, and activating regulatory T-cell responses to restrain potentially destructive inflammation) (161 - 166).
Conclusions
With the exception of invasive exogenous pathogens, microbes in and on humans assemble into spatially constrained heterotypic communities. Nutritional, signaling, and physical interactions among community participants drive the emergent properties of the community. Pairs of organisms can interact through multiple mechanisms, and the collective outcome, for example, increased or diminished nososymbiocity, varies according to context. In many cases, such as dental caries and periodontal diseases, it is the community that represents the fundamental etiological unit. Dysbiotic microbial communities fundamentally represent a quasi-organismal entity, where constituent organisms with functional specialization engage in intimate interactions within the community and with the host to maximize its pathogenic potential and outcompete health-compatible communities. The mutually reinforcing interactions between dysbiotic communities and inflammation not only drive periodontitis but, being self-sustained, may also contribute to the chronicity of this oral disease. In dental caries, host dietary sugars can modulate the dysbiosis and polymicrobial interactions in supragingival communities leading to highly structured and localized acidic microenvironments that shape the persistence and metabolic activity of the community to promote disease development and severity.
ACKNOWLEDGMENTS
Research in the authors’ laboratories is supported by NIH/NIDCR grants: DE028561, DE029436, DE026152, and DE031206 (to G.H.); DE025220, DE025848, and DE031491 (to H.K.); and DE011111, DE012505, DE023193, and DE031756 (to R.J.L.).
We thank Patrick Lane for drawing the figures. Due to space constraints, we regret that we could not cite all relevant studies.
Contributor Information
Richard J. Lamont, Email: rich.lamont@louisville.edu.
George Hajishengallis, Email: geoh@upenn.edu.
Hyun Koo, Email: koohy@upenn.edu.
Anthony R. Richardson, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
REFERENCES
- 1. Hajishengallis G, Lamont RJ. 2021. Polymicrobial communities in periodontal disease: their quasi-organismal nature and dialogue with the host. Periodontol 2000 86:210–230. doi: 10.1111/prd.12371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ. 2002. Communication among oral bacteria. Microbiol Mol Biol Rev 66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. 2007. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 71:653–670. doi: 10.1128/MMBR.00024-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang M, Whiteley M, Lewin GR, Kline KA. 2022. Polymicrobial interactions of oral microbiota: a historical review and current perspective. mBio 13:e0023522. doi: 10.1128/mbio.00235-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cugini C, Ramasubbu N, Tsiagbe VK, Fine DH. 2021. Dysbiosis from a microbial and host perspective relative to oral health and disease. Front Microbiol 12:617485. doi: 10.3389/fmicb.2021.617485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamont RJ, Koo H, Hajishengallis G. 2018. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16:745–759. doi: 10.1038/s41579-018-0089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim D, Koo H. 2020. Spatial design of polymicrobial oral biofilm in its native disease state. J Dent Res 99:597–603. doi: 10.1177/0022034520909313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, Dewhirst FE, Borisy GG. 2011. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci U S A 108:4152–4157. doi: 10.1073/pnas.1101134108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. 2016. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A 113:E791–800. doi: 10.1073/pnas.1522149113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perpich JD, Yakoumatos L, Stocke KS, Lewin GR, Ramos A, Yoder-Himes DR, Whiteley M, Lamont RJ. 2022. Porphyromonas gingivalis tyrosine kinase is a fitness determinant in polymicrobial infections. Infect Immun 90:e0017022. doi: 10.1128/iai.00170-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choo SW, Mohammed WK, Mutha NVR, Rostami N, Ahmed H, Krasnogor N, Tan GYA, Jakubovics NS. 2021. Transcriptomic responses to coaggregation between Streptococcus gordonii and Streptococcus oralis. Appl Environ Microbiol 87:e0155821. doi: 10.1128/AEM.01558-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hajishengallis G, Lamont RJ, Koo H. 2023. Oral polymicrobial communities: assembly, function, and impact on diseases. Cell Host Microbe 31:528–538. doi: 10.1016/j.chom.2023.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azimi S, Lewin GR, Whiteley M. 2022. The biogeography of infection revisited. Nat Rev Microbiol 20:579–592. doi: 10.1038/s41579-022-00683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shokeen B, Dinis MDB, Haghighi F, Tran NC, Lux R. 2021. Omics and interspecies interaction. Periodontol 2000 85:101–111. doi: 10.1111/prd.12354 [DOI] [PubMed] [Google Scholar]
- 15. Baty JJ, Stoner SN, Scoffield JA. 2022. Oral commensal streptococci: gatekeepers of the oral cavity. J Bacteriol 204:e0025722. doi: 10.1128/jb.00257-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreth J, Merritt J. 2023. Illuminating the oral microbiome and its host interactions: tools and approaches for molecular ecological studies. FEMS Microbiol Rev 47:fuac052. doi: 10.1093/femsre/fuac052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saha S, Lach SR, Konovalova A. 2021. Homeostasis of the gram-negative cell envelope. Curr Opin Microbiol 61:99–106. doi: 10.1016/j.mib.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng L, Yang B, Xu Y, Xiong Y, Wang F, Liu B, Yang W, Yao T, Wang L. 2022. Elucidation of a complete mechanical signaling and virulence activation pathway in enterohemorrhagic Escherichia coli. Cell Rep 39:110614. doi: 10.1016/j.celrep.2022.110614 [DOI] [PubMed] [Google Scholar]
- 19. Shimizu T, Ichimura K, Noda M. 2016. The surface sensor NlpE of enterohemorrhagic Escherichia coli contributes to regulation of the type III secretion system and flagella by the Cpx response to adhesion. Infect Immun 84:537–549. doi: 10.1128/IAI.00881-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Otto K, Silhavy TJ. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A 99:2287–2292. doi: 10.1073/pnas.042521699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marsh PD. 2003. Are dental diseases examples of ecological catastrophes? Microbiology (Reading) 149:279–294. doi: 10.1099/mic.0.26082-0 [DOI] [PubMed] [Google Scholar]
- 22. Hajishengallis G, Darveau RP, Curtis MA. 2012. The keystone-pathogen hypothesis. Nat Rev Microbiol 10:717–725. doi: 10.1038/nrmicro2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hajishengallis G, Lamont RJ. 2012. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamont RJ, Hajishengallis G. 2015. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med 21:172–183. doi: 10.1016/j.molmed.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuboniwa M, Lamont RJ. 2010. Subgingival biofilm formation. Periodontology 2000 52:38–52. doi: 10.1111/j.1600-0757.2009.00311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuboniwa M, Houser JR, Hendrickson EL, Wang Q, Alghamdi SA, Sakanaka A, Miller DP, Hutcherson JA, Wang T, Beck DAC, Whiteley M, Amano A, Wang H, Marcotte EM, Hackett M, Lamont RJ. 2017. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat Microbiol 2:1493–1499. doi: 10.1038/s41564-017-0021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamont RJ, Miller DP. 2022. Tyrosine kinases and phosphatases: enablers of the Porphyromonas gingivalis lifestyle. Front Oral Health 3:835586. doi: 10.3389/froh.2022.835586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ. 2003. Luxs-based signaling in Streptococcus gordonii: Autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J Bacteriol 185:274–284. doi: 10.1128/JB.185.1.274-284.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park T, Im J, Kim AR, Lee D, Jeong S, Yun CH, Han SH. 2021. Short-chain fatty acids inhibit the biofilm formation of Streptococcus gordonii through negative regulation of competence-stimulating peptide signaling pathway. J Microbiol 59:1142–1149. doi: 10.1007/s12275-021-1576-8 [DOI] [PubMed] [Google Scholar]
- 30. Hendrickson EL, Beck DAC, Miller DP, Wang Q, Whiteley M, Lamont RJ, Hackett M. 2017. Insights into dynamic polymicrobial synergy revealed by time-coursed RNA-Seq. Front Microbiol 8:261. doi: 10.3389/fmicb.2017.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mutha NVR, Mohammed WK, Krasnogor N, Tan GYA, Wee WY, Li Y, Choo SW, Jakubovics NS. 2019. Transcriptional profiling of coaggregation interactions between Streptococcus gordonii and Veillonella parvula by dual RNA-Seq. Sci Rep 9:7664. doi: 10.1038/s41598-019-43979-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu T, Yang R, Zhou J, Lu X, Yuan Z, Wei X, Guo L. 2021. Interactions between Streptococcus gordonii and Fusobacterium nucleatum altered bacterial transcriptional profiling and attenuated the immune responses of macrophages. Front Cell Infect Microbiol 11:783323. doi: 10.3389/fcimb.2021.783323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mutha NVR, Mohammed WK, Krasnogor N, Tan GYA, Choo SW, Jakubovics NS. 2018. Transcriptional responses of Streptococcus gordonii and Fusobacterium nucleatum to coaggregation. Mol Oral Microbiol 33:450–464. doi: 10.1111/omi.12248 [DOI] [PubMed] [Google Scholar]
- 34. He X, Hu W, Kaplan CW, Guo L, Shi W, Lux R. 2012. Adherence to streptococci facilitates Fusobacterium nucleatum integration into an oral microbial community. Microb Ecol 63:532–542. doi: 10.1007/s00248-011-9989-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohammed WK, Krasnogor N, Jakubovics NS. 2018. Streptococcus gordonii challisin protease is required for sensing cell--cell contact with Actinomyces oris. FEMS Microbiol Ecol 94. doi: 10.1093/femsec/fiy043 [DOI] [PubMed] [Google Scholar]
- 36. Jakubovics NS, Gill SR, Vickerman MM, Kolenbrander PE. 2008. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol Ecol 66:637–644. doi: 10.1111/j.1574-6941.2008.00585.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramsey MM, Whiteley M. 2009. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci U S A 106:1578–1583. doi: 10.1073/pnas.0809533106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. 2016. A commensal bacterium promotes virulence of an opportunistic pathogen via cross-respiration. mBio 7:e00782-16. doi: 10.1128/mBio.00782-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stacy A, Everett J, Jorth P, Trivedi U, Rumbaugh KP, Whiteley M. 2014. Bacterial fight-and-flight responses enhance virulence in a polymicrobial infection. Proc Natl Acad Sci U S A 111:7819–7824. doi: 10.1073/pnas.1400586111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramsey MM, Rumbaugh KP, Whiteley M. 2011. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog 7:e1002012. doi: 10.1371/journal.ppat.1002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duan D, Scoffield JA, Zhou X, Wu H. 2016. Fine-tuned production of hydrogen peroxide promotes biofilm formation of Streptococcus parasanguinis by a pathogenic cohabitant Aggregatibacter actinomycetemcomitans. Environ Microbiol 18:4023–4036. doi: 10.1111/1462-2920.13425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ślęzak P, Śmiga M, Smalley JW, Siemińska K, Olczak T. 2020. Porphyromonas gingivalis HmuY and Streptococcus gordonii GAPDH-novel heme acquisition strategy in the oral microbiome. Int J Mol Sci 21:4150. doi: 10.3390/ijms21114150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu B, Macleod LC, Newsome E, Liu J, Xu P. 2019. Aggregatibacter actinomycetemcomitans mediates protection of Porphyromonas gingivalis from Streptococcus sanguinis hydrogen peroxide production in multi-species biofilms. Sci Rep 9:4944. doi: 10.1038/s41598-019-41467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie H, Hong J, Sharma A, Wang B-Y. 2012. Streptococcus cristatus ArcA interferes with Porphyromonas gingivalis pathogenicity in mice. J Periodontal Res 47:578–583. doi: 10.1111/j.1600-0765.2012.01469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cugini C, Stephens DN, Nguyen D, Kantarci A, Davey ME. 2013. Arginine deiminase inhibits Porphyromonas gingivalis surface attachment. Microbiology (Reading) 159:275–285. doi: 10.1099/mic.0.062695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perera D, McLean A, Morillo-López V, Cloutier-Leblanc K, Almeida E, Cabana K, Mark Welch J, Ramsey M. 2022. Mechanisms underlying interactions between two abundant oral commensal bacteria. ISME J 16:948–957. doi: 10.1038/s41396-021-01141-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoare A, Wang H, Meethil A, Abusleme L, Hong BY, Moutsopoulos NM, Marsh PD, Hajishengallis G, Diaz PI. 2021. A cross-species interaction with a symbiotic commensal enables cell-density-dependent growth and in vivo virulence of an oral pathogen. ISME J 15:1490–1504. doi: 10.1038/s41396-020-00865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou P, Li X, Huang I-H, Qi F. 2017. Veillonella catalase protects the growth of Fusobacterium nucleatum in microaerophilic and Streptococcus gordonii-resident environments. Appl Environ Microbiol 83:e01079-17. doi: 10.1128/AEM.01079-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sakanaka A, Kuboniwa M, Shimma S, Alghamdi SA, Mayumi S, Lamont RJ, Fukusaki E, Amano A. 2022. Fusobacterium nucleatum metabolically integrates commensals and pathogens in oral biofilms. mSystems 7:e0017022. doi: 10.1128/msystems.00170-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Treerat P, Redanz U, Redanz S, Giacaman RA, Merritt J, Kreth J. 2020. Synergism between Corynebacterium and Streptococcus sanguinis reveals new interactions between oral commensals. ISME J 14:1154–1169. doi: 10.1038/s41396-020-0598-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duran-Pinedo AE, Paster B, Teles R, Frias-Lopez J. 2011. Correlation network analysis applied to complex biofilm communities. PLoS One 6:e28438. doi: 10.1371/journal.pone.0028438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Redanz S, Treerat P, Mu R, Redanz U, Zou Z, Koley D, Merritt J, Kreth J. 2020. Pyruvate secretion by oral streptococci modulates hydrogen peroxide dependent antagonism. ISME J 14:1074–1088. doi: 10.1038/s41396-020-0592-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang X, Kudo Y, Baker JL, LaBonte S, Jordan PA, McKinnie SMK, Guo J, Huan T, Moore BS, Edlund A. 2020. Cariogenic Streptococcus mutans produces tetramic acid strain-specific antibiotics that impair commensal colonization. ACS Infect Dis 6:563–571. doi: 10.1021/acsinfecdis.9b00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rued BE, Covington BC, Bushin LB, Szewczyk G, Laczkovich I, Seyedsayamdost MR, Federle MJ. 2021. Quorum sensing in Streptococcus mutans regulates production of tryglysin, a novel RaS-RiPP antimicrobial compound mBio:12–20. doi: 10.1128/mBio.02688-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rostami N, Shields RC, Serrage HJ, Lawler C, Brittan JL, Yassin S, Ahmed H, Treumann A, Thompson P, Waldron KJ, Nobbs AH, Jakubovics NS. 2022. Interspecies competition in oral biofilms mediated by Streptococcus gordonii extracellular deoxyribonuclease SsnA. NPJ Biofilms Microbiomes 8:96. doi: 10.1038/s41522-022-00359-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kreth J, Giacaman RA, Raghavan R, Merritt J. 2017. The road less traveled - defining molecular commensalism with Streptococcus sanguinis. Mol Oral Microbiol 32:181–196. doi: 10.1111/omi.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaspar JR, Lee K, Richard B, Walker AR, Burne RA. 2021. Direct interactions with commensal streptococci modify intercellular communication behaviors of Streptococcus mutans. ISME J 15:473–488. doi: 10.1038/s41396-020-00789-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang X, Li X, Ling J. 2017. Streptococcus gordonii Luxs/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans. J Basic Microbiol 57:605–616. doi: 10.1002/jobm.201700010 [DOI] [PubMed] [Google Scholar]
- 59. Ng HM, Kin LX, Dashper SG, Slakeski N, Butler CA, Reynolds EC. 2016. Bacterial interactions in pathogenic subgingival plaque. Microb Pathog 94:60–69. doi: 10.1016/j.micpath.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 60. Egland PG, Palmer RJ, Kolenbrander PE. 2004. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci U S A 101:16917–16922. doi: 10.1073/pnas.0407457101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu J, Wu C, Huang I-H, Merritt J, Qi F. 2011. Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology (Reading) 157:2433–2444. doi: 10.1099/mic.0.048314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huffines JT, Stoner SN, Baty JJ, Scoffield JA. 2022. Nitrite triggers reprogramming of the oral polymicrobial metabolome by a commensal streptococcus. Front Cell Infect Microbiol 12:833339. doi: 10.3389/fcimb.2022.833339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bradshaw DJ, Marsh PD, Watson GK, Allison C. 1998. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun 66:4729–4732. doi: 10.1128/IAI.66.10.4729-4732.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Diaz PI, Zilm PS, Rogers AH. 2002. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology (Reading) 148:467–472. doi: 10.1099/00221287-148-2-467 [DOI] [PubMed] [Google Scholar]
- 65. Jang YJ, Choi YJ, Lee SH, Jun HK, Choi BK. 2013. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol 58:17–27. doi: 10.1016/j.archoralbio.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 66. Smalley JW, Olczak T. 2017. Heme acquisition mechanisms of Porphyromonas gingivalis - strategies used in a polymicrobial community in a heme-limited host environment. Mol Oral Microbiol 32:1–23. doi: 10.1111/omi.12149 [DOI] [PubMed] [Google Scholar]
- 67. Byrne DP, Potempa J, Olczak T, Smalley JW. 2013. Evidence of mutualism between two periodontal pathogens: co-operative haem acquisition by the HmuY haemophore of Porphyromonas gingivalis and the cysteine protease interpain A (InpA) of Prevotella Intermedia. Mol Oral Microbiol 28:219–229. doi: 10.1111/omi.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tan KH, Seers CA, Dashper SG, Mitchell HL, Pyke JS, Meuric V, Slakeski N, Cleal SM, Chambers JL, McConville MJ, Reynolds EC, Schneider DS. 2014. Porphyromonas gingivalis and Treponema denticola exhibit metabolic symbioses. PLoS Pathog 10:e1003955. doi: 10.1371/journal.ppat.1003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kin LX, Butler CA, Slakeski N, Hoffmann B, Dashper SG, Reynolds EC. 2020. Metabolic cooperativity between Porphyromonas gingivalis and Treponema denticola. J Oral Microbiol 12:1808750. doi: 10.1080/20002297.2020.1808750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grenier D. 1992. Nutritional interactions between two suspected periodontopathogens, Treponema denticola and Porphyromonas gingivalis. Infect Immun 60:5298–5301. doi: 10.1128/iai.60.12.5298-5301.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meuric V, Martin B, Guyodo H, Rouillon A, Tamanai-Shacoori Z, Barloy-Hubler F, Bonnaure-Mallet M. 2013. Treponema denticola improves adhesive capacities of Porphyromonas gingivalis. Mol Oral Microbiol 28:40–53. doi: 10.1111/omi.12004 [DOI] [PubMed] [Google Scholar]
- 72. Fong KP, Chung WO, Lamont RJ, Demuth DR. 2001. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect Immun 69:7625–7634. doi: 10.1128/IAI.69.12.7625-7634.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sarkar J, McHardy IH, Simanian EJ, Shi W, Lux R, Gray CM. 2014. Transcriptional responses of Treponema denticola to other oral bacterial species. PLoS ONE 9:e88361. doi: 10.1371/journal.pone.0088361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fitzsimonds ZR, Liu C, Stocke KS, Yakoumatos L, Shumway B, Miller DP, Artyomov MN, Bagaitkar J, Lamont RJ. 2021. Regulation of olfactomedin 4 by Porphyromonas gingivalis in a community context. ISME J 15:2627–2642. doi: 10.1038/s41396-021-00956-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ohshima J, Wang Q, Fitzsimonds ZR, Miller DP, Sztukowska MN, Jung Y-J, Hayashi M, Whiteley M, Lamont RJ. 2019. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc Natl Acad Sci U S A 116:8544–8553. doi: 10.1073/pnas.1900101116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mans JJ, von Lackum K, Dorsey C, Willis S, Wallet SM, Baker HV, Lamont RJ, Handfield M. 2009. The degree of microbiome complexity influences the epithelial response to infection. BMC Genomics 10:380. doi: 10.1186/1471-2164-10-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shu Y, Upara C, Ding Q, Zhu M, Zeng E, Banas JA, Hong L. 2023. Spent culture supernatant of Streptococcus gordonii mitigates inflammation of human periodontal cells and inhibits proliferation of pathogenic oral microbes. J Periodontol 94:575–585. doi: 10.1002/JPER.22-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, Hashim A, Hoch S, Curtis MA, Nussbaum G, Lambris JD, Hajishengallis G. 2014. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 15:768–778. doi: 10.1016/j.chom.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taxman DJ, Swanson KV, Broglie PM, Wen H, Holley-Guthrie E, Huang M-H, Callaway JB, Eitas TK, Duncan JA, Ting JPY. 2012. Porphyromonas gingivalis mediates Inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J Biol Chem 287:32791–32799. doi: 10.1074/jbc.M112.401737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Polak D, Ferdman O, Houri-Haddad Y. 2017. Porphyromonas gingivalis capsule-mediated coaggregation as a virulence factor in mixed infection with Fusobacterium nucleatum. J Periodontol 88:502–510. doi: 10.1902/jop.2016.160397 [DOI] [PubMed] [Google Scholar]
- 81. Yang R, Liu T, Pang C, Cai Y, Lin Z, Guo L, Wei X. 2022. The regulatory effect of coaggregation between Fusobacterium nucleatum and Streptococcus gordonii on the synergistic virulence to human gingival epithelial cells. Front Cell Infect Microbiol 12:879423. doi: 10.3389/fcimb.2022.879423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. El-Awady A, de Sousa Rabelo M, Meghil MM, Rajendran M, Elashiry M, Stadler AF, Foz AM, Susin C, Romito GA, Arce RM, Cutler CW. 2019. Polymicrobial synergy within oral biofilm promotes invasion of dendritic cells and survival of consortia members. NPJ Biofilms Microbiomes 5:11. doi: 10.1038/s41522-019-0084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang G, Rudney JD. 2011. Streptococcus cristatus attenuates Fusobacterium nucleatum-induced cytokine expression by influencing pathways converging on nuclear factor-κB. Mol Oral Microbiol 26:150–163. doi: 10.1111/j.2041-1014.2010.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. 2013. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-κB RelA/P65. PLoS Pathog 9:e1003326. doi: 10.1371/journal.ppat.1003326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shin JE, Baek KJ, Choi YS, Choi Y. 2013. A periodontal pathogen Treponema denticola hijacks the Fusobacterium nucleatum-driven host response. Immunol Cell Biol 91:503–510. doi: 10.1038/icb.2013.35 [DOI] [PubMed] [Google Scholar]
- 86. Kim D, Barraza JP, Arthur RA, Hara A, Lewis K, Liu Y, Scisci EL, Hajishengallis E, Whiteley M, Koo H. 2020. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc Natl Acad Sci U S A 117:12375–12386. doi: 10.1073/pnas.1919099117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Karygianni L, Ren Z, Koo H, Thurnheer T. 2020. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 28:668–681. doi: 10.1016/j.tim.2020.03.016 [DOI] [PubMed] [Google Scholar]
- 88. Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10:497–506. doi: 10.1016/j.chom.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Daep CA, Novak EA, Lamont RJ, Demuth DR. 2011. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect Immun 79:67–74. doi: 10.1128/IAI.00361-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kageyama S, Furuta M, Takeshita T, Ma J, Asakawa M, Yamashita Y, Bello MGD. 2022. High-level acquisition of maternal oral bacteria in formula-fed infant oral microbiota. mBio 13:e0345221. doi: 10.1128/mbio.03452-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sulyanto RM, Thompson ZA, Beall CJ, Leys EJ, Griffen AL. 2019. The predominant oral microbiota is acquired early in an organized pattern. Sci Rep 9:10550. doi: 10.1038/s41598-019-46923-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Peterson SN, Snesrud E, Liu J, Ong AC, Kilian M, Schork NJ, Bretz W, Highlander SK. 2013. The dental plaque microbiome in health and disease. PLoS ONE 8:e58487. doi: 10.1371/journal.pone.0058487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rosan B, Lamont RJ. 2000. Dental plaque formation. Microbes Infect 2:1599–1607. doi: 10.1016/s1286-4579(00)01316-2 [DOI] [PubMed] [Google Scholar]
- 94. Hannig C, Hannig M, Rehmer O, Braun G, Hellwig E, Al-Ahmad A. 2007. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch Oral Biol 52:1048–1056. doi: 10.1016/j.archoralbio.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 95. Hajishengallis G, Chavakis T, Lambris JD. 2020. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol 2000 84:14–34. doi: 10.1111/prd.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou P, Liu J, Merritt J, Qi F. 2015. A YadA-like autotransporter, Hag1 in Veillonella atypica is a multivalent hemagglutinin involved in adherence to oral streptococci, Porphyromonas gingivalis, and human oral buccal cells. Mol Oral Microbiol 30:269–279. doi: 10.1111/omi.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hajishengallis G, Lamont RJ. 2016. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol 24:477–489. doi: 10.1016/j.tim.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, Lamont RJ, Jenkinson HF. 2013. Microbial interactions in building of communities. Mol Oral Microbiol 28:83–101. doi: 10.1111/omi.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yao ES, Lamont RJ, Leu SP, Weinberg A. 1996. Interbacterial binding among strains of pathogenic and commensal oral bacterial species. Oral Microbiol Immunol 11:35–41. doi: 10.1111/j.1399-302x.1996.tb00334.x [DOI] [PubMed] [Google Scholar]
- 100. Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. 2010. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 8:471–480. doi: 10.1038/nrmicro2381 [DOI] [PubMed] [Google Scholar]
- 101. Whitmore SE, Lamont RJ. 2011. The pathogenic persona of community-associated oral streptococci. Mol Microbiol 81:305–314. doi: 10.1111/j.1365-2958.2011.07707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Demuth DR, Irvine DC, Costerton JW, Cook GS, Lamont RJ. 2001. Discrete protein determinant directs the species-specific adherence of Porphyromonas gingivalis to oral streptococci. Infect Immun 69:5736–5741. doi: 10.1128/IAI.69.9.5736-5741.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ho M-H, Lamont RJ, Xie H. 2017. A novel peptidic inhibitor derived from Streptococcus cristatus ArcA attenuates virulence potential of Porphyromonas gingivalis. Sci Rep 7:16217. doi: 10.1038/s41598-017-16522-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Takahashi N, Saito K, Schachtele CF, Yamada T. 1997. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol Immunol 12:323–328. doi: 10.1111/j.1399-302x.1997.tb00733.x [DOI] [PubMed] [Google Scholar]
- 105. Duran-Pinedo AE, Baker VD, Frias-Lopez J. 2014. The periodontal pathogen Porphyromonas gingivalis induces expression of transposases and cell death of Streptococcus mitis in a biofilm model. Infect Immun 82:3374–3382. doi: 10.1128/IAI.01976-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Green JM, Hollandsworth R, Pitstick L, Carter EL. 2010. Purification and characterization of the folate catabolic enzyme p-aminobenzoyl-glutamate hydrolase from Escherichia coli. J Bacteriol 192:2407–2413. doi: 10.1128/JB.01362-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tran PV, Nichols BP. 1991. Expression of Escherichia coli pabA. J Bacteriol 173:3680–3687. doi: 10.1128/jb.173.12.3680-3687.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nowakowska Z, Madej M, Grad S, Wang T, Hackett M, Miller DP, Lamont RJ, Potempa J. 2021. Phosphorylation of major Porphyromonas gingivalis virulence factors is crucial for their processing and secretion. Mol Oral Microbiol 36:316–326. doi: 10.1111/omi.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Roky M, Tan J, Sztukowska MN, Trent JO, Demuth DR. 2020. Identification of small-molecule inhibitors targeting Porphyromonas gingivalis interspecies adherence and determination of their in vitro and in vivo efficacies. Antimicrob Agents Chemother 64:e00884–00820. doi: 10.1128/AAC.00884-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shetty S, Varshney U. 2021. Regulation of translation by one-carbon metabolism in bacteria and eukaryotic organelles. J Biol Chem 296:100088. doi: 10.1074/jbc.REV120.011985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ducker GS, Rabinowitz JD. 2017. One-carbon metabolism in health and disease. Cell Metab 25:27–42. doi: 10.1016/j.cmet.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Al-Hebshi NN, Borgnakke WS, Johnson NW. 2019. The microbiome of oral squamous cell carcinomas: a functional perspective. Curr Oral Health Rep 6:145–160. doi: 10.1007/s40496-019-0215-5 [DOI] [Google Scholar]
- 113. Lamont RJ, Fitzsimonds ZR, Wang H, Gao S, Hajishengallis G. 2022. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol 2000 89:154–165. doi: 10.1111/prd.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fitzsimonds ZR, Rodriguez-Hernandez CJ, Bagaitkar J, Lamont RJ. 2020. From beyond the pale to the pale riders: the emerging association of bacteria with oral cancer. J Dent Res 99:604–612. doi: 10.1177/0022034520907341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Redanz U, Redanz S, Treerat P, Prakasam S, Lin LJ, Merritt J, Kreth J. 2021. Differential response of oral mucosal and gingival cells to Corynebacterium durum, Streptococcus sanguinis, and Porphyromonas gingivalis multispecies biofilms. Front Cell Infect Microbiol 11:686479. doi: 10.3389/fcimb.2021.686479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhou Y, Sztukowska M, Wang Q, Inaba H, Potempa J, Scott DA, Wang H, Lamont RJ. 2015. Noncanonical activation of β-catenin by Porphyromonas gingivalis. Infect Immun 83:3195–3203. doi: 10.1128/IAI.00302-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Darveau RP, Belton CM, Reife RA, Lamont RJ. 1998. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun 66:1660–1665. doi: 10.1128/IAI.66.4.1660-1665.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. 2013. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun 81:2288–2295. doi: 10.1128/IAI.00264-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Morillo-Lopez V, Sjaarda A, Islam I, Borisy GG, Mark Welch JL. 2022. Corncob structures in dental plaque reveal microhabitat taxon specificity. Microbiome 10:145. doi: 10.1186/s40168-022-01323-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bertolini M, Dongari-Bagtzoglou A. 2019. The relationship of Candida albicans with the oral bacterial microbiome in health and disease. Adv Exp Med Biol 1197:69–78. doi: 10.1007/978-3-030-28524-1_6 [DOI] [PubMed] [Google Scholar]
- 121. Koo H, Andes DR, Krysan DJ. 2018. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog 14:e1007342. doi: 10.1371/journal.ppat.1007342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Du Q, Ren B, Zhou X, Zhang L, Xu X. 2022. Cross-kingdom interaction between Candida albicans and oral bacteria. Front Microbiol 13:911623. doi: 10.3389/fmicb.2022.911623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bamford CV, Nobbs AH, Barbour ME, Lamont RJ, Jenkinson HF. 2015. Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii. Microbiology (Reading) 161:18–29. doi: 10.1099/mic.0.083378-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xu H, Sobue T, Bertolini M, Thompson A, Dongari-Bagtzoglou A. 2016. Streptococcus oralis and Candida albicans synergistically activate μ-calpain to degrade E-cadherin from oral epithelial junctions. J Infect Dis 214:925–934. doi: 10.1093/infdis/jiw201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yang C, Scoffield J, Wu R, Deivanayagam C, Zou J, Wu H. 2018. Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol Oral Microbiol 33:283–291. doi: 10.1111/omi.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ren Z, Jeckel H, Simon-Soro A, Xiang Z, Liu Y, Cavalcanti IM, Xiao J, Tin NN, Hara A, Drescher K, Koo H. 2022. Interkingdom assemblages in human saliva display group-level surface mobility and disease-promoting emergent functions. Proc Natl Acad Sci U S A 119:e2209699119. doi: 10.1073/pnas.2209699119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai C-H, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, Koo H. 2014. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun 82:1968–1981. doi: 10.1128/IAI.00087-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H, Mitchell TJ. 2017. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog 13:e1006407. doi: 10.1371/journal.ppat.1006407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kim D, Liu Y, Benhamou RI, Sanchez H, Simón-Soro Á, Li Y, Hwang G, Fridman M, Andes DR, Koo H. 2018. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J 12:1427–1442. doi: 10.1038/s41396-018-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nobbs AH, Jenkinson HF. 2015. Interkingdom networking within the oral microbiome. Microbes Infect 17:484–492. doi: 10.1016/j.micinf.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sztajer H, Szafranski SP, Tomasch J, Reck M, Nimtz M, Rohde M, Wagner-Döbler I. 2014. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J 8:2256–2271. doi: 10.1038/ismej.2014.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vílchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H, Wagner-Döbler I. 2010. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 11:1552–1562. doi: 10.1002/cbic.201000086 [DOI] [PubMed] [Google Scholar]
- 133. Joyner PM, Liu J, Zhang Z, Merritt J, Qi F, Cichewicz RH. 2010. Mutanobactin A from the human oral pathogen Streptococcus mutans is a cross-kingdom regulator of the yeast-mycelium transition. Org Biomol Chem 8:5486–5489. doi: 10.1039/c0ob00579g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jarosz LM, Deng DM, van der Mei HC, Crielaard W, Krom BP. 2009. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell 8:1658–1664. doi: 10.1128/EC.00070-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jack AA, Daniels DE, Jepson MA, Vickerman MM, Lamont RJ, Jenkinson HF, Nobbs AH. 2015. Streptococcus gordonii comCDE (competence) operon modulates biofilm formation with Candida albicans. Microbiology (Reading) 161:411–421. doi: 10.1099/mic.0.000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Settem RP, El-Hassan AT, Honma K, Stafford GP, Sharma A. 2012. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect Immun 80:2436–2443. doi: 10.1128/IAI.06276-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Orth R-H, O’Brien-Simpson NM, Dashper SG, Reynolds EC. 2011. Synergistic virulence of Porphyromonas gingivalis and Treponema denticola in a murine periodontitis model. Mol Oral Microbiol 26:229–240. doi: 10.1111/j.2041-1014.2011.00612.x [DOI] [PubMed] [Google Scholar]
- 138. Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, Ebersole JL. 2007. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun 75:1704–1712. doi: 10.1128/IAI.00733-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, Houri-Haddad Y. 2009. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol 36:406–410. doi: 10.1111/j.1600-051X.2009.01393.x [DOI] [PubMed] [Google Scholar]
- 140. Makkawi H, Hoch S, Burns E, Hosur K, Hajishengallis G, Kirschning CJ, Nussbaum G. 2017. Porphyromonas gingivalis stimulates TLR2-Pi3K signaling to escape immune clearance and induce bone resorption independently of MyD88. Front Cell Infect Microbiol 7:359. doi: 10.3389/fcimb.2017.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Daep CA, James DM, Lamont RJ, Demuth DR. 2006. Structural characterization of peptide-mediated inhibition of Porphyromonas gingivalis biofilm formation. Infect Immun 74:5756–5762. doi: 10.1128/IAI.00813-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. 2010. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal 3:ra11. doi: 10.1126/scisignal.2000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. 2011. The C5A receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol 186:869–877. doi: 10.4049/jimmunol.1003252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hasturk H, Hajishengallis G, Lambris JD, Mastellos DC, Yancopoulou D, Forsyth Institute Center for Clinical and Translational Research staff . 2021. Phase IIa clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J Clin Invest 131:e152973. doi: 10.1172/JCI152973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kuboniwa M, Tribble GD, Hendrickson EL, Amano A, Lamont RJ, Hackett M. 2012. Insights into the virulence of oral biofilms: discoveries from proteomics. Expert Rev Proteomics 9:311–323. doi: 10.1586/epr.12.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Frias-Lopez J, Duran-Pinedo A. 2012. Effect of periodontal pathogens on the metatranscriptome of a healthy multispecies biofilm model. J Bacteriol 194:2082–2095. doi: 10.1128/JB.06328-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, Morell RJ, Freeman AF, Lazarevic V, Trinchieri G, Diaz PI, Holland SM, Belkaid Y, Hajishengallis G, Moutsopoulos NM. 2018. A dysbiotic microbiome triggers T(H)17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med 10:eaat0797. doi: 10.1126/scitranslmed.aat0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Payne MA, Hashim A, Alsam A, Joseph S, Aduse-Opoku J, Wade WG, Curtis MA. 2019. Horizontal and vertical transfer of oral microbial dysbiosis and periodontal disease. J Dent Res 98:1503–1510. doi: 10.1177/0022034519877150 [DOI] [PubMed] [Google Scholar]
- 149. Curtis MA, Diaz PI, Van Dyke TE, Mariano RJ. 2020. The role of the microbiota in periodontal disease. Periodontol 2000 83:14–25. doi: 10.1111/prd.12296 [DOI] [PubMed] [Google Scholar]
- 150. Herrero ER, Fernandes S, Verspecht T, Ugarte-Berzal E, Boon N, Proost P, Bernaerts K, Quirynen M, Teughels W. 2018. Dysbiotic biofilms deregulate the periodontal inflammatory response. J Dent Res 97:547–555. doi: 10.1177/0022034517752675 [DOI] [PubMed] [Google Scholar]
- 151. Teles F, Wang Y, Hajishengallis G, Hasturk H, Marchesan JT. 2021. Impact of systemic factors in shaping the periodontal microbiome. Periodontol 2000 85:126–160. doi: 10.1111/prd.12356 [DOI] [PubMed] [Google Scholar]
- 152. Stabholz A, Soskolne WA, Shapira L. 2010. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000 53:138–153. doi: 10.1111/j.1600-0757.2010.00340.x [DOI] [PubMed] [Google Scholar]
- 153. Emery DC, Cerajewska TL, Seong J, Davies M, Paterson A, Allen-Birt SJ, West NX. 2020. Comparison of blood bacterial communities in periodontal health and periodontal disease. Front Cell Infect Microbiol 10:577485. doi: 10.3389/fcimb.2020.577485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hajishengallis G, Chavakis T. 2021. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 21:426–440. doi: 10.1038/s41577-020-00488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li X, Wang H, Yu X, Saha G, Kalafati L, Ioannidis C, Mitroulis I, Netea MG, Chavakis T, Hajishengallis G. 2022. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185:1709–1727. doi: 10.1016/j.cell.2022.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N. 2020. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182:447–462. doi: 10.1016/j.cell.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tan L, Wang H, Li C, Pan Y. 2014. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J Periodontal Res 49:760–769. doi: 10.1111/jre.12159 [DOI] [PubMed] [Google Scholar]
- 158. Pan Y, Teng D, Burke AC, Haase EM, Scannapieco FA. 2009. Oral bacteria modulate invasion and induction of apoptosis in Hep-2 cells by Pseudomonas aeruginosa. Microb Pathog 46:73–79. doi: 10.1016/j.micpath.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 159. Li Q, Pan C, Teng D, Lin L, Kou Y, Haase EM, Scannapieco FA, Pan Y. 2014. Porphyromonas gingivalis modulates Pseudomonas aeruginosa-induced apoptosis of respiratory epithelial cells through the STAT3 signaling pathway. Microbes Infect 16:17–27. doi: 10.1016/j.micinf.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 160. Moutsopoulos NM, Konkel JE. 2018. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol 39:276–287. doi: 10.1016/j.it.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 341:569–573. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wang B, Lim JH, Kajikawa T, Li X, Vallance BA, Moutsopoulos NM, Chavakis T, Hajishengallis G. 2019. Macrophage β2-integrins regulate IL-22 by ILC3s and protect from lethal Citrobacter rodentium-induced colitis. Cell Rep 26:1614–1626. doi: 10.1016/j.celrep.2019.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, Doherty JM, Mills JC, Colonna M. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457:722–725. doi: 10.1038/nature07537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206:1465–1472. doi: 10.1084/jem.20082683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. doi: 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]