Abstract
INTRODUCTION
Pregestational smoking increases the risk of gestational diabetes mellitus (GDM) and is a common health problem during pregnancy, with its incidence on the rise worldwide, especially in China. This study is a meta-analysis of passive smoking as a risk factor associated with GDM.
METHODS
Two independent reviewers searched passive smoking and the risk of GDM in PubMed, Medline, Web of Knowledge, Science Direct, China National Knowledge Internet (CNKI) and Wanfang databases (up to May 2023). The authors extracted the study data independently and used the Newcastle–Ottawa scale (NOS) to evaluate the quality of the included articles. A meta-analysis was conducted using a random effects model depending on the size of the heterogeneity. Begg’s and Egger’s tests were performed to assess publication bias.
RESULTS
The overall relative risk for GDM caused by passive smoking was 1.47 (95% CI: 1.31–1.64), with moderate heterogeneity between studies (I2=41.7%, p=0.079). Subgroup and sensitivity analyses were stable, and no evidence of publication bias was found.
CONCLUSIONS
Passive smoking is a risk factor for GDM, even in those who are not active smokers. To eliminate the effects of other confounding factors, larger prospective cohort studies are required to clarify the relationship between passive smoking and the occurrence of GDM.
Keywords: passive smoking, gestational diabetes mellitus, GDM, metaanalysis
INTRODUCTION
Gestational diabetes mellitus (GDM), which refers to abnormal glucose metabolism first detected or occurring during pregnancy, is a prevalent complication1. Survey data show that more than 90% of diabetes in pregnant women is GDM2, which is increasing worldwide3. GDM has both short- and long-term health effects during pregnancy and subsequent generations. These women are at increased risk of type 2 diabetes4, and their offspring are at increased risk of childhood obesity5 and adult cardiovascular disease6. A meta-analysis has shown that active smoking during pregnancy is associated with an increased risk of GDM7 (OR=2.322; 95% CI: 1.359–3.967). However, many pregnant women choose to quit smoking during pregnancy, but passive smoking during pregnancy is also harmful. Studies have shown that passive smoking can increase the risk of type 2 diabetes8. However, there is insufficient research to confirm that passive smoking and GDM are associated. This study aims to clarify whether passive smoking is a risk factor for GDM through a systematic review and meta-analysis.
METHODS
Search strategy and selection criteria
This meta-analysis was performed according to the Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRISMA) guidelines9 (Supplementary file). Published articles were searched on passive smoking and GDM (up to May 2023). English articles were mainly searched in PubMed, Medline, Web of Knowledge, and Science Direct. Chinese articles were searched in the CNKI and Wanfang databases. The search terms were: ‘passive smoking’, ‘secondhand smoking’, ‘environmental smoking’, and ‘gestational diabetes mellitus or GDM’. To avoid omissions, the researchers reviewed references that met the study criteria.
Study selection and extraction criteria: 1) cohort study or case-control study; 2) diagnosis of gestational diabetes or GDM; 3) exposure to passive smoking; and 4) effect size (OR and relative risk, RR), CI, and any information that can be derived.
Exclusion criteria: 1) exposure factors were not identified as passive smoking in the study; 2) reviews, case reports, meetings, letters, and animal studies; and 3) studies without OR values or where the OR and 95% CI could not be calculated in the raw data provided.
Data extraction and quality assessment
Two researchers extracted authors (year of publication), study type, country, sample size, and number of GDM cases. The OR (RR, HR) and 95% CI were extracted to conduct a meta-analysis and adjust confounding factors. The selected articles were then assessed for quality using the NOS10. There are nine entries on this self-rating scale, each occupying 1 point. The quality of the article was independently assessed by HZ and EM based on previous studies; only those with NOS scores ≥5 were selected10.
Statistical analysis
Statistical analysis was performed using Stata 13.0. Judging heterogeneity by I2, a low heterogeneity was considered when I2 <25.0%. A fixed effects model analysis was used; otherwise, a random effects model was used to calculate the pooled OR11. Sources of heterogeneity between studies were explored by sensitivity analysis. Begg’s or Egger’s method and the funnel plot12 were used to test for publication bias.
RESULTS
Study selection
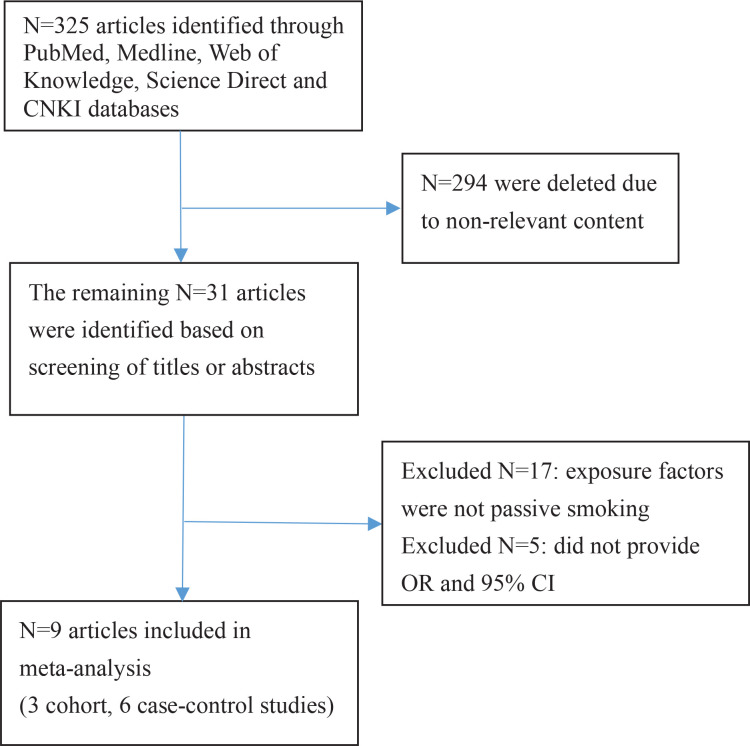
Figure 1 shows the search process. After reviewing the titles and abstracts of 325 articles, 316 articles that did not meet the inclusion criteria of content, study design, and target population were excluded. A total of nine articles13-21 were selected for this meta-analysis; these included 27654 pregnant women, 3730 of whom were diagnosed with GDM; three cohort studies13-15, six case-control studies16-21; four English articles13-16 and five Chinese articles17-21; eight study subjects in the Chinese population13,15-21, and one in the European population14. Seven studies indicated a positive correlation between passive smoking (who were currently exposed to passive smoke but did not actively smoke) and GDM13-19, and two did not20,21 (Table 1).
Figure 1.
Main characteristics of in cluded studies on the passive smoking and risk of GDM
Table 1.
Main characteristics of included studies on the passive smoking and risk of GDM
| Authors Year | City Country | Survey time | Language | Study | Sample size | GDM | OR 95 % CI | Adjustment factors | Score |
|---|---|---|---|---|---|---|---|---|---|
| Na et al.13 2022 | Beijing China | 2017–2020 | English | Cohort study | 3083 | 562 | 1.37 (1.11–1.70) | Age, BMI, ethnicity, education level, profession, parity | 7 |
| Morales et al.14 2022 | Valencia Spain | 2/2017–4/2020 | English | Cohort study | 1262 | 106 | 1.66 (1.15–2.38) | Age, BMI | 6 |
| Gao et al.15 2020 | Tianjin China | 10/2010–8/2012 | English | Cohort study | 19331 | 1485 | 1.36 (1.12–1.65) | Age, BMI, family history of diabetes, parity, education level, pressure, number of pregnancies, weight gain during pregnancy, drinking | 7 |
| Carroll et al.16 2018 | Beijing China | 1/2012–6/2014 | English | Case-control | 276/276 | 276/274 | 1.52 (1.05–2.20) 1.71 (1.14–2.56) |
Education level, profession, drinking, physical activities, total sleep time, number of pregnancies, family history of diabetes | 7 |
| Yang and Zhou17 2018 | Linyi China | 11/2013–6/2017 | Chinese | Case-control | 1018 | 302 | 1.571 (1.207–1.985) | Age, progestational BMI, number of pregnancies, education level, family history of diabetes, sleeping hours, weight gain during pregnancy, physical activities | 7 |
| Shi et al.18 2021 | Huzhou China | 3/2019–10/2019 | Chinese | Case-control | 300 | 200 | 1.571 (1.199–2.06) | Age, progestational BMI, number of pregnancies, dietary habit, education level, family history of diabetes, sleeping hours, weight gain during pregnancy, physical activities | 6 |
| Shu et al.19 2020 | Ningbo China | 1/2018–3/2019 | Chinese | Case-control | 1644 | 672 | 1.906 (1.501–2.421) | Age, education level, ethnicity, family history of diabetes, pre-pregnancy weight, number of pregnancies, abortion, pressure | 7 |
| Ou et al.20 2002 | Shanghai China | 10/1999–2/2001 | Chinese | Case-control | 262 | 85 | 0.99 (0.352–1.023) | Age, obesity during pregnancy, BMI, parity, family history of diabetes, physical activities, education level, cholesterol, trilaurin | 7 |
| Guo and Guo21 2020 | Zhengzhou China | 1/2020–12/2020 | Chinese | Case-control | 3343 | 603 | 1.135 (0.956–1.349) | Age, BMI, parity, abortion, exfetation, dietary habit, sleeping hours | 7 |
GDM: gestational diabetes mellitus.
Passive smoking and the risk of GDM
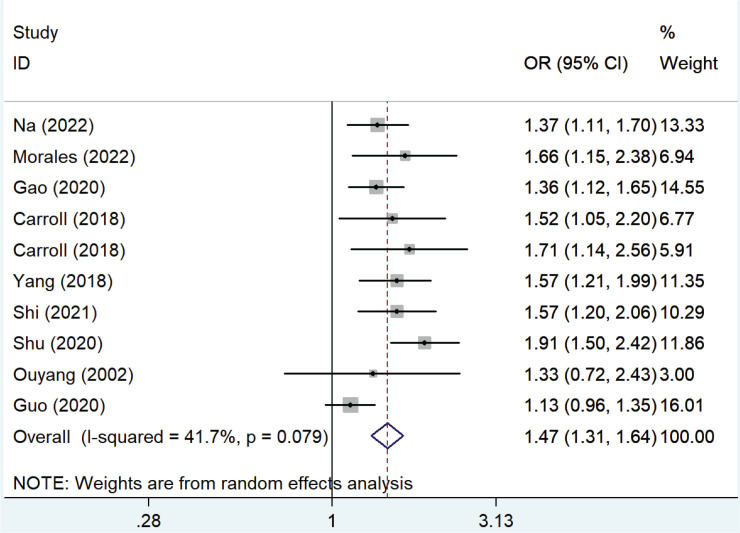
Figure 2 shows the pooled OR values from all studies showing that passive smoking was associated with the risk of developing GDM (OR=1.47; 95% CI: 1.31–1.64) with low heterogeneity (I2=41.7%).
Figure 2.
Forest plot of the relationship between passive smoking and GDM
Subgroup and sensitivity analysis
Subgroup analysis based on the study design, language, follow-up years, number of GDM cases, and adjustments to the OR score showed that the results remained similar. Based on study styles, OR values were 1.40 (95% CI: 1.23–1.60, n=3, I2=0.0%, p=0.616) for cohort studies and 1.43 (95% CI: 1.30–1.59, n=7, p=0.013) for case-control studies; based upon published in English, 1.44 (95% CI: 1.28–1.62, n=5, I2=0.0%, p=0.753), and Chinese, 1.41 (95% CI: 1.26–1.57, n=5, I2=73.7%, p=0.004); based upon follow-up years, ≥3 years, 1.48 (95% CI: 1.28–1.72, n=3, I2=0.0%, p=0.575) and <3 years, 1.40 (95% CI: 1.27–1.54, n=7, I2=61.6%, p=0.016); based upon the number of GDM cases ≥500, 1.36 (95% CI: 1.23–1.50, n=4, I2=74.8%, p=0.008) and <500, 1.54 (95% CI: 1.35–1.77, n=6, I2=0.0%, p=0.684).
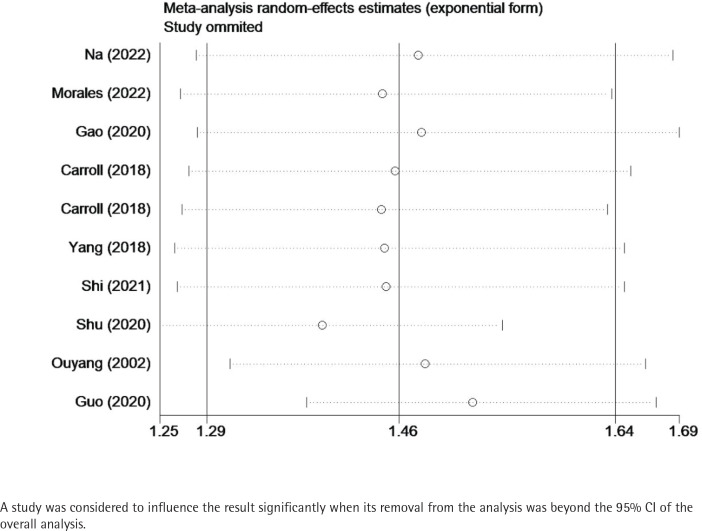
Sensitivity analysis confirmed that the results remained stable after the removal of one study at a time, in which no individual studies were found to affect the overall OR, and the pooled ORs ranged from 1.44 (95% CI: 1.30–1.60) to 1.55 (95% CI: 1.39–1.72). Table 2 and Figure 3 show the data from our subgroup and sensitivity analyses, respectively.
Table 2.
Subgroup and sensitivity analysis of the included studies
| Variables | Number of studies | Effect estimates | Heterogeneity | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | χ2 | p | I2 (%) | ||
| Study design | ||||||
| Cohort | 3 | 1.40 | 1.23–1.60 | 0.97 | 0.616 | 0.00 |
| Case-control | 7 | 1.43 | 1.30–1.59 | 16.16 | 0.013 | 62.9 |
| Language | ||||||
| English | 5 | 1.44 | 1.28–1.62 | 1.94 | 0.753 | 0.00 |
| Chinese | 5 | 1.41 | 1.26–1.57 | 15.24 | 0.004 | 73.7 |
| Follow-up years | ||||||
| ≥3 | 3 | 1.48 | 1.28–1.72 | 1.11 | 0.575 | 0.00 |
| <3 | 7 | 1.40 | 1.27–1.54 | 15.64 | 0.016 | 61.6 |
| Number of GDM | ||||||
| ≥500 | 4 | 1.36 | 1.23–1.50 | 11.90 | 0.008 | 74.8 |
| <500 | 6 | 1.54 | 1.35–1.77 | 3.11 | 0.684 | 0.00 |
| Score | ||||||
| High | 4 | 1.51 | 1.35–1.68 | 5.66 | 0.129 | 47.0 |
| Moderate | 6 | 1.33 | 1.18–1.50 | 9.30 | 0.098 | 46.2 |
GDM: gestational diabetes mellitus.
Figure 3.
Sensitivity analysis of the relationship between passive smoking and GDM
Publication bias
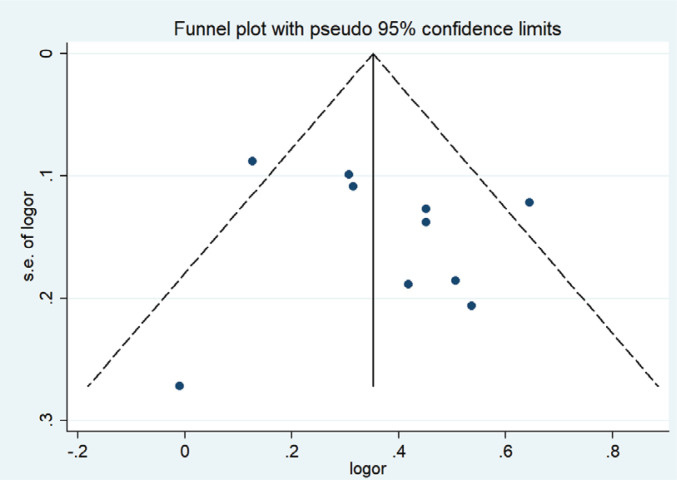
A funnel plot was used to evaluate publication bias. Begg’s (p= p0.602) and Egger’s (p=0.500) tests showed no publication bias, as shown in Figure 4.
Figure 4.
Funnel plot of the relationship between passive smoking and GDM
DISCUSSION
Our meta-analysis confirmed that passive smoking led to a 1.42 times higher risk of pregnant women developing GDM compared to those who had not been exposed to secondhand smoke (OR=1.42; 95% CI: 1.31–1.54, I2=47.7%). Because of the heterogeneity, we conducted a subgroup analysis. Sensitivity analysis confirmed that a single study did not alter the pooled OR, and the ORs ranged from 1.44 to 1.55. The global prevalence of GDM is about 1.8–31.0%, and about 20.3% in China22. Several epidemiological studies have shown that the etiology of GDM may be a combination of genetic and environmental factors. It is believed that the occurrence of GDM is related to the family history of diabetes, maternal pregnancy age, pre-pregnancy body mass index, and age at first pregnancy23,24. Our previous research8 confirms that passive smoking is a risk factor for type 2 diabetes mellitus even in those who are not active smokers, but how passive exposure to tobacco smoke leads to GDM is unclear. According to the ‘Chinese reported health hazards of smoking’, the passive smoking rate of fertile women in China was 51.9% in 201225. About 60–75% of non-smoking pregnant women are exposed to smoking environments during pregnancy26. A prospective cohort study of 193131 pregnant women in Tianjin15 found that 47.3% (9148/19331) of women were exposed to passive smoking during pregnancy, and the risk of GDM caused by passive smoking is 1.36 times higher than that caused by non-passive smoking. Previous studies have confirmed that long-term or passive smoking may affect glucose metabolism and increase the risk of developing diabetes in the population. The pathogenic mechanism is still unclear, but the reason may be that nicotine in tobacco can cause impaired insulin sensitivity and pancreatic islet β-cell function27. This results in a sympathetic excitation and increased catecholamine release to antagonize the secretory function of islets28. It could also be that the carbon monoxide produced by burning tobacco enters the bloodstream and binds to hemoglobin, leading to an increase in hemoglobin. Epidemiological findings show that women who smoke passively have elevated hemoglobin content and fasting blood glucose levels.
Limitations
There are some limitations to this study. Only one study reported the exposure of pregnant women to passive smoking in the workplace, and this may have led to an underestimation of the dangers of passive smoking. We did not stratify the analysis by age and weight, but all of the studies are adjusted for age and BMI. The studies used questionnaires to evaluate passive smoking, and self-reported methods could easily result in reporting bias.
CONCLUSIONS
This meta-analysis indicates that passive smoking increases the risk of developing GDM in non-smoking pregnant women.
Supplementary Material
CONFLICTS OF INTEREST
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. H. Zhang, L. Tian, J. Huang, and J. Yin report that since the initial planning of the work, this study was supported by the Shanxi Health Commission Key Laboratory of Nervous System Disease Prevention and Treatment (2020SY20) of the Sinopharm Tongmei General Hospital.
FUNDING
This work was supported by the Shanxi Health Commission Key Laboratory of Nervous System Disease Prevention and Treatment (2020SY20).
ETHICAL APPROVAL AND INFORMED CONSENT
Ethical approval and informed consent were not required for this study.
DATA AVAILABILITY
The data supporting this research are available from the authors on reasonable request.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer-reviewed.
REFERENCES
- 1.Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. 2019;48(3):479–493. doi: 10.1016/j.ecl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan H, Shang L. Prevalence status of gestational diabetes mellitus. Article in Chinese. Chinese Journal of Practical Gynecology and Obstetrics. 2015;(1):91–94. doi: 10.7504/fk2014120120. [DOI] [Google Scholar]
- 3.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Song C, Li C, Liu P, Sun Z, Yang X. Increased risk of cardiovascular disease in women with prior gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;140:324–338. doi: 10.1016/j.diabres.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 6.Kaseva N, Vääräsmäki M, Sundvall J, et al. Gestational diabetes but not prepregnancy overweight predicts for cardiometabolic markers in offspring twenty years later. J Clin Endocrinol Metab. 2019;104(7):2785–2795. doi: 10.1210/jc.2018-02743. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Xiao CM, Zhang Y, et al. Factors associated with gestational diabetes mellitus: a meta-analysis. J Diabetes Res. 2021;2021:6692695. doi: 10.1155/2021/6692695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei X, E M, Yu S. A meta-analysis of passive smoking and risk of developing Type 2 Diabetes Mellitus. Diabetes Res Clin Pract. 2015;107(1):9–14. doi: 10.1016/j.diabres.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na J, Chen H, An H, et al. Passive smoking and risk of gestational diabetes mellitus among nonsmoking women: a prospective cohort study in China. Int J Environ Res Public Health. 2022;19(8):4712. doi: 10.3390/ijerph19084712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales-Suárez-Varela M, Peraita-Costa I, Perales-Marín A, Llopis-Morales A, Llopis-González A. Risk of gestational diabetes due to maternal and partner smoking. Int J Environ Res Public Health. 2022;19(2):925. doi: 10.3390/ijerph19020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, Leng J, Liu H, et al. Development and validation of an early pregnancy risk score for the prediction of gestational diabetes mellitus in Chinese pregnant women. BMJ Open Diabetes Res Care. 2020;8(1):e000909. doi: 10.1136/bmjdrc-2019-000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll X, Liang X, Zhang W, et al. Socioeconomic, environmental and lifestyle factors associated with gestational diabetes mellitus: a matched case-control study in Beijing, China. Sci Rep. 2018;8(1):8103. doi: 10.1038/s41598-018-26412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XS, Zhou LX. Analysis of influencing factors of gestational diabetes mellitus. Article in Chinese. Chin J Family Plann Gynecotokol. 2018;10(5):92–96. doi: 10.3969/j.issn.1674-4020.2018.05.23. [DOI] [Google Scholar]
- 18.Shi W, Shen L, Cai L. Analysis of influencing factors of gestational diabetes mellitus and the intervention effect of individualized medical nutrition therapy. Article in Chinese. Chin. J. Woman Child Health Res. 2021;(3):417–421. [Google Scholar]
- 19.Shu M, Pan X, Zhang B, et al. The relationship between passive smoking and gestational diabetes mellitus. Article in Chinese. Preventive Medicine. 2020;32(7):726–729. doi: 10.19485/j.cnki.issn2096-5087.2020.07.020. [DOI] [Google Scholar]
- 20.Ou Y, Shen F, Jiang F, Hu H, Pan M. Risk factors in women with gestational diabetes mellitus. Article in Chinese. Chinese Journal of Preventive Medicine. 2002;(6):378–381. [PubMed] [Google Scholar]
- 21.Guo M, Guo W. Incidence and risk factors of gestational diabetes mellitus in pregnant women in a first-class hospital in 2020. Chinese Journal of Practical Medicine. 2022;49(6):30–34. doi: 10.3760/cma.j.cn115689-20211202-04396. [DOI] [Google Scholar]
- 22.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 23.Solarnon CG, Willet WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–1083. doi: 10.1001/jama.278.13.1078. [DOI] [PubMed] [Google Scholar]
- 24.Zhao WJ, Che QH, Jia LH. Case-control study on effect factors of gestational diabetes mellitus. Article in Chinese. Maternal and Child Health Care of China. 2009;24(25):3550–3552. [Google Scholar]
- 25.Han J, Chen X. A meta-analysis of cigarette smoking prevalence among adolescents in China: 1981-2010. Int J Environ Res Public Health. 2015;12(5):4617–4630. doi: 10.3390/ijerph120504617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao T, Lee AH, Mao Z. Potential unintended consequences of smoke-free policies in public places on pregnant women in China. Am J Prev Med. 2009;37(Suppl 2):S159–S164. doi: 10.1016/j.amepre.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury P, Rayford PL, Chang LW. Pathophysiological effects of nicotine on the pancreas. Proc Soc Exp Biol Med. 1998;218(3):168–173. doi: 10.3181/00379727-218-44284. [DOI] [PubMed] [Google Scholar]
- 28.Gu L, Li J, Pan G, et al. Effects of passive smoking on glycemic parameters and lipid profiles in a Chinese female population. Clin Lab. 2017;63(7):1147–1152. doi: 10.7754/Clin.Lab.2017.170102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this research are available from the authors on reasonable request.