Abstract
Background
Despite potential analgesic benefits from topical ophthalmic amides and esters, their outpatient use has become of concern because of the potential for abuse and ophthalmic complications.
Objectives
To assess the effectiveness and safety of topical ophthalmic anesthetics compared with placebo or other treatments in persons with corneal abrasions.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; Embase.com; Latin American and Caribbean Health Sciences (LILACS); ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), without restriction on language or year of publication. The search was performed on 10 February 2023.
Selection criteria
We included randomized controlled trials (RCTs) of topical ophthalmic anesthetics alone or in combination with another treatment (e.g. nonsteroidal anti‐inflammatory drugs (NSAIDs)) versus a non‐anesthetic control group (e.g. placebo, non‐treatment, or alternative treatment). We included trials that enrolled participants of all ages who had corneal abrasions within 48 hours of presentation.
Data collection and analysis
We used standard Cochrane methodology.
Main results
We included nine parallel‐group RCTs with a total of 556 participants (median number of participants per study: 45, interquartile range (IQR) 44 to 74), conducted in eight countries: Australia, Canada, France, South Korea, Turkey, New Zealand, UK, and USA.
Study characteristics and risk of bias
Four RCTs (314 participants) investigated post‐traumatic corneal abrasions diagnosed in the emergency department setting. Five trials described 242 participants from ophthalmology surgery centers with post‐surgical corneal defects: four from photorefractive keratectomy (PRK) and one from pterygium surgery. Study duration ranged from two days to six months, the most common being one week (four RCTs). Treatment duration ranged from three hours to one week (nine RCTs); the majority were between 24 and 48 hours (five RCTs). The age of participants was reported in eight studies, ranging from 17 to 74 years of age. Only one participant in one trial was under 18 years of age. Of four studies that reported funding sources, none was industry‐sponsored. We judged a high risk of bias in one trial with respect to the outcome pain control by 48 hours, and in five of seven trials with respect to the outcome complications at the furthest time point. The domain for which we assessed studies to be at the highest risk of bias was missing or selective reporting of outcome data.
Findings
The treatments investigated included topical anesthetics compared with placebo, topical anesthetic compared with NSAID (post‐surgical cases), and topical anesthetics plus NSAID compared with placebo (post‐surgical cases).
Pain control by 24 hours
In all studies, self‐reported pain outcomes were on a 10‐point scale, where lower numbers represent less pain. In post‐surgical trials, topical anesthetics provided a moderate reduction in self‐reported pain at 24 hours compared with placebo of 1.28 points on a 10‐point scale (mean difference (MD) −1.28, 95% confidence interval (CI) −1.76 to −0.80; 3 RCTs, 119 participants). In the post‐trauma participants, there may be little or no difference in effect (MD −0.04, 95% CI −0.10 to 0.02; 1 RCT, 76 participants). Compared with NSAID in post‐surgical participants, topical anesthetics resulted in a slight increase in pain at 24 hours (MD 0.82, 95% CI 0.01 to 1.63; 1 RCT, 74 participants).
One RCT compared topical anesthetics plus NSAID to placebo. There may be a large reduction in pain at 24 hours with topical anesthetics plus NSAID in post‐surgical participants, but the evidence to support this large effect is very uncertain (MD −5.72, 95% CI −7.35 to −4.09; 1 RCT, 30 participants; very low‐certainty evidence).
Pain control by 48 hours
Compared with placebo, topical anesthetics reduced post‐trauma pain substantially by 48 hours (MD −5.68, 95% CI −6.38 to −4.98; 1 RCT, 111 participants) but had little to no effect on post‐surgical pain (MD 0.41, 95% CI −0.45 to 1.27; 1 RCT, 44 participants), although the evidence is very uncertain.
Pain control by 72 hours
One post‐surgical RCT showed little or no effect of topical anesthetics compared with placebo by 72 hours (MD 0.49, 95% CI −0.06 to 1.04; 44 participants; very low‐certainty evidence).
Proportion of participants with unresolved epithelial defects
When compared with placebo or NSAID, topical anesthetics increased the number of participants without complete resolution of defects in trials of post‐trauma participants (risk ratio (RR) 1.37, 95% CI 0.78 to 2.42; 3 RCTs, 221 participants; very low‐certainty evidence). The proportion of placebo‐treated post‐surgical participants with unresolved epithelial defects at 24 to 72 hours was lower when compared with those assigned to topical anesthetics (RR 0.14, 95% CI 0.01 to 2.55; 1 RCT, 30 participants; very low‐certainty evidence) or topical anesthetics plus NSAID (RR 0.33, 95% CI 0.04 to 2.85; 1 RCT, 30 participants; very low‐certainty evidence).
Proportion of participants with complications at the longest follow‐up
When compared with placebo or NSAID, topical anesthetics resulted in a higher proportion of post‐trauma participants with complications at up to two weeks (RR 1.13, 95% CI 0.23 to 5.46; 3 RCTs, 242 participants) and post‐surgical participants with complications at up to one week (RR 7.00, 95% CI 0.38 to 128.02; 1 RCT, 44 participants). When topical anesthetic plus NSAID was compared with placebo, no complications were reported in either treatment arm up to one week post‐surgery (risk difference (RD) 0.00, 95% CI −0.12 to 0.12; 1 RCT, 30 participants). The evidence is very uncertain for safety outcomes.
Quality of life
None of the included trials assessed quality of life outcomes.
Authors' conclusions
Despite topical anesthetics providing excellent pain control in the intraoperative setting, the currently available evidence provides little or no certainty about their efficacy for reducing ocular pain in the initial 24 to 72 hours after a corneal abrasion, whether from unintentional trauma or surgery. We have very low confidence in this evidence as a basis to recommend topical anesthetics as an efficacious treatment modality to relieve pain from corneal abrasions. We also found no evidence of a substantial effect on epithelial healing up to 72 hours or a reduction in ocular complications when we compared anesthetics alone or with NSAIDs versus placebo.
Keywords: Adolescent; Adult; Aged; Humans; Middle Aged; Young Adult; Analgesics; Anesthetics, Local; Anti-Inflammatory Agents, Non-Steroidal; Anti-Inflammatory Agents, Non-Steroidal/therapeutic use; Corneal Injuries; Corneal Injuries/drug therapy; Pain, Postoperative
Plain language summary
What are the benefits and unwanted effects of topical anesthetics for corneal abrasions?
Key message(s)
1. We are very uncertain about the effectiveness of anesthetic eye drops for control of pain due to corneal abrasions (scratches).
2. We are very uncertain about the safety of anesthetic eye drops regarding speed of healing and complications.
3. To ensure trustworthy evidence, researchers should follow best‐practice guidance. Future research studies should include more people followed over a longer period of time after treatment ends.
What is a corneal abrasion?
A corneal abrasion is a scratch on the clear outer layer of the eye. These scratches can be caused by fingernails, dust, dirt, wood, twigs, thorns, or metal shavings blown or pushed into the eye. Improper use of contact lenses sometimes results in minor but painful scratches on the cornea. Some eye surgeries, like one type of laser refractive surgery, may require deliberately creating an abrasion. The symptoms of corneal abrasion include eye pain, blurred vision, grittiness, excessive tearing, redness, light sensitivity, or even headache.
How are corneal abrasions treated?
Non‐medicine‐based care of corneal abrasions includes eye rinse with clean water or normal saline and frequent blinking. Although most minor scratches on the cornea can heal on their own, they are typically treated with antibiotic eye drops or ointment to prevent infections. Sometimes, doctors prescribe topical painkillers to reduce eye pain, such as anesthetics (medications that lower the sense of pain) and nonsteroidal anti‐inflammatory drugs (NSAIDs).
What did we want to find out?
We assessed whether anesthetic eye drops reduce pain in people with corneal abrasions. We also examined whether anesthetic eye drops influence the healing of the corneal wound or cause unwanted effects on the eyes.
What did we do?
We performed a systematic review by searching for studies that compared anesthetic eye drops with no treatment, inactive eye drops, or a different medication. We summarized the review findings and reported results along with our confidence about the evidence based on the study design and method.
What did we find?
We found nine studies that had enrolled 556 people aged 17 years or older. Four studies took place in hospital emergency care settings and five took place in eye surgery settings. Most studies were one week long, but their length ranged from two days (one study) to six months (another study). Only four studies reported funding sources, none of which were drug companies.
In comparison with inactive treatment, anesthetic eye drops alone were effective in reducing eye pain up to 24 hours after treatment and may also be effective when combined with NSAIDs. When compared with NSAIDs, the anesthetic eye drops alone were slightly less effective at pain control. At 48 hours, anesthetics alone decreased eye pain relative to inactive eye drops but were no more effective at 72 hours. Anesthetic eye drops resulted in a slight delay in wound healing up to 72 hours after treatment. Other complications, such as infections, were slightly more frequent with anesthetics, but these complications were similar between groups up to one week after treatment. There were too few studies to know whether people responded to treatment differently when the abrasion was from an injury or from eye surgery. No study looked at quality of life.
The evidence for all outcomes in this review is very uncertain. Further research studies that enroll larger numbers of participants and follow them for at least one week are likely to change our findings.
What are the limitations of the evidence?
We are not confident of the conclusions suggested by the evidence found for this review of the effectiveness and safety of anesthetic eye drops because of the flawed collection and reporting of data, and the small size of the studies.
How up‐to‐date is this evidence?
The evidence is up‐to‐date as of 10 February 2023.
Summary of findings
Summary of findings 1. Topical ophthalmic anesthetic compared with placebo or NSAID.
| Topical ophthalmic anesthetic compared with placebo or NSAID | ||||||
|
Patients or population: corneal abrasion (post‐trauma or post‐surgical) Settings: emergency department or ophthalmology surgery Intervention: anesthetic (tetracaine, proparacaine, lidocaine) Comparison: placebo or NSAID (diclofenac, artificial tears, saline) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with comparator | Corresponding risk with anesthetic | |||||
|
Changes in mean participant‐reported ocular pain from baseline to 24 hours
Assessed with: VAS pain intensity 0 to 10 |
Placebo, post‐surgery | ⊕⊝⊝⊝ Very lowa,b |
Lower is better. The original VAS was from 0 to 100 in Waldman 2014, which compared tetracaine 1% with placebo in post‐trauma participants. |
|||
| The mean change in the comparison group was 3.63 (SD 1.00) | The mean change in the intervention group was 1.28 lower (0.80 to 1.76) | — | 119 (3 RCTs) | |||
| Placebo, post‐trauma (see comment) | ||||||
| The mean change in the comparison group was 0.11 (SD 0.13) | The mean change in the intervention group was 0.04 lower (0.10 lower to 0.02 higher) | — | 76 (1 RCT) |
|||
| NSAID, post‐surgery | ||||||
| The mean change in the comparison group was 2.09 (SD 1.77) | The mean change in the intervention group was 0.82 higher (0.01 to 1.63) | — | 74 (1 RCT) |
|||
|
Changes in mean participant‐reported ocular pain from baseline to 48 hours
Assessed with: VAS pain intensity 0 to 10 |
Placebo, post‐surgery | ⊕⊝⊝⊝ Very lowa,b,c |
Lower is better. Waldman 2014 was excluded from the analysis due to missing data. |
|||
| The mean change in the comparison group was 0.81 (SD 1.46) | The mean change in the intervention group was 0.41 higher (0.45 lower to 1.27 higher) | — | 44 (1 RCT) | |||
| Placebo, post‐trauma | ||||||
| The mean change in the comparison group was 7.23 (SD 1.95) | The mean change in the intervention group was 5.68 lower (4.98 lower to 6.38 lower) | — | 111 (1 RCT) | |||
|
Changes in mean participant‐reported ocular pain from baseline to 72 hours
Assessed with: VAS pain intensity 0 to 10 |
The mean change in the comparison group was 0.2 (SD 0.83) | The mean change in the intervention group was 0.49 higher (0.06 lower to 1.04 higher) | — | 44 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c,d |
Lower is better. Only Verma 1995 reported pain outcomes beyond 48 hours (at 64 hours). |
| Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours | Placebo, post‐trauma | ⊕⊝⊝⊝ Very lowb,c,e |
RR < 1 is better. Another post‐surgery trial (44 participants) reported no events in either arm. |
|||
| 152 per 1000 | 208 per 1000 (118 to 367) |
RR 1.37 (0.78 to 2.42) | 221 (3 RCTs) |
|||
| Placebo, post‐surgery | ||||||
| 200 per 1000 | 28 per 1000 (2 to 510) |
RR 0.14 (0.01 to 2.55) | 30 (1 RCT) |
|||
|
Proportion of participants with complications at longest time point Up to 2 weeks |
Placebo, post‐trauma | ⊕⊝⊝⊝ Very lowa,c,f |
RR < 1 is better. Two post‐surgical trials (75 participants) and one post‐trauma trial (33 participants) reported no events in either arm. |
|||
| 65 per 1000 | 73 per 1000 (15 to 355) |
RR 1.13 (0.23 to 5.46) | 242 (3 RCTs) |
|||
| Placebo, post‐surgery | ||||||
| 19 per 1000 (1 to 356) |
136 per 1000** | 7.00 (0.38 to 128.02) | 44 (1 RCT) |
|||
| *The basis for the assumed risk is the mean baseline risk from the studies in the meta‐analysis; the total number of events in the control group divided by the total number of participants in the control groups, scaled to 1000. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **The corresponding risk was the absolute risk (number of events divided by number of participants in the intervention group). The 95% CI was calculated using a binomial distribution. CI: confidence interval; MD: mean difference; NSAID: nonsteroidal anti‐inflammatory drug; RR: risk ratio; SD: standard deviation; VAS: visual analog scale | ||||||
|
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded for serious risk of bias (−2 levels). bDowngraded for imprecision (−1 level). cDowngraded for indirectness (−1 level). dDowngraded for risk of bias (−1 level). eDowngraded for inconsistency (−1 level). fDowngraded for extreme imprecision (−2 levels).
Summary of findings 2. Topical ophthalmic anesthetic plus NSAID compared with placebo.
| Topical ophthalmic anesthetic plus NSAID compared with placebo | ||||||
|
Patients or population: corneal abrasion (post‐surgical) Settings: ophthalmology surgery Intervention: anesthetic with NSAID (proparacaine plus diclofenac) Comparison: placebo (artificial tears) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with placebo | Corresponding risk with anesthetic plus NSAID | |||||
| Changes in mean participant‐reported ocular pain from baseline to 24 hours VAS (scale 0 to 10) | The mean change in the comparison group was 8.08 (SD 2.28) | The mean change in the intervention group was 5.72 lower (4.09 lower to 7.35 lower) | — | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
Lower is better. |
| Changes in mean participant‐reported ocular pain from baseline to 48 hours | — | — | — | — | — | — |
| Changes in mean participant‐reported ocular pain from baseline to 72 hours | — | — | — | — | — | — |
| Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours | 200 per 1000 | 66 per 1000 (8 to 570) | RR 0.33 (0.04 to 2.85) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
RR < 1 is better. |
|
Proportion of participants with complications at longest time point Up to 1 week |
No adverse events reported in either arm. | — | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
RR < 1 is better. | |
| *The basis for the assumed risk is the mean baseline risk from the studies in the meta‐analysis; the total number of events in the control group divided by the total number of participants in the control groups, scaled to 1000. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NSAID: nonsteroidal anti‐inflammatory; RR: risk ratio; SD: standard deviation; VAS: visual analog scale | ||||||
|
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded for risk of bias (−1 level). bDowngraded for extreme imprecision (−2 levels).
Background
Description of the condition
Corneal abrasions (also known as corneal epithelial defects) are lamellar losses of the corneal epithelium, the superficial, regenerative, squamous barrier of the cornea (Nishida 2022). Etiologies of abrasions include accidental trauma (mechanical, chemical, or phototoxic), ocular surgery, corneal dryness, exposure (inadequate eyelid coverage of the cornea), neurotrophic disease, ocular inflammation, infection, as well as a variety of other intrinsic ocular pathologies (Nishida 2022). Corneal abrasion is a common emergency, representing about 13% of eye‐related emergency department visits in the United States (Channa 2016; Vaziri 2016), with an estimated annual incidence of 3 per 1000 persons and a roughly two‐to‐one male predominance according to the National Ambulatory Medical Care Survey (NAMCS) (McGwin 2005). Globally an estimated 55 million eye injuries occur each year, with 750,000 requiring hospitalization (Négrel 1998).
Abrasions resulting from trauma may inoculate the eye with foreign matter and microbial organisms, leading to corneal infection. Symptoms of a corneal abrasion include intense pain, photophobia, redness, and tearing. Depending on the healthcare setting, corneal abrasions may be diagnosed and initially treated by primary care physicians, emergency medicine providers, or eye care specialists (Ahmed 2015). On clinical examination, corneal epithelial defects are best visualized by instilling fluorescein dye into the tear film, which adheres to bare stroma (but not intact epithelium), and emits a green fluorescence when illuminated with cobalt‐blue filtered light (Martonyi 2022). In an emergency setting, an examination is performed with a slit lamp biomicroscope or penlight to identify other complicating factors such as microbial infection (manifested as a corneal infiltrate), corneal laceration (deep injury beyond the corneal epithelium), or the presence of a foreign body (Hamill 2022).
In contrast to trauma, the creation of a corneal epithelial defect is the intended consequence of many commonly performed ocular surgeries, including photorefractive keratectomy (PRK, a laser refractive procedure), superficial keratectomy (removal of anterior corneal lesions), and the epithelium‐off variations of corneal cross‐linking (a treatment for keratoconus). Unlike accidental trauma, abrasions created in the setting of ocular surgery derive benefit from a sterile field. Often, adjunctive treatments such as intraoperative topical mitomycin‐C (an anti‐metabolite) and postoperative steroids are used to reduce postoperative inflammation and scarring. Bandage contact lenses are typically applied for patient comfort after these types of procedures and are removed when the cornea re‐epithelializes (Chuck 2018; Garcia‐Ferrer 2019).
The human cornea is one of the most densely innervated tissues, with an estimated density of approximately 7000 nerve terminals per square millimeter (Nishida 2022). Approximately 20% of corneal nociceptors are mechanoreceptors that generate acute pain (Shaheen 2014). Regardless of the cause of a corneal abrasion, the dense network of sensory nerve endings in the cornea may result in intense eye pain until the corneal epithelial defect is healed (Marfurt 2010; Nishida 2022). In a healthy eye, most such defects heal fully in 24 to 48 hours by peripheral migration of sheets of epithelial cells, ultimately derived from the limbal epithelial stem cells (Hamill 2022); topical antibiotic is almost always prescribed to prevent infection. Although healing often occurs without permanent damage to the cornea, potential complications include recurrent corneal erosions, infectious keratitis, corneal scarring, thinning of the corneal stroma, or corneal perforation. These events may require intensive medical or surgical management and can lead to vision loss or loss of the eye. The mainstays of treatment for a corneal abrasion are infection prophylaxis and pain control, coupled with close outpatient follow‐up (Hamill 2022).
Description of the intervention
Although it is standard practice to prescribe topical antimicrobial drops or ointments for corneal abrasions as prophylaxis against infection, there is variability in practice patterns for treatment of the pain (Hamill 2022; Sabri 1997). Ointments, bandage contact lenses, and patching of the eye closed under a gauze pad may decrease discomfort by reducing direct exposure of the defect and minimizing the mechanical irritation caused by repeated eyelid movement. It is theorized, however, that patching and bandage contact lenses could potentiate corneal infections by decreasing the cycling of tears over the ocular surface, thereby trapping microbes and impeding the action of host immune factors and antimicrobial medications (Hamill 2022). A Cochrane Review found that patching may not aid with healing or pain control. No conclusions, however, could be drawn about the relative risk of complications (Lim 2016). Bandage contact lenses are another modality for ameliorating pain through barrier coverage while allowing for blinking, normal cosmesis, and the ability to see. In the setting of ocular surface surgeries, such as corneal cross‐linking or PRK, the placement of a bandage contact lens is standard practice. Although the clinical efficacy and safety of bandage contact lenses have been established to some degree in the setting of traumatic corneal abrasions (Menghini 2013; Vandorselaer 2001), it is well‐known that extended contact lens wear increases the risk of infection and, therefore, contact lens use may be discouraged for corneal abrasions judged to be at high risk for microbial inoculation (Hamill 2022; Poggio 1989; Schein 1989).
In contrast to systemic analgesics such as oral acetaminophen, nonsteroidal anti‐inflammatory drugs (NSAIDs), gabapentin, and opioids for abrasions, topical pharmacologic analgesics have the most direct, local effect on ocular pain with limited systemic side effects. Classes of topical ophthalmic treatments for corneal pain include NSAIDs (ketorolac, diclofenac, indomethacin, bromfenac, flurbiprofen, nepafenac) as well as amide and ester analgesics (tetracaine, proparacaine, lidocaine). Amide and ester anesthetics act to inhibit electrical conduction on axons by blocking sodium channels on the inner wall of the cell membrane (Levine 2017). The duration of action of these medications is approximately 20 to 30 minutes, and they therefore require frequent dosing for use in the outpatient setting to be effective (Levine 2017). Analgesic intervention may be administered as an adjuvant to other treatments for corneal analgesia including bandage contact lenses, ointments, patching, topical NSAIDs, or oral analgesics. Here we study the efficacy and safety of topical amide and ester anesthetics for corneal abrasions.
How the intervention might work
Topical ophthalmic amide and ester medications act directly on sensory corneal nerve endings to relieve pain. In the clinical setting, these medications provide immediate relief to ocular surface pain to permit a thorough eye exam. Likewise, the immediate effectiveness of these medications also allows for excellent analgesia for a wide variety of ocular procedures (Levine 2017). These analgesic properties may be therapeutic over several days of outpatient use as a corneal epithelial defect heals. Accordingly, a recent systematic review found that topical NSAIDs for corneal abrasions significantly reduced pain and oral analgesia use without a difference in complications compared with control (Yu 2021).
Why it is important to do this review
Despite potential analgesic benefits from outpatient use of topical ophthalmic amides and esters, their use has become a topic of great controversy. Multiple published case reports and series have identified severe ocular complications associated with the outpatient use of topical anesthetic medications (Aksoy 2013; Ansari 2006; Ardjomand 2002; Chen 2004; Chern 1996; Dornic 1998; Epstein 1968; Katsimpris 2007; Khakshoor 2012; Kim 1997; Lee 2008; Pharmakakis 2002; Rosenwasser 1990; Varga 1997; Webber 1999; Willis 1970; Wu 2016; Yagci 2011). Reported complications include infection, corneal scarring, perforations, and severe ocular morbidities requiring evisceration or enucleation. In fact, topical anesthetic abuse seems to be a distinct entity with characteristic features such as persistent epithelial defects, corneal stromal ring infiltrates, disproportionate pain, and concurrent substance abuse disorder (Rosenwasser 1990). A person abusing one of these topical medications may have obtained it in a surreptitious way or may not admit to their use, making the diagnosis of abuse and treatment challenging. In support of these concerns, an intact corneal sensation from the trigeminal nerve is integral to the feedback loop that heals and maintains the ocular surface (Shaheen 2014). People with neurotrophic corneas (decreased or absent corneal sensation often from insults to the trigeminal nerve from herpes simplex virus, varicella‐zoster virus, ocular surgery, neurosurgery, diabetes, or other causes) have chronically high rates of dry eye, non‐healing epithelial defects, microbial keratitis, corneal scarring, corneal thinning, and corneal perforation (Chang 2022). Although the pain response to a corneal abrasion is severe, nociception is part of a protective sensory mechanism that includes increased tear production, the blink reflex, and the stimulation of growth factors important for healing (Chang 2022). The pain itself may serve as a harbinger of a complication, prompting timely presentation to medical care. In addition to interrupting the neural feedback loop, there is evidence that anesthetic medications may be directly cytotoxic to the corneal epithelium, although the full mechanism remains to be studied (Boljka 1994; Parsons 2022; Peyman 1994). Embracing many of these sentiments, a survey of 75 corneal specialists found universal opposition to the outpatient use of topical anesthetics (Lee 2019). However, there is a paucity of studies both designed and sufficiently powered to establish a causal relationship between outpatient topical anesthetic use and ocular complications. Although the collective body of case reports and series indicates a syndrome of topical anesthetic abuse (often with devastating consequences), it is unclear whether a strict prohibition is warranted, or whether these medications can be safely administered in a controlled and limited fashion in order to relieve suffering, in the same manner that topical steroids are prescribed for pain and inflammation despite potential for abuse and adverse events. Given the prevalence of both traumatic and surgically created corneal abrasions, an evidence‐based analgesic strategy for corneal epithelial defects will have broad implications for clinical care. Our goal is to review outcome data from randomized controlled trials (RCTs) of topical amide and ester anesthetics for the efficacy of analgesia and safety for corneal epithelial defects resulting from both trauma and ocular surface surgery. Since particularly devastating complications may be rare, pooling data from multiple studies provides more statistical power to estimate the benefits and risks of use more accurately and precisely than any single RCT.
Objectives
To assess the effectiveness and safety of topical ophthalmic anesthetics compared with placebo or other treatments in persons with corneal abrasions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in this review. We included all eligible trials irrespective of their publication status. We planned to include within‐person trials, where eyes had been allocated randomly to the intervention and comparator, but none were found.
Types of participants
We included trials that enrolled participants of all ages who had corneal abrasions within 48 hours of presentation, and from varying causes, including accidental trauma and ophthalmic surgery.
Types of interventions
We included trials that compared topical ophthalmic anesthetics (amide or ester class) with a non‐amide or non‐ester control group (either placebo, non‐treatment, or alternative treatment). We also included trials in which topical anesthetics with an NSAID were compared with a control group (see Differences between protocol and review). We excluded trials in which participants were given topical anesthetics only once after trauma‐ or surgery‐induced abrasion because of negligible clinical benefits or harms associated with the transient pharmacological effects of topical anesthetics.
Types of outcome measures
Primary outcomes
Critical outcomes
Pain control: change in participant‐reported ocular pain as measured using a pain scale that is continuous (e.g. 0 to 10 cm visual analog scale, VAS) or discrete (e.g. numerical rating scale 0 = "no pain" through 10 = "worst pain imaginable") from baseline to 24 hours, 48 hours, and 72 hours after treatment initiation. When the change scores were not available, we used pain scores measured at the above‐mentioned follow‐up time points.
Epithelial healing: proportion of participants without complete resolution of epithelial defects by 24 to 72 hours.
Complications: proportion of participants with adverse events (e.g. microbial keratitis or stromal infiltration, corneal stromal thinning, corneal perforation, surgical interventions) reported at the longest follow‐up time of the study. Complications that suggest abuse would be nonhealing epithelial defect, stromal infiltration, thinning, or perforation. The last two would be seen most likely after more than a week of frequent use of topical anesthetic.
Secondary outcomes
Important outcomes
Treatment failure: proportions of participants who required rescue oral analgesics by 72 hours after treatment initiation.
Quality of life: mean changes in quality of life as measured by a validated instrument for health‐related or vision‐related quality of life, or functions of daily activity as quantified by the 7 or 12 Instrumental Activities of Daily Living checklist, as defined in the original study. We planned to use data from the longest follow‐up time of the study. When the change scores were not available, we planned to use mean scores instead.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for potentially eligible RCTs and controlled clinical trials. There were no restrictions based on language or year of publication. The search was performed on 19 February 2022 and updated on 10 February 2023. Search details are provided in the specified appendices.
Cochrane Central Register of Controlled Trials (CENTRAL, which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (2023, Issue 2) (Appendix 1).
MEDLINE Ovid (All) (1946 to 10 February 2023) (Appendix 2).
Embase.com (Elsevier) (1947 to 10 February 2023) (Appendix 3).
PubMed (1948 to 10 February 2023) (Appendix 4).
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 10 February 2023) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp) (Appendix 7).
Searching other resources
We searched the reference lists of studies that were included following full‐text screening. We also searched the reference lists of systematic reviews and guidelines for additional trials missed by the electronic searches. We did not handsearch specific journals or conference proceedings as many eyes and vision conferences are included in Embase.
Data collection and analysis
Selection of studies
The Information Specialist performed electronic searches of the selected databases and removed duplicates. We worked in pairs (MS, KC, CI, SL, LL, IK) and independently screened the titles and abstracts resulting from the searches using the web‐based software Covidence. We resolved disagreements by discussion. We noted the number of citations considered not relevant in the selection of studies flow diagram (Figure 1). We obtained full‐text copies of reports from trials judged to be potentially relevant by either review author. We corresponded with study investigators to clarify study eligibility, as appropriate. For trial registration records and meeting abstracts with no full‐text report, we contacted the study investigators for desired information about study methods and any outcome data that were available. Whenever study investigators did not respond within two weeks, we proceeded with the information available.
1.
Study flow diagram
Working in pairs, review authors (MS, KC, CI, SL, LL, IK) independently assessed the full‐text copies of reports for inclusion by applying the Criteria for considering studies for this review. We resolved disagreements by discussion. For non‐English study reports, we used Google Translate for the initial translation of the Methods and Results sections of the report, which was sufficient to determine eligibility; we therefore did not enlist human translation. We were not masked to the names of the authors, institutions, or journal publications.
We listed all studies excluded during full‐text screening and provided a justification for exclusion (see Characteristics of excluded studies).
Data extraction and management
We piloted the data extraction form developed by Cochrane Eyes and Vision (CEV) in Covidence. We worked in pairs of review authors (MS, KC, CI, SL, LL, IK) to independently extract data. We resolved discrepancies through discussion.
We contacted trial investigators for desired data that had not been reported and allowed two weeks for a response before proceeding with the available data. All data were imported directly into RevMan Web by one author (LL) and one author (SL) verified the accuracy of the data imported.
For multi‐arm studies, we used data relevant to our intervention and comparator groups, taking care not to double‐count or omit participants. We planned, when two randomly allocated trial arms (interventions or comparators) contained relevant data, to combine data from them using the calculator within RevMan Web. Where data transformation was required (e.g. standard errors (SEs) from standard deviations (SDs), extracting data presented only in graphs or figures) we followed the guidance outlined in Chapter 5 and Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a; Li 2022). We extracted data available only in graphs or figures using browser‐based data extraction software (WebPlotDigitizer).
Assessment of risk of bias in included studies
We assessed the potential risk of bias in each included study using Cochrane's RoB 2 tool, as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022b). Working in pairs, review authors (MS, KC, CI, SL, LL, IK) assessed the risk of bias independently using the RoB 2 Excel tool (22 August 2019 version for individually randomized, parallel‐group trials; available from riskofbiasinfo.org). We compared judgments and resolved disagreements by discussion. We assessed bias for the 'intention‐to‐treat effect' for the efficacy outcome of pain control by 48 hours and the safety outcome of complications at the longest follow‐up time.
We considered and assessed risk of bias in the following domains:
bias due to the randomization process;
bias due to deviations from the intended intervention;
bias due to missing outcome data;
bias in measurement of the outcome; and
bias in selection of the reported result.
Based on these five domains, we assigned an overall risk of bias judgment of 'high', 'some concerns', or 'low' risk of bias.
Measures of treatment effect
We referred to the guidance outlined in Chapters 9 and 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022; McKenzie 2022). We calculated the risk ratio (RR) and 95% confidence intervals (95% CIs) for dichotomous outcomes (proportion of participants without full epithelial healing at 24 to 72 hours). We calculated the risk difference (RD) and 95% CI for dichotomous outcomes when one or more trials had zero events in both arms. We calculated the mean difference (MD) and 95% CIs for continuous outcomes (changes in pain scores from baseline to 24, 48, and 72 hours after treatment; risk of adverse events at longest time point). We had planned to calculate the standardized mean difference (SMD) and 95% CIs for continuous outcomes measured using different scales (e.g. mean change in quality of life or activities of daily living). Where possible, we checked for the skewness of continuous data (Altman 1996).
Unit of analysis issues
Where variations on RCTs were included, we referred to Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c). When both eyes of participants had been allocated to the same or different interventions, we extracted the results that accounted for the correlation between eyes. Whenever the investigators of a primary study had failed to consider the correlation between two eyes, or it was unclear whether they had, we excluded those studies in the sensitivity analysis.
Dealing with missing data
We requested missing data from study authors and allowed two weeks for a response before proceeding with the available data. We calculated missing standard deviations using P values, based on the methods outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). We did not impute missing data ourselves. When outcome data based on intention‐to‐treat (ITT) analysis were not available, we collected and combined, whenever feasible, data as reported by authors of the included trials based on either per‐protocol or complete‐case analysis. Either approach assumed that some outcome data were missing at random; we assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group when reported. This information was also used to assess potential risk of bias in individual trials (Higgins 2022b).
Assessment of heterogeneity
We examined the overall characteristics of the studies and participants (see Characteristics of included studies); in particular, we looked at the type of participants and types of interventions, to assess the extent to which the studies were similar enough to make pooling study results in meta‐analyses sensible.
We examined the forest plots of study results for consistency of effect estimates from individual studies; in particular, we considered the size and direction of effects and overlap of confidence intervals. We calculated the I2 statistic, which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2002). We considered I2 values over 75% to indicate considerable heterogeneity but also considered Chi2 and P values (Deeks 2022).
Assessment of reporting biases
We planned that, when there were 10 trials or more included in a meta‐analysis, we would construct funnel plots and consider tests for asymmetry to assess small study effects, which may be due to publication bias and other factors, according to Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Page 2022). Because there were only nine included trials that varied in the interventions and outcomes reported, we did not construct funnel plots. We examined selective reporting of results during the assessment of potential risk of bias.
Data synthesis
We referred to Chapters 9 and 10 of the Cochrane Handbook for Systematic Reviews of Interventions for data synthesis (Deeks 2022; McKenzie 2022). We pooled data using random‐effects models in RevMan Web when there were three or more trials reported on the same outcome. When data were sparse (fewer than three trials), we used a fixed‐effect model for meta‐analysis of outcomes.
Whenever there was substantial heterogeneity among individual study effect estimates, such that a combined result may not provide a good summary of the individual trial results, we did not pool the data but described the pattern of the individual study results. When there was evidence of statistical heterogeneity but all the effect estimates were in the same direction, such that a combined estimate would seem to provide a good summary of the individual trial results, we combined the data.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis if there were more than 10 trials (Sulewski 2022). Although only nine trials were included, we performed subgroup analysis on 'pain control', 'epithelial healing', or both, by the following covariates:
etiology of corneal abrasion: ocular surgery or trauma;
-
exposure to intervention medications:
duration of use (24 to 48 hours versus 48 hours or longer);
concentration of anesthetic (diluted versus standard concentration).
We were unable to analyze outcomes by gender or race as there were minimal demographic data in the study reports and no outcomes reported by treatment arm within the demographic subgroups. We did not perform subgroup analysis by frequency of use because all included trials had a frequency of use ≥ 4 times per day (see Differences between protocol and review).
Sensitivity analysis
We performed sensitivity analysis by:
excluding studies judged to be at an overall high risk of bias;
excluding within‐person studies that had not addressed the correlation of outcomes in pairs of eyes when reporting the trial results.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables to present relative and absolute risks estimated from the included studies based on interventions compared (Schünemann 2022). Working in pairs, review authors (MS, KC, CI, SL, LL, IK) independently graded the overall certainty of the evidence, resolving discrepancies by discussion, for the following efficacy and safety outcomes using the GRADE classification (Schünemann 2022):
Efficacy outcomes: changes in mean participant‐reported ocular pain from baseline to 24 hours, 48 hours, and 72 hours after treatment initiation.
-
Safety outcomes:
proportion of participants without complete resolution of epithelial defects by 24 to 72 hours;
proportion of participants with adverse events reported at the longest follow‐up time of the study.
We considered the following five elements when deciding to downgrade the certainty of the body of evidence from a high to a low level:
high risk of bias among included studies;
indirectness of evidence;
unexplained heterogeneity or inconsistency of results;
imprecision of results; or
high likelihood of publication bias.
We applied study‐level risk of bias assessments, based on responses to signaling questions in domains 1 to 3 of the RoB 2 tool, to provide judgment on risk of bias when we graded outcomes that we did not choose for complete RoB 2 assessment.
Results
Description of studies
Results of the search
The Information Specialist found 7980 records in the electronic databases and 263 records in trial registries on 19 February 2022. The search was run again on 10 February 2023, which retrieved a further 906 records from electronic databases (date limit January 2022 to 10 February 2023) and 319 records from trial registries. We did not identify any eligible studies through supplemental searches. After removing duplicates, we screened 7641 records from which we excluded 7602 records. We then assessed 39 full‐text articles for eligibility and included nine trials (21 records) in the current review (Figure 1). We labeled one study (Aseff 1997) as 'awaiting classification' because of incomplete data reported in the meeting abstract. We excluded 16 studies (17 records) and listed the reason for exclusion in the Characteristics of excluded studies.
Included studies
Types of trials
We give details of the nine included RCTs in the Characteristics of included studies table and summarize them in Table 3. The trials span the years 1994 to 2021, and they were conducted in multiple settings and locations. The median study length was seven days (interquartile range (IQR) 7 to 14 days; nine RCTs). The median study length was 11 days (IQR 7 to 18 days; 4 RCTs) for post‐trauma trials and seven days (IQR 3 to 7 days; 5 RCTs) for post‐surgical trials. The median anesthetic treatment duration was 24 hours (IQR 24 to 168 hours; nine RCTs). The median treatment duration was 36 hours (IQR 24 to 78 hours; four RCTs) for post‐trauma trials and 24 hours (IQR 24 to 168 hours; five RCTs) for post‐surgical trials.
1. Study characteristics.
| Study ID | Country | Etiology | Intervention(s) | Comparison(s) | Co‐intervention(s) | No. participants randomized/analyzed, intervention arm | No. participants randomized/analyzed, control arm | Intervention duration | Note |
| Ball 2010 | Canada | Trauma | Proparacaine 0.05% 2 to 4 drops, as needed, for 7 days, max dispensed 40 mL |
Saline drops (color and smell‐ matched) |
AB: gatifloxacin, 1 drop every 2 hours, for 7 days OA: 325 mg acetaminophen with 30 mg of codeine, 1 to 2 tablets every 4 hours if needed, for 7 days | NR/15 | NR/18 | 1 week | — |
| Lim 1999 | South Korea | PRK | 1) Proparacaine 0.05%
2) Diclofenac 0.1% + proparacaine 0.05% 1 drop every 4 hours for 7 days |
Artificial tears (Tears Natural) 1 drop every 4 hours for 7 days |
AB: ofloxacine, every 6 hours, for 1 week OA: mefenamic acid, as needed | 1) 15/15 2) 15/15 | 15/15 | 1 week | 3 of 7 treatment arms |
| Montard 1999 | France | PRK | Tetracaine 1% every 30 minutes for 24 hours | Diclofenac 0.1% every 4 hours for 3 days | AB: ofloxacine, every 6 hours, for 1 week OA: mefenamic acid, as needed Other: BCLs | 38/NR | 36/NR | 24 hours | — |
| Oksuz 2006 | Turkey | Pterygium | Lidocaine 2%c 1 mL every hour for 3 hours, starting 1 hour postop |
Artificial tearsc (Thilo‐Tears Jelly) 1 mL every hour for 3 hours, starting 1 hour postop |
AB: NR OA: none Other: patched | 23/NR | 22/NR | 3 hours | — |
| Shahinian 1997 | Canada and US | PRK | Proparacaine 0.05% 1 drop 4 times a day for 1 week |
Artificial tears (Hypotears) 1 drop 4 times a day for 1 week |
AB: 0.3% tobramycin and 0.1% dexamethasone, 4 times a day, over 1 week OA: acetaminophen and hydrocodone bitartrate, as needed, over 1 week Topical: topical diclofenac 0.1%, 4 times a day, for the first 48 hours Other: BCLs | 25 eyes/25 eyes | 23 eyes/23 eyes | 1 week | — |
| Shipman 2021 | US | Trauma | Tetracaine 0.5% 1 drop every 30 minutes as needed for 24 hours, max dispensed 2 mL |
Artificial tears (Systane) 1 drop every 30 minutes as needed for 24 hours, max dispensed 2 mL |
AB: polymyxin B sulfate/trimethoprim sulfate, 2 drops every 4 hours, max 24 hours OA: hydrocodone 7.5 mg/acetaminophen 325 mg, 1 to 2 tablets as needed every 6 hours, max 12 tablets | 59/56 | 59/55 | 24 hours | — |
| Ting 2009 | Australia | Trauma | Tetracaine 0.4% 1 drop every hour as needed for 48 hours |
Saline drops 0.9% 1 drop every hour as needed for 48 hours |
AB: topical antibiotics (unspecified)a OA: oral analgesics (unspecified) as needed for eye pain | 22/7 | 25/9 | 36 to 48 hours | — |
| Verma 1995 | UK | PRK | Tetracaine 1%d 1 drop every 30 minutes during waking hours for 24 hours, max dispensed 40 drops |
Saline dropsd (physiologic saline) 1 drop every 30 minutes during waking hours for 24 hours, max dispensed 40 drops |
AB: chloramphenicol 0.5%, topical, 1 drop every 6 hours, over 1 week OA: co‐proxamol, 2 tablets every 8 hours, over 2 daysb | 22/NR | 22/NR | 24 hours | — |
| Waldman 2014 | New Zealand | Trauma | Tetracaine 1% as needed, up to every 30 minutes for 24 hours, max dispensed 1.5 mL (50 drops) | Saline drops 0.9% as needed, up to every 30 minutes for 24 hours, max dispensed 1.5 mL (50 drops) | AB: preservative‐free chloramphenicol antibiotics 1%, topical ointment OA: paracetamol 500 mg, 2 tablets at 08:00, 12:00, 16:00, 20:00, oral, over 24 hoursb | 61/47 | 61/46 | 24 hours | — |
AB: topical antibiotics; BCLs: bandage contact lenses; NR: not reported; OA: oral anesthetic; PRK: photorefractive keratectomy aNot all participants received antibiotics (8/22 participants in the tetracaine group and 8/18 in the saline group received antibiotics). bThe study prescribed oral anesthetic to prevent breakthrough pain. cPrescribed at 1, 2, and 3 hours postop, inpatient setting. Not taken on an as‐needed basis. dPrescribed at a specific schedule. Not taken on an as‐needed basis.
Of the nine RCTs, four (44%) were conducted in the emergency department setting; three of these were single‐center trials conducted in New Zealand (Waldman 2014), Australia (Ting 2009), and the United States (Shipman 2021). Ball 2010 included two tertiary care emergency departments in Canada. Five RCTs (56%) were in ophthalmology surgical settings; Shahinian 1997 had two study sites, one in Canada and in the United States, while the other four RCTs were single‐center trials based in the United Kingdom (Verma 1995), Turkey (Oksuz 2006), France (Montard 1999), and South Korea (Lim 1999). Except for Oksuz 2006, which enrolled participants following pterygium surgery, all surgical trials enrolled patients who were status post photorefractive keratectomy (PRK).
The reporting of funding/sponsors varied; five trials did not have a statement regarding study funding, one reported there was no funding (Waldman 2014), and two stated partial support for authors from a foundation (Shipman 2021; Verma 1995). None of the trials reported industry funding. Five trials reported no conflict of interest among trial investigators; the author of one trial was a patent holder for the intervention (Shahinian 1997), and reports from the other trials did not include a disclosure statement.
All nine included trials had parallel‐group designs. Eight RCTs had two treatment arms, while Lim 1999 had seven. Eight trials randomized participants to treatment and analyzed data from one study eye. Shahinian 1997 randomized post‐PRK participants to treatments (N = 34); some participants had both eyes enrolled as study eyes, whereas others had a single study eye (N = 48 eyes). Four trials reported an a priori power calculation for the primary outcome of the trial; one stated the sample size for statistical significance without further detail (Verma 1995), and the remaining four trials did not state whether sample size calculations had been performed. Additionally, Waldman 2014 reported the power calculation for a secondary outcome. Four trials had associated trial registration records, one had been prospectively registered (Ting 2009), and three had been registered after the specified start date of participant enrollment (Ball 2010; Shipman 2021; Waldman 2014).
Types of participants
The nine included trials enrolled a total of 626 participants. Three of the seven treatment arms, one comparison and two separate interventions, of Lim 1999 were eligible for inclusion in this review (45/105 participants). We included in this review a total of 556 participants, with a median number of 45 participants per study (IQR 44 to 74).
Five trials described 242 participants (256 eyes) with post‐surgical corneal defects: four from PRK (Lim 1999; Montard 1999; Shahinian 1997; Verma 1995) and one from pterygium surgery (Oksuz 2006). Four trials analyzed abrasions of traumatic etiology in a total of 314 participants (314 eyes) (Ball 2010; Shipman 2021; Ting 2009; Waldman 2014). The majority of abrasions involved corneal foreign bodies (47%, 148 eyes) or direct trauma (19%, 61 eyes).
One eye of each participant had been treated and followed except in one trial, in which both eyes were allocated to the same treatment for four participants (eight eyes) who had bilateral surgery on the same day (Shahinian 1997). It is unclear whether this method of assignment was used for second eyes of participants who had sequential surgery on separate days.
Baseline characteristics of participants varied by age, gender, and ethnicity. One trial did not report the gender of the participants (Shahinian 1997). Three of the eight trials that reported the participant gender reported numbers in the overall trial but not within individual treatment groups (Lim 1999; Montard 1999; Verma 1995). For example, Lim 1999 reported that 71% of trial participants were women (75/105) but did not report gender by treatment arm. Among these eight trials, a higher proportion of women was seen in trials that had examined iatrogenic corneal abrasions from refractive or pterygium surgery (60%, 166/278; four RCTs) compared with trials of participants seen in emergency departments for corneal abrasions (21%, 65/314; four RCTs).
None of the trials reported racial demographics. One trial conducted in New Zealand reported ethnicity of participants, with 59% (69/116) 'European', 7% (8/116) 'Maori', 2% (2/116) 'Other', and 32% (37/116) 'Not Reported' (Waldman 2014).
One trial did not report the age of participants (Shahinian 1997). Of the eight trials that had reported participants’ ages at baseline, all participants were 18 years or older with one exception: Waldman 2014 enrolled one 17‐year‐old participant. Waldman 2014 also had the widest age range (17 to 74 years old). Seven trials reported either a mean or median age for participants, ranging from 27.8 to 47.9 years old.
Types of interventions
The nine included trials evaluated three of the commonly used topical anesthetics, of which types and concentrations varied: proparacaine (Ball 2010; Lim 1999; Shahinian 1997), tetracaine (Montard 1999; Shipman 2021; Ting 2009; Verma 1995; Waldman 2014), and lidocaine (Oksuz 2006). One study tested the amide anesthetic lidocaine 2% (Oksuz 2006). Two ester anesthetics of various concentrations were tested in the other trials: tetracaine (Montard 1999; Verma 1995; Waldman 2014 at 1%; Shipman 2021 at 0.5%; Ting 2009 at 0.4%) and proparacaine diluted from commercially available 0.5% to 0.05% (Ball 2010; Lim 1999; Shahinian 1997). Lim 1999 was a multi‐arm trial that tested both proparacaine 0.05% alone and its combination with topical diclofenac 0.1%.
Four trials enrolled post‐trauma patients who were sent home from the emergency department with topical anesthetics of varying concentration, frequency, duration, and total amount dispensed for self‐administration (Ball 2010; Shipman 2021; Ting 2009; Waldman 2014). In Waldman 2014, investigators prescribed tetracaine 1%, dosed as often as every 30 minutes for 24 hours (1.5 mL total volume dispensed). In Shipman 2021, they prescribed tetracaine 0.5%, one drop every 30 minutes as needed for 24 hours (2 mL total volume dispensed). In Ting 2009, they prescribed tetracaine 0.4%, one drop every hour as needed for 48 hours (1.5 mL total volume dispensed). In Ball 2010, they prescribed proparacaine 0.05%, two to four drops as needed for seven days (40 mL total volume dispensed). The authors stated no minimum time interval between doses "allowing patients unlimited use of the study drug" (Ball 2010).
The other five trials enrolled patients following ophthalmic surgery that had caused a corneal epithelial defect (Lim 1999; Montard 1999; Oksuz 2006; Shahinian 1997; Verma 1995). In Oksuz 2006, starting one hour after pterygium surgery, participants were administered lidocaine 2% hydrochloride gel, 1 mL every hour for three hours prior to hospital discharge. In Shahinian 1997, surgeons prescribed proparacaine 0.05%, one drop four times per day. In Verma 1995, they prescribed tetracaine 1%, one drop every 30 minutes "during waking hours" for 24 hours (40 drops total dispensed). In Montard 1999, they prescribed tetracaine 1%, every 30 minutes for 24 hours. In Lim 1999, they prescribed one group proparacaine 0.05%, one drop every four hours for seven days. The same dosing was used for the diclofenac 0.1% plus proparacaine 0.05% (Lim 1999).
Eight of the included trials compared topical anesthetics to placebo treatment, which included saline (Ting 2009; Verma 1995; Waldman 2014), artificial tears (Lim 1999; Oksuz 2006; Shahinian 1997; Shipman 2021), and a "colour‐ and smell‐matched" placebo, likely vehicle (Ball 2010). The only trial that used an active comparator was Montard 1999, in which the investigators compared tetracaine 1% with topical diclofenac 0.1%. Among the four trials that enrolled post‐trauma patients (Ball 2010; Shipman 2021; Ting 2009; Waldman 2014), Shipman 2021 was the only trial that had used artificial tears whereas the other three trials used saline as the comparator. In contrast, among the four trials that enrolled post‐surgical patients (Lim 1999; Montard 1999; Oksuz 2006; Shahinian 1997; Verma 1995), Verma 1995 was the only trial that used "physiologic saline" as the comparator. The other three trials used other brands of artificial tears; in Oksuz 2006 artificial tears in gel form were used. In all included trials, these placebo treatments were prescribed at the same frequency and duration as the respective study's topical anesthetic treatment arm. The only trial with different frequency and duration between treatment arms was Montard 1999, in which investigators prescribed tetracaine 1% every 30 minutes for 24 hours but allowed diclofenac 0.1% to be instilled every four hours for three days.
Oral analgesics were prescribed in all but one trial (Oksuz 2006). In two trials, oral analgesics were dosed on a schedule: two tablets of co‐proxamol (dextropropoxyphene 32.5 mg and paracetamol 325 mg) every eight hours for two days (Verma 1995) and two tablets of 500 mg paracetamol at 08:00, 12:00, 16:00, and 20:00 over 24 hours (Waldman 2014). Six of the RCTs prescribed various analgesics on an 'as‐needed' basis for breakthrough pain, including paracetamol‐noramidopyrine (Montard 1999), acetaminophen and codeine (Ball 2010), acetaminophen and hydrocodone (Shahinian 1997; Shipman 2021), and mefenamic acid (Lim 1999); one study did not specify the analgesic (Ting 2009).
The five surgical trials had different pre‐, peri‐, and postoperative protocols (see Characteristics of included studies for details). In two trials, bandage contact lenses were placed in post‐surgical eyes (Lim 1999; Shahinian 1997). All surgical eyes had occlusive patching in Oksuz 2006. Topical antibiotics were used in all RCTs except for Oksuz 2006. A variety of antibiotics were prescribed: chloramphenicol 0.5% (Verma 1995), chloramphenicol 1% (Waldman 2014), ofloxacin 0.3% (Montard 1999), and unspecified concentrations of ofloxacin (Lim 1999), polymyxin B sulfate/trimethoprim sulfate (Shipman 2021), and gatifloxacin (Ball 2010). One study used a combination of 0.3% tobramycin and 0.1% dexamethasone (Shahinian 1997). Antibiotics were not prescribed equally within and between groups in one study; 8/22 participants in the tetracaine group and 8/18 in the saline group received antibiotics (Ting 2009).
Critical outcomes
Pain control from baseline to 24 hours, 48 hours, and 72 hours after treatment initiation
All included nine trials assessed pain intensity using pain scoring systems where higher numbers represented higher pain intensity. Eight trials used a VAS, with two using 0‐ to 100‐point continuous scales (Ting 2009; Waldman 2014) and the others using a 0‐ to 10‐point continuous scales. Shahinian 1997 used a 0‐ to 10‐point continuous pain intensity scale but did not specify whether the instrument was a visual analog or numeric scale.
Baseline pain was recorded in two of the four trials of participants with traumatic corneal injuries (Shipman 2021; Waldman 2014). Some participants reported no pain at baseline and were analyzed in a mixed‐model to account for multiple measurements (Waldman 2014). Ting 2009 did not report baseline pain and Ball 2010 only reported the change score. In post‐surgical trials, the baseline pain following surgery was not reported. Shahinian 1997 only reported the mean pain before taking study drops. The earliest time point at which postoperative pain was reported was one hour (Montard 1999), four hours (Oksuz 2006), or the end of the day (Lim 1999). In Verma 1995, the baseline pain could not be extracted from the presented figure.
A clinically important difference in the VAS measurement of pain intensity was defined in three trials: 16 mm (SD 25 mm) on a 100 mm VAS (Waldman 2014), 2 cm (SD 2 cm) on a 10 cm VAS (Ball 2010), and 1.5 cm (SD 2.5 cm) on a 10 cm VAS (Shipman 2021). Waldman 2014 cited two observational studies that had validated the use of the VAS to assess acute (primarily abdominal) pain in the emergency department setting as the basis for selecting 16 mm. Ball 2010 used an informal survey of attending emergency department physicians. Shipman 2021 did not report how 1.5 cm was selected as a clinically important difference. Verma 1995 defined 3 cm as an acceptable level of pain on a 10 cm VAS but did not specify how this number was chosen.
We included data from six trials for pain outcomes reported at up to 24, 48, and 72 hours. Lim 1999 reported the mean pain intensity and P values comparing artificial tears versus anesthetic groups on day one but not on days two and three. We used the P value to determine the standard deviation for day one in order to include the data at 24 hours following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). Oksuz 2006 reported pain scores at four, seven, and 10 hours postoperatively, but we included only the 10‐hour follow‐up value in the analysis of pain control from baseline to 24 hours. Montard 1999 reported the pain profile at 24 hours based on a single factor analysis to extrapolate pain during sleeping hours.
The mean and standard error were extracted from figures for 24‐, 48‐, and 64‐hour time points reported in Verma 1995. Waldman 2014 used a mixed‐model to account for multiple measurements over the 48 hours and separately reported the mean difference for follow‐up durations of 24 and 48 hours. Shipman 2021 recorded the change in scores from pre‐ and two minutes post‐instillation of study drops and reported the overall pain rating at 24 to 48 hours follow‐up.
We did not include pain score data from three trials in the meta‐analysis (Ball 2010; Shahinian 1997; Ting 2009), because the timeframe of the trial results was outside the pre‐specified time windows or the trial results were reported as period averages. The authors of Shahinian 1997 reported averaged pain scores over the first postoperative week as documented by participants immediately before and one minute after applying the study eye drops (used as needed). Similarly, Ball 2010 reported the aggregated median change scores from all recorded pre‐ and five minutes post‐use of study drops over seven days. Ting 2009 reported the total pain burden over 36 hours.
Epithelial healing by 24 to 72 hours
Epithelial healing was assessed by slit lamp biomicroscopy in the majority of trials (67%; 6/9) (Lim 1999; Oksuz 2006; Shahinian 1997; Shipman 2021; Ting 2009; Waldman 2014); two other trials used digitized and computer‐assisted measurements (Montard 1999; Verma 1995). One trial stated only that "the ophthalmologist was directed to identify signs of delayed wound healing" (Ball 2010). Despite the variations in measurements of epithelial healing performed among the trials, we were not able to use all reported data in our analysis of this outcome. Three trials reported mean time to epithelial healing, but proportions of eyes with epithelial healing at 24 to 72 hours could not be derived (Montard 1999; Oksuz 2006; Shahinian 1997). Ball 2010 assessed eyes at three, five, and seven days after injury and reported, "no ocular complications or evidence of delayed wound healing in either group."
Complications reported at the longest follow‐up time
The median study length of seven days (post‐surgical trials) and 11 days (post‐trauma trials) gives an indication of the longest follow‐up. Methods of assessing complications included clinical assessment, such as slit lamp biomicroscopy, by an ophthalmologist or emergency medicine physician (Verma 1995; Waldman 2014). Other methods of assessing complications included the following: eliciting complaints from participants using a list of qualifying complications (Shipman 2021; Waldman 2014), eliciting complaints from participants without a list, judgment by an ophthalmologist who was asked to identify complications including any that appeared to be related to the initial injury or the use of study medications (Ball 2010), or no report of method used (Montard 1999). These subjective assessments were conducted at the time of clinical assessment, during telephone interview (Ball 2010; Shipman 2021; Waldman 2014), or in response to text messages (Waldman 2014). Most trials did not provide details on complications such as microbial keratitis or stromal infiltration, corneal stromal thinning, corneal perforation, or surgical interventions. Four trials reported specific adverse events (Shipman 2021; Ting 2009; Verma 1995; Waldman 2014). Three other trials stated only that there had been no adverse events (Ball 2010; Lim 1999; Oksuz 2006). The remaining two trials provided no information about complications (Montard 1999; Shahinian 1997).
Important outcomes
Treatment failure at 72 hours after treatment initiation
Investigators of one study reported the number of eyes that had required analgesia for breakthrough pain, from 24 hours to two weeks (Ting 2009). Reports from no other trial provided data for this outcome because our protocol specified the proportion of participants (or eyes) that were treatment failures rather than the amount of analgesia taken over the study period (e.g. median number of hydrocodone tablets over 48 hours). In addition, one indication of treatment failure as defined by our protocol was the use of rescue oral analgesics for pain not alleviated by topical anesthetic medication. Therefore, trials in which oral analgesics were prescribed to prevent breakthrough pain were not included in the analysis (Verma 1995; Waldman 2014).
Oksuz 2006 did not describe the use of oral analgesics. Lim 1999 assessed the number of oral analgesics used but did not report the results. Four of the included trials reported the amount of oral analgesia taken over the study period as a continuous measure, so we did not include these in the analysis (Ball 2010; Montard 1999; Shahinian 1997; Shipman 2021).
Quality of life
None of the included trials assessed health‐related, vision‐related, or function‐related quality of life outcome assessments.
Excluded studies
We documented reasons for exclusion of 16 studies in the table of Characteristics of excluded studies. We translated five non‐English articles using Google Translate; the original languages were French (Henrotte 1972), Portuguese (Ferreira 1992), Italian (Filippone 1967), and German (Steiner 1966; Weindler 2001). Further translation was not required to determine whether the trial was eligible for inclusion. We contacted the investigators of two trials that had registration records to inquire about trial status and data availability; one confirmed the trial had been halted, and data were not available (NCT02483897); the other investigator stated that the trial had never been initiated and had never enrolled participants (NCT02771392). For another study, we requested information regarding randomization but did not receive any response (Cherry 1996). Based on other published reports referenced in Cherry 1996, we determined that the study did not meet our eligibility criteria. Of the 16 excluded studies, eight were excluded for ineligible populations, four were excluded for ineligible study designs, three had ineligible interventions, and one had an ineligible comparison group.
Studies awaiting classification
We did not have enough information to confidently include or exclude a study reported only in a meeting abstract (Aseff 1997). Multiple contact attempts for all listed authors of the abstract were unsuccessful.
Risk of bias in included studies
We assessed the risk of bias for two outcomes: 1) the mean participant‐reported ocular pain from baseline to 48 hours (Figure 2), and 2) the proportion of participants with complications (Figure 3). We assessed the risk of bias in two trials that reported the first outcome (Shipman 2021; Verma 1995), and seven trials for the second outcome (Ball 2010; Lim 1999; Oksuz 2006; Shipman 2021; Ting 2009; Verma 1995; Waldman 2014). For the domain‐specific judgments, the domains for which we assessed trials to be at the highest risk of bias were missing outcome data or selective reporting of outcome data.
2.
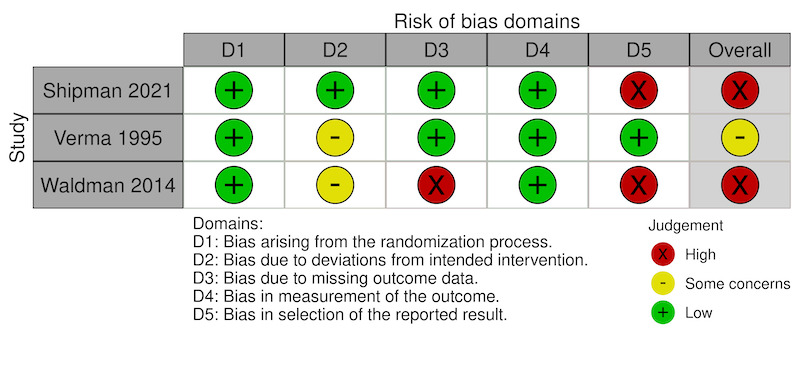
Risk of bias: Change in participant‐reported ocular pain from baseline to 48 hours
3.
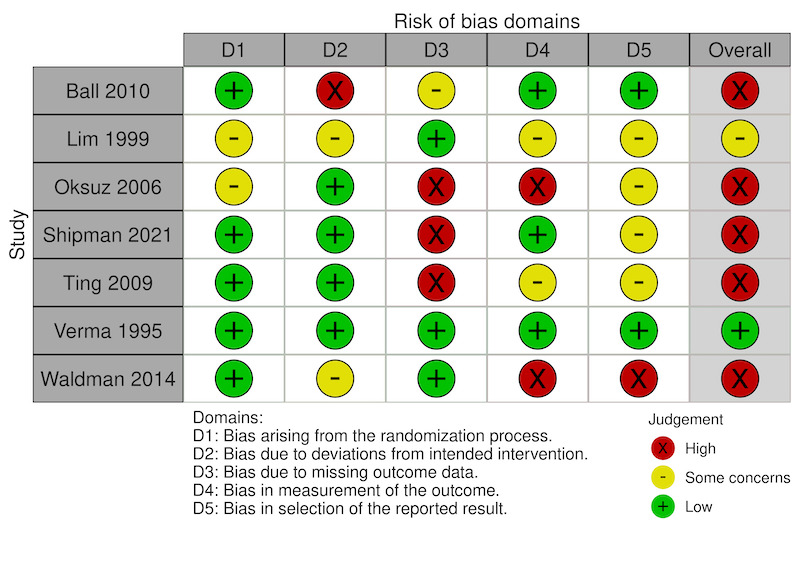
Risk of bias: Proportion of participants with complications at longest time point
Domain 1 ‐ Bias arising from the randomization process
We judged five trials to have low risk of bias as they described methods of randomized sequence generation and methods to conceal allocation, such as the use of sealed, opaque envelopes (Shipman 2021) and "research pharmacists not involved in the design or conduct of the study prepared identical, clear, minim packs" (Ting 2009). Neither Lim 1999 nor Oksuz 2006 described a method of allocation concealment, and few baseline characteristics of participants were provided. Therefore, we had some concerns about risk of bias for this domain.
Domain 2 ‐ Bias arising from deviations from intended interventions
Two trials addressed the primary outcome of pain at 48 hours (Shipman 2021; Verma 1995). We assessed one study as possessing low risk of bias because of deviations from intention‐to‐treat (Shipman 2021). For Verma 1995, we had some concerns about bias.
For adverse events, we assessed the evidence from four trials as indicating low risk of bias arising from deviations from intended interventions (Oksuz 2006; Shipman 2021; Ting 2009; Verma 1995). We had some concerns about two trials because of unclear post‐randomization exclusion of participants (Waldman 2014), no mention of adverse events (Lim 1999), and the potential unmasking of participants due to the burning sensation of tetracaine (Waldman 2014). We judged one study to have a high risk of bias because data were excluded for avoidable reasons, such as not having medication, not recording pain measurements, and loss to follow‐up (Ball 2010).
Domain 3 ‐ Bias due to missing outcome data
Regarding pain control by 48 hours, we judged one study as having a low risk of bias because of few missing outcome data (Shipman 2021), and one study as having high risk of bias because of differences in follow‐up rates between treatment groups (Verma 1995).
For adverse events at the longest follow‐up, we assessed three trials as having low risk of bias due to no missing data (Lim 1999; Waldman 2014; Verma 1995). We had some concerns about bias in one study because 20% of participants did not contribute adverse event data and the investigators did not provide any explanation (Ball 2010). We assessed three trials as possessing a high risk of bias because not all participants were required to follow up after 24 hours (Oksuz 2006), or had multiple follow‐up visits with varying attendance, and self‐reporting of adverse event data (Shipman 2021; Ting 2009). There was substantial loss to follow‐up for the clinical assessment at the 48‐hour (Waldman 2014) and one‐week (Shipman 2021) time points. In Waldman 2014, all participants who missed clinic follow‐up were successfully contacted by other methods (telephone, text messaging). In Shipman 2021, there was still missing data for 32% of participants after similar use of text messaging.
Domain 4 ‐ Bias in outcome measurement
For pain control by 48 hours, we assessed two included trials as having low risk of bias associated with measurement of the outcome (Shipman 2021; Verma 1995).
For collection of adverse events, we judged three trials as possessing low risk of bias (Ball 2010; Shipman 2021; Verma 1995). In two trials, there were some concerns about bias arising from examiners not being masked to treatment and sparse detail(s) of how adverse events were evaluated (Lim 1999; Ting 2009). We assessed two trials as having a high risk of bias for reasons such as disparity in the frequency of scheduled clinical assessments. In one study, delayed healing increased the frequency of assessment only for patients with incomplete healing (Oksuz 2006). In the second study, the baseline and follow‐up assessments in the emergency department were not described in detail, for example by gross physical exam, slit lamp biomicroscopy, or fluorescein dye uptake as visualized by under cobalt blue light, and relied on self‐reporting of adverse events (which may have gone undetected in the anesthetic group) (Waldman 2014).
Domain 5 ‐ Bias in selective reporting of outcome data
Concerning pain control by 48 hours, we assessed one study as having low risk of bias because all outcomes were reported (Verma 1995), and another study as having high risk of bias because reporting of results diverged from the statistical plan in the protocol (Shipman 2021).
For bias related to reporting adverse outcomes, we deemed two trials to have low risk of bias (Ball 2010; Verma 1995). We had some concerns about bias in four trials due to no study protocol being found or no definition of an adverse event (Lim 1999; Oksuz 2006; Ting 2009), multiple possible time points of measurement (Oksuz 2006), no data analysis plan, and multiple possible definitions used across the study (Shipman 2021). We considered one study at high risk of bias due to having no statistical analysis plan, a large cohort of participants excluded in a post hoc fashion for an unexpected result (i.e. persistent rust rings), and multiple time points with different numbers of participants at each (Waldman 2014).
Overall assessment of bias
In summary, for the outcome of pain control at 48 hours, we deemed one study to be at high risk of bias (Shipman 2021), and another study to raise some concerns about risk of bias (Verma 1995). For the outcome of adverse events at the last follow‐up time point, we judged one of the trials to be at low risk of bias, we had some concerns about risk of bias in one study, and we judged the remaining five trials to have high risk of bias.
Effects of interventions
We reported the effects of topical anesthetics in the following two comparisons: Comparison 1: Anesthetics alone versus placebo or NSAID (Table 1); Comparison 2: Anesthetics plus NSAID versus placebo (Table 2).
Comparison 1: Anesthetics versus placebo or NSAID
Critical outcomes
Pain control from baseline to 24 hours after treatment initiation
Three post‐surgical trials (Lim 1999; Oksuz 2006; Verma 1995) and one post‐trauma trial (Waldman 2014) comparing topical anesthetics with placebo reported pain control at this time point. One additional post‐surgical trial compared topical tetracaine with diclofenac (Montard 1999).
The combined estimate for pain scores reported by post‐surgical participants at 24 hours suggested that when compared with placebo, topical anesthetics reduced pain by 1.28 points on a 10‐point scale (MD −1.28, 95% CI −1.76 to −0.80; 3 RCTs, 119 participants; Figure 4) (Lim 1999; Oksuz 2006; Verma 1995). The authors of Waldman 2014 reported pain scores as model‐predicted values based on averaging multiple measurements over the first post‐intervention 24 hours. Based on the raw data provided by the author team, the single‐study estimate, converted from a 100‐point to a 10‐point scale, was derived from 76 participants (61% of 124 randomized) and showed no evidence of a difference in pain control by 24 hours (MD −0.04 points, 95% CI −0.10 to 0.02).
4.

Forest plot of comparison 1: topical anesthetic vs placebo, outcome: 1.1 Change in participant‐reported ocular pain from baseline to 24 hours
The investigators of Montard 1999 recruited 74 participants who had undergone PRK and reported this outcome by comparing topical tetracaine 1% (every 30 minutes for 24 hours) with diclofenac 0.1% (four times a day for three days). The single‐study estimate indicated an 0.82‐point higher pain score (on a 10‐point VAS) in the tetracaine group than in the diclofenac group (MD 0.82, 95% CI 0.01 to 1.63). We did not combine data across subgroups because of heterogeneity between them (I2 = 93%). The overall level of certainty of the effect estimates based on the available evidence is very low because of high risk of bias (−2 levels) associated with incomplete data and imprecision (−1 level) from small sample sizes.
Pain control from baseline to 48 hours after treatment initiation
One post‐surgical trial (Verma 1995) and two post‐trauma trials (Shipman 2021; Waldman 2014) compared pain control by 48 hours for tetracaine versus placebo. We did not include Waldman 2014 in this analysis given the substantial proportion of participants lost to follow‐up (and thus the risk of attrition bias); the data for only 43% of 124 randomized participants (tetracaine n = 26; saline n = 27) were reported, according to the information provided by the authors.
The single post‐surgical trial reported 0.41‐point greater pain in the anesthetic group on a 10‐point scale, showing little to no effect on pain control compared with placebo (MD 0.41, 95% CI −0.45 to 1.27; 44 participants; Figure 5) (Verma 1995). The post‐trauma trial had an estimated mean difference of 5.68 points (on a 10‐point scale), indicating that there may be a large reduction in pain when comparing topical anesthesia versus placebo (MD ‐5.68, 95% CI ‐6.38 to ‐4.98; 111 participants; Figure 5) (Shipman 2021), which is different in direction and magnitude from the estimate from Verma 1995. We did not pool these two trials for analysis because of substantial statistical heterogeneity (I² = 99%). The overall certainty of evidence was very low because of high risk of bias (−2 levels), due to missing data and selective outcome reporting, imprecision (−1 level), and inconsistency (−1 level).
5.

Forest plot of comparison 1: topical anesthetic vs placebo, outcome: 1.2 Change in participant‐reported ocular pain from baseline to 48 hours
Pain control from baseline to 72 hours after treatment initiation
Based on data from one post‐surgical trial, topical anesthetics had little to no effect on self‐reported pain measured on a 10‐point VAS at 64 hours (MD 0.49 point, 95% CI −0.06 to 1.04; 44 participants; Analysis 1.3) (Verma 1995). The overall certainty of evidence was very low because of risk of bias (−1 level), indirectness (−1 level), and imprecision (−1 level) from the small sample size.
1.3. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 3: Change in participant‐reported ocular pain from baseline to 72 hours
Pain control reported at other time points
One trial reported the mean cumulative pain score of 12 assessments over 36 hours for 55% (12/22) of tetracaine participants and 36% (9/25) of saline participants initially randomized (Ting 2009). The topical anesthetic group had a lower mean cumulative pain score of 404 (SD 75) compared with 629 (SD 172) for saline on a 100‐point scale.
Another two trials reported a seven‐day mean or median change in pain scores immediately before and after the use of study drops (Ball 2010; Shahinian 1997). Ball 2010 reported the seven‐day median change in pain score five minutes after using the study drops. The proparacaine group had a median improvement of 3.9 points on a 10‐point scale (IQR 1.5 to 5.1; n = 15) compared with a median of 0.6 points (IQR 0.2 to 2.0; n = 18) in the placebo group (P = 0.007, reported in Ball 2010). Shahinian 1997 reported the seven‐day mean change in pain score one minute after using study drops for 45 of 48 eyes. The proparacaine group had a larger decrease in mean pain (mean −1.75 points, SD 0.69) compared with placebo (mean −0.85 points, SD 0.98) on a 10‐point VAS (P < 0.002, reported in Shahinian 1997).
Epithelial healing by 24 to 72 hours
Five trials reported proportions of eyes without complete resolution of epithelial defects by 24 to 72 hours for anesthetics compared with placebo (Lim 1999; Shipman 2021; Ting 2009; Verma 1995; Waldman 2014). Three post‐trauma trials formed a subgroup with different concentrations of tetracaine; results of meta‐analysis for this subgroup provided no evidence of a difference in epithelial healing when tetracaine was compared with placebo (RR 1.37, 95% CI 0.78 to 2.42; 221 participants; I² = 0%; Figure 6) (Shipman 2021; Ting 2009; Waldman 2014). There were insufficient data to explore any dose‐response relationship.
6.

Forest plot of comparison 1: topical anesthetic vs placebo, outcome: 1.5 Proportion of eyes without complete resolution of epithelial defects by 24 to 72 hours
In one post‐surgical trial, all participants' corneal abrasions had healed by 24 to 72 hours (Verma 1995); there was no evidence of a difference in effect between treatment groups in the other post‐surgical trial (Lim 1999) (RR 0.14, 95% CI 0.01 to 2.55; 30 participants; Figure 6).
We calculated the risk difference (RD) to include the post‐surgical trial Verma 1995, in which epithelial healing had occurred in all eyes in both treatment groups. Analysis by the duration of anesthetic use showed no evidence of differences in the resolution of epithelial defects across trial settings (post‐surgical or post‐trauma) or treatment duration (Analysis 1.6). However, the evidence is of very low certainty because of risk of bias (−1 level), inconsistency (−1 level), and imprecision (−1 level).
1.6. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 6: Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours ‐ subgroup by duration of use (RD)
Complications reported by the longest follow‐up time
Seven trials reported the number or proportion of participants with complications by the longest follow‐up time for topical anesthetics compared with placebo (Ball 2010; Lim 1999; Oksuz 2006; Shipman 2021; Ting 2009; Verma 1995; Waldman 2014).
In the post‐surgical trials, no complications were reported in either treatment group for two trials: Lim 1999 up to one week and Oksuz 2006 up to 48 hours. In Verma 1995, the longest follow‐up was six months, although adverse events were reported at the week one postoperative clinical assessment. In Verma 1995, at one week, three participants in the tetracaine group experienced gritty sensation in the eye (n = 1), blurred vision (n = 1), and heaping of the epithelium (n = 1) compared with zero complications in the placebo group. The 95% CI of the estimated RR, despite crossing the line of no effect, is very asymmetric in favor of placebo (RR 7.00, 95% CI 0.38 to 128.02; 44 participants; Figure 7). The authors also stated that 20% of the trial participants experienced nausea and vomiting attributed to the oral analgesia (paracetamol/dextropropoxyphene), but no episodes were reported by the treatment group (Verma 1995).
7.

Forest plot of comparison 1: topical anesthetic vs placebo, outcome: 1.7 Proportion of individuals/eyes with complications at furthest time point
Among the post‐trauma trials, Ball 2010 reported no adverse events in either treatment arm. Ting 2009 reported complications at the two‐week phone interview. Meta‐analysis of complications reported from the other three post‐trauma trials provided an estimated RR of 1.13 (95% CI 0.23 to 5.46; 242, 3 RCTs; Figure 7) (Shipman 2021; Ting 2009; Waldman 2014). In the anesthetic group, the adverse events included infiltrate (n = 1), opaque scar (n = 1), worsening of the corneal abrasion (n = 2), and visual problems (n = 1). In the placebo group, the adverse events included recurrent corneal erosion (n = 1), worsening corneal abrasion (n = 2), residual foreign body (n = 2), uncontrolled pain (n = 2), and persistent redness and blurred vision or visual problems (n = 4).
We performed a separate analysis, including trials without events in either arm (see Differences between protocol and review). In Oksuz 2006, the authors stated, "we did not observe any corneal epithelial or ocular surface complications in either group" up to 48 hours. At one week, Ball 2010 reported "no ocular complications" and Lim 1999 stated "no keratitis was observed." The combined risk difference (RD) of 0.01 (95% CI −0.03 to 0.05; 7 RCTs, 394 participants; Analysis 1.8) indicated no evidence of a difference between topical anesthetic and placebo. Overall, the certainty of evidence was very low because of risk of bias (−1 level) and extreme imprecision (−2 levels).
1.8. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 8: Proportion of participants with complications at furthest time point (RD) ‐ subgroup by abrasion type
Important outcomes
Treatment failure by 72 hours after treatment initiation
One study had no treatment failures by 72 hours; no oral analgesia was used for eye pain in either treatment group from 24 hours to two weeks (0/17 in the tetracaine compared with 0/21 in the saline group; Ting 2009). The evidence was very low certainty because of risk of bias (−1 level) and imprecision (−2 levels) from the small sample size.
Quality of life
None of the included trials assessed this outcome.
Comparison 2: Anesthetics plus NSAID versus placebo
Critical outcomes
Pain control from baseline to 24 hours after treatment initiation
Only one post‐surgical trial compared an anesthetic plus an NSAID with placebo (Lim 1999). The single‐study effect estimate showed a large effect of the anesthetic plus NSAID on reducing pain scores at 24 hours when compared with placebo (MD ‐5.72 on a 10‐point scale, 95% CI ‐7.35 to ‐4.09; 30 participants; Analysis 2.1). The certainty of evidence was very low because of risk of bias (−1 level) and imprecision from the small sample size (−2 levels).
2.1. Analysis.

Comparison 2: Anesthetic + NSAID vs placebo, Outcome 1: Change in participant‐reported ocular pain from baseline to 24 hours
Pain control from baseline to 48 hours after treatment initiation
None of the included trials reported this outcome.
Pain control from baseline to 72 hours after treatment initiation
None of the included trials reported this outcome.
Epithelial healing by 24 to 72 hours
From data reported in Lim 1999, we found no evidence of an effect on epithelial healing when we compared proparacaine 0.05% plus diclofenac 0.1% with placebo (RR 0.33, 95% CI 0.04 to 2.85; 30 participants; Analysis 2.2). The certainty of evidence was very low because of risk of bias (−1 level) and imprecision (−2 levels).
2.2. Analysis.

Comparison 2: Anesthetic + NSAID vs placebo, Outcome 2: Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours
Complications reported by the longest follow‐up time
In Lim 1999, the authors stated that there were no incidences of keratitis during the one‐week treatment period. Data from Lim 1999 also indicated no evidence of a difference in ocular adverse effects between topical proparacaine 0.05% plus diclofenac 0.1% versus placebo (RD 0.00, 95% CI −0.12 to 0.12; 30 participants; Analysis 2.3). The certainty of evidence was very low because of risk of bias (−1 level) and imprecision (−2 levels).
2.3. Analysis.

Comparison 2: Anesthetic + NSAID vs placebo, Outcome 3: Proportion of participants with complications at furthest time point (RD)
Important outcomes
Treatment failure by 72 hours
None of the included trials reported this outcome.
Quality of life at the longest follow‐up time
None of the included trials reported this outcome.
Discussion
Summary of main results
In this review, we included nine randomized controlled trials (RCTs) that enrolled 314 post‐trauma participants (4 RCTs) and 242 post‐surgical participants (five RCTs) to evaluate the effectiveness and safety of topical anesthetics for relief of pain from corneal abrasion. For all outcomes of interest, the evidence that there was any important statistical or clinical difference in benefit or harm between topical anesthetic and placebo was of very low certainty because of risk of bias, outcomes that were not reported, and small sample sizes in the included trials.
The certainty of evidence that topical anesthetics have little or no effect on reducing participant‐reported ocular pain at 24 hours for corneal abrasion after surgery or after accidental trauma when compared with placebo or a nonsteroidal anti‐inflammatory drug (NSAID) was very low. Topical anesthetics combined with an NSAID may decrease pain at 24 hours relative to placebo in post‐surgical participants (one RCT; very low certainty). Very low‐certainty evidence suggested that topical anesthetics have little or no benefit over placebo in alleviating ocular pain by 48 or 72 hours in post‐surgical participants. Compared with placebo, topical anesthetics may improve pain at 48 hours in post‐trauma participants, based on evidence of very low certainty from one trial. Topical anesthetics alone or with an NSAID may not affect the resolution of epithelial defects by 24 to 72 hours after initiation of treatment (five RCTs; very low certainty). Of the seven trials that reported assessing adverse events, the longest follow‐up ranged from six months (post‐PRK) to 48 hours (post‐pterygium surgery) with complications reported at one to two weeks. Although the evidence was of very low certainty, we found no statistical difference in adverse events at longest follow‐up between anesthetic and placebo. There were insufficient data comparing the adverse effects of topical anesthetics plus NSAID with a placebo (one RCT).
Overall completeness and applicability of evidence
Overall, there was a paucity of evidence for the comparison of efficacy or safety of topical anesthetics compared with placebo for corneal epithelial defects of traumatic or post‐surgical origin. We included randomized trials that were characterized by clinically relevant participants, routinely prescribed interventions, and patient‐important outcomes, and found the applicability of the review findings to be limited. The evidence was of very low certainty for all outcomes primarily because of small sample sizes and high risk of bias in two of the five bias domains addressed (missing outcome data and selection of study outcomes for which results had been reported).
Population representativeness
We included trials that enrolled participants with corneal abrasions of various etiologies to optimize the applicability of the review findings. However, there were systematic differences in the direction and magnitude of effect estimates between trials of post‐trauma versus post‐surgical participants. Because there were too few trials for meaningful statistical subgroup analysis, we do not know the degree to which etiology may influence specific effects. For these reasons, we did not combine outcome data from post‐trauma participants with those from post‐surgical participants.
The findings are not applicable to pediatric populations as there was no participant younger than 17 years of age. We were interested in exploring treatment effects by gender and race, but none of the included trials provided sufficient information. There is an increasing awareness of the role that race and ethnicity may play in pain management (Booker 2023; Campbell 2012; Letzen 2022). Unfortunately, only one study reported the ethnicity of participants but did not report outcomes by ethnicity (Waldman 2014). The authors also discussed the influence of community culture on self‐reported pain but did provide further details (Waldman 2014).
For the comparison of anesthetic versus placebo, eight of the nine trials reported the gender of participants. The predominance of male participants across the four included trials in emergency departments was consistent with previously published US surveys (Channa 2016; McGwin 2005). In the only trial that tested the combination of anesthetic and NSAID against placebo, the authors did not report the gender ratio of participants within each treatment arm (Lim 1999). On average, there was a higher proportion of women enrolled in trials of post‐ophthalmic surgeries than in those conducted in the emergency department setting (60% versus 21%, respectively). Given the growing body of literature suggesting gender differences in pain perception and reporting (Racine 2012a; Racine 2012b), the difference in gender ratios across trials and between the two main etiologies of corneal abrasions in this review may have contributed to the heterogeneity of the observed treatment effect.
Interventions
The nine included trials evaluated three of the commonly used topical anesthetics: proparacaine (Ball 2010; Lim 1999; Shahinian 1997), tetracaine (Montard 1999; Shipman 2021; Ting 2009; Verma 1995; Waldman 2014), and lidocaine (Oksuz 2006). There was only one trial in which participants received an amide anesthetic (lidocaine) in three doses over three hours prior to discharge (Oksuz 2006). The other eight trials discharged participants with ester anesthetics: tetracaine at different concentrations with a duration of 24 to 48 hours and dilute proparacaine 0.05% for one week in three trials. The total amount dispensed and the frequency of use varied, but it was not always reported. Because of the very low certainty of evidence regarding critical outcomes, we cannot address concerns about anesthetic abuse from short‐term use of tetracaine by participants.
Co‐interventions in several trials may confound the effects of anesthetic use and contribute to the observed heterogeneity. Depending on the trial, oral analgesia was prescribed as needed for breakthrough pain, used on a schedule to prevent breakthrough pain, or simply not permitted. Antibiotic use differed across trials by type and duration. There were also differences in the use of bandage contact lenses, occlusive patches, and supplemental topical anesthetics.
The review findings are most applicable to ester anesthetics compared with placebo. We did not find trials comparing topical anesthetics with other common treatments, such as topical cycloplegics, and only one trial compared anesthetics with NSAIDs (Montard 1999). We found only one trial with dual therapy: NSAID plus anesthetic (Lim 1999).
Outcome measurement
None of the included trials assessed health‐related or vision‐related quality of life or functions of daily activity. Thus, although pain is multidimensional in nature, we cannot extrapolate the very low‐certainty evidence of topical anesthetic efficacy beyond the effects on pain intensity. There is concern that sole reliance on self‐reported pain intensity can result in an overestimation of the amount of treatment needed, which is a possible contributor to the overprescribing observed in the opioid epidemic (Pogatzki‐Zahn 2019; Sharfstein 2019).
All the pain intensity measurements could be converted to 10‐point scales, which are ubiquitous and commonly used in both clinical practice and randomized trials. Similar clinically important change in pain intensity was defined in three trials, ranging from 1.5 to 2 points on a 10‐point scale (Ball 2010; Shipman 2021; Waldman 2014). These definitions overlap with other reviews of interventions for acute pain, which have defined 'moderate' effects as a change of 1 to 2 points, and 'substantial' effects as greater than a change of 2 points on a 10‐point scale (Chou 2020). One trial noted that lower than 3 on a 10‐point scale is an accepted target for patients with a baseline of moderate to severe pain (Verma 1997). However, the trials included in this review were small, and only half reported participants' baseline pain, which makes it difficult to assess the sensitivity to detecting analgesic effects (Brinck 2018; McQuay 2012; Moore 2013).
Although the pain intensity scales used in the included trials are comparable, the timing of pain intensity measurements (e.g. at 24, 48 hours; change from before and one, three, or five minutes after use of study drops), method of aggregation (e.g. score at 10 hours; overall mean scores up to 24, 48 hours; total pain burden), and type of analysis (e.g. reported value, mixed‐model for multiple time points, single factor analysis) were different in each trial. Other Cochrane Reviews of treatments for corneal abrasions found similar issues with outcome specification and reporting (Algarni 2022; Lim 2016; Wakai 2017).
We sought to assess the proportion of participants who did not have sufficient pain control from topical anesthetics during the periods assumed to be most painful. All the included trials had the highest pain recorded between baseline and 72 hours, but only one trial reported treatment failure over that time period (Ting 2009).
The proportion of participants who did not return for follow‐up at emergency departments was much higher than the proportion of participants not followed up in the trials conducted in the setting of ophthalmic surgery. In some of the trials conducted in the emergency department, ophthalmologists assessed participants, but most assessments were performed by emergency physicians. These provider differences in outcome measurement and assessment (interpretation) may have contributed to the clinical heterogeneity associated with the different trial settings and etiology of abrasions. The authors of Ting 2009 state that only 34% (16/47) of the randomized participants returned for follow‐up at 36 to 48 hours and, therefore, the evidence remains "inconclusive" whether topical anesthetics prescribed for poor pain control after trauma could delay corneal epithelial healing. Most participants cited insignificant pain or no pain and absence of visual problems as the main reasons for noncompliance with return for follow‐up.
The majority of trials did not report adverse events beyond one week, and only three trials reported specific adverse events. In this review we sought to synthesize the evidence in light of ongoing concerns raised by case reports and case series regarding the risks of delayed healing and ocular morbidity due to topical anesthetic abuse. The majority of cases report adverse events at time points well beyond those of the trials in this review (Aksoy 2013; Ansari 2006; Ardjomand 2002; Chen 2004; Chern 1996; Dornic 1998; Epstein 1968; Katsimpris 2007; Khakshoor 2012; Kim 1997; Lee 2008; Pharmakakis 2002; Rosenwasser 1990; Varga 1997; Webber 1999; Willis 1970; Wu 2016; Yagci 2011). For example, in Turkey, where topical anesthetics were available over the counter until 2012, one study found that median drug use was 28 days (range: 10 to 112 days; seven patients) before admission to clinic for amniotic membrane transplantation (Burcu 2013). Another case series reported that epithelial healing took a median of 17 days (range: 6 to 50 days; 19 patients) in patients diagnosed with topical anesthetic abuse keratopathy (Yagci 2011). The findings of the current review cannot address whether these cases of anesthetic abuse are outliers or the norm since the majority of included trials did not clinically assess safety outcomes beyond one to two weeks.
The time from the corneal abrasion to participant randomization was longer in the acute trauma setting than the post‐surgical setting by 24 to 36 hours, depending on the trial. Post‐trauma trials varied in whether investigators included patients with rust rings, which has implications for potential risk of bias and for the applicability of findings. In the post‐surgical trials, the abrasion depth and area were more uniformly controlled and created in a sterile field. The baseline risk for healing time and adverse events such as microbial keratitis may differ systematically by setting.
Certainty of the evidence
We downgraded the certainty of evidence for most review outcomes because of risk of bias from missing outcome data and selection of the outcome results that had been reported, and because of imprecision of effect estimates based on small sample sizes. Aside from the clinical heterogeneity in the trial design and conduct (trial setting, dosage of intervention and co‐intervention, outcome measurement, method of aggregation, types of analysis, time of outcome assessment, and duration of follow‐up) and in patient characteristics (etiologies of abrasion, presence or absence of rust rings, which might have influenced dropout from post‐trauma trials), other reasons for the observed statistical heterogeneity included differences in the directions of effects between trials. The small number of trials limited our ability to explore these factors in depth. Of the nine included trials, we found trial registration records for only four trials; only one had been registered before participant enrollment began.
Potential biases in the review process
We aimed to minimize potential biases in the review process by applying standard Cochrane methodology. We contacted study authors during the screening of full‐text reports to minimize the exclusion of eligible trials. We clarified with investigators regarding conflicting information found in reports from the same study, trials halted prior to enrollment, and clarifications of issues regarding randomization. We did not exclude the one study for which we did not receive a response (Aseff 1997). We also received raw data for two outcome time points from one of the authors (Waldman 2014). We included trials regardless of language of publication and translated study reports to the extent necessary (Lim 1999; Montard 1999). One limitation of this review is that estimates of rates of serious adverse events may be under‐reported either because of rarity, the method of elicitation of reports of complications, or under‐reporting by the study investigators. This limitation prevented us from assessing the safety of the intervention to permit readers to balance efficacy and safety.
Agreements and disagreements with other studies or reviews
Three reviews that included multiple interventions for post‐PRK pain management have discussed the use of topical anesthetics for pain management (Garcia 2016; Golan 2018; Steigleman 2023). The Golan 2018 review recommended against the long‐term use of topical anesthetics, while Garcia 2016 stated that based on three studies (including Verma 1995 in our review), topical anesthetics did not delay epithelial healing. The conclusions of the authors of the third review were more measured and highlighted the need for careful monitoring (Steigleman 2023). Different inclusion criteria may account for these varying conclusions as both Golan 2018 and Steigleman 2023 included the same trials, while the Shahinian 1997 trial was omitted from Garcia 2016. We included two non‐English language trials that were excluded from the above reviews based on the publication language (Lim 1999; Montard 1999). Although their inclusion did not provide further certainty regarding the safety or efficacy of topical anesthetics, it highlights the potential for bias introduced by restricting studies included in reviews by language of publication.
A systematic review of RCTs evaluating the safety and efficacy of topical proparacaine and tetracaine for corneal abrasions (Swaminathan 2015) included several studies included in our review (Ball 2010; Waldman 2014; Verma 1995). These authors originally intended to review corneal abrasions seen in the emergency department. However, the selection was expanded to also include patients who underwent PRK because of a paucity of studies identified in their original search. Their conclusion was that topical anesthetics were safe and effective for corneal abrasions either from trauma or after PRK. It is important to note that these authors did not perform a statistical meta‐analysis, nor did they quantify the methodological rigor (e.g. risk of bias) of studies or the level of certainty in the data.
A more recent systematic review of RCTs and observational studies included several types of topical interventions for pain control (NSAIDs, bandage contact lenses, patching, topical anesthetics) compared with placebo or no treatment for traumatic corneal abrasions (Yu 2021). They explicitly excluded post‐surgical studies. For the comparison of topical anesthetics with placebo, Yu 2021 included the same four randomized controlled trials (RCTs) and one observational study that we excluded (Waldman 2018). Based on this trial, Yu 2021 noted that there may be differences in adverse outcomes based on corneal abrasion complexity (e.g. size of abrasion). Our review did not explore differences in outcomes based on corneal abrasion size. However, it is worth noting that the post‐surgical trials did report grouping by the abrasion size. Our review is in agreement with Yu 2021 on the absence of definitive evidence regarding safety outcomes, although our count of participants and complications differs. The reason for this difference may be from the review author's classification of complications and the specified time point. The authors of Yu 2021 recommend, "If topical anesthesia is given to the patient, we advocate for formulations such as dilute proparacaine as opposed to tetracaine, the latter being available in clinics largely to facilitate ocular examination." In our review, there was insufficient evidence, even with the inclusion of post‐surgical trials, to support or refute such a recommendation.
We focused solely on topical anesthetics. A number of treatment modalities for pain following PRK were highlighted in Steigleman 2023 but were not included in Yu 2021, possibly reflecting the inherent differences between diagnosis and management of post‐surgical epithelial defects and traumatic corneal abrasions.
Authors' conclusions
Implications for practice.
Corneal abrasions from trauma or epithelial defects created during ophthalmic surgery present commonly in clinical practice. Safely managing pain is therefore of great interest. With a growing opioid crisis in the United States, opioid‐sparing pain management is a favorable option. Although topical anesthetics provide excellent pain control in the intraoperative setting, they traditionally have not been prescribed for outpatient use.
Compared with placebo, topical anesthetics alone or combined with a nonsteroidal anti‐inflammatory drug (NSAID) have been shown to be effective in reducing pain up to 24 hours in post‐surgical patients. Compared with NSAIDs, topical anesthetics may be slightly less effective at pain control in post‐surgical patients. At 48 hours, topical anesthetics decreased pain relative to placebo but were no more effective at 72 hours. Very low‐certainty evidence regarding complications within seven days favored placebo in post‐surgical participants.
Despite a lack of evidence of efficacy or safety differences between topical anesthetic and placebo in accidental or surgically created corneal abrasions, use of anesthetic eye drops without close monitoring creates a potential for ocular morbidity. We wish to highlight that the studies in this review were characterized by very close follow‐up of participants, use of an often diluted anesthetic mixture, and dispensing of a very limited supply. Despite the fact that we found no differences in safety (e.g. complications at longest follow‐up) the evidence was of very low certainty and, therefore, we do not discount the case literature describing a typified pattern of abuse. Similar to the use of topical steroids or NSAIDs, the risk of adverse effects increases when anesthetic drops are used more frequently or for longer than recommended. It is not difficult to imagine that a practice pattern shift towards liberalization of topical anesthetic prescribing could lead to situations far beyond the controlled environments of the studies we reviewed: where larger quantities are dispensed, follow‐up is not ensured, or where other providers reflexively renew prescription refills. Furthermore, case series in the ophthalmic literature indicate that patients who abuse topical anesthetics may have a psychiatric disorder, history of drug abuse, or history of depression. It is possible that such patients demand anesthetic agents because of heightened pain awareness. However, it is not clear whether emergency rooms have the resources to screen patients for these disorders prior to prescribing topical anesthetics and to counsel them on the importance of follow‐up with an ophthalmologist. All trials included in this review were characterized by very short follow‐up, which may not have allowed such complications to manifest. Patients recruited in the emergency department setting had higher dropout rates than post‐surgery patients, which raises the potential for misuse compared with surgical patients who have close follow‐up by an ophthalmic surgeon for a defect created in a sterile field. The latter may be reasons for newer modalities to be used to treat post‐photorefractive keratectomy pain that may be inappropriate for use to relieve pain from abrasions that present in non‐surgical settings.
Implications for research.
Investigators planning future trials on the safety and efficacy of topical anesthetics should plan for a larger sample size of diverse participant populations and both shorter (e.g. first eight hours) and longer (e.g. over one week) follow‐up periods to assess critical outcomes and rare or non‐immediate complications. Sample size assumptions (effect size, power) should be justified. Traumatic corneal abrasions account for more than 10% of eye‐related emergency room visits, which would indicate the potential for RCTs with larger enrollment than the RCTs in this review. Attrition should be reported and investigated, with attempts made to contact such patients for possible risk of anesthetic abuse. One concern with topical anesthetics prescribed on an outpatient basis for corneal abrasions is that patients who obtain prescriptions from multiple emergency rooms and do not follow up with either an emergency room physician or an ophthalmologist are at risk for anesthetic abuse‐related corneal complications. The situation would be analogous to the opioid crisis where providers were not versed in prescribing opioids (or in some cases were incentivized) and patients became addicted.
Efficacy outcomes should include a more complete understanding of pain; participant‐reported functional and quality of life assessment should be used alongside pain levels at baseline and follow‐up. There are core outcome sets being developed that should be considered in future trials and reviews.
Investigators of future trials should collect data on adjunct opioid pain control, as there is a nationwide push for reduced opioid pain management due to the ongoing opioid addiction crisis.
Investigators of future trials should also avoid or reduce the potential sources of bias identified in this review, such as selection bias caused by differential attrition rates at follow‐up and information bias introduced by complete‐case analysis when the proportion of missing outcome data is substantial.
Investigators should report outcomes by treatment arm both overall and within sex/gender and race/ethnicity subgroups of participants.
Investigators should differentiate between abrasions with complications (e.g. rust rings) and abrasions without complications to increase the applicability of the evidence to difference patient groups.
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2023 | Amended | Amendment to the Summary of Findings Table 1, Outcome: Proportion of participants without complete resolution of epithelial defect by 24‐72 hours; Illustrative comparative risks (95% CI), Corresponding risk with anesthetic, Placebo, post‐surgery. The error did not impact the data or review findings or interpretation. The review did not use illustrative risk in any other sections. |
History
Protocol first published: Issue 5, 2022 Review first published: Issue 8, 2023
| Date | Event | Description |
|---|---|---|
| 10 February 2023 | New search has been performed | The original search on 19 February 2022 led to nine included studies and one study 'awaiting classification'. A top‐up search on 10 February 2023 yielded no new eligible studies. |
Risk of bias
Risk of bias for analysis 1.2 Change in participant‐reported ocular pain from baseline to 48 hours.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.2.1 Anesthetic vs placebo, VAS (0 to 10), post‐surgery | ||||||||||||
| Verma 1995 | Low risk of bias | "Patients were allocated drops based on a random‐number generator, and prenumbered containers were dispensed by the operating surgeon." Baseline characteristics of study participants were partially reported by refractive error groups, not by treatment group. | Some concerns | Eye drops were prenumbered and dispensed by the operating surgeon; the reference code was opened 3 months postoperatively. A p‐value was reported along with the pain scores but the authors did not report how the p‐value was derived. | Low risk of bias | Data for all 44 participants randomized were analyzed and reported. | Low risk of bias | All patients were asked to complete Visual Analogue Pain Charts which consisted of a series of horizontal lines 10 em in length with "no pain" written at one end and "worst pain imaginable" at the other. Both participants and the surgeon were likely masked to the eye drops patients received. All pain score data at pre‐specified time points were reported as mean and standard errors in a figure. | Low risk of bias | No study protocol for evaluation but pain scores were reported as a continuous measure. Data for all timepoints pre‐specified were reported in figures. | Some concerns | This trial was assessed to be at some concern for bias due to deviations from intended interventions. |
| Subgroup 1.2.2 Anesthetic vs placebo, VAS (0 to 10), post‐trauma | ||||||||||||
| Shipman 2021 | Low risk of bias | The allocation list was generated by a computer random‐number generator and randomization was performed with numbered, sealed, opaque envelopes issued in sequential order to the physician enrolling the patient in the study. | Low risk of bias | The envelopes were opaque to ensure enrolling physicians remained blinded despite the different packaging of the placebo and the tetracaine. The placebo and tetracaine were labeled for the patient as the “study drops” to maintain blinding. Intention‐to‐treat analysis used. | Low risk of bias | Loss to follow‐up for 3/59 (5.1%) tetracaine and 4/59 (6.8%) placebo. "Data for all participants who underwent random assignment were analyzed according to group assignment" | Low risk of bias | Patients were asked to record pain score measurements on a standard NRS (from 0 to 10 cm), which was appropriate. Both participants and physicians were masked to treatment assignment. Data were analyzed without showing patient ID, i.e. masked to the treatment assignment. | High risk of bias | In the methods section, primary outcome data were reported to be analyzed by the Wilcoxon signed‐rank test, whereas in the results section, pain scores were compared and reported as mean difference and its associated 95% CI. | High risk of bias | This trial was assessed to be at a high risk of bias due to bias in selection of the reported result. |
Risk of bias for analysis 1.7 Proportion of participants with complications at furthest time point.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.7.1 At 1 week, post‐surgery | ||||||||||||
| Verma 1995 | Low risk of bias | "Patients were allocated drops based on a random‐number generator, and prenumbered containers were dispensed by the operating surgeon." Baseline characteristics of study participants were partially reported by refractive error groups, not by treatment group. | Low risk of bias | Medications were contained in prenumbered containers and delivered by the operating surgeon who appeared to be masked too. ITT analysis of all participants. | Low risk of bias | The number of participants who lost to follow‐up was not reported by time point. It was unclear how many participants returned after post‐op week one. | Low risk of bias | The current outcome was self‐reported but was further verified by an objective surface exam (topography) and visual acuity examination for all participants who returned at week one follow‐up. The randomization code was not broken before post‐op month three. Outcome assessors were likely masked. | Low risk of bias | Data for this outcome were reported at week one, month one, and month six as pre‐defined, except for post‐op month three. There are multiple assessments, but the reporting appears to include the details of each case. The result is dichotomous and the details of the specific assessment results are reported. | Low risk of bias | The trial was assessed to be at a low risk of bias in all domains. |
| Subgroup 1.7.2 At 1 to 2 weeks, post‐trauma | ||||||||||||
| Ting 2009 | Low risk of bias | The method of randomization was not described beyond stating it is a randomized trial. The research pharmacists not involved in the design or conduct of the trial, prepared identical, clear minim packs that were "sent to the ED in no particular order." There are minimal differences between groups at baseline. | Low risk of bias | The containers were in clear, identical containers. The investigators did not indicate that toxicity (such as burning) prevented this. The study used a modified ITT analysis, excluding participants who had missing data at the 2 weeks assessment. | High risk of bias | Data were available for 83% of participants randomized: 17/22 in the amethocaine group and 21/25 in the saline group (post‐hoc p > 0.05). There is no evidence provided (such as a sensitivity analysis) to determine if there was biased data. The missing data could depend on its true value. During the week 2 phone interview followup, the reasons reported by participants for not attending the 36‐48 hr visit was pain relief and no visual problems. There is insufficient information to determine whether this was likely to be the case or if the missing data was due to complications. | Some concerns | The slitlamp assessment by an emergency department physician at 36 to 48 hours is appropriate. The phone interview assessment of complications at 2 weeks is not detailed in the article, though it may not be appropriate for determining many types of adverse events. | Some concerns | The trial registration does not specify assessment of complications. No trial protocol or statistical analysis plan was included. There is no information provided about how complications were assessed at the 36‐48 hr, 2 weeks, or throughout the study. There is insufficient detail to determine whether the result may have been selected on the basis of analysis. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data; some concerns in the measurement of the outcome and in selection of the reported result. |
| Shipman 2021 | Low risk of bias | The allocation list was generated by a computer random‐number generator and randomization was performed with numbered, sealed, opaque envelopes issued in sequential order to the physician enrolling the patient in the study. | Low risk of bias | The placebo and tetracaine were labeled for the patient as the “study drops” to maintain blinding. However, although we attempted to blind patients from their allocation group, the burning nature of the tetracaine may have unblinded the patients. Additionally, patients may have been unblinded as a result of the placebo having been packaged in 4 ampules versus the tetracaine in a single bottle. Data for all randomized participants were analyzed according to group assignment in an intention‐to‐treat fashion. | High risk of bias | Loss to follow‐up for 3/59 (5.1%) in the tetracaine groups and 4/59 (6.8%) in the placebo group. In the tetracaine group, of the 48 patients who did not complete all follow‐ups, 16 responded to a text message, reporting no study‐associated complications. In the placebo group, 4 patients did not attend the emergency department follow‐up visit and 45 did not return for their 1‐week follow‐up with the study ophthalmologist. Of these 49 patients who did not complete all follow‐ups, 6 responded to a text message, reporting no study‐associated complications. There is no evidence that it was not biased. The AEs could lead to loss to follow‐up with an ophthalmologist at 1 week. | Low risk of bias | The assessment method is not detailed. However, severe adverse events are assessed by ophthalmologists using standard methods (e.g., slitlamp). The assessment of severe adverse events occurred at set time points for both groups. The chart review would have different times for each participant if all the searches were performed on a specific day rather than a follow‐up time. Phone calls and passive collection of electronic medical record data could lead to diagnostic detection bias. Any patients found to have a significant complication were immediately referred to the study ophthalmologist, who was also blinded to the patient’s group allocation. | Some concerns | No data analysis plan was provided. There are multiple possible definitions used and vary across the study (text message, electronic medical record, phone call, emergency department assessment, ophthalmology assessment) without a description of how these were harmonized. There is insufficient information to determine if the results were selected on this basis. There are multiple ways the data may have been analyzed. There is insufficient information to determine if the results were selected on this basis. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data and some concerns in selection of the reported result. |
| Waldman 2014 | Low risk of bias | Computer generated, block randomized sequence. "Although a different size, the medications were packaged inside the questionnaire sheets, which were concealed inside a white envelope to disguise their identity." Minimal difference between groups for reported baseline characteristics. Median baseline pain was slightly higher in the tetracaine group; 54.6 mm (10–98) versus 48.0 mm (0–96). The etiology of corneal abrasion differed between groups but could be by chance. | Some concerns | The burning sensation of tetracaine may have unblinded participants. Saline was supplied in a 5mL single‐use plastic bullet. Tetracaine was supplied in three plastic 0.5mL commercially available vials. The presence of residual rust rings is not listed as an acceptable post‐randomization exclusion in the trial registration or Methods section. The exclusions are similar between groups so it is unlikely to have influenced the direction of the effect: "Of the patients with rust rings, fluorescein uptake was seen in four of the 10 in the saline group and seven of the 13 in the tetracaine group." | Low risk of bias | "We identified 11 patients who were noncompliant with the study protocols, four in the tetracaine group and seven in the saline group. Data analysis, however, was performed on all 116 patients enrolled into the study on an intention‐to‐treat analysis basis." There were 93. If the exclusion of participants with residual rust ring was post hoc, a number of participants had unreported outcome data. There is also unclear follow‐up beyond 48 hours. Compensating by other methods of follow‐up (call, text) reduced loss to follow‐up to 0 in both arms. The follow‐up contacts appear to have captured the missing data. The other missing data was for the retained rust rings. | High risk of bias | The assessment methods are not detailed. The clinical tests (e.g., slit lamp, cultures) are likely to be appropriate. The tetracaine may mask complications if they are only self‐reported. Because the objective methods are not detailed, this is unclear. The subjective method may be biased towards the intervention group. | High risk of bias | The trial registration did not exclude participants with rust rings, nor was this outlined in the Methods section. No protocol or statistical analysis plan in the registry. There were multiple time points with different participants in each. The source of collecting data may vary based on who is collecting data. The adverse events would be reported as a proportion. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data and bias in the measurement of the outcome, as well as some concerns due to deviations from intended interventions. |
Risk of bias for analysis 1.8 Proportion of participants with complications at furthest time point (RD) ‐ subgroup by abrasion type.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.8.1 Post‐surgery | ||||||||||||
| Oksuz 2006 | Some concerns | States the study was randomized. No information on sequence generation. No information was provided about allocation concealment. Based on the two reported baseline differences of age and sex, there is no indication of a problem with randomization. | Low risk of bias | The study was reported as "single‐masked" and a researcher who evaluated the self‐reported pain scores on a VAS scale "was masked to the ointment applied," rather than the participants. There was no evidence of deviations from the intervention under the trial context. An ITT analysis was performed. | High risk of bias | The authors did not report any numeric results for this outcome but simply stated that "we did not observe any corneal epithelial or ocular surface complications in either group." Not all participants were required to return after post‐op 24 hours, so there was evidence that results could be biased due to missing data. If participants dropped out for other complications but were not followed based on the epithelial healing, the missingness could depend on the true value. There are a number of participants not followed completely. | High risk of bias | After assessing re‐epithelialization at the post‐op 24 hours (visit 1), only participants with incomplete epithelial healing were re‐examined 12 hours following visit 1 (visit 2). Patients who still had incomplete epithelization were re‐examined on post‐op day 2 (visit 3). As such, not all participants were equally likely followed for the occurrence of ocular AE. The knowledge about the treatment received either by the participants or by the caregivers who followed the participants might provide a biased follow‐up plan consciously or unconsciously. There were differences in time points based on successive assessments. | Some concerns | There was no study protocol available for assessment. The authors did not report relevant definitions for ocular AE in the methods section either. There is no information about scales, assessments, or multiple time points. There is no information on how the data was analyzed. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data and measurement of the outcome with some concerns for bias in other domains. |
| Lim 1999 | Some concerns | "These subjects were randomly assigned to 7 groups of 15 eyes each according to the drug used fo the treatment of pain." However, no details were provided regarding allocation concealment or masking the participants or investigators. No information reported except for the reported mean age, age range, and sex distribution for the total study population. | Some concerns | "These subjects were randomly assigned to 7 groups of 15 eyes each according to the drug used of the treatment of pain." However, no details were provided regarding allocation concealment or masking the participants or investigators. No information reported except for the reported mean age, age range, and sex distribution for the total study population. | Low risk of bias | The authors did not report any numeric results for this outcome but simply stated that "No keratitis or corneal haze observed up to 1 week post‐op." There is no mention of missing data though it seems that all of the subjects were used for analysis. | Some concerns | Narrative only: "No keratitis or corneal haze observed up to 1 week post‐op." Blinding of assessors is unclear. The adverse events described are more objective than subjective. Knowledge would be less likely to bias the result | Some concerns | There were no study protocol available for assessment. The authors did not report relevant definitions for ocular AE in the methods section either. | Some concerns | The trial was assessed to be at some concerns of bias in four out of five domains: randomization process, deviations from intended interventions, outcome measurement, and selection of the reported result. |
| Verma 1995 | Low risk of bias | "Patients were allocated drops based on a random‐number generator, and prenumbered containers were dispensed by the operating surgeon." Baseline characteristics of study participants were partially reported by refractive error groups, not by treatment group. | Low risk of bias | Medications were contained in prenumbered containers and delivered by the operating surgeon who appeared to be masked too. ITT analysis of all participants. | Low risk of bias | The number of participants who lost to follow‐up was not reported by time point. It was unclear how many participants returned after post‐op week one. | Low risk of bias | The current outcome was self‐reported but was further verified by an objective surface exam (topography) and visual acuity examination for all participants who returned at week one follow‐up. The randomization code was not broken before post‐op month three. Outcome assessors were likely masked. | Low risk of bias | Data for this outcome were reported at week one, month one, and month six as pre‐defined, except for post‐op month three. There are multiple assessments, but the reporting appears to include the details of each case. The result is dichotomous and the details of the specific assessment results are reported. | Low risk of bias | The trial was assessed to be at a low risk of bias in all domains. |
| Subgroup 1.8.2 Post‐trauma | ||||||||||||
| Ting 2009 | Low risk of bias | The method of randomization was not described beyond stating it is a randomized trial. The research pharmacists not involved in the design or conduct of the trial, prepared identical, clear minim packs that were "sent to the ED in no particular order." There are minimal differences between groups at baseline. | Low risk of bias | The containers were in clear, identical containers. The investigators did not indicate that toxicity (such as burning) prevented this. The study used a modified ITT analysis, excluding participants who had missing data at the 2 weeks assessment. | High risk of bias | Data were available for 83% of participants randomized: 17/22 in the amethocaine group and 21/25 in the saline group (post‐hoc p > 0.05). There is no evidence provided (such as a sensitivity analysis) to determine if there was biased data. The missing data could depend on its true value. During the week 2 phone interview follow‐up, the reasons reported by participants for not attending the 36‐48 hr visit was pain relief and no visual problems. There is insufficient information to determine whether this was likely to be the case or if the missing data was due to complications. | Some concerns | The slitlamp assessment by an emergency department physician at 36 to 48 hours is appropriate. The phone interview assessment of complications at 2 weeks is not detailed in the article, though it may not be appropriate for determining many types of adverse events. | Some concerns | The trial registration does not specify assessment of complications. No trial protocol or statistical analysis plan was included. There is no information provided about how complications were assessed at the 36‐48 hr, 2 weeks, or throughout the study. There is insufficient detail to determine whether the result may have been selected on the basis of analysis. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data; some concerns in the measurement of the outcome and in selection of the reported result. |
| Ball 2010 | Low risk of bias | "Randomization key was generated via a computer using the random number function." "Staff at the hospital pharmacy diluted the proparacaine and filled numbered vials with either proparacaine or placebo. These vials were distinguishable only by number." Age and sex are the only reported baseline characteristics. | High risk of bias | "Patients were randomly assigned to groups receiving either 0.05% proparacaine or a colour‐ and smell‐ matched placebo." Staff at the hospital pharmacy diluted the proparacaine and filled numbered vials with either proparacaine or placebo. These vials were distinguishable only by number." There were 8 participants who either did not receive medications, record pain logs, or were lost to follow‐up and removed from ITT analysis. The breakdown of exclusions by reason and treatment arm is not reported. It is possible that it could have an impact on the effect estimate. | Some concerns | A total of 8/41 (20%) participants did not contribute adverse events data to the reported effect estimate. There was no description of the reasons for missing adverse event data. It is unlikely that participants would be lost to follow‐up because of serious adverse events and that be omitted from publication. | Low risk of bias | The assessments are standard and not inappropriate. Assessments were at the same timepoints. Any patients found to have a significant complication were immediately referred to the study ophthalmologist, who was also blinded to the patient’s group allocation. | Low risk of bias | The trial registration matches the reported outcome in the article. The outcome priorities are listed and reported. The reporting is narrative summary but is unlikely to be something other than a proportion. | High risk of bias | The trial was assessed to be at high risk of bias due to deviations from intended interventions and some concerns due to missing outcome data. |
| Shipman 2021 | Low risk of bias | The allocation list was generated by a computer random‐number generator and randomization was performed with numbered, sealed, opaque envelopes issued in sequential order to the physician enrolling the patient in the study. | Low risk of bias | The placebo and tetracaine were labeled for the patient as the “study drops” to maintain masking. However, although we attempted to blind patients from their allocation group, the burning nature of the tetracaine may have unblinded the patients. Placebo having been packaged in 4 ampules versus the tetracaine in a single bottle. Data for all randomized participants were analyzed according to group assignment in an intention‐to‐treat fashion. | High risk of bias | Loss to follow‐up for 3/59 (5.1%) in the tetracaine groups and 4/59 (6.8%) in the placebo group. In the tetracaine group, of the 48 patients who did not complete all follow‐ups, 16 responded to a text message, reporting no study‐associated complications. In the placebo group, 4 patients did not attend the emergency department follow‐up visit and 45 did not return for their 1‐week follow‐up with the study ophthalmologist. Of these 49 patients who did not complete all follow‐ups, 6 responded to a text message, reporting no study‐associated complications. There is no evidence that it was not biased. The AEs could lead to loss to follow‐up with an ophthalmologist at 1 week. | Low risk of bias | The assessment method is not detailed. However, severe adverse events are assessed by ophthalmologists using standard methods (e.g., slitlamp). The assessment of severe adverse events occurred at set time points for both groups. The chart review would have different times for each participant if all the searches were performed on a specific day rather than a follow‐up time. Phone calls and passive collection of electronic medical record data could lead to diagnostic detection bias. Any patients found to have a significant complication were immediately referred to the study ophthalmologist, who was also blinded to the patient’s group allocation. | Some concerns | No data analysis plan was provided. There are multiple possible definitions used and vary across the study (text message, electronic medical record, phone call, emergency department assessment, ophthalmology assessment) without a description of how these were harmonized. There is insufficient information to determine if the results were selected on this basis. There are multiple ways the data may have been analyzed. There is insufficient information to determine if the results were selected on this basis. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data and some concerns in selection of the reported result. |
| Waldman 2014 | Low risk of bias | Computer generated, block randomized sequence. "Although a different size, the medications were packaged inside the questionnaire sheets, which were concealed inside a white envelope to disguise their identity." Minimal difference between groups for reported baseline characteristics. Median baseline pain was slightly higher in the tetracaine group; 54.6 mm (10–98) versus 48.0 mm (0–96). The etiology of corneal abrasion differed between groups but could be by chance. | Some concerns | The burning sensation of tetracaine may have unblinded participants. Saline was supplied in a 5mL single‐use plastic bullet. Tetracaine was supplied in three plastic 0.5mL commercially available vials. The presence of residual rust rings is not listed as an acceptable post‐randomization exclusion in the trial registration or Methods section. The exclusions are similar between groups, so it is unlikely to have influenced the direction of the effect: "Of the patients with rust rings, fluorescein uptake was seen in four of the 10 in the saline group and seven of the 13 in the tetracaine group." | Low risk of bias | "We identified 11 patients who were noncompliant with the study protocols, four in the tetracaine group and seven in the saline group. Data analysis, however, was performed on all 116 patients enrolled into the study on an intention‐to‐treat analysis basis." There were 93. If the exclusion of participants with residual rust ring was post hoc, a number of participants had unreported outcome data. There is also unclear follow‐up beyond 48 hours. Compensating by other methods of follow‐up (call, text) reduced loss to follow up to 0 in both arms. The follow‐up contacts appear to have captured the missing data. The other missing data was for the retained rust rings. | High risk of bias | The assessment methods are not detailed. The clinical tests (e.g., slit lamp, cultures) are likely to be appropriate. The tetracaine may mask complications if they are only self‐reported. Because the objective methods are not detailed, this is unclear. The subjective method may be biased towards the intervention group. | High risk of bias | The trial registration did not exclude participants with rust rings, nor was this outlined in the Methods section. No protocol or statistical analysis plan in the registry. There were multiple time points with different participants in each. The source of collecting data may vary based on who is collecting data. The adverse events would be reported as a proportion. | High risk of bias | The trial was assessed to be at high risk of bias due to missing outcome data and bias in the measurement of the outcome, as well as some concerns due to deviations from intended interventions. |
Risk of bias for analysis 2.3 Proportion of participants with complications at furthest time point (RD).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lim 1999 | Some concerns | "These subjects were randomly assigned to 7 groups of 15 eyes each according to the drug used fo the treatment of pain." However, no details were provided regarding allocation concealment or masking the participants or investigators. No information reported except for the reported mean age, age range, and sex distribution for the total study population. | Some concerns | "These subjects were randomly assigned to 7 groups of 15 eyes each according to the drug used of the treatment of pain." However, no details were provided regarding allocation concealment or masking the participants or investigators. No information reported except for the reported mean age, age range, and sex distribution for the total study population. | Low risk of bias | The authors did not report any numeric results for this outcome but simply stated that "No keratitis or corneal haze observed up to 1 week post‐op." There is no mention of missing data though it seems that all of the subjects were used for analysis. | Some concerns | Narrative only: "No keratitis or corneal haze observed up to 1 week post‐op." Blinding of assessors is unclear. The adverse events described are more objective than subjective. Knowledge would be less likely to bias the result | Some concerns | There were no study protocol available for assessment. The authors did not report relevant definitions for ocular AE in the methods section either. | Some concerns | The trial was assessed to be at some concerns of bias in four out of five domains: randomization process, deviations from intended interventions, outcome measurement, and selection of the reported result. |
Acknowledgements
Acknowledgments from the authors
We thank Barbara Hawkins for her comments and methodology guidance on the protocol and review. We acknowledge the Cochrane Pain, Palliative and Supportive Care (PaPaS) resources on acute pain and thank Neil O'Connell, PaPaS Co‐ordinating Editor, and Andrew Moore, former PaPaS Senior Editor, for their comments on pain outcomes at the protocol development stage.
Editorial and peer reviewer contributions
CEV@US supported the authors in the development of this review. The following people conducted the editorial process for this review:
Sign‐off Editors (final editorial decision): Dr. Tianjing Li (University of Colorado Anschutz Medical Campus), Gianni Virgilli (Queen's University Belfast, Ireland; University of Florence, Italy), Barbara Hawkins (Johns Hopkins University)
Managing Editor and Assistant Managing Editor (selected peer reviewers, collated peer reviewer comments): Anupa Shah (Queen's University Belfast); Genie Han (Johns Hopkins University)
Information Specialist: Lori Rosman (Johns Hopkins University)
Copy Editor: Jenny Bellorini (Cochrane Central Production Service)
Peer reviewers: Miles Greenwald (Kellogg Eye Center), Chris Lim (Royal Melbourne Hospital)
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cornea] explode all trees #2 MeSH descriptor: [Corneal Diseases] explode all trees #3 MeSH descriptor: [Epithelium, Corneal] explode all trees #4 MeSH descriptor: [Keratectomy] explode all trees #5 MeSH descriptor: [Refractive Surgical Procedures] explode all trees #6 cornea* #7 (ocular NEXT/1 (surface* or epithelia*)) or keratectom* or keratoplast* or "cross linking" #8 MeSH descriptor: [Eye Injuries] explode all trees #9 Eye* NEXT/3 (injur* or abrasion* or erosion* or trauma* or wound* or (foreign NEXT/1 bod*) or (epithelial NEXT/1 defect*) or lesion* or laceration or surger* or surgical) #10 {OR #1‐#9} #11 MeSH descriptor: [Amides] explode all trees #12 MeSH descriptor: [Esters] explode all trees #13 amide OR amides OR ester OR esters #14 topical NEXT/2 (analgesic* or anesthetic* or anaesthetic*) #15 MeSH descriptor: [Tetracaine] explode all trees #16 "ak‐t‐caine" OR amethocaine OR ametocaine OR ametop OR anetaine OR anethaine OR butethanol OR butethol OR contralgin OR curtacaine OR decicain OR decicaine OR "dextrose‐pontocaine hcl" OR dicain OR dicaine OR fissucain OR gingicain OR intercain OR landocaine OR laudocaine OR meethobalm OR mucaesthin OR niphanoid OR pantocain OR pantocaine OR pontocaine OR rexocaine OR tetocaine OR tetracain OR tetracaine OR tetrakain OR tetrracaine OR tonexol OR uromucaesthin OR "136‐47‐0" OR "94‐24‐6" #17 alcaine OR anestalcon OR "chibro‐kerakain" OR kainair OR keracaine OR miraxil OR "ocu‐caine" OR oftetic OR ophthaine OR ophthetic OR opthetic OR "poen‐caina" OR proparacain OR proparacaine OR proporacaine OR proxymetacaine OR "499‐67‐2" OR "5875‐06‐9" #18 MeSH descriptor: [Lidocaine] explode all trees #19 akten OR "algrx 3268" OR algrx3268 OR alphacaine OR anestacaine OR anestacon OR anestacone OR aritmal OR betacaine OR cidancaina OR "col 1077" OR col1077 OR "corus 1030" OR corus1030 OR dalcaine OR dentipatch OR dolicaine OR duncaine OR dynexan OR "ela‐max" OR esracain OR esracaine OR farmacaina OR "gesicain jelly" OR "gesicain ointment" OR "gesicain viscous" OR glydo OR gravocain OR isicaine OR jetocaine OR jetokain OR "l‐caine" OR lecasin OR leostesin OR "lida mantle" OR lidbree OR lidocain OR lidocaine OR lidocaton OR lidocor OR lidocorit OR lidoderm OR lidonest OR lidopain OR lidopen OR lidorx OR lidothesin OR lignocaine OR lignostab OR lincaine OR liquocaine OR liris OR "ll 30" OR ll30 OR "lmx 4" OR "lmx 5" OR "lta ii kit" OR maricaine OR "neo novutox" OR neolidocaton OR Octocaine OR otipax OR "paediatric lta kit" OR "pediatric lta kit" OR penles OR radiaguard OR ralvo OR "remicaine gel" OR roxicaina OR rucaina OR ruciana OR solarcaine OR solcaine OR "sp 103" OR sp103 OR truxacaine OR "uad caine" OR vasocaine OR versatis OR xidocaine OR xilina OR xiline OR xilocaina OR "xilonest pomade" OR "xilotane gel" OR xilyne OR xylcaine OR xylestesin OR Xylesthesin OR xylocain OR xylocaina OR xylocaine OR xylocard OR xylocitin OR xyloctin OR xyloneural OR xylonor OR "xyloproct n" OR xyloton OR xylotox OR xylyne OR zingo OR ztlido OR "137‐58‐6" OR "24847‐67‐4" OR "56934‐02‐2" OR "73‐78‐9" #20 Oxybuprocaine OR benoxil OR benoxinate OR cebesine OR conjucain OR conjuncain OR dorsacain OR dorsacaine OR lacrimin OR novesin OR novesine OR oxibuprocainum OR oxibuprokain OR oxybucaine OR prescaina OR "5987‐82‐6" OR "99‐43‐4" #21 MeSH descriptor: [Cocaine] this term only #22 Cocaine OR cocain OR codrenine OR erythroxylin OR goprelto OR locosthetic OR neurocaine OR numbrino OR sterilocaine OR "50‐36‐2" OR "53‐21‐4" OR "5937‐29‐1" #23 {OR #11‐#22} #24 #10 AND #23 in Trials
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Cornea/ 13. exp Corneal Diseases/ 14. exp Epithelium, Corneal/ 15. exp Keratectomy/ 16. exp Refractive Surgical Procedures/ 17. cornea*.tw. 18. ((ocular adj1 (surface* or epithelia*)) or keratectom* or keratoplast* or "cross linking").tw. 19. exp eye injuries/ 20. (Eye* adj3 (injur* or abrasion* or erosion* or trauma* or wound* or foreign bod* or "epithelial defect*" or lesion* or laceration or surger* or surgical)).tw. 21. or/12‐20 22. exp Amides/ 23. exp Esters/ 24. (amide or amides or ester or esters).tw. 25. (topical adj2 (analgesic* or anesthetic* or anaesthetic*)).tw. 26. exp Tetracaine/ 27. ("ak‐t‐caine" or amethocaine or ametocaine or ametop or anetaine or anethaine or butethanol or butethol or contralgin or curtacaine or decicain or decicaine or "dextrose‐pontocaine hcl" or dicain or dicaine or fissucain or gingicain or intercain or landocaine or laudocaine or meethobalm or mucaesthin or niphanoid or pantocain or pantocaine or pontocaine or rexocaine or tetocaine or tetracain or tetracaine or tetrakain or tetrracaine or tonexol or uromucaesthin or "136‐47‐0" or "94‐24‐6").tw,rn. 28. (alcaine or anestalcon or "chibro‐kerakain" or kainair or keracaine or miraxil or "ocu‐caine" or oftetic or ophthaine or ophthetic or opthetic or "poen‐caina" or proparacain or proparacaine or proporacaine or proxymetacaine or "499‐67‐2" or "5875‐06‐9").tw,rn. 29. exp Lidocaine/ 30. (akten or "algrx 3268" or algrx3268 or alphacaine or anestacaine or anestacon or anestacone or aritmal or betacaine or cidancaina or "col 1077" or col1077 or "corus 1030" or corus1030 or dalcaine or dentipatch or dolicaine or duncaine or dynexan or "ela‐max" or esracain or esracaine or farmacaina or "gesicain jelly" or "gesicain ointment" or "gesicain viscous" or glydo or gravocain or isicaine or jetocaine or jetokain or "l‐caine" or lecasin or leostesin or "lida mantle" or lidbree or lidocain or lidocaine or lidocaton or lidocor or lidocorit or lidoderm or lidonest or lidopain or lidopen or lidorx or lidothesin or lignocaine or lignostab or lincaine or liquocaine or liris or "ll 30" or ll30 or "lmx 4" or "lmx 5" or "lta ii kit" or maricaine or "neo novutox" or neolidocaton or Octocaine or otipax or "paediatric lta kit" or "pediatric lta kit" or penles or radiaguard or ralvo or "remicaine gel" or roxicaina or rucaina or ruciana or solarcaine or solcaine or "sp 103" or sp103 or truxacaine or "uad caine" or vasocaine or versatis or xidocaine or xilina or xiline or xilocaina or "xilonest pomade" or "xilotane gel" or xilyne or xylcaine or xylestesin or Xylesthesin or xylocain or xylocaina or xylocaine or xylocard or xylocitin or xyloctin or xyloneural or xylonor or "xyloproct n" or xyloton or xylotox or xylyne or zingo or ztlido or "137‐58‐6" or "24847‐67‐4" or "56934‐02‐2" or "73‐78‐9").tw,rn. 31. (Oxybuprocaine or benoxil or benoxinate or cebesine or conjucain or conjuncain or dorsacain or dorsacaine or lacrimin or novesin or novesine or oxibuprocainum or oxibuprokain or oxybucaine or prescaina or "5987‐82‐6" or "99‐43‐4").tw,rn. 32. cocaine/ 33. (Cocaine or cocain or codrenine or erythroxylin or goprelto or locosthetic or neurocaine or numbrino or sterilocaine or "50‐36‐2" or "53‐21‐4" or "5937‐29‐1").tw,rn. 34. or/22‐33 35. 21 and 34 36. 11 and 35
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cornea'/exp #34 'cornea disease'/exp #35 'cornea epithelium'/exp #36 'cornea surgery'/exp #37 'refractive surgery'/exp #38 Cornea*:ab,ti,kw #39 (ocular NEXT/1 (surface* or epithelia*)):ab,ti,kw or (keratectom* or keratoplast* or "cross linking"):ab,ti,kw #40 'eye injury'/exp #41 (Eye* NEXT/3 (injur* or abrasion* or erosion* or trauma* or wound* or "foreign bod*" or "epithelial defect*" or lesion* or laceration or surger* or surgical)):ab,ti,kw #42 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 #43 'amide'/exp #44 'ester'/exp #45 (amide OR amides OR ester OR esters):ab,ti,kw #46 'tetracaine'/exp #47 ("ak‐t‐caine" OR amethocaine OR ametocaine OR ametop OR anetaine OR anethaine OR butethanol OR butethol OR contralgin OR curtacaine OR decicain OR decicaine OR "dextrose‐pontocaine hcl" OR dicain OR dicaine OR fissucain OR gingicain OR intercain OR landocaine OR laudocaine OR meethobalm OR mucaesthin OR niphanoid OR pantocain OR pantocaine OR pontocaine OR rexocaine OR tetocaine OR tetracain OR tetracaine OR tetrakain OR tetrracaine OR tonexol OR uromucaesthin OR "136‐47‐0" OR "94‐24‐6"):ab,ti,kw,tn #48 'proxymetacaine'/exp #49 (alcaine OR anestalcon OR "chibro‐kerakain" OR kainair OR keracaine OR miraxil OR "ocu‐caine" OR oftetic OR ophthaine OR ophthetic OR opthetic OR "poen‐caina" OR proparacain OR proparacaine OR proporacaine OR proxymetacaine OR "499‐67‐2" OR "5875‐06‐9"):ab,ti,kw,tn #50 'lidocaine'/exp #51 (akten OR "algrx 3268" OR algrx3268 OR alphacaine OR anestacaine OR anestacon OR anestacone OR aritmal OR betacaine OR cidancaina OR "col 1077" OR col1077 OR "corus 1030" OR corus1030 OR dalcaine OR dentipatch OR dolicaine OR duncaine OR dynexan OR "ela‐max" OR esracain OR esracaine OR farmacaina OR "gesicain jelly" OR "gesicain ointment" OR "gesicain viscous" OR glydo OR gravocain OR isicaine OR jetocaine OR jetokain OR "l‐caine" OR lecasin OR leostesin OR "lida mantle" OR lidbree OR lidocain OR lidocaine OR lidocaton OR lidocor OR lidocorit OR lidoderm OR lidonest OR lidopain OR lidopen OR lidorx OR lidothesin OR lignocaine OR lignostab OR lincaine OR liquocaine OR liris OR "ll 30" OR ll30 OR "lmx 4" OR "lmx 5" OR "lta ii kit" OR maricaine OR "neo novutox" OR neolidocaton OR Octocaine OR otipax OR "paediatric lta kit" OR "pediatric lta kit" OR penles OR radiaguard OR ralvo OR "remicaine gel" OR roxicaina OR rucaina OR ruciana OR solarcaine OR solcaine OR "sp 103" OR sp103 OR truxacaine OR "uad caine" OR vasocaine OR versatis OR xidocaine OR xilina OR xiline OR xilocaina OR "xilonest pomade" OR "xilotane gel" OR xilyne OR xylcaine OR xylestesin OR Xylesthesin OR xylocain OR xylocaina OR xylocaine OR xylocard OR xylocitin OR xyloctin OR xyloneural OR xylonor OR "xyloproct n" OR xyloton OR xylotox OR xylyne OR zingo OR ztlido OR "137‐58‐6" OR "24847‐67‐4" OR "56934‐02‐2" OR "73‐78‐9"):ab,ti,kw,tn #52 'oxybuprocaine'/exp #53 (Oxybuprocaine OR benoxil OR benoxinate OR cebesine OR conjucain OR conjuncain OR dorsacain OR dorsacaine OR lacrimin OR novesin OR novesine OR oxibuprocainum OR oxibuprokain OR oxybucaine OR prescaina OR "5987‐82‐6" OR "99‐43‐4"):ab,ti,kw,tn #54 'cocaine'/exp #55 (Cocaine OR cocain OR codrenine OR erythroxylin OR goprelto OR locosthetic OR neurocaine OR numbrino OR sterilocaine OR "50‐36‐2" OR "53‐21‐4" OR "5937‐29‐1"):ab,ti,kw,tn #56 #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 #57 #42 AND #56 #58 #32 AND #57
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 cornea*[tw] #3 (ocular[tw] AND (surface*[tw] OR epithelia*[tw])) OR keratectom*[tw] OR keratoplast*[tw] OR "cross linking"[tw] #4 (eye[tw] OR eyes[tw] OR eyelid*[tw]) AND (injur*[tw] OR abrasion*[tw] OR erosion*[tw] OR trauma*[tw] OR wound*[tw] OR "foreign bod*"[tw] OR "epithelial defect*"[tw] OR lesion*[tw] OR laceration*[tw] OR surger*[tw] OR surgical[tw]) #5 #2 OR #3 OR #4 #6 (amide[tw] OR amides[tw] OR ester[tw] OR esters[tw]) #7 ("ak‐t‐caine"[tw] OR amethocaine[tw] OR ametocaine[tw] OR ametop[tw] OR anetaine[tw] OR anethaine[tw] OR butethanol[tw] OR butethol[tw] OR contralgin[tw] OR curtacaine[tw] OR decicain[tw] OR decicaine[tw] OR "dextrose‐pontocaine hcl"[tw] OR dicain[tw] OR dicaine[tw] OR fissucain[tw] OR gingicain[tw] OR intercain[tw] OR landocaine[tw] OR laudocaine[tw] OR meethobalm[tw] OR mucaesthin[tw] OR niphanoid[tw] OR pantocain[tw] OR pantocaine[tw] OR pontocaine[tw] OR rexocaine[tw] OR tetocaine[tw] OR tetracain[tw] OR tetracaine[tw] OR tetrakain[tw] OR tetrracaine[tw] OR tonexol[tw] OR uromucaesthin[tw] OR "136‐47‐0"[tw] OR "94‐24‐6"[tw]) #8 (alcaine[tw] OR anestalcon[tw] OR "chibro‐kerakain"[tw] OR kainair[tw] OR keracaine[tw] OR miraxil[tw] OR "ocu‐caine"[tw] OR oftetic[tw] OR ophthaine[tw] OR ophthetic[tw] OR opthetic[tw] OR "poen‐caina"[tw] OR proparacain[tw] OR proparacaine[tw] OR proporacaine[tw] OR proxymetacaine[tw] OR "499‐67‐2"[tw] OR "5875‐06‐9"[tw]) #9 (akten[tw] OR "algrx 3268"[tw] OR algrx3268[tw] OR alphacaine[tw] OR anestacaine[tw] OR anestacon[tw] OR anestacone[tw] OR aritmal[tw] OR betacaine[tw] OR cidancaina[tw] OR "col 1077"[tw] OR col1077[tw] OR "corus 1030"[tw] OR corus1030[tw] OR dalcaine[tw] OR dentipatch[tw] OR dolicaine[tw] OR duncaine[tw] OR dynexan[tw] OR "ela‐max"[tw] OR esracain[tw] OR esracaine[tw] OR farmacaina[tw] OR "gesicain jelly"[tw] OR "gesicain ointment"[tw] OR "gesicain viscous"[tw] OR glydo[tw] OR gravocain[tw] OR isicaine[tw] OR jetocaine[tw] OR jetokain[tw] OR "l‐caine"[tw] OR lecasin[tw] OR leostesin[tw] OR "lida mantle"[tw] OR lidbree[tw] OR lidocain[tw] OR lidocaine[tw] OR lidocaton[tw] OR lidocor[tw] OR lidocorit[tw] OR lidoderm[tw] OR lidonest[tw] OR lidopain[tw] OR lidopen[tw] OR lidorx[tw] OR lidothesin[tw] OR lignocaine[tw] OR lignostab[tw] OR lincaine[tw] OR liquocaine[tw] OR liris[tw] OR "ll 30"[tw] OR ll30[tw] OR "lmx 4"[tw] OR "lmx 5"[tw] OR "lta ii kit"[tw] OR maricaine[tw] OR "neo novutox"[tw] OR neolidocaton[tw] OR Octocaine[tw] OR otipax[tw] OR "paediatric lta kit"[tw] OR "pediatric lta kit"[tw] OR penles[tw] OR radiaguard[tw] OR ralvo[tw] OR "remicaine gel"[tw] OR roxicaina[tw] OR rucaina[tw] OR ruciana[tw] OR solarcaine[tw] OR solcaine[tw] OR "sp 103"[tw] OR sp103[tw] OR truxacaine[tw] OR "uad caine"[tw] OR vasocaine[tw] OR versatis[tw] OR xidocaine[tw] OR xilina[tw] OR xiline[tw] OR xilocaina[tw] OR "xilonest pomade"[tw] OR "xilotane gel"[tw] OR xilyne[tw] OR xylcaine[tw] OR xylestesin[tw] OR Xylesthesin[tw] OR xylocain[tw] OR xylocaina[tw] OR xylocaine[tw] OR xylocard[tw] OR xylocitin[tw] OR xyloctin[tw] OR xyloneural[tw] OR xylonor[tw] OR "xyloproct n"[tw] OR xyloton[tw] OR xylotox[tw] OR xylyne[tw] OR zingo[tw] OR ztlido[tw] OR "137‐58‐6"[tw] OR "24847‐67‐4"[tw] OR "56934‐02‐2"[tw] OR "73‐78‐9"[tw]) #10 oxybuprocaine[tw] OR benoxil[tw] OR benoxinate[tw] OR cebesine[tw] OR conjucain[tw] OR conjuncain[tw] OR dorsacain[tw] OR dorsacaine[tw] OR lacrimin[tw] OR novesin[tw] OR novesine[tw] OR oxibuprocainum[tw] OR oxibuprokain[tw] OR oxybucaine[tw] OR prescaina[tw] OR "5987‐82‐6"[tw] OR "99‐43‐4"[tw] #11 cocaine[tw] OR cocain[tw] OR codrenine[tw] OR erythroxylin[tw] OR goprelto[tw] OR locosthetic[tw] OR neurocaine[tw] OR numbrino[tw] OR sterilocaine[tw] OR "50‐36‐2"[tw] OR "53‐21‐4"[tw] OR "5937‐29‐1"[tw] #12 #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 #1 AND #5 AND #12 #14 Medline[sb] #15 #13 NOT #14
Appendix 5. LILACS search strategy
(MH:A09.371.060.217$ OR MH:C11.204$ OR MH:A09.371.060.217.325$ OR MH:A10.272.510$ OR MH:E04.378$ OR MH:E04.540.825$ OR MH:C10.900.300.284.250$ OR MH:C11.297$ OR MH:C26.915.300.425.250$ OR cornea$ OR (ocular surface$) OR (epithelia$ surface$) OR keratectomy$ OR keratoplasty$ OR "cross linking" OR (Eye$ AND (injur$ OR abrasion$ OR erosion$ OR trauma$ OR wound$ OR "foreign body" OR "foreign bodies" OR "epithelial defect" OR lesion$ OR laceration OR surger$ OR surgical))) AND (Amides OR amidas OR MH:D02.065$ OR Esters OR esters OR MH:D02.241.400$ OR MH:SP4.097.036.654$ OR Tetracaine OR Tetracaina OR MH: D02.241.223.100.050.500.968$ OR MH: D02.455.426.559.389.127.020.937.968$ OR "ak‐t‐caine" OR amethocaine OR ametocaine OR ametop OR anetaine OR anethaine OR butethanol OR butethol OR contralgin OR curtacaine OR decicain OR decicaine OR "dextrose‐pontocaine hcl" OR dicain OR dicaine OR fissucain OR gingicain OR intercain OR landocaine OR laudocaine OR meethobalm OR mucaesthin OR niphanoid OR pantocain OR pantocaine OR pontocaine OR rexocaine OR tetocaine OR tetracain OR tetracaine OR tetrakain OR tetrracaine OR tonexol OR uromucaesthin OR "136‐47‐0" OR "94‐24‐6" OR proparacaine OR alcaine OR anestalcon OR "chibro‐kerakain" OR kainair OR keracaine OR miraxil OR "ocu‐caine" OR oftetic OR ophthaine OR ophthetic OR opthetic OR "poen‐caina" OR proparacain OR proporacaine OR proxymetacaine OR "499‐67‐2" OR "5875‐06‐9" OR Lidocaine OR Lidocaina OR MH:D02.065.199.092.500$ OR MH: D02.092.146.113.092.500$ OR akten OR "algrx 3268" OR algrx3268 OR alphacaine OR anestacaine OR anestacon OR anestacone OR aritmal OR betacaine OR cidancaina OR "col 1077" OR col1077 OR "corus 1030" OR corus1030 OR dalcaine OR dentipatch OR dolicaine OR duncaine OR dynexan OR "ela‐max" OR esracain OR esracaine OR farmacaina OR "gesicain jelly" OR "gesicain ointment" OR "gesicain viscous" OR glydo OR gravocain OR isicaine OR jetocaine OR jetokain OR "l‐caine" OR lecasin OR leostesin OR "lida mantle" OR lidbree OR lidocain OR lidocaine OR lidocaton OR lidocor OR lidocorit OR lidoderm OR lidonest OR lidopain OR lidopen OR lidorx OR lidothesin OR lignocaine OR lignostab OR lincaine OR liquocaine OR liris OR "ll 30" OR ll30 OR "lmx 4" OR "lmx 5" OR "lta ii kit" OR maricaine OR "neo novutox" OR neolidocaton OR Octocaine OR otipax OR "paediatric lta kit" OR "pediatric lta kit" OR penles OR radiaguard OR ralvo OR "remicaine gel" OR roxicaina OR rucaina OR ruciana OR solarcaine OR solcaine OR "sp 103" OR sp103 OR truxacaine OR "uad caine" OR vasocaine OR versatis OR xidocaine OR xilina OR xiline OR xilocaina OR "xilonest pomade" OR "xilotane gel" OR xilyne OR xylcaine OR xylestesin OR Xylesthesin OR xylocain OR xylocaina OR xylocaine OR xylocard OR xylocitin OR xyloctin OR xyloneural OR xylonor OR "xyloproct n" OR xyloton OR xylotox OR xylyne OR zingo OR ztlido OR "137‐58‐6" OR "24847‐67‐4" OR "56934‐02‐2" OR "73‐78‐9" OR Oxybuprocaine OR benoxil OR benoxinate OR cebesine OR conjucain OR conjuncain OR dorsacain OR dorsacaine OR lacrimin OR novesin OR novesine OR oxibuprocainum OR oxibuprokain OR oxybucaine OR prescaina OR "5987‐82‐6" OR "99‐43‐4" OR Cocaine OR cocain OR codrenine OR erythroxylin OR goprelto OR locosthetic OR neurocaine OR numbrino OR sterilocaine OR "50‐36‐2" OR "53‐21‐4" OR "5937‐29‐1")
Appendix 6. ClinicalTrials.gov search strategy
((cornea OR corneal OR ocular surface OR ocular epithelial OR ocular epithelium OR keratectomy OR keratoplasty OR "cross linking") OR (eye AND (injury OR abrasion OR erosion OR trauma OR wound OR "foreign body" OR "epithelial defect" OR lesion OR laceration OR surgery OR surgical))) AND (Amide OR ester OR tetracaine OR proparacaine OR lidocaine OR oxybuprocaine OR cocaine)
Appendix 7. WHO ICTRP search strategy
corneal AND amide OR corneal AND ester OR corneal AND tetracaine OR corneal AND proparacaine OR corneal AND lidocaine OR corneal AND oxybuprocaine OR corneal AND cocaine
cornea AND amide OR cornea AND ester OR cornea AND tetracaine OR cornea AND proparacaine OR cornea AND lidocaine OR cornea AND oxybuprocaine OR cornea AND cocaine
eye injury AND amide OR eye injury AND ester OR eye injury AND tetracaine OR eye injury AND proparacaine OR eye injury AND lidocaine OR eye injury AND oxybuprocaine OR eye injury AND cocaine
ocular surface AND amide OR ocular surface AND ester OR ocular surface AND tetracaine OR ocular surface AND proparacaine OR ocular surface AND lidocaine OR ocular surface AND oxybuprocaine OR ocular surface AND cocaine
keratectomy AND amide OR keratectomy AND ester OR keratectomy AND tetracaine OR keratectomy AND proparacaine OR keratectomy AND lidocaine OR keratectomy AND oxybuprocaine OR keratectomy AND cocaine
keratoplasty AND amide OR keratoplasty AND ester OR keratoplasty AND tetracaine OR keratoplasty AND proparacaine OR keratoplasty AND lidocaine OR keratoplasty AND oxybuprocaine OR keratoplasty AND cocaine
Data and analyses
Comparison 1. Anesthetic vs placebo or NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in participant‐reported ocular pain from baseline to 24 hours | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Anesthetic vs placebo, VAS (0 to 10), post‐surgery | 3 | 119 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.76, ‐0.80] |
| 1.1.2 Anesthetic vs placebo, VAS (0 to 10), post‐trauma | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.10, 0.02] |
| 1.1.3 Anesthetic vs NSAID, VAS (0 to 10), post‐surgery | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 0.82 [0.01, 1.63] |
| 1.2 Change in participant‐reported ocular pain from baseline to 48 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Anesthetic vs placebo, VAS (0 to 10), post‐surgery | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.45, 1.27] |
| 1.2.2 Anesthetic vs placebo, VAS (0 to 10), post‐trauma | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐5.68 [‐6.38, ‐4.98] |
| 1.3 Change in participant‐reported ocular pain from baseline to 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Proportion of post‐trauma participants without complete resolution of epithelial defects by 24 to 72 hours ‐ subgroup by tetracaine concentration | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.78, 2.42] |
| 1.4.1 Tetracaine 0.4% | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.29, 22.93] |
| 1.4.2 Tetracaine 0.5% | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.65, 4.27] |
| 1.4.3 Tetracaine 1% | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.53, 2.39] |
| 1.5 Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 24 to 48 hours, post‐trauma | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.78, 2.42] |
| 1.5.2 48 hours or longer, post‐surgery | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.55] |
| 1.6 Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours ‐ subgroup by duration of use (RD) | 5 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 24 to 48 hours, post‐trauma | 3 | 221 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.04, 0.16] |
| 1.6.2 24 to 48 hours, post‐surgery | 1 | 44 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.08, 0.08] |
| 1.6.3 48 hours or longer, post‐surgery | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | ‐0.20 [‐0.42, 0.02] |
| 1.7 Proportion of participants with complications at furthest time point | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 At 1 week, post‐surgery | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.38, 128.02] |
| 1.7.2 At 1 to 2 weeks, post‐trauma | 3 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.23, 5.46] |
| 1.8 Proportion of participants with complications at furthest time point (RD) ‐ subgroup by abrasion type | 7 | 394 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 1.8.1 Post‐surgery | 3 | 119 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.06, 0.11] |
| 1.8.2 Post‐trauma | 4 | 275 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.06] |
1.1. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 1: Change in participant‐reported ocular pain from baseline to 24 hours
1.2. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 2: Change in participant‐reported ocular pain from baseline to 48 hours
1.4. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 4: Proportion of post‐trauma participants without complete resolution of epithelial defects by 24 to 72 hours ‐ subgroup by tetracaine concentration
1.5. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 5: Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours
1.7. Analysis.

Comparison 1: Anesthetic vs placebo or NSAID, Outcome 7: Proportion of participants with complications at furthest time point
Comparison 2. Anesthetic + NSAID vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in participant‐reported ocular pain from baseline to 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Proportion of participants without complete resolution of epithelial defects by 24 to 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Proportion of participants with complications at furthest time point (RD) | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ball 2010.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (1 eye included) Study start date: 10 January 2005 Study end date: 9 January 2006 Participant follow‐up time: 1 week Treatment time: 1 week Time from abrasion to randomization (hours): 24 Power calculation: “We determined that 16 participants in each group would be needed to have an 80% chance of detecting a pain reduction of 2 cm on the visual analog scale between the 2 groups, assuming an α of 0.05, and a standard deviation of 2 cm. We chose 2 cm to represent a clinically meaningful difference based on an informal survey of attending emergency physicians at our hospital.” |
| Participants | Country/countries: Canada Setting: 2 tertiary care emergency departments Inclusion criteria: adult patients with acute (within 24 hours) traumatic corneal injuries Exclusion criteria: immunocompromised, known allergy to local anesthetic, unable to consent/follow instructions for dosing/go to follow‐up appointments, previous ocular pathology Reported a subgroup analyses (Y/N): no Total randomized (n): 43, not reported by group Exclusions and loss to follow‐up (n; reasons): 11, not reported by group; noncompliance with treatment Analyzed (n): 33 Proparacaine 0.05% group: 15 Placebo group: 18 Age (mean ± SD, range): no total Proparacaine 0.05% group: 38.0 (28.0 ± 47.0) Placebo group: 39.3 (27.0 ± 46.0) Gender (number, % female): 5 (15%) Proparacaine 0.05% group: 2 (13%) Placebo group: 3 (17%) Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): not reported Baseline pain (study scale): not reported |
| Interventions |
Interventions proparacaine 0.05% (diluted from proparacaine 0.5%), 2 to 4 drops as needed for 7 days, 40 mL total dispensed
Comparison: "colour‐ and smell‐matched placebo", 2 to 4 drops as needed for 7 days, 40 mL total dispensed
Co‐interventions:
|
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s):
Adverse event(s): ophthalmologist to assess for increased corneal thickness, corneal opacification, new corneal epithelial defects, or any other ocular pathology that could be related to either the initial injury or the use of study medication Measurement time points: all patients attended for follow‐up at an outpatient clinic on days 1, 3, 5, and 7 |
| Notes | Sponsorship source: not reported Conflicts of interest: "none" declared Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Ian Michael Ball Affiliated institution: Divisions of Emergency Medicine and Critical Care Medicine, Department of Medicine, London Health Sciences Centre Trial registration ID: NCT00620997 |
Lim 1999.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (1 eye included) Study start date: November 1997 Study end date: April 1998 Participant follow‐up time: 1 week Treatment time: 1 week Time from abrasion to randomization (hours): 0 Power calculation: not reported |
| Participants |
Country/countries: South Korea
Setting: ophthalmology surgery
Inclusion criteria: excimer laser PRK
Exclusion criteria: pregnant, acute systemic inflammation, hypersensitivity to eye drops, pre‐existing corneal disease, glaucoma, retinal disease, or a history of other ophthalmic surgeries; use of analgesic, systemic, or eye drop steroids within 48 hours before surgery
Reported a subgroup analysis (Y/N): no
Total randomized (n): 45*
Exclusions and loss to follow‐up (n; reasons): not reported
Age (mean ± SD, range): 27.8 (range 20 to 42), not reported by group
Race/ethnicity (study definition, n, %): not reported
Etiology of corneal abrasion (study definition, n, %): PRK (45, 100%)
Gender (number, % female): 75, 71%; not reported by group
Race/ethnicity (study definition, n, %): not reported
Etiology of corneal abrasion (study definition, n, %): PRK (45, 100%)
Baseline pain (study scale):
Proparacaine 0.05% group: not reported
Proparacaine 0.05% + diclofenac 0.1% group: not reported
Artificial tears group: not reported *Data from 60 participants randomized to ineligible comparisons were excluded from this review (15 participants in 4 groups) |
| Interventions |
Intervention 1: proparacaine 0.05% (diluted from Alcaine 0.5%, Alcon), 1 drop every 4 hours for 1 week
Intervention 2: diclofenac I 0.1% (Naclof, Cibar‐Geigy) plus proparacaine 0.05% (diluted from Alcaine 0.5%, Alcon), 1 drop every 4 hours for 1 week
Comparison: artificial tears (Tears Naturale, Alcon), 1 drop every 4 hours for 1 week
Co‐interventions:
Ineligible interventions excluded from this review:
|
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s): not reported Adverse event(s): keratitis or corneal clouding Measurement time points: days 1, 2, 3, 4, and 1 week. All patients were asked to visit every day until the epithelial defect was completely healed after surgery, and they were asked to visit again, 1 week after surgery |
| Notes | Sponsorship source: not reported Conflicts of interest: not reported Informed consent obtained?: unclear Ethics approval obtained?: unclear Investigator's name: Hyun Taek Lim Affiliated institution: Department of Ophthalmology, College of Medicine, University of Ulsan, Asan Medical Center, Seoul, Korea Trial registration ID: not reported |
Montard 1999.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (1 eye included) Study start date: not reported Study end date: not reported Participant follow‐up time: 3 days Treatment time: 24 hours Time from abrasion to randomization (hours): 0 Power calculation: not reported |
| Participants | Country/countries: France Setting: ophthalmology surgery Inclusion criteria: patients undergoing PRK Exclusion criteria: not reported Reported a subgroup analysis (Y/N): Yes; "sex" (male, female), myopia correction (low, medium, strong), previous PRK (first PRK, second eye, retreatment on an eye previously undergone PRK), size of epithelial defect Randomized (n): 74 Tetracaine 1% group: 38 Diclofenac 0.1% group: 36 Exclusions and loss to follow‐up (n): not reported Exclusion reasons: not reported Analyzed (n): not reported Age (mean ± SD, range): mean 30 years old; not reported by group Gender (number, % female): 42, 56.8%; not reported by group Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): PRK (74, 100%); 1st surgery (33), 2nd surgery on the other eye (32), 2nd surgery on the same eye/retreatment (9) Baseline pain: not reported |
| Interventions |
Intervention: tetracaine 1%, every 30 minutes for 24 hours
Comparison: diclofenac 0.1%, 4 times per day for 3 days
Co‐interventions:
|
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s): not reported Adverse event(s): not reported Measurement time points: pain assessed every hour for 30 hours; epithelial healing assessed on the day of surgery, 1 day and 3 days after surgery |
| Notes | Sponsorship source: not reported Conflicts of interest: not reported Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Montard, M Affiliated institution: Ophthalmology Department, Minjoz Hospital, Besançon, France Trial registration ID: not reported |
Oksuz 2006.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (1 eye included) Study start date: not reported Study end date: not reported Participant follow‐up time: 2 days Treatment time: 3 hours Time from abrasion to randomization (hours): 0 Power calculation: not reported |
| Participants | Country/countries: Turkey Setting: eye clinic Inclusion criteria: patients undergoing pterygium surgery Exclusion criteria: glaucoma, previous eye surgery, dementia or mental instability, deafness, hyperanxiety, communication barriers and the inability to complete the VAS Reported a subgroup analysis (Y/N): no Randomized (n): 45 Lidocaine gel 2% group: 23 Artificial tear gel group: 22 Exclusions and loss to follow‐up (n): not reported Exclusion reasons: not reported Analyzed (n): not reported Age (mean ± SD, range): overall not reported Lidocaine gel 2% group: 45.52 ± 9.15 Artificial tear gel group: 47.86 ± 9.74 Gender (number, % female): 20, 44% Lidocaine gel 2% group: 10, 43% Artificial tear gel group: 10, 45% Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): pterygium surgery (45, 100%) Baseline pain (study scale): not reported |
| Interventions | Intervention: lidocaine 2% gel (Xylocaine, AstraZeneca, Mississauga, Canada), 1 mL 1 hour after surgery, and every hour for 3 hours Comparison: artificial tear gel (Thilo‐Tears Jel, Alcon‐Couvreur, Puurs, Belgium), 1 mL 1 hour after surgery, and every hour for 3 hours Co‐interventions: eyes were patched from the very beginning of the operation to the completion of the corneal re‐epithelization |
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s): not reported Adverse event(s): side effects related to study drops, corneal epithelial, or ocular complications Measurement time points: hours 4, 7, 10, 24. Assessment after hour 24 based on incomplete re‐epithelialization, hours 36 and 48 |
| Notes | Sponsorship source: not reported Conflicts of interest: not reported Informed consent obtained?: yes Ethics approval obtained?: unclear Investigator's name: C. Tamer Affiliated institution: Mustafa Kemal University Trial registration ID: not reported |
Shahinian 1997.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (48 eyes of 34 persons) Study start date: not reported Study end date: not reported Participant follow‐up time: 1 week Treatment time: 1 week Time from abrasion to randomization (hours): 0 Power calculation: not reported |
| Participants | Country/countries: Canada; US Setting: ophthalmology surgery (PRK) Inclusion criteria: not reported Exclusion criteria: not reported Reported a subgroup analyses (Y/N): no Randomized (n): 48 eyes (34 patients); randomization unit was person Proparacaine 0.05% group: 25 eyes, number of patients not reported Artificial tears group: 23 eyes, number of patients not reported Exclusions and loss to follow‐up (n): not reported Exclusion reasons: not reported Analyzed (n): 48 eyes Proparacaine 0.05% group: 25 eyes Artificial tears group: 23 eyes Age (mean ± SD, range): not reported Gender (number, % female): not reported Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): PRK (48, 100%) Baseline pain (study scale): not reported |
| Interventions |
Intervention: proparacaine 0.05%, 1 drop 4 times a day for 1 week
Comparison: artificial tears, 1 drop 4 times a day for 1 week
Co‐interventions:
|
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s):
Adverse event(s): "After surgery, patients were observed daily with slit lamp examination until epithelial healing was reached. The epithelial defect size and any adverse effects were noted" Measurement time points: daily for 1 week |
| Notes | Sponsorship source: not reported Conflicts of interest: "Dr. Shahinian holds patent rights to either this or a competing drug. All other authors have no proprietary interest in the development or marketing of this or a competing drug." Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Lee Shahinian Jr Affiliated institution: Stanford University Department of Ophthalmology Trial registration ID: not reported Investigator contact: authors confirmed via email the multiple reports included interim results. We extracted data from the final publication (Shahinian 1997). |
Shipman 2021.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (one eye included) Study start date: 1 May 2015 Study end date: 30 September 2018 Participant follow‐up time: 1 week Treatment time: 24 hours Time from abrasion to randomization (hours): less than 36 hours Power calculation: “Calculations indicated that a sample of approximately 60 patients per group would have 95% power (at the 0.05 level) to detect a minimum clinical difference in pain scores of 1.5 cm on a 10‐cm NRS, given an SD of 2.5 cm” |
| Participants | Country/countries: US Setting: single‐center emergency department Inclusion criteria: 18 years to 80 years old; acute corneal abrasion from mechanical trauma or removal of a foreign body by the physician Exclusion criteria: contact lenses wearer; previous corneal surgery or transplant; more than 36 hours after injury, contaminated or retained foreign body or eye infection; pregnancy; penetrating injury; immunosuppression; allergy to study medication; unable to attend follow‐up; not fluent in English or Spanish; injury requiring urgent ophthalmologic evaluation (large or complicated abrasions with significant vision loss, corneal ulcers or lacerations) Reported a subgroup analyses (Y/N): no Randomized (n): 118 Tetracaine 0.5% group: 59 Artificial tear group: 59 Exclusions and loss to follow‐up (n; reasons): 7; did not attend 24‐ to 48‐hour follow‐up in ED Tetracaine 0.5% group: 3 Artificial tear group: 4 Analyzed (n): 111 Artificial tear group: 55 Tetracaine 0.5% group: 56 Age (median, IQR): overall not reported Tetracaine 0.5% group: 35 (28 to 43) Artificial tear group: 38 (27 to 47) Gender (number, % female): 48, 41% Tetracaine 0.5% group: 23, 39% Artificial tear group: 25, 42% Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): metallic foreign body (13, 11%); other foreign body (32, 27%); direct trauma (31, 26%); unknown (42, 36%) Tetracaine 0.5% group: metallic foreign body (8, 14%); other foreign body (17, 29%); direct trauma (11, 17%); unknown (23, 40%) Artificial tear group: metallic foreign body (5, 9%); other foreign body (15, 25%); direct trauma (20, 34%); unknown (19, 32%) Baseline pain (0 to 10 scale, median, IQR): overall not reported Tetracaine 0.5% group: 7 (6 to 7.5) Artificial tear group: 7 (6 to 8) |
| Interventions |
Intervention: tetracaine hydrochloride 0.5%, 1 drop every 30 minutes as needed up to 24 hours, dispensed in a single 2 mL bottle
Comparison: artificial tears (Systane, Alcon), 1 drop every 30 minutes as needed up to 24 hours, dispensed in 4 x 0.5 mL ampules
Co‐interventions:
|
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s):
Adverse event(s): adverse events at 1 week Measurement time points: baseline (ED, visit 1), 24 to 48 hours (ED, visit 2), 1 week (Ophthalmology, visit 3), chart review of all participants at study conclusion *Secondary outcome in trial registration NCT04187417 but not reported in the published article (Shipman 2021) |
| Notes | Sponsorship source: "The study was funded in part by a grant from the Foundation of Osteopathic Emergency Medicine Young Investigator Award" Conflicts of interest: "no such relationships exist" Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Stacia Shipman Affiliated institution: Department of Emergency Medicine, INTEGRIS Southwest Medical Center, Oklahoma City, OK Trial registration ID: NCT04187417 |
Ting 2009.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (1 eye included) Study start date: 2 May 2006 (anticipated date on trial registry; ACTRN012605000273684) Study end date: 2 May 2007 (calculated based on the reported duration of study) Participant follow‐up time: 2 weeks Treatment time: 36 to 48 hours Time from abrasion to randomization (hours): less than 36 hours; the mean hours from injury in the tetracaine group was 13.8 hours and 15.8 hours in the saline group Power calculation: power and sample size calculations were based on a two‐tailed difference of 25% to 50%, as there has been a large variation in corneal healing rates in previous studies |
| Participants | Country/countries: Australia Setting: emergency department of an urban hospital Inclusion criteria: traumatic superficial corneal abrasion with or without a retained foreign body, or keratitis from welding flash exposure Exclusion criteria: over 36 hours since corneal injury, under 18 years old, known adverse reaction to study medications, eye disease other than refractive error, contact lens use, pregnant or lactating, eye infection, functionally one‐eyed, requires urgent referral to ophthalmology (penetrating eye injury) Reported a subgroup analysis (Y/N): no Randomized (n): 47 Tetracaine 0.4% group: 22 Saline group: 25 Exclusions and loss to follow‐up (n; reasons): 31; did not attend follow‐up (22); retained foreign body/rust (2); data collected outside of follow‐up window (7) Tetracaine 0.4% group: 15; did not attend follow‐up (11); retained foreign body/rust (1); data collected outside of follow‐up window (3) Saline group: 16; did not attend follow‐up (11); retained foreign body/rust (1); data collected outside of follow‐up window (4) Analyzed (n): 16 Tetracaine 0.4% group: 7 Saline group: 9 Age (mean): overall not reported Tetracaine 0.4% group: 35.1 Saline group: 33.6 Gender (number, % female): 0 (0%) Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): corneal abrasion (15, 32%), corneal foreign body (20, 43%), welding flash burn (10, 21%), welding flash burn and corneal foreign body (2, 4%) Tetracaine 0.4% group: corneal abrasion (8, 36%), corneal foreign body (9, 41%), welding flash burn (4, 18%), welding flash burn and corneal foreign body (1, 5%) Saline group: corneal abrasion (7, 28%), corneal foreign body (11, 44%), welding flash burn (6, 24%), welding flash burn and corneal foreign body (1, 4%) Baseline pain (study scale): not reported |
| Interventions |
Intervention: amethocaine (tetracaine) 0.4%, 1 drop hourly as needed
Comparison: normal saline 0.9%, 1 drop hourly as needed
Co‐interventions:
*Not all participants received antibiotics. Discharged with topical antibiotics for subset of participants (8/22 amethocaine; 8/18 saline group) |
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s):
Adverse event(s): significant functional or clinical adverse Measurement time points: baseline, 36‐ to 48‐hour ED visit, 2‐week telephone interview |
| Notes | Sponsorship source: listed source of support listed differs between reports. Funding source name: Mater Foundation in trial registry (ACTRN012605000273684); no source of support reported in the full‐text publication (Ting 2009) Conflicts of interest: "none" declared Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Joseph Ting Affiliated institution: Department of Emergency Medicine, Mater Adults' Hospital, South Brisbane, Australia Trial registration ID: ACTRN012605000273684 |
Verma 1995.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (whether one or both eyes were included in the analysis was unclear) Study start date: not reported Study end date: not reported Participant follow‐up time: 6 months; pain scores were recorded for the first 4 days Treatment time: 24 hours Time from abrasion to randomization (hours): 0 Power calculation: "A total of 44 patients were recruited, which was deemed an appropriate sample size to give statistically significant results." |
| Participants | Country/countries: UK Setting: ophthalmology surgery Inclusion criteria: mean refraction diopters (D) at ‐3.00 ± 1.00 or at ‐6.00 ± 1.00, astigmatism (D) < −1.5, visual acuity > 20/30, age > 24 years Reported subgroups (if applicable): by mean preoperative refractive error. Group 1: Mean preoperative refractive error of ‐3.00 D (range, −2.75 to ‐4.00 D; 22 patients, 16 women and 6 men; age range, 26 to 54 years). Group 2: ‐6.00 D (range, ‐5.75 to ‐8.62 D; 22 patients, 13 women and 9 men; age range, 25 to 72 years) Randomized (n): 44 Tetracaine 1% group: 22 Physiologic saline group: 22 Exclusions and loss to follow‐up (n): not reported Exclusion reasons: not reported Analyzed (n): not reported Age (mean ± SD, range): range 25 to 72 years, not reported by group Gender (number, % female): 29 (66 %), not reported by group Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): PRK (44, 100%) Baseline pain (study scale): not reported |
| Interventions |
Intervention: tetracaine 1% without preservatives, 1 drop every 30 minutes during waking hours over 24 hours, dispensed 4 containers (40 drops)
Comparison: physiologic saline, 1 drop every 30 minutes during waking hours over 24 hours, dispensed 4 containers (40 drops)
Co‐interventions:
Other treatments: preoperative: 1 drop of 4% pilocarpine and 4 drops of 1% tetracaine instilled onto the cornea. Immediate postoperative: patients received mydriatic drops (2% homatropine and 10% phenylephrine) |
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s):
Adverse event(s): objective and subjective measurements of haze, glare, or halo; alteration in night vision postoperatively Measurement time points: baseline, daily until corneal full closure, subsequently at 1 week, and at 1, 3, and 6 months |
| Notes | Sponsorship source: "Iris Fund for the Prevention of Blindness research fellowship (Dr. Verma); Dr. Corbett received the Williams fellowship for medical and scientific research of the University of London, London, England. Prof. Marshall is a consultant to Summit Technology." Conflicts of interest: "Prof. Marshall is a consultant to Summit Technology" Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Seema Verma Affiliated institution: Department of Ophthalmology, St. Thomas' Hospital, London UK Trial registration ID: not reported |
Waldman 2014.
| Study characteristics | |
| Methods | Study design: parallel RCT Unit of randomization: individual (1 eye per person included in study) Study start date: 1 November 2011 Study end date: 31 October 2012 Participant follow‐up time: 1 month (telephone check) Treatment time: 24 hours Time from abrasion to randomization (hours): less than 36 hours Power calculation: “Allowing for a 30% dropout rate identified in prior studies, this would provide 126 patients or two groups of 63 patients. The binomial probability confidence interval (CI) states that the chance of not seeing any complications specifically attributed to tetracaine use in 63 patients would be 0% to 5.7% at the 95% CI. A sample of 63 patients per group would also have 95% power (at the 0.05 level) to detect a minimum clinical difference in pain scores of 16 mm, on a 100‐mm VAS, given a standard deviation of about 25 mm.” |
| Participants |
Country/countries: New Zealand
Setting: emergency department
Inclusion criteria: presented to emergency within 36 hours of injury, simple acute corneal abrasions from mechanical trauma, ultraviolet light, foreign body, or from removal of foreign body by the physician
Exclusion criteria: under 18 years old (one 17‐year‐old patient enrolled with parental consent), previous eye surgery or cataracts, wear contact lenses, injured both eyes, infectious or chemical conjunctivitis, grossly contaminated foreign body, ocular infection, allergic to study drugs, injury requiring urgent ophthalmologic evaluation (e.g. penetrating eye injuries, large or complicated corneal abrasions, or injuries causing a significant disruption of vision), deaf*, were unable to attend follow‐up in 48 hours*
Reported a subgroup analyses (Y/N): no
Randomized (n): 122
Tetracaine 1% group: 61
Saline group: 61
Exclusions and loss to follow‐up (n; reasons): 29; subsequently removed due to incorrect enrollment (n = 6, conjunctivitis (n = 2), chronic defect from eye surgery (n = 1), and large corneal lacerations (n = 3)). Excluded due to retained rust rings (n = 23)
Tetracaine 1% group: 14; subsequently removed due to incorrect enrollment (n = 4). Excluded due to retained rust rings (n = 10)
Saline group: 15; subsequently removed due to incorrect enrollment (n = 2). Excluded due to retained rust rings (n = 13)
Analyzed (n): 93
Tetracaine 1% group: 47
Saline group: 46
Age (median, range): overall not reported
Tetracaine 1% group: 37 (17 to 72)
Saline group: 38 (19 to 74)
Gender (number, % female): 12 (10%)
Tetracaine 1% group: 4 (6.8%)
Saline group: 8 (14%)
Race/ethnicity (study definition, n, %): not reported
Etiology of corneal abrasion (study definition, n, %): metallic foreign body (60, 51.7%), dirt foreign body (6, 5.2%), dust foreign body (15, 12.9%), wood foreign body (7, 6.0%), direct trauma (15, 12.9%), ultraviolet (3, 2.6%), unknown (8, 6.9%), other (2, 1.7%)
Tetracaine 1% group: metallic foreign body (30, 50.8%), dirt foreign body (1, 1.7%), dust foreign body (7, 11.9%), wood foreign body (5, 8.5%), direct trauma (8, 13.6%), ultraviolet (3, 5.1%), unknown (5, 5.8%), other (0, 0%)
Saline group: metallic foreign body (30, 52.6%), dirt foreign body (5, 8.8%), dust foreign body (8, 14.0%), wood foreign body (2, 3.5%), direct trauma (7, 12.3%), ultraviolet (0, 0%), unknown (3, 3.5%), other (3, 3.5%)
Baseline pain (study scale; median, range): overall not reported
Tetracaine 1% group: 0 to 100mm VAS; 48.0 mm (0 to 96 mm)
Saline group: 0 to 100 mm VAS; 54.6 mm (10 to 98 mm) *Exclusion not in trial registration (ACTRN12611000448943); exclusion added in published report (Waldman 2014) |
| Interventions |
Intervention: preservative‐free tetracaine hydrochloride 1%, taken as often as every 30 minutes for 24 hours, dispensed 1.5 mL (3 x 0.5 mL commercially available vials; approximately 50 drops total)*
Comparison: saline 0.9%, taken as often as every 30 minutes for 24 hours, dispensed 5 mL (one, single‐use plastic bullet)
Co‐interventions
*Trial registration specified 2 x 0.5 mL minims, approximately 30 drops (ACTRN12611000448943); 1.5 mL, approximately 50 drops in published report (Waldman 2014) |
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s):
Adverse event(s): delayed healing, enlarged abrasion, recurrent corneal ulceration, toxic keratitis, surface keratopathy, corneal storm infiltration, Candidal and bacterial keratitis, or uveitis Measurement time points: baseline, 48‐hour Emergency Department follow‐up, 1 week and 1 month telephone interview follow‐up |
| Notes | Sponsorship source: "There was no funding for the study, no sponsorship or involvement with the drug manufacturer, or other financial support." Conflicts of interest: "The authors have no conflicts of interest to report." Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Neil Waldman Affiliated institution: Emergency Department, Quality Risk and Education Unit, Southland Hospital, Invercargill, New Zealand Trial registration ID: ACTRN12611000448943 Trial discontinued: "The original trial registration was approved for 180 participants over a period of 6 months. Lower‐than‐expected recruitment rates resulted in an extension to the study to 1 year. After 1 year the trial was discontinued after recruiting 116 patients, rather than asking for an additional extension to recruit 10 more patients to reach the target of 126." Contacted authors: study authors provided raw data for VAS pain scores at 24 hours and 48 hours |
BCLs: bandage contact lenses CI: confidence interval D: diopters ED: emergency department ITT: intention‐to‐treat IV: inverse variance IQR: interquartile range MD: mean difference M‐H: Mantel‐Haenszel NSAID: nonsteroidal anti‐inflammatory drug NRS: numeric rating scale OR: odds ratio PRK: photorefractive keratectomy RCT: randomized controlled trial RR: risk ratio SD: standard deviation SE: standard error SMD: standardized mean difference VAS: visual analog scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2001 | Ineligible population: trial excluded participants with epithelial defects |
| Badalà 2004 | Ineligible intervention: no amide or ester anesthetic |
| Carruthers 1995 | Ineligible population: no participants with corneal abrasion |
| Castrén 1963 | Ineligible population and indication: participants under 18 years old, anesthetic used for foreign body removal |
| Chatziralli 2010 | Ineligible population: pre‐operative participants only |
| Cherry 1996 | Ineligible study design: non‐randomized trial |
| Ferreira 1992 | Ineligible population: pre‐operative participants only |
| Filippone 1967 | Ineligible population: no participants with corneal abrasion |
| Henrotte 1972 | Ineligible study design: case series |
| Kirwan 2008 | Ineligible intervention: anesthetics only administered once following surgery |
| NCT02483897 | Ineligible study: withdrawn because of "inability to recruit at required rate"; we confirmed with the investigators that no data were available |
| NCT02771392 | Ineligible study: trial never started and there was no enrollment; we confirmed with the investigators |
| NCT04283331 | Ineligible intervention: impregnated bandage soft contact lens, no topical anesthetic |
| Steiner 1966 | Ineligible population: randomized intervention study on animal models |
| Verma 1997 | Ineligible comparison: no non‐amide or non‐ester control group |
| Weindler 2001 | Ineligible population: pre‐operative participants only |
Characteristics of studies awaiting classification [ordered by study ID]
Aseff 1997.
| Methods | Study design: not reported Unit of randomization: not reported Study start date: not reported Study end date: not reported Participant follow‐up time: 3 days Treatment time: not reported Time from abrasion to randomization (hours): 0 Power calculation: not reported |
| Participants | Country/countries: Mexico Setting: ophthalmology surgery Randomized (n): 60 eyes Exclusions and loss to follow‐up (n): not reported Exclusion reasons: not reported Analyzed (n): not reported Age (mean ± SD, range): not reported Gender (number, % female): not reported Race/ethnicity (study definition, n, %): not reported Etiology of corneal abrasion (study definition, n, %): not reported Baseline pain: not reported Inclusion criteria: not reported Exclusion criteria: not reported Reported a subgroup analysis (Y/N): not reported |
| Interventions | Intervention: tetracaine Comparison: diclofenac |
| Outcomes |
Primary study outcome(s):
Secondary study outcome(s): not reported Adverse event(s): not reported Measurement time points: 24 and 72 hours |
| Notes | Sponsorship source: not reported Conflicts of interest: not reported Informed consent obtained?: yes Ethics approval obtained?: yes Investigator's name: Aseff A Affiliated institution: Department of Ophthalmology, Instituto Tecnologico y de Estudios Superiores de Monterrey‐Hospital San Jose, Monterrey, NL Address: Monterrey, NL, Mexico Trial registration ID: not reported Notes: we could not confirm the study design with the authors despite several attempts to contact them |
Differences between protocol and review
Types of interventions We expanded the intervention to allow for comparisons of anesthetics plus a second topical treatment (NSAIDs) with control.
Measures of treatment effect We also calculated the risk difference (RD) and 95% CI for dichotomous outcomes when an included study reported zero events in either comparison arm. We felt that the studies with zero events in both arms were informative, even if the baseline risk may differ across study populations.
Subgroup analysis
Subgroup analysis by gender or race was not performed due to the lack of stratified data reported.
Subgroup analysis by frequency of use (2 to 3 times versus ≥ 4 times per day) was not performed because all included trials examined the intervention with a dosing frequency at ≥ 4 times per day.
Summary of findings and assessment of the certainty of the evidence
We reported both safety outcomes rather than choosing only one based on data availability because both outcomes are clinically important.
We reported risks of adverse events per person, rather than per person‐time as planned in the protocol because not all included trials reported itemized adverse events.
We clarified the use of the study‐level risk of bias assessment for GRADE using domains 1 to 3 of the RoB 2 tool in the methods section.
Contributions of authors
All review authors contributed to the conception and design of the review, participated in study selection, data extraction, and/or analysis, drafted portions of the review, commented on drafts critically regarding intellectual content, and approved the final version for publication.
Sources of support
Internal sources
-
None, Other
No internal source of support.
External sources
-
National Eye Institute, National Institutes of Health, USA, USA
Cochrane Eyes and Vision US Project, supported by grant UG1EY020522 (PI: Tianjing Li, MD, MHS, PhD)
-
Queen’s University Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision’s work, is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland (ended in April 2023)
-
Public Health Agency, UK
The HSC Research and Development (R&D) Division of the Public Health Agency funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast (ended in April 2023)
Declarations of interest
Michael Sulewski: declared that he has no conflict of interest.
Cristos Ifantides: reported ownership of stock in Pfizer and consulting fees from Alcon. His partner works for AbbVie, a manufacturer of eye medications, but the partner does not work in the eye care space.
Louis Leslie: reported a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.
Kyongjin Cho: declared that he has no conflict of interest.
Su‐Hsun Liu: reported a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.
Irene C Kuo: reported consulting fees from Okogen (one‐time discussion re: therapy/structuring trials for treatment of adenoviral conjunctivitis) and Novan (one‐time phone conversation re: therapy for adenoviral conjunctivitis); personal payments.
Edited (no change to conclusions)
References
References to studies included in this review
Ball 2010 {published data only}
- Ball IM, Seabrook J, Desai N, Allen L, Anderson S. Dilute proparacaine for the management of acute corneal injuries in the emergency department. Canadian Journal of Emergency Medicine 2010;12(5):389‐96. [DOI] [PubMed] [Google Scholar]
- NCT00620997. Proparacaine vs placebo for corneal injuries. clinicaltrials.gov/show/NCT00620997 (first received 22 February 2008).
Lim 1999 {published data only}
- Lim HT, Kim YK, Tchah HW. Efficacy of topical NSAIDs and anesthetic drug in reducing post-PRK pain: comparative study [PRK 후 통증치료에 대한 점안 비스테로이드성 항염증약제들과 점안 마취제의 효과 비교]. Journal of the Korean Ophthalmological Society 1999;40(1):61‐9. [Google Scholar]
Montard 1999 {published data only}
- Montard M, Chopin C, Delbosc B, Muhieddine M. Tetracaine versus diclofenac in the treatment of pain following refractive photokeratectomy [Tétracaïne versus diclofénac dans le traitement de la douleur après photokératectomie réfractive]. Journal Francais d’Ophtalmologie 1999;22(1):14‐20. [PubMed] [Google Scholar]
Oksuz 2006 {published data only}
- Oksuz H, Tamer C. Pain relief after pterygium surgery with viscous lidocaine. Ophthalmologica 2006;220(5):323‐6. [DOI] [PubMed] [Google Scholar]
Shahinian 1997 {published data only}
- Jager R, Jain S, Sanislo S, Ackerman J, Miller, Shahinian L. The effect of topical dilute proparacaine on relief of postoperative PRK pain. Investigative Ophthalmology and Visual Science 1996;37:ARVO Abstract 2650. [Google Scholar]
- Shahinian L, Jain S, Jager RD, Lin DT, Sanislo SS, Miller JF. Dilute topical proparacaine for pain relief after photorefractive keratectomy. Ophthalmology 1997;104(8):1327‐32. [DOI] [PubMed] [Google Scholar]
- Shahinian L, Jain S, Jager RD, Sanislo SR, Lin DT, Miller JF. Dilute topical proparacaine for pain control following photorefractive keratectomy (PRK). In: American Academy of Ophthalmology Annual Meeting; 1996 Oct 27-31; Chicago (IL). 1996:116. [DOI] [PubMed]
Shipman 2021 {published data only}
- Garrison L, Shipman S, Keuchel M, Painter K. Short term topical tetracaine is safe and highly efficacious for the treatment of pain caused by corneal abrasions: a double-blind, randomized clinical trial. Annals of Emergency Medicine 2019;74(4):S100. [DOI] [PubMed] [Google Scholar]
- NCT04187417. Short term topical tetracaine is safe and highly efficacious for the treatment of pain caused by corneal abrasions. clinicaltrials.gov/show/NCT04187417 (first received 5 December 2019).
- Shipman S, Painter K, Keuchel M, Bogie C. Short-term topical tetracaine is highly efficacious for the treatment of pain caused by corneal abrasions: a double-blind, randomized clinical trial. Annals of Emergency Medicine 2021;77(3):338‐44. [DOI] [PubMed] [Google Scholar]
Ting 2009 {published data only}
- ACTRN12605000273684. A comparison of topical anaesthesia with placebo for the managment of minor corneal trauma [Topical anaesthesia (does / does not) delay re-epithelialisation after minor corneal trauma]. anzctr.org.au/ACTRN12605000273684.aspx (first received 31 August 2005).
- Ting JY, Barns KJ, Holmes JL. Management of Ocular Trauma in Emergency (MOTE) trial: a pilot randomized double-blinded trial comparing topical amethocaine with saline in the outpatient management of corneal trauma. Journal of Emergencies, Trauma, and Shock 2009;2(1):10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verma 1995 {published data only}
- Verma S, Corbett MC, Marshall J, Hamilton PAM. Pain control after PRK: do topical anesthetics have a role? American Academy of Ophthalmology 1995;1:102. [DOI] [PubMed] [Google Scholar]
- Verma S, Corbett MC, Marshall J. A prospective, randomized, double-masked trial to evaluate the role of topical anesthetics in controlling pain after photorefractive keratectomy. Ophthalmology 1995;102(12):1918‐24. [DOI] [PubMed] [Google Scholar]
- Verma S, Corbett MC, Marshall J. Topical anaesthesia and pain control after PRK: visual performance and cellular correlates. Investigative Ophthalmology and Visual Science 1995;36:ARVO Abstract 4892. [Google Scholar]
Waldman 2014 {published and unpublished data}
- ACTRN12611000448943. A prospective, randomized, double-blind trial to evaluate the role of topical anaesthetics in controlling pain after corneal abrasion in the emergency department. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12611000448943 (first received 5 March 2011).
- Pruet CM, Feldman RM, Kim G. Re: "Topical tetracaine used for 24 hours is safe and rated highly effective by patients for the treatment of pain caused by corneal abrasions: a double-blind, randomized clinical trial". Academic Emergency Medicine 2014;21(9):1062. [DOI] [PubMed] [Google Scholar]
- Tijunelis M, Tozer K. Good study design, but flawed conclusion in emergency department tetracaine use. Academic Emergency Medicine 2014;21(11):1302-3. [DOI] [PubMed] [Google Scholar]
- Waldman N, Densie IK, Herbison P. Topical tetracaine used for 24 hours is safe and rated highly effective by patients for the treatment of pain caused by corneal abrasions: a double-blind, randomized clinical trial. Academic Emergency Medicine 2014;21(4):374‐82. [DOI] [PubMed] [Google Scholar]
- Waldman N, Densie IK. Good study design, but flawed conclusion in emergency department tetracaine use. In reply. Academic Emergency Medicine 2014;21(11):1304-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2001 {published data only}
- Ahmed II, Breslin CW. Role of the bandage soft contact lens in the postoperative laser in situ keratomileusis patient. Journal of Cataract and Refractive Surgery 2001;27(12):1932‐6. [DOI] [PubMed] [Google Scholar]
Badalà 2004 {published data only}
- Badalà F, Fioretto M, Macrì A. Effect of topical 0.1% indomethacin solution versus 0.1% fluoromeholon acetate on ocular surface and pain control following laser subepithelial keratomileusis (LASEK). Cornea 2004;23(6):550-3. [DOI] [PubMed] [Google Scholar]
Carruthers 1995 {published data only}
- Carruthers JD, Sanmugasunderan S, Mills K, Bagaric D. The efficacy of topical corneal anesthesia with 0.5% bupivacaine eyedrops. Journal Canadien d’Ophtalmologie [Canadian Journal of Ophthalmology] 1995;30(5):264‐6. [PubMed] [Google Scholar]
Castrén 1963 {published data only}
- Castrén JA, Lavikainen P. Experiences with a new surface anesthetic. Acta Ophthalmologica 1963;41(6):702-7. [DOI] [PubMed] [Google Scholar]
Chatziralli 2010 {published data only}
- Chatziralli IP, Papazisis L, Sergentanis TN. Lidocaine 2% jelly versus lidocaine 2%-sodium hyaluronate 0.3% drops in phacoemulsification surgery. Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie [Graefe's archive for clinical and experimental ophthalmology] 2010;248(1):149‐50. [DOI] [PubMed] [Google Scholar]
Cherry 1996 {published data only}
- Cherry PM. The treatment of pain following excimer laser photorefractive keratectomy: additive effect of local anesthetic drops, topical diclofenac, and bandage soft contact. Ophthalmic Surgery Lasers 1996;27(5 Suppl):S477-80. [PubMed] [Google Scholar]
Ferreira 1992 {published data only}
- Ferreira AA, Horta AMS, Gomes MI, Luz CAB. Analgesia postoperative in ophthalmology: subconjuntival bupivacaine [Analgesia pós-operatória em oftalmologia: bupivacaína subconjuntival]. Arquivos do Instituto Penido Burnier 1992;34(2):106-9. [Google Scholar]
Filippone 1967 {published data only}
- Filippone C, Molinelli G. Comparative trials of corneal anesthesia, using instillation of an 0.5-percent solution of Rec 7-0518 as compared with a 4-percent solution of cocaine. Clinico-statistical study [Sperimentazione comparativa sull'anestesia corneale da instillazione tra una soluzione di Rec 7-0518 allo 0,5 percent ed una soluzione di cocaina al 4-percent. Studio clinico-statistico]. Annali di Ottalmologia e Clinica Oculistica 1967;93(2):212-21. [PubMed] [Google Scholar]
Henrotte 1972 {published data only}
- Henrotte J, Weekers JF. Clinical study of corneal lesions due to local and prolonged application of anesthetics [Etude clinique des lésions cornéennes dues à l'application locale et prolongée d'anesthésiques]. Archives d’Ophtalmologie et Revue Générale d’Ophtalmologie 1972;32(6):449-56. [PubMed] [Google Scholar]
Kirwan 2008 {published data only}
- Kirwan C, Mulqueen C, O'Keefe M. A double-blind randomized control study to determine the effect of Visthesia viscoelastic substance on pain following LASEK. Ophthalmologica 2008;222(4):229‐31. [DOI] [PubMed] [Google Scholar]
NCT02483897 {published data only}
- NCT02483897. Effect of tetracaine on pain management and corneal healing in patients with acute corneal abrasion. clinicaltrials.gov/show/NCT02483897 (first received 29 June 2015).
NCT02771392 {published data only}
- NCT02771392. Treatment of corneal abrasions with topical tetracaine: an evaluation of safety and efficacy. clinicaltrials.gov/show/NCT02771392 (first received 13 May 2016).
NCT04283331 {published data only}
- NCT04283331. Anesthetic impregnated bandage soft contact lens (BSCL) in pain management after photorefractive keratectomy (PRK). clinicaltrials.gov/show/NCT04283331 (first received 25 February 2020).
Steiner 1966 {published data only}
- Steiner L. Clinical experimental studies of the effects of various local anesthetics on the cornea [Klinisch-experimentelle Untersuchungen über die Wirkung einiger Oberflächenanästhetika auf die Hornhaut]. Klinische Monatsblätter für Augenheilkunde 1966;148(4):536-40. [PubMed] [Google Scholar]
Verma 1997 {published data only}
- Verma S, Corbett M, Patmore A, Heacock G, Marshall J. Topical anaesthesia and pain control after PRK: bupivacaine 0.75% vs amerthocaine 1%. Investigative Ophthalmology and Visual Science 1996;37:ARVO Abstract 919. [Google Scholar]
- Verma S, Corbett MC, Patmore A, Heacock G, Marshall J. A comparative study of the duration and efficacy of tetracaine 1% and bupivacaine 0.75% in controlling pain following photorefractive keratectomy (PRK). European Journal of Ophthalmology 1997;7(4):327‐33. [DOI] [PubMed] [Google Scholar]
Weindler 2001 {published data only}
- Weindler J. Cocaine eye drops or lidocaine-gel in addition to intra-chamber anesthesia in clear-corneal cataract surgery [Cocain-Augentropfen oder Lidocain-Gel zur Ergänzung der intrakameralen Anaesthesie bei der Clear-Cornea-Kataraktchirurgie?]. Ophthalmologe 2001;98(Suppl 1):S189. [Google Scholar]
References to studies awaiting assessment
Aseff 1997 {published data only}
- Aseff A, Valdez J, Vargas J, Vidaurri J. Wound healing and patient discomfort after excimer photorefractive keratectomy with topical diclofenac vs topic tetracaine. Investigative Ophthalmology and Visual Science 1997;38:ARVO Abstract 2465. [Google Scholar]
Additional references
Ahmed 2015
- Ahmed F, House RJ, Feldman BH. Corneal abrasions and corneal foreign bodies. Primary Care 2015;42(3):363-75. [DOI] [PubMed] [Google Scholar]
Aksoy 2013
- Aksoy A, Başkan AM, Aslan L, Aslankurt M. Topical proparacaine abuse resulting in evisceration. BMJ Case Reports 2013;2013:bcr2013009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Algarni 2022
- Algarni AM, Guyatt GH, Turner A, Alamri S. Antibiotic prophylaxis for corneal abrasion. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD014617. [DOI: 10.1002/14651858.CD014617.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansari 2006
- Ansari H, Garibaldi DC, Jun AS. Anaesthetic abuse keratopathy as a manifestation of ocular Munchausen's syndrome. Clinical and Experimental Ophthalmology 2006;34(1):81-3. [DOI: 10.1111/j.1442-9071.2006.01152.x] [DOI] [PubMed] [Google Scholar]
Ardjomand 2002
- Ardjomand N, Faschinger C, Haller-Schober EM, Scarpatetti M, Faulborn J. A clinico-pathological case report of necrotizing ulcerating keratopathy due to topical anaesthetic abuse [Nekrotisierende ulzerierende Keratopathie nach Lokalanasthetikamissbrauch Ein klinisch-pathologischer Fallbericht]. Ophthalmologe 2002;99(11):872-5. [DOI: 10.1007/s00347-002-0623-z] [DOI] [PubMed] [Google Scholar]
Boljka 1994
- Boljka M, Kolar G, Vidensek J. Toxic side effects of local anaesthetics on the human cornea. British Journal of Ophthalmology 1994;78(5):386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Booker 2023
- Booker SQ, Baker TA, Esiaka D, Minahan JA, Engel IJ, Banerjee K, et al. A historical review of pain disparities research: advancing toward health equity and empowerment. Nursing Outlook 2023 Apr 4 [Epub ahead of print];71(3):101965. [DOI: 10.1016/j.outlook.2023.101965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brinck 2018
- Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD012033. [DOI: 10.1002/14651858.CD012033.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burcu 2013
- Burcu A, Dogan E, Yalniz-Akkaya Z, Ornek F. Early amniotic membrane transplantation for toxic keratopathy secondary to topical proparacaine abuse: a report of seven cases. Cutaneous and Ocular Toxicology 2013;32(3):241-7. [DOI: 10.3109/15569527.2012.759959] [DOI] [PubMed] [Google Scholar]
Campbell 2012
- Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Management 2012;2(3):219-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2022
- Chang BH, Ewald MD, Groos EB. Neurotrophic keratitis. In: Mannis MJ, Holland EJ, editors(s). Cornea: Fundamentals, Diagnosis, and Management. 5th edition. Amsterdam (NL): Elsevier, 2022. [Google Scholar]
Channa 2016
- Channa R, Zafar SN, Canner JK, Haring RS, Schneider EB, Friedman DS. Epidemiology of eye-related emergency department visits. JAMA Ophthalmology 2016;134(3):312-9. [DOI] [PubMed] [Google Scholar]
Chen 2004
- Chen HT, Chen KH, Hsu WM. Toxic keratopathy associated with abuse of low-dose anesthetic: a case report. Cornea 2004;23(5):527-9. [DOI: 10.1097/01.ico.0000114127.63670.06] [DOI] [PubMed] [Google Scholar]
Chern 1996
- Chern KC, Meisler DM, Wilhelmus KR, Jones DB, Stern GA, Lowder CY. Corneal anesthetic abuse and candida keratitis. Ophthalmology 1996;103(1):37-40. [DOI] [PubMed] [Google Scholar]
Chou 2020
- Chou R, Wagner J, Ahmed AY, Blazina I, Brodt E, Buckley DI, et al. Treatments for acute pain: a systematic review. Comparative Effectiveness Review No. 240. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290- 2015-00009-I.) AHRQ Publication No. 20(21)-EHC006. Rockville, MD: Agency for Healthcare Research and Quality; December 2020. [DOI: 10.23970/AHRQEPCCER240] [DOI] [PubMed]
Chuck 2018
- Chuck RS, Jacobs DS, Lee JK, Afshari NA, Vitale S, Shen TT, et al. Refractive errors & refractive surgery Preferred Practice Pattern®. Ophthalmology 2018;125(1):P1-P104. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 28 July 2021. Available at covidence.org.
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Dornic 1998
- Dornic DI, Thomas JM, Lass JH. Topical diclofenac sodium in the management of anesthetic abuse keratopathy. American Journal of Ophthalmology 1998;125(5):719-21. [DOI: 10.1016/s0002-9394(98)00009-9] [DOI] [PubMed] [Google Scholar]
Epstein 1968
- Epstein DL, Paton D. Keratitis from misuse of corneal anesthetics. New England Journal of Medicine 1968;279(8):396-9. [DOI] [PubMed] [Google Scholar]
Garcia 2016
- Garcia R, Andrade DC, Teixeira MJ, Nozaki SS, Bechara SJ. Mechanisms of corneal pain and implications for postoperative pain after laser correction of refractive errors. Clinical Journal of Pain 2016;32(1):450-8. [DOI] [PubMed] [Google Scholar]
Garcia‐Ferrer 2019
- Garcia-Ferrer FJ, Akpek EK, Amescua G, Farid M, Lin A, Rhee MK, et al. Corneal Ectasia Preferred Practice Pattern®. Ophthalmology 2019;126(1):P170-215. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Golan 2018
- Golan O, Randleman JB. Pain management after photorefractive keratectomy. Current Opinion in Ophthalmology 2018;29:306-12. [DOI] [PubMed] [Google Scholar]
Hamill 2022
- Hamill MB. Mechanical injury. In: Mannis MJ, Holland EJ, editors(s). Cornea: Fundamentals, Diagnosis, and Management. 5th edition. Amsterdam (NL): Elsevier, 2022. [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2021c
- Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2022a
- Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Higgins 2022b
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Katsimpris 2007
- Katsimpris JM, Sarantoulakou M, Kordelou A, Petkou D, Petropoulos IK. Clinical findings in patients with topical anaesthetic abuse keratitis: a report of five cases. Klinische Monatsblatter für Augenheilkunde 2007;224(4):303-8. [DOI: 10.1055/s-2007-962933] [DOI] [PubMed] [Google Scholar]
Khakshoor 2012
- Khakshoor H, Moshirfar M, Simpson RG, Gharaee H, Vejdani AH, Christiansen SM, et al. Anesthetic keratopathy presenting as bilateral Mooren-like ulcers. Clinical Ophthalmology 2012;6:1719-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 1997
- Kim JY, Choi YS, Lee JH. Keratitis from corneal anesthetic abuse after photorefractive keratectomy. Journal of Cataract and Refractive Surgery 1997;23(3):447-9. [DOI: 10.1016/s0886-3350(97)80192-7] [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee JK, Stark WJ. Anesthetic keratopathy after photorefractive keratectomy. Journal of Cataract and Refractive Surgery 2008;34(10):1803-5. [DOI] [PubMed] [Google Scholar]
Lee 2019
- Lee MD, Driver TH, Seitzman GD. Cornea specialists do not recommend routine usage of topical anesthetics for corneal abrasions. Annals of Emergency Medicine 2019;74(3):463-6. [DOI] [PubMed] [Google Scholar]
Letzen 2022
- Letzen JE, Mathur VA, Janevic MR, Burton MD, Hood AM, Morais CA, et al. Confronting racism in all forms of pain research: reframing study designs. Journal of Pain 2022;23(6):893-912. [DOI: 10.1016/j.jpain.2022.01.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2017
- Levine LM, Brar VS, Goldstein MH, Kahana A, Katowitz MR, Law SK, et al. Ocular pharmacokinetics. In: Cantor LB, Rapuano CJ, Cioffi GA, editors(s). Basic and Clinical Science Course: Fundamental and Principles of Ophthalmology. American Academy of Ophthalmology, 2017. [Google Scholar]
Li 2022
- Li T, Higgins JPT, Deeks JJ. Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Lim 2016
- Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD004764. [DOI: 10.1002/14651858.CD004764.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marfurt 2010
- Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Experimental Eye Research 2010;90(4):478-92. [DOI] [PubMed] [Google Scholar]
Martonyi 2022
- Martonyi CL. Slit lamp examination and photography. In: Mannis MJ, Holland EJ, editors(s). Cornea: Fundamentals, Diagnosis, and Management. 5th edition. Amsterdam (NL): Elsevier, 2022. [Google Scholar]
McGwin 2005
- McGwin G Jr, Xie A, Owsley C. Rate of eye injury in the United States. Archives of Ophthalmology 2005;123(7):970-6. [DOI] [PubMed] [Google Scholar]
McKenzie 2022
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
McQuay 2012
- McQuay HJ, Derry S, Eccleston C, Wiffen PJ, Moore RA. Evidence for analgesic effect in acute pain - 50 years on. Pain 2012;153(7):1364-7. [DOI] [PubMed] [Google Scholar]
Menghini 2013
- Menghini M, Knecht PB, Kaufmann C, Kovacs R, Watson SL, Landau K, et al. Treatment of traumatic corneal abrasions: a three-arm, prospective, randomized study. Ophthalmic Research 2013;50(1):13-8. [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut-offs - 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400-12. [DOI] [PubMed] [Google Scholar]
Nishida 2022
- Nishida T, Saika S, Morishige N. Cornea and sclera: anatomy and physiology. In: Mannis MJ, Holland EJ, editors(s). Cornea: Fundamentals, Diagnosis, and Management. 5th edition. Amsterdam (NL): Elsevier, 2022. [Google Scholar]
Négrel 1998
- Négrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiology 1998;5(3):143-69. [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Parsons 2022
- Parsons MR. Factitious keratoconjunctivitis. In: Mannis MJ, Holland EJ, editors(s). Cornea: Fundamentals, Diagnosis, and Management. 5th edition. Amsterdam (NL): Elsevier, 2022. [Google Scholar]
Peyman 1994
- Peyman GA, Rahimy MH, Fernandes ML. Effects of morphine on corneal sensitivity and epithelial wound healing: implications for topical ophthalmic analgesia. British Journal of Ophthalmology 1994;78(2):138-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pharmakakis 2002
- Pharmakakis NM, Katsimpris JM, Melachrinou MP, Koliopoulos JX. Corneal complications following abuse of topical anesthetics. European Journal of Ophthalmology 2002;12(5):373-8. [DOI: 10.1177/112067210201200505] [DOI] [PubMed] [Google Scholar]
Pogatzki‐Zahn 2019
- Pogatzki-Zahn E, Schnabel K, Kaiser U. Patient-reported outcome measures for acute and chronic pain: current knowledge and future directions. Current Opinion in Anaesthesiology 2019;32(5):616-22. [DOI: 10.1097/ACO.0000000000000780] [DOI] [PubMed] [Google Scholar]
Poggio 1989
- Poggio EC, Glynn RJ, Schein OD, Seddon JM, Shannon MJ, Scardino VA, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. New England Journal of Medicine 1989;321(12):779-83. [DOI] [PubMed] [Google Scholar]
Racine 2012a
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain 2012;153(3):602-18. [DOI] [PubMed] [Google Scholar]
Racine 2012b
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012;153(3):619-35. [DOI] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- Review Manager Web (RevMan Web). Version 5.2.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rosenwasser 1990
- Rosenwasser GO, Holland S, Pflugfelder SC, Lugo M, Heidemann DG, Culbertson WW, et al. Topical anesthetic abuse. Ophthalmology 1990;97(8):967-72. [DOI] [PubMed] [Google Scholar]
Sabri 1997
- Sabri K, Pandit JC, Thaller VT, Evans NM, Crocker GR. Current management of corneal abrasions: evidence based practice? British Journal of Ophthalmology 1997;81(12):1116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schein 1989
- Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. New England Journal of Medicine 1989;321(12):773-8. [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Shaheen 2014
- Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Survey of Ophthalmology 2014;59(3):263-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharfstein 2019
- Sharfstein JM, Olsen Y. Lessons learned from the opioid epidemic. JAMA 2019;322(9):809-10. [DOI: 10.1001/jama.2019.9794] [DOI] [PubMed] [Google Scholar]
Steigleman 2023
- Steigleman WA, Rose-Nussbaumer J, Al-Mohtaseb Z, Santhiago MR, Lin CC, Pantanelli SM, et al. Management of pain after photorefractive keratectomy: a report by the American Academy of Ophthalmology. Ophthalmology 2023;130(1):87-98. [DOI: 10.1016/j.ophtha.2022.07.028] [DOI] [PubMed] [Google Scholar]
Swaminathan 2015
- Swaminathan A, Otterness K, Milne K, Rezaie S. The safety of topical anesthetics in the treatment of corneal abrasions: a review. Journal of Emergency Medicine 2015;49(5):810-5. [DOI] [PubMed] [Google Scholar]
Vandorselaer 2001
- Vandorselaer T, Youssfi H, Caspers-Valu L E, Dumont P, Vauthier L. Treatment of traumatic corneal abrasion with contact lens associated with topical nonsteroid anti-inflammatory agent (NSAID) and antibiotic: a safe, effective and comfortable solution [Traitement des érosions cornéennes traumatiques par l'association d'une lentille de contact, d'un collyre anti-inflammatoire non stéroïdien (AINS) et d'un collyre antibiotique: solution sûre, efficace et confortable]. Journal Français D'Ophtalmologie 2001;24(10):1025-33. [PubMed] [Google Scholar]
Varga 1997
- Varga JH, Rubinfeld RS, Wolf TC, Stutzman RD, Peele KA, Clifford WS, et al. Topical anesthetic abuse ring keratitis: report of four cases. Cornea 1997;16(4):424-9. [PubMed] [Google Scholar]
Vaziri 2016
- Vaziri K, Schwartz SG, Flynn HW Jr, Kishor KS, Moshfeghi AA. Eye-related emergency department visits in the United States, 2010. Ophthalmology 2016;123(4):917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakai 2017
- Wakai A, Lawrenson JG, Lawrenson AL, Wang Y, Brown MD, Quirke M, et al. Topical non-steroidal anti-inflammatory drugs for analgesia in traumatic corneal abrasions. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD009781. [DOI: 10.1002/14651858.CD009781.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waldman 2018
- Waldman N, Winrow B, Densie I, Gray A, McMaster S, Giddings G, et al. An observational study to determine whether routinely sending patients home with a 24-hour supply of topical tetracaine from the emergency department for simple corneal abrasion pain is potentially safe. Annals of Emergency Medicine 2018;71(6):767-78. [DOI] [PubMed] [Google Scholar]
Webber 1999
- Webber SK, Sutton GL, Lawless MA, Rogers CM. Ring keratitis from topical anaesthetic misuse. Australian and New Zealand Journal of Ophthalmology 1999;27(6):440-2. [DOI: 10.1046/j.1440-1606.1999.00246.x] [DOI] [PubMed] [Google Scholar]
WebPlotDigitizer [Computer program]
- WebPlotDigitizer. Version 4.5. Rohatgi A, accessed 20 October 2022. Available from: automeris.io/WebPlotDigitizer.
Willis 1970
- Willis WE, Laibson PR. Corneal complications of topical anesthetic abuse. Canadian Journal of Ophthalmology 1970;5(3):239-43. [PubMed] [Google Scholar]
Wu 2016
- Wu H, Hu Y, Shi XR, Xu F, Jiang CY, Huang R, et al. Keratopathy due to ophthalmic drug abuse with corneal melting and perforation presenting as Mooren-like ulcer: A case report. Experimental and Therapeutic Medicine 2016;12(1):343-6. [DOI: 10.3892/etm.2016.3296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yagci 2011
- Yagci A, Bozkurt B, Egrilmez S, Palamar M, Ozturk BT, Pekel H. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea 2011;30(5):571-5. [DOI: 10.1097/ico.0b013e3182000af9] [DOI] [PubMed] [Google Scholar]
Yu 2021
- Yu CW, Kirubarajan A, Yau M, Armstrong D, Johnson DE. Topical pain control for corneal abrasions: a systematic review and meta-analysis. Academic Emergency Medicine 2021;28(8):890-908. [DOI: 10.1111/acem.14222] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sulewski 2022
- Sulewski M, Ifantides C, Liu S-H, Leslie L, Kuo IC. Topical ophthalmic anesthetics for corneal abrasions. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD015091. [DOI: 10.1002/14651858.CD015091] [DOI] [PMC free article] [PubMed] [Google Scholar]


