Abstract
Platelets are a class of pluripotent cells that, in addition to hemostasis and maintaining vascular endothelial integrity, are also involved in tumor growth and distant metastasis. The tumor microenvironment is a complex and comprehensive system composed of tumor cells and their surrounding immune and inflammatory cells, tumor‐related fibroblasts, nearby interstitial tissues, microvessels, and various cytokines and chemokines. As an important member of the tumor microenvironment, platelets can promote tumor invasion and metastasis through various mechanisms. Understanding the role of platelets in tumor metastasis is important for diagnosing the risk of metastasis and prolonging survival. In this study, we more fully elucidate the underlying mechanisms by which platelets promote tumor growth and metastasis by modulating processes, such as immune escape, angiogenesis, tumor cell homing, and tumor cell exudation, and further summarize the effects of platelet−tumor cell interactions in the tumor microenvironment and possible tumor treatment strategies based on platelet studies. Our summary will more comprehensively and clearly demonstrate the role of platelets in tumor metastasis, so as to help clinical judgment of the potential risk of metastasis in cancer patients, with a view to improving the prognosis of patients.
Keywords: mechanism research, platelets, targeted therapies, tumor growth, tumor metastasis
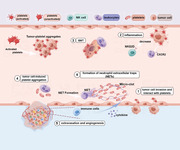
The summary of interactions between tumor cells and platelets. ①Platelets can facilitate the tumor cells invasion. ②The interaction can cause inflammation, causing the decrease of NKG2D on the surface of NK cells. ③ Leukocytes help the EMT. ④In addition, tumor cells can cause platelet aggregation. ⑤These can help the tumor cells extravasation and angiogenesis. ⑥In the blood vessels, neutrophil extracellular traps (NETs) can stimulate the migration and invasion of cancer cells. Immune cells in the tumor microenvironment can release cytokines to promote the growth of cancer. The figure is made using Figdraw. The figure is Graphic Abstract image.
1. INTRODUCTION
Cancer cells’ genetic mutations and a large number of protein mutations allow them to proliferate uncontrollably. The ability of cancer cells to persist, proliferate, and even metastasize in the body is significantly related to their ability to evade recognition and attack by the body's immune system through a variety of mechanisms. Previous studies have demonstrated that the body's immune cells and fibroblasts can be used by cancer cells. 1 , 2 , 3 A growing body of research suggested that platelets in the blood are also used by cancer cells to help them grow and become aggressive.
Cancer‐associated thrombosis is the second leading cause of death in patients with tumors. 4 The incidence of venous thromboembolism has been reported to be 2%–20% and arterial thromboembolism within 1%–4.7% in patients with malignancy. 5 , 6 , 7 Platelets, as important regulatory cells of thrombosis, play an important role in cancer‐associated thrombosis. Cancer cell‐induced platelet activation also facilitates the development and spread of tumors. Previous studies found that mean platelet volume and platelet distribution width are closely related to the prognosis of renal cell carcinoma, colorectal cancer (CRC), melanoma, and other tumors. 8 , 9 , 10
Previous studies confirmed that tumor angiogenesis and tumor cell evasion of body immunity are closely related to the tumor microenvironment (TME). 11 , 12 , 13 Numerous studies confirmed that platelets play an important role in tumor growth and metastasis, 14 greatly influencing the behavior of cancer cells, while the physiology and phenotype of platelets are also affected by cancer cells. In the TME, platelets are activated and aggregated by tumor cells 15 , 16 and aid in the immune escape of tumor cells through paracrine secretion and direct contact, thereby increasing the survival of tumor cells during metastasis. Platelets interact with tumor cells through surface receptors and help tumor metastasis. 17 The aggregation of platelets around tumor cells protects them from shear forces and natural killer (NK) cells in the blood, and provides a scaffold for tumor cells to adhere to the vessel wall. In addition, angiogenesis factors and growth factors secreted by activated platelets can promote tumor growth and angiogenesis, 18 thus, establishing a good microenvironment for tumor metastasis. At the same time, tumor cells will further activate platelets and stimulate platelets to release active substances to optimize the growth environment of the tumor itself (Figure 1). 19
FIGURE 1.
Presentation of the function of platelets in cancer growth and invasion. Platelets can release cytokines and other molecules to facilitate the growth of tumor cells. Reproduced with permission from reference 37.
Stromal cells and tumor‐infiltrating immune cells are the main cellular participants in TME. The tumor stroma is composed of different types of cells, including fibroblasts and endothelial cells. 20 Remarkably, we can observe platelet infiltration into the tumor stroma, 21 with increased blood platelet counts (PCs). 22 Tumor‐infiltrating platelets can interact with other stromal players of TME. Platelets can release growth factors acting on neoplastic epithelial cells to trigger epithelial‐mesenchymal transition (EMT), and platelets interact with epithelial cells to promote angiogenesis. Activated platelets and their secreted products contribute to the formation of cancer‐associated fibroblasts (CAFs). These contribute to tumor promotion and progression. 23 Platelets also adjust various angiogenesis regulators, which can turn on local angiogenesis in the TME. 24 Moreover, intratumoral platelet accumulation is associated with tumor progression. 25 In conclusion, platelets function as an important stromal component in the TME through interactions with other members.
Our article focuses on the function of platelets in the process of tumor metastasis, discusses the interaction between platelets and tumors, and more importantly, provides a reference for further research in this field by introducing the current platelet‐based tumor‐targeted therapies.
2. THE FORMATION AND CHARACTERISTICS OF PLATELETS
There are three main types of blood cells in human blood: red blood cells (the most numerous), platelets, and white blood cells (the least numerous). Platelets are generated in the bone marrow and circulate in the bloodstream. When bleeding or injury occurs somewhere in the body, they go to the wound and help stop the bleeding by gathering more platelets together with other clotting factors to form a clot.
Platelets are small, non‐nucleated cell fragments that originate from the largest megakaryocytes in the bone marrow. 26 Megakaryocytes are the important regulatory cells in the hematopoietic stem cell microenvironment 27 and are stimulated by thrombopoietin (TPO) to differentiate from hematopoietic stem cells in the bone marrow, which can migrate to the lungs to produce platelets. 28 To avoid activation of platelets before they enter the circulation, megakaryocytes mature and undergo cytoplasmic remodeling to produce blunt projections, forming proplatelets. The proplatelet morphology elongates and thins over time, extending into the lumen of the blood vessel 29 and remodeling into proplatelets in the blood under the shear stress of blood flow. Then, the proplatelets generate platelets by fission. 30
Normal platelets are concave, oval, or disc‐shaped in circulation, 31 and they are the smallest cells in the circulation. The number of platelets in the human body ranges from 150 × 10 9 to 400 × 10 9 per liter. 30 , 32 , 33 , 34 The outermost layer of platelets is coated with a variety of glycoproteins, which are either secreted by the internal granules of platelets or constitute the membrane components of platelets, and play a very important role in platelet adhesion and aggregation. 34 The platelet membrane (PM) and the platelet contractile protein system are encapsulated underneath these glycoproteins, which help to maintain the platelet's disc‐like morphology. Apart from the membrane glycoproteins, there are many receptors on the surface of PMs. PM receptors that support hemostasis and thrombosis include the integrin gene family, the leucine‐rich glycoprotein gene family, the selectin gene family, and the immunoglobulin gene family.
3. INTERACTION BETWEEN PLATELETS AND TUMORS
In this section, first, we will discuss how platelets and tumors interact to promote tumor cell growth and immune system escape and later, we will focus on the mechanisms of their interaction in promoting the formation of new blood vessels and aiding tumor cells from the primary site through the epithelial stroma to the new site.
3.1. Tumor cell homing and tumor cell growth
As the neoplastic organism formed by abnormal proliferation of local tissues, tumor cells do not grow in isolation, but create a suitable “soil” for their growth, that is, TME, and promote their own development by interacting with a variety of cells in the body. 35 Tumor cells and the microenvironment in which they reside are a functional whole. Tumor cells are regarded as seeds, while the microenvironment in which they reside is regarded as soil. Tumor cells and their microenvironment interact and coevolve to promote tumorigenesis.
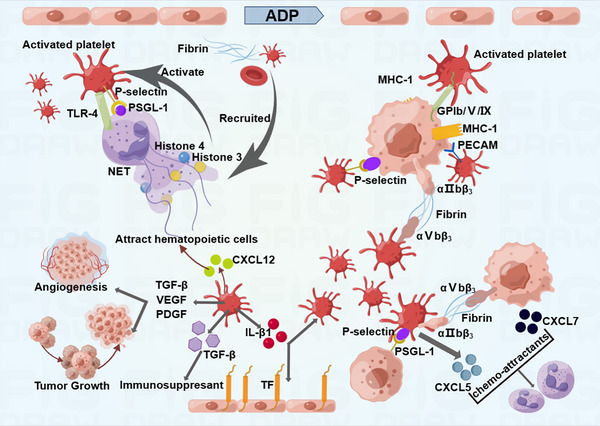
Under normal physiological conditions, circulating platelets do not interact with inactivated endothelial cells. When cancer occurs, tumor cells release cytokines and growth factors that activate vascular endothelial cells, thus promoting the proliferation of the tumor cells. High expression of vascular hemophilia factor, P‐selectin, integrin protein, and tissue factor (TF) are activated by endothelial cells, 36 which mediate platelet rolling along the endothelium by binding to adhesion receptors on the platelet surface. The interaction of platelets with activated endothelial cells induces complete activation of platelets and high expression of P‐selectin on their surfaces, allowing platelets to adhere firmly to the endothelium, thus facilitating local recruitment of platelets. 37
Fully activated platelets release proangiogenic factors, which further activate endothelial cells, support angiogenesis, 38 and regulate vascular permeability and vascular tone. 36 At the same time, the proangiogenic factors, together with the activated factors stored inside platelets, are released into TME, further promoting revascularization and inflammation (Figure 2). For instance, lysophosphatidic acid (LPA), which is produced by activated platelets, can bind to LPA receptors (e.g., LAP1) on breast cancer cells and this interaction contributes to the proliferation and osteolytic bone metastasis of cancer cells. 39 Apart from releasing contents, platelets also communicate with tumor cells via G protein‐coupled receptors on platelets’ surface and the loss of two critical G proteins (Gαi2 and Gα13) substantially reduced platelet extravasation and tumor growth. 40 A large number of platelets are present in the TME, 41 and these platelets help to build the TME by releasing platelet‐derived microparticles (PMPs), which support the overgrowth of tumors. 42 While accumulating evidence indicates that PMPs are associated with poor prognosis in various cancers including those of lung and stomach, 43 , 44 PMPs also can induce apoptosis of solid tumor cells through transferring platelet‐derived RNA, primarily miR‐24. 45 Mechanistically, platelet‐derived miR‐24 inhibited mitochondrial RNAs, resulting in growth impairment. 45 Besides, interactions between platelets and tumor cells not only trigger the secretion of microparticles derived from platelets, but also stimulate those derived from tumor cells and those with both tumor and platelet markers. All of these microparticles recruit macrophages and activate their tumor‐killing activities, eventually causing tumor cell‐cycle arrest. 46 Taken together, the role of platelets in tumor growth remains controversial and requires further investigation.
FIGURE 2.
Various mechanisms of platelet activation. Platelets interact with tumor cells to produce inflammations in cancer. Reproduced with permission from reference 38.
The platelet homing by tumors is selective. Chen et al. 47 observed that the proliferative environment of pancreatic cancer can reject platelets, but is not related to a strong “homing” affinity. In addition, different effects mediated by activated platelets were observed in breast cancer and pancreatic cancer models. 47 This suggests that the “homing” effect of platelets to different types of tumors may be highly correlated with the blood supply of tumors and influenced by the distinct microenvironments of various types of cancer. More than 90% of pancreatic cancers are composed of highly fibrotic collagen stroma, which greatly compress the microvessels in the tumor, leading to a significant decrease in effective blood perfusion. 48 This indicates that the high expression of stroma can reduce blood perfusion and thus, affect the homing effect of platelets. Besides, as different tumor types have an impact on platelet homing ability, 47 this limits the applicability of platelet‐based targeted therapeutic strategies for tumors.
3.2. Platelets and tumor cell metastasis
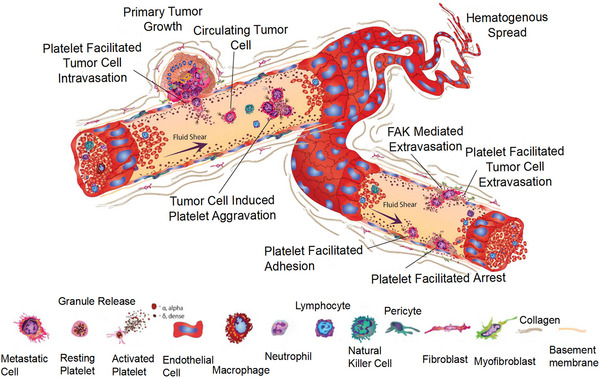
During tumor metastasis, individual tumor cells are unable to fight against the body's attack. The majority of tumor cells entering the bloodstream die within a short period of time under the action of the host immune system and the mechanical shear of the bloodstream. Only a very small number of tumor cells with high viability and metastatic potential interact with platelets and the coagulation system in the bloodstream can help form small cancer clots that escape immune clearance, thus metastasizing to distant sites. 49 In previous experiments with mice, it was found that the metastasis of cancer cells was significantly reduced when the number of platelets was reduced or lost, 18 suggesting that platelets may aid tumor cells to metastasize.
Anoikis is a special cell‐programmed death induced by cells breaking into contact with the extracellular matrix or adjacent cells. Overcoming the anoikis has a positive effect on tumor metastasis. Haemmerle et al. 50 demonstrated that platelets induce resistance to anoikis in vitro, which is critical for metastasis in vivo. Platelets can contact directly with tumor cells or release some bioactive molecules to facilitate metastasis. In the blood vessels, platelets can prolong the time that tumor cells stay. In addition, P‐selectin on platelets can bind to tumor cells along with fibrous proteins, which can cause tumor cell‐induced platelet aggregation (TCIPA). 51 The process can protect circulating tumor cells (CTCs) from clearance. In oral squamous cell carcinoma, podoplanin (PDPN) is a transmembrane glycoprotein on the surface of tumor cells. C‐type lectin‐like receptor‐2 (CLEC‐2) is located in the platelets which can combine with PDPN. The interaction between PDPN and CLEC‐2 can activate the platelets. The activated platelets can accumulate and enhance the invasion and metastasis of the tumor cells. 52 It is known that chemical signals, such as NF‐κB and transforming growth factor‐β (TGF‐β) signals released by platelets, can make the tumor cells more invasive. 53 In a recent research, the immune checkpoint molecule pair HLA‐E:CD94‐NKG2A mediated the interaction between CTCs and NK cells. RGS18 secreted from platelets improved the expression of HLA‐E via AKT‐GSK3β‐CREB signaling. RGS18 can facilitate the metastasis of tumor cells. 54
However, another experiment showed that platelets inhibited the growth of liver cancer in mice of nonalcoholic fatty liver disease. The antitumor function of platelets is mediated by P2Y12‐dependent CD40L release, which leads to the activation of CD8 T cells by the CD40 receptor. 55 Platelets can also inhibit tumor cell proliferation by blocking the cell cycle at the G0/G1 phase. 56 The role of platelets in tumor progression is complex and diverse, requiring further study.
3.2.1. Metastasis through immune escape
One of the ways in which cancer cells in the blood can eventually metastasize successfully is by allowing platelets to coat their surface, thus creating a barrier that makes the cancer cells invisible to cellular components of the immune system. Mechanistically, tumor cells enter the systemic circulation and release thrombin to rapidly recruit and activate platelets. By binding to P‐selectin, which is highly expressed on the surface of activated platelets, together with fibrin deposition, 57 tumor cells rapidly form a microthrombotic barrier, 58 a process known as TCIPA. 51 TCIPA is a prerequisite for the survival and metastasis of tumor cells in the vascular system. It induces platelet activation and aggregation mainly through the secretion of various active substances. This forms a layer of platelets wrapping the tumor cells, thus hiding them from being detected by immune cells and also avoiding injury from the high‐speed shear stress of blood flow. In addition to protecting the integrity of CTCs, platelets can also transfer major histocompatibility complex class I molecules to the surface of TCIPA aggregates by secreting exosomes, 59 which interferes with the recognition of circulating NK cells and helps CTCs evade immune surveillance. Platelets recruit monocytes and granulocytes to cancer cell blocking sites, which together shape a protumor environment conducive to metastasis, 14 thus allowing CTCs to successfully cross the endothelium into the early metastatic niche and subsequently form metastatic foci. The most widely used antiplatelet coagulation drug in oncology treatment is aspirin. Aspirin can improve the prognosis of mice with chronic hepatitis B and reduce the incidence of hepatocellular carcinoma, and also inhibit the growth and metastasis of osteosarcoma through the NF‐κB signaling pathway. In recent years, numerous clinical studies have shown that antiplatelet therapy can reduce tumor metastasis and prolong patient survival. 60 For instance, daily oral aspirin (≥75 mg/day) prevented distant tumor metastasis and reduced tumor morbidity and mortality and, furthermore, the survival of patients with CRC diagnosed with the PIK3CA variant can be prolonged by treating with routine oral aspirin (325 mg/day). 61 In conclusion, platelets are necessary in tumor growth, angiogenesis, metastasis, and evasion of host immune response through TCIPA. In the comprehensive treatment of tumors, antiplatelet agents can be applied to inhibit platelet activation, contributing to the reduction tumor metastasis. Antiplatelet agents may be considered in combination with surgery, local therapy, and radiotherapy to increase their efficacy in treating tumors, but a large number of clinical studies are still needed to confirm this.
TGF‐β released from platelets suppresses the activity of antitumor T cells and is a cytokine that exerts an immunosuppressive effect, enhancing the growth and metastasis of cancer cells. 62 Primarily synthesized as an inactive proprotein, TGF‐β requires GARP (glycoprotein‐A repetitions predominant), a docking receptor for latent TGF‐β, which can promote the furin‐dependent cleavage of TGF‐β proprotein, eventually leading to the TGF‐β maturation. 63 Previous research indicated that GARP expressed in tumor cells induced the activation of latent TGF‐β, and hence, matured TGF‐β could upregulate the expression of Foxp3 which rendered effector T cells immune‐suppressive. 64 This tolerogenic FoxP3/GARP/TGFβ axis, therefore, favors tumor immune evasion. TGF‐β suppresses the differentiation of cytotoxic T cells and increases the regulatory T cells which subsequently inhibit the activation of NK cells and T cells. 44
During the interaction between platelets and NK cells, tumor cells also express normal platelet surface receptors, which prevent NK cells from recognizing tumor cells, leading to tumor immune escape and increasing the early survival rate of metastatic cancer cells. 65 For instance, platelets express “a disintegrin and metalloproteinase” (ADAMs) on their surfaces, and one of the members called ADAM10 participates in the shedding of the stress‐induced NKG2D ligands such as MICA/B and ULBP2 on tumor cells. 66 , 67 PD‐1 and CTLA‐4 belonging to the immune checkpoint proteins can interact with TCIPA and Tregs to evade the immune system to achieve the goal of metastasis. 68 In normal conditions, NK cells with NKG2D (an activating immunoreceptor) on their surfaces can recognize NKG2D ligands expressed on tumor cells, thus exerting cytotoxic effects. 69 However, the cleavage of NKG2D ligands on tumor cells by ADAM10 damages this tumor‐lysis function. 69 The reactivity of NK cells is modulated by activating inhibitory surface receptors, such as tumor necrosis factor (TNF) receptors. 70 One of the TNF family members called glucocorticoid‐induced TNF‐related ligand is coexpressed with P‐selectin on platelets owing to platelet activation mediated by tumor cells. 71 Although this molecule does not affect the activation or function of platelets, it contributes to platelets coating with tumor cells and binds to glucocorticoid‐induced TNF‐related protein on NK cells, reducing Interferon‐γ (IFN‐γ) generation and NK cell cytotoxicity. 71 A study demonstrated that solid tumor cells induced platelets to upregulate the expression of the platelet‐derived receptor activator of NF‐κB ligand (RANKL), another member of the TNF family. 70 Receptor activator of NF‐κB (RANK) is displayed on NK cells, which weaken the NK tumor‐killing effects. 64 Individuals with solid tumors have NK cells with upregulation of RANK, which interact with RANKL, weakening the antitumor effects of NK cells, with a more prominent effect on IFN‐γ generation and a weaker effect on cytotoxicity. 64 This impairs NK cell immunosurveillance and aids tumor evasion. Moreover, TGF‐β can directly influence NK cells through reducing NK cells’ activating receptors and dampening IFN‐γ transcription. 72 , 73 Platelet‐secreted TGF‐β can also downregulate the expression of NKG2D, an activating receptor on the surface of NK cells, resulting in a decrease in the antitumor activity of NK cells. 74 Experiments in vivo showed that in thrombocytopenic mice, hematogenous metastasis of tumor cells was reduced. But when NK cells were precleared, hematogenous metastasis significantly increased. 63 Therefore, through weakening NK cells and other immune components by TGF‐β, platelets foster tumor metastasis. Overall, platelets can affect the antitumor effects of NK cells.
In some cases, platelets also mediate immune escape through activating immune‐inhibitory receptors. For instance, in myeloproliferative neoplasms where the bone marrow generates an excessive amount of blood cells and platelets, oncogenic JAK2V617F (Janus kinase 2) mutation leads to phosphorylation of STAT5 (signal transducer and activator of transcription 5) and STAT3, which upregulates the PD‐L1 expression in mutated cancer cells including platelets. 75 This results in PD‐L1‐mediated immune escape via suppressing cell cycle progression and metabolism of T cells. 75
3.2.2. Tumor−platelet interaction promotes metastasis through angiogenesis
Continuous tumor proliferation requires an adequate blood supply from new blood vessels to carry nutrients, oxygen, and metabolites necessary for growth. In addition to providing nutrients and water for tumor growth, new blood vessels spread tumor cells to distant areas and form new metastases in different parts of the body. The TME and stromal cells are the main factors that promote neovascularization, and these vascular networks provide the environment for tumor development, progression, and metastasis. 76
Tumor−platelet interaction promotes angiogenesis mainly through two ways. On the one hand, cancer cells usually secrete growth factors such as vascular endothelial growth factor (VEGF) to induce vascularization. Platelets can then absorb VEGF, lipids, protein hydrolases, and other components from the environment that are beneficial to vascular renewal, and then release them when being stimulated to promote vascular renewal. 38 In breast cancer, platelets sequester proangiogenic factors from aggressive tumor tissues and then send them to nonaggressive ones, hence facilitating vascularization in the indolent tumors. 77 Solid tumors can also express TF, which interacts with factor VII/VIIa, resulting in the formation of thrombin. 78 On the other hand, vasculature inside tumors plays a crucial role. The overexpression of TF by tumor endothelial cell system (ECs) is associated with increased expression of VEGF‐A and microvessel density (MVD). 79 Indeed, transfecting TF in cancer cell lines induced increased transcription of VEGF and reduced transcription of thrombospondin 1 (TSP1) and thrombospondin 2 (TSP2) secreted by platelets, indicating the proangiogenic role of TF. 70 Both TSP1 and TSP2 are related to antiangiogenesis and are endogenous angiogenesis inhibitors with reduced or no expression in a variety of tumor tissues. Moreover, the expression of TSP1 and TSP2 was negatively correlated with MVD. Besides, aggressive tumor‐activated bone marrow cells can upregulate CD24 expression on indolent tumor cells and enhance the infiltration of VEGFR2+ tumor‐associated endothelial cells. 77 This promotes the platelet‐cloaking of tumor cells and tumor vascularization. 77
Platelets are the main carriers of the proangiogenic factors VEGF, platelet‐derived growth factor (PDGF), and basic fibroblast growth factor (β‐FGF). PMPs can also secrete VEGF, PDGF, FGF, and matrix metalloproteinase (MMPs) to promote tumor angiogenesis, increasing the chance of distant metastasis of tumor cells. 38 VEGF is one of the most important angiogenic proteins transported and released by platelets. 80 PDGF is necessary for the growth of smooth muscle cells, fibroblasts, and glial cells. 81 Overexpression of PDGFs and platelet‐derived growth factor receptors (PDGFRs) is associated with human cancer and is significantly associated with malignant consequences. 82 It shows that anti‐VEGF drug therapy can inhibit the signaling pathways of growth factors and thus targeting angiogenesis and distant metastasis. 41 Through releasing α‐granules, platelets can also promote bone marrow‐derived cells to mobilize into the blood and facilitate their homing to tumor‐associated neovasculature in a VAMP‐8‐dependent way. 83 Studies have shown that nucleolin hUTP14a upregulated the transcription and secretion of PDGF, thereby contributing to increased MVD in colon cancer tissues. 42 Platelets may induce a “wound healing” response via degranulation of PDGF, CXCL12, and VEGF‐A. This subsequently recruits and stimulates CAF and myeloid cells, and leads to extracellular matrix (ECM) deposition, enhancing cancer angiogenesis. 84
Interestingly, apart from proangiogenic factors, platelets are also the sources of antiangiogenic molecules, such as TSP1, endostatin, and platelet factor‐4. 85 It indicates that simulators and inhibitors of angiogenesis are finely controlled and released through different α‐granules, depending on specific stimuli. 86 For example, ligation of protease‐activated receptor PAR1 triggers the selective release of granules containing VEGF, whereas PAR4 activation induces the release of granules loaded with endostatin but not those with VEGF. 86 However, an opposing opinion is raised that platelet secretion does not rely on the contents but is random, kinetically controlled, and is associated with agonist potency. 54
The molecules expressed on the platelets may also participate in angiogenesis. For example, CD40 ligand (CD40L) is a molecule expressed on the surface of platelets and it can be cleaved to form soluble CD40L. 87 This molecule stimulates the endothelial progenitor cells, increasing the secretion of MMP‐9 from these cells possibly via the p38 MAPK signaling pathway, which supports angiogenesis. 88 Soluble CD40L also binds CD40 expressed on platelets, further activating platelets. 87 Platelets can interact with host endothelial cells via GPIIb/IIIa, an integrin complex expressed on platelets, which plays a vital role in platelet‐mediated endothelial cell spouting. 89 Vitronectin receptor αvβ3, which are expressed on endothelial cells, tumor cells, and platelets, may also play a role in the angiogenesis mediated by the interaction among these cells, since blockade of this molecule (merely on ECs or tumor cells) remarkably inhibits cancer cell invasion. 89
Platelets are important in maintaining vascular integrity and hemostasis within tumors. Research has shown that induced thrombocytopenia causes hemorrhage in tumors, resulting in apoptosis of tumor cells and decreased cell proliferation. 90 Further, thrombocytopenia‐triggered bleeding of tumors was dampened by resting platelets but not by degranulated ones, which indicated the granular contents of platelets play a fundamental role in preventing intratumor bleeding by platelets. 90 For instance, some factors released by platelets, such as angiopoietin‐1, have the effect of maintaining microvascular integrity probably through counteracting with VEGF‐A secreted by tumor cells, which in turn reduces the invasion of immune cells into tumor tissue and contributes to tumor metastasis. 91 Besides, another study also suggested that intratumor hemorrhage can be induced by innate immune cells so platelets may hamper local tissue damage via regulating leukocyte recruitment, thus maintaining the integrity of blood vessels. 92 What is more, recent research showed that platelet‐derived growth factor B (PDGFB) promoted and maintained vascular integrity in the TME by promoting pericyte recruitment. 93
3.2.3. Metastasis through EMT
Platelets play an important role in the adhesion and extravasation of tumor cells to surrounding tissues and organs. By releasing cytokines, platelets induce the tumor cells to undergo EMT. Tumor cells with altered phenotype cross the basement membrane and vascular endothelium, transferring to the target organs. The activated blood cells simultaneously release ATP and MMPs, which induce the opening of the vascular endothelial barrier and degrade the vascular basement membrane structure, prompting the tumor cells to infiltrate the blood vessels and invade the surrounding environment. 94
PDGF is a basic protein that is isolated from human platelets and stored in platelet alpha granules. 95 In addition, PDGF can be synthesized and released by vascular smooth muscle cells, fibroblasts, macrophages, and so on. PDGF released from tumor cells can induce proliferation and migration of vascular endothelial cells, smooth muscle cells, and tumor cells, and inhibit their apoptosis, thus playing a direct and indirect role in tumor angiogenesis. 96 The role of PDGFs in tumor EMT was indicated in multiple studies. 97 For instance, through direct contact with tumor cells or secreting TGF‐β, platelets activate the TGF‐β/Smad and NF‐κB signaling pathways to induce EMT of colon cancer cells (Figure 3), 98 enhancing the invasive ability of tumor cells. Colon cancer cells treated with TGF‐β can express PDGF‐B, which is phosphorylated by platelets to cause EMT of tumor cells, but the ability of tumor cells to undergo distant metastasis is significantly reduced when the TGF‐β gene is knocked out. 99 PDGF produced by highly activated platelets can also increase MMP2/9 expression and stimulate the p38/MAPK pathway to trigger EMT in platelet‐stimulated cholangiocarcinoma. 100 This leads to tumor dissemination and metastasis. 100 Upregulation of PDGF‐D in endometrial cancer cells can lead to tumor metastasis via promoting MMP2/9 expression and EMT. 101 Direct contact of platelets with gastric cancer cells also contributed to the upregulation of EMT‐related genes, such as MMP9, leading to tumor migration. 102
FIGURE 3.
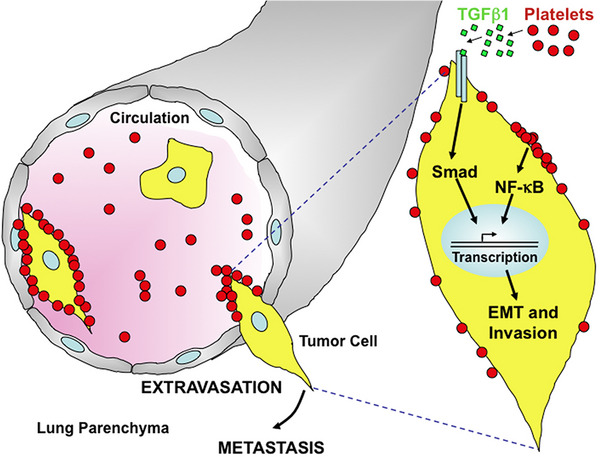
Platelets interact with tumor cells to facilitate EMT‐like transition. Platelets secrete TGFβ−1, which can activate signaling pathway in the tumor cells. Reproduced with permission from reference 95.
The interaction between platelets and endothelial cells plays an important role in promoting the EMT of cancer cells. For instance, platelet priming of tumor cells significantly increased colon cancer adhesion to endothelium and the crosstalk also induced EMT, which was indicated by upregulation of snail 1 and β‐catenin and downregulation of E‐cadherin in a time‐dependent way. 46 This eventually resulted in more metastatic sites in other organs. 46 In addition, microparticles derived from platelets or tumors or both may also prime the endothelial cells through increasing intercellular adhesion molecule 1 expression on ECs, thus fostering the adhesion of cancer cells at metastatic sites (Figure 4). 46 Besides, after platelets are activated, P‐selection stored in the platelet granules can be transferred to the platelet surface and then bound to P‐selectin ligands expressed on the tumor cells. 103 Increased P‐selectin expression enhances the ability of tumor cells to adhere to the vascular endothelium and contributes to tumor cell exudation.
FIGURE 4.
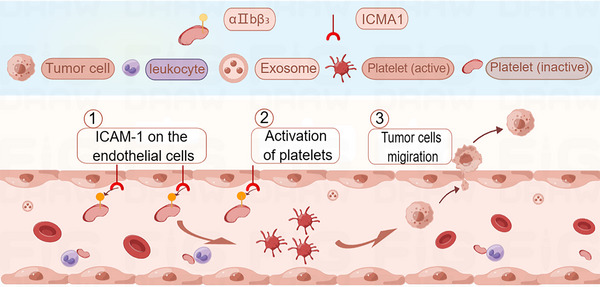
In the blood vessels, ICAM‐1 (intercellular adhesion molecule 1) expression on endothelial cells can activate the platelets, turning the inactive form into the active form. The activated platelets can foster the migration of cancer cells to form secondary tumors. In addition, platelets can interact with leukocytes. The figure is made using Figdraw.
Tumor cells are often characterized by expression of tumor‐specific antigens or overexpression of tumor‐associated antigens. These molecules may be involved in platelet‐induced tumor EMT. For instance, COX‐2 expression is upregulated in a variety of tumor cells, and its downstream metabolite TXA2 binds to the G‐protein‐coupled receptors P2Y and TBXA2R on the surface of platelets, inducing platelet activation to bind to tumor cells and promoting their hematogenous metastasis. 104 Ward et al. 105 demonstrated that the tumor‐associated antigen CD97 induces platelet activation and participates in the RHO‐GTP signaling pathway mediated by LPA, which induces EMT in tumors and improves the survival of tumor cells. Adams and Pang et al. 106 , 107 demonstrated that tumor cells can also express thrombin, and when tumor cells were coincubated with platelets activated by thrombin and transfused back to mice, the metastatic capacity of tumor cells was significantly increased.
Recent research showed that platelets can trigger tumor biosynthesis of 12S‐hydroxyeicosatetraenoic acid (12S‐HETE) and its esterification to plasma membrane phospholipids through tumor cell uptake of platelet‐derived extracellular vesicles expressing 12‐LoX. This may affect the expression of EMT marker genes and foster cancer metastasis. 108 TBK1, which is the abbreviation of TRAF (tumor necrosis factor receptor‐associated factor) family member‐associated NK‐κB activator (TANK)‐binding kinase 1, was found as a novel mediator of platelet‐induced NF‐κB signaling through activating NK‐κB subunit p65. 109 Thus, TBK1 contributed to EMT of mammary carcinoma cells, their invasiveness, and metastasis. 109
4. ASSOCIATION BETWEEN PC AND CANCER
More than a hundred years ago, scientists have observed that there seems to be a correlation between platelets and cancer, and many cancer patients have increased PCs by many times or even up to 20 times. Moreover, the higher the PC, the more severe the disease and the shorter the survival time of cancer patients. However, it is only recently that scientists are slowly understanding what is behind the cause.
The change of PC can be used as a reference index for tumor occurrence, development, curative effect, and prognosis. The blood of patients with advanced stage is in a state of hypercoagulation, which may be conducive to tumor growth and metastasis. 110 Increased PCs have been revealed to be a predictor of cancer in patients with occult malignancies 111 and are consistently associated with progression‐free and/or worse overall survival in ovarian, 112 , 113 colorectal, 114 lung cancer, 115 gastric cancer, 103 and breast cancer. 116 Platelets are involved in tumor angiogenesis and cancer progression. High PCs in lung, colon, stomach, kidney, and gynecologic cancers are associated with shorter disease survival. 103 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 Previously, scientists at the University of Exeter Medical School in the United Kingdom analyzed the records of 40,000 patients and found that more than 11% of men and 6% of women over the age of 40 with thrombocytosis were diagnosed with cancer within 1 year. 120 In addition, for patients with two elevated PCs within 6 months, the chance of cancer diagnosis increased to 18% for men and 10% for women. 120 In 2022, a nested case−control study, which involved more than 8 million subjects, reported that individuals with high PCs would have a diagnosis of cancer within 10 years after the blood test. 118 These cancers include colon, ovarian, stomach, and lung cancers and the extent of the correlation between the diagnosis and high PC varies among them. 118 In addition, PCs increased as these cancers approached the date of diagnosis. 121 Platelets influence disease burden and treatment outcome in cancer patients and are involved in steps, such as cancer metastasis. Platelets also play an important role in protecting cancer cells from chemotherapy‐induced apoptosis and in maintaining the integrity of the tumor vascular system. 122 In vitro tests have demonstrated that platelets increase resistance to 5‐fluorouracil and paclitaxel in colon and ovarian cancer cell lines. 123 Lower PCs strongly increase the sensitivity to paclitaxel in mouse models of breast or lung cancer. 123 This special function of tumor‐associated platelets (TAPs) provides a guarantee for tumors to maintain their rapid growth characteristics, so that tumor tissues will not be necrotic due to insufficient blood and nutrient supply.
The underlying mechanism of cancer‐induced increase in PC has been revealed. Cancer cells secrete various cytokines which may promote platelet formation. For instance, several types of tumor cells can secrete interleukin‐6 (IL‐6), 124 which stimulates liver cells to produce more TPO, which in turn promotes the maturation of platelet‐producing megakaryocytes, leading to higher PCs (Figure 5). 125 Besides, tumor cells can produce proteases, such as thrombin, cathepsin B, MMP‐2, and MMP‐14. These proteases may stimulate protease‐activated receptors (PARs), which mediate platelet activation. Specifically, through binding to PAR1, thrombin caused exposure of anionic phospholipids which facilitated platelet activation and blood clotting. 126 Thrombin can also activate PAR4 but the response was restricted to platelet aggregation, akin to the response of platelets triggered by epinephrine, adenosine diphosphate (ADP), or other weak agonists. 127
FIGURE 5.
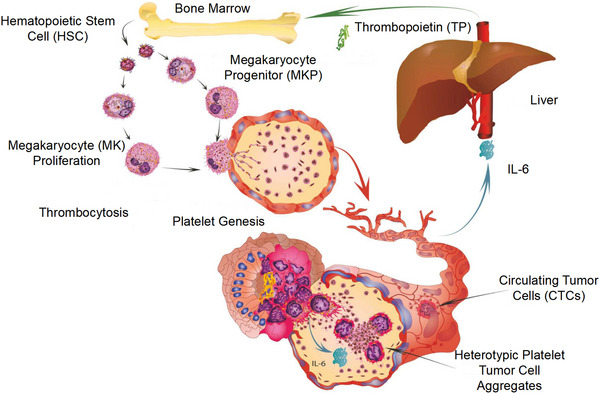
Thrombopoietin from the liver can enhance the production of platelets. In addition, IL‐6 from the tumor cells can stimulate the production of thrombopoietin, which can form a cycle. Reproduced with permission from reference 37.
5. CHARACTERISTICS OF THE INFLAMMATORY MICROENVIRONMENT MEDIATED BY PLATELETS INTERACTING WITH LEUKOCYTES AND STROMAL CELLS
Mutations in normal cells are necessary for tumorigenesis, but mutations alone are not enough. Inflammation plays an important role in initiating tumorigenesis by destroying tissues, and leukocytes are a key part of this process. 127 Study showed that platelet and leukocyte interactions mediate a range of inflammatory physiological and pathological processes. 128 Under physiological conditions, resting endothelial cells keep platelets in a resting state. However, during endothelial injury, proteins of the subendothelial matrix are exposed to the lumen and can activate platelets. Activated platelets can recruit more platelets to aggregate and seal the wound on the one hand; on the other hand, they interact with leukocytes, causing leukocyte recruitment, activation, extravasation, phenotypic switching, and a series of alterations in functions, further enhancing the degree of tumor inflammatory environment, including immunosuppression. 129
In the TME, platelet surface receptors and released active substances enhance platelet activation, forming TCIPA, which contributes to cancer thrombus formation and helps tumor cells escape immune attack and metastasis. Glycoprotein IIb‐IIIa is the platelet surface transmembrane receptor most involved in TCIPA. As early as 1987, it was observed that GPIIb/IIIa contributed to cancer thrombus formation during platelet aggregation (TCIPA) induced by lung cancer cells and glioma cells, helping tumor cells to escape the immune attack and thus tumor metastasis. 130 Amirkhosravi et al. 131 showed that oral or intravenous administration of XV454, an antagonist of GPIIb‐IIIa, to mice with lung metastases significantly reduced the binding of platelets to tumor cells and the occurrence of hematogenous metastasis of tumor cells. Cancer thrombus formation can block normal blood flow and the endothelial cells are activated, which promotes inflammation. Specifically, some receptors on endothelial cells will allow leukocytes, for example, neutrophils, to adhere to endothelial cells, migrate through blood vessel walls, and enter the tissues which trigger inflammation in distant organs in patients with cancer. 132 Platelets can also be activated, releasing a large number of proinflammatory factors that regulate the TME. This drives the recruitment of leukocytes, attracting more leukocytes to the site, thus inflammation is exacerbated. This process also contributes to the remodeling of the extracellular matrix and angiogenesis. Apart from releasing factors to foster inflammation, activated platelets also express receptors on their surfaces that can interact directly with immune cells, such as p‐selectin and CD40L. 133 , 134
It is shown that knockout of Ral GTPases, a modulator of cancer metastasis in platelets, remarkably reduces the platelet surface expression of P‐selectin, which subsequently leads to a substantial decrease in platelet−leukocyte interactions. 135 Thromboxane A2 (TXA2) is another substance derived from platelets that participate in controlling tumor metastasis. 136 Specifically, as a prostanoid product of cyclooxygenase‐1, TXA‐2 favors the formation of intravascular premetastatic niches and the recruitment of monocytes or macrophages that foster metastasis. 136 In addition, Zhang et al. 137 discovered that neutrophil‐lymphocyte ratio (NLR) and platelet‐lymphocyte ratio (PLR) were strongly associated with cancer metastasis to lymph nodes. NLR also served as an independent prognostic factor for the overall survival of patients with gastric cancer. 137
Platelets have recently been found to be involved in the formation of neutrophil extracellular traps (NETs), 138 which are web‐like structures composed of DNA and antimicrobial peptides from neutrophils. 139 On the front lines of the host defense response, the antimicrobial function of neutrophils has been adapted to fight infection and injury from different sources and degrees. In the past, the formation of NETs was thought to be an innate immune defense mechanism against severe bacterial infections. 140 Recent studies have found NETs in biopsies of human tumors. 141 NETs also stimulate the migration and invasion of cancer cells in vitro. 141 Ren et al. 142 demonstrated that surgical removement of tumors may cause stress that activated platelets via TLR4/ERK5/integrin GPIIb/IIIa signaling, fostering the aggregation of platelets with CTCs. These aggregates were then trapped by NETs which help the tumor cells to metastasize to distal sites. 142 Thus, by disrupting the cross‐talk between NETs and platelets, cancer metastasis may be prevented after resection. 142 Apart from cancer metastasis, NETs were also shown to induce procoagulant activity in patients with CRC. 143 It was found that CRC patients had neutrophils that were more prone to produce NETs whose level also elevated with cancer progression. 143 Subsequently, NETs promoted the exposure of phosphatidylserine on platelets and also induced the switch of endothelial cells to procoagulant phenotype. 143 These led to the increased risk of thrombosis in cancer patients. 143 In fact, there is a positive feedback loop between platelets and NETs. Specifically, NETs stimulate platelets via releasing substances, such as TFs, a platelet activator, from neutrophils. 144 In turn, activated platelets also facilitate the formation of NETs. 145
Generally, both neutrophils and platelets contribute to the construction of the TME. Patients with cancer tend to show an increase in the number of circulating neutrophils. 146 The number of neutrophils is usually small but these cells remain key regulators of tumor development and progression. 147 According to different properties and effects on tumor progression, neutrophils were divided into N1‐ and N2‐neutrophils. 148 N1 neutrophils have an immunoactivating ability and tumor cell cytotoxicity, while N2 neutrophils secrete reactive oxygen species (ROS), arginase and peroxidase, and other molecules, which inhibit T cell and NK cell function and promote tumor. 149 , 150 The switch from antitumor N1 neutrophils to protumor N2 neutrophils can be induced by TGF‐β which is mainly derived from platelets. 151
Neutrophils show a unique ability at each stage of cancer: from tumor initiation to primary tumor growth to metastasis. 152 Nevertheless, during these processes, neutrophils have different phenotypes and sometimes even show opposite functions, indicating their plasticity in the TME. 153 In addition, it is shown that platelets can form neutrophil‐platelet aggregates (NPAs) with neutrophils. The complex can boost the functions of neutrophils in the process of inflammation, which can worsen varieties of inflammatory diseases. 154 However, it was unclear if NPAs could play a similar role in the TME. According to recent studies, it is known that the NLR and PLR may have a role in predicting tumor response and prognosis. 155 , 156 Maybe the two markers can reveal a completely new approach for the treatment of cancer patients, which is required to be explored further.
In addition, in the TME, platelets interact with the endothelial cells to help the initiation, metastasis, and angiogenesis to facilitate the progression of tumors. It was discovered that by secreting granule contents, such as 5‐hydroxytryptamine, platelet factor IV, TGF‐β, or directly adhering to damaged blood vessels, TAPs are able to maintain the integrity of tumor vascular endothelium and prevent intratumor hemorrhage. 157 , 158 The significant finding can provide nutrients for blood vessels.
CAFs, as a major component in the TME, exert essential effects on the development of tumors. A number of evidence suggested that only CAFs cannot affect the tumor. The interaction between other cells and CAFs can facilitate the growth of tumors. CAFs can be activated by PDGF signaling mediated by platelets. Activated CAFs can secrete some factors to affect the status of platelets. 159 PDGFB released from platelets can enhance the accumulation of CAFs and deposition of extracellular matrix. 160 Abundant studies indicated that PDGF is indispensable for the function of the CAFs. In addition, TGF‐β secreted by platelets can improve the proliferative ability of fibroblasts, which enhance the malignancy of tumors. 161
6. PLATELETS AND CANCER TREATMENT
Platelet has been proven to be a biomarker for cancer prediction, detection, and prognosis in the future due to changes in its number, nucleic acid information, molecular expression, and transcriptional profile. Based on its interaction with tumors, scientists can perform anticancer treatments by disrupting their communication as well as using nanotechnology.
6.1. Platelets as a biomarker
The use of blood components as biomarkers for cancer prediction and detection is a promising development. In addition to CTCs and cell‐free DNA, cancer‐associated platelets are also promising biomarkers. 162 , 163 , 164 , 165 As platelets are the second most numerous blood cell in the blood, they can be isolated and counted easily. In addition, platelets also store various biomolecules released by cancer cells because they actively absorb and store proteins and nucleic acids from outside. Information on PCs, protein and mRNA profiles, and their activation status can be determined to monitor the presence and, to some extent, the location of tumors. However, as platelets are prone to contamination by white blood cells during isolation and platelet activation during isolation can affect the results of the analysis, establishing a stable methodological procedure for platelet purification would help to make platelets an official cancer biomarker.
PC was shown to be an independent prognostic factor predicting the survival of patients with breast cancer. 166 It was negatively correlated with overall survival and disease‐free survival in those patients. 166 Another research indicated that PC also had prognostic value in operable, nonmetastatic breast carcinoma and the preoperative PC can be combined with the level of CTCs to predict cancer progression. 167 A novel study indicated that platelet RNA may be a liquid biopsy biomarker for pan‐cancer at an early stage. This may compensate for the shortage of mutated cf DNA because its plasma level is very low during the early cancer stage. 168 Besides, nuclear receptor‐interacting protein 1 RNA, a specific circular RNA of platelets, can also be used for detecting non‐small cell lung cancer (NSCLC) due to its differential expression between NSCLC patients and healthy individuals. 169 When researchers decreased the number of platelets, the ability of chemotherapeutic drugs to poison cancer cells increased, indicating that platelets have an antichemotherapeutic drug effect. 123 , 170 Studies also found that cancer patients with higher PCs had poorer postchemotherapy outcomes and less effective treatment. 171 These findings seem to suggest that inhibiting the number or activity of platelets may improve the efficacy of chemotherapy drugs. For instance, it has been shown that the combination of multi‐kinase inhibitor sorafenib with resminostat (an inhibitor of histone deacetylase) significantly impeded platelet‐triggered invasion of hepatocellular carcinoma, with downregulated platelet‐induced CD44 expression (a cancer stem cell marker) and EMT genes. 172 Additionally, the efficacy of this combination treatment depends on PC. 172
Apart from nucleic acids and PC, molecules expressed on platelets have also been used to reflect cancer progression (Figure 6). For instance, in NSCLCs, PD‐L1 can be transferred from tumor cells to platelets and the PD‐L1 level of platelets can reflect the total expression of this molecule on tumor cells. 173 Thus, detecting platelet PD‐L1 level overcomes the difficulty in histological quantification of PD‐L1 level in tumor biopsies, and it can also predict the response of cancer cells to immune‐checkpoint inhibitor therapy. 173 Substances secreted by platelets may also reflect tumor development. For instance, there is a positive correlation between the levels of PDGFR and tumorigenesis. Thus, a novel biosensor for monitoring cancers was designed to detect the PDGFR based on a charge‐based affinity bait molecule, which was superior to using antigen‐antibody reactions for detection. 174 In glioblastoma (GMB), circulating platelets often express or produce various proangiogenic factors, such as P‐selectin and VEGFRs, differentiating patients with GME from healthy individuals. Thus, by measuring the expression level of these cargoes of platelets, the development of GBM may be monitored. 175 Platelets also produce microparticles which can be combined with neutrophil‐derived microparticles and neutrophil/lymphocyte ratio to construct a predictive model for NSCLC development. 176
FIGURE 6.
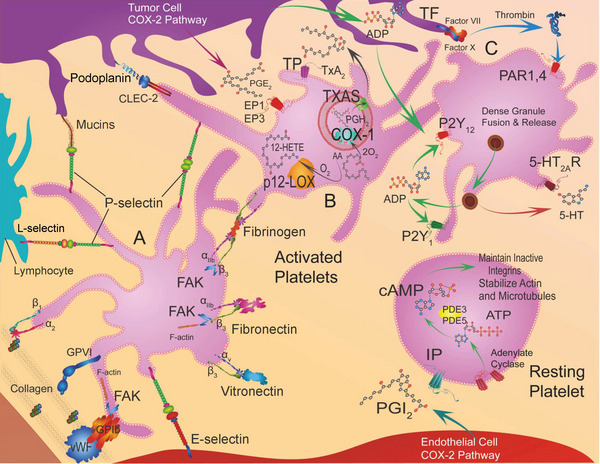
Presentation of platelet receptors and intracellular signaling among several kinds of cells. In platelet A, it shows that adhesion molecules on the platelets which can interact with endothelial cells, lymphocytes, other platelets. In platelet B, TXAS can generate TxA2, which can activate TP receptors. And cyclooxygenase‐1 (COX‐1) can synthesize PGH2, which can stimulate EP1 and EP3 receptors. In platelet C, the action of protease‐activated receptors (PAR1 and 4) can stimulate the platelets. Tumor cells can release ADP to activate P2Y1 or P2Y12 receptors. These targets provide possible therapies. Reproduced with permission from reference 37.
The transcriptional profile of normal platelets differs from that of platelets isolated from patients with platelet‐associated diseases. 177 Based on this evidence, platelets are an attractive platform for cancer diagnosis and prognosis. Specifically, scientists have focused on tumor‐educated blood platelets (TEPs) as these platelets changed their transcription profile during the cross‐talk with tumor cells. 178 It has been reported that through sequencing mRNA data of TEPs, multiple cancers can be diagnosed with accuracy reaching 90%. 178 Besides, this RNA information can also be used to identify locations of primary tumors and specific tumor mutations, such as KRAS and PIK3CA mutations. 178 Further, diagnostic and prognostic readout of TEP RNA sequencing can be optimized by swarm intelligence, which leads to accurate detection of early and late‐stage NSCLC and early CRC. 179 , 180 The expression of TEPs mRNAs is different between healthy individuals and cancer patients. For example, Xing et al. discovered that the integrin alpha 2b (ITGA2B) RNA level of TEPs can be used as a candidate marker for differentiating malignant lung nodules from benign ones, and diagnosing stage I NSCLC. 182
With the progress of the molecular biology of tumors, the study of platelet‐based RNA sequencing and its biological indicators will potentially provide a series of convenient and rapid molecular biology test markers for early diagnosis, prognosis determination, and follow‐up of clinical tumors (Table 1). It can provide a valuable platform where the diagnosis and prognosis can make progress.
TABLE 1.
Biomarkers of platelets in cancer diagnosis and monitoring.
| Biomarkers | Correlation with platelets | Application | Reference |
|---|---|---|---|
| Platelet counts | Intrinsic characteristics | Nonmetastatic breast carcinoma/drug response | 123 , 163 , 170 , 171 |
| NRIP1 RNA, circRNA | The differential expression of RNA in platelets between healthy people and cancer patients | Pan‐cancer/NSCLC | 168 , 169 |
| PD‐L1 | PD‐L1 can be transferred from tumor cells to platelets; PD‐L1 levels of platelets can reveal the therapeutic effects | NSCLCs/immune‐checkpoint inhibitor therapies | 173 |
| PDGFR | Platelet‐derived growth factor is a proangiogenic factor isolated from human platelets, platelet‐derived growth factor receptor is a member of the caseomic acid protein kinase family, which can promote the chemotaxis, division and proliferation of cells in the body, growth and development, wound repair | Monitoring | 174 |
| VEGFRs | Platelets express or produce various proangiogenic factors like VEGFRs | GMB | 175 |
| Microparticles | Produced by platelets to detect the progression of cancer | NSCLC development | 176 |
| mRNA profiles | The expression mRNA of platelets between healthy people and cancer patients | Diagnosis/identification, location, malignancy, stage, and mutation of tumors | 177 , 178 , 179 , 180 , 181 |
Abbreviations: circRNA, circular RNA; NRIP1, nuclear receptor‐interacting protein 1; PDGFR, platelet‐derived growth factor receptor; PD‐L1, programmed death ligand 1; VEGFR, vascular endothelial growth factor receptor.
6.2. Targeted therapy strategies based on platelet−tumor interactions
The close interaction between platelets and tumors makes the use of platelets as a drug delivery vehicle or platform extremely attractive. Therefore, platelet‐based targeted therapeutic strategies have been attracting the attention of researchers and are increasingly being applied in oncology treatment.
6.2.1. Disruption of the crosstalk between platelets and cancers
Due to the close relationship between platelets and cancer cells, platelets can serve as a target for antitumor treatments.
As most tumor cells require abundant blood supply and reside in highly vascularized environment, antiangiogenic treatment can be effective against cancer development. However, this treatment has drawbacks and may not improve prognosis. 182 Fortunately, focal adhesion kinase (FAK) in platelets was demonstrated to be a promising target, which modulated the platelet extravasation into TME, further inducing the platelet−tumor interaction. 183 It has been shown that after cessation of antiangiogenic therapy, the augmented infiltration of platelets in the tumor was associated with cancer rebound which can be strongly inhibited by FAK inhibitors or FAK‐deficient platelets. 183 Moreover, a combination of FAK inhibitors and antiangiogenic drugs, such as anti‐VEGF antibody bevacizumab, has strong effects in killing tumor and can prevent the negative effects caused by the withdrawal of antiangiogenic drugs. 183
In vitro studies showed the inhibition of specific molecules released by tumor cells can provide treatment. Human colon adenocarcinoma caco‐2 cell line released ADP and MMP‐2 to induce platelet activation and aggregation. 174 When ADP and MMP‐2 are inhibited, respectively, the binding of tumor cells and platelets is reduced.187 Therefore, when lack of these substances, the expression of platelet GpIIb/IIIa, GpIb, and P‐selectin can be downregulated. Another study indicated that α‐Hederin suppressed the platelet‐activating factor receptor (PTAFR), thus blocking the PAF/PTAFR pathway that normally induces tumor cell migration and invasion. 185 In addition, the expression of MMP‐2 can also be hampered by α‐Hederin via preventing the activation of STAT3 in platelet‐activating factor (PAF)‐stimulated tumor cells. 170 Therefore, by targeting platelet activation, tumor development can be hindered. The interaction between CLEC2−PDPN exerts significant effects on metastasis, which can be an underlying target in tumor therapy. By blocking the interaction, the invasion and metastasis can be prevented. 123
In recent decades, PDGFR has become a popular target in treating cancers. For instance, it has been shown that in osteosarcoma, specific inhibition of PDGFRα/β kinases by pyrimidine‐2,4‐diamine derivatives can significantly kill tumor cell lines. 186 Another study demonstrated that PDGFRC was overexpressed in triple‐negative breast cancer patients and was associated with the patients’ survival. 187 Additionally, after PDGFC and its receptors are inhibited, the drug effect of doxorubicin (DOX) can be enhanced, with more tumor apoptosis than only using DOX. 187 Nayeem et al. discovered that PDGFRα was overexpressed in prostate cancer and the phosphorylation of this molecule and its downstream effector such as Akt was associated with tumorigenesis. However, the PDGFRα/Akt axis can be inhibited by Imatinib, a tyrosine kinase inhibitor, suppressing the growth and metastasis of tumor cells. 188 In the therapy of hepatocellular carcinoma, JI‐MT, a novel indomethacin derivative, showed cytotoxic effect in the cancer cell line and reduced the liver cancer nodules in patients, via inhibiting PDGFR‐α. 189 The overexpression of PDGFR‐α has also been found in GMB multiforme and HIF1α inhibitor echinomycin can target HIF1α‐PDGFD/PDGFRα‐AKT pathway, promoting the apoptosis of tumor cells at normoxia or mild‐hypoxia. 190
Besides, as platelets can also assist tumors to evade the immune system, a related therapeutic strategy can be designed. For instance, a study indicated that platelets can increase the expression of PD‐L1 on lung and renal cancer cells, which resulted in tumor immune invasion. 191 However, this can be reversed by eptifibatide, an antiplatelet drug. This suggests that antiplatelet drugs may be used as adjuvant to immune checkpoint inhibitors to treat tumors. 191 The chimera antigen receptor (CAR) T cell therapy changed the synthesis of PAFs, which can be indicated by altered levels of lysophosphatidylcholines, a substance involved in the PAF synthesis, in multiple myeloma patients treated with CAR‐T. 170 This suggests that treatment of multiple myeloma by CAR‐T therapy may be promoted by targeting PAF remodeling. 170 The characteristics of platelets can provide directions for targeted therapies. Platelets can be involved in the process of coagulation. When blood vessels are damaged, TFs are released to initiate coagulation. During the process, platelets can accumulate. If the damage is on the tumor blood vessels, platelets with tumor‐killing drugs can also reach the damaged sites. These platelets were modified to target the tumor cells instead of normal cells. Immune checkpoint inhibitors can target the tumor cells by activating T cells. 68 It is known that immune checkpoint HLA‐E:CD94‐NKG2A can help the tumor cells to evade the immune system. By targeting the immune checkpoint, it can provide new clews for the targeted therapy. 69
6.2.2. Application of nano‐modified platelet carriers in anticancer therapy
In recent decades, nanotechnology has thrived and an increasing number of nanoparticles have been combined with platelets to achieve better therapy against cancers.
PMs have various molecules that can interact with cancer cells. Therefore, nanoparticles decorated with molecules derived from platelets have been explored. A recent study designed a biomimetic nanoparticle coated with PM, which contained resiquimod (R848), an agonist of toll‐like receptor, and can locally deliver the drug intratumorally. 192 Due to the biocompatibility of the PM, this R848‐loaded nanoparticle can stay in the tumor environment for a longer time with a higher affinity for cancer cells, which results in prolonged and enhanced antitumor immunity. 192 Likewise, PM‐based nanoparticles can also be loaded with apatinib, an antiangiogenic drug, and vascular disruption agents, which can amass in tumor tissues owing to adhesion molecules on the membrane attaching to impaired vessels. This can effectively damage vessels and disrupt tumor angiogenesis. 193 PM has also been applied to novel cancer treatment, such as glucose oxidase (GOx)‐based starvation therapy. 194 Specifically, GOx‐functionalized PMs have been made to coat manganese dioxide which is a nanoshell assembling DOX‐loaded nanoparticles. This results in enhanced and more targeted tumor‐killing effects. 194 Li et al. 195 discovered that compared with free DOX, DOX attached to nanodiamonds coated with PM showed a longer time staying in blood circulation and stronger inhibition of tumor growth. PM has been used to camouflage single‐atom nanozyme. For instance, mesoporous Fe single‐atom nanozyme coated with platelets has been made, which can destroy mitochondria via hampering the expression of heat shock proteins and thus strengthen the mild‐temperature photothermal therapy. 196 Interestingly, scientists have made a membrane combined with membranes of both platelets and tumor cells. This hybrid membrane was used to coat the β‐mangostin‐loaded nanoparticles, showing an improved ability targeting glioma. 197 Taken together, due to the coating by PM, drug‐loaded nanoparticles tend to accumulate in tumor sites and have stronger therapeutic potency, better biocompatibility, and less systemic side effects on essential organs.
Apart from PM, platelet vesicles have also been investigated in cancer treatment combined with nanotechnology. For example, platelet exosomes were used, together with photothermal sensitive liposomes, to incorporate GOx and ferric ammonium, thus constructing a nanosystem controlled by a laser (FG@PEL). 198 Photothermal effect can induce vascular damage, which activates platelet exosomes that often stick to tumor cells due to their biological property. 198 This allows FG@PEL to exert its cascade targeting effect against tumor. 198 For the treatment of pancreatic cancer, platelet vesicles were used to coat RSL‐3, a drug that can pass through the stroma and disrupt tumor angiogenesis. Due to platelet vesicles, the uptake of the drug by tumor cells increased, which enhanced tumor embolism and cell death. 199
The capture of platelets by P‐selectin was also explored. For example, nanoparticles coated with a P‐selectin‐targeting peptide (PSN peptide) were constructed, and the core of these nanoparticles was filled with ticagrelor or celecoxib. 200 Through capturing activated platelets, these PSN peptide‐modified nanocarriers have improved capability in accumulating at the primary tumor and premetastatic sites, hence robustly inhibiting platelet−tumor interaction and inflammation. 200 As TAPs are often captured by primary tumor cells to facilitate the development of premetastatic niches, these nanoparticles also inhibit tumor metastasis. 200
Without combination with nanotechnology, platelets themselves can act as carriers to deliver drugs. For instance, Bhandarkar et al. 201 designed a quercetin (QCT)‐loaded platelet which had high encapsulation efficiency due to the canalicular system in platelets that allowed the drug to be loaded into the cytoplasm of platelets. These QCT‐loaded platelets showed great ability to kill GMB cells in vitro and are promising to target GMB tumor in humans. 201 Also, platelets can be loaded with gold nanorods (AuNRs) carrying photothermal agents. 202 These AuNR‐loaded platelets can stay stable in the blood and target tumor cells precisely after laser irradiation. 202 Therefore, platelet inhibitors play an essential role in tumor therapy (Table 2).
TABLE 2.
Summary of the application of platelet inhibitors in tumor therapy.
| Platelet inhibitors | Detailed introduction | Mechanism | Reference |
|---|---|---|---|
| FAK inhibitors | FAK in platelets was demonstrated to be a promising target, further causing the platelet−tumor interaction | Controlling the platelet extravasation | 183 |
| ADP/MMP‐2 inhibitors | ADP and MMP‐2 released from tumor cells | Reducing the activation and aggregation of platelets | 184 |
| PTAFR inhibitors | The receptor of platelet‐activating factor (PAF). PAF can activate the platelets | Blocking PAF/PTAFR pathway | 185 |
| α‐Hederin | Expression of matrix metalloproteinase‐2 can be hampered by α‐Hederin | Inhibiting the activation of STAT3 | 185 |
| PDGFR inhibitors | PDGFR expressed in the tumor cells | Inhibiting PDGFRα/β kinases | 186 , 187 |
| Imatinib | Inhibit PDGFRα/Akt and disrupting the action of PDGF | Tyrosine kinase inhibitor | 188 |
| HIF1α | Inhibiting the expression of PDGFR in tumor cells | Inhibitor echinomycin | 190 |
| Eptifibatide | PD‐L1 inhibitor | Immune checkpoint inhibitors | 191 |
| CAR‐T therapy | Chimera antigen receptor (CAR) T cell therapy changes the synthesis of platelet‐activating factors (PAFs) | Targeting PAF remodeling | 170 |
| Targeting immune checkpoint HLA‐E:CD94‐NKG2A | Immune checkpoint HLA‐E:CD94‐NKG2A on the tumor cells and T cell | Inhibiting the immune checkpoint | 69 |
| R848‐loaded nanoparticle | Coated with platelet membrane, which contained resiquimod (R848) and the drug | Agonist of toll‐like receptor | 192 |
| Nanoparticle with apatiniband and VDAs | Platelet membrane‐based nanoparticles loaded with apatinib, an antiangiogenic drug | Damage vessels and disrupt tumor angiogenesis | 193 |
| Glucose oxidase (GOx)‐based starvation therapy | Application of platelet membrane | Improve the efficacy of killing tumors | 194 |
| Mesoporous Fe single‐atom nanozyme (Fe‐SAzyme) coated with platelets | Platelet membrane used to camouflage single‐atom nanozyme | Destroy mitochondria | 196 |
| β‐Mangostin‐loaded nanoparticles | The hybrid membrane of both platelets and tumor cells used to coat the β‐mangostin‐loaded nanoparticles | Improved ability targeting glioma | 197 |
| Platelet exosomes | Produced by exosomes | FG@PEL to exert its cascade targeting effect against tumor | 198 |
| Platelet vesicles with RSL‐3 | Platelet vesicles used to coat RSL‐3 | Pass through the stroma and disrupt tumor angiogenesis | 199 |
| A nanoparticle coated with PSN peptide | Nanoparticles coated with a P‐selectin‐targeting peptide | Inhibiting platelet−tumor interaction and inflammation | 200 |
| Quercetin (QCT)‐loaded platelet | Drug to be loaded into the cytoplasm of platelets | Target the glioblastoma tumor | 201 |
| AuNR‐loaded platelets | Platelets loaded using AuNR | Target tumor cells precisely | 202 |
Abbreviations: CART, chimera antigen receptor T cell therapy; FAK, focal adhesion kinase; Fe‐SAzyme, Fe single‐atom nanozyme; GOx, glucose oxidase; MMP‐2, matrix metalloprotein‐2; PDGFR, platelet‐derived growth factor; PTAFR, platelet‐activating factor receptor.
6.3. Current clinical trial progression
Nowadays, many current clinical trials about the function and regulation of platelets in cancer treatment have been in progression (Table 3). A research team values tumor‐educated platelets ITGA2B and selectin P (SELP) mRNA expression in pancreatic and biliary tract cancer. They take expression levels of ITGA2b and SELP on tumor‐educated platelets for diagnosis of pancreatic and biliary cancer as the standard to study the expression pattern of ITGA2b and SELP genes of tumor‐educated platelets in pancreatic and biliary tree cancer and its diagnostic value. Xing et al. 182 have found that TEP ITGA2B is an important marker to improve the recognition of patients with stage I NSCLC and differentiate malignant from benign lung nodules. D'Ambrosi et al. 203 indicate that further research on the RNA transfer mechanism between platelets and cancer cells can help us better understand and monitor the progression of cancer. What is more, the study showed the effects of inhibiting platelet function on circulating cancer cells for breast cancer patients. The purpose of this study is to determine the effects of Plavix and aspirin in women with metastatic breast cancer, and now the study is in phase 2. Platelet inhibition of CTCs is measured by the number of patients with detectable CTCs. 203 Elevated PC as prognostic factor in colorectal cancer with synchronous liver metastases is also a new study. The aim of this study was to evaluate the role of preoperative PC in patients with synchronous colorectal liver metastases. Pedrazzani et al. 204 research found that high PC values are significantly associated with overall survival and cancer‐related survival in TNM stage IV patients.
TABLE 3.
Current status of academic research on the function and regulation of platelets in cancer therapy.
| Interventions | Clinical registries | Status | Study results | Conditions | Study start | Study completion | Reference |
|---|---|---|---|---|---|---|---|
| Diagnostic test: mRNA expression | NCT05493878 | Not yet recruiting | No results available | Pancreatic and biliary tract cancer | 2023‐03 (estimated) | 2024‐05 (estimated) | None |
| Diagnostic test: mRNA expression | GZR2017‐186 | Completed | No results available | Non‐small‐cell lung cancer | Not reported | 2017 (actual) | 182 |
| Biological: A single 2 mL heparinized peripheral blood | NCT02758678 | Completed | No results available | Lung cancer | 2015‐11 (actual) | 2016‐10 (actual) | None |
| Biological: Blood samples containing K2‐EDTA | 42763‐CRINF‐1034 CESC | Completed | No results available | Colorectal cancer | Not reported | 204 | |
|
Drug: Plavix Drug: Aspirin |
NCT00263211 | Terminated | Has results | Breast neoplasms | 2006‐01 (actual) | 2009‐09 (actual) | None |
| Diagnostic test: Platelet count | NCT02211677 | Recruiting | No results available | Nasopharyngeal carcinoma | 2014‐08 (actual) | 2024‐12 (estimated) | None |
|
Drug: Pembrolizumab Drug: Clopidogrel Drug: Acetylsalicylic acid |
NCT03245489 | Recruiting | No results available | Recurrent or metastatic squamous cell carcinoma of the head and neck | 2017‐10‐20 (actual) | 2023‐12‐31 (estimated) | None |
Some researches evaluate the expression of P selectin on platelets of blood between serum samples from lung cancer and healthy individuals through serum separation and Raman spectroscopy analysis. Assessment of the concentration of P‐selectin in three groups in lung cancer patients and comparing the concentration of P‐selectin depends on tumor volume. Comparisons were made between the three groups and finally with the healthy population. Bendas and Borsig point out that integrins were shown to contribute to cancer progression. 205
Best et al. 179 characterize the platelet mRNA profiles of various crowds. They provide powerful evidence for the clinical relevance of blood platelets for liquid biopsy‐based molecular diagnostics in patients with different types of cancer.
7. CONCLUSION
With the in‐depth study of platelet function, the hypothesis that platelets regulate the TME to promote tumor cell proliferation and metastasis through various ways has been gradually confirmed. This includes the formation of TCIPA to assist tumor cell immune escape and promotion of neovascularization leading to tumor cell extravasation (Figure 7). In addition, platelets are involved in tumor growth and metastasis through various mechanisms.For example, platelets releasing a large amount of active substances can interact with tumor cells leading to the invasion and metastasis of tumor cells. Compared with the large amount of evidence that platelets promote tumor metastasis, some experiments have found that platelets can inhibit tumor metastasis. This process is affected by many factors, providing new clues for the treatment of tumor metastasis. However, the specific mechanism is still unclear, and further studies about the relationship between platelets and tumors are worthwhile in the future, so as to prevent the tumor metastasis and progression.
FIGURE 7.
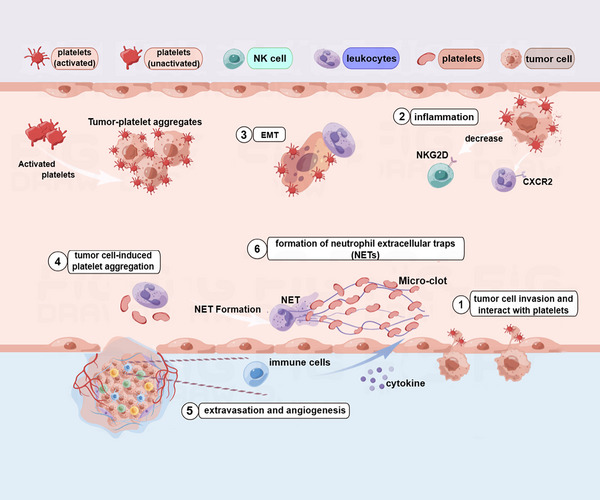
The summary of interactions between tumor cells and platelets. ① Platelets can facilitate the tumor cells invasion. ② The interaction can cause inflammation, causing the decrease of NKG2D on the surface of NK cells. ③ Leukocytes help the EMT. ④ In addition, tumor cells can cause platelet aggregation. ⑤ These can help the tumor cells extravasation and angiogenesis. ⑥ In the blood vessels, neutrophil extracellular traps (NETs) can stimulate the migration and invasion of cancer cells. Immune cells in the tumor microenvironment can release cytokines to promote the growth of cancer. The figure is made using Figdraw.
Currently, there are clinical observations on the effects of antiplatelet drugs on recurrence and metastasis in tumor patients. Focusing on the platelets, the decrease of platelets can promote treatment. Antiplatelet drugs, such as aspirin (pro‐oxygenase inhibitors), can contribute to the prevention, occurrence, and recurrence of tumors, especially liver disease. It may be due to the fact that the liver is the main organ producing coagulation proteins. However, it remains questionable whether the antitumor effect of these drugs is the result of platelet inhibition, and drug resistance research is also an urgent question that needs to be addressed. Targeting the molecules or specific proteins, such as enzymes, provides effective ideas for cancer treatment. In addition, disturbing any process in the interaction between platelets and tumor cells is useful, such as angiogenesis, immune escape, and so on. More in‐depth and detailed studies are needed because platelets are involved in every process of tumor hematogenous metastasis, and factors, such as treatment time, dose, tumor site, and type, need to be considered. Nowadays, platelets assisting tumor cell invasion and metastasis are receiving increasing attention from researchers and can be a key direction and area for future treatment of oncological diseases.
This review summarized the interactions between platelets and tumors and furtherexplained the potential mechanisms of tumor cell homing and growth, tumor cell metastasis (including immune escape, angiogenesis, EMT), TME, and other processes regulating tumor development. What is more, the role and significance of platelets as cancer biomarkers is also pointed out. On this basis, we outlined the application of platelet‐based targeted therapeutic strategies in tumor treatment, and discussed the research field and corresponding development applications. In the future, more and more experiments will be required to validate the antitumor effects. According to the characteristics of platelets, it is possible to explore more drugs. In addition, platelets can combine with other antitumor therapies, which may be a direction in cancer therapies.
AUTHOR CONTRIBUTIONS
Kaili Liao, Xue Zhang, and Wenyige Zhang wrote the main manuscript text and Qijun Yang, Feifei Teng, Yingcheng He, , Jie Liu, Daixin Guo, Gaoquan Cao, Yanmei Xu,Bo Huang and Yuxuan Xie searched the literature and prepared Figures 1−7. Jie Liu and Jinting Cheng participated in the writing and manuscript revision. Xiaozhong Wang reviewed and revised the manuscript and wrote guidance. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
There are no conflict of interest in this study.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This article does not contain any studies with patients or animals performed by any of the authors.
ACKNOWLEDGMENTS
Thanks to Figdraw for the drawing material.
Liao K, Zhang X, Liu J, et al. The role of platelets in the regulation of tumor growth and metastasis: the mechanisms and targeted therapy. MedComm. 2023;4:e350. 10.1002/mco2.350
Kaili Liao, Xue Zhang, Jie Liu, and Feifei Teng contributed equally for this work.
DATA AVAILABILITY STATEMENT
All data are available. Please contact us to access if it is needed.
REFERENCES
- 1. McLane LM, Abdel‐Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457‐495. [DOI] [PubMed] [Google Scholar]
- 2. Sharma N, Atolagbe OT, Ge Z, Allison JP. LILRB4 suppresses immunity in solid tumors and is a potential target for immunotherapy. J Exp Med. 2021;218(7):e2020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuzet S‐E, Gaggioli C. Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res. 2016;365(3):607‐619. [DOI] [PubMed] [Google Scholar]
- 4. Fernandes CJ, Morinaga LTK, Alves JL, et al. Cancer‐associated thrombosis: the when, how and why. Eur Respir Rev. 2019;28(151):180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kekre N, Connors JM. Venous thromboembolism incidence in hematologic malignancies. Blood Rev. 2019;33:24‐32. [DOI] [PubMed] [Google Scholar]
- 6. Mulder FI, Horváth‐Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137(14):1959‐1969. [DOI] [PubMed] [Google Scholar]
- 7. Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70(8):926‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakin A, Sahin S, Sakin A, et al. Mean platelet volume and platelet distribution width correlates with prognosis of early colon cancer. J BUON. 2020;25(1):227‐239. [PubMed] [Google Scholar]
- 9. Yun Z‐Y, Zhang X, Liu Y‐S, et al. Lower mean platelet volume predicts poor prognosis in renal cell carcinoma. Sci Rep. 2017;7(1):6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li N, Diao Z, Huang X, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep. 2017;7(1):2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu S‐K, Chiu C‐C, Dahms H‐U, et al. Unfolded protein response (UPR) in survival, dormancy, immunosuppression, metastasis, and treatments of cancer cells. Int J Mol Sci. 2019;20(10):2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Cao X. Characteristics and significance of the pre‐metastatic niche. Cancer Cell. 2016;30(5):668‐681. [DOI] [PubMed] [Google Scholar]
- 13. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer. 2016;138(9):2078‐2087. [DOI] [PubMed] [Google Scholar]
- 15. Oakes SA. Endoplasmic reticulum stress signaling in cancer cells. Am J Pathol. 2020;190(5):934‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartoszewska S, Collawn JF. Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell Mol Biol Lett. 2020;25:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walsh TG, Metharom P, Berndt MC. The functional role of platelets in the regulation of angiogenesis. Platelets. 2015;26(3):199‐211. [DOI] [PubMed] [Google Scholar]
- 18. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yap ML, McFadyen JD, Wang X, et al. Targeting activated platelets: a unique and potentially universal approach for cancer imaging. Theranostics. 2017;7(10):2565‐2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer‐associated fibroblasts. Nat Rev Cancer. 2020;20(3):174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qi C, Li B, Guo S, et al. P‐selectin‐mediated adhesion between platelets and tumor cells promotes intestinal tumorigenesis in Apc(Min/+) mice. Int J Biol Sci. 2015;11(6):679‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497‐500. [DOI] [PubMed] [Google Scholar]
- 23. Yan M, Jurasz P. The role of platelets in the tumor microenvironment: from solid tumors to leukemia. Biochim Biophys Acta. 2016;1863(3):392‐400. [DOI] [PubMed] [Google Scholar]
- 24. Yan M, Lesyk G, Radziwon‐Balicka A, Jurasz P. Pharmacological regulation of platelet factors that influence tumor angiogenesis. Semin Oncol. 2014;41(3):370‐377. [DOI] [PubMed] [Google Scholar]
- 25. Zhang SR, Yao L, Wang WQ, et al. Tumor‐infiltrating platelets predict postsurgical survival in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(13):3984‐3993. [DOI] [PubMed] [Google Scholar]
- 26. Nakeff A, Maat B. Separation of megakaryocytes from mouse bone marrow by velocity sedimentation. Blood. 1974;43(4):591‐595. [PubMed] [Google Scholar]
- 27. Day RB, Link DC. Megakaryocytes in the hematopoietic stem cell niche. Nat Med. 2014;20(11):1233‐1234. [DOI] [PubMed] [Google Scholar]
- 28. Lefrançais E, Ortiz‐Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson JL, Shivdasani RA, Boers C, Hartwig JH, Italiano JE. Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood. 2005;106(13):4066‐4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Machlus KR, Italiano JE. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du Y, Chen B. Combination of drugs and carriers in drug delivery technology and its development. Drug Des Devel Ther. 2019;13:1401‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponomareva AA, Nevzorova TA, Mordakhanova ER, Andrianova IA, Litvinov RI. [Structural characterization of platelets and platelet‐derived microvesicles]. Tsitologiia. 2016;58(2):105‐114. [PubMed] [Google Scholar]
- 33. Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36(2):195‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gremmel T, Frelinger AL, Michelson AD. Platelet physiology. Semin Thromb Hemost. 2016;42(3):191‐204. [DOI] [PubMed] [Google Scholar]
- 35. Fiorini E, Veghini L, Corbo V. Modeling cell communication in cancer with organoids: making the complex simple. Front Cell Dev Biol. 2020;8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaertner F, Massberg S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat Rev Immunol. 2019;19(12):747‐760. [DOI] [PubMed] [Google Scholar]
- 37. Haemmerle M, Stone RL, Menter DG, Afshar‐Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palacios‐Acedo AL, Mège D, Crescence L, Dignat‐George F, Dubois C, Panicot‐Dubois L. Platelets, thrombo‐inflammation, and cancer: collaborating with the enemy. Front Immunol. 2019;10:1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boucharaba A, Serre C‐M, Grès S, et al. Platelet‐derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114(12):1714‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cho MS, Li J, Gonzalez‐Delgado R, et al. The effect of platelet G proteins on platelet extravasation and tumor growth in the murine model of ovarian cancer. Blood Adv. 2021;5(7):1947‐1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li R, Ren M, Chen N, et al. Presence of intratumoral platelets is associated with tumor vessel structure and metastasis. BMC Cancer. 2014;14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ortiz‐Otero N, Mohamed Z, King MR. Platelet‐based drug delivery for cancer applications. Adv Exp Med Biol. 2018;1092:235‐251. [DOI] [PubMed] [Google Scholar]
- 43. Janowska‐Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752‐760. [DOI] [PubMed] [Google Scholar]
- 44. Kim HK, Song KS, Park YS, et al. Elevated levels of circulating platelet microparticles, VEGF, IL‐6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39(2):184‐191. [DOI] [PubMed] [Google Scholar]
- 45. Michael JV, Wurtzel JGT, Mao GF, et al. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood. 2017;130(5):567‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Plantureux L, Mège D, Crescence L, et al. The interaction of platelets with colorectal cancer cells inhibits tumor growth but promotes metastasis. Cancer Res. 2020;80(2):291‐303. [DOI] [PubMed] [Google Scholar]
- 47. Chen X, Wang Q, Liu L, et al. Double‐sided effect of tumor microenvironment on platelets targeting nanoparticles. Biomaterials. 2018;183:258‐267. [DOI] [PubMed] [Google Scholar]
- 48. Xie D, Xie K. Pancreatic cancer stromal biology and therapy. Genes Dis. 2015;2(2):133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu K‐Y, Ning Z‐C. Roles of platelets in tumor metastasis. Chin J Biochem Mol Biol. 2022;38(8):1006‐1014. [Google Scholar]
- 50. Haemmerle M, Taylor ML, Gutschner T, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Asghar S, Parvaiz F, Manzoor S. Multifaceted role of cancer educated platelets in survival of cancer cells. Thromb Res. 2019;177:42‐50. [DOI] [PubMed] [Google Scholar]
- 52. Suzuki ‐Inoue K. Roles of the CLEC‐2–podoplanin interaction in tumor progression. Platelets. 2018; 29 :786‐792. [DOI] [PubMed] [Google Scholar]
- 53. Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist‐responsive manner. Blood. 2012;120(26):5209‐5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu X, Song J, Zhang H, et al. Immune checkpoint HLA‐E:CD94‐NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41(2):272‐287. e9. [DOI] [PubMed] [Google Scholar]
- 55. Ma C, Fu Q, Diggs LP, et al. Platelets control liver tumor growth through P2Y12‐dependent CD40L release in NAFLD. Cancer Cell. 2022;40(9):986‐998. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pu F, Li X, Wang S, Huang Y, Wang D. Platelet supernatant with longer storage inhibits tumor cell growth. Transfus Apher Sci. 2021;60(2):103042. [DOI] [PubMed] [Google Scholar]
- 57. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362‐2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133(2):S63‐S69. [DOI] [PubMed] [Google Scholar]
- 59. Steinbichler TB, Dudás J, Riechelmann H, Skvortsova I‐I. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170‐181. [DOI] [PubMed] [Google Scholar]
- 60. Foss A, Muñoz‐Sagredo L, Sleeman J, Thiele W. The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin Exp Metastasis. 2020;37(1):47‐67. [DOI] [PubMed] [Google Scholar]
- 61. Tao DL, Tassi Yunga S, Williams CD, McCarty OJT. Aspirin and antiplatelet treatments in cancer. Blood. 2021;137(23):3201‐3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Flavell RA, Sanjabi S, Wrzesinski SH, Licona‐Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Metelli A, Salem M, Wallace CH, et al. Immunoregulatory functions and the therapeutic implications of GARP‐TGF‐β in inflammation and cancer. J Hematol Oncol. 2018;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Metelli A, Wu BX, Fugle CW, et al. Surface expression of TGFβ docking receptor GARP promotes oncogenesis and immune tolerance in breast cancer. Cancer Res. 2016;76(24):7106‐7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cluxton CD, Spillane C, O'Toole SA, et al. Suppression of natural killer cell NKG2D and CD226 anti‐tumour cascades by platelet cloaked cancer cells: implications for the metastatic cascade. PLoS One. 2019;14(3):e0211538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waldhauer I, Goehlsdorf D, Gieseke F, et al. Tumor‐associated MICA is shed by ADAM proteases. Cancer Res. 2008;68(15):6368‐6376. [DOI] [PubMed] [Google Scholar]
- 67. Chitadze G, Lettau M, Bhat J, et al. Shedding of endogenous MHC class I‐related chain molecules A and B from different human tumor entities: heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer. 2013;133(7):1557‐1566. [DOI] [PubMed] [Google Scholar]
- 68. Lee DY, Im E, Yoon D, et al. Pivotal role of PD‐1/PD‐L1 immune checkpoints in immune escape and cancer progression: their interplay with platelets and FOXP3+Tregs related molecules, clinical implications and combinational potential with phytochemicals. Semin Cancer Biol. 2022;86(3):1033‐1057. [DOI] [PubMed] [Google Scholar]
- 69. Maurer S, Kopp H‐G, Salih HR, Kropp KN. Modulation of immune responses by platelet‐derived ADAM10. Front Immunol. 2020;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clar KL, Hinterleitner C, Schneider P, Salih HR, Maurer S. Inhibition of NK reactivity against solid tumors by platelet‐derived RANKL. Cancers (Basel). 2019;11(3):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Placke T, Salih HR, Kopp H‐G. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol. 2012;189(1):154‐160. [DOI] [PubMed] [Google Scholar]
- 72. Kopp H‐G, Placke T, Salih HR. Platelet‐derived transforming growth factor‐beta down‐regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69(19):7775‐7783. [DOI] [PubMed] [Google Scholar]
- 73. Guo S‐W, Du Y, Liu X. Platelet‐derived TGF‐β1 mediates the down‐modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis. Hum Reprod. 2016;31(7):1462‐1474. [DOI] [PubMed] [Google Scholar]
- 74. Placke T, Kopp H‐G, Salih HR. The wolf in sheep's clothing: platelet‐derived “pseudo self” impairs cancer cell “missing self” recognition by NK cells. Oncoimmunology. 2012;1(4):557‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prestipino A, Emhardt AJ, Aumann K, et al. Oncogenic JAK2 causes PD‐L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med. 2018;10(429):eaam7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409‐426. [DOI] [PubMed] [Google Scholar]
- 77. Kuznetsov HS, Marsh T, Markens BA, et al. Identification of luminal breast cancers that establish a tumor‐supportive macroenvironment defined by proangiogenic platelets and bone marrow‐derived cells. Cancer Discov. 2012;2(12):1150‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70(5):1194‐1201. [DOI] [PubMed] [Google Scholar]
- 79. Bluff JE, Menakuru SR, Cross SS, et al. Angiogenesis is associated with the onset of hyperplasia in human ductal breast disease. Br J Cancer. 2009;101(4):666‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15(4):197‐204. [DOI] [PubMed] [Google Scholar]
- 82. Östman A. PDGF receptors in tumor stroma: biological effects and associations with prognosis and response to treatment. Adv Drug Deliv Rev. 2017;121:117‐123. [DOI] [PubMed] [Google Scholar]
- 83. Feng W, Madajka M, Kerr BA, et al. A novel role for platelet secretion in angiogenesis: mediating bone marrow‐derived cell mobilization and homing. Blood. 2011;117(14):3893‐3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sabrkhany S, Griffioen AW, Oude Egbrink MGA. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. 2011;1815(2):189‐196. [DOI] [PubMed] [Google Scholar]
- 86. Italiano JE, Richardson JL, Patel‐Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro‐ and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Freedman JE. CD40‐CD40L and platelet function: beyond hemostasis. Circ Res. 2003;92(9):944‐946. [DOI] [PubMed] [Google Scholar]
- 88. Bou Khzam L, Boulahya R, Abou‐Saleh H, Hachem A, Zaid Y, Merhi Y. Soluble CD40 ligand stimulates the pro‐angiogenic function of peripheral blood angiogenic outgrowth cells via increased release of matrix metalloproteinase‐9. PLoS One. 2013;8(12):e84289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trikha M, Zhou Z, Timar J, et al. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002;62(10):2824‐2833. [PubMed] [Google Scholar]
- 90. Ho‐Tin‐Noé B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68(16):6851‐6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li S, Zou H, Gong M, et al. Angiopoietin‐1 promotes the integrity of neovascularization in the subcutaneous matrigel of type 1 diabetic rats. BioMed Res Int. 2019;2019:2016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ho‐Tin‐Noé B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009;175(4):1699‐1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang Y, Cedervall J, Hamidi A, et al. Platelet‐specific PDGFB ablation impairs tumor vessel integrity and promotes metastasis. Cancer Res. 2020;80(16):3345‐3358. [DOI] [PubMed] [Google Scholar]
- 94. Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers. 2015;3(1‐2):e978720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet‐derived growth factor. Physiol Rev. 1999;79(4):1283‐1316. [DOI] [PubMed] [Google Scholar]
- 96. Zou X, Tang XY, Qu ZY, et al. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: a review. Int J Biol Macromol. 2022;202:539‐557. [DOI] [PubMed] [Google Scholar]
- 97. Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial‐mesenchymal‐like transition and promotes metastasis. Cancer Cell. 2011;20(5):576‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Steller EJA, Raats DA, Koster J, et al. PDGFRB promotes liver metastasis formation of mesenchymal‐like colorectal tumor cells. Neoplasia. 2013;15(2):204‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pan S, Hu Y, Hu M, et al. Platelet‐derived PDGF promotes the invasion and metastasis of cholangiocarcinoma by upregulating MMP2/MMP9 expression and inducing EMT via the p38/MAPK signalling pathway. Am J Transl Res. 2020;12(7):3577‐3595. [PMC free article] [PubMed] [Google Scholar]
- 101. Yoon H, Tang C‐M, Banerjee S, et al. Cancer‐associated fibroblast secretion of PDGFC promotes gastrointestinal stromal tumor growth and metastasis. Oncogene. 2021;40(11):1957‐1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Saito R, Shoda K, Maruyama S, et al. Platelets enhance malignant behaviours of gastric cancer cells via direct contacts. Br J Cancer. 2021;124(3):570‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kılınçalp S, Ekiz F, Başar O, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25(8):592‐594. [DOI] [PubMed] [Google Scholar]
- 104. Dovizio M, Maier TJ, Alberti S, et al. Pharmacological inhibition of platelet‐tumor cell cross‐talk prevents platelet‐induced overexpression of cyclooxygenase‐2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84(1):25‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ward Y, Lake R, Faraji F, et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018;23(3):808‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Adams GN, Rosenfeldt L, Frederick M, et al. Colon cancer growth and dissemination relies upon thrombin, stromal PAR‐1, and fibrinogen. Cancer Res. 2015;75(19):4235‐4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pang JH, Coupland LA, Freeman C, Chong BH, Parish CR. Activation of tumour cell ECM degradation by thrombin‐activated platelet membranes: potentially a P‐selectin and GPIIb/IIIa‐dependent process. Clin Exp Metastasis. 2015;32(5):495‐505. [DOI] [PubMed] [Google Scholar]
- 108. Contursi A, Schiavone S, Dovizio M, et al. Platelets induce free and phospholipid‐esterified 12‐hydroxyeicosatetraenoic acid generation in colon cancer cells by delivering 12‐lipoxygenase. J Lipid Res. 2021;62:100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang Y, Unnithan RVM, Hamidi A, et al. TANK‐binding kinase 1 is a mediator of platelet‐induced EMT in mammary carcinoma cells. FASEB J. 2019;33(7):7822‐7832. [DOI] [PubMed] [Google Scholar]
- 110. Zhou Q, Huang F, He Z, Zuo MZ. Clinicopathological and prognostic significance of platelet count in patients with ovarian cancer. Climacteric. 2018;21(1):60‐68. [DOI] [PubMed] [Google Scholar]
- 111. Aubron C, Flint AW, Bailey M, et al. Is platelet transfusion associated with hospital‐acquired infections in critically ill patients? Crit Care. 2017;21(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug‐eluting stents: results of the TRIGGER‐PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012;59(24):2159‐2164. [DOI] [PubMed] [Google Scholar]
- 113. Krenn‐Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet‐to‐lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524‐2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Baranyai Z, Krzystanek M, Jósa V, et al. The comparison of thrombocytosis and platelet‐lymphocyte ratio as potential prognostic markers in colorectal cancer. Thromb Haemost. 2014;111(3):483‐490. [DOI] [PubMed] [Google Scholar]
- 115. Calverley DC, Phang TL, Choudhury QG, et al. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin Transl Sci. 2010;3(5):227‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Liu S, Fang J, Jiao D, Liu Z. Elevated platelet count predicts poor prognosis in breast cancer patients with supraclavicular lymph node metastasis. Cancer Manag Res. 2020;12:6069‐6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, Hideghéty K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33(4):1725‐1729. [PubMed] [Google Scholar]
- 118. Giannakeas V, Kotsopoulos J, Cheung MC, et al. Analysis of platelet count and new cancer diagnosis over a 10‐year period. JAMA Netw Open. 2022;5(1):e2141633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Erdemir F, Kilciler M, Bedir S, Ozgok Y, Coban H, Erten K. Clinical significance of platelet count in patients with renal cell carcinoma. Urol Int. 2007;79(2):111‐116. [DOI] [PubMed] [Google Scholar]
- 120. Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract. 2017;67(659):e405‐e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Giannakeas V. Trends in platelet count among cancer patients. Exp Hematol Oncol. 2022;11(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pavlovic N, Rani B, Gerwins P, Heindryckx F. Platelets as key factors in hepatocellular carcinoma. Cancers (Basel). 2019;11(7):1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Radziwon‐Balicka A, Medina C, O'Driscoll L, et al. Platelets increase survival of adenocarcinoma cells challenged with anticancer drugs: mechanisms and implications for chemoresistance. Br J Pharmacol. 2012;167(4):787‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Riegel K, Yurugi H, Schlöder J, et al. ERK5 modulates IL‐6 secretion and contributes to tumor‐induced immune suppression. Cell Death Dis. 2021;12(11):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kaser A, Brandacher G, Steurer W, et al. Interleukin‐6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720‐2725. [DOI] [PubMed] [Google Scholar]
- 126. Andersen H, Greenberg DL, Fujikawa K, Xu W, Chung DW, Davie EW. Protease‐activated receptor 1 is the primary mediator of thrombin‐stimulated platelet procoagulant activity. Proc Natl Acad Sci USA. 1999;96(20):11189‐11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation‐induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759‐771. [DOI] [PubMed] [Google Scholar]
- 128. Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol. 2015;5(3):1265‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rossaint J, Zarbock A. Platelets in leucocyte recruitment and function. Cardiovasc Res. 2015;107(3):386‐395. [DOI] [PubMed] [Google Scholar]
- 130. Bastida E, Almirall L, Ordinas A. Tumor‐cell‐induced platelet aggregation is a glycoprotein‐dependent and lipoxygenase‐associated process. Int J Cancer. 1987;39(6):760‐763. [DOI] [PubMed] [Google Scholar]
- 131. Amirkhosravi A, Mousa SA, Amaya M, et al. Inhibition of tumor cell‐induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost. 2003;90(3):549‐554. [DOI] [PubMed] [Google Scholar]
- 132. Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694‐707. [DOI] [PubMed] [Google Scholar]
- 133. Lievens D, Zernecke A, Seijkens T, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116(20):4317‐4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449‐458. [DOI] [PubMed] [Google Scholar]
- 135. Wersäll A, Williams CM, Brown E, Iannitti T, Williams N, Poole AW. Mouse platelet Ral GTPases control P‐selectin surface expression, regulating platelet–leukocyte interaction. Arterioscler Thromb Vasc Biol. 2018;38(4):787‐800. [DOI] [PubMed] [Google Scholar]
- 136. Lucotti S, Cerutti C, Soyer M, et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet‐derived COX‐1/thromboxane A2. J Clin Invest. 2019;129(5):1845‐1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang L‐X, Wei Z‐J, Xu AM, Zang JH. Can the neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. 2018;56:320‐327. [DOI] [PubMed] [Google Scholar]
- 138. Jiao Y, Li W, Wang W. Platelet‐derived exosomes promote neutrophil extracellular trap formation during septic shock. Crit Care. 2020;24(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ravindran M, Khan MA, Palaniyar N. Neutrophil extracellular trap formation: physiology, pathology, and pharmacology. Biomolecules. 2019;9(8):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Berger‐Achituv S, Brinkmann V, Abed UA, Kühn LI, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cools‐Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ren J, He J, Zhang H, et al. Platelet TLR4‐ERK5 axis facilitates NET‐mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. 2021;81(9):2373‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang Y, Wang C, Yu M, et al. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb Res. 2019;180:87‐97. [DOI] [PubMed] [Google Scholar]
- 144. Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73(10):1854‐1863. [DOI] [PubMed] [Google Scholar]
- 145. Andrews RK, Arthur JF, Gardiner EE. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb Haemost. 2014;112(4):659‐665. [DOI] [PubMed] [Google Scholar]
- 146. Wu L, Zhang XHF. Tumor‐associated neutrophils and macrophages‐heterogenous but not chaotic. Front Immunol. 2020;11:553967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431‐446. [DOI] [PubMed] [Google Scholar]
- 148. Ohms M, Möller S, Laskay T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes. Front Immunol. 2020;11:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mihaila AC, Ciortan L, Macarie RD, et al. Transcriptional profiling and functional analysis of N1/N2 neutrophils reveal an immunomodulatory effect of S100A9‐blockade on the pro‐inflammatory N1 subpopulation. Front Immunol. 2021;12:708770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor‐associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20(3):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor‐beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258(11):7155‐7160. [PubMed] [Google Scholar]
- 152. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173‐187. [DOI] [PubMed] [Google Scholar]
- 153. Xue R, Zhang Q, Cao Q, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612(7938):141‐147. [DOI] [PubMed] [Google Scholar]
- 154. Vanichakarn P, Blair P, Wu C, Freedman JE, Chakrabarti S. Neutrophil CD40 enhances platelet‐mediated inflammation. Thromb Res. 2008;122(3):346‐358. [DOI] [PubMed] [Google Scholar]
- 155. Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mellor KL, Powell AGMT, Lewis WG. Systematic review and meta‐analysis of the prognostic significance of neutrophil‐lymphocyte ratio (NLR) after R0 gastrectomy for cancer. J Gastrointest Cancer. 2018;49(3):237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bishop PD, Lewis KB, Schultz J, Walker KM. Comparison of recombinant human thrombin and plasma‐derived human alpha‐thrombin. Semin Thromb Hemost. 2006;32(1):86‐97. [DOI] [PubMed] [Google Scholar]
- 158. Xiong YQ, Sun HC, Zhang W, et al. Human hepatocellular carcinoma tumor‐derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15(15):4838‐4846. [DOI] [PubMed] [Google Scholar]
- 159. Miyashita N, Saito A. Organ specificity and heterogeneity of cancer‐associated fibroblasts in colorectal cancer. Int J Mol Sci. 2021;22(20):10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhang Y, Manouchehri Doulabi E, Herre M, et al. Platelet‐derived PDGFB promotes recruitment of cancer‐associated fibroblasts, deposition of extracellular matrix and Tgf‐β signaling in the tumor microenvironment. Cancers (Basel). 2022;14(8):1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Razavi AS, Mohtashami M, Razi S, Rezaei N. TGF‐β signaling and the interaction between platelets and T‐cells in tumor microenvironment: foes or friends? Cytokine. 2022;150:155772. [DOI] [PubMed] [Google Scholar]
- 162. Castro‐Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. He Y, Shi J, Schmidt B, et al. Circulating tumor cells as a biomarker to assist molecular diagnosis for early stage non‐small cell lung cancer. Cancer Manag Res. 2020;12:841‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Poudineh M, Sargent EH, Pantel K, Kelley SO. Profiling circulating tumour cells and other biomarkers of invasive cancers. Nat Biomed Eng. 2018;2(2):72‐84. [DOI] [PubMed] [Google Scholar]
- 165. Zviran A, Schulman RC, Shah M, et al. Genome‐wide cell‐free DNA mutational integration enables ultra‐sensitive cancer monitoring. Nat Med. 2020;26(7):1114‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Li L, Wang J, Meng S, et al. Peripheral blood leukocytes and platelets serve as prognostic factors in breast cancer. Cancer Biother Radiopharm. 2021;36(2):167‐173. [DOI] [PubMed] [Google Scholar]
- 167. Bednarz‐Knoll N, Popęda M, Kryczka T, et al. Higher platelet counts correlate to tumour progression and can be induced by intratumoural stroma in non‐metastatic breast carcinomas. Br J Cancer. 2022;126(3):464‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wurdinger T, In ’t Veld SGJG, Best MG. Platelet RNA as pan‐tumor biomarker for cancer detection. Cancer Res. 2020;80(7):1371‐1373. [DOI] [PubMed] [Google Scholar]
- 169. D'Ambrosi S, Visser A, Antunes‐Ferreira M, et al. The analysis of platelet‐derived circRNA repertoire as potential diagnostic biomarker for non‐small cell lung cancer. Cancers (Basel). 2021;13(18):4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ke M, Kang L, Wang L, et al. CAR‐T therapy alters synthesis of platelet‐activating factor in multiple myeloma patients. J Hematol Oncol. 2021;14(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. D'Alessandro R, Refolo MG, Lippolis C, et al. Antagonism of sorafenib and regorafenib actions by platelet factors in hepatocellular carcinoma cell lines. BMC Cancer. 2014;14:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bottsford‐Miller J, Choi H‐J, Dalton HJ, et al. Differential platelet levels affect response to taxane‐based therapy in ovarian cancer. Clin Cancer Res. 2015;21(3):602‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hinterleitner C, Strähle J, Malenke E, et al. Platelet PD‐L1 reflects collective intratumoral PD‐L1 expression and predicts immunotherapy response in non‐small cell lung cancer. Nat Commun. 2021;12(1):7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rejeeth C, Sharma A, Kannan S, et al. Label‐free electrochemical detection of the cancer biomarker platelet‐derived growth factor receptor in human serum and cancer cells. ACS Biomater Sci Eng. 2022;8(2):826‐833. [DOI] [PubMed] [Google Scholar]
- 175. Boonyawan K, Hess KR, Yang J, et al. A relative increase in circulating platelets following chemoradiation predicts for poor survival of patients with glioblastoma. Oncotarget. 2017;8(52):90488‐90495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Campanella R, Guarnaccia L, Cordiglieri C, et al. Tumor‐educated platelets and angiogenesis in glioblastoma: another brick in the wall for novel prognostic and targetable biomarkers, changing the vision from a localized tumor to a systemic pathology. Cells. 2020;9(2):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Liu T, Wang J, Li T, et al. Predicting disease progression in advanced non‐small cell lung cancer with circulating neutrophil‐derived and platelet‐derived microparticles. BMC Cancer. 2021;21(1):939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Macaulay IC, Carr P, Gusnanto A, Ouwehand WH, Fitzgerald D, Watkins NA. Platelet genomics and proteomics in human health and disease. J Clin Invest. 2005;115(12):3370‐3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Best MG, Sol N, Kooi I, et al. RNA‐seq of tumor‐educated platelets enables blood‐based pan‐cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28(5):666‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Best MG, Sol N, In ’t Veld SGJG, et al. Swarm intelligence‐enhanced detection of non‐small‐cell lung cancer using tumor‐educated platelets. Cancer Cell. 2017;32(2):238‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Xu L, Li X, Li X, et al. RNA profiling of blood platelets noninvasively differentiates colorectal cancer from healthy donors and noncancerous intestinal diseases: a retrospective cohort study. Genome Med. 2022;14(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Xing S, Zeng T, Xue N, et al. Development and validation of tumor‐educated blood platelets integrin alpha 2b (ITGA2B) RNA for diagnosis and prognosis of non‐small‐cell lung cancer through RNA‐seq. Int J Biol Sci. 2019;15(9):1977‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190‐3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Haemmerle M, Bottsford‐Miller J, Pradeep S, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Medina C, Jurasz P, Santos‐Martinez MJ, et al. Platelet aggregation‐induced by caco‐2 cells: regulation by matrix metalloproteinase‐2 and adenosine diphosphate. J Pharmacol Exp Ther. 2006;317(2):739‐745. [DOI] [PubMed] [Google Scholar]
- 186. Cao L, Zhang Y, Mi J, et al. α‐Hederin inhibits the platelet activating factor‐induced metastasis of HCC cells through disruption of PAF/PTAFR axis cascaded STAT3/MMP‐2 expression. Pharmacol Res. 2022;178:106180. [DOI] [PubMed] [Google Scholar]
- 187. Chen X, Liu L, Liu P, et al. Discovery of potent and orally bioavailable platelet‐derived growth factor receptor (PDGFR) inhibitors for the treatment of osteosarcoma. J Med Chem. 2022;65(7):5374‐5391. [DOI] [PubMed] [Google Scholar]
- 188. Kim S, You D, Jeong Y, Yoon SY, Kim SA, Lee JE. Inhibition of platelet‐derived growth factor C and their receptors additionally increases doxorubicin effects in triple‐negative breast cancer cells. Eur J Pharmacol. 2021;895:173868. [DOI] [PubMed] [Google Scholar]
- 189. Nayeem MJ, Yamamura A, Hayashi H, et al. Imatinib mesylate inhibits androgen‐independent PC‐3 cell viability, proliferation, migration, and tumor growth by targeting platelet‐derived growth factor receptor‐α. Life Sci. 2022;288:120171. [DOI] [PubMed] [Google Scholar]
- 190. Patel S, Nanavati P, Sharma J, Chavda V, Savjani J. Functional role of novel indomethacin derivatives for the treatment of hepatocellular carcinoma through inhibition of platelet‐derived growth factor. Arch Med Res. 2021;52(5):483‐493. [DOI] [PubMed] [Google Scholar]
- 191. Peng G, Wang Y, Ge P, et al. The HIF1α‐PDGFD‐PDGFRα axis controls glioblastoma growth at normoxia/mild‐hypoxia and confers sensitivity to targeted therapy by echinomycin. J Exp Clin Cancer Res. 2021;40(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bahmani B, Gong H, Luk BT, et al. Intratumoral immunotherapy using platelet‐cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12(1):1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Li B, Chu T, Wei J, et al. Platelet‐membrane‐coated nanoparticles enable vascular disrupting agent combining anti‐angiogenic drug for improved tumor vessel impairment. Nano Lett. 2021;21(6):2588‐2595. [DOI] [PubMed] [Google Scholar]
- 194. Du Y, Wang S, Luan J, Zhang M, Chen B, Shen Y. GOx‐functionalized platelet membranes‐camouflaging nanoreactors for enhanced multimodal tumor treatment. Int J Nanomed. 2022;17:2979‐2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Li Q‐R, Xu H‐Z, Xiao R‐C, et al. Platelets are highly efficient and efficacious carriers for tumor‐targeted nano‐drug delivery. Drug Deliv. 2022;29(1):937‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Qi P, Zhang J, Bao Z, Liao Y, Liu Z, Wang J. A platelet‐mimicking single‐atom nanozyme for mitochondrial damage‐mediated mild‐temperature photothermal therapy. ACS Appl Mater Interfaces. 2022;14(17):19081‐19090. [DOI] [PubMed] [Google Scholar]
- 197. Wu L, Li Q, Deng J, et al. Platelet‐tumor cell hybrid membrane‐camouflaged nanoparticles for enhancing therapy efficacy in glioma. Int J Nanomed. 2021;16:8433‐8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ma Y, Zhang Y, Han R, et al. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282:121384. [DOI] [PubMed] [Google Scholar]
- 199. Zhang Y, Huang Z, Cheng J, et al. Platelet‐vesicles‐encapsulated RSL‐3 enable anti‐angiogenesis and induce ferroptosis to inhibit pancreatic cancer progress. Front Endocrinol (Lausanne). 2022;13:865655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Li S, Li L, Lin X, Chen C, Luo C, Huang Y. Targeted inhibition of tumor inflammation and tumor‐platelet crosstalk by nanoparticle‐mediated drug delivery mitigates cancer metastasis. ACS Nano. 2021;16(1):50‐67. [DOI] [PubMed] [Google Scholar]
- 201. Bhandarkar S, Prabhakar B, Shende P. Quercetin‐loaded platelets as a potential targeted therapy for glioblastoma multiforme cell line U373‐MG. Biotechnol J. 2021;16(12):e2100271. [DOI] [PubMed] [Google Scholar]
- 202. Rao L, Bu L‐L, Ma L, et al. Platelet‐facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem Int Ed Engl. 2018;57(4):986‐991. [DOI] [PubMed] [Google Scholar]
- 203. D'Ambrosi S, Nilsson RJ, Wurdinger T. Platelets and tumor‐associated RNA transfer. Blood. 2021;137(23):3181‐3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio and platelet count as predictors of long‐term outcome after R0 resection for colorectal cancer. Sci Rep. 2017;7(1):1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available. Please contact us to access if it is needed.


