Abstract
Brain tumors account for less than 2% of all malignancies. However, they are associated with the highest morbidity and mortality rates among all solid tumors. The most common malignant primary brain tumors are glioma or glioblastoma (GBM), which have a median survival time of about 14 months, often suffer from recurrence after a few months following treatment, and pose a therapeutic challenge. Despite recent therapeutic advances, the prognosis for glioma patients is poor when treated with modern therapies, including chemotherapy, surgery, radiation, or a combination of these. Therefore, discovering a new target to treat brain tumors, particularly glioma, might be advantageous in raising progression-free survival and overall survival (OS) rates. Statins, also known as competitive HMG-CoA reductase inhibitors, are effective medications for reducing cholesterol and cardiovascular risk. The use of statins prior to and during other cancer treatments appears to enhance patient outcomes according to preclinical studies. After surgical resection followed by concurrent radiation and treatment, OS for patients with GBM is only about a year. Statins have recently emerged as potential adjuvant medications for treating GBM due to their ability to inhibit cell growth, survival, migration, metastasis, inflammation, angiogenesis, and increase apoptosis in-vitro and in-vivo studies. Whether statins enhance clinical outcomes, such as patient survival in GBM, is still debatable. This study aimed to explore the effects of statin therapy in the context of cancer treatment, with a particular focus on GBM.
Keywords: brain, glioblastoma, glioma, statin, tumor
Introduction
Cancer is a major global public health problem. According to the WHO and the American Cancer Society (ACS), cancer is the second leading cause of death despite tremendous advances in tumor identification and therapy in recent years [1–4]. Cancer incidence and mortality are considered in the GLOBOCAN 2020 global cancer burden update [5]. Excluding non-melanoma skin cancer, it forecasts 19.3 million new cancer cases and more than 10 million deaths worldwide [5]. In addition, the ACS predicted that in 2021, there would be 24 690 new cases of brain cancer and other nervous system tumors diagnosed in the USA, with 18 000 deaths due to these tumors [6].
Brain cancer refers to various tumors that develop in the brain or other tissues [7]. Glioma or glioblastoma (GBM) is the most common type of brain cancer, which accounts for more than 80% of all malignant brain tumors [8]. Other brain cancers include meningioma, pituitary adenoma, and schwannoma [9]. The incidence and fatality rates of brain cancers vary depending on several factors, including age, gender, and geographic region [10]. For instance, brain cancer is most common in industrialized countries, although fatality rates in underdeveloped countries may be greater due to restricted access to treatment [11]. GBM is the most common highly invasive brain tumor among adults, affecting roughly 3-5 people per 100 000 persons per year [12]. Gliomas tend to affect males, elderly, Caucasian and those with certain rare genetic disorders [13]. Glioma is associated with poor prognosis and short survival time of about 12–15 months [8,14]. Recently, investigations for new management approaches focused on enhancing the GBM sensitivity toward apoptosis-induced therapy [15].
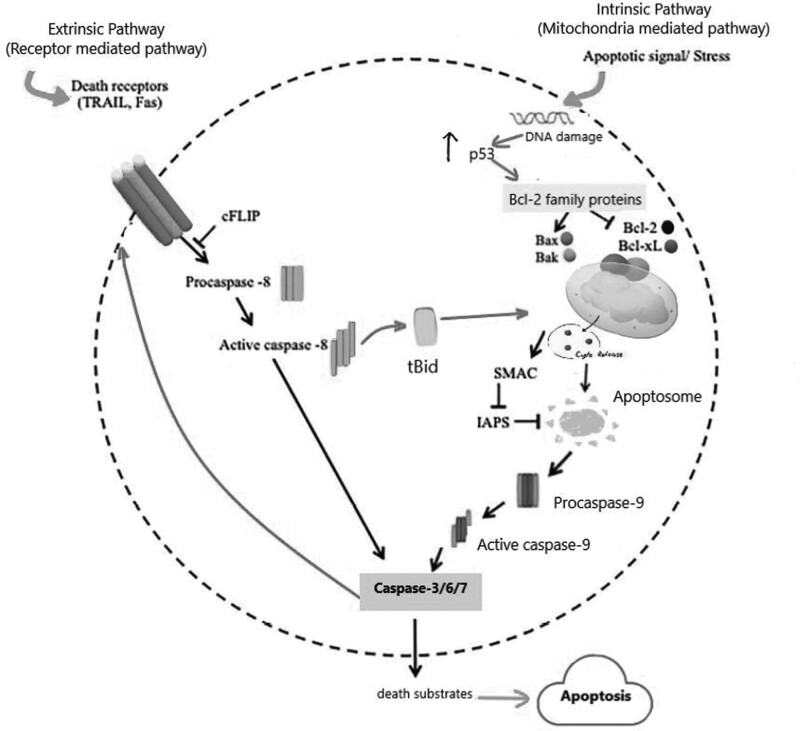
Apoptosis, also known as programmed cell death, is a highly regulated process that is involved in various physiological and pathological processes, including development, tissue homeostasis, and cancer [16,17]. The extrinsic and the intrinsic pathways are the two main apoptotic pathways, illustrated in Fig. 1. The intrinsic pathway is activated by intracellular signals, such as DNA damage, oxidative stress, or the lack of cell survival signals [15–17]. These signals activate proapoptotic proteins such as Bax and Bak and induce the release cytochrome c from the mitochondria [17–20]. Cytochrome c then binds to apoptotic protease-activating factor 1 (Apaf-1), forming the apoptosome, a caspase-activating complex [21,22]. The apoptosome subsequently activates caspase-9, which activates downstream effector caspases leading to cell death [23]. Both extrinsic and intrinsic pathways activate effector caspases, which cleave and activate a wide range of substrates, resulting in the morphological and biochemical alterations seen during apoptosis. The control of these pathways is complex and involves the interaction of proapoptotic and antiapoptotic proteins [16,24]. On the other hand, extrinsic pathway is activated by binding of extracellular signals to death receptors on the cell surface [16]. Activation of death receptors induces caspase-8 activation and eventually activation of downstream effector caspases such as caspase-3, -6, and -7 [16,24,25].
Fig. 1.
The intrinsic and extrinsic pathways of apoptosis. Death receptors (DRs), play a key role in the extrinsic route, which is activated by outside stimuli or ligand molecules. The intrinsic pathway is mediated by Bax/Bak insertion into mitochondrial membrane, followed by release of cytochrome c, which combines with Apaf-1 and procaspase-9 to create apoptosomes, which are then activated by caspase-3 to cause apoptosis. Bcl-2, B-cell lymphoma protein 2; Bcl-xL, Bcl-2 homolog splice variants; cFLIP, cellular FLICE inhibitory proteins; Cyt C, cytochrome; IAPs, proteins that suppress apoptosis; SMAC, second mitochondrial activator of caspases; tBid, truncated bid; TRAIL, TNF-related apoptosis-inducing ligand.
All mammalian cells produce cholesterol, one of the most significant biological lipid components, that is, widely dispersed throughout the body’s tissues [26,27]. Cholesterol is mainly found in cellular membranes, where it preserves the fluidity and integrity of cell membranes and composes membrane microstructures [26,27]. Several biological processes, including the cell immunological response, posttranslational modification of proteins, and cell signal transmission, are affected by cholesterol and its precursors or metabolites, which may play a role in promoting malignancy [28,29]. Additionally, cancer cells’ eternal growth accompanies by a rise in their need for cholesterol. Many different types of malignant tumors, including GBM, exhibit metabolic problems of cholesterol. This suggests that reprogramming the metabolic profile of cholesterol is a novel characteristic of cancer [30,31].
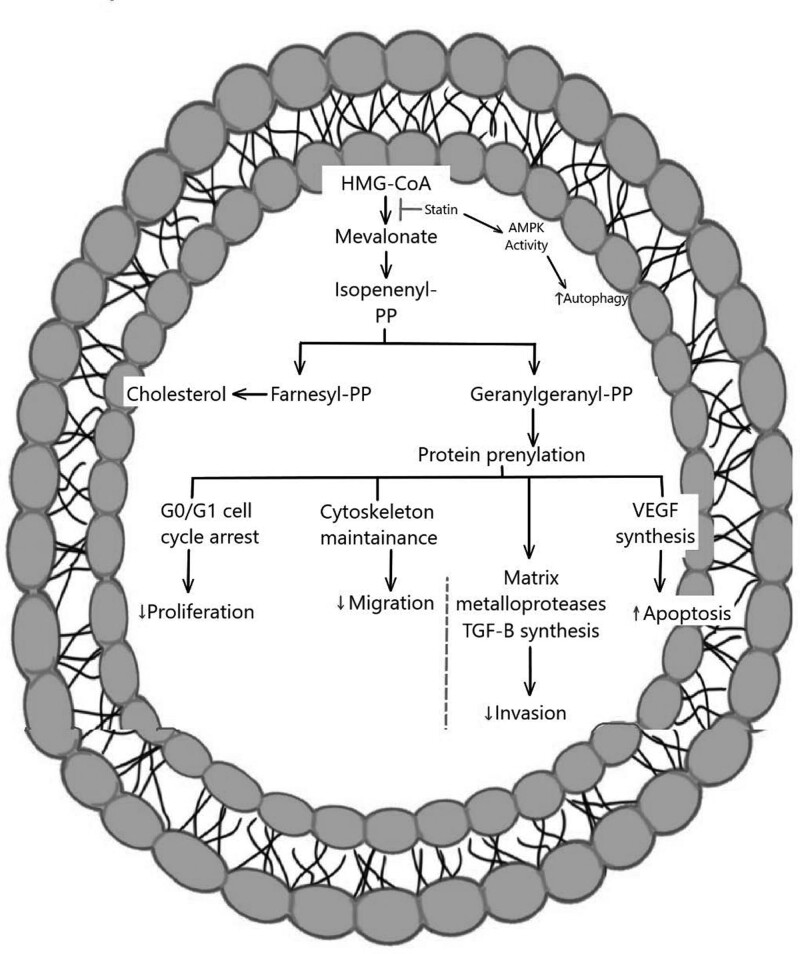
Statins, known as lipid-lowering agents, inhibit 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductases, the rate-limiting enzyme of the mevalonate pathway and cholesterol synthesis [32–34]. In addition, several clinical studies have demonstrated their efficiency in the primary and secondary prevention of myocardial infarction and cerebrovascular events [35,36]. However, statins’ use is associated, rarely, with muscular deterioration and malfunction, hepatic dysfunction, a higher chance of developing type 2 diabetes, and renal insufficiency. Additionally, the aforementioned side effects are not dose-dependent [37,38]. Thus, targeting cholesterol synthesis, by statin therapy, can prevent glioma tumors’ growth and can be a therapeutic approach in glioma or other types of brain cancers. The roles of statin therapy in glioma are illustrated in Fig. 2 and it will be explored in the later sections.
Fig. 2.
General molecular signaling pathways of statins in glioma.
Brain de-novo synthesis of cholesterol and statins penetration of the blood-brain barrier
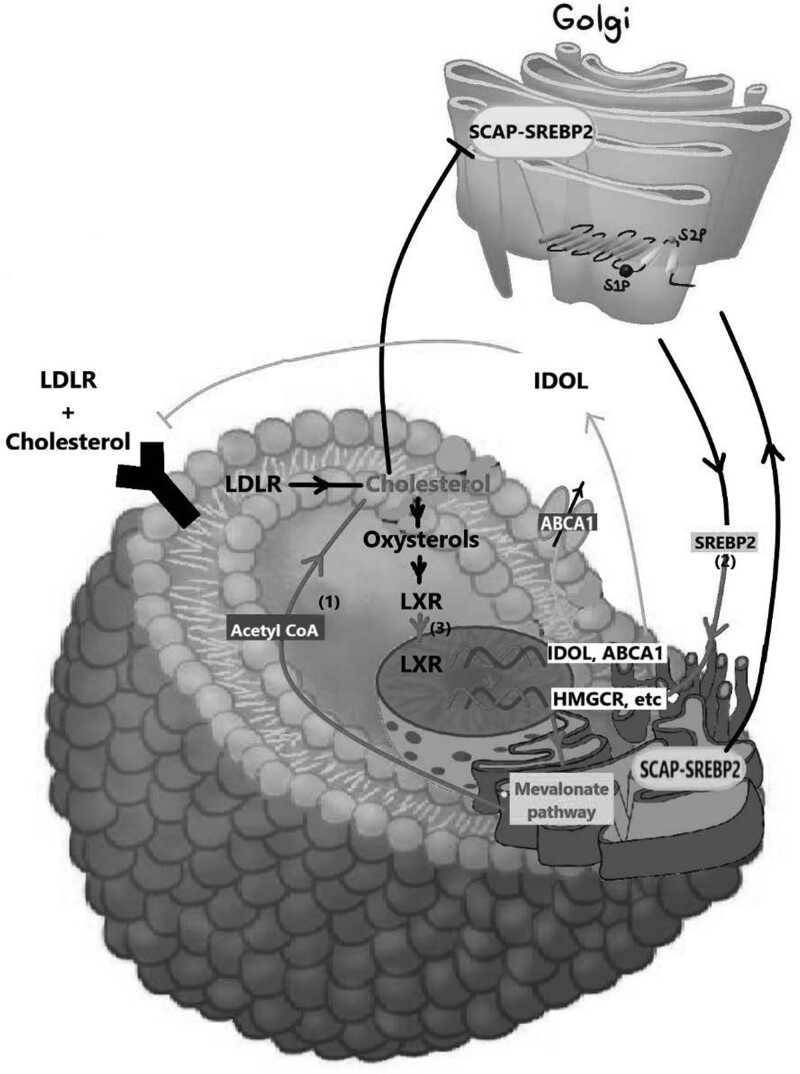
The blood-brain barrier (BBB) makes it difficult for the brain to absorb cholesterol from the periphery and hence the brain has a separate microenvironment for cholesterol metabolism [39,40]. De-novo synthesis of cholesterol, primarily by astrocytes and oligodendrocytes, is the primary source of cholesterol in the brain [41]. Myelin sheath surrounding the brain, which is mainly composed of cholesterol, is less permeable to ions and facilitates quick and precise electrical signal transmission during brain processes [42]. Simvastatin and lovastatin are the most lipophilic statins, and hence they penetrate the BBB most easily with high in-vivo BBB permeability coefficients [43]. There are two primary routes through which cells get cholesterol, either from endogenous synthesis and/or exogenous uptake, illustrated in Fig. 3. Cholesterol can get in the cells through low-density lipoprotein receptor (LDLR)-mediated endocytosis that allows cells to take up LDL from the periphery and is subsequently carried to the lysosome after entering the cell. The cholesterol ester is hydrolyzed in the lysosome to liberate unprocessed cholesterol [44]. Cells then use Acetyl-CoA and NADP (NADPH) as the precursors for the de-novo synthesis of cholesterol (also known as the mevalonate pathway) [45].
Fig. 3.
Cholesterol balance in healthy cells. Both de-novo synthesis from acetyl-CoA produced by glycolysis and exogenous absorption by low-density lipoprotein receptors (LDLR) are major sources of cholesterol for cells. Through [3], the inhibition of proteolytic processing and nuclear import of sterol regulatory element binding proteins (SREBP2), which results in a reduction in activity in the mevalonate pathway, or through its conversion to oxysterols that activate liver X receptors (LXRs), cholesterol can negatively regulate its own levels. By upregulating ABCA1 expression and activating IDOL transcription, an E3 ubiquitin ligase that ubiquitinates LDLR, LXRs reduce cellular cholesterol levels. ER, endoplasmic membrane; SCAP, SREBP cleavage-activating protein.
According to research, glioma cells may convert cholesterol into corticosteroids like progesterone, androstenedione, androstenediol, and androstenedione, which may speed up glioma progression [46]. Apo-E-carrying cholesterol is endocytosed by neurons, which are specialized in producing electrical activity and rely on adjacent astrocytes to transport cholesterol [47]. The cholesterol ester is hydrolyzed, and the esterified cholesterol enters the lysosome. Niemen-Pick Type C protein 1 (NPC1) transports the unverified cholesterol from the lysosome to the cell membrane or endoplasmic reticulum [48,49]. Two major signaling cascades regulate cholesterol homeostasis, which are liver X receptors (LXRs) that bind to sterol regulatory elements in transcription factors (SREBPs) [50,51]. SREBPs enter the nucleus and trigger the transcription of genes required for cholesterol production [50,51]. A cholesterol derivative called oxysterol is produced in more significant quantities in response to rising cellular cholesterol levels. The expression of genes involved in cholesterol uptake, efflux, and conversion to bile acids is stimulated by the activation of LXRs by oxysterols, eventually causing a drop in cholesterol concentration in cellular tissues. The body’s homeostasis of cholesterol is preserved by this feedback system. In the end, the LXR network regulatory mechanism lowers the level of free cholesterol. It has been demonstrated that lipid droplets (LDs) are actively gathered and used by GBM cells for their growth and cancerous activity. ACAT1, also referred to as acyl-CoA: cholesterol acyltransferase 1 (SOAT1), is an essential enzyme for producing LDs and cholesterol esterification. According to studies, SOAT1 is overexpressed in GBM cells, and this overexpression boosts LD formation and cholesterol esterification, which results in more lipid accumulation and tumor development.
Additionally, SOAT1 inhibition has been shown to decrease GBM cell proliferation and cause cell death, indicating that it may be a potential therapeutic target for treating GBM [52,53]. Blocking the production of LD by inhibiting cholesterol esterification through the targeting of SOAT1 also reduces the SREBP-1-regulated lipogenesis, suppressing the growth of GBM [52,53]. The SOAT1 inhibitor avasimibe can prevent the formation of cerebral gliomas in xenograft model mice and extend animal longevity [54]. Moreover, avasimibe limits the viability of the GBM cell line, EGFRvIII U87, without damaging astrocytes [54–56]. Moreover, avasimibe prevents the development of GBM cells by inducing cell cycle arrest and activating caspase-8-dependent apoptotic pathways [54]. Oxysterols, such as 24,25-epoxy cholesterol and 7-OHC, are an essential target in metabolic treatment because they serve as a conduit for cholesterol metabolism between the CNS and the peripheral nervous system [57,58]. It has been shown that preventing the release of cholesterol from lysosomes efficiently triggers proliferative autophagy in GBM cells [59].
These findings suggest that, compared with normal glial cells, the GBM cholesterol metabolic profile is significantly altered by LXR decoupling. Furthermore, as mentioned earlier, the data demonstrates that GBM mainly relies on outsourcing cholesterol for growth instead of de-novo synthesis. Therefore, it is tempting to hypothesize that the dependence of GBM cells on CNS-derived cholesterol enables them to direct their cellular NADPH, a key reducing agent in relatively short supply, towards buffering reactive oxygen species and synthesizing other macromolecules, as the mevalonate pathway consumes 26 reducing equivalents of NADPH [60,61]. Furthermore, to fulfill the vigorous proliferation requirements of tumors, GBM suppresses the synthesis of oxysterols, which uncouples LXR and causes an increase in cholesterol absorption, and decreases cholesterol efflux [61]. As a result, inhibiting cholesterol absorption by activating LXR has emerged as a possible strategy for treating GBM.
Satins effect on the growth of normal and cancerous brain cells in vitro
Besides the inhibitory effect of statins on cholesterol biosynthesis, they modify the level of critical intracellular molecules involved in various intracellular signaling pathways, allowing for a broad range of biological activities [62,63]. The use of statins was associated with a slightly lower overall cancer incidence compared with nonusers and those who took other lipid-lowering medications [64,65]. In-vitro and in-vivo studies revealed that statins might be effective in cancer prevention and/or treatment via inhibiting tumor growth and inducing apoptosis [66,67]. Further, statins showed cytotoxic and antiproliferative effects against in several human cancer cell lines [66,68,69]. Furthermore, statins treatment upregulated the expression of the proapoptotic proteins and downregulated the expression of antiapoptotic proteins in tumor cells [70]. Additionally, statins are thought to obstruct the two key isoprenoid metabolites, the geranylgeranyl pyrophosphate (GGPP) and farnesyl pyrophosphate synthesis within the mevalonate metabolic pathway, which in turn prevents the proliferation of malignant cells and finally leads to cell death [71,72]. The three statins on which the majority of research studies focused when addressing how statins affect glioma growth are simvastatin, atorvastatin, and fluvastatin.
Simvastatin and the growth of brain cells in vitro
Simvastatin inhibits GBM tumors development by enhancing tumor cell death and suppressing cell proliferation and migration [73]. Simvastatin induced apoptosis of neuronal cell line by decreasing the amount of cholesterol in the cell membrane, damaged the stability of lipid rafts, and then inhibited the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, and induced caspase-3 dependent apoptosis in GBM [72,74,75]. Moreover, simvastatin and fluvastatin suppressed GGPP synthesis, which prevented extracellular signal-regulated protein kinase (ERK1/2) and Akt activation and hence induced apoptosis in C6 glioma cells [76]. These studies pave the way for simvastatin to be used with other traditional anticancer medications as a more effective adjuvant chemotherapy agent.
Atorvastatin and the growth of brain cells in vitro
Yi and colleagues reported that atorvastatin decreased cell invasion and migration of human U87 primary GBM cell line by preventing the expression of microglial membrane type 1 matrix metalloproteinase (MT1-MMP), which is overexpressed in many cancer types and is associated with increased cancer invasion and metastasis [77]. Atorvastatin could prevent the production of microglial MT1-MMP by preventing the p38 mitogen-activated protein kinase pathway that regulates various cellular processes, including inflammation, apoptosis, cell differentiation, and cell cycle arrest [78]. Additionally, the apoptotic effect induced by atorvastatin was investigated [79,80]. It has been shown that atorvastatin affects the expression of crucial proteins associated with apoptosis, migration, and invasion of tumor cells in a 3D spheroids model of the U87 GBM cell line [79,80]. For instance, atorvastatin upregulated the expression of apoptotic factors (as caspase-3 and caspase-8) and reduced the expression of antiapoptotic proteins (as Bcl-2) on glioma spheroids [79,80]. Moreover, atorvastatin inhibited the proliferation of GBM cells in a dose range of 1–10 μM, in a dose-dependent manner, following 48h of incubation [79,80]. Furthermore, atorvastatin treatment reduced GBM cells’ invasion and migration [79,80].
Fluvastatin and the growth of brain cells in vitro
Fluvastatin decreased the viability of rat GBM cell line C6 in a dose-dependent manner without negatively affecting the number of normal neuron cells [81]. Even at high doses, fluvastatin exhibited a neurotrophic effect on normal neuron cells, consistent with a reported increase in neurite length and branching of rat hippocampal neurons after treatment with pravastatin [82,83]. Fluvastatin-induced apoptosis in glioma cells is based on the morphological changes such as the presence of rounded and shrunken cells with irregular and pycnotic nuclei and reduced length of cytoplasmic protrusions [81–84]. The underlying mechanism of fluvastatin-induced cytotoxicity is not entirely understood. However, molecular experiments revealed a crucial role of fluvastatin in inducing JNK1/2 phosphorylation while inhibiting ERK1/2 phosphorylation [81,84–86]. This modulatory effect of fluvastatin on ERK and JNK activation results in disruptive MAPK pathways in C6 cells [81], leading to apoptosis. Furthermore, Fluvastatin, at concentration of 2.5 to 5 μM, significantly inhibited matrix metalloproteinase 9 activity, by 48–56%, in C6 cells [81]. This might be responsible, at least in part, for the inhibitory effect of fluvastatin on cancer cell invasion and metastasis.
The relationship between the duration of statins therapy and the clinical outcome of reducing the risk of brain cancer, including glioma
The New England Veterans Integrated Service Network-1 pharmacy-epidemiology database found that statin users had a statistically significantly lower risk for all cancers compared with nonusers after adjusting for age and multiple confounders [87]. Simvastatin, lovastatin, atorvastatin, pravastatin, and rosuvastatin were the examined statins. Recent observational studies reported the unique benefits of statins when used for at least 5 years, to specific types of cancer, such as breast, lung, and pancreatic cancers [41,88]. However, long-term use of statins did not change the incidence of all cancers; even long-term users may benefit more from the protective impact of these medications, according to the extensive time interval found [89,90]. In the pooled dataset, there were significant inverse relationships between simvastatin and lovastatin and glioma incidence among individuals who used statins for longer than 5 years compared with nonusers [91,92]. However, the use of atorvastatin, pravastatin, and rosuvastatin did not show promising outcomes in glioma incidence [91,92]. This could be due to high in-vivo BBB permeability coefficients for lovastatin and simvastatin unlike the hydrophilic pravastatin [64,93,94]. In contrast, one cohort analysis found no link between taking statins (i.e. lovastatin and simvastatin) for more than 5 years and the chance of developing brain cancer [95]. The results of these contradictory studies may be causative, where the prospect of gendering age-specific effects might guide trials of tailored treatment interventions. Additionally, the incidence of brain tumors is rising due to improvements in diagnosing or managing primary brain tumors [95–101]. The currently available approaches for glioma treatment include surgery, radiation, chemotherapy, and pain management [12,102]. Additionally, it is a common practice to treat newly diagnosed patients of GBM with radiation and temozolomide simultaneously following maximal surgical resection. Ionizing radiation is the only known modifiable risk factor for glioma compared with other malignancies [102]. Surgical resection treats most malignant primary brain tumors, and progression-free survival and overall survival rates are associated with the degree of resection [103,104]. Thus, radiation and chemotherapy are used in conjunction with surgery. However, radiation and surgery are associated with the risk of causing cognitive deterioration, establishing new tumors, or growing more aggressive and treatment-resistant cancer strains [105,106]. Additionally, chemotherapy must be administered at higher doses for treating brain cancer than other malignancies to cross the BBB [107]. Thus, previous observational studies have failed to identify consistent anticancer benefits of statins use for longer duration of action in glioma [64].
Only a few clinical studies have looked at the risk of glioma among statin users [108–110]. Atorvastatin, a synthetic lipophilic statin approved in 1996, was chosen in a clinical trial for its high CNS bioavailability, low toxicity, and adequate capacity to decrease LDL cholesterol [108]. Atorvastatin was safely added to standard chemoradiation; although it was tolerated did not improve progression-free survival or overall survival [108]. Another study found a slight but statistically significant association between long-term statin use and increased survival, independent of oncological therapy [109]. However, a third study showed that statin’s effect is still debatable, and further investigation is probably unnecessary [110].
Statins and brain metastases, particularly metastatic breast cancer
The most frequent intracranial tumors in adults are brain metastases (BM), and many studies have investigated the possible impact of statins in preventing and treating BM from breast cancer [42,111,112]. Most molecular research studies on the risk of metastatic spread have been conducted on breast cancer with early staging and showed that markers such as p16 kinase inhibitor, which is a tumor suppressor protein, is linked to the development of BM with metastatic lymph nodes from breast cancer patients [113].
The findings of most studies about the possible impact of statins in preventing and treating BM from breast cancer have been inconsistent. In one study, women with breast cancer who were on statin therapy at the time of diagnosis showed a decreased risk of developing BM than those who were not on statins [114]. Another study found no significant link between statin therapy and the risk of BM in 30% of breast cancer participants [115]. However, further results of the same study demonstrated that clinically appropriate doses of simvastatin added to carboplatin and vinorelbine chemotherapy courses did not improve the overall survival in metastatic breast cancer (MBC) patients [114]. Additionally, simvastatin medication in MBC proved to be extremely well tolerated since there were no side effects from simvastatin or severe toxicity from chemotherapy [114].
Whole-brain radiation therapy (WBRT) is the cornerstone of BM treatment independent of the initial tumor histology [116]. However, trials have shown more significant toxicity and/or no tumor control or survival advantages associated with WBRT. Most patients with BM have a dismal prognosis, with an estimated survival measured in months using WBRT [117]. Statins might have possible radiosensitizing effect through inhibiting nuclear factor-B, and activating autophagy, among other mechanisms [44,118]. Therefore, simvastatin was added to WBRT to assess its efficacy and safety in patients with BM from breast, lung, and other cancers [115,119]. Simvastatin was chosen above other statins for its greater ability to cross the BBB, and they have potential neuroprotective effects [115]. However, simvastatin did not improve the radiological response 4 weeks after radiation and had no impact on the 1-year progression-free survival and 1-year overall survival rates, consistent with the results of the radiological response analysis [115]. Additionally, it is debatable whether simvastatin must be used for more extended periods, at greater dosages, or with shorter intervals between doses to manifest its radiosensitizing effect significantly [120]. Thus, clinical studies with various participants and dose schedules are required to evaluate the radiosensitizing impact of statins.
Statins and hypoxia-growth-dependent glioma
In addition to cholesterol levels and angiogenesis, the growth of many human malignancies is primarily influenced by hypoxia [121]. Hypoxia can result in increased tumor invasion, decreased apoptosis, chemo- and radio-resistance, and resistance to antiangiogenic therapy [122,123]. The effect of statin monotherapy on cancer treatment alone is limited. Consequently, it might be possible to restrict the tumor resistance from hypoxia by combining statin therapy with thiazolidinedione or pioglitazone [124–126] to allow for creating a potent chemotherapeutic regimen. To test this hypothesis, three malignant glioma cell lines from humans (U87, U138, and LN405) and one from rat (RG II) were examined [126]. Statin and pioglitazone combination therapy showed a considerable cytotoxic impact after 48 h, and after 144 h, it became significantly stronger [126]. This cytotoxicity proposed the upregulation of proapoptotic proteins like Bax and Bim in conjunction with a downregulation of antiapoptotic proteins such as Bcl-2. Additionally, activation of phosphorylation cascades as mitogen-activated protein kinase pathway, Ras, and Rho members of cell regulators occurred in malignant tissues [127–129]. Ras activation also activates other pathways necessary for malignant glioma growth, advancement through the cell cycle, and prevention of apoptosis [127–129].
Conclusion
Statins have been found to reduce the incidence of several cancers, such as glioma. However, only a few preclinical and clinical studies have looked at the risk of glioma among statin users for a longer period (i.e. for more than 5 years). Increasing evidence from in-vitro studies and a few clinical studies suggests that statins might be effective in inhibiting glioma growth and induction of apoptosis. For instance, statins have been linked to a slightly lower overall glioma incidence in statin users for less than 5 years compared with nonusers. However, previous research studies have failed to identify consistent anticancer benefits of statins use, either in short-or long-term use, in glioma. Hence, more clinical studies are needed to withdraw definitive conclusions about statins use in glioma. The lack of financial incentive to conduct large-scale randomized controlled trials may be a significant factor in the need for more studies with this focus.
Acknowledgements
A.Z.A. designed the review article and drafted and proofread the article critically. G.B.H. revised the review article and helped in writing Statins and Brain Metastases, Particularly Metastatic Breast Cancer, Statins and Hypoxia-Growth-Dependent Glioma, and the conclusion sections. Z.Y.A. helped in writing the introduction and Satins Effect on the Growth of Normal and Cancerous Brain Cells In Vitro section and revised the article critically. K.A. helped in writing the introduction and revised the article critically. A.F.A. helped in writing the Fluvastatin and the Growth of Brain Cells In Vitro section. A.K.A. helped in writing The Relationship Between the Duration of Statins Therapy and the Clinical Outcome of Reducing the Risk of Brain Cancer, Including Glioma section. N.S.A. assisted in drawing the included figures. All authors approved the final version of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Cancer Society. Cancer statistics center. 2022. http://cancerstatisticscenter.cancer.org. [Accessed 25 December 2022].
- 2.World Health Organization. WHO editorial style manual. World Health Organization; 2021. pp. 83–91. [Google Scholar]
- 3.Nagai H, Kim YH. Cancer prevention from the perspective of global cancer burden patterns. J Thorac Dis 2017; 9:448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando K, Hu Q, Kasagi Y, Oki E, Mori M. Recent developments in cancer research: expectations for a new remedy. Ann Gastroenterol Surg 2021; 5:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin 2021; 71:381–406. [DOI] [PubMed] [Google Scholar]
- 7.Hill JR, Kuriyama N, Kuriyama H, Israel MA. Molecular genetics of brain tumors. Arch Neurol 1999; 56:439–441. [DOI] [PubMed] [Google Scholar]
- 8.Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev 2017; 18:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black PM. Benign brain tumors. Meningiomas, pituitary tumors, and acoustic neuromas. Neurol Clin 1995; 13:927–952. [PubMed] [Google Scholar]
- 10.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, et al.; Brain Tumor Epidemiology Consortium. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer 2008; 113(7 Suppl):1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khazaei Z, Goodarzi E, Borhaninejad V, Iranmanesh F, Mirshekarpour H, Mirzaei B, et al. The association between incidence and mortality of brain cancer and human development index (HDI): an ecological study. BMC Public Health 2020; 20:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs 2016; 20(5 Suppl):S2–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efird JT. Season of birth and risk for adult onset glioma. Int J Environ Res Public Health 2010; 7:1913–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamimi AF, Juweid M. Chapter 8. epidemiology and outcome of glioblastoma. In: De Vleeschouwer S, editor. Glioblastoma. Codon Publications; 2017. https://www.ncbi.nlm.nih.gov/books/NBK470003/. [Accessed 25 January 2023] doi: 10.15586/codon.glioblastoma.2017.ch8. [PubMed] [Google Scholar]
- 15.Valdebenito S, D’Amico D, Eugenin E. Novel approaches for glioblastoma treatment: Focus on tumor heterogeneity, treatment resistance, and computational tools. Cancer Rep (Hoboken) 2019; 2:e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmore SA. A review of programmed cell death. Toxicol Pathol 2007; 35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull 2019; 9:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korsmeyer S, Wei M, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 2000; 7:1166–1173. [DOI] [PubMed] [Google Scholar]
- 19.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 2006; 13:1423–1433. [DOI] [PubMed] [Google Scholar]
- 20.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci 2010; 123(Pt 19):3209–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Mechanisms of expression of apoptotic protease activating factor-1 (Apaf-1) in nuclear, mitochondrial and cytosolic fractions of the cerebral cortex of newborn piglets. Neurosci Lett 2007; 415:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avrutsky MI, Troy CM. Caspase-9: a multimodal therapeutic target with diverse cellular expression in human disease. Front Pharmacol 2021; 12:701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 2013; 5:a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity 2019; 50:1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol 2010; 22:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cockcroft S. Mammalian lipids: structure, synthesis and function. Essays Biochem 2021; 65:813–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Zou T, Shen X, Nelson PJ, Li J, Wu C, et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020; 2:27–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vona R, Iessi E, Matarrese P. Role of cholesterol and lipid rafts in cancer signaling: a promising therapeutic opportunity? Front Cell Dev Biol 2021; 9:622908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res 2019; 9:219–227. [PMC free article] [PubMed] [Google Scholar]
- 31.Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol 2021; 14:101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willey JZ, Elkind MS. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the treatment of central nervous system diseases. Arch Neurol 2010; 67:1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotyla P. The role of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors (statins) in modern rheumatology. Ther Adv Musculoskelet Dis 2010; 2:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang SY, Li H, Tang JJ, Wang J, Luo J, Liu B, et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat Commun 2018; 9:5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Lessem J, Jha P, Lonn E. Primary and secondary prevention of myocardial infarction and strokes: an update of randomly allocated, controlled trials. J Hypertens Suppl 1993; 11:S61–S73. [PubMed] [Google Scholar]
- 36.Fleg JL, Forman DE, Berra K, Bittner V, Blumenthal JA, Chen MA, et al.; American Heart Association Committees on Older Populations and Exercise Cardiac Rehabilitation and Prevention of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic He. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation 2013; 128:2422–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin 2016; 32:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rendon LF, Tewarie IA, Cote DJ, Gabriel A, Smith TR, Broekman MLD, et al. Statins and gliomas: a systematic review of the preclinical studies and meta-analysis of the clinical literature. Drugs 2022; 82:293–310. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad F, Sun Q, Patel D, Stommel JM. Cholesterol metabolism: a potential therapeutic target in glioblastoma. Cancers (Basel) 2019; 11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015; 6:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orth M, Bellosta SC. its regulation and role in central nervous system disorders. Cholesterol 2012; 2012:292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poitelon Y, Kopec AM, Belin S. Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells 2020; 9:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Climent E, Benaiges D, Pedro-Botet J. Hydrophilic or lipophilic statins? Front Cardiovasc Med 2021; 8:687585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J Cell Biol 1997; 138:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mast N, Petrov AM, Prendergast E, Bederman I, Pikuleva IA. Brain Acetyl-CoA production and phosphorylation of cytoskeletal proteins are targets of CYP46A1 activity modulation and altered sterol flux. Neurotherapeutics 2021; 18:2040–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo X, Zhou S, Yang Z, Li ZA, Hu W, Dai L, et al. Cholesterol metabolism and its implication in glioblastoma therapy. J Cancer 2022; 13:1745–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SI, Jeong W, Lim H, Cho S, Lee H, Jang Y, et al. APOE4-carrying human astrocytes oversupply cholesterol to promote neuronal lipid raft expansion and Aβ generation. Stem Cell Rep 2021; 16:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi X, Liao G. Cholesterol in Niemann-Pick Type C disease. Subcell Biochem 2010; 51:319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feltes M, Gale SE, Moores S, Ory DS, Schaffer JE. Monitoring the itinerary of lysosomal cholesterol in Niemann-Pick Type C1-deficient cells after cyclodextrin treatment. J Lipid Res 2020; 61:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol 2018; 14:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu R, Ou Z, Ruan X, Gong J. Role of liver X receptors in cholesterol efflux and inflammatory signaling (review). Mol Med Rep 2012; 5:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng F, Guo D. Lipid droplets, potential biomarker and metabolic target in glioblastoma. Intern Med Rev 2017; 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kou Y, Geng F, Guo D. Lipid metabolism in glioblastoma: from de novo synthesis to storage. Biomedicines 2022; 10:1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu JY, Fu WQ, Zheng XJ, Li W, Ren LW, Wang JH, et al. Avasimibe exerts anticancer effects on human glioblastoma cells via inducing cell apoptosis and cell cycle arrest. Acta Pharmacol Sin 2021; 42:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY, et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin Cancer Res 2016; 22:5337–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Z-F, Li G-Z, Zhai Y, Pan C-Q, Wang D, Yu M-C, et al. EGFRvIII promotes the proneural–mesenchymal transition of glioblastoma multiforme and reduces its sensitivity to temozolomide by regulating the NF-κB/ALDH1A3 axis. Genes 2023; 14:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong J, Quinn CM, Brown AJ. Synthesis of the oxysterol, 24(S), 25-epoxycholesterol, parallels cholesterol production and may protect against cellular accumulation of newly-synthesized cholesterol. Lipids Health Dis 2007; 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Björkhem I. Do oxysterols control cholesterol homeostasis? J Clin Invest 2002; 110:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanati M, Binabaj MM, Ahmadi SS, Aminyavari S, Javid H, Mollazadeh H, et al. Recent advances in glioblastoma multiforme therapy: a focus on autophagy regulation. Biomed Pharmacother 2022; 155:113740. [DOI] [PubMed] [Google Scholar]
- 60.Villa GR, Hulce JJ, Zanca C, Bi J, Ikegami S, Cahill GL, et al. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell 2016; 30:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fracassi A, Marangoni M, Rosso P, Pallottini V, Fioramonti M, Siteni S, et al. Statins and the brain: more than lipid lowering agents? Curr Neuropharmacol 2019; 17:59–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies JT, Delfino SF, Feinberg CE, Johnson MF, Nappi VL, Olinger JT, et al. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights 2016; 9:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf 2010; 9:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbalata CI, Tefas LR, Achim M, Tomuta I, Porfire AS. Statins in risk-reduction and treatment of cancer. World J Clin Oncol 2020; 11:573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor ML, Wells BJ, Smolak MJ. ‘Statins and cancer: a meta-analysis of case-control studies’. Eur J Cancer Prev 2008; 17:259–268. http://www.jstor.org/stable/45051787. [DOI] [PubMed] [Google Scholar]
- 66.Mengual D, Medrano LE, Villamizar-Villamizar W, Osorio-Llanes E, Mendoza-Torres E, Bolívar S. Novel effects of statins on cancer via autophagy. Pharmaceuticals (Basel) 2022; 15:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maj M, Czajkowski R, Zegarska B, Kowaliszyn B, Pokrywczynska M, Drewa T. Anti-proliferative and cytotoxic activity of rosuvastatin against melanoma cells. Postepy Dermatol Alergol 2016; 33:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torres CG, Olivares A, Stoore C. Simvastatin exhibits antiproliferative effects on spheres derived from canine mammary carcinoma cells. Oncol Rep 2015; 33:2235–2244. [DOI] [PubMed] [Google Scholar]
- 69.Wood WG, Igbavboa U, Muller WE, Eckert GP. Statins, Bcl-2, and apoptosis: cell death or cell protection? Mol Neurobiol 2013; 48:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerra B, Recio C, Aranda-Tavío H, Guerra-Rodríguez M, García-Castellano JM, Fernández-Pérez L. The mevalonate pathway, a metabolic target in cancer therapy. Front Oncol 2021; 11:626971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiarella E, Nisticò C, Di Vito A, Morrone HL, Mesuraca M. Targeting of mevalonate-isoprenoid pathway in acute myeloid leukemia cells by bisphosphonate drugs. Biomedicines 2022; 10:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu H, Jiang H, Lu D, Xiong Y, Qu C, Zhou D, et al. Effect of simvastatin on glioma cell proliferation, migration, and apoptosis. Neurosurgery 2009; 65:1087–96; discussion 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werner M, Sacher J, Hohenegger M. Mutual amplification of apoptosis by statin-induced mitochondrial stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br J Pharmacol 2004; 143:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ponce J, de la Ossa NP, Hurtado O, Millan M, Arenillas JF, Dávalos A, et al. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke 2008; 39:1269–1275. [DOI] [PubMed] [Google Scholar]
- 75.Yanae M, Tsubaki M, Satou T, Itoh T, Imano M, Yamazoe Y, et al. Statin-induced apoptosis via the suppression of ERK1/2 and Akt activation by inhibition of the geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. J Exp Clin Cancer Res 2011; 30:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yongjun Y, Shuyun H, Lei C, Xiangrong C, Zhilin Y, Yiquan K. Atorvastatin suppresses glioma invasion and migration by reducing microglial MT1-MMP expression. J Neuroimmunol 2013; 260:1–8. [DOI] [PubMed] [Google Scholar]
- 77.Altwairgi AK. Statins are potential anticancerous agents (Review). Oncol Rep 2015; 33:1019–1039. [DOI] [PubMed] [Google Scholar]
- 78.Bayat N, Ebrahimi-Barough S, Norouzi-Javidan A, Saberi H, Tajerian R, Ardakan MMM, et al. Apoptotic effect of atorvastatin in glioblastoma spheroids tumor cultured in fibrin gel. Biomed Pharmacother 2016; 84:1959–1966. [DOI] [PubMed] [Google Scholar]
- 79.Bayat N, Izadpanah R, Ebrahimi-Barough S, Norouzi Javidan A, Ai A, Mokhtari Ardakan MM, et al. The anti-angiogenic effect of atorvastatin in glioblastoma spheroids tumor cultured in fibrin gel: in 3D in vitro model. Asian Pac J Cancer Prev 2018; 19:2553–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sławińska-Brych A, Zdzisińska B, Kandefer-Szerszeń M. Fluvastatin inhibits growth and alters the malignant phenotype of the C6 glioma cell line. Pharmacol Rep 2014; 66:121–129. [DOI] [PubMed] [Google Scholar]
- 81.Pooler AM, Xi SC, Wurtman RJ. The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons. J Neurochem 2006; 97:716–723. [DOI] [PubMed] [Google Scholar]
- 82.Cerezo-Guisado MI, Alvarez-Barrientos A, Argent R, Garci´a-Mari´n LJ, Bragado MJ, Lorenzo MJ. c-Jun N-terminal protein kinase signalling pathway mediates lovastatin-induced rat brain neuroblast apoptosis. Biochim Biophys Acta 2007; 1771:164–176. [DOI] [PubMed] [Google Scholar]
- 83.Murinson BB, Haughey NJ, Maragakis NJ. Selected statins produce rapid spinal motor neuron loss in vitro. BMC Musculoskelet Disord 2012; 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long C, Yuan L, Wei W, Li J. Overcoming chemoresistance in glioblastoma by fluvastatin via prenylation-dependent inhibition of Ras signaling. Hum Exp Toxicol 2022; 41:9603271221125934. [DOI] [PubMed] [Google Scholar]
- 85.Yano M, Matsumura T, Senokuchi T, Ishii N, Murata Y, Taketa K, et al. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res 2007; 100:1442–1451. [DOI] [PubMed] [Google Scholar]
- 86.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst 2008; 100:134–139. [DOI] [PubMed] [Google Scholar]
- 87.Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res 2021; 40:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer 2011; 11:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joharatnam-Hogan N, Alexandre L, Yarmolinsky J, Lake B, Capps N, Martin RM, et al. Statins as potential chemoprevention or therapeutic agents in cancer: a model for evaluating repurposed drugs. Curr Oncol Rep 2021; 23:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaist D, Andersen L, Hallas J, Sørensen HT, Schrøder HD, Friis S. Use of statins and risk of glioma: a nationwide case-control study in Denmark. Br J Cancer 2013; 108:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cote DJ, Rosner BA, Smith-Warner SA, Egan KM, Stampfer MJ. Statin use, hyperlipidemia, and risk of glioma. Eur J Epidemiol 2019; 34:997–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saheki A, Terasaki T, Tamai I, Tsuji A. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res 1994; 11:305–311. [DOI] [PubMed] [Google Scholar]
- 93.Chen BK, Chiu HF, Yang CY. Statins are associated with a reduced risk of brain cancer: a population-based case-control study. Medicine (Baltim) 2016; 95:e3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsieh HC, Hsu JC, Lu CY. 10-year trends in statin utilization in Taiwan: a retrospective study using Taiwan’s National Health Insurance Research Database. BMJ Open 2017; 7:e014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raparelli V, Pannitteri G, Todisco T, Toriello F, Napoleone L, Manfredini R, et al. Treatment and response to statins: gender-related differences. Curr Med Chem 2017; 24:2628–2638. [DOI] [PubMed] [Google Scholar]
- 97.Bandyopadhyay S, Bayer AJ, O’Mahony MS. Age and gender bias in statin trials. QJM 2001; 94:127–132. [DOI] [PubMed] [Google Scholar]
- 98.Xiao A, Brenneman B, Floyd D, Comeau L, Spurio K, Olmez I, et al. Statins affect human glioblastoma and other cancers through TGF-β inhibition. Oncotarget 2019; 10:1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walker D, Hamilton W, Walter FM, Watts C. Strategies to accelerate diagnosis of primary brain tumors at the primary-secondary care interface in children and adults. CNS Oncol. 2013; 2:447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thakkar P, Greenwald BD, Patel P. Rehabilitation of adult patients with primary brain tumors: a narrative review. Brain Sci 2020; 10:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ransohoff J. Surgical management of malignant brain tumors. Natl Cancer Inst Monogr 1977; 46:145–150. [PubMed] [Google Scholar]
- 102.Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol 2012; 14:1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rossi M, Gay L, Ambrogi F, Conti Nibali M, Sciortino T, Puglisi G, et al. Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol 2021; 23:812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Povoski SP, Neff RL, Mojzisik CM, O'Malley DM, Hinkle GH, Hall NC, et al. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol 2009; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barisano G, Bergamaschi S, Acharya J, Rajamohan A, Gibbs W, Kim P, et al. Complications of radiotherapy and radiosurgery in the brain and spine. Neurographics 2018; 8:167–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mo F, Pellerino A, Soffietti R, Rudà R. Blood-brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci 2021; 22:12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doolittle ND, Muldoon LL, Culp AY, Neuwelt EA. Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol 2014; 71:203–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Altwairgi AK, Alghareeb WA, AlNajjar FH, Alhussain H, Alsaeed E, Balbaid AAO, et al. Atorvastatin in combination with radiotherapy and temozolomide for glioblastoma: a prospective phase II study. Invest New Drugs 2021; 39:226–231. [DOI] [PubMed] [Google Scholar]
- 109.Gaist D, Hallas J, Friis S, Hansen S, Sørensen HT. Statin use and survival following glioblastoma multiforme. Cancer Epidemiol. 2014; 38:722–727. [DOI] [PubMed] [Google Scholar]
- 110.Happold C, Gorlia T, Nabors LB, Erridge SC, Reardon DA, Hicking C, et al.; EORTC Brain Tumor Group and on behalf of the CENTRIC and CORE Clinical Trial Groups. Do statins, ACE inhibitors or sartans improve outcome in primary glioblastoma? J Neurooncol 2018; 138:163–171. [DOI] [PubMed] [Google Scholar]
- 111.Watson R, Tulk A, Erdrich J. The link between statins and breast cancer in mouse models: a systematic review. Cureus 2022; 14:e31893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campos S, Davey P, Hird A, Pressnail B, Bilbao J, Aviv RI, et al. Brain metastasis from an unknown primary, or primary brain tumour? A diagnostic dilemma. Curr Oncol 2009; 16:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furet E, El Bouchtaoui M, Feugeas JP, Miquel C, Leboeuf C, Beytout C, et al. Increased risk of brain metastases in women with breast cancer and p16 expression in metastatic lymph-nodes. Oncotarget 2017; 8:37332–37341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alarfi H, Youssef LA, Salamoon M. A prospective, randomized, placebo-controlled study of a combination of simvastatin and chemotherapy in metastatic breast cancer. J Oncol 2020; 2020:4174395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Hamamsy M, Elwakil H, Saad AS, Shawki MA. A randomized controlled open-label pilot study of simvastatin addition to whole-brain radiation therapy in patients with brain metastases. Oncol Res 2016; 24:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev 2017; 9:CD006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brachman DG, Pugh SL, Ashby LS, Thomas TA, Dunbar EM, Narayan S, et al. Phase 1/2 trials of Temozolomide, Motexafin Gadolinium, and 60-Gy fractionated radiation for newly diagnosed supratentorial glioblastoma multiforme: final results of RTOG 0513. Int J Radiat Oncol Biol Phys 2015; 91:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gorabi AM, Kiaie N, Aslani S, Sathyapalan T, Jamialahmadi T, Sahebkar A. Implications on the therapeutic potential of statins via modulation of autophagy. Oxid Med Cell Longev 2021; 2021:9599608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sheikholeslami K, Ali Sher A, Lockman S, Kroft D, Ganjibakhsh M, Nejati-Koshki K, et al. Simvastatin induces apoptosis in medulloblastoma brain tumor cells via mevalonate cascade prenylation substrates. Cancers (Basel) 2019; 11:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Na E, Cho S, Kim DJ, Choi J, Han E. Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: a retrospective cohort study. Cardiovasc Diabetol 2020; 19:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2011; 2:1117–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015; 3:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bouleftour W, Rowinski E, Louati S, Sotton S, Wozny AS, Moreno-Acosta P, et al. A review of the role of hypoxia in radioresistance in cancer therapy. Med Sci Monit 2021; 27:e934116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tilija Pun N, Jeong CH. Statin as a potential chemotherapeutic agent: current updates as a monotherapy, combination therapy, and treatment for anti-cancer drug resistance. Pharmaceuticals (Basel) 2021; 14:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee JY, Cha BS. Effects of combination therapy of statin and thiazolidinedione on vascular inflammation. Korean Circ J 2018; 48:602–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tapia-Pérez JH, Kirches E, Mawrin C, Firsching R, Schneider T. Cytotoxic effect of different statins and thiazolidinediones on malignant glioma cells. Cancer Chemother Pharmacol 2011; 67:1193–1201. [DOI] [PubMed] [Google Scholar]
- 127.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 2011; 75:50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 2012; 16:103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci 2010; 86:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]