Abstract
Objective
To assess whether brief mindfulness-based cognitive behavioral therapy (MBCBT) could enhance the benefits of total knee arthroplasty (TKA) in improving pain and pain-related disability. Specifically, to determine 1) whether patients who received MBCBT differed from matched controls who received treatment-as-usual with regard to postsurgical pain outcomes and 2) whether changes in pain catastrophizing, depression, or anxiety explained the potential effects of MBCBT on pain outcomes.
Design
Pilot clinical trial.
Setting
An academic teaching hospital serving a large urban and suburban catchment area surrounding the Boston, Massachusetts metropolitan region.
Subjects
Sample of 44 patients undergoing TKA. Patients who completed a brief MBCBT intervention (n = 22) were compared with age-, race-, and sex-matched controls who received treatment-as-usual (n = 22).
Methods
The MBCBT intervention included four 60-minute sessions delivered by a pain psychologist in person and via telephone during the perioperative period. Participants were assessed at baseline and at 6 weeks, 3 months, and 6 months after surgery.
Results
Compared with matched controls, patients who received MBCBT had lower pain severity and pain interference at 6 weeks after surgery. Group differences in outcomes were mediated by changes in pain catastrophizing but not by changes in depression or anxiety. The MBCBT group had similar reductions in pain severity and interference as the control group did at 3 and 6 months after surgery.
Conclusions
This work offers evidence for a safe and flexibly delivered nonpharmacological treatment (MBCBT) to promote faster recovery from TKA and identifies change in pain catastrophizing as a mechanism by which this intervention could lead to enhanced pain-related outcomes.
Keywords: Knee Osteoarthritis, Total Knee Arthroplasty, Cognitive Behavioral Therapy, Mindfulness, Pain Catastrophizing
Introduction
The knee is one of the joints most commonly affected by osteoarthritis [1, 2], with approximately 24% of the global population experiencing symptomatic knee osteoarthritis (KOA) [3–5]. As the age distribution shifts with increased life expectancy, the proportion of individuals 65 years of age or older continues to rise both globally and locally [6]; given that age is a major risk factor for osteoarthritis, the number of people suffering from KOA is also likely to increase [7]. Total knee arthroplasty (TKA) is the most common surgical treatment for patients with end-stage KOA and, like KOA cases, is expected to increase in prevalence. In the United States alone, the number of knee arthroplasties performed reached more than 700,000 in 2012 and is projected to increase several-fold by 2050 [8]. TKA is generally considered a safe and effective treatment for KOA, with the majority of patients reporting substantial pain relief and improved functional status [9].
Although most patients benefit from TKA, approximately 10% to 34% experience unfavorable long-term outcomes such as persistent postoperative pain, despite clinical and radiological indications of successful surgery [10]. Persistent pain after TKA is associated with patient dissatisfaction, as well as with increased health care and personal burden [11, 12]. Studies have shown that preoperative psychological distress (depression and anxiety) and pain catastrophizing (negative cognitive and emotional responses to actual or anticipated pain) contribute to worse pain-related outcomes in patients undergoing TKA [13–22]. In response to these findings and to the risks associated with opioids and sedatives [23], recent efforts have focused on testing nonpharmacological, psychological interventions to safely enhance recovery from surgery and prevent the transition from acute postsurgical pain to chronic pain.
Psychological or mind–body interventions have historically been included as part of multidisciplinary treatment for chronic pain and are designed to reduce pain intensity, psychological distress, and pain-related disability. Such mind–body approaches, which have been effectively integrated into the treatment of patients with established chronic pain, are being adapted for the perioperative period [24, 25]. In the context of TKA, there is some evidence that perioperative cognitive behavioral therapy (CBT) and mindfulness-based interventions improve postoperative pain and functioning [26–33]. Mindfulness-based CBT (MBCBT), which combines components of CBT (e.g., cognitive restructuring, activity pacing, sleep hygiene) and mindfulness-based interventions (e.g., mindfulness exercises) [34, 35], might confer even greater benefits on pain-related outcomes than those provided by either approach alone [36–39]. To date, no studies have tested the effects of MBCBT on postoperative outcomes after TKA.
The purpose of this pilot trial was to assess whether brief MBCBT could enhance the efficacy of TKA in improving pain and pain-related disability. Specifically, we examined whether patients who received MBCBT differed from matched controls who received treatment as usual (TAU) with regard to postsurgical outcomes (pain severity and interference at 6 weeks, 3 months, and 6 months after TKA) (Aim 1) and whether changes in pain catastrophizing, depression, or anxiety explained the potential effects of MBCBT on postsurgical pain outcomes (Aim 2).
Methods
Study Design
This was a pilot clinical trial comparing pain outcomes of patients undergoing TKA who completed a brief MBCBT intervention with the outcomes of age-, race-, and sex-matched controls from a larger parent study who received TAU. The parent study examined bio-behavioral risk factors associated with the development of persistent postsurgical pain after TKA [40]. Additional studies based on the parent study do not overlap with the present study with regard to aims or data presented [41–46]. All study-related procedures were approved by the Brigham and Women’s Hospital Institutional Review Board, and the study was registered on ClinicalTrials.gov (NCT04328701). Informed consent was obtained from each participant.
Participants
All participants (N = 44) were recruited from Brigham and Women’s Hospital through posted flyers, advertisement letters mailed to patients scheduled for TKA, advertisements in local orthopedic clinics, and announcements on the hospital research website, as well as directly from orthopedic surgery clinics at Brigham and Women’s Hospital. The parent study for this pilot trial evaluated 6-month outcomes after TKA [40], enrolling patients from 2012–2019. Participants enrolled in the MBCBT arm (n = 22) were recruited specifically for the present pilot study, which had its own Institutional Review Board approval, ClinicalTrials.gov registration, and consent form; otherwise, all recruitment and assessment procedures matched the parent study. Participants in the MBCBT arm were demographically matched to 22 participants in the parent study who underwent surgery as usual. Inclusion criteria included 1) age >45 years; 2) meeting American College of Rheumatology diagnostic criteria for KOA; 3) scheduled TKA; 4) English proficiency; and 5) stable medication dosage for at least 1 month before study enrollment. Exclusion criteria included 1) use of opioids in the previous 30 days; 2) recent history of substance abuse disorder; 3) presence of a sleep disorder, systemic inflammatory disorder, or autoimmune disorder; 4) pregnancy; 5) Raynaud’s disease; 6) current infection; 7) moderate-to-severe peripheral neuropathy; 8) history of myocardial infarction or other serious cardiovascular condition in the prior 12 months; 9) current use of oral steroids; and 10) delirium, dementia, psychosis, or other cognitive impairment that would prevent completion of study procedures.
Mindfulness-Based Cognitive Behavioral Therapy
The four-session MBCBT protocol used in this study was adapted from CBT and mindfulness-based stress reduction protocols used in a study of chronic low back pain [47]. The protocol was adapted for use in the perioperative period by maximizing flexibility to better accommodate surgical patients [25] (e.g., shortened from eight sessions to four sessions, allowed for remote sessions via telephone), and included both presurgical and postsurgical sessions. The first and fourth sessions were conducted in person during the baseline and 6-week follow-up visits, whereas the second and third sessions were conducted via telephone. Participants were flexibly able to schedule the second and third sessions, one before and one after surgery. Each MBCBT session lasted approximately 60 minutes and was delivered by a clinical pain psychologist (SMM). All 22 participants in the MBCBT arm completed all four sessions. Session content is outlined in Table 1.
Table 1.
Summary of MBCBT session content
| Session | Topics Covered |
|---|---|
| Session 1 (in-person) |
|
| Session 2 (telephone) |
|
| Session 3 (telephone) |
|
| Session 4 (in-person) |
|
Measures
All measures were administered at baseline (before MBCBT and/or TKA) and at 6 weeks, 3 months, and 6 months after surgery.
Primary Outcomes
Pain severity and interference. The Brief Pain Inventory [48] is a self-report measure of pain severity and interference. Participants were asked to indicate the level of their worst, least, average, and current pain on numeric rating scales from 0 (no pain) to 10 (worst pain you can imagine). The mean of these scores was used as a measure of pain severity. Participants were asked to indicate the degree to which pain interfered with seven daily activities. The mean of these scores was used as a measure of pain interference. Higher scores on the Brief Pain Inventory are indicative of greater pain severity and pain interference. The Brief Pain Inventory is a widely used, well-validated, and reliable measure of pain severity and interference across many chronic pain populations [49].
Potential Mediators
Pain catastrophizing. The Pain Catastrophizing Scale is a self-report measure assessing three dimensions of negative pain-related cognitions: rumination, magnification, and helplessness [50]. Items were summed, with higher total scores indicative of greater catastrophizing. The Pain Catastrophizing Scale is a well-validated and widely used measure of catastrophic thinking associated with pain [51].
Depression and anxiety. The Patient-Reported Outcomes Measurement Information System Short Forms were used to assess depression and anxiety symptoms [52]. The depression scale consists of eight items, and the anxiety scale consists of seven items, each rated from 1 to 5, with higher scores indicative of greater symptoms. The Patient-Reported Outcomes Measurement Information System has shown good reliability and validity in patients with osteoarthritis [53].
Statistical Analysis
All data were analyzed in IBM SPSS Statistics for Windows, Version 28.0 (IBM Corp., Armonk, NY, USA). Two-sample t and chi-squared tests were used to compare MBCBT participants and matched controls on sociodemographic variables and baseline levels of outcome and mediator variables. To examine group differences in postsurgical outcomes (Aim 1), two-way mixed-design analyses of variance (mixed-design ANOVAs) were conducted, with time (baseline and 6 weeks, 3 months, and 6 months after surgery) as the within-subject factor, group (MBCBT and TAU) as the between-subjects factor, and group × time as the interaction term. These analyses were followed with simple main-effects analyses. All tests were two tailed, with alpha set at 0.05. Between-group effect sizes for ANOVAs were calculated with partial eta squared (ηp2); effect sizes are generally considered small at ηp2 = 0.01, medium at ηp2 = 0.06, and large at ηp2 = 0.14 [54].
Potential mediators of improvements in pain outcomes (Aim 2) were examined with the SPSS MEMORE macro [55], which assesses mediation in repeated-measures designs. We first conducted 1) mixed-design ANOVAs to examine group differences in potential mediators (i.e., pain catastrophizing, depression, and anxiety) and 2) bivariate Pearson correlations between changes in potential mediators and changes in pain severity and interference in the full sample. We then included those variables in the mediation models that were related to the predictor variable (MBCBT vs TAU) and outcome variables (changes in pain severity and interference).
Results
Preliminary Analyses
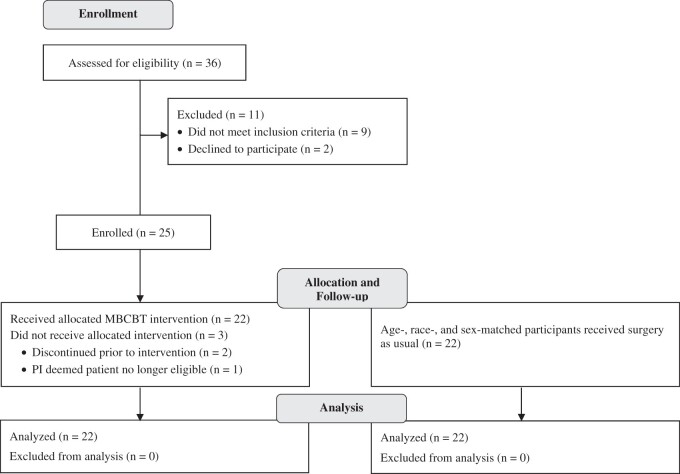
Figure 1 depicts patient flow through the study. Results of t and chi-squared tests confirmed that the matching system was successful; there were no significant differences between the MBCBT group (n = 22) and matched controls (n = 22) on any of the baseline sociodemographic variables (Table 2). The full sample of 44 participants was predominantly female (55%), middle- to older-aged (mean [M] = 67 years, standard deviation [SD] = 7 years; sample age range: 52–84 years), and White (86%). The MBCBT and TAU arms did not differ significantly on any of the outcome or potential mediator variables at baseline (Table 2). All participants completed the entirety of the baseline, 6-week, 3-month, and 6-month assessments, resulting in minimal missing data (<0.5%). Participants with missing data on a given measure at one or more time point(s) were excluded from analyses using that measure and time point(s).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram displaying study enrollment, allocation, participation, and follow-up.
Table 2.
Group comparisons on sociodemographic, outcome, and mediator variables at baseline
| Variable | Full sample (N = 44) | MBCBT (n = 22) | TAU (n = 22) | t/χ2 | P |
|---|---|---|---|---|---|
| Age, years, M ± SD | 66.8 ± 7.0 | 67.6 ± 7.2 | 65.9 ± 6.9 | 0.79 | 0.432 |
| Sex, n (%) | 0.00 | 1.000 | |||
| Male | 20 (45.5) | 10 (45.5) | 10 (45.5) | ||
| Female | 24 (54.5) | 12 (54.5) | 12 (54.5) | ||
| Race, n (%) | 2.11 | 0.349 | |||
| White | 38 (86.4) | 18 (81.8) | 20 (90.9) | ||
| Black | 4 (9.1) | 2 (9.1) | 2 (9.1) | ||
| Declined to answer | 2 (4.5) | 2 (9.1) | 0 (0.0) | ||
| Outcome variables, M ± SD | |||||
| BPI severity | 3.5 ± 2.1 | 4.0 ± 2.3 | 2.9 ± 1.9 | 1.69 | 0.098 |
| BPI interference | 3.6 ± 2.2 | 4.1 ± 2.3 | 3.1 ± 2.1 | 1.36 | 0.181 |
| Mediator variables, M ± SD | |||||
| PCS | 12.9 ± 10.3 | 15.1 ± 11.8 | 10.7 ± 8.2 | 1.43 | 0.161 |
| PROMIS depression | 46.1 ± 6.8 | 47.3 ± 5.7 | 45.0 ± 7.6 | 1.09 | 0.282 |
| PROMIS anxiety | 50.4 ± 7.9 | 50.1 ± 7.5 | 50.7 ± 8.4 | 0.26 | 0.797 |
BPI = Brief Pain Inventory; PCS = Pain Catastrophizing Scale; PROMIS = Patient-Reported Outcomes Measurement Information System. All tests were two tailed.
Comparison of the MBCBT and TAU Groups on Postsurgical Outcomes
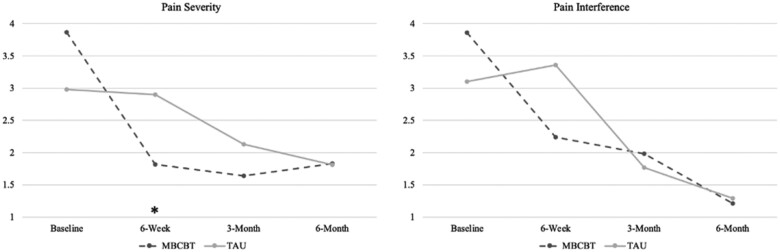
Mean pain severity and interference ratings for each group at baseline and at 6 weeks, 3 months, and 6 months after surgery are depicted in Figure 2. Table 3 displays the results of two-way mixed-design ANOVAs, including main effects of time (baseline, 6 weeks, 3 months, 6 months) and interaction effects of group (MBCBT, TAU) and time for each outcome variable. Table 4 shows the results of simple main-effects analyses.
Figure 2.
Mean pain severity and interference ratings at baseline and at 6 weeks, 3 months, and 6 months after surgery by group.
Table 3.
Main effects of time and interaction effects of group and time on pain outcomes
| Pain Outcome | F | df | P | ηp2 |
|---|---|---|---|---|
| Pain severity | ||||
| Time | 12.43 | 3, 108 | <0.001 | 0.26 |
| Group × time | 3.93 | 3, 108 | 0.010 | 0.10 |
| Pain interference | ||||
| Time | 18.36 | 3, 105 | <0.001 | 0.34 |
| Group × time | 2.94 | 3, 105 | 0.037 | 0.08 |
All tests were two tailed. The P values are bold when they are less than the significance level cutoff of 0.05. Effect sizes were calculated with partial eta squared (ηp2).
Table 4.
Simple main effects of time on pain outcomes by group
| Pain Outcome | Mean Difference | SE | F | df | P | ηp2 |
|---|---|---|---|---|---|---|
| MBCBT | ||||||
| Pain severity | ||||||
| Baseline to 6 weeks | –2.05 | 0.51 | 15.98 | 1, 36 | <0.001 | 0.31 |
| Baseline to 3 months | –2.22 | 0.48 | 21.77 | 1, 36 | <0.001 | 0.38 |
| Baseline to 6 months | –2.04 | 0.46 | 19.74 | 1, 36 | <0.001 | 0.35 |
| Pain interference | ||||||
| Baseline to 6 weeks | –1.62 | 0.53 | 9.37 | 1, 35 | 0.004 | 0.21 |
| Baseline to 3 months | –1.88 | 0.55 | 11.82 | 1, 35 | 0.002 | 0.25 |
| Baseline to 6 months | –2.65 | 0.48 | 30.90 | 1, 35 | <0.001 | 0.47 |
| TAU | ||||||
| Pain severity | ||||||
| Baseline to 6 weeks | –0.08 | 0.51 | 0.02 | 1, 36 | 0.878 | 0.00 |
| Baseline to 3 months | –0.85 | 0.48 | 3.17 | 1, 36 | 0.083 | 0.08 |
| Baseline to 6 months | –1.17 | 0.46 | 6.51 | 1, 36 | 0.015 | 0.15 |
| Pain interference | ||||||
| Baseline to 6 weeks | 0.26 | 0.52 | 0.25 | 1, 35 | 0.621 | 0.01 |
| Baseline to 3 months | –1.33 | 0.53 | 6.26 | 1, 35 | 0.017 | 0.15 |
| Baseline to 6 months | –1.81 | 0.46 | 15.14 | 1, 35 | <0.001 | 0.30 |
All tests were two tailed. The P values are bold when they are less than the significance level cutoff of 0.05. Effect sizes were calculated with partial eta squared (ηp2).
Mixed-design ANOVAs showed overall significant group differences (group × time interactions) in pain severity (medium to large effect size) and pain interference (medium effect size). The MBCBT group had significantly lower pain severity at 6 weeks (M = 1.82, SD = 1.19) than did the TAU group (M = 2.90, SD = 1.77) (F[1,36] = 4.89, 95% confidence interval [CI] = 0.09 to 2.07, P = 0.033, ηp2 = 0.12 [medium to large effect size]). Though the result was nonsignificant, the MBCBT group also reported lower pain interference at 6 weeks (M = 2.24, SD = 1.77) than did the TAU group (M = 3.36, SD = 2.02) (F[1,35] = 3.20, 95% CI = –0.15 to 2.39, P = 0.082, ηp2 = 0.08 [medium effect size]). Simple main-effects analyses indicated that participants in the MBCBT group had significant reductions in pain severity and interference from baseline to 6 weeks after surgery (large effect sizes), whereas those who received TAU showed no improvements in pain severity or interference at 6 weeks after surgery. The groups did not significantly differ on pain outcomes at any other time point (P for all > 0.40, ηp2 = 0.00–0.02). The MBCBT group had significant reductions in pain severity and interference from baseline to 3 and 6 months after surgery (large effect sizes), and the TAU group had significant reductions in pain severity from baseline to 6 months (large effect size) and in pain interference from baseline to 3 and 6 months after surgery (large effect sizes).
Mediation Analyses
Given that the MBCBT and TAU arms differed in outcomes at 6 weeks after surgery only, this time point was used in mediation analyses. Results of mixed-design ANOVAs showed a medium-magnitude group difference in pain catastrophizing from baseline to 6 weeks, though it was not statistically significant in this small sample (Table 5). The MBCBT group had significant reductions in catastrophizing from baseline to 6 weeks (M = –6.37, standard error [SE] = 2.57, 95% CI = –11.57 to –1.17, P = 0.018), whereas the TAU group did not (M = –1.14, SE = 2.39, 95% CI = –5.97 to 3.70, P = 0.637). There were no group × time interaction effects, or main effects of time, for depression or anxiety. Results of Pearson correlations indicated that a change in pain catastrophizing from baseline to 6 weeks was associated with changes in pain severity (r = 0.51, P < 0.001) and pain interference (r = 0.36, P = 0.025), but changes in depression or anxiety were not associated with changes in pain severity or pain interference (Table 6). Thus, only pain catastrophizing was included in mediation analyses.
Table 5.
Main effects of time and interaction effects of group and time on potential mediators
| Potential Mediator | F | df | P | ηp2 |
|---|---|---|---|---|
| Pain catastrophizing | ||||
| Time | 4.57 | 1, 39 | 0.039 | 0.11 |
| Group × time | 2.22 | 1, 39 | 0.144 | 0.05 |
| Depression | ||||
| Time | 1.59 | 1, 39 | 0.214 | 0.04 |
| Group × time | 0.52 | 1, 39 | 0.476 | 0.01 |
| Anxiety | ||||
| Time | 0.95 | 1, 39 | 0.335 | 0.02 |
| Group × time | 0.92 | 1, 39 | 0.342 | 0.02 |
All tests were two tailed. The P values are bold when they are less than the significance level cutoff of 0.05. Effect sizes were calculated with partial eta squared (ηp2).
Table 6.
Correlations between changes in potential mediators and changes in outcome measures at 6 weeks after surgery
| Potential Mediators | Change in BPI Severity | Change in BPI Interference |
|---|---|---|
| Change in PCS | 0.51*** | 0.36* |
| Change in PROMIS depression | 0.18 | 0.11 |
| Change in PROMIS anxiety | 0.02 | –0.01 |
BPI = Brief Pain Inventory; PCS = Pain Catastrophizing Scale; PROMIS = Patient-Reported Outcomes Measurement Information System.
P < 0.05;
P < 0.01;
P < 0.001; correlations are bold when they are significant at the P < 0.05 level.
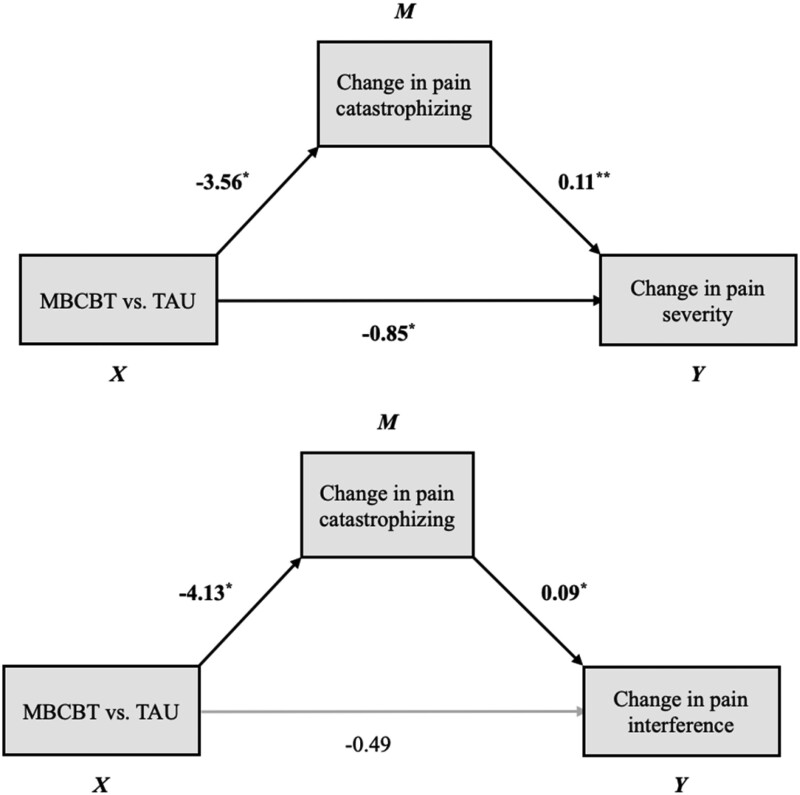
Results of mediation analyses indicated significant indirect effects of MBCBT on pain outcomes through changes in pain catastrophizing (Figure 3). Participants who underwent MBCBT experienced a decrease in catastrophizing, which in turn was associated with improved pain at 6 weeks after surgery (b = –0.40, 95% CI = –1.03 to –0.02). Similarly, MBCBT led to decreased catastrophizing, which was associated with improvements in pain interference at 6 weeks after surgery (b = –0.36, 95% CI = –0.98 to –0.02).
Figure 3.
Direct and indirect effects of MBCBT on pain severity and interference at 6 weeks after surgery through change in pain catastrophizing. *P < 0.05; **P < 0.01.
Discussion
Patients in this pilot clinical trial who received a four-session MBCBT appeared to improve more rapidly after TKA than did matched controls who received TAU, with significantly lower pain severity and pain interference at 6 weeks after surgery in the MBCBT group. The MBCBT group subsequently had reductions similar to those of matched controls in pain severity and interference at 3 and 6 months after surgery. These findings add to the growing body of evidence [26–33] that a nonpharmacological, psychological intervention flexibly delivered during the perioperative period could safely and effectively promote faster recovery from TKA. This is potentially crucial for patients, as faster recovery could result in earlier return to work and physical activity and lower reliance on opioids and other pain medications.
In addition, this study identified change in pain catastrophizing as a mechanism by which perioperative MBCBT might lead to enhanced pain-related outcomes. When left untreated, catastrophic responses to actual or anticipated pain can lead to fear and avoidance of activity, which in turn leads to deconditioning, increased pain, and distress [56–58]. Our findings suggest that brief MBCBT can effectively reduce catastrophizing in patients undergoing TKA and in turn can help them recover more quickly from surgery in terms of pain and pain interference. These findings are consistent with literature showing that pain catastrophizing augments pain processing and strongly contributes to the development and maintenance of chronic pain, including chronic postsurgical pain [13, 59, 60], and can be reduced in patients undergoing TKA through psychological intervention [61].
Unexpectedly, MBCBT did not result in greater reductions in anxiety or depression at 6 weeks after surgery, and changes in anxiety and depression were not related to changes in pain outcomes, despite prior literature showing that psychological distress is associated with increased postoperative pain in patients undergoing TKA [62] and can be reduced through nonpharmacological adjunctive interventions [63]. One possible explanation is that participants in the present study had, on average, low baseline levels of anxiety and depression that remained consistently low during the study period. Thus, psychological distress might not have been a significant contributing factor to patients’ pain in this sample. It is also possible that surgery-related impact on mood and functioning caused depression and anxiety to persist or even increase during the immediate recovery period. Thus, although patients’ depressive and anxiety symptoms did not improve at 6 weeks after surgery, their mood symptoms might have improved at subsequent time points (e.g., 3 months or 6 months after surgery), particularly those who received perioperative psychological intervention.
Limitations and Future Directions
A notable shortcoming of this study is the lack of a randomized design. Although experimental participants were compared with age-, race-, and sex-matched controls, the lack of randomization precludes concluding definitively that MBCBT, compared with TAU, improved pain-related outcomes in patients undergoing TKA. It is possible that the observed group differences were instead due to uncontrolled processes. That is, participants who elected to participate in the pilot intervention program might have differed systematically from the controls in the parent observational cohort study. Indeed, the MBCBT group generally reported more severe pain and higher levels of distress before surgery, though these differences did not reach statistical significance. In addition, the fourth and final MBCBT session was delivered during patients’ 6-week visit, which could have acutely influenced self-reported outcomes that were assessed at that visit. Finally, the study sample was relatively small and homogenous (mostly White), limiting the generalizability of our findings. Overall, more rigorous randomized controlled trials with larger and diverse samples are needed.
In addition, quality improvement studies are needed to enhance this relatively novel nonpharmacological approach to preventing chronic postsurgical pain. To potentially increase or prolong the benefits of psychological interventions, future work could experiment with alternative evidenced-based approaches (e.g., acceptance and commitment therapy, biofeedback), format (e.g., in person, by telephone, or virtual face to face, and group vs individual sessions), and the structure and timing of sessions (e.g., single sessions, booster sessions, presurgical and postsurgical timing of treatment). Future research in this area should also examine additional mechanisms to explain how these brief perioperative interventions confer benefits in surgical patients. Our findings suggest that pain catastrophizing might be a crucial target for intervention in this patient population and can be improved through MBCBT. By identifying additional mechanisms (e.g., mindfulness, pain acceptance, perceived support, increased physical activity, reduced opioid use), we can refine these interventions to optimize outcomes and patient satisfaction while reducing provider burden. It is also of interest to determine what elements of the intervention might contribute most robustly to its benefits. At least one large, multisite trial of a CBT-oriented treatment did not report significant benefit on pain-related outcomes [64], and it is possible that the mindfulness-based elements of the present protocol provided additional pain-reducing effects at the 6-week time point. Recently, hospital systems have begun implementing Enhanced Recovery After Surgery (ERAS) protocols to optimize hydration, nutrition, and pain control, leading to faster, safer, and more comfortable recovery from surgery [65]. Findings from the present study and similar work could be used to inform the integration of psychological treatment (e.g., CBT, mindfulness-based interventions) into ERAS protocols to further enhance the efficacy of a second generation of ERAS programs.
Conclusions
This pilot clinical trial provides further support for the efficacy of perioperative psychological interventions in enhancing postoperative pain outcomes. Specifically, our findings offer evidence for a safe and flexibly delivered nonpharmacological treatment (MBCBT) to promote faster recovery from TKA, and our findings identify change in pain catastrophizing as a mechanism by which this intervention might lead to enhanced pain-related outcomes. Rigorous randomized controlled trials with larger samples are needed to enhance the long-term benefits for more patients and could experiment with different therapeutic approaches, format, and structure and timing of treatment. Future work should also identify additional core mechanisms that can be targeted by interdisciplinary health care providers (e.g., health psychologists, physical therapists, surgeons) to safely optimize outcomes and satisfaction in surgical patients and to prevent long-term sequelae of chronic pain.
Acknowledgments
We thank the patients who participated in this study and the surgeons who conducted the knee replacement surgeries.
Contributor Information
Bethany D Pester, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Jenna M Wilson, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Jihee Yoon, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Asimina Lazaridou, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Kristin L Schreiber, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Marise Cornelius, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Claudia M Campbell, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michael T Smith, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jennifer A Haythornthwaite, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Robert R Edwards, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Samantha M Meints, Department of Anesthesiology and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Funding sources: This research was supported by the U.S. National Institutes for Health (NIH) National Institutes for Arthritis and Musculoskeletal and Skin Diseases (NIAMS; R01-AG034982) to RRE, CMC, MTS, and JAH.
Conflicts of interest: We have no known conflict of interest to disclose.
Trial registration: ClinicalTrials.gov ID: NCT04328701.
Data availability: The data that support the study findings are available from the corresponding author upon reasonable request.
References
- 1. Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73(9):1659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turkiewicz A, Petersson IF, Björk J, et al. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthritis Cartilage 2014;22(11):1826–32. [DOI] [PubMed] [Google Scholar]
- 3. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira D, Peleteiro B, Araújo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthritis Cartilage 2011;19(11):1270–85. [DOI] [PubMed] [Google Scholar]
- 5. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: Literature update. Curr Opin Rheumatol 2018;30(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Census Bureau. Projections of the size and composition of the U.S. population: 2014 to 2060. Current Population Reports. 2014. Available at: https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf (accessed April 18, 2022).
- 7. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26(3):355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States—an alternative projection model. Osteoarthritis Cartilage 2017;25(11):1797–803. [DOI] [PubMed] [Google Scholar]
- 9. Katz JN, Mahomed NN, Baron JA, et al. Association of hospital and surgeon procedure volume with patient-centered outcomes of total knee replacement in a population-based cohort of patients age 65 years and older. Arthritis Rheum 2007;56(2):568–74. [DOI] [PubMed] [Google Scholar]
- 10. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2(1):e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gunaratne R, Pratt DN, Banda J, et al. Patient dissatisfaction following total knee arthroplasty: A systematic review of the literature. J Arthroplasty 2017;32(12):3854–60. [DOI] [PubMed] [Google Scholar]
- 12. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 2014;10(7):437–41. [DOI] [PubMed] [Google Scholar]
- 13. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag 2009;14(4):307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJL, Gross M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: Two-year follow-up. Pain Res Manag 2008;13(4):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: A systematic review and meta-analysis. Br J Anaesth 2015;114(4):551–61. [DOI] [PubMed] [Google Scholar]
- 16. Pan X, Wang J, Lin Z, Dai W, Shi Z. Depression and anxiety are risk factors for postoperative pain-related symptoms and complications in patients undergoing primary total knee arthroplasty in the United States. J Arthroplasty 2019;34(10):2337–46. [DOI] [PubMed] [Google Scholar]
- 17. Pavlin DJ, Sullivan MJL, Freund PR, Roesen K. Catastrophizing: A risk factor for postsurgical pain. Clin J Pain 2005;21(1):83–90. [DOI] [PubMed] [Google Scholar]
- 18. Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthopaed Relat Res 2010;468(3):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorel JC, Veltman ES, Honig A, Poolman RW. The influence of preoperative psychological distress on pain and function after total knee arthroplasty: A systematic review and meta-analysis. J Bone Joint Surg 2019;101-B(1):7–14. [DOI] [PubMed] [Google Scholar]
- 20. Stephens MAP, Druley JA, Zautra AJ. Older adults’ recovery from surgery for osteoarthritis of the knee: Psychosocial resources and constraints as predictors of outcomes. Health Psychol 2002;21(4):377–83. [DOI] [PubMed] [Google Scholar]
- 21. Terradas-Monllor M, Navarro-Fernández G, Ruiz MA, et al. Postoperative psychosocial factors in health functioning and health-related quality of life after knee arthroplasty: A 6-month follow up prospective observational study. Pain Med 2021;22(9):1905–15. [DOI] [PubMed] [Google Scholar]
- 22. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: Prevalence, sensory qualities, and postoperative determinants. Pain 2011;152(3):566–72. [DOI] [PubMed] [Google Scholar]
- 23. Lawal OD, Gold J, Murthy A, et al. Rate and risk factors associated with prolonged opioid use after surgery: A systematic review and meta-analysis. JAMA Netw Open 2020;3(6):e207367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aglio LS, Mezzalira E, Mendez-Pino L, et al. Surgical prehabilitation: Strategies and psychological intervention to reduce postoperative pain and opioid use. Anesth Analg 2022;134(5):1106–11. [DOI] [PubMed] [Google Scholar]
- 25. Pester BD, Edwards RR, Martel MO, Gilligan CJ, Meints SM. Mind-body approaches for reducing the need for post-operative opioids: Evidence and opportunities. J Clin Anesth Intensive Care 2022;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 26. Burns JW, Gerhart JI, Bruehl S, et al. Anger arousal and behavioral anger regulation in everyday life among people with chronic low back pain: Relationships with spouse responses and negative affect. Health Psychol 2016;35(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai L, Gao H, Xu H, et al. Does a program based on cognitive behavioral therapy affect kinesiophobia in patients following total knee arthroplasty? A randomized, controlled trial with a 6-month follow-up. J Arthroplasty 2018;33(3):704–10. [DOI] [PubMed] [Google Scholar]
- 28. Dindo L, Zimmerman MB, Hadlandsmyth K, et al. Acceptance and commitment therapy for prevention of chronic postsurgical pain and opioid use in at-risk veterans: A pilot randomized controlled study. J Pain 2018;19(10):1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dowsey M, Castle D, Knowles S, et al. The effect of mindfulness training prior to total joint arthroplasty on post-operative pain and physical function: A randomised controlled trial. Complement Ther Med 2019;46:195–201. [DOI] [PubMed] [Google Scholar]
- 30. Geng X, Wang X, Zhou G, et al. A randomized controlled trial of psychological intervention to improve satisfaction for patients with depression undergoing TKA: A 2-year follow-up. J Bone Joint Surg 2021;103(7):567–74. [DOI] [PubMed] [Google Scholar]
- 31. Hanley AW, Gililland J, Erickson J, et al. Brief preoperative mind-body therapies for total joint arthroplasty patients: A randomized controlled trial. Pain 2021;162(6):1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanley AW, Gililland J, Garland EL. To be mindful of the breath or pain: Comparing two brief preoperative mindfulness techniques for total joint arthroplasty patients. J Consult Clin Psychol 2021;89(7):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyer VM, Beydoun HA, Gyenai L, et al. The effect of preoperative behavioral intervention on pain, anxiety, opioid use, and function in patients undergoing total knee arthroplasty: A randomized controlled study. Military Med 2021;usab424. [DOI] [PubMed] [Google Scholar]
- 34. Day MA. Mindfulness-Based Cognitive Therapy for Chronic Pain: A Clinical Manual and Guide. Chichester, West Sussex; Malden, MA: John Wiley & Sons Inc.; 2017. [Google Scholar]
- 35. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. 2nd edition. New York, NY: Guilford Press; 2013. [Google Scholar]
- 36. Day MA, Thorn BE, Rubin NJ. Mindfulness-based cognitive therapy for the treatment of headache pain: A mixed-methods analysis comparing treatment responders and treatment non-responders. Complement Ther Med 2014;22(2):278–85. [DOI] [PubMed] [Google Scholar]
- 37. Day MA, Ward LC, Ehde DM, et al. A pilot randomized controlled trial comparing mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Pain Med 2019;20(11):2134–48. [DOI] [PubMed] [Google Scholar]
- 38. Day MA, Ward LC, Thorn BE, et al. Mechanisms of mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Clin J Pain 2020;36(10):740–9. [DOI] [PubMed] [Google Scholar]
- 39. Parra-Delgado M, Latorre-Postigo JM. Effectiveness of mindfulness-based cognitive therapy in the treatment of fibromyalgia: A randomised trial. Cogn Ther Res 2013;37(5):1015–26. [Google Scholar]
- 40. Edwards RR, Campbell C, Schreiber KL, et al. Multimodal prediction of pain and functional outcomes 6 months following total knee replacement: A prospective cohort study. BMC Musculoskelet Disord 2022;23(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abrecht CR, Cornelius M, Wu A, et al. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: Psychophysical and psychosocial factors. Pain Medicine 2019;20(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lazaridou A, Martel MO, Cornelius M, et al. The association between daily physical activity and pain among patients with knee osteoarthritis: The moderating role of pain catastrophizing. Pain Med 2019;20(5):916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mun CJ, Letzen JE, Nance S, et al. Sex differences in interleukin-6 responses over time following laboratory pain testing among patients with knee osteoarthritis. J Pain 2020;21(5-6):731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nandi M, Schreiber KL, Martel MO, et al. Sex differences in negative affect and postoperative pain in patients undergoing total knee arthroplasty. Biol Sex Differ 2019;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paschali M, Lazaridou A, Paschalis T, et al. Individual variation in diurnal cortisol in patients with knee osteoarthritis: Clinical correlates. Int J Psychophysiol 2021;167:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Speed TJ, Mun CJ, Smith MT, et al. Temporal association of pain catastrophizing and pain severity across the perioperative period: A cross-lagged panel analysis after total knee arthroplasty. Pain Med 2021;22(8):1727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zgierska AE, Burzinski CA, Garland EL, et al. Mindfulness-based therapy compared to cognitive behavioral therapy for opioid-treated chronic low back pain: Protocol for a pragmatic randomized controlled trial. Contemp Clin Trials 2021;110:106548. [DOI] [PubMed] [Google Scholar]
- 48. Cleeland CS. The Brief Pain Inventory. Houston, TX: Pain Research Group; 1991. [Google Scholar]
- 49. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5(2):133–7. [DOI] [PubMed] [Google Scholar]
- 50. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 51. Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum 2006;55(2):325–32. [DOI] [PubMed] [Google Scholar]
- 52. Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Katz P, Pedro S, Michaud K. Performance of the Patient-Reported Outcomes Measurement Information System 29-item profile in rheumatoid arthritis, osteoarthritis, fibromyalgia, and systemic lupus erythematosus. Arthritis Care Res 2017;69(9):1312–21. [DOI] [PubMed] [Google Scholar]
- 54. Cohen J. Statistical Power Analysis for the Behavioral Science. 2nd edition. Hove; London: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 55. Montoya AK, Hayes AF. Two-condition within-participant statistical mediation analysis: A path-analytic framework. Psychol Methods 2017;22(1):6–27. [DOI] [PubMed] [Google Scholar]
- 56. Craner JR, Sperry JA, Evans MM. The relationship between pain catastrophizing and outcomes of a 3-week comprehensive pain rehabilitation program. Pain Med 2016;17(11):2026–35. [DOI] [PubMed] [Google Scholar]
- 57. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: Catastrophizing as a maladaptive strategy. Pain 1989;37(1):51–6. [DOI] [PubMed] [Google Scholar]
- 58. Wertli MM, Eugster R, Held U, et al. Catastrophizing—a prognostic factor for outcome in patients with low back pain: A systematic review. Spine J 2014;14(11):2639–57. [DOI] [PubMed] [Google Scholar]
- 59. Edwards RR, Cahalan C, Calahan C, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7(4):216–24. [DOI] [PubMed] [Google Scholar]
- 60. Linton SJ, Nicholas MK, MacDonald S, et al. The role of depression and catastrophizing in musculoskeletal pain. Eur J Pain 2011;15(4):416–22. [DOI] [PubMed] [Google Scholar]
- 61. Sun J-N, Chen W, Zhang Y, et al. Does cognitive behavioral education reduce pain and improve joint function in patients after total knee arthroplasty? A randomized controlled trial. Int Orthopaed 2020;44(10):2027–35. [DOI] [PubMed] [Google Scholar]
- 62. Kazarian GS, Anthony CA, Lawrie CM, Barrack RL. The impact of psychological factors and their treatment on the results of total knee arthroplasty. J Bone Joint Surg 2021;103(18):1744–56. [DOI] [PubMed] [Google Scholar]
- 63. Chen W, Sun J-N, Hu Z-H, et al. Cognitive behavioral therapy cannot relieve postoperative pain and improve joint function after total knee arthroplasty in patients aged 70 years and older. Aging Clin Exp Res 2021;33(12):3293–302. [DOI] [PubMed] [Google Scholar]
- 64. Riddle DL, Keefe FJ, Ang DC, et al. Pain coping skills training for patients who catastrophize about pain prior to knee arthroplasty: A multisite randomized clinical trial. J Bone Joint Surg 2019;101(3):218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weber M, Chao M, Kaur S, Tran B, Dizdarevic A. A look forward and a look back: The growing role of ERAS protocols in orthopedic surgery. Clin Sports Med 2022;41(2):345–55. [DOI] [PubMed] [Google Scholar]