Abstract
Cancer and cardiovascular diseases (CVD) often share common risk factors, and patients with CVD who develop cancer are at high risk of experiencing major adverse cardiovascular events. Additionally, cancer treatment can induce short- and long-term adverse cardiovascular events. Given the improvement in oncological patients’ prognosis, the burden in this vulnerable population is slowly shifting towards increased cardiovascular mortality. Consequently, the field of cardio-oncology is steadily expanding, prompting the need for new markers to stratify and monitor the cardiovascular risk in oncological patients before, during, and after the completion of treatment. Advanced non-invasive cardiac imaging has raised great interest in the early detection of CVD and cardiotoxicity in oncological patients. Nuclear medicine has long been a pivotal exam to robustly assess and monitor the cardiac function of patients undergoing potentially cardiotoxic chemotherapies. In addition, recent radiotracers have shown great interest in the early detection of cancer-treatment-related cardiotoxicity. In this review, we summarize the current and emerging nuclear cardiology tools that can help identify cardiotoxicity and assess the cardiovascular risk in patients undergoing cancer treatments and discuss the specific role of nuclear cardiology alongside other non-invasive imaging techniques.
Keywords: cardio-oncology, nuclear cardiology, PET, scintigraphy, FDG, myocardial perfusion imaging, CMR, echocardiography, CTRCD
Graphical Abstract
Graphical Abstract.
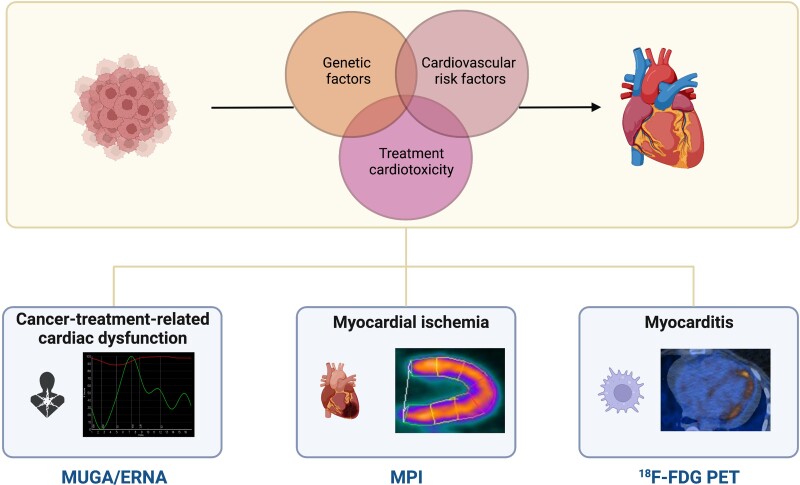
Pathophysiological pathways interconnecting cancers and CVD: genetic predispositions, cardiovascular risk factors, and cancer-treatment-related cardiotoxicity. CVD in cancer patients (and corresponding nuclear cardiology tools) consist mainly of cancer-treatment-related cardiac dysfunction (explored with MUGA/ERNA), myocardial ischaemia (with nuclear MPI), and myocarditis (with 18F-FDG PET). Abbreviations: 18F-FDG, fluor-18-radiolabelled fluorodeoxyglucose; CVD, cardiovascular diseases; ERNA, equilibrium radionuclide angiography; MPI, myocardial perfusion imaging; MUGA, multigated acquisition; PET, positron emission tomography.
Introduction
Cancer and cardiovascular diseases (CVD), leading mortality causes in high-income countries,1 are interconnected by common pathophysiological mechanisms2 and risk factors.3,4 Consequently, patients with cancer have an increased risk of CVD and major adverse cardiovascular events (MACE). Vice versa, cardiovascular risk factors (CVRFs) increase cancer risk.5–7 Additionally, cancer treatments induce short- and long-term cardiotoxicity.8,9 The prognostic improvement of oncological patients is slowly shifting their burden from cancer to cardiovascular mortality.10 Hence, cardio-oncology is a steadily expanding field, as evidenced by the recent publication of the first European Society of Cardiology (ESC) cardio-oncology guidelines,11 prompting the need for cardiovascular risk stratification markers in oncological patients.12,13 Despite being challenged by echocardiography and cardiac magnetic resonance (CMR),14 nuclear imaging remains a contemporary modality in patients receiving cardiotoxic therapies.
In this article, we briefly summarize the central mechanisms responsible for cancer-treatment-induced cardiotoxicity, review the main established and emergent nuclear cardiology tools useful in cancer settings, and discuss the role of nuclear medicine alongside echocardiography and CMR. Although also beneficial for managing cardiac tumours,15,16 this review will not cover this topic.
Mechanisms of interaction between cancer and CVD
CVD and cancer are two sides of the same coin,17 sharing identical pathophysiological pathways18 (Figure 1).
Figure 1.
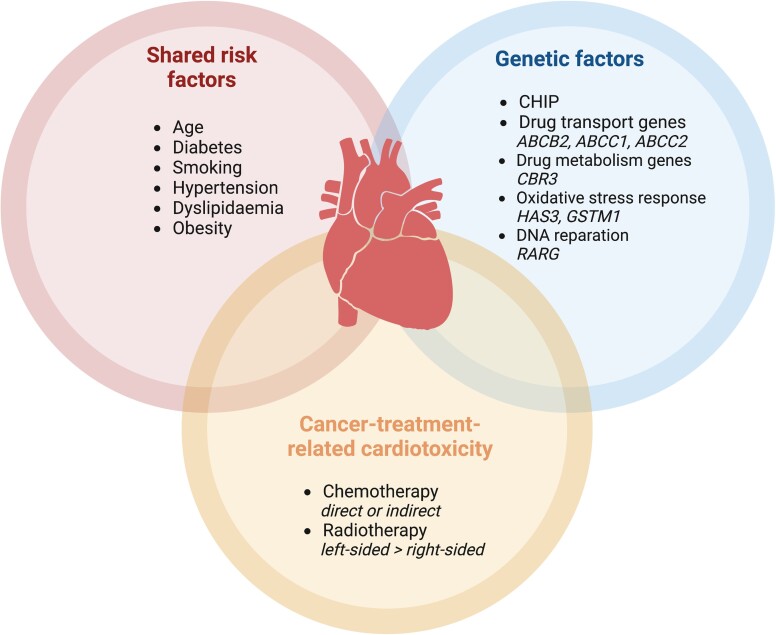
Mechanisms of CVD in cancer patients. Abbreviations: ABCB2, ABC transporter B family member 2 gene; ABCC1, ATP-binding cassette subfamily C member 1 gene; ABCC2, ATP-binding cassette subfamily C member 2 gene; CBR3, carbonyl reductase 3 gene; CHIP, clonal haematopoiesis of indeterminate potential; GSTM1, glutathione S-transferase mu 1 gene; HAS3, hyaluronan synthase 3 gene; RARG, retinoic acid receptor gene.
Risk factors
Typical CVRFs include age, diabetes, hypertension, smoking, dyslipidaemia, and overweight,19 all of which concomitantly increase cancer risk.20 By promoting inflammation and oxidative stress, diabetes favours a pro-oncogenic environment.21 Similarly, epidemiological data suggest a correlation between hypertension and dyslipidaemia on the one hand and cancer genesis on the other.22 Smoking promotes atherosclerosis and cancer,23,24 and a plethoric adipose tissue triggers oncogenic inflammatory molecules.25
Genetic factors
Intrinsic factors also predispose to CVD and cancer.26 For instance, specific age-related somatic mutations, labelled clonal haematopoiesis of indeterminate potential (CHIP), increase the risk of haematological malignancy27 and CVD.28 Other genes involved in drug delivery and metabolism modulate the risk of cancer-therapy-induced cardiotoxicity,26 either by increasing it, such as ATP-binding cassette transporters ABCB4 and ABCC, or by decreasing it, for example, ATP-binding transporters (ABCB1) and solute carriers (SLC28A3).26
Cancer-treatment-related cardiotoxicity
Cancer-treatment-induced cardiotoxicity is a critical contributor to CVD18 (Table 1). Cancer-treatment-related cardiac dysfunction (CTRCD), i.e. left ventricular (LV) dysfunction induced by cancer treatment, is the most common cardiotoxicity type.8 Two types of CTRCD are distinguished.29,30 Type I CTRCD, classically caused by anthracyclines, induces direct cumulative, dose-related, and usually irreversible cardiomyocyte damage. Type II CTRCD, traditionally induced by trastuzumab,29 is a reversible and dose-independent myocardial dysfunction without structural alterations. Cancer treatment can also induce coronary artery disease (CAD), notably vasospasm and arterial thrombosis.31,32 Likewise, chest radiotherapy favours atherosclerosis and fibrosis via inflammatory cascades in the coronary vessels.6,33
Table 1.
Main types of cancer treatments and related toxic effects
| Therapeutic class | Main treatment-induced toxicity mechanisms |
|---|---|
| Anthracyclines | Induction of oxidative stress, impaired autophagy, type II topoisomerase poisoning |
| Trastuzumab | Inhibition of epidermal growth factor receptor 2 |
| Fluoropyrimidines | Induction of oxidative stress in cardiomyocytes, vasospasm by favouring endothelial and smooth cell dysfunction, coronary artery thrombosis |
| Platinum drugs | Induction of oxidative stress and of direct damage to cardiomyocytes and mitochondria, platelet aggregation |
| Taxanes | Direct cardiomyocyte and mitochondrial damage, alteration of cell division and microtubule dysfunction, oxidative stress, platelet aggregation, endothelial injury, haemorrhagic myopericarditis |
| Vascular endothelial growth factor (VEGF) inhibitors (tyrosine kinase inhibitors, monoclonal antibodies) | Arterial and venous thrombosis |
| Immune checkpoint inhibitors | Increased CD4 and CD8 lymphocyte infiltration inducing myopericarditis and arrhythmia |
| Radiotherapy | Coronary atherosclerosis and fibrosis by triggering acute and long-term coronary inflammation |
Lately, the introduction of immune checkpoint inhibitors (ICI) to the cancer armamentarium was accompanied by increasing reports of immune-related adverse events (IRAEs),34,35 including myocarditis.36
Role of imaging for the early detection of CVD in cancer patients
The European Society for Medical Oncology guidelines highlight the need for an early screening of CVRFs and close cardiovascular monitoring of cancer patients.13 This assessment includes a baseline evaluation of LV ejection fraction (LVEF) to guide the cancer treatment choice and the need for cardioprotective therapies.13 However, LVEF alone can prove insufficient, since an LVEF drop is often a late-stage manifestation of cardiac damage.37,38 Global longitudinal strain (GLS) assessment using echocardiography or CMR is a more sensitive marker of cardiac dysfunction and is, therefore, recommended.14 Nonetheless, GLS is limited by scarce reproducibility,39 prompting the need for alternative tools.
Nuclear medicine imaging and particularly multigated acquisition (MUGA) scintigraphy have historically been at the frontline of LV monitoring in oncological patients.40,41 Although challenged by CMR,42 nuclear cardiology provides critical information for diagnosing, monitoring, and risk-stratifying cancer patients15,16,43–45 (Table 2). In the following part, we will review how nuclear cardiology can detect cardiac complications in oncological patients and discuss its role alongside echocardiography and CMR.
Table 2.
Main types of cancer-treatment-related cardiotoxicities and main cardiac cancers with the corresponding nuclear imaging diagnostic tools
| Type of toxicity/disease | Most common toxic agents | Imaging tools | Comments | |
|---|---|---|---|---|
| Cardiotoxicity | CTRCD | Anthracyclines, alkylating agents, TKI, proteasome inhibitors | MUGA (ERNA SPECT for RV function) ±First-pass 18F-FDG PET |
Diagnosis and monitoring of LV dysfunction |
| Coronary artery disease | Alkylating-like agents, fluoropyrimidine (vasospasm) taxanes, radiotherapy, hormonotherapy (Arimidex, Aromasin, Femara) | SPECT MPI PET MPI |
CACS derivable from hybrid CT imaging CMVD with PET MPI LVEF from SPECT and PET MPI |
|
| Myocarditis | Alkylating agents, immune checkpoint inhibitors |
18F-FDG PET ±68Ga-SSTR PET ±68Ga-FAPI PET ±89Zr-DFO-CD4 and 89Zr-DFO-CD8a PET |
Potential role for hybrid PET/CMR | |
| Specific disease | Cardiac tumours | NA |
18F-FDG for aggressive primary tumours and NECs 68Ga-SSTR for low-grade NETs |
Diagnosis and staging |
Abbreviations: ±, optional or used in research studies; 18F-FDG, fluor-18-radiolabelled fluorodeoxyglucose; 68Ga-FAPI, gallium-68-radiolabelled fibroblast activation protein inhibitors; 68Ga-SSTR, gallium-68-radiolabelled somatostatin receptor; 89Zr-DFO-CD4, zirconium-89-radiolabelled desferrioxamine-CD4; 89Zr-DFO-CD8a, zirconium-89-radiolabelled desferrioxamine-CD8a; 99mTc, technetium-99m; 123I-MIBG, iodine-123 metaiodobenzylguanidine; ATTR, transthyretin amyloidosis; CA, cardiac amyloidosis; CACS, coronary artery calcium score; CMVD, coronary microvascular dysfunction; CMR, cardiac magnetic resonance; CT, computed tomography; CTRCD, cancer-treatment-related cardiac dysfunction; ERNA, equilibrium radionuclide angiography; LVEF, left ventricular ejection fraction; MPI, myocardial perfusion imaging; MUGA, multigated acquisition; NA, not applicable; NEC, neuroendocrine carcinoma; NET, neuroendocrine tumour; PET, positron emission tomography; SPECT, single-photon emission computed tomography; TKI, tyrosine kinase inhibitors.
Diagnosis of cancer-treatment-related toxicity
CTRCD and LV systolic dysfunction
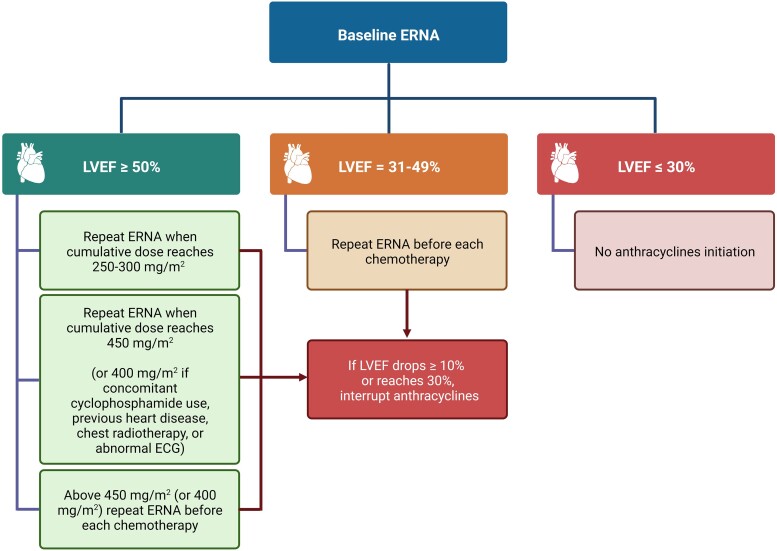
The ESC defines CTRCD as (i) a ≥10% LVEF decrease from baseline to below 50%, (ii) with a GLS drop of ≥15% from baseline, confirmed by a 2–3-week repeat study, in the context of cancer treatment.14 While only echocardiography and CMR can estimate GLS,46 MUGA robustly determines LVEF.47,48 In MUGA, cardiac volumes are derived from heart-centred images of the patient’s own radiolabelled erythrocytes49 and are therefore not influenced by geometric assumptions about the myocardial wall.50 Three types of MUGA are distinguished: (i) first-pass MUGA, (ii) planar equilibrium radionuclide angiography (ERNA), and (iii) single-photon emission computed tomography (SPECT) ERNA. In practice, first-pass MUGA is limited to specific indications [right ventricular ejection fraction (RVEF) and shunt assessment49,51], and only ERNA is used to assess CTRCD. Planar ERNA is acquired when the radiotracer has reached equilibrium and allows measuring LVEF52 (Figure 2), not RVEF, because of the superposition of heart structures. However, ERNA can also be performed with three-dimensional (3D) gated SPECT, which enables the delineation of both LVEF and RVEF.49,53–56 Overall, MUGA displays a high inter- and intra-observer reproducibility for LVEF measurement,57 which is crucial for serial follow-up during anticancer treatment.14,58 MUGA also helps select patients who can safely tolerate higher cumulative anthracycline doses, i.e. asymptomatic patients with LVEF > 40% and a drop in LVEF < 10%,13,41 significantly reducing heart failure occurrence.59 Although in good agreement,60 LVEF tends to be higher with SPECT than with planar ERNA,61 which needs to be taken into consideration for monitoring.14 Similarly, in breast cancer patients, MUGA gives slightly lower LVEF values than CMR.62 As such, when using MUGA, for an LVEF threshold of 50%, this difference could result in 35% more patients being diagnosed with CTRCD than with CMR.62 Hence, given that LV volumes tend to shrink and LVEF to increase after menopause,63 CTRCD thresholds might need to be adapted in women.64 Regarding surveillance, the European and American nuclear medicine societies recently issued an expert consensus for monitoring LVEF by ERNA for patients receiving anthracyclines,65 which has been summarized in Figure 3.
Figure 2.
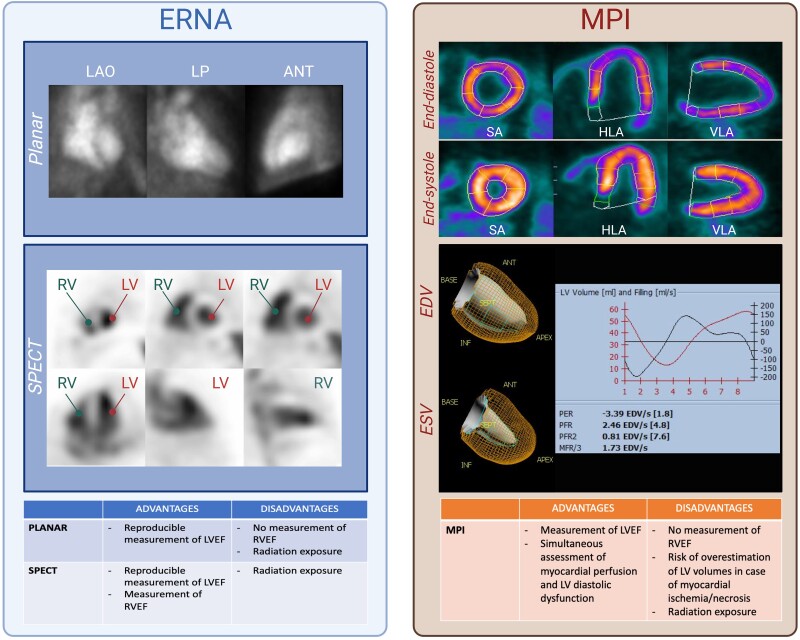
LV function assessment with nuclear cardiology. Left panel: ERNA techniques for LVEF assessment based on radiolabelled erythrocytes’ activity. Planar ERNA: end-diastolic and end-systolic LV volumes derived from LAO projections. Additional incidences include LP and anterior projections. SPECT ERNA: 3D reconstructions allowing LVEF/RVEF measurement. Right panel: NH3 PET MPI during end-diastole and end-systole enabling EDV/ESV estimation. Accurate volume measurement with MPI necessitates preserved myocardial perfusion. Diastolic (D) function can also be studied. Abbreviations: ANT, anterior; CHIP, clonal haematopoiesis of indeterminate potential; EDV, end-diastolic volume; ERNA, equilibrium radionuclide angiography; ESV, end-systolic volume; HLA, horizontal long axis; LAO, left anterior oblique; LP, left profile; LV, left ventricle; LVEF, left ventricular ejection fraction; MFR, mean filling rate during the first third of diastole; mL, millilitres; mL/s, millilitres per second; MPI, myocardial perfusion imaging; PER, peak ejection rate; NH3, ammonium; PET, positron emission tomography; PFR, peak filling rate; PFR/2, peak filling rate during the first half of diastole; RV, right ventricle; RVEF, right ventricular ejection fraction; SA, short axis; SPECT, single-photon emission computed tomography; VLA, vertical long axis.
Figure 3.
SNMMI/EANM Guidelines for ERNA-based LVEF monitoring in anthracycline-treated patients. Abbreviations: EANM, European Association of Nuclear Medicine; ECG, electrocardiogram; ERNA, equilibrium radionuclide angiography; LVEF, left ventricular ejection fraction; SNMMI, Society of Nuclear Medicine and Molecular Imaging.
A major drawback of MUGA is radiation exposure. Indeed, MUGA requires the injection of 555–1110 MBq (7–15 MBq/kg in children) of radiotracers,65 which in case of serial follow-up increases theoretically (albeit minimally) cancer risk.66–68 Cadmium-zinc-telluride (CZT)-based cameras, which detectors are more sensitive than conventional sodium iodide (NaI) ones, enable a two- to three-fold reduction in injected activity without altering image quality,60,69,70 hence decreasing the radiation burden. CZT-derived LVEF highly correlates with the one obtained from planar NaI detectors.71 CZT-based SPECT ERNA is also in high agreement with CMR for RVEF.72 Interestingly, LVEF can be obtained from fluor-18-radiolabelled fluorodeoxyglucose positron emission tomography (18F-FDG PET) gated first-pass acquisitions, showing excellent concordance with planar ERNA.73 Given that 18F-FDG PET is the mainstay for cancer staging, this elegant approach allows simultaneously measuring LVEF with no additional radiation exposure. However, first-pass cardiac 18F-FDG acquisitions result in a prolonged acquisition time (5 min), reducing the available scanning time for other patients.
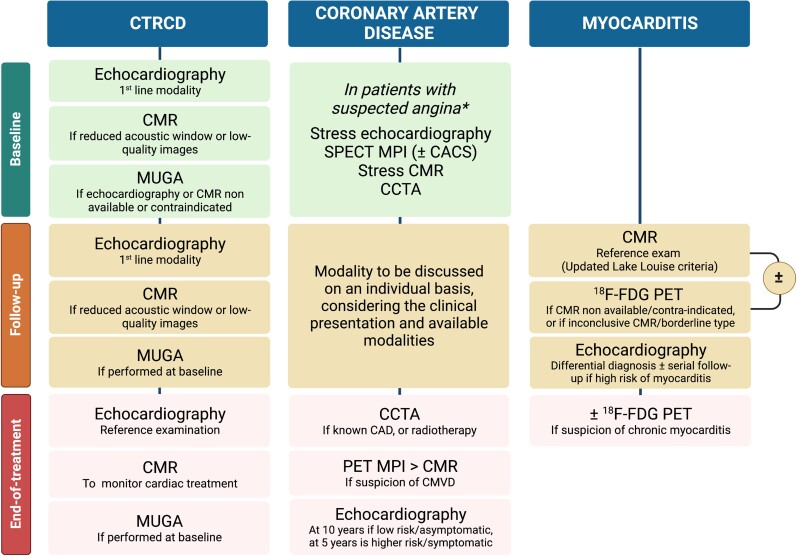
In practice, MUGA has long been supplanted by the more readily available and non-irradiating echocardiography and CMR (Figure 4). Transthoracic echocardiography (TTE) is the frontline risk stratification exam, owing to its wide availability, harmfulness, ability to assess morphology (including valves), function, and GLS. Whenever available, 3D echocardiography is preferred over 2D, given its higher reproducibility for LVEF and GLS assessment.74–76 GLS detects early signs of systolic dysfunction before any LVEF drop, with a change in GLS ≥ 15% predicting the risk of CTRCD.46 Importantly, a GLS-based cardioprotective strategy reduces the rate of CTRCD in patients undergoing anthracycline.77 Nonetheless, echography strain measurements lack inter-device standardization, which limits their routine use.78 In case of reduced acoustic window or low image quality, CMR is recommended as a second-line technique.11,75 CMR is considered the reference exam to calculate cardiac volumes and function and can detect even minor LVEF impairments and volume changes.75 The latter is particularly important in patients undergoing anticancer treatments, in whom CTRCD can manifest as an isolated LV end-diastolic volume reduction.79 Moreover, CMR accurately determines RVEF, which can be asymptomatically reduced in cancer survivors.80 Besides volumes and strain assessment, CMR is a promising tool for the early detection of cancer-treatment-related myocardial oedema and fibrosis via T1/T2 mapping and extracellular volume (ECV) measurement.81 Increased T1/T2 relaxation times hold promise to predict subsequent CTRCD,81 although there is a significant overlap between mapping parameters of patients who develop CTRCD and those who do not.82
Figure 4.
Algorithm proposal for non-invasive imaging in patients undergoing anticancer treatment. Abbreviations: 18F-FDG, fluor-18-radiolabelled fluorodeoxyglucose; CACS, coronary artery calcium score; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; CTRCD, cancer-treatment-related cardiac dysfunction; LVEF, left ventricular ejection fraction; SNMMI, Society of Nuclear Medicine and Molecular Imaging; MPI, myocardial perfusion imaging; MUGA, multigated acquisition; PET, positron emission tomography; SPECT, single-photon emission computed tomography. *The choice of imaging modality should be based on symptoms, known CAD, pretest probability, local availability and expertise, and patient characteristics.
During cancer treatment, echocardiography is the preferred modality for monitoring cardiac function.14 Surveillance frequency depends on a cardiotoxicity risk profile based on patient- and treatment-related factors.14 Importantly, given the inter-imaging variability, it is crucial to perform follow-up using the same modality.83 Indeed, minor LVEF variations are essential to detect, as they could be an early sign of cardiac toxicity. Compared with CMR, 2D and 3D TTE tend to underestimate LV volumes.84 Similarly, limits of agreement between MUGA and CMR often exceed ±10%,48 which could lead to incorrectly classifying patients with CTRCD. In this regard, MUGA’s radiation exposure argues against its systematic use for the follow-up of patients undergoing anticancer treatment.
After the end of treatment, patients who developed CTRCD should be monitored using echocardiography. In patients in whom a cardiac medication was introduced to mitigate treatment side effects, CMR is an option to assess treatment response.14
In summary, the 2022 ESC Guidelines on cardio-oncology only recommend MUGA as a third-line technique to assess LVEF, i.e. if TTE and CMR are unavailable or in case of CMR contraindication.11,14 Of note, the guidelines mention the potential interest of assessing the myocardial 18F-FDG uptake during intercourse PET/computed tomography (CT), as its increase could indicate an LVEF decline85 and, therefore, trigger LVEF assessment.14
Coronary artery disease
Cancer is a prothrombotic condition associated with enhanced platelet reactivity and circulating procoagulant products, which increase the atherosclerotic burden.86 Additionally, cancer treatments themselves (particularly chest radiotherapy) induce endothelial injuries, favouring vasospasm and thrombosis.87–89 Hence, screening for ischaemic heart diseases (IHD) is recommended in patients with intermediate-to-high pre-test likelihood46 undergoing heart-damaging cancer therapy,8 especially anthracyclines and chest radiotherapy.90,91 Such screening can be done with SPECT myocardial perfusion imaging (MPI), a mainstay in this indication.92
SPECT myocardial perfusion abnormalities can appear either during radiotherapy93 or later, up to 20 years after treatment completion.94 Most perfusion abnormalities develop in the apical territory,89,95 indicating left anterior descending artery damage.96,97 Accordingly, myocardial perfusion impairment is more prevalent in left-sided than right-sided chest cancer,89,98 a risk that linearly correlates with cardiac exposure volume.94,99 In patients with left-sided breast cancer, an irradiated cardiac volume of >5% is associated with significantly higher rates of perfusion abnormalities than with lower volumes.100 Interestingly, in cancer patients, SPECT-detected myocardial ischaemia does not correlate well with underlying obstructive CAD,97 highlighting the importance of coronary microvascular dysfunction (CMVD) and coronary spasm in this population.101–103
PET MPI is the reference non-invasive modality to diagnose CMVD, using 13N-ammonia (13N-NH3), 82Rubidium (82Rb), and 15O-water (15O-H2O) radiotracers.104 PET MPI allows for measuring myocardial blood flow (MBF) and coronary flow reserve (CFR), which are central to CMVD diagnosis.105 In patients undergoing chest radiotherapy, PET MPI shows an inverse correlation between the mean radiation dose to the heart and CMVD.106,107 Moreover, MBF could have prognostic value, with low CFR values being associated with an increased cumulative incidence of MACE in breast cancer patients.108
Another prognostic parameter is the coronary artery calcium score (CACS). CACS is obtained from a non-enhanced CT and quantifies the degree of coronary artery calcification, expressed with the Agatston score.109 CACS = 0 in asymptomatic patients is associated with a very low prevalence of severe coronary stenosis and high-risk plaque features.110 Conversely, an Agatston score of >400 is predictive of MACE, even for normal MPI.111 CACS can easily be yielded from the low-dose CT of PET/CT and SPECT/CT cameras, showing high agreement with the one obtained from standard non-enhanced scans.112–114 Since 18F-FDG PET/CT is part of routine oncological work-up, cardiovascular risk stratification with CACS could simultaneously be performed without additional radiation or cost.115
Beyond myocardial perfusion, nuclear MPI can also estimate LVEF116,117 (Figure 2). However, contrary to MUGA, MPI indirectly estimates cardiac volumes based on myocardial wall motion. In case of infarction, the necrotic segment is devoid of signal, leading to overestimating LV volumes.118 Another limitation of SPECT MPI is its inability to assess RVEF. Although more accurate,119–121 CZT cameras give lower values than conventional SPECT cameras,122 stressing the importance of performing serial follow-up using the same modality.
Nuclear MPI is only one of the tools available for myocardial ischaemia screening alongside stress echocardiography, and CMR. Additionally, contrast-enhanced coronary computed tomography angiography (CCTA) is an alternate tool which provides information on coronary plaque burden and coronary stenosis assessment.11,14 Although the recent European guidelines on cardio-oncology do not give strict recommendations on which modality to prefer in which setting,11 echocardiography and CMR remain the frontline techniques in this setting.14 Overall, three scenarios can be distinguished: baseline screening, follow-up during treatment, and end-of-treatment surveillance14 (Figure 4).
Baseline screening should always be considered in the oncological population, given their increased CAD risk.14 CACS assessment is an easy and minimally invasive way of characterizing the baseline CAD risk. If CACS = 0, the risk of dying from CAD within 5 years of cancer diagnosis remains below the mortality risk from cancer itself; conversely, if CACS > 300, the 5-year CAD mortality risk exceeds the cancer mortality risk,123 prompting more aggressive management.14 As abovementioned, CACS can be extracted from 18F-FDG PET’s low-dose CT without additional scanning time, cost, or radiation.115 As the mainstay baseline staging exam of most cancer types, 18F-FDG-PET-based CACS appears as a reasonable option for baseline CAD risk assessment. Advanced explorations should be preferred in patients with a higher baseline CAD risk. In nononcological settings, non-enhanced CT and CCTA are the first-line exam for detecting coronary calcifications and coronary stenosis in patients with low-to-intermediate CAD risk.92 Given the increased CAD risk in oncological patients, detection of coronary stenosis using CCTA can be discussed in symptomatic patients with no CAD history.14 However, this comes at the expense of increased radiation exposure.124 Stress echocardiography is indicated in patients with intermediate-to-high CAD probability undergoing ischaemia-inducing chemotherapies, such as fluorouracil, bevacizumab, sorafenib, and sunitinib.125 In addition to ischaemia, stress echocardiography could unveil patients at risk of developing CTRCD,126,127 a 5-unit fall in LV contractile reserve during dobutamine echocardiography predicting the subsequent LVEF drop.128 Myocardial perfusion CMR imaging using pharmacological stress is also an option, but its use for systematic screening is conflicted by its relatively low availability.14 SPECT MPI is a well-validated and widely accessible modality that additionally provides CACS in case of hybrid SPECT/CT.113
During treatment, there is no clear recommendation as to which modality to prioritize and the exploration frequency, which will depend on the clinical presentation and the available modalities.
After treatment completion, CCTA is an option for CAD identification,14 particularly in patients with known CAD, whose plaque progression can be accelerated by anticancer treatment, and in young patients treated with chest radiotherapy, i.e. at risk of perivascular fibrosis.14,90 Radiotherapy can also induce valve leaflet calcification, which can be assessed by CT.90 A limitation of CCTA is for the routine detection of microvascular dysfunction, although dynamic CT MPI is promising in this regard.129–132 Conversely, CMR detects both segmental ischaemia and CMVD,133 with the advantage over nuclear MPI of being devoid of radiation exposure. Still, CMR assessment of MBF remains in the research realm,134 and PET MPI is the reference exam for CMVD,104 displaying higher accuracy, reproducibility, and prognostic value than CMR.135,136 This favours PET in patients at risk of CMVD, particularly women with breast cancer108,137 and patients who underwent chest radiotherapy.106,138
Myocarditis
The last years have witnessed the development of immunotherapy, a new class of anticancer treatment that leverages the immune system to harness cancer progression. The primarily used class of immunotherapy is ICI. Immune checkpoints are T-lymphocyte-expressed receptors that recognize ligands at the surface of normal cells. The receptor–ligand binding inhibits the T-cell, preventing it from targeting normal cells.139 Some cancer cells express immune-checkpoint-binding ligands and can thus trick and inhibit T-lymphocytes. ICI block the receptor–ligand bond, allowing T-cells to recognize and attack cancer cells.139 The downfall of lifting T-cell inhibition is that this may unleash IRAEs.140 Cardiovascular IRAEs occur with an incidence ranging from 1.14 to 5%140 and include notably myocarditis, pericarditis, vasculitis, and Takotsubo cardiomyopathy.141,142
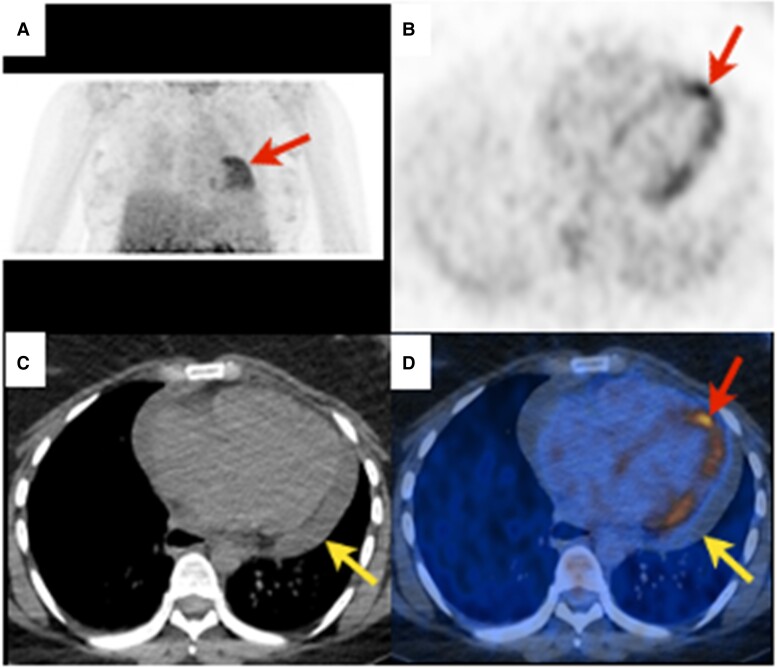
Diagnosing ICI-related myocarditis is challenging because of the various presentations143 and the prolonged interval between drug administration and symptom onset.140 While CMR is the reference exam,144 PET can also orient the diagnosis. Due to its availability and high uptake in inflammatory cells, 18F-FDG is a natural candidate in this indication,145 classically displaying focal or diffuse patchy myocardial 18F-FDG uptake with no vascular systematization146 (Figure 5). Despite a good spatial agreement between 18F-FDG uptake and T2 hyperintensity/late gadolinium enhancement (LGE), the diagnostic accuracy of 18F-FDG PET/CT in myocarditis is low.148 Several factors might explain this, such as an inadequate high-fat/low-carbohydrate diet, the initiation of immunosuppressive treatment, and the delay between myocarditis onset and image acquisition. Acquisition timing is indeed critical, with a small series showing a 100% sensitivity when 18F-FDG PET was performed within 14 days of disease onset vs. 20% when performed later.146 In 2019, Bonaca et al.147 proposed a definition of ICI-related myocarditis that includes 18F-FDG PET, with myocarditis deemed as possible in any ‘scenario meeting criteria for possible myocarditis (i.e. not explained by any other diagnosis such as acute coronary syndrome, trauma or Takotsubo cardiomyopathy on CMR, ultrasound, and cardiac biomarkers) with 18F-FDG PET showing patchy cardiac 18F-FDG uptake without another explanation’.
Figure 5.
Myocarditis. Twenty-seven-year-old dyspnoeic woman with widespread concave ST elevation on ECG and increased C-reactive protein (131 mg/L, N < 4), suggestive of myocarditis. 18F-FDG PET revealing diffuse heterogeneous (‘patchy’) myocardial 18F-FDG uptake [red arrows, (A) maximal intensity projection, (B), and (D) axial slices]. Non-enhanced CT showing pericardial effusion [(C), yellow arrow] without 18F-FDG uptake [(D), yellow arrow] related to pericarditis. According to the Bonaca et al.147 criteria, possible myocarditis was retained.
Other promising radiotracers target somatostatin receptors (SSTRs) overexpressed at the surface of vascular macrophages.149 The lower albeit variable150 physiologic myocardial uptake of SSTR radiotracers reduces the risk of false positives. In a small population of nine patients, gallium-68-radiolabelled DOTA0-D-Phe1-Tyr3-octreotide (68Ga-DOTATOC) PET/CT had a 100% sensitivity to diagnose ICI-related myocarditis, despite the initiation of steroid and immunosuppressive therapy.151 Recently, a 68Ga-radiolabelled tracer targeting fibroblast activation protein inhibitors (68Ga-FAPI) was introduced in oncological diseases.152,153 In three patients fulfilling the Bonaca et al.147 criteria for definite ICI-related myocarditis, focal myocardial uptake of 68Ga-FAPI identified cardiac remodelling territories.154 Similarly, the upregulation of translocator protein-18 kDa (TSPO) and chemokine receptors types 4 and 12 in inflammatory cells suggests a role for radiolabelled TSPO and 68Ga-pentixafor in myocarditis,155,156 although dedicated studies still need to be performed. Along the same line, radiotracers targeting the C-X-C motif chemokine receptor 4 overexpressed by leucocytes represent an exciting approach to diagnosing myocardial inflammation.157 Finally, novel inflammation radiotracers targeting CD4 and CD8 cells, zirconium-89-radiolabelled desferrioxamine-CD4 (89Zr-DFO-CD4) and 89Zr-DFO-CD8a, are under investigation and hold the potential to image myocarditis.158 Their high specificity could prove particularly useful in ICI-related myocarditis. Indeed, the reference treatment for myocarditis is steroids, which alleviate the antitumour effect of ICI.159 Therefore, establishing the diagnosis with certainty might reduce unnecessary immunosuppressive therapies or withholding ICI.
In practice, however, the guidelines recommend echocardiography and CMR as first-line examinations in suspected ICI-associated myocarditis and recommend cardiac PET only if CMR is non-available or contraindicated11 (Figure 4). Echocardiography’s primary role is to rule out non-inflammatory cardiac diseases and serve as a reference exam for LVEF monitoring.160 Serial echocardiography could also be discussed in patients at high risk of myocarditis, i.e. patients undergoing a combination of ICI, ICI with another cardiotoxic regimen, or in case of pre-existing CVD.14 The mainstay examination for diagnosing myocarditis remains CMR, using the Lake Louise criteria,161 updated in 2018 with the implementation of mapping techniques.160 The Lake Louise criteria consist of a triad combining oedema (as assessed by T2-weighted acquisitions), hyperaemia [reflected by early gadolinium enhancement (EGE)], and necrosis (set by LGE). The presence of ≥2 out of 3 criteria in a suggestive context establishes the diagnosis of myocarditis with high sensitivity and specificity.144 Mapping techniques improve intra- and inter-observer diagnostic confidence, the specificity for detecting active inflamamtion and edema, and improve the detection of milder forms of myocarditis.160 Additionally, reduced GLS and global circumferential strain could help risk-stratify patients with ICI myocarditis, the magnitude of strain reduction being predictive of MACE.162 Nonetheless, the updated Lake Louise criteria might not be as performant in ICI myocarditis. Indeed, recent data show the sensitivity of CMR to be lower in the latter, possibly because of reduced LGE in the early phase.163,164 Detecting LGE is particularly challenging in borderline forms of myocarditis,165 which display less necrotic insult and patchy distribution. Such patients might benefit from 18F-FDG PET, given the increased 18F-FDG uptake in myocarditis areas devoid of LGE, which could also guide potential myocardial biopsies.148 However, no dedicated study has assessed the diagnostic performance of 18F-FDG PET in this specific subgroup. 18F-FDG PET could also help distinguish chronic myocarditis from the scarred non-inflammatory myocardium, i.e. healed myocarditis.166 Indeed, LGE and strain do not clearly differentiate between chronic and healed myocarditis,167,168 whereas 18F-FDG uptake decreases in the latter,166 a feature that could help monitor treatment response to immunosuppressive therapy.169 Given their complementary diagnostic values, studies have evaluated the value of hybrid 18F-FDG PET/CMR in myocardial inflammatory diseases,170,171 showing an incremental detection of cases with hybrid PET/CMR over single modalities alone.172
Early signs of cardiac dysfunction
Numerous efforts aim at detecting early-stage cardiac impairment, i.e. when anticancer treatment is still modifiable or cardioprotective measures can be introduced8 (Figure 6).
Figure 6.
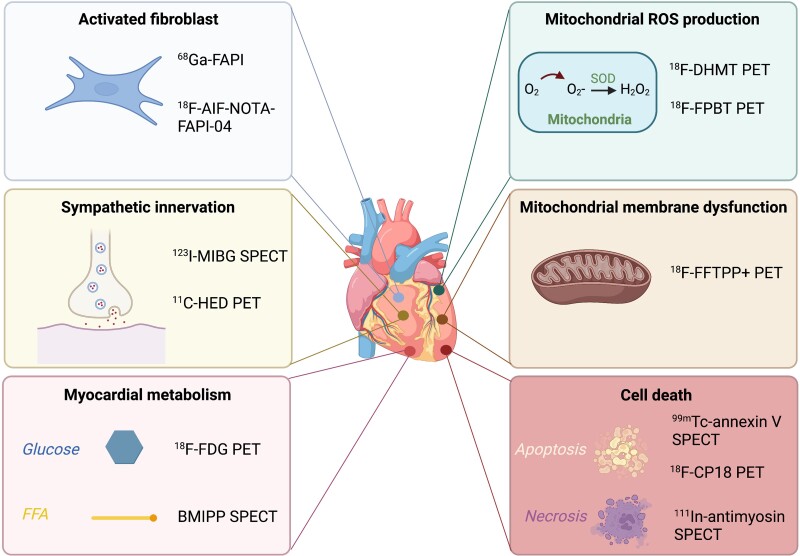
Main metabolic targets of early cardiac toxicity and corresponding radiotracers. Abbreviations: 11C-HED, carbon-11-radiolabelled hydroxyephedrine; 18F-AIF-NOTA-FAPI-04, fluor-18-labelled 1,4,7-triazacyclononane-N,N′,N″-triacetic acid-conjugated FAP inhibitor 04; 18F-CP18, fluor-18-radiolabelled caspase 3 substrate; 18F-DHMT, fluor-18-radiolabelled 6-(4-((1-(2-fluoroethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-5-methyl-5,6-dihydrophenanthridine-3,8-diamine; 18F-FDG, fluor-18-radiolabelled fluorodeoxyglucose; 18F-FTPP+, fluor-18-radiolabelled (4-fluorophenyl)triphenylphosphonium; 18F-FPBT, fluor-18-radiolabelled 3-(3-fluoropropyl)-2-phenyl-2,3-dihydrobenzo[d]thiazole; 68Ga-FAPI, gallium-68-radiolabelled fibroblast activation protein inhibitor; 99mTc, technetium-99m; 111In, indium-111; 123I-MIBG, iodine-123 metaiodobenzylguanidine; BMIPP, beta-methyl-iodine-123 phenylpentadecanoic acid; H2O2, hydrogen peroxide; O2, oxygen; O2-, ion oxide; PET, positron emission tomography; ROS, reactive oxygen species; SOD, superoxide dismutase; SPECT, single-photon emission computed tomography.
Cardiac diastolic function
Diastolic dysfunction is a potential early marker of LV dysfunction,173–175 which MUGA can assess. MUGA-derived diastolic function parameters include the peak filling rate, time-to-peak filling rate, and first third filling fraction57,176–178 that deteriorate before treatment-induced systolic dysfunction.177,179 However, the inter- and intra-observer reproducibility of ERNA-based diastolic function is moderate,57 questioning its utility in the early detection of CTRCD. CZT-based MUGA is promising, providing a highly reproducible assessment of diastolic function in cancer patients.180 Still, MUGA is not routinely used to assess diastolic function, which can easily be obtained from echocardiography.78 However, echocardiography is strongly operator-dependent, hampering its interest in surveillance.74 CMR can also assess diastolic function based on LV mass and hypertrophy, LA size and function, mitral inflow and pulmonary venous velocity profiles, as well as myocardial deformation imaging with strain. Additionally, T1 mapping and ECV can be used.181 CMR presents the advantage over echocardiography of highly reproducible and accurate volume measurements without geometrical or flat profile assumptions.181 CMR’s downsides are its restricted availability and the length of sequence acquisitions and image post-processing, limiting its routine use.181
Cardiac sympathetic innervation
Iodine-123 metaiodobenzylguanidine (123I-MIBG) reflects the uptake, storage, and release of norepinephrine in the synaptic cleft,182 hence allowing cardiac sympathetic innervation imaging.183 The main parameter is the heart-to-mediastinum ratio (HMR),184,185 i.e. the ratio between cardiac 123I-MIBG uptake and a mediastinal reference region of interest. A diminished HMR indicates cardiac sympathetic denervation, either functional (downregulation of post-synaptic β-adrenergic receptors) or due to direct damage (for example, after toxic treatments186).
In patients receiving anthracycline, the HMR drops before LVEF,187,188 highlighting 123I-MIBG’s potential role in early damage detection. Additionally, serial follow-up with cardiac 123I-MIBG scintigraphy shows a slight dose-dependent sympathetic impairment following anthracycline administration,187,189,190 suggesting a role in damage quantification.
PET radiotracers can also assess cardiac sympathetic activity,191,192 notably 6-fluoro-18F-L-dihydroxyphenylalanine (18F-DOPA), an analogue of L-dihydroxyphenylalanine (L-DOPA) routinely used to investigate neuroendocrine tumours.193 Another norepinephrine analogue is carbon-11-radiolabelled hydroxyephedrine (11C-HED),194 in which the need for on-site production limits clinical use. To date, however, no study has specifically studied these radiotracers to diagnose CTRCD.
Myocardial metabolism
Cardiac metabolism is a balance between various fuels, depending on the substrate’s bloodstream availability, dietary conditions, and underlying myocardial conditions.195 Under physiologic conditions, free fatty acids (FFAs) and glucose represent the primary cardiac energy sources.196 Myocardial glucose consumption can be imaged with 18F-FDG and FFA uptake with beta-methyl-iodine-123 phenylpentadecanoic acid (BMIPP).197 In the fasting phase, FFAs are abundantly available to the heart,196 rendering BMIPP more advantageous for assessing cardiac metabolism than 18F-FDG.198 Nonetheless, BMIPP is only routinely used in Japan,199 and one study reported BMIPP uptake reduction in patients receiving taxanes.200 Conversely, 18F-FDG PET is largely available and part of the routine oncological assessment. In patients treated with doxorubicin, an increased LV 18F-FDG uptake from baseline to end-of-treatment PET is associated with a subsequent LVEF drop85 and MACE.201 Moreover, increased RV 18F-FDG uptake predicts a higher cardiotoxicity risk.202 Similarly, in chest radiotherapy patients, focal cardiac 18F-FDG uptake is associated with myocardial damage,203–205 a study pointing towards a relation between the radiotherapy dose and the intensity of 18F-FDG uptake.206 Focal 18F-FDG cardiac uptake in cancer patients correlates highly with perfusion abnormalities on SPECT MPI,207 giving potential mechanistic insights for the subsequent cardiotoxicity. Still, a significant drawback of 18F-FDG PET is the high variability of cardiac uptake with diet and insulinaemia,208 which could be reduced by prolonged fasting.209 Additionally, 18F-FDG myocardial uptake increases in the ischaemic myocardium, which, although limiting the specificity of 18F-FDG patterns, could identify ischaemia onset.210
Alternatively, carbon-11 (11C) radiotracers can be used to image myocardial metabolism. 11C-acetate is taken up by cardiomyocytes and converted to acetyl-CoA, a substrate for energy production via the tricarboxylic acid cycle.211 The rate of 11C-acetate uptake is a marker of myocardial oxidative metabolism.212 In a pre-clinical model of mice undergoing treatment by tyrosine kinase inhibitors, the myocardium showed a decrease in 11C-acetate uptake concomitantly to an increase in 18F-FDG uptake.213 The short half-life of 11C (∼20 min), although interesting from a radiation exposure perspective, is the main factor limiting its routine use, as 11C requires an on-site cyclotron.211
Mitochondrial metabolism
The bottleneck of all cellular energy pathways is the mitochondrial production of ATP. Several chemotherapies affect ATP production and lead to cell death, generally by increasing reactive oxygen species (ROS) production.198 A PET radiotracer targeting ROS has recently been developed, named 18F-6-(4-((1-(2-fluoroethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-5-methyl-5,6-dihydrophenanthridine-3,8-diamine (18F-DHMT). In a pre-clinical rodent model of anthracycline-induced cardiotoxicity, 18F-DHMT evidenced an increased myocardial ROS production before any LV drop.214 Another ROS-targeting radiotracer is 18F-3-(3-fluoropropyl)-2-phenyl-2,3-dihydrobenzo[d]thiazole (18F-FPBT), in which myocardial uptake is also increased in rats receiving anthracycline.215 Deregulation of cardiomyocyte homeostasis by chemotherapy can manifest as mitochondrial membrane dysfunction, which can be explored with 18F(4-fluorophenyl)triphenylphosphonium (18F-FTPP+). In a swine model receiving intracoronary infusions of anthracycline, 18F-FTPP+ showed a partial mitochondrial depolarization in myocardial areas distal to the infused vessel.216 Recently, a radiotracer targeting TSPO, a translocator protein expressed in mitochondrial-activated microglia, has been validated in a model of myocardial infarction.217 This pre-clinical study showed that an early myocardial uptake of 18F-radiolabelled TSPO on PET predicted the subsequent LVEF reduction.
Cell death
A hallmark apoptosis feature is the exposition of phosphatidylserine at the cellular surface.218 Technetium-99m (99mTc)-radiolabelled annexin V is a phosphatidylserine ligand that detects apoptotic cardiomyocytes.219 In rats receiving doxorubicin, 99mTc-radiolabelled annexin V evidenced drug-induced toxicity in a dose-dependent manner before any functional impairment on echography.220 Recently, PET apoptosis radiotracers have also been developed.221 In a mouse model of experimentally induced anthracycline cardiotoxicity, 18F-CP18, a substrate of the caspase 3 enzyme present in apoptotic cells,222 evidenced apoptosis before any LVEF drop.223 Another target is myosin, externalized by necrotic cells after membrane rupture. Preliminary clinical studies showed that increased myocardial uptake of an indium-111 (111In)-radiolabelled antimyosin antibody preceded LVEF modifications in patients receiving anthracycline.189,224,225
Myocardial fibrosis
The cardiomyocyte loss induced by anticancer treatments is accompanied by myocardial fibroblast activation, leading to fibrotic ventricular remodelling, a condition of increased risk for heart failure.226 Although echocardiography and CMR can detect cardiac fibrosis, even at an early stage with mapping techniques,227,228 fibrosis still indicates myocardial damage. Therefore, detecting the onset of fibrotic replacement could help initiate cardiac treatments at an early and reversible stage.227 Fibroblast activation protein (FAP) is a transmembrane protease with enhanced expression in activated fibroblasts.229 Recently, pre-clinical findings evidenced intense 68Ga-FAPI myocardial uptake in areas of activated fibroblasts, conversely to no uptake in areas of advanced fibrosis.230–232 Similar incidental cases of 68Ga-FAPI cardiac uptake have been reported in cancer patients, unveiling myocardial ischaemia.233 This suggests that 68Ga-FAPI PET, likely to be used for cancer staging, could help simultaneously detect early stages of myocardial fibrosis. Moreover, 68Ga-FAPI myocardial uptake could pre-date any LVEF decrease, suggesting a potential role in cardiotoxicity prediction.234 Similarly, an 18F-radiolabelled FAPI tracer (18F-AlF-NOTA-FAPI-04) detects radiation-induced myocardial ischaemia before LVEF decreases, comforting the potential role of FAPI radiotracers for the early identification of cardiac damage.235
Future directions
One next step is to stratify the cardiotoxicity risk before treatment initiation. Predictive scores based on CVRFs and biological markers94,236,237 could be augmented by non-invasive imaging. For example, myocardial 18F-FDG uptake obtained from routine staging 18F-FDG PET can help stratify the cardiovascular risk with no additional cost or radiation burden.207 Cardiovascular risk stratification could also benefit from hybrid PET/CMR by combining CMR mapping techniques with the prognostic value of myocardial 18F-FDG uptake to predict the MACE risk.238,239
Artificial intelligence (AI) is a potential game changer in cardio-oncology.240,241 In 2619 cancer-free patients explored with SPECT MPI, a machine learning analysis combined with clinical data outperformed human analysis for MACE prediction.242 Moreover, the higher reproducibility of machine learning could improve diagnostic confidence in uncertain myocarditis patterns, such as patchy 18F-FDG myocardial uptake. AI also improves the characterization of several types of malignant masses,243–245 which might benefit cardiac tumour characterization.
In the era of precision medicine, where similar phenotypes arise from different genomic, metabolomic, and proteomic profiles, it will be crucial to tailoring the diagnosis to the tumour’s ‘-omic signature’.246 As a metabolic tool targeting specific pathophysiological pathways, nuclear imaging will most certainly play a central role in precision cardio-oncology.246 In addition to mapping cardiotoxicity, these probes might play a theranostic role, as with SSTR radiotracers, which help select patients in whom peptide receptor radionuclide therapy is indicated.247 An unsuccessful attempt in this sense has been made with 111In-labelled trastuzumab scintigraphy to predict cardiotoxicity from trastuzumab.248 Yet, the theranostic field is still in its infancy, and the wideness of metabolic targets assessable with nuclear radiotracers renders this goal within reach.
Conclusion
The progress in anticancer treatment is progressively turning cancer into a chronic condition. Consequently, the new challenge in this population is slowly shifting towards tackling other mortality causes, particularly CVD. Nuclear imaging allows for diagnosing various cardiac complications of anticancer therapies, even at an early stage, is useful for disease monitoring, and is a promising tool for the risk stratification of patients receiving cardiotoxic treatments. In addition, nuclear imaging has the unique ability to target specific metabolic links in the cardiotoxicity cascade for either diagnosis or treatment. Leveraging radiotracers already used routinely in patients with cancer, such as 18F-FDG and MPI tracers, could benefit this population with no additional cost or radiation exposure. Consequently, in the expanding field of cardio-oncology, nuclear medicine remains a central player that will most certainly remain at the forefront of the diagnostic armamentarium alongside cross-sectional imaging.
Acknowledgements
The graphical abstract, as well as Figures 1, 2, 3, 5, and 6, were created on (or using elements/templates from) BioRender.com.
Contributor Information
Nidaa Mikail, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Renata Chequer, Department of Nuclear Medicine, Bichat University Hospital, AP-HP, University Diderot, 75018 Paris, France.
Alessio Imperiale, Nuclear Medicine, Institut de Cancérologie de Strasbourg Europe (ICANS), University Hospitals of Strasbourg, 67093 Strasbourg, France; Molecular Imaging-DRHIM, IPHC, UMR 7178, CNRS/Unistra, 67093 Strasbourg, France.
Alexander Meisel, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Kantonsspital Glarus, Burgstrasse 99, 8750 Glarus, Switzerland.
Susan Bengs, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Angela Portmann, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Alessia Gimelli, Imaging Department, Fondazione CNR/Regione Toscana Gabriele Monasterio, Via G. Moruzzi 1, 56124 Pisa, Italy.
Ronny R Buechel, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Cathérine Gebhard, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Department of Cardiology, University Hospital Inselspital Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Alexia Rossi, Department of Nuclear Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. (WHO) WHO. WHO. The top 10 causes of death. 2020 2020 [cited 2022 2022 April 14]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2. Masoudkabir F, Sarrafzadegan N, Gotay C, Ignaszewski A, Krahn AD, Davis MK et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017;263:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Handy CE, Quispe R, Pinto X, Blaha MJ, Blumenthal RS, Michos ED et al. Synergistic opportunities in the interplay between cancer screening and cardiovascular disease risk assessment: together we are stronger. Circulation 2018;138:727–34. [DOI] [PubMed] [Google Scholar]
- 4. Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol 2016;32:900–7. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Pawlikowski M, Olivo-Marston S, Williams KP, Bower JK, Felix AS. Ten-year cardiovascular risk among cancer survivors: the National Health and Nutrition Examination Survey. PloS One 2021;16:e0247919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau ES, Paniagua SM, Liu E, Jovani M, Li SX, Takvorian K et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol 2021;3:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van ‘t Klooster CC, Ridker PM, Cook NR, Aerts J, Westerink J, Asselbergs FW et al. Prediction of lifetime and 10-year risk of cancer in individual patients with established cardiovascular disease. JACC CardioOncol 2020;2:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9–42. [DOI] [PubMed] [Google Scholar]
- 9. Atkins KM, Rawal B, Chaunzwa TL, Lamba N, Bitterman DS, Williams CL et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol 2019;73:2976–87. [DOI] [PubMed] [Google Scholar]
- 10. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–361. [DOI] [PubMed] [Google Scholar]
- 12. Anker MS, Hadzibegovic S, Lena A, Belenkov Y, Bergler-Klein J, de Boer RA et al. Recent advances in cardio-oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019’. ESC Heart Fail 2019;6:1140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Čelutkienė J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:1504–24. [DOI] [PubMed] [Google Scholar]
- 15. Mikail N, Pisani A, El Hatimi S, Hentic O, Lebtahi R, Poitier B et al. Early diagnosis and treatment of myocardial neuroendocrine tumor metastasis: the cornerstone role of multimodality imaging. Circ Cardiovasc Imaging 2021;14:e011857. [DOI] [PubMed] [Google Scholar]
- 16. Mikail N, Males L, Hyafil F, Benali K, Deschamps L, Brochet E et al. Diagnosis and staging of cardiac masses: additional value of CMR with (18)F-FDG-PET compared to CMR with CECT. Eur J Nucl Med Mol Imaging 2022;49:2232–41. [DOI] [PubMed] [Google Scholar]
- 17. de Boer RA, Aboumsallem JP, Bracun V, Leedy D, Cheng R, Patel S et al. A new classification of cardio-oncology syndromes. Cardiooncology 2021;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tocchetti CG, Ameri P, de Boer RA, D’Alessandra Y, Russo M, Sorriento D et al. Cardiac dysfunction in cancer patients: beyond direct cardiomyocyte damage of anticancer drugs: novel cardio-oncology insights from the joint 2019 meeting of the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc Res 2020;116:1820–34. [DOI] [PubMed] [Google Scholar]
- 19. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. [DOI] [PubMed] [Google Scholar]
- 20. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res 2019;115:844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stocks T, Van Hemelrijck M, Manjer J, Bjørge T, Ulmer H, Hallmans G et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012;59:802–10. [DOI] [PubMed] [Google Scholar]
- 23. Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA 2005;294:1505–10. [DOI] [PubMed] [Google Scholar]
- 24. Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim Y, Seidman JG, Seidman CE. Genetics of cancer therapy-associated cardiotoxicity. J Mol Cell Cardiol 2022;167:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuster JJ, Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res 2018;122:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23:2900–2. [DOI] [PubMed] [Google Scholar]
- 30. Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 2005;23:7820–6. [DOI] [PubMed] [Google Scholar]
- 31. Leiva O, AbdelHameid D, Connors JM, Cannon CP, Bhatt DL. Common pathophysiology in cancer, atrial fibrillation, atherosclerosis, and thrombosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2021;3:619–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zaborowska-Szmit M, Krzakowski M, Kowalski DM, Szmit S. Cardiovascular complications of systemic therapy in non-small-cell lung cancer. J Clinical medicine 2020;9:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koutroumpakis E, Deswal A, Yusuf SW, Abe JI, Nead KT, Potter AS et al. Radiation-induced cardiovascular disease: mechanisms, prevention, and treatment. Curr Oncol Rep 2022;24:543–53. [DOI] [PubMed] [Google Scholar]
- 34. Park BC, Stone CA Jr, Dewan AK, Johnson DB. Hypersensitivity reactions and immune-related adverse events to immune checkpoint inhibitors: approaches, mechanisms, and models. Immunol Allergy Clin North Am 2022;42:285–305. [DOI] [PubMed] [Google Scholar]
- 35. Dehghani L, Mikail N, Kramkimel N, Soyer P, Lebtahi R, Mallone R et al. Autoimmune pancreatitis after nivolumab anti-programmed death receptor-1 treatment. Eur J Cancer 2018;104:243–6. [DOI] [PubMed] [Google Scholar]
- 36. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 2018;19:e447–e58. [DOI] [PubMed] [Google Scholar]
- 37. Čelutkienė J, Plymen CM, Flachskampf FA, de Boer RA, Grapsa J, Manka R et al. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1615–33. [DOI] [PubMed] [Google Scholar]
- 38. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–8. [DOI] [PubMed] [Google Scholar]
- 39. Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K et al. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr 2015;28:630–41. [DOI] [PubMed] [Google Scholar]
- 40. Alexander J, Dainiak N, Berger HJ, Goldman L, Johnstone D, Reduto L et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979;300:278–83. [DOI] [PubMed] [Google Scholar]
- 41. Choi BW, Berger HJ, Schwartz PE, Alexander J, Wackers FJ, Gottschalk A et al. Serial radionuclide assessment of doxorubicin cardiotoxicity in cancer patients with abnormal baseline resting left ventricular performance. Am Heart J 1983;106:638–43. [DOI] [PubMed] [Google Scholar]
- 42. Jafari F, Safaei AM, Hosseini L, Asadian S, Kamangar TM, Zadehbagheri F et al. The role of cardiac magnetic resonance imaging in the detection and monitoring of cardiotoxicity in patients with breast cancer after treatment: a comprehensive review. Heart Fail Rev 2021;26:679–97. [DOI] [PubMed] [Google Scholar]
- 43. Cadour F, Thuny F, Sourdon J. New insights in early detection of anticancer drug-related cardiotoxicity using perfusion and metabolic imaging. Front Cardiovasc Med 2022;9:813883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soufer A, Liu C, Henry ML, Baldassarre LA. Nuclear cardiology in the context of multimodality imaging to detect cardiac toxicity from cancer therapeutics: established and emerging methods. J Nucl Cardiol 2020;27:1210–24. [DOI] [PubMed] [Google Scholar]
- 45. Polomski ES, Antoni ML, Jukema JW, Kroep JR, Dibbets-Schneider P, Sattler MGA et al. Nuclear medicine imaging methods of radiation-induced cardiotoxicity. Semin Nucl Med 2022;52:597–610. [DOI] [PubMed] [Google Scholar]
- 46. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhir V, Yan AT, Nisenbaum R, Sloninko J, Connelly KA, Barfett J et al. Assessment of left ventricular function by CMR versus MUGA scans in breast cancer patients receiving trastuzumab: a prospective observational study. Int J Cardiovasc Imaging 2019;35:2085–93. [DOI] [PubMed] [Google Scholar]
- 48. Printezi MI, Yousif LIE, Kamphuis JAM, van Laake LW, Cramer MJ, Hobbelink MGG et al. LVEF by multigated acquisition scan compared to other imaging modalities in cardio-oncology: a systematic review. Curr Heart Fail Rep 2022;19:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Odak M, Kayani WT. MUGA Scan. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
- 50. Hesse B, Lindhardt TB, Acampa W, Anagnostopoulos C, Ballinger J, Bax JJ et al. EANM/ESC Guidelines for radionuclide imaging of cardiac function. Eur J Nucl Med Mol Imaging 2008;35:851–85. [DOI] [PubMed] [Google Scholar]
- 51. Paul AK, Nabi HA. Gated myocardial perfusion SPECT: basic principles, technical aspects, and clinical applications. J Nucl Med Technol 2004;32:179–87; quiz 88–9. [PubMed] [Google Scholar]
- 52. Zhao J, Peng M, Xu L. Equilibrium radionuclide angiocardiography for the evaluation of right ventricular ejection fraction in patients with cardiac disorders. Int J Clin Exp Med 2015;8:18144–50. [PMC free article] [PubMed] [Google Scholar]
- 53. Chin BB, Bloomgarden DC, Xia W, Kim HJ, Fayad ZA, Ferrari VA et al. Right and left ventricular volume and ejection fraction by tomographic gated blood-pool scintigraphy. J Nucl Med 1997;38:942–8. [PubMed] [Google Scholar]
- 54. Xie BQ, Tian YQ, Zhang J, Zhao SH, Yang MF, Guo F et al. Evaluation of left and right ventricular ejection fraction and volumes from gated blood-pool SPECT in patients with dilated cardiomyopathy: comparison with cardiac MRI. J Nucl Med 2012;53:584–91. [DOI] [PubMed] [Google Scholar]
- 55. Dercle L, Ouali M, Pascal P, Giraudmaillet T, Chisin R, Lairez O et al. Gated blood pool SPECT: the estimation of right ventricular volume and function is algorithm dependent in a clinical setting. J Nucl Cardiol 2015;22:483–92. [DOI] [PubMed] [Google Scholar]
- 56. Dercle L, Giraudmaillet T, Pascal P, Lairez O, Chisin R, Marachet MA et al. Is TOMPOOL (gated blood-pool SPECT processing software) accurate to diagnose right and left ventricular dysfunction in a clinical setting? J Nucl Cardiol 2014;21:1011–22. [DOI] [PubMed] [Google Scholar]
- 57. Sachpekidis C, Sachpekidis V, Kopp-Schneider A, Arsos G, Moralidis E. Equilibrium radionuclide angiography: intra- and inter-observer repeatability and reproducibility in the assessment of cardiac systolic and diastolic function. J Nucl Cardiol 2021;28:1304–14. [DOI] [PubMed] [Google Scholar]
- 58. Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Nucl Cardiol 2019;26:1392–413. [DOI] [PubMed] [Google Scholar]
- 59. Schwartz RG, McKenzie WB, Alexander J, Sager P, D’Souza A, Manatunga A et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med 1987;82:1109–18. [DOI] [PubMed] [Google Scholar]
- 60. Liu YH, Fazzone-Chettiar R, Sandoval V, Tsatkin V, Miller EJ, Sinusas AJ. New approach for quantification of left ventricular function from low-dose gated bloodpool SPECT: validation and comparison with conventional methods in patients. J Nucl Cardiol 2021;28:939–50. [DOI] [PubMed] [Google Scholar]
- 61. Groch MW, DePuey EG, Belzberg AC, Erwin WD, Kamran M, Barnett CA et al. Planar imaging versus gated blood-pool SPECT for the assessment of ventricular performance: a multicenter study. J Nucl Med 2001;42:1773–9. [PubMed] [Google Scholar]
- 62. Huang H, Nijjar PS, Misialek JR, Blaes A, Derrico NP, Kazmirczak F et al. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2017;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Luu JM, Gebhard C, Ramasundarahettige C, Desai D, Schulze K, Marcotte F et al. Normal sex and age-specific parameters in a multi-ethnic population: a cardiovascular magnetic resonance study of the Canadian Alliance for Healthy Hearts and Minds cohort. J Cardiovasc Magn Reson 2022;24:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mikail N, Rossi A, Bengs S, Haider A, Stähli BE, Portmann A et al. Imaging of heart disease in women: review and case presentation. Eur J Nucl Med Mol Imaging 2022;50:130–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Farrell MB, Galt JR, Georgoulias P, Malhotra S, Pagnanelli R, Rischpler C et al. SNMMI procedure standard/EANM guideline for gated equilibrium radionuclide angiography. J Nucl Med Technol 2020;48:126–35. [DOI] [PubMed] [Google Scholar]
- 66. Carpeggiani C, Rossi G, Landi P, Michelassi C, Brambilla M, Cortigiani L et al. Long-term outcome and medical radiation exposure in patients hospitalized for cardiovascular disease. Int J Cardiol 2015;195:30–6. [DOI] [PubMed] [Google Scholar]
- 67. Laskey WK, Feinendegen LE, Neumann RD, Dilsizian V. Low-level ionizing radiation from noninvasive cardiac imaging: can we extrapolate estimated risks from epidemiologic data to the clinical setting? JACC Cardiovasc Imaging 2010;3:517–24. [DOI] [PubMed] [Google Scholar]
- 68. Doukky R, Frogge N, Appis A, Hayes K, Khoudary G, Fogg L et al. Impact of appropriate use on the estimated radiation risk to men and women undergoing radionuclide myocardial perfusion imaging. J Nucl Med 2016;57:1251–7. [DOI] [PubMed] [Google Scholar]
- 69. Tissot H, Roch V, Morel O, Veran N, Perrin M, Claudin M et al. Left ventricular ejection fraction determined with the simulation of a very low-dose CZT-SPECT protocol and an additional count-calibration on planar radionuclide angiographic data. J Nucl Cardiol 2019;26:1539–49. [DOI] [PubMed] [Google Scholar]
- 70. Claudin M, Imbert L, Djaballah W, Veran N, Poussier S, Roch V et al. Routine evaluation of left ventricular function using CZT-SPECT, with low injected activities and limited recording times. J Nucl Cardiol 2018;25:249–56. [DOI] [PubMed] [Google Scholar]
- 71. Chen YC, Ko CL, Yen RF, Lo MF, Huang YH, Hsu PY et al. Comparison of biventricular ejection fractions using cadmium-zinc-telluride SPECT and planar equilibrium radionuclide angiography. J Nucl Cardiol 2016;23:348–61. [DOI] [PubMed] [Google Scholar]
- 72. Apert A, Canu M, Jankowski A, Riou L, Broisat A, Charlon C et al. Comparison of cadmium zinc telluride ECG-gated SPECT equilibrium radionuclide angiocardiography to magnetic resonance imaging to measure right ventricular volumes and ejection fraction in patients with cardiomyopathy. J Nucl Cardiol 2022;29:1647–56. [DOI] [PubMed] [Google Scholar]
- 73. Bouallègue FB, Maïmoun L, Kucharczak F, Le Fur P, Vauchot F, Hay B et al. Left ventricle function assessment using gated first-pass (18)F-FDG PET: validation against equilibrium radionuclide angiography. J Nucl Cardiol 2021;28:594–603. [DOI] [PubMed] [Google Scholar]
- 74. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 75. Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging 2018;11:1173–86. [DOI] [PubMed] [Google Scholar]
- 76. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61:77–84. [DOI] [PubMed] [Google Scholar]
- 77. Thavendiranathan P, Negishi T, Somerset E, Negishi K, Penicka M, Lemieux J et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol 2021;77:392–401. [DOI] [PubMed] [Google Scholar]
- 78. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–801. [DOI] [PubMed] [Google Scholar]
- 79. Meléndez GC, Sukpraphrute B, D’Agostino RB, Jordan JH, Klepin HD, Ellis L et al. Frequency of left ventricular end-diastolic volume-mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am J Cardiol 2017;119:1637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ylänen K, Poutanen T, Savikurki-Heikkilä P, Rinta-Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J Am Coll Cardiol 2013;61:1539–47. [DOI] [PubMed] [Google Scholar]
- 81. O’Quinn R, Ferrari VA, Daly R, Hundley G, Baldassarre LA, Han Y et al. Cardiac magnetic resonance in cardio-oncology: advantages, importance of expediency, and considerations to navigate pre-authorization. JACC CardioOncol 2021;3:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Altaha MA, Nolan M, Marwick TH, Somerset E, Houbois C, Amir E et al. Can quantitative CMR tissue characterization adequately identify cardiotoxicity during chemotherapy? : impact of temporal and observer variability. JACC Cardiovasc Imaging 2020;13:951–62. [DOI] [PubMed] [Google Scholar]
- 83. Pickett CA, Cheezum MK, Kassop D, Villines TC, Hulten EA. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: a meta-analysis. Eur Heart J Cardiovasc Imaging 2015;16:848–52. [DOI] [PubMed] [Google Scholar]
- 84. Hoffmann R, Barletta G, von Bardeleben S, Vanoverschelde JL, Kasprzak J, Greis C et al. Analysis of left ventricular volumes and function: a multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J Am Soc Echocardiogr 2014;27:292–301. [DOI] [PubMed] [Google Scholar]
- 85. Sarocchi M, Bauckneht M, Arboscello E, Capitanio S, Marini C, Morbelli S et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med 2018;16:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li J, Zhao J, Lei Y, Chen Y, Cheng M, Wei X et al. Coronary atherosclerotic disease and cancer: risk factors and interrelation. Front Cardiovasc Med 2022;9:821267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res 2018;164:S23–S8. [DOI] [PubMed] [Google Scholar]
- 88. van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Int Med 2015;175:1007–17. [DOI] [PubMed] [Google Scholar]
- 89. Pak S, Hawash AA, Linares J, Valencia D, Kilgore A, Valencia V et al. Myocardial damage on SPECT imaging among patients treated with radiotherapy for left-sided breast cancer: systematic review with meta-analysis and narrative synthesis. J BUON 2018;23:910–8. [PubMed] [Google Scholar]
- 90. Mitchell JD, Cehic DA, Morgia M, Bergom C, Toohey J, Guerrero PA et al. Cardiovascular manifestations from therapeutic radiation: a multidisciplinary expert consensus statement from the International Cardio-Oncology Society. JACC CardioOncol 2021;3:360–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Desai MY, Windecker S, Lancellotti P, Bax JJ, Griffin BP, Cahlon O et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;74:905–27. [DOI] [PubMed] [Google Scholar]
- 92. Saraste A, Barbato E, Capodanno D, Edvardsen T, Prescott E, Achenbach S et al. Imaging in ESC clinical guidelines: chronic coronary syndromes. Eur Heart J Cardiovasc Imaging 2019;20:1187–97. [DOI] [PubMed] [Google Scholar]
- 93. Zhang P, Hu X, Yue J, Meng X, Han D, Sun X et al. Early detection of radiation-induced heart disease using (99m)Tc-MIBI SPECT gated myocardial perfusion imaging in patients with oesophageal cancer during radiotherapy. Radiother Oncol 2015;115:171–8. [DOI] [PubMed] [Google Scholar]
- 94. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 95. Gyenes G, Fornander T, Carlens P, Glas U, Rutqvist LE. Detection of radiation-induced myocardial damage by technetium-99m sestamibi scintigraphy. Eur J Nucl Med 1997;24:286–92. [DOI] [PubMed] [Google Scholar]
- 96. Seddon B, Cook A, Gothard L, Salmon E, Latus K, Underwood SR et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol 2002;64:53–63. [DOI] [PubMed] [Google Scholar]
- 97. Bergom C, Bradley JA, Ng AK, Samson P, Robinson C, Lopez-Mattei J et al. Past, present, and future of radiation-induced cardiotoxicity: refinements in targeting, surveillance, and risk stratification. JACC CardioOncol 2021;3:343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 2007;25:3031–7. [DOI] [PubMed] [Google Scholar]
- 99. Kaidar-Person O, Zagar TM, Oldan JD, Matney J, Jones EL, Das S et al. Early cardiac perfusion defects after left-sided radiation therapy for breast cancer: is there a volume response? Breast Cancer Res Treat 2017;164:253–62. [DOI] [PubMed] [Google Scholar]
- 100. Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005;63:214–23. [DOI] [PubMed] [Google Scholar]
- 101. Quintero-Martinez JA, Cordova-Madera SN, Villarraga HR. Radiation-induced heart disease. J Clin Med 2021;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Galán-Arriola C, Vílchez-Tschischke JP, Lobo M, López GJ, de Molina-Iracheta A, Pérez-Martínez C et al. Coronary microcirculation damage in anthracycline cardiotoxicity. Cardiovasc Res 2022;118:531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fabin N, Bergami M, Cenko E, Bugiardini R, Manfrini O. The role of vasospasm and microcirculatory dysfunction in fluoropyrimidine-induced ischemic heart disease. J Clin Med 2022;11:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schindler TH, Dilsizian V. Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging 2020;13:140–55. [DOI] [PubMed] [Google Scholar]
- 105. Gould KL, Johnson NP. Coronary physiology beyond coronary flow reserve in microvascular angina: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:2642–62. [DOI] [PubMed] [Google Scholar]
- 106. Groarke JD, Divakaran S, Nohria A, Killoran JH, Dorbala S, Dunne RM et al. Coronary vasomotor dysfunction in cancer survivors treated with thoracic irradiation. J Nucl Cardiol 2021;28:2976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Żyromska A, Małkowski B, Wiśniewski T, Majewska K, Reszke J, Makarewicz R. (15)O-H(2)O PET/CT as a tool for the quantitative assessment of early post-radiotherapy changes of heart perfusion in breast carcinoma patients. Br J Radiol 2018;91:20170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Divakaran S, Caron JP, Zhou W, Hainer J, Bibbo CF, Skali H et al. Coronary vasomotor dysfunction portends worse outcomes in patients with breast cancer. J Nucl Cardiol 2022;29:3072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr 2017;11:74–84. [DOI] [PubMed] [Google Scholar]
- 110. Cainzos-Achirica M, Nasir K. Debates in cardiac CT: the force of data is with CAC—and it’s rock solid. J Cardiovasc Comput Tomogr 2022;16:286–9. [DOI] [PubMed] [Google Scholar]
- 111. Chang SM, Nabi F, Xu J, Peterson LE, Achari A, Pratt CM et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol 2009;54:1872–82. [DOI] [PubMed] [Google Scholar]
- 112. Einstein AJ, Johnson LL, Bokhari S, Son J, Thompson RC, Bateman TM et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol 2010;56:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sharma V, Mughal L, Dimitropoulos G, Sheikh A, Griffin M, Moss A et al. The additive prognostic value of coronary calcium score (CCS) to single photon emission computed tomography myocardial perfusion imaging (SPECT-MPI)-real world data from a single center. J Nucl Cardiol 2021;28:2086–96. [DOI] [PubMed] [Google Scholar]
- 114. McConachie P, McKay E, Crane A, Nguyen N, Quinn R, Butler SP. Accurate measurement of coronary artery calcium in cancer patients using the CT component of PET/CT scans. Nucl Med Commun 2022;43:159–65. [DOI] [PubMed] [Google Scholar]
- 115. Pope A, Thomson L, Cantu S, Setia G, Torosyan N, Merz NB et al. Detection of subclinical atherosclerosis from PET-CT in patients with breast cancer. J Cardiovasc Comput Tomogr 2022;16:189–90. [DOI] [PubMed] [Google Scholar]
- 116. de Amorim Fernandes F, Peix A, Giubbini R, Karthikeyan G, Massardo T, Patel C et al. Reproducibility of global LV function and dyssynchrony parameters derived from phase analysis of gated myocardial perfusion SPECT: a multicenter comparison with core laboratory setting. J Nucl Cardiol 2020;29:952–61. [DOI] [PubMed] [Google Scholar]
- 117. Bouallègue F B, Mariano-Goulart D, Agostini D, Manrique A. Feasibility of biventricular volume and function assessment using first-pass gated (15)O-water PET. EJNMMI Res 2018;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Manrique A, Faraggi M, Véra P, Vilain D, Lebtahi R, Cribier A et al. 201Tl and 99mTc-MIBI gated SPECT in patients with large perfusion defects and left ventricular dysfunction: comparison with equilibrium radionuclide angiography. J Nucl Med 1999;40:805–9. [PubMed] [Google Scholar]
- 119. Jensen MM, Schmidt U, Huang C, Zerahn B. Gated tomographic radionuclide angiography using cadmium-zinc-telluride detector gamma camera; comparison to traditional gamma cameras. J Nucl Cardiol 2014;21:384–96. [DOI] [PubMed] [Google Scholar]
- 120. Wu D, Zhang Z, Ma R, Guo F, Wang L, Fang W. Comparison of CZT SPECT and conventional SPECT for assessment of contractile function, mechanical synchrony and myocardial scar in patients with heart failure. J Nucl Cardiol 2019;26:443–52. [DOI] [PubMed] [Google Scholar]
- 121. Pelletier-Galarneau M, Finnerty V, Tan S, Authier S, Gregoire J, Harel F. Assessment of left ventricular ejection fraction with cardiofocal collimators: comparison between IQ-SPECT, planar equilibrium radionuclide angiography, and cardiac magnetic resonance. J Nucl Cardiol 2019;26:1857–64. [DOI] [PubMed] [Google Scholar]
- 122. Bailliez A, Lairez O, Merlin C, Piriou N, Legallois D, Blaire T et al. Left ventricular function assessment using 2 different cadmium-zinc-telluride cameras compared with a γ-camera with cardiofocal collimators: dynamic cardiac phantom study and clinical validation. J Nucl Med 2016;57:1370–5. [DOI] [PubMed] [Google Scholar]
- 123. Whelton SP, Al Rifai M, Dardari Z, Shaw LJ, Al-Mallah MH, Matsushita K et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC Consortium. Eur Heart J Cardiovasc Imaging 2019;20:389–95. [DOI] [PubMed] [Google Scholar]
- 124. Gimelli A, Achenbach S, Buechel RR, Edvardsen T, Francone M, Gaemperli O et al. Strategies for radiation dose reduction in nuclear cardiology and cardiac computed tomography imaging: a report from the European Association of Cardiovascular Imaging (EACVI), the Cardiovascular Committee of European Association of Nuclear Medicine (EANM), and the European Society of Cardiovascular Radiology (ESCR). Eur Heart J 2018;39:286–96. [DOI] [PubMed] [Google Scholar]
- 125. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–39. [DOI] [PubMed] [Google Scholar]
- 126. Cottin Y, L’Huillier I, Casasnovas O, Geoffroy C, Caillot D, Zeller M et al. Dobutamine stress echocardiography identifies anthracycline cardiotoxicity. Eur J Echocardiogr 2000;1:180–3. [DOI] [PubMed] [Google Scholar]
- 127. Jarfelt M, Kujacic V, Holmgren D, Bjarnason R, Lannering B. Exercise echocardiography reveals subclinical cardiac dysfunction in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2007;49:835–40. [DOI] [PubMed] [Google Scholar]
- 128. Civelli M, Cardinale D, Martinoni A, Lamantia G, Colombo N, Colombo A et al. Early reduction in left ventricular contractile reserve detected by dobutamine stress echo predicts high-dose chemotherapy-induced cardiac toxicity. Int J Cardiol 2006;111:120–6. [DOI] [PubMed] [Google Scholar]
- 129. Rossi A, Wragg A, Klotz E, Pirro F, Moon JC, Nieman K et al. Dynamic computed tomography myocardial perfusion imaging: comparison of clinical analysis methods for the detection of vessel-specific ischemia. Circ Cardiovasc Imaging 2017;10:e005505. [DOI] [PubMed] [Google Scholar]
- 130. de Knegt MC, Rossi A, Petersen SE, Wragg A, Khurram R, Westwood M et al. Stress myocardial perfusion with qualitative magnetic resonance and quantitative dynamic computed tomography: comparison of diagnostic performance and incremental value over coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2020;8:jeaa270. [DOI] [PubMed] [Google Scholar]
- 131. Kitagawa K, Nakamura S, Ota H, Ogawa R, Shizuka T, Kubo T et al. Diagnostic performance of dynamic myocardial perfusion imaging using dual-source computed tomography. J Am Coll Cardiol 2021;78:1937–49. [DOI] [PubMed] [Google Scholar]
- 132. Nous FMA, Geisler T, Kruk MBP, Alkadhi H, Kitagawa K, Vliegenthart R et al. Dynamic myocardial perfusion CT for the detection of hemodynamically significant coronary artery disease. JACC Cardiovasc Imaging 2022;15:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Rahman H, Scannell CM, Demir OM, Ryan M, McConkey H, Ellis H et al. High-resolution cardiac magnetic resonance imaging techniques for the identification of coronary microvascular dysfunction. JACC Cardiovasc Imaging 2021;14:978–86. [DOI] [PubMed] [Google Scholar]
- 134. Mathew RC, Bourque JM, Salerno M, Kramer CM. Cardiovascular imaging techniques to assess microvascular dysfunction. JACC Cardiovasc Imaging 2020;13:1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:2625–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rasmussen LD, Winther S, Eftekhari A, Karim SR, Westra J, Isaksen C et al. Second-line myocardial perfusion imaging to detect obstructive stenosis: head-to-head comparison of CMR and PET. JACC Cardiovasc Imaging 2023;16:642–55. [DOI] [PubMed] [Google Scholar]
- 137. Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation 2017;135:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Banister HR, Hammond ST, Parr SK, Sutterfield SL, Turpin VG, Treinen S et al. Lower endothelium-dependent microvascular function in adult breast cancer patients receiving radiation therapy. Cardiooncology 2021;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 140. Stein-Merlob AF, Rothberg MV, Ribas A, Yang EH. Cardiotoxicities of novel cancer immunotherapies. Heart 2021;107:1694–703. [DOI] [PubMed] [Google Scholar]
- 141. Malaty MM, Amarasekera AT, Li C, Scherrer-Crosbie M, Tan TC. Incidence of immune checkpoint inhibitor mediated cardiovascular toxicity: a systematic review and meta-analysis. Eur J Clin Invest 2022;52:e13831. [DOI] [PubMed] [Google Scholar]
- 142. Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging 2018;11:1187–90. [DOI] [PubMed] [Google Scholar]
- 143. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–76. [DOI] [PubMed] [Google Scholar]
- 145. Ederhy S, Devos P, Pinna B, Funck-Brentano E, Abbar B, Fenioux C et al. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography imaging for the diagnosis of immune checkpoint inhibitor-associated myocarditis. Arch Cardiovasc Dis 2022;115:114–6. [DOI] [PubMed] [Google Scholar]
- 146. Ozawa K, Funabashi N, Daimon M, Takaoka H, Takano H, Uehara M et al. Determination of optimum periods between onset of suspected acute myocarditis and ¹⁸F-fluorodeoxyglucose positron emission tomography in the diagnosis of inflammatory left ventricular myocardium. Int J Cardiol 2013;169:196–200. [DOI] [PubMed] [Google Scholar]
- 147. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Nensa F, Kloth J, Tezgah E, Poeppel TD, Heusch P, Goebel J et al. Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol 2018;25:785–94. [DOI] [PubMed] [Google Scholar]
- 149. Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV et al. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J Am Coll Cardiol 2017;69:1774–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lee H, Schubert EK, Vidula MK, Pryma DA, Marchlinski FE, Goldberg LR et al. Potential clinical utility of (68)Ga-DOTATATE PET/CT for detection and response assessment in cardiac sarcoidosis. J Nucl Cardiol 2022;30:1075–87. [DOI] [PubMed] [Google Scholar]
- 151. Boughdad S, Latifyan S, Fenwick C, Bouchaab H, Suffiotti M, Moslehi JJ et al. (68)Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. J Immunother Cancer 2021;9:e003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W et al. (68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 2019;60:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019;60:801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Finke D, Heckmann MB, Herpel E, Katus HA, Haberkorn U, Leuschner F et al. Early detection of checkpoint inhibitor-associated myocarditis using (68)Ga-FAPI PET/CT. Front Cardiovasc Med 2021;8:614997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Vorster M. Gallium-68 labelled radiopharmaceuticals for imaging inflammatory disorders. Semin Nucl Med 2023;53:199–212. [DOI] [PubMed] [Google Scholar]
- 156. Glasenapp A, Derlin K, Gutberlet M, Hess A, Ross TL, Wester HJ et al. Molecular imaging of inflammation and fibrosis in pressure overload heart failure. Circ Res 2021;129:369–82. [DOI] [PubMed] [Google Scholar]
- 157. Kircher M, Lapa C. Novel noninvasive nuclear medicine imaging techniques for cardiac inflammation. Curr Cardiovasc Imaging Rep 2017;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kristensen LK, Fröhlich C, Christensen C, Melander MC, Poulsen TT, Galler GR et al. CD4(+) And CD8a(+) PET imaging predicts response to novel PD-1 checkpoint inhibitor: studies of Sym021 in syngeneic mouse cancer models. Theranostics 2019;9:8221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Maslov DV, Tawagi K, Kc M, Simenson V, Yuan H, Parent C et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer 2021;9:e002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–76. [DOI] [PubMed] [Google Scholar]
- 161. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Quinaglia T, Gongora C, Awadalla M, Hassan MZO, Zafar A, Drobni ZD et al. Global circumferential and radial strain among patients with immune checkpoint inhibitor myocarditis. JACC Cardiovasc Imaging 2022;15:1883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cadour F, Cautela J, Rapacchi S, Varoquaux A, Habert P, Arnaud F et al. Cardiac MRI features and prognostic value in immune checkpoint inhibitor-induced myocarditis. Radiology 2022;303:512–21. [DOI] [PubMed] [Google Scholar]
- 164. Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chen W, Jeudy J. Assessment of myocarditis: cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep 2019;21:76. [DOI] [PubMed] [Google Scholar]
- 166. Palmisano A, Vignale D, Peretto G, Busnardo E, Calcagno C, Campochiaro C et al. Hybrid FDG-PET/MR or FDG-PET/CT to detect disease activity in patients with persisting arrhythmias after myocarditis. JACC Cardiovasc Imaging 2021;14:288–92. [DOI] [PubMed] [Google Scholar]
- 167. Krumm P, Brendel JM, Klingel K, Müller KAL, Kübler J, Gräni C et al. Using multiparametric cardiac magnetic resonance to phenotype and differentiate biopsy-proven chronic from healed myocarditis and dilated cardiomyopathy. J Clin Med 2022;11:5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J Am Coll Cardiol 2016;67:1800–11. [DOI] [PubMed] [Google Scholar]
- 169. Tanimura M, Dohi K, Imanaka-Yoshida K, Omori T, Moriwaki K, Nakamori S et al. Fulminant myocarditis with prolonged active lymphocytic infiltration after hemodynamic recovery. Int Heart J 2017;58:294–7. [DOI] [PubMed] [Google Scholar]
- 170. Abgral R, Dweck MR, Trivieri MG, Robson PM, Karakatsanis N, Mani V et al. Clinical utility of combined FDG-PET/MR to assess myocardial disease. JACC Cardiovasc Imaging 2017;10:594–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Köhler J et al. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med 2015;56:255–60. [DOI] [PubMed] [Google Scholar]
- 172. Hanneman K, Kadoch M, Guo HH, Jamali M, Quon A, Iagaru A et al. Initial experience with simultaneous 18F-FDG PET/MRI in the evaluation of cardiac sarcoidosis and myocarditis. Clin Nucl Med 2017;42:e328–e34. [DOI] [PubMed] [Google Scholar]
- 173. Upshaw JN, Finkelman B, Hubbard RA, Smith AM, Narayan HK, Arndt L et al. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. JACC Cardiovasc Imaging 2020;13:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Border WL, Sachdeva R, Stratton KL, Armenian SH, Bhat A, Cox DE et al. Longitudinal changes in echocardiographic parameters of cardiac function in pediatric cancer survivors. JACC CardioOncol 2020;2:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mincu RI, Lampe LF, Mahabadi AA, Kimmig R, Rassaf T, Totzeck M. Left ventricular diastolic function following anthracycline-based chemotherapy in patients with breast cancer without previous cardiac disease—a meta-analysis. J Clin Med 2021;10:3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lee BH, Goodenday LS, Muswick GJ, Yasnoff WA, Leighton RF, Skeel RT. Alterations in left ventricular diastolic function with doxorubicin therapy. J Am Coll Cardiol 1987;9:184–8. [DOI] [PubMed] [Google Scholar]
- 177. Klein R, Nadouri D, Osler E, Johnson C, Dent S, Dwivedi G. Diastolic dysfunction can precede systolic dysfunction on MUGA in cancer patients receiving trastuzumab-based therapy. Nucl Med Commun 2019;40:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Aggarwal A, Brown KA, LeWinter MM. Diastolic dysfunction: pathophysiology, clinical features, and assessment with radionuclide methods. J Nucl Cardiol 2001;8:98–106. [DOI] [PubMed] [Google Scholar]
- 179. Reuvekamp EJ, Bulten BF, Nieuwenhuis AA, Meekes MR, de Haan AF, Tol J et al. Does diastolic dysfunction precede systolic dysfunction in trastuzumab-induced cardiotoxicity? Assessment with multigated radionuclide angiography (MUGA). J Nucl Cardiol 2016;23:824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hansen NL, Haarmark C, Zerahn B. Ventricular peak emptying and filling rates measured by gated tomographic radionuclide angiography using a cadmium-zinc-telluride SPECT camera in chemotherapy-naïve cancer patients. J Nucl Cardiol 2020;27:1193–201. [DOI] [PubMed] [Google Scholar]
- 181. Chamsi-Pasha MA, Zhan Y, Debs D, Shah DJ. CMR in the evaluation of diastolic dysfunction and phenotyping of HFpEF: current role and future perspectives. JACC Cardiovasc Imaging 2020;13:283–96. [DOI] [PubMed] [Google Scholar]
- 182. Slart R, Tio RA, Elsinga PH, Schwaiger M. Autonomic Innervation of the Heart. New York City: Springer Publishing; 2015. p235–53. [Google Scholar]
- 183. Zelt JGE, deKemp RA, Rotstein BH, Nair GM, Narula J, Ahmadi A et al. Nuclear imaging of the cardiac sympathetic nervous system: a disease-specific interpretation in heart failure. JACC Cardiovasc Imaging 2020;13:1036–54. [DOI] [PubMed] [Google Scholar]
- 184. Laursen AH, Thune JJ, Hutchings M, Hasbak P, Kjaer A, Elming MB et al. (123) I-MIBG imaging for detection of anthracycline-induced cardiomyopathy. Clin Physiol Funct Imaging 2018;38:176–85. [DOI] [PubMed] [Google Scholar]
- 185. Gimelli A, Liga R, Agostini D, Bengel FM, Ernst S, Hyafil F et al. The role of myocardial innervation imaging in different clinical scenarios: an expert document of the European Association of Cardiovascular Imaging and Cardiovascular Committee of the European Association of Nuclear Medicine. Eur Heart J Cardiovasc Imaging 2021;22:480–90. [DOI] [PubMed] [Google Scholar]
- 186. Santos MJ D, da Rocha ET, Verberne HJ, da Silva ET, Aragon DC, Junior JS. Assessment of late anthracycline-induced cardiotoxicity by (123)I-mIBG cardiac scintigraphy in patients treated during childhood and adolescence. J Nucl Cardiol 2017;24:256–64. [DOI] [PubMed] [Google Scholar]
- 187. Lekakis J, Prassopoulos V, Athanassiadis P, Kostamis P, Moulopoulos S. Doxorubicin-induced cardiac neurotoxicity: study with iodine 123-labeled metaiodobenzylguanidine scintigraphy. J Nucl Cardiol 1996;3:37–41. [DOI] [PubMed] [Google Scholar]
- 188. Arrais TR, Cavalli GD, Dos Santos BT, Pereira GB, Migliavaca CB, Grossman GB et al. MIBG cardiac imaging compared to ejection fraction in evaluation of cardiotoxicity: a systematic review. J Nucl Cardiol 2021;29:2274–91. [DOI] [PubMed] [Google Scholar]
- 189. Carrió I, Estorch M, Berná L, López-Pousa J, Tabernero J, Torres G. Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J Nucl Med 1995;36:2044–9. [PubMed] [Google Scholar]
- 190. Valdés Olmos RA, ten Bokkel Huinink WW, ten Hoeve RF, van Tinteren H, Bruning PF, van Vlies B et al. Assessment of anthracycline-related myocardial adrenergic derangement by [123I]metaiodobenzylguanidine scintigraphy. Eur J Cancer 1995;31a:26–31. [DOI] [PubMed] [Google Scholar]
- 191. Kelly JM, Babich JW. PET tracers for imaging cardiac function in cardio-oncology. Curr Cardiol Rep 2022;24:247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Burger IA, Lohmann C, Messerli M, Bengs S, Becker A, Maredziak M et al. Age- and sex-dependent changes in sympathetic activity of the left ventricular apex assessed by 18F-DOPA PET imaging. PLoS One 2018;13:e0202302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur J Nucl Med Mol Imaging 2017;44:1588–601. [DOI] [PubMed] [Google Scholar]
- 194. Wang JZ, Moody JB, Kaps N, Britt D, Lavallee A, Renaud JM et al. Reproducible quantification of regional sympathetic denervation with [(11)C]meta-hydroxyephedrine PET imaging. J Nucl Cardiol 2021c;28:2745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res 2021;128:1487–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 2018;15:457–70. [DOI] [PubMed] [Google Scholar]
- 197. Peterson LR, Gropler RJ. Radionuclide imaging of myocardial metabolism. Circ Cardiovasc Imaging 2010;3:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Tong D, Zaha VG. Metabolic imaging in cardio-oncology. J Cardiovasc Transl Res 2020;13:357–66. [DOI] [PubMed] [Google Scholar]
- 199. Yoshinaga K, Tamaki N. Current status of nuclear cardiology in Japan: ongoing efforts to improve clinical standards and to establish evidence. J Nucl Cardiol 2015;22:690–9. [DOI] [PubMed] [Google Scholar]
- 200. Saito K, Takeda K, Imanaka-Yoshida K, Imai H, Sekine T, Kamikura Y. Assessment of fatty acid metabolism in taxan-induced myocardial damage with iodine-123 BMIPP SPECT: comparative study with myocardial perfusion, left ventricular function, and histopathological findings. Ann Nucl Med 2003;17:481–8. [DOI] [PubMed] [Google Scholar]
- 201. Cho SG, Kim YH, Park H, Park KS, Kim J, Ahn SJ et al. Prediction of cardiac events following concurrent chemoradiation therapy for non-small-cell lung cancer using FDG PET. Ann Nucl Med 2022;36:439–49. [DOI] [PubMed] [Google Scholar]
- 202. Kim J, Cho SG, Kang SR, Yoo SW, Kwon SY, Min JJ et al. Association between FDG uptake in the right ventricular myocardium and cancer therapy-induced cardiotoxicity. J Nucl Cardiol 2020;27:2154–63. [DOI] [PubMed] [Google Scholar]
- 203. Jingu K, Kaneta T, Nemoto K, Ichinose A, Oikawa M, Takai Y et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int J Radiat Oncol Biol Phys 2006;66:845–51. [DOI] [PubMed] [Google Scholar]
- 204. Unal K, Unlu M, Akdemir O, Akmansu M. 18F-FDG PET/CT findings of radiotherapy-related myocardial changes in patients with thoracic malignancies. Nucl Med Commun 2013;34:855–9. [DOI] [PubMed] [Google Scholar]
- 205. Evans JD, Gomez DR, Chang JY, Gladish GW, Erasmus JJ, Rebueno N et al. Cardiac ¹⁸F-fluorodeoxyglucose uptake on positron emission tomography after thoracic stereotactic body radiation therapy. Radiother Oncol 2013;109:82–8. [DOI] [PubMed] [Google Scholar]
- 206. Jo IY, Lee JW, Kim WC, Min CK, Kim ES, Yeo SG et al. Relationship between changes in myocardial F-18 fluorodeoxyglucose uptake and radiation dose after adjuvant three-dimensional conformal radiotherapy in patients with breast cancer. J Clin Med 2020;9:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Haider A, Bengs S, Schade K, Wijnen WJ, Portmann A, Etter D et al. Myocardial (18)F-FDG uptake pattern for cardiovascular risk stratification in patients undergoing oncologic PET/CT. J Clin Med 2020;9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Osborne MT, Hulten EA, Murthy VL, Skali H, Taqueti VR, Dorbala S et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol 2017;24:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ishida Y, Sakanaka K, Itasaka S, Nakamoto Y, Togashi K, Mizowaki T et al. Effect of long fasting on myocardial accumulation in 18F-fluorodeoxyglucose positron emission tomography after chemoradiotherapy for esophageal carcinoma. J Radiat Res 2018;59:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zampella E, Assante R, Acampa W, Gaudieri V, Nappi C, Mannarino T et al. Incremental value of (18)F-FDG cardiac PET imaging over dobutamine stress echocardiography in predicting myocardial ischemia in patients with suspected coronary artery disease. J Nucl Cardiol 2022;29:3028–38. [DOI] [PubMed] [Google Scholar]
- 211. Yoshii Y, Furukawa T, Saga T, Fujibayashi Y. Acetate/acetyl-CoA metabolism associated with cancer fatty acid synthesis: overview and application. Cancer Lett 2015;356:211–6. [DOI] [PubMed] [Google Scholar]
- 212. Wu KY, Dinculescu V, Renaud JM, Chen SY, Burwash IG, Mielniczuk LM et al. Repeatable and reproducible measurements of myocardial oxidative metabolism, blood flow and external efficiency using (11)C-acetate PET. J Nucl Cardiol 2018;25:1912–25. [DOI] [PubMed] [Google Scholar]
- 213. O’Farrell AC, Evans R, Silvola JM, Miller IS, Conroy E, Hector S et al. A novel positron emission tomography (PET) approach to monitor cardiac metabolic pathway remodeling in response to sunitinib malate. PLoS One 2017;12:e0169964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Boutagy NE, Wu J, Cai Z, Zhang W, Booth CJ, Kyriakides TC et al. In vivo reactive oxygen species detection with a novel positron emission tomography tracer, (18)F-DHMT, allows for early detection of anthracycline-induced cardiotoxicity in rodents. JACC Basic Transl Sci 2018;3:378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mota F, Pell VR, Singh N, Baark F, Waters E, Sadasivam P et al. A reactivity-based (18)F-labeled probe for PET imaging of oxidative stress in chemotherapy-induced cardiotoxicity. Mol Pharm 2022;19:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Detmer FJ, Alpert NM, Moon SH, Dhaynaut M, Guerrero JL, Guehl NJ et al. PET imaging of mitochondrial function in acute doxorubicin-induced cardiotoxicity: a proof-of-principle study. Sci Rep 2022;12:6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol 2018;71:263–75. [DOI] [PubMed] [Google Scholar]
- 218. Wang X, Feng H, Zhao S, Xu J, Wu X, Cui J et al. SPECT And PET radiopharmaceuticals for molecular imaging of apoptosis: from bench to clinic. Oncotarget 2017;8:20476–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Benali K, Louedec L, Azzouna RB, Merceron O, Nassar P, Al Shoukr F et al. Preclinical validation of 99mTc-annexin A5-128 in experimental autoimmune myocarditis and infective endocarditis: comparison with 99mTc-HYNIC-annexin A5. Mol Imaging 2014:13. doi: 10.2310/7290.2014.00049. [DOI] [PubMed] [Google Scholar]
- 220. Gabrielson KL, Mok GS, Nimmagadda S, Bedja D, Pin S, Tsao A et al. Detection of dose response in chronic doxorubicin-mediated cell death with cardiac technetium 99m annexin V single-photon emission computed tomography. Mol Imaging 2008;7:132–8. [PMC free article] [PubMed] [Google Scholar]
- 221. Van de Wiele C, Ustmert S, De Spiegeleer B, De Jonghe PJ, Sathekge M, Alex M. Apoptosis imaging in oncology by means of positron emission tomography: a review. Int J Mol Sci 2021;22:2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Su H, Chen G, Gangadharmath U, Gomez LF, Liang Q, Mu F et al. Evaluation of [(18)F]-CP18 as a PET imaging tracer for apoptosis. Mol Imaging Biol 2013;15:739–47. [DOI] [PubMed] [Google Scholar]
- 223. Su H, Gorodny N, Gomez LF, Gangadharmath U, Mu F, Chen G et al. Noninvasive molecular imaging of apoptosis in a mouse model of anthracycline-induced cardiotoxicity. Circ Cardiovasc Imaging 2015;8:e001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Carrió I, Lopez-Pousa A, Estorch M, Duncker D, Berná L, Torres G et al. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. J Nucl Med 1993;34:1503–7. [PubMed] [Google Scholar]
- 225. Estorch M, Carrió I, Berná L, Martínez-Duncker C, Alonso C, Germá JR et al. Indium-111-antimyosin scintigraphy after doxorubicin therapy in patients with advanced breast cancer. J Nucl Med 1990;31:1965–9. [PubMed] [Google Scholar]
- 226. Francis Stuart SD, De Jesus NM, Lindsey ML, Ripplinger CM. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol 2016;91:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Tadic M, Cuspidi C, Marwick TH. Phenotyping the hypertensive heart. Eur Heart J 2022;43:3794–810. [DOI] [PubMed] [Google Scholar]
- 228. Hassan S, Barrett CJ, Crossman DJ. Imaging tools for assessment of myocardial fibrosis in humans: the need for greater detail. Biophys Rev 2020;12:969–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Nagaraju CK, Dries E, Popovic N, Singh AA, Haemers P, Roderick HL et al. Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction. Sci Rep 2017;7:10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N et al. Molecular imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med 2019;60:1743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Qiao P, Wang Y, Zhu K, Zheng D, Song Y, Jiang D et al. Noninvasive monitoring of reparative fibrosis after myocardial infarction in rats using (68)Ga-FAPI-04 PET/CT. Mol Pharm 2022;19:4171–8. [DOI] [PubMed] [Google Scholar]
- 232. Song W, Zhang X, He S, Gai Y, Qin C, Hu F et al. (68)Ga-FAPI PET visualize heart failure: from mechanism to clinic. Eur J Nucl Med Mol Imaging 2023;50:475–85. [DOI] [PubMed] [Google Scholar]
- 233. Zhu W, Guo F, Wang Y, Ding H, Huo L. 68Ga-FAPI-04 accumulation in myocardial infarction in a patient with neuroendocrine carcinoma. Clin Nucl Med 2020;45:1020–2. [DOI] [PubMed] [Google Scholar]
- 234. Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging 2020;13:e010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wei Y, Sun Y, Liu J, Zhang G, Qin X, Xu S et al. Early detection of radiation-induced myocardial damage by [(18)F]AlF-NOTA-FAPI-04 PET/CT imaging. Eur J Nucl Med Mol Imaging 2023;50:453–64. [DOI] [PubMed] [Google Scholar]
- 236. Kim DY, Park MS, Youn JC, Lee S, Choi JH, Jung MH et al. Development and validation of a risk score model for predicting the cardiovascular outcomes after breast cancer therapy: the CHEMO-RADIAT score. J Am Heart Assoc 2021;10:e021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc 2014;3:e000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Muehlberg F, Funk S, Zange L, von Knobelsdorff-Brenkenhoff F, Blaszczyk E, Schulz A et al. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail 2018;5:620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Cheung E, Ahmad S, Aitken M, Chan R, Iwanochko RM, Balter M et al. Combined simultaneous FDG-PET/MRI with T1 and T2 mapping as an imaging biomarker for the diagnosis and prognosis of suspected cardiac sarcoidosis. Eur J Hybrid Imaging 2021;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Kwan JM, Oikonomou EK, Henry ML, Sinusas AJ. Multimodality advanced cardiovascular and molecular imaging for early detection and monitoring of cancer therapy-associated cardiotoxicity and the role of artificial intelligence and big data. Front Cardiovasc Med 2022;9:829553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Madan N, Lucas J, Akhter N, Collier P, Cheng F, Guha A et al. Artificial intelligence and imaging: opportunities in cardio-oncology. Am Heart J Plus 2022;15:100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Betancur J, Otaki Y, Motwani M, Fish MB, Lemley M, Dey D et al. Prognostic value of combined clinical and myocardial perfusion imaging data using machine learning. JACC Cardiovasc Imaging 2018;11:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Zhu Y, Li H, Guo W, Drukker K, Lan L, Giger ML et al. Deciphering genomic underpinnings of quantitative MRI-based radiomic phenotypes of invasive breast carcinoma. Sci Rep 2015;5:17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Young JD, Cai C, Lu X. Unsupervised deep learning reveals prognostically relevant subtypes of glioblastoma. BMC Bioinformatics 2017;18:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Grossmann P, Stringfield O, El-Hachem N, Bui MM, Rios Velazquez E, Parmar C et al. Defining the biological basis of radiomic phenotypes in lung cancer. Elife 2017;6:e23421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Dreyfuss AD, Bravo PE, Koumenis C, Ky B. Precision cardio-oncology. J Nucl Med 2019;60:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Park S, Parihar AS, Bodei L, Hope TA, Mallak N, Millo C et al. Somatostatin receptor imaging and theranostics: current practice and future prospects. J Nucl Med 2021;62:1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Perik PJ, Lub-De Hooge MN, Gietema JA, van der Graaf WT, de Korte MA, Jonkman S et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2006;24:2276–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.