Summary:
Acellular fish skin grafts (FSGs) are tissue-based products created by minimally processing the skin of the Atlantic cod (Gadus morhua). The FSG is rich in omega-3 and facilitates tissue regeneration by supporting revascularization and ingrowth in the proliferation and remodeling phases of wound healing. FSG is structurally more similar to human skin than antiviral-processed skin substitutes such as amniotic membrane, and there are no known prion, bacterial, or viral diseases that can be transmitted from North-Atlantic cod to humans. The FSG is processed using a proprietary method that preserves the structure and lipid composition of the skin. FSG is CE marked, and US Food and Drug Administration cleared for multiple clinical applications in partial and full-thickness wounds. FSG is currently the only acellular dermal matrix product that does not originate from mammalian tissues. For this narrative review, Medline and UpToDate were used to include a total of 21 articles published from 2015 to 2022 about fish skin graft use. We also reported a case of a 7-year-old boy who underwent treatment with FSG for abdominal wall dehiscence at our department of pediatric surgery, IRCCS Sant’Orsola-Malpighi, Alma Mater Studiorum, University of Bologna, University Hospital of Bologna. FSG provides a valuable and sustainable treatment that improves wound healing in both adult and pediatric populations. We described the first application of an FSG for wound dehiscence of the abdominal wall in a pediatric patient, reporting how FSG was completely reabsorbed and improved the skin’s repair.
Takeaways
Question: The role of fish skin as a graft in pediatric patients.
Findings: Review of literature and presentation of a case report.
Meaning: The fish skin graft provides promising results in tissue regeneration and in the management of chronic and acute wounds, pressure ulcers, vascular ulcers, diabetes-related ulcers, animal bites, and burns.
INTRODUCTION
Skin grafts are commonly used to facilitate skin closure in nonhealing wounds, which require a great deal of healthcare resources and represent a major issue for public health, associated with high morbidity and significant costs. The available grafts can be obtained from the same patient (autograft), a human donor (allograft), or from different species (xenograft). In 2013, Kerecis developed a xenograft derived from the skin of the Atlantic cod (Gadus morhua). Kerecis Omega3 Wound is an intact decellularized fish skin graft (FSG) used for the management of chronic and acute wounds, pressure ulcers, vascular ulcers, diabetes-related ulcers, trauma wounds, acute surgical wounds, and burns. The FSG sheet contains fats, proteins, elastin, glycans, and other natural skin elements, and they are particularly rich in omega-3. Such properties guarantee a better ingrowth compared with human allograft, with statistically proven evidence. Its enhanced cellularization process and unique biomechanical properties make acellular FSG ideal to be used as a protecting cover for severe trauma and burn wounds in the battlefield. Furthermore, the authors described the antibacterial nature of FSG; it can withstand bacterial invasion for up to 48–72 hours.1
The aim of this review is to summarize the uses of the acellular FSG described in literature and to present the first case of FSG use in the pediatric age, for the treatment of abdominal wound dehiscence.
METHODS
For this narrative review, Medline and UpToDate were used to search articles about FSG use. A total of 21 articles published from 2015 to 2022 were included in the study. (See table, Supplemental Digital Content 1, which shows the cited study. http://links.lww.com/PRSGO/C770.) We also reported a case of a 7-year-old boy who underwent treatment with FSG for abdominal wall dehiscence at our Department of Pediatric Surgery, IRCCS Sant’Orsola-Malpighi, Alma Mater Studiorum, University of Bologna, University Hospital of Bologna.
ACELLULAR FISH SKIN GRAFT
Acellular FSG is a wound dressing created by minimally processing the skin of the Atlantic cod (Gadus morhua). Kerecis Omega3 Wound is produced in Isafjordur, Iceland, according to Good Manufacturing Practice standards, FDA standards, and the European Medical Device Directive, and has therefore attained the CE marking. The product is ISO13485 certified. The clinical trial followed the ethical guidelines of the Declaration of Helsinki and of the World Health Organization.
This graft contains fats, collagen, fibrin, proteoglycans and glycosaminoglycans, and other natural skin elements. It is particularly rich in omega-3, promoting tissue regeneration by supporting revascularization and growth in the proliferation and remodeling phases of wound healing. Kotronoulas et al analyzed the lipidic composition of acellular fish skin graft in comparison with more frequently used wound grafting materials such as human cadaver skin and two different bovine collagen–based wound grafting materials. Their lipidic profile, in terms of the fatty acid composition, was outlined using targeted gas chromatography. They demonstrated that FSG was particularly rich in omega-3 fatty acids, whereas human cadaver skin showed a prevalence of omega-6 fatty acids, and the bovine collagen–based wound grafting materials mainly contained saturated and monounsaturated fatty acids.2
The FSG is processed using a proprietary method that preserves the structure and lipid composition of the skin. Such properties are maintained because viral inactivation is not strictly required for the purification of the tissue, as it intrinsically has very little risk of transmitting viral diseases to humans. In fact, viral inactivation is performed with detergents, which remove all soluble components from the tissue, including lipids, glycans, elastin, hyaluronic acid, soluble collagen, potentially beneficial to wound healing. Currently, there are no known prion, bacterial, or viral diseases that can be transmitted from North-Atlantic cod to humans such as bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease.
When compared with other grafts used such as mammalian acellular grafts (eg, porcine, bovine) and amniotic membrane, FSG seems to better mimic the three-dimensional human skin structure, and FSG is currently the only acellular dermal matrix product that does not originate from mammalian tissues.1
In the last years, FSG has been applied in different areas of interest: chronic diabetic foot ulcers, burns, chronic wounds (CWs), calciphylaxis wounds, necrotic angiodermatitis, or iatrogenic calcinosis cutis. Other additional challenges to its spread are represented by cultural and religious barriers compared with porcine and bovine derivatives.
The FGS is sterile, and before being applied, it must be soaked in saline 0.9% solution. After 2 minutes, the tissue is ready to be grafted. The FGS can also be meshed based on size of the wound and be applied using stiches or a medical patch. Pretesting for skin reactions is not necessary before the application, as there are no known adverse effects associated with the use of Kerecis Omega3 Wound.
MAIN APPLICATIONS
Diabetic Foot
The prevalence of diabetes is approximately 10.8% of the world population with an incidence of developing diabetic foot estimated around 6.3% in 2023.3 Generally, wound management strategies include active treatment such as vacuum-assisted closure (VAC) and skin graft or passive treatment (acellular dermal matrices). If a skin graft is needed, the new FSG could represent a valid alternative. Woodrow et al analyzed eight patients, who underwent FSG application for the management of postoperative diabetic foot wounds, showing how fish skin was well tolerated and potentially effective to accelerate wound healing.4 Lullove et al investigated the proportion of index ulcers healed at 12 weeks and the healing time in patients with ulcers for diabetic foot comparing FSG with the standard of care (SOC), represented by collagen alginate dressing. Their results revealed that in their larger cohorts, there was a significant difference in the proportion of healed wounds at 12 weeks, with the FSG group outperforming the SOC group.5 Zehnder et al treated all venous and diabetic wounds with SOC and FSG. They applied FSG on patients who did not show a reduction area more than 50% after 4 weeks. Among 42 patients, 21 needed FSG, which shows how patients treated with FSG had a minimum improvement of 25% over SOC in terms of the necessary time to obtain complete healing. They also reported the same cost for both treatments related to fewer weeks of treatment for patients who underwent fish skin grafting.6 Trinh et al reported their experience with complicated wounds secondary to an insufficient soft-tissue coverage after amputations in diabetic patients. They collected five patients treated with FSG, defining it as a viable treatment option in complicated wounds in the lower limb of diabetic patients to circumvent an otherwise necessary extension of the severed segment. Therefore, they refrained from making any comparison with other advanced medications.7 Michael et al, in their retrospective study, evaluated the efficacy of acellular FSG for the treatment of 58 full-thickness diabetic foot ulcers in 51 patients. They observed a rapid increase in wound healing, with a reduction of wound area by 87.57%, and 35 wounds (60.34%) fully healed after 16 weeks.8
Dardari et al reported a rare case of a CW due to necrotic angiodermitis in a patient with type 2 diabetes. They treated the patient with 10 applications of FSG, achieving accelerated scarring and pain relief, with complete wound healing in 10 weeks.9
Acute and Chronic Complex Wounds
It is estimated that 1%–2% of the population will experience a CW during their lifetime in developed countries. CW treatment is a challenge for the surgeon because of the difficult and slow healing, the impact on quality of life, and the costs they generate. At the state of art, there is no gold standard treatment described in the literature for these kinds of lesions. There are various debridement techniques available (surgical, biosurgical, enzymatic), as well as VAC and acellular dermal matrix. Kirsner et al compared the effect of FSG in acute full-thickness tissue injury to human amniotic membrane. In a double-blind, prospective randomized study, they showed that FSG is significantly superior in terms of healing time on acute wounds compared with human amniotic membrane, with a hazard ratio of 2.37 (95% CI: 1.75–3.2, P = 0.001).10 Likewise, Baldursson et al conducted a double blinded randomized controlled trial, investigating the healing time of whole thickness biopsy wounds treated with FSG (Kerecis Omega3 Wound) compared with porcine small intestinal submucosa extracellular matrix. They collected 82 patients, showing a statistically significant quicker healing at 28 days in the group treated with FSG (P = 0.041). Moreover, no significant statistically adverse reaction was noted, and there was no evidence of seroconversions of autoantibodies11 Badois et al proposed the use of FSG for the thin-skin donor site wounds in patients who underwent free flap reconstructions for head and neck cancer. They divided 21 patients in two groups: group 1 treated with SOC (paraffin gauze) and group 2 treated with FSG. The statistical analysis showed a significant decrease (P < 0.05) in the number of local infections and pain when using the fish skin matrix compared with the standard wound care protocol, although with higher costs for the FSG.12 Dorweiler et al used the Kerecis Omega3 wound to treat 21 patients with complicated diabetes-related limb wounds showing how FSG stimulates the granulation tissue and reepithelialization as well as having antibacterial and analgesic effects.7 Ciprandi et al13 reported their experiences in pediatric population with various lesions (six trauma-related, two wounds developed because of autoimmune diseases, three cases of surgical dehiscence, and four sacral ulcer wounds) undergoing treatment with FSG for definitive wound closure. Some of the posttraumatic lesions were caused by animal bites, which are very common in pediatrics. In their article, they described rapid wound healing in all patients, with a wound area coverage of 100% and complete healing in 95% of wounds. They also showed the important role of negative pressure wound therapy, the application length of which was halved to an average of 12 days from their standard experience of 21 days. Therefore, they concluded that FSG represents an innovative and sustainable solution for pediatric wound care, resulting in shorter surgery time and reduced hospital stays, with accelerated wound healing times.13 Yang et al14 in their study included 18 patients with full-thickness ulcers that were either more than 20 cm2 or that had been present for at least 52 weeks. They used the FSG and analyzed the odor, exudate, condition of the surrounding skin, sign of erythema, and adverse events, and compared these with the use of common extracellular matrices. They concluded that the FSG seems to be a promising and effective wound closing adjunctive to extracellular matrices.14
Burns
According to the World Health Organization, burns are a global public health problem, accounting for approximately 180,000 deaths annually. The correct management of burns represents a true challenge for patients and health-care providers. In deep dermal and full-thickness burn injuries, early excision and application of split-thickness skin grafts (STSGs) is the established main treatment to achieve early wound closure and avoid common complications, such as infections, cosmetically unacceptable scar formation, and incomplete wound healing. However, when facing large burns, autologous skin availability becomes a problem, and surgeons often rely heavily on allogenic and xenogeneic skin for temporary coverage after excision, using tissues from human cadaver and pig skin. In the last years, FSG has gained popularity in this field because of its properties, such as antisepsis, pain suppression, and rapid wound healing. Alam et al,15 in their series of 10 patients, used FSG for full-thickness burn injury, defining it as excellent, robust, and pliable xenograft that was easy to apply. So, limited to a small patient’s size, they concluded that both the analgesic effect noted and the relatively short average times until full reepithelialization were promising. At a molecular level, FSG provides an extracellular matrix composed of glycosaminoglycans, proteoglycans, fibronectin, and growth factors, promoting the migration of autologous cells to enhance the proliferative and epithelialization phases of the burn healing process. Wallner et al, in their retrospective study, compared the use of Suprathel, FSG (Kerecis Omega3 Wound), and STSG to assess the optimal burn management. They collected data related to objective wound healing and subjective quality of life for 12 months after. They showed a statistically significant reduction in the healing time for FSG-treated burn wounds compared with Suprathel-treated and STSG-treated wounds (P < 0.05). The scar quality, determined by the skin elasticity, hydration, and sebum content, in FSG-treated wounds was significantly better than in the Suprathel group. Therefore, the authors concluded that a potential improvement in the quality of life of burn patients can be envisioned using the FSG.16 Luze et al17 in their systematic review analyzed 14 studies investigating the reepithelialization time, concluding that treatment with FSG, not only resulted in at least equal outcomes, but potentially accelerated wound healing, assuring a significant antalgic effect compared with conventional approaches. Finally, they demonstrated that FSG is cost-effective, considering the reduction of necessary dressing changes and, therefore, of the inpatient treatment days, which translates into a general reduction of treatment-related costs. So, they consider FSG as the gold standard for early wound closure in deep dermal and full-thickness burn injuries, but they limit its use for selected cases of STSG.17 Stone et al18 studied the role of FSG in thermal injuries compared with other tissues in six female Yorkshire pigs, demonstrating the statistically superior wound healing properties of acellular FSG over its commercial competitor (fetal bovine dermis) in a preclinical porcine deep partial-thickness burn wound model. Their findings support the use of FSG for achieving enhanced wound closure, as established by quicker integration and reepithelialization without increased contraction.
OTHER APPLICATIONS
Neonatal Calcinosis Cutis
A case report of a rare neonatal calcinosis cutis induced by distant and delayed extravasation of intravenous calcium gluconate was described by Ahn et al.19 In their clinical case, after a surgical debridement of the defect (4 × 3 cm), they placed the FSG for secondary intention closure of the wound, achieving a complete wound healing within 2 weeks. No depression, contracture, or calcification at the wound site were found. Considering these results, Ahn et al stated the important role of FSG in the fast healing and in obtaining a great aesthetic result. Also, Tan et al20 used an FSG in a case of a calciphylaxis wound, recognizing an important role of this new graft in the healing of the wound.
Hidradenitis Suppurativa
Baldursson reported a series of three patients with hidradenitis suppurativa. Because of unaesthetic scars, the patients underwent a debridement of scar tissue with subsequent application of FSG. They showed a significant reduction in time to epithelialization, as early as 5–7 days, and wound closure was achieved on average after 21 days, compared with their SOC for these types of lesions, in which wound closure would require at least 1 month or more.21
CASE REPORT
A 7-year-old boy came to our observation for an enterocutaneous fistula developed after multiple surgical treatments for Hirschsprung disease. We approached the enterocutaneous fistula through a median laparotomy. After complete isolation of the fistula, we performed a resection of the ileal segment from which the fistula originated. Finally, we performed a termino-terminal ileo-ileal anastomosis. On postoperative day (POD) 7, the patient developed an abscess of the surgical wound with a wound dehiscence of 4 × 4 cm involving the skin, subcutaneous tissues, and the rectus muscles. Only the peritoneum was intact. After surgery, the patient made good clinical progress, with immediate, return of bowel function and the ability to pass stool. No fever was recorded, and no other systemic symptoms were referred. He had resumed oral feeding on POD 5, with immediate opening of the bowels to stool.
We treated the patient with advanced medication therapy until POD 76, achieving very poor results. Due to the lack of improvement of the granulation tissue and a reduction of the dimensions of the dehiscence of only 0.5 × 0.5 cm, we decided to place a graft to improve the skin repair.
Among the available grafts, we chose to use the acellular FSG Kerecis Omega3 Wound because, according to recent literature, it seemed an optimal solution for faster healing of the wound and to be less invasive, allowing the child to resume daily activities as soon as possible.13,17,18
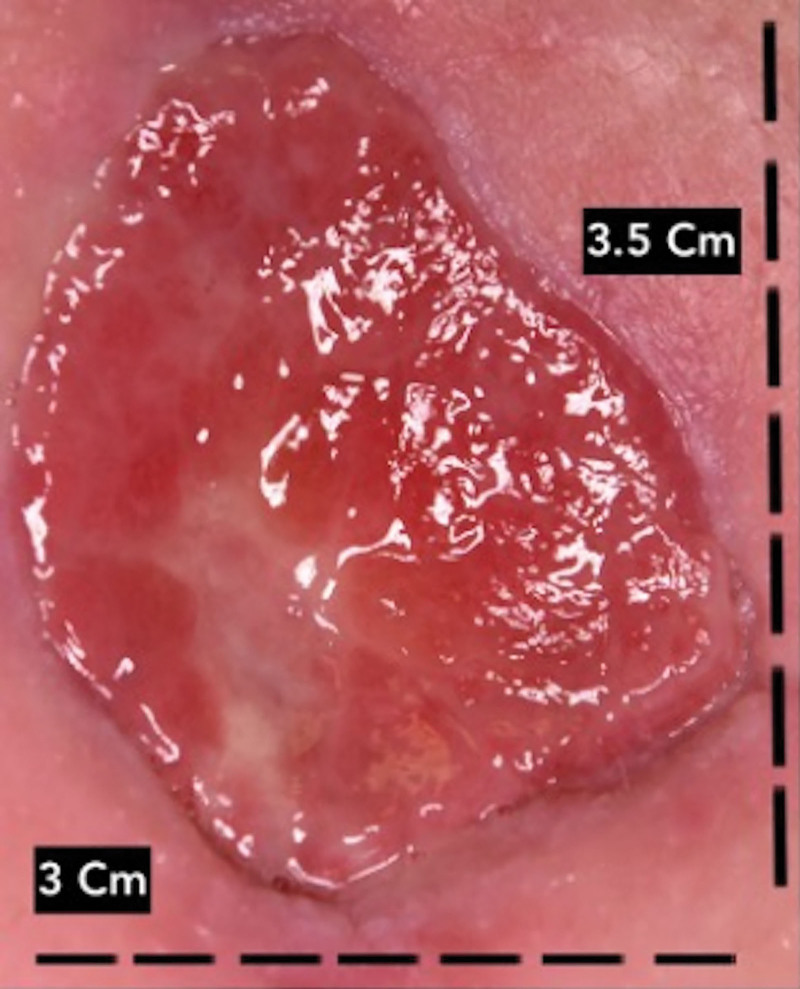
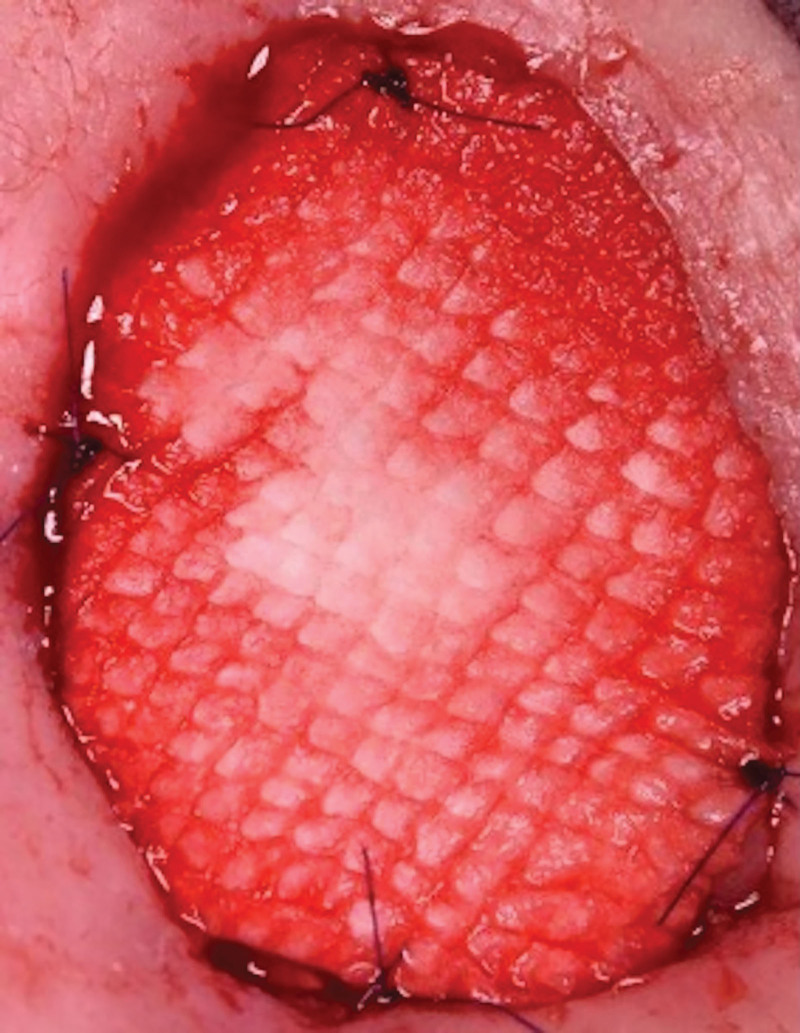
In the operating room, we prepared the wound bed, performing a debridement (Fig. 1) and causing bleeding of the wound bed. Then, the FSG was cut to the exact size of the wound and moisturized with room temperature saline for 1 minute. We applied the Kerecis Omega3 Wound on subcutaneous granulation tissue with four cardinal absorbable stitches as a fixation method (Fig. 2). At the end of procedure, we placed the VAC-device to obtain an optimal management of the exudate.
Fig. 1.
Wound debridement.
Fig. 2.
Application of FSG.
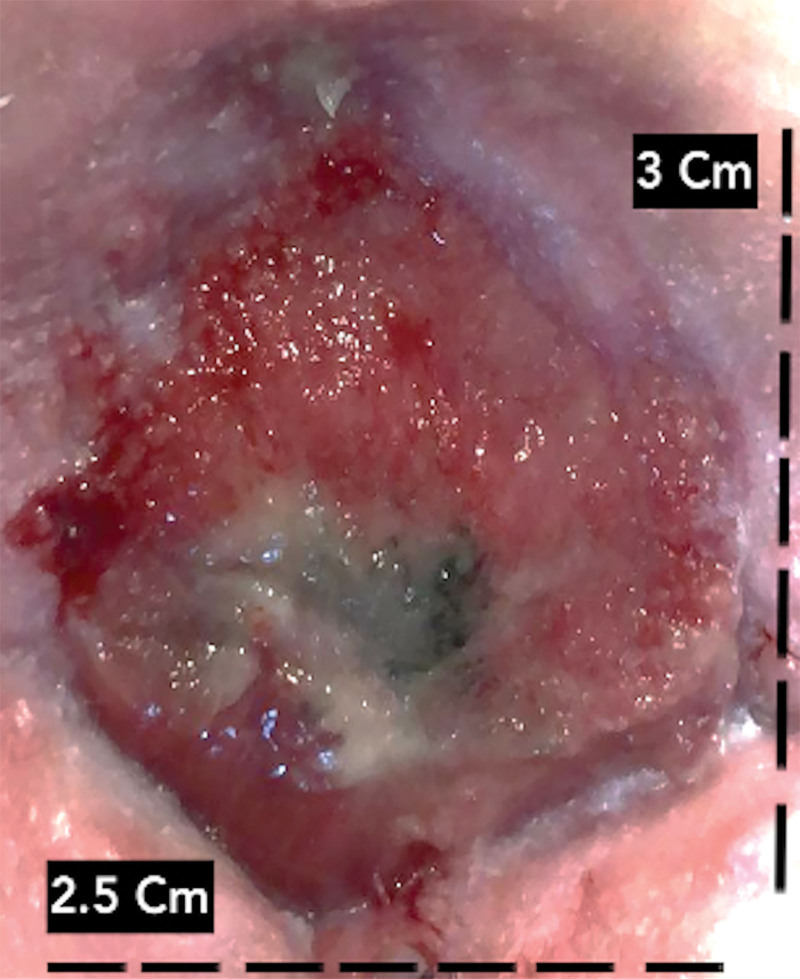
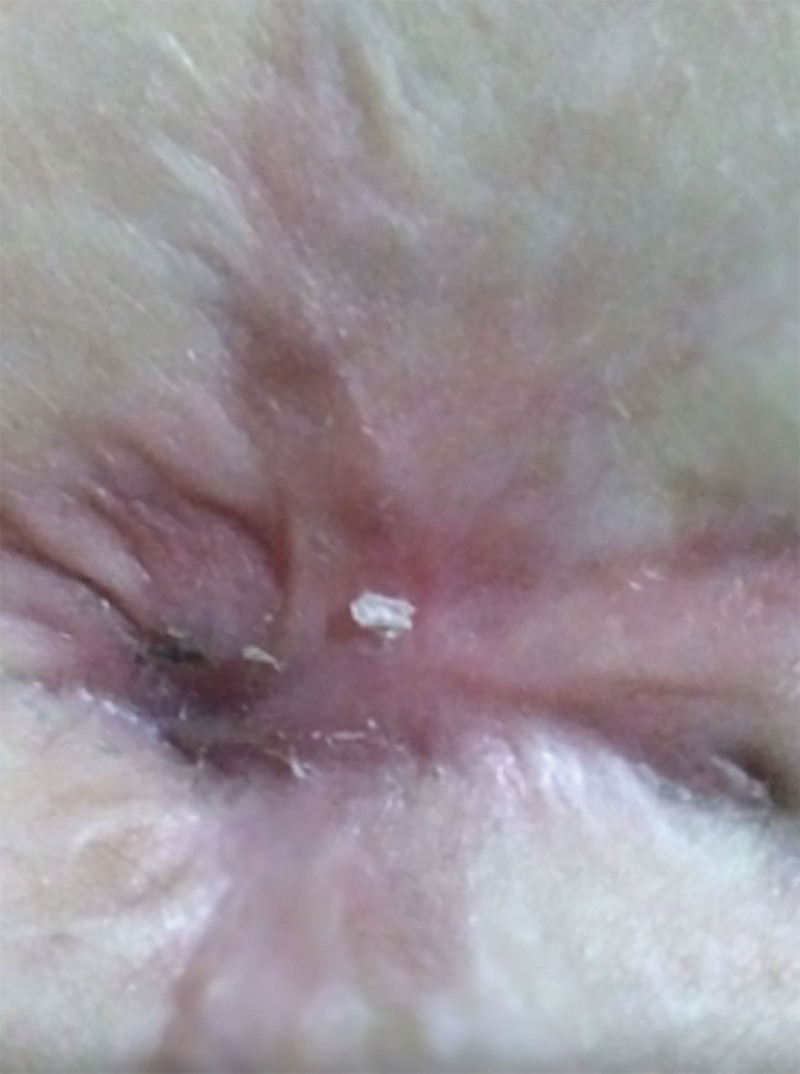
After 7 days, the patch was completely reabsorbed (Fig. 3), and the VAC-device was removed due to a considerable reduction of the exudate. We observed a reduction in the extension of the dehiscence of 0.5 × 0.5 cm. The wound was treated daily with saline and Biatain alginate until complete healing. After 30 days from the FSG placement, the wound dehiscence of 1.2 × 1.5 cm was substantially reduced. After 40 days, we witnessed complete healing (Fig. 4). The wound size was measured using Photoshop (Adobe Systems, Inc., San Jose, Calif.).
Fig. 3.
Resorption after 7 days and reduction in size.
Fig. 4.
Complete healing.
CONCLUSIONS
Kerecis omega3 Wound is an acellular FSG with promising results in tissue regeneration and in the management of chronic and acute wounds, pressure ulcers, vascular ulcers, diabetes-related ulcers, animal bites, and burns. FSG provides a valuable and sustainable treatment that improves wound healing in both adult and pediatric populations. According to our limited experience, the acellular FSG represents a valid alternative for promoting the healing of surgical wound dehiscence in the pediatric population. We could also speculate that FSG yields optimal results, faster than other advanced medications. To our knowledge, this is the first description of the application of an FSG for wound dehiscence of the abdomen in a pediatric patient, and this result is very encouraging.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 14 September 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Magnusson S, Baldursson BT, Kjartansson H, et al. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. Mil Med. 2017;182:383–388. [DOI] [PubMed] [Google Scholar]
- 2.Aristotelis K, Hulda SJ, Rósa SS, et al. Wound healing grafts: omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. Tissue Eng Regen Med. 2020;14:441–451. [DOI] [PubMed] [Google Scholar]
- 3.McDermott K, Fang M, Boulton AJM. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2023;46:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodrow T, Chant T, Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: a prospective evaluation. J Wound Care. 2019;28:76–80. [DOI] [PubMed] [Google Scholar]
- 5.Eric JL, Brock L, Patrick ME, et al. Evaluating the effect of omega-3-rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers: penultimate analysis of a multicenter, prospective, randomized controlled trial. Wounds. 2022;34:E34–E36. [PubMed] [Google Scholar]
- 6.Thomas Z, Marlise B. Faster than projected healing in chronic venous and diabetic foot ulcers when treated with intact fish skin grafts compared to expected healing times for standard of care: an outcome-based model from a Swiss hospital. Int J Low Extrem Wounds. 2022;15347346221096205. [DOI] [PubMed] [Google Scholar]
- 7.Dorweiler B, Trinh TT, Dünschede F, et al. The marine Omega3 wound matrix for treatment of complicated wounds: a multicenter experience report. Gefasschirurgie. 2018;23:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shannon M, Christopher W, Maliha K. Acellular fish skin graft use for diabetic lower extremity wound healing: a retrospective study of 58 ulcerations and a literature review. Wounds. 2019;31:262–268. [PubMed] [Google Scholar]
- 9.Dardari D, Lequint C, Jugnet AC, et al. Curing necrotic angiodermatitis with an intact fish skin graft in a patient living with diabetes. Medicina (Kaunas). 2022;58:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsner RS, Margolis DJ, Baldursson BT, et al. Fish skin grafts compared to human amnion/chorion membrane allografts: a double-blind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020;28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldursson BT, Kjartansson H, Konrádsdóttir F, et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds. 2015;14:37–43. [DOI] [PubMed] [Google Scholar]
- 12.Badois N, Bauër P, Cheron M, et al. Acellular fish skin matrix on thin-skin graft donor sites: a preliminary study. J Wound Care. 2019;28:624–628. [DOI] [PubMed] [Google Scholar]
- 13.Ciprandi G, Kjartansson H, Grussu F, et al. Use of acellular intact fish skin grafts in treating acute paediatric wounds during the COVID-19 pandemic: a case series. J Wound Care. 2022;31:824–831. [DOI] [PubMed] [Google Scholar]
- 14.Yang CK, Polanco TO, Lantis JC, II. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds. 2016;28:112–118. [PubMed] [Google Scholar]
- 15.Alam K, Jeffery SLA. Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: a case series. Mil Med. 2019;184:16–20. [DOI] [PubMed] [Google Scholar]
- 16.Wallner C, Holtermann J, Drysch M, et al. The use of intact fish skin as a novel treatment method for deep dermal burns following enzymatic debridement: a retrospective case-control study. Euro Burn J. 2022;3:43–55. [Google Scholar]
- 17.Luze H, Nischwitz SP, Smolle C, et al. The use of acellular fish skin grafts in burn wound management—a systematic review. Medicina (Kaunas). 2022;58:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone R, II, Saathoff EC, Larson DA, et al. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int J Mol Sci. 2021;22:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn KH, Park ES. A rare case report of neonatal calcinosis cutis induced by distant and delayed extravasation of intravenous calcium gluconate. Arch Plast Surg. 2021;48:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan SW, Wong J, Kee T, et al. Successful treatment of calciphylaxis in a renal transplant recipient with combination of intralesional sodium thiosulphate, intravenous sodium thiosulphate and fish skin graft. Australas J Dermatol. 2021;62:e358–e359. [DOI] [PubMed] [Google Scholar]
- 21.Baldursson B. The use of fragmented intact fish skin in patients with moderate to severe hidradenitis suppurativa for definitive lesion closure. Poster presented at: Symposium on Advanced Wound Care. Spring; 2022; April 6–10, 2002; Phoenix, Ariz. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.