Abstract
Despite improvements in HIV testing and earlier antiretroviral therapy (ART) initiation in children living with HIV through the years, a considerable proportion start treatment with advanced disease. We studied characteristics of children and adolescents living with HIV and their level of immunodeficiency at ART initiation using data from a multi-country Asian cohort. We included children and adolescents who were ART-naïve and <18 years of age at ART initiation from 2011 to 2020 at 17 HIV clinics in six countries. Incidence rates of opportunistic infections (OIs) in the first two years of triple-drug ART (≥3 antiretrovirals) was also reported. Competing risk regression analysis was performed to identify factors associated with first occurrence of OI. In 2,027 children and adolescents (54% males), median age at ART initiation increased from 4.5 years in 2011–2013 to 6.7 in 2017–2020, median CD4 count doubled from 237 cells/μl to 466 cells/μl, and proportion of children who initiated ART as severely immunodeficient decreased from 70% to 45%. During follow-up, 275 (14%) children who received triple-drug ART as first treatment and had at least one clinic visit, developed at least one OI in the first two years of treatment (9.40 per 100 person-years). The incidence rate of any first OI declined from 12.52 to 7.58 per 100 person-years during 2011–2013 and 2017–2020. Lower hazard of OIs were found in those with age at first ART 2–14 years, current CD4 ≥200 cells/μl, and receiving ART between 2017 and 2020. The analysis demonstrated increasing number of children and adolescents starting ART with high CD4 count at ART start. The rate of first OI markedly decreased in children who started ART in more recent years. There remains a clear need for improvement in HIV control strategies in children, by promoting earlier diagnosis and timely treatment.
Introduction
In 2022, an estimated 930,000 children aged less than 10 years and 1.65 million adolescents aged 10–19 years were living with HIV worldwide [1]. In that year, there were an estimated 270,000 children and adolescents with newly acquired HIV. Although countries have been placing more people on HIV treatment over the past decade, globally, only 57% of children aged 0–14 years and 65% of adolescents aged 10–19 years were receiving treatment in 2022 compared with 77% of adults [1–3]. Starting on antiretroviral therapy (ART) early is key to optimizing the health of people living with HIV (PLHIV) to achieve viral suppression and improved health outcomes, as opposed to the consequences of late presentation to care and delayed treatment [4, 5].
Since 2004, the World Health Organization (WHO) has regularly issued evidence-based guidelines for HIV treatment informed by expert opinion and community values and preferences. In 2010, WHO recommended ART initiation for all HIV-positive infants <24 months of age, regardless of CD4 level [6]. This was extended to all children <5 years of age in 2013 [7]. In 2016, WHO recommended starting all PLHIV on ART, regardless of their clinical or immune status [5]. Current international and local HIV management guidelines encourage immediate initiation of ART after HIV diagnosis, except for patients with specific opportunistic infections (OIs) for which immediate ART is contraindicated [8].
While there have been clear improvements in decreasing age, increasing CD4 count, and reduced disease severity at ART initiation in children and adolescents living with HIV [9–13], a substantial proportion still present with advanced symptomatic disease. In a global analysis conducted in 32 countries between 2004 to 2013, more than 40% of children in low- and middle-income countries and less than 20% in high income countries had severe immunodeficiency at ART initiation by 2013 [14]. In the Asia Pacific, most countries have expanded ART programs in line with WHO treatment recommendations. UNAIDS estimated that treatment coverage of children and younger adolescents (0–14 years) living with HIV in the region was 76% in 2021, higher than the 66% among adults [15]. However, progress has been uneven across countries. For example, pediatric HIV treatment coverage reported in 2021 was higher than 95% in Malaysia, but only 56% in Cambodia and 25% in Indonesia. Other countries in the region also reported relatively higher coverage at 75% in Thailand, 82% in Vietnam, and 95% in India [16]. Moreover, there have been concerning declines in maternal ART coverage in the region following the COVID-19 pandemic, falling from 58% in 2019 to 49% in 2021, which poorly compares to the 81% global average [1, 2]. With only half of pregnant women with HIV receiving ART for their health and to prevent vertical transmission, efforts to achieve elimination of pediatric HIV will stagnate or fail.
We used data from a regional longitudinal pediatric HIV cohort to describe characteristics of children and adolescents and their degree of immunodeficiency at ART initiation from 2011 to 2020, and assess whether improvements in ART coverage over time have resulted in decline in the incidence of OIs. This information is needed to further improve care and program strategies and, in turn, pediatric HIV treatment outcomes in the region.
Materials and methods
Study population
The TREAT Asia pediatric HIV Observational Database (TApHOD) is an observational clinical cohort of the International epidemiology Databases to Evaluate AIDS (IeDEA), and its methodology has been previously reported [17]. Briefly, TApHOD enrolls children and adolescents seen at 17 clinics in Cambodia (n = 1), India (n = 2), Indonesia (n = 2), Malaysia (n = 4), Thailand (n = 5) and Vietnam (n = 3). These sites are pediatric referral clinics within larger healthcare facilities (n = 13) or freestanding pediatric hospitals (n = 4). Demographic and clinical data are collected from medical records at each site, de-identified, and sent to the Kirby Institute, University of New South Wales, for aggregation, quality assessment and processing. Only site principal investigators (site physicians) could identify their patients during or after data collection. The database includes information on over 7400 children and adolescents who received care at the participating sites from 2001 to 2020. In this analysis, we included all children and adolescents (except for exposure to perinatal prophylaxis), who were ART-naïve and <18 years of age at ART initiation between 1 January 2011 and 31 December 2020. We used all available follow-up data up to December 2020.
Approval for the study was granted by the human research ethics committees at all participating sites, the data management and analysis center at the Kirby Institute (UNSW Sydney), and the coordinating center at TREAT Asia/amfAR (The Foundation for AIDS Research). Consent by parents or legal guardians and assent of the children and adolescents under care were not routinely obtained, unless required by the local ethics committee (i.e., in some sites in India, Malaysia, and Thailand).
Outcomes and key variable definitions
The primary outcomes analyzed were trends in patient characteristics at ART initiation (age, CD4 count, WHO clinical stage, and weight-for-age z score [WAZ]). The secondary outcome was incidence of first OI event within the first two years of ART. OI events were defined on the basis of diagnoses recorded in the medical records at each site. Adolescence were defined as 10 to 19 years of age, and children defined as <10 years. Children and adolescents who were enrolled younger than 15 years and have no other documented mode of infection reported were defined as perinatally acquired HIV. For laboratory and clinical measurements at treatment initiation, we used the closest values reported during a testing window of six months before to one week after ART start for CD4 and HIV viral load, and a window of six months before to four weeks after for WAZ, with priority given to the pre-ART measurement. For WHO clinical stage, we used the highest stage reported from six months before through the date of ART initiation. We defined severe HIV-associated immunodeficiency according to WHO criteria as the CD4 levels less than 25% (age <1 year), less than 20% (age 1 to <3 years), less than 15% (age 3 to 5 years), and less than 15% or less than 200 cells per mm3 (age ≥5 years) [18]. We converted weight measurements to WAZ using 2006 WHO standards [19] for children ≤10 years or Centers for Disease Control and Prevention standards [20] for children >10 years. We divided the year of ART start into three time periods (2011 to 2013, 2014 to 2016, and 2017 to 2020) to correspond to changes in WHO treatment guidelines.
Statistical analysis
We used descriptive statistics (number and percentage, median and interquartile range [IQR]) to summarize participants’ characteristics at ART initiation and by year of ART initiation (i.e., 2011–2013, 2014–2016, 2017–2020). In a subset of patients who had received at least three antiretrovirals as their initial treatment regimen and who had at least one follow-up visit after starting ART, we calculated the incidence rates of OI per 100 person-years (with 95% confidence intervals [CIs]) for the first occurrence of any OI, and for the first occurrence of each individual OI in the first two years of ART. The incidence rate was obtained by dividing the number of first OIs by person-years of observation and reported separately among patients who started ART in each time period. Follow-up was from the start of ART and ceased at the earliest of the following: the date of the OI being analyzed (i.e., first occurrence of any OI or the first occurrence of a specific OI), date of death, date of most recent visit, day prior to the patient’s 20th birthday, and at two years. To assess factors associated with the first occurrence of any OI, we used Fine and Gray’s subdistribution hazard model [21] to account for the competing effect of death. This model evaluates the effect of an exposure on the cause-specific hazard of the outcome of interest, while accounting for the presence of competing events. The following variables were included in the univariate analysis: sex, age, WAZ, severe immunodeficiency, and time period at first triple-drug ART, facility level, and country income group. We included CD4 count as a time-updated variable and if a CD4 count was missing, the previous CD4 count was carried forward. We did not use multiple imputation to replace missing data because there were relatively small numbers of covariates available. We also considered clinic as a random effect in the model. The initial multivariate model included all covariates with a P value of <0.20 on univariate analysis. We then used a backward stepwise approach and retained covariates with a P value of <0.05 in the final model. Crude and adjusted subdistribution hazard ratios (asHR) with 95% CI were reported as the measures of association. Missing data were included in the regression analyses as a separate category within each variable. The proportional hazards assumption was assessed using the estat phtest command in Stata. Data were analyzed using Stata version 12 (Stata Corporation, College Station, Texas, USA) and SAS Software (Version 9.4 for Windows).
Results
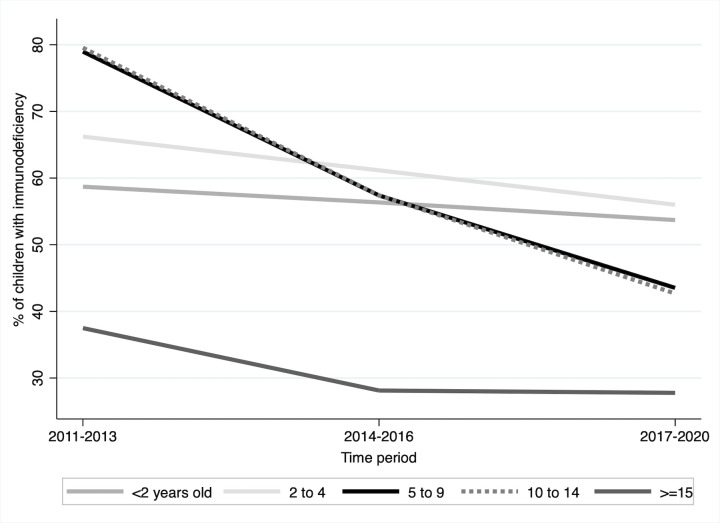
A total of 2027 (54% males) children and adolescents were eligible for inclusion in the analysis. Patient characteristics at first ART are summarized by period of first ART in Table 1. Median age at ART initiation increased from 4.5 years (IQR 1.7–7.9) in 2011–2013 to 6.7 (IQR 2.1–11.3) in 2017–2020, mainly driven by the increasing proportion of adolescents which was doubled over the same period. Overall, 56% of younger adolescents (10–14 years) and 60% of older adolescents (15–19 years) were male, with male accounting for 76% of older adolescents in 2017–2020. The percentage of children and adolescents with an available CD4 count at ART initiation declined over time from 87% in 2011–2013 to 64% in recent years. From 2011 to 2020, there was a doubling in median CD4 count at ART initiation overall from 237 cells/μl in 2011–2013 to 466 cells/μl in 2017–2020, and proportion of patients initiating ART with CD4 ≥ 500 cells/μl rose from 34% to 46%. Overall, there was a decrease in the proportion of patients who initiated treatment with severe immunodeficiency from 70% for those who initiated in 2011–2013 to 45% for those who initiated in 2017–2020. Among different age groups, the highest decrease in the proportion with severe immunodeficiency was observed in the age groups 5–9 years (79% to 43%) and 10–14 years (79% to 42%) and the lowest decrease was observed in the age group <2 years (59% to 52%) (Fig 1). Overall, half of the children and adolescents had advanced HIV disease, and the proportion of those who were severely underweight at ART start has remained largely unchanged (31%) over this period.
Table 1. Characteristics at ART start by period of ART initiation.
| Total | 2011–13 | 2014–16 | 2017–20 | |
|---|---|---|---|---|
| Patients (n) | 2027 | 747 | 739 | 541 |
| World Bank Country income group | ||||
| Upper-middle income | 302 (15) | 114 (15) | 103 (14) | 85 (16) |
| Lower-middle income | 1725 (85) | 633 (85) | 636 (86) | 456 (84) |
| Sex | ||||
| Male | 1100 (54) | 401 (54) | 407 (55) | 292 (54) |
| Female | 927 (46) | 346 (46) | 332 (45) | 249 (46) |
| Age, years | ||||
| Median (IQR) | 5.2 (1.8–9.9) | 4.5 (1.7–7.9) | 5.9 (1.9–10.1) | 6.7 (2.1–11.3) |
| <2 | 531 (26) | 211 (28) | 191 (26) | 129 (24) |
| 2 to 4 | 447 (22) | 201(27) | 147 (20) | 99 (18) |
| 5 to 9 | 569 (28) | 219 (29) | 212 (29) | 138 (26) |
| 10 to 14 | 372 (18) | 98 (13) | 145 (20) | 129 (24) |
| ≥15 | 108 (5.3) | 18 (2.4) | 44 (6.0) | 46 (8.5) |
| CD4 count, cells/μl | ||||
| Available data | 1597 (79) | 652 (87) | 598 (81) | 347 (64) |
| Median (IQR) | 338 (71–793) | 237 (39–752) | 355 (117–838) | 466 (141–809) |
| <200 | 616 (39) | 313 (48) | 199 (33) | 104 (30) |
| 200–349 | 194 (12) | 63 (9.7) | 98 (16) | 33 (9.5) |
| 350–499 | 162 (10) | 56 (8.6) | 57 (9.5) | 49 (14) |
| ≥500 | 625 (39) | 220 (34) | 244 (41) | 161 (46) |
| Median CD4 count by age (IQR) | ||||
| <2 years | 1108 (533–1848) | 1099 (495–1697) | 1140 (604–1839) | 1108 (458–2232) |
| Available data | 379 (71) | 170 (81) | 144 (75) | 65 (50) |
| 2 to 4 | 454 (114–843) | 379 (54–687) | 608 (193–906) | 527 (186–1086) |
| Available data | 373 (83) | 179 (89) | 124 (84) | 70 (71) |
| 5 to 9 | 142 (26–411) | 61 (22–243) | 226 (49–452) | 398 (35–398) |
| Available data | 463 (81) | 195 (89) | 180 (85) | 88 (64) |
| 10 to 14 | 180 (40–373) | 103 (16–211) | 204 (49–353) | 355 (97–578) |
| Available data | 290 (78) | 91 (93) | 114 (79) | 85 (66) |
| ≥15 | 351 (197–501) | 239 (163–527) | 293 (134–406) | 391 (229–519) |
| Available data | 92 (85) | 17 (94) | 36 (82) | 39 (85) |
| Severe HIV-associated immunodeficiency | ||||
| Available data | 1493 (74) | 611 (82) | 561 (76) | 321 (59) |
| No | 611 (41) | 186 (30) | 247 (44) | 178 (55) |
| Yes | 882 (59) | 425 (70) | 314 (56) | 143 (45) |
| HIV viral load, copies/ml | ||||
| Available data | 332 (16) | 131 (18) | 91 (12) | 110 (20) |
| Median log10 (IQR) | 5.4 (4.8–6.0) | 5.5 (5.1–6.1) | 5.4 (4.7–6.1) | 5.1 (4.6–5.9) |
| 400–999 | 5 (1.5) | 1 (0.8) | 0 | 4 (3.6) |
| 1,000–9,999 | 26 (7.8) | 6 (4.6) | 9 (9.9) | 11 (10) |
| ≥10,000 | 301 (91) | 124 (95) | 82 (90) | 95 (86) |
| Weight-for-age z-score | ||||
| Available data | 1783 (88) | 684 (92) | 623 (84) | 476 (88) |
| Median | -2.2 (-3.4 to -1.1) | -2.3 (-3.4 to -1.2) | -2.3 (-3.4 to -1.0) | -2.1 (-3.2 to -1.1) |
| <-3 | 585 (33) | 232 (34) | 207 (33) | 146 (31) |
| -3≤ to <-2 | 396 (22) | 160 (23) | 139 (22) | 97 (20) |
| -2≤ to <-1 | 398 (22) | 143 (21) | 126 (20) | 129 (27) |
| ≥-1 | 404 (23) | 149 (22) | 151 (24) | 104 (22) |
| WHO clinical stage | ||||
| Available data | 1230 (61) | 588 (79) | 387 (52) | 255 (47) |
| Stage I/II | 544 (44) | 251 (43) | 168 (43) | 125 (49) |
| Stage III | 498 (40) | 255 (43) | 153 (40) | 90 (35) |
| Stage IV | 188 (15) | 82 (14) | 66 (17) | 40 (16) |
| Initial ART regimen | ||||
| NNRTI-based | 1587 (78) | 648 (87) | 587 (79) | 352 (65) |
| PI-based | 362 (18) | 70 (9.4) | 133 (18) | 159 (29) |
| INSTI-based | 33 (1.6) | 2 (0.3) | 5 (0.7) | 26 (4.8) |
| NRTI | 37 (1.8) | 26 (3.5) | 11 (1.5) | 0 |
| Mono/dual | 8 (0.4) | 1 (0.1) | 3 (0.4) | 4 (0.7) |
Data are n (%), median (IQR, interquartile range). For weight-for-age z-score, the 2006 WHO standards was used for children ≤10 years and the Centers for Disease Control and Prevention standards for children >10 years. ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitor; mono/dual, single or two drugs.
Fig 1. Proportion of patients with severe immunodeficiency at ART start, by age group and calendar time period at ART start (n = 2027).
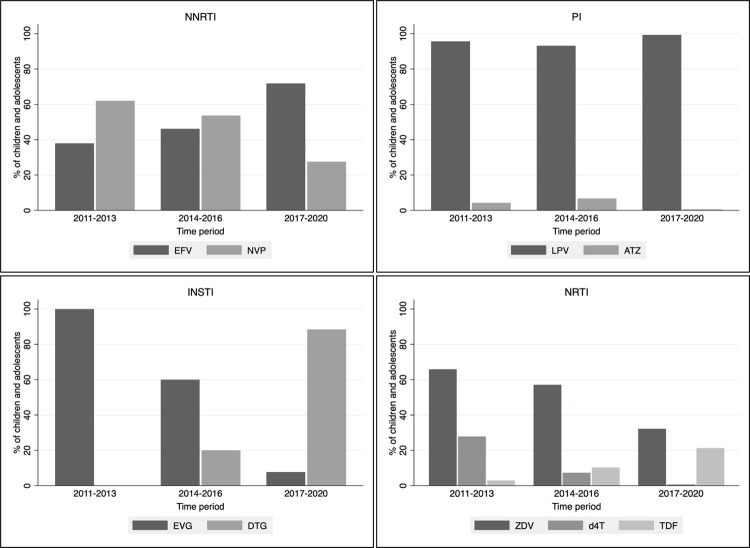
Between 2011 and 2020, most children and adolescents started a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen, but the use of NNRTIs decreased as protease inhibitor (PI)-based regimens increased after 2015 (Table 1). Efavirenz replaced nevirapine as the most commonly used NNRTI (from 38% in 2011–2013 to 72% in 2017–2020). The NRTIs zidovudine and stavudine were replaced by abacavir- and tenofovir-containing first-line regimen (Fig 2). There was negligible (<1%) use of a first-line integrase strand transfer inhibitor (INSTI)-based regimen prior to 2017, but 4.8% of patients received a INSTI-based initial regimen from 2017 to 2020. For the majority (92%) of patients, first INSTI-based contained Elvitegravir.
Fig 2. Proportion of children and adolescents on ART who used specific drug or drug combination by calendar period of initiation and drug class (n = 2019).
NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; INSTI = integrase strand transfer inhibitor; NRTI = nucleoside reverse transcriptase inhibitor; EFV = efavirenz; NVP = nevirapine; LPV = lopinavir/ritonavir; ATZ = atazanavir/ritonavir; EVG = elvitegravir; DTG = dolutegravir; ZDV = zidovudine; d4T = stavudine; TDF = tenofovir.
Incidence of OIs
Of 2027 children and adolescents, 1963 who had received triple-drug ART as their initial treatment and who had at least one clinic visit after starting ART were included in the OI analysis. Of the 64 excluded, two were on mono or dual therapy only, six were exposed to mono or dual therapy before starting triple-drug ART, and 56 did not have a clinic visit after starting ART (of which eight died, 18 transferred, 14 were lost to follow-up or LTFU, and 13 were not recorded as died, transferred, or LTFU). During 2011–2020, the total period of observation contributing to the first two years of ART was 3298 person-years with a median follow-up period of 2.00 years (IQR 1.68–2.00). Of 1963 children and adolescents, 275 experienced at least one OI resulting in a crude incidence rate of any first OI of 9.40 (95% CI 8.35–10.58) per 100 person-years (Table 2). When observation was restricted to the first year of ART only, the crude incidence rate of first OI was 13.95 (95% CI 12.25–15.90) per 100 person-years. The incidence rate of any first OI per 100 person-years was 12.52 in 2011–2013, 7.48 in 2014–2016, and 7.58 in 2017–2020. Recurrent upper respiratory tract infection and pulmonary tuberculosis (TB) remained the leading OIs, irrespective of the calendar period of starting ART, with an overall incidence of 4.26 (95% CI 3.59–5.05) and 1.39 (95% CI 1.04–1.86) per 100 person-years, respectively. These two infections and other common OIs, including severe recurrent bacterial pneumonia and lymphoid interstitial pneumonitis appeared much less common in children and adolescents who first received ART after 2014.
Table 2. Incidence rate per 100 person-years of the first occurrence of OIs and death in the first two years of ART, overall and by calendar time period of ART start.
| OIs | All | 2011–2013 | 2014–2016 | 2017–2020 |
|---|---|---|---|---|
| Number of patients | 1963 | 740 | 707 | 516 |
| All OIs | 402 | 223 | 113 | 66 |
| Person-years | 3298 | 1310 | 1252 | 736 |
| Incidence (95% CI) | 12.19 (11.05–13.44) | 17.02 (14.93–19.41) | 9.02 (7.50–10.85) | 8.97 (7.05–11.42) |
| Any first OI | 275 | 138 | 85 | 52 |
| Person-years | 2925 | 1101 | 1137 | 686 |
| Incidence (95% CI) | 9.40 (8.35–10.58) | 12.52 (10.60–14.80) | 7.48 (6.05–9.25) | 7.58 (5.77–9.94) |
| Deaths | 69 | 42 | 22 | 5 |
| Person-years | 3826 | 1415 | 1385 | 1027 |
| Incidence (95% CI) | 1.80 (1.42–2.28) | 2.97 (2.19–4.02) | 1.59 (1.05–2.41) | 0.49 (0.20–1.17) |
| First specific OI, WHO clinical stage 2 | ||||
| Recurrent upper respiratory tract infection | 133 | 79 | 39 | 15 |
| Person-years | 3124 | 1197 | 1205 | 722 |
| Incidence (95% CI) | 4.26 (3.59–5.05) | 6.60 (5.29–8.23) | 3.24 (2.36–4.43) | 2.08 (1.25–3.45) |
| Extensive wart virus infection, | 11 | 8 | 2 | 1 |
| Person-years | 3282 | 1299 | 1249 | 735 |
| Incidence (95% CI) | 0.34 (0.19–0.61) | 0.62 (0.31–1.23) | 0.16 (0.04–0.64) | 0.14 (0.02–0.97) |
| First specific OI, WHO clinical stage 3 | ||||
| Pulmonary tuberculosis | 45 | 22 | 16 | 7 |
| Person-years | 3236 | 1273 | 1235 | 729 |
| Incidence (95% CI) | 1.39 (1.04–1.86) | 1.73 (1.14–2.63) | 1.30 (0.79–2.12) | 0.96 (0.46–2.01) |
| Severe recurrent bacterial pneumonia | 28 | 19 | 6 | 3 |
| Person-years | 3256 | 1279 | 1243 | 735 |
| Incidence (95% CI) | 0.86 (0.59–1.25) | 1.49 (0.95–2.33) | 0.48 (0.22–1.07) | 0.41 (0.13–1.27) |
| Lymph node tuberculosis | 20 | 13 | 4 | 3 |
| Person-years | 3269 | 1290 | 1246 | 733 |
| Incidence (95% CI) | 0.61 (0.39–0.95) | 1.01 (0.58–1.73) | 0.32 (0.12–0.86) | 0.41 (0.13–1.27) |
| Lymphoid interstitial pneumonitis | 24 | 12 | 10 | 2 |
| Person-years | 3265 | 1294 | 1239 | 732 |
| Incidence (95% CI) | 0.73 (0.49–1.10) | 0.93 (0.53–1.63) | 0.81 (0.43–1.50) | 0.27 (0.07–1.09) |
| Unexplained persistent diarrhoea | 11 | 4 | 2 | 5 |
| Person-years | 3285 | 1304 | 1249 | 732 |
| Incidence (95% CI) | 0.33 (0.19–0.60) | 0.31 (0.12–0.82) | 0.16 (0.04–0.64) | 0.68 (0.28–1.64) |
| Persistent oral candidiasis | 10 | 3 | 5 | 2 |
| Person-years | 3279 | 1304 | 1243 | 732 |
| Incidence (95% CI) | 0.30 (0.16–0.57) | 0.23 (0.07–0.71) | 0. 40 (0.17–0.97) | 0.27 (0.07–1.09) |
| First specific OI, WHO clinical stage 4 | ||||
| Pneumocystis pneumonia | 15 | 3 | 6 | 6 |
| Person-years | 3278 | 1304 | 1242 | 731 |
| Incidence (95% CI) | 0.46 (0.28–0.76) | 0.23 (0.07–0.71) | 0.48 (0.22–1.08) | 0.82 (0.37–1.83) |
| Extrapulmonary or disseminated tuberculosis | 14 | 5 | 3 | 6 |
| Person-years | 3282 | 1304 | 1247 | 731 |
| Incidence (95% CI) | 0.43 (0.25–0.72) | 0.46 (0.21–1.02) | 0.24 (0.08–0.75) | 0.68 (0.28–1.64) |
| Cytomegalovirus retinitis or cytomegalovirus infection | 6 | 1 | 2 | 3 |
| Person-years | 3289 | 1308 | 1248 | 733 |
| Incidence (95% CI) | 0.18 (0.08–0.41) | 0.08 (0.01–0.54) | 0.16 (0.04–0.64) | 0.41 (0.13–1.27) |
| Oesophageal candidiasis | 2 | 2 | 0 | 0 |
| Person-years | 3294 | 1306 | 1252 | 736 |
| Incidence (95% CI) | 0.06 (0.02–0.24) | 0.15 (0.04–0.61) | 0 | 0 |
| Disseminated mycobacteriosis | 3 | 0 | 1 | 2 |
| Person-years | 3293 | 1310 | 1250 | 733 |
| Incidence (95% CI) | 0.09 (0.03–0.28) | 0 | 0.08 (0.01–0.57) | 0.27 (0.07–1.09) |
| Disseminated mycosis | 2 | 0 | 0 | 2 |
| Person-years | 3295 | 1310 | 1252 | 733 |
| Incidence (95% CI) | 0.07 (0.02–0.24) | 0 | 0 | 0.27 (0.07–1.09) |
95% CI, 95% confidence interval.
Risk factors for developing any first OIs in the first two years of ART start are shown in Table 3. In the final fitted multivariate model, lower hazard rates of OI were associated with age at first ART 2–14 years compared to <2 years (lowest asHR for 10–14 years 0.48 95% CI 0.28–0.82), current CD4 ≥200 cells/μl compared to <200 cells/μl (lowest asHR for 350–499 cells/μl 0.19 95% CI 0.10–0.37 and ≥500 cells/μl 0.19 95% CI 0.12–0.31), and receiving ART between 2017 and 2020 compared with between 2011 and 2013 (asHR 0.46 95% CI 0.22–0.97). Receiving care in regional, provincial, or university hospitals was found to be associated with an increased risk of developing first OI (vs. health centers; asHR 3.53 95% CI 1.37–9.07).
Table 3. Characteristics related to development of first OI within the first two years of ART start (where mortality is a competing event).
| Characteristics | Total (n = 1963) | Follow-up time (person-years) | First OI (n = 275) | Univariate analysis | Multivariate analysisc | ||
|---|---|---|---|---|---|---|---|
| SHR (95% CI) | p-value | aSHR (95% CI) | p-value | ||||
| Sex | 0.415 | ||||||
| Male | 1059 | 1606 | 144 | 1 | |||
| Female | 904 | 1319 | 131 | 1.08 (0.90–1.31) | |||
| Age at first ART (years) | 0.007 | 0.001 | |||||
| <2 | 515 | 741 | 96 | 1 | 1 | ||
| 2–4 | 437 | 676 | 60 | 0.71 (0.49–1.02) | 0.59 (0.38–0.90) | ||
| 5–9 | 559 | 847 | 75 | 0.70 (0.52–0.94) | 0.50 (0.34–0.72) | ||
| 10–14 | 353 | 502 | 40 | 0.62 (0.31–1.24) | 0.48 (0.28–0.82) | ||
| ≥15 | 99 | 159 | 4 | 0.20 (0.04–1.05) | 0.27 (0.05–1.35) | ||
| Current CD4 count (cells/ul) a | <0.001 | <0.001 | |||||
| <200 | 258 | 89 | 1 | 1 | |||
| 200–349 | 219 | 16 | 0.28 (0.19–0.42) | 0.25 (0.18–0.36) | |||
| 350–499 | 304 | 14 | 0.21 (0.11–0.38) | 0.19 (0.10–0.37) | |||
| ≥500 | 2009 | 118 | 0.29 (0.18–0.48) | 0.19 (0.12–0.31) | |||
| Missing data | 135 | 38 | 0.99 (0.52–1.88) | 0.93 (0.53–1.63) | |||
| Weight-for-age z-score at first ART | 0.014 | 0.422 | |||||
| <-3 | 558 | 737 | 95 | 1 | |||
| -3 ≤ to <-2 | 391 | 598 | 57 | 0.81 (0.64–1.02) | 1.00 (0.80–1.23) | ||
| ≥-2 | 786 | 1249 | 99 | 0.68 (0.48–0.96) | 0.85 (0.64–1.13) | ||
| Missing data | 228 | 340 | 24 | 0.59 (0.24–1.47) | 0.82 (0.34–1.96) | ||
| Severe HIV-associated immunodeficiency | <0.001 | 0.463 | |||||
| No | 591 | 975 | 48 | 1 | |||
| Yes | 866 | 1275 | 149 | 2.23 (1.67–2.98) | 1.17 (0.80–1.71) | ||
| Missing data | 506 | 674 | 78 | 2.13 (1.46–3.11) | 1.02 (0.61–1.71) | ||
| Year of ART start b | 0.299 | 0.124 | |||||
| 2011–2013 | 740 | 1102 | 138 | 1 | 1 | ||
| 2014–2016 | 707 | 1137 | 85 | 0.64 (0.35–1.15) | 0.66 (0.40–1.06) | ||
| 2017–2020 | 516 | 686 | 52 | 0.58 (0.25–1.33) | 0.46 (0.22–0.97) | ||
| Facility level | 0.026 | 0.009 | |||||
| Healthcare center | 781 | 1129 | 48 | 1 | 1 | ||
| Regional, provincial, or university hospital | 1182 | 1796 | 227 | 3.09 (1.14–8.39) | 3.53 (1.37–9.07) | ||
| Country income group | 0.606 | ||||||
| Lower-middle income | 1671 | 2456 | 244 | 1 | |||
| Upper-middle income | 292 | 469 | 31 | 0.70 (0.18–2.74) | |||
OI, opportunistic infection. Death (n = 45) was a competing event for first OI in this analysis. Total numbers include missing values. Missing values were included as a separate category in all analyses. Global p values have excluded missing categories. 95% CI, 95% confidence interval; asHR, adjusted subdistribution hazard ratio.
a CD4 count was considered time-dependent variable. Total number was not given as children and adolescents moved between categories.
b Year of ART start was our variable of interest and was included in the final model, even if non-significant in the univariate analysis.
c Adjusted for current CD4 count, age at ART start, year of ART start, and facility level (competing risks regression analysis with death as competing event); Weight-for-age z-score at first ART and severe HIV-associated immunodeficiency were not remained in the multivariate model. Their aSHR (shown in italics) were obtained by adding and removing them individually to the final model.
Discussion
In this study involving children and adolescents who started ART in the Asia region, we describe demographic and clinical characteristics at ART start across time periods. Between 2011 and 2020, the absolute numbers of children starting treatment decreased in the cohort, with improvements in the median CD4 counts at ART start and decline in the overall proportions with severe immunodeficiency. By 2017 to 2020, an increasing proportion of patients with CD4 cell counts >500 cells/μl were receiving early treatment. Furthermore, children and adolescents initiating ART after 2013 had almost half the risk of experiencing an OI in the first two years of treatment compared to those initiating ART in 2011–2013, and mortality decreased substantially.
The decline in the number and proportion of children <5 years at ART start may reflect local progress in implementing effective vertical prevention strategies in some of the countries represented in the cohort. In Asia, Thailand and Malaysia met WHO criteria for elimination of mother-to-child transmission (MTCT) of HIV in 2016 and 2018, respectively, with transmission rates of <2% [22, 23]. Reduction of infant transmission to less than 5% has also been reported in recent years in Cambodia [24] and Vietnam [25]. Furthermore, our study clinics are mainly located within tertiary facilities and the decentralization of HIV care to local primary care clinic may have also contributed to the overall reduction in the absolute number of children initiating ART [26]. In our cohort, there were more males in the older adolescent group, which was consistent with the recent report of UNAIDS in East Asia and Pacific where male adolescents were two times more likely than females to contract HIV. This pattern is different with sub-Saharan Africa, where adolescent females were three times more likely to contract HIV than males [27]. In general, evidence suggests that male adolescents are more likely to engage in risky behavior than their female counterparts, and in many countries in the Asia Pacific region, gender norms encourage early onset of sexual behaviour and high number of sexual partners. Together with inconsistent condom use, this could explain the high risk of HIV in male adolescents in the region [28]. Along with the two-fold increase in the proportion of younger adolescents and the three-fold increase in the proportion of older adolescents over the study period, their median CD4 count at ART start also increased from 2011–2013 and reached up to over 350 cells/μl in 2017–2020. This finding is consistent with that of Apondi et al who has shown similar increase in the proportion of older adolescents at ART start in their study in East Africa [29]. Implementation of the Treat All strategy, rise in HIV testing and linkage to care, education on the AIDS epidemic, and counselling are described by the authors as likely contributing factors to this increase [30, 31].
High proportions of children and adolescents living with HIV were severely immunosuppressed in our study (70% in 2011–2013 and 45% in 2017–2020) and we observed a modest decrease in the proportion of children <5 years of age who were immunodeficient (62% to 55%). Given the greater risk of rapid disease progression and worse HIV outcomes in children <5 years of age compared to older children [32], this finding highlights the need to ensure earlier diagnosis and ART initiation for infants with perinatally acquired HIV. In a previous global analysis, 42% to 64% of children <16 years living in low- and middle-income countries who received treatment in 2013 were severely immunodeficient [14]. In a more recent study, 55% of children <5 years in 2014–2017 started treatment with severe immunodeficiency [12]. Notably, an IeDEA study conducted in earlier years (2004–2013) found only a small decline in the proportion of children <1 year with severe immunodeficiency over time [14]. Likewise, the small decrease observed in the proportion of severely immunosuppressed in children <2 years from 2011–2013 to 2017–2020 could be partly explained by late diagnosis and delayed and poor engagement in care among those diagnosed. Poor access to and low coverage of MTCT in lower-middle income countries may explain the small reduction we observed in very young children. Indonesia is an example, where despite national efforts, only 28% of pregnant women were tested for HIV in 2020 [33].
In this study, 14% of children experienced at least one OI during the first two years of ART. Recurrent upper respiratory tract infection and pulmonary TB were the most common OIs over the study period. We observed a small decline in the incidence of TB over time. This is consistent with data from studies in sub-Saharan Africa demonstrating the ongoing risk of HIV-associated TB even among populations with high ART coverage [34, 35]. HIV infection increases the progression of TB infection to active disease and worsens disease severity [36]. This makes it essential that HIV programs strengthen their existing strategies of early detection of TB and monitoring the development of both diseases. In the multivariate analyses, higher hazard rate of OI is observed if ART is started at <2 years compared to initiating at 2 years or older. This can possibly attributed to the higher incidence of some of the OIs in the younger children, including recurrent or chronic upper respiratory tract infection (5.81 per 100 person-years in <2 years vs. 3.43 per 100 person-years in 2–14 years).
We used data from clinical settings collected through the course of routine care, there is the potential for incomplete and/or inconsistent data, which limits the reliability of our findings when applied to broader populations of children and adolescents with HIV. Notably, the shift to treat-all policies that are not reliant on CD4 counts has meant that there are fewer children starting ART with baseline CD4 tests. This is compounded by the variability in available methods for diagnosing OIs. Moreover, TApHOD sites are mainly university-based clinics and/or referral centers located in capital cities. While they represent the major pediatric HIV care and treatment centers in their geographic areas, the sites may not be fully representative of the greater population of children and adolescents living with HIV across the region. In addition, residual confounding in the multivariable analyses due to unmeasured covariates is of concern in observational studies and may have affected our findings.
Conclusions
Our analyses suggest that across the three time periods examined, there have been marked increases in the median CD4 count at ART initiation in our regional cohort, and substantial improvement in health outcomes, as measured by persistent reductions in the incidence rates of mortality and first OIs. An increasing proportion of children and adolescents did not have a baseline CD4 count, and a substantial proportion were still initiating treatment with severe immunodeficiency. Despite some of the recent successes in pediatric HIV control in the region, there is a clear need for further program enhancement, with earlier diagnosis and immediate treatment for children.
Supporting information
(DOCX)
(TIFF)
(DOCX)
Acknowledgments
The authors are grateful to the clinical sites who are part of the TREAT Asia pediatric HIV Observational Database (TApHOD), namely the following:
PS Ly, V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N Kumarasamy, E Chandrasekaran, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; A Kinikar, V Mave, S Nimkar, I Marbaniang, BJ Medical College and Sassoon General Hospitals, Maharashtra, India; DK Wati, D Vedaswari, IB Ramajaya, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati, D Muktiarti, Cipto Mangunkusumo–Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; SM Fong, M Lim, F Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff, P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; TJ Mohamed, MR Drawis, Department of Pediatrics, Women and Children Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy, KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk, V Sirisanthana, L Aurpibul, Department of Pediatrics, Faculty of Medicine, and Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; P Ounchanum, R Hansudewechakul, S Denjanta, A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon, P Kosalaraksa, P Tharnprisan, T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Puthanakit, S Anugulruengkit, W Jantarabenjakul, R Nadsasarn, Department of Pediatrics and Center of Excellence for Pediatric Infectious Diseases and Vaccines, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; K Chokephaibulkit, K Lapphra, W Phongsamart, S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; KH Truong, QT Du, CH Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do, TM Ha, VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen, DM Tran, HTT Tran, TTT Giang, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn, JL Ross, T Suwanlerk, TREAT Asia/amfAR—The Foundation for AIDS Research, Bangkok, Thailand; MG Law, A Kariminia, The Kirby Institute, UNSW Sydney, NSW, Australia. Lead author: AH Sohn, annette.sohn@treatasia.org.
Data Availability
The data that supported this study are not openly available due to their containing sensitive information (e.g., sex, date of birth, date of death) that could compromise the privacy of research participants. Data are formally owned by the 17 contributing clinical sites. De-identified aggregated data are, however, available with permission of the study Steering Committee and request to access will be subject to HREC review of all the participating sites prior to sharing. Data request can be sent to Ms. Tulathip Suwanlerk, TREAT Asia pediatric HIV Observational Database (TApHOD) Coordinator, TREAT Asia, Bangkok, Thailand (Ph: +66 2 663 7561).
Funding Statement
The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center, as part of the International epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNICEF. Global and regional trends [Internet]. 2023. [cited 2023 Aug 18]. Available from: https://data.unicef.org/topic/hivaids/global-regional-trends/ [Google Scholar]
- 2.UNAIDS. Global HIV statistics—Fact sheet 2023. [Internet]. [cited 2023 Aug 18]. Available from: https://www.unaids.org/en/resources/fact-sheet [Google Scholar]
- 3.UNICEF. Adolescent HIV treatment [Internet]. 2023. [cited 2023 Aug 18]. Available from: https://data.unicef.org/topic/hivaids/adolescent-hiv-treatment/ [Google Scholar]
- 4.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013/11/12 ed. 2013. Nov 9;382(9904):1555–63. doi: 10.1016/S0140-6736(13)61409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Internet]. 2016. [cited 2021 Jun 10]. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf [PubMed] [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach—2010 revision [Internet]. Geneva: World Health Organization; 2010. [cited 2023 Aug 18]. 194 p. Available from: https://apps.who.int/iris/handle/10665/164255 [PubMed] [Google Scholar]
- 7.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach [Internet]. Geneva: World Health Organization; 2013. [cited 2023 Aug 18]. 269 p. Available from: https://apps.who.int/iris/handle/10665/85321 [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. [Internet]. 2017. [cited 2021 Jun 8]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK475977/ [PubMed] [Google Scholar]
- 9.Adedimeji A, Edmonds A, Hoover D, Shi Q, Sinayobye J d’Amour, Nduwimana M, et al. Characteristics of HIV-Infected Children at Enrollment into Care and at Antiretroviral Therapy Initiation in Central Africa. Andrei G, editor. PLoS ONE. 2017. Jan 12;12(1):e0169871. doi: 10.1371/journal.pone.0169871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlucci JG, De Schacht C, Graves E, González P, Bravo M, Yu Z, et al. CD4 Trends With Evolving Treatment Initiation Policies Among Children Living With HIV in Zambézia Province, Mozambique, 2012–2018. J Acquir Immune Defic Syndr. 2022. Mar 1;89(3):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MA, Phiri S, Wood R, Wellington M, Cox V, Bolton-Moore C, et al. Temporal Trends in the Characteristics of Children at Antiretroviral Therapy Initiation in Southern Africa: The IeDEA-SA Collaboration. Beck EJ, editor. PLoS ONE. 2013. Dec 9;8(12):e81037. doi: 10.1371/journal.pone.0081037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyun V, Technau KG, Vinikoor M, Yotebieng M, Vreeman R, Abuogi L, et al. Variations in the characteristics and outcomes of children living with HIV following universal ART in sub-Saharan Africa (2006–17): a retrospective cohort study. Lancet HIV. 2021. Jun;8(6):e353–62. doi: 10.1016/S2352-3018(21)00004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koller M, Patel K, Chi BH, Wools-Kaloustian K, Dicko F, Chokephaibulkit K, et al. Immunodeficiency in Children Starting Antiretroviral Therapy in Low-, Middle-, and High-Income Countries. J Acquir Immune Defic Syndr. 2015. Jan 1;68(1):62–72. doi: 10.1097/QAI.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panayidou K, Davies MA, Anderegg N, Egger M, The IeDEA, COHERE, PHACS and IMPAACT 219C Collaborations Writing Group. Global temporal changes in the proportion of children with advanced disease at the start of combination antiretroviral therapy in an era of changing criteria for treatment initiation. J Int AIDS Soc. 2018. Nov;21(11):e25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNAIDS. Global HIV statistics—Fact sheet 2022 [Internet]. 2022. [cited 2022 Dec 15]. Available from: https://www.unaids.org/en/resources/fact-sheet [Google Scholar]
- 16.UNICEF. Global and regional trends [Internet]. 2023. [cited 2022 Dec 15]. Available from: https://data.unicef.org/topic/hivaids/global-regional-trends/ [Google Scholar]
- 17.Kariminia A, Chokephaibulkit K, Pang J, Lumbiganon P, Hansudewechakul R, Amin J, et al. Cohort Profile: The TREAT Asia Pediatric HIV Observational Database. Int J Epidemiol. 2011. Feb 1;40(1):15–24. doi: 10.1093/ije/dyp358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: Towards universal access [Internet]. 2006. [cited 2022 Dec 15]. Available from: https://apps.who.int/iris/handle/10665/43554 [Google Scholar]
- 19.World Health Organization. Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age; methods and development [Internet]. Onis M de, editor. Geneva: WHO Press; 2006. [cited 2022 Dec 15]. (WHO child growth standards). Available from: https://www.who.int/publications/i/item/924154693X [Google Scholar]
- 20.Centers for Disease Control. The SAS Program for CDC Growth Charts that Includes the Extended BMI Calculations [Internet]. 2022. [cited 2022 Dec 15]. Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999. Jun 1;94(446):496–509. [Google Scholar]
- 22.Lolekha R, Boonsuk S, Plipat T, Martin M, Tonputsa C, Punsuwan N, et al. Elimination of Mother-to-Child Transmission of HIV—Thailand. MMWR Morb Mortal Wkly Rep. 2016/06/10 ed. 2016. Jun 10;65(22):562–6. doi: 10.15585/mmwr.mm6522a2 [DOI] [PubMed] [Google Scholar]
- 23.Aziz MN. Malaysia’s success in eliminating mother-to-child transmission of HIV and syphilis. Malaysian Journal of Medicine and Health Sciences, 2019;15(104):24–24. 2019;15(104):24. [Google Scholar]
- 24.Samreth S, Keo V, Tep R, Ke A, Ouk V, Ngauv B, et al. Access to prevention of mother-to-child transmission of HIV along HIV services cascade through integrated active case management in 15 operational districts in Cambodia. J Int AIDS Soc. 2019/10/22 ed. 2019. Oct;22(10):e25388. doi: 10.1002/jia2.25388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen RN, Ton QC, Tran QH, Nguyen TKL. Mother-to-Child Transmission of HIV and Its Predictors Among HIV-Exposed Infants at an Outpatient Clinic for HIV/AIDS in Vietnam. HIV AIDS (Auckl). 2020/08/09 ed. 2020;12:253–61. doi: 10.2147/HIV.S259592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kredo T, Ford N, Adeniyi FB, Garner P. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Database Syst Rev. 2013/06/29 ed. 2013. Jun 27;(6):Cd009987. doi: 10.1002/14651858.CD009987.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNAIDS. The path that ends AIDS: UNAIDS Global AIDS Update 2023. 2023. [cited 2023 Aug 18]; Available from: https://www.unaids.org/en/resources/documents/2023/global-aids-update-2023 [Google Scholar]
- 28.UNFPA, UNESCO and WHO. Sexual and reproductive health of young people in Asia and the Pacific: A review of issues, policies and programmes. 2023. [cited 2023 Aug 18]; Available from: https://asiapacific.unfpa.org/en/publications/sexual-and-reproductive-health-young-people-asia-and-pacific [Google Scholar]
- 29.Apondi E, Humphrey JM, Sang E, Mwangi A, Keter A, Musick BS, et al. Trends Over Time for Adolescents Enrolling in HIV Care in Kenya, Tanzania, and Uganda From 2001–2014. J Acquir Immune Defic Syndr. 2018. Oct 1;79(2):164–72. doi: 10.1097/QAI.0000000000001796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sam-Agudu NA, Folayan MO, Ezeanolue EE. Seeking wider access to HIV testing for adolescents in sub-Saharan Africa. Pediatr Res. 2016/02/18 ed. 2016. Jun;79(6):838–45. doi: 10.1038/pr.2016.28 [DOI] [PubMed] [Google Scholar]
- 31.UNICEF. HIV and AIDS in adolescents: Turning the tide against AIDS will require more concentrated focus on adolescents and young people [Internet]. 2021. [cited 2022 Dec 15]. Available from: https://data.unicef.org/topic/hiv-aids/ [Google Scholar]
- 32.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev. 2013. Jul;254(1):143–69. doi: 10.1111/imr.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siregar KN, Hanifah L, Rikawarastuti, Wahyuniar L. Prevention of HIV Transmission from Mother to Child: Challenges to the Successful Program Implementation and Practice in Indonesia. J Int Assoc Provid AIDS Care. 2021. Jan 1;20:232595822110407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kay AW, Rabie H, Maleche-Obimbo E, Sekadde MP, Cotton MF, Mandalakas AM. HIV-Associated Tuberculosis in Children and Adolescents: Evolving Epidemiology, Screening Prevention and Strategies Management. Pathogens. 2022/01/22 ed. 2021. Dec 29;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandalakas AM, Kay AW, Bacha JM, Devezin T, Golin R, Simon KR, et al. Tuberculosis among Children and Adolescents at HIV Treatment Centers in Sub-Saharan Africa. Emerg Infect Dis. 2020/11/22 ed. 2020. Dec;26(12):2933–43. doi: 10.3201/eid2612.202245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry SH, Barnabas SL, Cotton MF. Tuberculosis and HIV-An Update on the “Cursed Duet” in Children. Front Pediatr. 2020/03/27 ed. 2019. Apr 25;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIFF)
(DOCX)
Data Availability Statement
The data that supported this study are not openly available due to their containing sensitive information (e.g., sex, date of birth, date of death) that could compromise the privacy of research participants. Data are formally owned by the 17 contributing clinical sites. De-identified aggregated data are, however, available with permission of the study Steering Committee and request to access will be subject to HREC review of all the participating sites prior to sharing. Data request can be sent to Ms. Tulathip Suwanlerk, TREAT Asia pediatric HIV Observational Database (TApHOD) Coordinator, TREAT Asia, Bangkok, Thailand (Ph: +66 2 663 7561).