Abstract
Pulmonary embolism (PE) is a common disease associated with high morbidity and mortality. Currently, guidelines recommend systemic thrombolysis in patients with haemodynamic instability (high-risk PE) or patients with intermediate–high-risk PE with haemodynamic deterioration. Nevertheless, more than half of high-risk PE patients do not receive systemic thrombolysis due to a perceived increased risk of bleeding. In these cases, percutaneous catheter-directed therapy (CDT) or surgical embolectomy should be considered. CDT has emerged and appears to be an effective alternative in treating PE, with a hypothetical lower risk of bleeding than systemic thrombolysis, acting directly in the thrombus with a much lower dose of thrombolytic drug or even without thrombolytic therapy. CDT techniques include catheter-directed clot aspiration or fragmentation, mechanical embolectomy, local thrombolysis, and combined pharmaco-mechanical approaches. A few observational prospective studies have demonstrated that CDT improves right ventricular function with a low rate of haemorrhage. Nevertheless, the evidence from randomised controlled trials is scarce. Here we review different scenarios where CDT may be useful and trials ongoing in this field. These results may change the upcoming guidelines for management and treatment of PE, establishing CDT as a recommended treatment in patients with acute intermediate–high-risk PE.
Tweetable abstract
In patients with high-risk PE with risk of bleeding, active bleeding or when systemic thrombolysis fails, percutaneous catheter-directed therapy (CDT) should be considered. Ongoing trials will analyse role of CDT in acute intermediate–high-risk PE. https://bit.ly/3JIc0mA
Introduction
Pulmonary embolism (PE) is a common disease associated with high morbidity and mortality worldwide [1]. The clinical presentation of PE ranges from asymptomatic to cardiogenic shock or sudden death [2, 3]. Although anticoagulant treatment is the cornerstone of therapy, risk stratification is essential to determine the most appropriate therapeutic strategy and the most proper location for the patient (i.e. intensive care unit, hospital ward or home) [4]. Stratification of acute PE comprises high-risk PE, intermediate-risk PE (intermediate–high or intermediate–low) and low-risk PE. High-risk PE involves haemodynamic instability, which is defined using any of the following criteria.
1) Cardiac arrest: need for cardiopulmonary resuscitation.
2) Obstructive shock: systolic blood pressure (BP) <90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite adequate filling status and end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate).
3) Persistent hypotension: systolic BP <90 mmHg or systolic BP drop ≥40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolaemia or sepsis.
To differentiate low-risk versus intermediate-risk PE we need to evaluate clinical signs of PE severity, serious comorbidity, and right ventricle (RV) dysfunction on transthoracic echocardiogram (TTE) or computed tomography pulmonary angiography (CTPA). Intermediate-risk PE contains pulmonary embolism severity index (PESI) ≥III, simplified PESI (sPESI) ≥1, Hestia criteria ≥1 or RV dysfunction on TTE or CTPA. By contrast, if PESI class risk <III, sPESI=0, no Hestia criteria and no findings of RV dysfunction, the patient is stratified as low-risk PE [4]. In low-risk PE, early discharge or outpatient management with standard anticoagulant therapy should be considered [4]. Intermediate-risk PE can be divided into intermediate–low-risk and intermediate–high-risk PE. Intermediate–high-risk PE is characterised by both RV dysfunction and elevated cardiac troponin, while intermediate–low-risk PE is confirmed if only one or none of those criteria are present [4]. Figure 1 shows risk stratification of acute PE.
FIGURE 1.
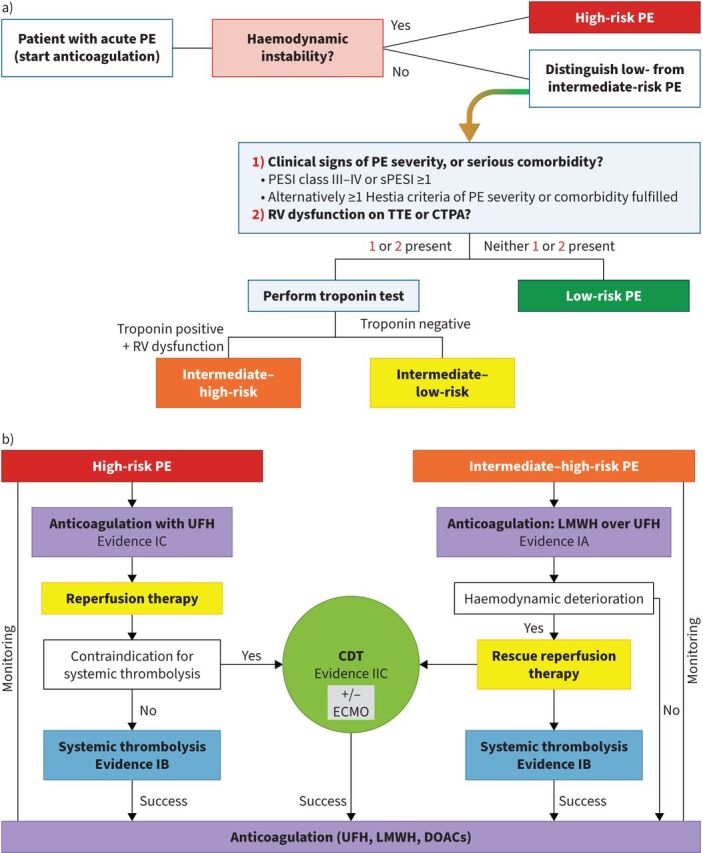
a) Prognostic stratification of acute pulmonary embolism (PE). Information from [4]. b) Proposed algorithm for catheter-directed therapies (CDT) in high-risk and intermediate-high risk PE. Reproduced and modified from [13] with permission. PESI: pulmonary embolism severity index; sPESI: simplified pulmonary embolism severity index; RV: right ventricular; TTE: transthoracic echocardiogram; CTPA: computed tomography pulmonary angiography; UFH: unfractionated heparin; LMWH: low-molecular weight heparin; ECMO: extracorporeal membrane oxygenation; DOAC: direct oral anticoagulant. GBP
PE therapy
Anticoagulant therapy is the basis of treatment for patients with PE, although in patients in high-risk PE or intermediate–high-risk PE with haemodynamic deterioration, rescue therapy with full-dose fibrinolysis or catheter-directed therapy (CDT) is required. Current guidelines on the management of PE recommend systemic thrombolysis therapy for high-risk PE (evidence IB) [4–6]. A meta-analysis that included 2057 patients with acute PE showed that thrombolytic therapy was associated with a significant reduction of overall mortality (OR 0.59, 95% CI 0.36–0.96) compared with anticoagulation alone [7]. Nevertheless, it has been estimated that more than half of high-risk PE patients do not receive systemic thrombolysis due to a perceived increased risk of bleeding or a contraindication to thrombolysis [8, 9]. The Pulmonary Embolism International THrOmbolysis (PEITHO) study confirmed the clinical efficacy of full-dose thrombolysis (using tenecteplase) in patients with intermediate–high risk [10]. However, stroke occurred in 12 patients (2.4%) in the thrombolysis arm (OR 12.10, 95% CI 1.57–93.39 versus heparin alone), being haemorrhagic in 10 cases [10]. Reduced-dose thrombolysis may be capable of improving safety while maintaining reperfusion efficacy. The PEITHO-3 study (ClinicalTrials.gov identifier: NCT04430569) is a randomised, placebo-controlled, trial with long-term follow-up that will assess the efficacy and safety of a reduced dosage of thrombolytic therapy (Alteplase 0.6 mg·kg−1) in patients with intermediate–high-risk acute PE. These results may lead to a change in the treatment of patients with intermediate-risk PE [11]. In these cases, percutaneous CDT or surgical embolectomy should be considered (evidence IIC) [5,6]. Kuo et al. [12] performed a systematic review and meta-analysis that evaluated the use of CDT for the treatment of massive PE in 594 patients. The pooled clinical success rate from CDT was 86.5% (95% CI 82.1–90.2%) and pooled risks of major procedural complications were 2.4% (95% CI 1.9–4.3%) [12]. In patients with intermediate–high-risk PE, parenteral or oral anticoagulation (without reperfusion techniques) is the appropriate treatment in most cases, but in those patients that develop haemodynamic deterioration it is suggested to use reperfusion therapy (thrombolytic therapy or CDT) (evidence IIC) [4–6, 10].
Although systemic fibrinolysis has been considered the treatment of choice in patients with high-risk or intermediate–high-risk PE who suffer haemodynamic deterioration during hospitalisation, there are conditions where thrombolysis cannot be used due to active bleeding, high risk of bleeding, or when systemic fibrinolysis failed. In this context, CDT has emerged, and appears to be an effective alternative in treating PE with a hypothetical lower risk of bleeding than systemic thrombolysis, acting directly in the thrombus with a much lower dose of thrombolytic drug or even without thrombolytic therapy [10, 12]. CDT techniques include catheter-directed clot aspiration or fragmentation, mechanical embolectomy, local thrombolysis, and combined pharmaco-mechanical approaches. Catheter-directed ultrasound-assisted thrombolysis (USAT) combines high-frequency, low-energy ultrasound waves to disintegrate the thrombus with local thrombolysis [13]. The most used technique is local thrombolysis using different catheter systems. However, evidence on the efficacy and safety of CDT has been limited to observational and small randomised trials. The ULTIMA trial randomised 59 patients with acute intermediate-risk PE to receive unfractionated heparin (UFH) or USAT. The mean±sd decrease in RV/left ventricle (LV) ratio from baseline to 24 h was significantly higher for the USAT group (0.30±0.20 versus 0.03±0.16; p<0.001) [14]. There were no differences in major and minor bleeding complications in both groups (10% in the USAT group and 3% in UFH group; p=0.61) [14]. In the SEATTLE II study (a prospective, single-arm, multicentre trial) that included 150 patients with high-risk and intermediate-risk PE, a significant decrease in RV/LV diameter ratio at 48 h post-procedure (1.55–1.13; mean difference: −0.42; p<0.0001) was observed, although there were 10% with major bleeding within 30 days of the procedure [15]. The most important limitation of this trial was the lack of a control group. Due to the high rate of bleeding, the OPTALYSE trial (randomised, prospective multicentre, parallel-group trial) evaluated if lowering the dose and procedure time would improve the safety of CDT without affecting the efficacy [16]. In this study, 101 patients with intermediate-risk PE were randomised to receive different regimens of tissue plasminogen activator (tPA) in which the dose ranged from 4 to 12 mg and the infusion duration from 2 to 6 h. ∼25% improvement in RV/LV diameter ratio was observed in all arms and the major bleeding rate was 4.0%, with two intracranial haemorrhage events (one attributed to tPA delivered by CDT) [16]. In the randomised SUNSET sPE (Standard versus Ultrasound-Assisted Catheter Thrombolysis for Submasssive Pulmonary Embolism) trial that included 81 patients, there was no significant difference in mean thrombus score reduction in patients undergoing USAT (EkoSonic System) compared with those undergoing combined catheter-directed treatment (fibrinolysis and thrombectomy) (cCDT) (UniFuse, angioDynamics or Cragg-McNamara, Medtronic) (p=0.76). Nevertheless, the small sample size prevents any conclusions [17].
Although the existing data appear favourable, it is necessary to have high-quality evidence from randomised controlled trials to define scenarios in which CDT may be considered. The FLowTriever for Acute Massive Pulmonary Embolism (FLAME) study that enrolled 115 patients with high-risk PE showed a lower mortality rate in patients undergoing mechanical thrombectomy with the FlowTriever system compared with those treated with other therapies in the context arm (1.9% versus 29.5%, respectively) [18]. A recent analysis of the prospective FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH) study that evaluated 800 patients with high-risk (7.9%) and intermediate–high-risk (76.7%) PE reported 0.3% at 48-h mortality and 0.8% at 30-day mortality, no device-related deaths [19]. The CANARY trial compared the long-term effect of CDT plus anticoagulation (alteplase, 0.5 mg per catheter per h for 24 h) versus anticoagulation monotherapy in 94 patients with acute intermediate–high-risk acute PE [20]. The proportion of patients with an RV/LV ratio >0.9 at 3-month follow-up was numerically fewer in the CDT group compared with the anticoagulation monotherapy group (4.3% versus 12.8%; OR 0.31, 95% CI 0.06–1.69; p=0.24) [20]. One nonfatal gastrointestinal major bleeding occurred in the CDT group, and two patients assigned to the anticoagulation monotherapy group died due to PE during the follow-up.
Ongoing trials to evaluate CDT in PE
Currently, several trials are ongoing that evaluate CDT in acute PE. The Higher-Risk Pulmonary Embolism Thrombolysis (HI-PEITHO) study compares CDT (EkoSonic Endovascular System) plus anticoagulation versus anticoagulation alone in 406 patients with intermediate–high-risk acute PE with elevated risk of early death and/or imminent haemodynamic collapse, defined by at least two of the following criteria: 1) heart rate ≥100 beats per min; 2) systolic blood pressure ≤110 mmHg; and 3) respiratory rate >20 breaths per min and/or oxygen saturation on pulse oximetry <90% (or partial arterial oxygen pressure <60 mmHg) at rest while breathing room air. The primary outcome is PE-related mortality, cardiorespiratory decompensation or collapse, or non-fatal symptomatic and objectively confirmed PE recurrence, within 7 days [21]. Likewise, the Pulmonary Embolism – Thrombus Removal With Catheter-Directed Therapy (PE-TRACT) trial evaluates CDT and anticoagulation versus anticoagulation alone in 500 patients with intermediate–high-risk acute PE [22]. It is estimated that 500 patients will be enrolled and the primary completion will be available in 2027. Recently, another clinical trial has been initiated: the Catheter-directed Thrombolysis in Intermediate-high Risk Acute Pulmonary Embolism (PRAGUE-26) study plans to include 558 patients with intermediate–high-risk acute PE [23]. The primary outcome of the study is a clinical composite of all-cause mortality, PE recurrence or cardiorespiratory decompensation, within 7 days of randomisation. This data will be available in 2026. If the results of these clinical trials confirm that the treatment arm is superior to the control arm, CDT will be established as treatment in patients with acute intermediate–high-risk PE. Table 1 shows the key studies (observational and randomised clinical trials) on CDT in patients with PE [14–22, 24–34].
TABLE 1.
Summary of studies using catheter-directed therapies (CDT) in patients with acute pulmonary embolism (PE)
| Trial name | Year | Sample Size | Population | Intervention | Control | Primary outcome | Efficacy | Safety |
| Randomised controlled trials testing the efficacy and safety of different modes of catheter-directed thrombolysis | ||||||||
| ULTIMA [14] | 2014 | 59 | Intermediate–high risk | USAT, EkoSonic Endovascular System (10 mg per 15 h) | Anticoagulation monotherapy | Change in 24-h TTE-based RV/LV ratio. | Mean RV/LV ratio was reduced 1.28 to 0.99 at 24 h (p<0.001) in USAT group versus 1.20 to 1.17 at 24 h (p=0.31) in heparin group | At 90 days, there was one death (control), no major bleeding, four minor bleeding episodes (three in the USAT group and one in the heparin group; p=0.61) |
| OPTALYSE PE [16] | 2018 | 100 | Intermediate risk | USAT, EkoSonic Endovascular System (4 mg per 2 h) (arm 1) | USAT, EkoSonic System (4 mg per 4 h) (arm 2) (6 mg per 6 h) (arm 3) (12 mg per 6 h) (arm 4) |
Change in the 48-h CT-based RV/LV ratio | Mean RV/LV diameter ratio: Arm 1: 0.40 (24.0%) Arm 2: 0.35 (22.6%) Arm 3: 0.42 (26.3%) Arm 4: 0.48 (25.5%) |
Major bleeding event rates: Arm 1: 0% Arm 2: 3.7% Arm 3: 3.6% Arm 4: 11.1% |
| SUNSET-sPE [17] | 2021 | 81 | Intermediate risk (submassive) | USAT, EkoSonic Endovascular System (8 mg per 8 h) | cCDT, UniFuse (AngioDynamics) or Cragg-McNamara (Medtronic) (8 mg per 8 h) |
Change in the 48-h CT-based thrombus burden | No significant difference in mean thrombus burden. Mean reduction in RV/LV ratio (p=0.01): USAT: 0.37 cCDT: 0.59 |
Major bleeds: intervention four versus control zero |
| CANARY [20] | 2022 | 94 | Intermediate-high risk | cCDT, Cragg-McNamara (Medtronic) (12 mg per 24 h) | Anticoagulation monotherapy | Proportion of patients with a TTE-based RV/LV ratio >0.9 at a 3-month follow-up | 4.3% in the cCDT group and 12.8% in the anticoagulation monotherapy group (p=0.24) Median RV/LV ratio at 3-month follow-up (p=0.01): cCDT: 0.7 Anticoagulation monotherapy: 0.8 |
One case of nonfatal major gastrointestinal bleeding occurred in the cCDT group |
| HI-PEITHO (NCT04790370) [21] | 406 | Intermediate–high risk with additional criteria of severity | USAT, EkoSonic Endovascular System (9 mg per 7 h) | Parenteral anticoagulation monotherapy | Composite of PE-related mortality, PE recurrence or cardiorespiratory decompensation or collapse, within 7 days of randomisation | Ongoing | Ongoing | |
| BETULA (NCT03854266) [24] | 60 | Intermediate–high risk | cCDT, Unifuse (AngioDynamics) (4 mg per 2 h) | Unfractionated heparin monotherapy | Improvement in RV/LV ratio at 24 h | Recruiting | Recruiting | |
| PE-TRACT (NCT05591118) [22] | 500 | Intermediate risk | CDT consisting of mechanical thrombectomy or cCDT | Anticoagulation monotherapy | Peak oxygen consumption at a 3-month follow-up | Ongoing | Ongoing | |
| PEERLESS (NCT05111613) [25] | 550 | Intermediate–high risk without absolute contraindication of thrombolysis | Mechanical thrombectomy, FlowTriever System | cCDT (any commercially available CDT system) | Composite clinical end-point of all-cause mortality, ICH, major bleeding, clinical deterioration, ICU admission, and ICU length of stay during hospitalisation | Recruiting | Recruiting | |
| STORM-PE (NCT05684796) [26] | 100 | Intermediate–high risk | Mechanical aspiration, Indigo Aspiration System | Anticoagulation monotherapy | Change in the 48-h CT-based RV/LV ratio | Recruiting | Recruiting | |
| STRATIFY (NCT04088292) [27] | 210 | Intermediate–high risk | USAT (20 mg per 6 h) | Anticoagulation monotherapy | Reduction in Miller score 48–96 h post-randomisation | Recruiting | Recruiting | |
| Peripheral Systemic Thrombolysis Versus Catheter Directed Thrombolysis for Submassive PE (NCT03581877) [28] | 2023 | 31 | Intermediate risk | Peripheral low-dose thrombolysis (24 mg per 12 h) | USAT, EkoSonic Endovascular System (24 mg per 12 h) | Change in the 48-h TTE-based RV/LV ratio | Completed | Completed |
| Registry of prospective studies testing the efficacy and safety of different modes of catheter-directed thrombolysis | ||||||||
| SEATTLE II [15] | 2015 | 150 | Massive (n=31) Submassive (n=119) |
USAT, EkoSonic Endovascular System (24 mg per 24 h) | Change in the 48-h CT-based RV/LV ratio | Mean RV/LV ratio decreased from 1.55 to 1.13 at 48 h (p<0.0001). | One GUSTO severe bleed; 16 GUSTO moderate bleed | |
| KNOCKOUT PE Registry [29] | 2018 | 1480 | Intermediate–high risk or high risk | USAT, EkoSonic Endovascular System | Change in 24–48 h TTE-based RV/LV ratio Persistence of pulmonary hypertension |
Ongoing | Ongoing | |
| PERFECT [30] | 2015 | 101 | Massive (n=28) Submassive (n=73) |
Massive PE: catheter-directed mechanical or pharmaco-mechanical thrombectomy Submassive PE: catheter-directed thrombolysis (UniFuse, EkoSonic or pig tail) |
Clinical success was defined as: stabilisation of haemodynamic, improvement in pulmonary hypertension and/or right heart strain, and survival to hospital discharge | Clinical success: Massive PE: 85.7% (95% CI 67.3–96.0%) Submassive PE: 97.3% (95% CI 90.5–99.7%) |
No major haemorrhages and no haemorrhagic strokes | |
| FLARE [31] | 2019 | 106 | Intermediate–high risk | Mechanical thrombectomy, FlowTriever System (Inari) | Change in the 48-h CT-based RV/LV ratio | Mean RV/LV ratio 1.53 reduced to 1.15 at 48 h | Four patients (3.8%) experienced six major adverse events | |
| EXTRACT-PE [32] | 2021 | 119 | Submassive PE | Mechanical aspiration, IndigoVac (Penumbra) | Change in the 48-h CT-based RV/LV ratio | Mean RV/LV ratio reduction was 0.43 (95% CI 0.38–0.47; p<0.0001) at 48 h | Two (1.7%) patients experienced three major adverse events | |
| FLAME [18] | 2023 | 115 | High-risk PE | Mechanical thrombectomy, FlowTriever System (Inari) | Context arm | Composite incidence of all-cause mortality, clinical deterioration, bailout, and major bleeding at 45 days | FlowTriever: 17% Context arm: 63.9% |
|
| FLASH interim results registry [33] | 2022 | 250 | Intermediate–risk (n=233) High risk (n= 17) |
Mechanical thrombectomy, FlowTriever System (Inari) | Composite of MAEs: device-related death, major bleeding, and intraprocedural device- or procedure-related adverse events at 48 h | MAEs: three (1.2%), all of which were major bleeds that resolved without sequelae All-cause mortality was 0.4% at 30 days, with a single death that was unrelated to PE |
||
| FLASH 6 months [34] | 2023 | 799 | High risk (n=64) Intermediate–high risk (n=616) Intermediate–low risk or unclassified (n=119) |
Mechanical thrombectomy, FlowTriever System (Inari) | Evaluate RV function, MMRCD, 6MWT distances, and PE QoL scores at 6 months | Normal RV function increased from 15.1% to 95.1% (p<0.0001) MMRCD score improved from 3.0 to 0.0 (p<0.0001) 6MWT distances increased from 180 m to 398 m (p <0.001) |
Prevalence of site-reported chronic thromboembolic pulmonary hypertension was 1.0% and chronic thromboembolic disease was 1.9% | |
| FLASH full cohort [19] | 2023 | 800 | High risk (n=63) Intermediate–high risk (n=614) |
Mechanical thrombectomy, FlowTriever System (Inari) | Composite of MAE: device-related death, major bleeding, device- or procedure-related adverse events | MAEs: 1.8% All-cause mortality was 0.3% at 48-h follow-up and 0.8% at 30-day follow-up, with no device-related deaths |
||
RV: right ventricular; LV: left ventricular; TTE: transthoracic echocardiogram; CT: computed tomography; cCDT: combined catheter-directed treatment (fibrinolysis and thrombectomy); GUSTO: Global Use of Streptokinase and t-PA for Occluded Coronary Arteries; ICH: intracerebral haemorrhage; ICU: intensive care unit; MAE: major adverse events; MMRCD: modified Medical Research Council dyspnoea scores; 6MWT: 6-min walk test; QoL: quality of life.
Currently, the results with CDT are promising, but the evidence on the efficacy and safety remain low and require a dedicated randomised controlled trial assessing clinical useful end-points and not surrogate end-points (RV/LV). However, a retrospective review of 341 patients with massive or submassive PE treated with systemic heparin or CDT reported that the cost associated with the initial admission was significantly higher in the CDT group when compared with heparin (p=0.001) with a difference of USD 31 000 [35]. However, the cost analysis shows the heparin group had a higher long-term cost of treatment [35]. In addition, patients treated with CDT had fewer bleeding complications (4.2% versus 13.8%, p=0.005) and a lower mortality rate (3.9% versus 6.9%, p=0.309) compared with patients receiving systematic heparin [35].
Current role for CDT in PE
Nowadays, a role for CDT is considered in patients with high-risk or intermediate–high-risk PE who suffer haemodynamic deterioration during hospitalisation and that have active bleeding, high risk of bleeding, or when systemic fibrinolysis failed. Jara-Palomares et al. [36] developed and validated a score to stratify the risk of major bleeding after systemic thrombolysis. Predictors for major bleeding were recent major Bleeding (3 points), Age >75 years (1 point), active Cancer (1 point), and Syncope (1 point) (BACS) [36]. Among 1172 patients, the overall 30-day major bleeding rate in those classified as low-risk (none of the variables present, 0 points) was 2.9% (95% CI 1.6–4.9%), compared with 44% (95% CI 14–79%) in the high-risk group (>3 points). These data were validated in another cohort of patients (n=190) [36].
Of note, in patients with a contraindication for anticoagulation, the placement of an inferior vena cava (IVC) filter should be considered [4]. The Prevention of Recurrent Pulmonary Embolism by Cava Interruption 2 (PREPIC2) study randomised 399 hospitalised patients with severe acute PE associated deep vein thrombosis to receive a retrievable IVC filter plus anticoagulation versus anticoagulation alone. There were no significant differences in symptomatic recurrent PE at 3 months between the two groups (3.0% versus 1.5% respectively; relative risk with filter 2.00, 95% CI 0.51–7.89; p=0 .50 [37]. Importantly, extracorporeal membrane oxygenation (ECMO) may be considered, in combination with surgical embolectomy or catheter-directed treatment, in refractory circulatory collapse or cardiac arrest (evidence IIB) [4]. A meta-analysis that compared mechanical embolectomy and other strategies (systematic thrombolysis, CDT or ECMO) showed that mechanical embolectomy yields favourable results regardless of the timing of ECMO implantation in the reperfusion timeline, independent of thrombolysis administration or cardiac arrest presentation [38]. Figure 1 shows an algorithm for acute PE and timelines of CDT.
Footnotes
Conflict of interest: L. Jara-Palomares reports grants or contracts from MSD and Leo Pharma, outside the submitted work; and payment for expert testimony from Johnson & Johnson, Pfizer, Bayer HealthCare Pharmaceuticals, ROVI, Leo Pharma, Bristol-Myers Squibb, and MSD, outside the submitted work. M. Barca has nothing to disclose.
References
- 1.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016; 41: 3–14. doi: 10.1007/s11239-015-1311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost 2010; 8: 1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x [DOI] [PubMed] [Google Scholar]
- 3.Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112: e28–e32. doi: 10.1161/CIRCULATIONAHA.105.551374 [DOI] [PubMed] [Google Scholar]
- 4.Konstantinides S V, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 5.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–352. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 6.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123: 1788–1830. doi: 10.1161/CIR.0b013e318214914f [DOI] [PubMed] [Google Scholar]
- 7.Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2015; 36: 605–614. doi: 10.1093/eurheartj/ehu218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein PD, Matta F, Hughes PG, et al. Nineteen-year trends in mortality of patients hospitalized in the united states with high-risk pulmonary embolism. Am J Med 2021; 134: 1260–1264. doi: 10.1016/j.amjmed.2021.01.026 [DOI] [PubMed] [Google Scholar]
- 9.Keller K, Hobohm L, Ebner M, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2020; 41: 522–529. doi: 10.1093/eurheartj/ehz236 [DOI] [PubMed] [Google Scholar]
- 10.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370: 1402–1411. doi: 10.1056/NEJMoa1302097 [DOI] [PubMed] [Google Scholar]
- 11.Sanchez O, Charles-Nelson A, Ageno W, et al. Reduced-dose intravenous thrombolysis for acute intermediate–high-risk pulmonary embolism: rationale and design of the pulmonary embolism international THrOmbolysis (PEITHO)-3 trial. Thromb Haemost 2022; 122: 857–866. doi: 10.1055/a-1653-4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009; 20: 1431–1440. doi: 10.1016/j.jvir.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 13.Pruszczyk P, Klok FA, Kucher N, et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC working group on pulmonary circulation and right ventricular function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2022; 18: E623–E638. doi: 10.4244/EIJ-D-22-00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129: 479–486. doi: 10.1161/CIRCULATIONAHA.113.005544 [DOI] [PubMed] [Google Scholar]
- 15.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8: 1382–1392. doi: 10.1016/j.jcin.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 16.Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv 2018; 11: 1401–1410. doi: 10.1016/j.jcin.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Avgerinos ED, Jaber W, Lacomis J, et al. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE trial. JACC Cardiovasc Interv 2021; 14: 1364–1373. doi: 10.1016/j.jcin.2021.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow DA, Bergmark BA. Outcomes in high-risk pulmonary embolism patients undergoing FlowTriever mechanical thrombectomy: the FLAME study in perspective. Eur Heart J Acute Cardiovasc Care 2023; 12: 222–223. doi: 10.1093/ehjacc/zuad022 [DOI] [PubMed] [Google Scholar]
- 19.Toma C, Jaber WA, Weinberg MD, et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention 2023; 18: 1201–1212. doi: 10.4244/EIJ-D-22-00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadeghipour P, Jenab Y, Moosavi J, et al. Catheter-directed thrombolysis vs anticoagulation in patients with acute intermediate–high-risk pulmonary embolism: the CANARY randomized clinical trial. JAMA Cardiol 2022; 7: 1189–1197. doi: 10.1001/jamacardio.2022.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klok FA, Piazza G, Sharp ASP, et al. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate–high-risk pulmonary embolism: Rationale and design of the HI-PEITHO study. Am Heart J 2022; 251: 43–53. doi: 10.1016/j.ahj.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov . Pulmonary Embolism - Thrombus Removal With Catheter-Directed Therapy (PE-TRACT). ClinicalTrials.gov Identifier: NCT05591118. Date last updated: 17 July 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- 23.ClinicalTrials.gov . Catheter-directed Thrombolysis in Intermediate-high Risk Acute Pulmonary Embolism (PRAGUE-26). ClinicalTrials.gov Identifier: NCT05493163. Date last updated: 8 November 2022. https://clinicaltrials.gov/ct2/show/NCT05493163
- 24.ClinicalTrials.gov . Low Dose Catheter Directed Thrombolysis for Acute Pulmonary Embolism (BETULA). ClinicalTrials.gov Identifier: NCT03854266. Date last updated: 28 April 2021. https://classic.clinicaltrials.gov/ct2/show/NCT03854266
- 25.ClinicalTrials.gov . The PEERLESS Study (PEERLESS). ClinicalTrials.gov Identifier: NCT05111613. Date last updated: 23 June 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05111613
- 26.ClinicalTrials.gov . Comparison of Two Pulmonary Embolism Treatments. ClinicalTrials.gov Identifier: NCT05684796. Date last updated: 31 May 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05684796
- 27.ClinicalTrials.gov . Low Dose Thrombolysis, Ultrasound Assisted Thrombolysis or Heparin for Intermediate High Risk Pulmonary Embolism (STRATIFY). ClinicalTrials.gov Identifier: NCT04088292. Date last updated: 26 October 2022. https://classic.clinicaltrials.gov/ct2/show/NCT04088292
- 28.ClinicalTrials.gov . Peripheral Systemic Thrombolysis Versus Catheter Directed Thrombolysis for Submassive PE. ClinicalTrials.gov Identifier: NCT03581877. Date last updated: 21 March 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03581877
- 29.ClinicalTrials.gov . An International Pulmonary Embolism Registry Using EKOS (KNOCOUT PE). ClinicalTrials.gov Identifier: NCT03426124. Date last updated: 22 February 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03426124
- 30.Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015; 148: 667–673. doi: 10.1378/chest.15-0119 [DOI] [PubMed] [Google Scholar]
- 31.Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv 2019; 12: 859–869. doi: 10.1016/j.jcin.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 32.Sista AK, Horowitz JM, Tapson VF, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv 2021; 14: 319–329. doi: 10.1016/j.jcin.2020.09.053 [DOI] [PubMed] [Google Scholar]
- 33.Toma C, Bunte MC, Cho KH, et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: interim results of the FLASH registry. Catheter Cardiovasc Interv 2022; 99: 1345–1355. doi: 10.1002/ccd.30091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khandhar S, Jaber W, Bunte MC, et al. Longer-term outcomes following mechanical thrombectomy for intermediate- and high-risk pulmonary embolism: 6-month FLASH registry results. J Soc Cardiovasc Angiogr Interv 2023; 2: 101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson K, VandenHull A, Remund T, et al. Short-term cost comparison of systemic heparin therapy vs. catheter directed thrombolysis for the treatment of massive and submassive pulmonaryembolism with long-term chronic pulmonary hypertension cost model. S D Med 2021; 4: 70–74. [PMC free article] [PubMed] [Google Scholar]
- 36.Jara-Palomares L, Jiménez D, Bikdeli B, et al. Derivation and validation of a clinical prediction rule for thrombolysis-associated major bleeding in patients with acute pulmonary embolism: the BACS score. Eur Respir J 2020; 56: 2002336. doi: 10.1183/13993003.02336-2020 [DOI] [PubMed] [Google Scholar]
- 37.Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA 2015; 313: 1627–1635. doi: 10.1001/jama.2015.3780 [DOI] [PubMed] [Google Scholar]
- 38.Chopard R, Nielsen P, Ius F, et al. Optimal reperfusion strategy in acute high-risk pulmonary embolism requiring extracorporeal membrane oxygenation support: a systematic review and meta-analysis. Eur Respir J 2022; 60: 2102977. doi: 10.1183/13993003.02977-2021 [DOI] [PubMed] [Google Scholar]


