Abstract
The growing epidemics of obesity, hypertension, and diabetes, in addition to worsening environmental factors such as air pollution, water scarcity, and climate change, have fueled the continuously increasing prevalence of cardiovascular diseases (CVDs). This has caused a markedly increasing burden of CVDs that includes mortality and morbidity worldwide. Identification of subclinical CVD before overt symptoms can lead to earlier deployment of preventative pharmacologic and non-pharmacologic strategies. In this regard, non-invasive imaging techniques play a significant role in identifying early CVD phenotypes. An armamentarium of imaging techniques including vascular ultrasound, echocardiography, magnetic resonance imaging (MRI), computed tomography (CT), non-invasive CT angiography, positron emission tomography (PET) and nuclear imaging, with intrinsic strengths and limitations can be utilized to delineate incipient CVD for both clinical and research purposes. In this paper, we review the various imaging modalities used for the evaluation, characterization, and quantification of early subclinical cardiovascular diseases.
INTRODUCTION
Cardiovascular diseases (CVDs), including coronary artery disease (CAD), heart failure (HF), stroke, and atrial fibrillation (AF), represent significant public health problems in terms of clinical disease burden, mortality, and costs to health care systems worldwide. 1-4 Epidemiologically, CVDs increase exponentially from middle age into later adult life. However, there is substantial evidence that subclinical atherosclerosis and subclinical cardiac dysfunction both begin in early life from the intersection of multiple environmental factors over and above genetic predisposition. 5,6 Atherosclerosis is an indolent disease process characterized by the response to endothelial damage and vasa vasorum inflammation with eventual plaque rupture. It accounts for >50% of silent coronary heart disease (CHDs). 3 Moreover, early exposure to cardiovascular (CV) risk factors can also play a significant role in the premature development of myocardial hypertrophy and stiffening due to interstitial fibrosis, with subsequent subclinical cardiac dysfunction and remodeling, leading to HF, AF and other CVDs. 7-10 With the increasing prevalence of CVDs, there is not only the need for optimization of traditional risk factor control but also for the identification of early subclinical disease markers to improve the stratification of individuals at risk, in parallel with the development of newer preventive strategies and treatment modalities. The identification and measurement of risk factors early in life are crucial to predicting the development of CVDs in middle age and later in life.
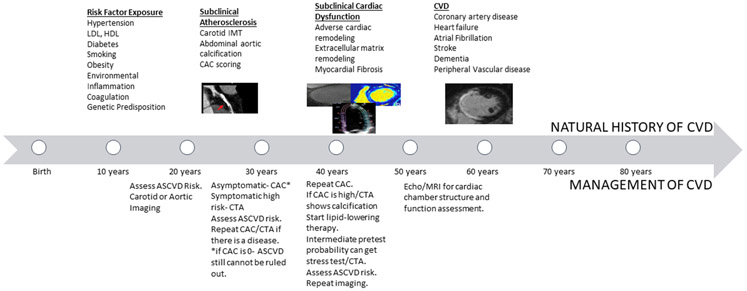
Our capacity to phenotype populations has increased exponentially due to the refinement of digital imaging and biomarker technology.11 Both non-invasive and invasive imaging technologies provide detailed information about the structure and function of the heart and vasculature. A recent increase in sensitivity and spatial resolution of imaging devices has led to a new era in diagnostic imaging, playing a pivotal role in early phenotyping, risk stratification, and disease management. 12The most prominent techniques studied include carotid ultrasound, coronary cardiac computed tomography (CT), echocardiography, magnetic resonance imaging (MRI) and positron emission tomography (PET). They are instrumental to identify and measure incipient atherosclerosis, myocardial perfusion abnormalities and subclinical cardiac dysfunction. Coronary artery calcium, carotid-intima-media thickness, and alteration of LV geometry and size offer the potential to characterize increased CVD risk in clinically asymptomatic individuals. 13-15 Therefore, quantitative structural and functional imaging provides an enhanced understanding of disease pathologic processes by elucidating the relationships among risk factors, subclinical phenotypes, and clinical outcomes, allowing for the development of personalized treatment strategies (Figure 1). In this paper, we review the utility and significance of multi-modality imaging techniques in characterizing the subclinical processes of atherosclerosis and cardiac dysfunction, underlying subclinical CVD development in early adult life.
Figure 1:
Natural History of CVD development
IMAGING ATHEROSCLEROSIS:
Pathogenesis
Emerging data show that the presence of triglyceride-rich lipoproteins and retinol-binding protein (a substrate for insulin resistance and metabolic syndrome), in addition to cholesterol15,16 and low-density lipoprotein (LDL) serum levels, 6,16-19 contribute to the development of subclinical atherosclerosis. 18,19 Being a chronic inflammatory disease, incipient atherosclerosis starts early life as a low-grade lesion,20-22 proceeding to overt clinical disease through mechanisms that include lipoprotein dysregulation, endothelial cell dysfunction, immune-cell activation, and plaque formation.17,23-25 This process of plaque formation can be categorized into different stages of evolution during the subclinical and clinical phases of CAD. In this regard, even asymptomatic individuals with subclinical atherosclerosis and plaque formation are at a higher mortality risk of up to 50% in men and 64% in women . 26 Risk factors associated with childhood and early adulthood atherosclerosis are the same as for CAD in later adulthood. 27-32The Young Finns Study, Childhood Determinants of Adult Health Study, as well as the Bogalusa and Muscatine studies, showed that an increase in childhood BMI, systolic and diastolic blood pressure, LDL levels, diabetes, presence of cigarette smoking and low levels of HDL were associated with greater atherosclerotic burden and advanced lesions in younger adults.27,33
Vulnerable atherosclerotic plaques have a thin fibrous cap with a necrotic center that contains macrophages and exhibits neovascularization. 24,34,35 Continued plaque growth results from plaque inflammation and a repeated rupture followed by local healing. This may lead to progressive impingement of the vessel lumen causing significant stenosis due to fibrosis and calcification. Therefore, macrophages, smooth muscle cell involvement and necrotic core lipidic infiltration play distinctive roles in progressive plaque formation leading to plaque rupture and calcification. Consequently, the majority of acute thrombus formations causing partial or total coronary obstruction derive from plaque rupture and luminal exposure of the lipid-rich core secondary to plaque erosion or disruption of the fibrous cap.25,34,35 Plaque calcification can occur early in plaque formation resulting from coronary intima and media inflammation followed by healing. It can be used as an indicator of subclinical atherosclerosis.36 37Intimal calcification is the main process associated with coronary atherosclerosis, while aortic medial calcification is primarily associated with diabetes, aging, and CKD, and is frequently mediated by calcium-phosphate disequilibrium.36,37
Calcification of the abdominal aorta (AAC) can precede the development of coronary artery calcification. 38 Prior autopsy studies have shown aortic atheroma formation by age 10 years in males.31 Moreover, abdominal atherosclerosis is strongly associated with coronary atherosclerosis. 22Wall shear stress varies in diverse vascular beds and different locations within the same arterial bed, accounting for different topographic atherogenic time courses and calcification rates. Accelerated disease, for example, is frequently seen at arterial bifurcations.39 Also, plaques formed in the carotid bulb are unique because of the presence of proteoglycan-rich lipid pools located deep in the intima, with speckled calcification in the absence of smooth muscle cells.40 In this regard, it is the creation of lipid pools by macrophages that lead to the development of advanced atheromatous plaques, with necrotic cores that release proteolytic enzymes and degrade surrounding tissue, therefore activating the apoptotic pathway.41,42 43Such necrotic core can be visualized and evaluated by vascular ultrasound, multi-detector CT and MRI.
In summary, an armamentarium of established imaging techniques can play a crucial role in the early diagnosis and risk stratification of subclinical CVD. 43,44 Such techniques can be used to measure luminal impingement and stenosis, vessel wall thickness, plaque, and necrotic core volume as well as coronary artery calcification. The latter is routinely used to localize and quantify the extent of atherosclerosis using computed tomography (CT), as detailed below.43
Coronary Computed Tomography
Coronary Artery Calcium Score (CAC)
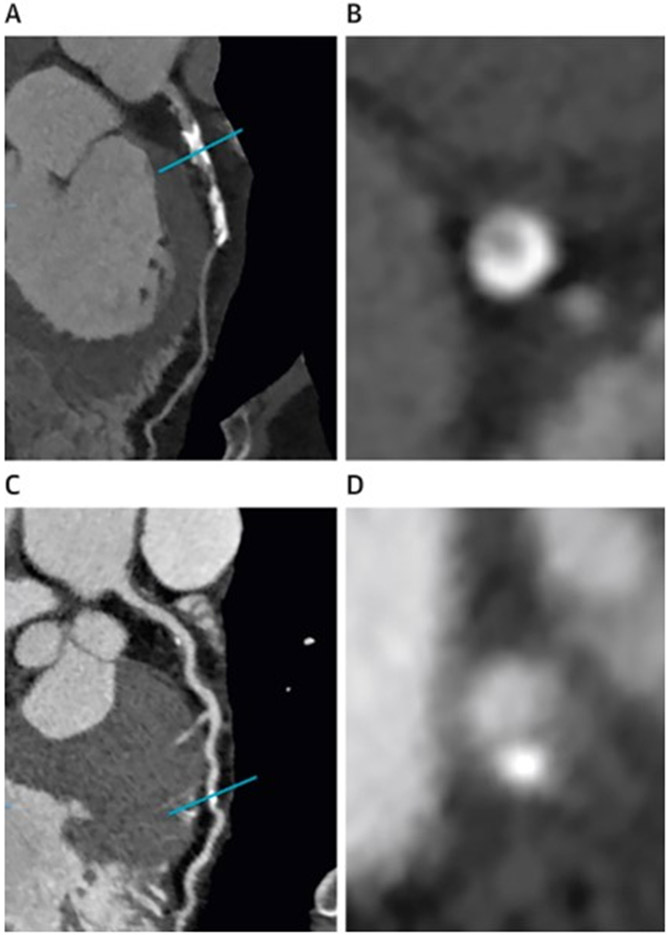
Although an indolent and regulated process, CAC is associated with arterial stiffness and an increased risk of clinically overt CVDs. Coronary artery calcium can be quantified by computed tomography and has evolved over the past few decades to play a significant role in CVD risk stratification and prediction of clinical outcomes. 45 Multiple-row detector CT scanners are currently used to assess subclinical and clinically overt atherosclerosis although originally, electron beam CT was used for this purpose. 46Using Agatston’s score method which is a sum of attenuation in Hounsfield units from areas containing diverse calcified lesions in the coronary arteries, CAC is routinely quantified clinically.46 CAC quantification has been correlated with atherosclerotic plaque burden for the entire heart, as well as for individual coronaries and different arterial segments of the coronary arterial tree (Figure 2).47-49
Figure 2:
A) curved multiplanar CT image of atherosclerotic plaque in the left anterior descending artery (LAD). B) cross-sectional image of coronary calcium of the same artery at the level of the blue line in the panel A. C) A curved multiplanar reformatted CT image of LAD of a different patient. D) Cross-sectional image of the same artery at the level of the blue line in the panel C.
Young adults have traditionally been classified as having a low risk for CVD, in part because the Framingham risk score is heavily weighted on age. 50,51 On the other hand, CAC scores from prospective studies, including the Multi-Ethnic Study of Atherosclerosis (MESA), have been employed as tools to assess the risk of CVD outcomes. 52. Among younger individuals, the Coronary Artery Risk Development in Young Adults (CARDIA) study enrolled 5115 young adults (18 to 30 years) with a 30-year follow-up, establishing the clinical utility of CAC scoring in young adulthood. The prevalence of CAC was found to be 10.2% at the CARDIA 15-year follow-up visit, with a mean Agatston score of 21.6, which was associated linearly with increasing age. The mean CAC score at follow-up year 25 increased to 244.4 on average. Moreover, the presence of any degree of CAC was associated with an increased risk of CHD (HR 5.0, 95% CI 2.8-8.7) and CVD (HR 3.0, 95% CI 1.9-4.7), and CAC >100 was associated with increased mortality. CARDIA also showed the prognostic value of a CAC risk score that included traditional risk factors which discriminate between low and high risk of developing CAC in middle age. This score was validated and found that it could help decrease the number of screened individuals needed to find one person with positive CAC from 3.5 to 2.2.53 Recently, a national-level consortium of prospective studies including CARDIA, CAC consortium and the Walter Reed Cohort composed of individuals 30-45 years old, showed an overall 21% prevalence of any degree of CAC. Males had higher mean CAC scores and a higher prevalence of nonzero CAC compared to females. 54 Moreover, white individuals had higher CAC prevalence than black individuals.54,55
Risk factors for CAC include age, gender, elevated blood pressure, diabetes, obesity, cigarette smoking, LDL and HDL.56,57CAC has also been shown to be associated with other atherosclerotic risk factors such as increased lipoprotein lipase A2, increased abdominal obesity and elevated fibrinogen among young adults. 58-60 Antiphospholipid positivity during young adulthood was associated with CAC,61 and higher telomerase activity accompanied by shorter telomeres has been shown to predict the presence of CAC. 62Moreover, CAC is also associated with adverse cardiac remodeling in middle-aged individuals, providing yet another dimension of its value as an early phenotype of atherosclerosis. Indeed, higher CAC is associated with greater left ventricular mass, higher LV end-diastolic volume, higher maximal left atrial volume (LA) and higher E/è ratio. Individuals with zero CAC from young adulthood to middle age have better LV function than those with detectable CAC. 63
In the CARDIA study, higher Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk scores at the baseline examination, year 5,and year 10 were associated with higher odds of CAC at exams conducted in years 15 and 25, showing that CAC reflects chronic risk exposure.64,65 CAC and abdominal aortic calcification are not only associated with subclinical CAD but have also been shown to associate with a reduced subclinical cognitive function such as lower processing speed, attention and working memory among middle-aged adults independent of other risk factors.66 Therefore, CAC in younger adults can be used as an early and effective imaging marker of subclinical CAD. However, the cumulative effect of radiation exposure associated with the number of individuals needed to screen for detection of CAC-positive young adults remains an important consideration limiting the usefulness of CAC as a routine screening tool in young adult populations.
Coronary CT Angiography
Coronary CT angiography (CTA) provides the highest diagnostic accuracy among non-invasive imaging modalities for visualizing the coronary anatomy, characterization of subclinical and flow-limiting CAD, and detecting significant stenosis when compared to invasive coronary angiography(ICA).67-69 CTA not only correlates well with ICA but also demonstrates a high negative predictive value and high per-patient sensitivity, especially in stable symptomatic patients.70,71 CTA is a reliable method for ruling out relevant plaques and stenosis in coronaries and quantifying the degree of specific stenotic lesions. 72-75 Moreover, although CAC is associated with significant atherosclerosis, the absence of CAC does not exclude obstructive CAD, especially in younger individuals. 74,76 With the advent of radiomics and machine learning techniques, CTA may identify coronary inflammation and help in the refinement of risk stratification.77,78 Recently, ACC/AHA guidelines established CTA as the non-invasive imaging modality of choice for a patient with acute or stable chest pain. In stable chest pain patients and negative CAC score, CTA has better diagnostic accuracy and can provide better prognostic information that includes coronary anatomy, assessing non-calcified plaque, characterization of plaque burden and plaque volume, which makes it a desirable tool to diagnose CAD in patients with chest pain or angina equivalent symptoms.79-81 However, excessive radiation exposure and cost-effectiveness make CTA undesirable as a screening tool among low-risk patients and populations.14 Table 1 describes the summary of selected studies that examine the role of coronary imaging in the development of atherosclerotic CVD .
Table 1:
Selected Studies that described the role of coronary artery imaging in ASCVD.
| First Author | Main Findings |
|---|---|
| Tota-Maharaj et al., 2012 53 | Diffuse CAC was associated with a higher mortality rate in the CAC >100. Left main CAC was associated with increased mortality risk. Increasing number of coronaries with CAC was associated with increased mortality risk. |
| Carr et al., 2017 54 | With 12.5 year follow-up , presence of CAC was associated with increased risk of fatal and nonfatal CHD. |
| Javaid et al., 2022 55 | Males had higher mean CAC scores and higher prevalence of nonzero CAC compared to females |
| Allen et al., 2014 58 | CAC of 100 HU was associated with 2.7%, 5%, 63% and 12.9% increase for moderate-stable, moderate-increasing, elevated stable and elevated increasing blood pressure trajectories. |
| Loria et al., 2007 57 | Adjusted odds-ratio of having CAC by 33-45 years were 1.5 per 10 cigarattes, 1.5 per 30mg/dl LDL, 1.3 per 10mmHG systolic blood pressure and 1.2 per 15mg/dl glucose at baseline. Young adults with above optimal risk factor levels were 2-3 times more likely to have CAC. |
| Green et al., 2009 59 | The age-, race-, and gender-adjusted prevalence of CAC with increasing quartiles of fibrinogen were 14.4%, 15.2%, 20.0%, and 29.1% |
| Iribarren et al., 2005 60 | The odds ratio (OR) of CAC per 1 standard deviation (SD) increment was 1.40 (95% CI, 1.17 to 1.67) and 1.39 (95% CI, 1.14 to 1.70) for Lp-PLA 2 mass and activity, respectively. |
| Lee et al., 2007 61 | The odds ratios (ORs) for CAC in the highest versus lowest tertiles of waist girth and WHR were 1.9 (95% CI: 1.36, 2.65) and 1.7 (1.23, 2.41), respectively |
| Majka et al., 2013 62 | Anti- b2-GPI IgM, IgG, IgA, and IgG positivity were associated with CAC [0 at year 15 after adjustment for traditional cardiovascular risk factors; [odds |
| Kroenke et al., 2012 63 | In multivariate-adjusted analysis, higher quartiles of telomerase were cross-sectionally associated with greater odds of prevalent CAC at year 15 (quartile 2: OR = 1.32, 95% CI: 0.54-3.23; quartile 3: OR = 1.40, 95% CI: 0.60-3.30; quartile 4: OR = 3.27, 95% CI: 1.39-7.71 compared with quartile 1, p-continuous = 0.012) and progressive CAC at year 20 |
| Yared et al., 2019 64 | Higher CAC was related to higher LV mass (β=1.218; adjusted P=0.007), higher LV end-diastolic volume (β=0.811; adjusted P=0.007), higher LV end-systolic volume (β=0.350; adjusted P=0.048), higher left atrial volume (β=0.214; adjusted P=0.009), and higher E/e' ratio (β=0.059; adjusted P=0.014) |
| Gidding et al., 2016 65 | For each 1 point higher PDAY score, the odds of CAC were 1.29; 95% confidence interval [CI], 1.25-1.33 |
| CT Angiography | |
| Arbab-Zadeh et al., 2015 68 | Sensitivity to identify patients with CAD was greater for CTA than SPECT-MPI (0.92 versus 0.62, respectively; P<0.001), resulting in greater overall accuracy (area under the receiver operating characteristic curve, 0.91 [95% confidence interval, 0.88-0.94] versus 0.69 [0.64-0.74]; P<0.001) |
| SCOT-HEART Trail 72 | Invasive coronary angiography was performed in 491 patients in the CTA group and in 502 patients in the standard-care group (hazard ratio, 1.00; 95% CI, 0.88 to 1.13), and coronary revascularization was performed in 279 patients in the CTA group and in 267 in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91 to 1.27) |
| Gottlieb et al., 2010 75 | From a total of 383 vessels without any coronary calcification, 47 (12%) presented with ≥50% stenosis; and from a total of 64 totally occluded vessels, 13 (20%) had no calcium. |
| Mortensen et al., 2022 77 | The presence of obstructive vs nonobstructive CAD among those with a CAC score of 0was associated with a multivariable adjusted hazard ratio of 1.51 (95% CI, 0.98-2.33) for myocardial infarction and all-cause death; however, this hazard ratio varied from 1.80 (95% CI, 1.02-3.19) in those who were younger than 60 years to 1.24 (95% CI, 0.64-2.39) in those who were 60 years or older. |
Abbreviations: CAC-coronary artery calcium; HU-Hounsfield unit;CAD- coronary artery disease; CTA- computed tomography angiography; SPECT-MPI-single photon emission CT- myocardial perfusion imaging; Ig- Immunoglobulin, Gp- glycoprotein.
Carotid Imaging
Carotid Ultrasound
As atherosclerosis is a subintimal process, the measurement of the distance between the lumen intima and media-adventitia interface by ultrasound captures the earliest manifestation of atherosclerosis and arterial fibrosis. In early clinical trials, carotid ultrasound was used to measure carotid intima-media thickness (IMT). 82,83Pignoli et al showed that carotid IMT measurements correlate well with atherosclerotic histology and are a biological marker of future CVD risk. 84 Carotid IMT has been established as a predictor of clinical cardiovascular outcomes. 85-88 In this regard, the Cardiovascular Health Study showed that a difference of 0.20mm in the Common Carotid IMT was associated with a 40% increase in MI and stroke. 89 Similarly, the Bogalusa Heart Study has shown that an increase in IMT of the common carotid and carotid bulb was associated with smoking, higher total cholesterol to HDL cholesterol ratio, high blood pressure, high insulin level and greater waist circumference in young adults. 90 Moreover childhood BMI and LDL levels predict carotid IMT in young adulthood.27,33,40-42
Generally, B-mode is preferred over M-mode using the linear-array transducer operating at a frequency of 13MHz to image the common carotid artery and 9MHz frequency to image the carotid artery bulb and proximal internal carotid arteries. 91,92Images are acquired at end-diastole with the image from each side obtained at the level of the common carotid before bifurcation. Subsequently, two images are acquired at the carotid arterial bulb and two images are obtained in the proximal 2cm of the internal carotid artery after the flow divider with the first image taken at 45° to horizontally, while the second obtained more vertically, at 20-25° angle. The maximum IMT is defined for the common carotid artery and the internal carotid artery as the mean of maximal IMT of the near and far walls on both the left and right sides of the carotids. Stenosis is assessed as any diminution of the lumen in any arterial segment separately for right and left carotids which indicates the development of atherosclerotic plaque of any type causing luminal narrowing.92,93 While these separate carotid IMT segments can be added as a risk score for predicting CVD and may have an association with traditional risk factors, these segments separately can have distinct associations with risk factors and outcomes.93 Shear stress at the carotid artery bifurcation are oscillatory and has a cyclically constant lumen to intimal gradient, acting as a substrate to foam cells that form typical cholesterol-rich plaques in that specific location.91
Measurements of carotid IMT by ultrasound have good reproducibility (70-80%) and are easily accessible. 94,95However, due to the typical anatomic distribution of carotid atherosclerotic plaques there are a few challenges. Fibrotic plaques and xanthomas are commonly found on ultrasound while progressive plaques are rare. Common carotid artery IMT might not be representative of atherosclerosis as plaques form mostly at low-shear stress segments such as the internal carotid artery. 91Additionally, the intima-media layers, in the absence of atherosclerosis, can thicken due to aging and hypertension. 96-98 Finally, information on plaque composition is limited by vascular ultrasound. Table 2 describes the summary of selected studies that examine the role of carotid imaging in the development of CVD.
Table 2:
Selected Studies that described the role of carotid and aortic artery imaging in ASCVD
| First Author | Main Findings |
|---|---|
| Bots et al., 1997 88 | Odds ratio of 0.163mm increase in IMT with stroke was 1.41 (95% CI, 1.25 to 1.82), myocardial infarction was 1.43 (95% CI, 1.16 to 1.78) When subjects with a previous myocardial infarction or stroke were excluded, odds ratios were 1.57 (95% CI, 1.27 to 1.94) for stroke and 1.51 (95% CI, 1.18 to 1.92) for myocardial infarction. |
| Urbina et al., 2002 91 | Race differences (blacks more than whites) were noted for the common carotid (p <0.001) and carotid bulb (bifurcation) IMT (women only, p <0.001). Men had a greater IMT in the common carotid (p <0.05), internal carotid (p <0.05), and carotid bulb (whites only, p <0.001). |
| Berry et al., 2009 89 | In young adults with a low 10 year risk, the IMT of common carotid (0.83mm) and internal carotid (0.85mm) was higher in high lifetime risk compared to low lifetime risk groups |
| Polak et al., 2010 94 | Carotid IMT was associated with LDL cholesterol, smoking and hypertension in all segments while CCA was associated strongly with fasting glucose and diastolic blood pressure and hypertension, diabetes and current smoking with bulb IMT, and LDL with ICA IMT |
| Nwabuo et al., 2020 99 | Carotid IMT has also been shown to be associated with variability of blood pressure due to the shear stress on the arterial scaffold resulting endothelial dysfunction, inflammation and oxidative stress leading arterial remodeling |
| Wilkins et al., 2014 | Family history of CVD was associated with carotid IMT >90% with an OR 1.93, 95% CI 1.10-3.4. |
| Raynor et al., 2013 | Lipid levels at young age have been shown to predict carotid IMT abnormalities in middle age. More than carotid stenosis, plaque area and volume showed greater association with traditional risk factors. |
| McMahan et al., 2005 22 | Odds ratios for a 1-unit increase in the risk scores were 1.18 (95% confidence interval, 1.14-1.22) for the CAC and 1.29 (95% confidence interval, 1.23-1.35) for the abdominal aorta. |
| Jurgens et al., 2021 114 | AAC scores tended to be much higher than CAC scores. AAC scores were higher in Black women than in White women. AAC predicted CVD with HR 1.77 (1.52– 2.06). AAC predicted incident CVD when CAC was 0. |
| Szulc, 2016 116 | People with any or more advanced AAC had higher risk of cardiovascular events (RR, 1.83; 95% CI, 1.40-2.39), fatal cardiovascular events (RR, 1.85; 95% CI, 1.44-2.39), and all-cause mortality (RR, 1.98; 95% CI, 1.55-2.53) |
Abbreviations: CI- confidence interval; IMT- intima-media thickness; CCA- common carotid artery; ICA-internal carotid artery;LDL-low-density lipoprotein; CBF-cerebral blood flow; AAC-abdominal aorta calcification; CAC-coronary artery calcification; CVD- cardiovascular disease; HR- hazard ratio;RR-relative risk;
Carotid Magnetic Resonance Imaging
High-resolution magnetic imaging resonance imaging (MRI) is the best tool to provide information on plaque volume and composition in the carotid arteries and abdominal aorta. 99-102Plaque characterization can be achieved through a combination of T1, T2 and proton density weight imaging. 103 Furthermore, an MRI of the carotid artery can describe and differentiate the components of a plaque–fibrous cap, necrotic core, presence of hemorrhage, and calcification. 100,102,104 Contrast-enhanced MRI can be used to better highlight the plaque fibrous cap.102High spatial resolution, high signal-to-noise imaging and improved plaque characterization make MRI a favorable imaging modality for atherosclerosis. However, there are several challenges to widespread use including imaging artifacts, claustrophobia, cost and accessibility.
Aortic Imaging
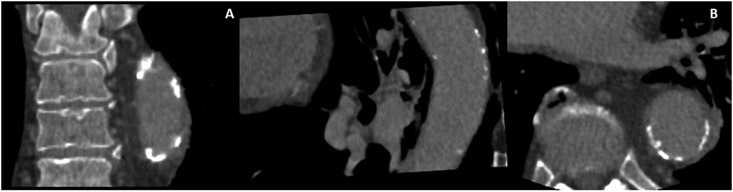
Epidemiological studies have shown that AAC is associated with CVD mortality, incident CHD, myocardial infarction and stroke. 105-108 Severe AAC is also a predictor of carotid atherosclerosis. Furthermore, there is evidence that coronary atherosclerosis is seven times more likely to occur if there is the presence of abdominal aortic atherosclerosis. 22The Progression of Early Subclinical Atherosclerosis (PESA) study showed that 25% of individuals with the subclinical disease had infrarenal atherosclerosis. Aortic medial calcification, like extra coronary calcification, is independent of atherosclerosis and related to aging, while intimal calcification correlates closely to atherosclerosis.109 110 In addition, similar to coronary atherosclerosis, abdominal atherosclerosis is associated with aging, smoking, hypertension and LDL cholesterol levels in young individuals. 22AAC can be quantified by computed tomography and assessed semi-quantitatively by X-ray and DEXA scanning. Electron beams or multidetector CT are the most accurate methods to facilitate the quantitative detection of aortic calcification. The Agatston score, corrected for slice thickness and attenuation threshold of >130 Hounsfield units is generally used for aortic calcification assessment (Figure 3).110
Figure 3:
CT image of descending aorta showing calcification and significant atherosclerosis. A) Orthogonal view of descending aorta. B) cross-sectional view of the descending aorta.
AAC increase with age and is associated with traditional risk factors, especially in smokers. Moreover, smoking cessation has been shown to reduce the deleterious effects of smoking on abdominal aortic atherosclerosis.111-113 In the CARDIA study, The AAC score was an early indicator of atherosclerosis as it reflects atherosclerotic CVD when CAC is zero.114 The prevalence of AAC was 53% among young adults,114 and AAC scores were generally found to be higher than CAC scores. CARDIA also showed that calcification magnitude in the abdominal aorta may have less CV prognostic impact compared with the same score in the coronaries (CAC). 114 AAC alters normal aortic physiology by inducing or augmenting aortic stiffness. 115 In this regard, greater ankle-branchial index and pulse wave velocity are associated with AAC. 115,116AAC also associates with adverse cardiac remodeling and diastolic dysfunction, especially in older age.
In summary, being an early indicator of CVD and easily detected by X-ray-based imaging technologies, AAC has advantages over other early markers of CV atherosclerosis as an accessible imaging marker. In addition, aortic atherosclerosis can also be detected by transesophageal echocardiography and MRI although radiography and computed tomography are the most commonly used techniques for AAC detection and diagnosis. AAC has however a lower prognostic predictive power for CVD when compared to CAC. Table 2 describes the summary of selected studies that examine the role of aortic imaging in the development of CVD .
Microvascular disease
Emerging data shows among patients that have angina without obstructive coronary disease, microvascular dysfunction (MVD) as a forerunner etiology, with a pooled prevalence of 41% affecting young women twice as much as men .117-119 MVD can coexist with obstructive/non-obstructive atherosclerosis and without macrovascular atherosclerosis. Studies have also shown MVD as a precursor to cardiomyocyte injury and stiffness leading to HF and even a potential role in takotsubo cardiomyopathy. 120-122 Being a syndrome of structural and functional abnormalities in coronary microcirculation, MVD is also associated with an increased risk of major cardiovascular events (MACE). 123 However diagnosis of MVD is quite challenging since patients have normal findings on physical examination. Disruption of adaptive mechanisms such as a change in the arterial diameter in response to flow changes causing shear stress, myogenic constriction in response to increased pressure and decreased microvascular resistance to maintain blood flow distally in the coronary circulatory system are potential mechanisms leading to MVD. 117,118 MVD encompasses the disruption of microvasculature – endothelial dysfunction, coronary spasm, inflammation, and atherosclerosis. 101 As the microcirculation is beyond the resolution of direct angiography- both invasive and non-invasive, there is a huge gap in understanding and defining the clinical phenotypes of MVD.
Coronary flow reserve (CFR) or myocardial perfusion reserve (MPR), which is a ratio of maximal hyperemic coronary flow to baseline coronary flow at rest, can reflect both epicardial stenosis and MVD.124,125 MPR can be best quantified using positron emission tomography (PET), although CT or MR perfusion by describing the kinetics of contrast material traversing the myocardium over time can also assess MPR.
MVD can also be evaluated by contrast echocardiography based on the assessment of injected microbubbles – those that get destroyed by ultrasound vs those that do not, containing or not high molecular weight gas. Using the mean velocity of the myocardial microbubble and cross-sectional area, myocardial blood flow can be quantified. Similarly, transthoracic Doppler echocardiography can also be used to estimate coronary flow velocity reserve (CFVR) which is obtained by pulsed-wave Doppler sampling of the proximal left anterior descending coronary artery and expressed as the ratio of coronary flow velocity during stress versus at rest. Although the method is inexpensive, entails no radiation exposure and abnormal CFVR has been associated with CVD, it is also highly operator dependent with limited clinical utility, making it challenging for widespread utilization.126-128
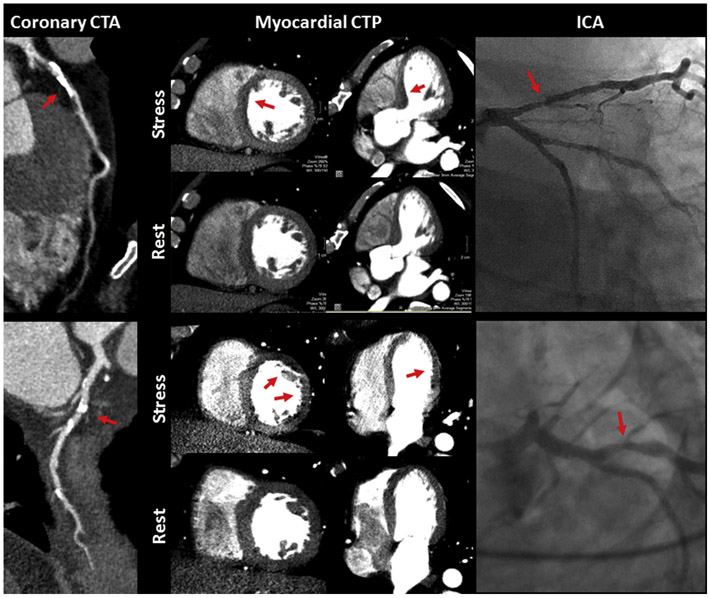
CT perfusion (CTP) combined with CT angiography is a non-invasive robust imaging modality that can evaluate MVD. CTP methods include static and dynamic CTP where static CTP provides semiquantitative or qualitative assessment, and dynamic CTP provides a quantitative assessment of myocardial perfusion (Figure 4). Images are obtained using ECG gating 30 seconds after contrast injection at rest and during vasodilator stress. Static CTP requires a single image at peak contrast opacification which is compared with images obtained at rest.75,129 In dynamic CTP, sequential images are obtained over time from contrast first pass, allowing quantitative perfusion assessment. 130,131CTA derived FFR (FFRCT) is calculated by simulating maximal hyperemia at a specified time point in a 3D coronary tree using mathematical modeling. FFRCT combined with CTA is more sensitive in the assessment of CAD compared to single photon emission CT (SPECT). The ratio of coronary luminal volume to mass has been shown to correlate with ischemia in non-obstructive CAD as well. Even though CT is the best tool to image atherosclerosis non-invasively, the radiation exposure and overestimation of coronary blood flow by dynamic CTP remain important limitations to using CTA combined with CTP in clinical practice.75,131-133
Figure 4:
The upper panel shows the presence of atherosclerotic plaque in the left anterior descending artery (LAD) which was not denoted as having significant CAD by CTA visual assessment but was denoted as significant CAD by CTA and semi-quantitative CTP metrics. Lower Panel shows the presence of atherosclerotic plaque in the lateral circumflex artery of a patient that was not denoted as significant CAD by CTA visual assessment, however, was denoted as having significant CAD by combined CTA and quantitative CTP metrics.
On the other hand, myocardial blood flow and MPR can also be assessed by cardiac MRI using adenosine as the vasodilator during stress. To determine MBF compartmental modeling, distributed parameter models can be used. 134-137 The CMR-derived MPR index can be used as a measure of MVD in nonobstructive CAD and is also correlated with native T1, suggesting an association with interstitial fibrosis. 138
Finally, cardiac PET remains the most accurate non-invasive modality for the quantification of MVD and uses radiotracers with high first-pass uptake.139,140 Post-processing software performs segmentation to compute the regional and global myocardial blood flow using arterial input function measurements. 141,142Comprehensive quantitative analysis of perfusion by PET includes stress flow at maximal vasodilation, CFR and transmural perfusion gradients which provide improved diagnostic accuracy for MVD. Limitations of PET include reduced temporal resolution, radiation exposure and limited camera sensitivity.139,140
IMAGING INCIPIENT HEART FAILURE
Globally, heart failure (HF) is rising in prevalence, increasing healthcare costs and expenditures.143 Heart failure with preserved ejection fraction (HFpEF) accounts for one-half of incident heart failure cases and is co-morbid with prevalent AF, diabetes, stroke and dementia.144-148 HFpEF has been associated with clinical phenotypes including aging, pulmonary hypertension, obesity and CAD.149 Several cardiac and non-cardiac mechanisms are known to be involved in the pathogenesis of HFpEF; left ventricular chamber (LV) and myocardial stiffening with accompanying rise in left ventricular diastolic filling pressures and subclinical systolic dysfunction characterize the HFpEF clinical syndrome. 150-153Moreover, excessive myocardial fibrosis, characterized by the accumulation of extracellular matrix in the myocardium, jeopardizes mechanical function and structural integrity, accompanied frequently by cardiomyocyte hypertrophy. 154-156The elucidation of mechanisms involved in the adverse extracellular matrix remodeling is critical not only to the epidemiology of HF but also AF.157-159
Cardiac imaging has evolved from volumetric analysis and now can offer granular and detailed information on the functional, mechanical, hemodynamic and tissue characterization of the cardiac chambers using echocardiography, MRI and nuclear imaging.153,160-162 The advent of non-invasive imaging techniques has demonstrated the complexities of the different phenotypes associated with HFpEF. Such complexities underscore the challenges of diagnosing the early stages of HFpEF. 174,175,174,175 In HFpEF, the progressive reduction in LV volumes, stroke volume (SV) and cardiac output is accompanied by a parallel reduction in contractile function as indexed by decreased myocardial strain, with compensatory increases in LV relative wall thickness, concentric remodeling, and torsion.163-169 These alterations are accompanied by improvement of LV ejection fraction to offset the LV cavity size and stroke volume reduction. 153,161,162 Hence, multi-modality non-invasive imaging is needed for accurate phenotyping in the diagnosis, and management of HF and identifying its early stages. 152,159-161 Table 4 describes the summary of selected studies that examine the role of imaging in the development of heart failure.
Echocardiography
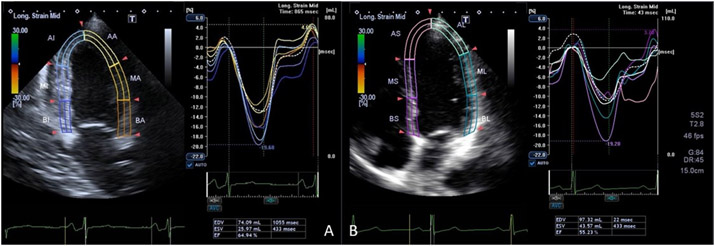
Echocardiography is the most commonly used, cost-effective and clinically established imaging tool for the diagnosis and clinical monitoring of cardiac remodeling. The LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV stroke volume, mass and ejection fraction are most often assessed by two-dimensional echocardiography (2D echo) according to the guidelines established by the American Society of Echocardiography. 169-171Moreover, speckle-tracking images for myocardial strain and strain rate are analyzed based on 16 segments of LV mid-wall layers using wall motion 2D tracking software. Strain is calculated as the change in segment length relative to its end-diastolic length from peak systolic values. Longitudinal strain (Ell) and strain rate curves can be assessed from 4-chamber views. Circumferential strain (Ecc) and strain rate can be assessed from the short-axis view at the mid-ventricular level. Global strain and strain rate values are calculated as the average of the peak segmental strain values (Figure 5). 170
Figure 5:
Two longitudinal orthogonal plane display of left ventricular with strain analysis in four chamber (A) and two chamber views (B).
In the CARDIA study, LV mass was highly correlated with BMI, subscapular skinfold thickness, height and systolic blood pressure among young adults. LV mass was greater in men than in women and greater in blacks than in whites independent of anthropometric and traditional risk factors. LV hypertrophy was found in 2% of the cohort. Blacks had higher LV wall thickness/diameter ratios. 171,172At the CARDIA Year 25 exam, greater exposure to cardiovascular risk factors entailed a greater chance of developing adverse LV remodeling. Over 25 years, LV end-systolic dimension and mass indexed to height were found to be associated with eccentric and concentric hypertrophy, diastolic dysfunction and incident clinical HF. 173 Moreover, longitudinal changes in LV mass index over 10 years were associated with a 15mmHg increase in systolic blood pressure and 20 pounds of weight gain. 174 Also, over 20 years, there was a significant decline in the prevalence of normal geometry from 84.2% to 69.7% among young adults in the CARDIA study. Change in cardiovascular risk traits in young adults predicted change in LV mass/geometry in middle age.174 Among young smokers, LV mass was increased, LV end-systolic stress was higher and pulmonary acceleration time was lower. 174,175
The left ventricular global function index (LVGFI) which is a validated measure of LV cardiac performance measured by cardiac MRI, can be assessed using echocardiography. LVGFI is defined as LV stroke volume/LV global volume where LV global volume is the sum of LVEDV + LVESV and myocardial volume (LV mass/density). Among young adults without clinically apparent CVD (CARDIA), LVGFI was a predictor of incident HF and CVD and showed prognostic value compared to LVEF, with higher LVGFI associated with HF (HR=0.70, 95%CI 0.54-0.91), CVD (HR=0.83, 95% CI 0.72-0.96). Moreover, worse LVGFI was associated with hypertension, obesity and smoking in young adults. 176
At the CARDIA year 25 examination, compared to women, men had greater LV volume and mass,177and black participants had larger LA volumes than whites. Among middle-aged adults, greater exposure to traditional risk factors such as diabetes, blood pressure variability and obesity in young adulthood was associated with adverse LV remodeling such as higher LV mass, lower LVEF, and impaired longitudinal and circumferential strain.172,178-183
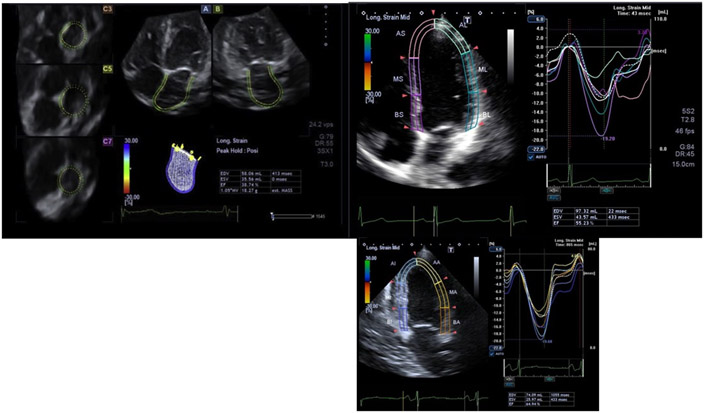
Left atrial function (LA) is characterized by 3 phases: LA reservoir function during LV systole, conduit function in early LV diastole and booster pump in late LV diastole. Recently, studies have highlighted the importance of LA function throughout human life and its alterations can be used as early CVD phenotypes. An increase in LA volume and pressure leads to chronic LA dilatation with an initial increase in the contractile function that worsens with CVD progression. Such LA remodeling has been associated with an increased risk of atrial fibrillation, stroke and heart failure.184-186 Given its thin walls and complex morphology, it may be challenging to visualize and evaluate LA structure and function. Echocardiography is the most frequently used imaging modality to assess LA phasic performance. Parasternal long-axis 2D views with or without M-mode echocardiography can be used for assessment of the left atrium (LA) measured at the point of maximum atrial volume (Figure 7). In 2D echocardiography, LA size and function are commonly evaluated using orthogonal 4 and 2 chamber views. Volumetric LA phasic function can be assessed by 3 DE where volumes are measured as maximum and minimum and also immediately before atrial contraction. Spectral Doppler can be used to assess the transmitral pulmonary venous flow. Myocardial strain and strain rate can be extracted using 2D speckly tracking.178 Among young adults, left atrial size indexed to body surface area was shown to be an independent predictor of clinical outcomes with an AUC of 0.77 for LA diameter and 0.78 for LA area. LA size indexed to BSA is a predictor of CVDs in the general population.187
Figure 7:
Multiplane display of LA 3d full volume showing three cross-sectional slices (C3, C5, and C7) and two longitudinal orthogonal planes (four chamber(A) and two-chamber (B) views).
Cardiac Magnetic Resonance Imaging
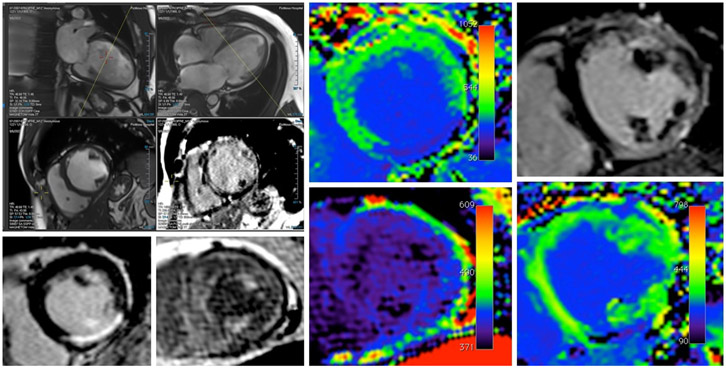
Since MRI is highly reproducible and has better spatial resolution and is less operator dependent than echocardiography, it has been considered the clinical gold standard for assessing cardiac structure and function. Images of the whole LV are used for the assessment and quantification of LV mass and volume. Short-axis images are segmented for endocardial and epicardial borders for quantification of LV volumes which is calculated using Simpson’s rule using two-chamber and four-chamber views in end-diastole and end-systole (Figure 6). However, MRI is expensive and its use as a screening tool for incipient structural alterations is currently limited. 188-192
Figure 6:
Left panel shows the MRI image of the left ventricle. Left upper panel shows the LV in 2 chamber and 4 chamber view. Left lower panel shows the LV in short axis where the scar in the myocardium can be appreciated. Right Panel shows the T1 mapping of LV myocardium.
Myocardial fibrosis can occur as either a replacement or interstitial fibrosis, and even though various imaging modalities can be used for the detection and visualization of myocardial fibrosis, contrast-enhanced MRI remains the gold standard for quantification with late-gadolinium enhancement (LGE) being used for accurate quantification of focal replacement fibrosis, while T1 mapping as the technique of choice for quantification of diffuse extracellular interstitial fibrosis. Replacement myocardial fibrosis expressed as myocardial scars is commonly secondary to coronary occlusion due to CAD or myocardial damage caused by other etiologies. Myocardial scars secondary to clinically overt or silent myocardial infarction are commonly quantified and used for late-gadolinium enhancement. In the non-ischemic state, myocardial collagenous scars are commonly secondary to toxic insults leading to localized necrosis or cellular apoptosis due to inflammation.155,193,194 This is seen most often in hypertensive heart disease, hypertrophic cardiomyopathy, amyloidosis and infiltrative interstitial disease that can also be diffused. In hypertension, pressure and volume overload, DM and obesity, the imbalance between myocyte growth and death governs the process of progressive interstitial myocardial fibrosis. This process is also commonly seen in association with aging. 154,193
Contrast-enhanced cardiac MRI provides an accurate method of detecting and quantifying interstitial and replacement myocardial fibrosis in a variety of pathologies. For example, late-gadolinium enhancement imaging can be used to identify the presence, pattern, and size of replacement fibrosis in ischemic cardiomyopathies. In such conditions, LGE is associated with LV adverse remodeling characterized by decreased ejection fraction, increased chamber volume and decreased mass. LGE quantified myocardial scar has been established as a predictor of cardiac mortality, HF hospitalization and malignant arrhythmias. Myocardial scars in non-ischemic cardiomyopathies are also associated with adverse LV remodeling of diverse types and magnitudes. On the other hand, T1 mapping allows for the quantitation of interstitial fibrosis due to ischemic and non-ischemic cardiac disease. In amyloidosis and sarcoidosis, increases in interstitial space can result in increased extracellular volume which can be quantified by T1 mapping.195,196 However, in patients with hypertension or significant obesity, T1 mapping may result in disparate extracellular volume determinations due to technical limitations associated with myocyte hypertrophy and/or reduced blood volume relative to body weight 197.
LA morphology and function are most accurately measured using Simpson’s rule quantified in multiple cross-sectional planes. However, as a simpler approach, LA can be quantified using orthogonal views as demonstrated by several groups of investigators.188,198 Using epicardial and endocardial contours in 2 and 4 chamber cine images, and using biplane are-length methods, LA volume curves can be generated during one cardiac cycle. Using the volume/time curve measurements for maximum LA volume, volume before atrial contraction and minimum volume can be obtained, allowing for LA passive and active emptying quantification. LA deformation parameters can also be assessed using feature tracking MRI. Global longitudinal strain curves can be created by averaging the strain measurements from all segments (Figure 8).158,159,185,186,189,198 Similar to LV myocardial scars, LA replacement fibrosis in association with LA dysfunction can be detected and assessed by contrast-enhanced cardiac MRI.157-159,186,198,199
Figure 8:
MRI image of LA showing contours drawn using Multimodality tissue tracking software at end- diastole and end systole.
More recently, Pezel T et al. have proposed the utilization of atrial-ventricular coupling expressed as the ratio of minimal LA volume to LV end-diastolic volume (LACI) as a measure of cardiac remodeling in patients with preserved or compromised LV ejection fraction.200,201 LACI has emerged as a powerful predictor of incident atrial fibrillation and heart failure in the multi-ethnic study of atherosclerosis, as well as in patients with myocardial infarction and other pathologies 200,202,204-207. LACI is a promising phenotype of the complex coupling of left atrial and ventricular function and may significantly enhance our armamentarium in characterizing early and established CVD.. Table 3 describes the summary of selected studies that examine the role of imaging in the development of cardiac remodeling and HF .
Table 3:
Imaging cardiac remodeling and heart failure
| First Author | Main Findings |
|---|---|
| Echocardiography | |
| Gardin et al., 1995a 172 | After adjustment for subscapular skinfold thickness, height, systolic and diastolic blood pressures, alcohol consumption, pulmonary function, smoking history, physical activity, total serum cholesterol, and family history of hypertension, LV mass remained higher in men than in women (P<.0001), in black men (167±43 g) than in white men (156±50 g, P<.0001), and in black women (142±49 g) than in white women (137±43 g, P<.002). |
| Perak et al., 2021 173 | Over 25 years, LV end systolic dimension and mass indexed to height was found to be associated with eccentric and concentric hypertrophy, diastolic dysfunction and incident clinical HF |
| Gidding et al., 2013 174 | Longitudinal changes of LV mass index over 10 year were associated with 15mmHg increase in systolic blood pressure and 20 pounds gain in weight |
| Gidding et al., 1995 175 | Among young smokers, LV mass was increased, LV end systolic stress was higher and pulmonary acceleration time was lower |
| Nwabuo et al., 2019 176 | LVGFI was a predictor of incident HF and CVD and showed prognostic value compared to LVEF, with higher LVGFI associated with HF (HR=0.70, 95%CI 0.54-0.91), CVD (HR=0.83, 95% CI 0.72-0.96). |
| Moreira et al., 2017 177 | At the CARDIA year 25 examination, compared to women, men had greater LV volume and mass |
| Kishi et al., 2014, 178 | High LV mass index was an independent predictor of systolic dysfunction (LVEF <50%) 20 years later (odds ratio 1.46, p = 0.0018) |
| Lin et al., 2021 179 | A 1-SD increment of intense glycemic exposure was significantly associated with worse target organs function after multivariable adjustment: left ventricular mass (β [SE], 5.468 [1.175]); global longitudinal strain (β [SE], 0.161 [0.071]); E/e’ ratio (β[SE], 0.192 [0.071]); CAC score (β [SE], 27.948 [6.116] |
| S. Liu et al., 2022 180 | Per 1-SD increase in cumulative systolic BP was associated with a higher risk of diastolic dysfunction, while an increase in cumulative diastolic BP was associated with a higher risk of systolic dysfunction and diastolic dysfunction. |
| Armstrong et al., 2014 187 | Among young adults, left atrial size indexed to body size area were shown to be independent predictors of clinical outcomes with an AUC of 0.77 for LA diameter and 0.78 for LA area. LA size indexed to BSA are predictors of CVDs in general population |
| Magnetic Resonance Imaging | |
| Habibi et al., 2014, 2015; 184,185 | Individuals with incident HF had greater maximal and minimal LA volume indexes (LAVImin) than control subjects (40 ± 13 mm3/m 2 vs. 33 ± 10 mm3/m2 [p <0.001] for maximal LA index and 25 ± 11 mm3/m2 vs. 17 ± 7 mm3/m2 [p <0.001] for LAVImin). In multivariable analysis, increased LGE was associated with lower LA passive emptying fraction, peak global longitudinal LA strain, systolic strain rate, early diastolic strain rate, and late diastolic strain rate |
| Markman et al., 2017; Raisi-Estabragh et al., 2022; 158, 186 | In the fully adjusted model, there was a significant association of minimum LAVI, LA total EF, LA passive EF and LA active EF with incident CVD (HR 1.12 per mm 3 /m 2 , P < 0.001; HR 0.95 per %, P < 0.001; HR 0.97 per %, P = 0.021; HR 0.98 per %, P < 0.027, respectively) |
| Zareian et al., 2015a, 2015b 198 | LA parameters with good-excellent (ICC; 0.88- 0.98, p < 0.001) intra-and inter reader reproducibility and fair-good (ICC; 0.44-0.82, p < 0.05-0.001) inter study reproducibility. |
| Imai et al., 2014 157 | Reduced LA regional and global function are related to both replacement |
| Petersen, et al., 2017 188 | BMI was the modifiable risk factor most consistently associated with subclinical changes to CMR parameters, particularly in relation to higher LV mass (+8.3% per SD [4.3 kg/m2], 95% CI: 7.6 to 8.9%), LV (EDV: +4.8% per SD, 95% CI: 4.2 to 5.4%); ESV: +4.4% per SD, 95% CI: 3.5 to 5.3%), RV (EDV: +5.3% per SD, 95% CI: 4.7 to 5.9%; ESV: +5.4% per SD, 95% CI: 4.5 to 6.4%) and LA maximal (+8.6% per SD, 95% CI: 7.4 to 9.7%) volumes. |
| Varadarajan et al., 2021 199 | Adverse LA remodeling over 10 years of follow-up strongly correlates with prolonged elevated levels of intracardiac stress, as assessed by NT-proBNP |
| Pezel, Venkatesh, et al., 2021 201 Pezel, Ambale-Venkatesh, et al., 2022 200 |
Greater LACI and ΔLACI were independently associated with HF (adjusted HR 1.44, 95% CI [1.25-1.66] and adjusted HR 1.55, 95% CI [1.30-1.85], respectively. Greater LACI and ΔLACI were independently associated with AF (hazard ratio, 1.69 [95% CI: 1.46, 1.96] and 1.71 [95% CI: 1.50, 1.94], respectively; both P , .001). |
| Germans et al., 2007 205 | changes in LA volume and function were age dependent and related to changes in LV mass-volume ratio. |
| Meucci et al., 2022 206 | Independent association between new-onset AF and LACI (hazard ratio [HR], 1.021; 95% CI, 1.017–1.026), LA maximum volume indexed (HR, 1.028; 95% CI, 1.017–1.039), LA minimum volume indexed (HR, 1.047; 95% CI, 1.037–1.060) and LA emptying fraction (HR, 0.967; 95% CI, 0.959–0.977, all p < 0.001). The inclusion of LACI in the multivariate model provided a larger improvement in the risk stratification for new-onset AF, as compared to conventional LA parameters. |
Abbreviations:LV-left ventricle; LVGFI- LV global function index;EDV-end-diastolic volume; ESV-end-systolic volume; RV-right ventricle; LA-left atrium; LACI-left atrial coupling index; AF- atrial fibrillation;HF- heart failure; CI- confidence interval; HR- hazard ratio;ICC-interclass correlation;AUC- area under the curve; BSA- body surface area; SE- standard error; SD- standard deviation; BP- blood pressure;CAC- coronary artery calcium; CARDIA- Coronary Artery Risk Development in Young Adults; LVEF- LV ejection fraction.
FUTURE PERSPECTIVES:
The ultimate goals of identifying early markers of atherosclerosis and heart failure include 1 - defining risk groups, and optimizing therapeutic and preventative interventions. 2 - reducing morbidity, and mortality due to CVD. 3 – reduce healthcare expenditures associated with the enormous CVD burden worldwide. 4- facilitate a deeper understanding of the pathogenetic mechanisms underlying the processes that lead to CVDs.
The advent of machine learning and artificial intelligence (AI)techniques applied to the analysis of massive longitudinal datasets have enabled the creation of neural networks and complex models which provide a much more nuanced and multifaceted phenotyping of early subclinical CVD. Machine learning methods, particularly random survival forest have been used in prediction of CVD risk which opens the possibility of discovering new relationships that are not hypothesis driven and without any prior assumptions.208-210 The ability to recognize the best predictors lead to biomarker discovery and antecedent imaging markers identification, especially in subclinical disease process.210-212 In the field of image acquisition and reconstruction, neural-network based solutions help focus on a faster image processing by improving overall image quality in CMR and drastically reduce radiation and contrast in CTA imaging. 78,213Artificial Intelligence based CAC scoring has shown to have reproducibility and agreement in a fraction of time. 214In future, these AI powered imaging will likely incorporate identification of plaque features from CTA tissue characterization and AI based segmentations in CMR leading to workflow efficiency, automated cardiac chamber quantification and better reproducibility.215,216 In a patient-centric clinical practice, imaging services can help refine risk stratification, facilitate the initiation of early preventative strategies and guide daily management decisions. Future, AI based integration of multimodality imaging data with medical records can be incorporated into an individual-specific cardiac risk model that provides personalized digital data for the individuals. Combined with AI based “omics” and biomarkers, imaging can provide a sophisticated diagnosis and management algorithm for enhanced precision in preventive CV medicine in clinical practice. Moreover, these early imaging markers can be used to design large-scale clinical trials to identify subclinical disease processes and show the impact of early life intervention on CV disease prevention. These clinical trials can enable a much greater refinement of personalized approaches in CV medicine.
CONCLUSIONS:
The appearance of atherosclerosis early in life reflecting exposure to hyperlipidemia, hypertension, obesity and smoking are strong markers of CVD in middle age. Even though frequently detected non-invasively as CT-defined CAC in the third and more frequently fourth decade of life (10 to 15 years later when compared to cardiac remodeling in populations), coronary atherosclerosis is a stronger predictor of mid-life cardiovascular events. Identifying young adults at risk using CAC aids in employing preventative measures to alter their atherosclerotic disease trajectories and risk factor exposures. Clinically, subclinical atherosclerosis and cardiac remodeling can be detected also as alterations of carotid and aortic wall thickness and calcification, myocardial perfusion abnormalities reflecting microvascular disease, and other early phenotypes particularly if more complex technologies based on MRI or PET are used. However, such technologies are not as accessible making ultrasound and CT the preferred tools for population screening and early identification of risks related to chronic exposure to hypertension, hyperlipidemia, obesity, cigarette smoking and diabetes. Newer preventive strategies as well as the discovery of novel pathways for preventing CVDs are sorely needed given the magnitude and burden of CVDs worldwide.
Lifelong low LDL-C is associated with low levels of atherosclerosis and CVD.217 Moreover, several clinical trials have shown that statin reduces CVD events and regresses coronary atherosclerosis. Genetic studies show that loss of function mutations in proprotein convertase subtilisin/kexin type 9(PSCSK −9) reduce LDL-C with significant reductions of CHD events over 15 years.218 However, Duke registry data and YOUNG-MI studies have documented a significant gap between young adults requiring statin therapy vs those who are actually on statin therapy. This has been attributed to hesitancy to initiate treatment due to a perceived lack of compelling evidence justifying therapy in young individuals. 219,220On the other hand, a modeling study based on NHANES predicted that the use of high-intensity statin for 30 years would be associated with a 51-71% reduction of premature CVD outcomes among 30-39-year-old adults. 221 Statin therapy alone is unlikely to reduce LDL-C, however, when added to PCSK-9, it is a 50-60% further reduction of LDL-C levels without prohibitive augmentation of serious adverse effects. 222Since atherosclerosis progresses over time, it may be beneficial to start lipid-lowering drugs in the fourth decade of life with revisiting every 5-10 years to limit the progression of atherosclerosis, reevaluate the risks of drug-related adverse effects and reduce health-related expenditures. 223However, before the implementation of such powerful preventive strategies, we need clinical trials that help us investigate the potential harms and benefits of early intervention, the ideal timing of treatment initiation and the expenditures associated with the large-scale implementation of such audacious strategies of personalized preventive CV medicine. We reviewed in this document the available data on the value of imaging markers that support early phenotyping of sub-clinical cardiovascular diseases.
Funding:
The CARDIA study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). The CARDIA study is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). The MESA research was supported by contracts N01-HC-95159, N01-HC- 95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC- 95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC- 95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- CVD
Cardiovascular disease
- CAD
coronary artery disease
- HF
heart failure
- AF
atrial fibrillation
- CHD
Coronary heart disease
- CV
cardiovascular
- CT
computed tomography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- LV
left ventricle
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- AAC
abdominal aorta calcification
- CAC
coronary artery calcification
- MESA
Multi-Ethnic Study of Atherosclerosis
- CARDIA
Coronary Artery Risk Development in Young Adults
- PDAY
Pathobiological Determinants of Atherosclerosis in Youth
- CTA
coronary CT angiography
- ICA
invasive coronary angiography
- ACC
American College of Cardiology
- AHA
American Heart Association
- IMT
intima-media thickness
- MI
myocardial infarction
- BMI
body mass index
- PESA
The Progression of Early Subclinical Atherosclerosis
- MVD
microvascular disease
- MACE
Major cardiovascular events
- CFR
coronary flow reserve
- MPR
myocardial perfusion reserve
- CFVR
coronary flow velocity reserve
- CTP
CT perfusion
- ECG
electrocardiogram
- FFR
fractional flow reserve
- SPECT
single photon emission CT
- HFpEF
Heart failure with preserved ejection fraction
- SV
Stroke Volume
- LVEDV
LV end-diastolic volume
- LVESV
LV end-systolic volume
- Ell
Longitudinal Strain
- Ecc
Circumferential Strain
- LVGFI
LV global function index
- LA
left atrium
- LGE
Late gadolinium enhancement
- LACI
left atrial coupling index
- AI
artificial intelligence
Footnotes
Disclose: None
Conflict of Interest: None
References
- 1.Al-Omary MS, Davies AJ, Khan AA, et al. Heart Failure Hospitalisations in the Hunter New England Area Over 10 years. A Changing Trend. Heart Lung Circ. 2017;26(6):627–630. doi: 10.1016/J.HLC.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Ambrosy AP, Fonarow GC, Butler J, et al. The Global Health and Economic Burden of Hospitalizations for Heart Failure: Lessons Learned From Hospitalized Heart Failure Registries. J Am Coll Cardiol. 2014;63(12):1123–1133. doi: 10.1016/J.JACC.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation. 2022;145(8):E153–E639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 4.Reddy KS. Cardiovascular Disease in Non-Western Countries. https://doi.org/101056/NEJMp048024. 2004;350(24):2438–2440. doi: 10.1056/NEJMP048024 [DOI] [PubMed] [Google Scholar]
- 5.Newman WPI, Freedman DS, Voors AW, et al. Relation of Serum Lipoprotein Levels and Systolic Blood Pressure to Early Atherosclerosis. http://dx.doi.org/101056/NEJM198601163140302. 2009;314(3):138–144. doi: 10.1056/NEJM198601163140302 [DOI] [PubMed] [Google Scholar]
- 6.Robinson JG, Williams KJ, Gidding S, et al. Eradicating the Burden of Atherosclerotic Cardiovascular Disease by Lowering Apolipoprotein B Lipoproteins Earlier in Life. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2018;7(20). doi: 10.1161/JAHA.118.009778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87(2):521–544. doi: 10.1152/PHYSREV.00032.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagaya Y, Weinberg EO, Ito N, Mochizuki T, Barry WH, Lorell BH. Glycolytic inhibition: effects on diastolic relaxation and intracellular calcium handling in hypertrophied rat ventricular myocytes. Journal of Clinical Investigation. 1995;95(6):2766. doi: 10.1172/JCI117980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377–1385. doi: 10.1093/EURHEARTJ/EHN153 [DOI] [PubMed] [Google Scholar]
- 10.Noel Bairey Merz C, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FitzGerald G, Botstein D, Califf R, et al. The future of humans as model organisms. Science (1979). 2018;361(6402):552–553. doi: 10.1126/SCIENCE.AAU7779/ASSET/5EC65A18-D123-47B7-91ED-45C618AA0229/ASSETS/GRAPHIC/361_552_F1.JPEG [DOI] [PubMed] [Google Scholar]
- 12.Douglas PS, Cerqueira M, Berman DS, et al. The Future of Cardiac Imaging: Report of a Think Tank Convened by the American College of Cardiology. JACC Cardiovasc Imaging. 2016;9(10):1211–1223. doi: 10.1016/J.JCMG.2016.02.027 [DOI] [PubMed] [Google Scholar]
- 13.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875 [DOI] [PubMed] [Google Scholar]
- 14.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. doi: 10.1016/j.jacc.2021.07.053 [DOI] [PubMed] [Google Scholar]
- 15.Mauger CA, Gilbert K, Suinesiaputra A, et al. Multi-Ethnic Study of Atherosclerosis: Relationship between Left Ventricular Shape at Cardiac MRI and 10-year Outcomes. https://doi.org/101148/radiol220122. Published online September 20, 2022. doi: 10.1148/RADIOL.220122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. The Lancet. 2014;384(9943):626–635. doi: 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 17.McGill HC, McMahan CA, Gidding SS. Preventing Heart Disease in the 21st Century. Circulation. 2008;117(9):1216–1227. doi: 10.1161/CIRCULATIONAHA.107.717033 [DOI] [PubMed] [Google Scholar]
- 18.Feng S, Zhu Y, Yan C, Wang Y, Zhang Z. Retinol binding protein 4 correlates with and is an early predictor of carotid atherosclerosis in type 2 diabetes mellitus patients. J Biomed Res. 2015;29(6):451–455. doi: 10.7555/JBR.29.20140087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhong Y, Chen H, et al. Retinol-Binding Protein-Dependent Cholesterol Uptake Regulates Macrophage Foam Cell Formation and Promotes Atherosclerosis. Circulation. 2017;135(14):1339–1354. doi: 10.1161/CIRCULATIONAHA.116.024503 [DOI] [PubMed] [Google Scholar]
- 20.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. New England Journal of Medicine. 1998;338(23):1650–1656. doi: 10.1056/NEJM199806043382302 [DOI] [PubMed] [Google Scholar]
- 21.Strong JP, Malcom GT, McMahan CA, et al. Prevalence and Extent of Atherosclerosis in Adolescents and Young Adults: Implications for Prevention From the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281(8):727–735. doi: 10.1001/JAMA.281.8.727 [DOI] [PubMed] [Google Scholar]
- 22.McMahan CA, Gidding SS, Fayad ZA, et al. Risk Scores Predict Atherosclerotic Lesions in Young People. Arch Intern Med. 2005;165(8):883–890. doi: 10.1001/ARCHINTE.165.8.883 [DOI] [PubMed] [Google Scholar]
- 23.Frostegård J Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11(1):117. doi: 10.1186/1741-7015-11-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMRA043430 [DOI] [PubMed] [Google Scholar]
- 25.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878 [DOI] [PubMed] [Google Scholar]
- 26.Kuller L, Borthani N, Furberg C, et al. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139(12):1164–1179. doi: 10.1093/OXFORDJOURNALS.AJE.A116963 [DOI] [PubMed] [Google Scholar]
- 27.Magnussen CG, Venn A, Thomson R, et al. The Association of Pediatric LDL-cholesterol and HDL-cholesterol Dyslipidemia Classifications and Change in Dyslipidemia Status with Carotid Intima-Media Thickness in Adulthood: Evidence from the Cardiovascular Risk in Young Finns Study, the Bogalusa Heart Study, and the Childhood Determinants of Adult Health (CDAH) Study. J Am Coll Cardiol. 2009;53(10):860. doi: 10.1016/J.JACC.2008.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juonala M, Magnussen CG, Venn A, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122(24):2514–2520. doi: 10.1161/CIRCULATIONAHA.110.966465 [DOI] [PubMed] [Google Scholar]
- 29.Urbina EM, Kieltkya L, Tsai J, Srinivasan SR, Berenson GS. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18(6):767–771. doi: 10.1016/J.AMJHYPER.2004.12.017 [DOI] [PubMed] [Google Scholar]
- 30.Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/JAMA.290.17.2277 [DOI] [PubMed] [Google Scholar]
- 31.Strong JP, Malcom GT, Oalmann MC. Environmental and genetic risk factors in early human atherogenesis: lessons from the PDAY study. Pathobiological Determinants of Atherosclerosis in Youth. Pathol Int. 1995;45(6):403–408. doi: 10.1111/J.1440-1827.1995.TB03476.X [DOI] [PubMed] [Google Scholar]
- 32.Gidding SS, Bookstein LC, Chomka EV. Usefulness of Electron Beam Tomography in Adolescents and Young Adults With Heterozygous Familial Hypercholesterolemia. Circulation. 1998;98(23):2580–2583. doi: 10.1161/01.CIR.98.23.2580 [DOI] [PubMed] [Google Scholar]
- 33.Li S, Chen W, Srinivasan SR, et al. Childhood Cardiovascular Risk Factors and Carotid Vascular Changes in Adulthood: The Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/JAMA.290.17.2271 [DOI] [PubMed] [Google Scholar]
- 34.Zaman AG, Helft G, Worthley SG, Badimon JJ. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis. 2000;149(2):251–266. doi: 10.1016/S0021-9150(99)00479-7 [DOI] [PubMed] [Google Scholar]
- 35.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25(10):2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18 [DOI] [PubMed] [Google Scholar]
- 36.Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94(5):1175–1192. doi: 10.1161/01.CIR.94.5.1175 [DOI] [PubMed] [Google Scholar]
- 37.Demer LL, Tintut Y. Vascular Calcification. Circulation. 2008;117(22):2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leow K, Szulc P, Schousboe JT, et al. Prognostic Value of Abdominal Aortic Calcification: A Systematic Review and Meta-Analysis of Observational Studies. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2021;10(2):1–19. doi: 10.1161/JAHA.120.017205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steenman M, Espitia O, Maurel B, et al. Identification of genomic differences among peripheral arterial beds in atherosclerotic and healthy arteries. Sci Rep. 2018;8(1):3940. doi: 10.1038/S41598-018-22292-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolodgie FD, Burke AP, Nakazawa G, Virmani R. Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscler Thromb Vasc Biol. 2007;27(5):986–989. doi: 10.1161/ATVBAHA.0000258865.44774.41 [DOI] [PubMed] [Google Scholar]
- 41.Kockx MM, De Meyer GRY, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97(23):2307–2315. doi: 10.1161/01.CIR.97.23.2307 [DOI] [PubMed] [Google Scholar]
- 42.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–1275. doi: 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 43.Tarkin JM, Dweck MR, Evans NR, et al. Imaging Atherosclerosis. Circ Res. 2016;118(4):750–769. doi: 10.1161/CIRCRESAHA.115.306247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.di Carli MF, Geva T, Davidoff R. The Future of Cardiovascular Imaging. Circulation. 2016;133(25):2640–2661. doi: 10.1161/CIRCULATIONAHA.116.023511 [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Bera K, Kikano E, et al. Coronary Artery Calcium Scoring: Current Status and Future Directions. Radiographics. 2022;42(4):947–967. doi: 10.1148/RG.210122/ASSET/IMAGES/LARGE/RG.210122.VA.JPEG [DOI] [PubMed] [Google Scholar]
- 46.Narula J, Chandrashekhar Y, Ahmadi A, et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2021;15(3):192. doi: 10.1016/J.JCCT.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–2162. doi: 10.1161/01.CIR.92.8.2157 [DOI] [PubMed] [Google Scholar]
- 48.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–133. doi: 10.1016/S0735-1097(97)00443-9 [DOI] [PubMed] [Google Scholar]
- 49.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol. 2018;72(4):434. doi: 10.1016/J.JACC.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary Calcium Independently Predicts Incident Premature Coronary Heart Disease Over Measured Cardiovascular Risk Factors: Mean Three-Year Outcomes in the Prospective Army Coronary Calcium (PACC) Project. J Am Coll Cardiol. 2005;46(5):807–814. doi: 10.1016/J.JACC.2005.05.049 [DOI] [PubMed] [Google Scholar]
- 51.Okwuosa TM, Greenland P, Ning H, Liu K, Lloyd-Jones DM. Yield of Screening for Coronary Artery Calcium in Early Middle-Age Adults Based on the 10-Year Framingham Risk Score: The CARDIA Study. JACC Cardiovasc Imaging. 2012;5(9):923–930. doi: 10.1016/J.JCMG.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33(23):2955–2962. doi: 10.1093/EURHEARTJ/EHS230 [DOI] [PubMed] [Google Scholar]
- 53.Carr JJ, Jacobs DR, Terry JG, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017;2(4):391–399. doi: 10.1001/JAMACARDIO.2016.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javaid A, Dardari ZA, Mitchell JD, et al. Distribution of Coronary Artery Calcium by Age, Sex, and Race Among Patients 30-45 Years Old. J Am Coll Cardiol. 2022;79(19):1873–1886. doi: 10.1016/j.jacc.2022.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bild DE, Folsom AR, Lowe LP, et al. Prevalence and Correlates of Coronary Calcification in Black and White Young Adults. Arterioscler Thromb Vasc Biol. 2001;21(5):852–857. doi: 10.1161/01.ATV.21.5.852 [DOI] [PubMed] [Google Scholar]
- 56.Loria CM, Liu K, Lewis CE, et al. Early Adult Risk Factor Levels and Subsequent Coronary Artery Calcification: The CARDIA Study. J Am Coll Cardiol. 2007;49(20):2013–2020. doi: 10.1016/J.JACC.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 57.Allen NB, Siddique J, Wilkins JT, et al. Blood Pressure Trajectories in Early Adulthood and Subclinical Atherosclerosis in Middle Age. JAMA. 2014;311(5):490–497. doi: 10.1001/JAMA.2013.285122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green D, Foiles N, Chan C, Schreiner PJ, Liu K. Elevated fibrinogen levels and subsequent subclinical atherosclerosis: the CARDIA Study. Atherosclerosis. 2009;202(2):623–631. doi: 10.1016/J.ATHEROSCLEROSIS.2008.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iribarren C, Gross MD, Darbinian JA, Jacobs DR, Sidney S, Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: The CARDIA study. Arterioscler Thromb Vasc Biol. 2005;25(1):216–221. doi: 10.1161/01.ATV.0000148322.89911.44 [DOI] [PubMed] [Google Scholar]
- 60.do Lee C, Jacobs DR, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86(1):48–54. doi: 10.1093/AJCN/86.1.48 [DOI] [PubMed] [Google Scholar]
- 61.Majka DS, Liu K, Pope RM, et al. Antiphospholipid antibodies and sub-clinical atherosclerosis in the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Inflamm Res. 2013;62(10):919–927. doi: 10.1007/S00011-013-0652-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kroenke CH, Pletcher MJ, Lin J, et al. Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis. 2012;220(2):506–512. doi: 10.1016/j.atherosclerosis.2011.10.041 [DOI] [PubMed] [Google Scholar]
- 63.Yared GS, Moreira HT, Ambale-Venkatesh B, et al. Coronary Artery Calcium From Early Adulthood to Middle Age and Left Ventricular Structure and Function. Circ Cardiovasc Imaging. 2019;12(6):e009228. doi: 10.1161/CIRCIMAGING.119.009228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gidding SS, Rana JS, Prendergast C, et al. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Risk Score in Young Adults Predicts Coronary Artery and Abdominal Aorta Calcium in Middle Age: The CARDIA Study. Circulation. 2016;133(2):139. doi: 10.1161/CIRCULATIONAHA.115.018042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gidding SS, McMahan CA, McGill HC, et al. Prediction of Coronary Artery Calcium in Young Adults Using the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Risk Score: The CARDIA Study. Arch Intern Med. 2006;166(21):2341–2347. doi: 10.1001/ARCHINTE.166.21.2341 [DOI] [PubMed] [Google Scholar]
- 66.Reis JP, Launer LJ, Terry JG, et al. Subclinical Atherosclerotic Calcification and Cognitive Functioning in Middle-Aged Adults: The CARDIA Study. Atherosclerosis. 2013;231(1):72–77. doi: 10.1016/J.ATHEROSCLEROSIS.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arbab-Zadeh A, Carli MFD, Cerci R, et al. Accuracy of Computed Tomographic Angiography and Single-Photon Emission Computed Tomography-Acquired Myocardial Perfusion Imaging for the Diagnosis of Coronary Artery Disease. Circ Cardiovasc Imaging. 2015;8(10). doi: 10.1161/CIRCIMAGING.115.003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neglia D, Rovai D, Caselli C, et al. Detection of Significant Coronary Artery Disease by Noninvasive Anatomical and Functional Imaging. Circ Cardiovasc Imaging. 2015;8(3). doi: 10.1161/CIRCIMAGING.114.002179/-/DC1 [DOI] [PubMed] [Google Scholar]
- 69.Bergström G, Persson M, Adiels M, et al. Prevalence of Subclinical Coronary Artery Atherosclerosis in the General Population. Circulation. 2021;144(12):916–929. doi: 10.1161/CIRCULATIONAHA.121.055340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167(6). doi: 10.1016/J.AHJ.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 71.CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. The Lancet. 2015;385(9985):2383–2391. doi: 10.1016/S0140-6736(15)60291-4 [DOI] [PubMed] [Google Scholar]
- 72.Meijboom WB, Meijs MFL, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135–2144. doi: 10.1016/J.JACC.2008.08.058 [DOI] [PubMed] [Google Scholar]
- 73.Eckert J, Schmidt M, Magedanz A, Voigtländer T, Schmermund A. Coronary CT Angiography in Managing Atherosclerosis. Int J Mol Sci. 2015;16(2):3740. doi: 10.3390/IJMS16023740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The Absence of Coronary Calcification Does Not Exclude Obstructive Coronary Artery Disease or the Need for Revascularization in Patients Referred for Conventional Coronary Angiography. J Am Coll Cardiol. 2010;55(7):627. doi: 10.1016/J.JACC.2009.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014;35(17):1120. doi: 10.1093/EURHEARTJ/EHT488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mortensen MB, Gaur S, Frimmer A, et al. Association of Age With the Diagnostic Value of Coronary Artery Calcium Score for Ruling Out Coronary Stenosis in Symptomatic Patients. JAMA Cardiol. 2022;7(1):1. doi: 10.1001/JAMACARDIO.2021.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolossváry M, Gerstenblith G, Bluemke DA, et al. Contribution of risk factors to the development of coronary atherosclerosis as confirmed via coronary CT angiography: A longitudinal radiomics-based study. Radiology. 2021;299(1):97–106. doi: 10.1148/RADIOL.2021203179/ASSET/IMAGES/LARGE/RADIOL.2021203179.FIG4.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang S, Koo BK, Hoshino M, et al. CT Angiographic and Plaque Predictors of Functionally Significant Coronary Disease and Outcome Using Machine Learning. JACC Cardiovasc Imaging. 2021;14(3):629–641. doi: 10.1016/J.JCMG.2020.08.025 [DOI] [PubMed] [Google Scholar]
- 79.Wieske V, Walther M, Dubourg B, et al. Computed tomography angiography versus Agatston score for diagnosis of coronary artery disease in patients with stable chest pain: individual patient data meta-analysis of the international COME-CCT Consortium. Eur Radiol. 2022;32(8):5233–5245. doi: 10.1007/S00330-022-08619-4/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torres FS, Venkatesh V, Nguyen ET, Jimenez-Juan L, Crean AM. Coronary Calcium Scan Acquisition Before Coronary CT Angiography: Limited Benefit or Useful Addition? http://dx.doi.org/102214/AJR128643. 2013;200(1):66–73. doi: 10.2214/AJR.12.8643 [DOI] [PubMed] [Google Scholar]
- 81.Kwon SW, Kim YJ, Shim J, et al. Coronary artery calcium scoring does not add prognostic value to standard 64-section CT angiography protocol in low-risk patients suspected of having coronary artery disease. Radiology. 2011;259(1):92–99. doi: 10.1148/RADIOL.10100886/ASSET/IMAGES/LARGE/100886FIG02.JPEG [DOI] [PubMed] [Google Scholar]
- 82.Thoenes M, Oguchi A, Nagamia S, et al. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int J Clin Pract. 2007;61(11):1942–1948. doi: 10.1111/J.1742-1241.2007.01597.X [DOI] [PubMed] [Google Scholar]
- 83.Crouse JR, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297(12):1344–1353. doi: 10.1001/JAMA.297.12.1344 [DOI] [PubMed] [Google Scholar]
- 84.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–1406. doi: 10.1161/01.CIR.74.6.1399 [DOI] [PubMed] [Google Scholar]
- 85.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–269. doi: 10.7326/0003-4819-128-4-199802150-00002 [DOI] [PubMed] [Google Scholar]
- 86.Yamasaki Y, Kodama M, Nishizawa H, et al. Carotid intima-media thickness in Japanese type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care. 2000;23(9):1310–1315. doi: 10.2337/DIACARE.23.9.1310 [DOI] [PubMed] [Google Scholar]
- 87.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–1437. doi: 10.1161/01.CIR.96.5.1432 [DOI] [PubMed] [Google Scholar]
- 88.Berry JD, Liu K, Folsom AR, et al. PREVALENCE AND PROGRESSION OF SUBCLINICAL ATHEROSCLEROSIS IN YOUNGER ADULTS WITH LOW SHORT-TERM BUT HIGH LIFETIME ESTIMATED RISK FOR CARDIOVASCULAR DISEASE: THE CARDIA AND MESA STUDIES. Circulation. 2009;119(3):382. doi: 10.1161/CIRCULATIONAHA.108.800235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103 [DOI] [PubMed] [Google Scholar]
- 90.Urbina EM, Srinivasan SR, Tang R, Bond MG, Kieltyka L, Berenson GS. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study). Am J Cardiol. 2002;90(9):953–958. doi: 10.1016/S0002-9149(02)02660-7 [DOI] [PubMed] [Google Scholar]
- 91.Finn A v., Kolodgie FD, Virmani R. Correlation Between Carotid Intimal/Medial Thickness and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(2):177–181. doi: 10.1161/ATVBAHA.108.173609 [DOI] [PubMed] [Google Scholar]
- 92.Loucks EB, Taylor SE, Polak JF, Wilhelm A, Kalra P, Matthews KA. CHILDHOOD FAMILY PSYCHOSOCIAL ENVIRONMENT AND CAROTID INTIMA MEDIA THICKNESS: THE CARDIA STUDY. Soc Sci Med. 2014;104:15. doi: 10.1016/J.SOCSCIMED.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Polak JF, Person SD, Wei GS, et al. Segment-Specific Associations of Carotid IMT with Cardiovascular Risk Factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke; a journal of cerebral circulation. 2010;41(1):9. doi: 10.1161/STROKEAHA.109.566596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joakimsen O, Bønaa KH, Stensland-Bugge E. Reproducibility of Ultrasound Assessment of Carotid Plaque Occurrence, Thickness, and Morphology. Stroke. 1997;28(11):2201–2207. doi: 10.1161/01.STR.28.11.2201 [DOI] [PubMed] [Google Scholar]
- 95.Velázquez F, Berná JD, Abellán JL, Serrano L, Escribano A, Canteras M. Reproducibility of sonographic measurements of carotid intima-media thickness. Acta radiol. 2008;49(10):1162–1166. doi: 10.1080/02841850802438520 [DOI] [PubMed] [Google Scholar]
- 96.van den Munckhof ICL, Jones H, Hopman MTE, et al. Relation between age and carotid artery intima-medial thickness: a systematic review. Clin Cardiol. 2018;41(5):698. doi: 10.1002/CLC.22934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Young W, Gofman JW, Tandy R, Malamud N, Waters ESG. The quantitation of atherosclerosis. II. Quantitative aspects of the relationship of blood pressure and atherosclerosis. Am J Cardiol. 1960;6(2):294–299. doi: 10.1016/0002-9149(60)90318-0 [DOI] [PubMed] [Google Scholar]
- 98.Nwabuo CC, Yano Y, Moreira HT, et al. Long-Term blood pressure variability in young adulthood and coronary artery calcium and carotid intima-media thickness in midlife: The cardia study. Hypertension. Published online 2020:404–409. doi: 10.1161/HYPERTENSIONAHA.120.15394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fayad ZA, Nahar T, Fallon JT, et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation. 2000;101(21):2503–2509. doi: 10.1161/01.CIR.101.21.2503 [DOI] [PubMed] [Google Scholar]
- 100.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106(11):1368–1373. doi: 10.1161/01.CIR.0000028591.44554.F9 [DOI] [PubMed] [Google Scholar]
- 101.Chan SK, Jaffer FA, Botnar RM, et al. Scan reproducibility of magnetic resonance imaging assessment of aortic atherosclerosis burden. J Cardiovasc Magn Reson. 2001;3(4):331–338. doi: 10.1081/JCMR-100108587 [DOI] [PubMed] [Google Scholar]
- 102.Kerwin WS, Hatsukami T, Yuan C, Zhao XQ. MRI of Carotid Atherosclerosis. AJR Am J Roentgenol. 2013;200(3):W304. doi: 10.2214/AJR.12.8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wüst RCI, Calcagno C, Daal MRR, Nederveen AJ, Coolen BF, Strijkers GJ. Emerging Magnetic Resonance Imaging Techniques for Atherosclerosis Imaging. Arterioscler Thromb Vasc Biol. 2019;39(5):841–849. doi: 10.1161/ATVBAHA.118.311756 [DOI] [PubMed] [Google Scholar]
- 104.Yuan C, Mitsumori LM, Beach KW, Maravilla KR. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology. 2001;221(2):285–299. doi: 10.1148/RADIOL.2212001612 [DOI] [PubMed] [Google Scholar]
- 105.Levitzky YS, Cupples LA, Murabito JM, et al. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101(3):326–331. doi: 10.1016/J.AMJCARD.2007.08.032 [DOI] [PubMed] [Google Scholar]
- 106.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DAM, Witteman JCM. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109(9):1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B [DOI] [PubMed] [Google Scholar]
- 107.Hoffmann U, Massaro JM, D’Agostino RB, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2). doi: 10.1161/JAHA.115.003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does Nonenhanced CT-based Quantification of Abdominal Aortic Calcification Outperform the Framingham Risk Score in Predicting Cardiovascular Events in Asymptomatic Adults? Radiology. 2019;290(1):108–115. doi: 10.1148/RADIOL.2018180562 [DOI] [PubMed] [Google Scholar]
- 109.Ibanez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) Study: JACC Focus Seminar 7/8. J Am Coll Cardiol. 2021;78(2):156–179. doi: 10.1016/J.JACC.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 110.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/RADIOL.2341040439/ASSET/IMAGES/LARGE/R05JA03G03X.JPEG [DOI] [PubMed] [Google Scholar]
- 111.Lv L, Wu S, Yang Y, Yue X. Modified effect of active or passive smoking on the association between age and abdominal aortic calcification: a nationally representative cross-sectional study. BMJ Open. 2021;11(10):e047645. doi: 10.1136/BMJOPEN-2020-047645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du Kim E, Kim JS, Kim SS, et al. Association of Abdominal Aortic Calcification with Lifestyle and Risk Factors of Cardiovascular Disease. Korean J Fam Med. 2013;34(3):213. doi: 10.4082/KJFM.2013.34.3.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jung JG, Wu LT, Kim JS, Du Kim E, Yoon SJ. Relationship between Smoking and Abdominal Aorta Calcification on Computed Tomography. Korean J Fam Med. 2019;40(4):248. doi: 10.4082/KJFM.17.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jurgens PT, Carr JJ, Terry JG, Rana JS, Jacobs DR, Duprez DA. Association of Abdominal Aorta Calcium and Coronary Artery Calcium with Incident Cardiovascular and Coronary Heart Disease Events in Black and White Middle-Aged People: The Coronary Artery Risk Development in Young Adults Study. J Am Heart Assoc. 2021;10(24). doi: 10.1161/JAHA.121.023037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bartstra JW, Mali WPTM, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol. 2021;28(12):1386–1391. doi: 10.1177/2047487320919895 [DOI] [PubMed] [Google Scholar]
- 116.Szulc P Abdominal aortic calcification: A reappraisal of epidemiological and pathophysiological data. Bone. 2016;84:25–37. doi: 10.1016/J.BONE.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 117.Camici PG, Crea F. Coronary Microvascular Dysfunction. https://doi.org/101056/NEJMra061889. 2007;356(8):830–840. doi: 10.1056/NEJMRA061889 [DOI] [PubMed] [Google Scholar]
- 118.Schelbert HR. Anatomy and physiology of coronary blood flow. Journal of Nuclear Cardiology. 2010;17(4):545–554. doi: 10.1007/S12350-010-9255-X/FIGURES/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mileva N, Nagumo S, Mizukami T, et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J Am Heart Assoc. 2022;11(7):23207. doi: 10.1161/JAHA.121.023207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galiuto L, de Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31(11):1319–1327. doi: 10.1093/EURHEARTJ/EHQ039 [DOI] [PubMed] [Google Scholar]
- 121.Elgendy IY, Pepine CJ. Heart Failure With Preserved Ejection Fraction: Is Ischemia Due to Coronary Microvascular Dysfunction a Mechanistic Factor? American Journal of Medicine. 2019;132(6):692–697. doi: 10.1016/j.amjmed.2018.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Y-Hassan S Coronary microvascular dysfunction in Takotsubo syndrome: cause or consequence. Am J Cardiovasc Dis. 2021;11(2):184. Accessed April 21, 2023. /pmc/articles/PMC8166587/ [PMC free article] [PubMed] [Google Scholar]
- 123.Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(21):2625. doi: 10.1016/J.JACC.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC Cardiovasc Imaging. 2015;8(2):210–220. doi: 10.1016/J.JCMG.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerber BL. Quantification of Myocardial Perfusion and Myocardial Perfusion Reserve by Positron Emission Tomography and Cardiovascular Magnetic Resonance Imaging. J Am Coll Cardiol. 2012;60(16):1556–1557. doi: 10.1016/J.JACC.2012.05.051 [DOI] [PubMed] [Google Scholar]
- 126.Gould KL, Johnson NP. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(21):2642–2662. doi: 10.1016/J.JACC.2018.07.106 [DOI] [PubMed] [Google Scholar]
- 127.Porter TR, Xie F, Kricsfeld D, Armbruster RW. Improved myocardial contrast with second harmonic transient ultrasound response imaging in humans using intravenous perfluorocarbon-exposed sonicated dextrose albumin. J Am Coll Cardiol. 1996;27(6):1497–1501. doi: 10.1016/0735-1097(96)00017-4 [DOI] [PubMed] [Google Scholar]
- 128.Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions Between Microbubbles and Ultrasound: In Vitro and In Vivo Observations. J Am Coll Cardiol. 1997;29(5):1081–1088. doi: 10.1016/S0735-1097(97)00029-6 [DOI] [PubMed] [Google Scholar]
- 129.George RT, Mehra VC, Chen MY, et al. Myocardial CT Perfusion Imaging and SPECT for the Diagnosis of Coronary Artery Disease: A Head-to-Head Comparison from the CORE320 Multicenter Diagnostic Performance Study. Radiology. 2014;272(2):407. doi: 10.1148/RADIOL.14140806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Danad I, Szymonifka J, Schulman-Marcus J, Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging. 2016;17(8):836–844. doi: 10.1093/EHJCI/JEW044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Branch KR, Haley RD, Bittencourt MS, Patel AR, Hulten E, Blankstein R. Myocardial computed tomography perfusion. Cardiovasc Diagn Ther. 2017;7(5):45262–45462. doi: 10.21037/CDT.2017.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.George RT, Jerosch-Herold M, Silva C, et al. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007;42(12):815–822. doi: 10.1097/RLI.0B013E318124A884 [DOI] [PubMed] [Google Scholar]
- 133.Sand NPR, Veien KT, Nielsen SS, et al. Prospective Comparison of FFR Derived From Coronary CT Angiography With SPECT Perfusion Imaging in Stable Coronary Artery Disease: The ReASSESS Study. JACC Cardiovasc Imaging. 2018;11(11):1640–1650. doi: 10.1016/J.JCMG.2018.05.004/SUPPL_FILE/MMC1.DOCX [DOI] [PubMed] [Google Scholar]
- 134.Ritter C, Brackertz A, Sandstede J, Beer M, Hahn D, Köstler H. Absolute quantification of myocardial perfusion under adenosine stress. Magn Reson Med. 2006;56(4):844–849. doi: 10.1002/MRM.21020 [DOI] [PubMed] [Google Scholar]
- 135.Hsu LY, Jacobs M, Benovoy M, et al. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging. 2018;11(5):697–707. doi: 10.1016/J.JCMG.2018.01.005/SUPPL_FILE/MMC1.DOCX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel AR, Antkowiak PF, Nandalur KR, et al. Assessment of Advanced Coronary Artery Disease: Advantages of Quantitative Cardiac Magnetic Resonance Perfusion Analysis. J Am Coll Cardiol. 2010;56(7):561–569. doi: 10.1016/J.JACC.2010.02.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Costa MA, Shoemaker S, Futamatsu H, et al. Quantitative Magnetic Resonance Perfusion Imaging Detects Anatomic and Physiologic Coronary Artery Disease as Measured by Coronary Angiography and Fractional Flow Reserve. J Am Coll Cardiol. 2007;50(6):514–522. doi: 10.1016/J.JACC.2007.04.053 [DOI] [PubMed] [Google Scholar]
- 138.Thomson LEJ, Wei J, Agarwal M, et al. Cardiac Magnetic Resonance Myocardial Perfusion Reserve Index Is Reduced in Women With Coronary Microvascular Dysfunction. Circ Cardiovasc Imaging. 2015;8(4). doi: 10.1161/CIRCIMAGING.114.002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salerno M, Beller GA. Noninvasive Assessment of Myocardial Perfusion. Circ Cardiovasc Imaging. 2009;2(5):412–424. doi: 10.1161/CIRCIMAGING.109.854893 [DOI] [PubMed] [Google Scholar]
- 140.Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2(4):343–358. doi: 10.1007/S40336-014-0070-2/FIGURES/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the Reproducibility of Baseline and Hyperemic Myocardial Blood Flow Measurements with 15O-Labeled Water and PET. Journal of Nuclear Medicine. 1999;40(11):1848–1856. Accessed October 18, 2022. https://jnm.snmjournals.org/content/40/11/1848 [PubMed] [Google Scholar]
- 142.el Fakhri G, Kardan A, Sitek A, et al. Reproducibility and Accuracy of Quantitative Myocardial Blood Flow Assessment with 82Rb PET: Comparison with 13N-Ammonia PET. Journal of Nuclear Medicine. 2009;50(7):1062–1071. doi: 10.2967/JNUMED.104.007831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–3287. doi: 10.1093/CVR/CVAC013 [DOI] [PubMed] [Google Scholar]
- 144.Kotecha D, Lam CSP, van Veldhuisen DJ, van Gelder IC, Voors AA, Rienstra M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol. 2016;68(20):2217–2228. doi: 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 145.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507–515. doi: 10.1038/nrcardio.2014.83 [DOI] [PubMed] [Google Scholar]
- 146.Packer M HFpEF Is the Substrate for Stroke in Obesity and Diabetes Independent of Atrial Fibrillation. JACC Heart Fail. 2020;8(1):35–42. doi: 10.1016/j.jchf.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 147.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine. 2006;355(3):251–259. doi: 10.1056/nejmoa052256 [DOI] [PubMed] [Google Scholar]
- 148.Faulkner KM, Dickson VV, Fletcher J, et al. Cognitive Impairment is Associated with Abnormal Cardiac Hemodynamics in Heart Failure with Preserved Ejection Fraction. J Card Fail. 2019;25(8):S4. doi: 10.1016/j.cardfail.2019.07.538 [DOI] [Google Scholar]
- 149.Samson R, Jaiswal A, Ennezat P v., Cassidy M, Jemtel THL. Clinical Phenotypes in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5(1):1–15. doi: 10.1161/JAHA.115.002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma K, Kass DA. Heart failure with preserved ejection fraction: Mechanisms, clinical features, and therapies. Circ Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pfeffer MA, Shah AM, Borlaug BA. Heart Failure with Preserved Ejection Fraction in Perspective. Circ Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Heerebeek L, Borbély A, Niessen HWM, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113(16):1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519 [DOI] [PubMed] [Google Scholar]
- 153.Smiseth OA, Morris DA, Cardim N, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23(2):e34–e61. doi: 10.1093/EHJCI/JEAB154 [DOI] [PubMed] [Google Scholar]
- 154.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: Functional significance and regulatory factors. Cardiovasc Res. 1993;27(3):341–348. doi: 10.1093/cvr/27.3.341 [DOI] [PubMed] [Google Scholar]
- 155.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cellular and Molecular Life Sciences. 2014;71(4):549–574. doi: 10.1007/s00018-013-1349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: The fibroblast awakens. Circ Res. 2016;118(6):1021–1040. doi: 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Imai M, Ambale Venkatesh B, Samiei S, et al. Multi-Ethnic Study of Atherosclerosis: Association between Left Atrial Function Using Tissue Tracking from Cine MR Imaging and Myocardial Fibrosis. Radiology. 2014;273(3):703–713. doi: 10.1148/radiol.14131971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Markman TM, Habibi M, Venkatesh BA, et al. Association of left atrial structure and function and incident cardiovascular disease in patients with diabetes mellitus: Results from multi-ethnic study of atherosclerosis (MESA). Eur Heart J Cardiovasc Imaging. 2017;18(10):1138–1144. doi: 10.1093/ehjci/jew332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Habibi M, Lima JAC, Khurram IM, et al. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation cardiac magnetic resonance study. Circ Cardiovasc Imaging. 2015;8(2). doi: 10.1161/CIRCIMAGING.114.002769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kalogirou F, Forsyth F, Kyriakou M, Mantle R, Deaton C. Heart failure disease management: a systematic review of effectiveness in heart failure with preserved ejection fraction. ESC Heart Fail. 2020;7(1):195–213. doi: 10.1002/EHF2.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nagueh SF, Chang SM, Nabi F, Shah DJ, Estep JD. Cardiac imaging in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10(9). doi: 10.1161/CIRCIMAGING.117.006547 [DOI] [PubMed] [Google Scholar]
- 162.Argulian E, Narula J. Advanced Cardiovascular Imaging in Clinical Heart Failure. Heart Fail. 2021;9(10):699–709. doi: 10.1016/J.JCHF.2021.06.016 [DOI] [PubMed] [Google Scholar]
- 163.Yoneyama K, Venkatesh BA, Bluemke DA, McClelland RL, Lima JAC. Cardiovascular magnetic resonance in an adult human population: Serial observations from the multi-ethnic study of atherosclerosis. Journal of Cardiovascular Magnetic Resonance. 2017;19(1):52. doi: 10.1186/s12968-017-0367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gjesdal O, Yoneyama K, Mewton N, et al. Reduced long axis strain is associated with heart failure and cardiovascular events in the multi-ethnic study of Atherosclerosis. Journal of Magnetic Resonance Imaging. 2016;44(1):178–185. doi: 10.1002/jmri.25135 [DOI] [PubMed] [Google Scholar]
- 165.Yoneyama K, Gjesdal O, Choi EY, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: The multi-ethnic study of atherosclerosis. Circulation. 2012;126(21):2481–2490. doi: 10.1161/CIRCULATIONAHA.112.093146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Venkatesh BA, Volpe GJ, Donekal S, et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: The Multi-Ethnic study of Atherosclerosis study. Hypertension. 2014;64(3):508–515. doi: 10.1161/HYPERTENSIONAHA.114.03697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JAC. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2009;2(3):191–198. doi: 10.1161/CIRCIMAGING.108.819938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Strait JB, Lakatta EG. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail Clin. 2012;8(1):143–164. doi: 10.1016/j.hfc.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rider OJ, Lewandowski A, Nethononda R, et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J. 2013;34(4):292–299. doi: 10.1093/eurheartj/ehs341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level-risk factors, screening, and outcomes. Nat Rev Cardiol. 2011;8(12):673–685. doi: 10.1038/nrcardio.2011.154 [DOI] [PubMed] [Google Scholar]
- 171.Gardin JM, Brunner D, Schreiner PJ, et al. Demographics and correlates of five-year change in echocardiographic left ventricular mass in young black and white adult men and women: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Coll Cardiol. 2002;40(3):529–535. doi: 10.1016/S0735-1097(02)01973-3 [DOI] [PubMed] [Google Scholar]
- 172.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of Cardiovascular Risk Factors to Echocardiographic Left Ventricular Mass in Healthy Young Black and White Adult Men and Women. Circulation. 1995;92(3):380–387. doi: 10.1161/01.CIR.92.3.380 [DOI] [PubMed] [Google Scholar]
- 173.Perak AM, Khan SS, Colangelo LA, et al. Age-Related Development of Cardiac Remodeling and Dysfunction in Young Black and White Adults: The Coronary Artery Risk Development in Young Adults Study. Journal of the American Society of Echocardiography. 2021;34(4):388–400. doi: 10.1016/J.ECHO.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gidding SS, Liu K, Colangelo LA, et al. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging. 2013;6(5):769–775. doi: 10.1161/CIRCIMAGING.112.000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gidding SS, Xie X, Liu K, Manolio T, Flack JM, Gardin JM. Cardiac function in smokers and nonsmokers: The CARDIA study. J Am Coll Cardiol. 1995;26(1):211–216. doi: 10.1016/0735-1097(95)00118-J [DOI] [PubMed] [Google Scholar]
- 176.Nwabuo CC, Moreira HT, Vasconcellos HD, et al. Left ventricular global function index predicts incident heart failure and cardiovascular disease in young adults: the coronary artery risk development in young adults (CARDIA) study. Eur Heart J Cardiovasc Imaging. 2019;20(5):533–540. doi: 10.1093/EHJCI/JEY123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moreira HT, Nwabuo CC, Armstrong AC, et al. Reference Ranges and Regional Patterns of Left Ventricular Strain and Strain Rate Using Two-Dimensional Speckle-Tracking Echocardiography in a Healthy Middle-Aged Black and White Population: The CARDIA Study. Journal of the American Society of Echocardiography. 2017;30(7):647–658.e2. doi: 10.1016/j.echo.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kishi S, Armstrong AC, Gidding SS, et al. Relation of Left Ventricular Mass at Age 23 to 35 Years to Global Left Ventricular Systolic Function 20 Years Later (from the Coronary Artery Risk Development in Young Adults Study). Am J Cardiol. 2014;113(2):377–383. doi: 10.1016/J.AMJCARD.2013.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin Y, Zhong X, Xiong Z, et al. Intensity of Glycemic Exposure in Early Adulthood and Target Organ Damage in Middle Age: The CARDIA Study. Front Physiol. 2021;12(June):1–9. doi: 10.3389/fphys.2021.614532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: The CARDIA study. J Am Coll Cardiol. 2015;65(25):2679–2687. doi: 10.1016/J.JACC.2015.04.042 [DOI] [PubMed] [Google Scholar]
- 181.Liu S, Liao Y, Zhu Z, et al. Association between cumulative blood pressure in early adulthood and right ventricular structure and function in middle age: The CARDIA study. Clin Cardiol. 2022;45(1):83–90. doi: 10.1002/CLC.23763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of Cardiovascular Risk Factors to Echocardiographic Left Ventricular Mass in Healthy Young Black and White Adult Men and Women. Circulation. 1995;92(3):380–387. doi: 10.1161/01.CIR.92.3.380 [DOI] [PubMed] [Google Scholar]
- 183.Reis JP, Allen N, Gibbs BB, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: The CARDIA study. Obesity. 2014;22(11):2434–2440. doi: 10.1002/OBY.20865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Habibi M, Samiei S, Venkatesh BA, et al. Cardiac magnetic resonance-measured left atrial volume and function and incident atrial fibrillation. Circ Cardiovasc Imaging. 2016;9(8). doi: 10.1161/CIRCIMAGING.115.004299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Habibi M, Chahal H, Opdahl A, et al. Association of CMR-measured la function with heart failure development: Results from the MESA study. JACC Cardiovasc Imaging. 2014;7(6):570–579. doi: 10.1016/j.jcmg.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Raisi-Estabragh Z, McCracken C, Condurache DG, et al. Left atrial structure and function are associated with cardiovascular outcomes independent of left ventricular measures: a UK Biobank CMR study. Eur Heart J Cardiovasc Imaging. 2022;23(9):1191. doi: 10.1093/EHJCI/JEAB266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Armstrong AC, Liu K, Lewis CE, et al. Editor’s choice: Left atrial dimension and traditional cardiovascular risk factors predict 20-year clinical cardiovascular events in young healthy adults: the CARDIA study. Eur Heart J Cardiovasc Imaging. 2014;15(8):893. doi: 10.1093/EHJCI/JEU018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Petersen SE, Aung N, Sanghvi MM, et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. Journal of Cardiovascular Magnetic Resonance. 2017;19(1):1–19. doi: 10.1186/S12968-017-0327-9/FIGURES/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Petersen SE, Sanghvi MM, Aung N, et al. The impact of cardiovascular risk factors on cardiac structure and function: Insights from the UK Biobank imaging enhancement study. PLoS One. 2017;12(10):e0185114–e0185114. doi: 10.1371/JOURNAL.PONE.0185114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Reis JP, Loria CM, Steffen LM, et al. Coffee, Decaffeinated Coffee, Caffeine, and Tea Consumption in Young Adulthood and Atherosclerosis Later in Life: The CARDIA Study. Arterioscler Thromb Vasc Biol. 2010;30(10):2059. doi: 10.1161/ATVBAHA.110.208280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grothues F, Smith GC, Moon JCC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. American Journal of Cardiology. 2002;90(1):29–34. doi: 10.1016/S0002-9149(02)02381-0 [DOI] [PubMed] [Google Scholar]
- 192.Myerson SG, Bellenger NG, Pennell DJ. Assessment of Left Ventricular Mass by Cardiovascular Magnetic Resonance. Hypertension. 2002;39(3):750–755. doi: 10.1161/HY0302.104674 [DOI] [PubMed] [Google Scholar]
- 193.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. doi: 10.1016/j.jacc.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10(1):15–26. doi: 10.1038/nrcardio.2012.158 [DOI] [PubMed] [Google Scholar]
- 195.Martinez-Naharro A, Kotecha T, Norrington K, et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc Imaging. 2019;12(5):810–819. doi: 10.1016/j.jcmg.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 196.Ambale-Venkatesh B, Lima JAC. Cardiac MRI: A central prognostic tool in myocardial fibrosis. Nat Rev Cardiol. 2015;12(1):18–29. doi: 10.1038/nrcardio.2014.159 [DOI] [PubMed] [Google Scholar]
- 197.Liu CY, Lai S, Lima JAC. MRI gadolinium dosing on basis of blood volume. Magn Reson Med. 2019;81(2):1157–1164. doi: 10.1002/MRM.27454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zareian M, Ciuffo L, Habibi M, et al. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: Validation and reproducibility assessment. Journal of Cardiovascular Magnetic Resonance. 2015;17(1). doi: 10.1186/s12968-015-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Varadarajan V, Ambale-Venkatesh B, Hong SY, et al. Association of Longitudinal Changes in NT-proBNP With Changes in Left Atrial Volume and Function: MESA. Am J Hypertens. 2021;34(6):626–635. doi: 10.1093/ajh/hpab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pezel T, Ambale-Venkatesh B, Quinaglia T, et al. Change in Left Atrioventricular Coupling Index to Predict Incident Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology. 2022;303(2):317–326. doi: 10.1148/RADIOL.210315/ASSET/IMAGES/LARGE/RADIOL.210315.VA.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pezel T, Venkatesh BA, De Vasconcellos HD, et al. Left Atrioventricular Coupling Index as a Prognostic Marker of Cardiovascular Events: The MESA Study. Hypertension. Published online 2021:661–671. doi: 10.1161/HYPERTENSIONAHA.121.17339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pezel T, Ambale Venkatesh B, Kato Y, et al. Left Atrioventricular Coupling Index to Predict Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Front Cardiovasc Med. 2021;0:1065. doi: 10.3389/FCVM.2021.704611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pezel T, Michos ED, Varadarajan V, et al. Prognostic value of a left atrioventricular coupling index in pre- and post-menopausal women from the Multi-Ethnic Study of Atherosclerosis. Front Cardiovasc Med. 2022;9:3378. doi: 10.3389/FCVM.2022.1066849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Backhaus SJ, Kowallick JT, Stiermaier T, et al. Atrioventricular mechanical coupling and major adverse cardiac events in female patients following acute ST elevation myocardial infarction. Int J Cardiol. 2020;299:31–36. doi: 10.1016/J.IJCARD.2019.06.036 [DOI] [PubMed] [Google Scholar]
- 205.Germans T, Götte MJW, Nijveldt R, et al. Effects of Aging on Left Atrioventricular Coupling and Left Ventricular Filling Assessed Using Cardiac Magnetic Resonance Imaging in Healthy Subjects. American Journal of Cardiology. 2007;100(1):122–127. doi: 10.1016/j.amjcard.2007.02.060 [DOI] [PubMed] [Google Scholar]
- 206.Meucci MC, Fortuni F, Galloo X, et al. Left atrioventricular coupling index in hypertrophic cardiomyopathy and risk of new-onset atrial fibrillation. Int J Cardiol. 2022;363:87–93. doi: 10.1016/J.IJCARD.2022.06.017 [DOI] [PubMed] [Google Scholar]
- 207.Vijiiac AE, Scarlatescu A, Verinceanu V, et al. Three-dimensional left and right atrioventricular coupling indices as prognostic markers in heart failure with reduced ejection fraction. Eur Heart J. 2022;43(Supplement_2). doi: 10.1093/EURHEARTJ/EHAC544.095 [DOI] [Google Scholar]
- 208.Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ambale-Venkatesh B, Yang X, Wu CO, et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ Res. 2017;121(9):1092–1101. doi: 10.1161/CIRCRESAHA.117.311312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ishwaran H, Kogalur UB, Gorodeski EZ, Minn AJ, Lauer MS. High-dimensional variable selection for survival data. J Am Stat Assoc. 2010;105(489):205–217. doi: 10.1198/jasa.2009.tm08622 [DOI] [Google Scholar]
- 211.Ambale-Venkatesh B, Yang X, Wu CO, et al. Cardiovascular Event Prediction by Machine Learning Novelty and Significance. Circ Res. 2017;121(9):1092–1101. doi: 10.1161/CIRCRESAHA.117.311312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weng S, Reps J, Kai J, Garibaldi J, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944. doi: 10.1371/journal.pone.0174944.t004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leiner T, Rueckert D, Suinesiaputra A, et al. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. Journal of Cardiovascular Magnetic Resonance. 2019;21(1):61. doi: 10.1186/s12968-019-0575-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Winkel DJ, Suryanarayana VR, Ali AM, et al. Deep learning for vessel-specific coronary artery calcium scoring: validation on a multi-centre dataset. Eur Heart J Cardiovasc Imaging. 2022;23(6):846–854. doi: 10.1093/EHJCI/JEAB119 [DOI] [PubMed] [Google Scholar]
- 215.Bhuva AN, Bai W, Lau C, et al. A Multicenter, Scan-Rescan, Human and Machine Learning CMR Study to Test Generalizability and Precision in Imaging Biomarker Analysis. Circ Cardiovasc Imaging. 2019;12(10). doi: 10.1161/CIRCIMAGING.119.009214 [DOI] [PubMed] [Google Scholar]
- 216.van Assen M, Razavi AC, Whelton SP, De Cecco CN. Artificial intelligence in cardiac imaging: where we are and what we want. Eur Heart J. 2023;44(7):541–543. doi: 10.1093/EURHEARTJ/EHAC700 [DOI] [PubMed] [Google Scholar]
- 217.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease . New England Journal of Medicine. 2006;354(12):1264–1272. doi: 10.1056/NEJMOA054013 [DOI] [PubMed] [Google Scholar]
- 219.Yang J, Biery DW, Singh A, et al. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am J Med. 2020;133(5):605. doi: 10.1016/J.AMJMED.2019.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zeitouni M, Nanna MG, Sun JL, Chiswell K, Peterson ED, Navar AM. Performance of Guideline Recommendations for Prevention of Myocardial Infarction in Young Adults. J Am Coll Cardiol. 2020;76(6):653. doi: 10.1016/J.JACC.2020.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pencina MJ, Pencina KM, Lloyd-Jones D, Catapano AL, Thanassoulis G, Sniderman AD. The Expected 30-Year Benefits of Early Versus Delayed Primary Prevention of Cardiovascular Disease by Lipid Lowering. Circulation. 2020;142(9):827–837. doi: 10.1161/CIRCULATIONAHA.120.045851 [DOI] [PubMed] [Google Scholar]
- 222.Davidson MH. Emerging low-density lipoprotein therapies: Targeting PCSK9 for low-density lipoprotein reduction. J Clin Lipidol. 2013;7(3):S11–S15. doi: 10.1016/J.JACL.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 223.Robinson JG, Gidding SS. Curing atherosclerosis should be the next major cardiovascular prevention goal. J Am Coll Cardiol. 2014;63(25 Pt A):2779–2785. doi: 10.1016/J.JACC.2014.04.009 [DOI] [PubMed] [Google Scholar]