Abstract
Kaposi’s sarcoma (KS) is the most common neoplasm in patients with AIDS. Epidemiologic evidence and the recent identification of herpesvirus-like DNA sequences in patients with KS have suggested a role for viral agents in the etiopathogenesis of this disease. It is unclear if these sequences are present in all types of KS and if the copy number of these sequences has a correlation with disease severity (staging). In order to clarify these issues, we retrospectively analyzed, by PCR and Southern blotting, formalin-fixed, paraffin-embedded biopsy specimens from 12 patients previously diagnosed with KS by histopathologic examination of these specimens between the years of 1977 and 1996. We also analyzed tissue samples from these patients taken from dermal sites without KS lesions and control tissues from healthy subjects. Of the 12 patients, 6 had classic KS, 5 had AIDS-associated KS, and 1 had the endemic type of KS. We tested the specimens for other herpesviruses, including cytomegalovirus, human herpesvirus 6 (HHV-6), HHV-7, HHV-8, and Epstein-Barr virus. Of 20 biopsy specimens from patients previously diagnosed with KS, 19 were positive for HHV-8 sequences (95%), while PCR for the DNAs of other herpesvirus agents was negative. Uninvolved tissue from these patients and control tissue from healthy subjects gave negative results for all viruses. Semiquantitative analysis of Southern blots showed higher levels of HHV-8 DNA in those patients with multicentric and visceral involvement than in those patients with localized involvement. In addition, in patients with localized skin disease, the nodular stage had higher levels of HHV-8 DNA than the patch or plaque stages. Our data confirmed that HHV-8 is involved in the etiopathogenesis of all types of KS and that there is a correlation between HHV-8 DNA load and the severity and staging of this disease.
Kaposi’s sarcoma (KS) is the most common neoplasm in patients with AIDS (4). Described in 1872 by Moritz Kaposi, KS is a complex tumor of uncertain histogenesis with a spindle-shaped cell proliferation and an inflammatory component. There is controversy whether this is a true neoplasm versus a hyperplastic lesion. Epidemiologic studies and behavorial and geographic risk factors suggest an infectious cause of KS; a sexually transmitted agent is probably the most likely etiologic factor involved in this tumor (14).
In 1972, Giraldo and coworkers (10) found herpesvirus-like particles in KS lesions. Nine years later (1981), the Centers for Disease Control (6) reported 26 cases of KS in homosexual men, which is now recognized as the beginning of the human immunodeficiency virus (HIV) epidemic. More recently, in 1994, Chang and colleagues (7) isolated herpesvirus-like DNA sequences from KS lesions from patients with AIDS (7). These sequences corresponded to a new herpesvirus that was called KSHV and that was later defined as human herpesvirus 8 (HHV-8). HHV-8, a rhadinovirus, shares close homology with other lymphotropic oncogenic gamma herpesviruses (Epstein-Barr virus [EBV] and herpesvirus saimiri).
It is not known whether these sequences are present in all types of KS and if the viral load in tissue specimens has a correlation with clinical severity and histologic staging of KS. In order to clarify these issues, we retrospectively analyzed by PCR and Southern blotting formalin-fixed, paraffin-embedded tissues from patients with KS. Twelve patients with biopsy-proven KS and whose KS was diagnosed at the Mayo Clinic between the years of 1977 and 1996 were included in this study. The performance of these assays was evaluated, and the results were correlated with histopathologic findings for biopsy specimens to assess the severity and staging of this disease.
MATERIALS AND METHODS
Clinical samples.
Formalin-fixed, paraffin-embedded biopsy specimens were obtained from 12 patients diagnosed with KS by histopathologic examination between 1977 and 1996. Histopathologic staging was performed by using a previously described classification (2). Of the 12 patients, 6 had classic KS, 5 had AIDS-associated KS, and 1 had the endemic type of KS. As positive controls, we used strains of cytomegalovirus (CMV), EBV, HHV-6, and HHV-7 obtained from the American Type Culture Collection, Rockville, Md., and HHV-8 from an infected BCBL-1 cell line (15).
Several negative controls were used: biopsy specimens from healthy skin or skin with other clinical conditions (psoriasis, dermatitis) from study patients with KS, similar tissue specimens from healthy patients, and liver and lung tissue from patients with no recognized infectious disease.
Extraction of nucleic acids, oligonucleotides, and PCR.
Nucleic acids were extracted from biopsy specimens by using a universal DNA isolation technique described previously (16).
PCR for the detection of CMV, EBV, HHV-6, HHV-7, and HHV-8 was performed by using the oligonucleotide primers described previously (Table 1). The probe corresponding to a region between these oligonucleotide primers was synthesized and labeled for chemiluminescence by using the enhanced chemiluminescence kit from Amersham (Arlington Heights, Ill.).
TABLE 1.
PCR primers and genome targets
As a control of internal consistency, for enzymatic amplification of target sequences, and for semiquantitative analysis of the PCR products, five reference standards were included in each PCR batch. For HHV-8, these standards were prepared by using known amounts of a purified plasmid pCR 2.1 (Invitrogen, Carlsbad, Calif.) constructed with a fragment with the same sequence as the PCR product and equivalent to approximately 10 to 100,000 genome equivalents. For objective evaluation, Southern blots were analyzed by a computer image processing system (NIH Image, National Institutes of Health, Bethesda, Md.).
RESULTS
Of 20 biopsy specimens from 12 patients with KS, for 19 (95%) HHV-8 target sequences were amplified by PCR (6 of 7 [86%] from patients with classic KS, 9 of 9 [100%] from patients with AIDS-associated KS, and 4 of 4 [100%] from patients with the endemic form of KS). All specimens were negative for CMV, EBV, HHV-6, and HHV-7 DNAs. Uninvolved tissue from these specimens and control tissues from healthy subjects (skin with other disorders [n = 9], healthy skin [n = 10], and healthy liver and lung specimens [n = 10]) gave negative PCR results for all viral DNA targets.
Semiquantitative analysis of gel electrophoresis and subsequent Southern blots showed higher levels of HHV-8 DNA in those patients with multicentric and visceral involvement than in those with localized disease (Table 2).
TABLE 2.
Demographic and clinical characteristics of patients with KS
| Patient no. | Age (yr) | Sexa | Type of KS | KS location | Histo- pathology staging | PCR scoreb |
|---|---|---|---|---|---|---|
| 1 | 32 | M | AIDS associated | Face, lips | Nodules | ++++ |
| 2 | 56 | M | AIDS associated | Legs, feet | Nodules | ++++ |
| 3 | 51 | M | Endemic | Colon | Nodules | ++++ |
| Lungs | Metastasis | ++++ | ||||
| 4 | 34 | M | AIDS associated | Feet | Nodules | ++++ |
| Lungs | Metastasis | ++++ | ||||
| 5 | 32 | M | AIDS associated | Cervical lymph nodes | Metastasis | +++ |
| 6 | 99 | M | Classic | Foot | Plaque | ++ |
| 7 | 64 | M | Classic | Foot | Plaque | ++ |
| 8 | 38 | M | AIDS associated | Face | Plaque | ++ |
| 9 | 57 | F | Classic | Leg | Patch | + |
| 10 | 55 | M | Classic | Thigh | Patch | + |
| 11 | 79 | F | Classic | Ear, arm | Patch | + |
| 12 | 84 | M | Classic | Foot | Patch | + |
M, male; F, female.
PCR score: +, 10 genome copies; ++, 100 genome copies; +++, 1,000 genome copies; ++++, 10,000 genome copies; +++++, 100,000 genome copies.
In the patients with localized skin disease, the nodular stage had higher levels of HHV-8 DNA than the patch or the plaque stages (Table 2; Fig. 1).
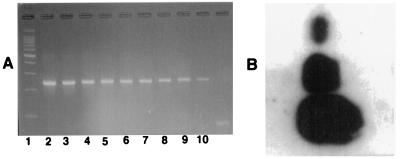
FIG. 1.
Molecular detection of HHV-8 DNA. (A) Gel electrophoresis with dilutions of HHV-8 target DNA of plasmid (pCR 2.1) representing 109 (lane 2) to 101 (lane 10) copies of the viral genome. (B) Southern blotting results for samples of tissue from KS lesions containing HHV-8 DNA. The dots are for the patch, plaque, and nodule stages, from top to bottom, respectively.
DISCUSSION
Epidemiologic studies performed in the past suggested that both the AIDS-associated and the non-AIDS-associated types of KS may be caused by an infectious agent (14). For example, it was shown that homosexual and bisexual males with AIDS were approximately 20 times more likely than hemophiliacs with AIDS to develop KS (3). These and other studies (1) showed that KS may be associated with behavioral and geographic risk factors most consistent with the tumor being caused by a sexually transmitted agent.
Application of novel molecular techniques revealed the strong association of HHV-8 with AIDS-related KS (7). Those investigators extended these findings to a search for HHV-8 DNA sequences in other tissues from KS patients, as well as in KS lesions from patients without HIV infection, and found that HHV-8 was presumably not solely an opportunistic infection in patients with AIDS but that the other three forms of KS seen in patients without HIV infection were caused by the same infectious agent (13).
At the Mayo Clinic, in archival biopsy specimens from patients with clinically diagnosed and histologically confirmed KS, we found HHV-8 DNA in 19 of 20 KS specimens (95%) from patients with different types of KS. Importantly, semiquantitative determination of viral load was directly correlated with the staging and dissemination of this tumor. These results have important clinical implications and diagnostic relevance and emphasize more than ever the pathogenic role of HHV-8 in this tumor. The HHV-8 DNA load could potentially have applications for the early diagnosis, staging, and monitoring of this disease. The identification of tumor growth under viral control, possibly a viral promoter, may lead to novel therapeutic interventions.
Rapid progress in the understanding of the relationship between HHV-8 and KS has been made. Despite this progress, a number of fundamental questions remain unanswered, such as those regarding the mechanisms of transmission, risk factors that place certain population groups such as homosexual men at an increased risk for KS compared to the risk for other groups, and effective interventions through the use of therapeutic agents and other interventions. Although recent studies have shown that HHV-8 is resistant to acyclovir but susceptible to ganciclovir, foscarnet, and cidofovir (11, 12), we still need to develop better and easier antiviral drug susceptibility assays, improve our ability to grow HHV-8 in cell cultures, and perform trials to determine the correlation of these results in clinical practice.
In conclusion, this study has shown that (i) HHV-8 is associated with all types of KS, (ii) higher levels of HHV-8 DNA are found in patients with multicentric and visceral KS than in those with localized skin disease, (iii) for mucocutaneous involvement, the nodular stage had higher levels of HHV-8 DNA than the patch or plaque stages, and (iv) there was an association between HHV-8 DNA load and the severity and staging of this disease. Importantly, our study suggests that understanding of the relationship between viral load and histopathologic stage needs to be expanded by investigations involving a large set of patient specimens examined by sophisticated quantitation techniques.
REFERENCES
- 1.Archibald C P, Schechter M T, Le T N, Craib K J P, Montaner J S G, O’Shaughnessy M V. Evidence for a sexually transmitted cofactor for AIDS-related Kaposi’s sarcoma in a cohort of homosexual men. Epidemiology. 1992;3:203–209. doi: 10.1097/00001648-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Armed Forces Institute of Pathology. Atlas of tumor pathology, 3rd series, fascicle 1. Washington, D.C: Armed Forces Institute of Pathology; 1991. Nonmelanocytic tumors of the skin; pp. 214–219. [Google Scholar]
- 3.Beral V, Bull D, Darby S, et al. Risk of Kaposi’s sarcoma and sexual practices associated with faecal contact in homosexual or bisexual men with AIDS. Lancet. 1992;339:632–635. doi: 10.1016/0140-6736(92)90793-3. [DOI] [PubMed] [Google Scholar]
- 4.Beral V, Peterman T A, Berkelman R L, Jaffe H W. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 5.Berneman Z N, Ablashi D V, Li G, Eger-Fletcher M, Reitz M S, Hung C L, Brus I, Komaroff A L, Gallo R C. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci USA. 1992;89:10552–10556. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Kaposi’s sarcoma and Pneumocystis carinii among homosexual men—New York City and California. Morbid Mortal Weekly Rep. 1981;30:305–307. [PubMed] [Google Scholar]
- 7.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 8.Drouet E, Michelson S, Denoyel G, Colimon R. Polymerase chain reaction detection of human cytomegalovirus in over 2000 blood specimens correlated with virus isolation and related to urinary virus excretion. J Virol Methods. 1993;45:259–276. doi: 10.1016/0166-0934(93)90112-5. [DOI] [PubMed] [Google Scholar]
- 9.Espy M J, Smith T F, Persing D H. Dependence of polymerase chain reaction product inactivation protocols on amplicon length and sequence composition. J Clin Microbiol. 1993;31:2361–2365. doi: 10.1128/jcm.31.9.2361-2365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraldo G, Beth E, Hagenan F. Herpes-type virus particles in tissue culture of Kaposi’s sarcoma from different geographic regions. J Natl Cancer Inst. 1972;49:1509–1526. doi: 10.1093/jnci/49.6.1509. [DOI] [PubMed] [Google Scholar]
- 11.Kedes D H, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. J Clin Invest. 1997;99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medveczky M M, Horvath E, Lund T, Medveczky P G. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. AIDS. 1997;11:1327–1332. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 14.Peterman T A, Jaffe H W, Friedman-Kien A E, Weiss R A. The aetiology of Kaposi’s sarcoma. Cancer Surv. 1991;10:23–27. [PubMed] [Google Scholar]
- 15.Renne R, Zhong W, Herndier B, Kedes D, McGrath M, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in B cell lymphoma cells in culture. Nature Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu G S, Kline B C, Stockman L, Roberts G D. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33:2913–2919. doi: 10.1128/jcm.33.11.2913-2919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt C A, Wilborn F, Weiss K, Brinkmann V, Oettle H, Lohmann R, Langrehr J M, Newhaur P, Siegert W. A prospective study of HHV-6 detected by polymerase chain reaction after liver transplantation. Transplantation. 1995;61:662–664. doi: 10.1097/00007890-199602270-00027. [DOI] [PubMed] [Google Scholar]