Abstract
In recent years, the development of bispecific antibodies (bsAbs) has been rapid, with many new structures and target combinations being created. The boom in bsAbs has led to the successive issuance of industry guidance for their development in the US and China. However, there is a high degree of similarity in target selection, which could affect the development of diversity in bsAbs. This review presents a classification of various bsAbs for cancer therapy based on structure and target selection and examines the advantages of bsAbs over monoclonal antibodies (mAbs). Through database research, we have identified the preferences of available bsAbs combinations, suggesting rational target selection options and warning of potential wastage of medical resources. We have also compared the US and Chinese guidelines for bsAbs in order to provide a reference for their development.
Key words: Bispecific antibody, Target selection, Structure, Regulatory guidance, Cancer immunotherapy, Clinical trials, Oncology, Mechanism
Graphical abstract
This review summarizes the developments of bispecific antibodies, with a focus on structure, target selection and regulatory guidance.
1. Introduction
Over the last three decades, therapeutic antibodies have become a key component of cancer treatment due to their specificity and sensitivity1. The first monoclonal antibody, Muromonab-CD3 (OKT3), was approved for marketing in 19862, Since then, antibody-based drugs have developed rapidly and have become one of the most important types of drugs. In oncology therapy, monoclonal antibody drugs have demonstrated excellent therapeutic effects, such as Rituximab (anti-CD20) and Trastuzumab (anti-HER2), which have been approved for the treatment of B-cell malignancies and breast cancer with promising results3,4.
Bispecific antibodies have been developed to address drug resistance and improve efficacy5. Combination therapies of monoclonal antibodies targeting different receptors or epitopes can enhance treatment efficacy and help to overcome drug resistance6. However, these therapies may also cause higher toxicity7,8. Bispecific antibodies can improve efficacy and safety by simultaneously recognizing and binding two different antigens or antigenic epitopes9. Additionally, they have the unique advantage of redirecting cytotoxic effector cells10.
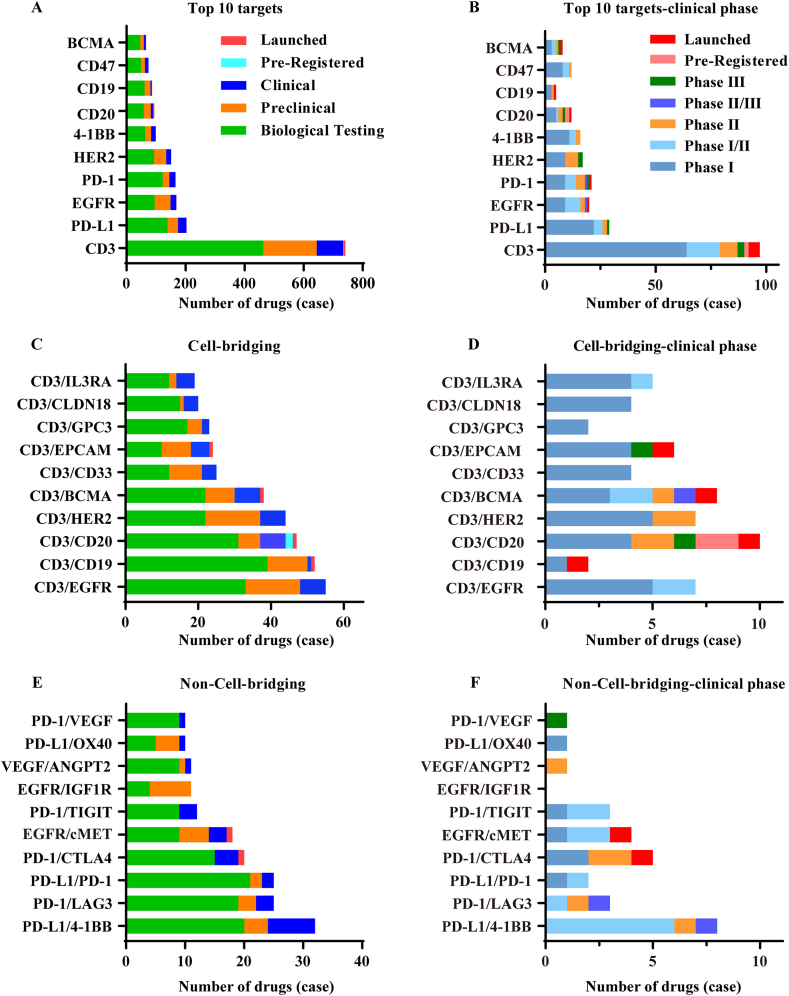
Bispecific antibodies have not been widely explored until the last decade, even though they have shown a specific benefit. Since the first bispecific antibody (Catumaxomab) was launched in 200911, nine bsAbs (seven for tumors) were approved for marketing, and five of them are coming to market in 2021 and 2022 (Table 1). Up to now, more than 200 drugs are being investigated in clinic, with 10 entering Phase III (Fig. 1) (https://www.cortellis.com/drugdiscovery/home)12. It can be expected that a large number of bispecific antibodies will come to market in the next 3–5 years, bringing the development of bispecific antibodies into a high-speed development period.
Table 1.
Approved bispecific antibodies for cancer therapy.
| Name | Targets | Developer | Time to market | Indication |
|---|---|---|---|---|
| Catumaxomab | CD3 × EpCAM | Trion pharma | 2009 (EMA) | Malignant ascites |
| Blinatumomab | CD3 × CD19 | Amgen | 2014(FDA), 2015(EMA) | ALL |
| Mosunetuzumab | CD3 × CD20 | Roche | 2022(EMA), 2022(FDA) | R/R FL |
| Tebentafusp | CD3 × gp100 | Immunocore | 2022(FDA), 2022(EU) | Uveal melanoma |
| Teclistamab | CD3 × BCMA | Janssen | 2022(EU), 2022(FDA) | R/R MM |
| Amivantamab | EGFR × cMET | Janssen | 2021(FDA) | NSCLC |
| Cadonilimab | PD-1 × CTLA-4 | Akeso | 2022(NMPA) | R/M CC |
ALL, acute lymphoblastic leukemia; NSCLC, non-small-cell lung carcinoma; R/M CC, relapsed or metastatic cervical cancer; R/R FL, relapsed or refractory follicular lymphoma; R/R MM, relapsed or refractory multiple myeloma.
Figure 1.
Preferred targets and combinations. (A) The top 10 most widely investigated targets. (B) The top 10 most widely investigated targets–clinical stage. (C) Top 10 selected target combinations of cell-bridging bsAbs and (D) their clinical phases. (E) Top 10 selected target combinations of non-cell-bridging bsAbs and (F) their clinical phases. Information was obtained from Cortellis Drug Discovery Intelligence (https://www.cortellis.com/drugdiscovery/home)12.
It is clear that the development of bispecific antibodies is in a rapid and early stage, and the market competition pattern is unclear. The similarity in target selection may lead to increased competition, but also limit therapeutic diversity and waste medical resources. To ensure a rational design and development strategy, it is important to summarize clinical data, analyze target selection, and clarify regulatory requirements. The FDA and NMPA have issued guidance on bsAbs in 2021 and 2022 respectively13,14, which may help to provide policy regulation.
2. Structure
2.1. Formats
The selection of format and target determines the therapeutic effect, pharmacokinetic characteristics and stability of bispecific antibodies15. The abundance of structural forms provides more solutions to technical problems in bispecific antibody research. We will briefly introduce the format design to provide a better understanding.
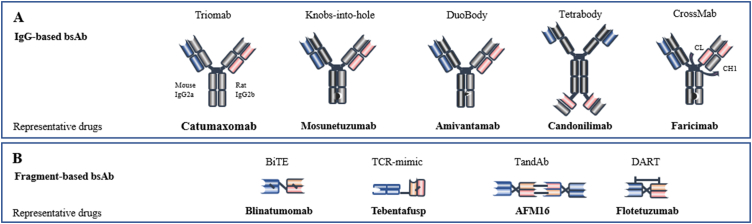
According to the existence of the Fc (fragment crystallizable) region, bispecific antibodies can be divided into two categories: IgG-Based bsAbs and Fragment-Based bsAbs (Fig. 2).
Figure 2.
Representative bispecific antibodies and their format. According to the existence of the Fc region, bispecific antibodies can be divided into two categories: (A) IgG-based bsAbs and (B) Fragment-based bsAbs. BiTE, bispecific T-cell engager; TandAb, tandem diabody; DART, dual affinity retargeting.
2.1.1. IgG-based bsAbs
IgG-Based bispecific antibodies are similar in structure to native antibodies, and all have Fc regions. The Fc region is associated with multiple activities of bispecific antibodies, such as antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cell phagocytosis (ADCP)16. Furthermore, the Fc region of bsAbs may contribute to an increase in half-life17. Additionally, the Fc region facilitates the purification of bsAbs and also promotes their stability and solubility18,19.
However, the IgG-Based bsAbs are also associated with various disadvantages, such as the side effects due to the off-target binding of active Fc domain to FcRs (Fc receptors)20, and the chain-associated issue21.
New formats are being developed to address these problems. For instance, the recently launched mosunetuzumab (anti-CD3/CD20 bispecific antibody) adopted the classic knobs-into-holes format to ensure to correct heavy chain assembly22. This technology has a large amino acid on one chain to create a “knob” and a smaller amino acid on the other chain to create a corresponding “hole”23, which is helpful for the correct assembly of two heterologous antibody heavy chains, thus solving the “chain-associated issue”. The mismatch between non-homologous heavy and light chains is another common problem. A new approach is the CrossMab format, which was created based on the Knobs-into-holes format and further solves the problem of light chain mispairing24. Faricimab (anti-ang-2/VEGF) is designed in this format and is currently approved for the treatment of diabetic macular edema and neovascular (wet) age-related macular degeneration (nAMD)25.
2.1.2. Fragment-based bsAbs
Fragment-based bsAbs are composed of the variable light and heavy domains from two antibodies, or the Fab units, and lack the Fc region which distinguishes them from IgG-Based bsAbs26,27. These fragments are bound together by linkers (e.g., disulfide bonds or non-covalent interactions) and different pharmacokinetic properties than the IgG-Based bsAbs28. Fragment-based bsAbs showed several advantages, including high yield, low cost, good tumor penetration, and the ability to overcome chain-related issues15,29,30. Due to their low molecular weight, BiTE (bispecific T-cell engager) antibodies are more readily metabolized in vivo, with a typical half-life of only 2–4 h31,32. To increase the half-life of fragment-based bsAbs, antibodies have been designed to be fused to an Fc region or albumin-binding molecules33. Half-Life Extended (HLE) BiTE is a novel format that builds upon the classical BiTE format by fusing it to an Fc domain, significantly increasing its serum half-life34. Studies have shown that CD19 HLE BiTE® is an effective treatment for CD19-positive malignancies, with a half-life of 210 h after a single intravenous injection, which could be suitable for once-weekly dosing35.
2.2. Affinity and valency
2.2.1. Affinity
The affinity of bispecific antibodies is a major factor influencing overall tolerability and cytokine release36. For CD3-targeting T-cell engagers, the affinity of the CD3 arm is a key factor in the success of T-cell bispecific antibodies (T-bsAbs). The CD3 arm with too high affinity would lead to excessive release of cytokines and affect the tissue distribution of bsAbs, limiting their reach to the target site37,38. In one study, PSMA/CD3 bispecific antibodies with lower CD3 affinity were reported to be more effective in killing tumor cells and reducing the incidence and severity of cytokine release syndrome (CRS) in prostate cancer patients compared to bsAbs with high CD3 affinity39. Thus, a proper affinity is essential for drug distribution and efficacy.
Additionally, bispecific antibodies can achieve high selectivity against tumor cells by decreasing the affinity of arms to tumor-specific antigens (TSA). HER2 T-cell-dependent bispecific antibody (TDB) is a bsAb with two low-affinity HER2 arms which has been reported to have high tumor specificity. It has a strong binding ability to cells with high HER2 expression, while the binding rate to low HER2-expressing cells is low. Clinical data has shown that this bsAb has better tolerability compared to CAR-T (chimeric antigen receptor T) cell therapies targeting HER240.
2.2.2. Valency
Valency refers to the number of binding sites in the antibody that can be used to bind antigens. It is another important factor in the design of bispecific antibodies, as it can affect the efficacy of the antibody31. Monovalent and multivalent designs can be used to achieve different levels of efficacy. Glofitamab is an example of a bsAb with a 2:1 valency against CD20 of B cells and CD3 of T cells. It has been shown to have 40-fold higher in vitro anti-tumor activity than 1:1 valency bsAbs41. This demonstrates the importance of considering all structural features when designing bispecific antibodies, as well as the need for a comprehensive screening process to obtain an optimal product42, 43, 44.
3. Classification of antibodies based on target selection
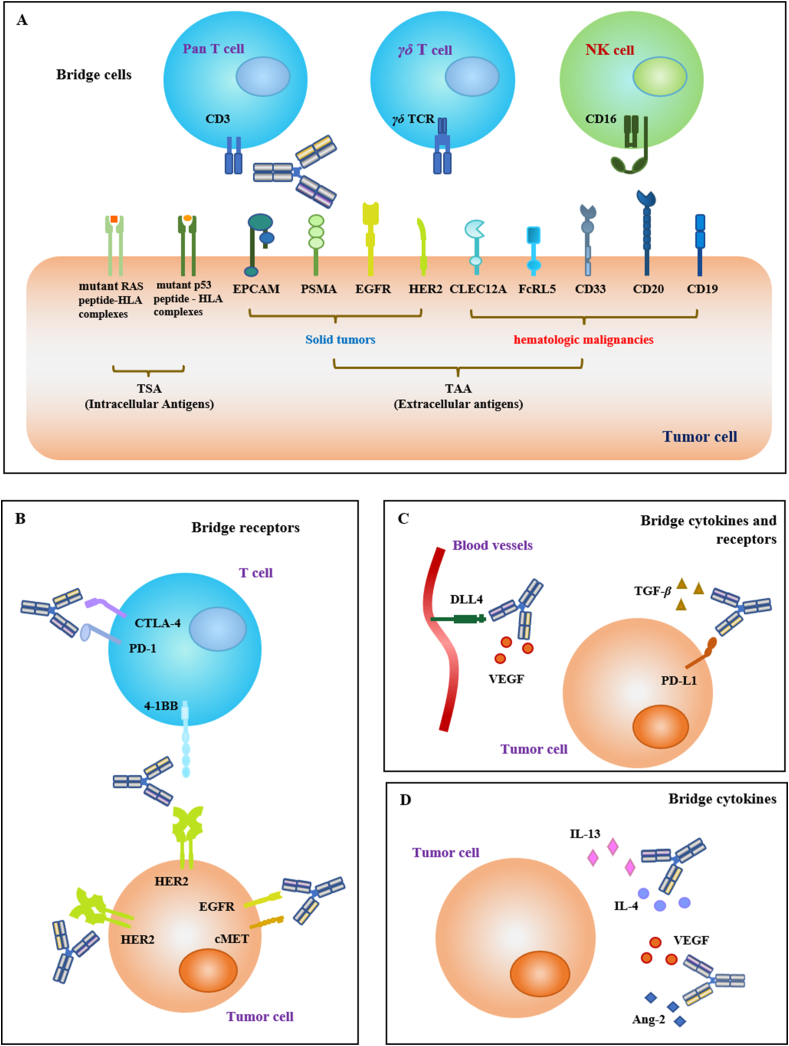
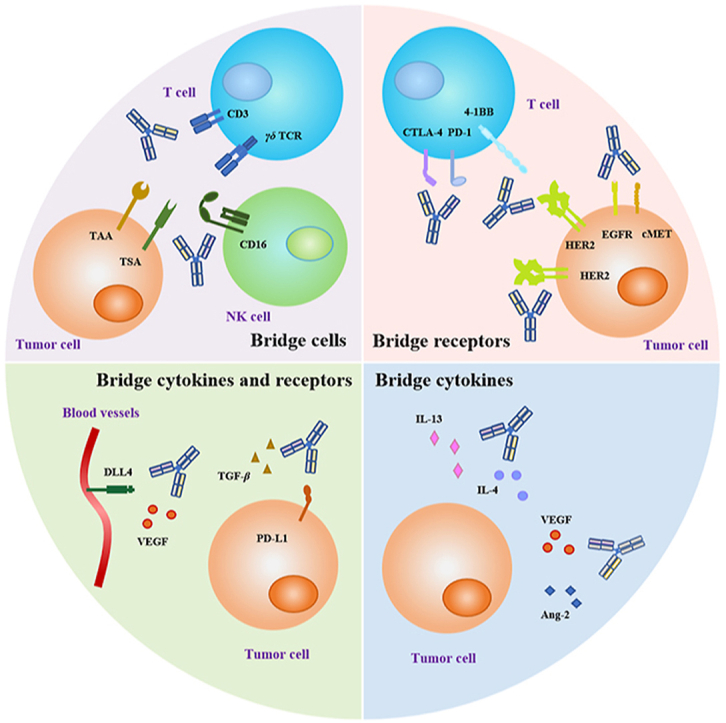
According to the NMPA guidelines, bispecific antibodies can be classified into three categories based on their mechanism of action: bridging cells, bridging receptors, and bridging cytokines. Additionally, the classification of bridging receptors and cytokines has been added to accommodate special bispecific antibodies, such as SHR-1701 (targeting TGF-β and PD-L1). All four types are shown in Fig. 3 to make it easier to understand.
Figure 3.
Bispecific antibodies in cancer therapy would be classified into four categories based on mechanism and target selection. (A) BsAbs that bridge immune effector cells to tumor cells, including pan T cells, γδ T cells and NK (natural killer) cells, etc. (B) BsAbs that bridge receptors from the same or different cells. (C) BsAbs that bridge cytokines and receptors. (D) BsAbs that bridge two cytokines.
3.1. Bridging cells
Bispecific antibodies (BsAb) can redirect cytotoxic effector cells to tumor cells. From a mechanistic perspective, this type of bispecific antibody can recruit immune cells (such as T cells and NK cells) to the tumor area to exert cytotoxic effects. One antigen-binding site of the BsAb binds to specific antigens expressed on tumor cells, while the other one bridges and activates effector cells such as macrophages and cytotoxic T lymphocytes (CTL)45,46. CD3 is the most common targeted protein expressed on effector cells, which can activate the anti-tumor activity of T cells. Some emerging target proteins are also classified into this category, such as TCR and CD16A (Table 2).
Table 2.
BsAbs bridge two cells in clinical stages.
| Bridge immune cell | Bridge tumor cell | Name | Indication | Phase | Clinical trial |
|---|---|---|---|---|---|
| CD3 | BCMA | BI836909 | R/R MM | Ⅰ | NCT03287908 |
| CD123 | APVO436 | AML | Ⅰ | NCT03647800 | |
| CD19 | AMG562 | DLBCL | Ⅰ | NCT03571828 | |
| CD20 | GEN3013 | DLBCL | Ⅰ/Ⅱ | NCT03625037 | |
| CD33 | GEM333 | AML | Ⅰ | NCT03516760 | |
| CD38 | GBR1342 | R/R MM | Ⅰ | NCT03309111 | |
| CEA | RG7802 | Solid tumors | Ⅰ | NCT02650713 | |
| CLEC12A | MCLA-117 | AML | Ⅰ | NCT03038230 | |
| DLL3 | AMG757 | AML | Ⅰ | NCT03541369 | |
| EGFR | AFM24 | Advanced solid tumor | Ⅰ/Ⅱ | NCT04259450 | |
| EpCAM | MT110 | Solid tumors | Ⅰ | NCT00635596 | |
| FcRH5 | RO7187797 | MM | Ⅰ | NCT03275103 | |
| FLT3 | AMG427 | AML | Ⅰ | NCT03541369 | |
| GD2 | NCT03541369 | SCLC | Ⅰ/Ⅱ | NCT04750239 | |
| Glypican-3 | ERY974 | Solid tumors | Ⅰ | NCT02748837 | |
| gpA33 | MGD007 | Colorectal carcinoma | Ⅰ | NCT02248805 | |
| GPRC5D | ERY974 | Solid tumors | Ⅰ | NCT02748837 | |
| HER2 | BTRC4017A | Solid tumors | Ⅰ | NCT03448042 | |
| MAGE-A4 (HLA-A∗02:01) | IMC-C103C | Select advanced solid tumors | Ⅰ/Ⅱ | NCT03973333 | |
| MUC17 | AMG199 | MUC17-positive solid tumors | Ⅰ | NCT04117958 | |
| MUC16 | REGN4018 | Recurrent ovarian cancer | Ⅰ/Ⅱ | NCT03564340 | |
| NY-ESO-1 (HLA-A∗02:01) | GSK01 | Select advanced solid tumors | Ⅰ/Ⅱ | NCT03515551 | |
| P-cadherin | PF-06671008 | Neoplasms | Ⅰ | NCT02659631 | |
| PRAME (HLA-A∗02:01) | IMC-F106C | Select advanced solid tumors | Ⅰ/Ⅱ | NCT04262466 | |
| PSCA | GEM3PSCA | NSCLC | Ⅰ | NCT03927573 | |
| PSMA | JNJ-63898081 | Neoplasms | Ⅰ | NCT03926013 | |
| SSTR2 | Xmab18087 | Neuroendocrine tumor | Ⅰ | NCT03411915 | |
| STEAP1 | AMG509 | Prostate cancer | Ⅰ | NCT04221542 | |
| 5T4 | GEN1044 | Malignant solid tumors | Ⅰ/Ⅱ | NCT04424641 | |
| γδTCR | CD1d | LAVA-051 | CLL | Ⅰ/Ⅱ | NCT04887259 |
| PSMA | LAVA-1207 | Metastatic castration resistant prostate cancer | Ⅰ/Ⅱ | NCT05369000 | |
| CD16A | BCMA | RO7297089 | R/R MM | Ⅰ | NCT04434469 |
| CD30 | AFM13 | NHL | Ⅰ/Ⅱ | NCT04074746 | |
| EGFR | AFM24 | Advanced solid tumor | Ⅰ/Ⅱ | NCT04259450 |
AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NSCLC, non-small-cell lung carcinoma; R/R MM, relapsed or refractory multiple myeloma; SCLC, small-cell carcinoma.
3.1.1. Targeting cytotoxic effector cells
3.1.1.1. CD3 targeting T cell engagers
Binding of T-bsAbs to CD3 has been shown to be a promising cancer therapy due to its ability to activate T cells without the restriction of the major histocompatibility complex (MHC) and directly induce tumor-associated antigens (TAA) and immune cells to form immune synapses (IS)47. Furthermore, they can induce tumor cell necrosis or apoptosis through the production of perforin and granzyme A/B47,48, as well as the stimulation of death ligands such as the Fas–FasL pathway49. However, they may also cause serious side effects. Catumaxomab, the first commercially available bispecific antibody for the treatment of malignant ascites, was withdrawn from the market in 2017 due to its potential to cause adverse events such as cytokine release syndrome (CRS) and T-cell-mediated hepatotoxicity11,50, 51, 52. Therefore, it is important to consider various factors when designing a bispecific antibody to ensure its safety and efficacy.
3.1.1.2. TCR targeting γδ T cell engagers
CD3 is widely distributed on the surface of T lymphocytes, and anti-CD3 bsAbs can activate the majority of T cells, including some immunosuppressive cells such as regulatory T cells (Tregs)53,54. Targeting specific T-cell subsets with bispecific antibodies is a promising approach to improve the efficacy and selectivity of T-bsAbs55. By selectively activating immune cells, it is possible to avoid the activation of immunosuppressive Tregs and reduce the risk of adverse events. For example, targeting Vγ9Vδ2 T cells, a small cell subpopulation (1%–10%) of the peripheral blood T cells, has shown promising therapeutic activity due to their conserved T-cell receptor (TCR) that recognizes malignant cells without relying on MHC56,57. 7D12-5 GS-6H4 is a novel bispecific antibody against Vγ9Vδ2 T cells and EGFR (epidermal growth factor receptor) that has been shown to induce activation of Vγ9Vδ2 T cells and promote apoptosis of colorectal cancer cells in a mouse xenograft model58. This novel therapy avoids the activation of immunosuppressive Tregs and is effective in killing tumors.
3.1.1.3. CD16A targeting NK cell engagers
AFM13 is a novel tetravalent bispecific antibody developed by Affimed that targets CD16A and CD3059. CD16A activates NK cells, increases the release of pro-inflammatory cytokines and chemokines, and enhances the anti-tumor capacity of NK cells60. The cytotoxicity of NK cells induced by AFM13 is strictly dependent on the presence of CD30. In a phase I clinical trial for the treatment of Hodgkin's lymphoma, AFM13 significantly induced activation of NK cells in peripheral blood, showing strong anti-tumor activity and good tolerability61. This demonstrates the potential of bispecific antibodies to selectively activate NK cells and provide more potent and durable anti-tumor activity.
3.1.2. Targeting tumor cells
3.1.2.1. Targeting tumor-associated antigens
Monoclonal antibodies targeting tumor-associated antigens (TAAs) such as CD19, CD20 or HER2 (human epidermal growth factor receptor 2) have shown good clinical efficacy in treating cancer62, 63, 64. However, due to the low expression of these targets on normal cells, the drugs can also cause the killing of normal cells during treatment. To reduce the risk of adverse effects, bispecific antibodies offer an advantage over monoclonal antibodies in terms of selectivity and specificity. By adjusting the affinity and valency of the antibody arms, bispecific antibodies can be designed to target TAAs on the surface of tumor cells while reducing damage to normal cells. This allows for more targeted and effective treatment of cancer with fewer side effects.
3.1.2.2. Targeting tumor-specific antigens
Distinct from tumor-associated antigens (TAAs), tumor-specific antigens (TSAs) are only expressed in tumor cells. Targeting TSAs theoretically avoids the toxicity to normal cells and has a higher safety profile. Mutant proteins expressed by mutated proto-oncogenes and tumor suppressor genes (e.g., RAS and p53) can become potential TSAs65,66. These mutated proteins are often intracellular proteins that are difficult to target directly by antibodies. However, it has been found that the hydrolyzed mutant proteins can bind to human leukocyte antigens (HLA) in the form of short peptides to form peptide-HLA (pHLA) complexes that present on the cell surface67. These peptides are also known as mutation-associated neoantigens (MANAs), which can be used as targets for bispecific antibody design.
Targeting these MANAs allows the design of bispecific antibodies with higher selectivity to redirect T cells to TSA-expressing tumor cells. TCR-mimic antibodies, also known as MANA-directed antibodies (MANAbodies), have been developed and have shown promising results in clinical trials. A MANA antibody targeting mutated RAS has been developed and has been shown to activate T cells and kill tumor cells in cancers with KRAS mutations, such as pancreatic, colorectal, and lung cancers68,69.
However, most MANAs are expressed at low levels on the cell surfaces, making the identification more difficult. When developing MANA antibodies, there are higher requirements for structures and valence optimization70.
3.2. Bridging receptors
BsAbs targeting two tumor receptors have been extensively studied due to their high efficacy and low toxicity. As previously mentioned, tumor-associated antigens (TAAs) are also expressed in normal tissues, leading to undesired toxicity. Targeting two TAAs or different epitopes of the same antigen would increase selectivity and reduce toxicity. In addition, dysregulation of multiple proteins is often observed in malignant tumors. Designing bispecific antibodies to inhibit compensatory pathways is beneficial for improving efficacy and overcoming resistance.
3.2.1. Receptors on tumor cells
3.2.1.1. Bridging two separate receptors
Simultaneously inhibiting two tumor-associated proteins can produce a stronger therapeutic effect because it can target multiple pathways involved in tumor growth and progression, thus providing a more comprehensive approach to treating cancer. Additionally, it can reduce the risk of drug resistance, as it is more difficult for the tumor to develop resistance to two drugs at once.
It is clear that EGFR inhibitors have shown promising results in the treatment of various cancers, including NSCLC (non-small-cell lung carcinoma) and colon cancer71, 72, 73, 74. However, mutations of EGFR and activation of compensatory pathways can lead to drug resistance75. To overcome this, the combination of two drugs to simultaneously block compensatory pathways has been developed, such as the combination of EGFR and cMET inhibitors, leading to the development of EGFR/cMET bsAbs76. Amivantamab (JNJ-61186372) is an example that has been approved by the FDA on May 21, 2021 for the treatment of adult patients with locally advanced or metastatic NSCLC (non-small-cell lung carcinoma)77, 78, 79.
3.2.1.2. Bridging different epitopes of the same receptor
Trastuzumab and pertuzumab are monoclonal antibody drugs targeting the HER2 protein, but they have different binding sites80,81. The combination of trastuzumab and pertuzumab has been shown to be effective in treating HER2-positive breast cancer, as it can target two different antigen-binding sites on the same receptor. This combination has been approved by the FDA for the treatment of HER2-positive advanced breast cancer, in combination with chemotherapy82. The use of this combination has been shown to be more effective than Trastuzumab alone, as it can block compensatory pathways that can lead to drug resistance.
Zanidatamab (ZW25) is a bispecific antibody that targets two epitopes of HER2, combining the binding sites of trastuzumab (HER2 ECD4) and pertuzumab (HER2 ECD2)83. It has shown promising results in the treatment of HER2-positive breast cancer and gastroesophageal adenocarcinoma (GEA). In a phase I clinical trial, ZW25 in combination with docetaxel had an overall response rate (ORR) of 90.5%, which was higher than the ORR of 80.2% in the standard first-line treatment group (pertuzumab, ttrastuzumab, and chemotherapy)84,85. The FDA has granted ZW25 fast-track designation in combination with standard chemotherapy for patients with high-HER2-expressed GEA86.
3.2.2. Receptors on immune cells
Immune cells have a variety of regulatory proteins on their surface, including a series of immune checkpoint proteins, which regulate the activation, proliferation and anti-tumor activity of immune cells87. Stimulating or inhibiting the relevant pathways in a rational manner can induce stronger immune clearance effects88,89. CTLA-4 and PD-1/PD-L1 are important immune checkpoint proteins, and the activation of these two pathways can significantly inhibit the activation of immune cells such as T cells, resulting in tumor cells “immune escape”90,91. CTLA-4 and PD-1/PD-L1 inhibitors promote the activation of immune cells in the tumor microenvironment (TME), which in turn leads to the apoptosis of tumor cells92,93. These immune checkpoint inhibitors (ICIs) have become important treatment options for tumors, however, drug resistance and side effects such as immune-related adverse events (IrAEs) are also present94. To address these issues, novel strategies such as combination therapies and novel drug delivery systems are being developed to improve the efficacy and reduce the toxicity of immune checkpoint inhibitors95.
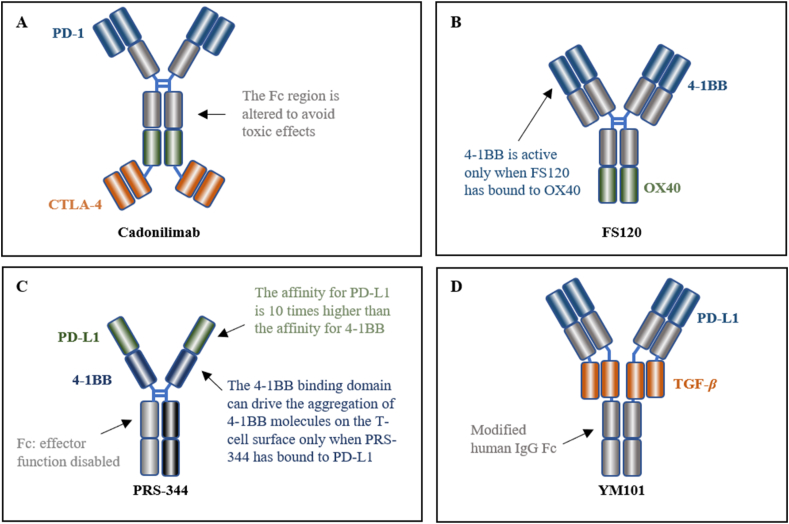
Cadonilimab is a bispecific antibody designed to target both PD-1 and CTLA-4, which is based on the Tetrabody format, providing enhanced efficacy and lower toxicity (Fig. 4A). The co-expression of CTLA-4 and PD-1 on tumor-infiltrating lymphocytes is widespread, while peripheral T cells are lacking. This reduces the tetravalent binding of cadonilimab to peripheral T cells and increases its enrichment in the TME. Additionally, the modified Fc region of cadonilimab helps to avoid Fc-mediated toxic effects, resulting in a higher specificity and lower toxicity96,97. On June 29, 2022, the NMPA approved its marketing for the treatment of patients with recurrent or metastatic cervical cancer (R/M CC) who have failed prior platinum-containing chemotherapy98.
Figure 4.
Representative bispecific antibodies with increased efficacy and reduced toxicity based on their unique structures.
Another excellent bsAb design is FS120, which is a dual agonistic targeting 4-1BB and OX40 with the tetravalent format (Fig. 4B)99. Activating the 4-1BB pathway stimulates the activation and proliferation of T cells100. However, monotherapy with agonist antibodies to 4-1BB may induce serious toxicities, limiting the development of 4-1BB mAbs101,102. In the design of FS120, the binding arm targeting 4-1BB can be activated only after the simultaneous binding of OX40, which will lead to increased selectivity and reduced toxicities. While ensuring safety, the antitumor effect of FS120 is improved compared to the combination of mAbs99.
3.2.3. Receptors on tumor and immune cells
Bispecific antibodies (bsAbs) can be used to target both immune cells and tumor cells (Table 3), activating the anti-tumor activity of the immune cells and directly acting on the tumor cells to induce apoptosis. The activated immune cells are usually tumor-infiltrating T cells that are already present in the TME9, and this bsAbs-induced slow and sustained immune response increases the specificity and safety of the drug.
Table 3.
BsAbs bridge two receptors in clinical stages.
| Classification | Target | Name | Indication | Phase | Clinical trial |
|---|---|---|---|---|---|
| Bridging two receptors on tumor cells | CD19 × CD47 | TG-1801 | B-cell lymphoma | Ⅰ | NCT03804996 |
| CD20 × CD47 | IMM0306 | B-NHL | Ⅰ | CTR20192612 | |
| EGFR × cMET | EMB-01 | Neoplasms | I/II | NCT05176665 | |
| EGFR × HER3 | Duligotuzumab | Head and neck cancer | Ⅰ | NCT01911598 | |
| EGFR × MET | LY3164530 | Neoplasms | Ⅰ | NCT02221882 | |
| HER2 × HER2 | Zanidatamab | HER2+/HR+ breast cancer | Ⅱ | NCT04224272 | |
| HER2 × HER3 | Zenocutuzumab | Solid tumours harboring NRG1 fusion | Ⅱ | NCT02912949 | |
| HER3 × IGF-1R | MM-141 | Pancreatic cancer | Ⅱ | NCT02538627 | |
| LRP5 × LRP6 | BI905677 | Neoplasms | Ⅰ | NCT03604445 | |
| PD-L1 × CD47 | IBI322 | Advanced malignant tumors lymphomas | Ⅰ | NCT04338659 | |
| Bridging two receptors on immune cells | CD40 × 4-1BB | GEN1042 | Malignant solid tumor | Ⅰ/Ⅱ | NCT04083599 |
| CTLA-4 × LAG-3 | Xmab22841 | Melanoma | Ⅰ | NCT03849469 | |
| CTLA-4 × OX40 | ATOR-1015 | Solid tumor | Ⅰ | NCT03782467 | |
| OX40 × 4-1BB | FS120 | Advanced cancer | Ⅰ | NCT04648202 | |
| PD-1 × CTLA-4 | AK104 | Cervical cancer | Ⅱ | NCT05227651 | |
| PD-1 × ICOS | Xmab23104 | Selected advanced solid tumors | Ⅰ | NCT03752398 | |
| PD-1 × LAG-3 | Tebotelimab | Gastric cancer | Ⅱ/Ⅲ | NCT04082364 | |
| PD-1 × TIM-3 | RG7769 | Solid tumors | Ⅰ | NCT03708328 | |
| Bridging receptors on tumor and immune cells | CD40 × MSLN | ABBV-428 | Advanced solid tumors cancer | Ⅰ | NCT02955251 |
| HER2 × 4-1BB | PRS-343 | HER2-positive solid tumors | Ⅰ | NCT03330561 | |
| PD-1 × PD-L1 | IBI318 | Advanced cutaneous squamous cell carcinoma | Ⅰ/Ⅱ | NCT04611321 | |
| PD-L1 × 4-1BB | MCLA-145 | Advanced cancer | Ⅰ | NCT03922204 | |
| PD-L1 × CTLA-4 | KN046 | Thymic carcinoma | Ⅱ | NCT04925947 | |
| PD-L1 × LAG-3 | FS118 | Advanced cancer | Ⅰ/Ⅱ | NCT03440437 | |
| PD-L1 × TIM-3 | LY3415244 | Solid tumor | Ⅰ | NCT03752177 | |
| PSMA × CD28 | REGN5678 | Metastatic castration-resistant prostate cancer | Ⅰ/Ⅱ | NCT03972657 |
PRS-344 is a tetravalent antibody that targets PD-L1 and 4-1BB, which are located on the surface of tumor cells and immune cells, respectively (Fig. 4C). PRS-344 is designed to bind to PD-L1 first, which then enables the 4-1BB binding domain to drive the aggregation of 4-1BB molecules on the surface of T cells103. Compared to a 4-1BB monoclonal antibody, it has shown a significant reduction in hepatotoxicity in the clinic104, and according to preclinical data, it has a better anti-tumor effect than the combination of two monoclonal antibodies103.
3.3. Bridging cytokines and receptors
Abnormal regulation of cytokines is highly correlated with the development and progression of tumors105. Therapeutic approaches targeting cytokines have been reported, however, their development is limited by factors such as short half-life and high immunogenicity106. To overcome these limitations, many cytokine-based therapies have been adopted in combination therapies to improve efficacy and reduce toxicity107, which is also the theoretical basis for designing bispecific antibodies (Table 4).
Table 4.
BsAbs bridge cytokines or cytokines/receptors in clinical stages.
| Classification | Target | Name | Indication | Phase | Clinical trial |
|---|---|---|---|---|---|
| Cytokines × receptors | TGF-β × CD73 | GS-1423 | Advanced solid tumors | Ⅰ | NCT03954704 |
| TGF-β × PD-L1 | SHR-1701 | Squamous cell carcinoma of head and neck | Ⅱ | NCT04650633 | |
| TGF-β × EGFR | BCA101 | Head and neck squamous cell carcinoma | Ⅰ | NCT04429542 | |
| VEGF × DLL4 | OMP-305B83 | Metastatic colorectal cancer | Ⅰ | NCT03035253 | |
| VEGF × PD-1 | AK112 | NSCLC | Ⅰ/Ⅱ | NCT04900363 | |
| Cytokines × cytokines | VEGF × Ang-2 | Vanucizumab | Advanced solid tumors | Ⅰ | NCT02665416 |
DLL4 is a receptor expressed in the vasculature and belongs to the Notch ligand family, which affects the formation of new vessels. Its expression is upregulated in various malignant tumors such as breast and bladder cancer108,109. However, DLL4 monoclonal antibodies have shown severe side effects in clinical trials110. To avoid its toxicity, navicixizumab was designed as a bispecific antibody targeting DLL4 and vascular endothelial growth factor (VEGF)111. This enables the antibody to better target the TME and has shown promising clinical activity and manageable toxicity in clinical trials for a range of solid tumors112. It has been granted a fast-track designation by the FDA for the treatment of heavily pretreated ovarian cancer113.
TGF-β is an important cytokine that can promote immune escape of tumor cells in advanced stages and inhibit immune cell function in a non-redundant manner in combination with PD-L1114,115. When TGF-β inhibitors are used in combination with ICI, anti-tumor activity is increased116. YM101 is the first bispecific antibody targeting PD-L1/TGF-β developed on the Checkbody platform. It can enhance T cell infiltration, alter the immune microenvironment, induce effective clearance of tumors by immune cells, and is superior to single anti-TGF-β or PD-L1 antibodies117.
3.4. Bridging two cytokines
Bispecific antibodies targeting two cytokines have been less studied in tumor therapy and remain to be further explored (Table 4). Vanucizumab is a promising new treatment for advanced solid tumors, as it has been shown to be effective in targeting both VEGF and Ang-2, two proteins that are involved in tumor growth and angiogenesis118. The safety and tolerability of the drug is comparable to other anti-VEGF or anti-Ang-2 inhibitors, making it a viable option for treating tumors such as breast cancer and gastric carcinoma119, 120, 121.
4. Innovative bispecific antibody drugs
Bispecific antibodies offer unique advantages over monoclonal antibodies, including increased selectivity and efficacy. This increased selectivity makes them ideal for therapies that require high specificity, such as antibody–drug conjugates (ADC) drugs and chimeric antigen receptor (CAR) T-cell therapy. Furthermore, multispecific antibodies, such as trispecific and tetraspecific antibodies, are being developed to further increase selectivity and efficacy.
4.1. Bispecific ADCs
ADC drugs are a promising new drug design strategy that combines the specificity of antibodies with the high toxicity of small molecules124. Bispecific antibodies are particularly well-suited for this type of drug, as they offer increased specificity and endocytosis ability compared to monoclonal antibodies. This increased specificity helps to reduce the toxic side effects of the small molecule payload, while the endocytosis ability allows for more efficient transmembrane delivery of the ADC drug125.
Bispecific ADCs targeting HER2 have been developed to improve the efficacy of HER2-targeted therapies126. ZW49, a bispecific antibody based on ZW25 (Fig. 5A), has demonstrated good antitumor activity and safety in clinical trials (ClinicalTrials.gov identifier: NCT03821233). Additionally, bsHER2xCD63his-ADC, a bispecific antibody targeting HER2 and CD63, has been designed to improve the internalization and antitumor ability of HER2-based ADCs127. CD63 has the ability to regulate the transportation of proteins via endocytosis128, which increases the endocytosis of ADC drugs and thus enhances their therapeutic efficacy129.
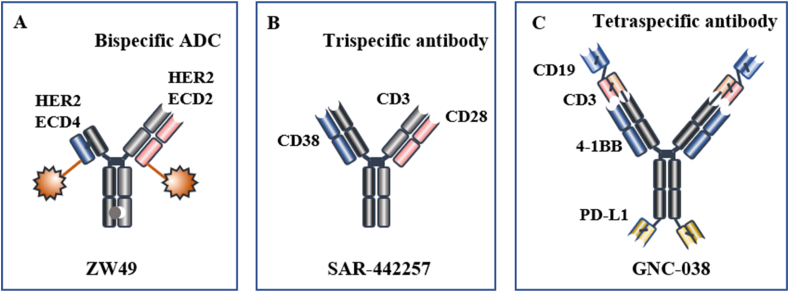
Figure 5.
Representative innovative bispecific antibody drugs. (A) ZW49 is a bispecific ADC122. (B) SAR-442257 is a trispecific antibody123. (C) GNC-038 is a tetraspecific antibody (ClinicalTrials.gov identifier: NCT05192486).
4.2. Trispecific/tetraspecific antibodies
Combination therapy targeting synergistic pathways is an essential strategy for enhancing the efficacy of cancer therapy. T cells also require multiple signals for activation. Trispecific/tetraspecific antibodies, derived from bispecific antibodies, are thought to have a greater therapeutic potential (Fig. 5B and C).
Trispecific antibodies possess three distinct antigen-binding sites that can effectively bridge cells and stimulate immune cells more efficiently. SAR-442257 is a trispecific antibody targeting CD3/CD28/CD38. CD3 can recruit and activate T cells, while CD28 can further activate T cells and extend the duration of the immune response. CD38 domains have the ability to guide T cells to myeloma cells130. SAR-442257 is currently undergoing Phase I clinical trials to evaluate its therapeutic effects in relapsed/refractory multiple myeloma (R/R MM) and non-classical Hodgkin's lymphoma (R/R NHL) (ClinicalTrials.gov identifier: NCT04401020).
Tetraspecific antibodies possess four distinct antigen-binding sites, offering more options for target selection. GNC-038 is the first tetraspecific antibody to enter clinical trials. GNC-038 contains four antigen-binding sites: CD19, CD3, PD-L1, and 4-1BB. The CD3 and 4-1BB arms respectively activate the first and second signals of T cells, and the anti-CD19 and anti-PD-L1 domains target tumor cells131. GNC-038 stimulates peripheral T cells and facilitates T cell infiltration into tumor sites. It can overcome the immunosuppression in the TME and display antitumor activity in vivo. GNC-038 is currently in Phase I/II clinical trials to assess its effectiveness in non-Hodgkin's lymphoma, diffuse large B-cell lymphoma, and other lymphomas (ClinicalTrials.gov identifier: NCT05192486).
5. Guidance from FDA and NMPA
5.1. Development of guidance
Research on bispecific antibodies is unique and an increasing number of research institutions are engaging in it, necessitating industry guidance principles to regulate research and development, pointing out potential challenges to ensure successful research outcomes.
The FDA first issued draft guidance for Bispecific Antibody Development Programs on April 19, 2019, followed by a final version on May 24, 202113. On April 11, 2022, the National Medicinal Products Administration (NMPA) published the Technical Guidelines for Clinical Development of Bispecific Antibody Class Antitumor Drugs (Draft for Comments), with the final version released on November 9, 202214. This marks the transition of bispecific antibodies from a “wild growth phase” to a more “scientific development phase” (Table 5). The European Union has yet to issue drug guidelines for bispecific antibody drugs, and the development of bispecific antibodies follows the guidelines for therapeutic protein drugs.
Table 5.
| Content | FDA | NMPA |
|---|---|---|
| Application scope | Bispecific antibodies, other types of bispecific protein products and multispecific products Not include antibody cocktails, polyclonal antibody products, or combination of monoclonal antibodies |
Bispecific and multispecific antibodies in cancer therapy |
| Classification | BsAbs that bridge two target cells BsAbs that do not bridge cells |
Classification by structure: Non-IgG based bsAbs; IgG based bsAbs Classification by target selection: Bridging cells; Bridging receptors; Bridging cytokines |
| Design of bsAbs | Not mentioned | Designing antibodies based on clinical needs, target selection and structure optimization |
| Scientific considerations | ||
| CMC quality considerations | The development of the manufacturing procedures should be carried out according to standard monoclonal antibody development practices. Studies should be conducted on quality characteristics such as antigen specificity; affinity and on- and off-rates; avidity; potency; product-related impurities, fragments, homodimers, other mispaired species; stability; and half-life | Not mentioned |
| Invitro tests | Required to carry out, in combination with pharmacological experiments to support the scientific principle of bispecific antibodies | |
| Non-clinical trials | Pharmacology and toxicology experiments: the range of studies is similar to that of monoclonal antibodies; target expression profile and specificity should be considered when selecting models | Referring to the relevant guidelines that have been published in China, non-clinical studies were conducted to further support the rationality of the topic of bsAbs |
| Risk control for first-in-human (FIH) trials of innovative drugs | Not mentioned | Develop and strictly implement a risk management plan during clinical trials; scientifically and appropriately set the starting dose of FIH, the magnitude and speed of dose escalation study; and rationally define the dose limit toxicity (DLT) |
| Clinical pharmacology | Similar to research on monoclonal antibodies and other therapeutic protein products | |
| Pharmacodynamics | Necessary to consider the binding and impact of each target | |
| Optimal drug delivery strategy | Extended dose exploration studies can be conducted with no less than two candidate dosing regimens within the determined safe dose range | Factors such as pharmacology, toxicology, and pharmacokinetics should be evaluated comprehensively; Early dose escalation studies should be performed |
| Control selection | Comparison with the standard of care or placebo in many situations. If monospecific products with the same antigens approved for the same indication exist, then a comparison is conducted with the monospecific products | Comparison with the best standard treatment |
| Clinical trial establishment | Clinical studies should inform the benefit-risk assessment and support approval based on the specific targets and other clinical considerations. Sponsors are encouraged to discuss product development plans with the FDA's appropriate clinical review division | BsAbs should perform functions that are not achieved by the mAbs or combinations of mAbs, and have the potential to provide clinical value |
| PK assessment | Choose the bispecific antibody conformation associated with the bispecific antibody PK assessment (biologically active or inactive forms) | Not mentioned |
| Immunogenicity | Detection of immunogenic reactions of different structural domains of bsAbs using multiple methods | Immunogenicity risk assessment should be conducted and a risk management plan should be developed before clinical studies; Integrates clinical PK, PD and safety data during development to fully assess immunogenicity; Develop an immunogenicity study strategy based on immunogenicity risk; Detection of immunogenic reactions of different structural domains of bsAbs using multiple methods |
| Development of biomarker | Not mentioned | Design and use of biomarkers based on factors such as the mechanism of action, biological relationships between targets, clinical applications and data |
5.2. Comments on the guidance
The guidance issued by the US and China suggest various aspects that should be taken into consideration during the development of bispecific antibodies, such as design strategy, preclinical studies, quality control, drug metabolism and toxicity. It is essential to compare the guidelines between the US and China.
The documents issued by the FDA and China are programmatic, providing strategic guidance for the majority of requirements, while specific research protocols should be developed on a case-by-case basis. Considering the complexity and technical challenges of bsAbs, the guidance principles of the FDA and NMPA are open to communication regarding trial design and trial process. It is encouraged for research and development organizations to communicate with regulatory authorities in order to ensure the successful development of bsAbs.
Both guidance documents from the two countries include stringent requirements for efficacy and safety testing, such as immunogenicity testing and safety assessment. The FDA has established fundamental standards for pharmacology, toxicology, and safety evaluation, while the NMPA lacks such evaluation standards. Additionally, the NMPA provides the foundation for the design, selection and use of biomarkers, which are not mentioned in the guidance provided by the FDA.
Furthermore, the guidance of the two countries differs in terms of the selection of control groups. The FDA recommends comparing with the standard of care or placebo, while if monospecific products with the same antigens are approved for the same indication, then a comparison should be made with the monospecific products. On the other hand, the NMPA recommends selecting the optimal treatment regimen as a control. BsAbs are required to achieve a function that cannot be achieved by related monoclonal antibodies or monoclonal antibody combination therapy, which can bring valuable clinical benefits to patients.
At present, the development of bsAbs is still in its early stages, and there are few published guidelines that can be consulted. The guidance issued by the FDA and NMPA are both very instructive for the development of the bispecific antibody market. For bsAbs that require approval from multiple countries, comprehensive considerations must be taken into account during the development stage to meet different regulatory requirements, such as pharmacodynamics, toxicology, and other in vitro tests.
6. Conclusions
Many successful bsAbs have been developed, providing a variety of successful templates and development experiences. Proper structural design and target selection are critical for ensuring the success of drug research, while maintaining the efficacy of combination therapy and reducing the corresponding toxicity, which is one of the core advantages of bispecific antibodies. Systematically understanding excellent examples of bispecific antibody design can help to develop novel therapeutic antibodies.
The development of bispecific antibodies has opened up new possibilities for the development of innovative drugs that can target multiple pathways simultaneously. The high selectivity of these antibodies makes them ideal for use as ADC drugs, which can improve selectivity against cancer cells and increase internalization for better clearance of tumor cells. Additionally, the development of trispecific and tetraspecific antibodies is expected to further enhance the anti-tumor effects of these drugs. Clinical trials are currently underway to evaluate the efficacy of these drugs, and the results of these trials will be eagerly awaited.
The collection of cases has revealed a high degree of similarity in target selection across multiple research institutions. CD3 is the most commonly chosen target for cell-bridging bsAbs, with 56 bispecific antibodies targeting both CD3 and CD19, including one marketed drug. For non-cell-bridging bsAbs, the most widely studied combination is PD-L1/4-1BB, with 32 bispecific antibodies. Bispecific antibodies offer the advantage of increased efficacy and reduced toxicity due to the rational combination of targets. However, the high similarity in target selection is unfavorable for study enrichment and may lead to wasted medical resources. Therefore, it is important to conduct a thorough review of the literature and market research before selecting a target for bispecific antibody development.
The rapid development of bispecific antibodies has led to an increased need for regulatory guidance. In 2021 and 2022, the US and China issued guidance to standardize the design strategy and drug evaluation of bsAbs, in order to maximize the unique benefits of bispecific antibodies. This guidance is intended to ensure the safety and efficacy of bsAbs, and to ensure that they are used in the most effective way. The global market for bispecific antibodies is growing rapidly, and sales of cancer-related bispecific antibodies are expected to reach $3.7 billion by 2027132. Despite the challenges that remain, bispecific antibodies offer unique advantages that make them a powerful therapeutic weapon. These advantages include increased efficacy, lower toxicity, and improved specificity, which can help to improve the effectiveness of cancer treatments.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2022YFA1303803), National Natural Science Foundation of China (82073701), and the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZ202209). This study was also supported by “Double First-Class” University Project (CPU2022PZQ07, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Chengliang Sun, Email: 616642273@qq.com.
Peng Yang, Email: pengyang@cpu.edu.cn.
Author contributions
Original draft preparation: Yanze Sun, and Chengliang Sun; review and editing: Chengliang Sun, and Peng Yang. All authors contributed to data collection and manuscript revision.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Goulet D.R., Atkins W.M. Considerations for the design of antibody-based therapeutics. J Pharmaceut Sci. 2020;109:74–103. doi: 10.1016/j.xphs.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emmons C., Hunsicker L.G. Muromonab-CD3 (orthoclone OKT3): the first monoclonal antibody approved for therapeutic use. Iowa Med. 1987;77:78–82. [PubMed] [Google Scholar]
- 3.Cameron D., Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the herceptin adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salles G., Barrett M, Foà R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torka P., Barth M., Ferdman R., Hernandez-Ilizaliturri F.J. Mechanisms of resistance to monoclonal antibodies (mAbs) in lymphoid malignancies. Curr Hematol Malig Rep. 2019;14:426–438. doi: 10.1007/s11899-019-00542-8. [DOI] [PubMed] [Google Scholar]
- 6.Scott A.M., Wolchok J.D., Old L.J. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 7.Boutros C., Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 8.Wolchok J.D., Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlén E., Veitonmäki N., Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother. 2018;6:3–17. doi: 10.1177/2515135518763280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R.P., Shinoda K, Rampuria P, Jin F, Bartholomew T, Zhao C, et al. Bispecific antibodies for immune cell retargeting against cancer. Expet Opin Biol Ther. 2022;22:965–982. doi: 10.1080/14712598.2022.2072209. [DOI] [PubMed] [Google Scholar]
- 11.Heiss M.M., Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intelligence DD. Drugs & biologics records for bispecific antibody. Cortellis Drug Discovery Intelligence. Accessed [November 7, 2022]. Available from: https://www.cortellis.com/drugdiscovery/result/7404fbb8-ea5d-1f4d-28ab-b703ee1116c8/drugs/productList?orderBy=drugTargets:desc&productListPage=1.
- 13.FDA . U.S. Food & Drug Administration; May 24, 2021. Bispecific antibody development programs guidance for industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bispecific-antibody-development-programs-guidance-industry Available from: [Google Scholar]
- 14.NMPA . Center for Drug Evaluation, NMPA; November 14, 2022. The technical guidelines for clinical research and development of bispecific antibody antineoplastic drugs.https://www.cde.org.cn/main/news/viewInfoCommon/e9e97adf7fd91fff6c49afac1320d233 Available from: [Google Scholar]
- 15.Wang Q., Chen Y, Park J, Liu X, Hu Y, Wang T, et al. Design and production of bispecific antibodies. Antibodies. 2019;8:43. doi: 10.3390/antib8030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuñez-Prado N., Compte M, Harwood S, Álvarez-Méndez A, Lykkemark S, Sanz L, et al. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20:588–594. doi: 10.1016/j.drudis.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Carter P.J. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 18.Kontermann R.E., Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Yu S., Li A, Liu Q, Yuan X, Xu H, Jiao D, et al. Recent advances of bispecific antibodies in solid tumors. J Hematol Oncol. 2017;10:155. doi: 10.1186/s13045-017-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D., Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 21.Milstein C., Cuello A.C. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537–540. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- 22.Kang C. Mosunetuzumab: first approval. Drugs. 2022;82:1229–1234. doi: 10.1007/s40265-022-01749-5. [DOI] [PubMed] [Google Scholar]
- 23.Ridgway J.B., Presta L.G., Carter P. 'Knobs-into-holes' engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9:617–621. doi: 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- 24.Strop P., Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012;420:204–219. doi: 10.1016/j.jmb.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Shirley M. Faricimab: first approval. Drugs. 2022;82:825–830. doi: 10.1007/s40265-022-01713-3. [DOI] [PubMed] [Google Scholar]
- 26.Ahamadi-Fesharaki R., Fateh A, Vaziri F, Solgi G, Siadat SD, Mahboudi F, et al. Single-chain variable fragment-based bispecific antibodies: hitting two targets with one sophisticated arrow. Mol Ther Oncolytics. 2019;14:38–56. doi: 10.1016/j.omto.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X., Sereno AJ, Huang F, Lewis SM, Lieu RL, Weldon C, et al. Fab-based bispecific antibody formats with robust biophysical properties and biological activity. mAbs. 2015;7:470–482. doi: 10.1080/19420862.2015.1022694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krah S., Sellmann C, Rhiel L, Schröter C, Dickgiesser S, Beck J, et al. Engineering bispecific antibodies with defined chain pairing. N Biotech. 2017;39:167–173. doi: 10.1016/j.nbt.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Fan G., Wang Z., Hao M., Li J. Bispecific antibodies and their applications. J Hematol Oncol. 2015;8:130. doi: 10.1186/s13045-015-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitragotri S., Burke P.A., Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labrijn A.F., Janmaat M.L., Reichert J.M., Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 32.Goebeler M.E., Bargou R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma. 2016;57:1021–1032. doi: 10.3109/10428194.2016.1161185. [DOI] [PubMed] [Google Scholar]
- 33.Kontermann R.E. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22:868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Arvedson T.L., Balazs M, Bogner P, Black K, Graham K, Henn A, et al. Abstract 55: generation of half-life extended anti-CD33 BiTE® antibody constructs compatible with once-weekly dosing. Cancer Res. 2017;77:55. [Google Scholar]
- 35.Lorenczewski G., Friedrich M, Kischel R, Dahlhoff C, Anlahr J, Balazs M, et al. Generation of a half-life extended anti-CD19 BiTE® antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood. 2017;130:2815. [Google Scholar]
- 36.Haber L., Olson K, Kelly MP, Crawford A, DiLillo DJ, Tavaré R, et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci Rep. 2021;11:14397. doi: 10.1038/s41598-021-93842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A., Dees S., Grewal I.S. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021;124:1037–1048. doi: 10.1038/s41416-020-01225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staflin K., Zuch de Zafra CL, Schutt LK, Clark V, Zhong F, Hristopoulos M, et al. Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI Insight. 2020;5:133757. doi: 10.1172/jci.insight.133757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang K., Castello G, Clarke SC, Li Y, Balasubramani A, Boudreau A, et al. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slaga D., Ellerman D, Lombana N, Vij R, Li J, Hristopoulos M, et al. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci Transl Med. 2018;10:eaat5775. doi: 10.1126/scitranslmed.aat5775. [DOI] [PubMed] [Google Scholar]
- 41.Bacac M., Colombetti S, Herter S, Sam J, Perro M, Chen S, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 42.Zheng S., Moores S, Jarantow S, Pardinas J, Chiu M, Zhou H, et al. Cross-arm binding efficiency of an EGFR x c-Met bispecific antibody. mAbs. 2016;8:551–561. doi: 10.1080/19420862.2015.1136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinmetz A, Vallée F, Beil C, Lange C, Baurin N, Beninga J, et al. CODV-Ig, a universal bispecific tetravalent and multifunctional immunoglobulin format for medical applications. mAbs. 2016;8:867–878. doi: 10.1080/19420862.2016.1162932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neijssen J., Cardoso RMF, Chevalier KM, Wiegman L, Valerius T, Anderson GM, et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem. 2021;296:100641. doi: 10.1016/j.jbc.2021.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clynes R.A., Desjarlais J.R. Redirected T cell cytotoxicity in cancer therapy. Annu Rev Med. 2019;70:437–450. doi: 10.1146/annurev-med-062617-035821. [DOI] [PubMed] [Google Scholar]
- 46.Maskalenko N.A., Zhigarev D., Campbell K.S. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov. 2022;21:559–577. doi: 10.1038/s41573-022-00413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Offner S., Hofmeister R., Romaniuk A., Kufer P., Baeuerle P.A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Renner C., Held G, Ohnesorge S, Bauer S, Gerlach K, Pfitzenmeier JP, et al. Role of perforin, granzymes and the proliferative state of the target cells in apoptosis and necrosis mediated by bispecific-antibody-activated cytotoxic T cells. Cancer Immunol Immunother. 1997;44:70–76. doi: 10.1007/s002620050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamakura D., Asano R., Kawai H., Yasunaga M. Mechanism of action of a T cell-dependent bispecific antibody as a breakthrough immunotherapy against refractory colorectal cancer with an oncogenic mutation. Cancer Immunol Immunother. 2021;70:177–188. doi: 10.1007/s00262-020-02667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Piskol R, Ybarra R, Chen Y-J, Li J, Slaga D, et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci Transl Med. 2019;11:eaax8861. doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 51.Maude S.L., Barrett D., Teachey D.T., Grupp S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borlak J., Länger F., Spanel R., Schöndorfer G., Dittrich C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget. 2016;7:28059–28074. doi: 10.18632/oncotarget.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhn C., Weiner H.L. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8:889–906. doi: 10.2217/imt-2016-0049. [DOI] [PubMed] [Google Scholar]
- 54.Ganesan R., Chennupati V., Ramachandran B., Hansen M.R., Singh S., Grewal I.S. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia. 2021;35:2274–2284. doi: 10.1038/s41375-021-01122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michalk I., Feldmann A, Koristka S, Arndt C, Cartellieri M, Ehninger A, et al. Characterization of a novel single-chain bispecific antibody for retargeting of T cells to tumor cells via the TCR co-receptor CD8. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schild H., Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, et al. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 57.de Weerdt I., Lameris R, Scheffer GL, Vree J, de Boer R, Stam AG, et al. A bispecific antibody antagonizes prosurvival CD40 signaling and promotes Vγ9Vδ2 T cell-mediated antitumor responses in human B-cell malignancies. Cancer Immunol Res. 2021;9:50–61. doi: 10.1158/2326-6066.CIR-20-0138. [DOI] [PubMed] [Google Scholar]
- 58.de Bruin R.C.G., Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. OncoImmunology. 2017;7 doi: 10.1080/2162402X.2017.1375641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J., Fu J., Zhang M., Liu D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J Hematol Oncol. 2015;8:96. doi: 10.1186/s13045-015-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capuano C., Pighi C., Battella S., De Federicis D., Galandrini R., Palmieri G. Harnessing CD16-mediated NK cell functions to enhance therapeutic efficacy of tumor-targeting mAbs. Cancers. 2021;13:2500. doi: 10.3390/cancers13102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothe A., Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory hodgkin lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson H.R., Qi J, Cook EM, Nichols C, Dadashian EL, Underbayev C, et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood. 2018;132:521–532. doi: 10.1182/blood-2018-02-830992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minson A., Dickinson M. Glofitamab CD20-TCB bispecific antibody. Leuk Lymphoma. 2021;62:3098–3108. doi: 10.1080/10428194.2021.1953016. [DOI] [PubMed] [Google Scholar]
- 64.de Melo Gagliato D., Jardim D.L., Marchesi M.S., Hortobagyi G.N. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016;7:64431–64446. doi: 10.18632/oncotarget.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levine A.J. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20:471–480. doi: 10.1038/s41568-020-0262-1. [DOI] [PubMed] [Google Scholar]
- 66.Bailey M.H., Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;174:1034–1035. doi: 10.1016/j.cell.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 68.Waters A, Der C. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect Med. 2017;8:a031435. doi: 10.1101/cshperspect.a031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Douglass J., Hsiue EH-C, Mog BJ, Hwang MS, DiNapoli SR, Pearlman AH, et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci Immunol. 2021;6:eabd5515. doi: 10.1126/sciimmunol.abd5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Leary K. Bispecifics target cancers' most wanted. Nat Rev Cancer. 2021;21:279. doi: 10.1038/s41568-021-00354-0. [DOI] [PubMed] [Google Scholar]
- 71.Tan L., Zhang J, Wang Y, Wang X, Wang Y, Zhang Z, et al. Development of dual inhibitors targeting epidermal growth factor receptor in cancer therapy. J Med Chem. 2022;65:5149–5183. doi: 10.1021/acs.jmedchem.1c01714. [DOI] [PubMed] [Google Scholar]
- 72.da Cunha Santos G., Shepherd F.A., Tsao M.S. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 73.Giaccone G., González-Larriba JL, van Oosterom AT, Alfonso R, Smit EF, Martens M, et al. Combination therapy with gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, gemcitabine and cisplatin in patients with advanced solid tumors. Ann Oncol. 2004;15:831–838. doi: 10.1093/annonc/mdh188. [DOI] [PubMed] [Google Scholar]
- 74.Petrelli F., Borgonovo K., Cabiddu M., Ghilardi M., Barni S. Cetuximab and panitumumab in KRAS wild-type colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2011;26:823–833. doi: 10.1007/s00384-011-1149-0. [DOI] [PubMed] [Google Scholar]
- 75.Turke A.B., Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Remon J., Hendriks L.E.L., Cardona A.F., Besse B. EGFR exon 20 insertions in advanced non-small cell lung cancer: a new history begins. Cancer Treat Rev. 2020;90 doi: 10.1016/j.ctrv.2020.102105. [DOI] [PubMed] [Google Scholar]
- 77.Vijayaraghavan S., Lipfert L, Chevalier K, Bushey BS, Henley B, Lenhart R, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Therapeut. 2020;19:2044–2056. doi: 10.1158/1535-7163.MCT-20-0071. [DOI] [PubMed] [Google Scholar]
- 78.Moores S.L., Chiu ML, Bushey BS, Chevalier K, Luistro L, Dorn K, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76:3942–3953. doi: 10.1158/0008-5472.CAN-15-2833. [DOI] [PubMed] [Google Scholar]
- 79.Park K., Haura EB, Leighl NB, Mitchell P, Shu CA, Girard N, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the chrysalis phase I study. J Clin Oncol. 2021;39:3391–3402. doi: 10.1200/JCO.21.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Exman P., Tolaney S.M. HER2-positive metastatic breast cancer: a comprehensive review. Clin Adv Hematol Oncol. 2021;19:40–50. [PubMed] [Google Scholar]
- 81.Swain S.M., Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardoso F., Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meric-Bernstam F., Hanna D, Beeram M, Lee KW, Kang YK, Chaves J, et al. Safety, anti-tumour activity, and biomarker results of the HER2-targeted bispecific antibody ZW25 in HER2-expressing solid tumours. Ann Oncol. 2019;30:v167–v168. [Google Scholar]
- 84.Zymeworks . Zymeworks; May 26, 2022. Clinical data demonstrating promising antitumor activity with zanidatamab in 1L setting of HER2-positive breast and gastroesophageal cancers to be presented at ASCO 2022.https://ir.zymeworks.com/news-releases/news-release-details/clinical-data-demonstrating-promising-antitumor-activity/ Available from: [Google Scholar]
- 85.Baselga J., Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Y., Zhu X., Wei X., Tang C., Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876 doi: 10.1016/j.bbcan.2021.188549. [DOI] [PubMed] [Google Scholar]
- 87.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Zhang X, Wang Y, Zhao W, Li H, Zhang L, et al. Application of immune checkpoint targets in the anti-tumor novel drugs and traditional Chinese medicine development. Acta Pharm Sin B. 2021;11:2957–2972. doi: 10.1016/j.apsb.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiaie S.H., Sanaei MJ, Heshmati M, Asadzadeh Z, Azimi I, Hadidi S, et al. Immune checkpoints in targeted-immunotherapy of pancreatic cancer: new hope for clinical development. Acta Pharm Sin B. 2021;11:1083–1097. doi: 10.1016/j.apsb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lei X., Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Sun C., Cheng Y, Liu X, Wang G, Min W, Wang X, et al. Novel phthalimides regulating PD-1/PD-L1 interaction as potential immunotherapy agents. Acta Pharm Sin B. 2022;12:4446–4457. doi: 10.1016/j.apsb.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Mercier I., Lines J.L., Noelle R.J. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun C., Yin M, Cheng Y, Kuang Z, Liu X, Wang G, et al. Novel small-molecule PD-L1 inhibitor induces PD-L1 internalization and optimizes the immune microenvironment. J Med Chem. 2023;66:2064–2083. doi: 10.1021/acs.jmedchem.2c01801. [DOI] [PubMed] [Google Scholar]
- 94.Gao L., Yang X., Yi C., Zhu H. Adverse events of concurrent immune checkpoint inhibitors and antiangiogenic agents: a systematic review. Front Pharmacol. 2019;10:1173. doi: 10.3389/fphar.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye J., Hou B, Chen F, Zhang S, Xiong M, Li T, et al. Bispecific prodrug nanoparticles circumventing multiple immune resistance mechanisms for promoting cancer immunotherapy. Acta Pharm Sin B. 2022;12:2695–2709. doi: 10.1016/j.apsb.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keam S.J. Cadonilimab: first approval. Drugs. 2022;82:1333–1339. doi: 10.1007/s40265-022-01761-9. [DOI] [PubMed] [Google Scholar]
- 97.Huang Z., Pang X, Zhong T, Chen N, He X, Xia D, et al. Cadonilimab, an anti-PD1/CTLA4 bi-specific antibody with Fc effector null backbone. J Immunother Cancer. 2021;9:A313. [Google Scholar]
- 98.NMPA . Center for Drug Evaluation, NMPA; June 29, 2022. The NMPA approved the marketing of cadunilimumab injection with conditions.https://www.nmpa.gov.cn/yaowen/ypjgyw/20220629135936153.html Available from: [Google Scholar]
- 99.Lakins M., Liao W, McConnell E, Kaka Q, Ofoedu J, Gradinaru C, et al. FS120, an OX40/CD137 tetravalent bispecific dual agonist antibody, synergistically increases the antitumor activity of anti-PD-1 in preclinical studies. J Immunother Cancer. 2021;9:A602. [Google Scholar]
- 100.Kim H.D., Park S, Jeong S, Lee YJ, Lee H, Kim CG, et al. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8+ T cells in hepatocellular carcinoma. Hepatology. 2020;71:955–971. doi: 10.1002/hep.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaspar M., Pravin J, Rodrigues L, Uhlenbroich S, Everett KL, Wollerton F, et al. CD137/OX40 bispecific antibody induces potent antitumor activity that is dependent on target coengagement. Cancer Immunol Res. 2020;8:781–793. doi: 10.1158/2326-6066.CIR-19-0798. [DOI] [PubMed] [Google Scholar]
- 102.Melero I., Hirschhorn-Cymerman D., Morales-Kastresana A., Sanmamed M.F., Wolchok J.D. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–1053. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peper-Gabriel J.K., Pavlidou M, Pattarini L, Morales-Kastresana A, Jaquin TJ, Gallou C, et al. The PD-L1/4-1BB bispecific antibody–anticalin fusion protein PRS-344/S095012 elicits strong T-cell stimulation in a tumor-localized manner. Clin Cancer Res. 2022;28:3387–3399. doi: 10.1158/1078-0432.CCR-21-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eskiocak U., Guzman W, Wolf B, Cummings C, Milling L, Wu HJ, et al. Differentiated agonistic antibody targeting CD137 eradicates large tumors without hepatotoxicity. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berraondo P., Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Propper D.J., Balkwill F.R. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 107.Montfort A., Filleron T, Virazels M, Dufau C, Milhès J, Pagès C, et al. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin Cancer Res. 2021;27:1037–1047. doi: 10.1158/1078-0432.CCR-20-3449. [DOI] [PubMed] [Google Scholar]
- 108.Kontomanolis E., Panteliadou M, Giatromanolaki A, Pouliliou S, Efremidou E, Limberis V, et al. Delta-like ligand 4 (DLL4) in the plasma and neoplastic tissues from breast cancer patients: correlation with metastasis. Med Oncol. 2014;31:945. doi: 10.1007/s12032-014-0945-0. [DOI] [PubMed] [Google Scholar]
- 109.Patel N.S., Dobbie MS, Rochester M, Steers G, Poulsom R, Le Monnier K, et al. Up-regulation of endothelial delta-like 4 expression correlates with vessel maturation in bladder cancer. Clin Cancer Res. 2006;12:4836–4844. doi: 10.1158/1078-0432.CCR-06-0285. [DOI] [PubMed] [Google Scholar]
- 110.Couch J.A., Zhang G, Beyer JC, de Zafra CL, Gupta P, Kamath AV, et al. Balancing efficacy and safety of an anti-DLL4 antibody through pharmacokinetic modulation. Clin Cancer Res. 2016;22:1469–1479. doi: 10.1158/1078-0432.CCR-15-1380. [DOI] [PubMed] [Google Scholar]
- 111.Jimeno A., Moore KN, Gordon M, Chugh R, Diamond JR, Aljumaily R, et al. A first-in-human phase 1a study of the bispecific anti-DLL4/anti-VEGF antibody navicixizumab (OMP-305B83) in patients with previously treated solid tumors. Invest N Drugs. 2019;37:461–472. doi: 10.1007/s10637-018-0665-y. [DOI] [PubMed] [Google Scholar]
- 112.Fu S., Corr B, Culm-Merdek K, Mockbee C, Youssoufian H, Stagg R, et al. Phase Ib study of navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2022;40:JCO2101801. doi: 10.1200/JCO.21.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mereo BioPharma . GlobeNewswire; October 7, 2019. Mereo biopharma receives FDA fast track designation for navicixizumab for the treatment of heavily pretreated ovarian cancer.https://www.globenewswire.com/news-release/2019/10/07/1925802/0/en/Mereo-BioPharma-Receives-FDA-Fast-Track-Designation-for-Navicixizumab-for-the-Treatment-of-Heavily-Pretreated-Ovarian-Cancer.html Available from: [Google Scholar]
- 114.Batlle E., Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gulley J.L., Schlom J, Barcellos-Hoff MH, Wang XJ, Seoane J, Audhuy F, et al. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol Oncol. 2022;16:2117–2134. doi: 10.1002/1878-0261.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Derynck R., Turley S.J., Akhurst R.J. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18:9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yi M., Zhang J, Li A, Niu M, Yan Y, Jiao Y, et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol. 2021;14:27. doi: 10.1186/s13045-021-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chae S.S., Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graaf AM, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16:3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sfiligoi C., de Luca A, Cascone I, Sorbello V, Fuso L, Ponzone R, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466–474. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 120.Etoh T., Inoue H., Tanaka S., Barnard G.F., Kitano S., Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [PubMed] [Google Scholar]
- 121.Hidalgo M., Martinez-Garcia M, Le Tourneau C, Massard C, Garralda E, Boni V, et al. First-in-human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin Cancer Res. 2018;24:1536–1545. doi: 10.1158/1078-0432.CCR-17-1588. [DOI] [PubMed] [Google Scholar]
- 122.Hamblett K., Hammond P, Barnscher S, Fung V, Davies R, Wickman G, et al. Abstract 3914: ZW49, a HER2-targeted biparatopic antibody–drug conjugate for the treatment of HER2-expressing cancers. Cancer Res. 2018;78:3914. [Google Scholar]
- 123.Garfall A.L., June C.H. Trispecific antibodies offer a third way forward for anticancer immunotherapy. Nature. 2019;575:450–451. doi: 10.1038/d41586-019-03495-3. [DOI] [PubMed] [Google Scholar]
- 124.Zhao P., Zhang Y., Li W., Jeanty C., Xiang G., Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B. 2020;10:1589–1600. doi: 10.1016/j.apsb.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shim H. Bispecific antibodies and antibody–drug conjugates for cancer therapy: technological considerations. Biomolecules. 2020;10:360. doi: 10.3390/biom10030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X., Huang AC, Chen F, Chen H, Li L, Kong N, et al. Novel development strategies and challenges for anti-Her2 antibody–drug conjugates. Antib Ther. 2022;5:18–29. doi: 10.1093/abt/tbac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Goeij B.E., Vink T, Ten Napel H, Breij EC, Satijn D, Wubbolts R, et al. Efficient payload delivery by a bispecific antibody–drug conjugate targeting HER2 and CD63. Mol Cancer Therapeut. 2016;15:2688–2697. doi: 10.1158/1535-7163.MCT-16-0364. [DOI] [PubMed] [Google Scholar]
- 128.Pols M.S., Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 129.Huang S., van Duijnhoven S.M.J., Sijts A., van Elsas A. Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J Cancer Res Clin Oncol. 2020;146:3111–3122. doi: 10.1007/s00432-020-03404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu L., Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Can. 2020;1:86–98. doi: 10.1038/s43018-019-0004-z. [DOI] [PubMed] [Google Scholar]
- 131.Systimmune GNC-038. Systimmune. December 1, 2022. https://systimmune.com/gnc-038 Available from:
- 132.Esfandiari A., Cassidy S., Webster R.M. Bispecific antibodies in oncology. Nat Rev Drug Discov. 2022;21:411–412. doi: 10.1038/d41573-022-00040-2. [DOI] [PubMed] [Google Scholar]