Abstract
Hepatocellular carcinoma (HCC) constitutes an extremely malignant form of primary liver cancer. Intricate connections linking to the immune system might be associated with the pathogenesis of HCC. Meanwhile, immunotherapy with immune checkpoint inhibitors has been established to be a favorable therapeutic possibility for advanced HCC. Although curative opportunities for advanced HCC are restricted, the immune checkpoint immunotherapy has developed as the main choice for treating HCC. However, patients with metabolic-associated fatty liver disease (MAFLD)-linked HCC might be less likely to benefit from the immunotherapy alone. The limitation of the effect of the immunotherapy might be owing to the impaired T cell activation in MAFLD patients, which could be well explained by a dysfunctional gut-liver axis. Gut microbiota and their metabolites including several bile acids could contribute to modulating the responses of the immune checkpoint immunotherapy. Roles of gut microbiota in the development of cancers have expected great interest in the latest studies. Here, an interplay between the gut and liver has been presented, which might suggest to affect the efficacy of immune checkpoint immunotherapy against HCC.
Keywords: Hepatocellular carcinoma, non-alcoholic fatty liver disease, metabolic-associated fatty liver disease, gut microbiota, probiotics, immune checkpoint inhibitors, T helper 17 cells, regulatory T cells
Introduction
Hepatocellular carcinoma (HCC) is the regular type of primary liver cancer approximately constituting 80% of cases [1]. HCC is an aggressive malignancy, which has been a healthcare burden worldwide [1]. Therapies for HCC are determined by the clinical stages of the disease. In case of early stage HCC, localized therapies such as ablation, radiation, and resection including hepatectomy are the typical treatment [2]. Among them, surgical management is the most imperative method for HCC patients to achieve long-term survival. In fact, the guidelines for the treatment of primary liver cancer had proposed surgical resection as the first choice for the treatment of HCC in stages with a spare of liver functional ability. However, these patients only represent about 20% of the total. In addition, the 5-year survival rate may not be satisfactory with more than 70% of patients unfortunately relapsing within 5 years [3]. Furthermore, HCC is frequently diagnosed in advanced and/or unresectable stages [4]. Therefore, HCC has high mortality and a high risk of recurrence even after drastic treatment. The majority of the patients with HCC diagnosed at advanced stages may miss the ideal time for remedial management. Then, there are few therapeutic treatments with limited possibilities and limited survival benefits, which is making the cure rate quite low [5]. At present, chemotherapy treatments for HCC are largely separated into targeted therapeutic medications and immunotherapeutic agents. Tactlessly, systematic palliative treatment may be also the option for most patients with advanced-stages of HCC [6].
While viral hepatitis with hepatitis virus such as hepatitis B virus (HBV) and/or hepatitis C virus (HCV) may characterize one of the most significant reason for HCC, alcoholic liver disease (ALD) and metabolic-associated fatty liver disease (MAFLD) are also the leading cause for the development of HCC (Figure 1). In particular, MAFLD may be a leading premonition of HCC [7]. The combination of a high fat diet and bacterial endotoxin could contribute to the activation of both innate and adaptive immune responses, which may be headmaster to the pathogenesis of MAFLD and HCC [8]. MAFLD-associated inflammatory, metabolic and/or endocrine mediators might play key roles in several tumorigenesis. However, the carcinogenesis to HCC may be multi factorial compelled by several other chronic inflammation such as colitis and/or pancreatitis. Major risk factors for HCC may also contain other metabolic disorders such as diabetes, liver fibrosis, aflatoxin-induced liver toxicity and immune-related diseases including autoimmune hepatitis [9, 10]. In addition, an imbalance in the composition of gut microbiota may lead to a disrupted intestinal barrier, which could direct to the translocation of bacteria and/or their products into the portal circulation, then increase hepatic exposure to harmful substances that might afterward result in chronic inflammation and/or the development of MAFLD or HCC [11, 12]. In other words, the balance of the gut microbiota might be indispensable for a physiological and appropriate functioning of the intestinal barrier to avoid the development of MAFLD or HCC.
Figure 1.
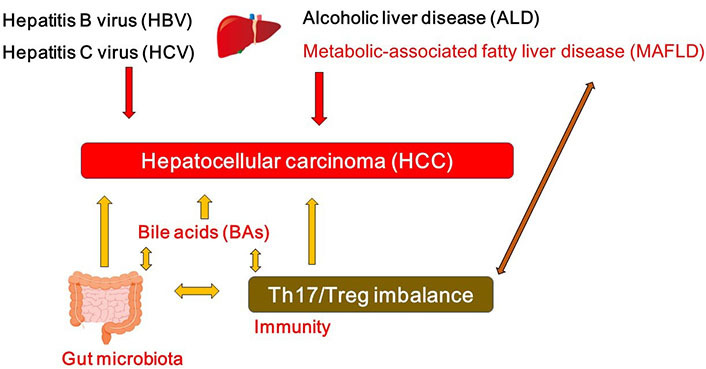
A hypothetical schematic representation and overview of the pathogenesis of HCC. HBV, HCV, ALD, and/or MAFLD as well as bile acids (BAs), T helper 17 (Th17)/regulatory T (Treg) imbalance, and/or gut microbiota could contribute to the pathogenesis of HCC. Arrowhead indicates stimulation whereas double-arrowheads suggests bidirectional stimulation. Note that several important activities such as cytokine-induction or anti-inflammatory reaction have been omitted for clarity
HCC and gut microbiota
Emerging evidence implicates a key role of gut microbiota in liver inflammation and the progress of HCC [11]. The gut-liver axis is the key mechanism by which the gut microbiome promotes various liver disease and/or HCC [12]. For example, Anaerotruncus enriched in the case of antibiotics usage are butyrate-producing bacteria with a positive correlation to the intake of saturated fatty acids and cholesterol, which may be involved in MAFLD-associated HCC [13]. Dysfunction of gut microbiota might provoke a neutrophil accumulation into the gut epithelium that alters the composition of inflammatory cytokine and/or chemokine, which might stimulate the Th17 cells [14]. The intra-tumoral regions of HCC are generally in an immunosuppressive state [15], which may include altered amount of effector to Treg cells and a profusion of immunosuppressive molecules developing a linkage to assist immune escaping [16]. Likewise, increased levels of Th17 cells have been observed in tumor tissue [17] and/or even in peripheral blood [18] of the patients with HCC, however, which might be interrelated with unfavorable disease consequences [17, 19]. Similar results have been also detected in animal models, whereby restrictive expansion of Th17 cells in tumor may reduce the growth of the transplanted liver tumor in animal models [20]. The gut might be an expected place for Th17 generation. It has been shown that gut microbiota could affect T cell differentiation of Th17 cells via regulating dendritic cells [21]. As a consequence, there appears to be a complex association among gut microbiota, the development of Th17 cells and the progression of HCC (Figure 1).
The source of tumor-associated Th17 cells may be also connected to the gut [22]. In addition, Th17 cells seem to be intensely linked with HCC progression, probably via assisting their angiogenesis [20, 23]. Interestingly, probiotic alteration of the gut microbiota could help prevent the occurrence of HCC [24]. Hence, the shift in the function of gut microbiota might accidentally support tumor formation and growth. In fact, bacterial metabolites in gut are known that play a role in carcinogenesis [25, 26]. Furthermore, modulation of gut microbiota may represent an effective procedure for strengthening anti-cancer immunity, which is suggested by various information that exhibit a close connection between gut microbiota and the pathogenic mechanisms of HCC [27]. The use of probiotics and/or the fecal microbiota transplantation (FMT) method could develop new paradigms with the potential effectiveness of several treatments available for HCC. In particular, Th17 cells seem to be an innovative therapeutic target against tumor-promoting inflammation. Strategies using probiotics and/or FMT to polarize the response away from Th17 cells might be useful to slow down the tumor growth of HCC.
HCC and BAs
Microbial products could closely affect the function of liver, whose derivatives including BAs could lead to carcinogenesis in liver [28]. Connection between BAs and HCC is progressively being established by several experiments. For example, the accumulation of toxic BAs might be related to the progression of HCC (Figure 1) [29]. In addition, experiments in which mice fed with a high-fat diet exhibit that long-term high-fat diet could induce liver tumors in mice along with the observation of considerably increased BAs in plasma and/or in liver [30]. BAs may be vigorously changed all the way to the impairment of the animal in the course of HCC progression. BA molecules have hydrophobic regions and hydrophilic regions in their configuration, which might provide them with interfacial activity to decrease the surface tension [31, 32]. In addition, BAs can extend the contact surface between lipids and lipase to speed up the digestion of various lipids [31]. BAs can also prevent the precipitation of cholesterol to inhibit the formation of cholesterol stones [31, 32]. Since BAs are great surfactants, bacteria in gut have to protect themselves against being degenerated by BAs. Accordingly, commensal bacteria in gut may have a complicated system to survive with BAs [32]. In addition, connections between BAs and gut microbiota may be bidirectional. For example, gut microbiota could convert some of the primary BAs to modulate the composition of the BAs [33]. In reverse, BAs could also affect the composition of the gut microbiota (Figure 1) [34]. Hence, BAs might work as potent controllers of gut microbiota [35]. Dysbiosis of gut microbiota associated with several cancers [36] could change the composition of BAs, which might eventually contribute to the carcinogenesis (Figure 1) [37, 38]. Oxidation and/or epimerization through the reactions of BAs might be interconnected to Firmicutes and/or Bacteroides species in the gut [39, 40].
Cell membrane perturbation by BAs might activate phospholipase A2 with the release of arachidonic acid from the plasma membrane, eventually leading to the production of increased levels of reactive oxygen species (ROS) in hepatocytes [41]. Therefore, BAs could damage the cell membrane, eventually leading to the increased levels of inflammation [41]. Apoptotic cells as a result of BAs might also initiate the inflammation. ROS may induce DNA damage in hepatocytes and contribute to the occurrence of HCC [42]. BAs could induce genetic instability scratched by the DNA damage via oxidative stress [43]. Therefore, the role of BAs has been involved in a wide range of cancers including HCC. For example, hydrophobic BAs could contribute to the growth of HCC [44]. In addition, elevated BAs in serum for a long-term has been identified in HCC patients by metabolomics [45]. Furthermore, conjugated BAs such as taurocholic acid and/or taurodeoxycholic acid could promote the progression of esophageal adenocarcinoma [46]. Acidic bile salts could stimulate epithelial to mesenchymal transition in Barrett’s cells [47]. High concentrations of BAs might be associated with an elevated risk of intestinal metaplasia [48], which could increase the risk of gastric cancer [49]. Additionally, BAs might be also involved in the initiation and/or the progression of pancreatic cancer. In fact, several BAs have shown an extreme increase in the patients with pancreatic cancer at multiple stages [50]. In pancreatic cancer cells, BAs could reduce the susceptibility of cancer cells to lead to apoptosis [51]. It has been shown that the damaging effect of BAs on colon epithelial cells might induce a compensatory renewal mechanism of the epithelium by inducing the epithelial cells to convert cancer stem cells [52]. It has been reported that both lithocholic acid and deoxycholic acid have tumor promoter activity on the cells at crypt of colon in the early phase of carcinogenesis [53]. In summary, BAs could contribute to the carcinogenesis of various cancers including HCC.
HCC treated with immune checkpoint inhibitors
Immune checkpoint inhibitors have been permitted for clinical usage for HCC treatment, which have revealed some efficacy in many clinical trials afterwards [54]. Up until now, cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1) and/or micro-environmental immune cells seem to be associated with the effectiveness. Each treatment appears definite efficacy and/or small toxicity profiles, being related to the tumor microenvironment of HCC [55]. By discharging the immune checkpoints which standstill the function of T-cells, each treatment could result in the re-activation of an anti-cancer immune system efficiently to attack the cancer cells [56]. As for advanced stages of HCC patients, a nearly complete response such as down-staging has been achieved by those treatments with immune checkpoint inhibition, while actual few has detected non-lethal and/or reversible adverse events [57, 58]. In addition, immune checkpoint inhibitors seem to increase overall survival among patients with advanced stages of HCC. More strategies should embrace a combination of immune checkpoint inhibitors with additional therapies to achieve the more vigorous responses [59].
The pathophysiological association among the PD-1/PD-L1 pathway, Th17 cells and Treg cells has been shown, suggesting an indispensable role of PD-1/PD-L1 in the regulation of Th17/Treg cells [60]. Similarly, CTLA-4 inhibitor therapy could also enhance Th17 cells [61]. In addition, blockade of CTLA-4 could also inhibit Treg cells [62]. Surprisingly, IL-17 and its primary source Th17 could upregulate PD-L1 expression and may hamper the efficacy of the immunotherapy [63]. Th17 cells are resistant to steroid therapy [64]. Similarly, the Th17 cells seem to be always associated with poor responses in cancer immunotherapy. In the mechanisms of immune checkpoint, interaction of PD-1 with PD-L1 might cause a reduction of phosphoinositide 3-kinase (PI3K) expression in T lymphocytes leading to the induction of Treg cells [65]. Th17 cells can also trans-differentiate into suppressive Treg cells [66], providing a source of tumor-associated Treg cells. On the contrary, elevated expression of PI3K/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) molecules may induce a conversion of Treg cells into Th17 cells in the situation of PD-1/PD-L1 dysfunction [65]. Hence, when the PD-1/PD-L1 pathway is congested by immune checkpoint inhibitors, the mTOR pathway might be activated. Accordingly, Th17 cells are abundant and Treg cells are less present. Excessive inflammation from Th17 cells may play significant consequences in several inflammation-associated carcinogenesis [17, 67]. Flexibility of Th17 cells according to the situation might provide a valuable strategy to enhance cancer immunotherapies.
This immune checkpoint inhibitory immunotherapy is deliberated a step frontward to the management of a diversity of malignancies, particularly in advanced stages of cancers. The immunotherapy could be pretty specific to the cancer cells without showing any negative effects on healthy cells and/or organs. The molecular mechanism is targeting at specific antigens presented in malignant cancer cells [68]. Subsequently, the research strategies to overwhelm the resistance of cancer cells seem to be heading toward an intensified strategy comprising better combination with different agents such as PD-1 plus CTLA-4 blockades. The gut-liver axis might be an anatomical and physiological connection between the liver and gut, which may help not only to maintain normal liver functions but also to protect the liver from the development of carcinogenesis [24]. Therefore, exposure of the liver to chronic lesions to bacterial metabolites may result in liver damage and eventually the development of HCC [24].
Therapeutic approach for patients with unresectable stages of HCC
As mentioned above, dysbiosis may influence the effectiveness of anticancer therapies including immune checkpoint inhibitors in some types of patients with cancers. Consistently, the intestinal microbiota could increase the effectiveness and/or the sensitivity of the treatment with anti PD-1 against HCC [69, 70], which suggests that the modulation of gut microbiota can manage to increase the activity of HCC treatments. Microbiota of patients who have responded to PD-1 immunotherapy exhibits increased certain gut bacterial species [70, 71]. In addition, germ-free mice transplanted with fecal samples from patients responding to anti-PD-1 and/or anti-PD-L1 immunotherapy have showed a reduction in the tumor growth and/or enhanced responses to anti-PD-1 and/or anti-PD-L1 treatment [71]. Reliably, the gut microbiota can affect the efficacy of immune checkpoint immunotherapy [72]. Specific bacteria possibly for enhancing the immune-stimulatory anti-cancer effects of PD-1 and/or CTLA-4 blockades have been shown in Table 1. In addition, it has been reported that modulation of gut microbiota may form a strong procedure of manipulation for the anti-tumor immunity [73]. Patients with immunoresistance to anti-PD-1 treatment of HCC may exhibit an enlarged amount of Ruminococcus spp. and Akkermansia muciniphila in their fecal samples [69]. In addition, intake of Bifidobacterium could improve the response to PD-L1 immune checkpoint immunotherapy against HCC [74]. Similarly, it has been reported that intake of Firmicutes and Faecalibacterium could augment the response to anti-CTLA-4 antibodies [74]. Interestingly, FMT in combination with oral supplementation of Akkermansia muciniphila could change the immune-resistance to the PD-1 immunotherapy [75]. Probiotics, prebiotics and FMT could shift the gut microbial community toward certain beneficial bacteria, which subsequently decreases the Th17 polarization and/or promotes the differentiation of anti-inflammatory Treg cells leading to slow down the growth of HCC tumor (Figure 2) [76]. On the other hand, microbial dysbiosis could direct to the disruption of the gut barrier, which may influence the production and construction of BAs, resulting in a carcinogenesis of a broad spectrum of malignant tumors including HCC [41]. In addition, the changed components of BAs might be connected with immune-resistance [77]. It has been shown that BAs might be enriched in cancer patients, which is associated with poor prognosis [78]. BAs could alter the gut microbial population [79], indicating that BAs might also affect the efficacy of immune checkpoint immunotherapy by gut bacterial alteration (Figure 2).
Table 1.
Microbial species which may enhance the effect of immune-checkpoint cancer therapies
| Immunotherapies | Microbial species | References |
|---|---|---|
| Anti PD-1/PD-L1 blockade |
Bacteroides cellulosilyticus Bifidobacterium dentium Coprococcus comes Lactobacillus oris Lactobacillus mucosae Streptococcus thermophilus |
[69, 74] |
| Anti CTLA-4 blockade |
Bacteroides thetaiotaomicron Bacteroides fragilis Burkholderia cepacia Faecalibacterium genus |
[74, 101] |
Figure 2.
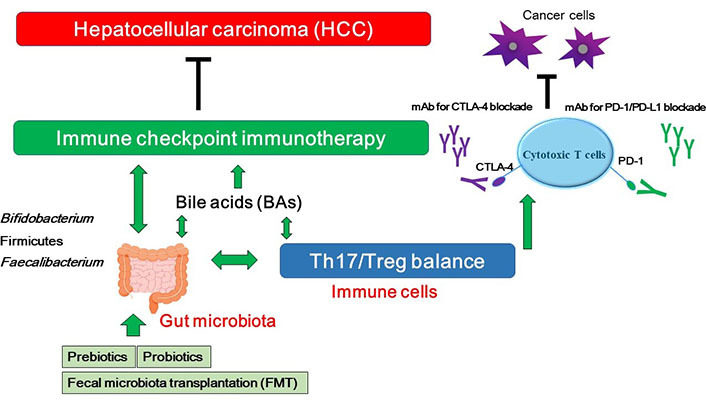
A hypothetical schematic representation and overview of the inhibition against HCC. BAs, Th17/Treg balance, and/or certain gut microbiota could contribute to the potentiation of the immune checkpoint immunotherapy with immune checkpoint inhibitors. Some kinds of probiotics, prebiotics, and FMT could contribute to the alteration of gut microbial community for playing valuable roles to the immune checkpoint therapy. Examples of certain beneficial microbial species with several effects on anti-cancer immune responses have been shown at the left side. Arrowhead indicates stimulation whereas double-arrowheads suggests bidirectional stimulation. Hammerhead shows inhibition. Note that several important activities such as cytokine-induction and/or anti-inflammatory reaction have been omitted for clarity. mAB: monoclonal antibody
Provided several significant results acquired from immunotherapy in patients with advanced HCC [69], it might be imperative to search the data relating to the gut microbiota whether there are the beneficial effects of the immunotherapy against HCC, or not. In addition, the possible modulation of the gut microbiota could help in overcoming any resistance to immunotherapy in patients with MAFLD-linked HCC [80]. Both fecal microbiota and BAs are related to consequences of immunotherapy for HCC [81]. These findings emphasize the possible role of gut microbiota and their metabolites to predict the consequences of immune checkpoint immunotherapy-treated HCC. Interestingly, a curcumin analog controls the metabolism of both Treg cells and tumor cells and could serve as a booster for immune checkpoint inhibitor therapies [82]. In the attempt to intensify the efficacy of an immunotherapy for HCC, double or triple checkpoints blockade such as anti PD-1 and anti CTLA-4 or anti PD-L1 and multi-target tyrosine kinase inhibitors have been suggested [83].
Future perspectives
The management of HCC has been radically changing. Usage of tyrosine kinase inhibitors and the introduction of the immunotherapy might improve the survival and the progression free period of the HCC even in advanced stages [84]. However, the efficacy of those strategies may be empowered by an adapted patient selection. Since patients with HCC are disposed to have more or less changes in gut microbiota, it may be striking to suppose that some modulation of gut microbiota could affect the efficacy of anticancer treatments in certain types of patients. And so, distinct bacterial species could control different immune responses. Gut microbiota-derived metabolites such as BAs and/or short-chain fatty acids (SCFAs) may be also involved in the regulation of inflammation and/or carcinogenesis [85]. In addition, these changes could be associated with an inflammatory and/or immune power shift. The life-style diet may be responsible for the dysbiosis, which is an important risk factor for carcinogenesis and/or influences the therapeutic outcomes, as for various anticancer treatments including the immune checkpoint immunotherapies [86]. In fact, several findings demonstrate that gut microbiota could affect the response to the immunotherapy as mentioned earlier. However, some bacterial products such as lipopolysaccharide (LPS) may stimulate the toll-like receptors (TLRs), in particular TLR-4, which in turn can activate the nuclear factor kappa B (NF-κB) pathway [87, 88]. The TLR-4/myeloid differentiation factor 88 (MyD88) pathway has been known as oncogenic signaling in human HCC and may be correlated with patients’ poor survival [89]. The immunomodulatory signaling with TLR-4 could induce the stimulation of mitogen-activated protein kinase (MAPK) following a mitogenic signal [89], which may be associated with an inhibition of programmed cell death. Therefore, prolonged stimulation of several TLRs with various bacterial substances in hepatocytes could promote the development of chronic liver diseases and/or HCC [90]. Consequently, the orientation of research towards the use of prebiotics, probiotics and/or FMT should guide to a new personalized treatment-paradigm with understanding the precise roles of gut microbiota for the more effective treatments available to HCC [91]. Prebiotics and/or probiotics could have a therapeutic effect against the chronic inflammation related cancers [91]. It remains to be clarified whether the gut microbiota in some types of malignant tumors could also be practical to the patients with HCC. Commensal bacteria are fundamental in coordinating antitumor responses in various tumor microenvironment [92–94]. In addition, BAs could also regulate adaptive immune responses by directly modifying the balance of Th17 cells and Treg cells, suggesting much wide-ranging roles of BAs in regulating inflammation and/or cancer [95–97]. Insertion of immune checkpoint inhibitors with the other combination therapy might achieve the benefit of therapeutic efficacy within this research approach, as this would allow patients to be directed to the most appropriate and safe treatment. For example, several objective therapeutic strategies for patients with unresectable advanced HCC have been proposed [98–100], which appear to be quite promising for the combination therapy with this immune-potentiation therapy. Despite convincing evidences regarding the roles of the gut-liver axis in the pathogenesis of HCC, however, putting this fact into a clinical practice is still a work in slow progress.
Conclusions
The tumor microenvironment of HCC might be intricate and dynamic. In some cases, the interplay between liver and gut could affect the efficacy of several anti-cancer treatments including immune checkpoint immunotherapy via the alteration of Th17 cells. Come to think of it, complicated interactions among HCC cells, suppressive immune cells, and the gut microbiome might produce a permissive microenvironment that facilitates immune evasion to approve HCC growth.
Abbreviations
- ALD
alcoholic liver disease
- BAs
bile acids
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- FMT
fecal microbiota transplantation
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- MAFLD
metabolic-associated fatty liver disease
- PD-1
programmed cell death 1
- PD-L1
programmed cell death ligand 1
- Th17
T helper 17
- TLRs
toll-like receptors
- Treg
regulatory T
Declarations
Author contributions
SY and SM: Conceptualization. SY, KT, HS, YI, TA, AT and SM: Writing—original draft, Writing—review & editing. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.
References
- 1.Perisetti A, Goyal H, Yendala R, Chandan S, Tharian B, Thandassery RB. Sarcopenia in hepatocellular carcinoma: current knowledge and future directions. World J Gastroenterol. 2022;28:432–48. doi: 10.3748/wjg.v28.i4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canale M, Ulivi P, Foschi FG, Scarpi E, De Matteis S, Donati G, et al. Clinical and circulating biomarkers of survival and recurrence after radiofrequency ablation in patients with hepatocellular carcinoma. Crit Rev Oncol Hematol. 2018;129:44–53. doi: 10.1016/j.critrevonc.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Choi JW, Lee JM, Lee DH, Yoon JH, Kim YJ, Lee JH, et al. Radiofrequency ablation using internally cooled wet electrodes in bipolar mode for the treatment of recurrent hepatocellular carcinoma after locoregional treatment: a randomized prospective comparative study. PLoS One. 2020;15:e0239733. doi: 10.1371/journal.pone.0239733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilagan CH, Goldman DA, Gönen M, Aveson VG, Babicky M, Balachandran VP, et al. Recurrence of hepatocellular carcinoma after complete radiologic response to trans-arterial embolization: a retrospective study on patterns, treatments, and prognoses. Ann Surg Oncol. 2022;29:6815–26. doi: 10.1245/s10434-022-12036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. RESORCE Investigators Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. Erratum in: Lancet. 2017;389:36. [DOI] [PubMed] [Google Scholar]
- 6.Hu WY, Wei HY, Li KM, Wang RB, Xu XQ, Feng R. LINC00511 as a ceRNA promotes cell malignant behaviors and correlates with prognosis of hepatocellular carcinoma patients by modulating miR-195/EYA1 axis. Biomed Pharmacother. 2020;121:109642. doi: 10.1016/j.biopha.2019.109642. [DOI] [PubMed] [Google Scholar]
- 7.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–7. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:637–44. doi: 10.1038/nrgastro.2013.146. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Desert R, Ge X, Han H, Song Z, Das S, et al. The matrisome genes from hepatitis B-related hepatocellular carcinoma unveiled. Hepatol Commun. 2021;5:1571–85. doi: 10.1002/hep4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behary J, Raposo AE, Amorim NML, Zheng H, Gong L, McGovern E, et al. Defining the temporal evolution of gut dysbiosis and inflammatory responses leading to hepatocellular carcinoma in Mdr2 -/- mouse model. BMC Microbiol. 2021;21:113. doi: 10.1186/s12866-021-02171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–20. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–74. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezasoltani S, Yadegar A, Asadzadeh Aghdaei H, Reza Zali M. Modulatory effects of gut microbiome in cancer immunotherapy: a novel paradigm for blockade of immune checkpoint inhibitors. Cancer Med. 2021;10:1141–54. doi: 10.1002/cam4.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Zheng L. Dynamic education of macrophages in different areas of human tumors. Cancer Microenviron. 2012;5:195–201. doi: 10.1007/s12307-012-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed F, Steele JC, Herbert JM, Steven NM, Bicknell R. Tumor stroma as a target in cancer. Curr Cancer Drug Targets. 2008;8:447–53. doi: 10.2174/156800908785699360. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Zhao F, Hoechst B, Gamrekelashvili J, Ormandy LA, Voigtländer T, Wedemeyer H, et al. Human CCR4+ CCR6+ Th17 cells suppress autologous CD8+ T cell responses. J Immunol. 2012;188:6055–62. doi: 10.4049/jimmunol.1102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells†. Hepatology. 2010;51:154–64. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 21.Michaelis L, Treß M, Löw HC, Klees J, Klameth C, Lange A, et al. Gut commensal-induced IκBζ expression in dendritic cells influences the Th17 response. Front Immunol. 2021;11:612336. doi: 10.3389/fimmu.2020.612336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung CY, Lee NP, El-Nezami H. Regulation of T helper 17 by bacteria: an approach for the treatment of hepatocellular carcinoma. Int J Hepatol. 2012;2012:439024. doi: 10.1155/2012/439024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin H, Yuan B, Huang W, Wang Y. Utilizing gut microbiota to improve hepatobiliary tumor treatments: recent advances. Front Oncol. 2022;12:924696. doi: 10.3389/fonc.2022.924696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikó E, Vida A, Bai P. Translational aspects of the microbiome—to be exploited. Cell Biol Toxicol. 2016;32:153–6. doi: 10.1007/s10565-016-9320-6. [DOI] [PubMed] [Google Scholar]
- 26.Kovács T, Mikó E, Vida A, Sebő É, Toth J, Csonka T, et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci Rep. 2019;9:1300. doi: 10.1038/s41598-018-37664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–39. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Feng J, Li J, Yu Q, Ji J, Wu J, et al. The gut microbiome-bile acid axis in hepatocarcinogenesis. Biomed Pharmacother. 2021;133:111036. doi: 10.1016/j.biopha.2020.111036. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Yang M, Zhao J, Li X, Xiao X, Zhang Y, et al. Bile salt (glycochenodeoxycholate acid) induces cell survival and chemoresistance in hepatocellular carcinoma. J Cell Physiol. 2019;234:10899–906. doi: 10.1002/jcp.27905. [DOI] [PubMed] [Google Scholar]
- 30.Xie G, Wang X, Huang F, Zhao A, Chen W, Yan J, et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer. 2016;139:1764–75. doi: 10.1002/ijc.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko CW, Qu J, Black DD, Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol. 2020;17:169–83. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- 32.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. Erratum in: Gut Microbes. 2016;7:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2017;16:S21–6. doi: 10.5604/01.3001.0010.5672. [DOI] [PubMed] [Google Scholar]
- 34.Tsuei J, Chau T, Mills D, Wan YJ. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med (Maywood) 2014;239:1489–504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Best N, Rolle-Kampczyk U, Schaap FG, Basic M, Olde Damink SWM, Bleich A, et al. Bile acids drive the newborn’s gut microbiota maturation. Nat Commun. 2020;11:3692. doi: 10.1038/s41467-020-17183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas RM, Jobin C. The microbiome and cancer: is the ‘Oncobiome’ mirage real? Trends Cancer. 2015;1:24–35. doi: 10.1016/j.trecan.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sipos A, Ujlaki G, Mikó E, Maka E, Szabó J, Uray K, et al. The role of the microbiome in ovarian cancer: mechanistic insights into oncobiosis and to bacterial metabolite signaling. Mol Med. 2021;27:33. doi: 10.1186/s10020-021-00295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiss B, Mikó E, Sebő É, Toth J, Ujlaki G, Szabó J, et al. Oncobiosis and microbial metabolite signaling in pancreatic adenocarcinoma. Cancers (Basel) 2020;12:1068. doi: 10.3390/cancers12051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridlon JM, Devendran S, Alves JM, Doden H, Wolf PG, Pereira GV, et al. The ‘in vivo lifestyle’ of bile acid 7α-dehydroxylating bacteria: comparative genomics, metatranscriptomic, and bile acid metabolomics analysis of a defined microbial community in gnotobiotic mice. Gut Microbes. 2020;11:381–404. doi: 10.1080/19490976.2019.1618173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vital M, Rud T, Rath S, Pieper DH, Schlüter D. Diversity of bacteria exhibiting bile acid-inducible 7α-dehydroxylation genes in the human gut. Comput Struct Biotechnol J. 2019;17:1016–9. doi: 10.1016/j.csbj.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–28. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang WJ, Chen SJ, Zhou SC, Wu SZ, Wang H. Inflammasomes and fibrosis. Front Immunol. 2021;12:643149. doi: 10.3389/fimmu.2021.643149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degirolamo C, Modica S, Palasciano G, Moschetta A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol Med. 2011;17:564–72. doi: 10.1016/j.molmed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen PT, Kanno K, Pham QT, Kikuchi Y, Kakimoto M, Kobayashi T, et al. Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:3255–68. doi: 10.1007/s00432-020-03374-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Zhou L, Yin P, Wang J, Lu X, Wang X, et al. A weighted relative difference accumulation algorithm for dynamic metabolomics data: long-term elevated bile acids are risk factors for hepatocellular carcinoma. Sci Rep. 2015;5:8984. doi: 10.1038/srep08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R, Li X, Hylemon PB, Zhou H. Conjugated bile acids promote invasive growth of esophageal adenocarcinoma cells and cancer stem cell expansion via sphingosine 1-phosphate receptor 2-mediated yes-associated protein activation. Am J Pathol. 2018;188:2042–58. doi: 10.1016/j.ajpath.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q, Agoston AT, Pham TH, Zhang W, Zhang X, Huo X, et al. Acidic bile salts induce epithelial to mesenchymal transition via VEGF signaling in non-neoplastic Barrett’s cells. Gastroenterology. 2019;156:130–44.E10. doi: 10.1053/j.gastro.2018.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuhisa T, Arakawa T, Watanabe T, Tokutomi T, Sakurai K, Okamura S, et al. Relation between bile acid reflux into the stomach and the risk of atrophic gastritis and intestinal metaplasia: a multicenter study of 2283 cases. Dig Endosc. 2013;25:519–25. doi: 10.1111/den.12030. [DOI] [PubMed] [Google Scholar]
- 49.Ni Z, Min Y, Han C, Yuan T, Lu W, Ashktorab H, et al. TGR5-HNF4α axis contributes to bile acid-induced gastric intestinal metaplasia markers expression. Cell Death Discov. 2020;6:56. doi: 10.1038/s41420-020-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rees DO, Crick PJ, Jenkins GJ, Wang Y, Griffiths WJ, Brown TH, et al. Comparison of the composition of bile acids in bile of patients with adenocarcinoma of the pancreas and benign disease. J Steroid Biochem Mol Biol. 2017;174:290–5. doi: 10.1016/j.jsbmb.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng HY, Chen YC. Role of bile acids in carcinogenesis of pancreatic cancer: an old topic with new perspective. World J Gastroenterol. 2016;22:7463–77. doi: 10.3748/wjg.v22.i33.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farhana L, Nangia-Makker P, Arbit E, Shango K, Sarkar S, Mahmud H, et al. Bile acid: a potential inducer of colon cancer stem cells. Stem Cell Res Ther. 2016;7:181. doi: 10.1186/s13287-016-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hori T, Matsumoto K, Sakaitani Y, Sato M, Morotomi M. Effect of dietary deoxycholic acid and cholesterol on fecal steroid concentration and its impact on the colonic crypt cell proliferation in azoxymethane-treated rats. Cancer Lett. 1998;124:79–84. doi: 10.1016/s0304-3835(97)00452-7. [DOI] [PubMed] [Google Scholar]
- 54.Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, et al. Immune checkpoint inhibitors in the treatment of HCC. Front Oncol. 2021;10:601240. doi: 10.3389/fonc.2020.601240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai E, Astara G, Ziranu P, Pretta A, Migliari M, Dubois M, et al. Introducing immunotherapy for advanced hepatocellular carcinoma patients: too early or too fast? Crit Rev Oncol Hematol. 2021;157:103167. doi: 10.1016/j.critrevonc.2020.103167. [DOI] [PubMed] [Google Scholar]
- 56.Greten TF, Lai CW, Li G, Staveley-O’Carroll KF. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology. 2019;156:510–24. doi: 10.1053/j.gastro.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katariya NN, Lizaola-Mayo BC, Chascsa DM, Giorgakis E, Aqel BA, Moss AA, et al. Immune checkpoint inhibitors as therapy to down-stage hepatocellular carcinoma prior to liver transplantation. Cancers (Basel) 2022;14:2056. doi: 10.3390/cancers14092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdelrahim M, Esmail A, Saharia A, Abudayyeh A, Abdel-Wahab N, Diab A, et al. Utilization of immunotherapy for the treatment of hepatocellular carcinoma in the peri-transplant setting: transplant oncology view. Cancers (Basel) 2022;14:1760. doi: 10.3390/cancers14071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hewitt DB, Rahnemai-Azar AA, Pawlik TM. Potential experimental immune checkpoint inhibitors for the treatment of cancer of the liver. Expert Opin Investig Drugs. 2021;30:827–35. doi: 10.1080/13543784.2021.1940948. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J, et al. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol Immunol. 2018;15:710–23. doi: 10.1038/cmi.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim ST, Chu Y, Misoi M, Suarez-Almazor ME, Tayar JH, Lu H, et al. Distinct molecular and immune hallmarks of inflammatory arthritis induced by immune checkpoint inhibitors for cancer therapy. Nat Commun. 2022;13:1970. doi: 10.1038/s41467-022-29539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okiyama N, Tanaka R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int. 2022;71:169–78. doi: 10.1016/j.alit.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Li S, Na R, Li X, Zhang Y, Zheng T. Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188758. doi: 10.1016/j.bbcan.2022.188758. [DOI] [PubMed] [Google Scholar]
- 64.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melin A, Routier É, Roy S, Pradere P, Le Pavec J, Pierre T, et al. Sarcoid-like granulomatosis associated with immune checkpoint inhibitors in melanoma. Cancers (Basel) 2022;14:2937. doi: 10.3390/cancers14122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, et al. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumour-associated Treg cells. Nat Commun. 2017;8:14649. doi: 10.1038/ncomms14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saenz SA, Local A, Carr T, Shakya A, Koul S, Hu H, et al. Small molecule allosteric inhibitors of RORγt block Th17-dependent inflammation and associated gene expression in vivo. PLoS One. 2021;16:e0248034. doi: 10.1371/journal.pone.0248034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39:7–18. doi: 10.1111/liv.14093. [DOI] [PubMed] [Google Scholar]
- 69.Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3:e202895. doi: 10.1001/jamanetworkopen.2020.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 73.Schwabe RF, Greten TF. Gut microbiome in HCC-mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230–8. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pierrard J, Seront E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy—a systematic review. Curr Oncol. 2019;26:395–403. doi: 10.3747/co.26.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306–15. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee NY, Suk KT. The role of the gut microbiome in liver cirrhosis treatment. Int J Mol Sci. 2021;22:199. doi: 10.3390/ijms22010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang J, et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12:102. doi: 10.1186/s13073-020-00796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–8. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. doi: 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor–treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004779. doi: 10.1136/jitc-2022-004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MaruYama T, Kobayashi S, Shibata H, Chen W, Owada Y. Curcumin analog GO-Y030 boosts the efficacy of anti-PD-1 cancer immunotherapy. Cancer Sci. 2021;112:4844–52. doi: 10.1111/cas.15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 84.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–93. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang X, Zheng J, Zhang S, Wang B, Wu C, Guo X. Advances in the involvement of gut microbiota in pathophysiology of NAFLD. Front Med (Lausanne) 2020;7:361. doi: 10.3389/fmed.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356–65. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 87.Hebeda CB, Pinedo FJ, Bolonheis SM, Ferreira ZF, Muscará MN, Teixeira SA, et al. Intracellular mechanisms of hydroquinone toxicity on endotoxin-activated neutrophils. Arch Toxicol. 2012;86:1773–81. doi: 10.1007/s00204-012-0886-3. [DOI] [PubMed] [Google Scholar]
- 88.Liu H, Wang J, Lin J, Sun J, Guo J, Liu Z, et al. LPS induced PCT production via TLR-4/NF-кB passway:it is the difference of G-/G+ bacteremia rats. Cytokine. 2021;137:155317. doi: 10.1016/j.cyto.2020.155317. [DOI] [PubMed] [Google Scholar]
- 89.Gao S, Chen T, Li L, Liu X, Liu Y, Zhao J, et al. Hypoxia-inducible ubiquitin specific peptidase 13 contributes to tumor growth and metastasis via enhancing the toll-like receptor 4/myeloid differentiation primary response gene 88/nuclear factor-κB pathway in hepatocellular carcinoma. Front Cell Dev Biol. 2020;8:587389. doi: 10.3389/fcell.2020.587389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie L, Huang Z, Meng H, Shi X, Xie J. Immunomodulation effect of polysaccharides from liquid fermentation of Monascus purpureus 40269 via membrane TLR-4 to activate the MAPK and NF-κB signaling pathways. Int J Biol Macromol. 2022;201:480–91. doi: 10.1016/j.ijbiomac.2022.01.045. [DOI] [PubMed] [Google Scholar]
- 91.Ikeda Y, Taniguchi K, Yoshikawa S, Sawamura H, Tsuji A, Matsuda S. A budding concept with certain microbiota, anti-proliferative family proteins, and engram theory for the innovative treatment of colon cancer. Explor Med. 2022;3:468–78. doi: 10.37349/emed.2022.00108. [DOI] [Google Scholar]
- 92.Chu H, Williams B, Schnabl B. Gut microbiota, fatty liver disease, and hepatocellular carcinoma. Liver Res. 2018;2:43–51. doi: 10.1016/j.livres.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukaida N. Intestinal microbiota: unexpected alliance with tumor therapy. Immunotherapy. 2014;6:231–3. doi: 10.2217/imt.13.170. [DOI] [PubMed] [Google Scholar]
- 94.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kou Y, Zheng X, Meng L, Liu M, Xu S, Jing Q, et al. The HVEM-BTLA immune checkpoint restrains murine chronic cholestatic liver injury by regulating the gut microbiota. Front Immunol. 2022;13:773341. doi: 10.3389/fimmu.2022.773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143–8. doi: 10.1038/s41586-019-1785-z. Erratum in: Nature. 2020;579:E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581:475–9. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo S, Li K, Hu B, Li C, Zhang M, Hussain A, et al. Membrane-destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Exploration (Beijing) 2021;1:35–49. doi: 10.1002/EXP.20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu M, Shi K, Huang R, Liu C, Yin L, Yong W, et al. Facile preparation of 177Lu-microspheres for hepatocellular carcinoma radioisotope therapy. Chin Chem Lett. 2022;33:3492–6. doi: 10.1016/j.cclet.2022.01.007. [DOI] [Google Scholar]
- 100.Chen H, Teng M, Zhang H, Liang X, Cheng H, Liu G, et al. Advanced radionuclides in diagnosis and therapy for hepatocellular carcinoma. Chin Chem Lett. 2022;33:3371–83. doi: 10.1016/j.cclet.2022.03.079. [DOI] [Google Scholar]
- 101.Miller PL, Carson TL. Mechanisms and microbial influences on CTLA-4 and PD-1-based immunotherapy in the treatment of cancer: a narrative review. Gut Pathog. 2020;12:43. doi: 10.1186/s13099-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


