Abstract
An emerging body of behavioural studies indicates that regular swimming in cold water has positive effects on mental health and wellbeing, such as reducing fatigue, improving mood, and lessening depressive symptoms. Moreover, some studies reported immediate effects of cold-water immersion (CWI) on elevating mood and increasing a positive emotional state. However, the neural mechanisms underlying these effects remain largely unknown. The lack of studies using neuroimaging techniques to investigate how a whole-body CWI affects neural processes has partly resulted from the lack of a tested experimental protocol. Previous protocols administered tonic limb cooling (1–10 °C) while recording functional magnetic resonance (fMRI) signals. However, using very low water temperature constitutes points of contrast to painful experiences that are different from what we experience after a whole-body head-out CWI. In our protocol, healthy adults unhabituated to cold water were scanned twice: immediately before (pre-CWI) and after (post-CWI) immersion in cold water (water temperature 20 °C) for 5 min. We recorded cardiac and ventilatory responses to CWI and assessed self-reported changes in positive and negative affects. Our protocol showed reliable changes in brain connectivity after a short exposure to cold water, thus enabling its use as a tested experimental framework in future studies.
Keywords: Whole-body cold-water immersion, Resting state, fMRI, Functional connectivity, Short exposure to cold water
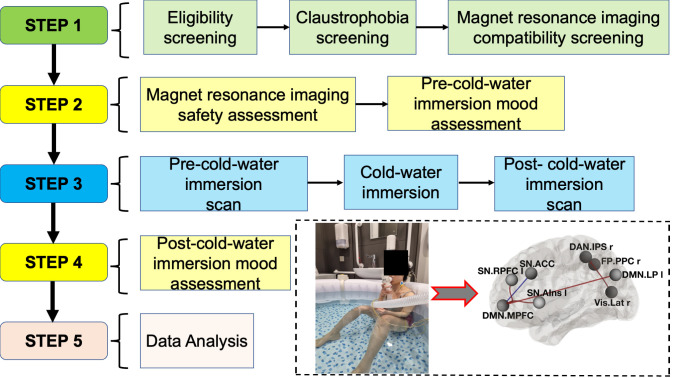
Graphical overview
Background
Cold-water swimming has become increasingly popular (Griffiths and Turner, 2022). With much evidence suggesting this activity has risks (Tipton, 1989; Tipton et al., 2017; Knechtle et al., 2020), the beneficial effects of cold-water immersion (CWI) have been linked to enhancing a positive mood state (Massey et al., 2022), higher perceived psycho-physical wellbeing (Huttunen, et al., 2004; Demori et al., 2021), and ameliorating mood disorders (Lindeman et al., 2002). It has been suggested that whole-body exposure to cold triggers a release of neurotransmitters such as serotonin, cortisol, dopamine, norepinephrine, and β-endorphin (Hirvonen et al., 2002), which play a crucial role in emotion (Gu et al., 2016; Canli and Lesch, 2007) and stress regulation (Godoy et al., 2018). Furthermore, emerging evidence indicates that the therapeutic benefits of cold water, such as increasing positive and decreasing negative affects, may be gained even from a single exposure (Massey et al., 2020; Kelly and Bird, 2022). This evidence was derived from behavioural studies using a questionnaire design and, as such, the neural mechanisms of the effects of CWI on affective processes remain largely unknown.
Only a few studies investigating how CWI changes brain activity have been conducted in humans (Jarrahi et al., 2017; Bitar et al., 2020; Grouper et al., 2022). However, these studies focused on assessing brain activity and its association to cognitive-affective aspects of pain, by using an experimental protocol of administered tonic limb cooling. It must be noted that physiological responses to limb cooling using very low water temperatures (1–10 °C) constitute points of contrast that result in painful experiences, which are different from what we experience after taking a cold shower or swimming in higher temperature open waters.
We developed and tested a novel protocol to examine the effects of short-term, head-out, whole-body CWI on changes in brain functional connectivity. This protocol can be adapted for other research questions focusing on brain-behaviour interactions [e.g., linking personality traits, cognitive functions such as decision making, memory, and perception to the brain's functional architecture (Cole et al., 2014)]. A tight control of experimental factors in our protocol makes it easy to replicate and minimise confounding effects.
Materials and reagents
Non-effervescent Haz-Tab (4.5 g NADDC per tablet) (Guest Medical, catalog number: H8801) made up to 1,000 ppm (one tablet per 2.5 L)
The Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988)
Appropriately sized hospital scrubs for each participant
Blankets
Equipment
MRI machine (3-Tesla Siemens Magnetom Lumina, 32-channels head-coil)
Water heater (Laz-y-spa, UK)
Water pump (Laz-y-spa, UK)
Water chiller unit (Grant Instruments, UK)
Three-lead electrocardiogram (ECG, Fukuda Denshi, UK)
ECG electrodes (Ambu® Blue Sensor P, Malaysia)
Electrodes covered with Tegaderm dressings (3M, Germany)
35 mm respiratory hose (Cranlea, UK)
Reusable nose clip (Cranlea, UK)
Two-way T-shape non-rebreathing valve (Hans Rudolf, US)
Respiratory turbine (K.L. Engineering, US)
Thermistor pod (ML309, AD Instruments, Australia)
Skin temperature thermistor (MLT422/A, Australia)
Digital recorder (Powerlab, AD Instruments, Australia)
Laptop connected to the Powerlab and programmed to a sampling rate of 400 Hz (Chart software, AD Instruments Australia)
Scanning parameters
Anatomical scan: T1-weighted MPRAGE images with the following parameters: repetition time (TR) = 1,900 ms, echo time (TE) = 2.74 ms, flip angle = 8°, field of view (FOV) = 256 × 256 mm2, voxel size = 1.0 × 1.0 × 1.0 mm3, and 192 axial slices.
Functional scan: blood oxygenation level–dependent (BOLD) contrast whole-brain functional images were acquired using a T2-weighted gradient-echo Echo Planar Imaging (EPI) sequence with a 32-channel head coil. Sequence duration 13.12 min. Acquisition parameters: TR = 2,680 ms; TE = 30 ms; matrix size = 64 × 64 mm2; slice thickness = 2.5 mm, spacing between slices = 2.5 mm, flip angle = 80°; 292 volumes with 48 axial slices were measured in interleaved slice order and positioned along a line to the anterior-posterior commissure (AC-PC orientation). An automated high-order shimming technique was used to maximise magnetic field homogeneity.
Software
MATLAB 2022a (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Natick, Massachusetts, United States)
CONN (v.2021a): A functional connectivity toolbox for correlated and anticorrelated brain networks (Whitfield-Gabrieli and Nieto-Castanon, 2012, http://www.nitrc.org/projects/conn)
JASP (JASP Team, 2022, version 0.16.3) (Computer software)
Procedure
-
Step 1. Participant screening
-
Eligibility screening
Provide interested volunteers with a participant information sheet containing sections explaining eligibility criteria, procedures, and risks.
Eligibility criteria: (i) age is between 18 and 45 years old; (ii) no history of medical, neurological, psychiatric, or substance use disorders; (iii) naïve to CWI (not exposed to cold water including cold shower, unheated swimming pool, or the sea) in the last 12–18 months, and (iv) free from chronic pain of any type (Figure 1 ).
If required, screen participants by telephone interview and undertake any additional pre-screening tests required by the medical officer to rule out any significant history of medical, neurological, psychiatric, or substance use disorders.
-
Claustrophobia screening
Siemens Lumina scanner has a relatively large tunnel, is fully lit, and is open at both ends. This dramatically lowers the experience of claustrophobia. Ask participants whether they have ever experienced claustrophobia (or fear of being in a closed space) in the past.
Ask participants whether they have previous experience with MRI scans.
Ask participants whether exposure to a feared situation have led to immediate anxiety or panic attacks in the past.
If the answer is yes to a. or c., the participant does not pass claustrophobia screening.
-
MRI compatibility screening
MRI compatibility screening should comply with the standard operation procedures approved for scanner use. The procedures can be slightly different across universities. Discuss the screening procedures with your local scanner manager.
Ask participants to answer questions assessing their eligibility for fMRI study: (i) Do you have a heart pacemaker or pacing wires? (ii) Have you had any recent surgery? (iii) Have you had any surgery to your head (including eyes/ears/brain)? (iv) Do you have any implanted devices (e.g., programmable hydrocephalus shunt, nerve stimulator, cochlea implant, aneurysm clip? (v) Have you ever sustained any injuries involving metal to your eyes or any parts of your body? (vi) Do you have any of the following: dentures with metal, body piercings, hearing aid, nitro patch, artificial limb or prothesis, tattoos? (vii) For women: do you have an IUD (coil)? Could you be pregnant?
If the answer to all questions is no, the participant will be taken to the next screening step. If the answer to one or several questions is yes, take this case to a radiographer.
Participants who passed Step 1 were scheduled to visit the Institute of Medical Imaging and Visualisation (IMIV) at Bournemouth University where the study took place. For the scheduled visit, ask participants to (i) refrain from eating food 2 h prior to the scanning session; (ii) bring a swimsuit/trunks or shorts (+vest top for women), a towel, a woollen jumper or sweatshirt with no metal, and slippers or flip flops.
-
-
Step 2. Pre-CWI assessments
-
MRI safety assessment
If the participant passes the MRI safety assessment, explain the experimental procedures and familiarise participants with the setup, including cold-water tub and monitoring equipment.
Answer any questions the participant may have and ask them to complete a written informed consent form.
-
Pre-CWI mood assessment
Provide a printed version of the PANAS questionnaire and instruct them to evaluate all items of the questionnaire, reflecting on how they feel as they complete it (in the moment).
Ask participants if they have any questions. Ensure that their clothes do not have any metal parts (or provide a set of scrubs and blankets).
-
-
Step 3. Experimental procedures
-
Pre-CWI scan
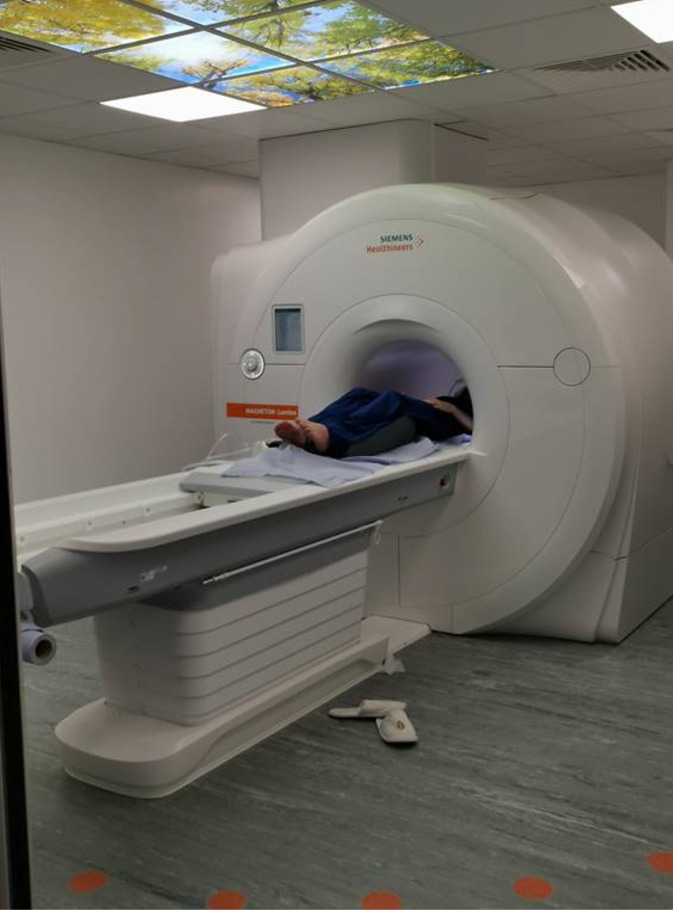
Position a participant in the scanner (Figure 2). Use MRI-compatible headphones to reduce scanner noise. Use pads to fixate the participant’s head to reduce head movements.
Provide an alarm button (by pressing the button, participants are able to stop the scanning at any time).
Ask participants to keep awake and keep their eyes open.
Start anatomical (structural) scan (4.5 min). Immediately after, start the functional scan (resting state) (13.12 min).
After completing the pre-CWI scanning procedures, take the participant to a changing room to change into swimwear.
-
CWI (cold-water immersion procedure)
Record participant’s height and mass.
Instrument with a three-lead ECG connected via ECG electrodes and waterproof the electrodes using Tegaderm dressings to improve the signal quality during immersion.
Provide the participants with a nose clip and mouthpiece and ask them to breathe freely throughout.
A minimum of 2 min of baseline data are collected on the Chart software with the participant seated next to the cold-water tank. The Chart program also collects water temperature, ECG, and ventilatory data throughout the immersion, and comments should be added to the chart software to highlight pre immersion, immersion, and post immersion phases.
Participant will stand, immediately enter the tank, and be seated in the water within a 10–15 s period. They will immerse to the depth of the axilla (see image in Graphical overview). If the immersion is too deep, ask them to sit at the shallow part; if it is too shallow, ask them to sit in a deeper part. This is done by putting weights on the bottom, which the participants could sit on.
Participants are encouraged to breathe freely through the mouthpiece and do not attempt to hold their breath.
Monitor the ECG during immersion for any ECG abnormalities that would necessitate withdrawal from the cold water.
Upon the completion of 5 min of immersion or if the participant requires earlier withdrawal, they stand up, exit the water tank onto a skid resistance surface, and the ECG and mouthpiece are removed.
Participants remove their swimwear and dry off and dress in private, as quickly as possible.
Record the duration of time from exiting the cold-water tub to the MRI chamber door closing, following the positioning of the participant in the scanner. The average time between exiting the cold-water tub and entering the scanner was M = 5.82 min, SD = 2.08 min (range: 2 min 25 s–10 min 30 s). It is not anticipated that the transition time between the tub and the scanner dramatically affects changes in the brain, as previous research reported the effects of CWI to last over a few days (Massey et al., 2022). However, we encouraged participants to dry off and dress quickly after the CWI. It must be noted that the cold-water tub should be set up as close to the MRI scanner as possible, to ensure the shortest transition time. In our study, the cold-water tub was next door to the scanner room.
-
Post-CWI scan
Position the participant in the scanner (Figure 2).
Use a lightweight cotton blanket to cover the participant’s body (to minimise shivering after CWI).
Switch off the built-in scanner ventilator (to minimise participant cooling and body movement due to cold air).
Immediately after, start the functional scan (resting state) (13.12 min).
Table 1 summarises the timeline for the experimental steps to provide a detailed estimation of the complete study per participant at the MRU suite.
-
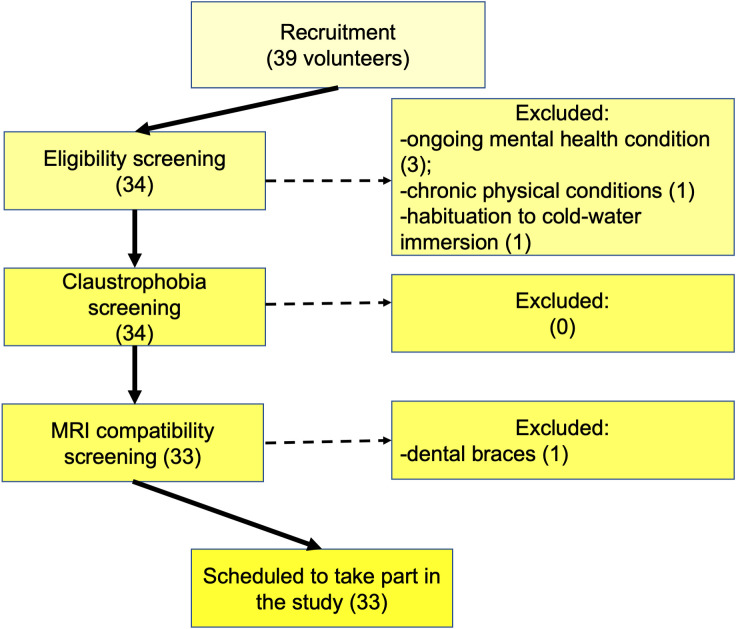
Figure 1. Participants selection consort diagram.
The diagram provides information about the selection steps (on the left) and the number of participants excluded in each selection step.
Figure 2. Participant’s position in the scanner.
Table 1. Detailed timeline for experimental steps B and C at the MRI suite.
| Steps | Sub steps | Additional time between sub steps | Time |
|---|---|---|---|
| B | MRI safety assessment (including explanation of CWI procedure and signing consent form) | 5 min | |
| Pre-CWI mood assessment | 2–3 min | ||
| Changing into MRI-compatible clothes (if required) | 1–3 min | ||
| C | Position participant in the scanner (pre-CWI) | 3 min | |
| Pre-CWI scanning | 4.5 min + 13.12 min = 17.62 min | ||
| Localise scan to position the brain | 1 min | ||
| Get participant from the scanner, change into swimwear | 3–4 min | ||
| Instrument with ECG electrodes, nose clip, and mouthpiece | 2–3 min | ||
| Taking baseline measurements | 2–3 min | ||
| Cold-water immersion | 5 min | ||
| Remove ECG, nose clip, and mouthpiece | 1 min | ||
| Drying and changing into MRI-compatible clothes | M = 5.83, SD = 2.09* | ||
| Position participant in the scanner (post-CWI) | 2–3 min | ||
| Post-CWI scanning | 13.12 min | ||
| Localise scan to position the brain | 1 min | ||
| Total time: | ~62 min** | ||
*This parameter ranged between 2 min 25 s and 11 min 15 s.
**Total time can be reduced if participants arrive in MRI-suitable clothes.
Data analysis
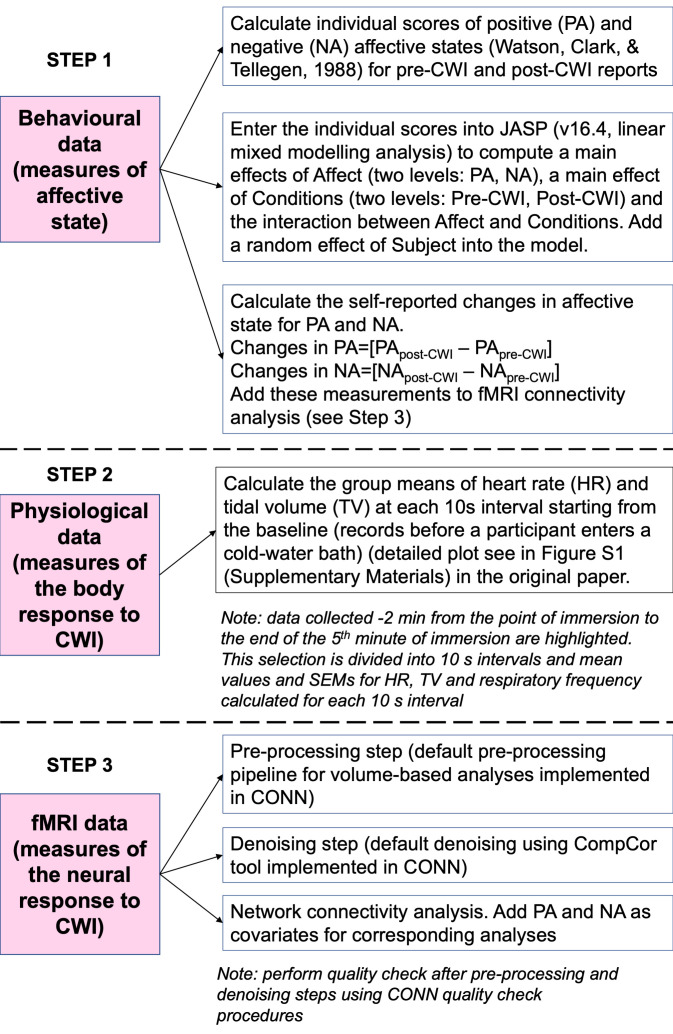
The original paper provides detailed data analysis for this study. The analytical steps are summarised in Figure 3.
Figure 3. Analytical steps in the present study.
Tips
To disinfect the mouthpieces, nose clips, and respiratory hose, two non-effervescent Haz-Tab were dissolved into 5 L of cold tap water. The tablet was allowed to dissolve completely before the equipment was dismantled and submerged in the liquid for 20 min.
The CWI tub does not need to be drained or disinfected between participants. It was checked twice a day for pH and free and combined chlorine and was dosed with chlorine daily in accordance with the manufacturer’s guidelines. The tub also had filters for trapping hair and detritus, which were cleaned daily.
Validation of protocol
Yankouskaya et al. (2023). Short-term head-out whole-body cold-water immersion facilitates positive affect and increases interaction between large-scale brain networks. Biology, 12(2): 211.
Acknowledgments
We wish to thank the participants and radiography team who are not authors (Carlo Vitale and Theophilus Akudjedu). This work was supported by the Institute of Medical Imaging and Visualisation (Bournemouth University, UK).
Competing interests
None of the authors has any conflicts of interest.
Ethics
The protocol requires approval from a local ethics committee. The study was reviewed and approved by the Departmental Research Board and Ethics Committee at Bournemouth University (Ethics ID 34976 17.02.2021) and conducted in accordance with the Declaration of Helsinki. Written informed consent from each participant was obtained before the study.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1.Bitar N., Dugré J. R., Marchand S. and Potvin S. (2020). Medial Orbitofrontal De-Activation During Tonic Cold Pain Stimulation: A fMRI Study Examining the Opponent-Process Theory . J. Pain Res. 13: 1335- 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canli T. and Lesch K. P. (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10(9): 1103- 1109. [DOI] [PubMed] [Google Scholar]
- 3.Cole M. W., Bassett D. S., Power J. D., Braver T. S. and Petersen S. E. (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron 83(1): 238-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demori I., Piccinno T., Saverino D., Luzzo E., Ottoboni S., Serpico D., Chiera M. and Giuria R. (2021). Effects of winter sea bathing on psychoneuroendocrinoimmunological parameters. Explore 17(2): 122-126. [DOI] [PubMed] [Google Scholar]
- 5.Godoy L. D., Rossignoli M. T., Delfino-Pereira P., Garcia-Cairasco N. and de Lima Umeoka E. H. (2018). A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 12: e00127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths S. and Turner Y. (2022). Trends in Outdoor Swimming. Second Edition. 2022. Available online: https://www.outdoorswimmershop.com/products/outdoor-swimmer-trends-report-22(accessed on 18 December 2022).
- 7.Grouper H., Löffler M., Flor H., Eisenberg E. and Pud D. (2022). Increased functional connectivity between limbic brain areas in healthy individuals with high versus low sensitivity to cold pain: A resting state fMRI study. PLoS One 17(4): e0267170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu S., Wang W., Wang F. and Huang J. H. (2016). Neuromodulator and Emotion Biomarker for Stress Induced Mental Disorders. Neural Plast. 2016: 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirvonen J., Lindeman S., Joukamaa M. and Huttunen P. (2002). Plasma catecholamines, serotonin and their metabolites and beta-endorphin of winter swimmers during one winter. Possible correlations to psychological traits. Int. J. Circumpolar Health 61(4): 363 -372. [DOI] [PubMed] [Google Scholar]
- 10.Huttunen P., Kokko L. and Ylijukuri V. (2004). Winter swimming improves general well-being . Int. J. Circumpolar Health 63(2 ): 140-144. [DOI] [PubMed] [Google Scholar]
- 11.Jarrahi B., Martucci K. T., Nilakantan A. S. and Mackey S. (2017). Investigating the BOLD spectral power of the intrinsic connectivity networks in fibromyalgia patients: A resting-state fMRI study. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2017: 497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly J. S. and Bird E. (2022). Improved mood following a single immersion in cold water. Lifestyle Medicine 3 (1): e53. [Google Scholar]
- 13.Knechtle B., Waśkiewicz Z., Sousa C. V., Hill L. and Nikolaidis P. T. (2020). Cold Water Swimming—Benefits and Risks: A Narrative Review. Int. J. Environ. Res. Public Health 17(23): 8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindeman S., Hirvonen J. and Joukamaa M. (2002). Neurotic psychopathology and alexithymia among winter swimmers and controls--a prospective study. Int J Circumpolar Health.61(2): 123 -130. [DOI] [PubMed] [Google Scholar]
- 15.Massey H., Gorczynski P., Harper C. M., Sansom L., McEwan K., Yankouskaya A. and Denton H. (2022). Perceived Impact of Outdoor Swimming on Health: Web-Based Survey. Interact. J. Med. Res . 11(1): e25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massey H., Kandala N., Davis C., Harper M., Gorczynski P. and Denton H. (2020). Mood and well‐being of novice open water swimmers and controls during an introductory outdoor swimming programme: A feasibility study. Lifestyle Medicine 1 (2): e12. [Google Scholar]
- 17.Tipton M. J. (1989). The Initial Responses to Cold-Water Immersion in Man. Clin. Sci. 77 (6): 581-588. [DOI] [PubMed] [Google Scholar]
- 18.Tipton M. J., Collier N., Massey H., Corbett J. and Harper M. (2017). Cold water immersion: kill or cure ? Exp. Physiol. 102(11): 1335-1355. [DOI] [PubMed] [Google Scholar]
- 19.Watson D., Clark L. A. and Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 54(6): 1063 -1070. [DOI] [PubMed] [Google Scholar]
- 20.Whitfield-Gabrieli S. and Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity 2(3): 125- 141. [DOI] [PubMed] [Google Scholar]