Abstract
The evolution of tissue engineering and 3D bioprinting has allowed for increased opportunities to generate musculoskeletal tissue grafts that can enhance functional and aesthetic outcomes in otolaryngology- head and neck surgery. Despite literature reporting successes in the fabrication of cartilage and bone scaffolds for applications in the head and neck, the full potential of this technology has yet to be realized. Otolaryngology as a field has always been at the forefront of new advancements and technology and is well poised to spearhead clinical application of these engineered tissues. In this review, we describe current 3D bioprinting methods and present an overview of potential cell types, bioinks, and bioactive factors available for musculoskeletal engineering using this technology. We review the otologic, nasal, tracheal, and craniofacial bone applications of 3D bioprinting with a focus on engineered graft implantation in animal models to highlight the status of functional outcomes in vivo; a necessary step to future clinical translation. Continued multidisciplinary efforts between material chemistry, biological sciences, and otolaryngologists will play a key in role in the translation of engineered, 3D bioprinted constructs for head and neck surgery.
Keywords: Tissue engineering, 3D-printing, bioprinting, otolaryngology, head and neck surgery
Graphical abstract

The ToC text should summarize your article in 50–60 words and should be written in the present tense and impersonal style. Please submit the text as a Word doc/docx file or a LaTeX file (with all necessary accompanying files).
3D bioprinting has emerged as a promising technique to produce patient-specific structures for regeneration of tissues relevant to the field of otolaryngology. Here, we describe the 3D bioprinting process which begins with imaging followed by bioink design, 3D bioprinting, and post-printing options. Reports describing in vivo implantation of 3D bioprinted scaffolds are highlighted and the advantages, disadvantages, and future outlook of the field are discussed.
1. Introduction
Three-dimensional (3D) printing is an additive manufacturing procedure that can fabricate 3D scaffolds through deposition of material in a layer-by-layer fashion. For decades, 3D printing has been widely used in business and production. This technology continues to evolve and become more accessible and affordable, and more recently, numerous papers in the international literature on its potential application in healthcare have been published [1,2]. Its application in healthcare and medicine has expanded with far-reaching consequences in medical education, surgical planning, tailored prosthesis and surgical graft creation [1,3–6]. Three-dimensional printing has the benefit of on-demand fabrication of medical devices or implants, making it attractive for production for in-house devices or for use in remote areas. Moreover, this technology has been applied to the field of tissue engineering, with the aim to develop functional tissue substitutes and fabricate personalized and complex, native-like cell-based tissue constructs with unprecedented shape and precision[7] which otherwise are not possible with conventional fabrication methods[1,8].
The capacity to fabricate tissue with defined architecture using 3D printing relies on similar principles and strategies as conventional tissue engineering. Seminal papers in the field of regenerative medicine by Langer and Vacanti in the 1990s and early 2000s [9,10] established a biomaterial-based concept of tissue engineering which aims to combine cells, signals and biomaterial scaffolds and which continues to remain applicable to 3D printing approaches. The engineered scaffold aims to support incorporation of multiple cell types for tissue regeneration, allow for sufficient transport of nutrients, and provide adequate mechanical support to mimic the desired tissue and allow for cells to adhere, proliferate, and differentiate in a 3D milieu [11]. Three-dimensional printing enhances these methods by implementing a more automated manufacturing process to generate scaffolds with novel and precise geometries and internal architectures, such as pore size which plays a critical role in tissue formation in vitro and in vivo [12,13], allowing for high precision and customization for various clinical applications[14]. In conventional 3D printing approaches, cells are typically seeded onto the 3D printed scaffold post-print and soluble signals are provided exogenously or incorporated within the scaffold to direct tissue formation. This approach is limited by inefficient and heterogenous distribution of cell density seeding with more cells being deposited on the scaffold periphery [11].
3D bioprinting, a subset of conventional 3D printing, has the potential to surpass current 3D printed tissue engineering strategies. As opposed to 3D printing of biomaterials alone, 3D bioprinting involves the deposition of cell-containing bioinks in specific patterns where they fuse and mature to form large-scale tissues [15–18]. The precise placement of live cells, proteins, genes, drugs, and biologically active particles has the potential to better guide tissue generation and formation. This is typically achieved using an aqueous based system of biologic materials (bioinks) that enables recapitulation of tissue with architectural complexity. The ability to spatially control the placement of the functional component (i.e., living cells) of tissue engineered scaffolds, can be used to create complex, heterocellular biological structures that can be co-printed with bioactive cues. This customization of the key anatomical features within the tissue, including the interconnected pores and the sizing and placement of blood vessels, can improve perfusion, neovascularization, and cellular communication to augment tissue development and enable the generation of larger tissues.
The capacity to engineer anatomically accurate, 3D bioprinted tissue grafts for surgical implantation, notably in otolaryngology, has significant potential to improve reconstructive outcomes for defects with complex shapes and sizes. Otolaryngologists are often at the forefront in adopting new technologies, and the recent push towards personalized medicine has driven advancement in several fields including regenerative medicine [3]. 3D bioprinting has the potential to address and circumvent current limitations in reconstructive surgery including the need for donor tissue, poor tissue match, and transplant rejection. The rapid growth of 3D bioprinting technologies may allow for anatomically accurate reconstructive options to generate tissues of the head and neck, notably musculoskeletal tissue such as cartilage and bone. To the best of our knowledge, this is the first review focused on 3D bioprinting in otolaryngology that accurately defines 3D bioprinting based on the printing of live cellular material. We will review the 3D bioprinting process and with it the myriad of cells, biomaterials, and bioactive molecules that can be utilized to generate 3D bioprinted musculoskeletal tissue and review the literature on 3D bioprinted scaffold implantation in animal models for application in the field of otolaryngology.
2. The 3D bioprinting process
3D bioprinting has the ability to precisely deposit different materials and cells to create personalized biological constructs [19]. The process of 3D bioprinting can be categorized into three steps: pre-bioprinting, bioprinting, and post-bioprinting [20,21]. Initially, the pre-bioprinting step involves generation of a computer assisted design (CAD) model of the desired tissue which can be designed using CAD software or obtained from patient-specific imaging such as Computed tomography (CT) or magnetic resonance imaging (MRI) in Digital Imaging and Communications in Medicine (DICOM) format. Standard Triangle Language (STL) format is used to provide the CAD image as input to the bioprinter. Therefore, the ability to create personalized and complex 3D printed constructs stems from the capacity to transform medical imaging (e.g., CT or MRI) into patient-specific 3D models.
The bioprinting process (Fig. 1) begins with bioink design and isolation and culture of suitable cells to obtain a large enough number of viable cells capable of producing the desired tissue [8]. Here, bioink is defined as the print “ink” that allows the printing of living cells [22]. Prior to printing, the cells are incorporated within the biomaterial of choice to generate the bioink with or without the addition of chemical signals or biological stimuli to augment tissue maturation or differentiation and then loaded onto the printer cartridge. The bioink is then dispensed to form the desired shape and the fabricated bioprinted construct is often then placed into an incubator for further culture and tissue maturation. This post-printing step contributes to the process of developing or maintaining the mechanical stability and functionality of the 3D construct [20]. Cells in the bioprinted construct need optimal nutrition and oxygen delivery as well as mechanical and chemical signals required for matrix remodeling and development of tissues for proper functionality of the construct. As noted, bioactive signaling molecules can be provided within the bioink or supplemented in the culture medium to direct cell growth or differentiation. Bioreactors can also be utilized to provide the ideal environment for the tissue to grow and mature [22,23]
Figure 1: The 3D bioprinting process.
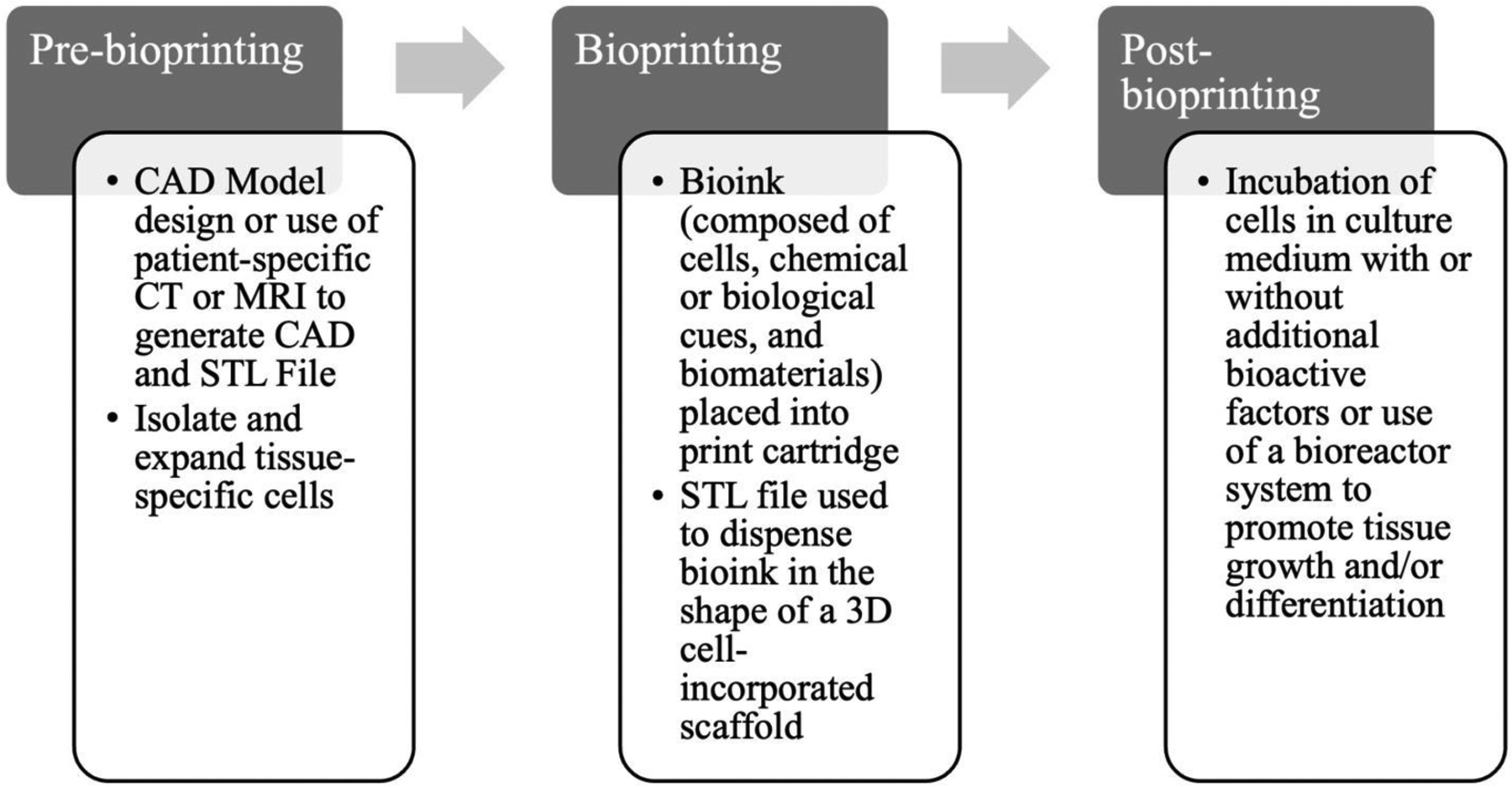
The 3D bioprinting process steps include pre-bioprinting, bioprinting, and post-bioprinting. Adapted from [18], no permissions required.
3. 3D Bioprinting for Tissue Regeneration in the Head and Neck
3.1. Bioinks
In otolaryngology, bioprinting techniques have focused on engineering bone and cartilage. Bone tissue is rigid, and the use of multiple hydrogel scaffolds for 3D bioprinting has been widely investigated [22,24]. Similarly, cartilage is tough but flexible with a high water content, making hydrogels an ideal material for cartilage engineering [25–27]. The choice of biomaterial scaffold facilitates the growth and differentiation of cells by creating a suitable microenvironment for cell attachment, migration, proliferation, and differentiation. Further discussion regarding the current status of bioink development have been reviewed elsewhere [15,24,28–32].
Bioinks used in 3D bioprinting strategies consist of a biomaterial solution in combination with cells. The biomaterial serves as a temporary support structure for cells and often involves biocompatible, biodegradable materials utilized in classic tissue engineering strategies. The selection of the proper biomaterial to generate the bioink is crucial for successful bioprinting. The material of choice in 3D bioprinting will provide the required properties for adequate printing fidelity, mechanical properties to ensure printability and long-term functionality, and can potentially affect tissue differentiation [33]. Key features of an ideal bioink scaffold include: (1) structural interconnectivity with an interconnected pore structure to enable cell growth, differentiation, and transport of supplements, (2) biocompatibility and biodegradability to promote cell interactions with a controllable degradation rate to coordinate tissue development and elaboration of extracellular matrix (ECM), and (3) similar mechanical properties of the tissues at the desired site of implantation [27,34]. Moreover, the printed constructs should be able to retain their structure for a defined period in vitro prior to implantation tp allow for engraftment within the host defect site in vivo.
Bioinks in 3D bioprinting strategies most commonly utilize hydrogels, which are highly hydrated crosslinked polymeric networks that mimic native ECM [15] and may have chondro- or osteo- conductive or inductive properties, such as collagen [35] and hyaluronic acid [36]. Hydrogels have been extensively studied in regenerative strategies as, aside from their ECM-like properties, they are highly permeable to oxygen, nutrients, and other water-soluble factors, allowing for ease of fabrication. Moreover, unlike seeding of cells onto polymeric scaffolds in more traditional tissue engineering strategies, cells in hydrogels can migrate and proliferate in a 3D fashion due to the porous network of the hydrogel[37]. These hydrogels tend to be naturally occurring polymers such as gelatin, alginate, collagen, hyaluronic acid, fibrin, and cellulose [15,29]. Despite batch-to-batch variability and potential immunogenicity, natural polymers are inherently biocompatible making them ideal for application as bioinks. Alternatively, synthetic hydrogels, including polyethylene glycol (PEG) and Pluronic, are abundant, have more reproducible properties, and can more readily be tailored in terms of molecular weight, mechanical strength, biodegradability, and block structure compared to naturally occurring polymers[38]. Because synthetic polymers have overall lower cell compatibility compared to natural polymers, they are often combined with natural polymers to generate tunable properties for bioprinting[29].
During the selection of the foundational components of the bioink (biomaterial, cells, bioactive signals), the bioink’s printability must be considered. There are multiple physicochemical properties that determine printability including viscosity, surface tension, and nozzle gauge as well as the crosslinking mechanism of the bioink [30,33,33]. The choice of the nozzle gauge in extrusion printing is one of the main factors that determines the resolution of the printed filament along with print speed which must be weighed with the shear stress imparted onto the suspended cells, which may be detrimental to cell viability. Viscosity, the resistance of a fluid upon application of stress, can be influenced by polymer type, concentration, and molecular weight as well as cell density of the suspended cells. Print fidelity generally increases with increased viscosity, yielding structures with increased stability. However, increased viscosity must be balanced with a likely concurrent increase in applied shear stress required for extrusion and effect on cell viability. Gelation and crosslinking of the printed bioink is necessary to maintain the 3D structure of the printed construct post-print but may interfere with printing and clogging of the nozzle if it occurs too rapidly. Hydrogels can be crosslinked via various methods including physical (i.e. ionic, or temperature controlled hydrophobic or hydrogen bonding), chemical (i.e. Schiff based or photo-crosslinking), or enzymatic methods [39]. Numerous hydrogels have been prepared by altering the chemical backbone of polymers to create photo-crosslinkable hydrogels [40,41]. Although physically crosslinked hydrogels are the most prominent hydrogel class used for bioprinting they have poor mechanical properties compared to chemically crosslinked hydrogels [33]. Ideally, the rheological properties of the printed scaffold allow the 3D printed structure to hold its shape, resulting in self-standing structures that can then be crosslinked post-print for further maintenance of the scaffold architecture.
It can be difficult to construct large free-form tissue structures when using hydrogels alone due to inadequate structural integrity and printability [42]. Alternative methods such as multi-material printing to generate hybrid, multi-material scaffolds composed of bioinks and biocompatible thermoplastics such as polylactic glycolic acid (PLGA), polylactic acid (PLA), and polycaprolactone (PCL) capable of forming complex geometries have been constructed [43,44]. The tunable selection of multiple biomaterials and/or bioinks can achieve a scaffold customized for the intended application. However, it is important to take into account the printing properties of these thermoplastics including increased melt temperature required to print which may affect cell viability due to close proximity of the printed filament to neighboring cells within the scaffold.
Bioactive inks, the addition of bioactive components to biomaterial scaffolds, has been developed in recent decades with the goal of driving cell development and differentiation. This has been described in the development of growth factor- and DNA-containing bioinks [45–47,47]. Bioinks with sustained release of growth factors direct cell fate. Localized release can also reduce the amount of growth factor required. Overall, the success of the printed scaffold is measured through short and long-term cell viability, cell proliferation, and ultimately functionality of the bioprinted construct.
3.2. Cell Sources
Selection of cell type is a major component of the 3D bioprinting process to generate the tissue of interest. Cell-based approaches for tissue engineering provide a source of regenerative cells upon transplantation to fill a tissue defect. Moreover, the ability of 3D bioprinting to create multicellular constructs of different cells type with spatial accuracy can more closely mimic human tissues that represent the cell type diversity of the tissue. Strategies applicable to the head and neck have focused on engineered bone and cartilage.
The cell source in biofabrication strategies depends on the anatomical and functional characteristics of the selected tissue. In addition, choice of cell type must be easily accessible, non-immunogenic and capable of producing the desired tissue. Moreover, these cells can either be allogeneic or autologous [48]. Although allogeneic sources provide the opportunity to create an “off-the-shelf” universal construct, they are plagued by the risk of immune rejection. Similarly, while cells derived from animal sources are readily available and could enable mass production of engineered tissue, the risk of disease transmission with xenogeneic material raises concerns regarding future surgical translation[49]. Autologous cells, especially for human applications, offer greater biocompatibility as well as the opportunity for personalization through the application of patient-specific cells [48]. However, the use of autologous cells can require additional patient procedures to acquire cells and with it the associated donor site morbidity and pain.
Mature cells
Debate remains regarding the use of mature cells or stem cells to produce musculoskeletal tissue. With regard to mature cells, the majority of applications for tissue engineering in the head and neck utilize chondrocytes and osteoblasts, cartilage and bone-specific cells, respectively, due to their ability to produce ECM. Primary osteoblasts and osteocytes can be directly isolated from bone tissue [50]. Osteoblasts appear to be an obvious source for bone engineering cells but are limited by availability and ability to obtain sufficient numbers [51]. Chondrocytes can be sourced from multiple sources in the head and neck including the auricle, septum, or nasal ala. In addition, complex tissue engineering approaches that aim to create multi-tissue constructs such as the trachea with an inner luminal mucosal layer have utilized epithelial cells [52,53]. Mature cells are typically expanded ex vivo to obtain sufficient cell numbers. However, primary cells have a limited lifespan. In the case of chondrocytes, expansion leads to dedifferentiation, limiting their ability to synthesize new matrix and diminishing their utility in regenerative applications [54].
Stem cells
Although few approaches incorporating stem cells and 3D bioprinting strategies for head and neck applications in animal models have been described, stem cells are commonly used in musculoskeletal tissue engineering applications. Stem cells for musculoskeletal regenerative approaches include undifferentiated cells such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), human amniotic fluid stem cells (hAFSCs), and mesenchymal stem cells. iPSCs, which are adult fibroblast cells transformed into an ESC-like state, circumvent ethical issues associated with ESCs and can be used to create patient-specific tissue [55,56]. iPSCs are advantageous for use in musculoskeletal engineering since harvesting of patient-specific cells is non-invasive and they possess the ability to differentiate down multiple lineages including mesenchymal lineages. Despite their promise, risks associated with viral transduction necessary to produce iPSCs, including insertional mutagenesis, tumorigenesis, and teratoma formation [57], must be further investigated prior to further clinical application. In addition, hAFSCs, sourced from cesarean births, can differentiate into multiple lineages including musculoskeletal lineages [44,58]. These cells also bypass controversy associated with ESCs as the amniotic fluid source would normally be discarded. These cells also do not require induction by a viral vector as is the case with iPSCs.
Mesenchymal stem cells (MSCs) are a commonly used in musculoskeletal regenerative approaches and have been shown to self-renew and can differentiate into multiple lineages including adipocytes, chondrocytes, osteocytes, myoblasts, and tendocytes [59,60]. MSCs were first isolated from bone marrow and have since been isolated from a variety of different sites such as periosteum, adipose tissue, infrapatellar fat pad, and synovium [60,61]. In addition, unlike MSCs from these more conventional sources which are of mesodermal origin, MSCs have also been isolated from the nasal turbinate which are of neural crest origin and likely have distinct properties [62]. Periodontal ligament stem cells [63], also known as periodontal ligament mesenchymal cells, are a cell population that exhibit similar characteristics to MSCs and have been used in the regeneration of the periodontal complex.
Of note, MSC secretion of trophic factors has led to regulatory approval for the use of MSCs clinically worldwide, although there is no current FDA-approved usage in the USA[64]. Due to their medicinal and immunomodulatory properties, distinct from their potential for differentiation, MSCs have been used to treat a range of diseases/ailments including graft vs-host, acute myocardial infarction, spinal cord injury, and amyotrophic lateral sclerosis [65,66]. This is due to MSC secretion of trophic factors that mediate anti-apoptosis, anti-scarring/anti-fibrosis, and angiogenesis [66–68]. MSCs have been described as immune evasive due to their secretion of immunosuppressive and anti-inflammatory cytokines that result in regulation of B- and T-cells, including T regulatory cells and natural killer cells, in an antigen-independent manner [69,70], lending themselves well to in vivo applications. However, evidence in the literature has pointed towards an increase in allogenic MSC immunogenicity following induced differentiation and further investigation into MSC-derived tissue transplantation is required [71].
3.3. Bioactive Factor Delivery
The 3D bioprinted scaffold can be designed to encapsulate or be chemically linked to desired bioactive factor(s) [72] to allow either for spatially controlled presentation to co-encapsulated cells or for local delivery to surrounding cells following implantation. These bioactive factors facilitate tissue formation by augmenting differentiation or maintaining homeostasis upon implantation into a defect site. Growth and differentiation factors are commonly used and carefully chosen as agents to drive specific cell responses. Growth factors used in osteogenic tissue engineering strategies most commonly include bone morphogenetic proteins (BMPs) −2, −4, and −7 [73,74]. Chondrogenic differentiation and maintenance of the chondrocyte phenotype have commonly employed TGF-ß1 [75,76] and TGF-ß3 [77]. A host of additional growth factors implicated in musculoskeletal regeneration have been investigated [78,79].
Additional signaling cues in the form of gene therapy have been explored in the tissue engineering arena and have yet to be incorporated into head and neck regeneration, offering promising future steps to generate more complex heterocellular tissue structures with defined tissue properties. These cues include plasmid DNA (pDNA) encoding growth factors [80], RNA interference [40,81], and messenger RNA (mRNA) [82] to control gene expression and related pathways relevant to tissue formation. As noted above, bioactive bioinks, which tether or encapsulate these signaling cues, hold great promise for developing a self-sustained scaffold without the need for prolonged exogenous delivery in the culture medium, allowing for earlier in vivo implantation. Further investigation into the complexity of tissue formation through use of multiple signaling cues and multiple tissue types is necessary.
4.1. Current methods for 3D bioprinting
There are many variations of 3D bioprinting techniques and, while the general description of these methods has become increasingly complex, here we review the most common methods using in 3D bioprinting: microextrusion, droplet, and laser-assisted 3D-bioprinting (Table 1).
Table 1: 3D bioprinting methods.
Adapted from [22]. No permission required.
| Bioprinters | Microextrusion-based Bioprinting | Droplet-based Bioprinting | Laser-Assisted Bioprinting |
|---|---|---|---|
| Bioink Viscosity | 6–30 × 107 mPa s−1 | 3.5–12 mPa s−1 | 1–300 mPa s−1 |
| Resolution | 100 μm− 1 mm | ≈ 20− 30 μm | ≈ 1 μm |
| Print Speed | Slow | Fast | Medium to Fast |
| Cell Viability | 40−80% | 80−90% | > 85− 95% |
4.2. Microextrusion 3D bioprinting
Microextrusion based bioprinting is the most used 3D printing technology and relies on the dispensing of biological materials and bioinks in the X–Y plane while the coordinated motion of a build platform or the dispensing nozzle moves in the Z direction [19,51]. This method employs pneumatic-, mechanical-, or solenoid-driven extrusion to dispense filaments of bioinks in a layer-by-layer fashion through microscale nozzles in accordance with the CAD software instruction. The dispensing quantity can be regulated by the air pressure level or the displacement of the pump or piston, depending on the method employed. The bioink in this method should then allow for rapid gelation of the bioink using physical or chemical polymeric crosslinking to maintain its shape without spreading [22].
The main advantages of extrusion-based bioprinting are its ability to deposit highly viscous bioinks (6–30 × 107 mPa s−1) with high cell density (including cell spheroids), ease of use, and its broad application range [51]. A disadvantage of this method is that cell survival is lower than it is with other bioprinting methods (e.g. cell viabilities of 40–80%) [19]. This could be addressed by increasing the nozzle diameter; however, this would then present the issue of lower resolution. Higher viscosity bioinks can provide increased structural support, whereas low viscosity bioinks can provide an environment for retaining cell viability and function; hence, a balance is essential.
One subset of the extrusion method is known as “embedded 3D printing” or “freeform reversible embedding” (FRESH method) in which extrusion occurs into an ionic support bath to maintain the printed structure until the deposited ink solidifies [83]. Specifically, the FRESH method [84] takes advantage of a support hydrogel as a temporary, thermoreversible support that can be washed away after printing, leaving behind the free-standing scaffold. The support hydrogel is composed of gelatin microparticles to create a gelatin-slurry which allows for biomaterials to be printed in complex geometries that otherwise would not have the structural integrity to be printed on their own without such a support bath.
4.3. Droplet 3D bioprinting
Droplet-based 3D bioprinting is a non-contact printing technique used to dispense droplets of cells and materials which are layered and deposited in precise locations [19,33]. This method utilizes energy sources such as electric, sound, and heat to generate droplets of controlled volumes of cells in the XYZ plane. Based on their droplet generation mechanisms, the droplet-based printing method can be classified as inkjet (thermal, piezoelectric), electro-hydrodynamic jetting, acoustic droplet ejection or micro-valve bioprinting.
The use of inkjet bioprinting technology has various major advantages including rapid bioprinting, low cost, and abundant availability. Furthermore, droplet based final parameters of inkjet bioprinting technology allow for high resolution printing and may attain an average diameter as small as ≈ 20–30 μm [19], equivalent to the diameter of the smallest blood artery, which is something that other currently existing bioprinting technologies cannot accomplish. Moreover, although droplet-based 3D bioprinting subjects cells to heat or force, adequate cell survival is often achieved (e.g., cell viabilities of 80–90%). In general, bioinks are of low viscosity (3.5– 12 mPa s−1) to allow for ease of flow without clogging the nozzle. Despite high resolution of this method, the main limitation is the low viscosity of the bioinks and associated low cell densities (<106 cells ml−1) which may not be biologically relevant, depending on the tissue of interest. The low viscosity of the bioink can also result in poor structural support of the 3D structure, and it can be difficult to control the size of the droplets in thermal inkjet bioprinting, resulting in uneven droplet distribution.
4.4. Light-based 3D bioprinting
Light-based bioprinting utilizes laser energy to deposit or solidify material, creating high-precision patterning of biomaterials and cells [85,86]. A subset of laser-assisted 3D printing includes stereolithography (SLA). Light-based 3D bioprinting methods typically utilize photocrosslinkable bioinks in which photocurable moieties such as acrylate or methacrylate groups are functionalized to the polymer backbone [40,41] to allow for UV-light mediated cross-linking.
Laser-assisted bioprinting
Laser-assisted bioprinting is based on the idea of laser-induced forward transfer which allows for precise printing of cells by use of a laser to transfer the bioink from a cartridge to the substrate, eliminating direct contact between the dispenser and the bioink [19,33,51]. Here, a glass plate is coated with an energy absorbing layer on one side and the bioink, containing cells, on the other side. The ink can then be deposited from the substrate when and where a laser pulse is applied to the energy absorbing layer. Rapid gelation of hydrogels is necessary to achieve high resolution of the printed pattern. The benefit of laser-assisted bioprinting is that it includes the use of a variety of printable bioinks with viscosity ranging from 1–300 mPa s−1, has high printing resolution, and due to the absence of direct contact with the dispenser and subsequent avoidance of shear stress, high cell viabilities (> 95%) can be achieved [19,51]. This method is limited to low cell density (<106 cells ml−1) applications which may not be biologically relevant depending on the tissue of interest and comes at a comparatively high financial cost.
Stereolithography (SLA) bioprinting
Similarly, SLA, is a 3D bioprinting tool that uses a pool of liquified cell-laden, UV-curable biopolymer in combination with laser energy to crosslink the polymer solution into the desired construct shape[87] . Precise movement of applied light causes the polymeric monomers to crosslink in a highly controlled manner to generate the desired tissue architecture from the bottom to the top layer using either a single-photon or multi-photon method. [88].
A variety of printable bioinks from low to high viscosities can be used with SLA bioprinting . The fast print speed of this method is a significant advantage along with high resolution (≈1 μm), and a nozzle-free approach which avoids the issue of nozzle clogging and prevents shear stress, resulting in high cell viability (>85%)[19]. In terms of drawbacks, the use of photocrosslinking requires a transparent and photosensitive bioink, limiting choice of additives and cell density (108 cells ml−1) [19]. Furthermore, laser-assisted bioprinting can be prohibitively expensive, limiting its applicability.
5. Post-bioprinting
Once the 3D bioprinted construct has been fabricated, the scaffold often requires further time to allow for tissue maturation. As noted, the presence of signaling cues, in the form of biochemical cues, can be tethered or encapsulated within a bioactive bioink or supplied exogenously in the culture medium to allow for tissue maturation or direct stem cell fate and achieve the desired functionality of the 3D construct [20]. However, static cell culture of the scaffold may not fully simulate the physiologic environment required for development of native musculoskeletal tissue and result in uneven delivery of nutrients and oxygen to cells within the scaffold. Moreover, static culture systems lack mechanical stimulation, an important component of native chondrogenic and osteogenic development [73,89].
Bioreactors, dynamic culture systems, can improve mass transfer with convective fluid flow or apply physiologically relevant physical signals such as shear stress, compression, pressure, and stretch to better recapitulate native development and augment tissue development. Bioreactor systems aim to enhance the physical environment using spinner flasks, rotating wall vessels, flow perfusion, magnetic, and ultrasonic bioreactors. Reviews on bioreactor systems for musculoskeletal engineering are detailed elsewhere [23,89–92].
Despite, the promise of bioreactors to achieve superior tissue maturation compared to static culture for otolaryngologic applications, several hurdles remain to be achieved prior to clinical implantation. For example, in the case of tracheal tissue engineering, early animal experiments with circumferential replacement of the trachea with engineered cartilage constructs demonstrated early death of animals. This was secondary to airway obstruction due to the in-growth of granulation tissue and/or sputum retention [93]. This was due to the need for epithelialization of the inner tracheal lumen, which was not supported with the cartilage-only constructs. Literature points towards the benefit of a vascularized wound surface to promote epithelialization; there is a need for both revascularization and epithelialization of engineered tracheas. Regarding respiratory epithelial cell growth, this is best achieved with an air-liquid-interface [94,95]. On the other hand, different culture conditions are required for neovascularization and promotion of cartilage maturation of the tracheal ring, pointing to the need to optimize unique bioreactor systems to promote multi-tissue development. Moreover, the need for neovascularization of in vitro cultured tissue limits the size and thickness of engineered constructs [14]. Upon in vivo implantation, angiogenesis can take several days, and perfusion is limited to a few hundred micrometers [96]. Without appropriate vascularization, larger biofabricated tissues can undergo cell death. Further optimization of construct-specific bioreactor systems will be essential to driving the application of bioengineered tissue in otolaryngologic practice.
6. 3D-Bioprinting Applications in Otolaryngology
Current advancement in the fields of tissue engineering, material sciences, and additive manufacturing provide the opportunity to augment approaches to reconstruction of head and neck structures. In fact, 3D printing has been applied to surgical training in otolaryngology, an example being the use of 3D printed temporal bones for surgical simulation [4,97], medical teaching [98,99], as well as customized surgical planning [100–103]. Moreover, clinical applications of 3D printing in otolaryngology have included the successful treatment of severe airway malacia using a 3D printed, personalized PCL external airway splint [104],[105]. Although clinical trials are in place to investigate the application of tissue engineering in the head and neck [106], none, to date, are underway that utilize 3D bioprinting with the direct printing of cells. From a technical perspective, 3D bioprinting has several advantages over traditional prosthesis or surgical grafts. For example, 3D bioprinting can solve issues related to misalignment of graft placement and reduce time of surgery and anesthesia in complex facial reconstruction [107]. As noted, compared to conventional tissue engineering, 3D bioprinting allows for more advanced and precise deposition of cells and biomaterials to create customized grafts with complex architecture [14]. This new technology has the capacity to advance surgical treatment in head and neck surgical cases.
It is additionally worth pointing out the discrepancy in the literature in defining 3D bioprinting[21,108]. Here, we refer to 3D bioprinting strategies in which cell-containing bioinks are directly printed to create complex, cellular 3D constructs rather than 3D printing of a polymeric scaffold to which cells are subsequently seeded manually with or without the use of hydrogels as a carrier or additional scaffolding. This review reports descriptions of 3D bioprinted cartilage and bone tissue for advancement in the fields of otology, nasal reconstruction, laryngology, and craniofacial bone reconstruction with a focus on the functional outcomes following in vivo implantation of these 3D bioprinted constructs.
6.1. 3D-Bioprinting for Otologic Applications
Tissue engineering for otologic applications has focused on cartilage regeneration for reconstruction of the auricle, or external ear, to treat patients with microtia and, less frequently, other anatomic units of the ear such as the tympanic membrane (TM) and bony ossicles.
Reconstruction of the external ear currently relies on prosthetic reconstruction or autologous cartilage grafts[109]. However, a prosthesis can potentially extrude, and cartilage autografts may result in donor site morbidity or be limited by size[110]. Cartilage tissue regeneration offers an alternative source for autologous cartilage to improve auricular reconstruction. The notion of tissue engineering for auricular reconstruction first captured attention in 1997 following publication of the Vacanti mouse [111], which evaluated the feasibility of growing cartilage on a biomaterial scaffold in the shape of a human auricle. Although several reports of 3D bioprinted cartilage-like scaffolds in the form of the auricle [112,113] have been described, few have investigated outcomes following in vivo implantation (Table 2).
Table 2: 3D bioprinting for otologic reconstructive applications.
PCL, Polycaprolactone.
| Anatomic Region | 3D Bioprinting Method | Scaffold Material | Cell Source | Animal Model | Orthotopic or Heterotopic Implantation | Ref |
|---|---|---|---|---|---|---|
| Auricle | Pneumatic Extrusion | Gelatin/fibrinogen/hyaluronic acid/PCL | Rabbit ear chondrocytes | Subcutaneous implantation in athymic mice | Heterotopic | [44] |
| Auricle | Pneumatic Extrusion | Alginate/PCL | Chondrocytes | Implantation in rabbit ear cartilage defect | Orthotopic | [114] |
| Auricle | Digital near-infrared photopolymerization | Gelatin methacrylate | Articular chondrocytes from newborn rat | Subcutaneous implantation in BALB/c nude mice | Heterotopic | [115] |
| Tympanic Membrane | Pneumatic extrusion | Alginate/fibrous PCL/collagen scaffold | Mesenchymal stem cells | Subacute tympanic membrane perforation implantation in Sprague-Dawley rats | Orthotopic | [119] |
Kang et. al. [44] constructed multi-material, patient-specific, human-scale ear-shaped cartilage scaffolds constructed with cell-laden composite hydrogels composed of gelatin, fibrinogen, hyaluronic acid and glycerol co-printed with PCL via extrusion-based 3D bioprinting (Fig. 2A). To create the external ear, rabbit ear chondrocytes mixed with the composite hydrogel were printed with PCL as a support scaffold to provide mechanical integrity along with Pluronic F-127 hydrogel as a sacrificial outer support layer prior to crosslinking of the hydrogel with thrombin. These constructs were implanted in the dorsal subcutaneous space of athymic mice for 1- or 2-months, and the implants were shown to maintain their shape. Histological analysis at these time points demonstrated formation of human-scale cartilage tissue scaffolds and the presence of microchannels between the printed filaments. The authors describe the potential for microchannels to allow for nutrient and oxygen diffusion with the implication for future vascularization upon implantation. Despite promising structural and functional characteristics, this group used nude mice in an ectopic site. Given the importance of monitoring graft tolerance, further work remains to investigate regeneration following implantation of these tissue constructs in an immunocompetent animal model that more closely mimics surgical correction of aural atresia or microtia.
Figure 2. 3D bioprinting for otologic applications.
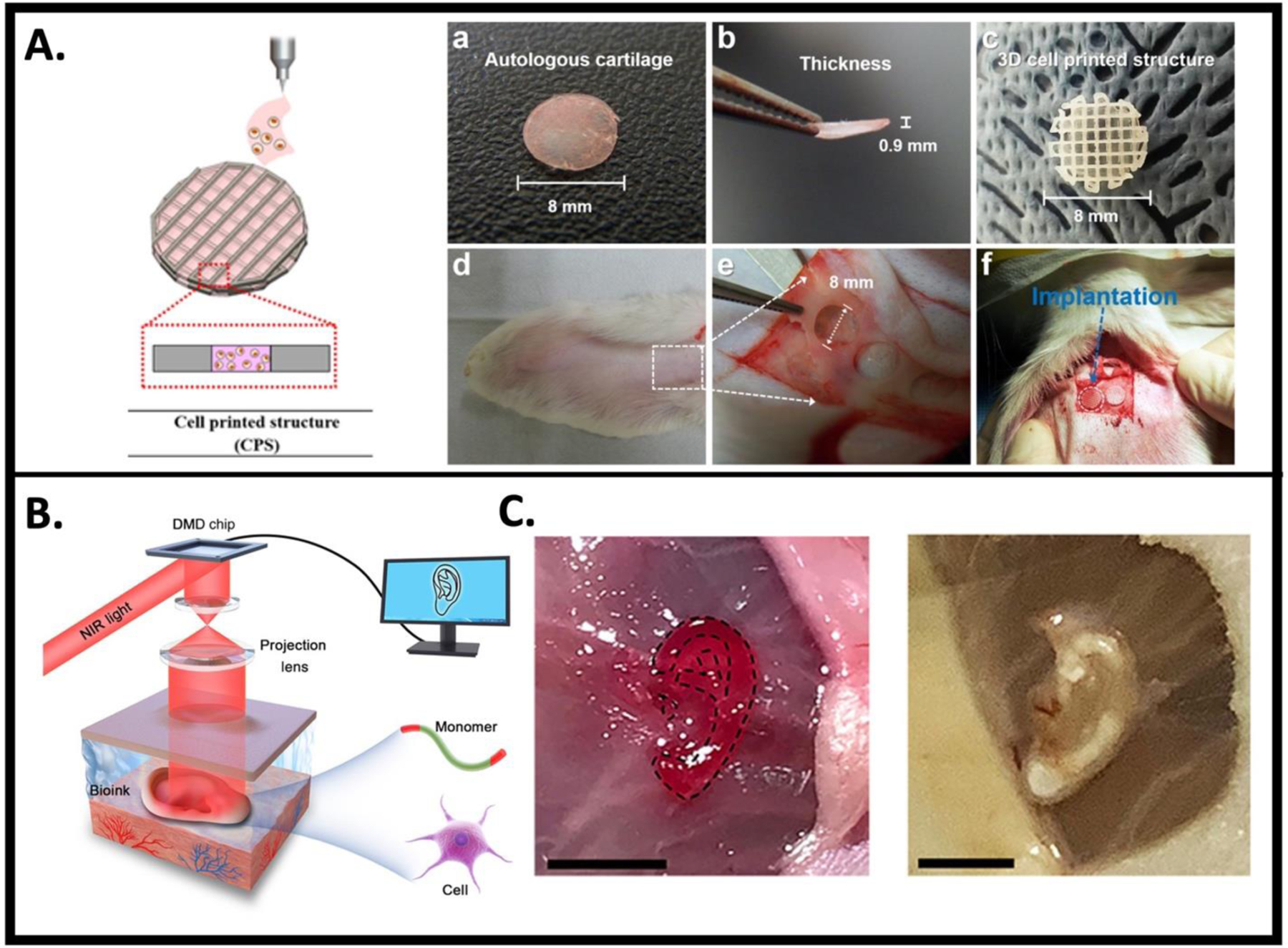
A. Schematic of cell-printed scaffold (CPS) composed of chondrocytes encapsulated in alginate bioink printed with PCL framework. (a, b) autologous cartilage, (c) CPS, (d) defect site on the rabbit ear, and (e) defect creation, (f) implantation of the grafts into the cartilage defect of rabbit model. Figure adapted from [114], permissions obtained. B. Schematic diagram of digital near-infrared (NIR) photopolymerization (DNP)-based noninvasive 3D bioprinting. Figure adapted from [115], permissions not required. C. DNP-based 3D bioprinted, ear-shaped construct printed subcutaneously in BALB/c nude mice. Figure adapted from [115], permissions not required. Scale bar, 5 mm.
Similarly, Park et al., [114] created cartilage-like scaffolds through the extrusion deposition of alginate bioink with encapsulated rabbit chondrocytes co-printed with PCL for cartilage repair in a rabbit model. These 3D bioprinted scaffolds were compared to manually seeding cells onto scaffolds resulting in enhanced cartilage regeneration both in vitro and following implantation in a rabbit ear cartilage defect model observed in the bioprinted group. In addition, complete integration of the graft into the surrounding native cartilage was observed in vivo, suggesting that 3D bioprinting can provide a successful method for auricular reconstruction.
A non-invasive in vivo 3D bioprinting system was created using digital near-infrared (NIR) photopolymerization (DNP) 3D bioprinting to initiate crosslinking of photopolymers within the body, with the rationale that NIR can penetrate deep tissue and allow for precise control over crosslinking [115] (Fig. 2B,C). In this system, a locally injected bioink composed of gelatin methacrylate (GelMA) with encapsulated articular rat chondrocytes was non-invasively crosslinked using NIR photopolymerization to create ear-like constructs within the subcutaneous space of BALB/c nude mice. Results demonstrated maintenance of the ear shape after 1 month and histological analysis showed chondrocyte morphology with collagen type II secretion, a marker of hyaline cartilage. This report provides evidence for the role of noninvasive in vivo 3D bioprinting technology to prepare complex tissues in situ.
TM regeneration in cases of perforation is another avenue for the application of 3D bioprinting for a common otologic presentation. Although various materials have been used as a scaffold including paper patch, gelfoam, and alloderm in acute TM perforation [116–118], chronic perforations are plagued by decreased regenerative capacity. As such, autologous tissue tympanoplasty with the grafts such as fascia or perichondrium serve as surgical options for treatment. These approaches can be invasive despite the use of endoscopic tympanoplasty, creating an opportunity for the use of 3D bioprinted grafts instead.
The first reported TM regenerative strategy using 3D bioprinting created composite scaffolds of PCL, collagen, and alginate with encapsulated mesenchymal stem cells (MSCs) [119]. A fibrous PCL micro/nanofiber structure was engineered using a centrifugal spinning process followed by collagen coating and freeze-lyophilization of the structure and subsequent cross-linking of the collagen. MSC-laden alginate bioink was printed onto the fibrous surface of the PCL/collagen scaffold and cross-linked with CaCl2. Implantation of the cell-laden composite scaffold allowed for complete healing of subacute TM perforations in Sprague-Dawley rats compared to cell-free scaffolds. No statistically significant differences in auditory brain reflex (ABR) thresholds at all frequencies or TM vibration velocity was observed in the 3D bioprinted, cell-laden scaffold group compared to normal TMs suggesting no effect on hearing. The authors propose this 3D bioprinted platform as an approach to heal less severe chronic TM perforations.
Few reports have investigated regenerative applications in the middle and inner ear structures associated with sound transduction, although 3D printing technology has been applied to the creation of custom bony ossicles. For example, human cadaveric CT images were used to accurately reconstruct 3D ossicular prostheses [120]. However, this study lacked functional outcomes in vivo, and to the best of our knowledge, there are no reports implementing 3D bioprinting techniques to study ossicular regeneration. Similarly, 3D printing has been used to create a biomimetic cochlea as a tool for predicting and optimizing stimulus signals [121]. These studies indicate the ability to 3D print complex anatomical shapes and lay the groundwork for future applications of complex 3D bioprinted middle and inner ear structures.
6.2. 3D-Bioprinting Applications in Nasal Reconstruction
The nasal cartilage represents an anatomic location of specialized connective tissue as nasal cartilage tissue is poorly vascularized and has poor regenerative ability. Nasal defects or deformities may arise due to congenital malformation, oncologic resection, or trauma, and result in functional or aesthetic deficits. Nasal reconstructive surgery, including septoplasty, functional rhinoplasty, and fracture repair, require the modification or application of cartilage grafts to restore nasal function and facial aesthetics. Current techniques employ autologous cartilage harvest which may suffer from donor-site morbidity, resorption issues, and be limited by the size and shape of the harvested cartilage [122,123]. Tissue engineered cartilage, namely 3D bioprinted grafts (Table 3), can potentially replace the need for autologous cartilage in nasal reconstructive methods. However, it is important to note that current approaches assessing in vivo nasal cartilage graft formation and viability typically utilize nasoseptal chondrocytes (NCs) in mono or co-culture followed by subcutaneous implantation in animal models, which may not fully reflect clinical aesthetic and functional outcomes.
Table 3.
3D bioprinting for nasal reconstructive applications.
| Anatomic Region | 3D Bioprinting Method | Scaffold Material | Cell Source | In Vivo Study Animal Model | Orthotopic or Heterotopic Implantation | Ref |
|---|---|---|---|---|---|---|
| Nasal cartilage | Pneumatic extrusion/FRESH method | Type I collagen | Human nasal chondrocytes | Subcutaneous implantation in nude mice | Heterotopic | [123] |
| Nasal cartilage | Pneumatic extrusion | Nanofibrillated cellulose/alginate | Human bone marrow-mesenchymal stem cells and human nasal chondrocytes | Subcutaneous implantation in nude female BALB/c mice | Heterotopic | [124] |
| Nasal cartilage | Pneumatic extrusion | Nanofibrillated cellulose/alginate | Human bone marrow-mesenchymal stem cells and human nasal chondrocytes | Subcutaneous implantation in nude mice | Heterotopic | [125] |
Lan et al. [123] investigated the effect of culture period on ECM formation and mechanical properties of 3D bioprinted scaffolds composed of collagen type I hydrogel with incorporated human nasoseptal chondrocytes (hNCs) with the goal to engineer scaffolds that allow for surgical suturing and resistance to contraction during scar formation (Fig. 3A,B). The FRESH method was used to print a patient-specific right lower lateral nasal cartilage generated from CT imaging and compared to controls composed of cells seeded onto a clinically approved collagen type I/III collagen membrane (Chondro-gide). Scaffolds were cultured in vitro for 3, 6, and 9 weeks in culture medium supplemented with TGFß-3. In vitro results demonstrated significant glycosaminoglycan (GAG)/DNA production which increased at weeks 6 and 9 compared to 3 weeks of in vitro culture along with significant Safranin-O and col II positive staining of the matrix. In addition, a suture test was performed with placement of a single 5–0 prolene suture on the edge of the construct and the scaffolds were observed for damage. Bioprinted constructs were only able to withstand suturing after 9 weeks of culture. The bending modulus of the engineered cartilage increased with increased culture time and to a greater degree in the bioprinted constructs compared to controls. In addition, the mechanical strength of 3D bioprinted scaffolds and controls increased following in vivo implantation, suggesting further remodeling upon implantation. This implies that the appropriate timing for implantation is one which allows for suturing and handling during reconstructive surgery given that future maturation will continue to occur post-operatively. Scaffolds were implanted in nude mice for 5 weeks following 9 weeks of culture in vitro and shown to maintain their gross morphology. Interestingly, both the cell-laden, 3D bioprinted constructs and control scaffolds showed peripheral loss of proteoglycan-rich matrix despite increased collagen deposition at the periphery. Further analysis demonstrated increased macrophage presence at the periphery, implicating a phagocytic role in proteoglycan loss which the authors propose may be secondary to secreted matrix metalloproteinases. This suggests that an immune response may affect tissue formation. Overall, this study showed strong evidence for the potential to engineer patient-specific human nasal cartilage grafts using 3D bioprinting via the FRESH method and raises questions regarding host immunologic response to implanted grafts and the effect on neo-tissue formation.
Figure 3. 3D bioprinting for nasal applications.

A. Gross morphology of FRESH printed nasal alar structure composed of collagen type I hydrogel with incorporated human nasal chondrocytes (hNCs). Image (top) shows 3D model of a right lower lateral nasal cartilage from CT (top left) and a preview of sliced nasal cartilage using Slic3r software (top right). Image (bottom) shows nasal alar cartilage 3D bioprinted in a gelatin support bath before (bottom left) and 30 min after incubation in 37°C with removal of support bath (bottom right). Figure adapted from [123], permissions not required. B. Demonstration of gross morphology of 3D bioprinted nasal alar constructs and commercially available control (chondro-gide) scaffolds across culture time. Figure adapted from [123], permissions not required. C. Photograph of hNC-laden nanofibrillated cellulose/alginate hydrogel after 14 days of implantation. Figure adapted from [124], permissions not required. D. Histological evaluation of glycosaminoglycan deposition of hNC and hNC/human bone marrow mesenchymal stem cell (hBMSC)-laden nanofibrillated cellulose/alginate hydrogel at days 14, 30, and 60 of implantation. Scale bar, 100 μm. Figure adapted from [124], permissions not required.
In addition, Moller et al. [124] (Fig. 3C,D) 3D bioprinted a nanofibrillated cellulose/alginate hydrogel with either hNCs, human bone marrow mesenchymal stem cells (hBM-MSCs), or co-culture (hNCs:hBM-MSCs, 20:80). These scaffolds supported cartilage-like tissue following subcutaneous implantation in BALB/c mice for 6 weeks. Histologic evidence of increasing GAG deposition was observed following subcutaneous implantation of the scaffolds into nude BALB/c mice in both the hNC only and co-culture groups as assessed with Alcian Blue and van Gieson staining which was increased in the co-culture group. Collagen type II (Col II) immunohistochemistry (IHC) confirmed deposition of chondrogenic extracellular matrix after 60 days of implantation in the co-culture group, while no evidence of chondrogenesis was observed in the hBMSC-only group. Due to this lack of chondrogenesis in the MSC group as well as evidence of hBMSC loss despite evidence of chondrogenesis over time in the co-culture group, the authors suggested the beneficial role of the MSCs to be a result of trophic factor release rather than chondrogenic differentiation of MSCs. The lack of robust chondrogenesis here may be secondary to lack of available differentiation or growth factors presented to the MSCs to guide differentiation towards the chondrogenic lineage. Results highlight the role MSCs have in trophic factor release which is distinct from their capacity for musculoskeletal differentiation.
Apelgren et al., [125] 3D bioprinted nanofibrillated cellulose and alginate in combination with hNCs and hMSCs either in mono- or co-culture (hNCs:hBM-MSCs, 20:80) followed by immediate subcutaneous implantation into nude mice. Results showed that the highest density of GAG-positive cells were obtained in scaffolds with monoculture of chondrocytes despite an increased overall number of cells in the mixed-cell scaffolds. In addition, the authors demonstrated that the chondrocyte clusters observed were derived from chondrocytes rather than from differentiation of MSCs towards the chondrogenic lineage, again, potentially due to the lack of instructive cues present in the system to initiate MSC chondrogenic differentiation. These results suggest that although co-culture of chondrocytes and stem cells results in increased chondrocyte proliferation, chondrocytes alone enhanced neo-cartilage formation in this system. Despite apparent differences in results regarding chondrocyte and MSC co-culture experiments, the literature suggests that 3D bioprinting can allow for engineering of multi-cellular constructs for cartilage engineering.
6.3. 3D Bioprinting Applications in Tracheal Reconstruction
3D-bioprinted scaffolds have focused attention on tracheal constructs as a potential solution for reconstruction of long segment tracheal defects where end-to-end anastomosis is not an appropriate surgical option. Such cases may be secondary to stenosis or tumor in the trachea necessitating long-segment resection [126]. The ideal long-segment tracheal graft aims to satisfy specific criteria for functionality including [6] : to allow for the flow of air to the lungs; to be airtight; to have mechanical properties to withstand pressures associated with respiration; and to be flexible enough to allow for the graft recipient to move without significant restriction. The progress of tissue engineering has made engineered tracheal grafts a promising option for tissue transplantation, and several regenerative approaches have focused on simultaneous regeneration of tracheal cartilage as well as epithelium to restore tracheal structure and function (Table 4).
Table 4. 3D bioprinting for tracheal reconstructive applications.
PCL, polycaprolactone.
| Anatomic Region | 3D Bioprinting Method | Scaffold Material | Cell Source | In Vivo Study Animal Model | Orthotopic or Heterotopic Implantation | Ref |
|---|---|---|---|---|---|---|
| Trachea | Pneumatic extrusion | PCL/alginate bioink | Rabbit epithelial cells and rabbit bone marrow- mesenchymal stem cells | Trachea scaffold implantation in a partial resection rabbit model | Orthotopic | [126] |
| Trachea | Pneumatic extrusion | PCL/atelocollagen bioink | Human nasal chondrocytes and human nasal turbinate stem cells | Subcutaneous implantation in BALB/c mice | Heterotopic | [52] |
| Trachea | Pneumatic extrusion | PCL/alginate bioink | Rabbit epithelial cells and rabbit chondrocytes | Trachea scaffold implantation in a partial resection rabbit model | Orthotopic | [129] |
| Trachea | Digital light processing/4D-bioprinting | Silk fibroin methacrylate | Rabbit auricular chondrocyte and rabbit nasal turbinate stem cells | Trachea scaffold implantation in a partial resection rabbit model | Orthotopic | [128] |
Bae et al., [126] biofabricated a multi-material, multi-cellular artificial trachea using 3D bioprinting to engineer an artificial trachea with incorporation of rabbit epithelial cells and chondrogenic-differentiated rabbit BM-MSCs (rBM-MSCs, Fig 4A). The artificial trachea was composed of PCL as a support layer which made up the first, third, and fifth layers of the cylindrically shaped construct. The innermost and outermost PCL layers were fabricated with pores to promote cell infiltration and neovascularization whereas the middle layers contained no pores to create a barrier between the epithelial cells and cartilage layer. The hydrogel bioink composed of alginate hydrogel was used to print the second and fourth layers with epithelial cells encapsulated within the second layer or rBM-MSCs within the fourth layer to create an inner lumen composed of epithelial cells and an outer layer composed of cartilage (Fig. 4A). MSCs were either implanted in an undifferentiated state (MSC) or following monolayer differentiation (d-MSC) in chondrogenic medium prior to incorporation within the scaffold. A half-pipe shaped partial tracheal resection was performed in New Zealand White Rabbits and the 3D bioprinted artificial trachea was implanted for 12 weeks. CT imaging prior to harvest showed good luminal contour of the trachea and no evidence of airway stenosis. In addition, bronchoscopic examination revealed that the tracheal defect was fully covered with epithelial cells which was verified by histopathology. Neovascularization was found around implants in all groups. However, neo-cartilage formation was limited in the d-MSC group and only to a localized area, potentially due to design parameters including cell seeding density. Alternatively, the approach to rBM-MSC chondrogenic differentiation may have affected the outcome because MSCs were induced to undergo chondrogenic differentiation in a 2D monolayer culture prior to incorporation within the scaffold. Previous studies have demonstrated de-differentiation of MSCs cultured in 2D [127] and the majority of studies to date utilize 3D systems for MSC chondrogenic differentiation, suggesting the potential need for an in vitro culture period within the scaffold prior to implantation or additional instructive cues available to cells upon implantation. Despite the limited cartilage formation in this system, initial results were promising given that the ability to maintain a patent airway is an important factor for tracheal graft success in vivo.
Figure 4. 3D bioprinting for tracheal applications.

A. 3D bioprinted artificial trachea, longitudinal and vertical view (top left). An SEM view of the five-layered structure (bottom left) shows (a,c,e) polycaprolactone (PCL) layers, (b) the alginate layer with mesenchymal stem cells (MSCs), and (d) the alginate layer with epithelial cells. Following implantation of the 3D bioprinted trachea in a rabbit model (top right), bronchoscopic images reveal fully epithelialized mucosa at 12 weeks post-surgery within the trachea inner lumen. Figure adapted from [126], permission not required. B. In vitro culture of a 4D-bioprinted trachea composed of hTBSCs to target the mucous membrane of the trachea as the base layer of the construct and hNCs for the hyaline cartilage ring to create a trachea-mimetic scaffold through shape morphing (top). Transplantation of the 4D bioprinted trachea into a partial defect rabbit trachea (bottom). Figure adapter from [128], permissions obtained.
Similarly, Park et al. [52] described a trachea-mimetic 3D bioprinted construct composed of a porous framework comprising separate cartilage rings in the outer grooves of the framework and an epithelium lining within the luminal surface of the framework. The framework was first printed with PCL followed by rotational printing of atelocollagen bioink containing hNCs to print cartilage rings on the outer framework. This was followed by rotational printing of the epithelial lining with atelocollagen bioink containing human nasal turbinate stem cells (hNTSCs) to print the epithelial lining within the inner lumen. This report indicates the potential to create extrusion-based 3D bioprinted constructs that avoid the typical approach to print-head positioning that is perpendicular to the build platform by taking advantage of a rotational printing system to shorten print time and create larger constructs. Results showed that varying cell densities of 1 × 106 cells/ml, 2 × 106 cells/ml, and 5 × 106 cells/ml could maintain their initial volume while a cell density of 1 × 107 cells/ml showed a significant volume contraction in both the cartilage and epithelial layers after gelation. In addition, gene expression markers of chondrogenic differentiation as well as epithelial differentiation markers were significantly higher at 5 × 106 cells/ml compared to other cell densities suggesting a cell concentration of 5 × 106 cells/ml was the most appropriate for the printing of both cartilage rings and epithelial lining of the tracheal construct in this system. Scaffolds composed of the PCL framework and atelocollagen-hNCs (5 × 106 cells/ml) to form the cartilage rings without the application of the epithelial layer were implanted subcutaneously in BALB/c nude mice for 8 weeks and implants retrieved. Scaffolds were implanted with or without the presence of a sinusoidal-patterned tubular mesh (SPTM) wrapped around the trachea-mimetic scaffold which served as a protective layer. Histological analysis revealed decreased cartilage formation without the presence of the SPTM compared to scaffolds with the SPTM, indicating that a protective barrier in this case prevented resorption of cartilage rings in vivo and provided a potential solution to the issue of macrophage-mediated proteoglycan loss presented earlier [123]. Despite evidence of cartilage formation, there was no evidence of a functional epithelial layer in this study and further work in an orthotopic model will be an important aspect of further clinical applications.
The same group [129] also 3D bioprinted a similar tracheal scaffold using the above-described tracheal construct with PCL framework and alginate bioink with encapsulated rabbit nasal epithelial cells on the inner layer and rabbit auricular chondrocytes on the outer hydrogel layer. The grafts were implanted in rabbits for 12 months using the partial tracheal resection rabbit model. Of note, three of the six control animals implanted with scaffolds without incorporated cells died in the first 3 months after implantation whereas one rabbit died in the experimental group during this period and one additional rabbit within the next 3 months. CT imaging at 12 months revealed that the inner diameter of the graft site was significantly reduced in the cell-free control group whereas the experimental group composed of cell-incorporated scaffolds showed no evidence of stenosis which was verified with bronchoscopic imaging. In addition, histopathology of the graft at 12 months revealed ciliated epithelial cells on the inner lumen of the airway in all rabbits over 3 months post-implantation, although only immature cartilage islets were observed at both 6 and 12 months in the experimental group. Further studies to optimize cell types and cell concentrations required to regenerate more robust neo-cartilage in these trachea-mimetic scaffolds will be necessary, given the promising results achieved so far.
The application of 4D bioprinting, which combines 3D bioprinting with the fourth dimension, time, was shown to be applicable to tracheal tissue engineering [128] (Fig 4B). In 4D bioprinting, a shape memory material is employed so that following 3D bioprinting, the properties of the material can be used to allow for the structure to morph into a planned shape over time following application of a stimulus which can include either osmotic pressure, magnetic stimulation, heat, or light. In this study, a multi-component 3D tracheal structure was fabricated through shape forming of a bilayer hydrogel using digital light processing bioprinting, a sub-type of SLA. Following 3D bioprinting of the bilayer hydrogel as a flat structure, the construct was able to shape morph and form a tubular structure using the swelling properties of the hydrogel as a stimulus. The hydrogel was composed of photocrosslinkable silk fibroin methacrylate (Sil-Ma) printed with encapsulated rabbit auricular cartilage chondrocytes onto the patterned side of the hydrogel to form the outer layer and rabbit turbinate-derived mesenchymal stem cells on the base layer to form an inner layer of respiratory mucosa. The 4D bioprinted trachea mimetic construct was placed into a rabbit trachea site with a 210° partial defect. Bronchoscopic examination at 6 weeks post-implantation revealed complete coverage of the scaffold with epithelial mucosa as well as mild stenosis, although the airway was described as patent. In addition, histopathologic analysis revealed stable integration of the graft and confirmed the presence of a respiratory epithelial layer covering the entirety of the implantation site. However, immature cartilage composed of several nests of chondrocytes was observed perimetrically along the implant site indicating additional need to augment neo-cartilage formation. Overall, the results of this 4D bioprinting model presents a platform to control the spatial organization of cells in a method that common digital light processing 3D bioprinting cannot easily achieve by creating a dynamic system.
6.4. 3D Bioprinting Applications in Craniofacial Bone Reconstruction
3D bioprinting technology can be used in oral, maxillofacial, and facial reconstructive surgery to bioprint pre-contoured grafts, develop prostheses and improve facial contour. As one can imagine, these defects require high precision which can be accomplished with 3D bioprinting [21]. A review of 3D printing in maxillofacial surgery showed that the highest number of clinical indications occur for reconstruction of the mandible and for dental implantations and has real life implications in improving patient outcomes [130]. Almost 300 publications from 35 countries showed that 2889 patient outcomes were improved by 3D printed objects secondary to improvement in precision and reduction of surgical time. 3D bioprinting has the potential to make similar improvements in maxillofacial surgical outcomes.
The development of 3D bioprinting technology may allow for fabrication of custom-made implants in the reconstruction of maxillofacial defects, notably following trauma or reconstruction for head and neck cancer. As noted in the otology section above, Kang et al. [44] developed not only cartilage scaffolds but also a custom 3D bioink which was applied to generate human sized mandible bone fragments (Fig 5). The bioink composed of gelatin, fibrinogen, hyaluronic acid, and glycerol was incorporated with hAFSCs and co-printed with PCL and tricalcium phosphate (TCP) along with Pluronic F127 as a sacrificial support. The CT image and CAD model-based mandible construct was used to generate a scaffold with inner architecture composed of PCL/TCP alternating with filaments of hAFSC-laden hydrogel, creating micro-channels within the scaffold that could promote vascularization and nutrient diffusion. In vitro analysis of osteogenic differentiation of the scaffold after 28 days demonstrated calcium deposition, a marker of bone formation. To study maturation of the bioprinted bone in vivo, 3D bioprinted discs with incorporated hAFSCs were cultured in osteogenic media for 10 days and implanted in a rat calvarial bone defect. Results showed that the scaffold formed newly vascularized bone tissue 5 months after in vivo implantation whereas the untreated defect and scaffold-only treated control groups showed fibrotic tissue ingrowth and minimal bone tissue formation as assessed with modified tetrachrome and hematoxylin/eosin stain. Further studies regarding application in a mandibular defect model are warranted for further application of this system.
Figure 5. 3D bioprinting for maxillofacial bone applications.

A. Three-dimensional computer assisted design (CAD) model generated from a CT image of mandibular bony defect to 3D bioprint the tissue defect site. The 3D bioprinted graft was composed of human amniotic fluid–derived stem cells (hAFSCs) mixed with composite hydrogel of gelatin, fibrinogen, hyaluronic acid and glycerol co-printed with polycaprolactone, tricalcium phosphate, and Pluronic F127 as a temporary support. Scaffolds were cultured in osteogenic medium for 28 days followed by Alizarin Red S staining, indicating calcium deposition, a marker of osteogenesis. Figure adopted from [44], permissions obtained.
In another study, Ma et al. [131] used 3D bioprinted hydrogels to explore the periodontal ligament stem cells (PDLSC)-ECM interaction and to screen for an appropriate ECM composition that would best allow for in vivo repair of an alveolar bone defect, a potential side effect of periodontitis. PDLSCs were encapsulated in injectable, photocrosslinkable composite hydrogels composed of GelMA and poly(ethylene glycol) dimethacrylate (PEGDA). Given that stem cell fate is affected by the microenvironment, varying compositions of hydrogels were 3D bioprinted to investigate how material composition affects cell behavior. PDLSC incorporated hydrogels composed of GelMA:PEGDA in a 4:1 ratio were found to be the optimal composition, taking into account cell viability and osteogenic differentiation. This hydrogel composition was applied to a rat model of alveolar bone defect. However, rather than implantation of the 3D bioprinted scaffold, the study here performed injection of the PDLSC-laden GelMa/PEG solution at the defect site followed by in situ UV crosslinking. These studies showed robust new bone formation in the defects treated with the PDLSC-laden hydrogel as compared to hydrogel or saline alone by 3- and 6-weeks post-implantation on micro-CT analysis and 6 weeks on histological analysis. Although the in vivo analysis of the 3D bioprinted scaffolds in this work did not utilize a 3D bioprinted scaffolds, this approach demonstrates the application of 3D bioprinting for not only functional tissue regeneration but also the study of cell−ECM interactions in 3D.
7. Advantages of 3D Bioprinting
3D bioprinting has emerged as a promising methodology to produce patient-specific structures for regeneration of tissues relevant to the field of otolaryngology. The emergence of this technology offers the ability to bridge the divergence between natural and artificial tissue using CAD software to print defect- and/or patient-specific structures. In addition to otolaryngology, multiple fields including neurology, cardiology, gastroenterology, pulmonology, and orthopedics [132] have benefited from the integration and rapid progress of nanotechnology, cell biology, material science, and engineering to rapidly expand the capabilities of tissue regeneration and transplantation medicine. 3D bioprinting has also shown promise in multiple arenas beyond tissue regeneration, including cancer research [133] and drug development and delivery [134,135]. The application of 3D bioprinting in otolaryngology has borrowed and built upon research in these areas, and several advantages are provided by 3D bioprinting compared to conventional tissue regeneration approaches. For example, 3D bioprinting has the advantage of rapid prototyping of complex shapes, precision placement of cells and bioinks, and control over construct microenvironment. Additional reviews regarding the advantages, disadvantages, and future of 3D bioprinting are available [132,136,137].
3D bioprinting allows for control over the external size and geometry of the cell-based constructs as well as the ability to design the anatomical structure using medical imaging data (CT, MRI, etc.), the latter of which allows for patient- or defect- specific tissue fabrication. Traditional tissue engineered methods require casting of molds to generate constructs of desired shapes and sizes which can be time consuming and lengthy to generate. With the use of CAD software, researchers can alter the software to generate tissue of various shapes and sizes, printed in a layer-by-layer fashion, to accelerate the pace of research. Therefore, 3D bioprinting enhances more traditional tissue engineering methods by implementing a more automated process [138]. This provides the ability to alter the design as needed relatively quickly, allowing for widespread application across different clinical scenarios.
In addition, 3D bioprinting allows for high precision and customization of printed constructs. This technology allows for spatial control over cell deposition and co-deposition within specific matrix materials, growth factors, proteins, and genes to produce tissue-like constructs composed of multiple cell types and materials with localized signaling cues. This can be used to generate complex tissues that more closely recapitulate native development. Bioactive inks may be able to create a self-sustained system without the need for lengthy in vitro culture to guide differentiation, allowing for earlier implantation [80]. Although none of the reports highlighted in this review took advantage of the opportunity of bioactive bioinks, co-deposition of cells with bioactive signaling cues such as growth factors to locally augment cell maturation and differentiation will be an important future step in creating functional tissues. Therefore, 3D bioprinted tissue can be designed with distinct properties based on the desired application.
The microstructure of the scaffold refers to the porosity and pore network within the construct which affects cell migration, proliferation, and differentiation as well as mass transport of oxygen and nutrients, key factors in guiding tissue formation. Conventional methods employ salt leaching, gas formation, phase separation, freeze-drying, fiber bonding and solvent casting to produce porous scaffolds [139]. However, control over pore size and pore interconnectivity is difficult to control with these techniques. 3D bioprinted scaffolds, on the other hand, can be engineered and bioprinted with specific pore size and pore network, which can also be used to control the mechanical properties of the scaffold. An increase in porosity will lead to a decrease in the mechanical properties of the scaffold; a balance between mechanical properties and function is necessary. Overall, 3D bioprinting for tissue regeneration demonstrates increased control over design and function of engineered tissue compared to earlier approaches.
8. Challenges and Future Outlook of 3D bioprinting
3D bioprinting represents the future of tissue regeneration. However, many challenges remain to be addressed before we are able to create fully functional tissue capable of clinical implantation including in otolaryngologic surgery. The major technical deficiencies in 3D bioprinting pertain to limitations in current bioinks, inadequate mechanical properties of the constructs, control over cell behavior, and the need for additional pre-clinical studies in suitable animal models of transplantation.
The selection of biomaterials employed in 3D bioprinting techniques is significantly dependent on biocompatibility and cellular growth as well as printing ability, such as viscosity, extrudability, and mechanical stability, which will ultimately affect cellular function and outcomes following implantation. Therefore, the success of fabrication of tissue constructs is limited in part by availability of versatile bioinks that exhibit all of the desired characteristics of a particular application [14]. Different bioink formulations with a range of biomechanical characteristics remain to be developed. Current bioprinting technologies must also be enhanced to increase resolution and the scalability of the printed structures.
The majority of 3D bioprinting reports use extrusion bioprinting methods. Hydrogel bioinks are commonly used which lack mechanical stability. This lack of mechanical stability is related to the chosen biomaterials and bioprinting platforms utilized. As elaborated above, current bioprinting platforms each have their own set of advantages and disadvantages which affect mechanical strength. Several steps have been attempted to improve mechanical properties of bioprinted structures. For example, Kang et al.[44] reported a multi-material system of cell-laden hydrogels and biodegradable polymers so as to overcome limitations associated with mechanical strength and size. While multimaterial bioinks have been the most extensively explored solution to mechanical stability concerns [44,112], additional combinations of polymers have yet to be examined. For example, creation of hybrid scaffolds composed of hyaluronic acid and methacrylated gelatin hydrogels showed significantly improved mechanical properties compared to their single component scaffolds [140]. Additionally, screening of biomaterials using 3D bioprinting might provide a platform to identify materials that best support the desired application [131].
Cell proliferation, maturation, and size of the scaffold post-printing will require engineering and optimization of bioreactor systems to augment in vitro culture of the bioprinted neo-tissue. In vivo studies of 3D bioprinted constructs in otolaryngologic surgery, to date, have not taken advantage of bioreactor systems prior to implantation. Similarly, the field has yet to take advantage of bioactive bioinks. Additionally, long-term data to support sufficient stability of the biofabricated tissue in vivo will be necessary prior to clinical translation [132]. This will require surgeon input and use of autologous cell constructs in appropriate animal models to investigate graft-vs-host compatibility without the need for immunosuppression. Ultimate translation of bioengineered tissue into the clinic will require FDA approval following the necessary testing and sterilization validation protocols. At the time of this review, more than 100 clinical trials of clinical applications of 3D printing were registered as in progress or recruiting in ClinicalTrials.gov, although no clinical trials of 3D bioprinting for tissue regeneration were registered. As of February 2023, only one cellular and gene therapy product is approved for musculoskeletal repair, the matrix-induced autologous chondrocyte implantation (MACI), which is approved for full thickness cartilage defects of the adult knee [141]. To date, no FDA approved implants using 3D bioprinting technology have been approved and no clinical trials investigating the use of 3D bioprinted tissue have been investigated.
9. Conclusion
3D bioprinting represents a new advanced technology within the realm of additive biomanufacturing, allowing the fabrication of on-demand, patient-specific structures for regeneration of tissues relevant to the field of otolaryngology. Despite the promise of 3D bioprinting to revolutionize tissue engineering approaches, continued development of biomaterials and printing techniques will be critical to the advancement and accessibility of the technology. Further investigation is needed to elucidate the specific microenvironmental factors including bioink properties, biochemical cues, and bioreactor systems that will best recapitulate native development to create large-scale tissue constructs of optimal mechanical integrity. Moreover, to date, reports often investigate heterotopic implantation of bioprinted tissue in small series animal models with limited data regarding long-term outcomes. The most promising systems for otolaryngologic application demonstrate in vivo implantation at orthotopic sites to better evaluate clinical applicability. However, no clinical trials have investigated 3D bioprinting approaches, leaving a gap in the research landscape. The partnering of tissue engineers with surgeon input will be necessary to move the field forward. Future endeavors to advance the utilization of 3D bioprinting clinically will require FDA-approved testing and sterilization validation protocols, which will remain costly to develop. Ultimately, the development of multidisciplinary healthcare teams will be critical to translating bioprinted structures to clinical practice.
Biographies

Dr. Alexandra McMillan, MD PhD, is a current NIH T32 research resident in the Department of Otolaryngology at the University of Iowa. After earning her B.S. in Molecular and Cellular Biology at Johns Hopkins University, she joined the MSTP at Case Western Reserve University where she completed her combined medical and research training. Her PhD involved development of novel strategies to deliver gene nanoparticles for tissue engineering applications. With primary mentorship from Prof. Aliasger Salem, PhD, her current research aims to engineer innovative 3D bioprinted cartilage scaffolds for airway reconstruction, with the overarching goal to apply tissue engineering techniques to improve clinical outcomes.

Prof. Aliasger Salem, PhD, is the Associate Vice President for Research for the University of Iowa and Bighley Chair and Professor of Pharmaceutical Sciences at the University of Iowa College of Pharmacy. He received his Ph.D. in pharmacy at the University of Nottingham, UK in 2002. He then received postdoctoral training at Johns Hopkins School of Medicine until 2004. His research interests include 3D bioprinting and development of scaffolds for tissue engineering.
Footnotes
There are no conflicts of interest to disclose.
References
- [1].Liaw C-Y, Guvendiren M, Biofabrication 2017, 9, 024102. [DOI] [PubMed] [Google Scholar]
- [2].Abdullah KA, Reed W, J Med Radiat Sci 2018, 65, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vaz VM, Kumar L, AAPS PharmSciTech 2021, 22, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McMillan A, Kocharyan A, Dekker SE, Kikano EG, Garg A, Huang VW, Moon N, Cooke M, Mowry SE, Ann Otol Rhinol Laryngol 2020, 129, 1168. [DOI] [PubMed] [Google Scholar]
- [5].Huang C-Y, Chang T-S, Hwang LA, Lin Y-S, J Chin Med Assoc 2022, DOI 10.1097/JCMA.0000000000000791. [DOI] [PubMed] [Google Scholar]
- [6].Frejo L, Goldstein T, Swami P, Patel NA, Grande DA, Zeltsman D, Smith LP, Int J Pediatr Otorhinolaryngol 2022, 155, 111066. [DOI] [PubMed] [Google Scholar]
- [7].Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV, Otolaryngol Head Neck Surg 2017, 156, 999. [DOI] [PubMed] [Google Scholar]
- [8].Mandrycky C, Wang Z, Kim K, Kim D-H, Biotechnol Adv 2016, 34, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Langer R, Vacanti JP, Science 1993, 260, 920. [DOI] [PubMed] [Google Scholar]
- [10].Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J, N Engl J Med 2001, 344, 1511. [DOI] [PubMed] [Google Scholar]
- [11].Mani MP, Sadia M, Jaganathan SK, Khudzari AZ, Supriyanto E, Saidin S, Ramakrishna S, Ismail AF, Faudzi AAM, Journal of Polymer Engineering 2022, 42, 243. [Google Scholar]
- [12].Nava MM, Draghi L, Giordano C, Pietrabissa R, J Appl Biomater Funct Mater 2016, 14, e223. [DOI] [PubMed] [Google Scholar]
- [13].Karageorgiou V, Kaplan D, Biomaterials 2005, 26, 5474. [DOI] [PubMed] [Google Scholar]
- [14].Saini G, Segaran N, Mayer JL, Saini A, Albadawi H, Oklu R, J Clin Med 2021, 10, 4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT, Biotechnol Adv 2017, 35, 217. [DOI] [PubMed] [Google Scholar]
- [16].Ozbolat IT, Journal of Nanotechnology in Engineering and Medicine 2015, 6, 024701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang X, Zhang Y, Cell Biochem Biophys 2015, 72, 777. [DOI] [PubMed] [Google Scholar]
- [18].Ramadan Q, Zourob M, Front. Med. Technol 2021, 2, 607648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Potyondy T, Uquillas JA, Tebon PJ, Byambaa B, Hasan A, Tavafoghi M, Mary H, Aninwene GE, Pountos I, Khademhosseini A, et al. , Biofabrication 2021, 13, DOI 10.1088/1758-5090/abc8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shafiee A, Atala A, Trends Mol Med 2016, 22, 254. [DOI] [PubMed] [Google Scholar]
- [21].Dwivedi R, Mehrotra D, J Oral Biol Craniofac Res 2020, 10, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang J, Wehrle E, Rubert M, Müller R, Int J Mol Sci 2021, 22, 3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yi T, Huang S, Liu G, Li T, Kang Y, Luo Y, Wu J, ACS Appl. Bio Mater 2018, 1, 193. [DOI] [PubMed] [Google Scholar]
- [24].Bakhtiary N, Liu C, Ghorbani F, Gels 2021, 7, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wei W, Ma Y, Yao X, Zhou W, Wang X, Li C, Lin J, He Q, Leptihn S, Ouyang H, Bioact Mater 2021, 6, 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ngadimin KD, Stokes A, Gentile P, Ferreira AM, Biomater. Sci 2021, 9, 4246. [DOI] [PubMed] [Google Scholar]
- [27].Hutmacher DW, Biomaterials 2000, 21, 2529. [DOI] [PubMed] [Google Scholar]
- [28].Ashammakhi N, Ahadian S, Xu C, Montazerian H, Ko H, Nasiri R, Barros N, Khademhosseini A, Mater Today Bio 2019, 1, 100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gopinathan J, Noh I, Biomater Res 2018, 22, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJA, Groll J, Hutmacher DW, Adv Mater 2013, 25, 5011. [DOI] [PubMed] [Google Scholar]
- [31].Schwab A, Levato R, D’Este M, Piluso S, Eglin D, Malda J, Chem. Rev 2020, 120, 11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chimene D, Lennox KK, Kaunas RR, Gaharwar AK, Ann Biomed Eng 2016, 44, 2090. [DOI] [PubMed] [Google Scholar]
- [33].Murphy SV, Atala A, Nat Biotechnol 2014, 32, 773. [DOI] [PubMed] [Google Scholar]
- [34].Cheng A, Schwartz Z, Kahn A, Li X, Shao Z, Sun M, Ao Y, Boyan BD, Chen H, Tissue Engineering Part B: Reviews 2019, 25, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rico-Llanos GA, Borrego-González S, Moncayo-Donoso M, Becerra J, Visser R, Polymers (Basel) 2021, 13, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS, Int J Biol Macromol 2016, 86, 917. [DOI] [PubMed] [Google Scholar]
- [37].Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, Dehghani F, Tissue Eng Part B Rev 2010, 16, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu J, Marchant RE, Expert Rev Med Devices 2011, 8, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].GhavamiNejad A, Ashammakhi N, Wu XY, Khademhosseini A, Small 2020, 16, e2002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McMillan A, Nguyen MK, Huynh CT, Sarett SM, Ge P, Chetverikova M, Nguyen K, Grosh D, Duvall CL, Alsberg E, Acta Biomater 2021, 124, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nguyen MK, McMillan A, Huynh CT, Schapira DS, Alsberg E, J Mater Chem B 2017, 5, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hoffman AS, Advanced Drug Delivery Reviews 2002, 54, 3. [DOI] [PubMed] [Google Scholar]
- [43].Ruiz-Cantu L, Gleadall A, Faris C, Segal J, Shakesheff K, Yang J, Mater Sci Eng C Mater Biol Appl 2020, 109, 110578. [DOI] [PubMed] [Google Scholar]
- [44].Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, Nat Biotechnol 2016, 34, 312. [DOI] [PubMed] [Google Scholar]
- [45].Gonzalez-Fernandez T, Rathan S, Hobbs C, Pitacco P, Freeman FE, Cunniffe GM, Dunne NJ, McCarthy HO, Nicolosi V, O’Brien FJ, et al. , J Control Release 2019, 301, 13. [DOI] [PubMed] [Google Scholar]
- [46].Freeman FE, Pitacco P, van Dommelen LHA, Nulty J, Browe DC, Shin J-Y, Alsberg E, Kelly DJ, Sci Adv 2020, 6, eabb5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bai X, Gao M, Syed S, Zhuang J, Xu X, Zhang X-Q, Bioactive Materials 2018, 3, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Marion NW, Mao JJ, Methods Enzymol 2006, 420, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yoon JK, Choi J, Lee HJ, Cho Y, Gwon YD, Jang Y, Kim S, Choi H, Lee JH, Kim YB, Transplant Proc 2015, 47, 2067. [DOI] [PubMed] [Google Scholar]
- [50].Ansari S, Ito K, Hofmann S, Curr Osteoporos Rep 2021, 19, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Murphy SV, Atala A, Bioessays 2013, 35, 163. [DOI] [PubMed] [Google Scholar]
- [52].Park JH, Ahn M, Park SH, Kim H, Bae M, Park W, Hollister SJ, Kim SW, Cho D-W, Biomaterials 2021, 279, 121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pan S, Shen Z, Xia T, Pan Z, Dan Y, Li J, Shi H, Am J Transl Res 2022, 14, 2910. [PMC free article] [PubMed] [Google Scholar]
- [54].Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG, Tissue Eng Part B Rev 2014, 20, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee H, Park J, Forget BG, Gaines P, Regen Med 2009, 4, 759. [DOI] [PubMed] [Google Scholar]
- [56].Lou X, Stem Cell Rev Rep 2015, 11, 645. [DOI] [PubMed] [Google Scholar]
- [57].Csobonyeiova M, Polak S, Zamborsky R, Danisovic L, J Adv Res 2017, 8, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Martin MM, Chan M, Antoine C, Bar-El L, Bornstein E, Young BK, Journal of Perinatal Medicine 2022, 0, DOI 10.1515/jpm-2022-0309. [DOI] [PubMed] [Google Scholar]
- [59].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Science 1999, 284, 143. [DOI] [PubMed] [Google Scholar]
- [60].Bruder SP, Jaiswal N, Haynesworth SE, J Cell Biochem 1997, 64, 278. [DOI] [PubMed] [Google Scholar]
- [61].Ullah I, Subbarao RB, Rho GJ, Biosci Rep 2015, 35, e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kim H-J, Shin S, Jeong S-Y, Lim S-U, Lee D-W, Kwon Y-K, Kang J, Kim S-W, Jung C-K, Lee C, et al. , J Clin Med 2021, 10, 1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Queiroz A, Albuquerque-Souza E, Gasparoni LM, de França BN, Pelissari C, Trierveiler M, Holzhausen M, World J Stem Cells 2021, 13, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wright A, Arthaud-Day ML, Weiss ML, Front Cell Dev Biol 2021, 9, 632717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Caplan AI, Stem Cells Transl Med 2017, 6, 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Caplan AI, Correa D, Cell Stem Cell 2011, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Haynesworth SE, Baber MA, Caplan AI, J Cell Physiol 1996, 166, 585. [DOI] [PubMed] [Google Scholar]
- [68].Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM, Blood 2002, 99, 3838. [DOI] [PubMed] [Google Scholar]
- [69].Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L, Blood 2008, 111, 1327. [DOI] [PubMed] [Google Scholar]
- [70].Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR, J Biomed Sci 2005, 12, 47. [DOI] [PubMed] [Google Scholar]
- [71].Lohan P, Treacy O, Griffin MD, Ritter T, Ryan AE, Front Immunol 2017, 8, 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chan BP, Leong KW, Eur Spine J 2008, 17 Suppl 4, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lieberman JR, Daluiski A, Einhorn TA, J Bone Joint Surg Am 2002, 84, 1032. [DOI] [PubMed] [Google Scholar]
- [74].Yun Y-R, Jang JH, Jeon E, Kang W, Lee S, Won J-E, Kim HW, Wall I, Regen Med 2012, 7, 369. [DOI] [PubMed] [Google Scholar]
- [75].Lin S, Li H, Wu B, Shang J, Jiang N, Peng R, Xing B, Xu X, Lu H, Cell Commun Signal 2022, 20, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Solorio LD, Vieregge EL, Dhami CD, Alsberg E, Tissue Eng Part B Rev 2013, 19, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dahlin RL, Ni M, Meretoja VV, Kasper FK, Mikos AG, Biomaterials 2014, 35, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Oliveira ÉR, Nie L, Podstawczyk D, Allahbakhsh A, Ratnayake J, Brasil DL, Shavandi A, Int J Mol Sci 2021, 22, E903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lee S-H, Shin H, Advanced Drug Delivery Reviews 2007, 59, 339. [DOI] [PubMed] [Google Scholar]
- [80].McMillan A, Nguyen MK, Gonzalez-Fernandez T, Ge P, Yu X, Murphy WL, Kelly DJ, Alsberg E, Biomaterials 2018, 161, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nguyen MK, Jeon O, Dang PN, Huynh CT, Varghai D, Riazi H, McMillan A, Herberg S, Alsberg E, Acta Biomater 2018, 75, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Patel S, Athirasala A, Menezes PP, Ashwanikumar N, Zou T, Sahay G, Bertassoni LE, Tissue Eng Part A 2019, 25, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shiwarski DJ, Hudson AR, Tashman JW, Feinberg AW, APL Bioeng 2021, 5, 010904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW, Sci. Adv 2015, 1, e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zheng Z, Eglin D, Alini M, Richards GR, Qin L, Lai Y, Engineering 2021, 7, 966. [Google Scholar]
- [86].Bertassoni LE, Advanced Materials 2022, 34, 2101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kumar H, Kim K, Methods Mol Biol 2020, 2140, 93. [DOI] [PubMed] [Google Scholar]
- [88].Zhang AP, Qu X, Soman P, Hribar KC, Lee JW, Chen S, He S, Adv. Mater 2012, 24, 4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fu L, Li P, Li H, Gao C, Yang Z, Zhao T, Chen W, Liao Z, Peng Y, Cao F, et al. , Stem Cells International 2021, 2021, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Harvestine JN, Gonzalez-Fernandez T, Sebastian A, Hum NR, Genetos DC, Loots GG, Leach JK, Sci. Adv 2020, 6, eaay2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Carpentier B, Layrolle P, Legallais C, Int J Artif Organs 2011, 34, 259. [DOI] [PubMed] [Google Scholar]
- [92].Mabvuure N, Hindocha S, Khan WS, CSCR 2012, 7, 287. [DOI] [PubMed] [Google Scholar]
- [93].Kojima K, Bonassar LJ, Roy AK, Vacanti CA, Cortiella J, The Journal of Thoracic and Cardiovascular Surgery 2002, 123, 1177. [DOI] [PubMed] [Google Scholar]
- [94].Pai AC, Lynch TJ, Ahlers BA, Ievlev V, Engelhardt JF, Parekh KR, Cells 2022, 11, 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dikina AD, Alt DS, Herberg S, McMillan A, Strobel HA, Zheng Z, Cao M, Lai BP, Jeon O, Petsinger VI, et al. , Adv Sci (Weinh) 2018, 5, 1700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Coulombe KLK, Murry CE, in 2014 40th Annual Northeast Bioengineering Conference (NEBEC), IEEE, Boston, MA, USA, 2014, pp. 1–2. [Google Scholar]
- [97].Mowry SE, Jammal H, Myer C, Solares CA, Weinberger P, Otol Neurotol 2015, 36, 1562. [DOI] [PubMed] [Google Scholar]
- [98].Sparks D, Kavanagh KR, Vargas JA, Valdez TA, Int J Pediatr Otorhinolaryngol 2020, 128, 109730. [DOI] [PubMed] [Google Scholar]
- [99].Chee J, Lin X, Lim WS, Loh WS, Thong M, Ng L, Oral Oncol 2021, 113, 105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Canzi P, Magnetto M, Marconi S, Morbini P, Mauramati S, Aprile F, Avato I, Auricchio F, Benazzo M, Acta Otorhinolaryngol Ital 2018, 38, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Suzuki M, Hagiwara A, Kawaguchi S, Ono H, Acta Otolaryngol 2005, 125, 29. [DOI] [PubMed] [Google Scholar]
- [102].Raos P, Klapan I, Galeta T, Coll Antropol 2015, 39, 667. [PubMed] [Google Scholar]
- [103].Numajiri T, Nakamura H, Sowa Y, Nishino K, Plast Reconstr Surg Glob Open 2016, 4, e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE, N Engl J Med 2013, 368, 2043. [DOI] [PubMed] [Google Scholar]
- [105].Les AS, Ohye RG, Filbrun AG, Ghadimi Mahani M, Flanagan CL, Daniels RC, Kidwell KM, Zopf DA, Hollister SJ, Green GE, Laryngoscope 2019, 129, 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Birchall M, ClinicalTrials.gov n.d. [Google Scholar]
- [107].Rwik S, Archives Oral Maxillofac Surg 2020, 3, DOI 10.36959/379/360. [DOI] [Google Scholar]
- [108].Frejo L, Grande DA, Bioelectron Med 2019, 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Baluch N, Nagata S, Park C, Wilkes GH, Reinisch J, Kasrai L, Fisher D, Plast Surg (Oakv) 2014, 22, 39. [PMC free article] [PubMed] [Google Scholar]
- [110].Niermeyer WL, Rodman C, Li MM, Chiang T, Laryngoscope Investig Otolaryngol 2020, 5, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA, Plast Reconstr Surg 1997, 100, 297. [DOI] [PubMed] [Google Scholar]
- [112].Jeon O, Lee YB, Jeong H, Lee SJ, Wells D, Alsberg E, Mater Horiz 2019, 6, 1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lee J-S, Hong JM, Jung JW, Shim J-H, Oh J-H, Cho D-W, Biofabrication 2014, 6, 024103. [DOI] [PubMed] [Google Scholar]
- [114].Park JY, Choi Y-J, Shim J-H, Park JH, Cho D-W, J Biomed Mater Res B Appl Biomater 2017, 105, 1016. [DOI] [PubMed] [Google Scholar]
- [115].Chen Y, Zhang J, Liu X, Wang S, Tao J, Huang Y, Wu W, Li Y, Zhou K, Wei X, et al. , Sci Adv 2020, 6, eaba7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Camnitz PS, Bost WS, Otolaryngol Head Neck Surg 1985, 93, 220. [DOI] [PubMed] [Google Scholar]
- [117].Azimi B, Milazzo M, Danti S, Front Bioeng Biotechnol 2021, 9, 669863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yang Z, Wu X, Chen X, Huang Y, Fang L, Li X, Zhang Y, Jia M, Acta Otolaryngol 2019, 139, 833. [DOI] [PubMed] [Google Scholar]
- [119].Jang CH, Ahn S, Lee JW, Lee BH, Lee H, Kim G, Mater Sci Eng C Mater Biol Appl 2017, 72, 456. [DOI] [PubMed] [Google Scholar]
- [120].Hirsch JD, Vincent RL, Eisenman DJ, 3D Print Med 2017, 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lei IM, Jiang C, Lei CL, de Rijk SR, Tam YC, Swords C, Sutcliffe MPF, Malliaras GG, Bance M, Huang YYS, Nat Commun 2021, 12, 6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chiesa-Estomba CM, Aiastui A, González-Fernández I, Hernáez-Moya R, Rodiño C, Delgado A, Garces JP, Paredes-Puente J, Aldazabal J, Altuna X, et al. , Tissue Eng Regen Med 2021, 18, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lan X, Liang Y, Vyhlidal M, Erkut EJ, Kunze M, Mulet-Sierra A, Osswald M, Ansari K, Seikaly H, Boluk Y, et al. , J Tissue Eng 2022, 13, 20417314221086370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Möller T, Amoroso M, Hägg D, Brantsing C, Rotter N, Apelgren P, Lindahl A, Kölby L, Gatenholm P, Plast Reconstr Surg Glob Open 2017, 5, e1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, Kölby L, PLoS One 2017, 12, e0189428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bae S-W, Lee K-W, Park J-H, Lee J, Jung C-R, Yu J, Kim H-Y, Kim D-H, Int J Mol Sci 2018, 19, E1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lee HJ, Choi BH, Min B-H, Park SR, Arthritis Rheum 2009, 60, 2325. [DOI] [PubMed] [Google Scholar]
- [128].Kim SH, Seo YB, Yeon YK, Lee YJ, Park HS, Sultan MT, Lee JM, Lee JS, Lee OJ, Hong H, et al. , Biomaterials 2020, 260, 120281. [DOI] [PubMed] [Google Scholar]
- [129].Park J-H, Yoon J-K, Lee JB, Shin YM, Lee K-W, Bae S-W, Lee J, Yu J, Jung C-R, Youn Y-N, et al. , Sci Rep 2019, 9, 2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Louvrier A, Marty P, Barrabé A, Euvrard E, Chatelain B, Weber E, Meyer C, J Stomatol Oral Maxillofac Surg 2017, 118, 206. [DOI] [PubMed] [Google Scholar]
- [131].Ma Y, Ji Y, Zhong T, Wan W, Yang Q, Li A, Zhang X, Lin M, ACS Biomater Sci Eng 2017, 3, 3534. [DOI] [PubMed] [Google Scholar]
- [132].Tan B, Gan S, Wang X, Liu W, Li X, J. Mater. Chem. B 2021, 9, 5385. [DOI] [PubMed] [Google Scholar]
- [133].Kang Y, Datta P, Shanmughapriya S, Ozbolat IT, ACS Appl Bio Mater 2020, 3, 5552. [DOI] [PubMed] [Google Scholar]
- [134].Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W, Biofabrication 2011, 3, 034112. [DOI] [PubMed] [Google Scholar]
- [135].Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT, Acta Biomaterialia 2017, 57, 26. [DOI] [PubMed] [Google Scholar]
- [136].Kačarević Ž, Rider P, Alkildani S, Retnasingh S, Smeets R, Jung O, Ivanišević Z, Barbeck M, Materials 2018, 11, 2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Muskan, Gupta D, Negi NP, Bioprinting 2022, 27, e00211. [Google Scholar]
- [138].Vijayavenkataraman S, Yan W-C, Lu WF, Wang C-H, Fuh JYH, Advanced Drug Delivery Reviews 2018, 132, 296. [DOI] [PubMed] [Google Scholar]
- [139].Haider A, Haider S, Rao Kummara M, Kamal T, Alghyamah A-AA, Jan Iftikhar F, Bano B, Khan N, Amjid Afridi M, Soo Han S, et al. , Journal of Saudi Chemical Society 2020, 24, 186. [Google Scholar]
- [140].Camci-Unal G, Cuttica D, Annabi N, Demarchi D, Khademhosseini A, Biomacromolecules 2013, 14, 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nordberg RC, Otarola GA, Wang D, Hu JC, Athanasiou KA, Sci. Transl. Med 2022, 14, eabp8163. [DOI] [PMC free article] [PubMed] [Google Scholar]


