Abstract
In order to reduce the diagnostic window between the time of human immunodeficiency virus (HIV) infection and laboratory diagnosis, new screening enzyme-linked immunosorbent assays (ELISAs) which permit the simultaneous detection of HIV antigen and antibody have been developed. Two fourth-generation assays, HIV DUO (Biomérieux) and HIV Combi (Boehringer Mannheim), for the combined detection of HIV antigen and antibody, were compared with a third-generation assay (HIV-1/HIV-2 3rd Generation Plus enzyme immunoassay [EIA]; Abbott) and a p24 antigen test (HIV-1 Ag monoclonal; Abbott). A total of 17 seroconversion panels, 15 cell culture supernatants infected with different HIV type 1 (HIV-1) subtypes, and 255 potentially cross-reactive serum samples were tested. Ten seroconversions were detected an average of 8.1 days earlier with HIV DUO and 7.5 days earlier with HIV Combi than with the third-generation ELISA. Overall, in the 17 seroconversion panels tested, HIV DUO detected HIV-1 infection an average of 4.8 days and HIV Combi detected infection an average of 4.4 days earlier than HIV-1/HIV-2 3rd Generation Plus EIA. HIV antigen was detected with HIV DUO and HIV Combi in all of the 15 cell culture supernatants infected with different HIV-1 subtypes, including subtype O. With fourth-generation assays, considerably fewer false-positive results (n = 4 to 6) were obtained, in comparison with the third-generation EIA (n = 18). Fourth-generation assays permit an earlier diagnosis of HIV infection than third-generation antibody screening assays through the detection of p24 antigen, which may be present in serum samples from individuals with recent HIV infection prior to seroconversion.
Since their introduction in 1985, the performance of human immunodeficiency virus (HIV) screening assays has continued to improve. The time between infection and antibody detection has been substantially shortened by using third-generation antigen (Ag) sandwich assays (17). The window between the presence of HIV type 1 (HIV-1) RNA in plasma and antibody seroconversion varies between 10.2 and 27.4 days, depending on the route of infection. HIV infection is detected between 9.4 and 17.4 days earlier by p24 Ag testing than with current third-generation assays (14). Additional screening for HIV Ag has not been introduced worldwide in blood banks for reasons of cost-effectiveness (1, 2). Although the prevalence and incidence of HIV infection in the general population in industrialized countries are relatively low, the residual risk of HIV transmission by blood donation (mostly by viremic but antibody-negative donors) is 1/493,000 per unit in the United States (2). By additional screening for p24 Ag, the risk of HIV infection may be reduced to 1/676,000 per unit.
Recently, fourth-generation assays, which permit the simultaneous detection of HIV Ag and antibody, have been developed, and the first of these are already available in Europe. Since the list price of these new tests will be similar to that of the third-generation assays, the cost per unit of blood should not increase. Provided that fourth-generation screening tests are of sensitivity comparable to that of traditional p24 Ag and HIV antibody assays, they would represent a major step towards improving the safety of donated blood.
In the present study, the sensitivity and specificity of two automated fourth-generation HIV screening tests are compared with those of a third-generation antibody assay (HIV-1/HIV-2 3rd Generation Plus enzyme immunoassay [EIA]; Abbott, Delkenheim, Germany) and with those of a p24 Ag detection assay (HIV-1 Ag monoclonal; Abbott).
MATERIALS AND METHODS
Enzymun-Test HIV Combi.
Enzymun-Test HIV Combi is an enzyme-linked immunosorbent assay for the simultaneous detection of HIV Ag and immunoglobulin G (IgG) and IgM antibodies to HIV-1 (including subtype O) and HIV-2. In the first reaction step, the patient’s sample is incubated in the presence of biotinylated and digoxenin-labelled HIV Ags (synthetic peptides gp41 and gp36 and recombinant reverse transcriptase [RT]) and biotinylated and digoxenin-labelled monoclonal anti-p24 antibody. After a first washing step, orthophenylenediamine-conjugated antidigoxenin antibody is added. After a second and final washing procedure, HIV Ag and/or antibody is detected by the addition of diammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) substrate. The minimum volume of sample required is 400 ml, and the total test time is 4 h. All of the assay steps are performed automatically by the Enzymun system (ES) 300 or ES 600/700. At the end of the assay, results are automatically calculated by the ES in relation to the cutoff (0.14 × extinction of positive calibrator + 1.0 × extinction of the negative calibrator). Samples with an index value (extinction of the sample divided by the cutoff value) of ≥1 are considered to be positive.
VIDAS HIV DUO.
VIDAS HIV DUO is an enzyme-linked fluorescent assay which permits the simultaneous detection of p24 Ag and IgG antibodies against HIV-1 (including subtype O) and HIV-2. The assay comprises two reactions. The first, for the detection of anti-HIV-1 and anti-HIV-2 IgG, is performed in the lower part of the solid-phase receptacle (SPR), which is coated with synthetic peptides (gp41 and gp36). Anti-human IgG labelled with alkaline phosphatase is used as the conjugate. The second reaction, for the detection of p24 Ag, is performed in the upper part of the SPR, which is coated with monoclonal anti-p24 antibodies. During incubation, p24 Ag is released through virus lysis and binds to the monoclonal antibodies on the SPR and also to the biotinylated anti-p24 antibodies. The antibody-Ag-antibody complex binds to the alkaline phosphatase-labelled streptavidin. The final detection step is the same for both reactions. The substrate (4-methylumbelliferyl phosphate) is catalyzed by the conjugate into a fluorescent product (4-methylumbelliferone). A sample volume of 200 ml is required, and the total test time is 100 min. All of the assay steps are performed automatically by the VIDAS instrument. At the end of the assay, results are automatically calculated by VIDAS in relation to a standard and printed. The test value is calculated by dividing the patient reference value by the reference value of the standard. A test value of ≥0.35 is considered to be positive. Values between 0.25 and 0.35 are borderline.
Comparative assays.
A third-generation assay (HIV-1/HIV-2 3rd Generation Plus EIA; Abbott), based on double-Ag sandwich EIA technology, was used for HIV antibody detection. The HIV-1 Ag monoclonal assay (Abbott) was used to detect p24 Ag. All the tests were performed and interpreted in accordance with the manufacturers’ recommendations.
Specimens.
The following specimens were tested to evaluate sensitivity. (i) Seventeen seroconversion panels were provided by different suppliers, including Boston Biomedica Inc. (BBI, West Bridgewater, Mass.; panels H, N, W, Y, Z, AC, AD, AE, AF, AG, AI, and AK), North American Biologicals Inc. (NABI; Boca Raton, Fla.; panels SV-0271-1, SV-0331, SV-0351, and SV-0361), and Laboratories Réunis Kutter-Lieners-Hastert (panel J). For seroconversion panels BBI Y, Z, AC, AD, AE, AF, AG, AK, and AI and NABI SV-271-1, -0331, -0351, and -0361, HIV-1 RNA detection was performed by BBI and NABI by quantitative PCR (Amplicor HIV-1 Monitor; Roche Diagnostics, Branchburg, N.J.) or qualitative transcription-mediated amplification (Gen-Probe, San Diego, Calif.). In addition, all of the seroconversion samples were tested for HIV-1 antibodies by Western blotting (Ortho/Cambridge; Cambridge Biotech, Worcester, Mass., or Dupont, Wilmington, Del.). The Western blot was interpreted according to Centers for Disease Control and Prevention (CDC) criteria (5). (ii) Five anti-HIV-1 subtype B IgM-positive samples from patients with recent HIV infection were used. Genotyping was performed by amplification and direct sequencing of the HIV-1 group-specific Ag (gag) p17 gene as described previously (10). The specific IgM against HIV-1 gp41 of the five samples was demonstrated by μ-capture EIA (8). (iii) One serum sample each from individuals infected with HIV-1 subtypes A, B, C, D, and E was used. These samples were serotyped by competitive EIA with HIV-1 subtype A- to E-specific gp120 V3 peptides as previously described (11). (iv) Dilutions of cell culture supernatants infected with different HIV-1 subtypes, including subtypes A, C, D, E, F, G, H, and O and mixed HIV-1 genotypes (National Institutes of Health, Bethesda, Md., and Hôpital Bichat, Paris, France), were tested in order to investigate the influence of the genetic variability of HIV on Ag detection. Virus isolates had been genotyped by sequencing of PCR-amplified fragments of the V3 genome region (13). All the supernatants were diluted in HIV-negative serum.
Two hundred fifty-five potentially cross-reacting samples were tested to challenge the specificity of the assays. These included samples from pregnant women and patients suffering from autoimmune diseases; samples which were positive for IgM antibodies against cytomegalovirus, herpes simplex virus, rubella virus, or Toxoplasma gondii; and samples positive for rheumatoid factor, hepatitis C virus antibody, or Epstein-Barr virus capsid Ag antibody, as well as sera that were HIV EIA reactive on tests different from those evaluated in the present study but Western blot negative, i.e., unconfirmed.
Statistical analysis.
The performance of VIDAS HIV DUO and Enzymun-Test HIV Combi was compared with that of HIV-1/HIV-2 3rd Generation Plus EIA for the seroconversion panels. The mean number of days by which the diagnostic window was reduced in comparison with the third-generation assay was determined for each fourth-generation test. The statistical significance of the reduction for each test was determined by the Wilcoxon test for matched pairs (4).
For the calculation of sensitivity and specificity, samples were considered to be HIV-1 positive if any of the following tests were positive: Western blot (interpreted according to CDC criteria [5]), HIV-1 p24 Ag assay, and HIV-1 RNA assay. Patients were considered HIV negative if all three screening assays (Enzymun-Test HIV Combi, VIDAS HIV DUO, and HIV-1/HIV-2 3rd Generation Plus EIA) were negative, or, in the case of the EIA-reactive samples, if they were negative or indeterminate on Western blots.
RESULTS
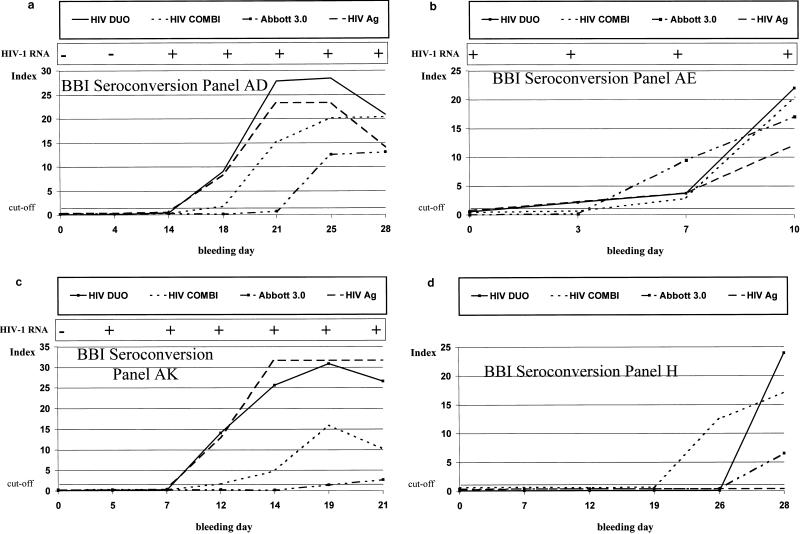
The results obtained for the 17 seroconversion panels are summarized in Table 1. Ten of the 17 seroconversions were detected between 2 and 20 days earlier with fourth-generation assays than with the HIV-1/HIV-2 3rd Generation Plus EIA. For these 10, the average reductions were 8.1 days for HIV DUO and 7.5 days for HIV Combi. Overall, in the 17 seroconversion panels tested, HIV DUO detected HIV-1 infection an average of 4.8 days and HIV Combi detected infection an average of 4.4 days earlier than HIV-1/HIV-2 3rd Generation Plus EIA. The performance of both fourth-generation assays was significantly better (P < 0.05) than that of the HIV-1/HIV-2 3rd Generation Plus EIA. There was no statistical difference in sensitivity between HIV DUO and HIV Combi. HIV Ag and antibody kinetics measured in four seroconversion panels are shown in Fig. 1. In those panels where HIV p24 Ag was present, VIDAS HIV DUO and HIV-1 Ag monoclonal antibody gave identical results. In three panels (BBI Z and AE and NABI SV-0331), HIV Combi detected p24 Ag between 2 and 4 days later than HIV DUO or HIV-1 Ag monoclonal. In one sample without Ag (panel H), HIV-1 seroconversion was detected 2 days earlier with HIV Combi than with HIV DUO. In 8 of 11 seroconversion panels where RT-PCR data was available from the suppliers (BBI and NABI), HIV-1 RNA was detected an average of 6.8 days (range, 3 to 13 days) earlier than was p24 Ag with HIV DUO or HIV-1 Ag monoclonal.
TABLE 1.
Comparison of the performance of HIV DUO, HIV Combi, HIV-1/HIV-2 3rd Generation Plus EIA, p24 Ag detection, and HIV-1 RNA RT-PCR in seroconversion panels
| Seroconversion panel | Bleeding day with first positive result in:
|
|||||
|---|---|---|---|---|---|---|
| HIV DUO | HIV Combi | HIV-1/HIV-2 3rd Generation Plus EIA | p24 Ag | RT-PCRa | Western blotb | |
| BBI H | 28 | 26 | 28 | Neg.c | NDe | 28 |
| BBI N | 0 | 0 | 0 | Neg.c | NDe | 2 |
| BBI W | 47 | 47 | 47 | 47 | NDe | 84 |
| BBI Y | 44 | 44 | 44 | 44 | 44 | Ind.d |
| BBI Z | 7 | 9 | 27 | 7 | 2 | 27 |
| BBI AC | 111 | 111 | 111 | 111 | 111 | 120 |
| BBI AD | 18 | 18 | 25 | 18 | 14 | Ind.d |
| BBI AE | 3 | 7 | 7 | 3 | 0 | Ind.d |
| BBI AF | 28 | 28 | 28 | 28 | 15 | 33 |
| BBI AG | 27 | 27 | 27 | 27 | 27 | 34 |
| BBI AK | 12 | 12 | 19 | 12 | 5 | Ind.d |
| BBI AI | 0 | 0 | 7 | 0 | 0 | 11 |
| NABI SV-0271-1 | 8 | 8 | 13 | 8 | NDe | 20 |
| NABI SV-0331 | 13 | 15 | 20 | 13 | 6 | Ind.d |
| NABI SV-0351 | 8 | 8 | 11 | 8 | 1 | 15 |
| NABI SV-0361 | 9 | 9 | 16 | 9 | 1 | 16 |
| J | 0 | 0 | 14 | 0 | NDe | 14 |
Data for HIV-1 RNA RT-PCR is from BBI and NABI.
Western blot results were interpreted according to CDC criteria.
Neg., no p24 antigenemia detected.
Western blotting was indeterminate (Ind.) at the last bleeding.
ND, not determined.
FIG. 1.
HIV Ag and antibody kinetics in four seroconversion panels: BBI AD (a), BBI AE (b), BBI AK (c), and BBI H (d).
All five HIV-1 IgM-positive samples, and all five samples from the five patients infected with different HIV-1 subtypes, were positive with both fourth-generation assays and with the HIV-1/HIV-2 3rd Generation Plus EIA (data not shown).
HIV-1 Ag was detected by both fourth-generation assays in all of the cell culture supernatants infected with different HIV-1 subtypes (Table 2). In serial dilutions of HIV-infected cell culture supernatants, HIV DUO detected HIV Ag at a twofold-higher dilution than HIV Combi in 8 of 15 samples tested.
TABLE 2.
End point titration of HIV-infected cell culture supernatants
| Culture supernatant | Highest reciprocal dilution with a positive result in:
|
|||
|---|---|---|---|---|
| HIV DUO | HIV Combi | HIV-1 Ag monoclonal | Calculated p24 Ag concn (pg/ml) | |
| HIV-1 subtype C | 5,120 | 640 | 5,120 | 97,000 |
| HIV-1 subtype E | 5,120 | 1,280 | 1,280 | 138,000 |
| HIV-1 subtype F | 2,560 | 640 | 5,120 | 176,000 |
| HIV-1 subtype G | 10,240 | 2,560 | 10,240 | 330,000 |
| HIV-1 subtype H | 2,560 | 640 | 5,120 | 147,000 |
| HIV-1 subtype A/A (92UG029) | 1,000 | <500 | 2,000 | 60,000 |
| HIV-1 subtype A/D (92UG035) | 1,000 | 500 | 2,000 | 23,000 |
| HIV-1 subtype A/D (92UG021) | 500 | 500 | 500 | 23,000 |
| HIV-1 subtype C/A (92RW009) | 500 | 500 | 500 | 21,500 |
| HIV-1 subtype E/A (92UG029) | 600 | 150 | 300 | 2,400 |
| HIV-1 subtype O (FAN) | 2,000 | 1,000 | 2,000 | 22,000 |
| HIV-1 subtype O (POC) | 4,000 | 1,000 | 2,000 | 65,000 |
| HIV-1 subtype O (LOB) | 4,000 | 1,000 | 4,000 | 43,000 |
| HIV-2 (MS/U937) | 50 | <50 | <50 | NDa |
| HIV-2 (ABI NIH-Z) | 2,000 | 8,000 | <500 | NDa |
ND, not determined.
The potentially cross-reacting samples showed four (1.6%) false-positive results with HIV DUO and six (2.4%) with HIV Combi. Three of the six HIV Combi false-positive results were with sera from hepatitis C virus-infected individuals. Higher numbers of false positives (n = 18 [7.1%]) were obtained with HIV-1/HIV-2 3rd Generation Plus EIA; 12 of these were in samples also reactive in an enzyme-linked immunosorbent assay from a manufacturer other than Abbott.
The calculations for sensitivity, specificity, and positive and negative predictive values are shown in Table 3. Since a relatively small number of positive samples were tested, and early seroconversion samples that were HIV-1 RNA positive were considered to be true positives, the values for sensitivity for the different screening EIAs ranged between 70.0 and 91.4%. Both fourth-generation assays were significantly more sensitive than HIV-1/HIV-2 3rd Generation Plus EIA. The specificity of HIV DUO was significantly better than that of HIV-1/HIV-2 3rd Generation Plus EIA, whereas there were no statistically significant differences in specificity between HIV Combi and HIV DUO and between HIV Combi and HIV-1/HIV-2 3rd Generation Plus EIA.
TABLE 3.
Sensitivities, specificities, and predictive values of three HIV screening assaysa
| Test | Result type | No. of samples
|
Sensitivity (95% CI) | Specificity (95% CI) | NPV (%) | PPV (%) | |
|---|---|---|---|---|---|---|---|
| HIV-1 positive (n = 93) | HIV-1 negative (n = 290) | ||||||
| HIV DUO | Positive | 85 | 5b | 91.4 (83.8–96.2) | 98.3 (96.0–99.4) | 97.3 | 94.4 |
| Negative | 8c | 285 | |||||
| Enzymun-Test HIV Combi | Positive | 82 | 8b | 88.2 (79.8–94.0) | 97.2 (94.6–98.8) | 96.2 | 91.1 |
| Negative | 11c | 282 | |||||
| HIV-1/HIV-2 3rd Generation Plus EIA | Positive | 49d | 19b | 70.0 (57.9–80.4) | 93.4 (90.0–96.0) | 92.8 | 72.1 |
| Negative | 21c | 271 | |||||
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Including seroconversion samples (BBI H and N) that were reactive in the screening EIAs but p24 and Western blot negative (n = 1 for HIV DUO and HIV-1/HIV-2 3rd Generation Plus EIA and n = 2 for Enzymun-Test HIV Combi).
Including early seroconversion samples (n = 8) that were positive only by RT-PCR.
Dilutions of virus lysates were not tested with HIV-1/HIV-2 3rd Generation Plus EIA.
DISCUSSION
Although the residual risk of HIV transmission by blood and blood products is very small (1, 7), a report from Couroucé et al. (6) indicated that the safety of donated blood could be improved by the combined use of a third-generation anti-HIV screening assay and the detection of p24 Ag. The results of our study demonstrate that fourth-generation assays permit an earlier diagnosis of HIV infection than third-generation double-Ag sandwich assays, by detecting p24 Ag which may be present in samples from individuals with recent HIV infection prior to seroconversion. In these cases, the diagnostic window may be reduced by an average of 9 days. Overall, in 17 seroconversion panels tested, HIV DUO and HIV Combi detected HIV-1 infection an average of 4.8 and 4.4 days earlier than the HIV-1/HIV-2 3rd Generation Plus EIA. Since the list price of fourth-generation assays is in the same range as that of third-generation anti-HIV screening assays, the safety of blood donor screening may be improved, without additional cost, provided that the specificity of fourth-generation EIAs is equivalent to that of third-generation assays.
In 8 of 11 seroconversion panels, the diagnostic window would be reduced by a further week by amplification of HIV-1 RNA by RT-PCR from plasma or serum. Cost-benefit analysis of expanded HIV testing protocols for donated blood has shown that RNA PCR testing would prevent eight more cases of transfusion-associated HIV infection annually than combined p24 Ag and antibody detection, at an additional cost of $96 million per year in the United States (2). Current commercially available PCR protocols are not adapted to large-scale screening of blood donations, and false-negative reactions have been reported for patients with low HIV-1 RNA or cDNA copy number, irrespective of the HIV-1 subtype (3).
Fourth-generation assays are more sensitive than any other test, including RT-PCR, since HIV-positive patients with a low viral load will have no detectable HIV-1 RNA, even if ultrasensitive PCR technology is used (unpublished data).
The genetic variability of HIV, particularly HIV-1, may represent a major challenge for Ag detection during primary HIV infection with fourth-generation assays. Tersmette et al. (15) have reported the failure of monoclonal antibody to detect p24 Ag from certain strains of HIV. HIV-1 subtype O (9), which is highly divergent from other HIV-1 subtypes known so far, may not be detected by assays using monoclonal antibodies for the capture of p24 Ag. Our results from dilutions of cell supernatants infected with different HIV-1 subtypes, including three subtype O isolates, show detection of p24 Ag at high dilutions by HIV DUO and HIV Combi, both of which use monoclonal antibodies for Ag capture.
Since fourth-generation EIAs combine two different test principles in one assay, the potential for nonspecific reactivity might be expected to be higher than that with third-generation antibody assays. The results obtained with potentially cross-reacting serum samples demonstrate that the prevalence of false-positive results is not increased with these new assays. In contrast, HIV-1/HIV-2 3rd Generation Plus EIA was less specific, especially with serum samples that were reactive in another third-generation EIA. Previous studies have shown that the HIV-1/HIV-2 3rd Generation Plus EIA gave a high frequency of false-positive results with potentially cross-reactive serum samples, particularly during pregnancy and in primary infection with cytomegalovirus and Epstein-Barr virus (16). Approximately 0.3% of blood donors who report no high-risk behavior for HIV infection at the time of donation have a repeatedly reactive EIA, but the majority are negative by Western blot analysis. Occasionally, repeated EIA reactivity with a negative or indeterminate Western blot may be observed during seroconversion (12). A second specimen drawn 1 to 2 weeks later usually resolves the indeterminate status of the patient.
In summary, the two fourth-generation HIV EIAs evaluated in this study offer the best sensitivities of any single tests, combined with still-high specificity, and will thus help to further improve the safety of the blood supply.
ACKNOWLEDGMENTS
We thank Walther Melchior (Boehringer Mannheim, Penzberg, Germany) and Nathalie Philippe (Biomérieux, Marcy l’Etoile, France) for providing test kits and financial support for purchasing commercially available seroconversion panels.
Footnotes
In memory of Nathalie Philippe, Marcy l’Etoile, France, 1961–1998.
REFERENCES
- 1.Alter H J, Epstein J S, Swenson S G, Van Raden M J, Ward J W, Kaslow R A, Menitove J E, Klein H G, Sandler S G, Sayers M H, Hewlett I K, Chernoff A I The HIV-Antigen Study Group. Prevalence of human immunodeficiency virus type 1 p24 antigen in U.S. blood donors—an assessment of the efficacy of testing in donor screening. N Engl J Med. 1990;323:1312–1317. doi: 10.1056/NEJM199011083231905. [DOI] [PubMed] [Google Scholar]
- 2.Aubuchon J P, Birkmeyer J D, Busch M P. Cost-effectiveness of expanded human immunodeficiency virus-testing protocols for donated blood. Transfusion. 1997;45:45–51. doi: 10.1046/j.1537-2995.1997.37197176950.x. [DOI] [PubMed] [Google Scholar]
- 3.Barlow K L, Tosswill J H C, Parry J V, Clewley J P. Performance of the Amplicor human immunodeficiency virus type 1 PCR and analysis of specimens with false-negative results. J Clin Microbiol. 1997;35:2846–2853. doi: 10.1128/jcm.35.11.2846-2853.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bünung H, Trenkler G. Nicht-parametrierbare Methoden. Berlin, Germany: Springer Verlag; 1978. [Google Scholar]
- 5.Centers for Disease Control. 1989. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. Morbid. Mortal. Weekly Rep. 38(Suppl. S-7):1–7.
- 6.Couroucé A M, Barin F, Maniez M, Janot C, Noel L, Elghouzzi M H the other members of the Retrovirus Study Group of the French Society of Blood Transfusion. Effectiveness of assays for antibodies to HIV and p24 antigen to detect very recent HIV infection in blood donors. AIDS. 1992;6:1548–1550. doi: 10.1097/00002030-199212000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Cumming P D, Wallace E L, Schorr J B, Dodd R Y. Exposure of patients to human immunodeficiency virus through the transfusion of blood components that test antibody-negative. N Engl J Med. 1989;321:941–946. doi: 10.1056/NEJM198910053211405. [DOI] [PubMed] [Google Scholar]
- 8.Faatz E, Schmitt U, Babiel R. Vergleichende Bewertung von 2 Anti-HIV 1+2 3. Gen. EIAS mit einem Anti-HIV-IgM Capture EIA in der Serokonversionsphase. 5. Deutscher AIDS Kongress, Hannover, Germany. AIDS-Forsch. 1994;9:624. [Google Scholar]
- 9.Gürtler L G, Hauser P H, Eberle J, von Brunn A, Knapp S, Zekeng L, Tsague J, Kaptue L. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J Virol. 1994;68:1581–1585. doi: 10.1128/jvi.68.3.1581-1585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasper P, Chalwatzis N, Duraisamy C, Ofenloch-Hähnle B, Faatz E. HIV-1 gag subtype A is predominant in Malaysian intravenous drug users. AIDS Res Hum Retroviruses. 1997;13:1251–1253. doi: 10.1089/aid.1997.13.1251. [DOI] [PubMed] [Google Scholar]
- 11.Kasper P, Smith A N, Duraisamy G, Ofenloch B, Faatz E. Infektionsepidemiologische Forschung. 1996. Korrelation zwischen HIV-1-Serotypisierung und-Genotypisierung: Möglichkeiten und Grenzen. 6. Deutscher AIDS-Kongreß, Munich, Germany, October 1996, abstr. V089; p. 32. [Google Scholar]
- 12.Kleinman S, Fitzpatrick L, Secord K, Wilke D. Follow-up testing and notification of anti-HIV Western blot atypical (indeterminant) donors. Transfusion. 1988;28:280–282. doi: 10.1046/j.1537-2995.1988.28388219161.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy E E, Korber B, Georges-Courbot M C, You B, Pinter A, Cook D, Kieny M P, Georges A, Mathiot C, Barré-Sinoussi F, Girard M. Diversity of V3 region sequences of human immunodeficiency viruses type 1 from the Central African Republic. AIDS Res Hum Retroviruses. 1993;9:997–1006. doi: 10.1089/aid.1993.9.997. [DOI] [PubMed] [Google Scholar]
- 14.Satten G A, Busch M P. 4th Conference on Retroviruses and Opportunistic Infections, Washington, D.C. 1997. Effect of transmission route on window period estimates, abstr. 122. [Google Scholar]
- 15.Tersmette M, Winkel I, Groenick M, Gruters R A, Spence R P, Saman E, van der Groen G, Miedema F, Huisman J G. Detection and subtyping of HIV-1 isolates with a panel of characterized monoclonal antibodies to HIV p24 gag. Virology. 1989;171:149–155. doi: 10.1016/0042-6822(89)90521-7. [DOI] [PubMed] [Google Scholar]
- 16.Weber B, Moshtaghi-Boronjeni M, Brunner M, Preiser W, Breiner M, Doerr H W. Evaluation of the reliability of 6 current anti-HIV-1/HIV-2 enzyme immunoassays. J Virol Methods. 1995;55:97–104. doi: 10.1016/0166-0934(95)00048-y. [DOI] [PubMed] [Google Scholar]
- 17.Zaaijer H L, Exel-Oehlers P V, Kraaijeveld T, Altena E, Lelie P N. Early detection of antibodies to HIV-1 by third-generation assays. Lancet. 1992;340:770–772. doi: 10.1016/0140-6736(92)92303-w. [DOI] [PubMed] [Google Scholar]