Abstract
We aimed to investigate the association between the presence of cutaneous urease‐producing bacteria and the development of incontinence‐associated dermatitis (IAD) using an original urea agar medium as a step toward developing advanced preventive measures. In previous clinical assessments, we developed an original urea agar medium to detect urease‐producing bacteria via the medium's colour changes. In a cross‐sectional study, specimens were collected via the swabbing technique at genital skin sites in 52 stroke patients hospitalised in a university hospital. The primary objective was to compare the presence of urease‐producing bacteria between the IAD and no‐IAD groups. Determining the bacterial count was the secondary objective. The prevalence of IAD was 48%. A significantly higher detection rate of urease‐producing bacteria was observed in the IAD group than in the no‐IAD group (P = .002) despite the total number of bacteria being equivalent between them. In conclusion, we discovered that there was a significant association between the presence of urease‐producing bacteria and IAD development in hospitalised stroke patients.
Keywords: bacterial flora, geriatric nursing, pH, prevalence, stroke
1. INTRODUCTION
Patients with urinary and faecal incontinence often develop incontinence‐associated dermatitis (IAD), which can predispose them to pressure injury if left untreated. 1 There is emerging evidence that IAD is highly prevalent, especially in geriatric care. 2 IAD is triggered by skin maceration because of urine and faeces, resulting in a compromised skin barrier, exposure to irritants (including bacteria), persistent inflammation, and tissue damage to the affected area (vulva, perineum, perianal region, and buttocks). 3 , 4 The prevalence of IAD in healthcare facilities varies in various care settings. In an Australian acute care hospital, the prevalence of IAD was 10% in the entire patient population and 42% in incontinent patients. 5 A survey reported the IAD prevalence among incontinent patients ranged from 8.4% in long‐term care facilities to 19% in acute care facilities. 6 IAD prevalence values as high as 9.4% and 5.9% were reported in a Brazilian teaching hospital and a Japanese long‐term medical facility, respectively. 7 , 8 Patients with IAD experience considerable discomfort with pain, burning, and itching in the affected areas, which all took a toll on their independence and quality of life. 4 , 9
The epidermis generates protective and defensive functions mediated by the stratum corneum, the end product of the differentiation of stem cells residing in the epidermis. 10 The most critical of these functions is the permeability barrier. The stratum corneum is a multilayered tissue composed of flattened, anucleate corneocytes surrounded by multiple planar lamellae sheets, enriched in ceramides, cholesterol, and free fatty acids. The localization of these highly hydrophobic lipids within the extracellular domains of the stratum corneum inhibits the outward movement of water. 11 Healthy skin provides a protective barrier via lipids and corneocytes. The mechanisms by which incontinence‐associated dermatitis disrupts the barrier are attributed to several factors, with the major of them being changes in pH and moisture levels. 12
The skin microbiota is kept within homeostatic balance when the pH of this organ remains in the acidic range. 4 , 5 , 6 The acidic range is an important aspect of the skin barrier function. When the skin pH moves into the alkaline range (pH > 7), the relative concentrations of pathogenic bacteria increase. The increase in skin pH is typically observed during incontinence in patients wearing diapers because of the regular contact they make with urine and faeces. This leads to reductions in the protective resident and damage to the skin barrier function. 13 Tissue‐engineered models of human skin infected with Staphylococcus aureus or Pseudomonas aeruginosa revealed heterogeneous pH microenvironments with the skin pH varying from 5 to 9. 14 Alkaline stress inhibited the growth of Staphylococcus epidermidis, a dominant bacterial strain of the human skin microbiota. 15 Larner et al. reported that the repeated application of alkaline urine on the arms of normal volunteers resulted in skin irritation. 16 On the other hand, Koudounas et al. reported that the effect of the 2‐hour application of synthetic urine with various pH values to healthy subjects' forearms on trans‐epidermal water loss, stratum corneum hydration, and skin surface pH was temporary, and these parameters were restored to baseline values within 5 minutes. 17 These results indicate that the pathophysiology of IAD is fragmentary and not fully understood, and it is still unclear how changes in skin pH due to exposure to urine and stool are associated with the development of IAD.
We focused on the effect of the presence of urease‐producing bacteria among pathogens related to skin injury. Urease is a nickel‐dependent metalloenzyme that catalyses the hydrolysis of urea into ammonia and carbon dioxide. 18 For some bacterial species, urease is an integral part of the bacterial acid response network, since the hydrolysis product, ammonia, is readily protonated into ammonium, during which process protons are consumed, resulting in an increase in pH. 19 Regarding cutaneous microorganisms, Staphylococci, including Staphylococcus aureus, are urease‐producing bacteria. 20
Hypothesizing that the presence of urease‐producing bacteria increases the skin pH and causes persistent inflammation at and around the genital skin site, this study aimed to investigate the association between the presence of cutaneous urease‐producing bacteria and the development of IAD towards its advanced preventive measures.
2. MATERIALS AND METHODS
2.1. Preparation of the urea agar medium
Prior to our assessment, the original urea agar medium was developed to detect urease‐producing bacteria on the skins of patients. The presence of urease‐producing bacteria was determined by observing the medium's colour changes induced by alkalization because of ammonia production. Christensen's urea agar medium has been commercially available to screen Helicobacter pylori infection, which is able to survive in the stomach by releasing urease. 21 However, per the results of our preliminary test, some of the urease production of urease‐producing strains delivered from the skin could not be detected using Christensen's urea agar medium (data not shown); therefore, the original urea agar medium was preferred in this study. A solution consisting of 1 g of yeast extract, 1 g of polypepone, 0.2 g of glucose, 1 g of sodium chloride, 0.4 g of potassium hypophosphite, 0.0024 g phenol red, and 3.0 g of agar, diluted with 200 mL of distilled water was prepared and autoclaved. Separately, 4 g of urea was dissolved in 10 g of distilled water (initial concentration: 6.67 mol/L) and stored for sterilisation in an ultraviolet chamber for 1 hour. The two solutions were mixed and adjusted to a pH of 6.7 ± 0.2 with a 1 M sodium hydroxide aqueous solution. After that, 10 g of the mixed solution was poured into a dish (diameter = 90 mm) and then the urea agar medium was completed. The final concentration of urea was 0.33 mol/L per dish. All chemicals used in preparing the urea agar medium were purchased from FUJIFILM Wako Pure Chemical Corp., Japan.
2.2. Evaluation of the urea agar medium
The urea agar medium was evaluated using urease chemical and standard strains. For the urease chemical, after dissolving 1 mg of purified jack bean urease (FUJIFILM Wako Pure Chemical Corp.) in 2 mL of phosphate‐buffered solution (PBS), the solution was diluted using a two‐fold dilution technique. Fifty microliters of each diluted solution were poured and spread onto the urea agar medium, and the medium was incubated aerobically at 37°C overnight. The final concentration of urease ranged from 0.6 units/mL to 37.5 units/mL per dish. In addition, the optical density at 620 nm was measured using a microplate reader (Multiscan FC, Thermo Fisher Scientific, USA) to quantify the (red) colour intensity of the medium.
For the standard strains, Staphylococcus aureus (ATCC 6538P) and Proteus mirabilis (ATCC 12453) and Escherichia coli (ATCC 25922) were used as positive and negative controls, respectively. 22 , 23 , 24 After being picked out from frozen stock at −80°C, all strains were cultured aerobically at 37°C in trypticase soy agar with 5% sheep blood (Becton Dickinson, USA). The colony of each bacterial strain was suspended in distilled water at a concentration of 0.5 McFarland. After a 100‐fold dilution of the suspension, 50 μL of the diluted solution was spread onto the agar. Each bacterial strain was incubated at 37°C overnight. The pH on the surface of the urea agar plate with standard strains was measured using a pH meter (LAQUA‐22 pH meter, HORIBA Ltd., Kyoto, Japan). PBS was also used as a control without any standard strains.
2.3. Study design
This cross‐sectional study was conducted in a Japanese university hospital from July 2022 to December 2022. This study was approved by the author's university ethics review board (approval number HM21‐222). Written informed consent was obtained from all participants or their legal representatives. All aspects of this research were performed per the principles set forth by the Declaration of Helsinki. All participants' data were kept confidential.
2.4. Patients
Eligible patients were hospitalised stroke patients with urinary incontinence who were using diapers. Patients with pressure injuries on or around the affected areas were excluded from this study.
2.5. Assessment of IAD
Skin assessments to diagnose IAD were carried out by one researcher who is a certified expert nurse in wounds, ostomy, and continence working with floor nurses during regular diaper changes for a day in the morning (around AM 9:00) using a standardised clinical examination in potentially‐affected areas, including the perianal region, intergluteal cleft, upper and lower buttock, genitalia (labia/scrotum), lower abdomen, and groin folds. 25 If IAD was observed, its severity, which consists of three categories (erythema, skin erosion, and ulcers) was determined by the researcher.
2.6. Collection of swabs
Skin bacteria were collected by the swabbing method. For patients with IAD, 1 × 3 cm squares of the area where the IAD was observed were gently swabbed with OptiSwab (SUGIYAMA‐GEN CO., LTD., Tokyo Japan). For patients without IAD, swabs were collected from the perianal or genital skin sites at a size of 1 × 3 cm squares. The collected swabs were immediately immersed in liquid media to maintain bacterial viability during transportation. The head of the immersed swab was then transferred to a 2‐mL aliquot of sterile PBS and vortexed for the urease assay as the primary outcome and the viable bacterial count as the secondary outcome.
2.7. Primary outcome
After the bacterial suspension containing the swabs was prepared, the presence of urease‐producing bacteria was assessed by observing the urea agar medium's colour change as described above. The degree of urease activity was categorised as “strong,” “weak,” or “almost none/none.” The determination of pH was performed to confirm that the pH on the surface of the agar medium increases as the colour of the agar medium changes with bacterial cultivation (including urease‐producing bacteria).
2.8. Secondary outcomes
For bacterial count measurements, the bacterial suspension containing the swabs was diluted via a 10‐fold dilution technique, and 50 μL of each dilution was spread on the trypticase soy agar medium (EIKEN CHEMICAL CO., LTD., Japan). After incubation at 37°C overnight, the viable bacterial count was determined by counting the number of visible bacteria (colony‐forming unit; CFU) per agar plate.
2.9. Demographic data collection
Demographic information on age, sex, type of stroke, clinical history, and functional disability was collected from patients' medical records. With the Japan‐specific long‐term care insurance system, functional disability was ranked from Rank J (requires partial support) to Rank C (remains in bed all the time and requires care for all activities of daily living).
2.10. Data analysis
The prevalence of IAD was calculated by dividing the total number of IAD cases in the sample by the total number of study participants. The patients with or without IAD were divided and assigned to the IAD group and the no‐IAD group, respectively, to compare primary and secondary outcomes between them. For the demographic characteristics, continuous variables were expressed as mean values ± standard deviations (SDs) and categorical variables were expressed as frequencies and percentages. Urease activity, as the primary outcome, was compared using the chi‐square test. As a secondary outcome, the nonparametric Mann‐Whitney U test was used for the number of CFUs. A P‐value of < .05 was considered statistically significant. All statistical analyses were conducted using the Statistical Package for the Social Sciences version 28.0 (IBM Corporation, Armonk, NY, USA).
3. RESULTS
3.1. Preparation and evaluation of urea agar medium
Examples of photos showing positive and negative results of urease activity testing are shown in Figure 1. A colour change from yellow to red indicates the presence of urease‐positive bacteria. If the urea in the broth decomposes and produces ammonia, an alkaline environment is created, and the medium turns red. A change in the colour of the medium was observed with the presence of urease in a concentration‐dependent manner (Figure 1A,B). As a result of the use of standard strains, the medium containing S. aureus and P. mirabilis showed urease positivity. On the contrary, E. coli hardly ever produced a colour change in the medium (Figure 1C). No colour change was represented in the agar medium with PBS. The pH on the surface of the agar medium after bacterial cultivation was 8.9 ± 0.02 with S. aureus, 9.4 ± 0.16 with P. mirabilis, 7.1 ± 0.08 with E. coli, and 6.7 ± 0.03 with PBS.
FIGURE 1.
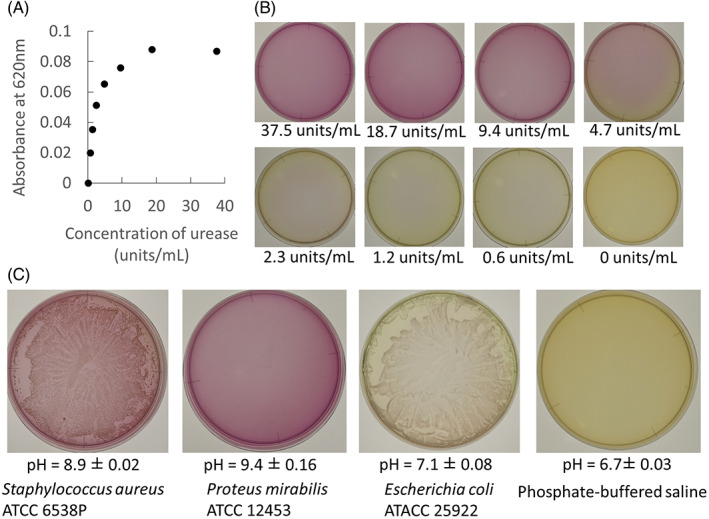
An original urea agar medium was evaluated in vitro. A, The absorbance at 620 nm of the urea agar medium was changed in addition to the urease solution in a concentration‐dependent manner. B, An example of photographs of the urea medium agar in addition to the urease solution in each concentration. C, Example of a photograph of the urea medium after the bacterial suspension was added and incubated aerobically at 37°C. Staphylococcus aureus and Proteus mirabilis and Escherichia coli were used as positive and negative controls, respectively. The pH on each agar plate was measured to confirm that urease‐producing bacteria increase the pH on agar surfaces in the presence of urea.
3.2. Patients' demographics
The demographics of patients in the IAD and no‐IAD groups are shown in Table 1. IAD was present in 48% (25/52) of the samples. Besides a clinical history of diabetes mellitus, there were no significant between‐group differences in participants' demographic characteristics.
TABLE 1.
Characteristics of patients with and without incontinence‐associated dermatitis.
| Characteristics | IAD (+) (n = 25) | IAD (−) (n = 27) | P‐value |
|---|---|---|---|
| Age in year, mean ± SD | 75.9 ± 10.8 | 71.2 ± 15.5 | .17 |
| Sex, male, n (%) | 13 (52.0) | 14 (51.9) | .99 |
| Type of stroke, n (%) | |||
| Cerebral infarction | 12 (48.0) | 9 (33.3) | .28 |
| Subarachnoid haemorrhage | 7 (28.0) | 7 (25.9) | .86 |
| Intracerebral haemorrhage | 6 (24.0) | 11 (40.7) | .2 |
| Clinical history, n (%) | |||
| Diabetes mellitus | 8 (32.0) | 2 (7.4) | .025 |
| Pressure injury | 4 (16.0) | 3 (11.1) | .61 |
| Urinary tract infection | 0 (0) | 0 (0) | N/A |
| Functional disability, n (%) | |||
| Rank B | 14 (56.0) | 15 (55.6) | .97 |
| Rank C | 11 (44.0) | 12 (44.4) | |
Abbreviation: SD, standard deviation.
3.3. Location and severity of IAD
The most common location for IAD formation was the perianal location, followed by the genitalia and the groin region. For the severity of IAD, there were 24 and 9 cases of erythema and skin erosion, respectively. No ulcers were detected in these cases (Table 2).
TABLE 2.
Locations and classifications of incontinence‐associated dermatitis. Thirty‐three cases of IAD were detected in 25 patients, with eight of them having IADs in two locations.
| Location | Erythema | Skin erosion | Total |
|---|---|---|---|
| Perianal skin, n | 16 | 3 | 19 |
| Intergluteal cleft, n | 0 | 0 | 0 |
| Lower buttock, n | 0 | 0 | 0 |
| Genitalia (labia/scrotum), n | 6 | 6 | 12 |
| Groin, n | 2 | 0 | 2 |
| Total, n | 24 | 9 | 33 |
3.4. Collection of swabs
For the IAD group, 33 swabs were collected from 25 patients because 8 out of 25 had IAD at two locations. For the no‐IAD group, 17 swabs were collected from 17 out of 27 patients. The remaining 10 patients missed their appointments for swab collection because of the busy schedules of floor nurses.
3.5. Urease assay
The photos show an example of the urea medium after bacterial cultivation (Figure 2A). The urease‐producing ability of the medium can be divided into three categories according to the degree of change in the colour of the medium, showing that the pH of the agar medium was 9.1 ± 0.11 in the strong, 7.9 ± 0.58 in the weak, and 6.8 ± 0.39 in almost none/none. Figure 2B indicates that the urease‐producing capacity of the IAD group was significantly stronger than that of the no‐IAD group (χ2 = 12.16. P = .002).
FIGURE 2.
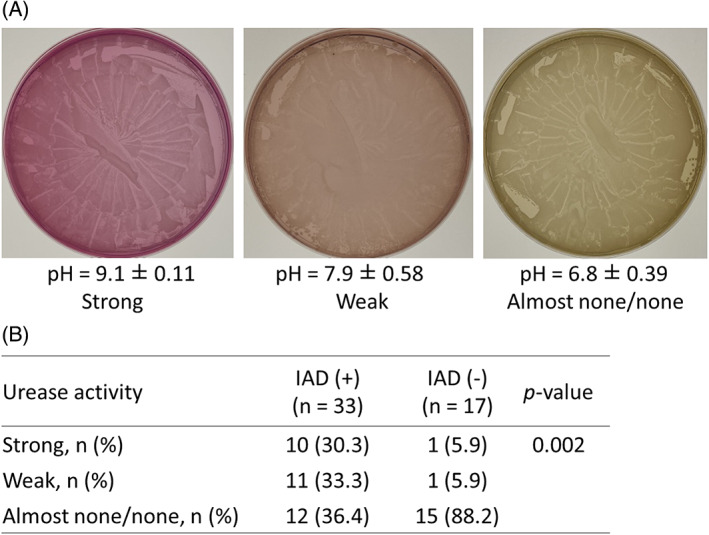
Swabs were collected from genitals and their surrounding skin surfaces in patients with and without incontinence‐associated dermatitis. A, The photos show an example of the urea medium after bacterial cultivation. To determine the urease activity in each swab, three classification strata (strong, weak, and almost none/none) were determined by the degree of colour change in the agar medium from yellow to red. B, The urease activity based on the three classification strata was compared between two groups.
3.6. CFU assay
Figure 3 shows that there were no significant differences in the number of CFUs in the IAD and no‐IAD groups (Z = 0.44, P = .56).
FIGURE 3.
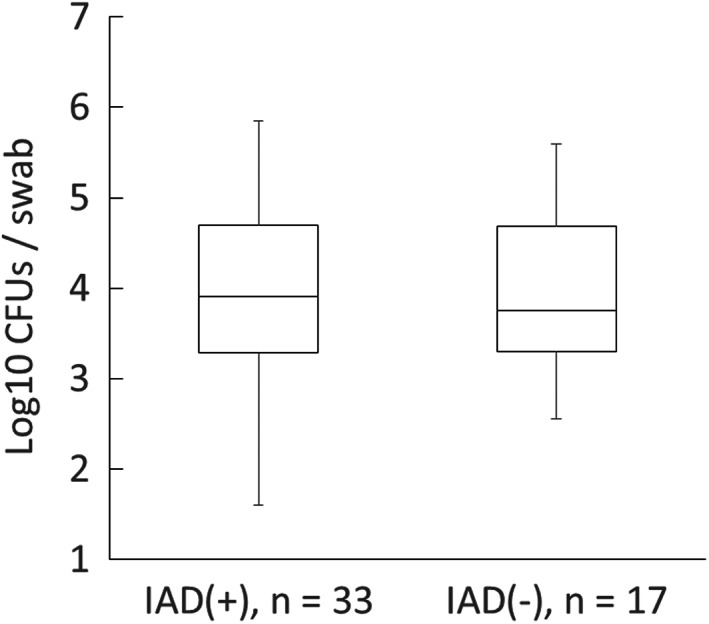
Comparison of bacterial growth. CFU, colony‐forming units.
4. DISCUSSION
Proper management of skin pH changes is a requirement for the successful achievement of the prevention of IAD development. The prophylactic use of superabsorbent pads and skin‐protective creams has been applied in clinical practice. 26 , 27 However, it remains unclear how skin pH changes contribute to IAD development in contemporary pathophysiological studies. To address this problem, we focused on urease‐producing bacterial strains on and around genital skin surfaces. To the best of our knowledge, this is the first study demonstrating that the presence of urease‐producing bacteria is associated with IAD development in hospitalised stroke patients using our original urea agar medium.
In this study, we prepared a novel agar medium in which the addition of urease chemicals or bacteria that can release urease results in colour changes in the medium to confirm the presence of urease‐producing bacteria in swabs collected from patients' genital skin surfaces with or without IAD. Our pH measurements showed that the surface pH of the medium containing the positive control strain was clearly alkalized while that of the medium without this strain was not (Figure 1). Our results indicated that the developed medium was capable of detecting urease‐producing bacteria.
The most significant finding of this study is that the detection rate of urease‐producing bacteria in the IAD group was significantly higher than that in the non‐IAD group (Figure 2), even though the total number of bacterial colonies was equivalent between them (Figure 3). These results suggest there was a significant association between urease‐producing bacterial colonisation and IAD development. Approximately 30.3% of swabs collected from the IAD group were categorised as having “strong” urease‐producing capacity while only 5.9% of those in the non‐IAD group did. The classification of the urease production capacity as “strong” or “weak” means that the surface of the medium has shifted from neutral to alkaline. This simulates the risk of homeostasis being compromised by the alkalization of the skin surface because of the contact between urine and urease‐producing bacteria.
At present, the evaluation of IADs depends on visual observation by healthcare providers, and point‐of‐care IAD assessment technology has not yet been developed. The skin pH is a widely‐used parameter in assessing the organ's physiological functions in clinical practice. 28 The challenge associated with measuring the skin pH is that its variables are typically affected by properties of the surrounding microclimate such as environmental humidity, temperature, and sweating; thus, such measurements should be performed under controlled conditions. 29 The urea agar medium developed in this study can be used irrespective of the surrounding microclimate, considering that it may be applicable to the identification of patients at high risk of developing IAD, as a novel point‐of‐care IAD assessment piece of technology.
The mechanism of the significant increase in the number of urease‐producing bacteria in patients with IAD cannot be determined from this study because we focused on the detection of urease‐producing bacteria in swabs collected from patients. The performance of bacteria on the medium to release urease during cultivation depends on the type of bacteria present and the number of viable bacteria as well. Therefore, the difference in urease activity between the IAD and non‐IAD groups in this study suggests that some sort of bacterial condition on the genital skin surface differed between the two groups. A previous study reported that the skin microbiome at the sacral area differed between bedridden elderly people and healthy individuals. 30 In addition, it is still challenging whether reducing the number of urease‐producing bacteria prevents IAD development or its severity. To clarify the mechanism, we plan to perform animal test to investigate the effect of the addition of urease‐producing bacteria on the development of skin damage such as IAD.
The prevalence of IAD in this study was 48%, suggesting that IAD is a prevalent and underappreciated skin condition in hospitalised stroke patients. IAD was typically observed in the perianal and genital areas, suggesting that more appropriate skin assessments and preventive measures against IAD development should be implemented for stroke patients on admission and during hospitalisation.
However, it is often difficult for clinicians to correctly identify IAD and to distinguish it from pressure injury. IAD is considered a significant risk factor for the development of pressure injuries and has been identified as a predictive factor significantly associated with the development of superficial pressure injuries. 31 In this study, identification of IAD was performed by one certified nurse with adequate experience in wound, ostomy, and incontinence. The presence of IAD were comprehensively assessed for patient history, symptoms, and location, in addition to visual observation of the colour, shape/edge, and depth of the dermatitis itself.
This study has two main limitations. The first was that we did not adjust for diabetes as a risk factor for IAD development (making it a possible confounder) because we prioritised the detection of urease‐producing bacteria. Patients' demographic data suggest that diabetes could be a risk factor for IAD development. However, it is difficult to explain why diabetes increases the number of urease‐producing bacteria or increases the skin pH from our findings or contemporary studies examining risk factors for IAD development. 2 , 32 Next, we could not establish a cause‐to‐effect relationship between the presence of urease‐producing bacteria and IAD development due to the cross‐sectional design of our study. Prospective study is needed to clarify whether urease‐producing bacteria are a cause of IAD development in all patients who are incontinent of urine with various diseases or aging as well as stroke. There also have a strong interest in investigating the association of urease‐producing bacteria with the existing risk factors and risk assessment scales of pressure injury development. This is because the friction and shear problems according to the Braden scale were reported to be strong covariates for the presence of IAD. 2 We think these would allow us to find a solution to the question of whether there be a case for the intervention in all patients who are incontinent of urine and at risk of IAD or pressure injury.
Nevertheless, we believe that our data provide important information on urease‐producing bacteria (a potential biological risk factor for IAD development) for healthcare providers. In the future, comparative prospective studies on the presence of urease‐producing bacteria will be needed. Studies in which patients' other baseline risk factors (including diabetes mellitus) are adjusted for in both groups will also be needed.
5. CONCLUSIONS
The presence of urease‐producing bacteria was associated with IAD development in hospitalised stroke patients when the original urea agar medium was used to detect urease‐producing bacteria at genital sites.
AUTHOR CONTRIBUTIONS
Kohta M, Koyanagi H, and Sugama J conceived and designed this study. Kohta M, Koyanagi H, Inagaki Y, Nishikawa K, Kobayashi N, Tamura S, Ishikawa M, and Banno Y collected data, analysed data, and interpreted the results. Kohta M and Sugama J drafted the manuscript, critically reviewed it for important intellectual content, and approved its final version to be published. All authors agreed to be accountable for all aspects of this work to ensure that questions related to the accuracy or integrity of any part of this work will be appropriately answered.
FUNDING INFORMATION
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers: 21K21151).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
ACKNOWLEDGEMENTS
We thank Professor Yohei Otaka, Professor Kazumitsu Sugiura, and Professor Shoji Matsumoto for their insightful advice during the study period. We also thank Enago (http://www.enago.jp) for its English language editing services.
Kohta M, Koyanagi H, Inagaki Y, et al. Selective detection of urease‐producing bacteria on the genital skin surface in patients with incontinence‐associated dermatitis. Int Wound J. 2023;20(8):3289‐3297. doi: 10.1111/iwj.14209
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gray M. Preventing and managing perineal dermatitis: a shared goal for wound and continence care. J Wound Osto Contin Nurs: Off Public Wound Osto Contin Nurs Soc. 2004;31(1):S2‐S9. quiz S10. [DOI] [PubMed] [Google Scholar]
- 2. Kottner J, Blume‐Peytavi U, Lohrmann C, Halfens R. Associations between individual characteristics and incontinence‐associated dermatitis: a secondary data analysis of a multi‐Centre prevalence study. Int J Nurs Stud. 2014;51(10):1373‐1380. [DOI] [PubMed] [Google Scholar]
- 3. Beeckman D, Schoonhoven L, Fletcher J, et al. Pressure ulcers and incontinence‐associated dermatitis: effectiveness of the pressure ulcer classification education tool on classification by nurses. Qual Saf Health Care. 2010;19(5):e3. [DOI] [PubMed] [Google Scholar]
- 4. Banharak S, Panpanit L, Subindee S, et al. Prevention and care for incontinence‐associated dermatitis among older adults: a systematic review. J Multidiscip Healthc. 2021;14:2983‐3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell JL, Coyer FM, Osborne SR. Incontinence‐associated dermatitis: a cross‐sectional prevalence study in the Australian acute care hospital setting. Int Wound J. 2016;13(3):403‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kayser SA, Phipps L, VanGilder CA, Lachenbruch C. Examining prevalence and risk factors of incontinence‐associated dermatitis using the international pressure ulcer prevalence survey. J Wound Osto Contin Nurs: Off Publi Wound Osto Contin Nurs Soc. 2019;46(4):285‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grden CRB, Martins AR, Cabral LPA, et al. Incontinence associated dermatitis in elderly people admitted to a university hospital. Rev Bras Enferm. 2020;73(Suppl 3):e20190374. [DOI] [PubMed] [Google Scholar]
- 8. Ichikawa Y, Sugama J. Prevalence of incontinence‐associated dermatitis and its relationship to nursing care and institutional structure in long‐term medical facilities. J Jpn WOCM. 2015;19(3):319‐326. [Google Scholar]
- 9. Bliss DZ, Funk T, Jacobson M, Savik K. Incidence and characteristics of incontinence‐associated dermatitis in community‐dwelling persons with fecal incontinence. J Wound Ost Contin Nurs: OffPublicWound Osto Contin Nurs Soc. 2015;42(5):525‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008;8(4):299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1‐26. [DOI] [PubMed] [Google Scholar]
- 12. McNichol LL, Ayello EA, Phearman LA, Pezzella PA, Culver EA. Incontinence‐associated dermatitis: state of the science and knowledge translation. Adv Skin Wound Care. 2018;31(11):502‐513. [DOI] [PubMed] [Google Scholar]
- 13. Rippke F, Berardesca E, Weber TM. pH and microbial infections. Curr Probl Dermatol. 2018;54:87‐94. [DOI] [PubMed] [Google Scholar]
- 14. Bullock AJ, Garcia M, Shepherd J, Rehman I, Sheila M. Bacteria induced pH changes in tissue‐engineered human skin detected non‐invasively using Raman confocal spectroscopy. Appl Spectrosc Rev. 2020;55(2):158‐171. [Google Scholar]
- 15. Ohkubo T, Matsumoto Y, Ogasawara Y, Sugita T. Alkaline stress inhibits the growth of Staphylococcus epidermidis by inducing TCA cycle‐triggered ROS production. Biochem Biophys Res Commun. 2022;588:104‐110. [DOI] [PubMed] [Google Scholar]
- 16. Larner J, Matar H, Goldman VS, Chilcott RP. Development of a cumulative irritation model for incontinence‐associated dermatitis. Arch Dermatol Res. 2015;307(1):39‐48. [DOI] [PubMed] [Google Scholar]
- 17. Koudounas S, Bader DL, Voegeli D. An exploratory study of the effects of the pH of synthetic urine on skin integrity in healthy participants. Skin Pharmacol Physiol. 2022;35(3):166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krajewska B, Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B: Enzym. 2009;59(1–3):9‐21. [Google Scholar]
- 19. Cotter PD, Hill C. Surviving the acid test: responses of gram‐positive bacteria to low pH. Microbiol Mol Biol Rev. 2003;67(3):429‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murchan S, Aucken HM, O'neill GL, Ganner M, Cookson BD. Emergence, spread, and characterization of phage variants of epidemic methicillin‐resistant Staphylococcus aureus 16 in England and Wales. J Clin Microbiol. 2004;42(11):5154‐5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christensen WB. Urea decomposition as a means of differentiating proteus and paracolon cultures from each other and from salmonella and Shigella types. J Bacteriol. 1946;52(4):461‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandecandelaere I, Van Nieuwerburgh F, Deforce D, Coenye T. Metabolic activity, urease production, antibiotic resistance and virulence in dual species biofilms of Staphylococcus epidermidis and Staphylococcus aureus. PLOS One. 2017;12(3):e0172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ranjbar‐Omid M, Arzanlou M, Amani M, Shokri Al‐Hashem SK, Amir Mozafari N, Peeri DH. Allicin from garlic inhibits the biofilm formation and urease activity of Proteus mirabilis in vitro. FEMS Microbiol Lett. 2015;362(9):fnv049. [DOI] [PubMed] [Google Scholar]
- 24. Maciejewska M, Adam D, Naômé A, et al. Assessment of the potential role of Streptomyces in cave moonmilk formation. Front Microbiol. 2017;8:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borchert K, Bliss DZ, Savik K, Radosevich DM. The incontinence‐associated dermatitis and its severity instrument: development and validation. J Wound Osto Contin Nurs: Off Public Wound Osto Contin Nurs Soc. 2010;37(5):527‐535. [DOI] [PubMed] [Google Scholar]
- 26. Sugama J, Sanada H, Shigeta Y, Nakagami G, Konya C. Efficacy of an improved absorbent pad on incontinence‐associated dermatitis in older women: cluster randomized controlled trial. B.M.C. Geriatrics. 2012;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kon Y, Ichikawa‐Shigeta Y, Iuchi T, et al. Effects of a skin barrier cream on management of incontinence‐associated dermatitis in older women: a cluster randomized controlled trial. J Wound Osto Contin Nurs: Off Public Wound Osto Contin Nurs Soc. 2017;44(5):481‐486. [DOI] [PubMed] [Google Scholar]
- 28. Stefaniak AB, Plessis J, John SM, et al. International guidelines for the in vivo assessment of skin properties in non‐clinical settings: part 1. pH. Skin research and technology: official journal of International Society for Bioengineering and the. Skin. 2013;19(2):59‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parra JL, Paye M, EEMCO Group . EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol. 2003;16(3):188‐202. [DOI] [PubMed] [Google Scholar]
- 30. Nagase S, Ogai K, Urai T, et al. Distinct skin microbiome and skin physiological functions between bedridden older patients and healthy people: a single‐center study in Japan. Front Med. 2020;7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demarre L, Verhaeghe S, Van Hecke A, Clays E, Grypdonck M, Beeckman D. Factors predicting the development of pressure ulcers in an at‐risk population who receive standardized preventive care: secondary analyses of a multicentre randomised controlled trial. J Adv Nurs. 2015;71(2):391‐403. [DOI] [PubMed] [Google Scholar]
- 32. Van Damme N, Clays E, Verhaeghe S, Van Hecke A, Beeckman D. Independent risk factors for the development of incontinence‐associated dermatitis (category 2) in critically ill patients with fecal incontinence: a cross‐sectional observational study in 48 ICU units. Int J Nurs Stud. 2018;81:30‐39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


