Abstract
Diabetic foot ulcer (DFU) is a major cause of morbidity, non‐traumatic lower limb amputation in diabetic patients and a high‐cost burden on the healthcare system. New therapeutic products are increasingly tested. Platelet‐rich plasma (PRP) and human platelet lysate (hPL) are reported to be useful. This trial was conducted to test whether the healing effect of hPL in chronic DFU was due to plasma or platelet lysates in a prospective double‐blind design. Autologous PRP was obtained from citrated blood, lysed, and used as drug 1 (active product). The platelet‐poor plasma (PPP) was used as a drug 2 (placebo). Ten patients were enrolled in arm 1 and 9 in arm 2. The drugs were injected perilesionally every 2 weeks for a total of sixinjections. Adverse events were recorded until Week 14. The DFUs were scored per the Texas and Wegner systems. No patient showed any major adverse events. Some reported local pain post‐injection. Wound healing was achieved in the hPL group in 9/10 of patients at a mean of 35.1 days. In the PPP group, no patient had healed by Day 84. The difference was statistically significant at P < 0.00001. We conclude that autologous hPL is safe and highly effective in healing chronic DFU and is superior to autologous PPP.
Keywords: diabetes, foot ulcers, platelet lysate, regenerative medicine, wound healing
1. INTRODUCTION
Diabetic foot ulcers (DFU) are commonly seen in diabetic patients, with lifetime prevalence reported to be from 15% to 25% and may reach up to 34% 1 , 2 , 3 DFU is a recurrent disease, and healing of an index wound may not mean a permanent remission if the appropriate preventive measures are not taken. 3 Approximately 40% to 65% of patients with DFUs experience a recurrence within 1 to 5 years. 1 , 4 , 5
Moreover, DFU is a major cause of morbidity and mortality in diabetic patients. It is the major cause of non‐traumatic lower limb amputation. 6 , 7 Five‐year mortality for DFU and major amputations is estimated to be 30.5%, and 56.6%, respectively, compared with 5‐year pooled mortality for all reported cancers, which is 31.0%. 8
The increased hospitalisation and supportive care for DFU represent a considerable economic burden. The EURODIALE study looked at 821 patients with DFU in several EU countries and analysed the direct and indirect annual costs, estimating a total annual cost per patient of €10 091, mostly for hospital costs. 5
In the United Kingdom, it was estimated that 20% of total spending on diabetes care is taken up by complications related to DFU 6 , 9 , 10 A study in the USA concluded that DFU imposes substantial additional costs on public and private payers. 10 , 11
For the treatment of DFU, new therapeutic products are increasingly tested and used for the in situ administration of bioactive molecules alone or with wound dressings. 12 It has been reported that platelet‐rich plasma (PRP) and human platelet lysate (hPL) could improve the healing of chronic wounds, including DFU. 9 , 13 , 14 , 15 , 16 , 17 , 18
We have previously published two cases of DFU who were treated and healed by autologous peri‐lesional hPL injections 18 as part of a larger trial, which we are reporting in this paper. Since autologous hPL uses a mixture of autologous plasma and autologous platelet lysates, the trial was set to test whether the healing effect was due to plasma or platelet lysates in a prospective double‐blind design.
2. PATIENTS AND METHODS
This is a prospective double‐blind, placebo‐controlled trial testing the use of an autologous hPL product with autologous platelet‐poor plasma (PPP) used as a placebo. This study was approved by the Institutional Review Board (IRB) at the Cell Therapy Center, University of Jordan. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. Inclusion and exclusion criteria and patient selection were previously published by us 18 and are outlined below.
2.1. Inclusion criteria
Patients with type II diabetes mellitus (DM) between the ages of 18 and 70 with an ulcer of at least 6 weeks duration.
HbA1c of less than 13%.
Index foot ulcer located on the plantar, medial, or lateral aspect of the foot (including all toe surfaces), and the wound area (length*width) measurement between 0.5 cm^ 2 and 20 cm^ 2, inclusive.
Wounds located under a Charcot deformity have to be free of acute changes and went through appropriate structural consolidation.
University of Texas stage A Grade II or III ulcer. 19
The protocol requires that post‐debridement, the ulcer would be free of necrotic debris, foreign bodies, or sinus tracts.
Non‐invasive vascular testing Ankle Brachial Index (ABI).
Otherwise medically free on physical examination (including a Semmes‐Weinstein monofilament test for neuropathy).
Results of blood tests within the accepted range, including a full blood count, liver and kidney function tests, hepatitis screening, and blood chemistry, including HbA1c.
Signed, approved, and informed consent from each patient.
2.2. Exclusion criteria
Patient currently enrolled or previously enrolled (within the last 30 days) in another investigative device or drug trial.
Ulcer area decreased by >50% during the 7‐day screening period.
Ulcer is due to a non‐diabetic aetiology or DFU not meeting inclusion criteria.
Evidence of gangrene in the ulcer or on any part of the foot.
Patient is currently receiving or has received radiation or chemotherapy within the last 3 months of randomization.
The patient has received growth factor therapy within 7 days of randomization.
Screening platelets count <100* 109/L, and or Hb less than <10 g/dL.
The patient is undergoing renal dialysis or has a known immune deficiency, known abnormal platelet activation disorder, liver disease, active cancer, eating/nutritional, hematologic, collagen vascular disease, rheumatic disease, or bleeding disorder.
History of peripheral vascular repair within 30 days of randomization.
Patient is known to have a physiological, developmental, physical, emotional, or social disorder or any other situation that may interfere with their compliance with the study requirements and/or the healing of the ulcer.
History of alcohol or drug abuse within the last year prior to randomization.
The patient has inadequate venous access for blood withdrawal.
Consenting patients were randomised in a double‐blind manner into 2 groups: one group was given hPL, and the second one was given PPP as a placebo.
2.3. hPL and PPP preparation
The method of preparation of hPL and PPP and their final concentration was as previously published. 18
Briefly, 25 mL of blood was acquired from each participant by venous phlebotomy into citrated tubes. PRP was obtained by centrifuging the citrated blood at 900g for 10 min and taking the supernatant.
For the hPL arm, the platelet count in the PRP preparation was adjusted at 1000 × 109/l before further processing. PRP was further processed by two freeze/thaw cycles at −80°C and 37°C, respectively, followed by centrifugation at 3060g for 20 min, and filtration of the supernatant using 0.22 μL filter (BD, USA).
For the PPP arm, PRP was centrifuged at 3060g for 20 min, and the supernatant was collected and filtered using a 0.22 μL filter (BD, USA). A platelet count was used to confirm the lack of platelets in the final preparation.
2.4. hPL and PPP injections
In group one, hPL was injected peri‐lesional at ulcer margins every 2 weeks for a maximum total of six injections. In group two, PPP was used in the same manner every 2 weeks for a maximum total of six injections. Each injection was composed of 5 mL of the material to be injected, distributed in five 1‐mL syringes. Adverse events were observed and recorded in a checklist form until Week 14.
The DFU was scored as per the Texas grading system and the Wegner scoring system. During each visit, the ulcer size was measured before the injection and recorded, then the surface area was calculated accordingly.
Wound healing was examined by a well‐trained surgeon with experience in DFU. Photographic images were obtained at baseline, 4 weeks, 12 weeks, and 14 weeks or when the wound has healed, whichever came first.
2.5. Outcome measures
The first outcome measure was treatment safety, determined by the number and severity of adverse events. Patients were monitored for 1 h post‐treatment, then phone interviewed at 24 h and 1 week. They were physically examined every 2 weeks. Blood tests were performed monthly for up to 6 months as part of our safety follow‐up policy.
The second outcome measure was efficacy, as measured by either a. complete healing at any given time point within 12 weeks b. partial healing measured by ulcer size reduction at 12 weeks. Treatment was considered a failure when neither a. nor b. above were reached at 12 weeks. In cases where ulcers fully healed before 12 weeks, the healing time was recorded.
3. RESULTS
Patient screening and enrollment are shown in Figure 1 below, which details the patients' selection, enrollment, and the reasons for those patients who did not complete the study. A total of 40 patients with chronic DFU were screened for this study; 30 patients met the inclusion criteria and were enrolled. They were randomised into two arms: hPL (active product) and PPP (placebo) arms. Both arms received the assigned treatment on top of the standard of care. The standard of care for DFU at our institution is as per the WIFI classification system 20 and it consists of the following: wound bed preparation, debridement as appropriate, moist wound bed dressing, and secondary dressing by absorptive fibre or foam with an antimicrobial agent. As for the off load it is according to the case biomechanics, consisting of the total contact cast or removable cast. In total, 10 patients in the (active product) arm and nine patients in the (placebo) arm completed the study, and their results were analysed.
FIGURE 1.
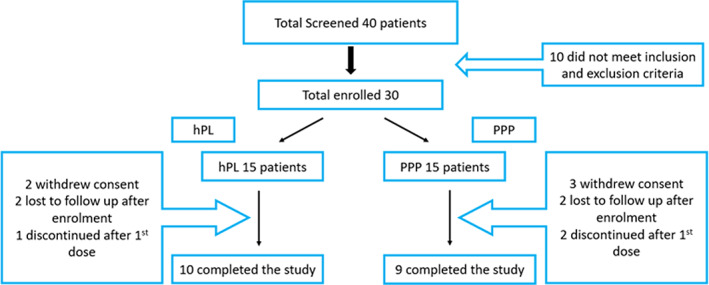
Patients screening and enrollment
In total, we enrolled six males and four females in the (active product) arm, with a mean age of 61.4 years, a mean ulcer duration of 27.6 weeks, and a mean ulcer size of 13.88 cm2 (Range: 2‐24.5 cm2); five males and four females in the (placebo) arm, with a mean age of 58.6 years, a mean ulcer duration of 26 weeks, and a mean ulcer size of 11.8 cm2 (Range: 1. 6‐22. 2 cm2). Other patients' epidemiology and characteristics are shown in Table 1.
TABLE 1.
Patients characteristics and DFU status
| n a | Age (years) | Hba1c | Duration of DFU (weeks) | Ulcer stage b (n) | |
|---|---|---|---|---|---|
| Mean/Median | Mean | ||||
| hPL | |||||
| Males | 6 | 62.5/62 | 8. 6 | 30 |
AI 1 AII 5 |
| Females | 4 | 60/62 | 8. 3 | 24 |
AI 1 AII 3 |
| Total | 10 | 61.4/62 | 8.48 | 27. 6 |
AI 2 AII 8 |
| PPP | |||||
| Males | 5 | 58. 2/61 | 8. 3 | 28 |
AI 1 AII 4 |
| Females | 4 | 59. 3/61.5 |
8 |
23.5 |
AI 1 AII 3 |
|
Total |
9 | 58. 6/61 | 8.12 |
26 |
AI 2 AII 7 |
n, number of patients.
As per the University of Texas staging system.
3.1. Treatment outcome and statistics
Haematology and chemistry blood tests done monthly showed no changes in both group. There were no significant adverse events observed either by physical examination or through patients' self‐reported questionnaires. Minor adverse events were documented following injections, including local pain, swelling, infection, bleeding, redness, and hotness. The total number of occurrences of these events throughout the full course of treatment was documented and charted in Table 2.
TABLE 2.
Total number of adverse events encountered during the whole course of treatment
| Group | Injection site pain | Local swelling | Local infection | Local bleeding | Redness and hotness |
|---|---|---|---|---|---|
| hPL | 36 | 10 | 6 | 5 | 9 |
| PPP | 28 | 7 | 4 | 1 | 6 |
Nine patients in the hPL group achieved full healing at or before the determined ending time point (12 weeks). One patient only showed partial healing (around 80% reduction in ulcer surface area) at Week 12. The treatment was considered a failure for that patient for the purposes of study analysis, but the patient was given two additional doses on a humanitarian basis. The ulcer showed full healing on day 110. Details are shown in Table 3A.
TABLE 3.
Details of the number of injections and treatment outcomes. (A) hPL group. (B) PPP group
| Patient number | Gender | Days needed for full healing | Number of injections for full healing | Healing percentage after hPL therapy |
|---|---|---|---|---|
| 1 | M | 78 days | 6 Injections | 100% |
| 2 | F | 110 days | 8 Injections | Partially healed at week 12 (80%) |
| 3 | M | 21 days | 2 Injections | 100% |
| 4 | M | 56 days | 4 Injections | 100% |
| 5 | F | 30 days | 3 Injections | 100% |
| 6 | M | 48 days | 4 Injections | 100% |
| 7 | F | 18 days | 2 Injections | 100% |
| 8 | M | 17 days | 2 Injections | 100% |
| 9 | F | 31 days | 3 Injections | 100% |
| 10 | M | 32 days | 3 Injections | 100% |
| Patient number | Gender | Days of follow‐up looking for healing | Number of injections given | % of DFU size reduction at week 12 |
|---|---|---|---|---|
| 1 | F | 84 | 6 | 10% |
| 2 | M | 84 | 6 | 0% |
| 3 | M | 84 | 6 | 5% |
| 4 | F | 84 | 6 | 15% |
| 5 | F | 84 | 6 | 0% |
| 6 | M | 84 | 6 | 3% |
| 7 | F | 84 | 6 | 5% |
| 8 | M | 84 | 6 | 0% |
| 9 | M | 84 | 6 | 0% |
None of the patients in the PPP group showed full healing at 12 weeks. Some patients showed ulcer size reduction, as detailed in Table 3B.
Using analysis of variance (ANOVA) one‐way test, the healing efficiency calculated based on ulcer surface area was 0.98 (SD ±0.0632) in the hPL arm and 0.0422 (SD ±0.0529) in the PPP arm. The difference between hPL and PPP arms was found to be statistically highly significant in favour of hPL arm with P < 0.00001.
Figure 2A shows representative photographs of DFU treated by autologous human platelet lysate (hPL) (Figure 2A), and representative photographs of DFU treated by autologous platelet‐poor plasma (PPP) (Figure 2B) at different time points as shown.
FIGURE 2.
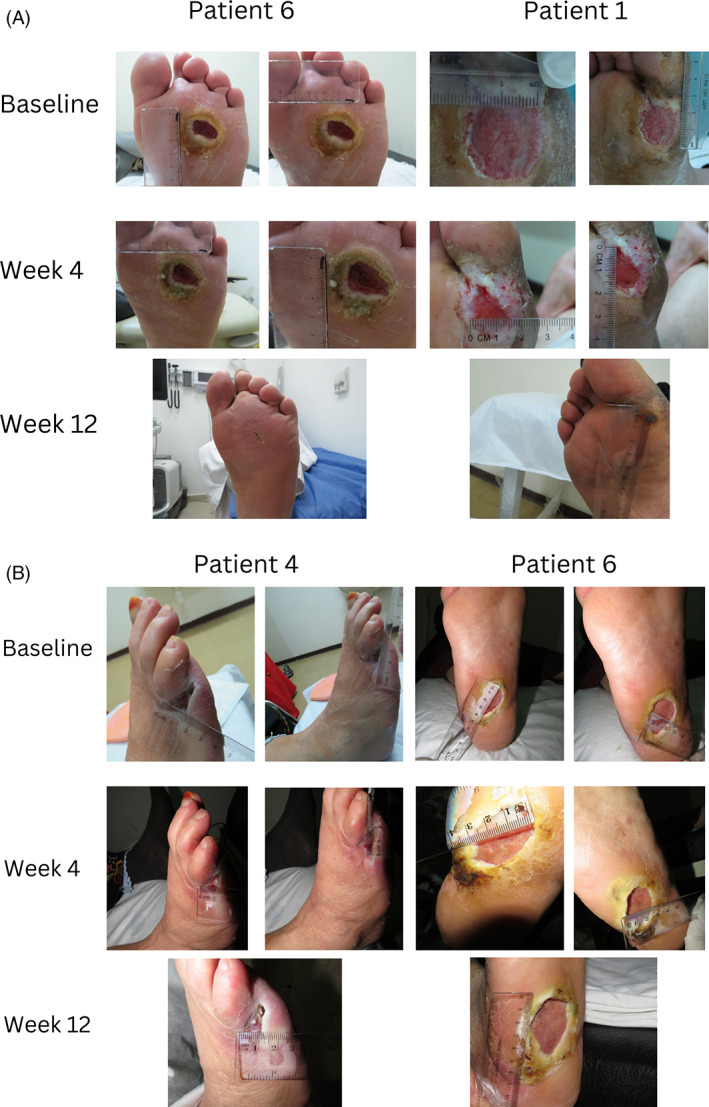
Representative photographs of DFU treated by autologous human platelet lysate (hPL) at different time points. Representative photographs of DFU treated by autologous platelet poor plasma (hPL) at different time points
4. DISCUSSION
DFU are a challenging problem in diabetes care since they are commonly seen, with a lifetime prevalence reported to be from 15% to 25% and may reach up to 34%. 3 In addition to their poor healing, DFU are known to recur, are costly to the health care budget, and do not have an effective, universally approved therapy. 1 , 10
New therapeutic products are being tested for in situ administration of bioactive molecules alone or with wound dressings. 12 Platelet‐based products, either in the form of PRP or hPL, have been tested with variable degrees of success. 9 , 13 , 14 , 15 , 16 , 17 , 18 In these papers, the contribution of platelet‐poor plasma (PPP) to DFU healing was not defined, and the question remains whether PPP may have contributed to the healing.
In this study, we aimed to explore the value of hPL versus PPP in a double‐blind prospective trial comparing the efficacy of perilesional injection of autologous hPL versus autologous PPP in chronic diabetic foot ulcers. As such, PPP was used as the control “placebo” arm since it has the same colour as hPL and has the same volume but lacks the “active” component of platelet lysates.
As can be seen from our results, the hPL has a superior therapeutic effect, and nine out of 10 patients healed within a short time, with a mean of 36.1 days from the start of treatment. We did not study the recurrence rate in these patients. We did not have any cases of worsening infection or uncontrolled infection, or amputation. We did not record any serious adverse events.
The statistically significant results of this pilot study demonstrated that hPL, when used as a peri‐lesional injection, has a favourable effect on the rate of healing of chronic DFU. The multiple regulatory proteins retained in hPL through the described preparation process seem to play a significant role in modulating the healing cascade to a semi‐acute state. Further specification of these factors is required before such definitive claims can be made. This is consistent with previous work done with PRP, 13 , 14 , 15 , 17 and limited work with hPL. 18 Topical PRP products have been reported to produce good therapeutic effects on DFU. 21
We are the first to report on perilesional hPL injections in DFU in a double‐blind, controlled study. Though our patient population is small, we believe it contributes to the concept because of the double‐blind design and comparison with plasma.
Furthermore, it seems that this modality is safe. We think the PPP has little, if any, therapeutic effect on DFU. Our work should stimulate a larger study to confirm these findings.
FUNDING INFORMATION
A. Awidi was supported by the Scientific Research Support Fund (SRF), Ministry of Higher Education/Jordan (January 1, 2009 year 2009.h). N. Younes was supported by the Deanship of Scientific Research/The University of Jordan.
Alhawari H, Jafar H, Al Soudi M, et al. Perilesional injections of human platelet lysate versus platelet poor plasma for the treatment of diabetic foot ulcers: A double‐blinded prospective clinical trial. Int Wound J. 2023;20(8):3116‐3122. doi: 10.1111/iwj.14186
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367‐2375. doi: 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 2. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493‐1498. doi: 10.1111/dme.13054 [DOI] [PubMed] [Google Scholar]
- 3. Ogurtsova K, Morbach S, Haastert B, et al. Cumulative long‐term recurrence of diabetic foot ulcers in two cohorts from centres in Germany and The Czech Republic. Diabetes Res Clin Pract. 2021;172:108621. doi: 10.1016/j.diabtes.2020.108621 [DOI] [PubMed] [Google Scholar]
- 4. Huang ZH, Li SQ, Kou Y, Huang L, Yu T, Hu A. Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta‐analysis. Int Wound J. 2019;16(6):1373‐1382. doi: 10.1111/iwj.13200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow‐up analysis in the Eurodiale subgroup. Int Wound J. 2013;10(5):555‐561. doi: 10.1111/j.1742-481X.2012.01022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217‐228. doi: 10.1001/jama.293.2.217 [DOI] [PubMed] [Google Scholar]
- 7. Rathnayake A, Saboo A, Malabu UH, Falhammar H. Lower extremity amputations and long‐term outcomes in diabetic foot ulcers: a systematic review. World J Diabetes. 2020;11(9):391‐399. doi: 10.4239/wjd.v11.i9.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five‐year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(16). doi: 10.1186/s13047-020-00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linertová R, Del Pino‐Sedeño T, Pérez LG, et al. Cost‐effectiveness of platelet‐rich plasma for diabetic foot ulcer in Spain. Int J Low Extrem Wounds. 2021;20(2):119‐127. doi: 10.1177/1534734620903239 [DOI] [PubMed] [Google Scholar]
- 10. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651‐658. doi: 10.2337/dc13-2176 [DOI] [PubMed] [Google Scholar]
- 11. Prompers L, Huijberts M, Schaper N, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale study. Diabetologia. 2008;51(10):1826‐1834. doi: 10.1007/s00125-008-1089-6 [DOI] [PubMed] [Google Scholar]
- 12. Eming SA, Martin P, Tomic‐Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez‐Zapata MJ, Martí‐Carvajal AJ, Solà I, et al. Autologous platelet‐rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;5:CD006899. doi: 10.1002/14651858.CD006899.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarvajnamurthy S, Suryanarayan S, Budamakuntala L, Suresh DH. Autologous platelet rich plasma in chronic venous ulcers: study of 17 cases. J Cutan Aesthet Surg. 2013;6:97‐99. doi: 10.4103/0974-2077.112671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non‐healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24:16. doi: 10.1186/s12929-017-0324-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romaldini A, Mastrogiacomo M, Cancedda R, Descalzi F. Platelet lysate activates human subcutaneous adipose tissue cells by promoting cell proliferation and their paracrine activity toward epidermal keratinocytes. Front Bioeng Biotechnol. 2018;21(6):203. doi: 10.3389/fbioe.2018.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elsaid A, El‐Said M, Emile S, Youssef M, Khafagy W, Elshobaky A. Randomized controlled trial on autologous platelet‐rich plasma versus saline dressing in treatment of non‐healing diabetic foot ulcers. World J Surg. 2020;44(4):1294‐1301. doi: 10.1007/s00268-019-05316-0 [DOI] [PubMed] [Google Scholar]
- 18. Jafar H, Hasan M, Al‐Hattab D, et al. Platelet lysate promotes the healing of long‐standing diabetic foot ulcers: a report of two cases and in vitro study. Heliyon. 2020;6(5):e03929. doi: 10.1016/j.heliyon.2020.e03929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong D, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855‐859. [DOI] [PubMed] [Google Scholar]
- 20. Mills JL Sr, Conte MS, Armstrong DG, et al. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220‐234. [DOI] [PubMed] [Google Scholar]
- 21. Hirase T, Ruff E, Surani S, Ratnani I. Topical application of platelet‐rich plasma for diabetic foot ulcers: a systematic review. World J Diabetes. 2018;9(10):172‐179. doi: 10.4239/wjd.v9.i10.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


