Abstract
Adequate blood supply, a prerequisite for flap survival after grafting, makes angiogenesis of the flap the biggest problem to be solved. Researches have been conducted around vascularisation in correlation with flap grafting. However, bibliometric analyses systematically examining this research field are lacking. As such, we herein sought to conduct comprehensive comparative analyses of the contributions of different researchers, institutions, and countries to this research space in an effort to identify trends and hotspots in angiogenesis and vascularisation in the context of flap grafting. Publications pertaining to angiogenesis and vascularisation in the context of flap grafting were retrieved from the Web of Science Core Collection. References were then analysed and plotted using Microsoft Excel 2019, VOSviewer, and CiteSpace V. In total, 2234 papers that were cited 40 048 times (17.63 citations/paper) were included in this analysis. The greatest number of studies were from the United States, with these studies exhibiting both the highest number of citations (13 577) and the greatest overall H‐index (60). For The institutions that published the greatest number of studies were WENZHOU MEDICAL UNIVERSITY (681), while UNIVERSITY OF ERLANGEN NUREMBERG has the highest number of citations (1458), and SHANGHAI JIAO TONG UNIVERSITY holds the greatest overall H‐index (20). The greatest number of studies in this research space were published by Gao WY, while Horch RE was the most commonly cited researcher in the field. The VOS viewer software clustered relevant keywords into three clusters, with clusters 1, 2, 3, and 4 corresponding to studies in which the keywords ‘anatomy’, ‘survival’, ‘transplantation’, ‘therapy’ most frequently appeared. The most promising research hotspot‐related terms in this field included ‘autophagy’, ‘oxidative stress’, ‘ischemia/reperfusion injury’, which exhibited a most recent average appearing year (AAY) of 2017 and after. Generally speaking, the results of this analysis indicate that the number of articles exploring angiogenesis and flap‐related research has risen steadily, with the United States and China being the two countries publishing the greatest proportion of studies in this field. The overall focus of these studies has shifted away from ‘infratest and tissue engineering’ towards ‘mechanisms’. In the future, particular attention should be paid to emerging research hotspots, which include ‘ischemia/reperfusion injury’ and treatments for promoting vascularization, such as ‘platelet‐rich plasma’. In light of these findings, funding agencies should continue increasing their investment in the exploration of the concrete mechanisms and interventional therapeutic relevance of angiogenesis during flap transplantation.
Keywords: bibliometric analysis, flap, global trends, regenerative medicine, vascularisation
1. BACKGROUND
Flap grafting is a significant technique during plastic and reconstructive surgery. While repairing tissue defects resulting from the removal of tumours, severe trauma 1 by transferring body tissue containing epithelial tissue, dermal tissue, subcutaneous tissue (which may include muscle or bone), and accompanied vascular tissue 2 from one site to another. The transposition of flaps includes ordinary‐tipped transfer flaps and free composite tissue flaps, etc.
Angiogenesis refers to the formation of new vessels from pre‐existing vascular networks guided by endothelial cells, which occurs primarily when the tissue requires an adequate supply of nutrients and oxygen and included steps, such as proliferation, migration, lumen formation, differentiation, and maturation, each involving multiple growth factors, receptors, and molecules. 3 Endothelial cells activated by signals from pro‐angiogenic mediators, such as angiogenic factors invade the adjacent domains, leading to new sprouts, and then gradually build up the lumen, thus lengthening the vessel wall, until the pro‐angiogenic signals diminish and a quiescent state is re‐established. 4 In adults, vessels are quiescent stable, rarely forming new branches under physiological conditions. Endothelial cells line the inner surface of the vessel to support tissue growth and repair, maintaining high plasticity in sensing angiogenic signals. Adequate vascularisation is a hinge step in many physiological or pathological processes, such as pregnancy, tumour metastasis, wound healing, and bone regeneration. 5
The extensive vascular network not only effectively removes metabolic waste and provides channels of action for immune monitoring and infiltration, but more importantly, it nourishes tissues as it supplies oxygen, nutrients, and growth factors to normal tissue, tumour tissue and especially traumatised tissue, such as skin flaps. 6 A prerequisite for flap survival is an adequate blood supply; however, blood supply is often limited during flap repair, and flap borders away from major vessels are prone to necrosis after grafting. Angiogenesis of the flap is the biggest problem to be solved, and by increasing the formation of new vessels and creating a new capillary network the blood supply can be improved, thus increasing the survival rate of the flap. 7 The design of any flap must therefore carefully consider the vascular structure and blood supply.
Inadequate blood perfusion, tissue ischaemia induced by inflammation and oxidative stress, and inadequate vascularisation may lead to failure of flap transfer. 8 Deheng et al. also demonstrated in a rat flap model that promoting vascular endothelial growth factor (VEGF) expression increased flap angiogenesis and that treated flaps had a greater viable tissue area and less oedema. 9 , 10 A growing number of studies have confirmed that the availability of appropriate vascularization is closely related to flap survival and quality. 11 , 12
Some preclinical studies have already focused on developing vascularisation strategies to stimulate regeneration of injured tissue, and the diversity of vascularisation signalling pathways allows it to be regulated at multiple levels. On the one hand, inadequate angiogenesis can lead to ischaemic conditions such as myocardial infarction, stroke, and poor wound healing, 13 while on the other hand, excessive vascularisation or abnormal remodelling can lead to accelerated infiltration of cancer and inflammation, for example. Preoperative and intraoperative application of VEGF has been shown to improve flap survival by increasing angiogenesis and blood supply, 14 and exosomes derived from human umbilical vascular endothelial cells (HUVECs) stimulated by oxidative stress significantly promote the pro‐angiogenic capacity of endothelial progenitor cells (EPCs) via the Wnt/β‐catenin signalling pathway, thereby improving randomised flap survival in vivo. flap survival; therefore, exosomes secreted by HUVECs after oxidative stress stimulation may also be considered as an alternative therapy to improve flap survival. 15
Good mastery of the vascularisation process has become a major, but challenging, goal. Many techniques are dedicated to promoting the progression of vascularisation, often using functional cells, such as adipose‐derived stem cells, 16 cytokines, such as VEGF, 17 and drugs such as deferoxamine 18 to promote the migration of various angiogenic cells to form microvessels, thereby improving blood supply and accelerating tissue regeneration to improve flap survival. The use of lasers to promote the expression of angiogenic markers such as VEGF and hypoxia‐inducible factor 1‐alpha (HIF‐1α) to promote angiogenesis after flap transfer is also commonly used. 19 However, the current methods that exist to stimulate angiogenesis in order to expect better flap survival have not yet achieved the desired results in clinical trials.
In summary, the importance of angiogenesis has stimulated the interest of researchers, and if this process can be captured well, it can greatly increase the chances of flap survival. Bibliometrics and visual analysis can analyse the selected documents from multiple dimensions, help to deeply understand the relationship between the retrieved documents, intuitively present the development rules and future trends of academic research in specific fields, and provide a reference and basis for academic research and innovation. 20 , 21 The aim of this paper is to explore the mechanistic link between vascularization and flaps with bibliometrics and seek better ways to develop angiogenesis in flaps, thereby increasing the chances of flap survival.
2. METHODS
2.1. Search strategies
Bibliometrics is one of the most common approaches for classifying bibliographic material. The Clarivate Analytics' ‘Web of Science Core Collection’ search function was employed to analyse the bibliometrics of vascularization and flap, which has a specific and precise search engine. All data used for the present study were downloaded on September 1, 2022. The literature search was conducted using the following search terminology: TS = (‘hem*tology’ OR ‘vascularization’ OR ‘vascularity’ OR ‘angiopoiesis’ OR ‘vas*formation’ OR ‘verifaction’ OR ‘angiogenesis’ OR ‘neovascularization’ OR ‘blood vessel formation’ OR ‘arteriogenesis’) and TS = (flap*). The Web of Science Core Collection covers 2234 relevant articles in this analysis published from 1999–2022 in English (Figure 1).
FIGURE 1.
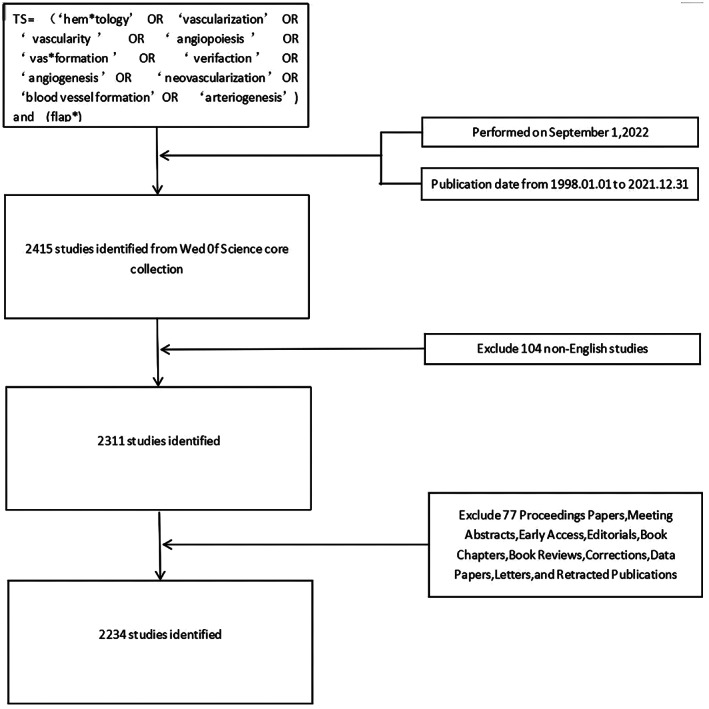
The search strategy used for the present bibliometric analysis. Searches of the Web of Science database were conducted with the following approach: TS = (‘hem*tology’ OR ‘vascularization’ OR ‘vascularity’ OR ‘angiopoiesis’ OR ‘vas*formation’ OR ‘verifaction’ OR ‘angiogenesis’ OR ‘neovascularization’ OR ‘blood vessel formation’ OR ‘arteriogenesis’) and TS = (flap*). Studies published from January 1st, 1998 to November 1st, 2022 were eligible for inclusion. Of the 2415 studies identified through this initial search, 104 non‐English studies were excluded, with 2234 of the remaining 2311 studies ultimately being included in the following analyses following the removal of 77 studies that did not meet with selected inclusion criteria. TS, topic search.
Based on the bibliometric analysis of those documents, various essential research indicators were downloaded and described, such as the most prominent authors in this theme, the year of publication, the most impactful articles, nationality, journals, and the institutions that have made the greatest contribution to this theme. All analyses were performed on a single day to avoid the impact of WoS database updates on the resultant analysis.
2.2. Data collection and analysis
Three authors (Tong X, Xiao ZH, and Li P) independently identified studies from the Web of Science Core Collection (WoSCC), which is often used in the bibliometric analysis, and extracted key data of interest like emerging trends, countries, institutions, important journals, authors, citation frequency, and the Hirsch index (H‐index) in this field. All collected data were imported into Microsoft Office 365 (Washington), VOSviewer (Leiden University, Leiden, the Netherlands), and CiteSpace V (Drexel University, Pennsylvania) for the subsequent bibliometric analyses. CiteSpace has become an important tool for bibliometric analysis in recent years and is always applied to produce a visual knowledge map for knowledge domain exploring. 22
3. RESULTS
3.1. Distribution of articles by publication years
This section presents the total number of studies found in WoS about vascularization and flap from 1999 to 2022, which is 2234. The overall publishing volume has fluctuated from 1999 to 2022, but the overall trend appears to be rising. The publications of 2020 is the highest (161), almost four times as many as the 41 publications in 1999. Based on these trends, the number of studies forecast to be published in 2022 in this field is 165. Given that 157 outputs have already been published in this field for the current year as of September 1, 2022, the actual output may exceed our forecasts. It is not difficult to draw the conclusion that the number of relevant studies in this field will continue to grow in the next 2 years, but we cannot rule out the existence of slight downward fluctuations.
3.2. Contribution of countries/regions and an analysis of international cooperation
Overall, the included studies were associated with 2058 institutions and 76 countries/regions. The research of the United States in this field has the highest number of studies, with 569 publications and leading citations which was 13 577, far exceeding those of subsequent countries. Peoples R China ranked second in the publication numbers with 315, followed by Germany (211 papers), Turkey (194 papers), Japan (173 papers), while Germany has the second highest citations which is 5225, followed by Peoples R China (3960 citations), Japan (3711 citations), Italy (2150 citations). It can be seen that the citation rate of articles in this field does not rank completely with the number of articles published.
The overall national output of the 10 most productive countries/regions is summarised in Table 1 and Figure 2B. Moreover, a large number of cooperation between many countries/regions were observed in Figure 3A, most notably between the United States and European countries.
TABLE 1.
The top 10 countries/regions with the greatest numbers of vascularization and flap relevant publications
| Rank | Country | Documents | Citations | H‐index |
|---|---|---|---|---|
| 1 | USA | 569 | 13 577 | 60 |
| 2 | PEOPLES R CHINA | 315 | 3960 | 30 |
| 3 | GERMANY | 211 | 5225 | 37 |
| 4 | TURKEY | 194 | 1826 | 22 |
| 5 | JAPAN | 173 | 3711 | 31 |
| 6 | ITALY | 117 | 2150 | 27 |
| 7 | FRANCE | 115 | 2038 | 23 |
| 8 | SOUTH KOREA | 101 | 1306 | 18 |
| 9 | SWITZERLAND | 76 | 1973 | 22 |
| 10 | BRAZIL | 64 | 778 | 16 |
FIGURE 2.

Trends of the publications number and analysis of countries in angiogenesis and flap‐related research. A, The annual worldwide publication output. B, Publication output growth trends of the top 10 countries.
FIGURE 3.
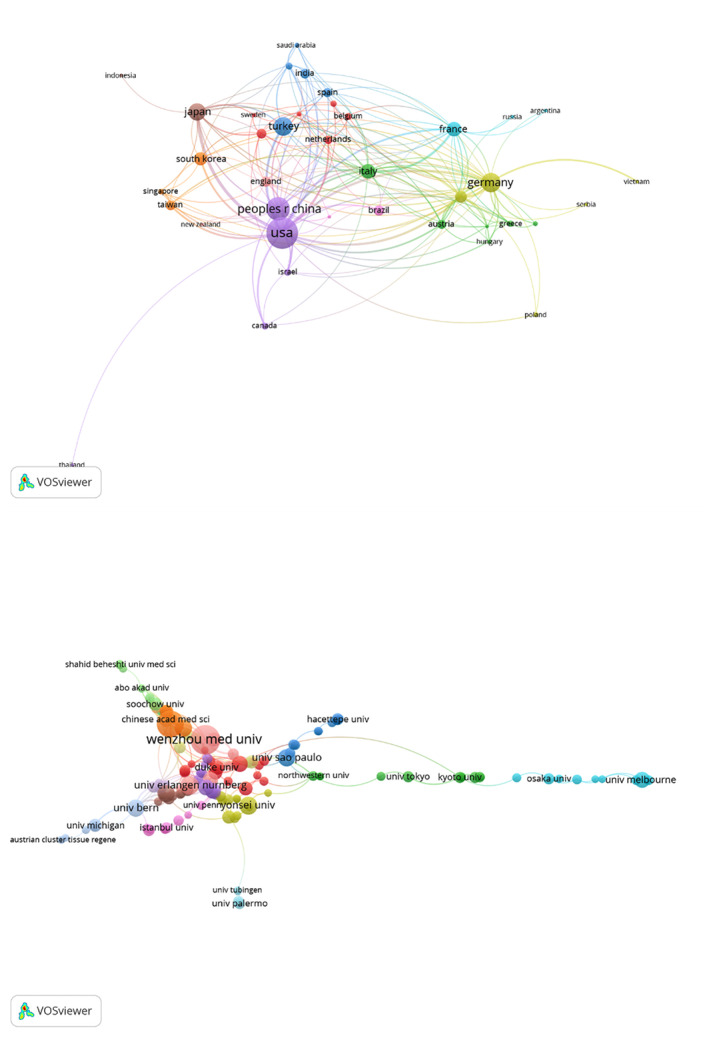
Cluster analysis of countries/regions and Institutions. A, A visualisation map was generated in which 39 countries/regions were grouped into 10 collaborative clusters, with each cluster having a specific colour. Nodes correspond to countries, with node size being proportional to the number of publications. Links indicate collaborations, with the distance and thickness of these links being related to the strength of that collaboration. B. A visualisation map was generated in which 106 institutions were grouped into 8 collaborative clusters.
A cluster analysis revealed that 39 countries/regions were represented over five times, and these were grouped into 10 clusters based upon the numbers of co‐authored articles (Figure 3A). The first cluster included Australia, Belgium, Finland, Iran, the Netherlands, and Sweden. The second cluster included Austria, Denmark, Greece, Hungary, Italy, and Romania. The third included Egypt, India, Saudi Arabia, Spain, and Turkey. The fourth cluster included Germany, Poland, Serbia, Switzerland, and Vietnam. The fifth cluster included Canada, Israel, Peoples R China, Thailand, United States. The sixth cluster included Argentina, France, and Russia. The seventh cluster included Singapore, South Korea, and Taiwan. The eighth cluster included Indonesia and Japan. The ninth cluster included Brazil and Portugal. The tenth cluster included England and New Zealand.
Overall, there was a number of collaborative research between the United States and Japan as well as the United States and Germany. Thailand, except for the United States, has poor collaborative research with other countries. In contrast to the United States, the second most productive country‐ Peoples R China tends to conduct research independently from other countries, whose reasons remain to be explored.
3.3. Institutional contributions
The 10 institutions that have contributed most substantially to this field are compiled in Table 2, and include the Wenzhou Medical University (81 papers), the Shanghai Jiao Tong University (66, 2.954%), the Udice French Research Universities (49, 2.193%), the University of Erlangen Nuremberg (41, 1.835%), and Harvard University (39, 1.746%). Similar to the data of countries/regions, the citation ranking of each institution is also quite different from the publication ranking. The University of Erlangen Nuremberg, the fourth largest publisher, has the highest number of citations which is 1485, while the University of Texas System, the seventh largest publisher, has the second highest number of citations which is 1386. We then used VOSviewer to conduct a co‐authorship analysis focused on institutions in an effort to elucidate collaborative relationships (Figure 3B). In this analysis, 106 institutions exhibited >10 documents, forming a co‐authorship network that was separated into 8 clusters. Among these institutions, the Chinese Academy of Sciences, Shanghai Jiao Tong University, and the University of Pennsylvania exhibited the closest cooperative relationships.
TABLE 2.
The top 10 institution with the greatest numbers of vascularization and flap relevant publications
| Rank | institution | Documents | Citations | H‐index |
|---|---|---|---|---|
| 1 | WENZHOU MEDICAL UNIVERSITY | 81 | 681 | 15 |
| 2 | SHANGHAI JIAO TONG UNIVERSITY | 66 | 1182 | 20 |
| 3 | UDICE FRENCH RESEARCH UNIVERSITIES | 49 | 698 | 16 |
| 4 | UNIVERSITY OF ERLANGEN NUREMBERG | 41 | 1458 | 19 |
| 5 | HARVARD UNIVERSITY | 39 | 899 | 14 |
| 6 | UNIVERSITY OF CALIFORNIA SYSTEM | 35 | 1131 | 16 |
| 7 | UNIVERSITY OF TEXAS SYSTEM | 31 | 1363 | 16 |
| 8 | EGYPTIAN KNOWLEDGE BANK EKB | 30 | 323 | 8 |
| 9 | YONSEI UNIVERSITY | 30 | 459 | 12 |
| 10 | CHANG GUNG MEMORIAL HOSPITAL | 29 | 670 | 13 |
We then used VOSviewer to conduct a co‐authorship analysis focused on institutions in an effort to elucidate collaborative relationships (Figure 3B). In this analysis, 106 institutions exhibited >10 documents, forming a co‐authorship network that was separated into eight clusters. Among these institutions, the Shanghai Jiao Tong University, Harvard Medical School, Columbia University, Stanford University, University of Bern, and New York University exhibited the closest cooperative relationships.
3.4. Leading journals in this field
In total, 607 total journals were found to have contributed to the identified research output in this field (Table 3), with the 10 most productive of these journals being compiled in Table 2. The greatest number of studies in this field were published in plastic and reconstructive surgery (177 publications, 6357 citations), followed by Annals of Plastic Surgery (151 publications, 2022 citations), and Journal of Plastic Reconstructive and Aesthetic Surgery (99 publications, 1506 citations) (Table 4).
TABLE 3.
The top 10 journals and authors with the largest number of angiogenesis and flap‐related research publications
| Rank | Journal | Documents | Citations | Rank | Author | Documents | Citations |
|---|---|---|---|---|---|---|---|
| 1 | PLASTIC AND RECONSTRUCTIVE SURGERY | 177 | 6357 | 1 | Gao WY | 31 | 295 |
| 2 | ANNALS OF PLASTIC SURGERY | 151 | 2022 | 2 | Lin DS | 30 | 350 |
| 3 | JOURNAL OF PLASTIC RECONSTRUCTIVE AND AESTHETIC SURGERY | 99 | 1506 | 3 | Horch RE | 29 | 1244 |
| 4 | JOURNAL OF RECONSTRUCTIVE MICROSURGERY | 80 | 926 | 4 | Zhang F | 27 | 793 |
| 5 | JOURNAL OF CRANIOFACIAL SURGERY | 66 | 477 | 5 | Lineaweaver WC | 24 | 753 |
| 6 | MICROSURGERY | 56 | 923 | 6 | Kneser U | 22 | 1142 |
| 7 | SURGICAL AND RADIOLOGIC ANATOMY | 35 | 524 | 7 | Arkudas A | 19 | 557 |
| 8 | JOURNAL OF SURGICAL RESEARCH | 33 | 574 | 8 | Ding J | 17 | 233 |
| 9 | AESTHETIC PLASTIC SURGERY | 32 | 249 | 9 | Li H | 17 | 268 |
| 10 | PLASTIC AND RECONSTRUCTIVE SURGERY GLOBAL OPEN | 30 | 72 | 10 | Morrison WA | 16 | 817 |
TABLE 4.
The top 10 publications with the highest citation of angiogenesis and flap‐related research articles
| Rank | Authors | Title | Journal | Year | Citations |
|---|---|---|---|---|---|
| 1 | Saint‐Cyr Michel, Wong Corrine, Schaverien Mark, Mojallal Ali, Rohrich Rod J. | The Perforasome Theory: Vascular Anatomy and Clinical Implications | PLASTIC AND RECONSTRUCTIVE SURGERY | 2009 | 436 |
| 2 | Kneser U., Schaefer D. J., Polykandriotis E., Horch R E., | Tissue engineering of bone: the reconstructive surgeon's point of view | JOURNAL OF CELLULAR AND MOLECULAR MEDICINE | 2006 | 390 |
| 3 | Kelly BT, Weiland DE, Schenker ML, Philippon MJ | Arthroscopic labral repair in the hip: Surgical technique and review of the literature | ARTHROSCOPY‐THE JOURNAL OF ARTHROSCOPIC AND RELATED SURGERY | 2005 | 297 |
| 4 | Nahabedian MY, Tsangaris T, Momen B | Breast reconstruction with the DIEP flap or the muscle‐sparing (MS‐2) free TRAM flap: Is there a difference? | PLASTIC AND RECONSTRUCTIVE SURGERY | 2005 | 228 |
| 5 | Yazar S, Lin CH, Wei FC | One‐stage reconstruction of composite bone and soft‐tissue defects in traumatic lower extremities | PLASTIC AND RECONSTRUCTIVE SURGERY | 2004 | 213 |
| 6 | Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS | Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis | FASEB JOURNAL | 1999 | 210 |
| 7 | Pflicke Holger, Sixt Michael | Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels | JOURNAL OF EXPERIMENTAL MEDICINE | 2009 | 202 |
| 8 | VandenDriessche T, Thorrez L, Naldini L, Follenzi A, Moons L, Berneman Z, Collen D, Chuah MKL | Lentiviral vectors containing the human immunodeficiency virus type‐1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen‐presenting cells in vivo | BLOOD | 2002 | 198 |
| 9 | Hwang Debby, Wang Hom Lay | Flap thickness as a predictor of root coverage: A systematic review | JOURNAL OF PERIODONTOLOGY | 2006 | 192 |
| 10 | Hacking SA, Bobyn JD, Toh KK, Tanzer M, Krygier JJ, | Fibrous tissue ingrowth and attachment to porous tantalum | JOURNAL OF BIOMEDICAL MATERIALS RESEARCH | 2000 | 179 |
3.5. Publication distributions by author
The 2234 identified publications associated with research focused on vascularization and flap were from 9453 authors, with the top 10 who contributed most to this field being shown in Table 2. Gao WY ranked first among these authors with 31 publications, followed by Lin DS (30 articles), Horch RE (29 articles), Zhang F (27 articles), Lineaweaver WC (24 articles), and Kneser U (22 articles). Horch RE and the team additionally exhibited the greatest number of citations in this analysis (1244) with an H‐index of 16, ranking them first in this research field. Kneser U was ranked sixth in the field, with a team that had published 22 articles, while their citations were ranked second, which cited 1142 times, and the H‐index was 14. Almost all of these authors had H‐index values greater than 10.
Highly cited articles in this research space included the most highly cited article by Saint‐Cyr Michel et al. published in Plastic and Reconstructive Surgery in 2009 with 436 citations, followed by a review article by Kneser U. et al. (390 citations) in the Journal of Cellular and Molecular Medicine.
3.6. Web of science categories
In a category analysis, 20 categories that appeared over 60 times were identified (Table 5). Among these categories, Surgery, Medicine Research Experimental, Dentistry Oral Surgery Medicine, Orthopaedics, Engineering Biomedical, Otorhinolaryngology, Radiology Nuclear Medicine Medical Imaging, Peripheral Vascular Disease, and Urology/Nephrology were respectively ranked 1, 2, 3, 5, 6, 8,12, 17, and 19.
TABLE 5.
The top 20 web of science categories with the largest number of vascularization‐related research in the context of flap articles
| Rank | Web of Science category | Documents | Rank | Web of Science category | Documents |
|---|---|---|---|---|---|
| 1 | Surgery | 1287 | 11 | Oncology | 59 |
| 2 | Medicine Research Experimental | 162 | 12 | Radiology Nuclear Medicine Medical Imaging | 58 |
| 3 | Dentistry Oral Surgery Medicine | 132 | 13 | Anatomy Morphology | 57 |
| 4 | Cell Biology | 118 | 14 | Materials Science Biomaterials | 57 |
| 5 | Orthopaedics | 112 | 15 | Medicine General Internal | 57 |
| 6 | Engineering Biomedical | 97 | 16 | Ophthalmology | 53 |
| 7 | Dermatology | 92 | 17 | Peripheral Vascular Disease | 52 |
| 8 | Otorhinolaryngology | 74 | 18 | Biotechnology Applied Microbiology | 49 |
| 9 | Cell Tissue Engineering | 70 | 19 | Urology Nephrology | 46 |
| 10 | Pharmacology Pharmacy | 61 | 20 | Multidisciplinary Sciences | 45 |
3.7. Keyword analysis
Next, the VOSviewer application was used to extract keywords from the titles of the abstracts of these 2234 studies, revealing 186 keywords that were present a minimum of 15 times. These keywords were subsequently grouped into four clusters based upon the number of articles in which they co‐occurred (Figure 4A). The first cluster (red) consisted of 71 keywords, the highest frequency of which were anatomy, reconstruction, flap, defects, and surgery. The second cluster (green) consisted of 55 keywords, the highest frequency of which were angiogenesis, survival, endothelial growth‐factor, model, expression and VEGF. The third cluster (blue) consisted of 45 keywords, the highest frequency of which were vascularization, tissue, transplantation, stem‐cells, tissue engineering, and regeneration. The fourth cluster (yellow) consisted of 15 keywords, the highest frequency of which were neovascularization, therapy, growth factor, wound healing, revascularization, hypoxia, and proliferation. The most current keywords in this network included autophagy, oxidative stress, indocyanine green, scaffold, random skin flap, outcomes, protection, ischemia/reperfusion injury, and adipogenesis (Figure 4B).
FIGURE 4.
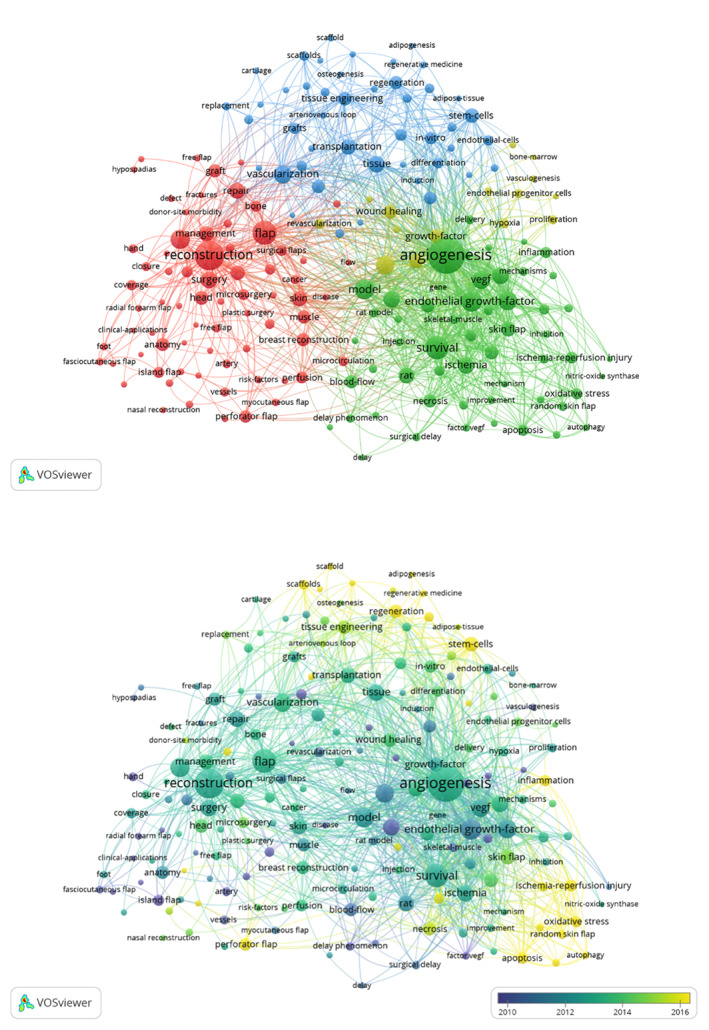
A co‐occurrence network visualisation map for keywords. A, A visualisation map grouped into five clusters consisting of nodes with the same colour. B, Node colour corresponds to average publication year associated with the indicated keywords. Nodes represent keywords, node size is proportional to publication number, and line thickness and the size between nodes is proportional to the relative strength of that collaborations. Yellow nodes respectively corresponding to earlier and more recent publications.
3.8. Co‐cited reference analysis
Using CiteSpace 5.6.R4 visualisation software, using the keyword as the node type, the items with the most references or appearances in the top 5% were selected to get a co‐occurrence map (Figure 5). Afterwards, we conducted a temporal co‐citation analysis (Figure 6), which revealed that the majority of the co‐cited references were published from 1999 to 2000. Perforator flap (cluster #0), tissue engineering (cluster #1), angiogenesis (cluster #2), graft (cluster #3), and repair (cluster #4) emerged as early and persistent hotspots in this field. Cluster #0 (perforator flap) contained the greatest number of articles.
FIGURE 5.
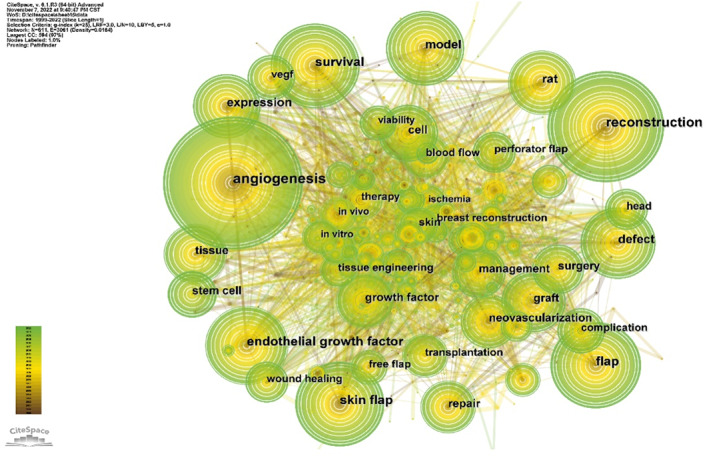
Co‐occurrence of keywords in research on the correlation between vascularization and flap.
FIGURE 6.
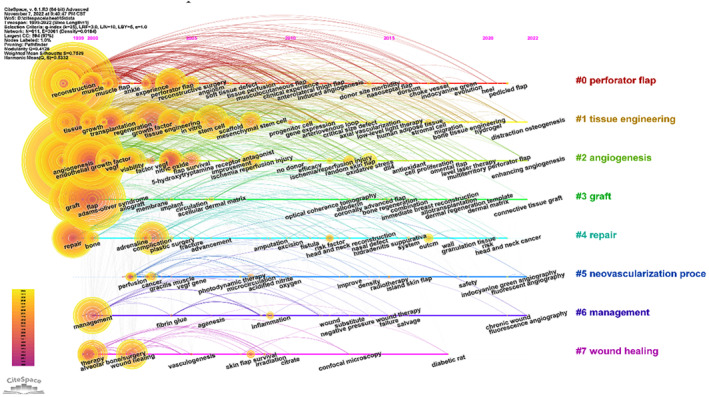
Timeline overview of co‐cited studies associated with angiogenesis and flap‐related research.
In order to better realise the value of literature retrieval and induction that plays a leading role in the research in this field, in the basis of using ‘timeline’ to show the keyword co‐occurrence map, the top 25 highlighted keywords were obtained (Figure 7). The highest intensity includes ‘oxidative stress’ (15.09, 2014–2022) and ‘gene therapy’ (12.13, 2004–2011). With the deepening of the research, the highlighted keywords gradually transformed into oxidative ‘oxidative stress’ (15.09, 2014–2022), ‘scaffold’ (9.3, 2007–2022), ‘protect’ (5.76, 2013–2022), ‘injury’ (5.19, 2002–2022), and ‘hydrogel’ (6.26, 2020–2022), all of which to some extent confirm the conclusion of diversification, specialisation and in‐depth of the research in this field in the stage of the research surge in recent years.
FIGURE 7.
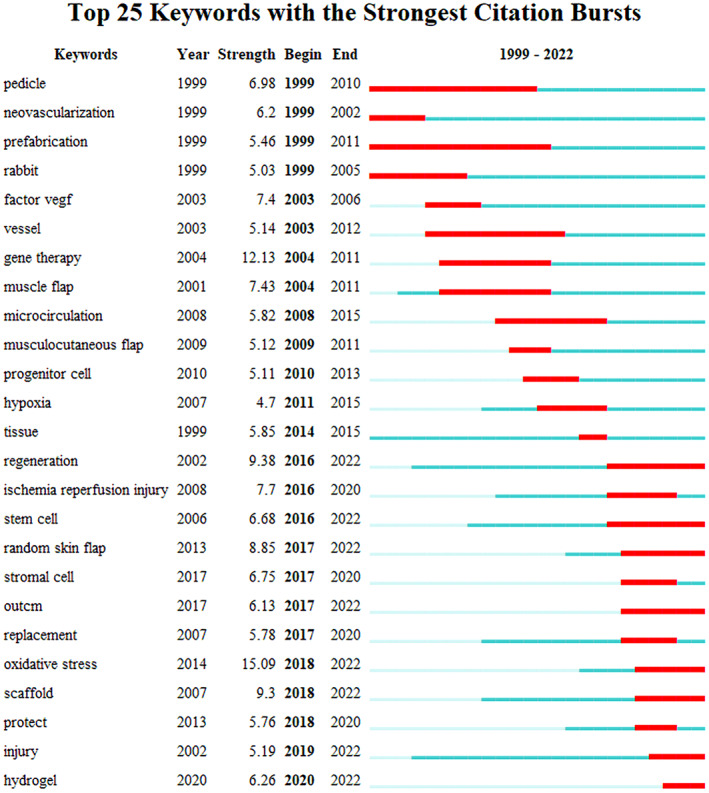
Burst keywords in research on the correlation between vascularization and flap.
4. DISCUSSION
4.1. Overview of the global quality and status of publications in this field
First, in the extraction of publications, we choose Web of Science as it provides precise and specific search engines for our selected fields. The selected literature were results not only based on the database used but also strongly influenced by the search strategy. Although constant revisions have been made to our search strategy, there were still some articles related to tumours and oral mucosal diseases, 23 , 24 which have no direct relationship with vascularization and skin flaps. Generally speaking, the bias caused by these individual articles is limited, while a large number of articles related to vascularization and flap are presented. The overall number of publications has grown steadily.
By evaluating the total number of citations produced by a given country, researchers will be able to judge the academic impact of that nation in a specific field. Currently, the United States has made the greatest impact on research pertaining to vascularization and flap (569 publications, 13 577 citations), followed by Germany (211, 5225), Peoples R China (315, 3960). The H‐index of the United States (60) was also far ahead of other countries, making it the only country with a total number of citations of more than 10 000. Both the H‐index and total number for citations of the United States were almost twice or even 20 times more than those of other top‐10 countries in publications. Thus, it can be inferred that the United States has been the overall leader in this field to date. In contrast, the H‐index for BRAZIL was relatively low (16), which was the last one in the top 10 countries, while the total number of citations for BRAZIL was also the lowest (778).
The growth in the number of publications in China is impressive in all fields, including vascularization and flap. Along with this, although the number of articles from China is second only to the United States in vascularization and flap research, the number and proportion of Chinese articles cited has not seen a significant increase, and even the number of citations was far lower than that of Germany. However, it is worth noting that the frequency of citations does not fully reflect the inherent scientific value of the article. The possibility of being cited may be reduced because the names of Chinese researchers have not been recognised by all countries and regions. At the same time, it does not rule out that the influence of China's articles in this field was lower than that of Germany and Japan, whose publication numbers were lower than China's.
The top 10 institutes publishing research on vascularization and flap were basically consistent with the top 10 countries in this research area. Chinese and American institutions account for half of the top 10 institutes. It is worth noting that the United States and China also ranked in the top two in the publication numbers, which illustrates the importance of focused research institutes as representative of the academic output for a given nation. Researchers such as Gao WY and Horch RE who had published higher numbers of studies generally possess more representation and a higher reputation in the field, while the contributions of Gao WY were mostly published in the last 5 years. Based on the existing research in the last 5 years on vascularization and flap, Lin DS and Ding J are also likely to further explore and discover this field.
When researchers consider selecting journals to publish research results, they usually hope that journals are relevant to the publication theme, and also hope that their research can reach a large number of interested readers. Plastic and Reconstructive Surgery and Annals of Plastic Surgery have published the greatest number of studies focused on vascularization and flap, followed by Reconstructive and Asthetic Surgery, Journai of Reconstructive Microsurgery Journal, and Craniofacial Surgery. Taking the quality of publications into consideration, Plastic and Reconstructive Surgery has published three of the top 10 highly‐cited publications, while journals in other fields such as Cellular and Molecular Medicine. It can be seen that the articles related to vascularization and flap were mainly published in the journals related to plastic surgery and reconstruction, 25 , 26 , 27 which makes it more convenient for researchers to query and read relevant journals. In the meantime, researchers in this field should pay attention to contributions from cytobiology and other journals.
4.2. Hotspot analysis of research pertaining to vascularization and flap
In this paper, we performed a bibliometric analysis of vascularization and flap, which has been shown to be a positive and rapidly developing scientific field with critical progress. In general, the number of publications was on the rise, the highest number in 2020 is almost four times that of 1999, but there were several fluctuations in the publications number. More than 76 countries and 2058 institutions have explored and contributed to this scientific field.
With the development of regenerative repair technology, flap transplantation, an important technique commonly used in reconstructive surgery, has developed more and more operation methods and new exploration. 28 , 29 , 30 Selection of flaps for wound coverage requires consideration of their inherent anatomical and vascular characteristics, as well as their immunogenicity and lymphatic properties and their ability to promote healing and improve the function of the recipient site. 31 , 32 , 33 Among them, the insurance of an adequate blood supply is particularly important for the survival and function of the flap to effectively promote angiogenesis, especially in the distal region of the flap, and prevent necrosis. During bibliometric analysis, we found that the improvement of flap operation was mainly through the exploration of anatomy and radiology, using autopsy, Doppler ultrasound, computed tomography, and vessel angiography to determine the key blood vessels of the flap. 34 , 35 Many studies focused on the molecular mechanisms and related pathways of stem cells and human umbilical vein endothelial cells in the hope of effectively promoting vascularization. 36 There have also been new postoperatively evaluated flap microcirculation and perfusion, such as fluorescence imaging techniques. 37 , 38 However, the safe and effective dose of fluorescence technology still needs to be further explored. 39 Many articles have evaluated the effectiveness of flap transplantation by conducting retrospective studies on wound size, degree of vascularization, surgical technique, and complications. 40 , 41 , 42 In addition, studies have shown that vascularization can also promote nerve regeneration and repair, providing a healthy tissue bed for the flap. 43 We found it interesting that in addition to the common pedicled flap, musculocutaneous flap, fascia flap, and flap, omentum flap, as a free flap, has become a valuable tool in reconstruction. Free omentum flaps can also be used for lymphedema treatment and vascularization, as well as for cerebrospinal fluid leakage. 44 , 45 Meanwhile, the arteriovenous (AV) loop model is considered an alternative to conventional free flaps as it permits the creation of significant volumes of axially vascularized tissue. 29
New research hotspots such as tissue engineering might bring a breakthrough to this certain field; scaffolds and bioengineered adipose tissue are being used as a promising substitute for autologous skin flaps for defect reconstruction. 46 While the survival of engineered tissue structures also depends on adequate vascularization, challenges remain in inducing vascularization for long‐term function. 47 Many high‐impact factor articles are also mainly related to tissue engineering technologies, reflecting the importance of translational medicine in flap repair and its future strategy and development direction. 48 , 49 , 50 Predicting the future research direction and development trend of vascularization and flap based on the current literature can effectively and accurately guide researchers towards further research. In view of these results, we believe that many detailed studies on vascularization and flap will be published in the coming years.
5. CONCLUSION
The research on vascularization and flaps is increasing, focusing on the flap operation, the molecular mechanism to promote flap vascularization, the methods to enhance the viability of the flap, and tissue engineering techniques so as to better promote the survival and functional maintenance of the flap. However, the international community is still faced with a great challenge to effectively improve flap angiogenesis. In summary, our bibliometric analysis provides a comprehensive overview of vascularization and flap research over the past 20 years, which may help researchers better understand the general profile and future trends in this field.
AUTHOR CONTRIBUTIONS
Xiao‐Fei Tong: Conceptualization, Methodology, Formal analysis, Writing Original draft preparation. Zhen‐Yang Xiao: Conceptualization, Visualisation, Formal analysis, Writing‐Original draft preparation. Pei‐Ting Li: Conceptualization, Methodology, Software, Writing‐Reviewing and Editing. Yang Hu: Investigation, Validation. Xiang‐Yu Chen: Methodology, Writing‐Reviewing and Editing. Ming‐Zhu Wang: Validation. Jian‐Da Zhou: Conceptualization, Writing‐Reviewing and Editing, Supervision. Xiao‐Fei Tong, Zhen‐Yang Xiao and Pei‐Ting Li Contributed equally.
FUNDING INFORMATION
This research was funded by Hunan Science and technology innovation plan, Grant/Award Number: 2018JJ2616, 2021SK53714; Key Research and Development Program of Hunan Province, Grant/Award Numbers: 2018SK2081, 2018SK2083, 2018SK2084; 2020 Li Ka Shing Foundation Cross‐Disciplinary Research Grant, Grant/ Award Numbers: 2020LKSFG18B, 2020LKSFG02E.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We thank the associate editor and the reviewers for their useful feedback that improved this paper, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript. All the contributors agree the publication of this paper.
Tong X‐F, Xiao Z‐Y, Li P‐T, et al. Angiogenesis and flap‐related research: A bibliometric analysis. Int Wound J. 2023;20(8):3057‐3072. doi: 10.1111/iwj.14181
Xiao‐Fei Tong, Zhen‐Yang Xiao, and Pei‐Ting Li contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data derived from public domain resources.
REFERENCES
- 1. Hudgins PA. Flap reconstruction in the head and neck: expected appearance, complications, and recurrent disease. Eur J Radiol. 2002;44(2):130‐138. [DOI] [PubMed] [Google Scholar]
- 2. Maciel‐Miranda A, Morris SF, Hallock GG. Local flaps, including pedicled perforator flaps: anatomy, technique, and applications. Plast Reconstr Surg. 2013;131(6):896e‐911e. [DOI] [PubMed] [Google Scholar]
- 3. Sajib S, Zahra FT, Lionakis MS, German NA, Mikelis CM. Mechanisms of angiogenesis in microbe‐regulated inflammatory and neoplastic conditions. Angiogenesis. 2018;21(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 4. Eelen G, Treps L, Li X, Carmeliet P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. 2020;127(2):310‐329. [DOI] [PubMed] [Google Scholar]
- 5. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409‐426. [DOI] [PubMed] [Google Scholar]
- 6. Menger MM, Laschke MW, Nussler AK, Menger MD, Histing T. The vascularization paradox of non‐union formation. Angiogenesis. 2022;25(3):279‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee JH, You HJ, Lee TY, Kang HJ. Current status of experimental animal skin flap models: ischemic preconditioning and molecular factors. Int J Mol Sci. 2022;23(9):5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He JB, Ma XY, Li WJ, Liu YY, Lin DS. Exenatide inhibits necrosis by enhancing angiogenesis and ameliorating ischemia/reperfusion injury in a random skin flap rat model. Int Immunopharmacol. 2021;90:107192. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida S, Yoshimoto H, Hirano A, Akita S. Wound healing and angiogenesis through combined use of a vascularized tissue flap and adipose‐derived stem cells in a rat hindlimb irradiated ischemia model. Plast Reconstr Surg. 2016;137(5):1486‐1497. [DOI] [PubMed] [Google Scholar]
- 10. Deheng C, Kailiang Z, Weidong W, et al. Salidroside promotes random skin flap survival in rats by enhancing angiogenesis and inhibiting apoptosis. J Reconstr Microsurg. 2016;32(8):580‐586. [DOI] [PubMed] [Google Scholar]
- 11. Lin J, Jia C, Wang Y, et al. Therapeutic potential of pravastatin for random skin flaps necrosis: involvement of promoting angiogenesis and inhibiting apoptosis and oxidative stress. Drug Des Dev Ther. 2019;13:1461‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang R, Lin C, Jiang C, Huang Z, Gao W, Lin D. Nobiletin enhances the survival of random pattern skin flaps: involvement of enhancing angiogenesis and inhibiting oxidative stress. Int Immunopharmacol. 2020;78:106010. [DOI] [PubMed] [Google Scholar]
- 13. Caporali A, Bäck M, Daemen MJ, et al. Future directions for therapeutic strategies in post‐ischaemic vascularization: a position paper from European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology. Cardiovasc Res. 2018;114(11):1411‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang T, Lineaweaver WC, Chen MB, Kisner C, Zhang F. Effects of vascular endothelial growth factor on survival of surgical flaps: a review of experimental studies. J Reconstr Microsurg. 2014;30(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 15. Guo L, Chen Y, Feng X, et al. Oxidative stress‐induced endothelial cells‐derived exosomes accelerate skin flap survival through Lnc NEAT1‐mediated promotion of endothelial progenitor cell function. Stem Cell Res Ther. 2022;13(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia P, Zhang K, Gong Y, Li G, Yan S, Yin J. Injectable stem cell laden open porous microgels that favor adipogenesis: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2017;9(40):34751‐34761. [DOI] [PubMed] [Google Scholar]
- 17. Akimoto M, Takeda A, Matsushita O, et al. Effects of CB‐VEGF‐A injection in rat flap models for improved survival. Plast Reconstr Surg. 2013;131(4):717‐725. [DOI] [PubMed] [Google Scholar]
- 18. Duscher D, Neofytou E, Wong VW, et al. Transdermal deferoxamine prevents pressure‐induced diabetic ulcers. Proc Natl Acad Sci U S A. 2015;112(1):94‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cury V, Moretti AI, Assis L, et al. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF‐1α and MMP‐2. J Photochem Photobiol B Biol. 2013;125:164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma L, Ma J, Teng M, Li Y. Visual analysis of colorectal cancer immunotherapy: a bibliometric analysis from 2012 to 2021. Front Immunol. 2022;13:843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan X, Chang C, Chen X, Li K. Emerging trends and focus of human gastrointestinal microbiome research from 2010‐2021: a visualized study. J Transl Med. 2021;19(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593‐608. [DOI] [PubMed] [Google Scholar]
- 23. Sakamoto H, Uematsu M, Morihana T, et al. Reconstruction of oral defects using free revascularized jejunum transfer. Gan no Rinsho Jpn J Cancer Clin. 1985;31(8):908‐913. [PubMed] [Google Scholar]
- 24. Amoh Y, Yang M, Li L, et al. Nestin‐linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 2005;65(12):5352‐5357. [DOI] [PubMed] [Google Scholar]
- 25. Fischer S, Lee TC, Krezdorn N, et al. First lower two‐thirds osteomyocutaneous facial allograft perfused by a unilateral facial artery: outcomes and vascularization at 1 year after transplantation. Plast Reconstr Surg. 2017;139(5):1175e‐1183e. [DOI] [PubMed] [Google Scholar]
- 26. Salibian AH, Menick FJ, Talley J. Microvascular reconstruction of the nose with the radial forearm flap: a 17‐year experience in 47 patients. Plast Reconstr Surg. 2019;144(1):199‐210. [DOI] [PubMed] [Google Scholar]
- 27. Kumar K, Jaffe W, London NJ, Varma SK. Free flap neovascularization: myth or reality? J Reconstr Microsurg. 2004;20(1):31‐34. [DOI] [PubMed] [Google Scholar]
- 28. Shandalov Y, Egozi D, Koffler J, et al. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci U S A. 2014;111(16):6010‐6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt VJ, Wietbrock JO, Leibig N, et al. Haemodynamically stimulated and in vivo generated axially vascularized soft‐tissue free flaps for closure of complex defects: evaluation in a small animal model. J Tissue Eng Regen Med. 2018;12(3):622‐632. [DOI] [PubMed] [Google Scholar]
- 30. Bonnel F. New concepts on the arterial vascularization of skin and muscle. Plast Reconstr Surg. 1985;75(4):552‐559. [DOI] [PubMed] [Google Scholar]
- 31. Hallock GG, Morris SF. Skin grafts and local flaps. Plast Reconstr Surg. 2011;127(1):5e‐22e. [DOI] [PubMed] [Google Scholar]
- 32. Novaes AB, Kon S, Ruben MP, Novaes AB Jr. Rebuilding of microvascularization following surgical gingival elimination by split flap. Study by perfusion and diaphanization. J Periodontol. 1976;47(4):217‐223. [DOI] [PubMed] [Google Scholar]
- 33. Djordjevic ML, Perovic SV, Slavkovic Z, Djakovic N. Longitudinal dorsal dartos flap for prevention of fistula after a Snodgrass hypospadias procedure. Eur Urol. 2006;50(1):53‐57. [DOI] [PubMed] [Google Scholar]
- 34. Carrasco‐López C, Julian Ibañez JF, Vilà J, et al. The anterior intercostal artery flap: anatomical and radiologic study. Plast Reconstr Surg. 2017;139(3):613e‐619e. [DOI] [PubMed] [Google Scholar]
- 35. Dominici C, Pacifici A, Tinti A, Cordellini M, Flamini FO. Preoperative and postoperative evaluation of latissimus dorsi myocutaneous flap vascularization by color flow duplex scanning. Plast Reconstr Surg. 1995;96(6):1358‐1365. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Q, Chiu Y, Chen Y, et al. Harnessing the synergy of perfusable muscle flap matrix and adipose‐derived stem cells for prevascularization and macrophage polarization to reconstruct volumetric muscle loss. Bioactive Mater. 2023;22:588‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li K, Zhang Z, Nicoli F, et al. Application of indocyanine green in flap surgery: a systematic review. J Reconstr Microsurg. 2018;34(2):77‐86. [DOI] [PubMed] [Google Scholar]
- 38. Capozzi VA, Ceni V, Sozzi G, et al. Endoscopic near infrared and indocyanine green to verify the viability of the subcutaneous flap for vulvar cancer. Gynecol Oncol. 2019;154(3):653‐654. [DOI] [PubMed] [Google Scholar]
- 39. Jiang J, Dong C, Zhai L, et al. Paeoniflorin suppresses TBHP‐induced oxidative stress and apoptosis in human umbilical vein endothelial cells via the Nrf2/HO‐1 signaling pathway and improves skin flap survival. Front Pharmacol. 2021;12:735530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delgove A, Weigert R, Casoli V. A new local muscle flap for elbow coverage‐the medial triceps brachii flap: anatomy, surgical technique, and preliminary outcomes. J Shoulder Elbow Surg. 2018;27(4):733‐738. [DOI] [PubMed] [Google Scholar]
- 41. Andermahr J, Helling HJ, Rehm KE, Koebke Z. The vascularization of the os calcaneum and the clinical consequences. Clin Orthop Relat Res. 1999;363:212‐218. [PubMed] [Google Scholar]
- 42. Schreiber A, Mattavelli D, Ferrari M, et al. The turbinal flap: an additional option for anterior skull base reconstruction. Cadaveric feasibility study and case report. Int Forum Allergy Rhinol. 2017;7(2):199‐204. [DOI] [PubMed] [Google Scholar]
- 43. Saffari TM, Bedar M, Hundepool CA, Bishop AT, Shin AY. The role of vascularization in nerve regeneration of nerve graft. Neural Regen Res. 2020;15(9):1573‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mazzaferro D, Song P, Massand S, Mirmanesh M, Jaiswal R, Pu LLQ. The omental free flap—a review of usage and physiology. J Reconstr Microsurg. 2018;34(3):151‐169. [DOI] [PubMed] [Google Scholar]
- 45. Fay LY, Lin YR, Liou DY, et al. The application of an Omentum graft or flap in spinal cord injury. Int J Mol Sci. 2021;22(15):7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mihaly E, Altamirano DE, Tuffaha S, Grayson W. Engineering skeletal muscle: building complexity to achieve functionality. Semin Cell Dev Biol. 2021;119:61‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qi D, Wu S, Kuss MA, et al. Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering. Acta Biomater. 2018;74:131‐142. [DOI] [PubMed] [Google Scholar]
- 48. Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv. 2016;34(2):112‐121. [DOI] [PubMed] [Google Scholar]
- 49. Cai L, Wang Q, Gu C, et al. Vascular and micro‐environmental influences on MSC‐coral hydroxyapatite construct‐based bone tissue engineering. Biomaterials. 2011;32(33):8497‐8505. [DOI] [PubMed] [Google Scholar]
- 50. Wang X, Yu Y, Yang C, Shang L, Zhao Y, Shen X. Dynamically responsive scaffolds from microfluidic 3D printing for skin flap regeneration. Adv Sci (Weinheim, Baden‐Wurttemberg, Germany). 2022;9(22):e2201155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data derived from public domain resources.


