Highlights
-
•
Innate immunity contributes to the primary defense against infections and new studies revealed that lncRNAs play a key role in these responses.
-
•
The expression levels of lncRNAs ANRIL and THRIL were significantly up-regulated in moderate and severe patients compared to the control group.
-
•
Higher levels of lncRNAs ANRIL and THRIL were observed in severe patients compared to moderate patients.
-
•
LncRNA NEAT1 levels in moderate and severe COVID-19 patients were significantly higher than the healthy group.
-
•
Blood levels of lncRNAs ANRIL and THRIL are linked with COVID-19 severity and can be considered as a circulating biomarker of disease progression.
Keywords: COVID-19, SARS-CoV-2, Long non-coding RNA, ANRIL, THRIL, NEAT1, MALAT1
Abstract
The current outbreak of coronavirus disease 2019 (COVID-19) is a global emergency, as its rapid spread and high mortality rate, which poses a significant threat to public health. Innate immunity plays a crucial role in the primary defense against infections, and recent studies have highlighted the pivotal regulatory function of long non-coding RNAs (lncRNAs) in innate immune responses. This study aims to assess the circulating levels of lncRNAs namely ANRIL, THRIL, NEAT1, and MALAT1 in the blood of moderate and severe SARS-CoV-2 infected patients, in comparison to healthy individuals. Additionally, it aims to explore the potential of these lncRNAs as biomarkers for determining the severity of the disease. The blood samples were collected from a total of 38 moderate and 25 severe COVID-19 patients, along with 30 healthy controls. The total RNA was extracted and qPCR was performed to evaluate the blood levels of the lncRNAs. The results indicate significantly higher expression levels of lncRNAs ANRIL and THRIL in severe patients when compared to moderate patients (P value = 0.0307, P value = 0.0059, respectively). Moreover, the expression levels of lncRNAs ANRIL and THRIL were significantly up-regulated in both moderate and severe patients in comparison to the control group (P value < 0.001, P value < 0.001, P value = 0.001, P value < 0.001, respectively). The expression levels of lncRNA NEAT1 were found to be significantly higher in both moderate and severe COVID-19 patients compared to the healthy group (P value < 0.001, P value < 0.001, respectively), and there was no significant difference in the expression levels of NEAT1 between moderate and severe patients (P value = 0.6979). The expression levels of MALAT1 in moderate and severe patients did not exhibit a significant difference compared to the control group (P value = 0.677, P value = 0.764, respectively). Furthermore, the discriminative power of ANRIL and THRIL was significantly higher in the severe patient group than the moderate group (Area under curve (AUC) = 0.6879; P-value = 0.0122, AUC = 0.6947; P-value = 0.0093, respectively). In conclusion, the expression levels of the lncRNAs ANRIL and THRIL are correlated with the severity of COVID-19 and can be regarded as circulating biomarkers for disease progression.
1. Introduction
COVID-19, caused by the SARS-CoV-2 virus (severe acute respiratory syndrome coronavirus 2), is a potentially lethal disease that poses a significant global public health threat (Nile et al., 2020). SARS-CoV-2 primarily targets the lower respiratory system and leads to a variable range of clinical manifestations, spanning asymptomatic and mild symptomatic cases to severe, life-threatening infections and fatalities (Shoraka et al., 2021; Shi et al., 2020).
It is crucial to identify precise and appropriate prognostic factors to distinguishing between mild, moderate and severe patients, predicting disease progression, determining outcomes, and anticipating morbidity and mortality among COVID-19 patients. Several reports have suggested that evaluating pattern changes in routine blood values (RBVs), including hematological, biochemical and immunological biomarkers, might be helpful in predicting the disease course and mortality. For instance, non-surviving patients exhibited increased leukocyte and neutrophil levels, as well as and decreased lymphopenia and eosinopenia levels. Furthermore, markers such as erythrocyte sedimentation rate (ESR), international normalized ratio (INR), prothrombin time (PT), C-reactive protein (CRP), d-dimer, and ferritin are significant factors associated with mortality (Huyut et al., 2022; Huyut and Ilkbahar, 2021; Huyut and Huyut, 2021; Mertoglu et al., 2021; Huyut and Huyut, 2023; Tahir Huyut et al., 2022).
However, COVID-19 is a complex disease influenced by multiple factors, such as age, comorbidities, and genetic background. It is widely accepted that the genetic background of the host plays a significant role in virus entry, immune responses, and viral infections (Debnath et al., 2020; Nguyen et al., 2023). Long non-coding RNAs (lncRNAs) are among the factors that regulate immune responses.
LncRNA are a subgroup of non-coding RNAs (ncRNA) with a length exceeding 200 nucleotides (Mendell et al., 2004). Various studies have demonstrated that lncRNAs can be classified based on their specific functions, including mediating chromatin modification and DNA methylation in the context of epigenetic regulation (Portela and Esteller, 2010), interactions with proteins (especially transcription factor) and DNA contributing to transcription regulation, mRNA processing during the post-transcriptional stage, as well as interactions with proteins to modulate protein translation and post-translation modifications (X Zhang et al., 2019; Bond et al., 2011; Wang and Chang, 2011).
LncRNAs play a crucial role in various essential biological processes, such as transcription, translation, gene expression regulation, immune responses, and more (Kornienko et al., 2013; Chen and Yan, 2013). Consequently, mutations and disruption of lncRNA regulation have been associated with a broad spectrum of human diseases, including cancer (van Poppel et al., 2012), cardiovascular diseases (Congrains et al., 2012), HBV-related cirrhosis (S Shoraka et al., 2021), and neurodegeneration diseases (Johnson, 2012). Evidence has revealed that lncRNAs play a regulatory role in the IFN signaling pathway, activation of JAK-STAT and NF-κB signaling pathway, as well as the production of cytokines and chemokines in respiratory viruses such as influenza A virus (IAV), respiratory syncytial virus (RSV) and SARS-CoV-2 (Kesheh et al., 2022; Pan et al., 2019; Wu et al., 2020; Wu et al., 2021). Given the involvement of lncRNAs in regulating innate immune response pathways against viruses, studying them can significantly contribute to the monitoring, control, and treatment of viral diseases (Liu and Ding, 2017).
The lncRNA antisense noncoding RNA in the INK4 locus (ANRIL) is transcribed from chromosome 9p21 (Zhou et al., 2016), which is a hotspot for coronary artery disease (CAD) (McPherson et al., 2007), This locus is also associated with open angle glaucoma, diabetes, periodontitis, and various cancers (Congrains et al., 2013). Another lncRNA, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), is located on chromosome 11q13.1. Studies have shown that MALAT1 is upregulated in many cancer tissues (Fang et al., 2016), as well as systemic lupus erythematosus (SLE) (Yang et al., 2017). Furthermore, the lncRNA nuclear-enriched abundant transcript 1 (NEAT1) is transcribed from the multiple endocrine neoplasia locus, which has been implicated in cancer development (Li et al., 2016). TNF-α and heterogeneous nuclear ribonucleoprotein L (hnRNPL)-related immunoregulatory lncRNA (THRIL) function as effective regulators of genes expression in immune responses. Recent studies have also demonstrated that THRIL plays a crucial role in controlling the production of various cytokines (Liang et al., 2020). Importantly, these lncRNAs are involved in the NF-κB signaling pathway (Zhou et al., 2016; Gong et al., 2020; Chen et al., 2018).
Due to the significant role of the NF-κB signaling pathway in COVID-19, our study aimed to assess the blood expression levels of ANRIL, THRIL, NEAT1, and MALAT1 in both moderate and severe COVID-19 patients. We compared these levels to those of a healthy control group, with the aim of identifying potential biomarkers that could serve as predictors of COVID-19 infection severity.
2. Material and methods
2.1. Study design and patients
In this study, we compared 38 moderate patients (15 females and 23 males) and 25 severe patients (7 females and 18 males) who were admitted to Taleghani and Imam Hossein medical and educational Hospitals of Shahid Beheshti University of Medical Sciences (SBMU), with clinically approved and laboratory-confirmed positive cases of COVID-19, detected through real-time PCR analysis of throat swab samples. Additionally, 30 healthy subjects (11 females and 19 males) were included for comparison purposes. The inclusion criteria for this study were as follows: hospitalization, age of at least 18 years old, positive test results for SARS-CoV-2, presence of pneumonia, and the presence or absence of comorbidities and cancers. Exclusion criteria consisted of pregnant women and patients under 18 years old. Disease severity was classified based on the clinical classification outlined in the WHO interim guidance (Organization, 2020). Moderate patients were adults with pneumonia but without severe pneumonia and oxygen saturation levels above 90%. On the other hand, severe patients included adults with severe pneumonia and oxygen saturation levels below 90% (Peng et al., 2020).
The study protocols received approval from the ethics committee of the Research Institute for Gastroenterology and Liver Disease (IR.SBMU.RIGLD.REC.1399.008, Tehran, Iran), and informed consent was obtained from all participants. Sample collection took place from March to September 2020.
2.2. RNA extraction and real-time PCR (RT-PCR)
The total RNA was extracted from the whole blood samples by Hybrid-R™ blood RNA extraction kit (GeneAll Biotechnology, South Korea) following the manufacturer's instructions. cDNA was synthesized using the Thermo Scientific RevertAid Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). qPCR was performed to quantify the expression profile of lncRNAs ANRIL, MALAT1, THRIL, and NEAT1 in whole blood of COVID-19 patients and healthy control group using the SYBR Green (RealQ plus 2x Master Mix Green, Ampliqon, Odense, Denmark) approach with relevant forward and reverse primers, the β2-Microglobulin was served as the internal reference gene. The qPCR was performed as follows steps: 95 °C for 15 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 60 s. The relative expression of RNA was computed based on the 2−ΔΔCT method. The primer sequence was as follows:
5′-TTATGCTTTGCAGCACACTGG-3′ (forward) and 5′-GTTCTGCCACAGCTTTGATCT-3′ (reverse) for ANRIL, 5′-CTTCCTCCCTTTAACTTATCCATTCAC-3′ (forward) and 5′-CTCT TCCTCCACCATTACCAACAATAC-3′ (reverse) for NEAT1, 5′-AAAGCAAGGTCTCCCCA CAA-3′ (forward) and 5′-GGTCTGTGCTAGATCAAAAGGCA-3′ (reverse) for MALAT1, 5′-CTGGGACTACAGATGCACCAC-3′ (forward) and 5′-GGAGGGAGCATGTCTGTTTCT-3′ (reverse) for THRIL, and 5′-TGCTGTCTCCATGTTTGATGTATCT-3′ (forward) and 5′-TCTC TGCTCCCCACCTCTAAGT-3′ (reverse) for β2-Microglobulin.
2.3. Statistical analysis
All statistical analyses were performed using Social Science Software Package 16 (SPSS Inc, Chicago, Illinois, USA).
Categorical variables were presented as frequency and percentage, while continuous variables were given as the mean ± standard deviation (Mean±SD).
Shapiro-Wilk test was used to verify the normality of distributions of quantitative variables. Tests of homogeneity of variances were performed using Levene's test. The normally distributed data were compared using the independent sample t-test, and those that were not normally distributed were analyzed with the Mann-Whitney U test. Categorical variables were analyzed with the χ2 test. For gene expression analysis, One-way ANOVA followed by the Tukey's HSD post-hoc test and also Kruskal-Wallis test followed by Dunn's post-hoc comparisons were utilized.
Receiver operating characteristic (ROC) curves were constructed for circulating lncRNAs, using the area under curve (AUC) as the global discrimination value measure. GraphPad Prism 8 was used to plot the charts. P< 0.05 was statistically significant.
3. Results
3.1. Baseline characteristics
In this study, we collected data from 38 patients with moderate COVID-19 symptoms, 25 patients with severe COVID-19 symptoms, and 30 healthy subjects. Out of the 63 infected patients, 9 (9.67%) severe patients died during hospitalization. The age range of severe patients was between 34 and 91 years, moderate patients between 29 and 84 years, and the healthy group between 23 and 50 years.
The demographic data of patients and control groups are presented in Table 1. Based on Table 1, there was no significant difference in the distribution of gender between patients and controls (p-value = 0.6395), and age as a confounding factor, was adjusted using regression analysis. The samples from moderate and severe patients were collected 10 days post-infection (dpi).
Table 1.
Demographic data of groups.
| Healthy control | Moderate patient | Severe patients | P-value | |
|---|---|---|---|---|
| Number of subjects | 30 | 38 | 25 | |
| Age (mean±SD) | 35.6 ± 6.66437 | 55.55±14.17401 | 65.64±15.54799 | 0.0027 |
| (SE) | 1.2167 | 2.2993 | 3.1096 | |
| Gender | 0.6395 | |||
| Male, n (%) | 19 (63.33) | 23 (60.52) | 18 (72) | |
| Female, n (%) | 11 (36.66) | 15 (39.47) | 7 (28) |
We classified the severity of the disease based on the clinical classification outlined in the WHO interim guidance (WHO, 2020). Moderate patients were defined as adults with pneumonia but no indication of severe pneumonia and oxygen saturation level of over 90%. On the other hand, severe patients referred to the adolescent or adults with severe pneumonia and oxygen saturation level below 90% (Peng et al., 2020).
The clinical features and laboratory information of moderate and severe patients are presented in Tables 2 and 3, respectively. Table 3 reveals that severe patients exhibited higher concentrations of LDH, and d-dimer, accompanied by reduced oxygen saturation and lymphocyte levels compared to moderate patients. These blood values were measured upon patients’ admission time to the hospital.
Table 2.
Clinical characteristics of patients with SARS-CoV-2 infection.
| symptoms | Moderate patients (%) (n = 38) | Severe patients (%) (n = 25) | P-value |
|---|---|---|---|
| Fever | 36.84% (14 of 38) | 40% (10 of 25) | 0.801 |
| cough | 39.47% (15 of 38) | 52% (13 of 25) | 0.328 |
| dyspnea | 44.73% (17 of 38) | 64% (16 of 25) | 0.134 |
| Myalgia | 23.68% (9 of 38) | 16% (4 of 25) | 0.461 |
| Chest pain | 10.52% (4 of 38) | 28% (7 of 25) | 0.074 |
| Diarrhea | 10.52% (4 of 38) | 16% (4 of 25) | 0.523 |
Table 3.
Clinicopathological data of patients with SARS-CoV-2 infection.
| Normal range | Moderate patients (n = 38) | Severe patients (n = 25) | P-value | |
|---|---|---|---|---|
| Laboratory findings (Mean±SD) | ||||
| O2 saturation (Spo2) | – | 93.20 ± 4.46 | 86.23 ± 7.87 | < 0.001 |
| WBC × 109 (U/L) | 4.5–10.5 × 109 | 7.75 ± 3.96 | 9.04 ± 3.69 | 0.223 |
| Lymphocyte × 109 (U/L) | 1.32–3.57 × 109 | 2.358 ± 9.29 | 1.540 ± 7.77 | 0.011 |
| PLT × 109 (U/L) | 150–400 × 109 | 187.05 ± 90.11 | 252.50 ± 82.65 | 0.115 |
| ALT (U/L) | 0–41 | 40.12 ± 36.21 | 85.75 ± 18.39 | 0.252 |
| AST (U/L) | 0–40 | 40.67 ± 32.66 | 74.88 ± 12.20 | 0.209 |
| Hb (g/ml) | 13–17.5 | 10.78 ± 2.63 | 11.28 ± 2.94 | 0.509 |
| LDH (U/L) | <248 | 513.16 ± 140.81 | 1107.12 ± 751.69 | 0.03 |
| ESR (mm/h) | 0–15 | 47.20 ± 37.01 | 26.43 ± 20.76 | 0.76 |
| D-dimer (mg/L) | 0–500 | 928.33 ± 1056.59 | 2270.81 ± 3541.12 | 0.01 |
| CRP (mg/L) | >10 | 31.83 ± 32.38 | 43.44 ± 39.72 | 0.247 |
| Comorbidity | ||||
| Chronic pulmonary diseases | 0% (0 of 38) | 12% (3 of 25) | 0.029 | |
| Diabetes | 12.43% (7 of 38) | 24% (6 of 25) | 0.241 | |
| Hypertension | 21% (8 of 38) | 32% (8 of 25) | 0.329 | |
| Cardiovascular diseases | 15.8% (6 of 38) | 28% (7 of 25) | 0.592 | |
| Chronic kidney diseases | 10.5% (4 of 38) | 8% (2 of 25) | 0.156 | |
| Cancers | 7.9% (3 of 38) | 16% (4 of 25) | 0.865 | |
| Metastatic Adenocarcinoma | 1 of 38 | 2 of 25 | – | |
| Lymphoma | 1 of 38 | 1 of 25 | – | |
| Hepatoblastoma | 1 of 38 | 1 of 25 | – |
Furthermore, we conducted an assessment of the association between LDH, d-dimer, and the following lncRNAs: ANRIL, THRIL, NEAT1, and MALAT1. However, no correlation was found between these laboratory factors and the mentioned lncRNAs. Out of the 38 moderate patients; 19 (50%) had comorbidities, while among the 25 severe patients; 15 (60%) had comorbidities. Among all comorbid conditions, only chronic pulmonary diseases exhibited significantly different frequencies between the moderate and severe groups. Additionally, among the nine severe patients who passed away, three had Adenocarcinoma and Hepatoblastoma (Table 3).
3.2. Blood levels of lncRNAs ANRIL, THRIL, NEAT1, and MALAT1 in moderate and severe COVID-19 patients and healthy controls
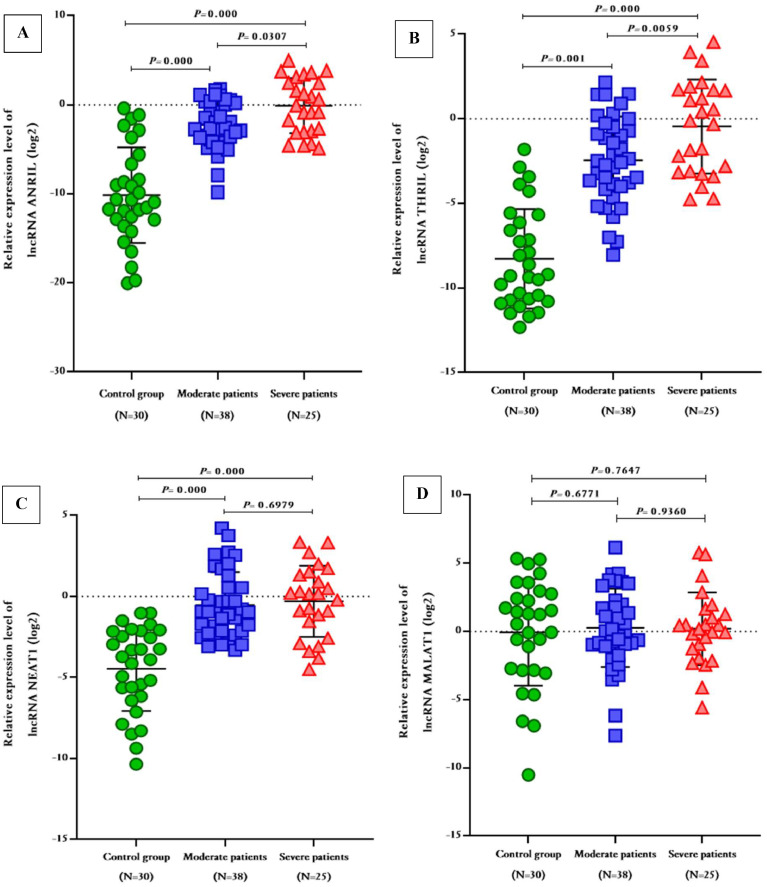
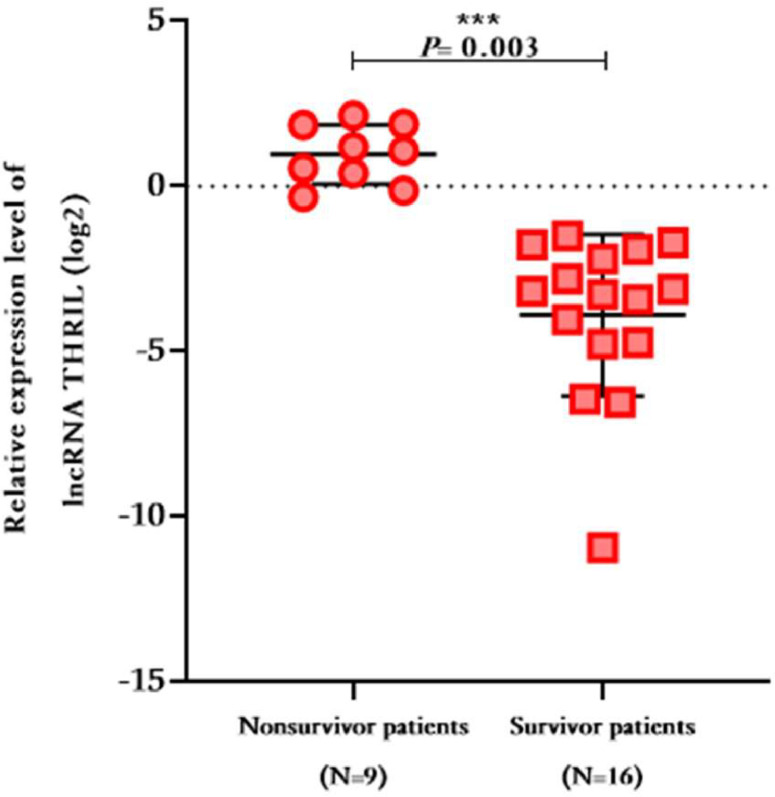
As depicted in Fig. 1, the expression levels of ANRIL and THRIL were significantly higher in severe patients compared to moderate patients (Fold change ANRIL = 2.163; 95% CI 0.2055 to 4.120; P-value = 0.0307, Fold change THRIL = 1.999; 95% CI 0.5896 to 3.409; P-value = 0.0059, respectively). Moreover, these aforementioned lncRNAs exhinited significant up-regulation in both moderate and severe patients when compared to the control group (Fold change ANRIL = 7.885; 95% CI 6.029 to 9.741; P-value = 0.000, 10.05; 95% CI 7.990 to 12.11; P-value = 0.000; respectively, Fold change THRIL = 5.821; 95% CI 4.484 to 7.158; P-value = 0.001; 7.820; 9% CI 6.338 to 9.303; P-value = 0.000; respectively). Additionally, the expression levels of lncRNA NEAT1 were significantly elevated in moderate and severe COVID-19 patients when compared to the healthy group (Fold change = 3.936; 95% CI 6.338 to 9.303; P-value = 0.000, 4.164; 95% CI 2.938 to 5.390; P-value = 0.000; respectively). However, there was no significant difference in NEAT1 levels between moderate and severe patients (Fold change = 0.2285; 95% CI -0.9373 to 1.394; P-value = 0.6979). On the other hand, the expression levels of MALAT1 patients did not show a significant difference in moderate and severe patients did not show a significant difference in comparison with the control group (Fold change = 0.3258; P-value = 0.677, 0.2596; P-value = 0.764, respectively), and there was no significant difference in expression level of MALAT1 in moderate and severe SARS-CoV-2 infected patients (Fold change = 0.0661, P-value = 0.9360). The expression level of these lncRNAs were compared between survivors and non-survivors within the severe patients, and it was observed that the mean expression level of THRIL was higher in non-survivors compared to survivors’ patients (Fold change = 3.808 ± 1.146, P-value = 0.003), whereas no other significant differences were found (Fig. 2).
Fig. 1.
The relative expression levels of lncRNAs ANRIL, THRIL, NEAT1, and MALAT1 in moderate and severe COVID-19 patients in comparison with healthy controls.
Fig. 2.
The relative expression level of THRIL in non-survivors compared to survivors in severe COVID-19 infected patients.
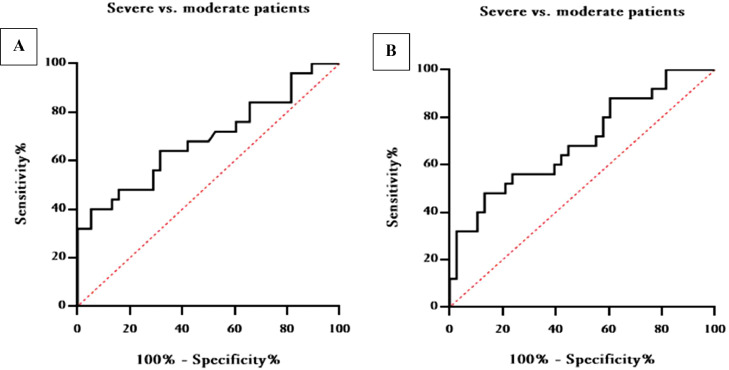
3.3. Prognostic value of lncRNAs ANRIL and THRIL levels in predict of COVID-19 severity
The receiver operating characteristic (ROC) curve was utilized to assess the specificity and sensitivity of lncRNAs ANRIL and THRIL as predictors of severity in patients infected with SARS-CoV-2. As shown in Table 4, the area under curve (AUC) of ANRIL in distinguishing severe from moderate patients was 0.6879 (95% CI 0.5480 to 0.8278, P-value = 0.0122), and the optimal cut off was calculated to be > 0.5645 (Sensitivity, 56%; Specificity, 71.05%) (Fig. 3A). Conversely, the AUC of THRIL in differentiating severe from moderate patients was 0.6947 (95% CI 0.5602 to 0.8293, P-value = 0.0093), and optimal cut off was also calculated to be > 0.7565 (Sensitivity, 56%; Specificity, 76.32%) (Fig. 3B).
Table 4.
Roc curve analysis of lncRNAs to differentiate moderate from severe patients.
| Variables | AUC | Sensitivity | Specificity | 95% CI | P-value |
|---|---|---|---|---|---|
| ANRIL (Cut off > 0.5645) | 0.6879 | 56% | 71.055% | 0.5480 to 0.8278 | 0.0122 |
| THRIL (Cut off > 0.7565) | 0.6947 | 56% | 76.32% | 0.5602 to 0.8293 | 0.0093 |
| NEAT1 (Cut off > 0.5378) | 0.5511 | 64% | 52.63% | 0.3994 to 0.7027 | 0.4956 |
| MALAT (Cut off > 1.529) | 0.5200 | 68% | 44.74% | 0.3739 to 0.6661 | 0.7895 |
Fig. 3.
ROC curve analysis of blood lncRNAs ANRIL (A) and THRIL (B) for prognosis of disease severity in COVID-19 infected patients.
4. Discussion
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially detected in Wuhan, China, in December 2019 (Makhmalbaf et al., 2022; Thijssen et al., 2020). This particular virus is falls under the category of highly pathogenic human coronavirus (HCoV) (Ye et al., 2020). The proposed mechanism behind COVID-19 involves the disruption of cytokine regulation, known as cytokine storm, which significantly impacts disease severity (Ghazavi et al., 2021). In fact, the cytokine storm is the main cause of acute respiratory distress syndrome (ARDS) and multiple organ failure (Chousterman et al., 2017). The persistent high morbidity and mortality associated with the SARS-CoV-2 pandemic pose a threat to global public health (Paniri and Akhavan-Niaki, 2020). Given this situation, the urgent need to develop therapeutic strategies with minimal side effects to tackle this virus has emerged (Ye et al., 2020). Therefore, effectively suppressing the cytokine storm plays a crucial role in preventing patient deterioration and saving lives (Wan et al., 2020).
Long noncoding RNAs (lncRNAs) are widely expressed in mammalian cells and play a vital role as RNA regulators in various cellular processes, including the activation of inflammatory signaling pathways (Heward and Lindsay, 2014; Wang et al., 2021). Thousands of lncRNAs are regulated by RNA or DNA viruses infections (Wapinski and Chang, 2011). The innate immune responses are the first line of host defense against viral infection stimulates innate immune responses (Hadjicharalambous and Lindsay, 2019). During virus invasion, host cells can sense and identify virus components as pathogen-associated molecular patterns (PAMPs) via pathogen recognition receptors (PRRs) on the cell surface [Toll-like receptor (TLR) 2 and 4]. TLR2 and TLR4 are demonstrated able to induce the different lncRNAs expressions. Additionally, PRR-dependent signaling pathways activate transcription factors (TFs) such as NF-κB, IRF-3, and IRF-7. Following this activation, interferons, chemokines, and cytokines are expressed (Schneider et al., 2014; Iwasaki and Pillai, 2014; Ouyang et al., 2016). Numerous studies have stated that NF-κB signaling pathways are strongly activated in COVID-19 patients (Amini-Farsani et al., 2021; Hadjadj et al., 2020; Sohn et al., 2020; García, 2020; Zhou et al., 2020) and many of the lncRNAs affect these pathway. However, the present study focused on ANRIL, THRIL, NEAT1, and MALAT1 which are involved in NF-κB signaling pathways regulation (Zhou et al., 2016; Gong et al., 2020; Chen et al., 2018; Chew et al., 2018; Xu et al., 2020; Kuai et al., 2021; Huang et al., 2022). Zhou et al. (Zhou et al., 2016) were the first to identify the connection between ANRIL and NF-κB signaling pathways, suggesting that ANRIL regulates the expression of IL6 and IL8 through binding with a transcriptional factor. The lncRNA THRIL can play a role in regulating of inflammatory response and TNF-α expression, whereas TNF is an activator of NF-κB signaling pathways (Newton and Manning, 2016; Li et al., 2014). NEAT1, is a proinflammatory lncRNA that promotes inflammation by inducing cytokines such as IL6 (Zhang et al., 2019; Rodrigues et al., 2021). IL6, IL8, and TNF-α play vital roles in the innate immune responses to SARS-CoV-2 infection. Therefore, identifying lncRNAs involved in the inflammatory response triggered by COVID-19 can serve as a prognostic biomarker and potential therapeutic target in SARS-CoV-2 infected patients (Zhang and Chu, 2019).
The broad spectrum of activities and various regulatory mechanisms of lncRNAs suggest that these transcripts are the main regulators of host immunity during viral infection. Limited studies have explored the involvement of specific lncRNAs in virus-associated cancers. For example, the lncRNA ANRIL has been investigated in Kaposi's sarcoma-associated herpesvirus (KSHV) infected cells (Sethuraman et al., 2017) and HTLV-1-induced (Song et al., 2018), while MALAT1 has been examined in HIV-1 infected cell line (Zhang et al., 2013; Qu et al., 2019), as well as in high-risk human papillomavirus (HR-HPV) (Jiang et al., 2014), HBV/HCV-hepatocellular carcinoma (HCC) (Lai et al., 2012; Lin et al., 2007), and in Epstein-Barr virus (EBV) positive cell lines (Zhang et al., 2019). Influenza, herpes simplex viruses (HSV) (Imamura et al., 2014), and HIV-1 (Zhang et al., 2013; Liu et al., 2018) has been linked to the induction of the lncRNA NEAT1. Additionally, the lncRNA THRIL shows upregulation in Zika virus (ZIKV) infected cells (Hu et al., 2017).
The present study was performed to evaluate the circulating blood levels of lncRNAs ANRIL, THRIL, NEAT1, and MALAT1 in moderate and severe COVID-19 patients and explore their potential roles as prognostic biomarkers that may predict COVID-19 severity.
According to our findings, the lncRNAs ANRIL and THRIL were found to be up-regulated in patients with severe COVID-19 compared to those with moderate symptoms. Additionally, both ANRIL and THRIL expression levels were higher in patients with COVID-19 compared healthy controls. The expression level of lncRNA NEAT1 was also increased in both severe and moderate COVID-19 patients when compared to the control group. On the other hand, there was no significant difference in the expression level of lncRNA MALAT1 between severe and moderate patients and healthy controls. In non-survivors of the severe group, the lncRNA THRIL exhibited higher expression levels compared to patients who survived. These findings suggest that ANRIL and THRIL might serve as a potential indicators of disease severity in COVID-19 patients. However, it is important to note that the golden for COVID-19 diagnosis remains the RT-PCR test, which detects the presence of SARS-CoV-2 genome in patients’ samples.
Recently, in silico analysis revealed that MALAT1 and NEAT1 expression levels increased in SARS-CoV-2 infected cells (Laha et al., 2021). Also, bioinformatics and computational evaluations determined the lncRNAs MALAT1 and NEAT1 upregulation in SARS-CoV-2 infected normal human bronchial epithelial cells (NHBE) (Vishnubalaji et al., 2020). Tang et al. (2020) demonstrated an elevation in the expression of NEAT1 and MALAT1 in the whole blood of moderate and severe COVID-19 patients when compared to healthy controls. Similarly, Rodrigues et al. (2021) found a significant increase in the levels of MALAT1 and NEAT1 in saliva and nasopharyngeal swab samples collected from COVID-19 patients. In another study, Abbasi-Kolli et al. (2022) observed that expression levels of THRIL and MALAT1 significantly increased in PBMC samples of acute COVID-19 patients compared to the healthy control and these groups did not show a significant difference in the NEAT1 expression level. Also, based on Huang et al. (2021) results, NEAT1 and MALAT1 have higher expression levels in severe case in comparison to mild COVID-19 infected patients.
Additionally, we found that patients with severe cases of COVID-19 were significantly older compared to those with less severity, which is consistent with current literature (Nabavi et al., 2021; Angioni et al., 2020). Furthermore, we observed a higher prevalence of chronic pulmonary diseases in severe cases. Clinical factors associated with increased disease severity included elevated LDH and d-dimer levels, as well as decreased oxygen saturation and lymphocyte count. Our findings align with previous studies that have reported lower oxygen saturation (Nabavi et al., 2021; Chen et al., 2020) and lymphocyte levels (Wang et al., 2020; Qin et al., 2020; Zhang et al., 2020), as well as elevated LDH and d-dimer levels (Chen et al., 2020; Zhang et al., 2020) in severe COVID-19 cases compared to moderate cases. It is important to note some limitations of this study, such as the inability to conduct long-term patient follow-ups and the unavailability of data on survival rates.
5. Conclusions
The recognition of lncRNAs involved in inflammatory responses to SARS-CoV-2 infection can be considered a novel approach to identify prognostic biomarkers and therapeutic targets for the treatment of COVID-19 patients. The present study specifically suggests that the circulating biomarkers lncRNAs ANRIL and THRIL exhibit good sensitivity in predicting the severity of COVID-19, thereby aiding in prognostic assessment. Additionally, further investigation into the role of lncRNAs in the progression of COVID-19 may provide valuable insights for the severity of disease severity and ultimately saving patients' lives.
Ethics statement
The study protocols were approved by the ethics committee of the Research Institute for Gastroenterology and Liver Disease (IR.SBMU.RIGLD.REC.1399.008, Tehran, Iran), and informed consent was collected from all participants.
Authors' contribution
ZR, SMH and SRM conceived the study, ENM, HM, SHSHA, MRN and HMA performed the sample collection, ZR, SRM and MSN carried out the laboratory and molecular tests, ZR, SHSHO and SRM carried out the interpretation and analyze of the data, ZR, SRM and SMH drafted the manuscript, HM, MRN, SHSHA and MRZ critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR-RIGLD-1110.2). The authors are grateful to the Institute laboratory staff for their valuable assistance in the present study, especially Mrs. Shabnam Kazemian.
Data availability
Data will be made available on request.
References
- Abbasi-Kolli M., Nahand J.S., Kiani S.J., Khanaliha K., Khatami A.R., Taghizadieh M., et al. The expression patterns of MALAT-1, NEAT-1, THRIL, and miR-155-5p in the acute to the post-acute phase of COVID-19 disease. Braz. J. Infect. Dis. 2022;26:102354–102362. doi: 10.1016/j.bjid.2022.102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Farsani Z., Yadollahi-Farsani M., Arab S., Forouzanfar F., Yadollahi M., Asgharzade S. Prediction and analysis of microRNAs involved in COVID-19 inflammatory processes associated with the NF-kB and JAK/STAT signaling pathways. Int. Immunopharmacol. 2021;100 doi: 10.1016/j.intimp.2021.108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angioni R., Sánchez-Rodríguez R., Munari F., Bertoldi N., Arcidiacono D., Cavinato S., et al. Age-severity matched cytokine profiling reveals specific signatures in COVID-19 patients. Cell Death Dis. 2020;11(11):1–12. doi: 10.1038/s41419-020-03151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A., Row P., Dudley E. Post-translation modification of proteins; methodologies and applications in plant sciences. Phytochemistry. 2011;72(10):975–996. doi: 10.1016/j.phytochem.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Chen X., Yan G.Y. Novel human lncRNA–disease association inference based on lncRNA expression profiles. Bioinformatics. 2013;29(20):2617–2624. doi: 10.1093/bioinformatics/btt426. [DOI] [PubMed] [Google Scholar]
- Chen Y., Qiu J., Chen B., Lin Y., Chen Y., Xie G., et al. RETRACTED: long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-κB pathway. Int. Immunopharmacol. 2018;59:252–260. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew C.L., Conos S.A., Unal B., Tergaonkar V. Noncoding RNAs: master regulators of inflammatory signaling. Trends Mol. Med. 2018;24(1):66–84. doi: 10.1016/j.molmed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K., Weber G.F. Springer; 2017. Cytokine Storm and Sepsis Disease pathogenesis. Seminars in Immunopathology. [DOI] [PubMed] [Google Scholar]
- Congrains A., Kamide K., Oguro R., Yasuda O., Miyata K., Yamamoto E., et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis. 2012;220(2):449–455. doi: 10.1016/j.atherosclerosis.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Congrains A., Kamide K., Ohishi M., Rakugi H. ANRIL: molecular mechanisms and implications in human health. Int. J. Mol. Sci. 2013;14(1):1278–1292. doi: 10.3390/ijms14011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath M., Banerjee M., Berk M. Genetic gateways to COVID-19 infection: implications for risk, severity, and outcomes. FASEB J. 2020;34(7):8787. doi: 10.1096/fj.202001115R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Zhang S., Wang Y., Shen S., Wang F., Hao Y., et al. Long non-coding RNA MALAT-1 modulates metastatic potential of tongue squamous cell carcinomas partially through the regulation of small proline rich proteins. BMC Cancer. 2016;16(1):1–10. doi: 10.1186/s12885-016-2735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137 doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.P., Zhang Y.W., Su X.Q., Gao H.B. Inhibition of long noncoding RNA MALAT1 suppresses high glucose-induced apoptosis and inflammation in human umbilical vein endothelial cells by suppressing the NF-κB signaling pathway. Biochem. Cell. Biol. 2020;98(6):669–675. doi: 10.1139/bcb-2019-0403. [DOI] [PubMed] [Google Scholar]
- Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjicharalambous M.R., Lindsay M.A. Long non-coding RNAs and the innate immune response. Noncoding RNA. 2019;5(2):34. doi: 10.3390/ncrna5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heward J.A., Lindsay M.A. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014;35(9):408–419. doi: 10.1016/j.it.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Huo Y., Yang L., Chen G., Luo M., Yang J., et al. ZIKV infection effects changes in gene splicing, isoform composition and lncRNA expression in human neural progenitor cells. Virol. J. 2017;14(1):1–11. doi: 10.1186/s12985-017-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, K.; Wang, C.; Vagts, C.; Raguveer, V.; Finn, P.W.; Perkins, D.L., LncRNAs NEAT1 and MALAT1 differentiate inflammation in severe COVID-19 patients. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- Huang K., Wang C., Vagts C., Raguveer V., Finn P.W., Perkins D.L. Long non-coding RNAs (lncRNAs) NEAT1 and MALAT1 are differentially expressed in severe COVID-19 patients: an integrated single-cell analysis. PLoS One. 2022;17(1) doi: 10.1371/journal.pone.0261242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyut M.T., Huyut Z. Forecasting of Oxidant/Antioxidant levels of COVID-19 patients by using Expert models with biomarkers used in the Diagnosis/Prognosis of COVID-19. Int. Immunopharmacol. 2021;100:108127. doi: 10.1016/j.intimp.2021.108127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyut M.T., Huyut Z.J.H. Effect of ferritin, INR, and d-dimer immunological parameters levels as predictors of COVID-19 mortality: a strong prediction with the decision trees. Heliyon. 2023;9(3) doi: 10.1016/j.heliyon.2023.e14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyut M.T., Ilkbahar F. The effectiveness of blood routine parameters and some biomarkers as a potential diagnostic tool in the diagnosis and prognosis of Covid-19 disease. Int. Immunopharmacol. 2021;98:107838. doi: 10.1016/j.intimp.2021.107838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyut M.T., Huyut Z., Ilkbahar F., Mertoğlu C. What is the impact and efficacy of routine immunological, biochemical and hematological biomarkers as predictors of COVID-19 mortality? Int. Immunopharmacol. 2022;105:108542. doi: 10.1016/j.intimp.2022.108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Imamachi N., Akizuki G., Kumakura M., Kawaguchi A., Nagata K., et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol. Cell. 2014;53(3):393–406. doi: 10.1016/j.molcel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014;14(5):315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Li Y., Fang S., Jiang B., Qin C., Xie P., et al. The role of MALAT1 correlates with HPV in cervical cancer. Oncol. Lett. 2014;7(6):2135–2141. doi: 10.3892/ol.2014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. Long non-coding RNAs in Huntington's disease neurodegeneration. Neurobiol. Dis. 2012;46(2):245–254. doi: 10.1016/j.nbd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kesheh M.M., Mahmoudvand S., Shokri S. Long noncoding RNAs in respiratory viruses: a review. Rev. Med. Virol. 2022;32(2):e2275. doi: 10.1002/rmv.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornienko A.E., Guenzl P.M., Barlow D.P., Pauler F.M. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11(1):1–14. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai F., Zhou L., Zhou J., Sun X., Dong W. Long non-coding RNA THRIL inhibits miRNA-24-3p to upregulate neuropilin-1 to aggravate cerebral ischemia-reperfusion injury through regulating the nuclear factor κB p65 signaling. Aging. 2021;13(6):9071. doi: 10.18632/aging.202762. (albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha S., Saha C., Dutta S., Basu M., Chatterjee R., Ghosh S., et al. In silico analysis of altered expression of long non-coding RNA in SARS-CoV-2 infected cells and their possible regulation by STAT1, STAT3 and interferon regulatory factors. Heliyon. 2021;7(3):e06395. doi: 10.1016/j.heliyon.2021.e06395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Chao T.C., Chang K.Y., Lin N., Patil V.S., Shimizu C., et al. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. 2014;111(3):1002–1007. doi: 10.1073/pnas.1313768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wei D., Yang C., Sun H., Lu T., Kuang D. Overexpression of long noncoding RNA, NEAT1 promotes cell proliferation, invasion and migration in endometrial endometrioid adenocarcinoma. Biomed. Pharmacother. 2016;84:244–251. doi: 10.1016/j.biopha.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Liang Y., Li H., Gong X., Ding C. Long non-coding RNA THRIL mediates cell growth and inflammatory response of fibroblast-like synoviocytes by activating PI3K/AKT signals in rheumatoid arthritis. Inflammation. 2020;43(3):1044–1053. doi: 10.1007/s10753-020-01189-x. [DOI] [PubMed] [Google Scholar]
- Lin R., Maeda S., Ca L., Karin M., Edgington T. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26(6):851–858. doi: 10.1038/sj.onc.1209846. [DOI] [PubMed] [Google Scholar]
- Liu W., Ding C. Roles of LncRNAs in viral infections. Front. Cell Infect. Microbiol. 2017;7:205. doi: 10.3389/fcimb.2017.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Hu P.W., Couturier J., Lewis D.E., Rice A.P. HIV-1 replication in CD4+ T cells exploits the down-regulation of antiviral NEAT1 long non-coding RNAs following T cell activation. Virology. 2018;522:193–198. doi: 10.1016/j.virol.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Yang Z., Zhou L., Zhu Q.Q., Xie H.Y., Zhang F., et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med. Oncol. 2012;29(3):1810–1816. doi: 10.1007/s12032-011-0004-z. [DOI] [PubMed] [Google Scholar]
- Makhmalbaf M., Hosseini S.M., Asadzadeh H., Saeedi Niasar M., Shoraka S., Yadegar B., et al. Detection of SARS-CoV-2 genome in stool and plasma samples of laboratory confirmed Iranian COVID-19 patients. Front. Mol. Biosci. 2022;9:865129–865137. doi: 10.3389/fmolb.2022.865129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson R., Pertsemlidis A., Kavaslar N., Stewart A., Roberts R., Cox D.R., et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.T., Sharifi N.A., Meyers J.L., Martinez-Murillo F., Dietz H.C. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 2004;36(10):1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- Mertoglu C., Huyut M.T., Arslan Y., Ceylan Y., Coban T.O.C., Investigation L. How do routine laboratory tests change in coronavirus disease 2019? Scand. J. Clin. Lab. Invest. 2021;81(1):24–33. doi: 10.1080/00365513.2020.1855470. [DOI] [PubMed] [Google Scholar]
- Nabavi S., Javidarabshahi Z., Allahyari A., Ramezani M., Seddigh-Shamsi M., Ravanshad S., et al. Clinical features and disease severity in an Iranian population of inpatients with COVID-19. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-87917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K., Manning G. Necroptosis and inflammation. Annu. Rev. Biochem. 2016;85:743–763. doi: 10.1146/annurev-biochem-060815-014830. [DOI] [PubMed] [Google Scholar]
- A. Nguyen, J. David, S. Maden Human leukocyte antigen susceptibility map for SARS-CoV-2 [published online ahead of print April 17, 2020]. J. Virol. 2023.510:20.
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is suspected: Interim guidance, 13 March 2020. [Google Scholar]
- Ouyang J., Hu J., Chen J.L. lncRNAs regulate the innate immune response to viral infection. Wiley Interdiscip. Rev. RNA. 2016;7(1):129–143. doi: 10.1002/wrna.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Zhao Z., Liao Y., Chiu S.H., Wang S., Chen B., et al. Identification of an interferon-stimulated long noncoding RNA (LncRNA ISR) involved in regulation of influenza A virus replication. Int. J. Mol. Sci. 2019;20(20):5118. doi: 10.3390/ijms20205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniri A., Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: role of lncRNAs in cytokine storm modulation. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F., Tu L., Yang Y., Hu P., Wang R., Hu Q., et al. Management and treatment of COVID-19: the Chinese experience. Can. J. Cardiol. 2020;36(6):915–930. doi: 10.1016/j.cjca.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A., Esteller M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D., Sun W.W., Li L., Ma L., Sun L., Jin X., et al. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res. 2019;47(6):3013–3027. doi: 10.1093/nar/gkz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A.C., Adamoski D., Genelhould G., Zhen F., Yamaguto G.E., Araujo-Souza P.S., et al. NEAT1 and MALAT1 are highly expressed in saliva and nasopharyngeal swab samples of COVID-19 patients. Mol. Oral Microbiol. 2021;36(6):291–294. doi: 10.1111/omi.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman S., Gay L.A., Jain V., Haecker I., Renne R. MicroRNA dependent and independent deregulation of long non-coding RNAs by an oncogenic herpesvirus. PLoS Pathog. 2017;13(7) doi: 10.1371/journal.ppat.1006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. Nature Publishing Group; 2020. COVID-19 Infection: The Perspectives on Immune Responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoraka S., Ferreira M.L.B., Mohebbi S.R., Ghaemi A. SARS-CoV-2 infection and Guillain-Barré syndrome: a review on potential pathogenic mechanisms. Front. Immunol. 2021;12:1636. doi: 10.3389/fimmu.2021.674922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoraka S., Mohebbi S.R., Hosseini S.M., Aghdaei H.A., Zali M.R. Identification of plasma lncRNA-ATB levels in hepatitis B virus-related cirrhosis and non-cirrhotic chronic hepatitis B patients. Virus Res. 2021;303 doi: 10.1016/j.virusres.2021.198503. [DOI] [PubMed] [Google Scholar]
- Sohn K.M., Lee S.G., Kim H.J., Cheon S., Jeong H., Lee J., et al. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 2020;35(38):343–359. doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Wu W., Chen M., Cheng W., Yu J., Fang J., et al. Long noncoding RNA ANRIL supports proliferation of adult T-cell leukemia cells through cooperation with EZH2. J. Virol. 2018;92(24):e00909–e00918. doi: 10.1128/JVI.00909-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir Huyut M., Velichko A., Belyaev M. Detection of risk predictors of COVID-19 mortality with classifier machine learning models operated with routine laboratory biomarkers. Appl. Sci. 2022;12(23):12180. [Google Scholar]
- Tang H., Gao Y., Li Z., Miao Y., Huang Z., Liu X., et al. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020;10(6):e200. doi: 10.1002/ctm2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen M., Devos T., Ejtahed H.S., Amini-Bavil-Olyaee S., Pourfathollah A.A., Pourkarim M.R. Convalescent plasma against COVID-19: a broad-spectrum therapeutic approach for emerging infectious diseases. Microorganisms. 2020;8(11):1733. doi: 10.3390/microorganisms8111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poppel H., Haese A., Graefen M., de la Taille A., Irani J., de Reijke T., et al. The relationship between prostate cancer gene 3 (PCA3) and prostate cancer significance. BJU Int. 2012;109(3):360–366. doi: 10.1111/j.1464-410X.2011.10377.x. [DOI] [PubMed] [Google Scholar]
- Vishnubalaji R., Shaath H., Alajez N.M. Protein coding and long noncoding RNA (lncRNA) transcriptional landscape in SARS-CoV-2 infected bronchial epithelial cells highlight a role for interferon and inflammatory response. Genes. 2020;11(7):760. doi: 10.3390/genes11070760. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID‐19) infected patients. Br. J. Haematol. 2020;189(3):428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Deng R., Gou L., Fu Z., Zhang X., Shao F., et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann. Transl. Med. 2020;8(9) doi: 10.21037/atm-20-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Yang N., Yang Y.H., Wen R., Liu C.F., Zhang T.N. Non-coding RNAs: master regulators of inflammasomes in inflammatory diseases. J. Inflamm. Res. 2021;14:5023. doi: 10.2147/JIR.S332840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wu W., Choi E.J., Lee I., Lee Y.S., Bao X. Non-coding RNAs and their role in respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) infections. Viruses. 2020;12(3):345. doi: 10.3390/v12030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhao T., Deng R., Xia X., Li B., Wang X. A study of differential circRNA and lncRNA expressions in COVID-19-infected peripheral blood. Sci. Rep. 2021;11(1):1–14. doi: 10.1038/s41598-021-86134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Jin X., Yang T., Zhang Y., Liu S., Wu L., et al. Upregulated lncRNA THRIL/TNF-α signals promote cell growth and predict poor clinical outcomes of osteosarcoma. Oncol. Targets Ther. 2020;13:119. doi: 10.2147/OTT.S235798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Liang N., Wang M., Fei Y., Sun J., Li Z., et al. Long noncoding RNA MALAT-1 is a novel inflammatory regulator in human systemic lupus erythematosus. Oncotarget. 2017;8(44):77400. doi: 10.18632/oncotarget.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the cytokine storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chu M. Targeting of IL-6-relevant long noncoding RNA profiles in inflammatory and tumorous disease. Inflammation. 2019;42(4):1139–1146. doi: 10.1007/s10753-019-00995-2. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Chen C.Y., Yedavalli V.S., Jeang K.T. NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1 posttranscriptional expression. mBio. 2013;4(1):10–1128. doi: 10.1128/mBio.00596-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang W., Zhu W., Dong J., Cheng Y., Yin Z., et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 2019;20(22):5573. doi: 10.3390/ijms20225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Cao L., Zhou R., Yang X., Wu M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 2019;10(1):1495. doi: 10.1038/s41467-019-09482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang S., Zuo L., Yue W., Li S., Xin S., et al. Differential expression profiling of lncRNAs related to Epstein-Barr virus infection in the epithelial cells. J. Med. Virol. 2019;91(10):1845–1855. doi: 10.1002/jmv.25516. [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir. Res. 2020;21(1):1–10. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Han X., Wittfeldt A., Sun J., Liu C., Wang X., et al. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway. RNA Biol. 2016;13(1):98–108. doi: 10.1080/15476286.2015.1122164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890. doi: 10.1016/j.chom.2020.04.017. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.