Abstract
Gnathostoma is a parasitic nematode that can infect a wide range of animal species, but human populations have become accidental hosts because of their habit of eating raw or undercooked meat from a wide variety of intermediate hosts. While gnathostomiasis is considered an endemic disease, cases of human gnathostomiasis have been increasing over time, most notably in nonendemic areas. There are several complexities to this parasitic disease, and this review provides an update on human gnathostomiasis, including the life cycle, diagnosis, treatment, and treatment strategies used to combat drug resistance. Even now, a definitive diagnosis of gnathostomiasis is still challenging because it is difficult to isolate larvae for parasitological confirmation. Another reason is the varying clinical symptoms recorded in reported cases. Clinical cases can be confirmed by immunodiagnosis. For Gnathosotoma spinigerum, the detection of IgG against a specific antigenic band with a molecular weight of 24 kDa from G. spinigerum advanced third-stage larvae (aL3), while for other species of Gnathostoma including G. binucleatum, the 33-kDa antigen protein is being used. This review also discusses cases of recurrence of gnathostomiasis and resistance mechanisms to two effective chemotherapeutics (albendazole and ivermectin) used against gnathostomiasis. This is significant, especially when planning strategies to combat anthelmintic resistance. Lastly, while no new chemotherapeutics against gnathostomiasis have been made available, we describe the management of recurrent gnathostomiasis using albendazole and ivermectin combinations or extensions of drug treatment plans.
Keywords: Gnathostomiasis, Anthelminthic resistance, Drug combination
Highlights
-
•
Gnathostomiasis is a nematode parasitic disease that can infect a wide variety of animals.
-
•
Humans are accidental hosts of Gnathostoma by ingestion of improperly cooked or raw infected meat.
-
•
Currently, there are only two effective chemotherapeutics for human gnathostomiasis i.e. albendazole and ivermectin.
-
•
Relapse of human gnathostomiasis is currently managed by drug combination or extension of treatment duration.
1. Introduction
Gnathostomiasis is a parasitic infection among humans and animals caused by the roundworm of the genus Gnathostoma (Daengsvang, 1981). Gnathostomiasis in humans mainly occurs because of the ingestion of undercooked or raw meat of definitive and/or paratenic hosts infected with the aL3 of different Gnathostoma sp. (Liu et al., 2020). In addition to this, ingestion of water contaminated with Cyclops harboring the aL3 and constant handling of aL3-infected meat barehanded are alternative routes of infection of gnathostomiasis infection in humans (Hale et al., 2003; Herman and Chiodini, 2009; Liu et al., 2020). Initially, gnathostomiasis was considered a unique parasitic disease in Asia because of the distribution of the causative agent and the eating habits of the people in endemic areas (Nawa and Nakamura-Uchiyama, 2004). Early records showed cases of human gnathostomiasis in Southeast Asian countries such as Vietnam, Philippines, Malaysia, Myanmar, Cambodia, Laos, Indonesia, and Thailand where high cases were also reported (Daengsvang, 1981). Other regions in Asia where cases of human gnathostomiasis were also reported are China, India, Japan, and Korea (Waikagul and Camacho, 2007; Liu et al., 2020). Nowadays, gnathostomiasis is considered important in travel medicine because usually the infected individuals in non-endemic areas were former travellers to or emigrants from endemic areas (Nawa and Nakamura-Uchiyama, 2004). For example in one case, a Peruvian patient living in Switzerland acquired the infection after visiting her home country in Peru (Chappuis et al., 2001). While another case described a Thai woman who has lived in the United States of America (US) since she was 12, and then visited her relatives in a rural area in Thailand for a month. While she had sought medical advice in Thailand, she was later referred to the US to consider further examination for parasitic infection. In the US, she was diagnosed with gnathostomiasis based on clinical grounds (Rusnak and Lucey, 1993). Another country known for the endemicity of gnathostomiasis is Mexico, wherein there are six states with a high prevalence of clinically-diagnosed human cases of gnathostomiasis and > 500 clinically-diagnosed patients between the years 1988–1996. Aside from Mexico, countries in the Latin America also consider human gnathostomiasis as a serious health problem for example in Ecuador, where an outbreak occurred (Ogata et al., 1998; Nawa and Nakamura-Uchiyama, 2004).
As tropical countries are visited annually by about 50 million residents from industrialized countries, there has also been a significant increase in tropical diseases among patients in non-endemic countries. This situation is further complicated because many clinicians are left in quandary as parasitic infections like gnathostomiasis, filariasis, and schistosomiasis to name a few are rarely seen in the temperate climates (Herman and Chiodini, 2009). For the case of gnathostomiasis intermittent migratory swellings, eosinophilia and a history of travel to Southeast Asia or other endemic countries is a classic triad to diagnose gnathostomiasis, but creeping eruption, which is an uncommon manifestation of cutaneous gnathostomiasis, could also be confused with larva migrans (Miyazaki, 1960; Caumes and Danis, 2004; Herman and Chiodini, 2009; Hamilton and Agranoff, 2018). Furthermore, while the etiology of eosinophilia could be narrowed down with the information on travel history, gnathostomiasis is one of the many other parasitic diseases that could be considered a probable cause (Schulte et al., 2002; O'Connell and Nutman, 2015). Identification of the worm provides a confirmed diagnosis for human gnathostomiasis, but this is not always practical because of the migrating nature of this particular parasite especially in cases of visceral gnathostomiasis where difficult-to-reach organs are affected (Daengsvang, 1981; Herman and Chiodini, 2009). For this, specific immunodiagnosis of human gnathostomiasis is invaluable. The study by Tapchaisri et al. (1991) showed the specific antigen with a molecular weight of about 24 kDa had no cross-reactivity with other parasitic infections. It should be noted that this data was from using total IgG and not specific IgG subclasses. This is because when a specific subclass of IgG was looked into, it was found that a lower sensitivity and specificity to 24 kDa band was observed at 92.9% and 93.4%, respectively (Anantaphruti et al., 2005).
In general, under-appreciation of the importance of parasitic diseases is a consequence of frivolous attention given to it, even though tropical diseases such as gnathostomiasis cause various impairments induced by the physiological and psychological symptoms; and intensity and severity of tropical diseases (Rosenfield et al., 1984). While Waikagul and Camacho (2007) mentioned that no disability-adjusted life years (DALYs) have been estimated for gnathostomiasis, it was mentioned in their study that gnathostomiasis still has an impact on the quality of life of a patient. The irritation, itching, and pain due to larval migration are uncomfortable for the patient and could affect their sleep and mental health which may also result in loss of interest, activity and work ability. While cutaneous gnathostomiasis is the common manifestation of infection in humans, migrating larvae can also invade the internal organs resulting in visceral gnathostomiasis which could be any of the following: ocular, auricular, pulmonary, gastrointestinal, genitourinary, and central nervous system (CNS) manifestations of human gnathostomiasis. Most importantly if the disease is not treated promptly, the parasite will continue to cause severe morbidity for up to 10 years with asymptomatic interval and recurrence of symptoms, because the worm can live in humans for several years and could even result in fatality when CNS is affected (Feinstein and Rodriguez-Valdes, 1984; Diaz, 2015; Dekumyoy and Watthanakulpanich, 2020). Currently, the chemotherapeutics effective against human gnathostomiasis are albendazole and/or ivermectin in combination with corticosteroids but they do not guarantee a cure rate of 100% and occasionally have recorded relapses (Waikagul and Camacho, 2007; Liu et al., 2020). Regional zoonotic reservoirs of Gnathostoma species and autochthonous gnathostomiasis may also be established in non-endemic regions for gnathostomiasis because of increasingly adventurous tastes among travellers and natives for the consumption of raw imported saltwater and freshwater species. The raw fish is usually prepared with lime juice or as sashimi or sushi (Diaz, 2015). This review therefore provides an update on human gnathostomiasis with a focus on the medical treatment of human gnathostomiasis and drug resistance of Gnathostoma spp. to currently available drugs which adds more complication to the treatment of human gnathostomiasis. The review also aims to provide additional information on the mechanism of action of available chemotherapeutics that is significant in formulating strategies to combat resistance.
2. Gnasthosoma as human foodborne nematode
2.1. Distribution of Gnathostoma species
Gnathostoma is a genus of parasitic nematode that infects many mammalian species. The genus Gnathostoma includes 18 reported species but only 13 species are considered valid; they are distributed worldwide. The valid species include: Gnathostoma socialis Leidy, 1858; G. trugidum Stossich, 1902; G. americanum Travassos, 1925; G. procyonis Chandler, 1942; G. miyazakii Anderson, 1964; G. binucleatum Almeyda-Artigas, 1991, G. lamothei Bertoni-Ruiz et al., 2005; G. spinigerum Owen, 1836; G. hispidum Fedchenco, 1872; G. doloresi Tubangui, 1925; G. nipponicum Yamaguti, 1941; G. malaysiae Miyazaki and Dunn, 1965 and G. vietnamicum Le-Van-Hoa, 1965 (Feinstein and Rodriguez-Valdes, 1984; Nawa and Nakamura-Uchiyama, 2004; Waikagul and Camacho, 2007; Chaicumpa, 2010). However only 6 species are known to infect humans i.e. G. spinigerum, G. hispidum, G. doloresi, G. nipponicum, G. binucleatum, and G. malaysiae (Dekumyoy and Watthanakulpanich, 2020; Liu et al., 2020; Thiangtrongjit et al., 2021). G. spinigerum is found in China, India, Japan, and Southeast Asia, and the latter is found in other parts of Asia, Australia and Europe. G. nipponicum distributes in Korea and Japan. G. binucleatum have been observed in Mexico and few South American countries (Diaz, 2015; Liu et al., 2020). Additionally, the most important causative agent of human gnathostomiasis is G. spinigerum that was first discovered in the stomach wall of a tiger that died in the London Zoological Gardens (Miyazaki, 1960). Mexico, Japan, Thailand and Vietnam are countries that reported a high prevalence of human gnathostomiasis while other countries in Asia with reported cases of human gnathostomiasis are Bangladesh, China, India and Indonesia. Other countries that reported human gnathostomiasis include Australia and Spain (Waikagul and Camacho, 2007).
2.2. Biology of Gnathostoma spp
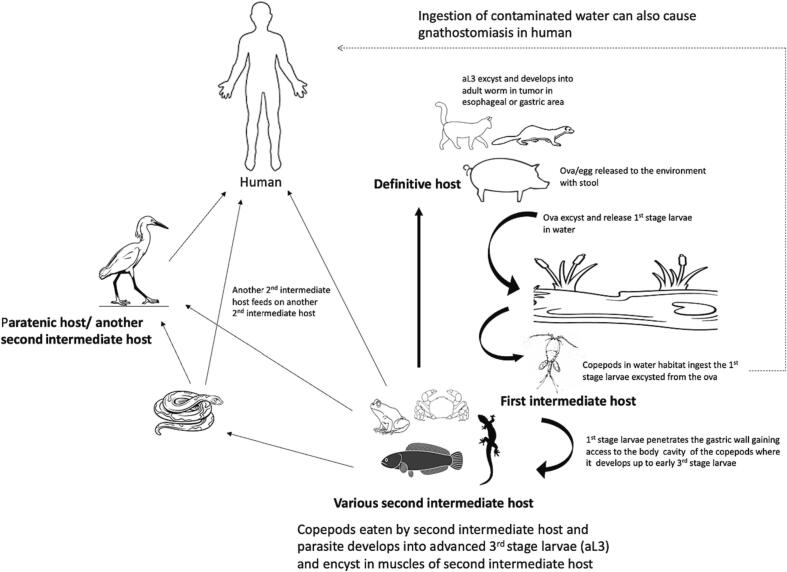
The life cycle of the Gnathostoma spp. typically requires two intermediate hosts and a definitive host. Definitive hosts are usually wild and domestic carnivores and other mammals such as pigs (Daengsvang, 1981; Ligon, 2005; Liu et al., 2020). The adult gnathostome worm resides within tumors in esophagus or stomach of the definitive host (Nawa and Nakamura-Uchiyama, 2004; Chaicumpa, 2010). The female worms shed eggs/ova via the host's stool into water. The first-stage larva (L1) develops and hatches from the eggs. The L1 is ingested by the first intermediate host, Cyclops spp. (Prommas and Daengsvang, 1933; Miyazaki, 1960; Daengsvang, 1981; Nawa and Nakamura-Uchiyama, 2004), and develops up to early third-stage larva(eL3). After the second intermediate host such as fish or amphibians feed on the first intermediate host, the eL3 develops further to become an aL3 then encysts within the muscles of the second intermediate host. The life cycle is completed when a definitive host eats the meat of an infected second intermediate host. However, in actual trophic settings, another second intermediate host may feed on a previously infected second intermediate hosts, but the larvae failed to become adult worms but encyst within the muscle of the animal now referred to as a paratenic host. Several species of animals could serve as second intermediate/paratenic hosts ranging from mammals, birds, and fish to reptiles and amphibians (Miyazaki, 1960; Nawa and Nakamura-Uchiyama, 2004; Liu et al., 2020). Humans are accidental hosts and acquire the disease when infected meat of the second intermediate host is eaten raw or undercooked (Daengsvang, 1981; Liu et al., 2020). While this is known to be the established route of infection, it was subsequently found that drinking contaminated water with infected Cyclops and constant processing of infected meat of the second intermediate host barehanded could be possible routes of infection in human (Waikagul and Camacho, 2007; Herman et al., 2009; Bravo and Gontijo, 2018). Furthermore, Dekumyoy and Watthanakulpanich (2020) also suggested perinatal transmission of the disease when a 3-day-old infant was found infected with the gnathostome parasite. An illustration of the life cycle of the Gnathostoma spp. is shown in Fig. 1.
Fig. 1.
A diagrammatic representation of the life cycle of Gnathostoma spp. showing the various intermediate hosts and definitive hosts and routes of gnathostomiasis infection in humans.
2.3. Epidemiology of gnathostomiasis
Gnathostomiasis is a foodborne zoonotic parasitic disease. The disease is endemic in areas where eating raw freshwater fish or shellfish is popular, and humans are considered accidental host of the parasite. Endemicity of the disease is mainly attributed to the habit of eating raw fish in endemic foci such as in Thailand and other Southeast Asian countries, China and Japan (Waikagul and Camacho, 2007; Liu et al., 2020). In these endemic foci, fish such as snakehead (Ophiocephalus striatus), swamp eel (Monopterus spp.) and loaches and other freshwater fish are some of the identified harbingers of the aL3, as presence of aL3 in these freshwater fish were detected (Daengsvang, 1949; Ando et al., 1988; Koga et al., 1996; Saksirisampant and Thanomsub, 2012; Chai et al., 2015). The first recorded case of human gnathostomiasis was recorded in Thailand in 1889, half a century after the parasite was first discovered by Richard Owen. Later, 16 additional cases were reported and then another 17 cases in between years 1942 to 1947. Because of the increasing number of reported cases of gnathostomiasis, the disease was suggested to be common in Thailand that should be examined carefully (Daengsvang, 1949). Meanwhile Li et al. (2009) mentioned that though other parts of China is presumed endemic for gnathostomiasis, but only sporadic cases have been reported. About 23 cases were recorded in 12 provinces of China, but it is possible that cases are higher than reported due to the high infection rates in animals and freshwater fish. In other parts of the world, the disease has also become a concern such as in Mexico and several other countries in South America like Ecuador that ascribed human gnathostomiasis to eating the local delicacy, ceviche, which is prepared by marinating fish and allowed to be pickled in lime juice only (Ligon, 2005; Herman and Chiodini, 2009). This local delicacy caused an outbreak in a fishing community in Sinaloa, Mexico where Dıaz Camacho et al. (2003) detected as high as 35% seroprevalence of antibodies against Gnathostoma in 309 individuals with 12 patients that had a history of chronic larva migrans. Likewise, the presence of aL3 encysted in meat of the fish caught in the three freshwater lakes and an estuary near the community were also detected. Aside from ingestion of raw fish, there were cases attributed to ingestion of raw snake meat as reported by Kurokawa et al. (1998) and raw chicken meat (Miyazaki, 1960).
Gnathostomiasis once confined in endemic areas have been found to cross borders because of increased international travel and immigration. Nonendemic regions such as Europe and Western countries have reported helminthic infections like gnathostomiasis (Moore et al., 2003; Ligon, 2005), and international travellers who tried exotic dishes in endemic areas became infected (Moore et al., 2003; Herman and Chiodini, 2009). The following cases of human gnathostomiasis involved individuals who visited gnathostomiasis endemic areas and returned back to their home country, which was non-endemic for the disease. The first case was described from a Peruvian woman who had been living in Switzerland for 3 years and visited her relatives in central Peru. The patient related eating ceviche on a daily basis during her stay with her relatives. The patient then presented with some complaints and noted migratory pruritic swelling that developed on her thigh. A positive serologic test was performed at Mahidol University in Bangkok followed by confirmation of gnathostomiasis infection, which the patient acquired from Peru (Chappuis et al., 2001). In another report, Moore et al. (2003) presented a list of patients, who were diagnosed with gnathostomiasis and treated at the Hospital for Tropical Diseases in London. Majority of these patients have travel history to endemic regions for gnathostomiasis, however the authors mentioned that dietary histories in these patients were not very informative for the diagnosis of gnathostomiasis because of inaccuracies, and the wide range of possible sources. This situation was evidenced in a most recent report of a returning Australian traveller, who reported no history of eating raw or undercooked meat, but had developed symptoms few weeks after returning from Thailand (Smith et al., 2021; Gora et al., 2022). Lastly, the report by Herman et al. (2009) described two British tourists, who visited Botswana for a fishing trip, diagnosed with gnathostomiasis and related history of eating raw fish caught in the area. It should be noted that Africa has been recently considered risk area for gnathostomiasis. Furthermore, the sporadic cases of gnathostomiasis in the African continent is indicative that gnathostomiasis is emerging in this region (Hale et al., 2003; Herman et al., 2009). This is further evidenced in the report by Frean (2020) where gnathostomiasis infection came from fish caught in Okavango river delta that caused the infection of an expatriate couple as well as an outbreak among passengers on a cruise traversing the Okavango river delta. Autochthonous infection may also emerge as importation of eels from Asia to the US could result in contamination of local species of eels, adding to this is the important role that wildlife plays as intermediate hosts (Bravo and Gontijo, 2018). Autochthonous infection was reported by Schimmel et al. (2020). In this report, the patient had no travel history, but did recall eating ceviche from a fish caught in a lake in Louisiana, USA.
Effective control of the disease is the prevention of eating raw or undercooked meat especially of fish. Improvede preparation of food to kill encysted aL3 in meat is also important and could also be achieved by freezing meat at −20 °C for 3–5 days or immersing it in either vinegar or soy sauce for 6 and 12 h, respectively. Water used for drinking should also be properly treated. Lastly, people who constantly handle meat, which may be infected with aL3 from a possible intermediate or paratenic host, should wear gloves or wash their hands frequently (Daengsvang, 1981; Rusnak and Lucey, 1993).
3. Signs and symptoms of gnathostomiasis
Mechanical damage caused by larval migration, inflammation and infection secondary to mechanical damage, combined effects of reactions to larval excretions and secretions with the activation of an immune response in the host mainly cause the signs and symptoms of gnathostomiasis (Daengsvang, 1981; Herman and Chiodini, 2009; Liu et al., 2020). Furthermore, the number of larvae ingested, the species of Gnathostoma, the organ and tissue that are affected by larval migration, are some of the factors that affect the clinical manifestations of human gnathostomiasis. As humans are accidental hosts, the larvae fail to reach adult stage in human hosts. However, the migratory nature of the larvae results to two types of clinical manifestations of gnathostomiasis namely cutaneous/external and visceral/internal. (Daengsvang, 1981; Waikagul and Camacho, 2007). Nonspecific symptoms of gnathostomiasis are low-grade fever, abdominal pain, tenderness and enlargement of the liver, pleurisy or pneumonitis and malaise (Dow et al., 1988; Rusnak and Lucey, 1993). Fever, anorexia, nausea, vomiting, abdominal pain, joint pain which may last for >2 weeks may also develop (Dow et al., 1988; Liu et al., 2020). Eosinophilia may also develop when the gastric and intestinal areas have been invaded by the larvae (Rusnak and Lucey, 1993).
Cutaneous/external gnathostomiasis is characterized by intermittent swellings of various sizes commonly observed on the torso with the appearance of migratory tracks from migrating larvae (Nawa and Nakamura-Uchiyama, 2004; Waikagul and Camacho, 2007). The direction of migration is unfixed and could last for 10 days, but one case was recorded to last for about 17 years (Waikagul and Camacho, 2007). Nawa and Nakamura-Uchiyama (2004) also added that the clinical features from the 5 species of Gnathostoma that cause infection in human could be divided into two groups in relation to cutaneous or external gnathostomiasis. The first group causes long-lasting recurrent infection in deeper parts of the skin of peripheral sites of the body and is exhibited by G. spinigerum and G. binucleatum. Creeping eruption, skin abscess or skin nodule also manifest in cutaneous gnathostomiasis, but are uncommon (Rusnak and Lucey, 1993). Occasionally, the creeping eruptions of Gnathostoma is confused with and attributed to cutaneous larva migrans (CLM) which is caused by Ancylostoma caninum and Strongyloides stercoralis (Waikagul and Camacho, 2007) or to inflammatory reaction such as deep-seated boil or ruptured cyst as the skin feels slightly infiltrated or even hard on palpation with no systemic symptoms such as fever or malaise. It should be taken into consideration that the most important clue for the diagnosis of gnathostomiasis is the migratory pattern of the recurrent nodule usually found in thoracic and breast areas, which is uncommonly seen in CLM (Bravo and Gontijo, 2018).
The second group of Gnathostoma spp. which includes G. hispidum, G. nipponicum and G. doloresi are known to preferentially migrate into the surface skin of the trunks with a short survival time. The second type of gnathostomiasis is described as internal/visceral and refers to the condition wherein the larvae invade or migrate to other organs which often cause severe symptoms depending on which type of organ is affected. Internal/visceral manifestations have been described as ocular, auricular, pulmonary, gastrointestinal, genitourinary and CNS. The description is derived according to which organ is affected: ocular, auricular, pulmonary, gastrointestinal, genitourinary and CNS. It is important to note that the fatality and deleterious effects are the results of migration of the parasitic larvae to vital organs of the human host (Nawa and Nakamura-Uchiyama, 2004). Details of reported manifestations of visceral gnathostomiasis are listed in Table 1.
Table 1.
Reported manifestations of visceral gnathostomiasis in humans according to the organ affected.
| Description | Organs affected | Manifestation reported | Reference |
|---|---|---|---|
| Ocular/ophthalmic | Eyes | Uveitis, iritis, intraocular haemorrhage, increased intraocular pressure, retinal scarring and detachment, blindness, occasional eye oedema, conjunctival pain and conjunctival erythema | Rusnak and Lucey (1993); Liu et al. (2020) |
| Auricular | Ear | Mastoiditis, sensorineural hearing loss and extrusion of the larva from the external auditory canal, soft palate, cheek, tip of tongue, and tympanic membrane, tinnitus, dizziness, hearing loss | Rusnak and Lucey (1993); Herman and Chiodini (2009); Liu et al. (2020) |
| Pulmonary | Respiratory and cardiac organ | Cough, pleuritic chest pain, haemoptysis, lobar consolidation or collapse, pleural effusions and pneumo- or hydropneumothorax, pericardial effusions, nodular densities | Rusnak and Lucey (1993); Herman and Chiodini (2009); Liu et al. (2020) |
| Gastrointestinal | Digestive system organs | Sharp abdominal pains or chronic mass in the right lower quadrant, anorexia, vomiting, indigestion, acute iliac fossa pain | Rusnak and Lucey (1993); Herman and Chiodini (2009); Liu et al. (2020) |
| Genitourinary | Bladder and other associated parts of the urinary system | Haematuria, passage of larvae in urine | Herman and Chiodini (2009); Liu et al. (2020) |
| Central nervous system | Brain, spinal cord, nerves | Radiculomyeloencephalitis, eosinophilic meningitis, subarachnoid haemorrhage, excruciating radicular pain, headache with subsequent paralysis of extremities, cranial nerve palsies, primary encephalitis, intracranial hematoma, transitory obstructive hydrocephalus | Rusnak and Lucey (1993); Herman and Chiodini (2009); Liu et al. (2020) |
3.1. Diagnosis of gnathostomiasis
Intermittent subcutaneous migratory swelling together with elevated blood eosinophil level, a relevant history of living in or traveling to endemic regions and eating raw or undercooked meat of fish, frog or chicken are bases for diagnosis of human gnathostomiasis. However, surgical removal of aL3 or identification of the worm in a tissue specimen along with eosinophilia could establish a confirmed diagnosis for gnathostomiasis (Daengsvang, 1981; Liu et al., 2020). Removal of the parasitic larva is not always possible especially in visceral gnathostomiasis. In this latter scenario, immunodiagnostic method by western blot analysis has been proven invaluable and is considered the gold standard method. The method detects the reactivity of IgG from the patient's serum or cerebrospinal fluid (CSF) to the 24-kDa antigen from aL3 of G. spinigerum (Tapchaisri et al., 1991; Waikagul and Camacho, 2007; Dekumyoy and Watthanakulpanich, 2020; Liu et al., 2020). However, only few laboratories perform serological testing for gnathostomiasis including Mahidol University and Swiss Tropical and Public Health Institute of Basel, Switzerland (Chaicumpa, 2010; Bravo and Gontijo, 2018). This limitation could be addressed by exploring other diagnostic markers such as in the study by Nuamtanong et al. (2019) wherein one serine protease inhibitor (serpin) named as GsSerp1 has immunodiagnostic potential and can also be produced by protein recombination. Furthermore, the study by Tinyou et al. (2020) that involved characterization of serpin of G. spinigerum further revealed that it is also a good candidate for drug target as its function is related to the parasite's homeostasis. In some endemic areas for gnathostomiasis, the causative agent of human gnathostomiasis could be other species of Gnathostosoma such as G. binucleatum. For this reason, antigenic markers for G. binucleatum were investigated and revealed a 40 kDa peptide from somatic antigen with specificity for diagnosis of human gnathostomiasis. Another study by Zambrano-Zaragoza et al. (2012) described a 33-kDa immunodominant antigen for G. binucleatum that still needs validation as a diagnostic tool. Although the antigen-based immunoblot assay for detecting G. binucleatum infection shows promising result, its implementation as part of routine diagnostics still needs to be validated (Neumayr et al., 2016). Clinical diagnosis of visceral gnathostomiasis can be assisted by medical imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography. These imaging results complemented with serological studies can be used for presumptive diagnosis of gnathostomiasis (Tapchaisri et al., 1991; Waikagul and Camacho, 2007; Diaz, 2015; Liu et al., 2020). In terms of CNS gnathostomiasis, Bravo and Gontijo (2018) suggested that infection by another nematode parasite, Angiostrongylus sp. should be the main differential diagnosis of CNS gnathostomiasis. Due to the severity that gnathostomiasis infection poses, there is an urgent need for a rapid detection tool which could aid not only in improving the point of care but also the ease of detection especially in areas with limited resources. These issues may be addressed by the recently developed immunochromatographic test (ICT) kits. Initially, the ICT utilized a recombinant protein from G. spinigerum larvae termed as rGslic18, which has strong reactivity with gnathostomiasis positive patients but not with other parasitic diseases. In the first screening of the ICT, serum samples were used, and then years later, the applicability of using whole blood sample was evaluated. Both serum and whole blood samples can be used for detection of specific antibodies against gnathostomiasis in this particular ICT (Janwan et al., 2016; Janwan et al., 2021).
4. Therapeutic management of human gnathostomiasis
4.1. Chemotherapeutic management of human gnathostomiasis
The most effective treatment for cutaneous gnathostomiasis is surgical removal of the larva; however, this is impractical for visceral gnathostomiasis cases (Kraivichian et al., 2004; Liu et al., 2020). Chemotherapy is a conventional strategy used to kill parasites in the human host, but this should be exercised with prudence, as dead parasites may induce inflammatory reactions that can be quite harmful to the host, for example when there is brain stem involvement (Suntharasamai et al., 1992). Several earlier clinical studies have demonstrated the efficacy of albendazole and ivermectin in treating human gnathostomiasis. Kraivichian et al. (1992) conducted a comparative study among symptomatic patients with gnathostomiasis, who were administered albendazole or placebo. The participants were randomly placed into three groups. Two groups received 400 mg albendazole for 21 days, with one group taking the drug once daily and the other group twice daily, and the third group was given a placebo drug. The study resulted in cure rates of 93.9% and 94.1%, respectively, in the albendazole groups. The disappearance of swellings and the return of normal eosinophilia were observed together with a reduction in enzyme-linked immunosorbent assay (ELISA) reaction to specific IgG in the patient's sera and a negative skin test as additional measures of chemotherapeutic effectiveness. The only side effects of albendazole reported in this study were nausea and dizziness. In another study by Nontasut et al. (2000), the cure rates of ivermectin administered at 0.2 mg/kg for 5 days and albendazole at 400 mg twice daily for 21 days were compared among symptomatic gnathostomiasis patients. The results revealed a cure rate of 95.2% among patients treated with ivermectin and a slightly lower cure rate of 93.8% among patients given albendazole. In this study, hypotension, dizziness, weakness and diuresis were side effects of ivermectin. While nausea, dizziness and increased alkaline phosphatase were some of the side effects reported by patients treated with albendazole. Additionally, superficial migration of the larvae was also observed in both ivermectin- and albendazole-treated groups.
In another comparative study of treatments for cutaneous gnathostomiasis, it was found that oral albendazole (400 mg) taken for 21 days had higher efficacy, with a 92% cure rate, compared with a single dose of ivermectin (200 μg/kg), which had a 76% cure rate, though the difference was not statistically significant. However, Kraivichian et al. (2004) emphasized on the safety and other advantages of using ivermectin over albendazole in terms of effective administration at a lower dosage and better compliance because of a shorter duration of treatment. Furthermore, neither drugs caused any major side effects among the participants of the study. The authors suggested that a second dose of ivermectin would probably increase the cure rate of human gnathostomiasis to 100%. This hypothesis was later explored in a study by Nontasut et al. (2005), who were concerned with the performance of ivermectin (0.2 mg/kg) administered for 2 days and albendazole (400 mg/kg) twice daily for 21 days. In this specific study, a cure was defined as the absence of migratory swellings and decreased ELISA titres after 1 year of treatment. The results showed a 100% cure rate among patients treated with ivermectin for 2 days, while a lower cure rate of 78.5% was observed among patients treated with albendazole. Dizziness was the only side effect reported in the group treated with ivermectin. Nausea, dizziness and increased alkaline phosphatase were reported by some patients treated with albendazole.
The tolerability of ivermectin in humans treated for gnathostomiasis was further looked into by Bussaratid et al. (2005). Their study involved 20 Thai patients with confirmed diagnosis of gnathostomiasis by western blotting. The study participants were divided into four groups, each group receiving different dosages of ivermectin: 50, 100, 150 and 200 μg/kg bodyweight. The results showed that, of the 20 patients in the study, four reported no adverse events. The other patients reported side effects that were most likely related to ivermectin. The side effects were experienced by approximatedly 5–30% of the remaining 16 patients and included malaise, myalgia, drowsiness, pruritus, nausea/vomiting, dizziness, diarrhea, a feeling of shortness of breath, palpitations, constipation, anorexia and headache. Additionally, transient microscopic haematuria, pyuria and mildly elevated liver enzymes were some of the laboratory abnormalities noted.
As involvement of the nervous system is the most severe form of gnathostomiasis because of the clinical syndromes caused by the migrating parasite (Wongpaiboonwatana and Nilaratanakul, 2020), therapeutic regimens for neurognathostomiasis have included corticosteroids, such as prednisolone and dexamethasone, as exemplified in the following case reports. The efficacy of corticosteroids as a chemotherapy is inconclusive, as no clinical trials have been conducted, and it is prescribed mainly to relieve cerebral and spinal edema (Suksathien et al., 2016; Wongpaiboonwatana and Nilaratanakul, 2020). In a case report presented by Suksathien et al. (2016), an 18-year-old Thai man presented with radicular pain and rapid progressive weakness of the lower extremities that led to paraplegia. Although the hallmark laboratory finding of eosinophilia was absent from this report, neurognathostomiasis was indicated by the presence of a haemorrhagic track along the thoracic cord in the MRI results. In this case, the patient was treated with oral dexamethasone, then pulse methylprednisolone together with albendazole and metronidazole. The patient reported improved sensation, although the lower extremities still showed weakness. Another recent case reported by Wongpaiboonwatana and Nilaratanakul (2020) presented a female Thai patient who had had previous diagnoses of traumatic subarachnoid haemorrhage, acute bacterial meningitis and ischemic stroke based on her signs and symptoms. However, upon examination of the cerebrospinal fluid with pleiocytosis and an MRI of the brain showing multiple sites of haemorrhaging in the bilateral hemispheres, neurognathostomiasis was suspected. A blood sample was sent for immunoblot testing, and positive reactivity to a 24-kDa G. spinigerum antigen was confirmed. A prescription of two doses of ivermectin (200 μg/kg) and dexamethasone (30 mg) daily, and then prednisolone (30 mg a day), were given to the patient and gradually resulted in an improvement of the patient's symptoms. Another case involved a male patient who had a history of touring Thailand who developed a slightly tender lesion on the skin on the right side of his groin associated with dysesthesia a week after his return to Spain. The patient was then treated with albendazole and dexamethasone on the suspicion of clinical parasitosis based on epidemiologic and cutaneous findings. In response, the patient reported complete recovery from the neurologic defects (de Górgolas et al., 2006).
4.2. Mechanism of action of albendazole and ivermectin
Currently, two of the five anthelminthics used to treat serious human parasitosis are effective against gnathostomiasis: albendazole and ivermectin (Albanese and Venturi, 2003). Albendazole is classified under the benzimidazole group of drugs. This class of drug is defined as a low-dose anthelminthic that has broad-spectrum activity and a high therapeutic index (Lacey, 1990; Albanese and Venturi, 2003). According to Fornelio et al. (1987), the mode of action of benzimidazole drugs is enigmatic because of their varying effects, such as the inhibition of several activities that include the succinate dehydrogenase-fumarate reductase complex, glucose uptake, acetylcholinesterase secretion, and lastly interference of cytoplasmic microtubules. In experimental studies, moderate inhibitory action at high doses was observed with regard to the succinate dehydrogenase-fumarate reductase complex, implying that this is not the primary site of anthelminthic action. Fornelio et al. instead concluded that the depletion of glucose is an important anthelminthic effect of benzimidazole, though this is related to pharmacokinetic factors because a decrease in glucose could not be observed in the in vitro experiment but presented in the in vivo experiment. A depletion of glucose was also observed in Trichinella spiralis larvae treated with both mebendazole and thiabendazole (Fornelio et al., 1987). These results are also in agreement with the results of a study by Arunyanart et al. (2009), who documented the ultrastructural effects of albendazole on aL3 of G. spinigerum after in vivo treatment with albendazole. Their results showed there was damage to the larva's body wall, with a statistically significant decrease in mitochondria. Additionally, the mitochondria appeared distorted and showed the formation of vacuoles, and the number of glycogen granules was significantly decreased. The action of benzimidazole is presumed to be the blockage of glucose uptake by the parasite, resulting in the loss of adenosine triphosphate (ATP) production, followed by depletion of energy source and death of the parasite (Albanese and Venturi, 2003). Most importantly, the primary mode of action of this class of drug is its interaction with the eukaryotic cytoskeletal protein, tubulin. Furthermore, this class of drug is known for its selective toxicity to helminths, which is central to the success of benzimidazoles (Lacey, 1990). Evidence for this includes the results of several experiments that showed the disappearance of cytoplasmic microtubules in the absorptive cells of benzimidazole-treated parasites. Furthermore, this selective toxicity was postulated to be due to differences in the number of protofilaments of nematode and mammalian microtubules, with the latter having 13 protofilament microtubules that the former do not possess (Enos and Coles, 1990). Albendazole sulfoxide and albendazole sulfone are two major metabolites of albendazole. The metabolism of albendazole appears to occur rapidly in patients, such that a 400 mg oral dose of albendazole results below detection of these two major metabolites in plasma, urine and bile according to study results presented by Marriner et al. (1986).
The second drug currently used for gnathostomiasis is ivermectin. Ivermectin is a broad-spectrum antiparasitic drug (Basáñez et al., 2008) that is a chemical derivative of the macrocyclic lactone avermectin (Campbell, 2011). Avermectin is produced by the actinomycete bacterium Streptomyces avermetilis. Its discovery is attributed to the joint efforts of Merck Sharp & Dohme Research Laboratories and the Kitasato Institute (Campbell, 1993). At first, ivermectin was introduced for use as an animal health antiparasitic product in 1981, and after 7 years, it was used to treat onchocerciasis (Crump and Ōmura, 2011). The idea that ivermectin could be used to treat human parasitosis was initiated by the promising results obtained from its efficacy against Onchocerca species in animals (Campbell, 2011).
The targets of ivermectin and related drugs are glutamate-gated chloride channels. The main effect of ivermectin on nematodes and insects is paralysis of the body-wall and pharyngeal muscle as a result of the increased permeability of the glutamate-gated chloride channels to chloride (James and Davey, 2009; Crump and Ōmura, 2011; Crump, 2017). The effect of ivermectin on the pharyngeal muscles of aL3 of G. spinigerum was demonstrated in a study by Anantaphruti et al. (2010). Abnormal changes were noted in the esophageal structures of larvae recovered from ivermectin-treated rats, and the epithelial tissues were found to have cells that were irregularly arranged, degenerated and dysfunctional, with destroyed esophageal gland ducts. While the most important and biologically relevant target of ivermectin is the glutamate-gated chloride channels, evidence for this appears to show this is applicable to adult gastrointestinal nematode worms but not the larvae. In larvae, the drug might act on a different target, for example the gamma-aminobutyric acid (GABA)-gated channels. An idea also surmised is the possibility of the involvement of host immunity in the activity of ivermectin. Larvae are known to elicit immunological reactions, as they excrete and secrete immunomodulatory proteins (Wolstenholme et al., 2016). However, in the case of G. spinigerum, Anantaphruti et al. (2010) explained that the abnormalities caused by ivermectin in the esophageal glands and tissues could result in the reduced production of esophageal toxins, which could lower eosinophil levels in gnathostomiasis patients. Because of the pharmacokinetics of ivermectin, the oral administration of ivermectin is the only approved route in humans. Enterohepatic recycling of ivermectin is suggested because of the tendency of a second rise in drug plasma levels. Ivermectin has high lipid solubility and is metabolized in the liver, where it is transformed into at least 10 metabolites and excreted with the feces (Canga et al., 2008).
5. Resistance of Gnathostoma to available therapeutics
For a variety of technical and ethical reasons, confirmation of anthelminthic resistance in human parasitic nematodes is difficult to obtain. However, the inefficacy of anthelminthics to some human nematode parasites has been reported. For example, there have been reports of reduced efficacy of benzimidazole anthelminthics against soil-transmitted nematodes in Mali and Zanzibar (Prichard, 2007). Treatment failures in imported gnathostomiasis have been described by Strady et al. (2009). In their study, eight of 13 patients reported a relapse after taking albendazole and ivermectin. Relapses were observed with those initially given albendazole, while only three patients treated with ivermectin reported relapse. In this study, a late relapse was also documented after 26 months of an apparently effective initial treatment. Recurrence in imported cutaneous gnathostomiasis has also been reported by Ménard et al. (2003). Their study described recurrences among travellers returning from an endemic area after they were considered cured of the infection. While albendazole is considered an effective drug for treatment against gnathostomiasis infections in Thailand, it seems that this is not the case for imported infections, as the recurrence of symptoms commonly occurs after treatment with albendazole. Furthermore, up to 2 years of follow-up is recommended to confirm an individual is cured of the infection.
While recurrence is more likely in imported cases than patients living in an endemic area because of the possibility of reinfection, there are also reports of the recurrence of cutaneous gnathostomiasis in endemic areas, including Thailand. In a case report by Sharma et al. (2017), recurrence was observed in a Thai patient who was treated with albendazole and then with ivermectin at 0.2 mg/kg/day for 2 consecutive days. The patient was also monitored with 3-month and 6-month follow-up appointments. In this report, reinfection was distinguished from recurrence when symptoms did not manifest within 4 weeks after infection. These previous reports did not describe the recovery of larvae, and recurrence was based on symptoms. This is in contrast to a study by Kanjanapruthipong et al. (2022), in which the surviving albendazole-treated immature larvae were recovered. Changes in the integument of surviving drug-treated immature stage (STIM) G. spinigerum were described and included an increased number of cephalic spines from the original four transverse rows in L3 to eight rows in STIM. There was also crooked cuticular spines at the caudal extremities of the STIM compared with the larger and smoother curved body of cuticular spines on G. spinigerum adults. These integumental changes demonstrated the effects of albendazole on worms, which could aid in our understanding of the occurrence of resistance to treatment.
No definitive mechanisms of resistance to available drugs have been described for G. spinigerum, however, the mechanisms of resistance exhibited by other nematodes to the drugs currently used against G. spinigerum has been thoroughly reviewed and studied. For example, specific mutations are correlated with benzimidazole-resistant phenotypes in several nematode species (Kaplan, 2004), and point mutations seemed to be relevant in benzimidazole resistance. The first mutation described was a tyrosine replacement of phenylalanine at amino acid 200 in the β-tubulin isotype 1. The second mutation was a deletion of the β-tubulin isotype 2 locus (Geerts and Gryseels, 2000). Evidence that benzimidazole resistance is closely linked to mutation(s) located in the β-tubulin gene was further supported in detail by Roos (1990), than the deletion because the generation of resistant populations to benzimidazole among the susceptible population showed homozygous individuals, which were later selected for with further drug usage. Heterozygosity for β-tubulin reactive fragments was absent in the resistant populations. This point mutation was also found in Wuchereria bancrofti and was observed in microfilariae from the blood of patients already given chemotherapy for 7 days (Prichard, 2007). Moreover, alterations to P-glycoprotein homology may cause resistance to ivermectin among nematodes. A supportive evidence for this is the higher level of P-glycoprotein mRNA observed in drug-selected strains of H. contortus; however, the role of the P-glycoprotein gene in drug resistance is also affected by factors such as the chemistry of the antiparasitic drug, subtypes of P-glycoprotein genes, and the history of drug selection (Xu et al., 1998). Another mechanism of ivermectin resistance in parasitic nematodes is the selection of specific alleles, as is the case for ATP-binding cassette (ABC) transporters and P-glycoproteins. Previous experiments provided evidence of reduced polymorphisms in ABC transporter genes in O. volvulus, namely OvABC-3, OvMDR-1 and OvABC-1, collected from patients given ivermectin and compared with a non-treated population. Selection of the β-tubulin isotype gene was another mechanism of ivermectin resistance observed in O. volvulus. There are 2 alleles of β-tubulin, allele A (wild-type) and allele B. Allele B differs from allele A by having three amino acid changes in the coding region (Met117Ala; Val120Ile;Val124Leu), also allele B is characterized by a 24-bp deletion of the intron adjacent to the coding region for the H3 helix. It was observed then that allele B was uncommonly observed among adult O. volvulus populations in a large part of West Africa and Uganda before the introduction of ivermectin (Prichard and Roulet, 2007). Reported cases of gnathostomiasis in the last 5 years are listed in Table 2.
Table 2.
List of recent cases of gnathostomiasis reported worldwide between 2017 and 2022.
| Year | Country | Sex of patient | Recurrence (Present/Absent) | Infection type | Treatment | Reference |
|---|---|---|---|---|---|---|
| 2022 | India | Male | Absent | Cerebral | Praziquantel | Pon et al. (2022) |
| 2022 | Thailand | Male | Absent | Ocular | Surgical removal | Kongwattananon et al. (2022) |
| 2022 | Not indicated | Female | Absent | Cutaneous | Not indicated | Makino et al. (2022) |
| 2022 | India | Male | Absent | Cutaneous, spinal (?) | Methylprednisolone and albendazole | Baskar et al. (2022) |
| 2021 | Australia | Male | Absent | Cerebral | Prednisolone | Smith et al. (2021) |
| 2021 | France | Female | Absent | Cutaneous | Ivermectin | Itani et al. (2021) |
| 2020 | Not indicated | Female | Absent | Ocular | Surgical removal, prednisolone | Agarwal et al. (2020) |
| 2020 | United States | Male | Absent | Cutaneous | Ivermectin and albendazole | Schimmel et al. (2020) |
| 2020 | Botswana | Male | Not indicated | Cutaneous | Not indicated | Frean (2020) |
| 2020 | Botswana | Female | Not indicated | Cutaneous | Not indicated | Frean (2020) |
| 2020 | Botswana | Male | Absent | Cutaneous | Albendazole | Frean (2020) |
| 2020 | Thailand | Male | Absent | Lung | Not indicated | Sivakorn et al. (2020) |
| 2020 | Brazil | Male | Absent | Cutaneous | Albendazole | Haddad Junior et al. (2020) |
| 2020 | Brazil | Male | Absent | Cutaneous | Albendazole | Haddad Junior et al. (2020) |
| 2019 | United States of America | Female | Absent | Cutaneous | Ivermectin | Sapp et al. (2019) |
| 2018 | Netherlands | Male | Absent | Cutaneous | Ivermectin and albendazole | Roach et al. (2018) |
| 2018 | India | Female | Absent | Ocular | Surgical removal | Sen et al. (2019) |
| 2017 | France | Female | Absent | Cutaneous | Ivermectin | Leroy et al. (2017) |
| 2017 | Thailand | Female | Present | Cutaneous | Albendazole and ivermectin | Sharma et al. (2017) |
6. Strategies to combat resistance of Gnathostoma
Currently, there are only 12 key anthelminthic drugs used against helminths in humans, the rest are being used for veterinary helminths. Of these 12 anthelminthic drugs, only 2 (albendazole and ivermectin) are effective against gnathostomiasis (Nontasut et al., 2005; Sepúlveda-Crespo et al., 2020). Relapse has been used to describe recurrence of gnathostomiasis rather than resistance. Nontasut et al. (2005) suggested using the other treatment if recurrence occurs with one of the two drugs. This is based on the results of their study wherein 3 patients treated with albendazole and then given a double-dose of ivermectin resulted in the disappearance of symptoms after about a month or two of treatment with ivermectin. This was also demonstrated in the study of Kraivichian et al. (2004) wherein a single dose of ivermectin was given when a relapse occur after receiving albendazole. This strategy is valid as the use of a mixture of molecules from different chemical families by drug combinations can control resistant parasites and slow the progression of further resistance according to Lanusse et al. (2018). Moreover, development of resistance could be slowed down when anthelminthic combinations at highest efficacy is utilized, because drug combinations decrease the likelihood of ineffective treatment in comparison to monotherapy (Sepúlveda-Crespo et al., 2020). This is also a high priority need for research in this field because optimization of the existing compounds is rational because of great difficulties associated with developing new anthelminthic drugs (Lanusse et al., 2018). This treatment approach was used Chappuis et al. (2001) wherein a patient was treated with albendazole 400 mg twice daily for 3 weeks and then developed cutaneous inflammatory plaques >2 weeks after finishing the albendazole therapy. The patient was then treated with ivermectin 12 mg daily for two consecutive days and considered cured after a 12-month follow-up examination. In another case report presented by Roach et al. (2018), A patient with recurrent migratory swelling for about 6 years was given with ivermectin 0.2 mg/kg once daily for two consecutive days that resulted in outward migration of the larva increased swelling and eosinophilia. The patient was then given albendazole 400 mg twice daily for 14 days, reported resolution of symptoms. A treatment approach that yield good response when relapse occurred after a a single dose of ivermectin (200 μg/kg) was a double dose of ivermectin of the same dosage as recommended by Kraivichian et al. (2004). The efficacy of this treatment regimen was confirmed in a study by Strady et al. (2009). Their studies revealed that in Thailand, a double dose of ivermectin showed 100% cure rate in comparison to a single dose of ivermectin. The success of this strategy could be related to the pharmacokinetic-based optimization of drug activity by increasing the exposure time of the parasite to anthelminthic (Lanusse et al., 2018). This treatment approach has been demonstrated in some recurrences of gnathostomiasis. In a case study presented (Del Giudice et al., 2001) a patient who travelled to Mexico and returned to France, complained of erythematous swellings was later found to have eosinophilia. She was then treated with albendazole 400 mg twice daily for 15 days but later reported cutaneous linear cord on the shoulder before the end of the therapy. The patient was then advised to continue the course of albendazole 400 mg twice daily for another 15 days. At a three-month follow-up, the patient reported no recurrent symptoms. In another case of gnathostomiasis reported in northern Western Australia, the patient was given 12 mg of ivermectin but after eight weeks reported recurrent symptoms and was then treated with ivermectin. After three months, the patient still had cutaneous symptoms and therefore was given another 3 doses of ivermectin. Over the next 6 years after treatment, the patient did not report any subsequent recurrence of symptoms (Jeremiah et al., 2011).
7. Concluding remarks
In conclusion, cases of gnathostomiasis worldwide have been found to be increasing. Reports are presented in this review not only in endemic areas but also in non-endemic regions. Although it is difficult to confirm gnathostomiasis infection, clinical confirmation and several diagnostic methods have been described. In terms of chemotherapeutics, there are three effective therapeutics that could be administered to treat gnathostomiasis, depending on the type of gnathostomiasis. Prednisolone, though not conventionally considered as anthelminthic, was found to be advantageous particularly in gnathostomiasis affecting the parasite's nervous system. While no additional chemotherapeutics have been suggested and tested for gnathostomiasis, the two widely used anthelminthic namely albendazole and ivermectin remain effective against recurrent symptoms of gnathostomiasis, when administered in combination or used for extended periods. This is a rational approach especially as there is difficulty in developing new anthelminthics. While this review does not present confirmed resistance mechanism of drug resistance in Gnathostoma, we provide additional information on available resistance mechanisms to benzimidazole and macrocyclic lactones, which are two drug families effective for gnathostomiasis. This knowledge can serve as a caveat on the limited available effective treatment for human gnathostomiasis.
Declaration of Competing Interest
The authors hereby declare no conflict of interest.
Acknowledgements
This research project is supported by Mahidol University (MU's Strategic Research Fund: 2023) to O.R.
References
- Agarwal M., Rajendran V., Biswas J., Cunningham E.T. Ocular gnathostomiasis presenting as branch retinal artery occlusion. Ocul. Immunol. Inflamm. 2020;30:619–622. doi: 10.1080/09273948.2020.1820532. [DOI] [PubMed] [Google Scholar]
- Albanese G., Venturi C. Albendazole: a new drug for human parasitoses. Dermatol. Clin. 2003;21:283–290. doi: 10.1016/S0733-8635(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Anantaphruti M.T., Nuamtanong S., Dekumyoy P. Diagnostic values of IgG4 in human gnathostomiasis. Trop. Med. Int. Health. 2005;10:1013–1021. doi: 10.1111/j.1365-3156.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- Anantaphruti M.T., Koga M., Nuamtanong S., Nintasen R. Esophageal deformation of Gnathostoma spinigerum in ivermectin-treated rats, and anthelminthic efficacy. Helminthologia. 2010;47:88–93. doi: 10.2478/s11687-010-0014-y. [DOI] [Google Scholar]
- Ando K., Tanaka H., Taniguchi Y., Shimizu M., Kondo K. Two human cases of gnathostomiasis and discovery of a second intermediate host of Gnathostoma nipponicum in Japan. J. Parasitol. 1988;74:623–627. doi: 10.2307/3282181. [DOI] [PubMed] [Google Scholar]
- Arunyanart C., Kanla P., Chaichun A., Intapan P., Maleewong W. Ultrastructural effects of albendazole on the body wall of Gnathostoma spinigerum third stage larvae. Southeast Asian J. Trop. Med. Public Health. 2009;40:1199–1207. https://www.tm.mahidol.ac.th/seameo/2009-40-6/07-4586.pdf [PubMed] [Google Scholar]
- Basáñez M.-G., Pion S.D.S., Boakes E., Filipe J.A.N., Churcher T.S., Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect. Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- Baskar D., Nashi S., Reddy A., Vangayalapati S., Arshad F., Srijithesh P., et al. A rare case of eosinophilic myelitis due to gnathostomiasis. Neurol. India. 2022;70:395–398. doi: 10.4103/0028-3886.338692. [DOI] [PubMed] [Google Scholar]
- Bravo F., Gontijo B. Gnathostomiasis: an emerging infectious disease relevant to all dermatologists. An. Bras. Dermatol. 2018;93:172–180. doi: 10.1590/abd1806-4841.20187498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussaratid V., Krudsood S., Silachamroon U., Looareesuwan S. Tolerability of ivermectin in gnathostomiasis. Southeast Asian J. Trop. Med. Public Health. 2005;36:644–649. https://www.tm.mahidol.ac.th/seameo/2005_36_3/17-3443.pdf [PubMed] [Google Scholar]
- Campbell W.C. Ivermectin, an antiparasitic agent. Med. Res. Rev. 1993;13:61–79. doi: 10.1002/med.2610130103. [DOI] [PubMed] [Google Scholar]
- Campbell W. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr. Pharm. Biotechnol. 2011;13:853–865. doi: 10.2174/138920112800399095. [DOI] [PubMed] [Google Scholar]
- Canga A., Sahagún Prieto A.M., Diez M., Fernandez N., Sierra M., Garcia J. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10:42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumes E., Danis M. From creeping eruption to hookworm-related cutaneous larva migrans. Lancet Infect. Dis. 2004;4:659–660. doi: 10.1016/S1473-3099(04)01178-8. [DOI] [PubMed] [Google Scholar]
- Chai J.-Y., Sohn W.-M., Na B.-K., Park J.-B., Jeoung H.-G., Hoang E.-H., et al. Larval Gnathostoma spinigerum detected in Asian swamp eels, Monopterus albus, purchased from a local market in Yangon, Myanmar. Korean J. Parasitol. 2015;53:619–625. doi: 10.3347/kjp.2015.53.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaicumpa W. Immunodiagnosis of gnathostomiasis. Siriraj Med. J. 2010;62:79–83. https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/243753 [Google Scholar]
- Chappuis F., Farinelli T., Loutan L. Ivermectin treatment of a traveler who returned from Peru with cutaneous gnathostomiasis. Clin. Infect. Dis. 2001;33:e17–e19. doi: 10.1086/322625. [DOI] [PubMed] [Google Scholar]
- Crump A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J. Antibiot. 2017;70:495–505. doi: 10.1038/ja.2017.11. [DOI] [PubMed] [Google Scholar]
- Crump A., Ōmura S. Ivermectin, ‘Wonder drug’ from Japan: the human use perspective. Proc. Jpn. Acad. B: Phys. Biol. Sci. 2011;87:13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daengsvang S. Human gnathostomiasis in Siam with reference to the method of prevention. J. Parasitol. 1949;35:116–121. doi: 10.2307/3273112. [DOI] [PubMed] [Google Scholar]
- Daengsvang S. Gnathostomiasis in Southeast Asia. Southeast Asian J. Trop. Med. Public Health. 1981;12(3):319–332. https://www.tm.mahidol.ac.th/seameo/1981-12-3/1981-12-3-319.pdf [PubMed] [Google Scholar]
- de Górgolas M., Santos-O’Connor F., Gárate T., Guarch Troyas R.M., Unzú A.L., Grobusch M.P., et al. Cutaneous and medullar gnathostomiasis in travelers to Mexico and Thailand. J. Travel Med. 2006;10:358–361. doi: 10.2310/7060.2003.9373. [DOI] [PubMed] [Google Scholar]
- Dekumyoy P., Watthanakulpanich D. In: Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th edition. Ryan E.T., et al., editors. Elsevier; London: 2020. 121 - Gnathostomiasis; pp. 888–890. [Google Scholar]
- Del Giudice P., Dellamonica P., Durant J., Rahelinrina V., Grobusch M.P., Janitschke K., et al. A case of gnathostomiasis in a European traveller returning from Mexico. Br. J. Dermatol. 2001;145:487–489. doi: 10.1046/j.1365-2133.2001.04384.x. [DOI] [PubMed] [Google Scholar]
- Diaz J.H. Gnathostomiasis: an emerging infection of raw fish consumers in Gnathostoma nematode-endemic and nonendemic countries. J. Travel Med. 2015;22:318–324. doi: 10.1111/jtm.12212. [DOI] [PubMed] [Google Scholar]
- Dıaz Camacho S.P., Willms K., de la Cruz Otero M.d.C., Zazueta Ramos M.L., Gaxiola S.B., Velázquez R.C., et al. Acute outbreak of gnathostomiasis in a fishing community in Sinaloa, Mexico. Parasitol. Int. 2003;52:133–140. doi: 10.1016/S1383-5769(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Dow C., Chiodini P.L., Haines A.J., Michelson S.M.C. Human gnathostomiasis. J. Infect. 1988;17:147–149. doi: 10.1016/S0163-4453(88)91711-2. [DOI] [PubMed] [Google Scholar]
- Enos A., Coles G.C. Effect of benzimidazole drugs on tubulin in benzimidazole resistant and susceptible strains of Caenorhabditis elegans. Int. J. Parasitol. 1990;20:161–167. doi: 10.1016/0020-7519(90)90096-6. [DOI] [PubMed] [Google Scholar]
- Feinstein R.J., Rodriguez-Valdes J. Gnathostomiasis, or larva migrans profundus. J. Am. Acad. Dermatol. 1984;11:738–740. doi: 10.1016/S0190-9622(84)70232-5. [DOI] [PubMed] [Google Scholar]
- Fornelio A.C., Caabeiro F.R., Gonzalez A.J. The mode of action of some benzimidazole drugs on Trichinella spiralis. Parasitology. 1987;95:61–70. doi: 10.1017/S0031182000057541. [DOI] [PubMed] [Google Scholar]
- Frean J. Gnathostomiasis acquired by visitors to the Okavango Delta, Botswana. Trop. Med. Infect. Dis. 2020;5:39. doi: 10.3390/tropicalmed5010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts S., Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin. Microbiol. Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora H., Smith S., Wilson I., Preston-Thomas A., Ramsamy N., Hanson J. The epidemiology and outcomes of central nervous system infections in Far North Queensland, tropical Australia; 2000-2019. PloS One. 2022;17 doi: 10.1371/journal.pone.0265410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad Junior V., Oliveira Í.F., Bicudo N.P., Marques M.E.A. Gnathostomiasis acquired after consumption of raw freshwater fish in the Amazon region: a report of two cases in Brazil. Rev. Soc. Bras. Med. Trop. 2020;54 doi: 10.1590/0037-8682-0127-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale D.C., Blumberg L., Frean J. Case report: Gnathostomiasis in two travelers to Zambia. Am. J. Trop. Med. Hyg. 2003;68:707–709. doi: 10.4269/ajtmh.2003.68.707. [DOI] [PubMed] [Google Scholar]
- Hamilton W., Agranoff D.D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.S., Chiodini P.L. Gnathostomiasis, another emerging imported disease. Clin. Microbiol. Rev. 2009;22:484–492. doi: 10.1128/CMR.00003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.S., Wall E.C., van Tulleken C., Godfrey-Faussett P., Bailey R.L., Chiodini P.L. Gnathostomiasis acquired by British tourists in Botswana. Emerg. Infect. Dis. 2009;15:594. doi: 10.3201/eid1504.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani O., Monsel G., Mrad M., Ruf T., Paris L., Caumes E. Gnathostomiasis in a traveller returning from Madagascar. J. Travel Med. 2021;28 doi: 10.1093/jtm/taab039. [DOI] [PubMed] [Google Scholar]
- James C.E., Davey M.W. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 2009;39:213–220. doi: 10.1016/j.ijpara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Janwan P., Intapan P.M., Yamasaki H., Rodpai R., Laummaunwai P., Thanchomnang T., et al. Development and usefulness of an immunochromatographic device to detect antibodies for rapid diagnosis of human gnathostomiasis. Parasit. Vectors. 2016;9:14. doi: 10.1186/s13071-016-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janwan P., Intapan P.M., Sadaow L., Rodpai R., Yamasaki H., Boonroumkaew P., et al. Development of immunochromatographic test kit for rapid detection of specific IgG4 antibody in whole-blood samples for diagnosis of human gnathostomiasis. Diagnostics. 2021;11:862. doi: 10.3390/diagnostics11050862. https://www.mdpi.com/2075-4418/11/5/862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremiah C.J., Harangozo C.S., Fuller A.J. Gnathostomiasis in remote northern Western Australia: the first confirmed cases acquired in Australia. Med. J. Aust. 2011;195:42–44. doi: 10.5694/j.1326-5377.2011.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Kanjanapruthipong T., Ampawong S., Thaenkham U., Tuentam K., Watthanakulpanich D. Survival of immature pre-adult Gnathostoma spinigerum in humans after treatment with albendazole. PloS One. 2022;17 doi: 10.1371/journal.pone.0264766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Koga M., Ishii Y., Lou Y., Higo H., Fujino T., Huang W., et al. A survey of Gnathostoma larvae in freshwater fish in the valley of the Yangtze river and morphological characteristics of the recovered larvae. Southeast Asian J. Trop. Med. Public Health. 1996;27:542–547. https://www.tm.mahidol.ac.th/seameo/1996-27-3/1996-27-3-542.pdf [PubMed] [Google Scholar]
- Kongwattananon W., Wiriyabanditkul T., Supwatjariyakul W., Somkijrungroj T. Intracameral gnathostomiasis: a case report and literature review. Ocul. Immunol. Inflamm. 2022;1-5 doi: 10.1080/09273948.2022.2073239. [DOI] [PubMed] [Google Scholar]
- Kraivichian P., Kulkumthorn M., Yingyourd P., Akarabovorn P., Paireepai C.-C. Albendazole for the treatment of human gnathostomiasis. Trans. R. Soc. Trop. Med. Hyg. 1992;86:418–421. doi: 10.1016/0035-9203(92)90248-B. [DOI] [PubMed] [Google Scholar]
- Kraivichian K., Nuchprayoon S., Sitichalernchai P., Chaicumpa W., Yentakam S. Treatment of cutaneous gnathostomiasis with ivermectin. Am. J. Trop. Med. Hyg. 2004;71:623–628. doi: 10.4269/ajtmh.2004.71.623. [DOI] [PubMed] [Google Scholar]
- Kurokawa M., Ogata K., Sagawa S., Miyaoka Y., Noda S., Nawa Y. Cutaneous and visceral larva migrans due to Gnathostoma doloresi infection via an unusual route. Arch. Dermatol. 1998;134:638–639. doi: 10.1001/archderm.134.5.638. https://jamanetwork.com/journals/jamadermatology/article-abstract/189025 [DOI] [PubMed] [Google Scholar]
- Lacey E. Mode of action of benzimidazoles. Parasitol. Today. 1990;6(4):112–115. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- Lanusse C., Canton C., Virkel G., Alvarez L., Costa-Junior L., Lifschitz A. Strategies to optimize the efficacy of anthelmintic drugs in ruminants. Trends Parasitol. 2018;34:664–682. doi: 10.1016/j.pt.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Leroy J., Cornu M., Deleplancque A.S., Loridant S., Dutoit E., Sendid B. Sushi, ceviche and gnathostomiasis - a case report and review of imported infections. Travel Med. Infect. Dis. 2017;20:26–30. doi: 10.1016/j.tmaid.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Li D.M., Chen X.R., Zhou J.S., Xu Z.B., Nawa Y., Dekumyoy P. Short report: case of gnathostomiasis in Beijing, China. Am. J. Trop. Med. Hyg. 2009;80:185–187. doi: 10.4269/ajtmh.2009.80.185. [DOI] [PubMed] [Google Scholar]
- Ligon B.L. Gnathostomiasis: a review of a previously localized zoonosis now crossing numerous geographical boundaries. Semin. Pediatr. Infect. Dis. 2005;16:137–143. doi: 10.1053/j.spid.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Liu G.-H., Sun M.-M., Elsheikha H.M., Fu Y.-T., Sugiyama H., Ando K., et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit. Vectors. 2020;13:616. doi: 10.1186/s13071-020-04494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino T., Sugiyama H., Oshima M., Mizawa M., Shimizu T. Cutaneous gnathostomiasis caused by Gnathostoma spinigerum. Br. J. Dermatol. 2022;186:e198–e199. doi: 10.1111/bjd.21007. [DOI] [PubMed] [Google Scholar]
- Marriner S.E., Morris D.L., Dickson B., Bogan J.A. Pharmacokinetics of albendazole in man. Eur. J. Clin. Pharmacol. 1986;30:705–708. doi: 10.1007/BF00608219. [DOI] [PubMed] [Google Scholar]
- Ménard A., Dos Santos G., Dekumyoy P., Ranque S., Delmont J., Danis M., et al. Imported cutaneous gnathostomiasis: report of five cases. Trans. R. Soc. Trop. Med. Hyg. 2003;97:200–202. doi: 10.1016/S0035-9203(03)90119-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki I. On the genus Gnathostoma and human gnathostomiasis, with special reference to Japan. Exp. Parasitol. 1960;9:338–370. doi: 10.1016/0014-4894(60)90040-0. [DOI] [PubMed] [Google Scholar]
- Moore D.A.J., McCrodden J., Dekumyoy P., Chiodini P.L. Gnathostomiasis: an emerging imported disease. Emerg. Infect. Dis. 2003;9:647. doi: 10.3201/eid0906.020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Nakamura-Uchiyama F. An overview of gnathostomiasis in the world. Southeast Asian J. Trop. Med. Public Health. 2004;35:87–91. https://www.tm.mahidol.ac.th/seameo/2004-35-suppl-1/15-087.pdf [Google Scholar]
- Neumayr A., Ollague J., Bravo F., Gotuzzo E., Jimenez P., Norton S.A., et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in Western Blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am. J. Trop. Med. Hyg. 2016;95:413–416. doi: 10.4269/ajtmh.16-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nontasut P., Bussaratid V., Chullawichit S., Charoensook N., Visetsuk K. Comparison of ivermectin and albendazole treatment for gnathostomiasis. Southeast Asian J. Trop. Med. Public Health. 2000;31:374–377. https://www.tm.mahidol.ac.th/seameo/2000_31_2/29-2490.pdf [PubMed] [Google Scholar]
- Nontasut P., Claesson B., Dekumyoy P., Pakdee W., Chullawichit S. Double-dose ivermectin vs albendazole for the treatment of gnathostomiasis. Southeast Asian J. Trop. Med. Public Health. 2005;36:650–652. https://www.tm.mahidol.ac.th/seameo/2005_36_3/18-3539.pdf [PubMed] [Google Scholar]
- Nuamtanong S., Reamtong O., Phuphisut O., Chotsiri P., Malaithong P., Dekumyoy P., et al. Transcriptome and excretory–secretory proteome of infective-stage larvae of the nematode Gnathostoma spinigerum reveal potential immunodiagnostic targets for development. Parasite. 2019;26:34. doi: 10.1051/parasite/2019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell E.M., Nutman T.B. Eosinophilia in infectious diseases. Immunol. Allergy Clin. North Am. 2015;35:493–522. doi: 10.1016/j.iac.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K., Nawa Y., Akahane H., Diaz Camacho S.P., Lamothe-Argumedo R., Cruz-Reyes A. Short report: gnathostomiasis in Mexico. Am. J. Trop. Med. Hyg. 1998;58:316–318. doi: 10.4269/ajtmh.1998.58.316. [DOI] [PubMed] [Google Scholar]
- Pon A.G., AV R.T., Reddy H.K., Nashi S., Mangalore S., Srijithesh P.R., et al. Worm in the brain: a case of CNS gnathostomiasis. Neurol. India. 2022;70:2458–2460. doi: 10.4103/0028-3886.364071. [DOI] [PubMed] [Google Scholar]
- Prichard R.K. Markers for benzimidazole resistance in human parasitic nematodes? Parasitology. 2007;134:1087–1092. doi: 10.1017/s003118200700008x. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Roulet A. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitology. 2007;134:1123–1132. doi: 10.1017/s0031182007000091. [DOI] [PubMed] [Google Scholar]
- Prommas C., Daengsvang S. Preliminary report of a study on the life-cycle of Gnathostoma spinigerum. J. Parasitol. 1933;19:287–292. doi: 10.2307/3271586. [DOI] [Google Scholar]
- Roach R.E.J., van Doorn R., de Bruïne F.T., Arend S.M., Visser L.G. A recurrent migratory swelling. Lancet Infect. Dis. 2018;18:1045. doi: 10.1016/s1473-3099(18)30128-2. [DOI] [PubMed] [Google Scholar]
- Roos M.H. The molecular nature of benzimidazole resistance in helminths. Parasitol. Today. 1990;6:125–127. doi: 10.1016/0169-4758(90)90229-W. [DOI] [PubMed] [Google Scholar]
- Rosenfield P.L., Golladay F., Davidson R.K. The economics of parasitic diseases: research priorities. Soc. Sci. Med. 1984;19:1117–1126. doi: 10.1016/0277-9536(84)90317-4. [DOI] [PubMed] [Google Scholar]
- Rusnak J.M., Lucey D.R. Clinical gnathostomiasis: case report and review of the English-language literature. Clin. Infect. Dis. 1993;16:33–50. doi: 10.1093/clinids/16.1.33. [DOI] [PubMed] [Google Scholar]
- Saksirisampant W., Thanomsub B.W. Positivity and intensity of Gnathostoma spinigerum infective larvae in farmed and wild-caught swamp eels in Thailand. Korean J. Parasitol. 2012;50:113–118. doi: 10.3347/kjp.2012.50.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp S.G.H., Kaminski M., Abdallah M., Bishop H.S., Fox M., Ndubuisi M., et al. Percutaneous emergence of Gnathostoma spinigerum following praziquantel treatment. Trop. Med. Infect. Dis. 2019;4:145. doi: 10.3390/tropicalmed4040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel J., Chao L., Luk A., Grafton L., Kadi A., Boh E. An autochthonous case of gnathostomiasis in the United States. JAAD Case Rep. 2020;6:337–338. doi: 10.1016/j.jdcr.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte C., Krebs B., Jelinek T., Nothdurft H.D., von Sonnenburg F., Löscher T. Diagnostic significance of blood eosinophilia in returning travelers. Clin. Infect. Dis. 2002;34:407–411. doi: 10.1086/338026. [DOI] [PubMed] [Google Scholar]
- Sen P., Dutta Majumder P., Biswas J., Rao C., Das K. Role of ultra-wide-field imaging in the diagnosis of intravitreal gnathostomiasis: a case report. Ocul. Immunol. Inflamm. 2019;27:380–382. doi: 10.1080/09273948.2018.1498525. [DOI] [PubMed] [Google Scholar]
- Sepúlveda-Crespo D., Reguera R.M., Rojo-Vázquez F., Balaña-Fouce R., Martínez-Valladares M. Drug discovery technologies: Caenorhabditis elegans as a model for anthelmintic therapeutics. Med. Res. Rev. 2020;40:1715–1753. doi: 10.1002/med.21668. [DOI] [PubMed] [Google Scholar]
- Sharma C., Piyaphanee W., Watthanakulpanich D. Case report: clinical features of intermittent migratory swelling caused by gnathostomiasis with complete follow-up. Am. J. Trop. Med. Hyg. 2017;97:1611–1615. doi: 10.4269/ajtmh.17-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakorn C., Promthong K., Dekumyoy P., Viriyavejakul P., Ampawong S., Pakdee W., et al. Case report: the first direct evidence of Gnathostoma spinigerum migration through human lung. Am. J. Trop. Med. Hyg. 2020;103:1129–1134. doi: 10.4269/ajtmh.20-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Wilson I., Starck L., Binotto E., Ho J., Hanson J. Cerebral gnathostomiasis. Med. J. Aust. 2021;215:164–165.e161. doi: 10.5694/mja2.51189. [DOI] [PubMed] [Google Scholar]
- Strady C., Dekumyoy P., Clement-Rigolet M., Danis M., Bricaire F.o., Caumes E. Long-term follow-up of imported gnathostomiasis shows frequent treatment failure. Am. J. Trop. Med. Hyg. 2009;80:33–35. doi: 10.4269/ajtmh.2009.80.33. [DOI] [PubMed] [Google Scholar]
- Suksathien R., Kunadison S., Wongfukiat O., Ingkasuthi K. Spinal gnathostomiasis: a case report with magnetic resonance imaging and electrophysiological findings. J. Med. Assoc. Thai. 2016;99:1367–1371. https://www.thaiscience.info/journals/Article/JMAT/10987841.pdf [PubMed] [Google Scholar]
- Suntharasamai P., Riganti M., Chittamas S., Desakorn V. Albendazole stimulates outward migration of Gnathostoma spinigerum to the dermis in man. Southeast Asian J. Trop. Med. Public Health. 1992;23:716–722. https://www.tm.mahidol.ac.th/seameo/1992-23-4/1992-23-4-716.pdf [PubMed] [Google Scholar]
- Tapchaisri P., Nopparatana C., Chaicumpa W., Setasuban P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomiasis. Int. J. Parasitol. 1991;21:315–319. doi: 10.1016/0020-7519(91)90033-4. [DOI] [PubMed] [Google Scholar]
- Thiangtrongjit T., Nogrado K., Ketboonlue T., Malaitong P., Adisakwattana P., Reamtong O. Proteomics of gnathostomiasis: a way forward for diagnosis and treatment development. Pathogens. 2021;10 doi: 10.3390/pathogens10091080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinyou A., Chaimon S., Phuphisut O., Kobpornchai P., Malaithong P., Poodeepiyasawat A., et al. Molecular cloning and characterization of serine protease inhibitor from food-borne nematode, Gnathostoma spinigerum. Acta Trop. 2020;204 doi: 10.1016/j.actatropica.2019.105288. [DOI] [PubMed] [Google Scholar]
- Waikagul J., Camacho S.P.D. In: Food-Borne Parasitic Zoonoses: Fish and Plant-Borne Parasites. Murrell K.D., Fried B., editors. Springer US; Boston, MA: 2007. Gnathostomiasis; pp. 235–261. [Google Scholar]
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B. How do the macrocyclic lactones kill filarial nematode larvae? Invert. Neurosci. 2016;16:1–9. doi: 10.1007/s10158-016-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongpaiboonwatana S., Nilaratanakul V. Neurognathostomiasis presented with multiple episodes of intracerebral hemorrhage: a case report. J. Infect. Dis. Antimicrob. Agents. 2020;37:85–90. https://www.idthai.org/2015/journal/_file_ar_fulltext/file_ar08419be897405321542838d77f855226.pdf [Google Scholar]
- Xu M., Molento M., Blackhall W., Ribeiro P., Beech R., Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998;91:327–335. doi: 10.1016/S0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- Zambrano-Zaragoza J.F., Durán-Avelar M.d.J., Messina-Robles M., Vibanco-Pérez N. Characterization of the humoral immune response against Gnathostoma binucleatum in patients clinically diagnosed with gnathostomiasis. Am. J. Trop. Med. Hyg. 2012;86:988–992. doi: 10.4269/ajtmh.2012.11-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]