Summary
Adoptive cell therapy using allogeneic γδ-T cells is a promising option for off-the-shelf T cell products with a low risk of graft-versus-host disease (GVHD). Long-term persistence may boost the clinical development of γδ-T cell products. In this study, we found that genetically modified Vγ9+Vδ2+ T cells expressing a tumor antigen-specific αβ-TCR and CD8 coreceptor (GMC) showed target-specific killing and excellent persistence. To determine the mechanisms underlying these promising effects, we investigated metabolic characteristics. Cytokine secretion by γδ-TCR-stimulated nongene-modified γδ-T cells (NGMCs) and αβ-TCR-stimulated GMCs was equally suppressed by a glycolysis inhibitor, although the cytokine secretion of αβ-TCR-stimulated GMCs was more strongly inhibited by ATP synthase inhibitors than that of γδ-TCR-stimulated NGMCs. Metabolomic and transcriptomic analyses, flow cytometry analysis using mitochondria-labeling dyes and extracellular flux analysis consistently suggest that αβ-TCR-transduced γδ-T cells acquire superior mitochondrial function.
In conclusion, αβ-TCR-transduced γδ-T cells acquire superior mitochondrial function with promising persistence.
Subject areas: Cellular therapy, Molecular biology, Immunology
Graphical abstract

Highlights
-
•
Long-term persistence may boost the clinical development of γδ-T cell products
-
•
αβ-TCR transduction into γδ-T cells showed promising persistence
-
•
αβ-TCR transduced γδ-T cells could utilize more mitochondrial energy metabolism
Cellular therapy; Molecular biology; Immunology
Introduction
A new era of immunotherapy for cancer has arrived with the application of immune checkpoint inhibitors and adoptive cell therapy with genetically modified T cells directed against cancer antigens. Chimeric antigen receptor-engineered T cells (CAR-T cells) targeting CD19 have shown clinical benefits in clinical trials1,2,3 and are an available standard therapy for B cell malignancies. T cell receptor (TCR)-engineered T cells (TCR-T cells) are less developed than CAR-T cells, but no conclusion has been reached as to whether CAR-T cells or TCR-T cells are superior. One of the characteristics of TCR-T cells is their ability to target intracellularly expressed antigens. New York esophageal squamous cell carcinoma 1 (NY-ESO-1) is an intracellularly expressed cancer-testis antigen with a wide range of expression in tumors but a limited range of expression in normal tissues.4,5,6 NY-ESO-1 is the most studied antigen in TCR-T cell clinical trials.7 Our group conducted clinical trials of TCR-T cells targeting NY-ESO-1-expressing tumors8,9 using the G50A + A51E TCR, an NY-ESO-1-specific TCR recognizing the NY-ESO-1p157-165 peptide (NE1p157) and HLA-A∗02:01 complex. This TCR had two amino acid replacements in the CDR2 region of the TCRβ chain to produce high affinity,10 which differs from the one reported by Robbins et al.11,12,13 G50A + A51E TCR-T cells showed tolerable toxicity and promising efficacy.8,9
Despite the successes described previously, there are two main issues with adoptive T cell therapy using αβ-T cells: mispairing of endogenous and engineered αβ-TCRs14 and difficulties in using allogeneic αβ-T cells for rapid and chemotherapy-damage-free cell product preparation due to graft-versus-host disease (GVHD). To address these issues, we focused on γδ-T cells. αβ-TCR-transduced γδ-T cells targeting cancer antigens have already been studied.15,16,17 γδ-T cells recognize tumors in an MHC-independent manner18,19 and have no risk of mispairing endogenous and engineered αβ-TCRs. Tumor infiltration by γδ-T cells is a good prognostic marker for many cancers.20 Although adoptive cell transfer of nongene-modified γδ-T cells (NGMCs) has been studied,21,22,23,24,25 these cells have not achieved a remarkable improvement in survival. Redirection of γδ-T cells by αβ-TCR gene transduction may yield antitumor activities against NGMC-resistant tumors and resolve the two aforementioned issues with αβ-T cell products.
In this study, we evaluated genetically modified Vγ9+Vδ2+ T cells expressing NY-ESO-1-specific αβ-TCR and the CD8 coreceptor (NE1-GMCs), with a specific focus on their metabolic preferences in association with their effector function and persistence.
Results
Characteristics of αβ-TCR-transduced γδ-T cells
γδ-T cells induced by a novel bisphosphonate prodrug, tetrakis-pivaloyloxymethyl 2-(thiazole-2-ylamino) ethylidene-1,1-bisphosphonate (PTA),26 exhibited a Vγ9+Vδ2+ phenotype (data not shown). Since coexpression of CD8αβ enhanced the antitumor effect of NY-ESO-1-specific αβ-TCR (G50A + A51E TCR)-transduced γδ-T cells (Figures S1A–S1D), we produced a retroviral vector encoding the G50A + A51E TCR gene and the human CD8αβ gene and used it in this study (Figure 1A). In NE1-GMCs, there were two distinct populations: Vδ2highCD8low and Vδ2lowCD8high cells (Figure 1B). Transduced NY-ESO-1-specific αβ-TCR was mainly observed in NE1-GMC Vδ2lowCD8high cells (Figures 1C and 1D), and there was a positive correlation between NY-ESO-1-specific αβ-TCR and CD8 expression (Figure S2A). Transduction of αβ-TCR had no obvious effect on the γδ-T cell phenotype, TOX expression and cytokine production (Figures S2B–S2D). NE1-GMCs tended to have lower PD-1 expression (Figure S2C). NE1-GMCs induced antigen-specific apoptosis in NE1p157-pulsed target cells and NY-ESO-1+HLA-A2+ tumor cells (Figures S3A–S3C). To assess in vivo persistence, we used NY-ESO-1-expressing tumor-bearing mice. NY-ESO-1+HLA-A2+ tumors regressed after NE1-GMC transfer with total-body irradiation (Figure 1E). NE1-GMCs were observed in the peripheral blood of mice 60 days after infusion (Figures 1F and S3D). In the control group, observation was terminated 65 days after tumor inoculation because of tumor growth. In contrast, in the NE1-GMC-infused group, no tumor growth was observed 90 days after tumor inoculation, and there was no tumor growth after reinoculation of the tumor. Vδ2highCD8low NE1-GMCs secrete IFN-γ in response to PTA. In contrast, Vδ2lowCD8high NE1-GMCs secreted IFN-γ in response to NE1-pMHC (Figure 1G).
Figure 1.
Characteristics of γδ-T cells in this study
(A) Construction of a retroviral vector to transduce the NY-ESO-1 TCR used in this study.
(B) Mean frequency (upper) and representative data (lower) of Vδ2 and CD8 expression in nongene-modified T cells (NGMCs, left, n = 5) and NY-ESO-1-TCR gene-modified T cells (NE1-GMCs, right, n = 5). Dot plots show representative data of the expression of Vδ2 and CD8 in NGMCs (left) and NE1-GMCs (right).
(C) Mean frequencies of αβTCR+ cells in NGMC Vδ2+ (left, n = 4), NE1-GMC Vδ2highCD8low and NE1-GMC Vδ2lowCD8high cells (right, n = 4).
(D) Mean frequency of NE1-tetramer-positive cells in NE1-GMC Vδ2lowCD8high cells (n = 3). The error bar shows the standard deviation.
E) Tumor size change in NW-MEL-38 melanoma cell (HLA-A2+, NY-ESO-1+)-bearing NOG mice. Mice were treated with total body irradiation (TBI) (2 Gy) and then infused with (dotted lines, n = 3) or without (solid lines, n = 3) NE1-GMCs. The frequency of CD8+NE1-Tetramer+ cells in infused NE1-GMCs was 62.2%.
(F) Frequency of human CD8+NE1-tetramer+ cells in human CD45+ cells. Sixty days after NE1-GMC infusion, peripheral blood was obtained from 3 mice, as shown in Figure 2A, and examined.
(G) Frequencies of IFN-γ-secreting NGMC Vδ2+, NE1-GMC Vδ2highCD8low and NE1-GMC Vδ2lowCD8high cells: no stimulation (white), stimulated with PTA (gray), or NE1-pMHC (black).
Effector function and metabolism dependency of IFN-γ secretion in NE1-GMCs
In cultures without cytokine supplementation, cell growth of PTA-treated γδ-T cells was suppressed (Figures S4A and S4B). PTA-treated NGMCs functioned as antigen-presenting cells, and the possibility of mutual cytotoxicity could not be ruled out (Figure S4C). In contrast, compared to NE1-GMCs, TCRγδ-specific agonistic mAb (clone IMMU510)-stimulated NGMCs remained at a similar frequency (Figure S4A) and showed similar cell proliferation (Figure S4D). For the aforementioned reasons, IMMU510 was used to stimulate NGMCs in subsequent experiments.
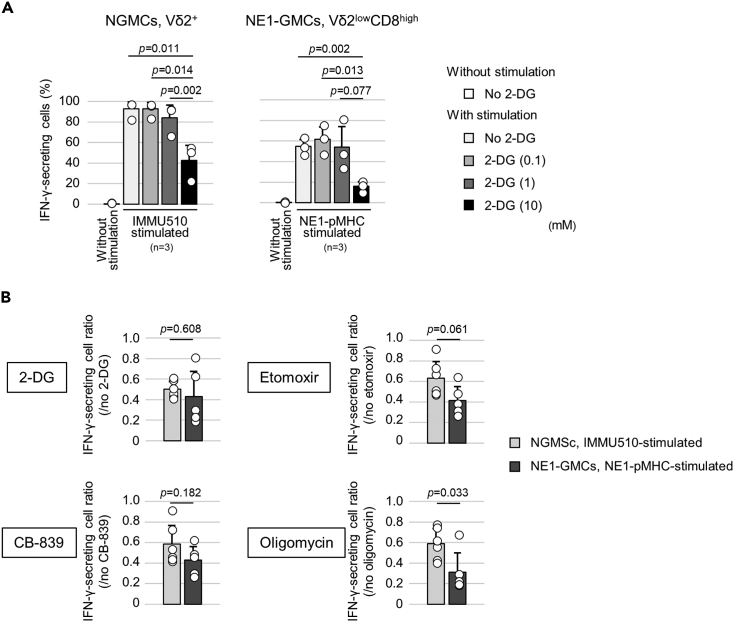
To assess the dependence on glucose metabolism, examinations of cytokine secretion by γδ-T cells using metabolic inhibitors were performed. IFN-γ secretion by NGMCs and NE1-GMCs decreased with increasing concentrations of 2-deoxy-D-glucose (2-DG), a glycolysis inhibitor (Figure 2A). Compared with that of IMMU510-stimulated NGMCs, the IFN-γ secretion of NE1-pMHC-stimulated NE1-GMCs was more strongly suppressed by oligomycin (an ATP synthase inhibitor) (Figure 2B and Table S1). The same trend of inhibition of IFN-γ secretion by oligomycin was observed for p40Tax-pMHC-stimulated p40Tax-specific αβ-TCR27 and CD8-transduced GMCs (p40Tax-GMCs) (Figure S5). Etomoxir (a fatty acid oxidation inhibitor) and CB-839 (a glutaminase inhibitor) also tended to inhibit IFN-γ secretion more in αβTCR-transduced GMCs, suggesting that αβTCR-transduced GMCs utilize more mitochondrial functions.
Figure 2.
NY-ESO-1-specific response and persistence of NE1-GMCs and comparison of the effects of metabolic inhibition on IFN-γ secretion between NGMCs and NE1-GMCs
(A) Frequencies of IFN-γ-secreting cells in NGMC Vδ2+ cells and NE1-GMC Vδ2lowCD8high cells in media with different 2-DG concentrations. The means of 3 experiments using γδ-T cells induced from 2 healthy donors are shown in the bar graph.
(B) Frequencies of IFN-γ-secreting cell ratios determined by comparing IMMU510-stimulated NGMC Vδ2+ cells (6 experiments using γδ-T cells induced from 3 healthy donors) and NE1-pMHC-stimulated NE1-GMC Vδ2lowCD8high cells (6 experiments using γδ-T cells induced from 3 healthy donors). The concentrations of metabolic inhibitors were 2-DG (10 mM), etomoxir (100 μM), CB-839 (2 μM), or oligomycin (2 μg/mL). The error bar shows the standard deviation.
Metabolomic analysis of NGMCs and NE1-GMCs
Metabolites from unstimulated and stimulated NGMCs and NE1-GMCs were analyzed using a combination of capillary electrophoresis and Fourier transform mass spectrometry (CE-FTMS), and 357 metabolites (cationic:195, anionic:162) were detected (Figure 3A). Metabolites involved in the TCA cycle and OXPHOS were strongly correlated with PC1, which separated unstimulated NE1-GMCs from unstimulated NGMCs (Figure 3B and Table S2). NE1-GMCs contained more metabolites of the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) in both unstimulated and stimulated states (Figure 3C). The frequencies of Vδ2+ cells in NGMCs and CD8+ cells in NE1-GMCs used in the study were similar (Figures S6A and S6B).
Figure 3.
Metabolomic analysis of NGMCs and NE1-GMCs
Three samples from three different healthy donors per group were assessed. The Roman numerals (I, II, and III) at the top of each sample name indicate that the T cells were derived from the same healthy donor and induced on the same day.
(A) Heatmap of 357 metabolites (cationic:195, anionic:162) detected by CE-FTMS compared among unstimulated NGMCs (blue; Ⅰ-1, Ⅱ-1, and Ⅲ-1), unstimulated NE1-GMCs (green; Ⅰ-2, Ⅱ-2, and Ⅲ-2), IMMU510-stimulated NGMCs (red; Ⅰ-3, Ⅱ-3, and Ⅲ-3), and NE1-pMHC-stimulated NE1-GMCs (orange; Ⅰ-4, Ⅱ-4, and Ⅲ-4). The bars on the right show the range of metabolites involved in glycolysis (lower panel) and the TCA cycle and OXPHOS (upper panel).
(B) Principal component analysis of 357 detected metabolites. Each circle indicates unstimulated NGMCs (blue), unstimulated NE1-GMCs (green), IMMU510-stimulated NGMCs (red), and NE1-pMHC-stimulated NE1-GMCs (orange).
(C) Bar graph of the metabolites involved in glycolysis, the TCA cycle, and OXPHOS in unstimulated and stimulated states. Each bar represents the mean relative areas of the indicated metabolites. Error bars indicate the standard deviation.
Transcriptomic analysis
RNA microarray analysis was performed using cells from the four groups as in the metabolomic analysis. Cells were newly induced on different days from those used for metabolomic analysis. Gene set enrichment analysis (GSEA) showed a trend toward increased expression of genes related to mitochondria in NE1-GMCs compared to NGMCs. Gene expression associated with the “electron transport chain: oxphos system in mitochondria” of WikiPathways was higher in NE1-GMCs than in NGMCs under both unstimulated and stimulated conditions (Figure 4A and Table 1). Gene expression associated with the “oxidative phosphorylation” of WikiPathways was higher in NE1-GMCs than in NGMCs in the stimulated state (Figures 4B and Table 1).
Figure 4.
Transcriptomic analysis of NGMCs and NE1-GMCs
Gene set enrichment analysis (GSEA) between NE1-GMCs purified with CD8 microbeads and NGMCs under unstimulated and stimulated conditions was performed.
(A) Heatmap of rank metric scores and plots of the enrichment scores for the “ WP electron transport chain: oxphos system in mitochondria” gene set in the unstimulated and TCR-stimulated states are shown.
(B) Heatmap of rank metric scores and plots of the enrichment scores for the “WP oxidative phosphorylation” gene set in the TCR-stimulated state are shown. The “unstimulated” rank metric score shows unstimulated NE1-GMCs versus unstimulated NGMCs, and the “stimulated” rank metric score shows NE1-pMHC-stimulated NE1-GMCs versus IMMU510-stimulated NGMCs. Rank metric score >0 (red) means higher in NE1-GMCs, <0 (blue) means lower in NE1-GMCs, compared with NGMCs. One sample per group was analyzed.
Table 1.
Summary of gene set enrichment analysis (GSEA): detected mitochondrial metabolism-related gene sets
| Gene set | SIZE | Unstimulated NE1-GMCs per unstimulated NGMCs |
NE1-pMHC-stimulated NE1-GMCs per IMMU510-stimulated NGMCs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ES | NES | NOM p value | FDR q-value | ES | NES | NOM p value | FDR q-value | ||
| WP_MITOCHONDRIAL_COMPLEX_III_ASSEMBLY | 15 | 0.69 | 1.44 | 0.057 | 1.000 | 0.53 | 1.07 | 0.401 | 1.000 |
| WP_ELECTRON_TRANSPORT_CHAIN_OXPHOS_SYSTEM_IN_MITOCHONDRIA | 90 | 0.45 | 1.33 | 0.042 | 1.000 | 0.55 | 1.57 | 0.004 | 1.000 |
| WP_GLYCOGEN_SYNTHESIS_AND_DEGRADATION | 40 | 0.50 | 1.30 | 0.109 | 1.000 | 0.52 | 1.31 | 0.117 | 1.000 |
| WP_TCA_CYCLE_AND_DEFICIENCY_OF_PYRUVATE_DEHYDROGENASE_COMPLEX_PDHC | 16 | 0.61 | 1.29 | 0.153 | 1.000 | 0.66 | 1.36 | 0.113 | 1.000 |
| WP_MITOCHONDRIAL_COMPLEX_I_ASSEMBLY_MODEL_OXPHOS_SYSTEM | 49 | 0.46 | 1.23 | 0.134 | 1.000 | 0.38 | 1.00 | 0.401 | 1.000 |
| WP_OXIDATIVE_PHOSPHORYLATION | 52 | 0.42 | 1.13 | 0.230 | 1.000 | 0.58 | 1.54 | 0.006 | 1.000 |
| WP_MITOCHONDRIAL_COMPLEX_IV_ASSEMBLY | 31 | 0.47 | 1.13 | 0.272 | 1.000 | 0.55 | 1.31 | 0.112 | 1.000 |
| WP_TCA_CYCLE_AKA_KREBS_OR_CITRIC_ACID_CYCLE | 18 | 0.50 | 1.09 | 0.346 | 1.000 | 0.57 | 1.22 | 0.194 | 1.000 |
ES, Enrichment Score; FDR, False discovery rate; NE1-GMCs, genetically modified γδ-T cells expressing an NY-ESO-1-specific αβ-TCR and the CD8 coreceptor; NES, Normalized Enrichment Score; NE1-pMHC, NY-ESO-1p157-165 peptide and HLA-A∗02:01 complex; NGMCs, non-gene-modified γδ-T cells; NOM, Nominal.
Mitochondrial phenotype assessed by flow cytometry
The mitochondrial phenotype of NGMCs and NE1-GMCs was assessed using MitoTracker Green and tetramethylrhodamine methyl ester (TMEM). NE1-GMCs had higher mitochondrial mass and mitochondrial membrane potential in the unstimulated state (Figure 5A). Another αβ-TCR-transduced GMCs, p40Tax-GMCs, also showed a similar mitochondrial phenotype (Figure S7A).
Figure 5.
Mitochondrial phenotype assessment by flow cytometry using mitochondria-labeling dyes and extracellular flux analysis of NGMCs and NE1-GMCs
(A) The mitochondrial phenotype of NGMCs and NE1-GMCs was assessed by flow cytometry using mitochondria-labeling dyes. The bar graph shows the average of five measurements of the mean fluorescence intensity (MFI) of MitoTracker Green and TMRM.
(B) Boxplots (median with the 25th and 75th percentiles) of the basal OCR/basal ECAR ratio comparing unstimulated NGMCs (total 18 measurements from 2 experiments) and unstimulated CD8+ cells selected from NE1-GMCs (total 18 measurements from 3 experiments). The median basal OCR/basal ECAR ratios were 0.95 and 0.65, respectively.
(C) Boxplots (median with the 25th and 75th percentiles) of the basal OCR/basal ECAR ratio of IMMU510-stimulated NGMCs (light gray, total 12 measurements from 2 experiments) and NE1-pMHC-stimulated CD8+ cells purified from NE1-GMCs (dark gray, total 15 measurements from 2 experiments). The median basal OCR/basal ECAR ratios were 0.92 and 2.33, respectively.
Metabolic status analyzed by extracellular flux
Cellular metabolic status was assessed by extracellular flux analysis, measuring the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), with the results representing the levels of mitochondrial respiration and glycolysis, respectively. In the absence of TCR stimulation, the median basal OCR/basal ECAR ratio of NGMCs was similar to or higher than that of NE1-GMCs (NGMCs: 0.95 vs. NE1-GMCs: 0.65) (Figures 5B and S7B). With TCR stimulation, the median basal OCR/basal ECAR ratio increased only in NE1-GMCs, indicating enhanced mitochondrial metabolism in NE1-GMCs upon αβ-TCR stimulation (NGMCs: 0.92 vs. NE1-GMCs: 2.33) (Figure 5C). A similar change was observed in p40Tax-GMCs (Figure S7C).
Discussion
In a study on TCR-T cells, an autoreactive TCR consisting of endogenous TCR and engineered TCR caused lethal toxicity in a mouse model.14 Considering the use of allogeneic γδ-T cells for αβ-TCR-transduced T cell therapy, T cell products would be safe because genetically engineered T cells are at least dual receptor-like, with monoclonal αβ-TCR and γδ-TCR. Acute rejection due to HLA incompatibility can be controlled by prior lymphodepleting chemotherapy. Therefore, if αβ-TCR-transduced γδ-T cells persist, the clinical development of off-the-shelf αβ-TCR-transduced allogeneic γδ-T cell products will advance greatly. The in vivo persistence of αβ-T cells is thought to be related to antitumor efficacy, and mainly in the context of CAR-T cell development, incorporation of 4-1BB (CD137) signaling28,29,30 and cytokines such as IL-7 and IL-1531 into T cells has been studied. Vγ9+Vδ2+ T cells are an innate-like T cell population.32 Clinical trials on these cells have not shown a remarkable survival benefit. The reason for this lack of efficacy is unclear33; however, their short persistence after activation may be a cause. In our study, αβ-TCR-transduced γδ-T cells showed promising persistence in vitro and in vivo. Lope et al. reported that IFN-γ secretion by γδ-T cells was glycolysis-dependent,34 which is consistent with our data. Mitochondrial function is useful for assessing the persistence of αβ-T cells35,36 and may be relevant for γδ-T cells. Here, we found that αβ-TCR-transduced γδ-T cells could utilize more mitochondrial energy metabolism.
In our study, αβ-TCR transduction increased the mitochondrial mass and mitochondrial membrane potential of γδ-T cells. αβ-TCR-transduced γδ-T cells contained more metabolites in the TCA cycle and OXPHOS and had higher gene expression of the mitochondrial electron transport chain in both unstimulated and stimulated states. In the stimulated state, IFN-γ production was more dependent on ATP synthesis, which was supported by the higher OXPHOS gene expression observed in αβ-TCR-transduced γδ-T cells. Although not high on the list in our gene analysis, our examinations using metabolic inhibitors suggest that energy through glutamine and fatty acid metabolism might also be important for the characteristics of αβ-TCR-transduced γδ-T cells. The metabolism of glutamine and fatty acids in αβ-TCR-transduced γδ-T cells requires further study.
Evaluation of spare respiratory capacity (SRC) is a useful method for assessing mitochondrial function in T cells.37,38 However, it was difficult to identify differences in SRC between TCR-stimulated NGMCs and NE1-GMCs. In contrast to SRC, the basal OCR/basal ECAR ratio can compensate for individual differences.39 An increased basal OCR/basal ECAR ratio was observed in NE1-pMHC-stimulated NE1-GMCs, which might reflect an inclination toward mitochondrial energy metabolism. Similar results were obtained for p40Tax-pMHC-stimulated p40Tax-GMCs. In a mouse model, a relationship between γδ-T cell subsets classified by cytokine secretion (IFN-γ or IL-17) and mitochondrial function was reported,34 but there were no obvious differences in cytokine profiles between NGMCs and NE1-GMCs induced from human donors in our study. The small sample size is one of the limitations of our study and requires further validation. However, our experiments consistently suggest that αβ-TCR-transduced γδ-T cells acquire superior mitochondrial function.
Recently, Stenger et al. reported the importance of the presence of αβ-TCR for αβ-T cell persistence by showing that knock out of endogenous TCR in CD19 CAR-T cells resulted in a lack of persistence, although the mechanism remains unclear.40 Mitochondria play an important role in the sustained killing of cytotoxic T cells.41 In our study, αβ-TCR and CD8 transduction to γδ-T cells yielded promising T cell persistence with higher utilization of mitochondrial function. CD8 is necessary to stabilize αβ-TCR recognition of the peptide epitope present on MHC molecules.16 The recruitment of CD8-associated Lck is essential for the augmentation of TCR signal transduction42 and in vivo persistence of αβ-T cells.43 These results support the enhanced antitumor activity of αβ-TCR and CD8 gene-cotransduced γδ-T cells. The interaction of αβ-TCR with nonstimulatory pMHC is known as coagonism.44,45,46,47 Appropriate Lck activation seems to be important for CD8+ T cell effector function and proliferation.
In conclusion, αβ-TCR transduction into γδ-T cells showed promising in vitro and in vivo persistence. As a mechanism, γδ-T cells could utilize more mitochondrial energy metabolism due to the acquisition of αβ-TCR and CD8.
Limitations of the study
The study has several limitations: small sample size and reporting from a single group.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-human TCR αβ-biotin | Miltenyi Biotec | Cat#130-113-529 |
| antibiotin microbeads | Miltenyi Biotec | Cat#130-090-485 |
| CD8 microbeads | Miltenyi Biotec | Cat#130-045-201 |
| Human TruStain FcX | BioLegend | Cat#422302 |
| anti-human TCR Vδ2 | BioLegend | Cat#331418, 331428, 331406 |
| anti-human CD8a | BioLegend | Cat#301006, 301036 |
| anti-human CD3ε | BioLegend | Cat#344815 |
| anti-human CD45 | BioLegend | Cat#304015 |
| anti-human CD45RA | BD Biosciences | Cat#561216 |
| anti-human CD27 | BioLegend | Cat#302810 |
| anti-human IFN-γ | BioLegend | Cat#502532 |
| anti-Ki67 | Beckman Coulter | Cat#B68180 |
| TCRγδ-specific agonistic mAb | Beckman Coulter | Cat# PN IM1349 |
| Chemicals, peptides, and recombinant proteins | ||
| tetrakis-pivaloyloxymethyl 2-(thiazole-2-ylamino) ethylidene-1,1-bisphosphonate | Tanaka, Y. et al.23 | N/A |
| RetroNectin | Takara Bio | Cat#T100A |
| GolgiStop Protein Transport Inhibitor | BD Biosciences | Cat#554724 |
| Live or dead fixable dead cell staining kit NIR fluorescence | AAT Bioquest | Cat#22605 |
| CytoTell Red 650 | AAT Bioquest | Cat#22255 |
| Apotracker Green | Biolegend | Cat#427402 |
| 2-deoxy-D-glucose | Sigma–Aldrich | Cat#D8375 |
| Etomoxir | Cayman | Cat#11969 |
| CB-839 | Cayman | Cat#22038 |
| Oligomycin | Abcam | Cat#141829 |
| MitoTracker Green | Invitrogen | Cat#M7514 |
| Tetramethylrhodamine (TMRM) | FUJIFILM | Cat#4987481499720 |
| Critical commercial assays | ||
| Combination of capillary electrophoresis and Fourier transform mass spectrometry (CE-FTMS) | Human Metabolome Technologies | N/A |
| Agilent RNA 6000 Pico Kit | Agilent Technologies | N/A |
| SurePrint G3 Human GE Microarray 8 × 60 K Ver3.0 | Agilent Technologies | N/A |
| SureScan Microarray scanner | Agilent Technologies | N/A |
| Seahorse XF Cell Mito Stress Test Kit | Agilent Technologies | Cat#103010-100 |
| Deposited data | ||
| Raw RNA data | This paper | GEO: GSE216880 |
| Experimental models: Cell lines | ||
| NW-MEL-38 | Memorial Sloan Kettering Cancer Center | RRID:CVCL_S591 |
| T2 | ATCC | RRID:CVCL_2211 |
| SW982 | ATCC | RRID:CVCL_1734 |
| Fuji | ATCC | RRID:CVCL_D880 |
| Experimental models: Organisms/strains | ||
| NOD/Shi-scid/IL-2Rγnull | the Central Institute for Experimental Animals | N/A |
| Software and algorithms | ||
| Gene set enrichment analysis (GSEA) version 4.3.2. | Broad Institute, Inc., Massachusetts Institute of Technology, and Regents of the University of California. | https://www.gsea-msigdb.org/gsea/index.jsp |
| SPSS Statistics version 26 | IBM Japan | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Mikiya Ishihara (mishihara@med.mie-u.ac.jp).
Materials availability
This study did not generate new unique reagents.
Experimental models and study participants
Animals
NOD/Shi-scid/IL-2Rγnull (NOG) female mice were purchased from the Central Institute for Experimental Animals (Kawasaki, Japan). Mice were fed a standard diet, housed under specific pathogen-free conditions, and used at 6–8 weeks of age. Five days after subcutaneous NW-MEL-38 melanoma cell inoculation, the mice were treated with total body irradiation (TBI) (2 Gy) and then infused with or without NE1-GMCs. The number of infused NE1-GMCs was 5×106 cells/mouse. All animal experiments were conducted according to protocols approved by the Animal Care and Use Committee of the Mie University Life Science Center.
Cell lines
T2 cells (HLA-A2+, TAP-deficient, Epstein–Barr virus-transformed lymphoblastoid cell line), SW982 cells (NY-ESO-1+/HLA-A2+ synovial sarcoma cell line), Fuji cells (NY-ESO-1-/HLA-A2+ synovial sarcoma cell line), and NW-MEL-38 cells (HLA-A2+, NY-ESO-1+ melanoma cell line) were cultured in RPMI-1640 medium supplemented with 25 mM HEPES, 10% fetal calf serum (FCS), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Mycoplasma contamination of the cell lines was checked, but not detected.
Method details
Vector construction and preparation of αβ-TCR-engineered γδ-T cells
αβ-TCR-engineered γδ-T cells were prepared as follows: Lymphocytes were isolated from the peripheral blood of healthy donors. None of the healthy donors had a history of an active autoimmune disease or malignancy. All samples were collected after obtaining written informed consent as part of a study approved by the Ethics Committee of Mie University Hospital. After negative selection using anti-human TCR αβ-biotin and antibiotin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), TCR αβ-negative cells were cultured in modified Yssel’s medium with 10% human type AB serum and PTA (day 0).26 The culture medium was replaced with fresh medium containing IL-7 and IL-15 on day 1, and half of the medium was changed every 2–3 days. For GMCs, proliferating lymphocytes were infected with retroviral vectors on RetroNectin (Takara Bio Inc., Shiga, Japan)-coated plates on days 4 and 5. For NE1-GMCs, retroviral vectors encoding the G50A + A51E TCR (NE1p157/HLA-A∗02:01-specific TCR-α and TCR-β chains with G50A + A51E amino acid replacements) and human CD8 (Figure 1A) were used. An NY-ESO-1-specific TCR has been reported by Schmid et al.10 On day 8, γδ-T cells were harvested and frozen with CELLBANKER1 (Takara Bio) until use, except for metabolomic and transcriptome analyses. Each experiment was performed within two months of γδ-T-cell freezing. Thawed γδ-T cells were cultured in RPMI 1640 medium with 10% FCS for 4–6 h and then used in each experiment.
All procedures performed in this study involving human participants were conducted in accordance with the Japanese law of Ethical Guidelines for Medical and Biological Research Involving Human Subjects.
In vitro functional assay based on flow cytometry
Cytokine secretion and TCR expression in γδ-T cells were measured using flow cytometry. γδ-T cells were cultured with Fc Receptor Blocking Solution Human TruStain FcX (BioLegend) prior to antibody staining. Fluorescently labeled anti-human TCR Vδ2 monoclonal antibody (mAb) (Clone B6), anti-human CD8a mAb (clone RPA-T8), anti-human CD3ε mAb (clone SK7), anti-human CD45 mAb (clone HI30), anti-human IFN-γ mAb (clone 4S. B3) were purchased from BioLegend, Inc., and anti-Ki67 mAb was purchased from Beckman Coulter, Inc. (Brea, CA, USA). The live or dead fixable dead cell staining kit NIR fluorescence (AAT Bioquest, Inc., Sunnyvale, CA, USA) was used to identify the live cells. For T cell stimulation, γδ-T cells were cultured in PTA (1.0 μM)-containing medium on a TCRγδ-specific agonistic mAb (clone IMMU510) (Beckman Coulter) (1.0 μg/mL)-coated plate or NE1p157/HLA-A∗02:01 complex (NE1-pMHC) (1.0 ng/μL)-coated plate. For the intracellular staining of cytokines, γδ-T cells were cultured for 5–6 h with a GolgiStop Protein Transport Inhibitor (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan). For metabolic inhibition experiments, RPMI 1640 (glucose-free) medium, 2-DG (Sigma–Aldrich Co. LLC, St. Louis, MO), etomoxir, CB-839 (Cayman Chemical, Ann Arbor, MI), and oligomycin (Abcam, Cambridge, UK) were used. For mitochondrial phenotype assessment, MitoTracker Green (Invitrogen) and tetramethylrhodamine (TMRM) (FUJIFILM) were used.
Metabolomic analysis
Comprehensive analysis of hydrophilic and ionic metabolites using a combination of capillary electrophoresis and Fourier transform mass spectrometry (CE-FTMS) was performed by Human Metabolome Technologies, Inc. (Yamagata, Japan). NE1-GMCs were purified using CD8 Microbeads (Miltenyi Biotec) on day seven. Metabolic extracts were prepared from 2.0-3.5 × 106 cells per sample, with three samples from three different healthy donors included in each group, and methanol containing an internal standard solution after washing with mannitol-containing solution on day 8. Prior to extraction, T cells were cultured for 6 h in RPMI 1640-10% FCS medium with or without TCR stimulation. TCRγδ-specific agonistic mAb- and NE1-pMHC-coated plates were used for NGMCs and NE1-GMCs, respectively.
Transcriptomic analyses
RNA extracts were prepared from 3.1 × 105 cells per sample from a healthy donor using a QIAamp RNA Blood Mini kit (QIAGEN K.K., Tokyo, Japan). Prior to RNA extraction, NGMCs and NE1-GMCs purified with CD8 microbeads were cultured with or without TCR stimulation, as in metabolomic analysis. RNA microarray analysis was performed by Agilent Technologies, Inc. Briefly, the RNA integrity of the samples was determined using an Agilent 2100 Bioanalyzer and Agilent RNA 6000 Pico Kit. All samples showed an RNA integrity number (RIN) higher than eight and qualified for further processing. Total RNA was reverse-transcribed to cDNA and then synthesized into Cy3-labeled cRNA by in vitro transcription. Labeled samples were hybridized to a SurePrint G3 Human GE Microarray 8 × 60 K Ver3.0. Scanning was performed using a SureScan Microarray scanner. Gene set enrichment analysis (GSEA)48 was performed using GSEA software version 4.3.2. (https://www.gsea-msigdb.org/gsea/index.jsp). The log2-transformed, normalized and validated gene data by Agilent Technologies, Inc. was used. The genes were ranked based on the gene set ‘WikiPathways subset of Canonical Pathways’.
Measurement of the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR)
OCR and ECAR were measured with a Seahorse XF HS Mini Analyzer using a Seahorse XF Cell Mito Stress Test Kit (Agilent Technologies Inc., Santa Clara, CA, USA). Briefly, after thawing, γδ-T cells were cultured in RPMI 1640-10% FCS medium. The TCR stimulation time was 72 h. After washing with assay medium, γδ-T cells were plated at 2.0–3.0 × 105 cells/well, with the same number of cells included in each experiment, in a cell culture plate with assay medium and cultured for a total of 50–60 min at 37°C in a CO2-free incubator until analysis. The final concentrations of oligomycin, FCCP, and rotenone/antimycin A were 1.5, 1.0 μM and 0.5 μM, respectively. The basal OCR/basal ECAR ratio was calculated using the equation: (pmol/min)/(mpH/min).39
Quantification and statistical analysis
For cytokine inhibition studies, a t test was used if Levene’s test showed a value above the significance level; otherwise, Welch’s t test was used. For metabolic status analysis using Seahorse, the Mann–Whitney U test was used. Calculations were performed using SPSS Statistics version 26 (IBM Japan, Ltd., Tokyo, Japan). For the metabolomic analysis, we described the results of Welch’s test reported by Human Metabolome Technologies, Inc. p values less than 0.05 were considered statistically significant (∗p < 0.05, ∗∗p < 0.01).
Acknowledgments
The authors thank Ms. J. Nakamura, Ms. C. Amaike, and Ms. T Hayashi for their technical assistance. The Department of Personalized Cancer Immunotherapy, Mie University Graduate School of Medicine, is an endowment department supported by a grant from T-Cell Nouveau, Inc.
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 23K06740 and 20K07656 to M.I., 19K07751 to H.M., and 18K08361 to H.F.) and Japan Agency for Medical Research and Development (Grant Number 21bk0104115 to H.F.).
Author contributions
M.I. and H.M. designed this study. M.I. performed experiments under the supervision of H.M., H.F., Y.A., T.K., I.T., and H.S. Y.A. designed and purified the retroviral vector. Y.T. purified PTA. M.I. wrote the manuscript with input from all the authors. All authors contributed to data interpretation, reviewed the manuscript, and agreed with the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: August 31, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107802.
Contributor Information
Mikiya Ishihara, Email: mishihara@med.mie-u.ac.jp.
Hiroshi Miwa, Email: hmiwa@med.mie-u.ac.jp.
Supplemental information
Data and code availability
-
•
The RNA data generated in this study have been deposited at Gene Expression Omnibus (GEO) and are publicly available as of the date of publication.
-
•
Accession number is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Maude S.L., Teachey D.T., Porter D.L., Grupp S.A. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.T., Scanlan M.J., Sahin U., Türeci O., Gure A.O., Tsang S., Williamson B., Stockert E., Pfreundschuh M., Old L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jungbluth A.A., Chen Y.T., Stockert E., Busam K.J., Kolb D., Iversen K., Coplan K., Williamson B., Altorki N., Old L.J. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int. J. Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 6.Gnjatic S., Nishikawa H., Jungbluth A.A., Güre A.O., Ritter G., Jäger E., Knuth A., Chen Y.T., Old L.J. NY-ESO-1: review of an immunogenic tumor antigen. Adv. Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J., Wang L. The Emerging World of TCR-T Cell Trials Against Cancer: A Systematic Review. Technol. Cancer Res. Treat. 2019;18 doi: 10.1177/1533033819831068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara M., Kitano S., Kageyama S., Miyahara Y., Yamamoto N., Kato H., Mishima H., Hattori H., Funakoshi T., Kojima T., et al. NY-ESO-1-specific redirected T cells with endogenous TCR knockdown mediate tumor response and cytokine release syndrome. J. Immunother. Cancer. 2022;10:e003811. doi: 10.1136/jitc-2021-003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishihara M., Nishida Y., Kitano S., Kawai A., Muraoka D., Momose F., Harada N., Miyahara Y., Seo N., Hattori H., et al. A phase 1 trial of NY-ESO-1-specific TCR-engineered T-cell therapy combined with a lymph node-targeting nanoparticulate peptide vaccine for the treatment of advanced soft tissue sarcoma. Int. J. Cancer. 2023;152:2554–2566. doi: 10.1002/ijc.34453. [DOI] [PubMed] [Google Scholar]
- 10.Schmid D.A., Irving M.B., Posevitz V., Hebeisen M., Posevitz-Fejfar A., Sarria J.C.F., Gomez-Eerland R., Thome M., Schumacher T.N.M., Romero P., et al. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J. Immunol. 2010;184:4936–4946. doi: 10.4049/jimmunol.1000173. [DOI] [PubMed] [Google Scholar]
- 11.Robbins P.F., Morgan R.A., Feldman S.A., Yang J.C., Sherry R.M., Dudley M.E., Wunderlich J.R., Nahvi A.V., Helman L.J., Mackall C.L., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K., Goloubeva O., Vogl D.T., Lacey S.F., Badros A.Z., Garfall A., Weiss B., Finklestein J., et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Angelo S.P., Melchiori L., Merchant M.S., Bernstein D., Glod J., Kaplan R., Grupp S., Tap W.D., Chagin K., Binder G.K., et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018;8:944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendle G.M., Linnemann C., Hooijkaas A.I., Bies L., de Witte M.A., Jorritsma A., Kaiser A.D.M., Pouw N., Debets R., Kieback E., et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16:565–570. doi: 10.1038/nm.2128. 1p following 570. [DOI] [PubMed] [Google Scholar]
- 15.van der Veken L.T., Hagedoorn R.S., van Loenen M.M., Willemze R., Falkenburg J.H.F., Heemskerk M.H.M. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66:3331–3337. doi: 10.1158/0008-5472.Can-05-4190. [DOI] [PubMed] [Google Scholar]
- 16.Hiasa A., Nishikawa H., Hirayama M., Kitano S., Okamoto S., Chono H., Yu S.S., Mineno J., Tanaka Y., Minato N., et al. Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes. Gene Ther. 2009;16:620–628. doi: 10.1038/gt.2009.6. [DOI] [PubMed] [Google Scholar]
- 17.Harrer D.C., Simon B., Fujii S.I., Shimizu K., Uslu U., Schuler G., Gerer K.F., Hoyer S., Dörrie J., Schaft N. RNA-transfection of gamma/delta T cells with a chimeric antigen receptor or an alpha/beta T-cell receptor: a safer alternative to genetically engineered alpha/beta T cells for the immunotherapy of melanoma. BMC Cancer. 2017;17:551. doi: 10.1186/s12885-017-3539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng J., Yin H. Gamma delta (gammadelta) T cells in cancer immunotherapy; where it comes from, where it will go? Eur. J. Pharmacol. 2022;919:174803. doi: 10.1016/j.ejphar.2022.174803. [DOI] [PubMed] [Google Scholar]
- 19.Dong R., Zhang Y., Xiao H., Zeng X. Engineering gammadelta T Cells: Recognizing and Activating on Their Own Way. Front. Immunol. 2022;13:889051. doi: 10.3389/fimmu.2022.889051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., Nair V.S., Xu Y., Khuong A., Hoang C.D., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida Y., Nakajima J., Wada H., Kakimi K. gammadelta T-cell immunotherapy for lung cancer. Surg. Today. 2011;41:606–611. doi: 10.1007/s00595-010-4478-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T., Chen J., Niu L., Liu Y., Ye G., Jiang M., Qi Z. Clinical Safety and Efficacy of Locoregional Therapy Combined with Adoptive Transfer of Allogeneic gammadelta T Cells for Advanced Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Vasc. Intervent. Radiol. 2022;33:19–27.e3. doi: 10.1016/j.jvir.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm M., Smetak M., Schaefer-Eckart K., Kimmel B., Birkmann J., Einsele H., Kunzmann V. Successful adoptive transfer and in vivo expansion of haploidentical gammadelta T cells. J. Transl. Med. 2014;12:45. doi: 10.1186/1479-5876-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki T., Matsushita H., Hoshikawa M., Hasegawa K., Kokudo N., Kakimi K. Adjuvant combination therapy with gemcitabine and autologous gammadelta T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy. 2017;19:473–485. doi: 10.1016/j.jcyt.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y., Xiang Z., Alnaggar M., Kouakanou L., Li J., He J., Yang J., Hu Y., Chen Y., Lin L., et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell. Mol. Immunol. 2021;18:427–439. doi: 10.1038/s41423-020-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y., Murata-Hirai K., Iwasaki M., Matsumoto K., Hayashi K., Kumagai A., Nada M.H., Wang H., Kobayashi H., Kamitakahara H., et al. Expansion of human gammadelta T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Sci. 2018;109:587–599. doi: 10.1111/cas.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiwara H., Okumura S., Miyahara Y., Wan L., Tawara I., Jo T., Tanaka Y., Tanaka Y., Ikeda H., Shiku H. Novel Cellular Immunotherapy Using Allogeneic Vγ9/δ2-T Cells Gene-Modified to Express HTLV-1 P40Tax-Specific TCR for the Treatment of Adult T Cell Leukemia. Blood. 2019;134:3216. doi: 10.1182/blood-2019-122412. [DOI] [Google Scholar]
- 28.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G., et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G., Plotkin J., Sadelain M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y., Zhang M., Ramos C.A., Durett A., Liu E., Dakhova O., Liu H., Creighton C.J., Gee A.P., Heslop H.E., et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gully B.S., Rossjohn J., Davey M.S. Our evolving understanding of the role of the gammadelta T cell receptor in gammadelta T cell mediated immunity. Biochem. Soc. Trans. 2021;49:1985–1995. doi: 10.1042/BST20200890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyashita M., Shimizu T., Ashihara E., Ukimura O. Strategies to Improve the Antitumor Effect of gammadelta T Cell Immunotherapy for Clinical Application. Int. J. Mol. Sci. 2021;22:8910. doi: 10.3390/ijms22168910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes N., McIntyre C., Martin S., Raverdeau M., Sumaria N., Kohlgruber A.C., Fiala G.J., Agudelo L.Z., Dyck L., Kane H., et al. Distinct metabolic programs established in the thymus control effector functions of gammadelta T cell subsets in tumor microenvironments. Nat. Immunol. 2021;22:179–192. doi: 10.1038/s41590-020-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krug A., Martinez-Turtos A., Verhoeyen E. Importance of T, NK, CAR T and CAR NK Cell Metabolic Fitness for Effective Anti-Cancer Therapy: A Continuous Learning Process Allowing the Optimization of T, NK and CAR-Based Anti-Cancer Therapies. Cancers. 2021;14:183. doi: 10.3390/cancers14010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins Y., Zabkiewicz J., Ottmann O., Jones N. Tinkering under the Hood: Metabolic Optimisation of CAR-T Cell Therapy. Antibodies. 2021;10:17. doi: 10.3390/antib10020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Windt G.J.W., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., Pearce E.L. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn K.M., Hussain T., Kraus F., Formosa L.E., Lam W.K., Dagley M.J., Saunders E.C., Assmus L.M., Wynne-Jones E., Loh L., et al. Metabolic characteristics of CD8(+) T cell subsets in young and aged individuals are not predictive of functionality. Nat. Commun. 2020;11:2857. doi: 10.1038/s41467-020-16633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho J., de Moura M.B., Lin Y., Vincent G., Thorne S., Duncan L.M., Hui-Min L., Kirkwood J.M., Becker D., Van Houten B., Moschos S.J. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol. Cancer. 2012;11:76. doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenger D., Stief T.A., Kaeuferle T., Willier S., Rataj F., Schober K., Vick B., Lotfi R., Wagner B., Grünewald T.G.P., et al. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood. 2020;136:1407–1418. doi: 10.1182/blood.2020005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisci M., Barton P.R., Randzavola L.O., Ma C.Y., Marchingo J.M., Cantrell D.A., Paupe V., Prudent J., Stinchcombe J.C., Griffiths G.M. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science (New York, N.Y.) 2021;374:eabe9977. doi: 10.1126/science.abe9977. [DOI] [PubMed] [Google Scholar]
- 42.Artyomov M.N., Lis M., Devadas S., Davis M.M., Chakraborty A.K. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc. Natl. Acad. Sci. USA. 2010;107:16916–16921. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zareie P., Szeto C., Farenc C., Gunasinghe S.D., Kolawole E.M., Nguyen A., Blyth C., Sng X.Y.X., Li J., Jones C.M., et al. Canonical T cell receptor docking on peptide-MHC is essential for T cell signaling. Science. 2021;372 doi: 10.1126/science.abe9124. [DOI] [PubMed] [Google Scholar]
- 44.Yachi P.P., Ampudia J., Gascoigne N.R.J., Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat. Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yachi P.P., Lotz C., Ampudia J., Gascoigne N.R.J. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J. Exp. Med. 2007;204:2747–2757. doi: 10.1084/jem.20062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juang J., Ebert P.J.R., Feng D., Garcia K.C., Krogsgaard M., Davis M.M. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J. Exp. Med. 2010;207:1223–1234. doi: 10.1084/jem.20092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoerter J.A.H., Brzostek J., Artyomov M.N., Abel S.M., Casas J., Rybakin V., Ampudia J., Lotz C., Connolly J.M., Chakraborty A.K., et al. Coreceptor affinity for MHC defines peptide specificity requirements for TCR interaction with coagonist peptide-MHC. J. Exp. Med. 2013;210:1807–1821. doi: 10.1084/jem.20122528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The RNA data generated in this study have been deposited at Gene Expression Omnibus (GEO) and are publicly available as of the date of publication.
-
•
Accession number is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.