Abstract
β-Carotene, because it is the precursor of vitamin A and has versatile biological roles, has been applied as a feed additive in the poultry industry for a long time. In this study, we investigated the deposition and bioconversion of β-carotene in laying hens. A total of 600 Hy-line brown laying hens at 40 wk of age were randomly divided into 5 dietary treatments, each group's dietary supplemental levels of β-carotene were 0, 15, 30, 60, 120 mg/kg feed, and the vitamin A levels were all 8,000 IU/kg. After 14-wk trial, samples were collected, then carotenoids and different forms of vitamin A were detected using the novel method developed by our laboratory. We found that dietary β-carotene treatment had no significant effects on laying hens’ production performance and egg quality (P > 0.05), except the yolk color. The deposition of β-carotene in the body gradually increased (P < 0.01) with the supplemental dose, whereas the contents of lutein and zeaxanthin decreased (P < 0.05). When the β-carotene supplemental level was above 30 mg/kg in the diet, the different forms of vitamin A in in serum, liver, ovary, and yolks were increased compared to the control group (P < 0.05). However, these indicators decreased when the additional dose was 120 mg/kg. Moreover, the mRNA levels of the genes involved in β-carotene absorption, bioconversion, and negative feedback regulation in duodenal mucosa and liver were upregulated after long-term feeding (P < 0.05). Histological staining of the ovaries indicated that the deposition of β-carotene led to a lower rate of follicle atresia (P < 0.05), and this positive effects may be related to the antioxidant function of β-carotene, which caused a reduction of oxidation products in the ovary (P < 0.05). Altogether, β-carotene could accumulate in laying hens intactly and exert its biological functions in tissue. Meanwhile, a part of β-carotene could also be converted into vitamin A but this bioconversion has an upper limit and negative feedback regulation.
Key words: β-carotene, vitamin A, supercritical fluid chromatography, laying hen, antioxidant
INTRODUCTION
β-Carotene is a symmetrical C40 isoprenoid compound containing 2 β-ionone rings, which means it has the highest provitamin A activity in carotenoids theoretically and a lot of beneficial physiological functions (Bohn et al., 2019; Nie et al., 2019). On account of animals being unable to synthesize β-carotene in vivo, it is important to obtain it through food intake for the health benefits (Meléndez-Martínez, 2019). In addition to being consumed as a nutritional supplement in the human diet, this bioactive compound has been applied as a feed additive for livestock and poultry production. However, most studies in recent years have mainly focused on the positive effects of dietary β-carotene for animals (Hui et al., 2020; Yuan et al., 2020; Yang et al., 2021; Fawzy et al., 2022), such as antioxidant action, immune response, development of intestinal barriers, and modulation of gut microbiota. There are limited reports on the characteristics of β-carotene absorption, deposition, and bioconversion into vitamin A in different animals.
In general, the small intestine is the major site for the absorption and bioconversion of carotenoids (Von Lintig et al., 2020), especially in the proximal parts of it. After ingestion, the β-carotene is released from the food matrix and taken up by the enterocytes. There are some membrane proteins involved in facilitating this process, including the scavenger receptor class B type I (SR-B1) as well as the cluster of differentiation 36 (CD36). Next, the uptake of β-carotene can undergo 3 main metabolic pathways (Shete and Quadro, 2013; Rodriguez-Concepcion et al., 2018; Bohn et al., 2019; Von Lintig et al., 2020): First, a part of β-carotene can be packaged into chylomicrons entering the bloodstream directly and deposited in multiple tissues and organs intactly after transportation. Second, β-carotene can be cleaved symmetrically to yield 2 molecules of retinal, this bioconversion process is mainly catalyzed by the key enzyme β-carotene-15,15′-oxygenase (BCO1). As the important intermediate product of the retinoids’ metabolism, retinal can be reversibly reduced to retinol, then the retinol is further converted by lecithin: retinol acyl transferase (LRAT) to retinyl esters as the main storage form and distributed in the body. Third, the β-carotene can also be asymmetrically dissociated into apocarotenoids, such as β-apo-10′-carotenal by the β-carotene 9′, 10′-oxygenase (BCO2), which is located in mitochondria. Moreover, it should be mentioned that to maintain the optimum vitamin A status in the body and avoid hypervitaminosis A, there is a negative feedback control of β-carotene bioconversion via the intestine-specific homeodomain transcription factor (ISX), this transcription factor activation represses the gene encoding SR-B1 and BCO1 to control the vitamin A production (Lobo et al., 2010; Ramkumar et al., 2021).
Nowadays, there is still little research for the deposition and bioconversion situation of β-carotene in the body of poultry. Laying hens always receive a high vitamin A level in feed under the modern culture pattern (Lima and Souza, 2018; Chen et al., 2022), therefore, the metabolic fate of β-carotene, especially whether it would be converted into vitamin A, needs to be further investigated. Previous research of the effects of β-carotene on vitamin A in poultry or eggs only focused on changes in retinol contents, thereby ignoring other forms of vitamin A, such as retinal and different retinyl esters (Jiang et al., 1994; Heying et al., 2014; Díaz-Gómez et al., 2017a,b). Retinol, however, did not fully reflect the alteration of vitamin A status in the body after supplementation with β-carotene because retinyl esters as the main stable forms account for a major proportion of all vitamin A in the body (Debelo et al., 2017).
Given the characteristics of β-carotene, an appropriate method for detecting the amounts of β-carotene and several kinds of retinoids in different samples of laying hens simultaneously was an important basis for investigating the deposition and bioconversion of β-carotene in laying hens. Supercritical fluid chromatography (SFC) has been regarded as a powerful technique in recent years for rapid and accurate analysis of carotenoids and fat-soluble vitamins (Bernal et al., 2013; Pilařová et al., 2019). Importantly, our previous studies have built a novel SFC-DAD-MS/MS method for the simultaneous determination of various carotenoids and different forms of vitamin A in egg yolk samples specifically (Miao et al., 2023), this can help us to further develop detection method for serum and tissue samples. Therefore, based on the determination method, the main objective of this study was to explore the deposition and bioconversion law of β-carotene in laying hens after supplementing it with different levels for the long term under adequate vitamin A status in the diet.
MATERIALS AND METHODS
Animal Ethics Statement
All animal experimental procedures in this study were approved by the Animal Care and Use Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China (IAS 2021-103).
Animal Treatments and Sample Collection
A total of 600 Hy-line brown laying hens at 40 wk of age were randomly divided into 5 dietary treatments with 8 replicates of 15 birds each and managed following the procedures described in the Hy-line brown laying hens husbandry manual. The control group was fed a basal diet, and the ingredients and nutrient composition are shown in Table 1. Except the metabolizable energy and available phosphorus, the data of main nutrients in feed were all measured values. There was no β-carotene supplementation in the basal diet, and the other 4 groups (BC15, BC30, BC60, and BC120) were additionally supplemented with 15, 30, 60, and 120 mg/kg β-carotene powder, respectively, which was produced by Blakeslea trispora fermentation (the raw material was purchased from Wuhan Stars Modern Bio-engineering Co., Ltd). The feeding period lasted 14 wk, and at the end of the entire experiment 1 hen was randomly selected from each repetition for sample collection after the 12 h fasting period. Blood samples (10 mL) were collected from the brachial wing vein before the hens were sacrificed and then centrifuged at 4,000 rpm for 10 min to separate the serum. The liver was dissected out and a representative sample was procured. A part of the ovarian tissues was harvested and fixed in 4% paraformaldehyde for histologic examination, and the remaining tissue was immediately frozen in liquid nitrogen together with the liver samples and stored at −80°C until further analysis.
Table 1.
Ingredients and nutrient compositions of the basal diet (as-fed basis).
| Ingredients | Amount (%) | Nutrient levels2 | Amount |
|---|---|---|---|
| Corn | 61.00 | ME (MJ/kg)3 | 11.14 |
| Soybean meal | 26.80 | CP (%) | 17.35 |
| Soybean oil | 0.70 | Ca (%) | 3.61 |
| Limestone | 9.00 | Total phosphorus (%) | 0.45 |
| CaHPO4 | 1.00 | Available phosphorus (%) | 0.37 |
| NaCl | 0.30 | Lysine (%) | 0.88 |
| Choline | 0.20 | Methionine (%) | 0.41 |
| Premix1 | 0.14 | Methionine + Cystine (%) | 0.72 |
| DL-Met | 0.17 | ||
| Zeolite powder | 0.69 | ||
| Total | 100.00 |
Premix provided per kilogram of diet: retinyl acetate for vitamin A, 8,000 IU; cholecalciferol for vitamin D3, 1,600 IU; DL-α-tocopheryl acetate for vitamin E, 10 IU; menadione sodium bisulfite for vitamin K3, 0.5 mg; thiamine for vitamin B1, 2.45 mg; riboflavin for vitamin B2, 4.0 mg; pyridoxine for vitamin B6, 3.0 mg; cobalamin for vitamin B12, 0.01 mg; D-pantothenic acid, 30 mg; nicotinic acid, 34.5 mg; folic acid, 0.4 mg; biotin, 0.2 mg; Zn (as zinc sulfate), 80 mg; Fe (as ferrous sulfate), 60 mg; Cu (as copper sulfate), 8 mg, Mn (as manganese sulfate), 60 mg; I (as potassium iodide), 0.5 mg; Se (as sodium selenite), 0.3 mg; phytase 300 mg.
Except the metabolizable energy and available phosphorus, the data of main nutrients in feed were all measured values.
ME = metabolizable energy.
Production Performance and Egg Quality
During the trial, we monitored the data of the total number of eggs, egg weight, broken eggs and abnormal eggs each day, and the feed consumption was calculated weekly. At the end of the trial, 3 eggs were collected from each replicate for egg quality determination. Eggshell strength was measured using an eggshell strength tester (ORKA Food Technology Ltd., Ramat HaSharon, Israel). The albumen height, Haugh unit, and Roche egg yolk color score were determined using a SONOVA egg-quality analyzer (ORKA Food Technology Ltd.). CIELAB values (L*, a*, b*) of yolk color were measured using a CR-400 (Konica Minolta Inc., Tokyo, Japan) colorimeter. Then, the egg yolk was separated and mixed to uniformity. The homogenized yolk was placed in culture dishes and freeze dried into yolk powder and stored at −80°C until the further determination of carotenoids and vitamin A.
Chemicals and Reagents
Methanol, n-hexane, dichloromethane, isopropanol, formic acid, acetic acid, and 2,6-di-tert-butyl-4-methylphenol (BHT, C15H24O) used in this study were all the chromatographic grade and obtained from Thermo Fisher Scientific (Pittsburgh, PA). Ultra-pure water (18.2 MΩ) was obtained from a Milli-Q water purification system (Millipore, Bradford, MA). There were 3 isotope internal standards used in the present study, β-carotene (β-carotene 0,10′,11,11′-13C4), retinol (vitamin A-[19,19,19,20,20,20-d6]), and retinyl palmitate (vitamin A palmitate-[10,19,19,19-d4]). Other analytical standards such as retinal, retinyl stearate, lutein, and zeaxanthin were purchased from Sigma-Aldrich (St Louis, MO).
Sample Preparation for Measuring Carotenoids and Vitamin A
The extraction buffer and pretreatment procedure for different samples were optimized in our previous research (Miao et al., 2023), the final sample preparation protocol was described briefly as follows and all of the operations were protected from light:
For the serum sample, 100 μL of serum was placed in a centrifuge tube, then 300 μL precooled methanol with 1% formic acid was added and mixed to precipitate protein. Next, the supernatant was collected and transferred into a new centrifuge tube and added 400 μL n-hexane which contained BHT at a concentration of 1% (w/v). The mixed solution was centrifuged at 14,000 rpm for 15 min at 4°C after being vortexed. Finally, 90 μL supernatant was transferred to the injection vial, with 10 μL of the 3 internal standard isotope solutions at a concentration of 2.5 μg/mL. The mix was vortexed and injected for SFC–DAD–MS/MS analysis. For liver and ovary tissues, the sample weight to be tested was 100 μg, and the extraction solution was 1.2 mL methanol and dichloromethane (1:1, v/v) with 1% (w/v) BHT. Then, the remaining steps were the same as for serum samples. For egg yolk samples, the procedure followed our previous research (Miao et al., 2023).
SFC–DAD–MS/MS Analysis
SFC coupled with a 6465 mass spectrometer (Agilent Technologies, Palo Alto, CA) was used to analyze carotenoids and vitamin A in serum, tissues and egg yolks. The Agilent Mass Hunter workstation was used to run the data acquisition and data analysis. Multiple reaction monitoring was conducted in the positive-ion electrospray ionization mode. The chromatography conditions, MS parameters, and MS/MS parameters can all be found in the previous paper (Miao et al., 2023).
Real-Time PCR Analysis
The mRNA expressions of critical genes related to the β-carotene metabolism pathway were detected using the real-time quantitative PCR. Total RNA was isolated from duodenal mucosa and liver samples using the RNAprep pure tissue kit (Tiangen Biotech Co., Ltd., Beijing, China) following the manufacturer's instructions. Agarose gel electrophoresis and microspectrophotometry (NanoDrop, Technologies, Wilmington, DE) were used to assess the RNA integrity and concentration. cDNA was transcribed via reverse transcription kits (PrimeScript RT reagent kit with gDNA Eraser, RR047 A, Takara, Beijing, China). The RT-PCR reactions were carried out using an Applied Biosystems QuantStudio Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA), with SYBR Green Master Mix (TB Green Premix ExTaq, RR420 A, Takara). Primer sequences used for analysis are presented in Table 2. The relative fold changes of mRNA expression levels were calculated according to the 2−ΔΔCt method with β-actin as an internal control.
Table 2.
Specific gene primers used for real-time PCR.
| Gene | Gene ID | Primers sequences (5′–3′) | Product size (bp) |
|---|---|---|---|
| SR-B1 | 416814 | F: TTGACCCCAGCAGCATC | 119 |
| R: CTTTGGCTTCGCTCCCT | |||
| CD36 | 417730 | F: TGTCTGCACCCTGTCAAA | 102 |
| R: GAACTCCTCCAAAGATGGC | |||
| BCO1 | 395346 | F: CTTCTCGCTCCCTGCTC | 80 |
| R: TGGCTGCTCTATGAACACA | |||
| BCO2 | 419792 | F: TCAGTTGCAGTGGCTTTG | 50 |
| R: TTGTTCCCTCCTTGTGCT | |||
| LRAT | 422403 | F: TAGCCTGCTCTGGAACAACT | 123 |
| R: ACGCTCCTCTGGTCACGAAT | |||
| RDH5 | 395452 | F: CAAGACAGCCGTGACCAA | 102 |
| R: AAGAACTCCTCGCCGTAGC | |||
| RDH10 | 420183 | F: CGCCACCATTGTCCTTT | 139 |
| R: CATCGCATCTTCTGGTTGT | |||
| DHRS3 | 419480 | F: CGGGTGCCATTGACTACT | 128 |
| R: CTCTGTGCTCGTGTGGAA | |||
| ISX | 418060 | F: TGCTTGCTCTTCAACATCC | 105 |
| R: GCTCCTCCATTTCCTCCT | |||
| RBP4 | 396166 | F: CTGGGATGTCTGTGCTGATATG | 138 |
| R: TCGTAATCTGTGTCCACTACCC | |||
| TTR | 396277 | F: TTACTGGAAGGGACTTGGC | 107 |
| R: GACTGAGGAGAGCAGCGA | |||
| β-actin | 396526 | F: CTCTATCCTGGCCTCCCT | 127 |
| R: GGGTGTGGGTGTTGGTAA |
Histological Analysis
The ovary tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated, embedded in conventional paraffin and cut into serial sections (5 μm). Then, the sections were stained with hematoxylin and eosin, dehydrated with an ethanol gradient, cleared with xylene, and mounted in neutral gum. We examined tissue morphology under a light microscope and used an image processing and analyzing system. The main characteristics of the atretic follicle are vacuolization, blurring, or disappearance of the egg cells in the follicle and granulosa cell degeneration. Primary follicles and secondary follicles are mainly judged according to the number and morphology of granulosa cells. The follicular atresia rate is the ratio of atresia follicles to all kinds of follicles.
Redox Indicators Measurement
Ovary tissues were homogenized in a 9-fold volume of precooled normal saline. The homogenates were centrifuged at 4,000 rpm for 10 min at 4°C, and the resultant supernatants were collected for protein assays and measurement of redox parameters. The protein carbonyl (PC) was determined using commercial kits from Beijing Solarbio Science and Technology Co., Ltd. The contents of hydrogen peroxide (H2O2), malondialdehyde (MDA), glutathione (GSH), and the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in the ovary were measured using the kits according to the manufacturer's instructions from the Nanjing Jiancheng Bioengineering Institute of China. In addition, all indicators were normalized to protein concentrations, which were measured with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, San Jose, CA).
Statistical Analyses
Statistical analyses were carried out using the SAS version 9.4 software (SAS Institute, Cary, NC). Data were tested for normality with the Shapiro–Wilk test, and Levene's test was applied to test the homogeneity of variances. One-way analysis of variance followed by Duncan's multiple comparison tests was further used for statistical evaluation of the results. The results are expressed as the mean ± standard error of measurements on samples from 8 replicates in each group setting, with P < 0.05 being set as the criterion for statistical significance.
RESULTS
Production Performance and Egg Quality
As shown in Tables 3 and 4, we found that dietary β-carotene with different levels in the diet for 14 wk had no significant effect on laying hens' production performance and egg quality (P > 0.05), except for the egg yolk color. In Table 5, the Roche egg yolk color score was improved with the laying hens uptake of β-carotene (P < 0.05), but the effect was more obvious in the high-dose addition group. Additionally, the CIELAB L* color values were significantly decreased (P < 0.05) and CIELAB a* color values were extremely significantly increased (P < 0.01) with the addition of β-carotene, whereas the b* value showed no significant change in each group.
Table 3.
Effect of dietary β-carotene supplementation on laying performance of hens.
| Amount of β-carotene (mg/kg diet) | Egg production (%) | Egg weight (g) | Feed intake (g/hen/d) | Feed/egg (g/g) |
|---|---|---|---|---|
| 0 (basal diet) | 92.13 ± 1.50 | 64.66 ± 1.57 | 124.93 ± 3.60 | 2.17 ± 0.06 |
| 15 | 92.34 ± 3.14 | 64.44 ± 0.58 | 124.14 ± 3.04 | 2.15 ± 0.10 |
| 30 | 92.35 ± 2.88 | 64.68 ± 0.54 | 125.32 ± 1.59 | 2.17 ± 0.08 |
| 60 | 92.70 ± 1.45 | 64.39 ± 1.06 | 124.90 ± 2.08 | 2.15 ± 0.03 |
| 120 | 92.88 ± 2.01 | 64.95 ± 0.73 | 125.39 ± 4.76 | 2.14 ± 0.08 |
| P value | 0.9725 | 0.8881 | 0.9342 | 0.8025 |
Table 4.
Effect of dietary β-carotene supplementation on egg quality.
| Amount of β-carotene (mg/kg diet) | Albumen height (mm) | Haugh unit (HU) | Eggshell strength (kg) | Eggshell thickness (mm) |
|---|---|---|---|---|
| 0 (basal diet) | 6.74 ± 0.59 | 82.40 ± 3.48 | 4.18 ± 0.15 | 34.67 ± 0.60 |
| 15 | 6.75 ± 0.40 | 82.71 ± 2.01 | 4.23 ± 0.13 | 34.17 ± 0.31 |
| 30 | 6.83 ± 0.67 | 82.81 ± 2.62 | 4.21 ± 0.30 | 34.46 ± 0.57 |
| 60 | 6.81 ± 0.62 | 82.62 ± 2.74 | 4.25 ± 0.12 | 34.47 ± 0.21 |
| 120 | 6.80 ± 0.59 | 82.84 ± 2.93 | 4.31 ± 0.32 | 34.46 ± 0.34 |
| P value | 0.9885 | 0.9988 | 0.7765 | 0.2254 |
Table 5.
Effect of dietary β-carotene supplementation of egg yolk color.
| Amount of β-carotene mg/kg diet |
Roche egg yolk color score | L* Lightness score |
a* Redness score |
b* Yellowness score |
|---|---|---|---|---|
| 0 (basal diet) | 5.18 ± 0.41b | 66.28 ± 1.45a | −4.27 ± 0.17c | 52.00 ± 2.44 |
| 15 | 5.46 ± 0.69b | 64.76 ± 1.99ab | −3.63 ± 1.03bc | 50.54 ± 1.50 |
| 30 | 5.31 ± 0.56b | 64.81 ± 1.30ab | −3.63 ± 0.53bc | 50.74 ± 2.56 |
| 60 | 5.63 ± 0.72ab | 64.04 ± 0.75b | −3.44 ± 0.32b | 50.15 ± 1.06 |
| 120 | 6.17 ± 0.55a | 63.70 ± 1.03b | −2.77 ± 0.36a | 50.55 ± 2.14 |
| P value | 0.0392 | 0.0157 | 0.0003 | 0.5010 |
Data points with different letters are significantly different (1-way analysis of variance) at the level of P < 0.05 by Duncan's multiple comparison test.
Method Validation
For the detection method, our laboratory developed an SFC–DAD–MS/MS method to determine carotenoids and vitamin A in egg yolk samples in a previous study (Miao et al., 2023). On this basis, we further extended the method aim at serum and tissue samples in this study. The Figure S1 and S2 are the chromatographic spectra of analytes in serum and tissue samples, which showed that the compounds under test were separated efficiently by the SFC. Because the composition of tissue was complex, the matrix effect was very large, so we focused on 3 compounds with isotope internal standards (β-carotene, retinol, and retinyl palmitate). Furthermore, the developed method was validated by determining the limit of detection (LOD, S/N = 3), limit of quantitation (LOQ, S/N = 10), linearity, recovery, and repeatability. The method validation data for the serum and tissue samples are shown in Supplemental Tables S1 and S2, respectively. All the results demonstrated that this method was appropriate to detect carotenoids and vitamin A in different samples.
The Contents of Carotenoids and Different Forms of Vitamin A in Serum
The changes of different analytes in laying hens’ serum caused by long-term supplementation of β-carotene are shown in Table 6. The β-carotene in serum of laying hens from the control group was below the limit of quantification (0.5 ng/mL) of the developed detection method. The contents of the other 4 groups were all gradually increased with the supplemental dose (P < 0.01), the highest level reached 403.35 ng/mL in the BC120 group. The levels of different forms of vitamin A, including retinal, retinol, retinyl palmitate, and retinyl stearate, were elevated compared with the control group (P < 0.05). It should be noted that the data reached a peak when the supplemental level of β-carotene was 60 mg/kg (except retinal), while the supplemental level of 120 mg/kg led to a decrease in vitamin A content. In addition, we also found zeaxanthin in serum decreased significantly after β-carotene was supplemented in the diet for 14 wk (P < 0.05) even in the BC15 group, there was a similar trend of lutein.
Table 6.
Effect of dietary β-carotene with different supplemental levels on the content of carotenoids and retinoids in serum sample.
| Compounds (ng/mL serum) | Control | BC15 | BC30 | BC60 | BC120 |
|---|---|---|---|---|---|
| β-Carotene | -1 | 21.47 ± 3.01c | 64.31 ± 4.41c | 155.21 ± 16.95b | 403.55 ± 50.86a |
| Retinal | 162.44 ± 16.04b | 180.50 ± 27.17b | 223.42 ± 23.17b | 247.97 ± 19.78a | 368.34 ± 17.50a |
| Retinol | 763.01 ± 54.30c | 888.93 ± 33.96c | 911.73 ± 29.19bc | 1140.01 ± 81.44a | 1159.80 ± 59.06b |
| Retinyl palmitate | 364.89. ± 41.32c | 418.04 ± 48.56c | 724.82 ± 62.75ab | 828.58 ± 66.49a | 647.18 ± 34.44ab |
| Retinyl stearate | 61.28 ± 9.35c | 68.51 ± 4.18c | 70.15 ± 9.70c | 149.16 ± 13.74a | 118.79 ± 10.22b |
| Lutein | 99.96 ± 9.47a | 80.28 ± 11.67ab | 73.50 ± 11.61ab | 78.80 ± 12.63ab | 48.28 ± 7.94a |
| Zeaxanthin | 690.18 ± 76.38a | 464.56 ± 26.37b | 465.12 ± 51.16b | 478.71 ± 58.10b | 505.81 ± 33.29b |
Means the content of β-carotene was below the limit of quantification of the detection method.
Data points with different letters are significantly different (1-way analysis of variance) at the level of P < 0.05 by Duncan's multiple comparison test.
The Contents of β-Carotene, Retinol, and Retinyl Palmitate in the Liver and Ovary
As shown in Table 7, the amounts of β-carotene in the BC15 and BC30 groups showed no significant difference, whether in the liver or the ovary, but the rate of increase was very clear when the supplement level was above 60 mg/kg (P < 0.05). For retinol, its amounts in the liver and ovary were also increased significantly under β-carotene supplementation due to the bioconversion (P < 0.05). Moreover, the amounts of retinyl palmitate in the liver were increased gradually among groups (P < 0.05), while its contents in laying hens’ ovaries were the highest in the BC60 group. Interestingly, under the same level of β-carotene addition, the deposition of β-carotene and retinol in the liver was 20 to 40 times as much as that in the ovary. The difference in retinyl palmitate between the 2 tissues was greater, and the maximum gap could reach more than 3,000 times.
Table 7.
Effect of dietary β-carotene with different supplemental levels on the content of carotenoids and retinoids in tissue sample.
| Compounds | Control | BC15 | BC30 | BC60 | BC120 |
|---|---|---|---|---|---|
| Liver (μg/g) | |||||
| β-Carotene | -1 | 1.67 ± 3.01c | 2.30 ± 0.25c | 7.97 ± 0.61b | 16.90 ± 1.64a |
| Retinol | 4.55 ± 0.41b | 5.64 ± 0.37ab | 5.72 ± 0.30a | 5.84 ± 0.48a | 4.76 ± 0.21ab |
| Retinyl palmitate | 671.86 ± 53.45d | 1009.90 ± 141.68cd | 1533.77 ± 230.92c | 2964.74 ± 189.29b | 3606.41 ± 276.08a |
| Ovary (ng/g) | |||||
| β-Carotene | -1 | 27.04 ± 1.34c | 62.00 ± 4.36c | 70.76 ± 7.34b | 179.61 ± 13.05a |
| Retinol | 193.64 ± 10.93b | 269.70 ± 14.14a | 263.27 ± 35.28a | 269.13 ± 13.24a | 265.24 ± 15.86a |
| Retinyl palmitate | 502.64 ± 85.59d | 729.68 ± 38.63cd | 853.76 ± 108.99bc | 1349.50 ± 125.22a | 1114.43 ± 85.38ab |
Means the content of β-carotene was below the limit of quantification of the detection method.
Data points with different letters are significantly different (1-way analysis of variance) at the level of P < 0.05 by Duncan's multiple comparison test.
The Determined Compounds in Egg Yolks
The data of each compound in egg yolks can be seen in Table 8. The increase of β-carotene in egg yolks was not unexpected with the elevated supplemental levels (P < 0.05), where the amounts in the 4 treatment groups were 1.42, 1.98, 4.72, and 10.30 μg/g egg yolk. The variation tendency of retinol, retinyl palmitate and retinyl stearate in egg yolks of different groups was almost consistent. The supplementation of β-carotene in feed could give rise to an increase of these 3 forms of retinoids in yolks compared with the control group (P < 0.05), but there was little difference between the group of BC60 and BC120 (P > 0.05). Meanwhile, the enrichment concentration of lutein and zeaxanthin in egg yolk was markedly higher than β-carotene, but their amounts were also decreased in egg yolks with the deposition of β-carotene (P < 0.05).
Table 8.
Effect of dietary β-carotene with different supplemental levels on the content of carotenoids and retinoids in egg yolk samples.
| Compounds (μg/g egg yolk) | Control | BC15 | BC30 | BC60 | BC120 |
|---|---|---|---|---|---|
| β-Carotene | -1 | 1.42 ± 0.12c | 1.98 ± 0.15c | 4.72 ± 0.26b | 10.30 ± 0.74a |
| Retinol | 4.71 ± 0.15c | 5.29 ± 0.20b | 5.45 ± 015b | 6.17 ± 0.19a | 6.35 ± 0.19a |
| Retinyl palmitate | 0.79 ± 0.04c | 1.18 ± 0.04b | 1.32 ± 0.08b | 1.80 ± 0.10a | 1.94 ± 0.09a |
| Retinyl stearate | 0.59 ± 0.04c | 0.79 ± 0.03b | 0.95 ± 0.12ab | 1.06 ± 0.07a | 1.10 ± 0.05a |
| Lutein | 23.85 ± 0.72a | 21.85 ± 0.92ab | 23.69 ± 1.25ab | 20.90 ± 1.07b | 17.47 ± 0.50c |
| Zeaxanthin | 43.18 ± 1.57a | 39.98 ± 2.11a | 42.21 ± 2.30a | 37.96 ± 2.58ab | 33.00 ± 1.21b |
Means the content of β-carotene was below the limit of quantification of the detection method.
Data points with different letters are significantly different (1-way analysis of variance) at the level of P < 0.05 by Duncan's multiple comparison test.
The Change of the Relative mRNA Expressions Related to β-Carotene and Vitamin A Metabolism in Duodenal Mucosa and Liver
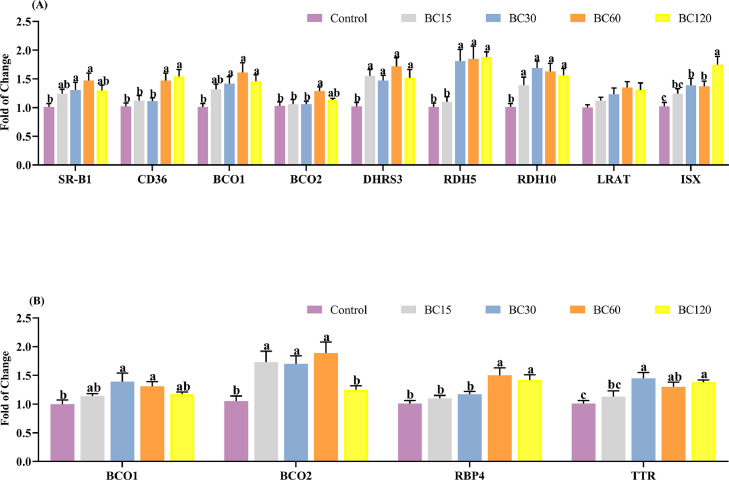
Figure 1 shows the results of the real-time PCR analysis. The relative mRNA expressions of genes involved in β-carotene absorption and bioconversion in duodenal mucosa were significantly upregulated after long-term feeding of it (except the LRAT, with a P value = 0.07), although most of them did not increase linearly with increasing supplemental dose. In particular, the highest elevated levels of SR-B1, BCO1, and BCO2 were all in the BC60 group, which were more than 47, 61, and 30% compared to the control group, respectively. Furthermore, there was an increased expression of ISX (P < 0.05), this important transcription factor was upregulated nearly 40% in the BC30 and BC60 groups, and more than 75% in the BC120 group, which meant activation of the negative feedback for β-carotene bioconversion. In addition, the relative mRNA expressions of BCO1 and BCO2 in the liver were also suppressed in BC120 group. The retinol-binding protein 4 (RBP4) and transthyretin (TTR) which were mainly expressed in the liver, and are responsible for the transportation of retinol from liver to peripheral tissues, were also significantly increased after β-carotene supplementation for a long time (P < 0.05).
Figure 1.
Effect of dietary β-carotene with different supplemental levels (basal diet supplementation with 0, 15, 30, 60, and 120 mg/kg β-carotene, n = 8) on the relative mRNA expressions for β-carotene and vitamin A metabolic pathways in laying hens' (A) duodenum mucosa and (B) liver. Values are means ± SEM, different lowercase letters represent significant differences for different groups (P < 0.05).
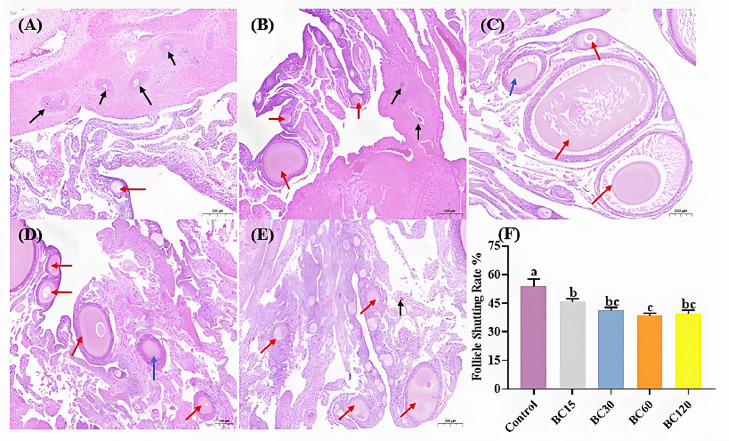
Effects of Dietary β-Carotene on Follicular Atresia Rate
The representative images of histological staining of laying hens’ ovaries in Figure 2 show that supplementation with β-carotene decreased (P < 0.05) the atresia rate of the follicle, and BC60 group had the lowest atresia rate. Specifically, as shown in Figure 2A, there were more atresia follicles in the ovary of laying hens without β-carotene supplementation, which are characterized by the reduction of the granulosa cell layer, the appearance of vacuoles and the pyknosis of oocyte nuclei. With β-carotene supplementation, more complete primary and secondary follicles could be seen in the section, while the number of atresia follicles in laying hens’ ovaries was also decreased.
Figure 2.
Effect of dietary β-carotene with different supplemental levels (basal diet supplementation with 0, 15, 30, 60, and 120 mg/kg β-carotene, n = 8) on the follicular atresia of laying hens ovaries. (A–E) Representative images of hematoxylin and eosin stained sections of the laying hens’ ovary with different group. Black arrows: atretic follicles. Red arrows: primary follicles. Blue arrows: secondary follicles. Scale bar = 200 μm. (F) Analysis of follicular atresia rate in different groups. Values are means ± SEM, different lowercase letters represent significant differences for different groups (P < 0.05).
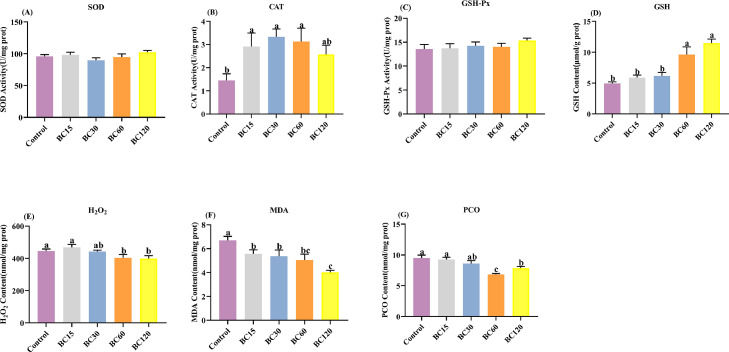
Redox Indicators in the Ovary
The activity of SOD, CAT, and GSH-Px in the ovaries of laying hens is shown in Figure 3. The results indicate that the activity of CAT was significantly increased in β-carotene treatment groups (P < 0.05), but SOD and GSH-Px activity was not affected by β-carotene (P > 0.05). The contents of GSH in the ovaries of laying hens gradually increased with the β-carotene supplemental dose (P < 0.05). As for the oxidative stress biomarker, H2O2, MDA, and PCO in ovary were all decreased on account of β-carotene acting as an antioxidant (P < 0.05), especially when the supplement level was above 60 mg/kg.
Figure 3.
Effect of dietary β-carotene with different supplemental levels (basal diet supplementation with 0, 15, 30, 60, and 120 mg/kg β-carotene, n = 8) on the redox status of laying hens ovaries. (A) superoxide dismutase activity, (B) catalase activity, (C) glutathione peroxidase activity, (D) glutathione amount, (E) hydrogen peroxide amount, (F) malondialdehyde amount, (G) protein carbonyl. Values are means ± SEM, different lowercase letters represent significant differences for different groups (P < 0.05).
DISCUSSION
Carotenoids are a group of widespread lipophilic pigments, which can be classified into 2 types according to their chemical composition, one is the carotenes formed exclusively by carbon and hydrogen atoms, β-carotene is a typical representative, whereas the other is xanthophylls containing oxygen atoms in addition to carbon and hydrogen (Ribeiro et al., 2018). Traditionally, xanthophylls such as lutein, canthaxanthin, and astaxanthin are applied more extensively in the laying hens’ industry than carotenes to improve yolk color and egg nutrient fortification. This study found that dietary supplementation with β-carotene for laying hens could also accumulate in egg yolks as well as serum and tissues without compromising the performance of laying hens or egg quality. Also, the changing trend of production performance and egg quality indicators in each group after dietary β-carotene in laying hens’ diets for 14 wk was basically consistent with the results of feeding for 7 wk (Miao et al., 2023). However, compared to the previous study on astaxanthin by our group, the coloring capacity of β-carotene was obviously weaker than that of astaxanthin under the same deposition contents in yolks (Dansou et al., 2021), in which the egg yolk enrichment in astaxanthin shows obvious red color.
With laying hens ingesting more β-carotene, the mRNA expression of SR-B1 and CD36 in duodenal mucosa increased significantly compared to the control group to promote β-carotene absorption. The deposition law of β-carotene was nearly identical in different samples. With long-term and graded doses of β-carotene supplementation in laying hens’ diets the amount of it in serum, liver, ovary, and egg yolk all increased gradually. The deposition law of β-carotene is basically consistent with that of other nonprovitamin A activity carotenoids, such as lutein and zeaxanthin in egg yolks (Skřivan et al., 2016), or astaxanthin in the liver of laying hens (Magnuson et al., 2018). In addition, the results of our study showed that the deposition of β-carotene did not reach a plateau when the supplementation level was 120 mg/kg in feed, which meant that β-carotene had the potential to accumulate further in laying hens’ bodies or eggs. Furthermore, we also found that there was to a certain extent a competitive relationship between the deposition of β-carotene and lutein or zeaxanthin in laying hens’ bodies. This phenomenon has also been reported in previous studies of humans and mice (Molldrem et al., 2004; Mamatha and Baskaran, 2011), which could be related to competition between carotenoids for micellar incorporation in the gastrointestinal tract occurring at high-dose uptake (Shilpa et al., 2020), however, the detailed mechanism need further research.
In addition to intact deposition, a part of β-carotene in feed could also be converted into vitamin A and distributed in laying hens tissues and egg yolk. Upregulation of BCO1 mRNA expressions accelerated the cleavage of β-carotene, increasing the contents of retinol in serum. Subsequently, retinal can undergo reversible reduction to retinol, dehydrogenase/reductase 3 (DHRS3) and retinol dehydrogenase (RDH5 and RDH10 are examples of this family) participated in this process (Shannon et al., 2017). Then, the retinol would be esterified and retinyl esters generated under the action of LRAT. The mRNA levels of these genes and the retinoids determined in different samples all had an increased tendency with β-carotene supplementation in the diet of laying hens. However, there was still an upper limit of β-carotene bioconversion when the dietary level was too high. In fact, multiple factors could modulate provitamin A carotenoids’ absorption and bioconversion, the vitamin A intake and status was the crucial one (Lietz et al., 2010; Green et al., 2021). It is generally believed that the provitamin A bioconversion potential of β-carotene was higher in the case of vitamin A deficiency, but lower when vitamin A was in excess (Condron et al., 2014; Harari et al., 2020; Green et al., 2021). Nowadays, synthetic retinyl esters are the main source of vitamin A in poultry nutrition, and the added level in the diet is usually at a high level of 8,000 to 10,000 IU/kg. This is done to maintain the vitamin A status in the body for improving animal immunity and alleviating stress (Abd El-Hack et al., 2019; Shojadoost et al., 2021). Hence, we set the content of vitamin A in the diet at 8,000 IU/kg, which could maintain an adequate vitamin A status for laying hens at the age of 40 wk. The β-carotene had a limited bioconversion efficiency in our study when the supplemental level was 120 mg/kg in the diet, and ISX played an important role in regulating SR-B1 and BCO1 for controlling the production of vitamin A.
As we all know, liver is the main storage site of carotenoids and vitamin A, and hepatic stellate cells can store a large amount of vitamin A as retinyl palmitate in lipid droplets in the cytoplasm (Senoo et al., 2010; Bohn et al., 2019). We also found that retinyl palmitate in laying hens’ livers was several orders of magnitude higher than that in other samples. In addition, the liver were able to synthesize and secrete RBP4, which could mobilize the retinol from the vitamin A store, and then bind to retinol and TTR to deliver retinol to peripheral tissue (Blaner, 2019). The results of increasing vitamin A in the ovaries and yolks in this research may be derived from this metabolic pathway.
Although only a small part of β-carotene could be converted in the body of laying hens under adequate vitamin A status in the diet, the deposition of β-carotene in target organs still played an important biological role, especially in the ovary. For laying hens, the state of the ovarian follicle directly correlates with production performance, and many ovarian diseases are mainly caused by disorders of atrophy and atresia of ovarian follicles in most cases (He et al., 2022). In our research, dietary β-carotene alleviated the follicular atresia rate compared with the control group, which was beneficial to laying hens ovarian health. Furthermore, oxidative stress is one of the common inducing factors of follicular atresia in chickens (Wang et al., 2021), whereas various kinds of carotenoids including β-carotene are thought to possess direct antioxidant activities and scavenge free radicals in animals’ bodies (Tvrdá et al., 2016; Rotondo Dottore et al., 2018; Gao et al., 2021; He et al., 2023a). As such, we focus on the redox status of ovary, and the results of improving antioxidant capacity and decreasing oxidative stress biomarker were not surprising because the supplementation of β-carotene in laying hens’ diet has been used for a long time. This is also very similar to our group's previous study involved in the effect of astaxanthin on follicular atresia in laying hens (He et al., 2023b). However, whether this antioxidant mechanism was mediated by β-carotene alone or combined with an increase in vitamin A needs further study.
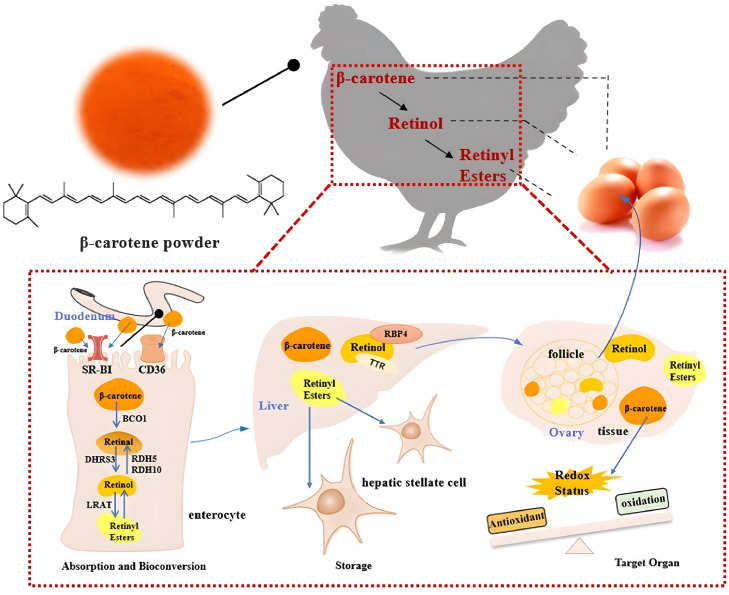
In conclusion, under adequate vitamin A status in the diet, the deposition of β-carotene in laying hens’ bodies was increased gradually with the dietary β-carotene levels (within 120 mg/kg) after supplementing it for 14 wk, and a part of the β-carotene converted into vitamin A at the same time. Hence, the contents of different forms of vitamin A (retinal, retinol, retinyl palmitate, and retinyl stearate) were all increased, but there was still a certain upper limit of the bioconversion. Meanwhile, the absorption and bioconversion of β-carotene mainly took place in the duodenum, whereas the liver is the main storage site, in which retinyl palmitate is the main storage form. The β-carotene deposited in the ovary after being transported could show an antioxidant function, which had a positive impact on alleviating follicular atresia in laying hens. Therefore, based on all the above results, a brief metabolic pathway of β-carotene in laying hens body was shown in Figure 4, and this research would provide a reference for further β-carotene application to the poultry industry.
Figure 4.
Graphical summary of the β-carotene metabolism in laying hens’ bodies.
ACKNOWLEDGMENTS
This work was supported by the China Agriculture Research Systems (CARS-40-K11); Beijing Agriculture Innovation Consortium (BAIC06-2023-G05); and the Agricultural Science and Technology Innovation Program (ASTIP-IAS-12).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103046.
Appendix. Supplementary materials
REFERENCES
- Abd El-Hack M.E., Alagawany M., Mahrose K.M., Arif M., Saeed M., Arain M.A., Soomro R.N., Siyal F.A., Fazlani S.A., Fowler J. Productive performance, egg quality, hematological parameters and serum chemistry of laying hens fed diets supplemented with certain fat-soluble vitamins, individually or combined, during summer season. Animal Nutr. 2019;5:49–55. doi: 10.1016/j.aninu.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J.L., Martín M.T., Toribio L. Supercritical fluid chromatography in food analysis. J. Chromatogr. A. 2013;1313:24–36. doi: 10.1016/j.chroma.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Blaner W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019;197:153–178. doi: 10.1016/j.pharmthera.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn T., Desmarchelier C., El S.N., Keijer J., van Schothorst E., Rühl R., Borel P. β-Carotene in the human body: metabolic bioactivation pathways – from digestion to tissue distribution and excretion. Proc. Nutr. Soc. 2019;78:68–87. doi: 10.1017/S0029665118002641. [DOI] [PubMed] [Google Scholar]
- Chen Q., Han X., Zhu H., Liu Y., Xu X. A comparison of two supplementary doses of vitamin A on performance, intestine and immune organ development, as well as gene expression of inflammatory factors in young Hy-line brown laying pullets. Animals. 2022;12:1271. doi: 10.3390/ani12101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condron K., Lemenager R., Claeys M., Lipkie T., Schoonmaker J. Supplemental β-carotene I: effect on plasma vitamin A, growth, performance, and carcass characteristics of feedlot cattle. Meat Sci. 2014;98:736–743. doi: 10.1016/j.meatsci.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Dansou D.M., Wang H., Nugroho R.D., He W., Zhao Q., Tang C., Zhang H., Zhang J. Effects of duration and supplementation dose with astaxanthin on egg fortification. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debelo H., Novotny J.A., Ferruzzi M.G. Vitamin A. Adv. Nutr. 2017;8:992–994. doi: 10.3945/an.116.014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Gómez J., Moreno J.A., Angulo E., Sandmann G., Zhu C., Capell T., Nogareda C. Provitamin A carotenoids from an engineered high-carotenoid maize are bioavailable and zeaxanthin does not compromise β-carotene absorption in poultry. Transgenic Res. 2017;26:591–601. doi: 10.1007/s11248-017-0029-y. [DOI] [PubMed] [Google Scholar]
- Díaz-Gómez J., Moreno J., Angulo E., Sandmann G., Zhu C., Ramos A., Capell T., Christou P., Nogareda C. High-carotenoid biofortified maize is an alternative to color additives in poultry feed. Anim. Feed Sci. Technol. 2017;231:38–46. [Google Scholar]
- Fawzy S., Wang W., Zhou Y., Xue Y., Yi G., Wu M., Huang X. Can dietary β-carotene supplementation provide an alternative to astaxanthin on the performance of growth, pigmentation, biochemical, and immuno-physiological parameters of Litopenaeus vannamei? Aquacult. Rep. 2022;23 [Google Scholar]
- Gao S., Heng N., Liu F., Guo Y., Chen Y., Wang L., Ni H., Sheng X., Wang X., Xing K. Natural astaxanthin enhanced antioxidant capacity and improved semen quality through the MAPK/Nrf2 pathway in aging layer breeder roosters. J. Anim. Sci. Biotechnol. 2021;12:1–15. doi: 10.1186/s40104-021-00633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.H., Ford J.L., Green J.B. A compartmental model describing the kinetics of β-carotene and β-carotene-derived retinol in healthy older adults. J. Nutr. 2021;151:434–444. doi: 10.1093/jn/nxaa306. [DOI] [PubMed] [Google Scholar]
- Harari A., Melnikov N., Kandel Kfir M., Kamari Y., Mahler L., Ben-Amotz A., Harats D., Cohen H., Shaish A. Dietary β-carotene rescues vitamin A deficiency and inhibits atherogenesis in apolipoprotein E-deficient mice. Nutrients. 2020;12:1625. doi: 10.3390/nu12061625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Geng H., Qin Y., Yang P., Wang W., Mai K., Song F. Dietary xanthophyll improved growth, antioxidant, pigmentation and meat quality in the southern catfish (Silurus soldatovi meridionalis Chen) Animal Nutr. 2023;13:101–115. doi: 10.1016/j.aninu.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Li D., Tian Y., Wei Q., Amevor F.K., Sun C., Yu C., Yang C., Du H., Jiang X. miRNA sequencing analysis of healthy and atretic follicles of chickens revealed that miR-30a-5p inhibits granulosa cell death via targeting Beclin1. J. Anim. Sci. Biotechnol. 2022;13:1–22. doi: 10.1186/s40104-022-00697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Wang H., Tang C., Zhao Q., Zhang J. Dietary supplementation with astaxanthin alleviates ovarian aging in aged laying hens by enhancing antioxidant capacity and increasing reproductive hormones. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heying E.K., Tanumihardjo J.P., Vasic V., Cook M., Palacios-Rojas N., Tanumihardjo S.A. Biofortified orange maize enhances β-cryptoxanthin concentrations in egg yolks of laying hens better than tangerine peel fortificant. J. Agric. Food Chem. 2014;62:11892–11900. doi: 10.1021/jf5037195. [DOI] [PubMed] [Google Scholar]
- Hui J., Li L., Li R., Wu M., Yang Y., Wang J., Fan Y., Zheng X. Effects of supplementation with β-carotene on the growth performance and intestinal mucosal barriers in layer-type cockerels. Anim. Sci. J. 2020;91:e13344. doi: 10.1111/asj.13344. [DOI] [PubMed] [Google Scholar]
- Jiang Y., McGeachin R., Bailey C. α-Tocopherol, β-carotene, and retinol enrichment of chicken eggs. Poult. Sci. 1994;73:1137–1143. doi: 10.3382/ps.0731137. [DOI] [PubMed] [Google Scholar]
- Lietz G., Lange J., Rimbach G. Molecular and dietary regulation of β, β-carotene 15, 15′-monooxygenase 1 (BCMO1) Arch. Biochem. Biophys. 2010;502:8–16. doi: 10.1016/j.abb.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Lima H., Souza L. Vitamin A in the diet of laying hens: enrichment of table eggs to prevent nutritional deficiencies in humans. World's Poult. Sci. J. 2018;74:619–626. [Google Scholar]
- Lobo G.P., Hessel S., Eichinger A., Noy N., Moise A.R., Wyss A., Palczewski K., Von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β, β-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson A., Sun T., Yin R., Liu G., Tolba S., Shinde S., Lei X. Supplemental microalgal astaxanthin produced coordinated changes in intrinsic antioxidant systems of layer hens exposed to heat stress. Algal Res. 2018;33:84–90. [Google Scholar]
- Mamatha B.S., Baskaran V. Effect of micellar lipids, dietary fiber and β-carotene on lutein bioavailability in aged rats with lutein deficiency. Nutrition. 2011;27:960–966. doi: 10.1016/j.nut.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Meléndez-Martínez A.J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201801045. [DOI] [PubMed] [Google Scholar]
- Miao Q., Yang Y., Du L., Tang C., Zhao Q., Li F., Yao X., Meng Y., Qin Y., Zhang J. Development and application of a SFC–DAD–MS/MS method to determine carotenoids and vitamin A in egg yolks from laying hens supplemented with β-carotene. Food Chem. 2023;414 doi: 10.1016/j.foodchem.2022.135376. [DOI] [PubMed] [Google Scholar]
- Molldrem K.L., Li J., Simon P.W., Tanumihardjo S.A. Lutein and β-carotene from lutein-containing yellow carrots are bioavailable in humans. Am. J. Clin. Nutr. 2004;80:131–136. doi: 10.1093/ajcn/80.1.131. [DOI] [PubMed] [Google Scholar]
- Nie M., Zhang Z., Liu C., Li D., Huang W., Liu C., Jiang N. Hesperetin and hesperidin improved β-carotene incorporation efficiency, intestinal cell uptake, and retinoid concentrations in tissues. J. Agric. Food Chem. 2019;67:3363–3371. doi: 10.1021/acs.jafc.9b00551. [DOI] [PubMed] [Google Scholar]
- Pilařová V., Plachká K., Khalikova M.A., Svec F., Nováková L. Recent developments in supercritical fluid chromatography–mass spectrometry: is it a viable option for analysis of complex samples? TrAC Trends Anal. Chem. 2019;112:212–225. [Google Scholar]
- Ramkumar S., Moon J., Golczak M., von Lintig J. LRAT coordinates the negative-feedback regulation of intestinal retinoid biosynthesis from β-carotene. J. Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro D., Freitas M., Silva A.M., Carvalho F., Fernandes E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018;120:681–699. doi: 10.1016/j.fct.2018.07.060. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D., Limon M.C., Meléndez-Martínez A.J., Olmedilla-Alonso B., Palou A. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Rotondo Dottore G., Ionni I., Menconi F., Casini G., Sellari-Franceschini S., Nardi M., Vitti P., Marcocci C., Marinò M. Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves’ orbitopathy (GO) J. Endocrinol. Invest. 2018;41:815–820. doi: 10.1007/s40618-017-0809-5. [DOI] [PubMed] [Google Scholar]
- Senoo H., Yoshikawa K., Morii M., Miura M., Imai K., Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative – past, present and future. Cell Biol. Int. 2010;34:1247–1272. doi: 10.1042/CBI20100321. [DOI] [PubMed] [Google Scholar]
- Shannon S.R., Moise A.R., Trainor P.A. New insights and changing paradigms in the regulation of vitamin A metabolism in development. Wiley Interdiscip. Rev.: Dev. Biol. 2017;6:e264. doi: 10.1002/wdev.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shete V., Quadro L. Mammalian metabolism of β-carotene: gaps in knowledge. Nutrients. 2013;5:4849–4868. doi: 10.3390/nu5124849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilpa S., Shwetha H.J., Raju M., Lakshminarayana R. Pages 41–73 in Carotenoids: Properties, Processing and Applications, Galanakis C.M. Elsevier; Amsterdam, Netherlands: 2020. Factors affecting bioaccessibility and bio-efficacy of carotenoids. [Google Scholar]
- Shojadoost B., Yitbarek A., Alizadeh M., Kulkarni R.R., Astill J., Boodhoo N., Sharif S. Centennial review: effects of vitamins A, D, E, and C on the chicken immune system. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skřivan M., Marounek M., Englmaierova M., Skřivanová E. Effect of increasing doses of marigold (Tagetes erecta) flower extract on eggs carotenoids content, colour and oxidative stability. J. Anim. Feed Sci. 2016;25:58–64. [Google Scholar]
- Tvrdá E., Kováčik A., Tušimová E., Paál D., Mackovich A., Alimov J., Lukáč N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016;7:1–13. doi: 10.1186/s40104-016-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lintig J., Moon J., Lee J., Ramkumar S. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2020;1865 doi: 10.1016/j.bbalip.2019.158580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jia R., Gong H., Celi P., Zhuo Y., Ding X., Bai S., Zeng Q., Yin H., Xu S. The effect of oxidative stress on the chicken ovary: involvement of microbiota and melatonin interventions. Antioxidants. 2021;10:1422. doi: 10.3390/antiox10091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., He Z., Hu R., Yan J., Zhang Q., Li B., Yuan X., Zhang H., He J., Wu S. Dietary β-carotene on postpartum uterine recovery in mice: crosstalk between gut microbiota and inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.744425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Yan J., Hu R., Li Y., Wang Y., Chen H., Hou D.-X., He J., Wu S. Modulation of gut microbiota and oxidative status by β-carotene in late pregnant sows. Front. Nutr. 2020;7 doi: 10.3389/fnut.2020.612875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.