Abstract
Background
Plasma refill rates can be estimated by combining measurements of urine output with relative blood volume profiles. Change in plasma refill rates could guide decongestive loop diuretic therapy in acute heart failure. The objective of the study was to assess average relative blood volume profiles generated from 2 or 3 follow-up measurements obtained hours after loop diuretic administration in subjects with vs without baseline congestion.
Methods
A systematic review was conducted of articles written in English, French, Spanish, and German, using MEDLINE (1964 to 2019), Cochrane Reviews (1996 to 2019), and Embase (1974 to 2019). Search terms included the following: diuretics, hemoconcentration, plasma volume, and blood volume. We included studies of adults given a loop diuretic with at least one baseline and one follow-up measurement. A single author extracted subject- or group-level blood volume measurements, aggregated them when needed, and converted them to relative changes.
Results
Across all 16 studies that met the prespecified inclusion criteria, relative blood volume maximally decreased 9.2% (6.6% to 12.0%) and returned to baseline after 3 or more hours. Compared to subjects without congestion, those with congestion experienced smaller decreases in relative blood volume across all follow-up periods (P = 0.001) and returned to baseline within the final follow-up period.
Conclusions
Single doses of loop diuretics produce measurable changes in relative blood volume that follow distinct profiles for subjects with vs without congestion. Measured alongside urine output, these profiles may be used to estimate plasma refill rates—potential patient-specific targets for decongestive therapy across serial diuretic doses.
Graphical abstract
Résumé
Contexte
Le taux de remplissage plasmatique peut être estimé en combinant les mesures de la diurèse et les profils volémiques relatifs. Chez les personnes atteintes d’insuffisance cardiaque aiguë, une variation du taux de remplissage plasmatique pourrait guider un traitement décongestif par un diurétique de l’anse. L’étude avait pour objectif d’évaluer les profils volémiques relatifs moyens obtenus dans le cadre de deux ou trois mesures de suivi réalisées quelques heures après l’administration d’un diurétique de l’anse à des sujets présentant ou non une congestion initiale.
Méthodologie
Une revue systématique d’articles rédigés en anglais, en français, en espagnol et en allemand a été effectuée au moyen des bases de données MEDLINE (1964 à 2019), Cochrane Reviews (1996 à 2019) et Embase (1974 à 2019). Les termes de recherche comprenaient : diurétiques, hémoconcentration, volume plasmatique et volume sanguin. Nous avons inclus des études portant sur des adultes ayant reçu un diurétique de l’anse chez qui au moins une mesure initiale et une mesure de suivi avaient été effectuées. Un seul auteur a recueilli des mesures du volume sanguin individuelles ou de groupe, les a regroupées, au besoin, et converties en variations relatives.
Résultats
Parmi les 16 études qui répondaient aux critères d’inclusion prédéfinis, le volume sanguin relatif a diminué de 9,2 % (de 6,6 % à 12,0 %) et est revenu aux valeurs initiales après trois heures ou plus. Les sujets qui présentaient une congestion ont connu des diminutions du volume sanguin relatif inférieures à celles de ceux n’en présentant pas lors de toutes les périodes de suivi (p = 0,001); le volume sanguin relatif est revenu aux valeurs initiales durant la période finale de suivi.
Conclusions
Des doses uniques de diurétique de l’anse produisent des changements mesurables du volume sanguin relatif selon des profils distincts chez les sujets présentant une congestion, comparativement à ceux n’en présentant pas. Utilisés en association avec les mesures de la diurèse, ces profils peuvent servir à estimer le taux de remplissage plasmatique, qui constitue potentiellement une cible particulière au patient qui reçoit une série de doses d’un diurétique comme traitement décongestif.
Congestion in the setting of heart failure is a maladaptive accumulation of extracellular salt and water.1 Although common, it manifests differently among individual patients, especially in terms of the amount of interstitial (or tissue) congestion.2 Patient responses to the treatment of congestion (“decongestion”) also vary. Some patients tolerate complete decongestion, whereas others with a greater burden of disease must “compromise” and accept residual congestion.3 Determining an individual patient’s tolerance to diuretic treatment is challenging. A normal physiological elevation in serum creatinine level, for example, easily can be misinterpreted as kidney injury4 and lead to unjustified and unnecessary reduction of neurohormonal antagonist therapy, particularly renin-angiotensin system blockade. Better monitoring tools for tailoring decongestive therapy to the individual characteristics of each patient’s heart failure are needed.5, 6, 7
Monitoring for hemoconcentration relative to the start of decongestive therapy has been proposed as a practical way to determine when decongestion has progressed enough to lead to a contracted blood volume.8 Although repurposing routine daily laboratory tests in this way would be convenient, infrequent measurements cannot establish whether the “dynamic equilibria between interstitial and plasma fluid” are at steady state or in transition.9 A blood volume that appears to be “dry” and hemoconcentrated at one moment in time may not, in fact, reflect a diminished extracellular fluid volume, rather than slow intravascular-to-interstitial redistribution. Plasma refill from a “wet” interstitium may be ongoing.
Although steady-state estimates of plasma volume are not useful,10 repeated measurements of blood volume and urine output over hours—timed to assess the response to a perturbation—can distinguish between these 2 scenarios.11 When they are begun at the onset of a discrete episode of fluid removal, such as a single loop diuretic dose, serial measurements of relative blood volume change over the course of hours can be combined with the amount of fluid removed to calculate the plasma refill rate.12 Unlike plasma volume—which only reflects the intravascular compartment and correlates poorly with congestion13—plasma refill reflects overall extracellular volume status: both the intravascular and the interstitial compartments.14 As long as the perturbation (fluid removal) and its response (blood volume change) are known, plasma refill can be estimated without directly measuring plasma volume. Plasma refill rates have been successfully used to guide perturbations from mechanical fluid removal (eg, ultrafiltration) for decades.15 But unlike mechanical fluid removal, which uses an extracorporeal circuit equipped with inline sensors of hematocrit or protein concentration, high frequency measurements of hemoconcentration are not available to estimate plasma refill rates during diuretic-induced fluid removal.16 If plasma refill rates are to guide diuretic therapy across serial perturbations (information that is currently not being harnessed for its potential diagnostic value),17 investigators need a better understanding of whether plasma refill rates can be feasibly estimated after individual diuretic doses.
Therefore, we conducted a systematic review of the existing literature to generate average relative blood volume profiles within hours of a loop diuretic dose, and tested the hypothesis that the profile of relative blood volume change—representing the degree and duration of blood volume change from a single diuretic dose—differs as a function of the degree of congestion. We also present a method for how, in theory, serial relative blood volume profiles could be combined with urine output to estimate serial plasma refill rates at the bedside of a patient receiving loop diuretic therapy.
Methods
Overview
We conducted a systematic review, and upon retrieving multiple articles that focused on blood volume after single loop diuretic doses, we proceeded to a meta-analysis. Given that articles included a mix of both subject-level (‘individual patient’) and group-level (‘aggregate’) data, we conducted a 2-step meta-analysis to avoid potential biases related to the availability of subject-level data.18 After aggregating subject-level data, we combined it with existing group-level data using random-effects meta-analysis with inverse variance weighting.19 Our report complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)20 and Meta-Analysis of Observational Studies In Epidemiology (MOOSE) guidelines.21 Our protocol was not registered.
Search strategy and eligible studies
A medical librarian retrieved article titles and abstracts from MEDLINE (January 1964 to April 2019), Embase (January 1974 to April 2019), and Cochrane Reviews (January 1996 to April 2019) using the search strings listed in the Supplemental Methods. We identified additional articles by searching reference lists. An investigator reviewed all potentially relevant articles in 2 steps—first by title and abstract together, and then if appropriate, by evaluation of the full text.
We included studies that reported on adults who (i) were given an oral or intravenous loop diuretic that is currently approved by the US Food and Drug Administration (bumetanide, ethacrynic acid, furosemide, or torsemide) and (ii) had both a baseline and at least one follow-up measurement (within 12 hours) that allowed calculation of relative blood volume change (including a standard error or standard deviation if only group-level data were presented).
We considered studies within an article to be distinct when results were reported by groups that were defined by different experimental methods or baseline characteristics. For example, in the article by Samet et al.22 one study had follow-up measurements after 2 hours, and another after 3 hours; in the article by Ramirez and Abelmann,23 one study comprised subjects with acute heart failure and the other healthy controls.
Given our interest in determining whether baseline volume status determines blood volume change, we grouped each study by “congestion” vs “no congestion.” We used the operational definition of congestion within each paper: subjects with congestion had characteristics (signs, symptoms, and recent history) consistent with decompensated heart failure. The specific characteristics that led to the grouping of subjects as congested or not within each study are listed in Table 1. Because the article by Haug34 collapsed subjects with and without congestion into a single group, without providing subject-level data, we excluded this study from our subgroup analysis.
Table 1.
Characteristics of 16 studies by congestion subgroup
| Study | Year | n | Diuretic dose and route | Measurement(s) (h after dose) | Rationale for subgrouping or exclusion |
|---|---|---|---|---|---|
| Baseline congestion—included in analysis | |||||
| Ramìrez and Abelmann23 (acute heart failure) | 1968 | 5∗ | Ethacrynic acid 1 to 2 mg per kg IV | Plasma volume and hematocrit† (1.4) | Class IV heart failure, “considerable fluid retention” |
| Sigurd et al.24 | 1974 | 10 | Bumetanide 3 mg IV | Percent change in plasma volume (2) | Signs of congestive heart failure, diuretics held for 24 h |
| Austin et al.25 | 1976 | 27 | Furosemide 40 to 60 mg IV (n = 9) or ethacrynic acid 25 to 50 mg IV (n = 18) | Plasma volume‡ (1) | Class III heart failure with elevated pulmonary artery pressure (mean of 36 mm Hg) |
| Figueras and Weil26 | 1978 | 21 | Furosemide 40 to 160 mg IV | Plasma volume and hematocrit† (7.4) | Acute pulmonary cardiogenic edema |
| Schuster et al.27 | 1984 | 21∗ | Furosemide 40 to 80 mg IV | Blood volume (4.4) | Persistent clinical signs of mild to moderate pulmonary edema |
| No baseline congestion—included in analysis | |||||
| Davidov et al.28 (plasma volume) | 1967 | 5∗ | Furosemide 80 to 160 mg IV | Blood volume (2) | Edema cleared after treatment with diuretics, previous heart failure |
| Davidov et al.28 (hematocrit) | 1967 | 25∗ | Furosemide 80 to 160 mg IV | Hematocrit (2) | Edema cleared after treatment with diuretics, previous heart failure |
| Davidov et al.29,§ (short term) | 1967 | 32∗ | Furosemide 40 to 300 mg IV | Plasma volume and red cell volume (2) | Hypertensive patients with no signs of heart failure |
| Samet and Bernstein22 (2-h) | 1968 | 25∗ | Ethacrynic acid 200 mg PO | Blood volume (2) | No current signs of heart failure, previous history of heart failure |
| Samet and Bernstein22 (3-h) | 1968 | 5∗ | Ethacrynic acid 200 mg PO | Blood volume (3) | No current signs of heart failure, previous history of heart failure |
| Ramìrez and Abelmann23 (healthy controls) | 1968 | 6∗ | Ethacrynic acid 1 to 2 mg per kg IV | Plasma volume and hematocrit† (1.5) | No known heart or kidney disease |
| Rosenthal30 | 1968 | 10 | Furosemide 0.5 to 0.6 mg per kg IV | Blood volume (1,2) | No known heart or kidney disease |
| Olesen31 | 1970 | 10 | Furosemide 120 mg PO | Hemoglobin concentration (2,4) | Recumbent for 4 h despite no diuretic treatment, history of heart disease |
| Scholz et al.32 | 1970 | 6 | Furosemide 30 mg IV | Plasma volume‡ (3) | 2 with hyperthyroidism but no reported heart or kidney disease |
| Baylis and De Beer33,|| | 1981 | 5 | Furosemide 40 mg IV | Hematocrit¶ (2,3) | No heart or kidney disease |
| Not included in subgroup analysis | |||||
| Haug34 | 1976 | 7 | Furosemide 80 mg PO | Hematocrit¶ (1,3) | Inseparable mix of undertreated heart failure and healthy subjects |
IV, intravenous; PO, per os (oral).
Individual patient data were aggregated to perform the meta-analysis.
Total blood volume was estimated from plasma volumes and hematocrits using the formula in the Supplemental Methods.
Total blood volume was estimated from plasma volume and 45% as the hematocrit value, using the formula in the Supplemental Methods.
Davidov et al.29 reported a study of blood volume measurements obtained at 2, 24, and 48 hours after furosemide 100 mg IV was administered in 6 subjects; however, this study was not included because the authors provided no measure of dispersion (standard deviation or standard error) to generate an inverse variance weight.
Baylis and De Beer33 reported studying piretanide 12 mg IV in the same 5 subjects; however, this study was not included because piretanide is not approved by the US Food and Drug Administration.
Extracted manually from graphic images with digitization software.
Meta-analysis
Retrieving subject-level data for the original articles was not possible, as they were published between 1967 and 1984 (Table 1). So we conducted a 2-step meta-analysis19 by first aggregating subject-level data,22,23,27, 28, 29 grouping subjects by baseline volume status, as described above.
One investigator extracted absolute volume measurements (indicator and dye-dilution techniques) or relative volume measurements (venous hematocrits),35 and the number of subjects and level of precision for each measurement (standard deviation or standard error). Two articles presented graphical data,33,34 and digitization software (Digitizelt, version 2.3.3, Braunschweig, Germany; http://www.digitizeit.de/) was used to extract measurements.
We used the following formula36 to estimate blood volume from plasma volume and venous hematocrit:
where Hct% is the venous hematocrit. The constants 0.96 and 0.91 reflect, respectively, correction factors for “trapped plasma”37 and the Fahreus effect—venous hematocrits are higher than “total body” hematocrits.36 For studies that reported only plasma volume, we used 45% as the hematocrit value.37, 38 For one study that presented group-level data on both plasma volume and hematocrit,26 we combined the standard errors from each group-level measurement using the formula provided in the Supplemental Methods.
Blood volume change was calculated relative to the most proximate baseline measurement obtained just before administration of a diuretic. We segregated follow-up measurements into 3 periods delineated by the opposing forces of diuresis and plasma refill.39 These 2 processes act concurrently but slightly out of phase—depending on the degree of interstitial congestion.40 During the first period, which includes the first 2 hours after dosing, diuresis peaks, and in the absence of interstitial congestion, it outpaces plasma refill. During the second period, which includes the third hour after dosing, diuresis wanes, whereas plasma refill may (depending on the degree of congestion) continue to replenish the intravascular space. Finally, during the third period, which occurs after the third hour, the dynamic equilibrium between the intravascular and interstitial compartments stabilizes. Although rates of diuresis may vary among patients with different degrees of congestion, and follow-up measurements varied among studies, these time intervals encompass the time of maximum diuresis and maximum relative change in blood volume.
We converted group-level average blood volume measurements to relative change at each follow-up period by subtracting the follow-up measurement from the baseline measurement and dividing by the baseline measurement. For articles that reported neither blood volumes nor plasma volumes, we used hematocrit to determine relative changes in blood volume. Red blood cells are neither removed nor added during diuresis; therefore, a relative increase in a hematocrit (hemoconcentration) indicates a relative decrease in blood volume according to the following formula41:
Statistical analysis
Some studies reported more than one follow-up measurement, and we conducted a longitudinal random-effects meta-analysis to account for correlations between repeated measurements within the same studies.42 We weighted each group-level blood volume change using study-specific inverse variances and covariances43 that were obtained from the standard errors of relative blood volume change, as described in detail in the Supplemental Methods.
Results
We identified 13 eligible articles that reported results from 16 unique studies (Supplemental Fig. S1). Table 1 shows the characteristics of these 16 studies. Studies included 220 subjects overall—89 with congestion and 131 without. Most authors provided scant additional details about subjects’ baseline characteristics, such as left ventricular ejection fraction, the nature of the right side of the heart, baseline renal function, and urine output.
Loop diuretic doses, types, and routes of administration varied widely among studies. Four different types of loop diuretics were used: furosemide (n = 10); ethacrynic acid (n = 4); furosemide or ethacrynic acid (n = 1); and bumetanide (n = 1). Additionally, diuretics were given intravenously in 12 studies and orally in 4 studies. Some studies used a fixed dose (n = 7), whereas others titrated doses to subject responses (n = 9). For example, Scholz et al.32 administered a fixed 30-mg intravenous dose of furosemide, and Davidov et al.29 titrated intravenous furosemide to doses as high as 300 mg.
Methods of blood volume measurements also varied among studies. Although 5 studies reported absolute blood volumes, the remaining 11 used indirect estimates of blood volume, as follows: hemoglobin concentrations (n = 1); percentage change in plasma volumes (n = 1); plasma volumes (n = 2); hematocrits (n = 3); or a combination of plasma volumes and hematocrits or red blood cell volumes (n = 4). The timing of follow-up measurements ranged from 1 to 7.4 hours after a diuretic dose; the most common timing was 2 to 3 hours after. Twelve studies reported a single follow-up measurement; 4 studies made repeated follow-up measurements.
Despite variable methods, we calculated a –7.2% change in average blood volume in the first 2 hours after diuretic dosing (95% confidence interval [CI] –10.4% to –4.1%) and a –9.2% change in the third hour (–12.0% to –6.6%; Supplemental Fig. S2). After the third hour, the average blood volume change for all subjects was not different from baseline (–6.3% to +0.7%; P = 0.11).
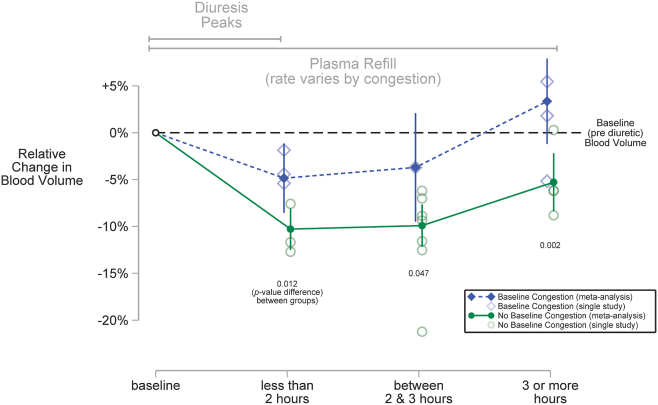
When grouped by baseline congestion, 2 statistically distinct profiles of average blood volume change emerged (Fig. 1). The shapes of these profiles differed in 2 important respects. First, the profile for subjects without baseline congestion was farther from baseline than the profile for subjects with baseline congestion. During each follow-up period, subjects without congestion experienced a 5% or greater reduction in relative blood volume change than subjects with congestion. Second, although average blood volume among subjects with congestion returned to baseline in the third hour after diuretic administration (–3.7% [95% CI –9.4% to +2.0%], P = 0.2), it remained lower than baseline beyond the third hour (–3.4% [95% CI –7.9% to –1.1%]) among subjects without congestion.
Figure 1.
Profiles of relative blood volume change after single loop diuretic dose by baseline congestion status. Each profile is a plot of the average change in blood volume relative to baseline (black horizontal dashed line). The grey horizontal lines at the top of the figure represent periods delineated by the opposing forces of diuresis and plasma refill; these 2 processes act concurrently but slightly out of phase, depending on the degree of interstitial congestion. The profile of solid diamonds that are connected by a dashed line represents 84 subjects with baseline congestion, and the profile of solid circles that are connected by a solid line represents 129 subjects with no baseline congestion. The vertical lines centred on each solid diamond or circle represent 95% confidence intervals generated from a mixed-effects longitudinal meta-analysis (details provided in the text). The profiles are statistically distinct during each follow-up period. Compared to subjects with congestion, subjects without congestion had an average blood volume that was 5.4% lower (1.2% to 9.7%, P = 0.012) in the first 2 hours after diuretic administration, 6.2% lower (0.1% to 12.3%, P = 0.047) in the third hour after diuretic administration, and 8.6% lower (3.2% to 14.1%, P = 0.002) beyond the third hour. Relative blood volume changes from individual studies are plotted with shapes that correspond to their profile: hollow diamonds are changes among subjects with baseline congestion, and hollow circles are changes among subjects without baseline congestion.
Discussion
Blood volume change after loop diuretic administration was studied decades ago; the papers included in this meta-analysis were all published between 1968 and 1984 (Table 1). The findings then were not conclusive, however. Many early papers, such as those by Davidov et al. (1967)28,29 and Samet and Bernstein (1968),22 reported large decreases in blood volume after loop diuretic administration. In later papers, blood volume was found, paradoxically, to be increased. When explaining this inconsistency in 1984 (the year of the most recent paper in our meta-analysis), Schuster et al. opined that “the preponderant evidence now does not support the concept that blood volume is depleted after furosemide.”27 Our findings suggest otherwise. When all eligible studies were combined, the average subject’s blood volume decreased by more than 5% during the first 2 hours, and by more than 9% during the third hour after diuretic administration (Supplemental Fig. S2). These findings remain provisional, given the heterogeneity of loop diuretic dosing and relative blood volume measurement. But our meta-analysis is the only comprehensive review of these data; as such, it provides the strongest evidence that loop diuretics cause measurable decreases in blood volume within hours of a single dose.
The relationship between a loop diuretic dose and blood volume change is nuanced. We found that subjects’ baseline congestion level determines just how much a dose decreases blood volume. Subjects who were not congested at baseline experienced 5% or greater decrease in relative blood volume change at each follow-up period, compared to subjects with congestion (Fig. 1). This difference persisted after the period of diuresis in those who were not congested, as they did not return to their baseline volume status during the follow-up periods studied. The finding that baseline congestion dampens the impact of a loop diuretic dose on blood volume likely explains some of the discrepant findings seen by earlier investigators, as studies enrolled subjects with variable amounts of congestion, which may have masked the tendency of a loop diuretic dose to decrease blood volume (Table 1). More important than resolving a decades-old question about the impact of loop diuretics on blood volume, the clinical implications of our findings may yield insights into how congestion can be better monitored and treated by measuring relative blood volume change, along with urine output, to potentially track plasma refill rates within patients after they have received serial doses of loop diuretics.
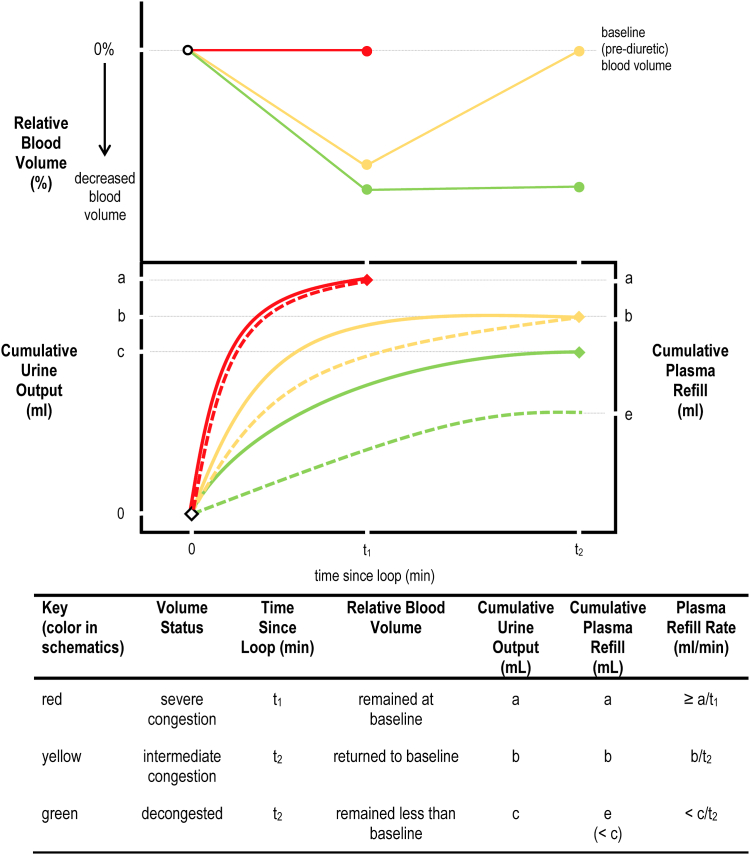
Congestion accumulates in 2 separate extracellular fluid compartments that exist in dynamic equilibrium: the intravascular compartment (plasma) and the extravascular compartment (interstitium). Although distribution across these compartments can make congestion difficult to assess, studies of mechanical fluid removal, such as ultrafiltration, have shown that the rate at which extravascular fluid replenishes intravascular fluid— that is, the plasma refill rate—correlates with the degree of interstitial congestion. Other things being equal, a greater level of interstitial congestion is associated with a brisker plasma refill rate.12 The plasma refill rate is the net amount of fluid that crosses into the intravascular compartment per unit of time, usually expressed as milliliters per minute. Although the volume of fluid crossing into the intravascular compartment cannot be directly measured, it can be inferred from 2 measurements: (i) the amount of fluid removed from the intravascular compartment (such as the ultrafiltrate volume during ultrafiltration or the urine output during diuresis); and (ii) the relative blood volume change associated with that removal. Note that estimates of plasma refill rate do not depend on measurements of plasma volume, which are cumbersome. As shown in Figure 2, the plasma refill rate is the volume removed, divided by the time it takes for the blood volume to return to baseline. And so, if the blood volume decreases and then returns to baseline, the plasma refill rate is the amount of fluid removed divided by the duration of the period of removal (expressed in mL per minute).
Figure 2.
Schematic profiles of relative blood volume used to estimate plasma refill rate.
The plasma refill rate can be estimated from cumulative urine output if the relative blood volume profile is known. Three different plasma refill rates are depicted in the schematic; these represent either different patients or different volume states within the same patient. In the top panel, the solid red line represents severe congestion—relative blood volume remains at baseline despite diuresis. The solid yellow line represents intermediate congestion—relative blood volume is initially negative but returns to baseline. The solid green line represents a decongested volume status—relative blood volume is persistently negative throughout follow-up. In the bottom panel, the solid red, yellow, and green lines represent cumulative urine output after a loop diuretic is administered, while the dashed red, yellow, and green lines represent the cumulative plasma refill. The lines are coded to correspond to the relative blood volume profiles in the top panel. In the case of severe congestion (red lines), just a single measurement of cumulative urine output is needed at the first follow-up to generate a lower-bound estimate for the plasma refill rate (≥ a/t1). For intermediate congestion (yellow lines), the plasma refill rate is estimated as the cumulative urine output divided by the time since loop diuretic administration at the second follow-up (b/t2). Last, for the decongested state, blood volume does not return to baseline. So the cumulative plasma refill (e) is not measurable and, therefore, only an upper bound (< c/t2) for the plasma refill rate can be specified.
The difficulty in measuring plasma refill rate during diuresis—an idea proposed by Boyle and Sobotka over 15 years ago17—is not in measuring the urine output, but in knowing what is happening to the blood volume during diuretic fluid removal. Our findings suggest that 3 follow-up blood volume measurements after a loop diuretic dose—during the first 2 hours, during third hour, and after the third hour—would be sufficient, along with careful urine output measurements, to make the plasma refill rate estimable.
These findings are subject to several important limitations. The first, and most important limitation, is the amount of methodological heterogeneity across studies, as depicted in the fourth and fifth columns of Table 1. Studies varied in how loop diuretics were administered; diuretic type, route, and dose certainly impact the extent and timing of diuresis and, hence, relative blood volume changes. Studies also varied in how and when blood volume was measured. In some cases, we used simplifying assumptions to estimate blood volume from other measurements (eg, absolute plasma volume and hematocrit); doing so may have introduced biases or added imprecision to our estimates. Collapsing the timing of follow-up measurements into 3 “categorical” periods is also a simplification that may obscure important differences among subjects. Nevertheless, such heterogeneity (and remedies to address it) tend to bias to the null: flat profiles statistically indistinguishable from baseline. A discernable signal strengthens our findings because despite the methodological differences among studies, clear, statistically significant differences in relative blood volume changes emerged when congested subjects were compared to noncongested subjects. Second, the original papers provided scant information about subjects’ baseline characteristics beyond the authors’ clinical impressions. We were unable to explore how clinical profiles that are known to impact the dynamics of decongestion, such as the dominant “sidedness” of elevated filling pressures (left vs right) and the systolic function of the left ventricle (preserved or not),3 may impact relative blood volume changes. This lack of data made our assignment by baseline congestion status difficult to corroborate. Yet the pre-diuretic state of congestion is the simplest pathophysiological explanation of the divergent blood volume changes. Third, some studies reported urine output, but many did not. Although this prevented us from adjusting for diuretic responsiveness in our models (as well as estimating plasma refill rates), 4 out of 5 studies of subjects with congestion titrated the diuretic dose to achieve an adequate urine output (Table 1), suggesting that diuretic responsiveness was achieved and that the lack of blood volume change in the congested group is not simply from inadequate diuresis. Fourth, original papers were published more than 4 decades ago; contemporary management of heart failure patients, particularly with neurohormonal antagonist therapies, may influence relative blood volume change. Fifth, our findings apply to only intermittent dosing of loop diuretics. Fluid removal with continuous intravenous infusions may produce a more steady change in relative blood volume from baseline that may, therefore, not be measurable. Last, opinions differ about how to combine longitudinal data for a meta-analysis with both subject-level and group-level data. Nevertheless, to avoid potential biases introduced by using only subject-level data,18 we included both types of data, using a method that has outperformed others in simulation studies (Supplemental Methods).
The limitations of our meta-analysis could be addressed in a contemporary study of patients hospitalized for decongestive diuretic therapy using diuretic doses that ensure that some minimum effective urine output is achieved.44 Careful measurement of urine output will be needed to interpret relative blood volume profiles (without urine output data, a flat profile, for example, cannot distinguish between brisk refill and no diuresis) and generate estimates of plasma refill rates, as depicted in Figure 2. To ensure the feasibility of measuring plasma refill rates in real-world scenarios, routine serial hematocrits could be used to measure relative blood volume.45 The measurement error of repeat hematocrits obtained on stable patients46 (the so-called reference change value47) is smaller than the changes in relative blood volume we observed (Fig. 1), indicating that serial measurements of hematocrits would have sufficient precision to allow confidence in detection of clinically useful changes in blood volume following diuretic administration.
The most critical issue to be addressed by future studies is whether, as with ultrafiltration,12 relative blood volume change and plasma refill rates are feasible to measure, and responsive to volume status changes across serial doses within individual patients.48 If they are feasible to measure and responsive to meaningful clinical changes, plasma refill rates could be used to monitor decongestive therapy across serial diuretic doses. Our expectation is that, were such a study to be done, plasma refill rates would decline alongside congestion, and change in plasma refill rate would serve as an objective measure of change in a patient’s clinical trajectory.3 For example, a change from a “wet” to a “dry” (fast to slow) plasma refill rate may help confirm that a patient whose decongestive therapy has “stalled” after initial improvement is actually optivolemic and ready for discharge.3 Similarly, an increasing plasma refill rate may uncover the emergence of otherwise subclinical congestion in the outpatient setting.49
Acknowledgements
The authors thank our medical librarian, Loretta Grikis. The contents of this publication do not represent the views of the US Department of Veterans Affairs or the US Government.
Data Availability
The data that support the findings of this study are available from the corresponding author, B.P.L., upon reasonable request.
Ethics Statement
The authors confirm that this research has adhered to relevant ethical guidelines.
Patient Consent
The authors confirm that patient consent is not applicable to this meta-analysis.
Funding Sources
Support for the medical librarian was provided in kind from the White River Junction VA Medical Center. The authors have no other funding sources to declare.
Disclosures
The authors have no conflict of interest to disclose.
Footnotes
See page 648 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.05.003.
Supplementary Material
References
- 1.Boorsma E.M., Ter Maaten J.M., Damman K., et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol. 2020;17:641–655. doi: 10.1038/s41569-020-0379-7. [DOI] [PubMed] [Google Scholar]
- 2.Miller W.L., Mullan B.P. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail. 2014;2:298–305. doi: 10.1016/j.jchf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Hollenberg S.M., Warner Stevenson L., Ahmad T., et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;75:132. doi: 10.1016/j.jacc.2019.08.001. J Am Coll Cardiol2019;74:1966-2011. Erratum. [DOI] [PubMed] [Google Scholar]
- 4.Damman K., Tang W.H., Testani J.M., McMurray J.J. Terminology and definition of changes renal function in heart failure. Eur Heart J. 2014;35:3413–3416. doi: 10.1093/eurheartj/ehu320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullens W., Verbrugge F.H. Increasing diuresis in congestive heart failure: ready for prime time? J Am Coll Cardiol. 2013;62:1184–1186. doi: 10.1016/j.jacc.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Katz S.D. In search of euvolemia in heart failure. J Am Coll Cardiol HF. 2014;2:306–307. doi: 10.1016/j.jchf.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Zalawadiya S., Stevenson L.W. Getting to dry. Circulation. 2018;137:1684–1687. doi: 10.1161/CIRCULATIONAHA.118.032691. [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M., Greene S.J., Fonarow G.C., et al. Hemoconcentration-guided diuresis in heart failure. Am J Med. 2014;127:1154–1159. doi: 10.1016/j.amjmed.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Guyton A.C., Taylor A.E., Granger H.J. In: Circulatory Physiology II: Dynamics and the Control of the Body Fluids. Guyton A.C., Taylor A.E., Granger H.J., editors. Saunders; Philadelphia: 1975. Extracellular, intracellular, and insterstitial fluids; pp. 10–17. [Google Scholar]
- 10.Swolinsky J.S., Tuvshinbat E., Leistner D.M., et al. Discordance between estimated and measured changes in plasma volume among patients with acute heart failure. ESC Heart Fail. 2022;9:66–76. doi: 10.1002/ehf2.13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimondini A., Cipolla C.M., Della Bella P., et al. Hemofiltration as short-term treatment for refractory congestive heart failure. Am J Med. 1987;83:43–48. doi: 10.1016/0002-9343(87)90495-5. [DOI] [PubMed] [Google Scholar]
- 12.Marenzi G., Lauri G., Grazi M., et al. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol. 2001;38:963–968. doi: 10.1016/s0735-1097(01)01479-6. [DOI] [PubMed] [Google Scholar]
- 13.Cuthbert J.J., Pellicori P., Rigby A.S., et al. Are non-invasive estimations of plasma volume an accurate measure of congestion in patients with chronic heart failure? Eur Heart J Qual Care Clin Outcomes. 2023;9:281–292. doi: 10.1093/ehjqcco/qcac035. [DOI] [PubMed] [Google Scholar]
- 14.Fauchald P. Effects of ultrafiltration on body fluid volumes and transcapillary colloid osmotic gradient in hemodialysis patients. Contrib Nephrol. 1989;74:170–175. doi: 10.1159/000417488. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo M.R., Ronco C., Abraham W.T., et al. Extracorporeal ultrafiltration for fluid overload in heart failure: current status and prospects for further research. J Am Coll Cardiol. 2017;69:2428–2445. doi: 10.1016/j.jacc.2017.03.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas B.P., D'Addio A., Block C., et al. Limited agreement between two noninvasive measurements of blood volume during fluid removal: ultrasound of inferior vena cava and finger-clip spectrophotometry of hemoglobin concentration. Physiol Meas. 2019;40 doi: 10.1088/1361-6579/ab21af. [DOI] [PubMed] [Google Scholar]
- 17.Boyle A., Sobotka P.A. Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate. J Card Fail. 2006;12:247–249. doi: 10.1016/j.cardfail.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Stewart L.A., Tierney J.F. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof. 2002;25:76–97. doi: 10.1177/0163278702025001006. [DOI] [PubMed] [Google Scholar]
- 19.Riley R.D., Simmonds M.C., Look M.P. Evidence synthesis combining individual patient data and aggregate data: a systematic review identified current practice and possible methods. J Clin Epidemiol. 2007;60:431–439. doi: 10.1016/j.jclinepi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate heathcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Samet P., Bernstein W.H. Acute effects of oral ethacrynic acid upon total blood volume. Am Heart J. 1968;75:288–290. doi: 10.1016/0002-8703(68)90084-7. [DOI] [PubMed] [Google Scholar]
- 23.Ramírez A., Abelmann W.H. Hemodynamic effects of diuresis by ethacrynic acid in normal subjects and in patients with congestive heart failure. Arch Intern Med. 1968;121:320–327. doi: 10.1001/archinte.1968.03640040014003. [DOI] [PubMed] [Google Scholar]
- 24.Sigurd B., Hesse B., Valentin N. Bumetanide: a new potent diuretic. The effects of a single intravenous dose in patients with congestive heart failure. Dan Med Bull. 1974;21:63–67. [PubMed] [Google Scholar]
- 25.Austin S.M., Schreiner B.F., Kramer D.H., Shah P.M., Yu P.N. The acute hemodynamic effects of ethacrynic acid and furosemide in patients with chronic postcapillary pulmonary hypertension. Circulation. 1976;53:364–369. doi: 10.1161/01.cir.53.2.364. [DOI] [PubMed] [Google Scholar]
- 26.Figueras J., Weil M.H. Blood volume prior to and following treatment of acute cardiogenic pulmonary edema. Circulation. 1978;57:349–355. doi: 10.1161/01.cir.57.2.349. [DOI] [PubMed] [Google Scholar]
- 27.Schuster C.J., Weil M.H., Besso J., Carpio M., Henning R.J. Blood volume following diuresis induced by furosemide. Am J Med. 1984;76:585–592. doi: 10.1016/0002-9343(84)90281-x. [DOI] [PubMed] [Google Scholar]
- 28.Davidov M., Kakaviatos N., Finnerty F.A., Jr. Intravenous administration of furosemide in heart failure. JAMA. 1967;200:824–829. [PubMed] [Google Scholar]
- 29.Davidov M., Kakaviatos N., Finnerty F.A., Jr. Antihypertensive properties of furosemide. Circulation. 1967;36:125–135. doi: 10.1161/01.cir.36.1.125. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal J., Boucher R., Nowaczynski W., Genest J. Acute changes in plasma volume, renin activity, and free aldosterone levels in healthy subjects following furosemide administration. Can J Physiol Pharmacol. 1968;46:85–91. doi: 10.1139/y68-015. [DOI] [PubMed] [Google Scholar]
- 31.Olesen K.H. Dynamic aspects of volume and osmoregulation in congestive heart failure during furosemide diuresis. Angiology. 1970;21:35–48. doi: 10.1177/000331977002100107. [DOI] [PubMed] [Google Scholar]
- 32.Scholz H.R., Demiroglu C., Ritzl F. Gesamtkörperwasser, Rhodanidraum und 131-jod-albuminraum unter akuter furosemid-wirkung [Total body water, rhodanide space and I-131-albumin space under the acute effect of furosemide] Arzneimittelforschung. 1970;20:1249–1251. [in German] [PubMed] [Google Scholar]
- 33.Baylis P.H., De Beer F.C. Human plasma vasopressin response to potent loop-diuretic drugs. Eur J Clin Pharmacol. 1981;20:343–346. doi: 10.1007/BF00615403. [DOI] [PubMed] [Google Scholar]
- 34.Haug T.O. Time course of changes in concentration of some plasma components after frusemide. Br Med J. 1976;2:622. doi: 10.1136/bmj.2.6036.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ertl A.C., Diedrich A., Raj S.R. Techniques used for the determination of blood volume. Am J Med Sci. 2007;334:32–36. doi: 10.1097/MAJ.0b013e318063c6d1. [DOI] [PubMed] [Google Scholar]
- 36.Gregersen M.E., Rawson R.A. Blood volume. Physiol Rev. 1959;39:307–342. doi: 10.1152/physrev.1959.39.2.307. [DOI] [PubMed] [Google Scholar]
- 37.Chaplin H., Jr., Mollison P.L. Correction for plasma trapped in the red cell column of the hematocrit. Blood. 1952;7:1227–1238. [PubMed] [Google Scholar]
- 38.Gibson J.G., Evans W.A. Clinical studies of the blood volume. II. The relation of plasma and total blood volume to venous pressure, blood velocity rate, physical measurements, age and sex in ninety normal humans. J Clin Invest. 1937;16:317–328. doi: 10.1172/JCI100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brater D.C. In: Diuretic Agents: Clinical Physiology and Pharmacology. Seldin D., Giebisch G., editors. Academic Press; San Diego: 1997. Diuretic pharmacokinetics and pharmacodynamics; pp. 193–195. [Google Scholar]
- 40.Mitsides N., Pietribiasi M., Waniewski J., Brenchley P., Mitra S. Transcapillary refilling rate and its determinants during haemodialysis with standard and high ultrafiltration rates. Am J Nephrol. 2019;50:133–143. doi: 10.1159/000501407. [DOI] [PubMed] [Google Scholar]
- 41.Fleming S.J., Wilkinson J.S., Aldridge C., et al. Dialysis-induced change in erythrocyte volume: effect on change in blood volume calculated from packed cell volume. Clin Nephrol. 1988;29:63–68. [PubMed] [Google Scholar]
- 42.Ishak K.J., Platt R.W., Joseph L., Hanley J.A., Caro J.J. Meta-analysis of longitudinal studies. Clin Trials. 2007;4:525–539. doi: 10.1177/1740774507083567. [DOI] [PubMed] [Google Scholar]
- 43.Bagos P.G. Meta-analysis in Stata using gllamm. Res Synth Methods. 2015;6:310–332. doi: 10.1002/jrsm.1157. [DOI] [PubMed] [Google Scholar]
- 44.Costanzo M.R., Negoianu D., Jaski B.E., et al. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail. 2016;4:95–105. doi: 10.1016/j.jchf.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Dill D.B., Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 46.Coskun A., Braga F., Carobene A., et al. Systematic review and meta-analysis of within-subject and between-subject biological variation estimates of 20 haematological parameters. Clin Chem Lab Med. 2019;58:25–32. doi: 10.1515/cclm-2019-0658. [DOI] [PubMed] [Google Scholar]
- 47.McCormack J.P., Holmes D.T. Your results may vary: the imprecision of medical measurements. BMJ. 2020;368:m149. doi: 10.1136/bmj.m149. [DOI] [PubMed] [Google Scholar]
- 48.Bell K., Craig J., Irwig L. In: Evidence-based Medical Monitoring: From Principles to Practice. Glasziou P.P., Irwig L., Aronson J.K., editors. Blackwell; Malden, MA: 2008. Monitoring the initial response to treatment; pp. 75–89. [Google Scholar]
- 49.Gheorghiade M., Vaduganathan M., Fonarow G.C., Bonow R.O. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, B.P.L., upon reasonable request.