Abstract
The rapid pace of contemporary environmental change puts many species at risk, especially rare species constrained by limited capacity to adapt or migrate due to low genetic diversity and/or fitness. But the ability to acclimate can provide another way to persist through change. We compared the capacity of rare Boechera perstellata (Braun's rockcress) and widespread B. laevigata to acclimate to change. We investigated the phenotypic plasticity of growth, biomass allocation, and leaf morphology of individuals of B. perstellata and B. laevigata propagated from seed collected from several populations throughout their ranges in a growth chamber experiment to assess their capacity to acclimate. Concurrently, we assessed the genetic diversity of sampled populations using 17 microsatellite loci to assess evolutionary potential. Plasticity was limited in both rare B. perstellata and widespread B. laevigata, but differences in the plasticity of root traits between species suggest that B. perstellata may have less capacity to acclimate to change. In contrast to its widespread congener, B. perstellata exhibited no plasticity in response to temperature and weaker plastic responses to water availability. As expected, B. perstellata also had lower levels of observed heterozygosity than B. laevigata at the species level, but population‐level trends in diversity measures were inconsistent due to high heterogeneity among B. laevigata populations. Overall, the ability of phenotypic plasticity to broadly explain the rarity of B. perstellata versus commonness of B. laevigata is limited. However, some contextual aspects of our plasticity findings compared with its relatively low genetic variability may shed light on the narrow range and habitat associations of B. perstellata and suggest its vulnerability to climate warming due to acclimatory and evolutionary constraints.
Keywords: acclimation, adaptation, Boechera laevigata (smooth rockcress), Boechera perstellata (Braun's rockcress), Brassicaceae, conservation, genetic diversity, plasticity, rare species
The question of why some species are rare while others are common is an enduring one with implications for ecological theory and the conservation of biodiversity. Our research reveals some limited differences in plasticity between rare B. perstellata and widespread B. laevigata that are dependent on various abiotic factors. These differences could help to explain the narrow range and habitat specificity of B. perestellata, while highlighting its potential vulnerability to future environmental change.

1. INTRODUCTION
Biodiversity is at risk worldwide due to dramatic rates of environmental change, often as a result of anthropogenic activities (Knapp et al., 2021; Malhi et al., 2020). Rare species are especially susceptible to extinction in the face of such change (Mouillot et al., 2013), and as such, rare species often drive declines in the biodiversity of communities and systems (Dee et al., 2019). Species can persist through environmental change via migration to more suitable habitat (Chen et al., 2011; Crickenberger & Wetheym, 2018; Hickling et al., 2006; Parmesan, 2006), adaptation to changed conditions in existing habitat (Hamann et al., 2020; Jump & Peñuelas, 2005; Sheth et al., 2018), and/or acclimation to changed conditions (Chevin et al., 2010; Nicotra et al., 2010; Seebacher et al., 2015). But relative to more common species, the ability of rare species to migrate and/or adapt could be impeded by their low fitness (Boyd, Anderson, et al., 2022; Iverson et al., 2004) and/or genetic diversity (Cole, 2003; Gitzendanner & Soltis, 2000; Leimu & Fischer, 2008). Given these constraints, the ability to acclimate to relatively rapid environmental change through phenotypic plasticity could be an important pathway to persistence for rare species.
Relative to other taxonomic groups, plants are generally characterized by high levels of plasticity (Sultan, 2000). But plant species, populations, and individuals can exhibit dramatic differences in plasticity that may influence their responses to environmental change in different ways (Balaguer et al., 2001; Cleavitt, 2002; Dangremond et al., 2015; Godoy et al., 2012; Nicotra & Davidson, 2010; Osunkoya & Swanborough, 2001; Pohlman et al., 2005; Stamp & Hatfield, 2020; Sultan, 2000; Valladares et al., 2000, 2007). Plasticity has been underexplored in the context of the causes and consequences of plant species rarity (Boyd, Anderson, et al., 2022; but see Boyd, Odell, et al., 2022; Hirst et al., 2017; Liao et al., 2006; Lovell & McKay, 2015; Rutherford et al., 2017), although it has been long suggested that rare species could be constrained by low plasticity relative to more common species (Murray et al., 2002). Such constraints could explain both the narrow ranges and habitat specificity of many rare species, as well as their declines in the face of environmental change (Nicotra et al., 2010). Studies that compare the plasticity of rare and common species in the context of environmental change could test the hypothesis that geographically restricted species and/or habitat specialists may be limited by an inability to acclimate to broader environmental conditions (Boyd, Anderson, et al., 2022) and provide guidance for rare species conservation during a time of rapid environmental change (Bevill & Louda, 1999). Congeneric comparisons could be especially useful in advancing our understanding of the causes and consequences of species rarity by controlling for differences in life history and phylogeny (Combs et al., 2013; Farnsworth, 2006; Godt & Hamrick, 2001; Kunin & Gaston, 1997; Murray et al., 2002).
Boechera perstellata (E. L. Braun) Al‐Shehbaz (Braun's rockcress) is a rare endemic plant species with a disjunct distribution consisting of a small number of populations along wooded limestone outcrops in central Tennessee and north‐central Kentucky, USA (USFWS, 1995, 1997, 2004) separated by a ~250‐km gap (Figure 1a). Previous research revealed that B. perstellata has very low levels of genetic diversity (Baskauf et al., 2014) but that study did not include relative comparisons with more common species. In contrast, widespread B. laevigata (Muhl. Ex Willd.) Al‐Shehbaz (smooth rockcress) occurs throughout much of the eastern United States (Figure 1b) as well as southern Quebec in habitat that is largely similar to that of B. perstellata (Al‐Shehbaz & Windham, 2010; Bloom et al., 2002) but across a broader range of substrates (Kiefer et al., 2009). Populations of B. laevigata also have been reported along railroad tracks suggesting that its distribution in some locations may have been influenced by human activities (Kiefer et al., 2009).
FIGURE 1.

Maps of the distribution of rare Boechera perstellata (a) and widespread B. laevigata (b) in the eastern United States at the county level (green shaded areas) and locations of the natural populations of each species from which seeds were collected (yellow dots). While B. perstellata only occurs in the United States, the range of B. laevigata extends into southeastern Canada.
To investigate the role of acclimation as a cause and/or consequence of species rarity, we compared the phenotypic plasticity of rare B. perstellata and widespread B. laevigata in response to a suite of abiotic factors—light, temperature, and water availability. We also compared the evolutionary potential of these species through investigations of their population‐level genetic diversity. Given its narrow geographic distribution and less diverse habitat associations than B. laevigata, we hypothesized that rare B. perstellata would have lower plasticity and genetic diversity than its more common congener. In particular, we expected B. perstellata to have comparably lower plasticity in biomass allocation traits associated with light and water acquisition in the context of the availability of these resources. In response to warming, we expected B. perstellata to exhibit lower plasticity in traits associated with size and leaf morphology, which have been found to be particularly responsive to temperature (see Stotz et al., 2021). We also expected that populations of B. perstellata would exhibit less intraspecific variation in plasticity and within‐population genetic diversity—as causal and/or consequential to rarity—than would populations of B. laevigata, although we expected some significant divergence between the disjunct Tennessee and Kentucky populations of B. perstellata.
2. MATERIALS AND METHODS
2.1. Study system
Boechera perstellata is a rare species restricted to just three counties each in the Central Basin region of Tennessee, USA and the Bluegrass region of north‐central Kentucky, USA (USFWS, 2004, 2018). This rare species is associated with almost dry to moderately moist, shady, steep slopes on limestone outcrops, often in sheltered areas with limited competition from surrounding vegetation (USFWS, 2004, 2018). At present, there are 47 occurrences of B. perstellata known from throughout its range with the vast majority occurring in Kentucky (USFWS, 2018). While most occurrences consist of few individuals, some are comprised of >1000 individuals (NatureServe, 2005). Given its small geographic range and narrow habitat specificity (i.e., limestone outcrops) but large size of some populations, we categorize B. perstellata as “endemic” (see Rabinowitz, 1981), which is the most common type of species rarity (May, 1988; Rabinowitz, 1981). Given its endemism and associated conservation concerns, B. perstellata is listed as federally endangered (USFWS, 2018). Like its rare congener, widespread B. laevigata often occurs on limestone rock outcrops, but this species also can be found growing on open rocky or gravel areas throughout a wider range that encompasses much of eastern North America (Al‐Shehbaz, 1988).
Despite their phylogenetic relatedness, comparisons of Boechera species can be complicated by differences in their life histories and reproductive and ploidy patterns. While the genetic diversity of B. perstellata has been compared with that of short‐lived perennials in particular (Baskauf et al., 2014), its lifespan is not well known beyond the persistence of woody old growth for several years (Braun, 1956). Depending on plant size and resource availability, B. laevigata has been described as being able to function as a short‐lived perennial or biennial (Bloom et al., 2002, 2003). While most Boechera species are sexual diploids, some species are polyploid, and some reproduce asexually through seed (apomixis; Dobeš et al., 2006). Both B. perstellata and B. laevigata are typically diploid, sexually reproducing species, and B. laevigata is known to be capable of reproducing by both outcrossing and selfing (Bloom, 1988). Recent evidence also suggests that a few populations of this species are reproducing by apomixis (Carman et al., 2019; M. Windham, personal communication). The model organism Arabidopsis thaliana is in the same family (Brassicaceae) as Boechera species, and the Boechera genus has become a model system for testing ecological and evolutionary questions given its relatively small genomes and occurrence in natural habitats throughout North America (Rushworth et al., 2011; Schranz et al., 2007).
2.2. Seed collection and propagation
Several populations were sampled from throughout the range of each Boechera species to represent a range of genetic and phenotypic variation within each species. Seeds of B. perstellata were collected from one population each in Rutherford County and Smith County, Tennessee, USA (referred to as TN1 and TN2, respectively) and two populations from Franklin County, Kentucky, USA (KY1, KY2; Figure 1a; Table S1 in Supporting Information for this article). The sites for collection of B. perstellata seed were determined in cooperation with the USFWS given the federally protected status of this species. Sampled populations in Tennessee were separated by ~50 km while sampled populations in Kentucky were separated by ~12 km such that gene flow between populations would have been unlikely. Seeds from parent individuals of B. laevigata were collected from three populations: Cheatham County, Tennessee, USA (TN); Cook County, Illinois, USA (IL); Clarion County, Pennsylvania, USA (PA; Figure 1b; Table S1). All B. perstellata seeds and B. laevigata seeds from the Tennessee population were collected by the authors, laboratory personnel, and/or professional contacts. Vouchers for the B. laevigata populations were deposited in the Austin Peay State University Herbarium (ASPC). From each population of each species, we collected numerous seeds from each of 12–16 distinct parent individuals in separate paper bags to retain maternal information. All seed was collected from the field in spring/summer 2018. All collected seeds were stratified for 4 months prior to planting.
Following stratification, we sowed 6–8 seeds representing half to full siblings per maternal parent from each population into multiple 7‐cm2, 8.5‐cm‐deep (~0.4 L) pots filled with a commercial potting medium (Pro‐Mix Bx Biofungicide + Mycorrhizae; Premier Tech Horticulture). Although this growth environment differs from the limestone outcrops with which B. perstellata is generally associated, our use of potting mix was informed by our previously successful protocol for growing rare species associated with rock substrates and with consideration of our limited seed supply given the endangered status of this species (see Boyd, Odell, et al., 2022). Using a rich and common potting medium for both B. perstellata and B. laevigata also allowed us to control for the influence of edaphic factors on measured outcomes. Four pots from each parent individual were randomly assigned such that one pot was housed in each of four growth chambers (PGR 15, Conviron Controlled Environments Limited, Winnipeg, Manitoba, Canada). During a 1‐month germination period, all chambers were to set to provide a 12‐h photoperiod at constant 25°C, and we watered pots as needed to maintain moist soil. Following the germination period, we thinned each pot so that it included the single individual that exhibited the earliest third leaf development. These individuals were then transplanted into separate 11‐cm2, 9.5‐cm‐deep (~1.1 L) pots containing the same potting medium to minimize the potential for plants to become root‐bound for the duration of the project (n values reported in Table S2).
2.3. Environmental treatments
Following transplantation, we programmed the four growth chambers to allow us to assess plasticity of B. perstellata and B. laevigata in response to light, temperature, and water as modified from methods previously described by Boyd, Odell, et al. (2022). We generally aimed to impose contrasting levels of these abiotic factors across which plasticity could be tested that were also informed by the general direction and specifics of likely environmental change when possible, although this determination was more apparent for some factors than others. We programmed an “ambient” chamber to be used in all of the plasticity assessments to provide temperature of 20–30°C (nighttime–daytime) based on regional weather records and a 12‐h photoperiod with a maximum daily light level of 250 μmol photons/m2/s in accordance with our field measurements of photosynthetically active radiation (PAR) in B. perstellata habitat during the growing season. The availability of water in B. perstellata habitat is a complex abiotic factor to assess due to the potential for small‐scale temporal variation associated with precipitation events. But given the generally well‐drained substrate associated with B. perstellata, we imposed a level of water stress as a baseline in our plasticity experiments by watering pots in the ambient chamber to 50% field capacity every 2 days as determined by weighing a subset of pots of each species in accordance with previously described methods (see Boyd, Odell, et al., 2022). The other three growth chambers were programmed to provide the same conditions as the “ambient” chamber but each with a contrasting level of a single condition (light, temperature, or water) across which plasticity could be assessed. Specifically, in one chamber, we doubled light availability to a maximum daily level of 500 photons μmol/m2/s to represent a dramatic increase in light, as could be associated with timber harvesting and/or other land‐use change associated with deforestation. In another chamber, we simulated average projections of global temperature increase for this century (Pörtner et al., 2022) by exposing plants to 22–32°C (nighttime–daytime). In the final chamber, we doubled water availability by watering plants to 100% field capacity every 2 days. Projections suggest that precipitation amounts and heavy precipitation events will increase in eastern North America (IPCC, 2023); however, we concede that the effects of such change on water availability will be complicated by edaphic factors and effects on evapotranspiration. As such, while allowing us to test for plasticity, the “real world” translation of our water treatment levels is limited. We grew all individuals in the growth chambers for 4 months following treatment initiation during which time growth data were collected. To minimize any effects of chamber and pseudoreplication (Gibson, 2014), we reassigned treatment levels to each chamber monthly and moved all plants accordingly. Within chambers, we rotated the positions of pots each week to control for spatial differences in environmental conditions.
2.4. Growth, allocation, and leaf morphology measures
We recorded plant height and counted the numbers of leaves of each individual at 4 months after treatment initiation. We then harvested all individuals to yield measures of growth and biomass allocation. We quantified root length (cm) as the distance from the tip of the longest root to the beginning of the green shoot when freshly harvested plants were held upright. The single youngest fully expanded leaf from each individual was removed, scanned to determine its area, and dried in a laboratory oven to calculate specific leaf area (SLA; cm2/g) for each individual. Concurrently, we separated the remains of each harvested plant into shoots and roots, which were also dried to determine shoot dry mass (g), root dry mass (g), and total dry mass (g). We included the dry mass of leaf material removed for SLA measurements and genetic investigations (described in later subsections) in our determinations of shoot dry mass. We calculate mass‐based root‐to‐shoot ratio (RSRmass; g/g) by dividing root dry mass by shoot dry mass, length‐based root‐to‐shoot ratio (RSRlength; cm/cm2) by dividing root length by shoot length, and specific root length (SRL; cm/g) by dividing root length by root dry mass.
2.5. Analyses of survival and trait plasticity
We analyzed the probability of survival to harvest as a function of species, growth chamber treatment, and their interaction in a generalized linear mixed model framework with a binomial distribution and a random effect for source population in the glmer function of the lme4 R package.
To analyze plasticity of growth, allocation, and leaf morphological traits, we fit linear mixed‐effects models using the lmer function of the R package lme4 (Bates et al., 2015) with species, abiotic treatment (i.e., light, temperature, and water), and their interaction as explanatory factors. We included population as a random effect in these models. A significant interaction indicated that B. perstellata and B. laevigata responded differently to a change in the environmental factor (i.e., that the two species exhibited differences in plasticity; see Boyd, Odell, et al., 2022). To account for the numerous traits analyzed to assess plasticity, we used corrected p‐values to minimize the false discovery rate (FDR; Benjamini & Hochberg, 1995). Results of statistical tests were considered significant if FDR‐corrected p ≤ .05. When there was a significant species × abiotic treatment interaction, we contrasted estimated means of each species between abiotic treatment levels with the R package emmeans (ver. 1.8.7).
We used a relative distance plasticity index (RDPI; see Valladares et al., 2006) to calculate plasticity of traits in the context of our light, temperature, and water treatments as previously detailed in these contexts by Boyd, Odell, et al. (2022). The RDPI, which can range from 0 (no plasticity) to 1 (maximum plasticity), is based on the absolute phenotypic distances of 1.8.7 genotypes across different environments and allows for statistical comparison of plasticity for species and populations (Valladares et al., 2006). To assess the potential influence of plasticity on fitness, we conducted across‐environment multivariate genotypic selection analysis (Stinchcombe et al., 2004; Van Kleunen & Fischer, 2001) with the lmer (linear mixed model) function of the R package lme4 (ver. 1.1‐21; Bates et al., 2015). We focused these analyses on traits with significant effects of abiotic factor or abiotic factor × species interactions (i.e., traits for which we found significant evidence for plasticity). We analyzed fitness as a function of mean trait values, RDPI, species, and two‐way interactions between mean trait values and species and RDPI and species in separate models for each manipulated environmental condition (i.e., temperature and water). The average total dry biomass calculated across ambient and manipulated environmental conditions (e.g., ambient and increased temperature) for each maternal line (i.e., mean total biomass) served as the fitness proxy in these regressions because total biomass was measurable for all individuals included in our experiment and is generally associated positively with reproductive output (Weiner et al., 2009). A significant effect of RDPI in a trait on fitness suggests selection for plasticity in both species if the slope is positive and selection against plasticity if the slope is negative. A significant interaction between species and RDPI suggests that the magnitude or direction of selection differs between species. Because we conducted two separate analyses of selection on plasticity, we used a Bonferroni‐corrected α = 0.025 (= 0.05/2) to assess significance and control for type I errors. We used the predictorEffects function of the R package effects (ver. 4.2‐0; Fox & Weisberg, 2018) to visualize selection landscapes from these multiple regression models as partial residual plots.
2.6. DNA extraction and microsatellite genotyping
Leaf tissue was collected from all B. perstellata and B. laevigata individuals included in the growth chamber experiments prior to harvest. Collected leaf tissue was dried on silica gel and ground in a bead mill (MM301; Retsch). DNA was extracted from leaf material with the E.Z.N.A. SP Plant DNA Kit (Omega Bio‐Tek, Inc.). Previously designed microsatellite primers were initially evaluated using touchdown polymerase chain reaction (PCR) analysis (see Baskauf et al., 2014), except for ICE4, F03, and H06, for which constant annealing temperatures of 50, 50, and 55°C, respectively, were used. Fluorescent labeling protocols followed Schuelke (2000) as modified by Baskauf et al. (2014), except that six primers (F03, G03, G06, G08, G09, and H06) had a short tag (GTTTCTT) attached to the 5′ end of the reverse primer. PCR products were multiplexed, and fragment analysis was completed with an ABI 3130XL DNA Analyzer (Applied Biosystems) by the University of Tennessee Health Science Center (Memphis, Tennessee, USA). Individuals were genotyped using GeneMarker v1.97 (SoftGenetics, LLC). Alleles were manually identified and verified by two people.
2.7. Analysis of genetic diversity
In total, 185 B. perstellata and 151 B. laevigata individuals were included in most analyses. We used GenAlEx v6.503 (Peakall & Smouse, 2006, 2012) to calculate standard genetic diversity statistics, including percentage of polymorphic loci (P), alleles per locus (A), observed (H o), and Nei's (1978) unbiased expected heterozygosity (H e). Examination of alleles indicated that the Illinois population (IL) of B. laevigata is likely apomictic and that the Pennsylvania population (PA) of this species experiences high levels of inbreeding. To account for possible apomixis, we used the R package Poppr v4.0.3 (Kamvar et al., 2014), generating a unique multilocus genotype dataset with a clonal threshold of 0 for an analysis of clonal diversity. In addition to the number of unique multilocus genotypes (MLG), clonal diversity measures included the expected number of multilocus genotypes (eMLG), the Shannon–Wiener index of MLG diversity using natural logarithm (H; Shannon, 1948), and Simpson's (1949) index (D). To assess population structure and grouping of individuals in the study, we used a discriminant analysis of principal components (DAPC; Jombart et al., 2010; Machado et al., 2021) with the R package adegenet v2.1.3 (Jombart, 2008). DAPC is a nonparametric multivariate analysis that combines principal component analysis, K‐means clustering, and discriminant analysis and that does not require assumptions of populations being in Hardy–Weinberg equilibrium or the absence of linkage disequilibrium (Alhusain & Hafez, 2018). We used the randomization‐based testing of FSTAT ver. 2.9.4 (Goudet, 1995, 2003) to compare the genetic diversity of B. perstellata and B. laevigata in terms of allelic richness (Rs, a measure of the number of alleles independent of sample size) and sample‐size weighted estimates of observed heterozygosity and expected heterozygosity (H s, “gene diversity”; Nei, 1987) using 1000 permutations. For the FSTAT comparison of the species, a unique‐MLG‐only dataset was used for the presumed apomictic IL population of B. laevigata while full datasets were used for the sexually reproducing populations of both species (as detailed by Lindsay, 2021).
3. RESULTS
3.1. Survival
Across all treatments, 84.5% of B. perstellata individuals and 92% of B. laevigata individuals used in our plasticity experiment survived to harvest (Table S2), but the probability of survival varied as a function of treatment. Specifically, individuals across both species had a significantly lower probability of survival with increased watering than in ambient conditions (Figure S1). In contrast, the probability of survival did not differ between species across treatments. The interaction of species and treatment also did not significantly affect survival. No individuals of either species flowered during the experiment.
3.2. Species growth, allocation, and leaf morphology
There were significant differences in several traits of the rare and widespread Boechera species across levels of abiotic treatments (Table S3). Specifically, in all three comparisons (i.e., ambient vs. increased light, ambient vs. increased temperature, ambient vs. increased water), B. perstellata individuals had more leaves and greater SLA than B. laevigata individuals (Table S4). In the ambient versus increased water comparison only, B. laevigata had greater root mass and RSRmass than B. perstellata individuals across treatment levels (Table S4).
3.3. Phenotypic plasticity
We found no evidence for phenotypic plasticity of either B. perstellata or B. laevigata in response to light availability (see results for main effects of abiotic factor and species × abiotic factor interaction in Table S3). However, there were some differences in the plasticity of root growth and allocation of rare and widespread Boechera species in response to both warming and increased water availability (see results for species × abiotic factor interaction in Table S3). In response to warming, widespread B. laevigata allocated less of its total biomass to roots (i.e., lower RMRmass) but had greater SRL (Figure 2). In contrast, B. perstellata did not exhibit plasticity of these or any other any measured traits in the context of temperature change (Figure 2). In response to increased water availability, individuals of both B. perstellata and B. laevigata produced less root biomass and allocated less of their total biomass to roots (Figure 3). In addition, increased water availability was associated with greater SLA of rare B. perstellata but no significant change in the SLA of B. laevigata (Figure 3). Selection via biomass did not operate significantly on trait plasticity under temperature manipulation (Table S5), but selection did operate significantly on trait plasticity of RSRmass under water manipulation (Table S6). Specifically, there was selection for plasticity of RSRmass (i.e., plasticity of this trait was adaptive) in both Boechera species (Figure S2).
FIGURE 2.
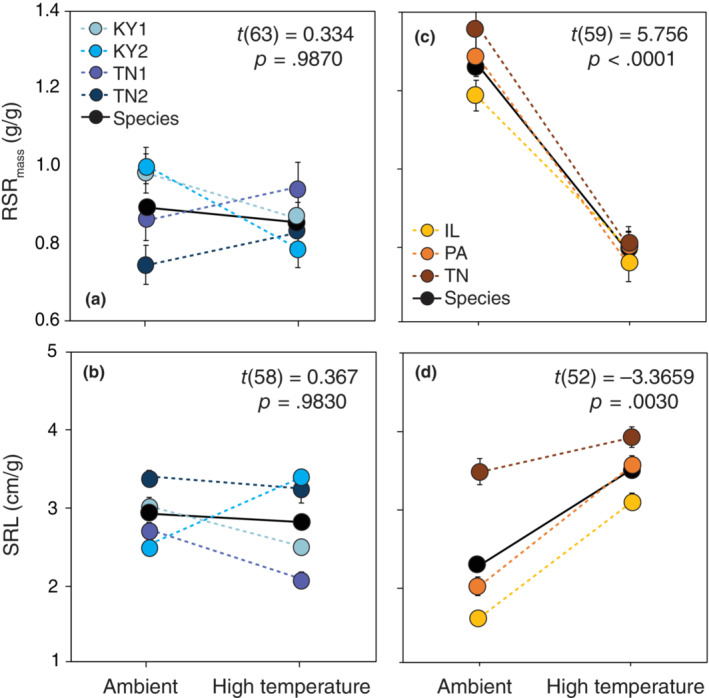
Reaction norms of the means of mass‐based root‐shoot ratio (RSRmass) and specific root length (SRL) of rare Boechera perstellata (a, b) and widespread B. laevigata (c, d) grown in ambient conditions of B. perstellata habitat and with increased temperature. Solid lines and symbols depict species‐level means and norms; dashed lines and colored symbols depict population‐level means and norms. Error bars represent ±1 standard error of the mean; p‐values denote the significance of differences in species means between abiotic treatment levels (i.e., species‐level plasticity).
FIGURE 3.

Reaction norms of the means of root mass, mass‐based root‐shoot ratio (RSRmass), and specific leaf area (SLA) of individuals of rare Boechera perstellata (a–c) and widespread B. laevigata (d– f) grown with water stress and with increased water availability. Solid lines and symbols depict species‐level means and norms; dashed lines and colored symbols depict population‐level means and norms. Error bars represent ±1 standard error of the mean; p values denote the significance of differences in species means between abiotic treatment levels (i.e., species‐level plasticity).
3.4. Genetic diversity
A total of 17 microsatellite loci amplified in all populations for both species and were polymorphic in at least one of the species (Table S7). Standard genetic diversity measures for plants from the growth chamber experiments indicated that, on average, rare B. perstellata is less variable than widespread B. laevigata (Table 1). Across populations, mean alleles per locus and percent polymorphic loci were about twice as high, H e was more than four times as high, and H o was two orders of magnitude higher for B. laevigata than for B. perstellata. However, the sampled populations of B. laevigata were not homogenous in their genetic diversity, with two populations having relatively high diversity but one population exhibiting almost no diversity. As a result, the FSTAT analysis indicated that only H o differed significantly between the rare and widespread Boechera species (p = .0280), while allelic richness and gene diversity (the sample‐size weighted H e) did not vary significantly between species (Table 2). At the species level, the three B. laevigata populations represented in these experiments revealed a total of 86 alleles across all the sampled loci, while the four populations of B. perstellata totaled 40 alleles across the same loci.
TABLE 1.
Measures of genetic diversity of individuals grown in growth chambers from seed collected from four natural populations of rare Boechera perstellata and three populations of widespread B. laevigata.
| Population or species | N | Alleles Per locus | Private alleles | Polymorphic loci | H o | H e |
|---|---|---|---|---|---|---|
| KY1 | 40.0 ± 0.0 | 1.2 ± 0.1 | 1 | 17.7 | 0.001 ± 0.001 | 0.055 ± 0.036 |
| KY2 | 41.0 ± 0.0 | 1.3 ± 0.2 | 3 | 17.7 | 0.003 ± 0.003 | 0.052 ± 0.036 |
| TN1 | 51.9 ± 0.1 | 1.4 ± 0.2 | 12 | 23.5 | 0.003 ± 0.003 | 0.087 ± 0.042 |
| TN2 | 54.9 ± 0.1 | 1.4 ± 0.1 | 4 | 29.4 | 0.002 ± 0.001 | 0.084 ± 0.040 |
| B. perstellata | 47.0 ± 0.8 | 1.3 ± 0.1 | 5 ± 2.4 | 22.1 ± 2.8 | 0.002 ± 0.000 | 0.070 ± 0.019 |
| IL | 60.0 ± 0.0 | 2.3 ± 0.2 | 11 | 82.4 | 0.804 ± 0.094 | 0.430 ± 0.050 |
| PA | 38.0 ± 0.0 | 1.0 ± 0.0 | 1 | 0.0 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| TN | 53.9 ± 0.1 | 3.6 ± 0.7 | 46 | 82.4 | 0.224 ± 0.034 | 0.469 ± 0.061 |
| B. laevigata | 50.6 ± 1.3 | 2.3 ± 0.3 | 19.3 ± 13.6 | 54.9 ± 27.5 | 0.343 ± 0.058 | 0.300 ± 0.040 |
Note: Measures include the number of alleles per locus, number of private alleles, percentage of polymorphic loci, direct count of heterozygosity (H o), and Nei's, 1978 unbiased estimate of mean expected heterozygosity (H e). Values shown are means across 17 microsatellite loci ± standard errors (when applicable). Populations of B. perstellata: Franklin County, Kentucky, USA (KY1, KY2); Rutherford County, Tennessee, USA (TN1); Smith County, Tennessee (TN2). Populations of B. laevigata: Cook County, Illinois, USA (IL); Clarion County, Pennsylvania, USA (PA); Cheatham County, Tennessee, USA (TN).
TABLE 2.
Statistical comparison of several genetic diversity statistics averaged across 17 microsatellite loci for four Boechera perstellata and three Boechera laevigata populations, using FSTAT.
| Statistic | B. perstellata | B. laevigata | p‐Value |
|---|---|---|---|
| Allelic richness (R S ) | 1.223 | 2.071 | .1430 |
| Observed heterozygosity (H o) | 0.003 | 0.186 | .0370 |
| Gene diversity (H s) | 0.073 | 0.289 | .1430 |
Note: For the presumed apomictic population of B. laevigata, only unique MLGs (n = 9) were included in the analysis. R S is the expected number of alleles per locus at the smallest number of sampled individuals at a locus. H o and H s (Nei, 1987) are weighted by sample size.
Clonal diversity analyses revealed that although widespread B. laevigata has a greater number of observed and expected multilocus genotypes across populations than rare B. perstellata, the mean values of diversity indices D and H were lower for the widespread species than for the rare species (Table 3). Once again, clonal diversity statistics differed greatly among populations of widespread B. laevigata. While the TN population of B. laevigata exhibited greater D and H values than any of the populations of rare B. perstellata, the IL and PA populations of B. laevigata had lower values than any of the B. perstellata populations (Table 3). DAPC clustering also revealed greater genetic heterogeneity among populations of widespread B. laevigata relative to populations of rare B. perstellata (Figure S3). Specifically, all but one of the B. perstellata populations clustered together with relatively minor genetic differences, while the three B. laevigata populations were more widely spaced in the analysis output. Each population was its own genetic cluster, except that the relatively high diversity TN population of B. laevigata contained three genetic clusters and TN1 population of B. perstellata contained two clusters.
TABLE 3.
Clonal diversity statistics using a clonal threshold of 0 for individuals grown in growth chambers from seed collected from four natural populations of rare Boechera perstellata and three populations of widespread B. laevigata.
| Population or species | N | Multilocus genotypes | Expected multilocus genotypes | H | D |
|---|---|---|---|---|---|
| KY1 | 40 | 6 | 5.90 | 1.27 | 0.65 |
| KY2 | 41 | 9 | 8.63 | 1.55 | 0.72 |
| TN1 | 52 | 15 | 12.77 | 2.22 | 0.85 |
| TN2 | 55 | 11 | 9.64 | 1.97 | 0.84 |
| B. perstellata | 47 | 10.3 ± 1.89 | 9.2 ± 1.41 | 1.75 ± 0.87 | 0.765 ± 0.05 |
| IL | 60 | 9 | 6.74 | 0.93 | 0.384 |
| PA | 38 | 1 | 1.00 | 0.00 | 0.000 |
| TN | 53 | 52 | 37.49 | 3.94 | 0.999 |
| B. laevigata | 50.3 | 20.7 ± 15.8 | 15.1 ± 11.3 | 1.62 ± 1.19 | 0.461 ± 0.29 |
Note: Statistics include the number of multilocus genotypes, expected number of multilocus genotypes at the least common sample size ≥10 based on rarefaction, Shannon‐Wiener index of multilocus genotype diversity (H), and Simpson's index of diversity (D). Values shown are means across 17 microsatellite loci ± standard errors (when applicable). Populations of B. perstellata: Franklin County, Kentucky, USA (KY1, KY2); Rutherford County, Tennessee, USA (TN1); Smith County, Tennessee (TN2). Populations of B. laevigata: Cook County, Illinois, USA (IL); Clarion County, Pennsylvania, USA (PA); Cheatham County, Tennessee, USA (TN).
4. DISCUSSION
Phenotypic plasticity can influence organismal fitness and species performance by facilitating expansion across diverse abiotic environments and/or persistence in locations experiencing environmental change (Godoy et al., 2012; Nicotra & Davidson, 2010). For rare endemic species, limited phenotypic plasticity could constrain their geographic range and/or habitat specificity, while widespread species with broader habitat breadths could be facilitated by relatively high phenotypic plasticity (Murray et al., 2002). However, our findings suggest that the ability of phenotypic plasticity to elucidate the relative rarity and commonness of B. perstellata and B. laevigata, respectively, is limited. Specifically, we found no evidence of plasticity of any measured traits for either species within the context of light and limited differences in plasticity between species within the context of temperature and water. But we suggest that the differences that we did detect in the plasticity of root traits of rare B. perstellata and widespread B. laevigata in response to those abiotic factors could help to explain differences in their geographic range sizes and habitat associations.
Previous research suggested that temperature plasticity in particular could be especially influential to range size of Boechera species (Lovell & McKay, 2015), although other studies of plasticity of rare and common species within the context of temperature did not reveal similar trends (Hirst et al., 2017). Our significant plasticity findings suggest that widespread B. laevigata may be able to alter its root allocation in response to warming in ways that B. perstellata cannot. The specific responses of B. laevigata to warming revealed by our study could indicate increased investment in longer but thinner roots, which could increase the acquisition of belowground resources when they are limited with less total root biomass investment (see Ostonen et al., 2007). Although we did not measure soil moisture of pots in our high‐temperature treatment level, it is plausible that warming would be associated with reduced soil moisture and that plasticity of root traits could facilitate the distribution of B. laevigata across a broader latitudinal range than the constrained range of B. perstellata. In contrast to their warming responses, root traits of B. perstellata and B. laevigata both responded to changes in water availability in similar directions. Their shared reductions in root biomass and allocation in response to increased watering could indicate a shift away from strategies that would enhance the acquisition of belowground resources when those resources become more plentiful, which could facilitate their association with habitats ranging from fairly dry to moist. We suggest that the stronger plasticity of root traits in response to water availability of B. laevigata could elucidate its association with a wider range of substrates than rare B. perstellata. The only trait for which rare B. perstellata exhibited more plasticity than B. laevigata was SLA in response to increased water availability, but in a direction that contrasts other reported responses of SLA to wetter conditions (see Dwyer et al., 2014; Rosas et al., 2019).
In the face of environmental change, limited plasticity could impede species persistence, especially when migration and/or adaptation are also impeded by low fitness and genetic diversity as is characteristic of many rare species (Boyd, Anderson, et al., 2022). In contrast, phenotypic plasticity could provide a pathway to persistence through change, especially relatively rapid environmental change that may outpace the ability of species to adapt to new conditions (Chevin et al., 2010; Snell‐Rood et al., 2018). Habitat loss due to disturbances such as development and timber harvesting remains an ongoing threat to rare B. perstellata given its small number of extant occurrences (USFWS, 2004). A lack of phenotypic plasticity of B. perstellata in the context of light (which is shared by widespread B. laevigata) could limit its ability to respond to such change. Our findings also suggest that climatic warming could threaten the persistence of B. perstellata given its limited plasticity in response to warming (Figure 3). Although there were some observable differences in plasticity among populations of both Boechera species (Figures 2 and 3), the insignificance of these differences could reflect overall similarities given their shared evolutionary history or be due to relatively small sample sizes within populations that reduced our statistical power to detect population‐level differences compared with differences at the species level.
Importantly, phenotypic plasticity is not always adaptive (Bonser, 2021; Hendry, 2016; Palacio‐López et al., 2015), but research on the adaptive nature of phenotypic plasticity in plant traits has been limited (Arnold et al., 2019; Wei et al., 2021), including within the context of rarity (Boyd, Anderson, et al., 2022). While our selection analyses revealed that plasticity was not always influential to fitness (i.e., plasticity was most neutral), we did find evidence that plasticity in RSRmass across Boechera species within the context of water availability is adaptative. Plasticity in root allocation as a response among plants to changes in the availability of water and other belowground resources (Eriz et al., 2017) is a common example of the optimal partitioning theory (see Bloom et al., 1985). Although there is generally a positive association between vegetative size and reproductive output in plant individuals of the same age and species (Weiner et al., 2009), we concede that the use of total biomass as a fitness proxy in our selection analyses may not have resolved the actual fitness consequences of plasticity. Biomass allocation, in particular, involves trade‐offs in investment toward functions including maintenance, growth, and reproduction (Weiner et al., 2009), and we were unable to resolve the relationship between vegetative and reproductive biomass of Boechera species in our experiments due to time and space constraints associated with the growth chambers. We suggest that future longer‐term research experiments that perhaps utilize garden and/or field settings include assessments of more direct and lifetime fitness consequences of phenotypic plasticity (Anderson et al., 2021; Baythavong et al., 2011; Baythavong & Stanton, 2010; Van Buskirk & Steiner, 2009).
In contrast to our plasticity results, our analysis of the species‐level genetic diversity of rare B. perstellata and widespread B. laevigata largely supported our hypothesis about the comparative genetic diversity of these rare and common species, which aligns with the results of previous comparative syntheses (Boyd, Anderson, et al., 2022; Cole, 2003; Gitzendanner & Soltis, 2000; Leimu & Fischer, 2008). Specifically, there were more than twice as many alleles present across sampled loci for widespread B. laevigata compared with rare B. perstellata even though fewer B. laevigata populations were sampled. However, our hypothesis that the widespread species would have higher levels of genetic diversity was not consistently supported at the population level given that the three sampled B. laevigata populations diverged greatly in genetic diversity, probably because they represent a range of reproductive modes. Nearly every individual of the highly heterozygous B. laevigata population from Illinois shared an identical heterozygote genotype (i.e., fixed heterozygosity) for each polymorphic locus, resulting in a low number of multilocus genotypes and suggesting that this population is engaging in apomixis rather than sexual reproduction. High levels of heterozygosity tend to be associated with apomixis in Boechera species (Beck et al., 2012; Li et al., 2017; Rushworth et al., 2011, 2018) and are thought to be the result of hybridization between genetically distinct lineages (Alexander et al., 2015; Beck et al., 2012; Dobeš et al., 2006; Li et al., 2017; Windham et al., 2015). Although B. laevigata is known to reproduce sexually via both outcrossing and selfing (Bloom, 1988), apomixis has been previously reported in this species (Carman et al., 2019). In contrast to apomictic lineages, sexual Boechera species are usually highly inbred due to selfing and thus have very low levels of heterozygosity (Dobeš et al., 2006; Rushworth et al., 2011, 2018; Windham et al., 2015). The lack of genetic diversity of the Pennsylvania population of B. laevigata suggests that this is likely a sexual but highly inbreeding population that may have gone through one or more severe genetic bottlenecks resulting in the loss of alleles. The relatively high diversity population of B. laevigata from Tennessee also appears to be reproducing sexually because it lacks fixed heterozygosity, and while this population appears to be inbreeding (as its observed heterozygosity levels are much lower than what would be expected for an outcrossing population), its relatively high measures of genetic diversity indicate that it has not experienced the degree of allele loss through random genetic drift that the Pennsylvania population appears to have experienced. Regardless of the diversity measure examined, this population of B. laevigata had much higher genetic diversity than any population of rare B. perstellata. Additionally, the wide range of reproductive modes evidenced for B. laevigata could facilitate its ability adapt to different population situations. For example, it has been suggested that asexual reproduction (e.g., apomixis) might aid in range expansion (Hörandl, 2006; Meloni et al., 2013; Windham et al., 2015).
It has been broadly hypothesized that species that can tolerate a wide range of environmental conditions (i.e., species with broad niche breadths) would be associated with relatively wide geographic range sizes and diverse habitat types (Brown, 1984; Cardillo et al., 2019; Gaston, 2000; Slatyer et al., 2013). Previous research on rare species reported a positive association between phenotypic plasticity (within the context of edaphic factors) and niche breadth (Rutherford et al., 2017), and this association has been more broadly demonstrated with congeneric comparisons beyond the context of rarity species (e.g., Bell & Sultan, 1999; Griffith & Sultan, 2005; Sultan, 2001). While the plasticity of rare endemic B. perstellata in response to water suggests that its fundamental niche may be broader than its current distribution, which could facilitate its persistence in the face of some types of abiotic disturbance, its limited genetic diversity and limited plasticity in the context of temperature could suggest that it is less able to adapt and/or acclimate to the range of temperatures experienced by widespread B. laevigata across its broader range. Although the growth chamber‐based approach of our research allowed for tight controls of environmental conditions across which plasticity could be assessed, future transplant experiments of narrowly distributed rare species such as B. perstellata beyond their natural ranges could be used to further assess their fundamental niche size compared with more common congeners in “real world” conditions. Such transplants combined with replacement and/or additive experiments also could elucidate the potential role of local edaphic factors and biotic factors such as competition in the restriction of B. perstellata to its narrow range and habitat type, especially given the potential for invasive non‐native and prolific native species to encroach into its habitat in response to disturbance (USFWS, 2004). Although our study was focused on just one pair of congeneric species, our experimental approach combined with “real world” transplants could be applied to other rare species toward elucidating broader understanding of species rarity. Such work would need to consider the protected status and imperiled nature of many rare species and integrate careful plans for both research and protection.
AUTHOR CONTRIBUTIONS
Jennifer Nagel Boyd: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (lead); writing – original draft (lead); writing – review and editing (lead). Carol Baskauf: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); supervision (lead); writing – original draft (supporting); writing – review and editing (supporting). Annie Lindsay: Data curation (equal); writing – review and editing (supporting). Jill T. Anderson: Conceptualization (equal); formal analysis (equal); writing – original draft (supporting); writing – review and editing (supporting). Jessica Brzyski: Conceptualization (equal); funding acquisition (equal); writing – review and editing (supporting). Jennifer Cruse‐Sanders: Conceptualization (equal); funding acquisition (equal); writing – original draft (supporting); writing – review and editing (supporting).
OPEN RESEARCH BADGES
This article has earned an Open Data badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://datadryad.org/stash/share/AlNwOP2bbXWggvCmMrnrCl8R3X6pvYjC‐eWOCNl‐ViM.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation Awards 1655762 and 1655521, an Austin Peay State University (APSU) Graduate Research Support Grant, and the APSU Department of Biology. The authors thank J. Odell and W. Rogers for field collections of seeds of B. perstellata and B. laevigata; E. Bach, S. Baskauf, L. Deason, K. England, E. Faulkner, C.D. Fitch, Z. Irick, B. Isaac, J. Isaac, A. Malcom, L. Malcom, E. Raskin, M. Robinson, J. Simons, T. Witsell, and J. Whittle for field collections of B. laevigata leaves and/or seeds; J. Odell for assistance with the growth chamber experiments; C.D. Finch, S. Thrasher, L. Deason, and C. Kelly for assistance with genetics work; G. Call, T. Crabtree, K. McDonald, and T. Littlefield for facilitation of site locations and access for seed collections.
Boyd, J. N. , Baskauf, C. , Lindsay, A. , Anderson, J. T. , Brzyski, J. , & Cruse‐Sanders, J. (2023). Phenotypic plasticity and genetic diversity shed light on endemism of rare Boechera perstellata and its potential vulnerability to climate warming. Ecology and Evolution, 13, e10540. 10.1002/ece3.10540
Jennifer Nagel Boyd and Carol Baskauf contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Dryad repository: https://datadryad.org/stash/share/AlNwOP2bbXWggvCmMrnrCl8R3X6pvYjC‐eWOCNl‐ViM.
REFERENCES
- Alexander, P. J. , Windham, M. D. , Beck, J. B. , Al‐Shehbaz, I. A. , Allphin, L. , & Bailey, C. D. (2015). Weaving a tangled web: Divergent and reticulate speciation in Boechera fendleri sensu lato (Brassicaceae: Boechereae). Systematic Botany, 40, 572–596. [Google Scholar]
- Alhusain, L. , & Hafez, A. M. (2018). Nonparametric approaches for population structure analysis. Human Genomics, 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Shehbaz, I. A. (1988). The genera of Arabideae (Cruciferae; Brassicaceae) in the southeastern United States. Journal of the Arnold Arboretum, 5269, 85–166. [Google Scholar]
- Al‐Shehbaz, I. A. , & Windham, M. D. (2010). Boechera laevigata. In Flora of North America Editorial Committee (Ed.), Flora of North America north of Mexico (Vol. 7, p. 386). Oxford University Press. [Google Scholar]
- Anderson, J. T. , Jameel, M. I. , & Geber, M. A. (2021). Selection favors adaptive plasticity in a long‐term reciprocal transplant experiment. Evolution, 75, 1711–1726. [DOI] [PubMed] [Google Scholar]
- Arnold, P. A. , Nicotra, A. B. , & Kruuk, L. E. B. (2019). Sparse evidence for selection on phenotypic plasticity in response to temperature. Philosophical Transactions of the Royal Society B, 374, 20180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer, I. , Martínez‐Ferri, E. , Valladares, F. , Pérez‐Corona, F. , Baquédano, F. J. , Castillo, F. J. , & Manrique, E. (2001). Population divergence in the plasticity of the response of Quercus coccifera to the light environment. Functional Ecology, 15, 124–135. [Google Scholar]
- Baskauf, C. J. , Jinks, N. C. , Mandel, J. R. , & McCauley, D. E. (2014). Population genetics of Braun's Rockcress (Boechera perstellata, Brassicaceae), and endangered plant with a disjunct distribution. Journal of Heredity, 104, 124–137. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Baythavong, B. S. , Agrawal, A. , & McPeek, M. A. (2011). Linking the spatial scale of environmental variation and the evolution of phenotypic plasticity: Selection favors adaptive plasticity in fine‐grained environments. American Naturalist, 178, 75–87. [DOI] [PubMed] [Google Scholar]
- Baythavong, B. S. , & Stanton, M. L. (2010). Characterizing selection on phenotypic plasticity in response to natural environmental heterogeneity. Evolution, 64, 2904–2920. [DOI] [PubMed] [Google Scholar]
- Beck, J. B. , Alexander, P. J. , Allphin, L. , Al‐Shehbaz, I. A. , Rushworth, C. , Bailey, C. D. , & Windham, M. D. (2012). Does hybridization drive the transition to asexuality in diploid Boechera? Evolution, 66, 985–995. [DOI] [PubMed] [Google Scholar]
- Bell, D. L. , & Sultan, S. E. (1999). Dynamic phenotypic plasticity for root growth in Polygonum: A comparative study. American Journal of Botany, 86, 807–819. [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. [Google Scholar]
- Bevill, R. L. , & Louda, S. M. (1999). Comparisons of related rare and common species in the study of plant rarity. Conservation Biology, 13, 493–498. [Google Scholar]
- Bloom, A. J. , Chapin, F. S. , & Mooney, H. A. (1985). Resource limitation in plants – An economic analogy. Annual Review of Ecology and Systematics, 16, 363–392. [Google Scholar]
- Bloom, T. C. (1988). The life history of Arabis laevigata var. laevigata (Muhl.) Poir (Brassicaceae) (M.S. thesis). University of Kentucky. [Google Scholar]
- Bloom, T. C. , Baskin, J. M. , & Baskin, C. C. (2002). Ecological life history of the facultative woodland biennial Arabis laevigata variety laevigata (Brassicaceae): Survivorship. Journal of the Torrey Botanical Society, 128, 93–108. [Google Scholar]
- Bloom, T. C. , Baskin, J. M. , & Baskin, C. C. (2003). Ecological life history of the facultative woodland biennial Arabis laevigata (Brassicaceae): Effects of leaf litter cover, herbivory, and substrate‐type on bolting and fecundity. Journal of the Torrey Botanical Society, 130, 12–22. [Google Scholar]
- Bonser, S. P. (2021). Misinterpreting the adaptive value of phenotypic plasticity in studies on plant adaptation to new and variable environments. Plant Biology, 23, 683–685. [DOI] [PubMed] [Google Scholar]
- Boyd, J. N. , Anderson, J. T. , Brzyski, J. , Baskauf, C. , & Cruse‐Sanders, J. (2022). Eco‐ evolutionary causes and consequences of rarity in plants: A meta‐analysis. New Phytologist, 235, 1272–1286. [DOI] [PubMed] [Google Scholar]
- Boyd, J. N. , Odell, J. , Cruse‐Sanders, J. , Rogers, W. , Anderson, J. T. , Baskauf, C. , & Brzyski, J. (2022). Phenotypic plasticity and genetic diversity elucidate rarity and vulnerability of endangered riparian plant. Ecosphere, 13, e3996. [Google Scholar]
- Braun, E. L. (1956). Growth habits of Arabis perstellata . Rhodora, 58, 292–295. [Google Scholar]
- Brown, J. H. (1984). On the relationship between abundance and distribution of species. American Naturalist, 124, 255–279. [Google Scholar]
- Cardillo, M. , Dinnage, R. , & McAlister, W. (2019). The relationship between environmental niche breadth and geographic range size across plant species. Journal of Biogeography, 46, 97–109. [Google Scholar]
- Carman, J. G. , Mateo de Arias, M. , Gao, L. , Zhao, X. , Kowallis, B. M. , Sherwood, D. A. , Srivastava, M. K. , Dwivedi, K. K. , Price, B. J. , Watts, L. , & Windham, M. D. (2019). Apospory and diplospory in diploid Boechera (Brassicaceae) may facilitate speciation by recombination‐driven apomixis‐to‐sex reversals. Frontiers in Plant Science, 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, I.‐C. , Hill, J. K. , Ohlemueller, R. , Roy, D. B. , & Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science, 333, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Chevin, L.‐M. , Lande, R. , & Mace, G. M. (2010). Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biology, 8, e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleavitt, N. L. (2002). Stress tolerance of rare and common moss species in relation to their occupied environments and asexual dispersal potential. Journal of Ecology, 90, 785–795. [Google Scholar]
- Cole, C. T. (2003). Genetic variation in rare and common plants. Annual Review of Ecology, Evolution, and Systematics, 34, 213–237. [Google Scholar]
- Combs, J. K. , Lambert, A. M. , & Reichard, S. H. (2013). Predispersal seed predation is higher in a rare species than its widespread sympatric congeners (Astragalus, Fabaceae). American Journal of Botany, 100, 2149–2157. [DOI] [PubMed] [Google Scholar]
- Crickenberger, S. , & Wetheym, D. S. (2018). Annual temperature variation as a time machine to understand to effects of long‐term climate change on a poleward range shift. Global Change Biology, 24, 3804–3819. [DOI] [PubMed] [Google Scholar]
- Dangremond, E. M. , Feller, I. C. , & Sousa, W. P. (2015). Environmental tolerances of rare and common mangroves along light and salinity gradients. Oecologia, 179, 1187–1198. [DOI] [PubMed] [Google Scholar]
- Dee, L. E. , Cowles, J. , Isbell, F. , Pau, S. , Gaines, S. D. , & Reich, P. (2019). When do ecosystem services depend on rare species? Trends in Ecology and Evolution, 34, 746–758. [DOI] [PubMed] [Google Scholar]
- Dobeš, C. , Koch, M. , & Sharbel, T. F. (2006). Embryology, karyology, and modes of reproduction in the North American genus Boechera (Brassicaceae): A compilation of seven decades of research. Annals of the Missouri Botanical Garden, 93, 517–534. [Google Scholar]
- Dwyer, J. M. , Hobbs, R. J. , & Mayfield, M. M. (2014). Specific leaf area responses to environmental gradients through space and time. Ecology, 95, 399–410. [DOI] [PubMed] [Google Scholar]
- Eriz, A. , Yan, Z. , Tian, D. , Han, W. , Tang, Z. , & Fang, J. (2017). Drought effect on plant biomass allocation: A meta‐analysis. Ecology and Evolution, 24, 11002–11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth, E. J. (2006). Plant life history traits of rare versus frequent plant taxa of sandplains: Implications for research and management trials. Biological Conservation, 136, 44–52. [Google Scholar]
- Fox, J. , & Weisberg, S. (2018). Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. Journal of Statistical Software, 87, 1–27. [Google Scholar]
- Gaston, K. J. (2000). Global patterns in biodiversity. Nature, 405, 220–227. [DOI] [PubMed] [Google Scholar]
- Gibson, D. J. (2014). Methods in comparative plant ecology. Oxford University Press. [Google Scholar]
- Gitzendanner, M. A. , & Soltis, P. S. (2000). Patterns of genetic variation in rare and widespread plant congeners. American Journal Botany, 87, 783–792. [PubMed] [Google Scholar]
- Godoy, O. , Valladares, F. , & Castro‐Díez, P. (2012). The relative importance for plant invasiveness of trait means, and their plasticity and integration in a multivariate framework. New Phytologist, 195, 912–922. [DOI] [PubMed] [Google Scholar]
- Godt, M. J. , & Hamrick, W. J. L. (2001). Genetic diversity in rare southeastern plants. Natural Areas Journal, 21, 61–70. [Google Scholar]
- Goudet, J. (1995). FSTAT (version 1.2): A computer program to calculate F‐statistics. Journal of Heredity, 86, 485–486. [Google Scholar]
- Goudet, J. (2003). FSTAT (ver. 2.9.4), a program to estimate and test population genetics parameters. Computer program and documentation distributed by the author . http://www.t‐de‐meeus.fr/Programs/Fstat294.zip
- Griffith, T. M. , & Sultan, S. E. (2005). Shade tolerance plasticity in response to neutral vs. green shade cues in Polygonum species of contrasting ecological breadth. New Phytologist, 166, 141–148. [DOI] [PubMed] [Google Scholar]
- Hamann, E. , Pauli, C. , Joly‐Lopez, Z. , Groen, S. C. , Rests, J. S. , Kane, N. C. , Purugganan, M. D. , & Franks, S. J. (2020). Rapid evolutionary changes in gene expression in response to climate fluctuations. Molecular Ecology, 30, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry, A. P. (2016). Key questions on the role of phenotypic plasticity in ecoevolutionary dynamics. Journal of Heredity, 107, 25–41. [DOI] [PubMed] [Google Scholar]
- Hickling, R. , Roy, D. B. , Hill, J. K. , Fox, R. , & Thomas, C. D. (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology, 12, 450–455. [Google Scholar]
- Hirst, M. J. , Griffin, P. C. , Sexton, J. P. , & Hoffmann, A. A. (2017). Testing the niche‐breadth– Range size hypothesis: Habitat specialization vs. performance in Australian alpine daisies. Ecology, 98, 2708–2724. [DOI] [PubMed] [Google Scholar]
- Hörandl, E. (2006). The complex causality of geographical parthenogenesis. New Phytologist, 171, 525–538. [DOI] [PubMed] [Google Scholar]
- IPCC . (2023). Climate change 2023: Synthesis report. A report of the intergovernmental panel on climate change. In Core Writing Team , Lee H., & Romero J. (Eds.), Contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change. IPCC. [Google Scholar]
- Iverson, L. R. , Schwartz, M. W. , & Prasad, A. M. (2004). How fast and far might tree species migrate in the eastern United States due to climate change? Global Ecology and Biogeography, 13, 209–219. [Google Scholar]
- Jombart, T. (2008). Adegenet: An R package for the multivariate analysis of genetic markers. Bioinformatics, 24, 1403–1405. [DOI] [PubMed] [Google Scholar]
- Jombart, T. , Devillard, S. , & Balloux, F. (2010). Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genetics, 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump, A. S. , & Peñuelas, J. (2005). Running to stand still: Adaptation and the response of plants to rapid climate change. Ecology Letters, 8, 1010–1020. [DOI] [PubMed] [Google Scholar]
- Kamvar, Z. N. , Tabima, J. F. , & Grünwald, N. J. (2014). Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ, 4, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer, C. , Dobeš, C. , & Koch, M. A. (2009). Boechera or not? Phylogeny and phylogeography of eastern North American Boechera species (Brassicaceae). Taxon, 58, 1109–1121. [Google Scholar]
- Knapp, W. M. , Frances, A. , Noss, R. , Naczi, R. F. C. , Weakley, A. , Gann, G. D. , Baldwin, B. G. , Miller, J. , McIntyre, P. , Mishler, B. D. , Moore, G. , Olmstead, R. G. , Strong, A. , Kennedy, K. , Heidel, B. , & Gluesenkamp, D. (2021). Vascular plant extinction in the continental United States and Canada. Conservation Biology, 35, 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin, W. E. , & Gaston, K. (1997). The biology of rarity: Causes and consequences of rare‐ common differences. Springer. [Google Scholar]
- Leimu, R. , & Fischer, M. (2008). A meta‐analysis of local adaptation in plants. PLoS One, 3, e4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. W. , Rushworth, C. A. , Beck, J. B. , & Windham, M. D. (2017). Boechera microsatellite website: An online portal for species identification and determination of hybrid parentage. Database, 2017, baw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, J.‐X. , Zou, X.‐Y. , Ge, Y. , & Chang, J. (2006). Effects of light intensity on growth of four Mosla species. Botanical Studies, 47, 403–408. [Google Scholar]
- Lindsay, A. (2021). Population genetics of the widespread Boechera laevigata (Brassicaceae) and comparisons with its rare congener B. perstellata (M.S. thesis). Austin Peay State University. [Google Scholar]
- Lovell, J. T. , & McKay, J. K. (2015). Ecological genetics of range size variation in Boechera spp. (Brassicaceae). Ecology and Evolution, 5, 4962–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, R. M. , de Oliveira, F. A. , de Matos Alves, F. , de Souza, A. P. , & Forni‐Martins, E. R. (2021). Population genetics of polyploid complex Psidium cattleyanum Sabin (Myrtaceae): Preliminary analyses based on new species‐specific microsatellite loci and extension to other species of the genus. Biochemical Genetics, 59, 219–234. [DOI] [PubMed] [Google Scholar]
- Malhi, Y. , Franklin, J. , Seddon, N. , Solan, M. , Turner, M. G. , Field, C. B. , & Knowlton, N. (2020). Climate change and ecosystems: Threats, opportunities and solutions. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 375, 20190104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R. M. (1988). How many species are there on earth? Science, 214, 1441–1449. [DOI] [PubMed] [Google Scholar]
- Meloni, M. , Reid, A. , Caujapé‐Castells, J. , Marrero, Á. , Fernández‐Palacios, J. M. , Mesa‐ Coelo, R. A. , & Conti, E. (2013). Effects of clonality on the genetic variability of rare, insular species: The case of Ruta microcarpa from the Canary Islands. Ecology and Evolution, 3, 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillot, D. , Bellwood, D. R. , Baraloto, C. , Chave, J. , Galzin, R. , Harmelin‐Vivien, M. , Kulbicki, M. , Lavergne, S. , Lavorel, S. , Mouquet, N. , Paine, C. E. T. , Renaud, J. , & Thuiller, W. (2013). Rare species support vulnerable functions in high diversity ecosystems. PLoS Biology, 11, e1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, B. R. , Thrall, P. H. , Gill, M. , & Nicotra, A. (2002). How plant life‐history and ecological traits relate to species rarity and commonness at varying spatial scales. Austral Ecology, 27, 291–310. [Google Scholar]
- NatureServe . (2005). NatureServe explorer, Borodinia perstellata, Braun's Rockcress . https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.130490/Borodinia_perstellata
- Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M. (1987). Molecular evolutionary genetics. Columbia University Press. [Google Scholar]
- Nicotra, A. , & Davidson, A. (2010). Adaptive phenotypic plasticity and plant water use. Functional Plant Biology, 37, 117–127. [Google Scholar]
- Nicotra, A. B. , Atkin, O. K. , Bonser, S. P. , Davidson, A. M. , Finnegan, E. J. , Mathesius, U. , Poot, P. , Purugganan, M. D. , Richards, C. L. , Valladares, F. , & van Kleunen, M. (2010). Plant phenotypic plasticity in a changing climate. Trends in Plant Science, 15, 684–692. [DOI] [PubMed] [Google Scholar]
- Ostonen, I. , Püttsepp, Ü. , Biel, C. , Alberton, O. , Bakker, M. R. , Lõhmus, K. , Majdi, H. , Metcalfe, D. , Olsthoorm, A. F. M. , Pronk, A. , Vanguelova, E. , Weih, M. , & Brunner, I. (2007). Specific root length as an indicator of environmental change. Plant Biosystems – An International Journal Dealing with all Aspects of Plant Biology, 141, 426–442. [Google Scholar]
- Osunkoya, O. O. , & Swanborough, P. W. (2001). Reproductive and ecophysiological attributes of the rare Gardenia actinocarpa (Rubiaceae) compared with its common co‐occurring congener, G. ovularis . Austalian Journal of Botany, 49, 471–478. [Google Scholar]
- Palacio‐López, K. , Beckage, B. , Scheiner, S. , & Molofsky, J. (2015). The ubiquity of phenotypic plasticity in plants: A synthesis. Ecology and Evolution, 5, 3389–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics, 37, 637–669. [Google Scholar]
- Peakall, R. , & Smouse, P. E. (2006). GENALEX 6: Genetic analysis in excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall, R. , & Smouse, P. E. (2012). GenAlEx 6.5: Genetic analysis in excel. Population genetic software for teaching and research‐ an update. Bioinformatics, 28, 2537–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman, C. L. , Nicotra, A. B. , & Murray, B. R. (2005). Geographic range size, seedling ecophysiology and phenotypic plasticity in Australian Acacia species. Journal of Biogeography, 2005(32), 341–351. [Google Scholar]
- Pörtner, H.‐O. , Roberts, D. C. , Tignor, M. , Poloczanska, E. S. , Mintenbeck, K. , Alegría, K. A. , Craig, M. , Langsdorf, S. , Löschke, S. , Möller, V. , Okem, A. , & Rama, B. (Eds.). (2022). Climate change 2022: Impacts, adaptation and vulnerability, contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press. [Google Scholar]
- Rabinowitz, D. (1981). Seven forms of rarity. In Synge H. (Ed.), The biological aspects of rare plant conservation (pp. 205–217). John Wiley & Sons, Hoboken. [Google Scholar]
- Rosas, T. , Mencuccini, M. , Barba, J. , Cochard, H. , Saura‐Mas, S. , & Martínez‐Vilata, J. (2019). Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytologist, 223, 632–646. [DOI] [PubMed] [Google Scholar]
- Rushworth, C. A. , Song, B. H. , Lee, C. R. , & Mitchell‐Olds, T. (2011). Boechera, a model system for ecological genomics. Molecular Ecology, 20, 4843–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth, C. A. , Windham, M. D. , Keith, R. A. , & Mitchell‐Olds, T. (2018). Ecological differentiation facilitates fine‐scale coexistence of sexual and asexual Boechera . American Journal of Botany, 105, 2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, S. , Bonser, S. P. , Wilson, P. G. , & Rossetto, M. (2017). Seedling response to environmental variability: The relationship between phenotypic plasticity and evolutionary history in closely related Eucalyptus species. American Journal of Botany, 104, 840–857. [DOI] [PubMed] [Google Scholar]
- Schranz, M. E. , Windsor, A. J. , Song, B. H. , Lawton‐Rauh, A. , & Mitchell‐Olds, T. (2007). Comparative genetic mapping in Boechera stricta, a close relative of Arabidopsis. Plant Physiology, 144, 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuelke, M. (2000). An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology, 18, 233–234. [DOI] [PubMed] [Google Scholar]
- Seebacher, F. , White, C. R. , & Franklin, C. E. (2015). Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Climate Change, 56, 61–66. [Google Scholar]
- Shannon, C. E. (1948). A mathematical theory of communication. Bell System Technical Journal, 37, 379–423. [Google Scholar]
- Sheth, S. N. , Kulbaba, M. W. , Pain, R. E. , & Shaw, R. G. (2018). Expression of additive genetic variance for fitness in a population of partridge pea in two field sites. Evolution, 72, 2537–2545. [DOI] [PubMed] [Google Scholar]
- Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 88. [Google Scholar]
- Slatyer, R. A. , Hirst, M. , & Sexton, J. P. (2013). Niche breadth predicts geographical range size: A general ecological pattern. Ecology Letters, 16, 1104–1114. [DOI] [PubMed] [Google Scholar]
- Snell‐Rood, E. , Kobiela, M. E. , Sikkink, K. L. , & Shephard, A. M. (2018). Mechanisms of plastic rescue in novel environments. Annual Review of Ecology, Evolution, and Systematics, 49, 331–354. [Google Scholar]
- Stamp, M. A. , & Hatfield, J. D. (2020). The relative importance of plasticity versus genetic differentiation in explaining between‐population differences; a meta‐analysis. Ecology Letters, 23, 1432–1441. [DOI] [PubMed] [Google Scholar]
- Stinchcombe, J. R. , Dorn, L. A. , & Schmitt, J. (2004). Flowering time plasticity in Arabidopsis thaliana: A reanalysis of Westerman & Lawrence (1970). Journal of Evolutionary Biology, 17, 197–207. [DOI] [PubMed] [Google Scholar]
- Stotz, G. C. , Salgado‐Luarte, C. , Escobedo, V. M. , Valladares, F. , & Gianoli, E. (2021). Global trends in phenotypic plasticity of plants. Ecology Letters, 24, 2267–2281. [DOI] [PubMed] [Google Scholar]
- Sultan, S. E. (2000). Phenotypic plasticity for plant development, function and life history. Trends in Plant Science, 5, 537–542. [DOI] [PubMed] [Google Scholar]
- Sultan, S. E. (2001). Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology, 82, 328–343. [Google Scholar]
- United States Fish and Wildlife Service (USFWS) . (1995). Endangered and threatened wildlife and plants; determination of endangered status for Arabis perstellata . Federal Register, 60, 56–61. [Google Scholar]
- United States Fish and Wildlife Service (USFWS) . (1997). Recovery plan for Arabis perstellata (Braun's Rockcress). USFWS. [Google Scholar]
- United States Fish and Wildlife Service (USFWS) . (2004). Endangered and threatened wildlife and plants; designation of critical habitat for Arabis perstellata (Braun's rock‐cress). Federal Register, 69, 31459–31496. [Google Scholar]
- United States Fish and Wildlife Service (USFWS) . (2018). Braun's Rockcress (Arabis perstellata) 5‐year review: Summary and evaluation. USFWS. [Google Scholar]
- Valladares, F. , Gianoli, E. , & Gómez, J. M. (2007). Ecological limits to plant phenotypic plasticity. New Phytologist, 176, 749–763. [DOI] [PubMed] [Google Scholar]
- Valladares, F. , Wright, S. J. , Lasso, E. , Kitajima, K. , & Pearcy, R. W. (2000). Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology, 81, 1925–1936. [Google Scholar]
- Valladares, F. A. , Sanchez‐Gomez, D. , & Zavala, M. A. (2006). Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology, 94, 1103–1116. [Google Scholar]
- Van Buskirk, J. , & Steiner, U. K. (2009). The fitness costs of developmental canalization and plasticity. Journal of Evolutionary Biology, 22, 852–860. [DOI] [PubMed] [Google Scholar]
- Van Kleunen, M. , & Fischer, M. (2001). Adaptive evolution of plastic foraging responses in a clonal plant. Ecology, 82, 3309–3319. [Google Scholar]
- Wei, G.‐W. , Chen, Y.‐H. , Sun, X.‐S. , Matsubara, S. , Luo, F.‐L. , & Yu, F.‐H. (2021). Elevation‐ dependent selection for plasticity in leaf and root traits of Polygonum hydropiper in response to flooding. Environmental and Experimental Botany, 182, 104331. [Google Scholar]
- Weiner, J. , Campbell, L. G. , Pino, J. , & Echarte, L. (2009). The allometry of reproduction within plant populations. Journal of Ecology, 97, 1220–1233. [Google Scholar]
- Windham, M. D. , Beck, J. B. , Li, F. W. , Allphin, L. , Carman, J. G. , Sherwood, D. A. , Rushworth, C. A. , Sigel, E. , Alexander, P. J. , Bailey, C. D. , & al‐Shehbaz, I. A. (2015). Searching for diamonds in the apomictic rough: A case study involving Boechera lignifera (Brassicaceae). Systematic Botany, 40, 1031–1044. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Data Availability Statement
The data that support the findings of this study are available in the Dryad repository: https://datadryad.org/stash/share/AlNwOP2bbXWggvCmMrnrCl8R3X6pvYjC‐eWOCNl‐ViM.


