Abstract
Purpose:
Myelofibrosis (MF) is a clonal myeloproliferative neoplasm characterized by systemic symptoms, cytopenias, organomegaly, and bone marrow fibrosis. JAK2 inhibitors afford symptom and spleen burden reduction but do not alter the disease course and frequently lead to thrombocytopenia. TGFβ, a pleiotropic cytokine elaborated by the MF clone, negatively regulates normal hematopoiesis, downregulates antitumor immunity, and promotes bone marrow fibrosis. Our group previously showed that AVID200, a potent and selective TGFβ 1/3 trap, reduced TGFβ1-induced proliferation of human mesenchymal stromal cells, phosphorylation of SMAD2, and collagen expression. Moreover, treatment of MF mononuclear cells with AVID200 led to increased numbers of progenitor cells (PC) with wild-type JAK2 rather than JAK2V617F.
Patients and Methods:
We conducted an investigator-initiated, multicenter, phase Ib trial of AVID200 monotherapy in 21 patients with advanced MF.
Results:
No dose-limiting toxicity was identified at the three dose levels tested, and grade 3/4 anemia and thrombocytopenia occurred in 28.6% and 19.0% of treated patients, respectively. After six cycles of therapy, two patients attained a clinical benefit by IWG-MRT criteria. Spleen and symptom benefits were observed across treatment cycles. Unlike other MF-directed therapies, increases in platelet counts were noted in 81% of treated patients with three patients achieving normalization. Treatment with AVID200 resulted in potent suppression of plasma TGFβ1 levels and pSMAD2 in MF cells.
Conclusions:
AVID200 is a well-tolerated, rational, therapeutic agent for the treatment of patients with MF and should be evaluated further in patients with thrombocytopenic MF in combination with agents that target aberrant MF intracellular signaling pathways.
Translational Relevance.
Increased levels of TGFβ1 have been implicated as a pivotal mediator of myelofibrosis (MF) disease activity. AVID200, a TGFβ 1/3 protein trap was evaluated in a phase Ib trial in 21 patients with advanced MF. Dose-limiting toxicities were not observed. Two patients attained clinical benefit by IWG/MRT criteria at 24 weeks. Spleen and symptom responses were observed across treatment cycles. Remarkably, platelet counts increased in 81% of treated patients and normalized in three patients, a therapeutic effect not observed with other MF therapies. AVID200 resulted in suppression of plasma TGFβ1 levels and MF mononuclear cell p-pSMAD2 levels. All patients had megakaryocytes (MK) with reduced levels of GATA1, a marker of impaired MK maturation, that was reversed in MK from patients with increased platelet numbers following therapy. These data indicate that AVID200 treatment promotes MK maturation and platelet production in MF by interrupting the effects of TGFβ1.
Introduction
Myelofibrosis (MF) is a progressive myeloproliferative neoplasm (MPN), resulting in diminished quality of life and limited survival due to the consequences of symptomatic splenomegaly, systemic symptoms, ineffective hematopoiesis, bone marrow fibrosis, and eventual evolution to acute myeloid leukemia (AML; ref. 1). MF is associated with increased JAK/STAT signaling due to driver mutations in JAK2 (JAK2V617F), calreticulin (CALR), and the thrombopoietin receptor (MPL). A risk-adapted treatment approach to MF is currently considered the standard of care, which is centered on alleviating spleen size and systemic symptom burdens with JAK2 inhibitors that downregulate the JAK/STAT signaling pathways, which promote cell proliferation and inflammatory cytokine expression (2). Although these agents have collectively improved the quality of life of patients with MF, they frequently exacerbate disease-related anemia and thrombocytopenia, which limit dose optimization and consequently compromise the possibility to achieve bone marrow histopathologic remissions or halt disease progression (3). Allogeneic hematopoietic cell transplantation, which is used in approximately 10% of patients with advanced forms of MF, is currently the only modality of therapy that provides patients with a chance for long-term maintenance-free survival (4).
A major hallmark of MF is a marked elevation in proinflammatory cytokines (5). These cytokines are elaborated by the malignant clone and modify the bone marrow microenvironment, thereby promoting malignant hematopoiesis. TGFβ is a pleiotropic cytokine implicated in the promotion of angiogenesis, tumor growth, collagen fibrosis, metastatic spread, and downregulation of antitumor immunity. TGFβ levels have been shown by numerous investigators to be increased in patients with MF and to play a central role in the development of progressive bone marrow fibrosis, which contributes to a dysregulated marrow microenvironment (6, 7). Active forms of TGFβ1 bind to a membrane receptor serine/threonine kinase complex that phosphorylates receptor SMADs (SMAD2 and SMAD3), which accumulate in the nucleus where they complex with SMAD4 to regulate target gene expression (8). TGFβ can also signal through noncanonical pathways, including ERK, p38 MAPK, and JNK activation (9, 10). TGFβ1 and TGFβ3 have additional effects, including their ability to inhibit normal hematopoiesis through the canonical SMAD-dependent signaling pathway, which induces normal HSC quiescence (11–13). In MF, the excessive production of TGFβ has largely been attributed to its increased production by megakaryocytes (14) and monocytes (15). TGFβ also negatively regulates the proliferation of hematopoietic stem/progenitor cells (12, 16). Although megakaryocytic hyperplasia is a pathologic hallmark of MF, thrombocytopenia frequently accompanies advanced forms of MF, which compromises the use of JAK2 inhibitors and contributes to an increased risk of developing bleeding events (17). Up to 25% of patients with MF have platelet counts below 100 × 109/L at presentation. Patients with thrombocytopenia are more likely to be anemic and transfusion-dependent, as well as have high-risk disease characteristics and a poor overall survival rate. This myelofibrosis presentation has also been described as myelodepletive phenotype (18).
TGFβ has been thought to play a role in the development of MF-associated thrombocytopenia due to its well-known inhibitory effects on several stages of megakaryocyte development (19, 20). Because of the multiple pivotal roles of TGFβ in the development of MF, we considered this cytokine as a potential target for therapeutic intervention (21). Therefore, we explored the use of AVID200, a potent and highly specific trap for two isoforms of TGFβ, TGFβ1, and β3 with an IC50s of ∼5 pmol/L to treat patients with MF (22). We previously reported that TGFβ1 was the predominant TGFβ isoform elaborated by MF megakaryocytes (21). AVID200 notably does not affect TGFβ2 levels, which is produced by cardiomyocytes and acts autonomously on myocardium and via paracrine signaling on AV cushions, which are required for heart development (23). Although cardiac toxicity was not observed in the participants of several clinical trials with galunisertib, a small-molecule pan-TGFβ inhibitor that acts by inhibiting signaling through TGFβ receptor I. This concern led to the interruption of further clinical development of this agent (24–30). The inability of AVID200 to affect TGFβ2 levels provided a means of avoiding the potential for cardiac toxicity, therefore, making it a promising approach to interrupt TGFβ signaling in patients with MF. A phosphorylated form of SMAD2 (pSMAD2) has been used to explore endogenous activation of TGFβ signaling in normal donor or MF cells. Robust expression of pSMAD2 has been previously observed in the absence of an exogenous source of TGFβ, and addition of AVID200 to MF-MK has been reported to decrease pSMAD2 levels without affecting total SMAD2/SMAD3 levels, indicating that AVID200 blocks the effects of TGFβ produced in a paracrine/autocrine fashion in MF (16).
The possible effectiveness of AVID200 was also demonstrated by its ability in vitro to reduce TGFβ1 levels, reduce TGFβ1-induced proliferation of human mesenchymal stromal cells, decrease phosphorylation of SMAD2, and to induce collagen expression (22). Moreover, treatment of MF mononuclear cells with AVID200 led to the appearance of increased numbers of assayable progenitor cells with wild-type JAK2 rather than mutated JAK2. To evaluate the toxicity and possible efficacy of AVID200 in patients with MF, we conducted a first-in-human phase Ib study of AVID200 at three dose levels.
Patients and Methods
This investigator-initiated clinical trial (NCT03895112) was sponsored by the NCI Myeloproliferative Neoplasm Research Consortium (MPN-RC) and was conducted in strict accordance with the Principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Institutional ethical review board approval of the protocol was required at each participating institution, and written informed consent was obtained from all patients prior to screening. J.M. held the investigational new drug application.
Enrolled subjects had a confirmed diagnosis of primary MF (PMF) or postessential thrombocythemia/polycythemia vera MF using WHO criteria. Subjects were required to have intermediate-2, or high-risk disease according to the IWG-MRT Dynamic International Prognostic Scoring System (DIPSS; ref. 31) and at least grade 2 bone marrow fibrosis (BMF) as quantitated using the European Consensus on Grading of Bone Marrow Fibrosis (32). Subjects were not eligible for therapy with the JAK2 inhibitor ruxolitinib, had been previously treated and experienced a lack or loss of response on ruxolitinib as determined by the investigator, or chose not to receive ruxolitinib. Baseline platelet counts of ≥25 × 109/L were required.
Cohorts of three patients were followed for at least one cycle and evaluated for dose-limiting toxicity (DLT). A modified toxicity probability interval (mTPI) dose-escalation design (33) was used with three dose levels and a target toxicity rate of 30%. Following conclusion of the dose escalation, dose levels 2 and 3 were expanded to include a total of nine patients at each dose level. Subjects received intravenous AVID200 (lots A and B) in dose cohorts of 180 mg (A), 550 mg (A), 180 mg (B) on day 1 of a 21-day cycle (Supplementary Fig. S1). Upon exhaustion of the first lot and completion of additional pharmacokinetic (PK) studies, subjects enrolled in dose level 3 and the expansion phase were treated with lot B of AVID200. All subjects treated with lot B received a lower calculated dose to match the exposure of the original doses administered with lot A. Therefore, subjects enrolled in the dose-escalation phase received 70 mg (B) and 180 mg (B) for dose levels 2 and 3. Additional information regarding lot B dose selection based on estimated exposure in humans is provided in the Supplementary materials.
AVID200 was infused on day 1 of a 21-day cycle. Patients who completed six cycles and maintained at least stable disease (SD) by International Working Group/European Leukemia Net (IWG/ELN) criteria (34) with at least one grade reduction in bone marrow fibrosis were allowed to continue treatment with repeat response assessment after cycle 12. After six cycles, if the subject did not attain the minimum response, they were removed from treatment.
The primary objective was to estimate the MTD of AVID200 treatment in MF. Secondary objectives included characterization of the safety of AVID200 and estimation of response by IWG/ELN criteria and change in bone marrow fibrosis after cycles 6 and 12. Exploratory objectives included evaluation of quality of life (QOL) and patient-reported symptoms using the Myelofibrosis Symptom Assessment Form (MF-SAF) version 4.0 and European Organisation for Research and Treatment of Cancer Quality of Life questionnaire-C30 (EORTC QLQ-C30) and to conduct correlative studies to measure biomarkers of TGFβ activation or inhibition and explore biomarkers of AVID200 therapeutic response. PK profiling was also performed for lot A of study drug.
Toxicity was assessed according to the NCI Common Toxicity Criteria for Adverse Events (CTCAE), version 5.0. DLTs were defined as an absolute neutrophil count <500/mm3 for ≥7 days, a platelet count <20 × 109/L with a documented hemorrhagic event, or other nonhematologic adverse events of grade ≥ 3 occurring in cycle 1. Criteria for AVID200 dose modifications after cycle 1 for hematologic and nonhematologic Treatment Emergent Adverse Events (TEAE) regardless of causality were specified in the protocol.
Response assessment was the primary objective of the dose-expansion phase and was based on the proportion of treated subjects that attained either a complete response (CR), partial response (PR), or clinical improvement (CI) as defined by the revised IWG/ELN (34), or a decrease in bone marrow fibrosis by ≥1 grade with otherwise stable disease (SD) after cycle 6.
Pharmacokinetic data were available for the first six patients treated [three at DL1 (180 mg/m2) and DL2 (550 mg/m2) using lot A of study drug]. PK time points per the protocol were prior to infusion, end of infusion, +2 hours, +4 hours, +24 hours (day 2), day 8, and day 15 for cycle 1. PK data are presented as μg/mL. The R package PKNCA was used to perform noncompartmental analysis for PK data (R version 4.1.2).
Plasma TGFβ1 levels measured at baseline and after AVID200 treatment were quantified by MesoScale Discovery (MSD) U-PLEX Platform (N05228A-1 Rockville). Briefly, stored plasma was thawed, and the levels of TGFβ1 were determined according to the manufacturer's instructions by an electro-chemiluminescent signal. MSD assays work on the same principle as ELISA assays but have a higher sensitivity, less matrix interference, and high consistency between runs (35, 36).
Levels of pSMAD2 were determined with a commercial Elisa (catalog No. MBS 269933, M BioSource Life Technology; ref. 37) or by Western blot analyses with pSMAD2 and SMAD2 antibodies. Activation of p38-MAPK was determined by comparing levels of pp38 and total p38 in blood mononuclear cells as assessed by Western blotting (38) at baseline and weekly during the first cycle and then day 1 of every cycle for the first six cycles. Canonical and noncanonical TGFβ molecular signaling targets pSMAD2 and pp38 were measured in mononuclear cells at baseline and at the end of AVID200 treatment by Western blotting. Whole cell extracts were prepared in NP40 buffer plus proteases and phosphatase inhibitors (Roche). Fifty micrograms of total proteins was separated on SDS-PAGE gels and transferred to nitrocellulose membranes. Transferred proteins were detected with primary antibodies pSMAD2 (No. 3108); SMAD2/SMAD3 (No. 3102), pp38 (No. 4511), pERK1/2 (No. 9101; Cell Signaling Technology); GAPDH (No. CB1001, Calbiochem); and then with appropriate horseradish peroxidase–coupled secondary antibodies (Calbiochem). Immune complexes were detected with an Enhanced Chemiluminescence Kit (Amersham) or LUM-LIGHTPlus (Roche).
qPCR was used to assess the variant allele frequency (VAF) of the patient's MPN driver mutation at baseline and day 1 of cycles 7 and 13. Results were compared with baseline for determination of absolute and relative decrease in VAF of the driver mutation in granulocytes. Changes in histomorphologic features in the bone marrow after six and 12 cycles were also assessed by central blinded pathology review.
Next-generation sequencing, using targeted panels that encompass 167 genes associated with hematologic malignancies, was utilized on samples from baseline and response timepoints or end of treatment (whichever occurred later). Sequencing was performed using an Illumina ILLUMINA-HISEQ-4000 with 2×101-bp paired-end reads, yielding an average depth of ∼500×. The sequencing reads were then aligned to the human genome (hg19) using the WA-MEM algorithm (v. 1–14–0; ref. 39), and their quality was evaluated using FastQC. Variant calling was conducted using an in-house pipeline that incorporates three variant callers (CAVEMAN (1.7.4; ref. 40), Mutect (4.0.1.2; ref. 41), Strelka (2.9.1; ref. 42), and PINDEL (1.5.4; refs. 41, 43). All variants were annotated using the Ensembl Variant Effect Predictor (VEP, version 86; ref. 44) and OncoKb (45). A subset of the candidate variants, specifically those that were called by at least two callers or matched known somatic mutations, was selected for further manual annotation. The variants reported in this study were identified as pathogenic or likely pathogenic based on the outcome of the manual annotation process.
In addition, sections of paraffin-embedded bone marrow biopsies from three patients who experienced increases in platelet numbers (platelet-responsive) and three platelet-unresponsive patients at baseline and at the end of therapy (matched) were provided as deidentified material (Supplementary Table S1). Bone marrow sections from untreated patients with PMF, ET, and from patients with hematopoietic malignancies but without fibrosis provided were analyzed in parallel as control. The 3-μm sections were incubated for 1 hour with a decalcifying solution (Osteodec; Bio-Optica) and subjected to treatment with EDTA buffer pH 8 for 20 minutes in a pressure cooker (110–120°C, high pressure) for antigen retrieval. The sections were then sequentially incubated with appropriate primary rabbit monoclonal anti-CD42b (Ab183345, 1:150, Abcam) and rat monoclonal anti-GATA1 (SC-265; 1:100, Santa Cruz Biotechnology) antibodies for 1.5 hours, respectively, at room temperature. The binding of the antibodies was revealed by incubation with secondary Alexa Fluor 488 and/or Alexa Fluor 568-conjugated donkey anti-rabbit and anti-rat antibodies (Invitrogen, both 1:200) for 1 hour. The sections were observed blindly by two independent investigators with the Nikon A1 Confocal laser Microscope System (Nikon). Fluorescent images were acquired with the Imaging Software NIS-Elements (Nikon) and processed with the Fiji software (NIH). Events were quantified by examination of images acquired at 60× from at least 10 randomly selected areas per section. Results are presented as mean (±SD) of at least three separate patients per group. Results obtained at baseline and after posttreatment were analyzed by paired t test and differences considered statistically significant with a P < 0.05. Results from controls were compared by ANOVA for multiple comparisons.
Data Availability
The data generated in this study are not publicly available but are available upon reasonable request from the corresponding author.
Results
Twenty-two subjects were enrolled (one withdrew before receiving treatment) and nine patients (three at each dose level) were treated with AVID200 during the dose-escalation phase and 12 during the dose expansion phase (Table 1). After discontinuing ruxolitinib, patients had a median time to start AVID200 therapy of 6.0 months (range, 0.5–59.9), and 90.5% of patients had previously received ruxolitinib treatment. Supplementary Table S2 specifies all the treatments administered prior to the initiation of AVID200 and the time from discontinuation (when known) to C1D1 of AVID20O in each case. According to DIPSS classification, patients with intermediate-2 risk and high-risk disease accounted for 61.9% and 38.1%, respectively. Severe bone marrow fibrosis was present in 17/21 (81%) of patients. An abnormal karyotype was present in 50% of patients. Baseline somatic mutational profiles of the 21 subjects enrolled in the study demonstrated 71% with JAK2, 29% TET2, 24% ASXL1, and 19% CALR mutations (Supplementary Fig. S2). The median TSS at baseline was 14 (range, 3–39). Representativeness of study participants is described in Supplementary Table S3.
Table 1.
Patient demographics and clinical characteristics by study phase.
| Dose escalation (n = 9) | Dose expansion (n = 12) | Total (n = 21) | |
|---|---|---|---|
| Age (y) | 69 (51–81) | 74 (51–85) | 73 (51–85) |
| Sex, F | 2 (22.2%) | 3 (25.0%) | 5 (23.8%) |
| Prior ruxolitinib | 8 (88.9%) | 11 (91.7%) | 19 (90.5%) |
| DIPSS | |||
| Int-2 | 6 (66.7%) | 7 (58.3%) | 13 (61.9%) |
| High | 3 (33.3%) | 5 (41.7%) | 8 (38.1%) |
| JAK2V617 | 6 (66.7%) | 9 (75.0%) | 15 (71.4%) |
| VAF% | 55 (5–84) | 47 (21–96) | 47 (5–96) |
| CALR | 3 (33.3%) | 1 (8.3%) | 4 (19.0%) |
| Spleen length cm | 10 (0–19) | 11 (0–24) | 10 (0–24) |
| Platelet count × 109/L | 114 (42–290) | 107 (27–695) | 114 (27–695) |
| Platelet count <100 × 109/L | 4 (44.4%) | 6 (50.0%) | 10 (47.6%) |
| Platelet count <50 × 109/L | 1 (11.1%) | 3 (25.0%) | 4 (19.0%) |
| RBC transfusion dependent | 5 (55.6%) | 9 (75.0%) | 14 (66.7%) |
| PB blasts % | 1 (0–21) | 3 (0–8) | 2 (0–21) |
| BMF grade | |||
| Mild | — | 1 (8.3%) | 1 (4.8%) |
| Moderate | — | 3 (25.0%) | 3 (14.3%) |
| Severe | 9 (100%) | 8 (66.7%) | 17 (81.0%) |
| TSS | 10.5 (3–26) | 16.5 (3–39) | 14.0 (3–39) |
| Normal karyotype | 4 (44.4%) | 6 (54.5%) | 10 (50.0%)a |
| Abnormal karyotype | 5 (55.6%) | 5 (45.5%) | 10 (50.0%)a |
| Adverse Karyotype | 0 | 3 (27.3%) | 3 (15%)a |
Note: Median (range) or n (%) values are presented.
Abbreviations: DIPSS, dynamic international prognostic score; PB, peripheral blood; RBC, red blood cell; VAF, variant allele frequency.
aBaseline karyotype available in 20/21 patients (escalation cohort: 9, expansion cohort: 11).
No DLTs were observed during the dose-escalation phase, and therefore the dose-expansion phase was conducted for both dose levels 2 and 3 with six patients at each dose level. Across all cycles, grade 3 or higher adverse events were observed in 16/21 (76.2%) patients. Grade 3/4 nonhematologic AE were observed in nine (42.9%) subjects and included one subject in each case with epistaxis, mucositis, extraocular muscle paresis, fatigue, rash, duodenal hemorrhage, gastric hemorrhage, urinary tract infection, and syncope. Grade 3/4 hematologic AE were anemia (six, 28.6%) and thrombocytopenia (four, 19.0%) (Table 2). No fatal events were seen in this trial. No cardiac adverse events were observed.
Table 2.
Adverse events across all dose levels and cycles (regardless of attribution) occurring in more than 10% of patients or grade 3 or higher events.
| Adverse event term | Grade 1–2, n (%) | Grade 3–4, n (%) | All grades, n (%) | |
|---|---|---|---|---|
| Hematologic | Anemia | 1 | 6 | 7 (33%) |
| Platelet count decreased | 3 | 4 | 7 (33%) | |
| Neutrophil count decreased | 3 | 3 (14%) | ||
| White blood cell decreased | 2 | 2 (10%) | ||
| Leukocytosis | 1 | 1 | 2 (10%) | |
| Nonhematologic | Pruritus | 9 | 9 (43%) | |
| Fatigue | 7 | 1 | 8 (38%) | |
| Abdominal pain | 7 | 7 (33%) | ||
| Diarrhea | 6 | 6 (29%) | ||
| Edema limbs | 5 | 5 (24%) | ||
| Nausea | 5 | 5 (24%) | ||
| Epistaxis | 4 | 1 | 5 (24%) | |
| Hyperuricemia | 5 | 5 (24%) | ||
| Gastrointestinal disorders | 2 | 2 | 4 (19%) | |
| Creatinine increased | 4 | 4 (19%) | ||
| AST increased | 3 | 3 (14%) | ||
| Pain | 4 | 4 (19%) | ||
| Dizziness | 4 | 4 (19%) | ||
| Cough | 3 | 3 (14%) | ||
| Dyspnea | 3 | 3 (14%) | ||
| Hypomagnesmia | 3 | 3 (14%) | ||
| Hypocalcemia | 2 | 1 | 3 (14%) | |
| Headache | 3 | 3 (14%) | ||
| Muscle cramps | 3 | 3 (14%) | ||
| Anorexia | 3 | 3 (14%) | ||
| Hyponatremia | 3 | 3 (14%) | ||
| Vomiting | 3 | 3 (14%) | ||
| Palpitations | 3 | 3 (14%) | ||
| Rash maculopapular | 2 | 1 | 3 (14%) | |
| Mucositis | 2 | 1 | 3 (14%) | |
| Urinary tract infection | 1 | 1 | 2 (10%) | |
| Duodenal hemorrhage | 1 | 1 (5%) | ||
| Gastric hemorrhage | 1 | 1 (5%) | ||
| Extraocular muscle paresis | 1 | 1 (5%) | ||
| Syncope | 1 | 1 (5%) |
The median number of cycles administered was six (range, 1–22), and eight (38.1%) patients received more than six cycles. For dose levels 2 to 3 at cycle 7, two patients attained CI [TSS: one, spleen/anemia/TSS: one], five patients had SD, four had progressive disease (PD), and seven were not evaluable (Supplementary Fig. S3). Four patients received 12 or more cycles of treatment. Following cycle 12, three of the four attained CI (TSS: two, spleen/anemia/TSS: one). One patient (dose level 1) was not evaluable due to proceeding with HSCT after four cycles of AVID200. Reasons for discontinuation included disease progression (n = 9), lack of response (n = 5), study completion (n = 4), other (n = 2), patient decision (n = 1).
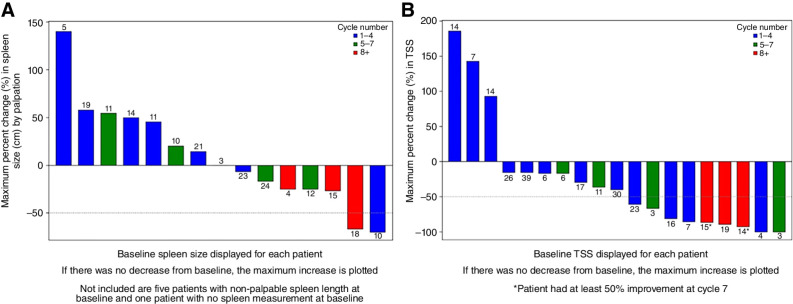
Median percent change in palpable spleen length was 0.0% (range, −70% to +140%), and TSS reduction was 40% across all cycles of treatment (−100% to +185.7%), (Figs. 1A and B). Two (13.3%) of the 15 patients with baseline palpable splenomegaly attained 50% reduction in spleen length. Five (33.3%) patients attained a 25% reduction in palpable spleen length at any time during the study treatment. Nine (47.4%) patients achieved >50% decrease in TSS across all cycles.
Figure 1.
Change in spleen length and total symptom score with AVID200 therapy. A, Greatest percent change from baseline to post-baseline in spleen length for patients with palpable spleen at baseline. Cycle number where the greatest change occurred is shown in the legend. Baseline spleen size is displayed numerically for each patient above the change bar. B, Greatest percentage change from baseline to post-baseline in total symptom score (TSS). Cycle number where the greatest change occurred is shown in the legend. Baseline TSS is displayed numerically for each patient above the change bar.
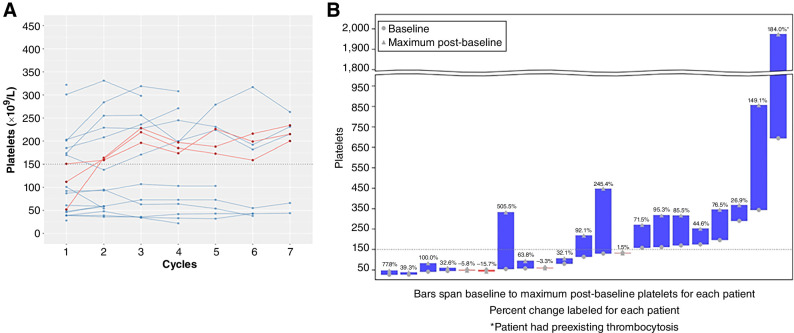
The median platelet count at baseline was 114 × 109/L (range, 27–695) and 215 × 109/L (range, 44–263) after cycle 6 in eight subjects (Fig. 2A). The median maximum change in platelets from baseline across all cycles was +71.5% (range, −15.7% to 505.5%) in all subjects (Fig. 2B). Seventeen subjects had an increase in platelets during treatment. Three subjects normalized their platelet counts. In these patients, the time from ruxolitinib discontinuation to initiation of AVID200 was 1, 16, and 28 months. Prior ruxolitinib was received by 19 of 21 patients (Table 1) before trial enrollment, and median time since discontinuation prior to AVID200 treatment was 6 months (range, 1–60).
Figure 2.
Change in platelet count with AVID200 therapy. A, Longitudinal plot by cycle for platelet count for cycles 1 to 7. Patients with platelets ≤150 × 109/L at baseline who later normalized are displayed in red. B, Best percent change from baseline to post-baseline platelets for each patient. Baseline and maximum post-baseline platelet value is displayed on the y-axis, and percentage increase is shown above each bar.
Comparison of somatic mutation profile of samples available from baseline and at cycle 7 demonstrated a median change in JAK2V617F VAF of +0.83% (n = 5 cases) and a median change in CALR VAF of +3% (n = 4 cases). No new somatic mutations were identified during the course of treatment (Supplementary Fig. S4).
Paired BM biopsy samples for 16 subjects were available for central histopathologic review and failed to show a reduction in bone marrow fibrosis, overall cellularity, or MK number. Consistent with previous reports, CD42bpos MK from BM of patients with MF showed barely detectable levels of GATA1 (Supplementary Fig. S5). BM sections from both platelet nonresponder and responder patients at baseline showed increased numbers of CD42bpos cells, which barely expressed detectable levels of GATA1 (Fig. 3A; Supplementary Fig. S6). After treatment, GATA1 expression level was significantly decreased in the platelet nonresponder group but was strongly positive in the CD42bpos cells from patients that experienced an increase in platelet numbers (Fig. 3B).
Figure 3.
AVID200 increases GATA1 content and maturation state of the megakaryocytes in the bone marrow of patients who had an increase in platelet numbers. A, Merged confocal microscopy analysis for CD42b (red), GATA1 (green), and Hoechst (blue) of representative fields from the bone marrow of one platelet nonresponder and one platelet responder MF patient at baseline and posttreatment, as indicated. The CD42b-positive cells indicated by rectangles were also acquired in zoom mode, and the images are presented on the right. Megakaryocytes with immature or mature morphology are indicated as iMK and mMK, respectively. Plt indicates the presence of platelets. The presence or absence of green (GATA1) signal in the nucleus is indicated with a + or a –. Signals acquired in the individual red, green, and blue channels are shown in Supplementary Fig. S6A, for clarity. Similar results were obtained with two additional patients per group (see the additional images presented in Supplementary Figs. S6B and S6C). B, Frequency of CD42b-positive cells, of CD42b-positive cells with the morphology of mature megakaryocytes, of CD42b-positive cells containing some GATA1 or strongly positive for GATA1 in the bone marrow sections from nonresponder and responder patients at baseline and posttreatment, as indicated. In B, results are presented as mean (±SD) and per individual patient (each symbol represents a patient). The degree of statistical significance is indicated within the quadrant.
Elevated TGFβ1 levels were detected (mean, 6,433 pg/mL) in the plasma isolated from the trial participants prior to treatment and were significantly decreased post-AVID200 treatment (P < 0.05; Supplementary Fig. S7). TGFβ1 levels were not significantly different at baseline (P = 0.12) and after treatment in nonresponder and responder groups (P = 0.25; Supplementary Fig. S8).
We next examined the effects of AVID200 on TGFβ downstream signaling events (canonical and noncanonical pathways) in MNC isolated from four patients at baseline and after AVID200 treatment. According to platelet response to AVID200 therapy, MF cells isolated from those four patients were identified as nonresponders (n = 2) or responders (n = 2). Four patients from those groups were analyzed for TGFβ1 levels (Supplementary Fig. S8) and TGFβ downstream targets (Fig. 4). All samples expressed similar levels of pSMAD2 at baseline, whereas following AVD200 treatment, pSMAD2 protein levels, a canonical TGFβ downstream signaling event, were decreased (Fig. 4A and B).
Figure 4.
Western blotting analyses of pSMAD2, SMAD2/SMAD3, pp38, and pERK1/2 in patients with MF treated with AVID200. A, Total cell extracts isolated from MF mononuclear cells (MNC) from patients who responded to AVID200 with platelet count elevations and nonresponders who failed to experience an increase in platelet numbers were examined by Western blotting at baseline and after AVID200 treatment as indicated. GAPDH was used as loading control. B, Relative expression of pSMAD2 on the corresponding total SMAD2/3 previously normalized toward GAPDH. pp38 and pERK1/2 were normalized toward GAPDH by Image studio Lite 5.2.
MEK–ERK pathways have been identified as contributing to JAK2 inhibitor resistance (46, 47); TGFβ is known to activate MEK/ERK signaling. TGFβ has been shown to also activate SRC signaling in myofibroblasts (48), and overactivation of ERK1/2 causes a reduction in pro-platelet formation by human MK (49). In MNCs from both responsive and nonresponsive patients, pp38 and pERK1/2 MAP kinase, noncanonical TGFβ downstream signaling events, were phosphorylated to a greater extent at baseline than pSMAD2, but these levels were not affected by AVID200 treatment (Fig. 4A and B).
PK data for dose levels 1 and 2 (lot A) were consistent with known characteristics of the study drug (Supplementary Table S4). Maximum concentration was achieved within the first few hours with a median half-life of 65.3 and 77.7 hours for the two dose levels, respectively (Supplementary Fig. S9).
Discussion
The results of this investigator-initiated multicenter phase 1b study of AVID200 in MF demonstrated both safety and tolerability at the three doses levels tested. No deaths were attributable to AVID200 therapy. Pharmacodynamic studies indicated that AVID200 was effective in interrupting TGFβ signaling. Elevated TGFβ plasma levels were reduced following AVID200 therapy and pSMAD2 levels, which are downstream of canonical TGFβ signaling were also reduced in MF MNC indicating the on-target effects of this agent.
In this high-risk patient population, clinical responses with single-agent AVID200 therapy were observed. Two of the 12 subjects treated in the dose-expansion phase at the two highest doses attained a CI by IWG/MRT criteria after six cycles of therapy. Improvements seen across all cycles of treatment included spleen size reduction of at least 50% in two patients with large baseline spleen sizes. Due to financial constraints, spleen volume assessment by imaging was not feasible. Nine patients reported a ≥50% reduction in symptom burden, which occurred within four cycles in four of the treated subjects. Notably three of the four patients who received up to 12 cycles of therapy attained a CI, which suggested that TGF-β inhibitor monotherapy might require a longer duration of treatment to elicit clinical responses in this advanced disease setting. These responses, however, were not uniform in that 67% of patients discontinued participation in the trial due to lack of response or disease progression, and none of the patients attained any measurable reduction in their driver mutation VAF.
The therapies that are currently approved for treating advanced phase MF are frequently associated with progressive thrombocytopenia, which could be drug induced or a consequence of the underlying disease. Remarkably, AVID200 therapy was associated with clinically significant and durable improvements in platelet counts. Although platelet increases were observed across baseline platelet counts, the greatest platelet response with AVID200 treatment was observed in patients with baseline platelet counts >150 × 109/L, and there were no obvious clinical or laboratory features including TGFβ level that were associated with this observation. This profound effect on thrombopoiesis could not be attributed to a rebound effect from prior ruxolitinib therapy since most patients had a substantial drug holiday prior to receiving AVID200. Patients generally did not experience worsening platelet counts with more prolonged administration of this agent. Currently, only pacritinib (CTI Bio) a JAK2/IRAK1 inhibitor is approved for patients with MF and a platelet count <50 × 109/L based on phase 3 clinical data demonstrating clinical activity in the form of spleen and symptom benefit without significant worsening of platelet level (50). However, this agent is not associated with normalization of the platelet count, which usually remains suppressed despite an anemia response rate of 25%. It is important to emphasize that the present IWG/MRT criteria that are universally used to assess responses to MF-directed therapies do not include improvement in platelet numbers. Because these response criteria were used in the present study, the true potential clinical utility of AVID200 may be underestimated due to the limitations of these response criteria.
TGFβ is a pleiotropic cytokine, which has long been known to be produced by many tissues but the elevated levels in MF are largely due to its increased production by MK and monocytes. TGFβ is a known inhibitor of megakaryopoiesis reducing the numbers and ploidy of MK and acting at the MK progenitor cell level as well as impairing maturation of differentiated MK (12). TGFβ1 is also known to modulate thrombopoietin-mediated effects on megakaryocytic proliferation by interfering with thrombopoietin-induced signal transduction, particularly by reducing the activities of MAPK, ERK1/ERK2, and STAT5 (38). The effect of AVID200 on thrombopoiesis was, therefore, not unanticipated. The increase of GATA1 staining in the MK of patients who experienced a substantial increase in platelet numbers is likely due to the reversal of the effect of TGFβ1 on MK maturation. Whether the reduction of TGFβ1 signaling associated with AVID200 treatment led to greater numbers of MK progenitor cells or improved thrombopoietin signaling remains the subject of speculation since sufficient cells were not available to address these alternative mechanisms. However, our group has demonstrated that in vitro treatment of MF MK with AVID200 also reduced pSMAD2 levels and led to an increase in MK numbers (22).
Our group previously evaluated fresolimumab (GC1008, Sanofi-Aventis) a human immunoglobulin G4 (IgG4) kappa mAb that neutralizes TGFβ1, 2, and 3 (51). This phase I single-institution trial in patients with intermediate/high-risk MF was terminated early due to corporate decision and only enrolled three patients without objective spleen or symptom response after a total of 12 cycles of fresolimumab therapy in two patients. Despite potent suppression of elevated baseline levels of TGFβ1, a reduction in bone marrow fibrosis was not observed. Durable transfusion independence was attained in one patient.
TGFβ has also been demonstrated to exert immunosuppressive effects on a variety of immune cells including dendritic cells, tumor-associated macrophages and neutrophils, natural killer cells myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg), and cytotoxic T cells (52). In a phase I dose-escalation study of AVID200 in solid malignancies, on-target suppression of TGFβ and pSMAD signaling was accompanied by biomarker evidence of immune activation (53). Increasing evidence suggests cooperation between TGFβ and PD-1 immunosuppressive pathways in cancer. TGFβ can compromise PD-1 inhibitor resistance through CD14+ monocyte commitment and expansion of MDSC that also promote Treg phenotype (54, 55). Dual blockade of TGFβ and PD-1 in a murine model of fibroblast-associated immune-excluded phenotype, resulted in increased cytotoxic T-cell infiltration and tumor regression and provides rationale for this therapeutic strategy. This concept has translated into the clinic with multiple therapeutic approaches, the most mature of which is the first-in-class bifunctional fusion protein, bintrafusp alfa, composed of the extracellular domain of the TGFβ receptor II fused to a human immunoglobulin G1 antibody blocking PD-L1 (56, 57).
The TGFβ receptor type 1 kinase (ALK5) inhibitor, galunisertib (Eli Lily), has also been evaluated in a multicenter trial of 41 patients with lower-risk myelodysplastic syndrome (MDS; ref. 26). Hematologic improvement erythroid response (Hi-E) was observed in 24.4% of treated patients, and one patient with baseline thrombocytopenia (<100 × 109/L) attained a platelet increase of >30 × 109/L for >8 weeks on study. Notably, three of 11 patients with baseline bone marrow fibrosis had a documented reduction in this biomarker. This drug has not been evaluated in patients with MF due to lack of accessibility of the agent from the manufacturer.
TGFβ1 is also a potent inducer of collagen synthesis and has been universally implicated in pathologic fibrosis observed in MF patient BM. Our group has previously demonstrated treatment of mesenchymal stromal cells with AVID200 reduced their proliferation, levels of pSMAD2 and collagen expression (22). It is not surprising that the administration of AVID200 to patients with MF as a single agent did not result in reduction in BM fibrosis within a 6-month period. This lack of effect of a TGFβ1 trap on MF BM fibrosis may be due to the relatively short time the drug was administered and the possibility, which additional cytokines including PDGF, fibroblast growth factor, IL8, and lipocalin 2 also contribute to the development of BMF in MF (58).
An alternative explanation for the lack of effect of AVID200 on BMF was provided by a report by Ishikawa working in our laboratory and the recent report of Yao and colleagues (59, 60). We reported that marrow cells from patients with MF expressed a noncanonical TGFβ signature, which included activation of ID1, c-Jun N-terminal kinase (JUN), GADD45b, and genes with binding motifs for the JUN transcriptional complex AP1. Subsequently, Yao and colleagues using a mouse model of MF reported that SMAD4-independent TGFβ signaling mediated the bone marrow fibrosis but was not required for the disruption of hematopoietic niches in MF. Specifically, the loss of TGFβ signaling in mesenchymal stem cells did not prevent the suppression of key marrow niche factors, including KIT ligand and CXCL12, or prevent the development of extramedullary hematopoiesis or reduction in the numbers of bone marrow hematopoietic stem cells. Furthermore, treatment with a JNK inhibitor prevented the development of the MF phenotype suggesting that the signals that regulate niche gene expression in bone marrow mesenchymal stem cells were distinct from those that induce the fibrosis program. These authors, however, did not explore whether JNK inhibitors were capable of reversing the total MF phenotype after it had been established. These data suggest that the degree of depletion of TGFβ levels achieved with intermittent AVID200 therapy might not be sufficient to diminish JNK-dependent TGFβ signaling to the degree needed to reverse bone marrow fibrosis and its hematologic sequelae beyond the observed increase in platelet numbers. These reports provide a rationale for combining AVID200 with a JNK inhibitor to treat patients with MF. We are currently exploring the availability of tolerable JNK inhibitors such as CC-90001, which is a selective JNK inhibitor, 12.9-fold more potent for JNK1 inhibition, and associated with acceptable safety in patients with pulmonary fibrosis to treat patients with MF in combination with AVID200 (61–63).
AVID200 monotherapy in a population of patients with an advanced stage of MF resulted in limited toxicity as well as improvements in spleen size, symptom benefit, and platelet counts. We did not identify evidence of improvement in BM fibrosis as assessed by histomorphologic assessment; however, longer follow-up for bone marrow evaluation was not performed in most of the treated patients. The improvement in platelet counts following AVID200 treatment provides a distinct advantage, as the currently approved therapeutic approaches and those undergoing clinical development in MF are universally associated with treatment-related thrombocytopenia. We propose that future trials of AVID200 should include patients at an earlier stage of their disease to determine if AVID200 is capable of preventing the progression of bone marrow fibrosis. We also suggest that administration of AVID200 be combined with other agents that target noncanonical TGFβ pathways or other MF dysregulated intracellular signaling pathways that are currently not employed due to preexisting MF-associated thrombocytopenia.
Supplementary Material
MPN-RC 118 phase 1b study schema
Baseline mutational profile of enrolled patients.
Duration of treatment and response status.
Oncoprint Analysis of Serial Samples from Phase 1b Trial of AVID200
The bone marrow from patients with MF contain more CD42pos cells than patients with ET or with hematopoietic malignancies without fibrosis (normal control).
GATA1 expression in megakaryocytes from patients treated with AVID200
Effects of AVID200 therapy on plasma TGFβ levels.
AVID200 induced similar decrements in the plasma levels of TGF-β in the three patients with and without platelet response
PK data for Cycle 1 (first 24 hours only) for A) 180 mg/m2 dose level and B) 550 mg/m2 dose level during dose escalation phase I.
Clinical info on the platelets-responsive and non-responsive patients
Patient listing of prior myelofibrosis treatment and time since discontinuation if known
Representativeness of Study Participants.
Individual patient data for AUC, Cmax and Tmax for dose levels 1 and 2 (lot A). Summary measures for DL 1 and DL2 are shown in red.
Acknowledgments
This trial was supported by the PO1 grant entitled Myeloproliferative Neoplasms Research Consortium (MPN-RC) 5P01CA108671–14 and in part by funding through the Cancer Center Support Grants (CCSG) for NCI-designated Cancer Centers to Tisch Cancer Institute 5P30CA196521–07. We would like to acknowledge all the patients and their caregivers for participating in this clinical trial.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J. Mascarenhas reports grants and personal fees from Celgene/BMS during the conduct of the study; grants and personal fees from Incyte, Geron, AbbVie, CTI Bio, Karyopharm, Kartos, GSK, and PharmaEssentia; personal fees from Morphosys, Pfizer, Imago, Roche, and Merck; and grants from Galecto outside the submitted work. A. Migliaccio reports grants and other support from Dompè Farmaceutics outside the submitted work. J.M. Palmer reports other support from CTI, Morphosys, Incyte, and Pharmessentia outside the submitted work. A. Kuykendall reports personal fees from Incyte, AbbVie, Novartis, Imago, Geron, and CTI Biopharma; and grants and personal fees from MorphoSys, GSK, Protagonist, and BMS outside the submitted work. R.A. Mesa reports personal fees from Incyte, BMS, Novartis, CTI, Telios, Geron, GSK, and Sierra; and grants and personal fees from Morphosys outside the submitted work. R.K. Rampal reports grants and personal fees from Incyte, Stemline, Morphosys, and Zentalis; personal fees from Galecto, Pharmessentia, BMS, Blueprint, AbbVie, Cogent, Sumitomo Dainippon, Kartos, Servier, and Karyopharm; CTI fees from Sierra Oncology/GSK; and grants from Ryvu outside the submitted work. A. Gerds reports personal fees from CTI Biopharma, Incyte, GlaxoSmithKline, Morphosus, PharmaEssentia, Bristol Myers Squibb, AbbVie, and Kartos/Telios outside the submitted work. A. Yacoub reports personal fees from Incyte, CTI Pharma, Novartis, Servier, CTI Pharma, Pharmaessentia, AbbVie, Apellis, Gilead, Notable Labs, and Pfizer outside the submitted work. K. Pettit reports personal fees from AbbVie Inc., Sierra Oncology, Incyte, CTI Biopharma, and Blueprint Medicines outside the submitted work. M. Talpaz is an advisory board member for Sierra Oncology, BMS, and Sumitomo. R.S. Komrokji reports grants and personal fees from BMS and personal fees from AbbVie, CTI, Geron, JAZZ, Pharma Essentia, Servio, Taiho, and Rigel outside the submitted work. M. Kremyanskaya reports grants from NCI and BMS during the conduct of the study; personal fees from Morphosys, Incyte, CTI BioPharma, AbbVie, and Protagonist outside the submitted work. K. Johnson reports grants from NIH during the conduct of the study; personal fees from GSK and CTI Biopharma; and nonfinancial support from Protagonist Therapeutics outside the submitted work. M. Dougherty reports grants from NIH during the conduct of the study. E.M. McGovern reports grants from NIH during the conduct of the study. A.M. Vannucchi reports participation with advisory boards and lectures for Novartis, Incyte, BMS, and GSK. C. Mead-Harvey reports grants from NCI during the conduct of the study. R. Hoffman reports grants from Formation Biologics during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
J. Mascarenhas: Conceptualization, supervision, funding acquisition, investigation, writing–original draft, writing–review and editing. A.R. Migliaccio: Conceptualization, resources, formal analysis, supervision, validation, methodology, writing–original draft, writing–review and editing. H. Kosiorek: Data curation, formal analysis, validation, methodology, writing–original draft, writing–review and editing. R. Bhave: Investigation, writing–review and editing. J. Palmer: Investigation, writing–review and editing. A. Kuykendall: Investigation, writing–review and editing. R. Mesa: Investigation, writing–review and editing. R.K. Rampal: Data curation, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. A.T. Gerds: Investigation, writing–review and editing. A. Yacoub: Investigation, writing–review and editing. K. Pettit: Investigation, writing–review and editing. M. Talpaz: Investigation, writing–review and editing. R. Komrokji: Investigation, writing–review and editing. M. Kremyanskaya: Investigation, writing–review and editing. A. Gonzalez: Methodology, writing–review and editing. F. Fabris: Data curation, writing–review and editing. K. Johnson: Investigation, writing–review and editing. M. Dougherty: Data curation, supervision, writing–review and editing. E. McGovern: Data curation, methodology, writing–review and editing. J. Arango Ossa: Methodology, writing–review and editing. D. Domenico: Data curation. N. Farnoud: Data curation, software, formal analysis, methodology, writing–review and editing. R.S. Weinberg: Methodology, writing–review and editing. A. Kong: Validation, methodology. V. Najfeld: Methodology, writing–review and editing. A.M. Vannucchi: Resources, methodology, writing–review and editing. F. Arciprete: Formal analysis, methodology, writing–review and editing. M. Zingariello: Methodology, writing–review and editing. M. Falchi: Formal analysis. M.E. Salama: Formal analysis, methodology, writing–original draft, writing–review and editing. C. Mead-Harvey: Formal analysis, methodology, writing–review and editing. A. Dueck: Conceptualization, data curation, supervision, validation, writing–review and editing. L. Varricchio: Data curation, formal analysis, validation, methodology, writing–original draft, writing–review and editing. R. Hoffman: Conceptualization, supervision, funding acquisition, investigation, writing–original draft, project administration, writing–review and editing.
References
- 1. Mascarenhas J, Gleitz HFE, Chifotides HT, Harrison CN, Verstovsek S, Vannucchi AM, et al. Biological drivers of clinical phenotype in myelofibrosis. Leukemia 2023;37:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waksal JA, Mascarenhas J. Novel therapies in myelofibrosis: beyond JAK inhibitors. Curr Hematol Malig Rep 2022;17:140–54. [DOI] [PubMed] [Google Scholar]
- 3. Mascarenhas JO, Verstovsek S. The clinical dilemma of JAK inhibitor failure in myelofibrosis: predictive characteristics and outcomes. Cancer 2022;128:2717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. England J, Gupta V. Novel therapies vs hematopoietic cell transplantation in myelofibrosis: who, when, how? Hematology Am Soc Hematol Educ Program 2021;2021:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev 2013;24:133–45. [DOI] [PubMed] [Google Scholar]
- 6. Blank U, Karlsson S. TGF-beta signaling in the control of hematopoietic stem cells. Blood 2015;125:3542–50. [DOI] [PubMed] [Google Scholar]
- 7. Martinaud C, Desterke C, Konopacki J, Pieri L, Torossian F, Golub R, et al. Osteogenic potential of mesenchymal stromal cells contributes to primary myelofibrosis. Cancer Res 2015;75:4753–65. [DOI] [PubMed] [Google Scholar]
- 8. Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 2000;1:169–78. [DOI] [PubMed] [Google Scholar]
- 9. Funaba M, Zimmerman CM, Mathews LS. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem 2002;277:41361–8. [DOI] [PubMed] [Google Scholar]
- 10. Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther 2005;315:1005–12. [DOI] [PubMed] [Google Scholar]
- 11. Fortunel N, Hatzfeld J, Kisselev S, Monier MN, Ducos K, Cardoso A, et al. Release from quiescence of primitive human hematopoietic stem/progenitor cells by blocking their cell-surface TGF-beta type II receptor in a short-term in vitro assay. Stem Cells 2000;18:102–11. [DOI] [PubMed] [Google Scholar]
- 12. Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood 2000;96:2022–36. [PubMed] [Google Scholar]
- 13. Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 2009;113:1250–6. [DOI] [PubMed] [Google Scholar]
- 14. Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 2007;110:986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verstovsek S, Manshouri T, Pilling D, Bueso-Ramos CE, Newberry KJ, Prijic S, et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med 2016;213:1723–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hino M, Tojo A, Miyazono K, Urabe A, Takaku F. Effects of type beta transforming growth factors on haematopoietic progenitor cells. Br J Haematol 1988;70:143–7. [DOI] [PubMed] [Google Scholar]
- 17. Sastow D, Mascarenhas J, Tremblay D. Thrombocytopenia in patients with myelofibrosis: pathogenesis, prevalence, prognostic impact, and treatment. Clin Lymphoma Myeloma Leuk 2022;22:e507–e20. [DOI] [PubMed] [Google Scholar]
- 18. Marcellino BK, Verstovsek S, Mascarenhas J. The myelodepletive phenotype in myelofibrosis: clinical relevance and therapeutic implication. Clin Lymphoma Myeloma Leuk 2020;20:415–21. [DOI] [PubMed] [Google Scholar]
- 19. Vannucchi AM, Bianchi L, Paoletti F, Pancrazzi A, Torre E, Nishikawa M, et al. A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis. Blood 2005;105:3493–501. [DOI] [PubMed] [Google Scholar]
- 20. Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood 2002;100:3495–503. [DOI] [PubMed] [Google Scholar]
- 21. Ceglia I, Dueck AC, Masiello F, Martelli F, He W, Federici G, et al. Preclinical rationale for TGF-beta inhibition as a therapeutic target for the treatment of myelofibrosis. Exp Hematol 2016;44:1138–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varricchio L, Iancu-Rubin C, Upadhyaya B, Zingariello M, Martelli F, Verachi P, et al. TGF-beta1 protein trap AVID200 beneficially affects hematopoiesis and bone marrow fibrosis in myelofibrosis. JCI Insight 2021;6:e145651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhattacharya A, Al-Sammarraie N, Gebere MG, Johnson J, Eberth JF, Azhar M. Myocardial TGFbeta2 is required for atrioventricular cushion remodeling and myocardial development. J Cardiovasc Dev Dis 2021;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reiss KA, Wattenberg MM, Damjanov N, Prechtel Dunphy E, Jacobs-Small M, Lubas MJ, et al. A pilot study of galunisertib plus stereotactic body radiotherapy in patients with advanced hepatocellular carcinoma. Mol Cancer Ther 2021;20:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harding JJ, Do RK, Yaqubie A, Cleverly A, Zhao Y, Gueorguieva I, et al. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med 2021;10:3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santini V, Valcarcel D, Platzbecker U, Komrokji RS, Cleverly AL, Lahn MM, et al. Phase II study of the ALK5 inhibitor galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin Cancer Res 2019;25:6976–85. [DOI] [PubMed] [Google Scholar]
- 27. Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, et al. TGFbeta receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol 2019;83:975–91. [DOI] [PubMed] [Google Scholar]
- 28. Ikeda M, Takahashi H, Kondo S, Lahn MMF, Ogasawara K, Benhadji KA, et al. Phase 1b study of galunisertib in combination with gemcitabine in Japanese patients with metastatic or locally advanced pancreatic cancer. Cancer Chemother Pharmacol 2017;79:1169–77. [DOI] [PubMed] [Google Scholar]
- 29. Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol 2016;18:1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujiwara Y, Nokihara H, Yamada Y, Yamamoto N, Sunami K, Utsumi H, et al. Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2015;76:1143–52. [DOI] [PubMed] [Google Scholar]
- 31. Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010;115:1703–8. [DOI] [PubMed] [Google Scholar]
- 32. Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005;90:1128–32. [PubMed] [Google Scholar]
- 33. Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose-finding trials. Clin Trials 2010;7:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: international working group-myeloproliferative neoplasms research and treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013;122:1395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pellicciotta I, Marciscano AE, Hardee ME, Francis D, Formenti S, Barcellos-Hoff MH. Development of a novel multiplexed assay for quantification of transforming growth factor-beta (TGF-beta). Growth Factors 2015;33:79–91. [DOI] [PubMed] [Google Scholar]
- 36. Kruse N, El-Agnaf OM, Mollenhauer B. Validation of electrochemiluminescence assays for highly sensitive and reproducible quantification of alpha-synuclein in cerebrospinal fluid. Bioanalysis 2017;9:621–30. [DOI] [PubMed] [Google Scholar]
- 37. Farrington DL, Yingling JM, Fill JA, Yan L, Qian YW, Shou J, et al. Development and validation of a phosphorylated SMAD ex vivo stimulation assay. Biomarkers 2007;12:313–30. [DOI] [PubMed] [Google Scholar]
- 38. Desterke C, Bilhou-Nabera C, Guerton B, Martinaud C, Tonetti C, Clay D, et al. FLT3-mediated p38-MAPK activation participates in the control of megakaryopoiesis in primary myelofibrosis. Cancer Res 2011;71:2901–15. [DOI] [PubMed] [Google Scholar]
- 39. Li H. Exploring single-sample SNP and INDEL calling with whole-genome de novo assembly. Bioinformatics 2012;28:1838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453–74. [DOI] [PubMed] [Google Scholar]
- 41. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013;31:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 2012;28:1811–7. [DOI] [PubMed] [Google Scholar]
- 43. Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 2009;25:2865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, et al. The ensembl variant effect predictor. Genome Biol 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol 2017;2017:PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stivala S, Codilupi T, Brkic S, Baerenwaldt A, Ghosh N, Hao-Shen H, et al. Targeting compensatory MEK/ERK activation increases JAK inhibitor efficacy in myeloproliferative neoplasms. J Clin Invest 2019;129:1596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brkic S, Stivala S, Santopolo A, Szybinski J, Jungius S, Passweg JR, et al. Dual targeting of JAK2 and ERK interferes with the myeloproliferative neoplasm clone and enhances therapeutic efficacy. Leukemia 2021;35:2875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abdalla M, Thompson L, Gurley E, Burke S, Ujjin J, Newsome R, et al. Dasatinib inhibits TGFbeta-induced myofibroblast differentiation through Src-SRF pathway. Eur J Pharmacol 2015;769:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turro E, Greene D, Wijgaerts A, Thys C, Lentaigne C, Bariana TK, et al. A dominant gain-of-function mutation in universal tyrosine kinase SRC causes thrombocytopenia, myelofibrosis, bleeding, and bone pathologies. Sci Transl Med 2016;8:328ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mascarenhas JO. Pacritinib in patients with myelofibrosis-Reply. JAMA Oncol 2018;4:1787. [DOI] [PubMed] [Google Scholar]
- 51. Mascarenhas J, Li T, Sandy L, Newsom C, Petersen B, Godbold J, et al. Anti-transforming growth factor-beta therapy in patients with myelofibrosis. Leuk Lymphoma 2014;55:450–2. [DOI] [PubMed] [Google Scholar]
- 52. Gulley JL, Schlom J, Barcellos-Hoff MH, Wang XJ, Seoane J, Audhuy F, et al. Dual inhibition of TGF-beta and PD-L1: a novel approach to cancer treatment. Mol Oncol 2022;16:2117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yap T, Araujo D, Wood D, Denis JF, Gruosso T, Tremblay G, et al. AVID200, first-in-class TGF-BETA1 and beta3 selective inhibitor: results of a phase 1 monotherapy dose escalation study in solid tumors and evidence of target engagement in patientsy. J Immunother Cancer 2020;8:A6-A7. [Google Scholar]
- 54. Gonzalez-Junca A, Driscoll KE, Pellicciotta I, Du S, Lo CH, Roy R, et al. Autocrine TGFbeta is a survival factor for monocytes and drives immunosuppressive lineage commitment. Cancer Immunol Res 2019;7:306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barlesi F, Isambert N, Felip E, Cho BC, Lee DH, Peguero J, et al. Bintrafusp Alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with non-small cell lung cancer resistant or refractory to immune checkpoint inhibitors. Oncologist 2023;28:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spira A, Wertheim MS, Kim EJ, Tan B, Lenz HJ, Nikolinakos P, et al. Bintrafusp Alfa: a bifunctional fusion protein targeting PD-L1 and TGF-beta, in patients with pretreated colorectal cancer: results from a phase I trial. Oncologist 2023;28:e124–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zahr AA, Salama ME, Carreau N, Tremblay D, Verstovsek S, Mesa R, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica 2016;101:660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ishikawa G, Fujiwara N, Hirschfield H, Varricchio L, Hoshida Y, Barosi G, et al. Shared and tissue-specific expression signatures between bone marrow from primary myelofibrosis and essential thrombocythemia. Exp Hematol 2019;79:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao JC, Oetjen KA, Wang T, Xu H, Abou-Ezzi G, Krambs JR, et al. TGF-beta signaling in myeloproliferative neoplasms contributes to myelofibrosis without disrupting the hematopoietic niche. J Clin Invest 2022;132:e154092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nagy MA, Hilgraf R, Mortensen DS, Elsner J, Norris S, Tikhe J, et al. Discovery of the c-Jun N-terminal kinase inhibitor CC-90001. J Med Chem 2021;64:18193–208. [DOI] [PubMed] [Google Scholar]
- 62. Popmihajlov Z, Sutherland DJ, Horan GS, Ghosh A, Lynch DA, Noble PW, et al. CC-90001, a c-Jun N-terminal kinase (JNK) inhibitor, in patients with pulmonary fibrosis: design of a phase 2, randomised, placebo-controlled trial. BMJ Open Respir Res 2022;9:e001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Greenberg S, Horan G, Bennett B, Blease K, Ye Y, Azaryan A, et al. Late breaking abstract—evaluation of the JNK inhibitor, CC-90001, in a phase 1b pulmonary fibrosis trial. Eur Respir J 2017;50:OA474. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MPN-RC 118 phase 1b study schema
Baseline mutational profile of enrolled patients.
Duration of treatment and response status.
Oncoprint Analysis of Serial Samples from Phase 1b Trial of AVID200
The bone marrow from patients with MF contain more CD42pos cells than patients with ET or with hematopoietic malignancies without fibrosis (normal control).
GATA1 expression in megakaryocytes from patients treated with AVID200
Effects of AVID200 therapy on plasma TGFβ levels.
AVID200 induced similar decrements in the plasma levels of TGF-β in the three patients with and without platelet response
PK data for Cycle 1 (first 24 hours only) for A) 180 mg/m2 dose level and B) 550 mg/m2 dose level during dose escalation phase I.
Clinical info on the platelets-responsive and non-responsive patients
Patient listing of prior myelofibrosis treatment and time since discontinuation if known
Representativeness of Study Participants.
Individual patient data for AUC, Cmax and Tmax for dose levels 1 and 2 (lot A). Summary measures for DL 1 and DL2 are shown in red.
Data Availability Statement
The data generated in this study are not publicly available but are available upon reasonable request from the corresponding author.