Abstract
Ryanodine receptors (RyRs) are Ca2+ release channels, gated by Ca2+ in the cytosol and the sarcoplasmic reticulum lumen. Their regulation is impaired in certain cardiac and muscle diseases. Although a lot of data is available on the luminal Ca2+ regulation of RyR, its interpretation is complicated by the possibility that the divalent ions used to probe the luminal binding sites may contaminate the cytoplasmic sites by crossing the channel pore. In this study, we used Eu3+, an impermeable agonist of Ca2+ binding sites, as a probe to avoid this complication and to gain more specific information about the function of the luminal Ca2+ sensor. Single-channel currents were measured from skeletal muscle and cardiac RyRs (RyR1 and RyR2) using the lipid bilayer technique. We show that RyR2 is activated by the luminal addition of Ca2+, whereas RyR1 is inhibited. These results were qualitatively reproducible using Eu3+. The luminal regulation of RyR1 carrying a mutation associated with malignant hyperthermia was not different from that of the wild-type.
RyR1 inhibition by Eu3+ was extremely voltage dependent, whereas RyR2 activation did not depend on the membrane potential. These results suggest that the RyR1 inhibition site is in the membrane’s electric field (channel pore), whereas the RyR2 activation site is outside. Using in silico analysis and previous results, we predicted putative Ca2+ binding site sequences. We propose that RyR2 bears an activation site, which is missing in RyR1, but both isoforms share the same inhibitory Ca2+ binding site near the channel gate.
Significance
Answering the pathologically relevant, long-standing question of how the cardiac- and skeletal muscle-type ryanodine receptors are regulated by the Ca2+ content of the sarcoplasmic reticulum lumen is complicated by the technical issue that Ca2+ flowing through the channel interferes with its cytoplasmic Ca2+ regulation. An impermeable Ca2+ binding site agonist (Eu3+) allowed us to selectively study the luminal Ca2+ regulation of the channels. Our electrophysiological measurements gained new information about the function and location of the intrinsic luminal Ca2+ binding sites in both ryanodine receptor isoforms.
Introduction
In striated muscles, the sequence of events linking electrical excitation to contraction is called excitation-contraction coupling. The key step of excitation-contraction coupling is when the action potential activates dihydropyridine receptors, thereby inducing Ca2+ release from the sarcoplasmic reticulum (SR) by opening the ryanodine receptor Ca2+ channels (RyR). As a result, the intracellular [Ca2+] increases and triggers a contraction. The way Ca2+ release is triggered by dihydropyridine receptors fundamentally differs in skeletal and cardiac muscles, but the skeletal muscle and cardiac RyR isoforms (RyR1 and RyR2, respectively) share the same ligand gating mechanisms (1). The most important ligand that controls RyR gating is cytoplasmic Ca2+, which activates RyR1 with an EC50 of ∼10 μM. There is an additional, functionally inhibiting, low affinity (IC50 ∼ 250 μM) Ca2+ binding site on the cytoplasmic side of the channel (2,3,4). RyR2 is more sensitive to cytoplasmic Ca2+ activation (EC50 ∼ 1 μM) (5,6,7,8).
It has been long known that the Ca2+ stored in the SR lumen also regulates both RyR isoforms. Studies on cardiomyocytes demonstrated that RyR2 opens if the SR Ca2+ load exceeds a certain threshold, resulting in diastolic Ca2+ release (9,10,11,12,13,14,15). This process is referred to as store-overload-induced Ca2+ release (SOICR) (16). RyR2 was investigated at the single-channel level using the lipid bilayer technique, which showed that increasing the [Ca2+] on the luminal side of the channel enhanced its probability of opening (8,17,18,19,20,21). The luminal activation mechanism of RyR2 has been extensively studied in the past three decades, because inherited cardiac arrhythmias, such as catecholaminergic polymorphic ventricular tachycardia (CPVT), are associated with a lower SOICR threshold, which is thought to be the underlying mechanism of the disease (16,22,23,24). Despite intensive research, the regulation of RyR by the SR Ca2+ content and the molecular mechanism of SOICR are still disputed. According to one proposal, luminal Ca2+ activation is initiated when Ca2+ binds to a distinct binding site on the luminal side of the channel (25,26). Another proposal suggests that Ca2+ passes through the RyR channel during a short-lived, random opening event and activates channel opening by binding to the known cytoplasmic, activation binding sites (self-flux, or feedthrough regulation) (20). Of the more recent contributions, Laver’s single-channel experiments are particularly important as they suggested a unified model, in which RyR activation results from a successive series of events, called “luminal-triggered Ca2+ feedthrough activation” (8,27,28,29). In this model, Ca2+ first binds to a luminal Ca2+ binding site, which triggers brief openings, allowing Ca2+ to reach the activating binding site on the cytoplasmic side of the channel.
The regulation of RyR1 by luminal Ca2+ is more controversial, and its role in the main RyR1-related muscle disorder, malignant hyperthermia syndrome (MHS), is also obscure. MHS is an idiosyncratic drug reaction that develops in genetically susceptible individuals when exposed to volatile anesthetics, such as halothane. MHS is linked to RyR1 point mutations that make the channel sensitive to activation by halothane. Symptoms include muscle contracture, consequent hyperthermia, acidosis, and hyperkalemia, leading to death unless immediately treated with the RyR1 inhibitor dantrolene (30,31,32,33,34). HEK cells expressing MHS-RyR1 display a low threshold for SOICR (35,36,37). Furthermore, halothane was shown to trigger MH by further decreasing the SOICR threshold, suggesting that MHS has a similar pathomechanism as that described for CPVT (38).
On the other hand, other studies have found that increasing the [Ca2+] on the luminal side of RyR1 actually inhibits the channel (20,39). Because this inhibition was dependent on the membrane potential, the effect was attributed to luminal Ca2+ crossing the pore and reaching the inhibiting Ca2+ binding site on the cytoplasmic side of the channel. In fact, it is known that Ca2+ can actually pass through the channel pore and binds to the activating and inhibiting binding sites on the cytoplasmic side of the channel (40,41). This prevents the Ca2+ binding sites on the luminal side of the channel, if present, from being selectively studied using Ca2+ (and other divalents) and can complicate interpreting the results of these experiments. Appropriately investigating the putative luminal Ca2+ sensing mechanism of RyRs (and isolating the role of SOICR in the pathomechanisms) is also hindered by the fact that the Ca2+ flux rate (and thus the involvement of the cytoplasmic sites in these processes) is highly dependent on the luminal [Ca2+] (i.e., the overall concentration gradient).
The selective and specific investigation of a luminal Ca2+ sensor requires a Ca2+ binding site agonist that does not permeate through the channel. Considering that lanthanides (Ln3+) were commonly used as probes of Ca2+ binding proteins and were shown not to penetrate through Ca2+ channels, we investigated the interaction of Eu3+ with RyR and identified Eu3+ as such an ideal research tool (42,43,44,45,46). We used it here to help find the luminal Ca2+ binding sites of RyR1 and RyR2.
Here, we present experimental data obtained by measuring single RyR channel currents reconstituted in lipid bilayers. During our purification procedure RyR is expected to lose any regulatory proteins attached to it, including calsequestrin (CSQ), a Ca2+ buffer protein that regulates RyR depending on the SR luminal Ca2+ content (47,48,49,50). Our RyRs are therefore suitable for investigating the function of the intrinsic luminal Ca2+ sensors of the channel, without CSQ interference. Our experiments were designed to learn 1) how luminal Ca2+ binding sites regulate the function of RyR1 and RyR2 and 2) whether SOICR is a relevant mechanism in MHS.
We find that the luminal Ca2+ sensors in RyR1 and RyR2 are functionally different, being stimulatory in RyR2 but inhibitory in RyR1. Furthermore, we show that the sensitivity of MHS-RyR1 (Y524S) to luminal Ca2+ is the same as the wild-type and that altered luminal regulation of RyR1 is therefore not a relevant factor in the pathomechanism of MHS (at least not in Y524S).
Although recent cryo-EM studies revealed the structure of the cytoplasmic Ca2+ binding sites (and those of other ligands), the structure of the luminal Ca2+ sensor was not determined (51,52,53). Here, using our functional data and in silico analysis, we identify RyR sequences, which may contain the luminal Ca2+ sensors.
Materials and methods
Animals and ethical approval
All experiments complied with the Hungarian Animal Welfare Act, the 2010/63/EU guideline of the European Union, and were approved by the Animal Welfare Committee of the University of Debrecen (22/2012/DEMÁB). Skeletal muscle was collected from rabbits and mice. Rabbits (n = 5) were killed by using a guillotine.
Transgenic C57Bl/6 mice carrying the mutation Y524S in the RyR1 gene were also used in this study (n = 20) (this mutation is analogous to the human mutation Y522S associated with MHS). As the homozygous mice die in the early stage of their intrauterine life, heterozygous MHS mice were used. Wild-type littermates were used as controls (n = 18). Mice were killed by CO2 inhalation, which was followed by cervical dislocation.
RyR2 was purified from canine ventricular myocardium (n = 5). Adult mongrel dogs were anesthetized with i.m. injections of 10 mg/kg ketamine hydrochloride (Calypsol, Richter Gedeon, Hungary) +1 mg/kg xylazine hydrochloride (Sedaxylan, Eurovet Animal Health BV, the Netherlands) according to a protocol approved by the local Animal Care Committees (9/2015/DEMÁB).
Materials
Phospholipids were obtained from Avanti Polar Lipids (Alabaster, AL). All other chemicals, if not specified, were purchased from Sigma-Aldrich (St. Louis, MO).
Sarcoplasmic reticulum vesicle isolation
SR vesicles were isolated from rabbit or mouse skeletal muscle by differential centrifugation as described in the literature (42,54,55). All the steps were performed on ice or at 4°C in the presence of protease inhibitors. After homogenization in 100 mM NaCl, 20 mM EGTA, 20 mM Na-HEPES (pH 7.5), cell debris was pelleted at 3500 × g, for 35 min using a tabletop centrifuge. Crude microsomes were collected from the supernatant by centrifugation in a Ti45 rotor at 40,000 × g, for 30 min. To dissolve the actomyosin content, the pellet was resuspended in 600 mM KCl, 10 mM K-PIPES, 250 mM sucrose, 1 mM EGTA, 0.9 mM CaCl2 (pH 7.0). After incubation for 1 h at 4°C, the microsomes were centrifuged at 109,000 × g for 30 min, and then the pellet was resuspended in 300 mM sucrose, 10 mM K-PIPES (pH 7.0). Vesicles were aliquoted, snap-frozen in liquid nitrogen, and stored in a deep freezer until processed in RyR purification.
SR vesicles were also isolated from dog myocardium using the same method.
RyR purification
Skeletal muscle or myocardial HSR vesicles (30–35 mg protein) were solubilized for 2 h in 1% CHAPS, 1 M NaCl, 100 μM EGTA, 150 μM CaCl2, 5 mM AMP, 0.45% phosphatidylcholine, 20 mM Na-PIPES (pH 7.2) at 4°C. The sample was loaded onto a 10%–28% linear sucrose gradient and centrifuged overnight at 90,000 × g in an SW27 rotor. This method yields RyRs free of CSQ. RyR-containing fractions of the gradient were identified, snap-frozen in liquid nitrogen, and stored at −70°C in small aliquots (42,54,55).
RyR reconstitution and single-channel recording
Purified RyR1 and RyR2s were incorporated into planar lipid bilayers. Bilayers were formed across an aperture with a diameter of 200 μm drilled in the wall of a Delrin cap (Warner Instruments, Hamden, CT). The lipid mixture contained phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in a ratio of 5:4:1, dissolved in n-decane in a final lipid concentration of 20 mg/mL. The bilayer separated two chambers. Both chambers contained 250 mM KCl, 50 or 1 μM CaCl2, 10 mM HEPES (pH 7.2). Some experiments were performed in a Ca2+-free cytosolic recording medium, which did not contain added Ca2+ (estimated [Ca2+] of ∼2 μM). In these experiments, luminal [Ca2+] was set to 50 μM in control. Most RyRs were incorporated into the bilayer so that their cytosolic foot region faced toward the measuring electrode. The bilayer currents were recorded under voltage clamp conditions, using an Axopatch 200 amplifier and pCLAMP 6.03 (Axon Instruments, Sunnyvale, CA) software. The holding potentials are indicated in the figure legends. Currents were filtered at 1 kHz through an eight-pole low-pass Bessel filter and digitized at 3 kHz (42,54,55).
Transmembrane voltages are referred to ground (trans to cis). Data points of the dose-response curves were obtained by progressively adding Ca2+ or Eu3+ to a single RyR. Cumulative dose-response curves are shown.
Statistics
More than 140 RyRs were involved in the analysis. Open probabilities (Po) were determined using the pClamp software suite (Molecular Devices, Sunnyvale, CA). The threshold for an open event was set at 50% of the maximum open current. Low Po recordings were analyzed using at least 3-min-long traces. Statistical analysis was performed in Origin 7.0 (OriginLab, Northampton, MA) and in Excel (Microsoft, Redmond, WA). Results are expressed as mean ± SE. Relative Po data were calculated by normalizing each set of datapoints to their own control. Statistical significance of differences was evaluated using one-way ANOVA with Tukey’s honest significant difference post test. Some datasets were evaluated using independent two-sample t-test. Differences were considered significant when p-value was less than 0.05. The number of channels investigated (n) is given in the figure legends.
Eu3+ binding site prediction
Five different methods were employed to identify likely metal ion binding sites. These included Placevent and Fold-X, which rely on biophysical considerations, MIB, which compares a structure to a library of templates of known binding sites, IonCom, which combines sequence and structural data, and bindEmbed21, which predicts sites based only on the amino acid sequence (56,57,58,59,60).
Methods based on structural information are often sensitive to the particular conformation of the input structure. For this reason, we used seven different PDB structures of rabbit RyR1 as input for Placevent, Fold-X, MIB, and IonCom: 3J8H, 5T15, 5GKY, 6M2W, 7TZC, 7TDI, and 7T64 (53,61,62,63,64,65). These structures were chosen to get a representative sample of the possible conformations of the luminal surface loops. Only the luminal-facing part of the channel was considered for analysis; this part included residues 4561–4663 and 4824–4935 from all four monomers. In order to ensure that all methods considered the entire structure rather than individual chains, the four separate chains were fused into a single chain for analysis.
These structures were used directly by the MIB webserver (http://bioinfo.cmu.edu.tw/MIB/) and by the stand-alone versions of IonCom and Fold-X. To run Placevent, the integral-equation-based reference interaction site model (RISM) as implemented in the rism1d and rism3d programs in AmberTools22 was first run to generate correlations, and the sites were then predicted using Placevent itself (66). The Eu3+ parameters for RISM calculations were taken from Li et al. (67).
The electrostatic surface areas were calculated using APBS over a fragment of RyR1 containing the entire channel region but without most of the cytosolic region (residues 12–545, 3668–4253, 4540–4587, and 4626–5037) (68). All structural visualization was carried out using PyMOL 2.6 (Schrödinger, 2021).
For bindEmbed21, the entire human, rabbit, and rat RyR1 sequences (UniProt identifiers P21817, P11716, and F1LMY4, respectively) were used as input.
Results
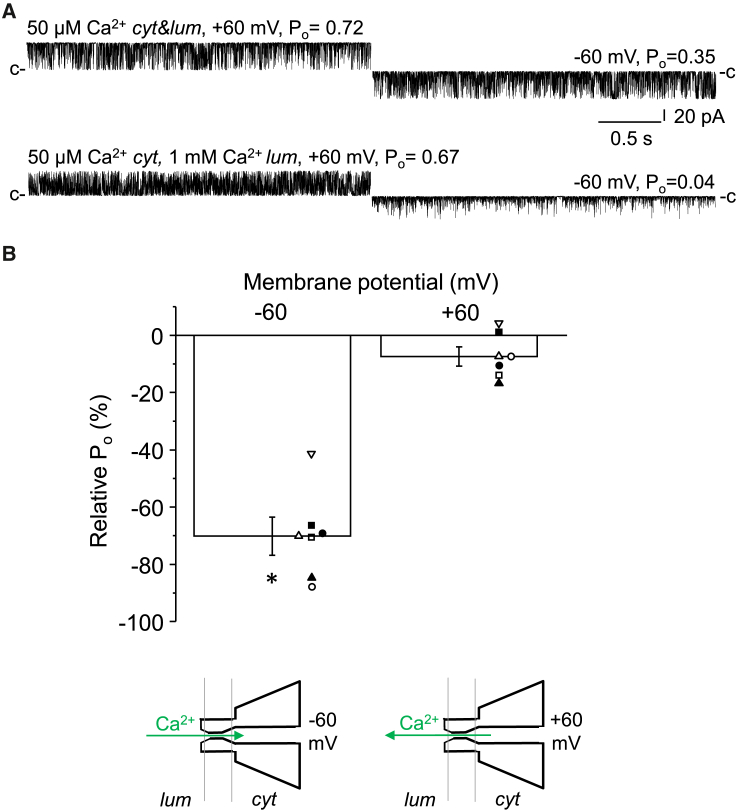
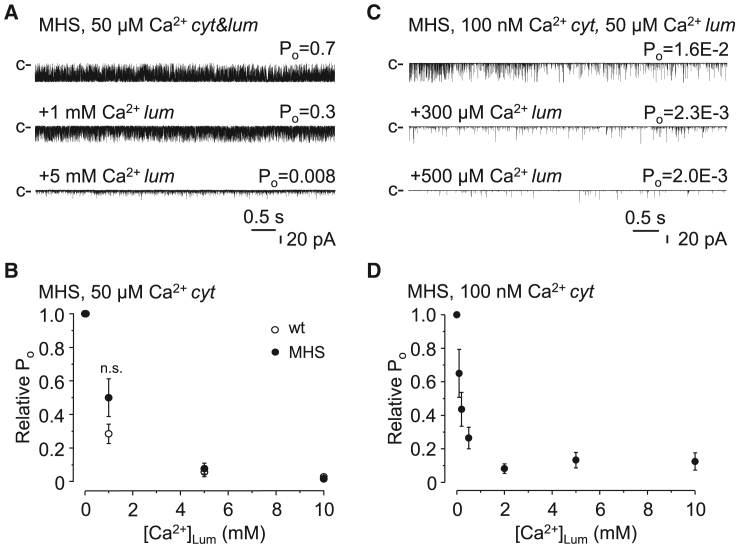
Before we began to investigate the role of the luminal Ca2+ binding sites of RyR1 using Eu3+, we wanted to see how luminal Ca2+ worked in rabbit RyR1 under our experimental conditions. Since the results on the luminal regulation of RyR2 are consistent in the literature, we performed parallel experiments on RyR2, which served as a good internal control to verify the specificity of our RyR1 treatments. Fig. 1 shows representative single-channel current traces of RyRs demonstrating that at the cytosolic [Ca2+]s that saturate the activating Ca2+ binding sites and thus permit maximal open probability (50 μM for RyR1 and 5 μM for RyR2), RyR1 (rabbit) was strongly inhibited by increasing luminal [Ca2+] (Fig. 1 A), whereas RyR2 (dog) was not affected (Fig. 1 B). In the millimolar range of [Ca2+]Lum, the single-channel current amplitude substantially decreased, indicating that Ca2+ became a relevant charge carrier. At low (100 nM) cytosolic [Ca2+], increasing luminal [Ca2+] activated RyR2, as expected (Fig. 1 C), but it still inhibited RyR1 (Fig. 1 D). These RyR2 results agree with previously published results, and the RyR1 data are also in accordance with some of the previously published RyR1 results (8,18,19,20,21,22,39,69). Quantified data from multiple RyR1 channels are presented on the right side of Fig. 1. These data raise the question of why the responses of RyR1 and RyR2 are so different. One possible answer is that Ca2+ crosses through the pore of RyR1 and interacts with the cytosolic, inhibitory Ca2+ binding site (the activating binding site is unavailable, as it is saturated with 50 μM cytoplasmic Ca2+), as suggested earlier by Meissner (20). If this is correct, then it would be the lower affinity of the cytoplasmic inhibitory binding site of RyR2 that prevented RyR2 from being inhibited. This “feedthrough inhibition” hypothesis is supported by the fact that the inhibitory effect of luminal Ca2+ on RyR1 was extremely voltage dependent, with a preference for negative membrane potentials, which would force luminal cations across the pore (Fig. 2 A and B, see illustration at the bottom). Accordingly, increasing luminal [Ca2+] should activate RyR1 when the cytosolic binding sites are unsaturated (feedthrough activation). But surprisingly, high luminal [Ca2+] at 100 nM cytoplasmic Ca2+ did not enhance RyR1 activity at any negative membrane potentials. This result indicates that under our experimental conditions the Ca2+ flux was not significant enough to influence the cytoplasmic Ca2+ binding sites, at least in RyR1. Thus, these data seem to disprove the “feedthrough inhibition” hypothesis proposed by Meissner and suggest that the inhibition by luminal Ca2+ is mediated directly through a putative luminal Ca2+ binding site. In this case, the extreme voltage dependence of the inhibition would suggest that the binding site resides in the channel pore, where the voltage drops.
Figure 1.
Luminal Ca2+ activates RyR2 but inhibits RyR1. (A–D) Representative single-channel current traces of RyR1 (rabbit) or RyR2 (dog) channels are displayed on the left. Recording conditions, cytoplasmic (cyt) and luminal (lum) Ca2+ concentrations are indicated in the headline. The closed state current levels are labeled by “c”. Downward deflections represent channel openings. Open probability (Po) values are indicated in the top right corners. Calibration lines are displayed at the bottom. Corresponding mean ± SE values of relative open probabilities (Po) plotted as the function of luminal Ca2+ concentrations ([Ca2+]Lum) are shown on the right. (n = 5–7, A; n = 3–6, B; n = 3, C; n = 5–19, D). Values are expressed relative to the control (50 μM luminal Ca2+ for RyR1 or 1 μM luminal Ca2+ for RyR2). Cytoplasmic [Ca2+]s were 50 μM or 100 nM for RyR1 and 5 μM or 100 nM for RyR2, respectively, as indicated.
Figure 2.
Voltage-dependent effect of luminal Ca2+. (A) Representative single-channel current traces of RyR1 recorded at +60 (left) and −60 mV (right). Top traces were recorded under control conditions, in symmetric 50 μM Ca2+. Bottom traces were recorded at 50 μM cytoplasmic (cyt) and 1 mM luminal (lum) Ca2+. The closed state current levels are labeled with “c” on the sides. (B) Relative Po % values are expressed as (Po 1 mM lum/Po 50 μM lum) – 1 and plotted as the function of the membrane potential (n = 7). Columns and error bars represent mean ± SE. Asterisk (∗) indicates p < 0.05. The illustration in the inset depicts the movement of Ca2+ directed by the driving force at −60 mV. To see this figure in color, go online.
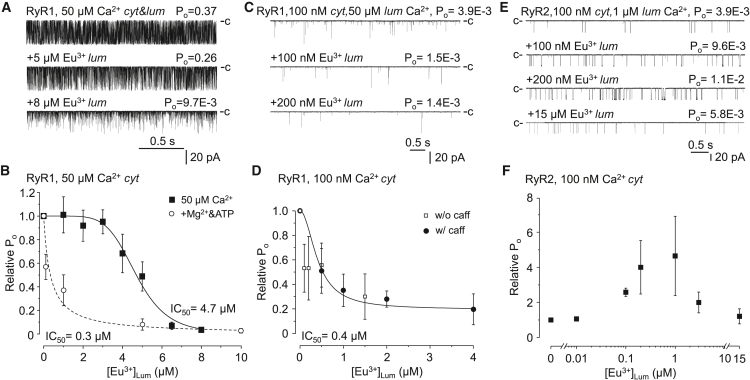
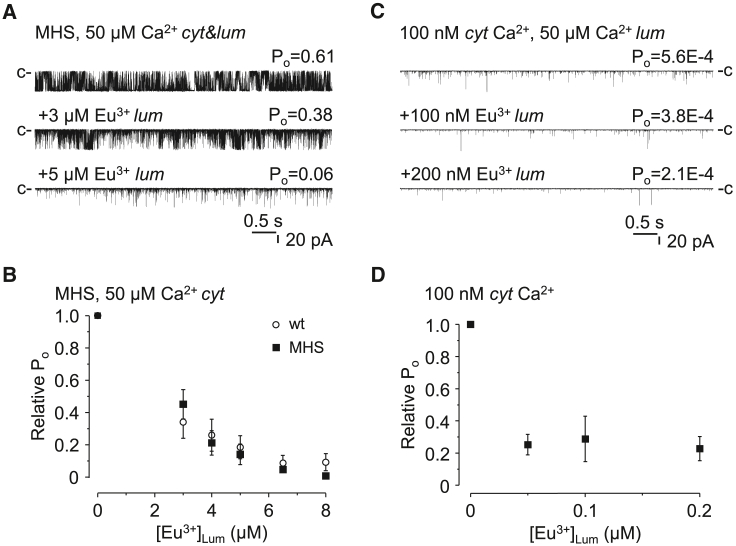
To specifically test the hypothesis that RyR1 carries a genuine inhibitory Ca2+ binding site on the luminal side of the protein and to avoid “self-flux” effects, we needed to choose a research tool to selectively probe the putative luminal binding site. Previously, we demonstrated that Eu3+ is a specific and strong agonist of the cytoplasmic Ca2+ binding sites of RyR1 and does not permeate through the pore (42). Making use of these properties in this study, we tested the effects of Eu3+ again, but this time on the luminal side of the channel to determine whether Eu3+ is a specific, nonpermeable Ca2+ binding site agonist of the luminal sites as well. First, the cytoplasmic [Ca2+] was set to 50 μM, and Eu3+ was added to the luminal side of rabbit RyR1 (in addition to 50 μM Ca2+). Luminal Eu3+ significantly inhibited RyR1 activity in a concentration-dependent manner with an IC50 of 4.7 μM (Fig. 3 A and B).
Figure 3.
Luminally applied Eu3+ inhibits RyR1. (A) Representative single-channel current traces of RyR1 recorded at 50 μM cytoplasmic (cyt) and luminal (lum) Ca2+ and in the presence of Eu3+ as indicated, which was added in addition to 50 μM Ca2+. Closed state current level is labeled by “c”. Downward deflections represent channel openings. Calibration lines are shown at the bottom. (B) Pooled data of currents, like shown in (A). Average values (±SE) of relative open probabilities (Po). Po values obtained from Eu3+-treated RyR1s were normalized to control (untreated) values and plotted as the function of luminal Eu3+ concentration ([Eu3+]Lum) (n = 4–19). Both the cytoplasmic and luminal [Ca2+] were 50 μM. Empty dots represent averaged data obtained in a recording medium containing 50 μM Ca2+, 1 mM Mg2+ (both sides), and 3 mM ATP in the cytoplasmic chamber. (C) Representative single-channel current traces of RyR1 recorded at 100 nM cytoplasmic (cyt) Ca2+ under control conditions and during additional Eu3+ treatment. The luminal [Ca2+] was 50 μM. (D) Average values of relative Pos of RyR1s obtained under conditions shown in (C). Po values of Eu3+ were normalized to control (untreated) values in the absence (white squares, w/o caff) or presence of 2 mM caffeine (black spheres, w/caff) (n = 3–11 and n = 4). Cytoplasmic [Ca2+] = 100 nM, and luminal = 50 μM. (E) Biphasic response of RyR2 to luminal Eu3+. Representative single-channel current traces of RyR2 recorded at 100 nM cytoplasmic (cyt) Ca2+ and during Eu3+ treatment. The closed state level of the channel is labeled by “c”. Luminal [Ca2+] was 5 μM. (F) Average values of relative Pos of multiple RyR2s. Po values of Eu3+ were normalized to control (untreated) values (n = 5).
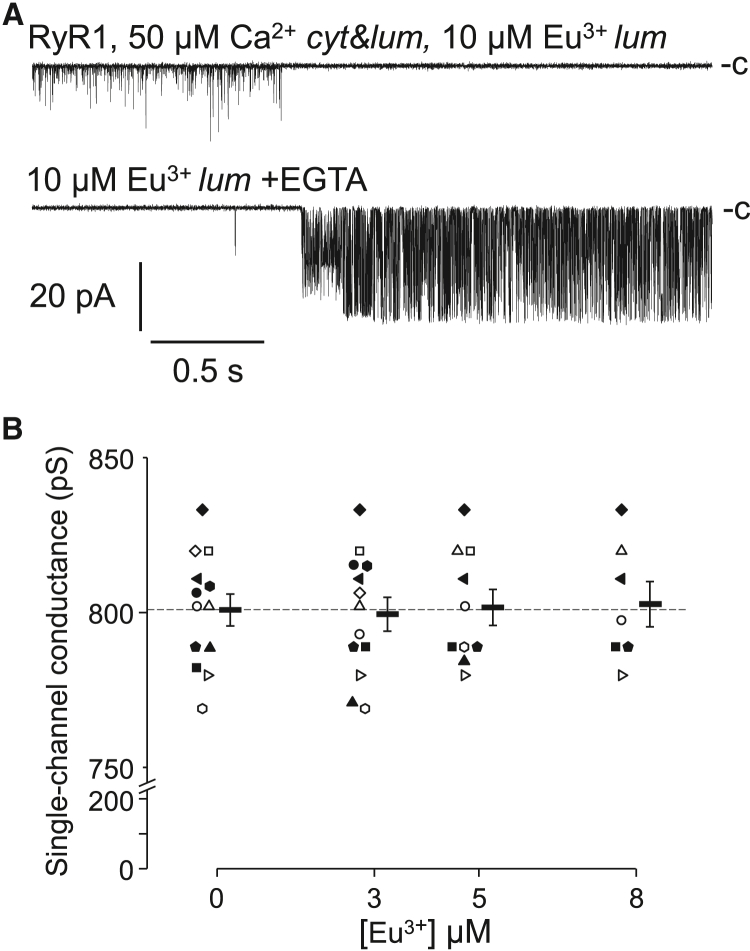
At membrane potentials that push Eu3+ inside the pore, a qualitatively different state of RyR1 was also observed only at high concentrations of the ion, as a long-lasting, permanent closed state appeared abruptly during the course of Eu3+ treatment, indicating that the ion occluded the channel pore. This state was limited only to ≥5 μM Eu3+ and highly negative membrane potentials. The block was canceled by the chelation of Eu3+ with EGTA (Fig. 4 A); otherwise, it was irreversible. The long (several seconds) latency of EGTA’s effect indicated that Eu3+ strongly bound inside the conductive pore of the RyR, causing permanent physical occlusion, as reported for the L-type Ca2+ channel channels earlier (70). The conductances of RyRs were approximately 800 pS and were not altered by Eu3+ (Fig. 4 B). These data imply that Eu3+ cannot permeate the channel and therefore cannot reach the cytosolic binding sites. In addition, the analogy between the effects of Eu3+ and Ca2+ provides indirect evidence that Eu3+ is an agonist of the luminal Ca2+ binding sites.
Figure 4.
Eu3+ occludes the pore. (A) Single-channel recordings demonstrate that RyR1 occasionally but permanently closes during 10 μM Eu3+ treatment, which is canceled by the chelation of Eu3+ using equimolar EGTA. EGTA was added ∼15 s before this trace. (B) Single-channel conductance as the function of [Eu3+]. Individual data points and mean ± SE are shown.
In the next series of experiments, we wanted to learn more about the function of the putative luminal Ca2+ binding sites of RyR1 and RyR2, and we tested the effect of luminal Eu3+ at low (nonsaturating) cytoplasmic [Ca2+]. First, the cytoplasmic [Ca2+] was set to 100 nM, and RyR1 channels were treated with Eu3+. Luminal Eu3+ still inhibited RyR1 activity (IC50 = 0.4 μM) (Fig. 3 C and D). As some investigators reported that RyR was only activated by luminal Ca2+ when the channel was preactivated by caffeine, we also performed some experiments in the presence of 2 mM caffeine, but the inhibitory effect of Eu3+ was even more evident (Fig. 3 D). In summary, it should be emphasized that no combination of cytoplasmic and luminal Ca2+ and Eu3+ concentrations (and caffeine) was able to activate RyR1, and Eu3+ appeared to be an even more potent inhibitor in 100 nM cytoplasmic Ca2+, when the baseline Po is significantly lower, than in 50 μM. For better comparability, find the overlapping curves in Fig. S1.
Because Mg2+ is always present in physiological solutions and since Mg2+ binds to Ca2+ binding sites (and shields itself from self-flux regulation by doing so) (40), it seems necessary to test the sensitivity to luminal Eu3+ in the presence of Mg2+. RyR1 currents were reconstituted in symmetric 50 μM Ca2+ as before, but 1 mM Mg2+ was added to both sides of the bilayer. To improve channel activity, 3 mM ATP was also added to the cytoplasmic side, resulting in a baseline Po of 0.54 ± 0.1 (similar to that measured in 50 μM Ca2+ only). Compared with 50 μM Ca2+ alone, the Kd of Eu3+ was significantly lower (4.7 versus 0.3 μM) (Fig. 3 B), which is very similar to the value observed at 100 nM cytoplasmic [Ca2+] (Fig. 3 D) (0.4 μM). This indicates that the effect of luminal regulation is stronger when the cytoplasmic Ca2+ binding site is not occupied by Ca2+ (i.e., 100 nM Ca2+) (note that Mg2+ is a competitive antagonist of the cytoplasmic Ca2+ binding site). Apparently, the luminal inhibition becomes less effective when the channel is fully activated by cytoplasmic Ca2+, implying that there is a cooperative interaction between the cytoplasmic and luminal Ca2+ binding sites.
Experiments were also performed with RyR2 (dog) in 100 nM cytoplasmic Ca2+, showing that the effect of Eu3+ was biphasic: it activated RyR2 at submicromolar concentrations but inhibited it at higher concentrations (Fig. 3 E and F). These results clearly demonstrate that the regulation of both channels by Ca2+ and Eu3+ is qualitatively similar (i.e., luminal Eu3+ at high concentrations inhibit both), suggesting that both RyR1 and RyR2 carry genuine luminal Ca2+ binding sites, but that these sites function in opposite ways in the two isoforms. In addition, the fact that Eu3+’s effect was analogous to Ca2+ in both RyR1 and RyR2 provides evidence that Ca2+ and Eu3+ are qualitatively equivalent ligands of the Ca2+ binding sites, and thus, these results validate our experimental approach.
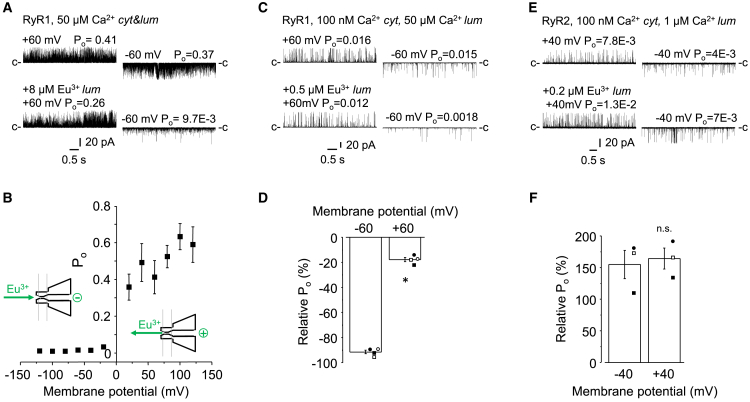
The voltage dependence of the effect of luminal Ca2+ on RyR1 mentioned above raises the question of whether the effect of Eu3+ is also similarly voltage dependent. Examples of current recordings at +/− 60 mV in the presence or absence of 8 μM Eu3+ are shown in Fig. 5 A, and Fig. 5B shows an analysis of Pos at different membrane potentials in 50 μM cytoplasmic Ca2+. These data show that the inhibitory effect of Eu3+ was highly polarized with a preference for negative voltages, which drive Eu3+ into the channel pore (see illustration in Fig. 5 B, insets). Under positive membrane potentials, the decrease in the driving force between +20 and +120 mV was accompanied by gradually weaker inhibition. The effect of Eu3+ also remained voltage dependent in low (100 nM) cytoplasmic [Ca2+], as the inhibition was significantly stronger at −60 than +60 mV. This is shown in Fig. 5 C and D, so that Pos measured during Eu3+ treatment under different membrane potentials was normalized to the Pos of the same, but untreated channels at the corresponding voltages and expressed as a percentage of change in Po.
Figure 5.
Voltage dependence of luminal Eu3+. (A and C) Representative single-channel current traces of RyR1 recorded at +60 (top) and −60 mV (bottom) under indicated ionic concentrations (50 μM and 100 nM cyt Ca2+, 50 μM lum Ca2+). Bottom traces were recorded after Eu3+ was added to the luminal face of the same channel in indicated concentrations. Closed state levels of the currents are labeled by “c”. (B) Pooled data of experiments similar to the sample recording in (A). Mean Po±SE at 8 μM Eu3+ plotted as the function of the membrane potential. [Ca2+]s were as in (A). The drawings in the inset illustrate the direction of Eu3+ movement at corresponding membrane potentials (n = 5). (C) Representative RyR1 currents recorded at +60 and −60 mV Ca2+ and Eu3+ concentrations, and Po values are indicated in the headline. (D) Relative Po values are expressed as (Po 1 mM lum/Po 50 μM lum) – 1 using recordings similar in (C). Mean ± SE values were plotted as the function of the membrane potential (n = 3 and n = 3). Asterisk (∗) indicates p<0.05 (E) Representative RyR2 currents recorded at +40 and −40 mV Ca2+ and Eu3+ concentrations, and Po values are indicated in the headline. (F) Relative Po values of RyR2 currents similar to that in (E) are expressed as (Po 1 mM lum/Po 5 μM lum) – 1. Mean ± SE values were plotted as the function of the membrane potential (n = 3 and n = 3). n.s., non-significant. To see this figure in color, go online.
The voltage dependence of the activating action of Eu3+ on RyR2 was also analyzed at 100 nM cytoplasmic Ca2+. As shown in Fig. 5 E and F, the activation of RyR2 by Eu3+ was independent of membrane potential, as it was equally about twofold at both −40 and +40 mV.
As RyR is impermeable to Eu3+, the voltage-dependent inhibition of RyR1 could not be due to self-flux effects. Therefore, these results indicate that the inhibition sites of RyR1 and RyR2 should reside in the channel pore, but that the RyR2 activation site should lie outside the electrical field of the channel pore.
Similar experiments were performed using rat RyR2 channels with similar results (data not shown).
It should be pointed out that our results were obtained in rabbit and mouse RyR1 and canine and rat RyR2; therefore, these results may only be true for rabbit and RyR1 and canine and rat RyR2, as interspecies differences in luminal Ca2+ regulation may occur.
So far, our results that Ca2+ and Eu3+ inhibit RyR1 function do not seem to support the previously proposed molecular mechanism of MHS (i.e., that a reduced SOICR threshold is responsible for initiating malignant hyperthermia). However, hypothetically, luminal Ca2+-triggered Ca2+ release in MHS skeletal muscle could also occur if the inhibitory potential of luminal Ca2+ was weaker in MHS-RyR1. This possibility was tested using mouse Y524S MHS-RyR1s, and the results are presented in Fig. 6. The experimental design was the same as before: luminal Ca2+ was tested when the [Ca2+] of the cytoplasmic compartment of the bilayer chamber was set to 50 μM or 100 nM Ca2+. Under control conditions, the Po of MHS channels was significantly higher than the Po of wild-type channels at 100 nM cytoplasmic Ca2+, as expected from the MHS phenotype (Po wt 100 nM = 0.0031 ± 0.001 (n = 5), Po MHS 100 nM = 0.02 ± 0.007 (n = 13)) (71,72). Otherwise, the results were qualitatively consistent with the data shown in Fig. 1 A and D; that is, the Y524S MHS-RyR1 was inhibited by luminal Ca2+ the same way as the wild-type (Fig. 6 A and B). The two datasets in Fig. 6 B were not statistically different. We must emphasize that strong inhibition was also observed at 100 nM cytoplasmic Ca2+ as well, with a tendency toward slight activation above 5 mM Ca2+, indicating that self-activation by Ca2+ flux could barely be seen in our experiments (despite the higher sensitivity of MHS-RyR1 to cytoplasmic Ca2+) (Fig. 6 C and D). This is in accordance with previous studies showing that self-flux regulation is not significant when the main charge carrier is other than Ca2+ (40).
Figure 6.
MHS RyR1 is inhibited by luminal Ca2+. (A and C) Single-channel current traces of Y524S MHS RyR1 recorded at 50 μM or 100 nM cytoplasmic (cyt) and different concentrations of luminal (lum) Ca2+. Closed state level of the currents are labeled by “c”. (B) Values were expressed relative to control. Mean relative Po ±SE of wild-type RyR1 (white spheres) and Y524S MHS RyR1(black squares) recorded at 50 μM cytoplasmic (cyt) Ca2+ were plotted as the function of luminal Ca2+ concentration ([Ca2+]Lum) (n = 5–7 and n = 6–8). The [Ca2+]Lum in baseline conditions was 50 μM. (D) Mean relative Po ±SE of Y524S MHS RyR1 recorded at 100 nM cytoplasmic (cyt) Ca2+ plotted as the function of luminal Ca2+ concentration ([Ca2+]Lum) (n = 4–22). The [Ca2+]Lum in baseline conditions was 50 μM.
The Eu3+ inhibition of MHS-RyR1 was similar to that of the wild-type channels at both (50 μM and 100 nM) cytoplasmic [Ca2+]s (Fig. 7). These data argue against the previously published conclusions that increased sensitivity to SOICR would be a relevant pathomechanism in MHS (at least in the case of Y524S) (38).
Figure 7.
MHS RyR1 is inhibited by luminal Eu3+. (A and C) Single-channel current traces of Y524S MHS mouse RyR1 recorded at 50 μM or 100 nM cytoplasmic (cyt) Ca2+ and different concentrations of luminal (lum) Eu3+. The [Ca2+]Lum in baseline conditions was 50 μM. Closed state current levels are labeled by “c”. Channel openings are represented by downward deflections. (B) Pooled data of multiple RyR currents, similar to the example current shown in (A). Mean relative Po ±SE values of wild-type RyR1 (white spheres) and Y524S MHS RyR1 (black squares) recorded at 50 μM cytoplasmic (cyt) Ca2+ were plotted as the function of luminal (lum) Eu3+ concentration ([Eu3+]Lum) (n = 4–19 and (n = 6–8). (D) Pooled data of multiple RyR currents, similar to that of (C). Mean relative Po ±SE of Y524S MHS RyR1 recorded at 100 nM cytoplasmic (cis) Ca2+ plotted as the function of luminal Eu3+ concentration ([Eu3+]Lum) (n = 3–8).
Discussion
It has been long known that the Ca2+ content of the SR regulates Ca2+ release, but the exact mechanism is still unclear. Although there are many studies available in the literature, forming a uniform opinion is difficult, because of issues such as self-flux-regulation. We have compared the intrinsic luminal Ca2+ regulation of the two RyR isoforms in the same study for the first time, to our knowledge, while avoiding these difficult issues by using Eu3+, a specific agonist of the luminal Ca2+ binding sites. We demonstrate that 1) RyR2 is activated by luminal Ca2+, whereas RyR1 is inhibited, 2) these results were qualitatively reproducible using Eu3+, 3) under our experimental conditions, the impact of flux regulation is below the detection limit, 4) the inhibitory effect of Eu3+ on RyR1 was highly voltage dependent, whereas its activating effect on RyR2 was voltage-independent, and 5) the luminal regulation of the Y524S MHS-RyR1 channel is not different from the wild-type. The fact that depolarizing the membrane potential attenuates the inhibition and that the inhibition is stronger at negative voltages is explained as the movement of Eu3+ under the influence of the membrane field on its way to its binding site (see the illustration in Fig. 8 C).
Figure 8.
Eu3+ binding site prediction on the luminal side of RyR2. Representatives of the two most likely metal ion binding sites predicted for RyR2. The view in panels (A) and (B) is down the RyR2 pore from the luminal side of the channel; PDB structure 5T15 is shown. (A) The two sites are indicated using colored sticks. The spheres show the possible binding locations for the Eu3+ ions as predicted by Placevent. The site comprised of residues 4893–4903 (the selectivity filter site) is colored yellow. D4917, which was also predicted to potentially interact with the bound ion by IonCom, is colored orange. Eu3+ is labeled with a yellow sphere. The S1-S2 luminal loop site (aa 4538–4550) is not labeled, as it is not resolved in the EM structure. (B) The electrostatic surface area of the luminal pore area. The electrostatic potential surface is ramped from −10 (red) to +10 (blue) kT/e. It can be seen that the luminal mouth, where the selectivity filter site is located, has a much greater overall negative charge than the surface loops. Eu3+ is colored yellow. (C) Schematic drawing illustrating the location of the putative inhibitory Ca2+ binding sites near the gate in RyR1. The yellow arrow shows the movement of Eu3+ in the transmembrane electric field at −60 mV membrane potential. Eu3+ is colored yellow. (D) Illustration of the location of the putative inhibitory Ca2+ binding sites near the gate and the putative activating binding site in the S1-S2 loop (in purple) in RyR2. (E) Sequence alignment of the luminal loop putative site (top) and the luminal pore site (bottom) of RyR1 and RyR2 of different species. The EF-hand-like sequence is labeled with red and the pore binding site with green letters. (F) Sequence of the putative luminal binding sites. To see this figure in color, go online.
Impaired regulation results in uncontrolled Ca2+ release (SOICR), which is thought to be the cause of arrhythmias, for example in heart failure or CPVT, or in the muscle disease MHS (16,22,23,24,38). Halothane triggers an MH crisis by activating a susceptible mutant RyR (72,73). Recently, an alternative mechanism was proposed by Chen’s group in which halothane promotes Ca2+ release in MHS by reducing the threshold for SOICR (38). Unfortunately, as triggering SOICR in skeletal muscle fibers is not possible, all their Ca2+ imaging data was obtained using a heterologous expression system. Chen et al. could elicit SOICR from 12 different MHS-RyR1-overexpressing HEK cells (including Y523S-rabbit sequence, identical to Y524S in the mouse), in high Ca2+-containing extracellular solutions, but only when the cells were treated with 2 mM caffeine, a RyR agonist known to sensitize RyR to cytoplasmic Ca2+. Although bilayer experiments are missing in that study, the porcine R615C that was included in their experiments was shown previously to be activated by millimolar luminal [Ca2+] in lipid bilayer experiments (36,38). In contrast, our results in the present study show that both wild-type and Y524S MHS-RyR1 are inhibited by both Ca2+ and Eu3+ under similar experimental conditions, thereby refuting their idea. This discrepancy may arise from the fact that our RyRs were prepared from heterozygous mice, so our preparation contains RyR tetramers in which wild-type and Y524S MHS-RyR1 monomers are combined. Even though luminal Ca2+ was shown to activate MHS-RyR1 channels in that study, the fact that our RyRs are inhibited by luminal Ca2+ but our heterozygous mice still exhibited a strong MHS phenotype argues against the hypothesis that enhanced SOICR propensity is the underlying mechanism of MHS (72,74). The results by Chen et al. might be explained by self-flux activation of the MHS-RyRs. All in all, we conclude that low SOICR threshold is not a relevant mechanism in MHS (at least in the case of Y524S); thus CPVT and MHS do not share a common pathomechanism.
One important conclusion of this study is that canine RyR2 can be directly (i.e., in the absence of CSQ) activated by luminal Ca2+ (just like in (8,17,18,19,20,21)). Qin et al. showed that when CSQ was peeled off, the RyR2 channel was not sensitive to luminal Ca2+, which disagrees with our results (75). However, they tested 1 μM cytoplasmic Ca2+, whereas we showed that when cytosolic Ca2+ binding sites were not occupied by Ca2+, the effect of luminal Eu3+ was stronger. This could explain why Qin et al. did not show any luminal Ca2+ effect in 1 μM cytoplasmic Ca2+ when CSQ was removed, but we did at diastolic [Ca2+] (100 nM).
Eu3+ binding site prediction
Due to the pathological significance of the luminal binding site, it is of crucial importance to find the luminal Ca2+ sensor. Although, a cryo-EM reconstruction of RyR revealed the structural basis of gating and ligand-dependent activation, and several lines of evidence show that a luminal Ca2+ binding site exists, its exact location remains unknown. However, a popular mechanistic model of the process was created based on mutational analysis studies from Chen’s group. Investigating the role of amino acid residues near the gate of RyR2 (I4867) showed that the luminal Ca2+ regulation was disrupted by mutations of the amino acids E4872 and A4860. E4872 faces toward the cytoplasmic side of the gate, whereas A4860 is on the luminal side of the gate. Based on structural analysis, they proposed that a ring formed by glutamines (Q4863) provides a site for Ca2+ binding on the luminal side of the gate. Ca2+ binding to this site initiates the opening of RyR2, which is linked to the formation of a new binding site formed partially by E4872 on the cytoplasmic side of the gate. This model would perfectly describe the mechanical choreography of the “luminal-triggered Ca2+ feedthrough activation” mechanism, but unfortunately, it is incompatible with our current results. As the effect of luminal Ca2+ on RyR2 and RyR1 is opposite, but the primary sequences of the two isoforms in this region are identical, the model appears unlikely. Nevertheless, it is not surprising that the gate region, where all the conformational changes converge together to decide the channel state, is so sensitive to mutations.
Our finding that the effect of Eu3+ was biphasic on RyR2 seems to correlate well with previous data, for example that published by Ching et al., who showed that RyR2 was activated under control experimental conditions but inhibited after the channel was treated with trypsin (26). This result implies that there are two functionally distinct regulatory binding sites on the luminal face of RyR2, and that the function of the inhibitory site becomes unmasked when the activating site is destroyed by trypsinization.
Following this idea, Gaburjakova and Gaburjakova designed elegant experiments to show that Sr2+, Mg2+, and Ba2+ compete with Ca2+ for the same luminal binding site in RyR2, and their affinities displayed a strong correlation with that of the EF-hand motif, the most common sequence for Ca2+ binding in Ca2+ binding proteins. They performed an EF-hand pattern prediction analysis on the trypsin-accessible regions of the luminal RyR2 structure, which revealed an EF-hand like sequence within the intraluminal loop connecting the transmembrane helices 1 and 2 (S1-S2 loop, 4538–4550) (Fig. 8 E, top sequence alignment, in red) (76). Unfortunately, the loop is unresolved in the cryo-EM structure; thus it is hard to tell anything about its relationship to the transmembrane helices.
Comparing the loop primary amino acid sequences in the aligned RyR2 and RyR1 sequences, it is apparent that they are very different, and that RyR1 has a 10 amino acid residue insertion in the middle. We propose that this EF-hand like sequence is the luminal, activating Ca2+ binding site in RyR2, which is disrupted in RyR1. Our functional result, that the activation of RyR2 is voltage independent, is compatible with this model, as the EF-hand containing loop is not part of the pore; therefore it does not fall in the voltage drop (Fig. 8 D).
Since the inhibition of RyR1 was highly voltage dependent, we searched for the inhibiting binding site in the pore. We attempted to identify this site computationally using seven of the known structures of RyR1 together with five different computational methods (see materials and methods). Placevent, MIB, and IonCom identified possible binding sites in all seven structures. Placevent and MIB suggested a possible binding location including residues 4893–4903, which includes the selectivity filter (GGGIG, residues 4894–4898) (Fig. 8 A and B). Placevent tended to bind Eu3+ at the lowest concentration examined (10 mM). The most frequently identified sites by IonCom were D4899 and E4900 followed by D4917 and D4877. The first two are in the selectivity filter site, and the third is adjacent to it, whereas the fourth lies between the two sites. Fold-X found a high affinity binding site in only one structure, 3J8H, where Eu3+ was again found associated with residues 4899–4902 (Fig. 8 D). Finally, bindEmbed21, a method that makes use of only amino acid sequence information, also predicted that E4900 was involved in Ca2+ binding. bindEmbed21 was also able to locate the two EF-hand motifs of RyR1, the Zn2+ binding site in the C-terminal domain, and one residue in the known cytosolic Ca2+ binding site, so its prediction of E4900 is likely to be reliable. Moreover, the residues within the putative selectivity filter site (4893–4903) are close enough to the intersubunit boundaries that residues from more than one chain may be involved in binding a given ion, which might promote some rearrangement of the transmembrane helices. Finally, as will be described below, the presence of D4917, either as a component of the selectivity filter binding site or as an adjacent residue, also suggests a possible mechanism by which the binding of either Eu3+ or Ca2+ might affect channel gating. Previous studies have shown that D4899 and E4900 have important roles in ion permeation and selectivity (77,78) One of these studies also showed that D4917, one of the residues predicted by IonCom to bind Ca2+, is also important for conductance and the Ca2+ regulation of RyR1 (77). D4917 lies in helix S6, whose movement is crucial to channel gating. It is close enough to the selectivity filter that it could presumably form part of the Ca2+ binding site directly or could interact with the other residues that do so, thereby providing a mechanism by which channel opening might be triggered (51).
A recent molecular dynamics study also highlighted the importance of residues 4899 and 4900 and showed that saturating the selectivity filter with divalent Ca2+ prevented the permeation of K+ ions through a previously described charge/space mechanism (79,80). Presumably, a similar mechanism might also account for the ability of Eu3+ to effectively block the channel.
The amino acid sequence (4893–4908) is very conserved between the two isoforms and among species, indicating that the two isoforms share the same inhibiting binding site, as also suggested by our results (Fig. 8 E, bottom sequence alignment, in green).
The sequence of the putative luminal binding sites is given in the table of Fig. 8 F.
Almost every residue in this region of RyR1 (4893–4903 + 4917) has at least one disease-associated mutation (MHS, central core disease, and myopathy). Interestingly enough, the same area in RyR2 does not have nearly so many disease-associated mutations in this area (only a few, CPVT).
Also, our results showed that direct binding to the channel pore (Q4933) is also possible, which is consistent with the observation that Eu3+ causes complete pore block at high concentrations (Fig. S2).
Role of SR Ca2+ in regulating Ca2+ release
Since SR Ca2+ does not merely regulate its own release by interacting with the intrinsic RyR Ca2+ binding sites, our results need to be put into a wider physiological context for their importance to be properly evaluated. The best way to do this is to answer the questions “if luminal Ca2+ inhibits RyR1, how do Ca2+ sparks develop in skeletal muscle fibers?” and “if luminal Ca2+ activates RyR2, what makes Ca2+ release refractory in diastole? (81,82,83,84)” To answer these questions, we need to consider two things: that increasing the SR Ca2+ content may affect RyR gating, and that it certainly increases the force driving Ca2+ across the SR membrane. Since both of these are expected to influence Ca2+ release at the same time, their relative significance is difficult to distinguish experimentally. Here, we summarize some studies that attempted to separate these two factors.
In skeletal muscle fibers (in contrast to cardiomyocytes), significant SR depletion is associated with increased SR membrane permeability (84,85,86), which is consistent with the observation that a constant amount of Ca2+ is released at different SR Ca2+ concentrations (87,88). Launikonis et al. systematically investigated the question of how Ca2+ spark frequency depends on SR Ca2+ content and concluded that SR Ca2+ concentration is only a minor determinant of spark frequency (in contrast to cardiomyocytes, where increased SR load increases spark frequency) (87). Both of these observations are in line with our observations that luminal Ca2+ inhibits RyR1. Altogether, we can form a general model in which the SR [Ca2+] (which is around 400 μM (89)) does not reach concentrations high enough to reduce the Po to 0; we can therefore expect RyR opening events in fibers prepared for spark measurements. And whereas RyR1 is inhibited by luminal Ca2+, an increasing SR load would be linked to an increasing driving force, resulting in a larger Ca2+ flux when a RyR spontaneously opens in a Ca2+ release unit, thereby promoting a stronger CICR in the other RyRs in the same unit. If the increased flux rate overcomes the influence of luminal inhibition, the resulting effect will be a larger mean flux and slightly increased spark frequency at higher SR Ca2+ concentrations.
In cardiomyocytes, elevation of the SR Ca2+ content increases spark frequency more significantly (10). Fill’s group investigated the role of luminal Ca2+ in the local regulation of sparks using Tris, a large organic cation that interferes with the Ca2+ current (90). In the presence of Tris, increasing SR load was associated with reduced Ca2+ spark frequency; therefore they concluded that CICR predominantly depends on the magnitude of the RyR current and not on luminal Ca2+ directly gating the RyR through luminal binding sites. Thus, spark frequency increases with SR load because spontaneous RyR openings produce a larger Ca2+ current (due to a higher driving force), which serves as a stronger CICR trigger.
These experiments give a reasonable answer for the long-standing question of how Ca2+ release is terminated: CICR appears to be a positive feedback mechanism and should theoretically last until the SR completely empties; however a single Ca2+ release event still leaves an ample Ca2+ reserve in the SR (91), meaning that the release does not end because the SR has completely emptied. The authors argued that the RyR current collapses during Ca2+ release and stops feeding CICR with Ca2+, leading to sudden spark termination. They suggested that the current becomes insufficient well before the Ca2+ is significantly depleted from the SR (90).
From the perspective of our results, their findings mean that a high SR Ca2+ concentration does not affect RyR gating directly, raising the question of whether luminal regulation plays a significant role in cardiac physiology at all. Nevertheless, in pathological conditions that are associated with the oversensitivity of RyR2 to SR Ca2+ (such as CPVT and CHF), SOICR is thought to be the underlying mechanism of the disease (16,23,92). The next related question is how is it that SR Ca2+ release is suppressed in resting skeletal muscle fibers or during diastole, whereas SR [Ca2+] constantly increases? That is, “what is the mechanism of RyR2 refractoriness? (81,82,83)” The low RyR2 current at low SR load may explain refractoriness as well, but another possibility also exists. It has been observed that Ca2+ release terminates at 60% residual SR Ca2+, suggesting that this reduction in the driving force might be enough to sufficiently attenuate the RyR current in a Ca2+ buffered system to cause the collapse of a spark (91,93). A mathematical model by Sobie et al. supported the idea that SR Ca2+ depletion is coupled to a Ca2+-dependent change in RyR2 gating, which contributes to Ca2+ release termination and subsequent RyR2 refractoriness (94). This hypothesis was confirmed by further studies (95,96,97,98). Although there is a consensus that RyR2 gating is directly controlled by luminal Ca2+, there is also an alternative mechanism that is widely accepted (16,23,92), which holds that the sensitivity of RyR2 to luminal Ca2+ is mediated by the accessory protein CSQ (93,99,100).
The role of calsequestrin in regulating RyR activity
CSQ is the Ca2+ buffer protein of the SR, which also regulates RyR activity as the SR [Ca2+] changes. The most convincing evidence supporting the significance of CSQ in RyR2 regulation is the finding that CSQ mutants are linked to CPVT independent of their Ca2+ buffering properties (101,102). This phenotype is consistent with earlier bilayer studies showing that CSQ inhibits channel activity. CSQ regulation is dynamic and relies on the Ca2+-dependent conformational change of CSQ. 10 μM Ca2+ causes compaction of the CSQ structure, whereas higher [Ca2+] (10–100 μM) induces CSQ polymerization, which stabilizes at 1 mM Ca2+ (103,104). These polymers bind to RyR through a macromolecular complex formed by triadin and junction, and this complex inhibits the channel activity, reaching maximal inhibition at 1 mM Ca2+. At >5 mM Ca2+, CSQ dissociates from RyR, thereby freeing it from inhibition (47,48,103,104,105). Since this [Ca2+] is expected to directly activate RyR2, a spontaneous Ca2+ release event will then occur. This model is consistent with the observation that overexpression of CSQ causes a large increase in Ca2+ content and release (106,107,108). The importance of the intrinsic RyR2 Ca2+ binding sites was shown in a CSQ KO mouse, which exhibited CPVT (109). In the CSQ KO animal, there is no CSQ inhibition, which leads to abnormal Ca2+ release and Ca2+ waves during diastole and, thus, triggered activity (110,111). CSQ makes the heart CPVT resistant, because it helps suppress spontaneous Ca2+ release during diastole (adjusts the threshold [Ca2+]SR for Ca2+-release termination) (112,113).
Interestingly, the effect of CSQ was reported to depend on the presence of MgATP on the cytosolic face of RyR2. Bilayer experiments by Chen et al. clearly showed that when the cytoplasmic recording medium contained 1 μM Ca2+ and was supplemented with MgATP, CSQ addition led to channel inhibition. In contrast, when 1 μM Ca2+ alone was present, CSQ addition resulted in channel activation (114). Two other studies also reported activation by CSQ when only cytoplasmic Ca2+ was present in the recording medium (75,115). The presence of MgATP is physiological, and therefore the effect of CSQ under these conditions is more relevant.
In skeletal muscle, CSQ was shown to accelerate Ca2+ flux during the middle of a Ca2+ release event, which was explained as arising from the additional Ca2+ released by CSQ as it depolymerizes (116). This also has the effect of lifting the CSQ inhibition from RyR1, which may also help maintain the flux rate over the course of the Ca2+ release.
The different degrees of spark frequency response to enhanced SR load in skeletal muscle (mild increase) and cardiomyocytes (robust increase) may be explained by the inhibitory action of luminal Ca2+ on RyR1. CSQ is believed to buffer the [Ca2+] to near 1 mM in the vicinity of RyR. As this [Ca2+] was shown to strongly inhibit RyR1, we suggest that this inherent Ca2+ inhibition prevails, leaving only a complementary, minor role for CSQ in suppressing Ca2+ release in the resting skeletal muscle.
CSQ does appear to play an essential role in the regulation of Ca2+ release, but its function has not been fully elucidated. Further understanding of its function would be facilitated by testing it on RyRs resistant to luminal Ca2+.
Conclusion
Based on this structural analysis and our functional data, we propose that RyR2 carries two binding sites: an activating site in the S1-S2 loop and an inhibiting site located in the pore (see the cartoon in Fig. 8 C and D). The activating site dominates over the function of the inhibiting site, whose significance is negligible. In RyR1, in contrast, the activating binding site is missing, and the inhibiting site affinity is high enough to help significantly suppress RyR1’s activity in resting muscle, where the average [Ca2+] of the SR falls in the range applied during this study (89). During Ca2+ release, when the [Ca2+] is decreasing, RyR1 is gradually freed from inhibition, compensating for the decreasing flux rate due to diminishing driving forces.
Author contributions
Z.É.M.: investigation, formal analysis, visualization, and writing – original draft. J.B.: investigation, conceptualization, visualization, writing – original draft, and writing – review and editing. V.B.-H.: investigation, conceptualization, and visualization. I.J.: writing – review and editing, J.G.: validation and writing – review and editing. M.G.: validation and writing – review and editing. J.A.: conceptualization, investigation, formal analysis, visualization, writing – original draft, supervision, and funding acquisition.
Acknowledgments
We thank József Orosz for his technical assistance. This work was funded by the National Research, Development and Innovation Fund of Hungary provided to J.A. (NKFIH FK144576). J.A. is supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences. Z.É.M. was supported by the New National Excellence Program (ÚNKP-22-4-I-DE-28) of the Ministry for Innovation and Technology of Hungary.
This research was also funded by VEGA, the Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences (VEGA 2/0131/20 and VEGA 2/0008/20), and Interreg SK-AT StruBioMol ITMS: 305011X666.
Declaration of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Editor: Eleonora Grandi.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2023.07.029.
Supporting material
Data and code availability
All data are contained within the article and available upon request.
References
- 1.Fill M., Copello J.A. Ryanodine Receptor Calcium Release Channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 2.Sárközi S., Szegedi C., et al. Jóna I. Regulation of the rat sarcoplasmic reticulum calcium release channel by calcium. J. Muscle Res. Cell Motil. 2000;21:131–138. doi: 10.1023/a:1005630321863. [DOI] [PubMed] [Google Scholar]
- 3.Szigeti G.P., Almássy J., et al. Jóna I. Alterations in the calcium homeostasis of skeletal muscle from postmyocardial infarcted rats. Pflügers Archiv. 2007;455:541–553. doi: 10.1007/s00424-007-0298-z. [DOI] [PubMed] [Google Scholar]
- 4.Meissner G. Ryanodine Receptor/Ca2+ Release Channels and Their Regulation by Endogenous Effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 5.Ashley R.H., Williams A.J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J. Gen. Physiol. 1990;95:981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copello J.A., Barg S., et al. Fleischer S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys. J. 1997;73:141–156. doi: 10.1016/S0006-3495(97)78055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laver D.R., Roden L.D., et al. Dulhunty A.F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J. Membr. Biol. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- 8.Laver D.R. Regulation of the RyR channel gating by Ca2+ and Mg2. Biophys. Rev. 2018;10:1087–1095. doi: 10.1007/s12551-018-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabiato A. Two Kinds of Calcium-Induced Release of Calcium from the Sarcoplasmic Reticulum of Skinned Cardiac Cells. Adv. Exp. Med. Biol. 1992:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- 10.Satoh H., Blatter L.A., Bers D.M. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol Circ Physiol. 1997;272:H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H., Lederer M.R., et al. Cannell M.B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 12.Shannon T.R., Ginsburg K.S., Bers D.M. Potentiation of Fractional Sarcoplasmic Reticulum Calcium Release by Total and Free Intra-Sarcoplasmic Reticulum Calcium Concentration. Biophys. J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terentyev D., Viatchenko-Karpinski S., et al. Györke S. Luminal Ca 2+ Controls Termination and Refractory Behavior of Ca 2+ -Induced Ca 2+ Release in Cardiac Myocytes. Circ. Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 14.Lukyanenko V., Györke I., Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Archiv. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- 15.Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D., Xiao B., et al. Chen S.R.W. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc. Natl. Acad. Sci. USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitsapesan R., Williams A.J. Regulation of current flow through ryanodine receptors by luminal Ca2+ J. Membr. Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- 18.Sitsapesan R., Williams A.J. The gating of the sheep skeletal sarcoplasmic reticulum Ca(2+)-release channel is regulated by luminal Ca2+ J. Membr. Biol. 1995;146:133–144. doi: 10.1007/BF00238004. [DOI] [PubMed] [Google Scholar]
- 19.Xu L., Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys. J. 1998;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathy A., Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones P.P., Guo W., Chen S.R.W. Control of cardiac ryanodine receptor by sarcoplasmic reticulum luminal Ca2. J. Gen. Physiol. 2017;149:867–875. doi: 10.1085/jgp.201711805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang D., Wang R., et al. Chen S.R.W. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ. Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 23.Jiang D., Chen W., et al. Chen S.R.W. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc. Natl. Acad. Sci. USA. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones P.P., Jiang D., et al. Chen S.R.W. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem. J. 2008;412:171–178. doi: 10.1042/BJ20071287. [DOI] [PubMed] [Google Scholar]
- 25.Györke I., Györke S. Regulation of the Cardiac Ryanodine Receptor Channel by Luminal Ca2+ Involves Luminal Ca2+ Sensing Sites. Biophys. J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ching L.L., Williams A.J., Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 27.Laver D.R. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys. J. 2007;34:889–896. doi: 10.1111/j.1440-1681.2007.04708.x. [DOI] [PubMed] [Google Scholar]
- 28.Laver D.R. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin. Exp. Pharmacol. Physiol. 2007;34:889–896. doi: 10.1111/j.1440-1681.2007.04708.x. [DOI] [PubMed] [Google Scholar]
- 29.Laver D.R., Honen B.N. Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: cytoplasmic and luminal regulation modeled in a tetrameric channel. J. Gen. Physiol. 2008;132:429–446. doi: 10.1085/jgp.200810001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLennan D., Phillips M. Malignant hyperthermia. Science. 1992;256:789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- 31.MacLennan D.H., Duff C., et al. Worton R.G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343:559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- 32.Kolb M., Horne M., Martz R. Dantrolene in Human Malignant Hyperthermia A Multicenter Study. Anesthesiology. 1982;56:254–262. doi: 10.1097/00000542-198204000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg H., Davis M., et al. Stowell K. Malignant hyperthermia. Orphanet J. Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riazi S., Kraeva N., Hopkins P.M. Malignant Hyperthermia in the Post-Genomics Era: New Perspectives on an Old Concept. Anesthesiology. 2018;128:168–180. doi: 10.1097/ALN.0000000000001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson T.E., Lin M., Volpe P. Evidence for intraluminal Ca++ regulatory site defect in sarcoplasmic reticulum from malignant hyperthermia pig muscle. J. Pharmacol. Exp. Therapeut. 1991;256:645–649. [PubMed] [Google Scholar]
- 36.Jiang D., Chen W., et al. Chen S.R.W. Reduced threshold for luminal Ca2+ activation of RyR1 underlies a causal mechanism of porcine malignant hyperthermia. J. Biol. Chem. 2008;283:20813–20820. doi: 10.1074/jbc.M801944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacLennan D.H., Chen S.R.W. Store overload-induced Ca 2+ release as a triggering mechanism for CPVT and MH episodes caused by mutations in RYR and CASQ genes. J. Physiol. 2009;587:3113–3115. doi: 10.1113/jphysiol.2009.172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W., Koop A., et al. Chen S.R.W. Reduced threshold for store overload-induced Ca2+ release is a common defect of RyR1 mutations associated with malignant hyperthermia and central core disease. Biochem. J. 2017;474:2749–2761. doi: 10.1042/BCJ20170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrmann-Frank A., Lehmann-Horn F. Regulation of the purified Ca2+ release channel/ryanodine receptor complex of skeletal muscle sarcoplasmic reticulum by luminal calcium. Pflügers Archiv. 1996;432:155–157. doi: 10.1007/s004240050117. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y., Porta M., et al. Fill M. Flux regulation of cardiac ryanodine receptor channels. J. Gen. Physiol. 2010;135:15–27. doi: 10.1085/jgp.200910273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laver D.R. Luminal Ca(2+) activation of cardiac ryanodine receptors by luminal and cytoplasmic domains. Eur. Biophys. J. 2009;39:19–26. doi: 10.1007/s00249-009-0417-1. [DOI] [PubMed] [Google Scholar]
- 42.Sárközi S., Komáromi I., et al. Almássy J. Lanthanides Report Calcium Sensor in the Vestibule of Ryanodine Receptor. Biophys. J. 2017;112:2127–2137. doi: 10.1016/j.bpj.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadad N., Zable A.C., et al. Shoshan-Barmatz V. Ca2+ binding sites of the ryanodine receptor/Ca2+ release channel of sarcoplasmic reticulum. Low affinity binding site(s) as probed by terbium fluorescence. J. Biol. Chem. 1994;269:24864–24869. [PubMed] [Google Scholar]
- 44.Pidcock E., Moore G.R. Structural characteristics of protein binding sites for calcium and lanthanide ions. J. Biol. Inorg. Chem. 2001;6:479–489. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 45.Switzer M.E. The lanthanide ions as probes of calcium ion binding sites in biological systems. Sci. Prog. 1978;65:19–30. [PubMed] [Google Scholar]
- 46.dos Remedios C.G. Lanthanide ion probes of calcium-binding sites on cellular membranes. Cell Calcium. 1981;2:29–51. [Google Scholar]
- 47.Beard N.A., Sakowska M.M., et al. Laver D.R. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beard N.A., Casarotto M.G., et al. Dulhunty A.F. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys. J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Györke I., Hester N., et al. Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szegedi C., Sárközi S., et al. Varsányi M. Calsequestrin: more than “only” a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 1999;337:19–22. [PMC free article] [PubMed] [Google Scholar]
- 51.des Georges A., Clarke O.B., et al. Frank J. Structural Basis for Gating and Activation of RyR1. Cell. 2016;167:145–157.e17. doi: 10.1016/j.cell.2016.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng W., Shen H., et al. Yan N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science. 2016;354:aah5324. doi: 10.1126/science.aah5324. [DOI] [PubMed] [Google Scholar]
- 53.Nayak A.R., Samsó M. Ca2+ inactivation of the mammalian ryanodine receptor type 1 in a lipidic environment revealed by cryo-EM. Elife. 2022:11. doi: 10.7554/eLife.75568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geyer N., Diszházi G., et al. Almássy J. Bile acids activate ryanodine receptors in pancreatic acinar cells via a direct allosteric mechanism. Cell Calcium. 2015;58:160–170. doi: 10.1016/j.ceca.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Altafaj X., France J., et al. Ronjat M. Maurocalcine interacts with the cardiac ryanodine receptor without inducing channel modification. Biochem. J. 2007;406:309–315. doi: 10.1042/BJ20070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Littmann M., Heinzinger M., et al. Rost B. Protein embeddings and deep learning predict binding residues for various ligand classes. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-03431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Y.-F., Cheng C.-W., et al. Lu C.-H. MIB: Metal Ion-Binding Site Prediction and Docking Server. J. Chem. Inf. Model. 2016;56:2287–2291. doi: 10.1021/acs.jcim.6b00407. [DOI] [PubMed] [Google Scholar]
- 58.Schymkowitz J.W.H., Rousseau F., et al. Serrano L. Prediction of water and metal binding sites and their affinities by using the Fold-X force field. Proc. Natl. Acad. Sci. USA. 2005;102:10147–10152. doi: 10.1073/pnas.0501980102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sindhikara D.J., Yoshida N., Hirata F. Placevent: an algorithm for prediction of explicit solvent atom distribution-application to HIV-1 protease and F-ATP synthase. J. Comput. Chem. 2012;33:1536–1543. doi: 10.1002/jcc.22984. [DOI] [PubMed] [Google Scholar]
- 60.Hu X., Dong Q., et al. Zhang Y. Recognizing metal and acid radical ion-binding sites by integrating ab initio modeling with template-based transferals. Bioinformatics. 2016;32:3260–3269. doi: 10.1093/bioinformatics/btw396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan Z., Bai X., et al. Yan N. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517:50–55. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai X.-C., Yan Z., et al. Yan N. The Central domain of RyR1 is the transducer for long-range allosteric gating of channel opening. Cell Res. 2016;26:995–1006. doi: 10.1038/cr.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma R., Haji-Ghassemi O., et al. Yuchi Z. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020;16:1246–1254. doi: 10.1038/s41589-020-0627-5. [DOI] [PubMed] [Google Scholar]
- 64.Melville Z., Dridi H., et al. Marks A.R. A drug and ATP binding site in type 1 ryanodine receptor. Structure. 2022;30:1025–1034.e4. doi: 10.1016/j.str.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Iyer K.A., Hu Y., et al. Samsó M. Molecular mechanism of the severe MH/CCD mutation Y522S in skeletal ryanodine receptor (RyR1) by cryo-EM. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2122140119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koski J.P., Moore S.G., et al. Modine N.A. Water in an External Electric Field: Comparing Charge Distribution Methods Using ReaxFF Simulations. J. Chem. Theor. Comput. 2022;18:580–594. doi: 10.1021/acs.jctc.1c00975. [DOI] [PubMed] [Google Scholar]
- 67.Li P., Song L.F., Merz K.M. Parameterization of highly charged metal ions using the 12-6-4 LJ-type nonbonded model in explicit water. J. Phys. Chem. B. 2015;119:883–895. doi: 10.1021/jp505875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jurrus E., Engel D., et al. Baker N.A. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018;27:112–128. doi: 10.1002/pro.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sitsapesan R., Williams A.J. Regulation of Current Flow through Ryanodine Receptors by Luminal Ca 2+ J. Membr. Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- 70.Lansman J.B. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J. Gen. Physiol. 1990;95:679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng W., Barrientos G.C., et al. Pessah I.N. Functional and biochemical properties of ryanodine receptor type 1 channels from heterozygous R163C malignant hyperthermia-susceptible mice. Mol. Pharmacol. 2011;79:420–431. doi: 10.1124/mol.110.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chelu M.G., Goonasekera S.A., et al. Hamilton S.L. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. Faseb. J. 2006;20:329–330. doi: 10.1096/fj.05-4497fje. [DOI] [PubMed] [Google Scholar]
- 73.MacLennan D., Phillips M. Malignant hyperthermia. Science. 1992;256:789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- 74.Lanner J.T., Georgiou D.K., et al. Hamilton S.L. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat. Med. 2012;18:244–251. doi: 10.1038/nm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin J., Valle G., et al. Fill M. Luminal Ca2+ Regulation of Single Cardiac Ryanodine Receptors: Insights Provided by Calsequestrin and its Mutants. J. Gen. Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaburjakova J., Gaburjakova M. Cardiac ryanodine receptor: Selectivity for alkaline earth metal cations points to the EF-hand nature of luminal binding sites. Bioelectrochemistry. 2016;109:49–56. doi: 10.1016/j.bioelechem.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Gao L., Balshaw D., et al. Meissner G. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca(2+) release channel (ryanodine receptor) activity and conductance. Biophys. J. 2000;79:828–840. doi: 10.1016/S0006-3495(00)76339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y., Xu L., et al. Meissner G. Probing the role of negatively charged amino acid residues in ion permeation of skeletal muscle ryanodine receptor. Biophys. J. 2005;89:256–265. doi: 10.1529/biophysj.104.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heinz L.P., Kopec W., et al. Fink R.H.A. In silico assessment of the conduction mechanism of the Ryanodine Receptor 1 reveals previously unknown exit pathways. Sci. Rep. 2018;8:6886. doi: 10.1038/s41598-018-25061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillespie D. Energetics of divalent selectivity in a calcium channel: the ryanodine receptor case study. Biophys. J. 2008;94:1169–1184. doi: 10.1529/biophysj.107.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sobie E.A., Song L.-S., Lederer W.J. Local recovery of Ca 2+ release in rat ventricular myocytes. J. Physiol. 2005;565:441–447. doi: 10.1113/jphysiol.2005.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramay H.R., Liu O.Z., Sobie E.A. Recovery of cardiac calcium release is controlled by sarcoplasmic reticulum refilling and ryanodine receptor sensitivity. Cardiovasc. Res. 2011;91:598–605. doi: 10.1093/cvr/cvr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belevych A.E., Terentyev D., et al. Györke S. Shortened Ca 2+ Signaling Refractoriness Underlies Cellular Arrhythmogenesis in a Postinfarction Model of Sudden Cardiac Death. Circ. Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pape P.C., Carrier N. Effect of Sarcoplasmic Reticulum (SR) Calcium Content on SR Calcium Release Elicited by Small Voltage-Clamp Depolarizations in Frog Cut Skeletal Muscle Fibers Equilibrated with 20 mM EGTA. J. Gen. Physiol. 1998;112:161–179. doi: 10.1085/jgp.112.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pape P.C., Jong D.-S., Chandler W.K. Effects of Partial Sarcoplasmic Reticulum Calcium Depletion on Calcium Release in Frog Cut Muscle Fibers Equilibrated with 20 mM EGTA. J. Gen. Physiol. 1998;112:263–295. doi: 10.1085/jgp.112.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pizarro G., Ríos E. How Source Content Determines Intracellular Ca2+ Release Kinetics. Simultaneous Measurement of [Ca2+] Transients and [H+] Displacement in Skeletal Muscle. J. Gen. Physiol. 2004;124:239–258. doi: 10.1085/jgp.200409071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Launikonis B.S., Zhou J., et al. Ríos E. The Changes in Ca2+ Sparks Associated with Measured Modifications of Intra-store Ca2+ Concentration in Skeletal Muscle. J. Gen. Physiol. 2006;128:45–54. doi: 10.1085/jgp.200609545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Posterino G.S., Lamb G.D. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J. Physiol. 2003;551:219–237. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ziman A.P., Ward C.W., et al. Bloch R.J. Quantitative measurement of Ca2(+) in the sarcoplasmic reticulum lumen of mammalian skeletal muscle. Biophys. J. 2010;99:2705–2714. doi: 10.1016/j.bpj.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo T., Gillespie D., Fill M. Ryanodine Receptor Current Amplitude Controls Ca 2+ Sparks in Cardiac Muscle. Circ. Res. 2012;111:28–36. doi: 10.1161/CIRCRESAHA.112.265652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bassani J.W., Yuan W., Bers D.M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am. J. Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 92.Priori S.G., Chen S.R.W. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Györke S., Belevych A.E., et al. Radwański P.B. The role of luminal Ca regulation in Ca signaling refractoriness and cardiac arrhythmogenesis. J. Gen. Physiol. 2017;149:877–888. doi: 10.1085/jgp.201711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sobie E.A., Dilly K.W., et al. Jafri M.S. Termination of Cardiac Ca2+ Sparks: An Investigative Mathematical Model of Calcium-Induced Calcium Release. Biophys. J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kubalova Z., Terentyev D., et al. Györke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl. Acad. Sci. USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Picht E., DeSantiago J., et al. Bers D.M. Cardiac Alternans Do Not Rely on Diastolic Sarcoplasmic Reticulum Calcium Content Fluctuations. Circ. Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 97.Belevych A., Kubalova Z., et al. Györke S. Enhanced Ryanodine Receptor-Mediated Calcium Leak Determines Reduced Sarcoplasmic Reticulum Calcium Content in Chronic Canine Heart Failure. Biophys. J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zima A.V., Picht E., et al. Blatter L.A. Termination of Cardiac Ca 2+ Sparks. Circ. Res. 2008;103 doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Györke S., Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 100.Faggioni M., Knollmann B.C. Calsequestrin 2 and arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1250–H1260. doi: 10.1152/ajpheart.00779.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Houle T., Ram M., Cala S. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc. Res. 2004;64:227–233. doi: 10.1016/j.cardiores.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Terentyev D., Nori A., et al. Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 103.Wei L., Varsányi M., et al. Beard N.A. The Conformation of Calsequestrin Determines Its Ability to Regulate Skeletal Ryanodine Receptors. Biophys. J. 2006;91:1288–1301. doi: 10.1529/biophysj.106.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beard N.A., Laver D.R., Dulhunty A.F. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog. Biophys. Mol. Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Gaburjakova M., Bal N.C., et al. Periasamy M. Functional interaction between calsequestrin and ryanodine receptor in the heart. Cell. Mol. Life Sci. 2013;70:2935–2945. doi: 10.1007/s00018-012-1199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones L.R., Suzuki Y.J., et al. Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J. Clin. Invest. 1998;101:1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sato Y., Ferguson D.G., et al. Kranias E.G. Cardiac-specific Overexpression of Mouse Cardiac Calsequestrin Is Associated with Depressed Cardiovascular Function and Hypertrophy in Transgenic Mice. J. Biol. Chem. 1998;273:28470–28477. doi: 10.1074/jbc.273.43.28470. [DOI] [PubMed] [Google Scholar]
- 108.Terentyev D., Viatchenko-Karpinski S., et al. Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc. Natl. Acad. Sci. USA. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Knollmann B.C., Chopra N., et al. Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Postma A.V., Denjoy I., et al. Guicheney P. Absence of Calsequestrin 2 Causes Severe Forms of Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 111.Liu B., Walton S.D., et al. Györke S. Gene Transfer of Engineered Calmodulin Alleviates Ventricular Arrhythmias in a Calsequestrin-Associated Mouse Model of Catecholaminergic Polymorphic Ventricular Tachycardia. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kornyeyev D., Petrosky A.D., et al. Escobar A.L. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J. Mol. Cell. Cardiol. 2012;52:21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Terentyev D., Kubalova Z., et al. Gyorke S. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys. J. 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen H., Valle G., et al. Volpe P. Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J. Gen. Physiol. 2013;142:127–136. doi: 10.1085/jgp.201311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qin J., Valle G., et al. Fill M. Ryanodine Receptor Luminal Ca2+ Regulation: Swapping Calsequestrin and Channel Isoforms. Biophys. J. 2009;97:1961–1970. doi: 10.1016/j.bpj.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Manno C., Figueroa L.C., et al. Rios E. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. USA. 2017;114:E638–E647. doi: 10.1073/pnas.1620265114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article and available upon request.