Abstract
As the rate of discovery of drug-resistant Helicobacter pylori cases increases worldwide, the relevant societies have updated their guidelines for primary eradication regimens. A promising strategy against drug-resistant H. pylori is tailored therapy based on the results of an antibiotic susceptibility test; however, it is difficult to apply this strategy to all cases. Although culture-based antibiotic susceptibility tests can assess resistance to any antimicrobial agent, their greatest disadvantage is the time required to draw a conclusion. In contrast, molecular-based methods, such as polymerase chain reaction, can rapidly determine the presence of resistance, although a single test can only test for one type of antimicrobial agent. Additionally, the limited availability of facilities for molecular-based methods has hindered their widespread use. Therefore, low-cost, minimally invasive, simple, and effective primary regimens are needed. Several studies have compared the efficacy of the latest primary eradication regimens against that of tailored therapies, and their results have shaped guidelines. This article reviews the latest research on empirical and tailored treatments for H. pylori infections. Evidence for the superiority of tailored therapy over empirical therapy is still limited and varies by region and treatment regimen. A network meta-analysis comparing different empirical treatment regimens showed that vonoprazan triple therapy provides a superior eradication effect. Recently, favorable results towards vonoprazan dual therapy have been reported, as it reached eradication levels similar to those of vonoprazan triple therapy. Both vonoprazan dual therapy and tailored therapy based on antibiotic susceptibility tests could contribute to future treatment strategies.
Keywords: Helicobacter pylori, Antibiotics, Drug resistance, Bacterial susceptibility test, Vonoprazan
INTRODUCTION
Helicobacter pylori is a cause of gastritis and peptic ulcers, as well as a major cause of gastric carcinogenesis.1,2 Eradication of H. pylori has been shown to reduce metachronous carcinogenesis.3 Over the past 25 years, the number of infected patients4,5 and new cases of gastric cancer6 had decreased worldwide owing to the widespread adoption of eradication therapy and improved sanitation. In the 1990s, triple therapy (which combined a proton pump inhibitor [PPI] with clarithromycin, amoxicillin, or metronidazole) showed a high eradication success rate and became popular worldwide. This led to a growth in the number of clarithromycin-resistant strains and more frequent failures of empirical primary treatment. In Asia, the prevalence of clarithromycin-resistant strains has been reported to be 30% in Japan, 50% in China, 40% in Korea, and approximately 15% in Taiwan.7 The gold standard eradication therapy for H. pylori infection is tailored therapy, which is based on the selection of antimicrobial agents according to antibiotic susceptibility test (AST) results8 as well as other common bacterial infectious diseases. However, AST has several disadvantages and is not widely used.9 This review presents the latest results of empirical and AST-based therapies for H. pylori to inform future treatment strategies.
STANDARD TREATMENT REGIMENS BASED ON CURRENT GUIDELINES
The primary treatment regimens recommended by current guidelines are listed in Table 1. For areas where clarithromycin-resistant strain rate is 15% to 20% or higher, the Maastricht VI/Florence Consensus Report recommends bismuth-containing quadruple therapy (BQT), consisting of a PPI and two antimicrobial agents plus bismuth, or quadruple concomitant therapy using a PPI and three antimicrobial agents (amoxicillin, clarithromycin, and nitroimidazole) for the same duration as the primary treatment.10 In contrast, PPI-based standard triple therapy (STT) is recommended only in areas with a clarithromycin-resistant strain rate of ≤15%. In addition, for both treatment options, a 14-day duration of eradication was reported to increase the eradication success rate when compared to that of a shorter duration.11,12 This supports the 14-day duration of eradication recommended by the guidelines.10 Similarly, the Toronto consensus guidelines recommend selecting empirical primary eradication according to local clarithromycin resistance rates.13
Table 1.
Comparison of Recommended Eradication Regimens Based on Guidelines
| Guideline | First-line therapy | Salvage therapy |
|---|---|---|
| Maastricht VI/Florence Consensus Report (2022)10 | Area of clarithromycin resistance <15%: PPI-based triple therapy for 14 days |
Bismuth-containing quadruple therapy for 14 days |
| Area of clarithromycin resistance ≥15%: Bismuth-containing quadruple therapy for 14 days Quadruple concomitant therapy for 14 days |
Fluoroquinolone-containing quadruple or triple therapy for 14 days | |
| Tailored therapy based on the result of AST (third line) | ||
| Toronto Consensus (2016)13 | Area of clarithromycin resistance <15%: PPI-based triple therapy for 14 days |
Bismuth-containing quadruple therapy for 14 days |
| Area of clarithromycin resistance ≥15%: Bismuth-containing quadruple therapy for 14 days Quadruple concomitant therapy for 14 days |
Fluoroquinolone-containing triple therapy for 14 days | |
| ACG Clinical Guideline (2016)18 | Area of clarithromycin resistance <15%, and no history of clarithromycin use: PPI-based triple therapy for 14 days |
Bismuth-containing quadruple therapy for 14 days |
| Fluoroquinolone-containing quadruple or triple therapy for 14 days | ||
| Area of clarithromycin resistance ≥15%, or history of clarithromycin use: Bismuth-containing quadruple therapy for 10–14 days Quadruple concomitant therapy for 10–14 days |
Quadruple concomitant therapy for 10 days | |
| Rifabutin-containing triple therapy for 10 days | ||
| High-dose dual therapy for 14 days | ||
| Fifth Chinese National Consensus Report (2018)19 | Bismuth-containing quadruple therapy for 10–14 days | No statement |
| Guideline in Korea (2021)21 | PPI-based triple therapy for 14 days | Bismuth-containing quadruple therapy for 10–14 days |
| Quadruple sequential therapy for 10 days | ||
| Quadruple concomitant therapy for 10 days | Fluoroquinolone-containing triple therapy for 14 days | |
| PPI-based triple therapy for 7 days (after clarithromycin resistance testing) | ||
| Guideline in Japan (2019)20 | PPI-based triple therapy for 7 days | PPI-based triple therapy for 7 days |
| Vonoprazan-based triple therapy for 7 days | Vonoprazan-based triple therapy for 7 days |
PPI, proton pump inhibitor; AST, antibiotic susceptibility test.
Studies have shown that a patient's history of macrolide or fluoroquinolone use is associated with the prevalence of resistant strains.14-16 A history of macrolide use for more than two weeks has also been shown to decrease the success rate of eradication with STT, including that of clarithromycin.17 Therefore, the American College of Gastroenterology guidelines suggest that when estimating of the proportion of clarithromycin-resistant strains in a region is difficult, treatment should be selected based on the patient's history of macrolide use.18 Specifically, 14-day triple therapy, including clarithromycin, should be limited to patients in areas with less than 15% clarithromycin-resistant strains and no history of macrolide use, while BQT or quadruple concomitant therapy for 10 to 14 days is recommended for other patients. As first-line treatments, the guidelines also allow quadruple sequential therapy (PPI+amoxicillin for 5 days, followed by PPI+clarithromycin+metronidazole for 5 days), quadruple hybrid therapy (PPI+amoxicillin for 7 days, followed by PPI+amoxicillin+clarithromycin+metronidazole for 7 days), and levofloxacin triple therapy.
Furthermore, the Fifth Chinese National Consensus Report also states that STT should be selected after confirming antimicrobial susceptibility, and BQT for 10 to 14 days is recommended as empirical treatment.19 However Japanese20 and Korean21 guidelines contain regimens that include clarithromycin for primary eradication, despite expressing concerns about the increase in clarithromycin-resistant strains.
The availability of the potassium-competitive acid blocker (P-cab) vonoprazan in Japan since 2015 has led to changes in the Japanese guideline.20 Vonoprazan has more potent and longer-lasting antacid effects than PPIs22,23 and can achieve 24 hours with pH >4 ratio of 100%,24 which is important in the eradication of H. pylori and is expected to have greater eradication success than that of conventional PPI-based triple therapy. In the eradication therapy of H. pylori, antimicrobial agents are active mainly when the intragastric pH exceeds 4.25 Because vonoprazan can achieve intragastric pH >4 earlier than PPI after oral administration, it is suggested that the time for the antimicrobial agent to act on H. pylori can be prolonged, which leads to a higher eradication success rate.25 The vonoprazan-based triple therapy (vonoprazan+clarithromycin+amoxicillin for 7 days) is also expected to be effective against clarithromycin-resistant strains.26-28 A meta-analysis integrating eight randomized controlled trials (RCTs) showed that vonoprazan-based triple therapy had a higher eradication success rate than that of PPI-based triple therapy (pooled eradication rates 79.2% vs 45.8%: risk ratio [RR], 1.66; 95% confidence interval [CI], 1.08 to 2.54; p=0.02).29 For first-line therapies, the Japanese guidelines recommend vonoprazan-based triple therapy or PPI-based triple therapy, which have the highest eradication success rate among the regimens covered by the Japanese insurance system. Most reports of vonoprazan efficacy have originated in Japan (Table 2), since it was first launched there; however, recently Singapore and Thailand have also conducted RCTs comparing 7-day vonoprazan-based triple therapy to 14-day conventional PPI-based triple therapy (Table 2).30,31 In these studies, eradication success rates were comparable; 87.4% to 96.7% success rate was recorded for 7-day vonoprazan-based triple therapy and 88.0% to 88.5% for 14-day PPI-based triple therapy.
Table 2.
Randomized Controlled Trials Comparing P-cab-Based Regimen and PPI-Based Regimen as First-Line Therapy
| Author (year) | Country | CLA-resistant strain, % | P-cab-based regimen | PPI-based regimen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | ITT analysis | PP analysis | Regimen | ITT analysis | PP analysis | ||||||||||
| No. | Eradication rate, % (95% CI) | No. | Eradication rate, % (95% CI) | No. | Eradication rate, % (95% CI) | No. | Eradication rate, % (95% CI) | ||||||||
| Murakami et al. (2016)26 | Japan | 30.4 | VPZ/AMO/CLA, 7 days | 324 | 92.6 (89.2–95.2) | NA | NA | LPZ/AMO/CLA, 7 days | 320 | 75.9 (70.9–80.5) | NA | NA | |||
| Maruyama et al. (2017)27 | Japan | NA | VPZ/AMO/CLA, 7 days | 72 | 95.8 (88.3–99.1) | 70 | 95.7 (88.0–99.1) | LPZ or RPZ/AMO/CLA, 7 days | 69 | 69.6 (57.3–80.1) | 63 | 71.4 (58.7–82.1) | |||
| Ang et al. (2022)30 | Singapore | 12.7 | VPZ/AMO/CLA, 7 days | 119 | 87.4 (80.1–92.3) | 108 | 96.3 (90.5–98.8) | RPZ or OPZ or EPZ/AMO/CLA, 14 days | 125 | 88.0 (81.0–92.7) | 117 | 94.0 (87.9–97.2) | |||
| Bunchorntavakul et al. (2021)31 | Thailand | NA | VPZ/AMO/CLA, 7 days | 61 | 96.7 (88.0–99.7) | 60 | 98.3 (90.1–100) | OPZ/AMO/CLA, 14 days | 61 | 88.5 (77.8–94.6) | 58 | 93.1 (83.0–97.7) | |||
| Choi et al. (2022)35 | Korea | 30.3 | TPZ/AMO/CLA, 7 days | 175 | 62.9 (55.5–69.6) | 175 | 69.3 (53.2–67.5) | LPZ/AMO/CLA, 7 days | 175 | 60.6 (61.5–76.1) | 150 | 67.3 (59.4–74.3) | |||
P-cab, potassium-competing acid blocker; PPI, proton pump inhibitor; CLA, clarithromycin; ITT, intension-to-treat; PP, per-protocol; CI, confidence interval; VPZ, vonoprazan; AMO, amoxicillin; NA, not applicable; LPZ, lansoprazole; RPZ, rabeprazole; OPZ, omeprazole; EPZ, esomeprazole.
In Korea, clarithromycin-resistant strains have also risen in frequency over the past decade, although the success rate of eradication with STT has been declining; as of 2016, the success rate was approximately 70%.32,33 In light of this situation, the Korean guidelines issued in 202021 proposed the following first-line therapies: (1) PPI-based triple therapy for 14 days; (2) quadruple sequential therapy; (3) quadruple concomitant therapy; and (4) STT after clarithromycin resistance testing. However, BQT, which is positioned as first-line therapy in Europe, the United States, and China, is not listed because of its rate of side effects and its potential as a second-line therapy option. The guidelines issued by the Korean College of Helicobacter and Upper Gastrointestinal Research in 2022 make similar recommendations.34 Tegoprazan, the other P-cab product, was launched in Korea in 2018 and is now available for H. pylori treatment. Tegoprazan has been shown to achieve pH >4 in the stomach 2 hours after administration, with a rapid onset of effect comparable to that of vonoprazan.36 In vitro, tegoprazan has been shown to improve minimum inhibitory concentrations (MICs) of clarithromycin, fluoroquinolone, metronidazole, and amoxicillin by 46.3%, 46.7%, 55.6%, and 34.5%, respectively.37 Thus far, few studies have examined the efficacy of tegoprazan in the treatment of H. pylori, but one recent RCT found that 7-day triple therapy combining tegoprazan with amoxicillin and clarithromycin was non-inferior to 7-day lansoprazole-based triple therapy (62.9% vs 60.1%, non-inferiority test, p=0.009) (Table 2).35 The possible reasons why tegoprazan-based regimen was not superior to PPI in a first-line therapy are as follows: (1) insufficient dose of tegoprazan used in the trial; (2) differences in the MIC distributions for clarithromycin-resistant strains compared with those reported in the Japanese trials; (3) pharmacological differences between vonoprazan and tegoprazan; and (4) insufficient eradication treatment period compared to 14 days of treatment.38 In fact, in a retrospective case-controlled study, tegoprazan-based triple therapy was reported to be more effective in a 14-day regimen compared to a 7-day regimen (eradication rate: 78.6% vs 63.9%).39 The efficacy of tegoprazan as first-line therapy will need to be validated in the future.
IMPACT OF ANTIMICROBIAL RESISTANCE ON H. PYLORI TREATMENT OUTCOME
1. Clarithromycin resistance
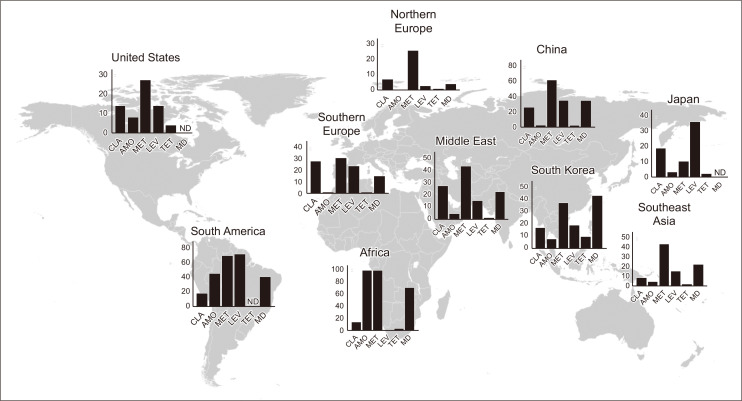
The acquisition of drug resistance in H. pylori is accelerated mainly by chromosomal mutations, physiological changes (such as impaired regulation of drug uptake and/or efflux), biofilms, coccoid formation, and other factors.40 Clarithromycin resistance has a particularly large impact on eradication success. When STT was used as a primary regimen, a 90% success rate against clarithromycin-susceptible strains and a 22% success rate against clarithromycin-resistant strains were reported.41 Accurate prediction and diagnosis of this clarithromycin-resistant strain are key to successful primary eradication. H. pylori is known to form cross-resistance to macrolide antimicrobial agents,42,43 and a history of macrolide use is closely associated with the prevalence of clarithromycin-resistant strains. In fact, as reported in Taiwan, the rate of clarithromycin-resistant strains decreases when macrolide antimicrobial use is restricted.44 The proportion of clarithromycin-resistant strains is increasing worldwide, but varies by region (Fig. 1).45-54
Fig. 1.
Global comparison of the proportion of antimicrobial-resistant Helicobacter pylori strains. Data were obtained from reports published since 2012.45-54 Multidrug-resistant strains were defined as those resistant to at least two antimicrobial agents.
CLA, clarithromycin; AMO, amoxicillin; MET, metronidazole; LEV, levofloxacin; TET, tetracycline; MD, multidrug resistance; ND, no data.
2. Metronidazole resistance
In addition, metronidazole-resistant H. pylori strains cannot be ignored. A meta-analysis calculated the proportion of H. pylori strains with potential drug resistance, heteroresistance,55 by integrating and analyzing 22 studies.56 In this study, heteroresistance to clarithromycin had a weighted pooled prevalence of 6.8% (95% CI, 5.1% to 8.6%), whereas heteroresistance to metronidazole was more common, with a weighted pooled prevalence of 13.8% (95% CI, 8.9% to 18.6%). Heteroresistance cannot be ignored, as it has been shown that subpopulations of strains confirmed to be heteroresistant in vitro are also clinically resistant to antimicrobial agents.57 Although most drug-resistant H. pylori strains that pose clinical challenges are presently clarithromycin-resistant strains, there is a concern that metronidazole-resistant strains may become more common as metronidazole use increases owing to changing guidelines.
3. Resistances to other antimicrobials
In the context of eradication regimens, H. pylori strains resistant to levofloxacin, amoxicillin, and tetracycline can also be problematic. Emerging multidrug resistance to various types of antimicrobials has become a serious problem. The acquisition of multidrug resistance mainly occurs from biofilm formation and the expression of efflux pumps.49,58 Special attention should be paid to multidrug resistance, as it can cause the failure of multiple rounds of empirical therapy. The emergence of multidrug-resistant H. pylori strains in more than 20% of cases has been noted in Southeast Asia (Fig. 1),59 with inadequate durations of treatment with multiple antimicrobial agents noted as a cause of multidrug resistance.16
DETECTION METHODS FOR H. PYLORI ANTIMICROBIAL RESISTANCE
For AST, there are two main methods to detect antimicrobial resistance: MIC measurement using culture-based methods and molecular tests using polymerase chain reaction (PCR). Each method has their own advantages and disadvantages (Table 3).
Table 3.
Comparison of Culture-Based and Molecular-Based Methods
| Culture-based method | Molecular-based method | |
|---|---|---|
| Method details | Agar dilution | Clarithromycin-resistance |
| E-test | PCR-based method: real-time PCR, multiplex PCR, PCR-restriction fragment length polymorphism | |
| Disk diffusion | Fluorescence in situ hybridization | |
| Broth microdilution | Fluoroquinolone-resistance | |
| PCR-based method: real-time PCR, allele-specific PCR | ||
| Metronidazole | ||
| PCR-based method: real-time PCR, multiplex allele-specific PCR | ||
| Amoxicillin | ||
| PCR-based method: real-time PCR | ||
| Type of sample required | Gastric biopsy sample | Gastric biopsy sample |
| Stool sample | ||
| Determinable resistance of antimicrobial agent | All antimicrobial agents | Clarithromycin |
| Metronidazole | ||
| Fluoroquinolone | ||
| Amoxicillin | ||
| Time required to determine | Long (7–14 days) | Short (1–2 days) |
| Cost | Low | High |
PCR, polymerase chain reaction.
1. Culture-based AST
The measurement of MIC by culturing gastric biopsy samples has a long history as an AST.60 These methods are further classified as agar dilution, E-test, disk diffusion, and broth microdilution. Among these, agar dilution is the gold standard for AST, despite being labor-intensive and time-consuming.61 Although the E-test is widely used in clinical practice because of its simplicity, it is limited by difficulties in assessing susceptibility to metronidazole.62 The disk diffusion technique, by contrast, is as simple as the E-test but can evaluate susceptibility to a wide range of antimicrobial agents, including levofloxacin, clarithromycin, and metronidazole.63 The broth microdilution technique is reported to be accurate and easy to perform.64 Although both culture-based tests have the disadvantage of being labor- and time-intensive in the laboratory, their greatest advantage is that they can reliably assess resistance to all antimicrobial agents present.
2. Molecular-based methods
When drug susceptibility testing in culture is unavailable owing to time constraints, real-time PCR,65,66 multiplex PCR,67,68 fluorescence in situ hybridization,69 and PCR-restriction fragment length polymorphism (PCR-RFLP) using gastric biopsy samples,70,71 or PCR using stool samples72,73 can be used to evaluate drug resistance to specific antimicrobial agents.
Clarithromycin exerts its antibacterial activity by binding to the peptidyl transferase region of the 23S rRNA of H. pylori.74 Point mutations of the rrl gene, which encodes the binding region of 23S rRNA (especially A2142G, A2143G, or A2144G point mutation), contribute to clarithromycin resistance,75,76 and these point mutations are detected by PCR or other methods. Molecular tests, including PCR, can detect drug resistance faster than culture methods and have been found to be valid.77 A report compared the detection rate of A2144 or A2143 from PCR-RFLP with that of MIC measurement by the agar culture method, and they found that the diagnostic performance was nearly equivalent.78 Another study compared the diagnostic accuracy of real-time PCR against MIC measurements by culture test, and it showed a high concordance rate; 84.6% of subjects diagnosed with clarithromycin-resistant infections by the culture method also had point mutations in 23S rRNA.79
DNA gyrase, which is used to break the DNA double helix structure during DNA replication, is required for H. pylori to proliferate. DNA gyrase exists as a tetramer formed by the subunits encoded by the gyrA and gyrB genes. Fluoroquinolones exert antimicrobial activity by selectively inhibiting this tetramer. Point mutations in the quinolone resistance-determining region (QRDR) of gyrA and gyrB have been associated with fluoroquinolone resistance.76,80 The strength of quinolone resistance varies by the number of mutated codons encoding the QRDR;80,81 therefore, it is important to identify the mutated region. Allele-specific PCR82 and fluorescence resonance energy transfer-based real-time PCR66 have been developed to rapidly detect gyrA mutations.
Because the mechanism of resistance acquisition differs among antimicrobial agents, a single molecular test can usually evaluate only a single antimicrobial resistance. To overcome this disadvantage, GenoType HelicoDRⓇ was developed, a revolutionary genotyping test that can simultaneously detect QRDR mutations and 23S rRNA mutations within 6 hours.83 The sensitivity and specificity of the HelicoDRⓇ test were reported to be 98.2% and 80.0% for gyrA and 94.9% and 87.1% for 23S rRNA mutations, respectively.84 However, the types of molecular tests that can be used in real clinical practice are limited, and it is difficult to evaluate more than three types of drug resistance simultaneously. Limited access to molecular-based AST is a major limitation at this stage.
3. Next-generation sequencing
Genetic mutations associated with specific antimicrobial resistance do not coincide with the acquisition of other antimicrobial resistance.74 Therefore, to examine mutations in multiple regions, a comprehensive study of the drug resistance profile of infectious H. pylori strains is required. Next-generation sequencing (NGS) of DNA was devised to address this shortcoming.85 NGS can comprehensively examine mutations for specific antimicrobials, such as in genes for clarithromycin resistance,86,87 amoxicillin resistance,88 metronidazole resistance,89 and levofloxacin resistance.90 Furthermore, it can also simultaneously detect mutations associated with multiple antimicrobial resistance. In fact, reports have documented the use of NGS for increasing eradication success rates. A study comprehensively investigated mutations in gyrA, 23S rRNA, and 16S rRNA genes using NGS in gastric mucosal specimens from 126 H. pylori-infected patients. It showed a strong association between the eradication success rate and the number of mutations.91 While some studies have shown the efficacy of NGS, a study found that this approach was inapplicable to some antimicrobials. This study compared the concordance rates of antimicrobial resistance to clarithromycin, amoxicillin, metronidazole, levofloxacin, rifabutin, and tetracycline using NGS and agar dilution, and the authors found that NGS accurately assessed resistance to clarithromycin, levofloxacin, rifabutin, and tetracycline; however, NGS results were in poor agreement with those of culture-based methods for amoxicillin and metronidazole resistance.92 A major disadvantage of NGS is the higher cost compared with culture-based methods and molecular-based tests. Yet, as the cost of NGS decreases, it is expected to become easier to apply in clinical practice.
H. PYLORI ERADICATION OUTCOMES OF TAILORED THERAPY VERSUS THOSE OF EMPIRICAL THERAPY
To summarize, the main challenges in evaluating drug resistance are the time-consuming nature of the culture-based method, the inability to investigate specific drug resistances, and the high cost of molecular-based tests. We will explore whether tailored therapy can sufficiently compensate for these shortcomings.
1. Clinical outcomes of tailored therapy
Based on the results of AST, the possibility of clinically applying tailored therapy with individually-selected susceptible antimicrobial agents is being explored.93 Several RCTs showed the superiority of tailored therapy over STT, when comparing the efficacy of tailored therapy and empirical therapy in detecting clarithromycin-resistant strains using molecular-based methods73,94-97 as well as using culture-based methods (Table 4).98-103 However, RCTs that compared the efficacy of tailored therapy and empirical therapy using BQT, quadruple concomitant, or fluoroquinolone-containing regimens failed to show the superiority of tailored therapy (Table 4).104-113 Whereas a meta-analysis integrating 16 RCTs compared the efficacy of empirical therapy and tailored therapy and concluded that tailored therapy was slightly more effective, this study found no difference in efficacy between tailored therapy and BQT (RR, 1.02; 95% CI, 0.92 to 1.13; p=0.759).114 BQT, in fact, is recommended as an empirical first-line therapy in the United States19 and European guidelines.11 The efficacy of BQT was further supported by a meta-analysis that integrated five studies to compare its efficacy against that of tailored therapy. The pooled eradication rate of BQT was significantly higher (86% vs 78%, p<0.05).115 A recent meta-analysis combined 54 clinical studies to examine the efficacy of tailored therapy as first- and second-line therapy.116 In this study, tailored therapy had a significantly higher eradication success rate than that of empirical therapy in an integrated analysis that included all eradication regimens (86% vs 76%: RR, 1.12; 95% CI, 1.08 to 1.17). However, there were no differences within the group of primary treatments, and no differences within the group of secondary treatments. Furthermore, the efficacy of tailored therapy in second-line treatment and third-line treatment has been reported to vary in the range of 60% to 98% (Table 4).109,117-119 Differences in resistance rates by region and time precluded easy comparisons; yet, they also suggested that tailored therapy may not be effective in all cases.
Table 4.
Randomized Controlled Trial Comparing the Efficacy of Empirical Therapy versus Tailored Therapy
| Author (year) | Country | Empirical therapy | Tailored therapy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Regimen | Eradication rate, % (95% CI)* | No. | Method | Target antimicrobials | Eradication rate, % (95% CI)* | |||
| First-line | |||||||||
| Furuta et al. (2007)95 | Japan | 150 | PPI/AMO/CLA, 7 days | 70.0 (69.5–76.7) | 150 | SISAR | CLA | 96.0 (91.3–98.3) | |
| Kawai et al. (2008)73 | Japan | 35 | PPI/AMO/CLA, 7 days | 71.4 (54.7–83.7) | 35 | Nested PCR (stool) | CLA | 94.3 (80.2–99.3) | |
| Lee et al. (2013)94 | Korea | 308 | PPI/AMO/CLA, 7 days | 75.9 (70.5–80.5) | 616 | DPO-PCR | CLA | 91.2 (86.2–94.5) | |
| 308 | PPI/AMO/MET, 7 days | 79.1 (73.9–83.4) | |||||||
| Ong et al. (2019)104 | Korea | 196 | PPI/AMO/CLA/MET, 14 days | 86.2 (80.6–90.4) | 201 | DPO-PCR | CLA | 81.6 (75.6–86.3) | |
| Delchier et al. (2019)96 | France | 208 | PPI/AMO/CLA, 7 days | 73.1 (66.6–78.6) | 207 | PCR/reverse hybridization | CLA/LEV | 85.5 (80.0–89.7) | |
| Choi et al. (2021)105 | Korea | 107 | PPI/AMO/CLA/MET, 10 days | 82.2 (73.8–88.4) | 110 | DPO-PCR | CLA | 82.7 (74.5–88.7) | |
| Cha et al. (2021)106 | Korea | 161 | PPI/BIS/TET/MET, 7 days | 88.2 (82.2–92.4) | 147 | DPO-PCR | CLA | 80.3 (73.0–85.9) | |
| Kim et al. (2022)107 | Korea | 145 | PPI/AMO/CLA/MET, 14 days | 82.8 (75.7–88.1) | 145 | DPO-PCR | CLA | 85.8 (78.8–90.4) | |
| Cho et al. (2022)108 | Korea | 141 | PPI/BIS/AMO/CLA, 14 days | 85.8 (79.0–90.7) | 141 | DPO-PCR | CLA | 80.9 (73.5–86.5) | |
| Hsieh et al. (2022)97 | Taiwan | 91 | PPI/AMO/CLA, 7 days | 75.8 (66.0–83.5) | 91 | PCR-RLFP (gastric juice) | CLA | 89.0 (80.8–94.1) | |
| Toracchio et al. (2000)101 | Italy | 56 | PPI/CLA/TIN, 10 days | 75.0 (62.1–84.5) | 53 | Agar dilution | CLA/TIN | 90.6 (79.2–96.2) | |
| Romano et al. (2000)102 | Italy | 40 | PPI/CLA/MET, 7 days | 77.5 (64.6–90.4) | 40 | E-test | CLA/AMO/MET/TET | 95.0 (88.2–100) | |
| Neri et al. (2003)98 | Italy | 116 | PPI/AMO/CLA, 7 days | 67.2 (58.2–75.1) | 116 | E-test | CLA/AMO/TIN | 75.9 (67.3–82.7) | |
| Romano et al. (2003)103 | Italy | 75 | PPI/CLA/MET, 7 days | 77.3 (66.9–85.7) | 75 | E-test | CLA/AMO/MET/TET | 94.6 (87.6–98.3) | |
| Marzio et al. (2006)109 | Italy | 39 | PPI/AMO/LEV, 10 days | 92.3 (78.8–98.0) | 41 | Agar dilution | CLA/AMO/LEV/RIF | 95.1 (82.8–99.4) | |
| Park et al. (2014)99 | Korea | 57 | PPI/AMO/CLA, 7 days | 71.9 (59.0–81.9) | 57 | Agar dilution | CLA | 94.7 (84.9–98.7) | |
| Martos et al. (2014)100 | Spain | 54 | PPI/AMO/CLA, 10 days | 66.7 (53.3–77.7) | 55 | E-test | CLA | 94.5 (84.4–98.6) | |
| Zhou et al. (2016)110 | China | 350 | PPI/BIS/AMO/CLA, 10 days | 77.4 (73.1–82.0) | 318 | E-test | CLA | 88.7 (85.2–92.1) | |
| 350 | PPI/AMO/CLA/MET, 10 days | 87.0 (83.0–90.7) | |||||||
| Chen et al. (2019)111 | China | 96 | PPI/BIS/AMO/MET, 14 days | 85.4 (78.4–92.5) | 286 | Agar dilution | CLA/MET/LEV | 91.6 (88.4–94.8) | |
| Pan et al. (2020)112 | China | 157 | PPI/BIS/AMO/CLA, 14 days | 63.7 (55.9–70.8) | 310 | Agar dilution | CLA/MET/LEV/AMO/FR | 76.8 (71.7–81.1) | |
| Li et al. (2022)113 | China | 67 | PPI/AMO/FR, 10 days | 85.1 (74.5–91.9) | 134 | E-test | CLA | 80.6 (73.0–86.5) | |
| Second-line | |||||||||
| Miwa et al. (2003)118 | Japan | 39 | PPI/AMO/MET, 10 days | 92.4 (79.0–98.0) | 38 | Dry plate | CLA/MET | 81.6 (66.0–92.0) | |
| Lamouliatte et al. (2003)119 | France | 57 | PPI/AMO/CLA, 7 days | 47.4 (34.4–60.3) | 113 | E-test | CLA/AMO/MET | 74.3 (65.0–82.4) | |
| 58 | PPI/AMO/CLA, 14 days | 34.5 (22.2–46.7) | |||||||
| 57 | PPI/AMO/MET, 14 days | 63.2 (50.6–75.7) | |||||||
| Marzio et al. (2006)109 | Italy | 32 | PPI/AMO/LEV, 10 days | 81.2 (63.5–92.7) | 51 | Agar dilution | CLA/AMO/TIN/RIF/LEV | 98.0 (89.5–99.9) | |
CI, confidence interval; PPI, proton pump inhibitor; AMO, amoxicillin; CLA, clarithromycin; SISAR, serial invasive signal amplification reaction; PCR, polymerase chain reaction; MET, metronidazole; DPO-PCR, dual-priming oligonucleotide-based PCR; LEV, levofloxacin; TET, tetracycline; BIS, bismuth; PCR-RFLP, PCR-restriction fragment length polymorphism; TIN, tinidazole; RIF, rifabutin; FR, furazolidone.
*Eradication rate in intension-to-treat analysis.
2. Cost-effectiveness of tailored therapy
For clinically applying tailored therapy, cost-effectiveness is very important for the allocation of facility resources. Tailored therapy methods have been mainly culture-based, but in recent years, molecular-based methods have been established for tailored therapy. The methods of AST targeted by cost-effectiveness analysis used to be dominated by culture-based methods, but in recent years there has been a shift to molecular-based methods; thus, we cannot compare old reports with recent ones. It is necessary to always keep abreast of the latest literature when considering cost-effectiveness; the recommended methods for first-line therapies have changed, which prevents meta-analyses from comparing studies conducted at different times. The first report evaluating the cost-effectiveness of tailored therapy was published in 1999, but it covered culture-based methods, such as AST.120 Using molecular-based methods, a study compared the detection of clarithromycin resistance using dual-priming oligonucleotide-based PCR (DPO-PCR) between STT and tailored therapy. They found that tailored therapy was more effective, and cost-effectiveness was comparable or even slightly better.121,122 This was supported by a recent study comparing the first-line regimens as recommended by U.S. and European guidelines (14-day triple therapy, sequential therapy, and BQT) with tailored therapy, for which clarithromycin resistance was detected by multiplex PCR. They also concluded that tailored therapy was more cost-effective.123 In contrast, compared to 14-day pantoprazole, amoxicillin, metronidazole, and bismuth combination therapy, tailored therapy based on clarithromycin resistance detection by multiplex PCR was less cost-effective (average cost per patient: $340.70 vs $263.90).108 When compared against BQT, tailored therapy based on clarithromycin resistance detection by DPO-PCR was less cost-effective (average cost per patient: $406.50 vs $503.50), while having comparable eradication success rates.124 As this study suggests, the superiority of tailored therapy may remain unsupported if the control group is a regimen that is highly effective in eradicating H. pylori, even with mixed clarithromycin-resistant strains.
FUTURE ERADICATION STRATEGIES INCORPORATING TAILORED THERAPY
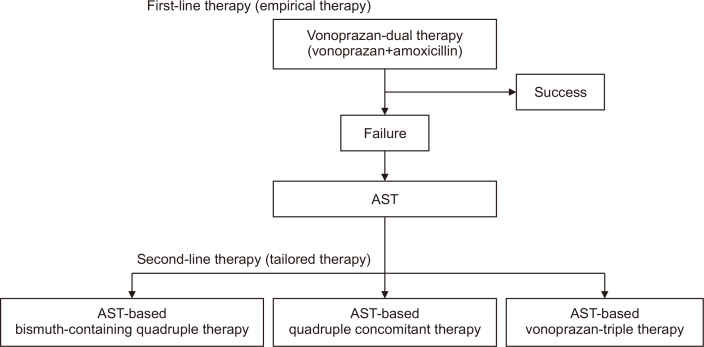
Finally, we discuss the potential position of tailored therapy when vonoprazan-based regimens are incorporated into future eradication strategies. Recently, the efficacy of a two-drug therapy combining vonoprazan and amoxicillin has been reported. Limiting the number of drugs used for eradication to two is expected to improve adherence to medication, and using only one antimicrobial agent is expected to avoid the acquisition of new drug resistance, including resistance to clarithromycin. Several reports from Japan showed that eradication rates of vonoprazan-based dual therapy were similar to vonoprazan-based triple therapy.125-127 In a meta-analysis integrating these studies, the pooled eradication rate of vonoprazan-based dual therapy was similar to that of vonoprazan-based triple therapy (87.5% vs 89.6%: RR, 0.99; 95% CI, 0.93 to 1.05; p=0.65).128 Vonoprazan-based dual therapy performed comparably to vonoprazan-based triple therapy, indicating application potential. Recently, an RCT of vonoprazan-based dual therapy versus STT was conducted in the United States and Europe.129 Vonoprazan-based dual therapy eradicated H. pylori in 78.5% of cases, compared to 84.7% for vonoprazan-based triple therapy and 78.8% for lansoprazole-based triple therapy, demonstrating competitive efficacy. Furthermore, for vonoprazan-based triple therapy, the success rate of eradication against clarithromycin-resistant strains was also superior to that of lansoprazole-based triple therapy (69.6% vs 31.9%, p<0.001). Based on these results, vonoprazan-based triple therapy and dual therapy have been approved and are now available in the United States.130 Since vonoprazan-based dual therapy can eradicate H. pylori while minimizing impact on H. pylori antimicrobial resistance, it may be acceptable to apply vonoprazan-based dual therapy as an empirical treatment for primary therapy. Subsequently, we propose the strategy of vonoprazan-based dual therapy as a primary treatment, followed by a tailored treatment based on AST. Our proposed strategy could reduce both the consumption of antimicrobials used in the overall H. pylori treatment and medical resources for AST, including cost, labor, and materials (Fig. 2). However, knowledge of vonoprazan-based dual therapy is insufficient in the field.131 The specific duration of vonoprazan dual therapy and dosage of amoxicillin have not yet been determined. In fact, it has been reported that the effectiveness of vonoprazan dual therapy is higher in patients with smaller body surface area.132 Further research is required to establish the new strategy.
Fig. 2.
Flowchart outlining future eradication strategies incorporating tailored therapy. The first-line therapy is vonoprazan-dual therapy, without considering clarithromycin-resistant strains. If eradication fails, an antibiotic susceptibility test (AST) is performed. A second-line therapy with a modified regimen of quadruple therapy may be needed depending on AST results.
CONCLUSION
This review describes the performance of empirical therapy within the current guidelines and the latest AST-based tailored therapies. Empirical therapy, recommended as the primary eradication regimen in current guidelines, is a reasonable strategy with outcomes comparable to those of tailored therapy. Tailored therapy is currently considered for secondary and tertiary eradication. An ideal treatment against H. pylori is, as stated in the first edition of the Maastricht Consensus Report,133 “a simple, well-tolerated regimen with good compliance and cost-effectiveness.” A potential candidate treatment consistent with this philosophy is a vonoprazan-based dual therapy and subsequent tailored therapy as first-line and second-line therapies, respectively. Further research is required to support this strategy.
Footnotes
CONFLICTS OF INTEREST
F.I. received honoraria for lectures from Takeda Pharmaceutical Co., Ltd., AstraZeneca PLC, Otsuka Pharmaceutical Co., Ltd., AbbVie GK, Zeria Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Inc., and EA Pharma Co., Ltd. S.S. received honoraria for lectures from Takeda Pharmaceutical Co., Ltd. Except for that, no potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomized controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Watari J, Tomita T, Tozawa K, Oshima T, Fukui H, Miwa H. Preventing metachronous gastric cancer after the endoscopic resection of gastric epithelial neoplasia: roles of Helicobacter pylori eradication and aspirin. Gut Liver. 2020;14:281–290. doi: 10.5009/gnl19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakiuchi T, Matsuo M, Endo H, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: a preliminary report. J Gastroenterol. 2019;54:699–707. doi: 10.1007/s00535-019-01559-9. [DOI] [PubMed] [Google Scholar]
- 5.Kusano C, Gotoda T, Ishikawa H, Moriyama M. The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer. 2017;20(Suppl 1):16–19. doi: 10.1007/s10120-017-0688-7. [DOI] [PubMed] [Google Scholar]
- 6.Sekiguchi M, Oda I, Matsuda T, Saito Y. Epidemiological trends and future perspectives of gastric cancer in Eastern Asia. Digestion. 2022;103:22–28. doi: 10.1159/000518483. [DOI] [PubMed] [Google Scholar]
- 7.Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki S, Kusano C, Horii T, Ichijima R, Ikehara H. The ideal Helicobacter pylori treatment for the present and the future. Digestion. 2022;103:62–68. doi: 10.1159/000519413. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Esaki M, Kusano C, Ikehara H, Gotoda T. Development of Helicobacter pylori treatment: how do we manage antimicrobial resistance? World J Gastroenterol. 2019;25:1907–1912. doi: 10.3748/wjg.v25.i16.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71:1724–1762. doi: 10.1136/gutjnl-2022-327745. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337. doi: 10.1002/14651858.CD008337.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallone CA, Barkun AN, Szilagyi A, et al. Prolonged treatment duration is required for successful Helicobacter pylori eradication with proton pump inhibitor triple therapy in Canada. Can J Gastroenterol. 2013;27:397–402. doi: 10.1155/2013/801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic resistance of Helicobacter pylori among male United States veterans. Clin Gastroenterol Hepatol. 2015;13:1616–1624. doi: 10.1016/j.cgh.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139:463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 16.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 17.Lim SG, Park RW, Shin SJ, et al. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig Liver Dis. 2016;48:385–390. doi: 10.1016/j.dld.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 19.Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. doi: 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. 2019;24:e12597. doi: 10.1111/hel.12597. [DOI] [PubMed] [Google Scholar]
- 21.Jung HK, Kang SJ, Lee YC, et al. Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Korean J Intern Med. 2021;36:807–838. doi: 10.3904/kjim.2020.701.e7e56635bb194296a6dfc13ec5cd9b98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41:636–648. doi: 10.1111/apt.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects: a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719–730. doi: 10.1111/apt.13325. [DOI] [PubMed] [Google Scholar]
- 24.Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43:1048–1059. doi: 10.1111/apt.13588. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol. 2019;9:1560. doi: 10.3389/fphar.2018.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama M, Tanaka N, Kubota D, et al. Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: a randomized controlled trial. Can J Gastroenterol Hepatol. 2017;2017:4385161. doi: 10.1155/2017/4385161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noda H, Noguchi S, Yoshimine T, et al. A novel potassium-competitive acid blocker improves the efficacy of clarithromycin-containing 7-day triple therapy against Helicobacter pylori. J Gastrointestin Liver Dis. 2016;25:283–288. doi: 10.15403/jgld.2014.1121.253.7hp. [DOI] [PubMed] [Google Scholar]
- 29.Yang C, Li S, Huang T, et al. Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2022;47:897–904. doi: 10.1111/jcpt.13637. [DOI] [PubMed] [Google Scholar]
- 30.Ang D, Koo SH, Chan YH, et al. Clinical trial: seven-day vonoprazan- versus 14-day proton pump inhibitor-based triple therapy for first-line Helicobacter pylori eradication. Aliment Pharmacol Ther. 2022;56:436–449. doi: 10.1111/apt.17070. [DOI] [PubMed] [Google Scholar]
- 31.Bunchorntavakul C, Buranathawornsom A. Randomized clinical trial: 7-day vonoprazan-based versus 14-day omeprazole-based triple therapy for Helicobacter pylori. J Gastroenterol Hepatol. 2021;36:3308–3313. doi: 10.1111/jgh.15700. [DOI] [PubMed] [Google Scholar]
- 32.Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016;21:266–278. doi: 10.1111/hel.12279. [DOI] [PubMed] [Google Scholar]
- 33.Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci. 2016;31:1246–1253. doi: 10.3346/jkms.2016.31.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung HK, Kang SJ, Lee YC, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021;15:168–195. doi: 10.5009/gnl20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YJ, Lee YC, Kim JM, et al. Triple therapy-based on tegoprazan, a new potassium-competitive acid blocker, for first-line treatment of Helicobacter pylori infection: a randomized, double-blind, phase III, clinical trial. Gut Liver. 2022;16:535–546. doi: 10.5009/gnl220055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han S, Choi HY, Kim YH, et al. Comparison of pharmacodynamics between tegoprazan and dexlansoprazole regarding nocturnal acid breakthrough: a randomized crossover study. Gut Liver. 2023;17:92–99. doi: 10.5009/gnl220050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JW, Kim N, Nam RH, et al. Efficacy of tegoprazan for improving the susceptibility of antimicrobial agents against antibiotic-resistant Helicobacter pylori. Gut Liver. 2021;15:53–60. doi: 10.5009/gnl20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J. Role of tegoprazan in Helicobacter pylori eradication therapy. Gut Liver. 2022;16:493–494. doi: 10.5009/gnl220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung YS, Kim S, Kim HY, Noh SJ, Park JH, Park CH. 7-day versus 14-day tegoprazan-based triple therapy to treat Helicobacter pylori infection: real-world evidence. J Gastroenterol Hepatol. 2022;37:1911–1918. doi: 10.1111/jgh.15939. [DOI] [PubMed] [Google Scholar]
- 40.Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance: from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613–629. doi: 10.1038/s41575-021-00449-x. [DOI] [PubMed] [Google Scholar]
- 41.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 42.Gerrits MM, van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6:699–709. doi: 10.1016/S1473-3099(06)70627-2. [DOI] [PubMed] [Google Scholar]
- 43.Xia HX, Buckley M, Keane CT, O'Morain CA. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. J Antimicrob Chemother. 1996;37:473–481. doi: 10.1093/jac/37.3.473. [DOI] [PubMed] [Google Scholar]
- 44.Liou JM, Chang CY, Chen MJ, et al. The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors: a nationwide study. PLoS One. 2015;10:e0124199. doi: 10.1371/journal.pone.0124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SE, Hwang JH. Management of Helicobacter pylori infection: a comparison between Korea and the United States. Gut Liver. 2022;16:503–514. doi: 10.5009/gnl210224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 47.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bujanda L, Nyssen OP, Vaira D, et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013-2020: results of the European registry on H. pylori management (Hp-EuReg) Antibiotics (Basel) 2021;10:1058. doi: 10.3390/antibiotics10091058.e2f4ed15a9cc483084ffd3596f765d09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyanova L, Hadzhiyski P, Kandilarov N, Markovska R, Mitov I. Multidrug resistance in Helicobacter pylori: current state and future directions. Expert Rev Clin Pharmacol. 2019;12:909–915. doi: 10.1080/17512433.2019.1654858. [DOI] [PubMed] [Google Scholar]
- 50.Kouitcheu Mabeku LB, Eyoum Bille B, Tepap Zemnou C, Tali Nguefack LD, Leundji H. Broad spectrum resistance in Helicobacter pylori isolated from gastric biopsies of patients with dyspepsia in Cameroon and efflux-mediated multiresistance detection in MDR isolates. BMC Infect Dis. 2019;19:880. doi: 10.1186/s12879-019-4536-8.863b41e8ea93440db169120101f2c854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shokrzadeh L, Alebouyeh M, Mirzaei T, Farzi N, Zali MR. Prevalence of multiple drug-resistant Helicobacter pylori strains among patients with different gastric disorders in Iran. Microb Drug Resist. 2015;21:105–110. doi: 10.1089/mdr.2014.0081. [DOI] [PubMed] [Google Scholar]
- 52.Lyu T, Cheung KS, Ni L, et al. High prevalence and risk factors of multiple antibiotic resistance in patients who fail first-line Helicobacter pylori therapy in southern China: a municipality-wide, multicentre, prospective cohort study. J Antimicrob Chemother. 2020;75:3391–3394. doi: 10.1093/jac/dkaa315. [DOI] [PubMed] [Google Scholar]
- 53.Park JY, Shin TS, Kim JH, Yoon HJ, Kim BJ, Kim JG. The prevalence of multidrug resistance of Helicobacter pylori and its impact on eradication in Korea from 2017 to 2019: a single-center study. Antibiotics (Basel) 2020;9:646. doi: 10.3390/antibiotics9100646.a3bbf33e30d94ed6b953b475dc83d145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olano M, Chu M, Guzmán J, Castillo D, Sauvain M. Diagnostic performance of the culture and susceptibility of Helicobacter pylori in peruvian patients: results from a sentinel laboratory. Rev Peru Med Exp Salud Publica. 2021;38:406–411. doi: 10.17843/rpmesp.2021.383.7256.2c9a826c65c64687a8a88a6a485e0d76 [DOI] [PubMed] [Google Scholar]
- 55.The Editors, author. Hetero-resistance: an under-recognised confounder in diagnosis and therapy? J Med Microbiol. 2001;50:1018–1020. doi: 10.1099/0022-1317-50-12-1018. [DOI] [PubMed] [Google Scholar]
- 56.Kouhsari E, Sadeghifard N, Khadiv A, et al. Heteroresistance to clarithromycin and metronidazole in patients with a Helicobacter pylori infection: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2022;21:19. doi: 10.1186/s12941-022-00509-3.25ccf930b1b24b50b12d2fa17ff4a87c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizvanov AA, Haertlé T, Bogomolnaya L, Talebi Bezmin Abadi A. Helicobacter pylori and its antibiotic heteroresistance: a neglected issue in published guidelines. Front Microbiol. 2019;10:1796. doi: 10.3389/fmicb.2019.01796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye L, Meng F, Mao X, et al. Using next-generation sequencing to analyze Helicobacter pylori clones with different levofloxacin resistances from a patient with eradication failure. Medicine (Baltimore) 2020;99:e20761. doi: 10.1097/MD.0000000000020761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukri A, Lopes BS, Hanafiah A. The emergence of multidrug-resistant Helicobacter pylori in Southeast Asia: a systematic review on the trends and intervention strategies using antimicrobial peptides. Antibiotics (Basel) 2021;10:1061. doi: 10.3390/antibiotics10091061.13efbc78b3f44c068b86024f01400c88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osato MS. Antimicrobial susceptibility testing for Helicobacter pylori: sensitivity test results and their clinical relevance. Curr Pharm Des. 2000;6:1545–1555. doi: 10.2174/1381612003399059. [DOI] [PubMed] [Google Scholar]
- 61.Valdivieso-García A, Imgrund R, Deckert A, et al. Cost analysis and antimicrobial susceptibility testing comparing the E test and the agar dilution method in Campylobacter jejuni and Campylobacter coli. Diagn Microbiol Infect Dis. 2009;65:168–174. doi: 10.1016/j.diagmicrobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217–1220. doi: 10.1001/archinte.161.9.1217. [DOI] [PubMed] [Google Scholar]
- 63.Tang X, Shen Y, Hu R, et al. Re-assessment of the disk diffusion technique for routine antimicrobial susceptibility testing for Helicobacter pylori. Helicobacter. 2020;25:e12703. doi: 10.1111/hel.12703. [DOI] [PubMed] [Google Scholar]
- 64.Weseler A, Geiss HK, Saller R, Reichling J. A novel colorimetric broth microdilution method to determine the minimum inhibitory concentration (MIC) of antibiotics and essential oils against Helicobacter pylori. Pharmazie. 2005;60:498–502. [PubMed] [Google Scholar]
- 65.Oleastro M, Ménard A, Santos A, et al. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 2003;41:397–402. doi: 10.1128/JCM.41.1.397-402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glocker E, Kist M. Rapid detection of point mutations in the gyrA gene of Helicobacter pylori conferring resistance to ciprofloxacin by a fluorescence resonance energy transfer-based real-time PCR approach. J Clin Microbiol. 2004;42:2241–2246. doi: 10.1128/JCM.42.5.2241-2246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woo HY, Park DI, Park H, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 2009;14:22–28. doi: 10.1111/j.1523-5378.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 68.Binmaeil H, Hanafiah A, Mohamed Rose I, Raja Ali RA. Development and validation of multiplex quantitative PCR assay for detection of Helicobacter pylori and mutations conferring resistance to clarithromycin and levofloxacin in gastric biopsy. Infect Drug Resist. 2021;14:4129–4145. doi: 10.2147/IDR.S325056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of H pylori infection and determination of clarithromycin resistance in H pylori eradication therapy. World J Gastroenterol. 2007;13:671–675. doi: 10.3748/wjg.v13.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Booka M, Okuda M, Shin K, et al. Polymerase chain reaction: restriction fragment length polymorphism analysis of clarithromycin-resistant Helicobacter pylori infection in children using stool sample. Helicobacter. 2005;10:205–213. doi: 10.1111/j.1523-5378.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 71.Umegaki N, Shimoyama T, Nishiya D, Suto T, Fukuda S, Munakata A. Clarithromycin-resistance and point mutations in the 23S rRNA gene in Helicobacter pylori isolates from Japan. J Gastroenterol Hepatol. 2000;15:906–909. doi: 10.1046/j.1440-1746.2000.02072.x. [DOI] [PubMed] [Google Scholar]
- 72.Vécsei A, Innerhofer A, Binder C, et al. Stool polymerase chain reaction for Helicobacter pylori detection and clarithromycin susceptibility testing in children. Clin Gastroenterol Hepatol. 2010;8:309–312. doi: 10.1016/j.cgh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Kawai T, Yamagishi T, Yagi K, et al. Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J Gastroenterol Hepatol. 2008;23 Suppl 2:S171–S174. doi: 10.1111/j.1440-1746.2008.05408.x. [DOI] [PubMed] [Google Scholar]
- 74.Arslan N, Yılmaz Ö, Demiray-Gürbüz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol. 2017;23:2854–2869. doi: 10.3748/wjg.v23.i16.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mégraud F. Resistance of Helicobacter pylori to antibiotics. Aliment Pharmacol Ther. 1997;11 Suppl 1:43–53. doi: 10.1046/j.1365-2036.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 76.Zanotti G, Cendron L. Structural aspects of Helicobacter pylori antibiotic resistance. Adv Exp Med Biol. 2019;1149:227–241. doi: 10.1007/5584_2019_368. [DOI] [PubMed] [Google Scholar]
- 77.Saruuljavkhlan B, Yamaoka Y. Benefits of a molecular-based method for the detection of clarithromycin-resistant Helicobacter pylori. Gut Liver. 2021;15:487–489. doi: 10.5009/gnl210278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussein RA, Al-Ouqaili MT, Majeed YH. Detection of clarithromycin resistance and 23SrRNA point mutations in clinical isolates of Helicobacter pylori isolates: phenotypic and molecular methods. Saudi J Biol Sci. 2022;29:513–520. doi: 10.1016/j.sjbs.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noh JH, Ahn JY, Choi J, et al. Real-time polymerase chain reaction for the detection of Helicobacter pylori and clarithromycin resistance. Gut Liver. 2023;17:75–381. doi: 10.5009/gnl220076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu Y, Zhang M, Lu B, Dai J. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter. 2016;21:349–363. doi: 10.1111/hel.12299. [DOI] [PubMed] [Google Scholar]
- 81.Cattoir V, Nectoux J, Lascols C, et al. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents. 2007;29:389–396. doi: 10.1016/j.ijantimicag.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Nishizawa T, Suzuki H, Umezawa A, et al. Rapid detection of point mutations conferring resistance to fluoroquinolone in gyrA of Helicobacter pylori by allele-specific PCR. J Clin Microbiol. 2007;45:303–305. doi: 10.1128/JCM.01997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cambau E, Allerheiligen V, Coulon C, et al. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol. 2009;47:3600–3607. doi: 10.1128/JCM.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JW, Kim N, Nam RH, et al. GenoType HelicoDR test in the determination of antimicrobial resistance of Helicobacter pylori in Korea. Scand J Gastroenterol. 2014;49:1058–1067. doi: 10.3109/00365521.2014.894117. [DOI] [PubMed] [Google Scholar]
- 85.Pohl D, Keller PM, Bordier V, Wagner K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol. 2019;25:4629–4660. doi: 10.3748/wjg.v25.i32.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Ye L, Jin L, et al. Application of next-generation sequencing to characterize novel mutations in clarithromycin-susceptible Helicobacter pylori strains with A2143G of 23S rRNA gene. Ann Clin Microbiol Antimicrob. 2018;17:10. doi: 10.1186/s12941-018-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iwamoto A, Tanahashi T, Okada R, et al. Whole-genome sequencing of clarithromycin resistant Helicobacter pylori characterizes unidentified variants of multidrug resistant efflux pump genes. Gut Pathog. 2014;6:27. doi: 10.1186/1757-4749-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qureshi NN, Gallaher B, Schiller NL. Evolution of amoxicillin resistance of Helicobacter pylori in vitro: characterization of resistance mechanisms. Microb Drug Resist. 2014;20:509–516. doi: 10.1089/mdr.2014.0019. [DOI] [PubMed] [Google Scholar]
- 89.Binh TT, Suzuki R, Trang TT, Kwon DH, Yamaoka Y. Search for novel candidate mutations for metronidazole resistance in Helicobacter pylori using next-generation sequencing. Antimicrob Agents Chemother. 2015;59:2343–2348. doi: 10.1128/AAC.04852-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miftahussurur M, Shrestha PK, Subsomwong P, Sharma RP, Yamaoka Y. Emerging Helicobacter pylori levofloxacin resistance and novel genetic mutation in Nepal. BMC Microbiol. 2016;16:256. doi: 10.1186/s12866-016-0873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nezami BG, Jani M, Alouani D, Rhoads DD, Sadri N. Helicobacter pylori mutations detected by next-generation sequencing in formalin-fixed, paraffin-embedded gastric biopsy specimens are associated with treatment failure. J Clin Microbiol. 2019;57:e01834–18. doi: 10.1128/JCM.01834-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hulten KG, Genta RM, Kalfus IN, Zhou Y, Zhang H, Graham DY. Comparison of culture with antibiogram to next-generation sequencing using bacterial isolates and formalin-fixed, paraffin-embedded gastric biopsies. Gastroenterology. 2021;161:1433–1442. doi: 10.1053/j.gastro.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wenzhen Y, Yumin L, Quanlin G, et al. Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med. 2010;49:1103–1109. doi: 10.2169/internalmedicine.49.3031. [DOI] [PubMed] [Google Scholar]
- 94.Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013;208:1123–1130. doi: 10.1093/infdis/jit287. [DOI] [PubMed] [Google Scholar]
- 95.Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521–528. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]
- 96.Delchier JC, Bastuji-Garin S, Raymond J, et al. Efficacy of a tailored PCR-guided triple therapy in the treatment of Helicobacter pylori infection. Med Mal Infect. 2020;50:492–499. doi: 10.1016/j.medmal.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Hsieh MS, Kuo FC, Wu MC, et al. Tailored susceptibility-guided therapy via gastric juice PCR for the first-line H. pylori eradication, a randomized controlled trial. J Formos Med Assoc. 2022;121:1450–1457. doi: 10.1016/j.jfma.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 98.Neri M, Milano A, Laterza F, et al. Role of antibiotic sensitivity testing before first-line Helicobacter pylori eradication treatments. Aliment Pharmacol Ther. 2003;18:821–827. doi: 10.1046/j.1365-2036.2003.01757.x. [DOI] [PubMed] [Google Scholar]
- 99.Park CS, Lee SM, Park CH, et al. Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol. 2014;109:1595–1602. doi: 10.1038/ajg.2014.222. [DOI] [PubMed] [Google Scholar]
- 100.Martos M, Bujanda L, Salicio Y, et al. Clarithromycin for first-line treatment of Helicobacter pylori infection after culture in high-resistance regions. Eur J Gastroenterol Hepatol. 2014;26:1380–1384. doi: 10.1097/MEG.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 101.Toracchio S, Cellini L, Di Campli E, et al. Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1639–1643. doi: 10.1046/j.1365-2036.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- 102.Romano M, Iovene MR, Montella F, Vitale LM, De Simone T, Del Vecchio Blanco C. Pretreatment antimicrobial-susceptibility testing in the eradication of H. pylori infection. Am J Gastroenterol. 2000;95:3317–3318. doi: 10.1111/j.1572-0241.2000.03317.x. [DOI] [PubMed] [Google Scholar]
- 103.Romano M, Marmo R, Cuomo A, et al. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol. 2003;1:273–278. doi: 10.1016/S1542-3565(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 104.Ong S, Kim SE, Kim JH, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: a multicenter randomized controlled trial. Helicobacter. 2019;24:e12654. doi: 10.1111/hel.12654. [DOI] [PubMed] [Google Scholar]
- 105.Choi YI, Chung JW, Kim KO, et al. Tailored eradication strategy vs concomitant therapy for Helicobacter pylori eradication treatment in Korean patients. World J Gastroenterol. 2021;27:5247–5258. doi: 10.3748/wjg.v27.i31.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cha B, Bang BW, Shin JB, et al. Bismuth containing quadruple therapy versus tailored therapy as first-line treatments for Helicobacter pylori infection in a high clarithromycin resistance area. Scand J Gastroenterol. 2021;56:1017–1022. doi: 10.1080/00365521.2021.1948606. [DOI] [PubMed] [Google Scholar]
- 107.Kim SJ, Jee SR, Park MI, et al. A randomized controlled trial to compare Helicobacter pylori eradication rates between the empirical concomitant therapy and tailored therapy based on 23S rRNA point mutations. Medicine (Baltimore) 2022;101:e30069. doi: 10.1097/MD.0000000000030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho JH, Jin SY, Park S. Comparison of tailored Helicobacter pylori eradication versus modified bismuth quadruple therapy in Korea: a randomized controlled trial. Expert Rev Anti Infect Ther. 2022;20:923–929. doi: 10.1080/14787210.2022.2017280. [DOI] [PubMed] [Google Scholar]
- 109.Marzio L, Coraggio D, Capodicasa S, Grossi L, Cappello G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter. 2006;11:237–242. doi: 10.1111/j.1523-5378.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 110.Zhou L, Zhang J, Song Z, et al. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: a randomized trial. Helicobacter. 2016;21:91–99. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 111.Chen Q, Long X, Ji Y, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther. 2019;49:1385–1394. doi: 10.1111/apt.15273. [DOI] [PubMed] [Google Scholar]
- 112.Pan J, Shi Z, Lin D, et al. Is tailored therapy based on antibiotic susceptibility effective? A multicenter, open-label, randomized trial. Front Med. 2020;14:43–50. doi: 10.1007/s11684-019-0706-8. [DOI] [PubMed] [Google Scholar]
- 113.Li P, Jin J, Chen Y, Ma J, Du Q, Han Y. Susceptibility-guided vs. empirical 10-day quadruple treatment for Helicobacter pylori-infected patients: a prospective clinical trial of first-line therapy. Front Microbiol. 2022;13:973975. doi: 10.3389/fmicb.2022.973975.db7d579614e54d1496fa15bb4a81759b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gingold-Belfer R, Niv Y, Schmilovitz-Weiss H, Levi Z, Boltin D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:2649–2658. doi: 10.1111/jgh.15575. [DOI] [PubMed] [Google Scholar]
- 115.Ouyang Y, Zhang W, He C, Zhu Y, Lu N, Hu Y. Susceptibility-guided therapy vs. bismuth-containing quadruple therapy as the first-line treatment for Helicobacter pylori infection: a systematic review and meta-analysis. Front Med (Lausanne) 2022;9:844915. doi: 10.3389/fmed.2022.844915.5537500dcb0645d99b2898bfcbf68776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nyssen OP, Espada M, Gisbert JP. Empirical vs. susceptibility-guided treatment of Helicobacter pylori infection: a systematic review and meta-analysis. Front Microbiol. 2022;13:913436. doi: 10.3389/fmicb.2022.913436.652a36f75db449c590606295496b219f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liou JM, Lee YC, Wu MS Taiwan Gastrointestinal Disease and Helicobacter Consortium, author. Treatment of refractory Helicobacter pylori infection-tailored or empirical therapy. Gut Liver. 2022;16:8–18. doi: 10.5009/gnl20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miwa H, Nagahara A, Kurosawa A, et al. Is antimicrobial susceptibility testing necessary before second-line treatment for Helicobacter pylori infection? Aliment Pharmacol Ther. 2003;17:1545–1551. doi: 10.1046/j.1365-2036.2003.01541.x. [DOI] [PubMed] [Google Scholar]
- 119.Lamouliatte H, Mégraud F, Delchier JC, et al. Second-line treatment for failure to eradicate Helicobacter pylori: a randomized trial comparing four treatment strategies. Aliment Pharmacol Ther. 2003;18:791–797. doi: 10.1046/j.1365-2036.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- 120.Breuer T, Graham DY. Costs of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost effective? Am J Gastroenterol. 1999;94:725–729. doi: 10.1111/j.1572-0241.1999.00943.x. [DOI] [PubMed] [Google Scholar]
- 121.Gweon TG, Kim JS, Kim BW. An economic modeling study of Helicobacter pylori eradication: comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. 2018;12:648–654. doi: 10.5009/gnl18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cho JH, Jeon SR, Kim HG, Jin SY, Park S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol. 2019;34:700–706. doi: 10.1111/jgh.14383. [DOI] [PubMed] [Google Scholar]
- 123.Choe AR, Shim KN, Park Y, Song EM, Tae CH, Jung SA. Cost-effectiveness, efficacy, and safety analysis of tailored therapy in patients with Helicobacter pylori infection. J Clin Med. 2021;10:2619. doi: 10.3390/jcm10122619.e4cf5d3bce7847d1b4becd214d10e4d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang YW, Shin GY, Kim JW, et al. Cost-effectiveness of empirical bismuth-based quadruple therapy and tailored therapy after clarithromycin resistance tests for Helicobacter pylori eradication. Dig Dis Sci. 2022;67:1222–1230. doi: 10.1007/s10620-021-06938-y. [DOI] [PubMed] [Google Scholar]
- 125.Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019–1026. doi: 10.1136/gutjnl-2019-319954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gotoda T, Kusano C, Suzuki S, Horii T, Ichijima R, Ikehara H. Clinical impact of vonoprazan-based dual therapy with amoxicillin for H. pylori infection in a treatment-naïve cohort of junior high school students in Japan. J Gastroenterol. 2020;55:969–976. doi: 10.1007/s00535-020-01709-4. [DOI] [PubMed] [Google Scholar]
- 127.Furuta T, Yamade M, Kagami T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion. 2020;101:743–751. doi: 10.1159/000502287. [DOI] [PubMed] [Google Scholar]
- 128.Ouyang Y, Wang M, Xu YL, Zhu Y, Lu NH, Hu Y. Amoxicillin-vonoprazan dual therapy for Helicobacter pylori eradication: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37:1666–1672. doi: 10.1111/jgh.15917. [DOI] [PubMed] [Google Scholar]
- 129.Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology. 2022;163:608–619. doi: 10.1053/j.gastro.2022.05.055. [DOI] [PubMed] [Google Scholar]
- 130.U.S. Food and Drug Administration, author. Novel Drug Approvals for 2022 [Internet] FDA; Silver Spring: c2022. [cited 2022 Nov 7]. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022 . [Google Scholar]
- 131.Sue S, Maeda S. Is a potassium-competitive acid blocker truly superior to proton pump inhibitors in terms of Helicobacter pylori eradication? Gut Liver. 2021;15:799–810. doi: 10.5009/gnl20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eto H, Suzuki S, Kusano C, et al. Impact of body size on first-line Helicobacter pylori eradication success using vonoprazan and amoxicillin dual therapy. Helicobacter. 2021;26:e12788. doi: 10.1111/hel.12788. [DOI] [PubMed] [Google Scholar]
- 133.Current European concepts in the management of Helicobacter pylori infection, author. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]