Abstract
Background
Transarterial chemoembolization (TACE) is the standard treatment for hepatocellular carcinoma (HCC); the value of its combination with systemic therapy is worthy of further exploration. This study aimed to investigate the efficacy and safety of TACE combined with tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitor (ICI) in the treatment of unresectable HCC.
Methods
In this retrospective observational, single-center study, 147 patients with unresectable HCC were divided into a TACE group (n=98) and a non-TACE group (n=49) based on whether TACE was performed during TKI plus ICI therapy. The survival outcomes and adverse events (AEs) of the two groups were compared.
Results
Data from patients with unresectable HCC who received TKI plus ICI treatment between July 2017 and April 2020 were collected. The median intrahepatic tumor size was 8.7 cm [interquartile range (IQR), 5.9–12.4 cm]. At data cut-off, overall survival (OS) of the TACE group was significantly longer than that of the non-TACE group (19.5 and 10.8 months, respectively, P=0.005). In the high-risk cohort (with main or contralateral portal vein tumor thrombi and/or bile duct invasion and/or a tumor burden >50% of liver), the OS of the TACE group was still longer than that of the non-TACE group (14.9 and 8.7 months, respectively, P=0.031). Major AEs were tolerated in both groups, and there was no significant difference in their incidence (34.7% and 30.6%, respectively, P=0.621).
Conclusions
TACE treatment combined with TKI plus ICI regime resulted in longer OS than treatment with TKI plus ICI alone for patients with unresectable HCC.
Keywords: Hepatocellular carcinoma (HCC), transarterial chemoembolization (TACE), tyrosine kinase inhibitor (TKI), immune checkpoint inhibitor (ICI), combination therapy
Highlight box.
Key findings
• Transarterial chemoembolization (TACE) treatment combined with tyrosine kinase inhibitor (TKI) plus immune checkpoint inhibitor (ICI) resulted in longer overall survival (OS) than treatment with TKI plus ICI without TACE for patients with unresectable hepatocellular carcinoma (HCC).
What is known and what is new?
• TKI plus ICI are the main therapeutic choice for unresectable HCC, but there are still a large proportion of patients who are not sensitive to this treatment.
• Patients receiving TACE combined with TKI and ICI had a better OS than patients receiving TKI plus ICI alone for the treatment of unresectable HCC. Moreover, TACE did not lead to more severe adverse events in TKI plus ICI therapy.
What is the implication, and what should change now?
• Addition of TACE improves outcome of TKI plus ICI regime in patients with unresectable HCC, and has an acceptable safety profile.
• These results provide hypothesis-generating data for the design of prospective, randomized, phase 2 efficacy trials.
Introduction
The systematic treatment of unresectable hepatocellular carcinoma (HCC) has undergone profound changes in the past decade. Tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitor (ICI) are the main therapeutic drugs for unresectable HCC (1,2). Recently, several studies have demonstrated the great potential of TKI combined with ICI in the treatment of HCC. The IMbrave150 trial (3) showed that atezolizumab plus bevacizumab led to a higher survival rate than sorafenib in patients with untreated unresectable HCC [overall survival (OS) 19.2 vs. 13.4 months, respectively, P<0.001], representing the first further improvement of OS in patients with unresectable HCC compared with sorafenib. Nevertheless, the tumor response rate remains at a low level of 33.2%, similar to the rates found in other clinical trials of combination therapy (4-6). Moreover, the prognosis of high-risk HCC patients such as those with main or contralateral portal vein tumor thrombus and/or bile duct invasion and/or tumor burden greater than 50% of the liver remains poor (7).
Transarterial chemoembolization (TACE) has been recommended as one of the common treatments for HCC by most guidelines (8-10). In 2020, the TACTICS trial (11) compared TACE plus sorafenib with TACE alone and reported a major improvement in progression-free survival (PFS) based on a new definition of untreatable progression. The results of TACTICS-L trials showed that the objective response rate (ORR) of TACE plus lenvatinib in the treatment of unresectable HCC was as high as 88.7% (12). TACE may promote immunogenic cell death and induce a tumor-associated antigen-specific response, thus enhancing the tumor response to ICI (13). Therefore, TACE may augment the efficacy of TKI plus ICI in unresectable HCC.
To date, “CHANCE001” and other few studies have reported the efficacy of TACE combined with TKI and ICI in the treatment of unresectable HCC. However, the contribution of TACE to the combination of TKI and ICI remains unclear (14-16). In this study, the survival outcomes of TACE combined with TKI plus ICI in patients with unresectable HCC were compared and observed, as were any unexpected toxicities. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-486/rc).
Methods
Study design and population
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Review Committee of The Third Affiliated Hospital of Sun Yat-sen University (No. 02-126-01), and individual consent for this retrospective analysis was waived. The diagnosis of HCC met the requirements of the AASLD guidelines (17). Data from patients with unresectable HCC who received TKI plus ICI treatment between July 2017 and April 2020 were collected. Follow-up data were obtained from patient follow-up phone calls, outpatient periodic review and readmission case data. The follow-up period was up to November 2021.
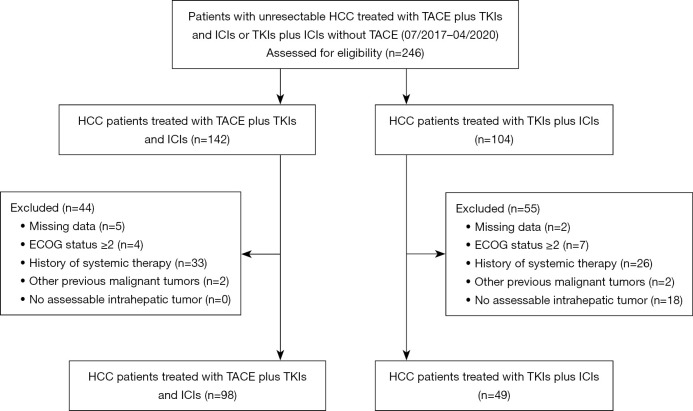
The inclusion criteria for patients were as follows: (I) 18–75 years old; (II) Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0–1; (III) Child-Pugh class A or B7-9 liver disease; and (IV) adequate hematologic, hepatic, and renal functions. Patients were excluded from our study if they (I) had missing follow-up data; (II) had any prior systemic therapy; (III) had other previous malignant tumors; or (IV) had no assessable intrahepatic tumor.
From July 2017 to April 2020, a total of 147 eligible patients with unresectable HCC received TKI plus ICI, including 98 patients who received TACE during combination therapy (hereafter, TACE group) and 49 who received combination therapy without TACE (hereafter, non-TACE group), as shown in the flow chart (Figure 1).
Figure 1.
Flowchart of patient inclusion and outcomes. HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; ICI, immune checkpoint inhibitor; ECOG, Eastern Cooperative Oncology Group.
The baseline characteristics of the two groups included age, sex, ECOG-PS, hepatitis B surface antigen (HBsAg), tumor size, tumor number, tumor involvement, main portal vein tumor thrombosis (PVTT), macrovascular invasion, extrahepatic metastasis, ascites, albumin (ALB), total bilirubin (TBIL), platelet count (PLT), prothrombin time (PT), α-fetoprotein (AFP) level, Child-Pugh score, Barcelona Clinic Liver Cancer (BCLC) stage, history of locoregional therapy, and time from initial diagnosis.
TACE treatment was performed according to the evaluation of the attending physician and the patient’s willingness. Before TACE, the attending physician explained the procedure, treatment outcome, possible side effects, and expected post-operative course.
High-risk patients were defined as those with main portal vein or contralateral portal branch tumor invasion and/or biliary duct invasion and/or tumor burden greater than 50% (7).
TACE procedure
TACE was performed under the guidance of digital subtraction angiography (DSA) by interventional radiologists with more than 10 years of experience in endovascular interventional therapy. If TACE was performed ≤1 week prior to systemic therapies, TACE could also be counted as concurrent therapy. The chemotherapeutic agents, embolization materials used in TACE, and detailed TACE procedures were as described in previously published articles (18). In short, a 2.8-F microcatheter (Renegade HiFlo Straight, Boston Scientific, Natick, MA, USA; Progreat, Terumo, Tokyo, Japan) was advanced as selectively as possible into the tumor feeding arteries. The lobaplatin concentration was 0.5 mg/mL and the total dose was 0.5 mg/kg. Lobaplatin was mixed with lipiodol at a ratio of 1:2 or 1:3. After administration of the chemotherapeutic/lipiodol mixture, 300 µm polyvinyl alcohol particles (PVA; Cook, Bloomington, IN, USA) were injected into the tumor-feeding arteries. Post embolization, occlusion of tumor supply was confirmed by angiography. Repeat TACE was allowed if the 1 month follow-up imaging demonstrated residual tumor and liver function was maintained.
TKI management
The TKI options included sorafenib or lenvatinib. The initial oral dose of sorafenib was 400 mg twice daily, and lenvatinib was 12 mg (if bodyweight ≥60 kg) or 8 mg (if bodyweight <60 kg) once per day. The initial dose was maintained and continued if there was no intolerable toxicity or radiographic disease progression. Patients in the TACE group resumed their prescribed dose of TKI 3–5 days after TACE. If the adverse events (AEs) of TKI were not tolerated at the standard dose, the dose was reduced by half. If AEs persisted despite dose reduction, treatment as suspended until the AEs disappeared.
ICI management
The options for ICI included nivolumab, pembrolizumab, and camrelizumab. They were administered on the same day as TKI. These ICI were given intravenously every 3–4 weeks at a standard dose, with each infusion lasting for at least half an hour until disease progression changed or intolerable toxicity associated with ICI appeared.
Assessment of tumor response
All patients were assessed for tumor response by abdominal contrast-enhanced computed tomography (CT) or magnetic resonance (MR) imaging before each TACE and TKI plus ICI treatment. If the patient’s disease is effectively controlled, follow-up is extended to every 3 months until data cutoff, death, or loss of follow-up. Response to treatment was assessed according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) (19) and modified RECIST (mRECIST) (20). RECIST 1.1 has been validated and provides more conservative estimates of response rates than mRECIST. It also allows comparison of response data with pivotal studies in patients with HCC, such as the Imbrave150 and KEYNOTE-240 trial. mRECIST has not been prospectively validated or evaluated in immuno-oncology therapy (21). However, traditional criteria like RECIST 1.1, which are based on tumor diameter reduction, underestimate the efficacy and do not predict survival in HCC patients treated with TACE (22). Therefore, the mRECIST has been developed to measure the reduction in viable tumor burden, which is considered to be more preferred for the evaluation of tumors treated with TACE. Given all this, the simultaneous use of mRECIST and RECIST1.1 evaluation criteria is more suitable for this study.
Two radiologists with more than 15 years of experience in abdominal imaging independently evaluated the target lesions, and any inconsistencies in the evaluation results were resolved by consensus. Tumor evaluation indices included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR included CR and PR, whereas the disease control rate (DCR) was defined as the sum of CR, PR, and SD.
OS in both groups was defined as the time period from the start of TKI plus ICI treatment to the patient’s death or survival to the cutoff date (1 November 2021). PFS was defined as either radiologic evidence of tumor progression at the time of TKI plus ICI therapy initiation or patient death, whichever occurred first.
Safety assessment
AEs that occurred during the follow-up were evaluated in terms of classification, incidence, and severity according to the National Cancer Institute Common Terminology Criteria Version 4.03 (NCI CTCAE v4.03) at each follow-up.
Study population
By the cutoff date, 59 (60.2%) of the 98 patients in the TACE group and 37 (75.5%) of the 49 patients in the non-TACE group had died. There were 5 (5.1%) patients in the TACE group and 3 (6.1%) patients in the non-TACE group who continued TKI plus ICI therapy.
Statistical analyses
The population baseline characteristics were determined using descriptive statistical methods, with categorical variables represented by median and quaternary intervals and continuous variables represented by median and 95% confidence interval (CI). Categorical variables were tested by the χ2 test or Fisher’s exact test, and continuous variables were tested by the t-test or U test. Kaplan-Meier survival analysis was used for PFS and OS, whereas a log-rank test was used to test for differences between groups. Cox proportional hazard regression models were utilized for univariate and multivariate analyses of OS in the total population of patients. The dichotomous variables P≤0.1 from univariate analyses were included in the multivariate analysis to describe the prognostic correlation of the potential survival predictors. The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was employed for all statistical analyses, and bilateral tests were used for all statistical tests. A 2-sided P<0.05 indicated statistical significance.
Results
Patient characteristics
A total of 147 patients with unresectable HCC who received TKI plus ICI with or without TACE were evaluated in this study. Among them, 98 patients who underwent TACE during TKI plus ICI treatment were included in the TACE group [median age, 52 years; interquartile range (IQR), 42–62 years; 87 men], whereas the other 49 patients were included in the non-TACE group (median age, 53 years; IQR, 47–63 years; 47 men). The baseline characteristics of the two groups are shown in Table 1. There was no significant difference between the two groups of independent variables (all comparisons, P>0.05; Table 1).
Table 1. Baseline patient characteristics.
| Variable | TACE group (n=98) | Non-TACE group (n=49) | P value |
|---|---|---|---|
| Median age (years) | 52 [42–62] | 53 [47–63] | 0.108 |
| Sex, n (%) | 0.150 | ||
| Male | 87 (88.8) | 47 (95.9) | |
| Female | 11 (11.2) | 2 (4.1) | |
| ECOG-PS, n (%) | 0.636 | ||
| 0 | 42 (42.9) | 21 (36.7) | |
| 1 | 56 (57.1) | 28 (63.3) | |
| Hepatitis B surface antigen, n (%) | 0.862 | ||
| Positive | 85 (86.7) | 43 (87.8) | |
| Negative | 13 (13.3) | 6 (12.2) | |
| Median intrahepatic tumor size (cm) | 8.8 [6.4–12.4] | 7.8 [3.8–12.6] | 0.209 |
| No. of intrahepatic tumors, n (%) | 0.692 | ||
| ≤3 | 27 (27.6) | 12 (24.5) | |
| >3 | 71 (72.4) | 37 (75.5) | |
| Tumor distribution, n (%) | 0.806 | ||
| Unilobar | 34 (34.7) | 16 (32.7) | |
| Bilobar | 64 (65.3) | 33 (67.3) | |
| Main portal vein tumor thrombus, n (%) | 0.744 | ||
| Yes | 14 (14.3) | 8 (16.3) | |
| No | 84 (85.7) | 41 (83.7) | |
| Macrovascular invasion†, n (%) | 0.098 | ||
| Yes | 73 (74.5) | 30 (61.2) | |
| No | 25 (25.5) | 19 (38.8) | |
| Extrahepatic metastasis, n (%) | 0.726 | ||
| Yes | 49 (50.0) | 26 (53.1) | |
| No | 49 (50.0) | 23 (46.9) | |
| Ascites, n (%) | 0.468 | ||
| Yes | 34 (34.7) | 20 (40.8) | |
| No | 64 (65.3) | 29 (59.2) | |
| AFP (ng/mL), n (%) | 0.554 | ||
| >200 | 59 (60.2) | 27 (55.1) | |
| ≤200 | 39 (39.8) | 22 (44.9) | |
| Child-Pugh liver function class, n (%) | 0.234 | ||
| A5–A6 | 75 (76.5) | 33 (67.3) | |
| B7–B9 | 23 (23.5) | 16 (32.7) | |
| BCLC stage, n (%) | 0.728 | ||
| B | 12 (12.2) | 7 (14.3) | |
| C | 86 (87.8) | 42 (85.7) | |
| History of locoregional therapy, n (%) | 69 (70.4) | 40 (81.6) | 0.143 |
| Median time from initial diagnosis (months) | 5.3 [1.4–11.2] | 7.7 [1.4–12.65] | 0.301 |
Data are shown as median [interquartile range] or n (%). †, some patients had hepatic venous invasion and no portal vein invasion (5 in the TACE group and 1 in the non-TACE group). ECOG-PS, Eastern Collaborative Oncology Group performance status; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization. Non-TACE group refers to patients who received systemic combination therapy without TACE.
The median follow-up time was 19.0 months (IQR, 12.4–26.4 months) in the TACE group and 10.8 months (IQR, 8.0–20.0 months) in the non-TACE group. Details of the different combinations of TKIs and ICIs are shown in Table S1. Seventy-four (77.6%) patients in TACE group and only 27 (58.3%) in non-TACE group had new hepatic foci. The sequential treatment of patients in the two groups after TKI plus ICI treatment is presented in Table 2.
Table 2. Sequential treatment after TKI plus ICI therapy.
| Variable | TACE group (n=46), n (%) | Non-TACE group (n=14), n (%) |
|---|---|---|
| Downstaging hepatectomy | 1 (2.2) | 1 (7.1) |
| Downstaging ablation | 2 (4.3) | 1 (7.1) |
| TACE | 19 (41.3) | 4 (28.6) |
| HAIC | 4 (8.7) | 3 (21.4) |
| Radiotherapy | 4 (8.7) | 1 (7.1) |
| Replace TKI | 23 (50.0) | 9 (64.3) |
| Best supportive care | 5 (10.9) | 1 (7.1) |
TKI, tyrosine kinase inhibitor; ICI, immune checkpoint inhibitor; TACE, transarterial chemoembolization; HAIC, hepatic artery infusion chemotherapy.
Treatment outcomes and survival analysis in the overall cohort
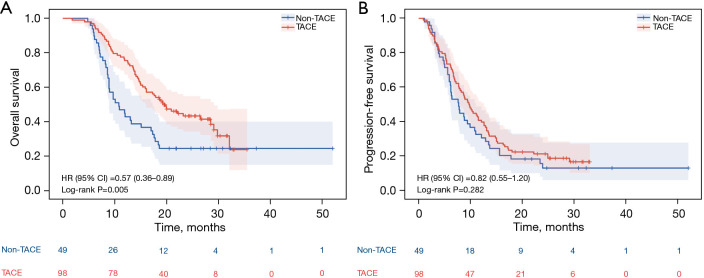
The median OS times of the TACE group and the non-TACE group were 19.5 months (95% CI: 15.2–23.8 months) and 10.8 months (95% CI: 7.5–14.1 months), respectively. The TACE group had a significantly longer OS time (P=0.005) (Figure 2A). The median PFS was 9.7 months (95% CI: 8.0–11.4 months) for the TACE group, and 7.7 months (95% CI: 5.5–9.9 months) (P=0.282) for the non-TACE group (Figure 2B).
Figure 2.
OS and PFS in overall population at final analysis. (A) Cumulative OS curves; (B) Cumulative PFS curves. HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; OS, overall survival; PFS, progression-free survival.
Subgroup analysis of OS was favorable for TACE plus TKI plus ICI (Figure 3), and patients with positive HBsAg, more intrahepatic foci, BCLC C stage, intrahepatic tumor diameter ≤7 cm and/or without main PVTT benefitted from TACE plus TKI plus ICI.
Figure 3.
Subgroup analysis of OS in overall population at final analysis. HR, hazard ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; ICI, immune checkpoint inhibitor; OS, overall survival.
The radiological evaluation outcomes of the two groups of patients with unresectable HCC are shown in Table 3. According to the RECIST 1.1, the ORR was 38.8% (38/98) for the TACE group and 26.5% (13/49) for the non-TACE group (P=0.141). The DCR was 90.8% (89/98) for the TACE group and 69.4% (34/49) for the non-TACE group (P=0.001). According to mRECIST, the ORR and DCR of the TACE group were significantly higher than those of the non-TACE group (ORR 74.4% vs. 40.8%, P<0.001; DCR 90.8% vs. 73.5%, P=0.005).
Table 3. Outcomes in patients with unresectable HCC treated with TACE group and non-TACE group.
| Variable | RECIST 1.1 | mRECIST | |||||
|---|---|---|---|---|---|---|---|
| TACE group (n=98) | Non-TACE group (n=49) | P value | TACE group (n=98) | Non-TACE group (n=49) | P value | ||
| Best overall response, n (%) | – | – | |||||
| CR | 0 (0.0) | 0 (0.0) | 22 (22.4) | 4 (8.2) | |||
| PR | 38 (38.8) | 13 (26.5) | 51 (52.0) | 16 (32.7) | |||
| SD | 51 (52.0) | 21 (42.9) | 16 (16.3) | 16 (32.7) | |||
| PD | 9 (9.2) | 15 (30.6) | 9 (9.2) | 13 (26.5) | |||
| ORR (CR + PR) | 38 (38.8) | 13 (26.5) | 0.141 | 73 (74.4) | 20 (40.8) | <0.001 | |
| DCR (CR + PR + SD) | 89 (90.8) | 34 (69.4) | 0.001 | 89 (90.8) | 36 (73.5) | 0.005 | |
Outcomes were determined according to the Response Evaluation Criteria in Solid Tumors, version 1.1 and modified Response Evaluation Criteria in Solid Tumors. Non-TACE group refers to patients who received systemic combination therapy without TACE. HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease; ORR, objective response rate; DCR, disease control rate.
TACE combined with TKI and ICI in high-risk and non-high-risk cohorts
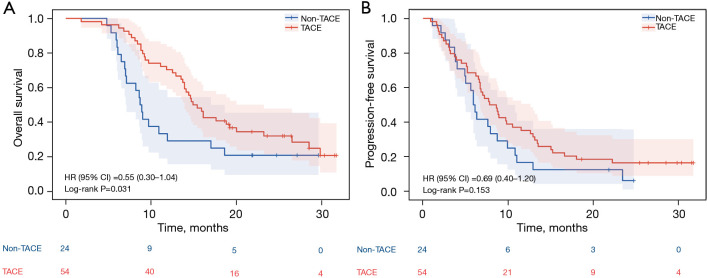
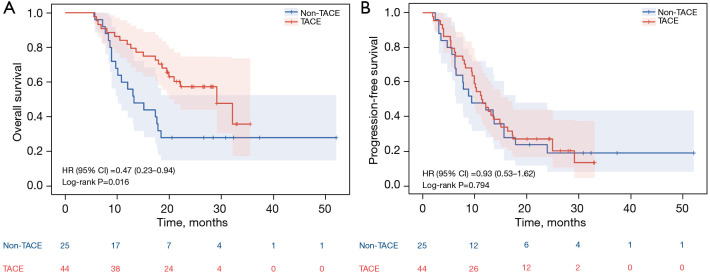
The median OS in the high-risk cohort (patients with high-risk factors of Vp-4 and/or tumor involvement >50% of the liver) was 14.9 months (95% CI: 13.2–16.7 months) in the TACE group compared with 8.7 months (95% CI: 7.7–9.7 months) in the non-TACE group (P=0.031) (Figure 4A). There was no significant difference in PFS in the high-risk cohort [PFS 7.8 months (95% CI: 5.6–10.0 months) vs. 5.9 months, respectively (95% CI: 5.1–6.7 months), P=0.153] (Figure 4B). In the non-high-risk cohort (44 patients in TACE group and 25 in non-TACE group), TACE combined with TKI and ICI showed a more pronounced advantage in OS [29.1 months (95% CI: 19.2–39.0 months) vs. 13.2 months without TACE (95% CI: 8.1–18.3 months), P=0.016] (Figure 5A). However, there was still no significant difference in PFS between the two groups in the non-high-risk cohort [PFS 11.3 months (95% CI: 8.6–14.0 months) vs. 9.4 months (95% CI: 2.4–16.4 months), respectively, P=0.794] (Figure 5B).
Figure 4.
OS and PFS in high-risk cohort population at final analysis. (A) Cumulative OS curves; (B) Cumulative PFS curves. HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; OS, overall survival; PFS, progression-free survival.
Figure 5.
OS and PFS in non-high-risk cohort population at final analysis. (A) Cumulative OS curves; (B) Cumulative PFS curves. HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; OS, overall survival; PFS, progression-free survival.
Complications
The median treatment duration of TKI plus ICI for the TACE group was 5.6 months (95% CI: 3.0–11.2 months), and it was 3.4 months (95% CI: 0.9–5.8 months) for the non-TACE group. Patients in the TACE group received a total of 224 TACE procedures, with a median of 3.0 (IQR, 1.0–3.0) procedures per patient.
AEs in both groups for all grades and grades 3–4 with an incidence greater than 10% are shown in Table 4. The incidence of grade 3–4 toxicities in the overall population was 29.9% (44/147). A total of 96.9% (95/98) and 34.7% (34/98) of patients in the TACE group and 98.0% (48/49) and 30.6% (15/49) of patients in the non-TACE group experienced any grade of AEs and grade 3–4 AEs at least once, respectively. No patients discontinued TKI plus ICI treatment due to TACE-related toxic side effects. There were no deaths due to TACE or TKI plus ICI therapy. Totals of 35 (35.7%) patients from the TACE group and 16 (32.7%) patients from the non-TACE group were prescribed a dose change or interruption due to treatment-related toxicity of systemic combination therapy, respectively. The overall incidence of grade 3–4 AEs in the TACE group was similar to that in the non-TACE group (34.7% vs. 30.6%, P=0.621).
Table 4. Adverse events with an incidence of more than 10% in either group.
| Event | TACE group (n=98), n (%) | Non-TACE group (n=49), n (%) | |||
|---|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | ||
| AST/ALT increased | 55 (56.1) | 4 (4.1) | 12 (24.5) | 1 (2.0) | |
| Constipation | 35 (35.7) | 0 (0.0) | 13 (26.5) | 0 (0.0) | |
| Pyrexia | 34 (34.7) | 7 (7.1) | 14 (28.6) | 2 (4.1) | |
| Hypertension | 31 (31.6) | 9 (9.2) | 13 (26.6) | 4 (8.2) | |
| PLT decreased | 26 (26.5) | 3 (3.1) | 20 (40.9) | 2 (4.1) | |
| Fatigue | 24 (24.5) | 7 (7.1) | 15 (26.5) | 1 (2.0) | |
| Weight decreased | 24 (24.5) | 0 (0.0) | 13 (26.5) | 0 (0.0) | |
| Diarrhea | 22 (22.4) | 0 (0.0) | 7 (14.4) | 0 (0.0) | |
| Rash | 21 (21.4) | 3 (3.1) | 10 (20.4) | 1 (2.0) | |
| Pneumonitis | 13 (13.3) | 2 (2.0) | 5 (10.2) | 2 (4.1) | |
| Nausea | 11 (11.2) | 2 (2.0) | 6 (12.2) | 0 (0.0) | |
| Proteinuria | 11 (11.2) | 0 (0.0) | 6 (12.2) | 1 (2.0) | |
| TBIL increased | 10 (10.2) | 2 (2.0) | 8 (16.3) | 2 (4.1) | |
Non-TACE group refers to patients who received systemic combination therapy without TACE. AST, aspartate transaminase; ALT, alanine aminotransferase; PLT, platelet; TBIL, total bilirubin; TACE, transarterial chemoembolization.
Discussion
In this study, the OS of patients receiving TKI plus ICI therapy with TACE was significantly longer than that of patients receiving TKI plus ICI therapy without TACE (19.5 vs. 10.8 months, P=0.005). According to the RECIST 1.1 and mRECIST criteria, the DCRs of the TACE group were higher than those of the non-TACE group (RECIST 1.1 90.8% vs. 69.4%, P=0.001; mRECIST 90.8% vs. 73.5%, P=0.005). These results provide hypothesis-generating data for the design of prospective, randomized, phase 2 efficacy trials.
Resection or liver transplantation is primary curative modalities to improve survival outcomes of HCC patients. However, it is estimated that up to 70% of patients may not be eligible for curative intent therapy, and thus systemic therapy with TKI and ICI is an integral component of HCC therapy in unresectable patients (23). It remains unclear if addition of TACE to systemic therapy confers survival advantage and our results have shown that TACE improves OS. It is worth noting that there was no statistical difference in PFS of patients receiving TACE combined with TKI and ICI compared with that of patients receiving treatment without TACE (9.7 vs. 7.7 months, P=0.282). A potential reason for this result may be that more patients in the TACE group were defined as having disease progression due to new hepatic foci (77.6% vs. 58.3%) rather than the enlargement of the primary tumor. Although these patients were defined as having tumor progression, they experienced improved OS and intrahepatic lesion control after repeat TACE. The progression was due to new intrahepatic lesions and may not necessarily have been due to the failure of TACE, which is a locoregional therapy (24).
Due to the breakthrough extended PFS and OS efficacy demonstrated by IMbrave150, atezolizumab plus bevacizumab was quickly approved as the first-line treatment for unresectable HCC in many countries. However, the OS of the high-risk cohort in the IMbrave150 study was only 7.6 months (25). A question worth raising is whether TACE can improve the survival time of high-risk patients. Notably, the effect of TACE in high-risk populations significantly enhanced the efficacy of TKI plus ICI therapy. Survival outcomes in the non-TACE group were similar to those in the survival in the IMbrave150 high-risk group. The TACE group survival in this study was significantly prolonged, suggesting that the primary cause of death in the high-risk group is intrahepatic tumor progression and that TACE may improve outcomes by intrahepatic tumor local control. Moreover, TACE plus TKI and ICI also significantly improved the OS (29.1 vs. 13.2 months, P=0.016) in the non-high-risk population. Such a remarkable therapeutic effect is rare in studies of unresectable HCC.
The results of our study are not entirely unexpected as TACE involves giving different pharmaceutical agent and thus potentially having additive or synergic effect to systemic therapeutic agents. TACE plus sorafenib has been shown to have superior PFS to sorafenib alone (11). The clinical effect of TACE appears to be at least additive if not synergistic to TKI and ICI therapy. TACE induces cell death and releases tumor antigens (13,26,27), and TKI can reprogram the immunosuppressive tumor microenvironment into an immunostimulatory environment (28,29), both of which may improve the effect of immunotherapy. However, the mechanism of action of how TACE promotes the efficacy of TKI and ICI remains unclear, and further basic studies are required to elucidate the pathophysiology.
In this study, the treatment of TACE plus TKI and ICI was validated to be clinically effective and safe. The classification, incidence, and severity of AEs in the whole population were also divided into two groups according to whether TACE was performed during systemic combination therapy for comparative analysis. All types of AEs were similar in both groups and were generally consistent with safety conclusions from other known trials, and TACE did not lead to more severe AEs associated with TKI plus ICI therapy.
There are several limitations in this study. First, this was a retrospective study with a limited sample size and relatively short follow-up time, and there was an inevitable selection bias affecting the results. Second, choice of agent, its duration and dose variations of TKI and ICI were involved in this study, which also leads to bias. Thirdly, the survival estimates in the study may be somewhat limited by the duration of treatment. Fourthly, our demographic population involves predominantly hepatitis B related HCC and the results may not be generalizable to other centers.
Conclusions
In summary, patients receiving TACE combined with TKI and ICI had a better OS than patients receiving TKI plus ICI alone for the treatment of unresectable HCC. Moreover, adding TACE to TKI and ICI regime was safe and did not result in additional complication.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Dr. Vishal G. Shelat (Tan Tock Seng Hospital, Singapore) and Dr. Tanios S. Bekaii-Saab (Mayo Clinic Cancer Center, USA) for their critical comments and valuable advice on this study.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Review Committee of The Third Affiliated Hospital of Sun Yat-sen University (No. 02-126-01), and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-486/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-486/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-486/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-486/coif). The authors have no conflicts of interest to declare.
References
- 1.Su GL, Altayar O, O’Shea R, et al. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022;162:920-34. 10.1053/j.gastro.2021.12.276 [DOI] [PubMed] [Google Scholar]
- 2.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 4.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Kudo M, Merle P, et al. LBA34 - Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 2022;33:S808-69. 10.1016/j.annonc.2022.08.031 [DOI] [Google Scholar]
- 6.Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008. 10.1016/S1470-2045(22)00326-6 [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Qin S, Ikeda M, et al. Abstract CT009: IMbrave150: Updated efficacy and safety by risk status in patients (pts) receiving atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first-line treatment for unresectable hepatocellular carcinoma (HCC). Cancer Res 2021;81:CT009. 10.1158/1538-7445.AM2021-CT009 [DOI] [Google Scholar]
- 8.Kudo M, Kawamura Y, Hasegawa K, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181-223. 10.1159/000514174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. 10.1016/j.annonc.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr 2020;9:452-63. 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueshima K, Ishikawa T, Saeki I, et al. Transcatheter arterial chemoembolization therapy in combination strategy with lenvatinib in patients with unresectable hepatocellular carcinoma (TACTICS-L) in Japan: Final analysis. J Clin Oncol 2022;40:4_suppl,:417. 10.1200/JCO.2022.40.4_suppl.417 [DOI] [Google Scholar]
- 13.Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer 2021;9:e003311. 10.1136/jitc-2021-003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng L, Fang S, Wu F, et al. Efficacy and Safety of TACE Combined With Sorafenib Plus Immune Checkpoint Inhibitors for the Treatment of Intermediate and Advanced TACE-Refractory Hepatocellular Carcinoma: A Retrospective Study. Front Mol Biosci 2021;7:609322. 10.3389/fmolb.2020.609322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Yang F, Zhang Y, et al. Prognostic efficacy and prognostic factors of TACE plus TKI with ICIs for the treatment of unresectable hepatocellular carcinoma: A retrospective study. Front Oncol 2022;12:1029951. 10.3389/fonc.2022.1029951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther 2023;8:58. 10.1038/s41392-022-01235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 18.Zhu K, Huang J, Lai L, et al. Medium or Large Hepatocellular Carcinoma: Sorafenib Combined with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology 2018;288:300-7. 10.1148/radiol.2018172028 [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 21.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronot M, Bouattour M, Wassermann J, et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist 2014;19:394-402. 10.1634/theoncologist.2013-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SH, Mei J, Cheng Y, et al. Postoperative Adjuvant Hepatic Arterial Infusion Chemotherapy With FOLFOX in Hepatocellular Carcinoma With Microvascular Invasion: A Multicenter, Phase III, Randomized Study. J Clin Oncol 2023;41:1898-908. 10.1200/JCO.22.01142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruix J, Reig M, Rimola J, et al. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology 2011;54:2238-44. 10.1002/hep.24670 [DOI] [PubMed] [Google Scholar]
- 25.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 26.Heinrich S, Castven D, Galle PR, et al. Translational Considerations to Improve Response and Overcome Therapy Resistance in Immunotherapy for Hepatocellular Carcinoma. Cancers (Basel) 2020;12:2495. 10.3390/cancers12092495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greten TF, Mauda-Havakuk M, Heinrich B, et al. Combined locoregional-immunotherapy for liver cancer. J Hepatol 2019;70:999-1007. 10.1016/j.jhep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AX, Abbas AR, de Galarreta MR, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med 2022;28:1599-611. 10.1038/s41591-022-01868-2 [DOI] [PubMed] [Google Scholar]
- 29.Esteban-Fabró R, Willoughby CE, Piqué-Gili M, et al. Cabozantinib Enhances Anti-PD1 Activity and Elicits a Neutrophil-Based Immune Response in Hepatocellular Carcinoma. Clin Cancer Res 2022;28:2449-60. 10.1158/1078-0432.CCR-21-2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as