Abstract
Objective
Aneurysmal subarachnoid hemorrhage (aSAH) is a major cause of death and disability worldwide and imposes serious burdens on society and individuals. However, predicting the long‐term outcomes in aSAH patients requiring mechanical ventilation remains challenging. We sought to establish a model utilizing the Least Absolute Shrinkage and Selection Operator (LASSO)‐penalized Cox regression to estimate the prognosis of aSAH patients requiring mechanical ventilation, based on regularly utilized and easily accessible clinical variables.
Methods
Data were retrieved from the Dryad Digital Repository. Potentially relevant features were selected using LASSO regression analysis. Multiple Cox proportional hazards analyses were performed to develop a model using the training set. Receiver operating characteristics and calibration curves were used to assess its predictive accuracy and discriminative power. Kaplan–Meier and decision curve analyses (DCA) were used to evaluate the clinical utility of the model.
Results
Independent prognostic factors, including the Simplified Acute Physiology Score 2, early brain injury, rebleeding, and length of intensive care unit stay, were identified and included in the nomogram. In the training set, the area under the curve values for 1‐, 2‐, and 4‐year survival predictions were 0.82, 0.81, and 0.80, respectively. In the validation set, the nomogram exhibited excellent discrimination ability and good calibration. Moreover, DCA demonstrated that the nomogram was clinically beneficial. Finally, a web‐based nomogram was constructed (https://rehablitation.shinyapps.io/aSAH).
Interpretation
Our model is a useful tool for accurately predicting long‐term outcomes in patients with aSAH who require mechanical ventilation and can assist in making individualized interventions by providing valuable information.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH), a subtype of hemorrhagic stroke that is associated with high morbidity and mortality, involves the spontaneous rupture of an intracranial aneurysm. 1 , 2 It is estimated that over 500,000 people annually develop aSAH, 3 accounting for over 70% of all forms of SAH. 4 Despite significant improvements in the clinical treatment of aSAH, patient outcomes remain unsatisfactory. 5 aSAH has a 45% mortality rate within 30 days, and almost half of those who survive, experience irreversible brain damage from stroke complications, including cephaledema, vasospasm, hydrocephalus, and seizure. 6 It is crucial to manage patients with aSAH appropriately during the acute phase, prior to aneurysm securement, to improve their survival and functional outcomes. 7 Research has shown that aSAH patients' outcomes depend on the time required for and the type of aneurysm repair, fluid management methods, and endovascular treatment of delayed cerebral ischemia, among other factors. 8
Hypoxia can develop because of inadequate oxygenation and ventilation in the brain, which can exacerbate ischemic damage to the brain. 9 In several studies, low brain oxygenation has been linked to poor neurologic outcomes and mortality following aSAH. 10 , 11 An individual with a Glasgow Coma Scale (GCS) score ≤8 is typically incapable of maintaining a patent airway and therefore requires intubation and mechanical ventilation to ensure adequate oxygenation. 12 It has been reported that 38.5–65% of all aSAH patients require mechanical ventilation. 13 According to some clinicians, aSAH patients requiring prolonged mechanical ventilation have a poor prognosis. 14 Therefore, it is important to identify early prognostic predictors for patients with aSAH, as part of their management. 15 However, the long‐term clinical outcomes of aSAH patients requiring mechanical ventilation remain unpredictable, and no prognostic model has been developed.
Consequently, it is necessary to refine a model for predicting the prognosis of aSAH patients and for guiding clinical treatment. The current study aimed to establish a model utilizing the Least Absolute Shrinkage and Selection Operator (LASSO)‐penalized Cox regression to estimate the prognosis of aSAH patients requiring mechanical ventilation, based on regularly utilized and easily accessible clinical variables. Univariate and multivariate analyses were conducted to identify independent risk variables. A visualization model was created using a nomogram and a web‐based calculator, and the estimated performance was assessed based on discrimination, calibration, and clinical value.
Methods
Data source
The datasets analyzed are available from the Dryad Digital Repository (www.datadryad.org), which is an open‐resource database that provides a broad range of discoverable, freely reusable, reference research data. All authors have waived their copyright to these original research data.
Patients
A secondary retrospective analysis was performed based on a cohort study, which included patients with aSAH hospitalized in the neuro‐intensive care unit (ICU) between January 2010 and December 2015. 16 Only patients who required mechanical ventilation were included in this study. All patients underwent computed tomography angiography upon admission to confirm subarachnoid hemorrhage caused by aneurysm rupture. Patients with iatrogenic aneurysm ruptures and those lost to follow‐up were excluded from the analysis.
Data collection
Promising predictive indicators were selected, including age, sex, tobacco use, alcohol abuse, diabetes, cardiovascular disease, anemia localization, GCS score, Simplified Acute Physiology Score 2 (SAPS2), World Federation of Neurological Surgeons Scale (WFNS) score, Fisher's score, intracerebral hemorrhage, early brain injury, external ventricular drain, rebleeding, angiographic vasospasm, delayed cerebral ischemia, length of mechanical ventilation, and length of ICU stay. Furthermore, the vital status and follow‐up duration for each patient with dysphagia were determined.
Selection of predictive variables and development of the prediction model
To determine the subset of predictors, a univariate Cox regression analysis and LASSO‐penalized Cox regression were performed to select optimal predictors from the risk factors in the training cohort using the “glmnet” package. 17 Furthermore, based on the results of the LASSO Cox regression analysis, 18 a multivariate Cox regression model was constructed to predict the outcomes. To enhance its clinical utilization, a web‐based calculator visualization tool was developed. 19
Validation of the prediction model
We assessed the predictive ability of our model for survival using the area under the receiver operating characteristic (ROC) curve (AUC) values from the ROC analysis. The training and verification sets were calibrated to evaluate the performance of the novel model. 20 Decision curve analysis (DCA) was used to evaluate the clinical usefulness of the model. 21 These assessments were conducted using the training and validation sets.
Statistical analysis
Normally distributed data are expressed as mean ± standard deviation, whereas non‐normally distributed data are expressed as medians (interquartile ranges). The chi‐square test was used to compare categorical variables between the training and validation sets, whereas the t‐test was used to compare continuous variables. Statistical significance was set at p < 0.05. Statistical analyses were performed using SPSS (v24.0; IBM SPSS Inc., Armonk, NY, USA) and R (v3.6.2; https://www.r‐project.org/) software.
Results
Baseline characteristics and outcomes of subjects
After removing incomplete data, 211 participants were enrolled in this study, of which 60% (N = 128) were randomized to the training set and the remaining 40% (N = 83) to the validation set (Table S1). The baseline characteristics of each group are presented in Table 1. The median follow‐up period was 638 days. During the follow‐up period, 51 deaths occurred and 77 patients survived in the training dataset, whereas 41 deaths occurred and 42 patients survived in the validation dataset.
Table 1.
Baseline demographics and clinical characteristics of patients in the training set and validation set.
| Variables | All patients (n = 211) | Training set (n = 128) | Validation set (n = 83) | p‐value |
|---|---|---|---|---|
| Age, years | 55.46 ± 12.84 | 55.04 ± 13.19 | 56.12 ± 12.34 | 0.551 |
| Gender | 0.428 | |||
| Male | 82 (38.9%) | 47 (36.7%) | 35 (42.2%) | |
| Female | 129 (61.1%) | 81 (63.3%) | 48 (57.8%) | |
| Tobacco use | 0.083 | |||
| No | 143 (67.8%) | 81 (63.3%) | 62 (74.7%) | |
| Yes | 68 (32.2%) | 47 (36.7%) | 21 (25.3%) | |
| Alcohol abuse | 0.796 | |||
| No | 192 (91.0%) | 117 (91.4%) | 75 (90.4%) | |
| Yes | 19 (9.0%) | 11 (8.6%) | 8 (9.6%) | |
| Diabetes | 0.249 | |||
| No | 205 (97.2%) | 123 (96.1%) | 82 (98.8%) | |
| Yes | 6 (2.8%) | 5 (3.9%) | 1 (1.2%) | |
| Cardiovascular disease | 0.952 | |||
| No | 175 (82.9%) | 106 (82.8%) | 69 (83.1%) | |
| Yes | 36 (17.1%) | 22 (17.2%) | 14 (16.9%) | |
| Anevrism localisation | 0.221 | |||
| Anterior | 176 (83.4%) | 110 (85.9%) | 66 (79.5%) | |
| Posterior | 35 (16.6%) | 18 (14.1%) | 17 (20.5%) | |
| Intracerebral hemorrhage | ||||
| No | 109 (51.7%) | 67 (52.3%) | 42 (50.6%) | 0.805 |
| Yes | 102 (48.3%) | 61 (47.7%) | 41 (49.4%) | |
| Early brain injury | 0.024 | |||
| No | 59 (28.0%) | 43 (33.6%) | 16 (19.3%) | |
| Yes | 152 (72.0%) | 85 (66.4%) | 67 (80.7%) | |
| External ventricular drain | 0.461 | |||
| No | 70 (33.2%) | 40 (31.3%) | 30 (36.1%) | |
| Yes | 141 (66.8%) | 88 (68.7%) | 53 (63.9%) | |
| Rebleeding | 0.674 | |||
| No | 183 (86.7%) | 110 (85.9%) | 73 (88.0%) | |
| Yes | 28 (13.3%) | 18 (14.1%) | 10 (12.0%) | |
| WFNS score, n (%) | 0.218 | |||
| III | 22 (10.4%) | 12 (9.4%) | 10 (12.0%) | |
| IV | 84 (39.8%) | 57 (44.5%) | 27 (32.5%) | |
| V | 105 (49.8%) | 59 (46.1%) | 46 (55.4%) | |
| Fisher score, n (%) | 0.465 | |||
| I | 1 (0.5%) | 0 (0.0%) | 1 (1.2%) | |
| II | 4 (1.9%) | 3 (2.3%) | 1 (1.2%) | |
| III | 40 (18.9%) | 22 (17.2%) | 18 (21.7%) | |
| IV | 166 (78.7%) | 103 (80.5%) | 63 (76.0%) | |
| Angiographic vasospasm | 0.743 | |||
| No | 137 (64.9%) | 82 (64.1%) | 55 (66.3%) | |
| Yes | 74 (35.1%) | 46 (35.9%) | 28 (33.7%) | |
| Delayed cerebral Ischemia | 0.894 | |||
| No | 154 (73.0%) | 93 (72.7%) | 61 (73.5%) | |
| Yes | 57 (27.0%) | 35 (27.3%) | 22 (26.5%) | |
| Length of mechanical ventilation, days | 18.56 ± 17.40 | 19.52 ± 17.85 | 17.10 ± 16.68 | 0.325 |
| Length of stay, days | 25.48 ± 23.47 | 26.66 ± 23.94 | 23.66 ± 22.74 | 0.365 |
WFNS, World Federation of Neurological Surgeons Scale.
Selection and design of prognostic predictors
Univariate analysis was performed on the training set to identify factors associated with aSAH prognosis. Five valuable factors (SAPS2, early brain injury, rebleeding, length of mechanical ventilation days, and length of ICU stay) were used as prognostic factors for poor outcomes (p < 0.05; Table 2). The five clinical features were then entered into the LASSO regression for 1000 bootstrap iterations, and four features with non‐zero coefficients and a minimum lambda value were selected (Fig. S1). According to the multivariate Cox regression analysis, SAPS2, early brain injury, rebleeding, and length of ICU stay were independent prognostic factors (Table 2).
Table 2.
Univariate and multivariable Cox hazards analysis to screen the potential prognostic factors based on the training cohort.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| Age, years | 1.02 (0.99–1.04) | 0.161 | ||
| Gender | 1.42 (0.82–2.48) | 0.215 | ||
| Tobacco use | 0.67 (0.37–1.23) | 0.201 | ||
| Alcohol abuse | 0.98 (0.35–2.72) | 0.969 | ||
| Diabetes | 0.38 (0.05–2.78) | 0.343 | ||
| Cardiovascular disease | 1.07 (0.54–2.14) | 0.841 | ||
| Anevrism localization | 1.19 (0.56–2.53) | 0.657 | ||
| GCS | 0.95 (0.88–1.04) | 0.299 | ||
| SAPS II | 1.03 (1.00–1.05) | 0.026 | 1.04 (1.02–1.07) | 0.001 |
| WFNS | 1.36 (0.87–2.13) | 0.181 | ||
| Fisher | 1.00 (0.55–1.83) | 0.990 | ||
| Intracerebral hemorrhage | 0.86 (0.50–1.49) | 0.594 | ||
| Early brain injury | 3.04 (1.48–6.25) | 0.003 | 3.13 (1.47–6.63) | 0.003 |
| External ventricular drain | 0.92 (0.51–1.66) | 0.770 | ||
| Rebleeding | 2.93 (1.52–5.62) | 0.001 | 2.55 (1.28–5.08) | 0.008 |
| Angiographic vasospasm | 0.62 (0.34–1.16) | 0.134 | ||
| Delayed cerebral ischemia | 1.01 (0.55–1.87) | 0.971 | ||
| Length of mechanical ventilation | 0.98 (0.96–1.00) | 0.016 | ||
| Length of stay | 0.97 (0.96–0.99) | 0.002 | 0.96 (0.95–0.98) | 0.000 |
GCS, Glasgow Coma Scale; SAPS II, Simplified Acute Physiology Score 2; WFNS, World Federation of Neurological Surgeons Scale.
Development of multivariate prognostic nomogram
A multivariate Cox regression model was used to develop a nomogram to discriminate survival (Fig. 1A). A straight line was drawn upward corresponding to each predictor axis, whereas a straight line denoted the total survival axis. Additionally, this prognostic nomogram exhibited good calibration based on the calibration curve (Fig. 1B).
Figure 1.
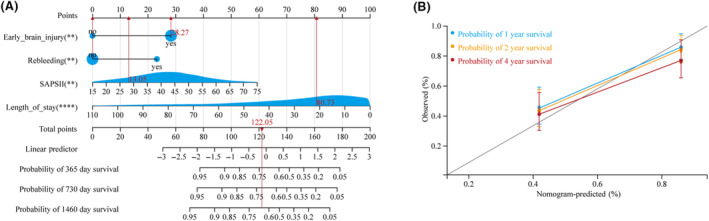
Nomogram of clinical features for predicting survival of aneurysmal subarachnoid hemorrhage (aSAH) patients requiring mechanical ventilation. (A) Nomogram constructed to predict the 1‐, 2‐, and 4‐year survival in the training cohort. (B) Calibration curves for predicting overall survival rate by the nomogram in the training and validation sets.
Following the development of this classification model, we stratified 128 trained patients into low‐ and high‐risk groups of 64 patients each. Figure 2A,B show the distribution of the risk scores and survival statuses ranked by the risk scores in the training set. Survival analysis revealed that the high‐risk group had a significantly lower survival rate than the low‐risk group (Fig. 2C). The AUCs for the 1‐, 2‐, and 4‐year survival predictions were 0.82, 0.81, and 0.80, respectively (Fig. 2D), indicating that the model was effective in predicting survival. The nomogram was thus capable of providing valuable and informed prognostic judgment based on DCA (Fig. S2).
Figure 2.
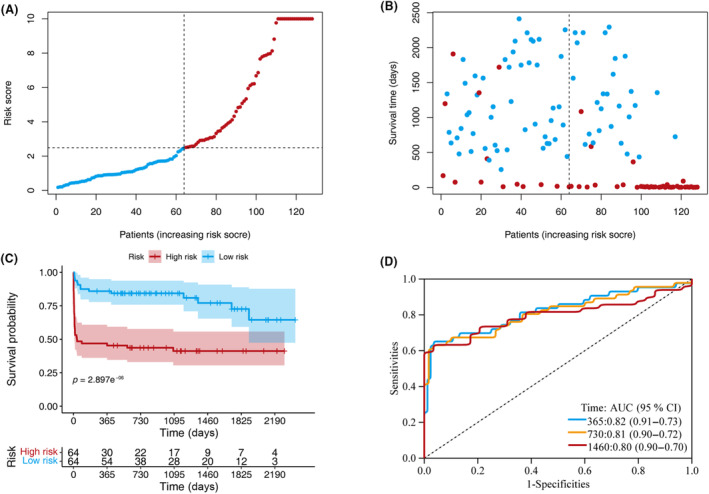
Model discrimination and performance in the training set. (A) Distribution of participants in the low‐ and high‐risk score categories based on survival status. (B) Comparison of survival risk between the two groups. (C) Overall survival curves were separated by groups with low‐ and high‐risk scores. (D) Time‐dependent receiver operating characteristic curves of the scoring system in the training set.
Performance of the model in the validation set
A cutoff value was used in the training set to stratify the patients into low‐ (n = 39) and high‐risk (n = 44) groups in the validation set. Figure 3A,B show the distribution of the risk scores and the survival status ranked by the risk scores in the validation set. Survival analysis revealed that the high‐risk group exhibited significantly worse survival than did the low‐risk group (Fig. 3C). The AUCs for the 1‐, 2‐, and 4‐year survival predictions were 0.72, 0.75, and 0.73, respectively (Fig. 3D).
Figure 3.
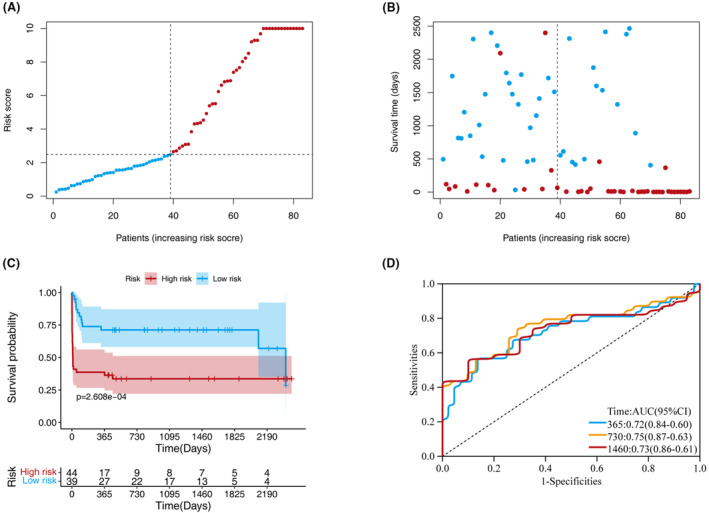
Model discrimination and performance in the validation set. (A) Distribution of participants in the low‐ and high‐risk score categories based on survival status. (B) Comparison of survival risk between the two groups. (C) Overall survival curves were separated by groups with low‐ and high‐risk scores. (D) Time‐dependent receiver operating characteristic curves of the scoring system in the validation set.
Establishment of a web‐based calculator
A web‐based prognostic model was developed for easy clinical use and visualization (https://rehablitation.shinyapps.io/aSAH/) of predicted aSAH prognosis based on the established nomogram (Fig. 4). A participant's survival probability can be estimated by entering the details of the aSAH participant into this web‐based program.
Figure 4.
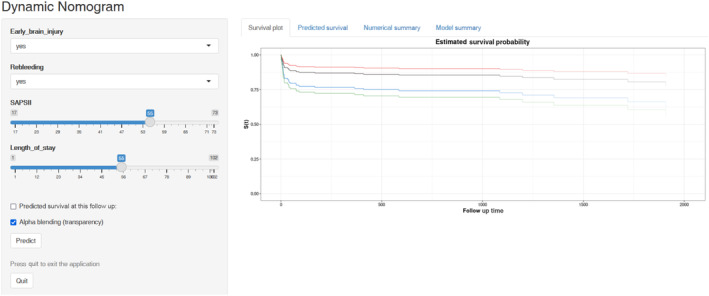
Web‐based dynamic nomogram for prediction of all‐cause mortality probability (https://rehablitation.shinyapps.io/aSAH/). By entering the specific information of the patients with aneurysmal subarachnoid hemorrhage (aSAH) in the web‐based online tool, we could obtain the corresponding all‐cause mortality probability for an aSAH patient requiring mechanical ventilation.
Discussion
SAH is a major cause of death and disability worldwide. 22 The poor outcome of aSAH can be attributed to the initial catastrophic event or to numerous acute or delayed neurological complications, such as cerebral ischemia, hydrocephalus, or rebleeding. 23 , 24 No reliable biomarkers exist for predicting complications and prognoses at clinically relevant timepoints that would allow identification of patients who need aggressive treatment or for determining when a neurological injury can be effectively treated and managed. 25 , 26 Recently, a growing number of mathematical models, based on multiple markers, have been developed in medicine using analytical approaches. 27 This approach involves combining a series of significant parameters to construct a model that performs better in terms of predicting prognosis. 28 In the present study, clinical data and demographic information of individuals with aSAH were used to estimate the prognostic risk using LASSO‐Cox regression analysis. The SAPS2, early brain injury, rebleeding, and the length of ICU stay were included in the multivariate model. Model performance was validated using discrimination and calibration curves, and DCA in the validation set. The model demonstrated a high degree of predictive power in both the training and validation sets. Furthermore, clinical decision‐making was facilitated with the help of a novel nomogram and a corresponding web‐based calculator. Unlike previous nomograms that calculated estimates, our dynamic nomogram produces exact results.
The predictive power of the model was also evaluated. The predictive validity of the model was assessed by dividing the raw data into training and testing sets. Kaplan–Meier analysis revealed that the high‐ and low‐risk groups had significantly different prognoses. For the training set, the AUCs for 1‐, 2‐, and 4‐year survival predictions were 0.82, 0.81, and 0.80, respectively, while for the validation set, these AUCs were 0.72, 0.75, and 0.73, respectively. In addition, a calibration curve and DCA were used to evaluate the model. The calibration curve and DCA results demonstrated that the method performed well. However, as no similar previous study exists, the performance of our model could not be compared and evaluated owing to the lack of comparable data.
Several studies have investigated biomarkers and constructed nomograms to predict the outcomes in patients with aSAH. Lu et al. developed a nomogram that predicted the risk of 6‐month unfavorable outcomes (by the modified Rankin score) in older patients with aSAH after endovascular coiling. 29 Hu et al. developed an online prediction tool based on a random forest model to identify patients at a high risk of delayed cerebral ischemia after aSAH. 30 Fang et al. reported that elevated D‐dimer levels on admission were associated with short‐ and long‐term mortality. 31 Wiśniewski et al. found that glucose‐6‐phosphate dehydrogenase and 8‐iso‐prostaglandin F2α are potential predictors of delayed cerebral ischemia after aSAH. 32 However, most of these previous studies focused mainly on neurological complications, and few studies have established survival models. In the present study, we focused on the prognosis of aSAH cases requiring mechanical ventilation, classified as high‐risk after complete resection, and constructed a nomogram to predict mortality in these treated patients.
We recommend the wide application of this novel scoring model in most hospitals for rapid prediction of the prognosis for aSAH patients, as this system is based on four easily accessible clinical variables. Our scoring model could provide an important reference for clinical management, which would be helpful for the selection of therapeutic regimens and the prediction of prognosis.
Among the independent prognostic factors in our model, we should notice that the length of stay is a confounder. It is generally considered that the less length of stay in the hospital seems to be associated with a better prognosis. However, there may be other scenarios that we could not ignore. Some patients stay less time in the hospital because they are dying, and the alive patients had more length of stay. This situation leads to quite different results. Further, well‐designed clinical trials are needed to overcome all the confounders.
This study had several strengths. First, our data suggested that a model is a good option for effectively predicting the survival of patients with aSAH with high accuracy. Second, the features included in this novel model are easy to obtain. Moreover, we developed an easy‐to‐operate web‐based calculator that would be favorable for promoting the precise prevention and personalized health management of aSAH, to maximize cost‐effectiveness.
Limitations
This study had a few limitations. First, although the performance of the model was good in both the training and validation sets, a multicenter clinical application is required to evaluate the utility of this model externally. Second, this retrospective analysis was based on an open‐access public database, which may have entailed several inherent selection biases. Third, a limited number of covariates were available for investigation and other novel biomarkers were not available. Therefore, a multicenter validation of the scoring system in a large study population is needed to obtain high‐level evidence for the clinical application of the model.
Conclusion
This study identified and validated a model incorporating four clinical characteristics (SAPS2, early brain injury, rebleeding, and length of ICU stay) to predict long‐term mortality in patients with aSAH. The model performed well in predicting the survival of patients with aSAH. This clinically useful tool can help to improve the prognostic management of aSAH patients.
Author Contributions
Wan Xichen and Wu Xiao collected and analyzed the data; Kang Junwei and Fang Longjun analyzed and interpreted the data; Tang Yunliang conceived the study.
Funding Information
This work was supported by the National Natural Science Foundation of China (Nos. 82202811 and 82201271), the Youth Talent Cultivation Project of First Affiliated Hospital of Nanchang University (No. YFYPY202116), the Natural Science Foundation of Jiangxi Province (No. 20224BAB216033), and the Science and Technology Project of Jiangxi Provincial Health Commission (No. 202210362).
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Supporting information
Figure S1
Table S1
Acknowledgements
We would like to thank Kevin Chalard et al. for sharing their data.
Funding Statement
This work was funded by National Natural Science Foundation of China grants 82201271 and 82202811; Natural Science Foundation of Jiangxi Province grant 20224BAB216033; Science and technology Project of Jiangxi Provincial Health grant 202210362; Youth Talent Cultivation Project of First Affiliated Hospital of Nanchang University grant YFYPY202116.
Data Availability Statement
All the data used in this study were obtained from the Dryad Digital Repository database (http://www.datadryad.org/).
References
- 1. Rinkel GJ, Algra A. Long‐term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349‐356. [DOI] [PubMed] [Google Scholar]
- 2. Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Magnesium for aneurysmal subarachnoid haemorrhage. Lancet. 2012;380(9851):1381 author reply 1382. [DOI] [PubMed] [Google Scholar]
- 3. Hughes JD, Bond KM, Mekary RA, et al. Estimating the global incidence of aneurysmal subarachnoid hemorrhage: a systematic review for central nervous system vascular lesions and meta‐analysis of ruptured aneurysms. World Neurosurg. 2018;115:430‐447.e7. [DOI] [PubMed] [Google Scholar]
- 4. Roquer J, Cuadrado‐Godia E, Guimaraens L, et al. Short‐ and long‐term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology. 2020;95(13):e1819‐e1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimada M, Rose JD. Takotsubo cardiomyopathy secondary to intracranial hemorrhage. Int J Emerg Med. 2014;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711‐1737. [DOI] [PubMed] [Google Scholar]
- 7. Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2010;9(5):504‐519. [DOI] [PubMed] [Google Scholar]
- 8. de Winkel J, van der Jagt M, Lingsma HF, et al. International practice variability in treatment of aneurysmal subarachnoid hemorrhage. J Clin Med. 2021;10(4):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang CH, Ni SY, Lu HY, Huang AP, Kuo LT. Predictors of prolonged mechanical ventilation among patients with aneurysmal subarachnoid hemorrhage after microsurgical clipping. Neurol Ther. 2022;11(2):697‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gouvea Bogossian E, Diaferia D, Ndieugnou Djangang N, et al. Brain tissue oxygenation guided therapy and outcome in non‐traumatic subarachnoid hemorrhage. Sci Rep. 2021;11(1):16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rass V, Helbok R. Early brain injury after poor‐grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep. 2019;19(10):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aneja S, Sharma S. Diagnosis and management of acute encephalitis in children. Indian J Pediatr. 2019;86(1):70‐75. [DOI] [PubMed] [Google Scholar]
- 13. Towner JE, Rahmani R, Zammit CG, et al. Mechanical ventilation in aneurysmal subarachnoid hemorrhage: systematic review and recommendations. Crit Care. 2020;24(1):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aytuluk HG, Basaran S, Dogan NO, Demir N. Comparison of conventional intensive care scoring systems and prognostic scores specific for intracerebral hemorrhage in predicting one‐year mortality. Neurocrit Care. 2021;34(1):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bobeff EJ, Bukowiecka‐Matusiak M, Stawiski K, et al. Plasma amino acids may improve prediction accuracy of cerebral vasospasm after aneurysmal subarachnoid haemorrhage. J Clin Med. 2022;11(2):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chalard K, Szabo V, Pavillard F, et al. Long‐term outcome in patients with aneurysmal subarachnoid hemorrhage requiring mechanical ventilation. PloS One. 2021;16(3):e0247942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1‐22. [PMC free article] [PubMed] [Google Scholar]
- 18. Tibshirani R. The lasso method for variable selection in the cox model. Stat Med. 1997;16(4):385‐395. [DOI] [PubMed] [Google Scholar]
- 19. Jalali A, Alvarez‐Iglesias A, Roshan D, Newell J. Visualising statistical models using dynamic nomograms. PloS One. 2019;14(11):e0225253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Post R, Germans MR, Tjerkstra MA, et al. Ultra‐early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2021;397(10269):112‐118. [DOI] [PubMed] [Google Scholar]
- 23. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306‐318. [DOI] [PubMed] [Google Scholar]
- 24. Svedung Wettervik T, Hånell A, Ronne‐Engström E, Lewén A, Enblad P. Temperature changes in poor‐grade aneurysmal subarachnoid hemorrhage: relation to injury pattern, intracranial pressure dynamics, cerebral energy metabolism, and clinical outcome. Neurocrit Care. 2023. PMID: 36922474. doi: 10.1007/s12028-023-01699-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Q, Zhang G, Wang L, et al. Clinical value and prognosis of C reactive protein to lymphocyte ratio in severe aneurysmal subarachnoid hemorrhage. Front Neurol. 2022;13:868764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nie Z, Lin F, Li R, Chen X, Zhao Y. A pooled analysis of preoperative inflammatory biomarkers to predict 90‐day outcomes in patients with an aneurysmal subarachnoid hemorrhage: a single‐center retrospective study. Brain Sci. 2023;13(2):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng Z, Zou K, Qing C, Wang J, Tang Y. Predicting mortality in acute kidney injury patients undergoing continuous renal replacement therapy using a visualization model: a retrospective study. Front Physiol. 2022;13:964312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang Y, Wang J, Chen G, Ye W, Yan N, Feng Z. A simple‐to‐use web‐based calculator for survival prediction in Parkinson's disease. Aging (Albany NY). 2021;13(4):5238‐5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu W, Tong Y, Zhang C, et al. A novel visual dynamic nomogram to online predict the risk of unfavorable outcome in elderly aSAH patients after endovascular coiling: a retrospective study. Front Neurosci. 2022;16:1037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu P, Li Y, Liu Y, et al. Comparison of conventional logistic regression and machine learning methods for predicting delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a multicentric observational cohort study. Front Aging Neurosci. 2022;14:857521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang F, Wang P, Yao W, et al. Association between D‐dimer levels and long‐term mortality in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2022;52(3):E8. [DOI] [PubMed] [Google Scholar]
- 32. Wiśniewski K, Popęda M, Price B, et al. Glucose‐6‐phosphate dehydrogenase and 8‐iso‐prostaglandin F2α as potential predictors of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2023;1‐10. PMID: 36640097. doi: 10.3171/2022.12.JNS222332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Data Availability Statement
All the data used in this study were obtained from the Dryad Digital Repository database (http://www.datadryad.org/).


