This nonrandomized controlled trial of patients diagnosed with non–small cell lung cancer assesses whether individualizing stereotactic ablative radiotherapy dose and fractionation by tumor size, location, and histological characteristics may be associated with local tumor control and the rate of toxic effects.
Key Points
Question
Is individualizing dose and fractionation of stereotactic ablative radiotherapy by tumor size, location, and histological characteristics associated with local control in the treatment of lung tumors?
Findings
In this nonrandomized controlled trial of 217 unique patients with 285 treated tumors, rates of freedom from local recurrence at 1 year were 97% for first non–small cell lung cancers (NSCLCs), 94% for a new or multiple NSCLCs, and 96% for lung metastases from NSCLC or another tumor; these results met the primary end point of 90% or higher. The proportion of patients with grade 3 to 5 toxic effects was low, at 5%, with a single possibly treatment-associated grade 5 event.
Meaning
The findings of this study suggest that an individualized dosing regimen, including doses lower than those routinely administered, is associated with excellent tumor control.
Abstract
Importance
Stereotactic ablative radiotherapy (SABR) is used for treating lung tumors but can cause toxic effects, including life-threatening damage to central structures. Retrospective data suggested that small tumors up to 10 cm3 in volume can be well controlled with a biologically effective dose less than 100 Gy.
Objective
To assess whether individualizing lung SABR dose and fractionation by tumor size, location, and histological characteristics may be associated with local tumor control.
Design, Setting, and Participants
This nonrandomized controlled trial (the iSABR trial, so named for individualized SABR) was a phase 2 multicenter trial enrolling participants from November 15, 2011, to December 5, 2018, at academic medical centers in the US and Japan. Data were analyzed from December 9, 2020, to May 10, 2023. Patients were enrolled in 3 groups according to cancer type: initial diagnosis of non–small cell lung cancer (NSCLC) with an American Joint Committee on Cancer 7th edition T1-3N0M0 tumor (group 1), a T1-3N0M0 new primary NSCLC with a history of prior NSCLC or multiple NSCLCs (group 2), or lung metastases from NSCLC or another solid tumor (group 3).
Intervention
Up to 4 tumors were treated with once-daily SABR. The dose ranged from 25 Gy in 1 fraction for peripheral tumors with a volume of 0 to 10 cm3 to 60 Gy in 8 fractions for central tumors with a volume greater than 30 cm3.
Main outcome
Per-group freedom from local recurrence (same-lobe recurrence) at 1 year, with censoring at time of distant recurrence, death, or loss to follow-up.
Results
In total, 217 unique patients (median [IQR] age, 72 [64-80] years; 129 [59%] male; 150 [69%] current or former smokers) were enrolled (some multiple times). There were 240 treatment courses: 79 in group 1, 82 in group 2, and 79 in group 3. A total of 285 tumors (211 [74%] peripheral and 74 [26%] central) were treated. The most common dose was 25 Gy in 1 fraction (158 tumors). The median (range) follow-up period was 33 (2-109) months, and the median overall survival was 59 (95% CI, 49-82) months. Freedom from local recurrence at 1 year was 97% (90% CI, 91%-99%) for group 1, 94% (90% CI, 87%-97%) for group 2, and 96% (90% CI, 89%-98%) for group 3. Freedom from local recurrence at 5 years ranged from 83% to 93% in the 3 groups. The proportion of patients with grade 3 to 5 toxic effects was low, at 5% (including a single patient [1%] with grade 5 toxic effects).
Conclusions and Relevance
The results of this nonrandomized controlled trial suggest that individualized SABR (iSABR) used to treat lung tumors may allow minimization of treatment dose and is associated with excellent local control. Individualized dosing should be considered for use in future trials.
Trial Registration
ClinicalTrials.gov Identifier: NCT01463423
Introduction
Stereotactic ablative radiotherapy (SABR), or stereotactic body radiotherapy, is standard of care for medically inoperable early-stage non–small cell lung cancer (NSCLC) and is also becoming established for pulmonary oligometastases from various primaries.1,2,3,4 Optimal dose regimens have not been fully established, particularly for central tumors, which have an increased risk of toxic effects, and guidelines5,6,7 recommend multiple schedules. The Radiation Therapy Oncology Group 0236 trial8 of SABR for peripherally located NSCLC used a high dose of 60 Gy in 3 fractions (around 54 Gy in 3 fractions using modern planning with heterogeneity corrections). Substantial toxic effects were seen, with a 27% rate of grade 3 to 4 toxic effects. SABR for centrally located tumors carries the risk of life-threatening toxic effects such as pulmonary hemorrhage.9,10
Despite toxic effects from higher dose regimens, there has been concern about lowering the dose due to reports of high local recurrence risk from lower doses, with a threshold of a 100 Gy biologically effective dose (BED) with α/β = 10 (BED10) cited.5,11,12,13 Most more recent lung SABR trials4,8,10,14,15 have used a dose higher than 100 Gy BED10 regardless of tumor size. Studies16,17,18,19 have shown a positive association between tumor size and volume and local recurrence, possibly due to increased hypoxia in larger tumors. Our group found in an analysis16 of patients mainly from a phase 1 dose escalation trial19 that, while large tumors require high SABR doses, smaller tumors can be controlled with lower doses (less than 100 Gy BED10). In a retrospective study20 of tumor volume-adapted dosing, in which small tumors received single-fraction treatment with BED10 less than 100 Gy and larger tumors received BED10 greater than 100 Gy in 3 to 4 fractions, high control rates were seen in both groups. In the current trial, which we named the iSABR trial (for individualized SABR), we prospectively individualized lung SABR dose and fractionation based on tumor size, location, and histological characteristics, including use of lower dose regimens with BED10 less than 100 Gy for small tumors. We hypothesized that this strategy would be associated with excellent local control and low toxic effects.
Methods
Eligibility Criteria
This nonrandomized controlled trial was a single-arm phase 2 study conducted at Stanford University, Stanford, California, and Hokkaido University, Sapporo, Japan. It was approved by each center’s institutional review board, and all participants provided written informed consent. Participants were enrolled from November 15, 2011, to December 5, 2018. Data were analyzed from December 9, 2020, to May 10, 2023.
Patients 18 years or older were enrolled in 3 groups according to cancer type (Figure 1). Patients in group 1 had an initial diagnosis of a single NSCLC. Patients in group 2 had a new primary NSCLC with history of prior NSCLC, or multiple synchronous NSCLCs. For groups 1 and 2, the American Joint Committee on Cancer 7th edition stage was T1-3N0M0. Patients in group 3 had lung metastases from NSCLC or another solid tumor, without known uncontrolled extrathoracic metastases. Biopsy confirmation of malignant tumor was encouraged, but when biopsy was not feasible it could be diagnosed with strict imaging criteria (present on ≥3 serial scans and ≥1 of the following: growing, increasing solid component, or increasing 18F-fluorodeoxyglucose avidity). If 1 tumor was treated, the sum of 3 orthogonal diameters had to be 20 cm or less; if multiple tumors were treated, the sum had to be 15 cm or less for each tumor. Patients could be re-enrolled if they developed a new tumor requiring treatment. Full inclusion and exclusion criteria are provided in the Trial Protocol in Supplement 1. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Figure 1. Patient Flowchart.
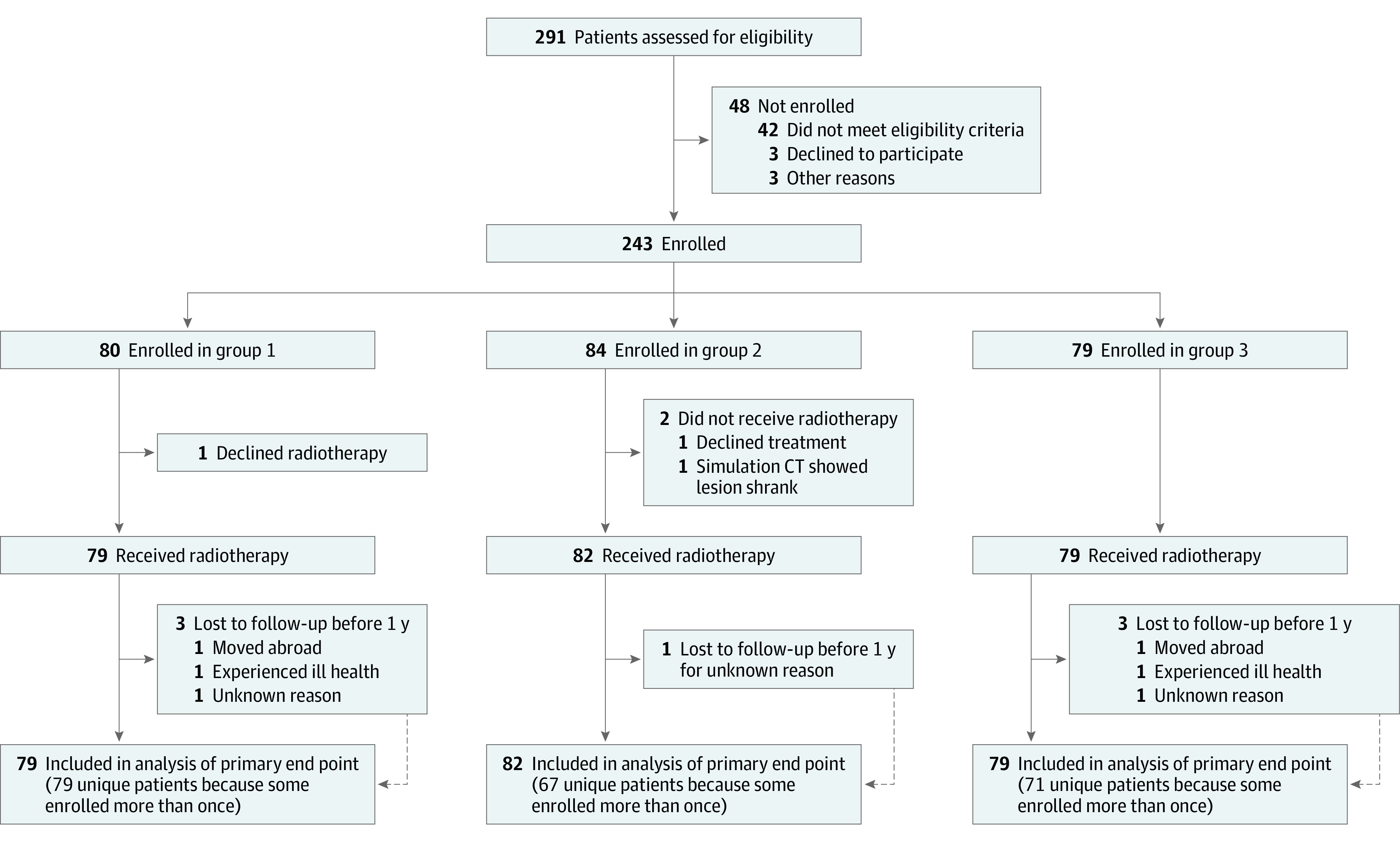
CT indicates computed tomography.
Procedures
Staging was performed with chest computed tomography (CT) and optionally positron emission tomography with CT (PET/CT). Patients with potentially operable tumors were recommended to see a thoracic surgeon before enrollment for discussion of resection as an alternative to SABR. Invasive mediastinal nodal staging was not required.
Up to 4 tumors were treated with once-daily SABR. Five dose-and-fractionation schedules were used, depending on tumor volume and location (Figure 2). Central tumors were within 2 cm of the proximal bronchial tree or had planning target volume (PTV) overlapping the great vessels, esophagus, trachea, pericardium, or brachial plexus (similar to the NRG Oncology/RTOG 0813 Trial10). Larger tumors received a higher dose, and central tumors generally received a lower dose per fraction. The dose ranged from 25 Gy in 1 fraction to 60 Gy in 8 fractions. All regimens had BED10 greater than 100 except for tumors with a volume of 10 cm3 or less with BED10 of 80 to 87.5 Gy. Colorectal cancer metastases received at least 50 Gy in 4 fractions due to previously documented radioresistance.21,22 Heterogeneity corrections were used, and 95% of the PTV received the prescription dose. The maximum dose was 120% or more of the prescription dose. Centralized plan review was performed.
Figure 2. Tumor Location and Volume Categories and Example Tumors.
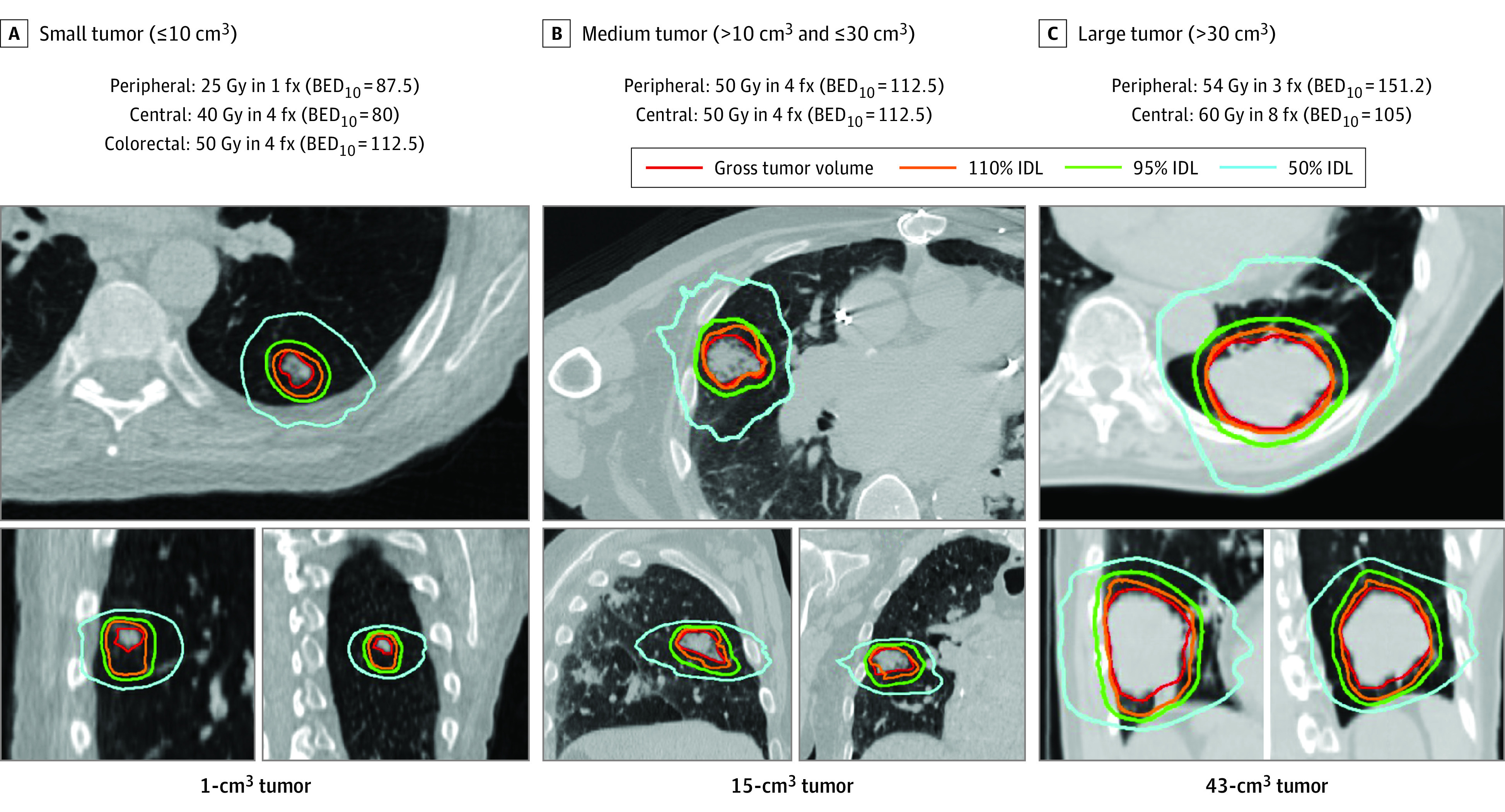
BED10 indicates biologically effective dose with α/β = 10; fx, fractions; and IDL, isodose line.
Simulation included 4-dimensional CT and expiratory breath-hold CT. Patients were treated in free breathing (when tumor motion was ≤5 mm), amplitude-based expiratory gating, or deep inspiration breath hold. For breath-hold CT, patients matched their breathing to a visual target using a biofeedback system.23,24 No margin was added for microscopic extension, and the final PTV was a 5-mm uniform expansion from the internal target volume. Most patients were treated on a linear accelerator with cone beam CT scanner (Varian Truebeam STx), without fiducial markers.
Patients were seen for physical examination, toxic effects assessment, and imaging (usually CT, sometimes PET/CT) every 3 months for 1 year, every 6 months for 2 more years, and then annually. This standardized follow-up protocol was used for all 3 groups. Patients lost to follow-up were contacted by phone to update data.
Outcomes
The primary end point was per-tumor 1-year freedom from local recurrence (recurrence in same lobe, excluding new primary cancers), censored at the time of distant recurrence, death, or loss to follow-up. We also performed an analysis in which distant recurrence and death were treated as competing risks because distant recurrence would usually be followed by systemic therapy and changes to imaging schedule, which could alter local recurrence risk. To isolate the influence of the radiation dose, we also recorded treated-tumor recurrence (TTR; recurrence with epicenter within 1 cm of the PTV). These definitions correspond to those used in NRG Oncology studies10,15,25 of SABR for NSCLC.
Secondary outcomes included overall survival (OS), regional and distant recurrence, recurrence-free survival, and toxic effects. Regional recurrence was nodal recurrence in the hila, mediastinum, or supraclavicular fossae. Distant recurrence was any recurrence not meeting the definition of local or regional recurrence. Recurrence was confirmed with biopsy when possible. Local vs distant recurrence was distinguished as follows: A new nodule(s) only in the treated lobe, consistent with progression of the same tumor, was scored as local recurrence. A tumor in the treated lobe outside the radiation field along with multiple other lesions all consistent with metastatic disease was scored as distant recurrence only. A new primary tumor (eg, with different histological characteristics or molecular profile) was not scored as recurrence. Equivocal cases were reviewed by a panel of 3 thoracic radiation oncologists (M.F.G., B.W.L., and M.D.).
Toxic effects were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (grade 2 = moderate, 3 = severe, 4 = life threatening, and 5 = death). No distinction was made between acute and late toxic effects, since many lung SABR toxic effects can occur in either period.
Statistical Analysis
All analyses were performed in the as-treated population: patients receiving at least 1 radiotherapy fraction. The primary end point was assessed using Kaplan-Meier estimates separately in the 3 patient groups, as they were expected to have varying risks of local and distant recurrence. We wanted to reject a 1-year freedom from local recurrence less than 80% for each group, so if the lower 90% CI of the observed value was 80% or higher, this would be considered a positive result. Assuming a true freedom from local recurrence rate of 90% and 1 tumor treated per course, 82 treatment courses per group would be required to have an 80% probability of the CI lower bound being 80% or higher. The sample size calculation used a simplified scenario of finding a binomial CI for the proportion of patients meeting the end point (as opposed to a Kaplan-Meier estimate).
Enrollment was closed due to slowing accrual after the planned 82 patients were treated in group 2 but 79 had been treated in groups 1 and 3. As patients could be re-enrolled and have multiple treatment courses and more than 1 tumor could be treated in each course, there was clustering on the treatment course and patient levels. However, since more than 90% of patients were enrolled once and most courses included treatment of only 1 tumor, clustering effects were minimal and nonclustered analyses were used.
Outcomes were calculated from the first treatment day. For competing risks analysis, local recurrence and TTR, death and distant recurrence were competing events. For regional and distant recurrence, only death was a competing event. Groups were compared using the Gray test (2-sided).
Toxic effects were analyzed on a per-patient basis, since if a patient was enrolled multiple times, it could be difficult to attribute toxic effects to each treatment course. For OS and regional and distant control, time from the first treatment was used because it could be difficult to attribute a regional or distant recurrence to a specific treatment course. The study database was locked in August 2021. The threshold for statistical significance was 2-tailed P = .05. Analysis was performed in SAS version 9.4 (SAS Institute) and R, version 4.2.2 (R Foundation for Statistical Computing).
Results
Patient Characteristics
A total of 243 patients (median [IQR] age, 72 [65-80] years; 144 [59%] male) were enrolled and 240 received protocol treatment (Figure 1). Among these 240 treatment courses, 79 were in group 1, 82 in group 2, and 79 in group 3. As some of these patients were re-enrolled for treatment of a new tumor, 217 unique patients (median [IQR] age, 72 [64-80] years; 129 [59%] male; 150 [69%] current or former smokers) were treated: 79 in group 1, 67 in group 2, and 71 in group 3. In total, 198 patients (91%) were treated once, 15 (7%) were treated twice, and 4 (2%) were treated 3 times. Three patients were enrolled at Hokkaido University and the remaining 214 at Stanford. Table 1 shows patient characteristics. In 214 of 240 treatment courses (89%), the cancer was biopsy proven. Pretreatment PET/CT was performed in 212 of 240 treatment courses (88%). In the 240 treatment courses, 285 tumors were treated (range, 1-3 tumors per course). Of these, 211 tumors (74%) were peripheral and 74 (26%) were central; of the central tumors, 9 (3%) were ultracentral (tumor contacting proximal bronchial tree or trachea).26
Table 1. Patient Characteristics.
| Characteristic | Total study cohort, No. (%) (N = 217) | Group 1: first primary NSCLC, No. (%) (n = 79) | Group 2: new primary or multiple synchronous NSCLC, No. (%) (n = 67) | Group 3: lung metastases, No. (%) (n = 71) |
|---|---|---|---|---|
| Age, median (IQR), y | 72 (64-80) | 74 (68-81) | 73 (66-81) | 65 (56-75) |
| Sex | ||||
| Male | 129 (59) | 45 (57) | 44 (66) | 40 (56) |
| Female | 88 (41) | 34 (43) | 23 (34) | 31 (44) |
| ECOG performance status | ||||
| 0 | 40 (20) | 5 (7) | 19 (31) | 16 (24) |
| 1 | 112 (56) | 37 (52) | 32 (52) | 43 (63) |
| 2 | 36 (18) | 20 (28) | 10 (16) | 6 (9) |
| 3 | 12 (6) | 9 (13) | 0 (0) | 3 (4) |
| Unknown | 17 (8) | 8 (10) | 6 (9) | 3 (4) |
| Smoking history | ||||
| Current | 26 (12) | 16 (20) | 6 (9) | 4 (6) |
| Former | 124 (57) | 53 (67) | 46 (69) | 25 (35) |
| Never | 58 (27) | 8 (10) | 14 (21) | 36 (51) |
| Unknown | 9 (4) | 2 (3) | 1 (1) | 6 (8) |
| Treatment courses, No. | 240 | 79 | 82 | 79 |
| Biopsy-proven | 214 (89) | 76 (96) | 68 (83) | 70 (89) |
| Medically operablea | N/A | 17 (22) | N/A | N/A |
| Histological characteristics | ||||
| Squamous cell carcinoma | 41 (17) | 17 (22) | 11 (13) | 13 (16) |
| Adenocarcinoma | 145 (60) | 50 (63) | 55 (67) | 40 (51) |
| Large cell carcinoma | 3 (1) | 2 (3) | 0 (0) | 1 (1) |
| Other | 41 (17) | 7 (9) | 10 (12) | 24 (30) |
| Unknown | 10 (4) | 3 (4) | 6 (7) | 1 (1) |
| Primary site, No. | N/A | All lung | All lung | Lung, 31; colorectal, 17; head and neck, 5; breast, 3; skin, 2; other, 21 |
| Tumors, No. | 285 | 79 | 103 | 103 |
| Location, size (dose)b | ||||
| Peripheral, 0-10 cm3 (25 Gy in 1 fx) | 159 (56) | 35 (44) | 72 (70) | 52 (50) |
| Peripheral, 0-10 cm3 colorectal (50 Gy in 4 fx) | 10 (4) | 0 (0) | 0 (0) | 10 (10) |
| Peripheral, 10.1-30 cm3 (50 Gy in 4 fx) | 31 (11) | 16 (20) | 7 (7) | 8 (8) |
| Peripheral, >30 cm3 (54 Gy in 3 fx) | 11 (4) | 7 (9) | 2 (2) | 2 (2) |
| Central, 0-10 cm3 (40 Gy in 4 fx) | 34 (12) | 8 (10) | 14 (14) | 12 (12) |
| Central, 0-10 cm3 colorectal (50 Gy in 4 fx) | 7 (2) | 0 | 0 | 7 (7) |
| Central, 10.1-30 cm3 (50 Gy in 4 fx) | 25 (9) | 8 (10) | 6 (6) | 11 (11) |
| Central, >30 cm3 (60 Gy in 8 fx) | 8 (3) | 5 (6) | 2 (2) | 1 (1) |
| AJCC 7th edition stage | ||||
| T1aN0M0 | N/A | 29 (37) | 66 (64) | N/A |
| T1bN0M0 | N/A | 26 (33) | 24 (23) | N/A |
| T2aN0M0 | N/A | 22 (28) | 9 (9) | N/A |
| T2bN0M0 | N/A | 2 (3) | 4 (4) | N/A |
Abbreviations: AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; fx, fractions; NSCLC, non–small cell lung cancer.
Operable status was identified by whether a patient was fit to receive anatomical resection with or without lymph node dissection, using pulmonary function test results and functional status.
Three patients received a dose different from the assigned dose for their location and size combination; see Results section.
The most common dose was 25 Gy in 1 fraction (158 tumors [55%]). Treatment plan dosimetric data are given in eTable 1 in Supplement 2. All treated patients completed radiotherapy. Three patients had protocol violations due to the dose: 1 patient received a central regimen to a peripheral tumor with volume greater than 30 cm3 (60 Gy in 8 fractions), 1 central tumor with a volume of 10 cm3 or less received 50 Gy in 4 fractions (rather than 40 Gy in 4 fractions), and 1 peripheral tumor with volume of 10 cm3 or less received 50 Gy in 4 fractions (rather than 25 Gy in 1 fraction) because it was treated in the same plan with a nearby larger tumor.
Disease Outcomes
The median (range) follow-up was 33 (2-109) months. The median OS was 59 (95% CI, 49-82) months. For groups 1, 2, and 3, this was 57 (95% CI, 43 to infinity), 82 (95% CI, 46 to infinity), and 57 (95% CI, 40 to infinity) months, respectively (Figure 3A). Sites of first recurrence are given in eTable 2 in Supplement 2. Of 285 treated tumors, 26 (9%) had a local recurrence. Details of equivocal local recurrences reviewed by a physician panel are given in eTable 3 in Supplement 2. Freedom from local recurrence at 1 year for group 1 was 97% (90% CI, 91%-99%); for group 2 was 94% (90% CI, 87%-97%), and for group 3 was 96% (90% CI, 89%-98%). Based on the lower 90% CIs, this met the primary end point and we concluded that the 1-year local recurrence risk was less than 20% for each group. Freedom from local recurrence at 2 years ranged from 90% in group 1 to 95% in group 3; at 5 years, it ranged from 83% in group 1 to 93% in group 2 (eTable 4 in Supplement 2). Results when including only the first enrollment per patient were similar (eTable 5 in Supplement 2). Figure 3B shows cumulative incidence of local recurrence.
Figure 3. Disease Outcomes.
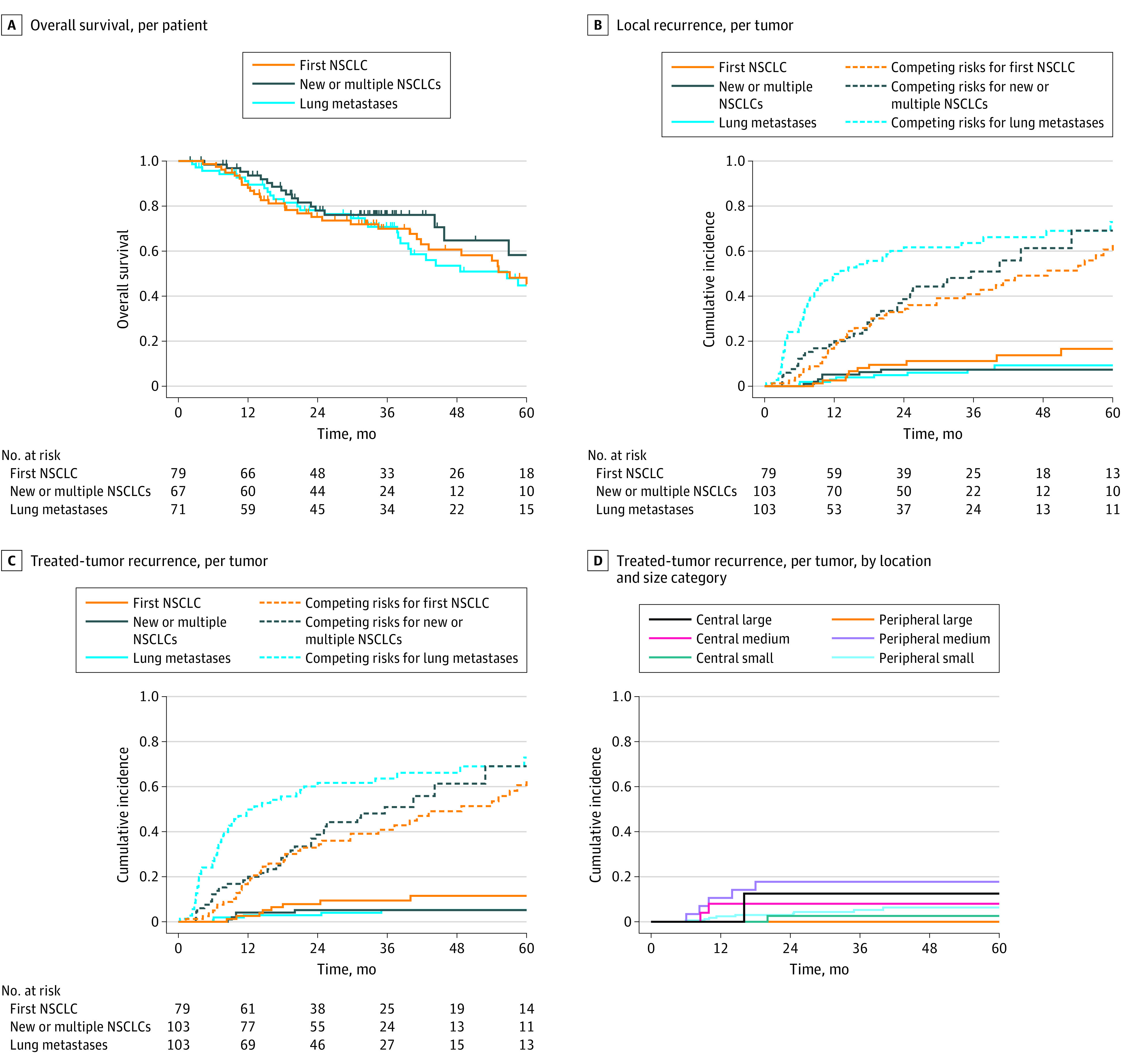
A, Tick marks indicate censored patients. B and C, Competing risks were death and distant recurrence. D, Due to the large number of groups, competing risk curves are not shown in this panel. Numbers of patients with time to recurrence vs competing events are provided in eTable 7 in Supplement 2. NSCLC indicates non–small cell lung cancer.
Of the 26 local recurrences, 17 (65%) were in field, 3 (12%) were marginal, and 6 (23%) were elsewhere in the lobe. The cumulative incidence of TTR for the whole cohort at 1 year was 3% (95% CI, 2%-6%), at 2 years was 5% (95% CI, 3%-8%), and at 5 years 7% (95% CI, 4%-11%). Figure 3C, eFigure 1 in Supplement 2, and eTable 6 in Supplement 2 show TTR by group.
We analyzed TTR by 6 tumor categories indicating location (central or peripheral) and size (small, medium, or large) (Figure 3D; eTable 7 in Supplement 2). In particular, small tumors (central or peripheral) treated with less than 100 Gy BED10 had a TTR rate less than 10%. The difference between the 6 groups was not statistically significant. For patients in groups 1 and 2 (ie, primary NSCLC), T1 and T2 tumors had similar TTR (eFigure 2 in Supplement 2; eTable 9 gives the TTR data for T1 vs T2 tumors), but there was a difference between histological characteristics with significantly more TTR in squamous cell carcinomas (SCC; eFigure 3 in Supplement 2). For group 3 patients, regarding primary tumor site, there were no local recurrences in 17 patients with colorectal tumors. Results for other primary tumor sites are not shown due to small patient numbers.
Other outcomes including site of first recurrence and rates of regional or distant recurrence are shown in eFigures 4, 5, 6, and 7 and eTables 8, 9, and 10 in Supplement 2.
Toxic Effects
Toxic effects were analyzed at the patient level (Table 2). The rate of CTCAE grade 2 or higher pneumonitis was 16 of 217 (7%) and grade 3 or higher pneumonitis was 3 of 217 (1%). The rate of grade 2 or higher chest pain (generally due to chest wall pain or rib fracture) was 13 of 217 (6%). One patient had a grade 5 adverse event, developing pulmonary hemorrhage possibly related to radiotherapy, without known local recurrence, 17 months after treatment of an ultracentral NSCLC greater than 30 cm3 (eFigure 8 in Supplement 2). Of the other 8 patients with ultracentral tumors, 3 had grade 2 or higher toxic effects, including grade 3 hemoptysis, grade 3 pneumonitis, and grade 2 pneumonitis. The proportion of patients with grade 3 to 5 toxic effects was 5% (including a single grade 5 event, 1%). The risk of grade 3 to grade 5 toxic effects was higher in patients with ultracentral tumors compared with that in others: 3 of 9 patients (33.3%) with ultracentral tumors had grade 3 to grade 5 toxic effects and 7 of 208 (3%) patients with non-ultracentral tumors had grade 3 to grade 4 toxic effects (P = .005 by Fisher exact test).
Table 2. Toxic Effects for the Total Study Cohort.
| Toxic effect | CTCAE grade, No. (%) (N = 217)a | |||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| Worst overall | 28 (13) | 7 (3) | 2 (1) | 1 (1) |
| Pneumonitis | 13 (6) | 2 (1) | 1 (1) | 0 |
| Noncardiac chest painb | 11 (5) | 2 (1) | 0 | 0 |
| Pleural effusion | 4 (2) | 1 (1) | 0 | 0 |
| Esophagitis | 3 (1) | 0 | 0 | 0 |
| Otherc | 5 (2) | 3 (1)d | 1 (1) | 1 (1) |
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
CTCAE version 4.0: grade 2 = moderate, 3 = severe, 4 = life threatening, and 5 = death.
Chest pain was generally due to chest wall pain or rib fracture.
The grade 5 toxic effect was a pulmonary hemorrhage possibly related to radiotherapy, and the grade 4 toxic effect was pneumonia probably related to radiotherapy.
One patient had grade 3 chest wall necrosis related to cryoablation for a lung tumor not treated on this study. The area of necrosis extended into the study radiation field but was graded as unrelated to study treatment since it was centered at the cryoablation site, outside the radiation field, and happened shortly after cryoablation.
Discussion
In this nonrandomized controlled trial, individualized SABR was associated with excellent local control and low rates of toxic effects. The 5-year cumulative incidence of TTR (recurrence within 1 cm of target) was low, at 7%. The proportion of patients with CTCAE grade 3 to grade 5 toxic effects was also low, at 5%, with a single possibly treatment-related grade 5 event. To our knowledge, this is the largest reported prospective trial using SABR for lung tumors, and it supports the use of SABR for a variety of scenarios and tumor sizes and for both central and peripheral tumors.
A main feature was the use of a lower dose for smaller tumors and higher dose for larger tumors or colorectal metastases. Most lung SABR trials2,8,15 enrolled patients with mainly small and/or T1 tumors. Larger tumors could require higher radiation dose due to factors such as hypoxia.16 Recent studies support this. A meta-analysis18 found that a higher SABR dose is required to control T2 vs T1 NSCLC. A retrospective analysis17 found that patients with local recurrence had higher tumor diameter. Some prior trials used higher doses for larger tumors. In an Indiana University study,9 60 Gy in 3 fractions was used for T1 tumors, and 66 Gy in 3 fractions for T2 tumors. A trial27 from Nagoya City University used doses of 44 or 48 or 52 Gy in 4 fractions depending on tumor diameter. Trials28,29 in other disease sites reversed this strategy, with larger tumors receiving lower dose to reduce the toxic effects. Given the difficulty in modeling outcomes for high dose-per-fraction radiotherapy, it is important to test volume-adapted dosing in trials. Our local control outcomes were similar to those from other trials of SABR for primary NSCLC8,27 and lung metastases.4,14
The most frequently used dose in our study was 25 Gy in 1 fraction (158 of 285 tumors), which was used for small peripheral noncolorectal tumors. This dose has a BED10 of 87.5 Gy, which is an apparent deescalation compared with most trials8,10,14,15 of lung SABR, which used prescription doses greater than 100 Gy BED10 for all tumor sizes. We observed a rate of TTR of less than 10% for small tumors treated with less than 100 Gy BED10, suggesting that more than 100 Gy BED10 may not be needed for small tumors when prescribing to the PTV (rather than isocenter, as in the dose-response analysis by Onishi et al11) and an inhomogeneous dose distribution with a high maximum dose and careful motion management are used. With large fraction sizes, such as 25 Gy, there may be multiple mechanisms of tumor control, such as vascular damage and immune activation, that are difficult to model.30 Two small randomized trials15,31 testing single vs multifraction SABR for primary NSCLC also showed high rates of local control with the single-fraction regimen (although doses were higher, at 30-34 Gy). A randomized clinical trial4 of SABR used to treat lung metastases found a numerically lower local control rate with 28 Gy in 1 fraction compared with 48 Gy in 4 fractions, although the difference was not statistically different. An exploratory analysis in that study4 suggested that many of the local failures in the single-fraction group were in patients with colorectal disease, which is consistent with prior observations21,22 that colorectal metastases are radioresistant. Our use of high-dose multifraction regimens for all colorectal metastases may have helped maintain high local control rates. We observed a higher rate of local recurrence in SCC lung cancers, consistent with a prior study.32 We speculate that this may be associated with the higher incidence of activating mutations in NFE2L2 in SCC compared with adenocarcinomas, which have been shown to be associated with radioresistance.33,34,35,36 Mutational profiling may be useful in the future for personalizing the prescription dose in lung SABR.
We observed a low rate of toxic effects. Patients with ultracentral tumors had more severe toxic effects, including 1 possible grade 5 toxic effect (pulmonary hemorrhage). Life-threatening toxic effects are a known possibility with SABR to central tumors.37 In the NRG Oncology/RTOG 0813 Trial,10 5 of 92 patients (5%) had grade 5 toxic effects, and in the HILUS trial38 of SABR for ultracentral tumors, 10 of 65 patients (15%) had treatment-related death. Our observation of 1 grade 5 event out of 74 central tumors may reflect a lower treatment-associated mortality than prior studies of central tumors owing to a treatment technique using lower doses for small tumors, sharp dose gradients, and respiratory motion management, although this would need to be validated in a larger study.
Limitations
This study has several limitations. The primary outcome was 1-year local recurrence, and although many recurrences are expected to occur within 1 year, local recurrences in SABR-treated NSCLC can occur later than this (nearly all by 2 years).39,40 This time was chosen since 1 of the groups of the trial included patients with lung metastases, for whom 1 year was considered the best compromise due to their overall poor prognosis. However, the median follow-up was 33 months and secondary analyses included local recurrence at 2 and 5 years, which had similarly low rates. Additionally, it can be difficult to differentiate local recurrence from fibrosis after SABR.41 We mitigated this by recommending biopsy of suspected local recurrences and having a panel review all equivocal local recurrences. The patients with lung metastases were mainly treated for oligometastatic disease, but the total number of metastases was not recorded, so we do not have precise data on treatment indications for this group. Finally, this single-arm study lacked a uniform dosing control group. It is therefore not possible to directly compare the outcomes of our individualized strategy with a uniform dosing approach. Nevertheless, the strategy used achieved excellent outcomes in line with prior uniform dosing studies and thus appears to be a promising approach for maximizing local control, safety, and patient convenience.
Conclusions
The results of this nonrandomized controlled trial suggest that individualized SABR used to treat lung cancers may allow minimization of treatment dose and may be associated with excellent local control and a low rate of toxic effects. Individualized dosing should be considered for use in future trials.
Trial Protocol
eTable 1. Treatment Plan Data, Grouped by Assigned Dose.
eTable 2. Site of First Recurrence, Patient Level
eTable 3. Details of Cases of Equivocal Local Recurrence Reviewed by Panel
eTable 4. Local Recurrence by Patient Group (Kaplan-Meier Method)
eTable 5. Local Recurrence by Patient Group, First Enrollment Per Patient Only (Kaplan-Meier Method)
eTable 6. Treated Tumor Recurrence By Patient Group (Cumulative Incidence Method)
eTable 7. Treated Tumor Recurrence vs Competing Events by Tumor Size/Location
eTable 8. Distant Recurrence by Patient Group
eTable 9. Treated Tumor Recurrence by T Stage (Primary NSCLC Only, so Groups 1-2 Only)
eTable 10. Treated Tumor Recurrence by Histology (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 1. Treated Tumor Recurrence, per Tumor, Kaplan-Meier Plot
eFigure 2. Treated Tumor Recurrence by T stage, per Tumor (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 3. Treated Tumor Recurrence By Histology, per Tumor (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 4. Treated Tumor Recurrence by Primary Tumor Site, per Tumor (All Patients)
eFigure 5. Cumulative Incidence of Regional Recurrence, per Patient (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 6. Cumulative Incidence of Distant Recurrence, per Patient
eFigure 7. Recurrence-Free Survival, per Patient
eFigure 8. Patient With Possible Grade 5 Toxicity
Data Sharing Statement
References
- 1.Ball D, Mai GT, Vinod S, et al. ; TROG 09.02 CHISEL investigators . Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non–small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494-503. doi: 10.1016/S1470-2045(18)30896-9 [DOI] [PubMed] [Google Scholar]
- 2.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non–small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637. doi: 10.1016/S1470-2045(15)70168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyman J, Hallqvist A, Lund JÅ, et al. SPACE—a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1-8. doi: 10.1016/j.radonc.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 4.Siva S, Bressel M, Mai T, et al. Single-fraction vs multifraction stereotactic ablative body radiotherapy for pulmonary oligometastases (SAFRON II): the Trans Tasman Radiation Oncology Group 13.01 phase 2 randomized clinical trial. JAMA Oncol. 2021;7(10):1476-1485. doi: 10.1001/jamaoncol.2021.2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non–small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7(5):295-301. doi: 10.1016/j.prro.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 6.Guckenberger M, Andratschke N, Dieckmann K, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non–small cell lung cancer. Radiother Oncol. 2017;124(1):11-17. doi: 10.1016/j.radonc.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology non–small cell lung cancer version 1.2022.. Accessed February 22, 2022 https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 8.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076. doi: 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833-4839. doi: 10.1200/JCO.2006.07.5937 [DOI] [PubMed] [Google Scholar]
- 10.Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non–small-cell lung cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37(15):1316-1325. doi: 10.1200/JCO.18.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non–small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7)(suppl 3):S94-S100. doi: 10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 12.Guckenberger M, Wulf J, Mueller G, et al. Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys. 2009;74(1):47-54. doi: 10.1016/j.ijrobp.2008.06.1939 [DOI] [PubMed] [Google Scholar]
- 13.Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol. 2012;7(9):1394-1399. doi: 10.1097/JTO.0b013e3182614bf3 [DOI] [PubMed] [Google Scholar]
- 14.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579-1584. doi: 10.1200/JCO.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 15.Videtic GM, Paulus R, Singh AK, et al. Long-term follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): a randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103(5):1077-1084. doi: 10.1016/j.ijrobp.2018.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JM, Diehn M, Loo BW Jr. Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys. 2010;78(2):323-327. doi: 10.1016/j.ijrobp.2010.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klement RJ, Sonke J-J, Allgäuer M, et al. Correlating dose variables with local tumor control in stereotactic body radiation therapy for early-stage non–small cell lung cancer: a modeling study on 1500 individual treatments. Int J Radiat Oncol Biol Phys. 2020;107(3):579-586. doi: 10.1016/j.ijrobp.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Loo BW Jr, Biswas T, et al. Local control after stereotactic body radiation therapy for stage I non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2021;110(1):160-171. doi: 10.1016/j.ijrobp.2019.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Q-T, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1(8):802-809. doi: 10.1097/01243894-200610000-00008 [DOI] [PubMed] [Google Scholar]
- 20.Trakul N, Chang CN, Harris J, et al. Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. Int J Radiat Oncol Biol Phys. 2012;84(1):231-237. doi: 10.1016/j.ijrobp.2011.10.071 [DOI] [PubMed] [Google Scholar]
- 21.Helou J, Thibault I, Poon I, et al. Stereotactic ablative radiation therapy for pulmonary metastases: histology, dose, and indication matter. Int J Radiat Oncol Biol Phys. 2017;98(2):419-427. doi: 10.1016/j.ijrobp.2017.02.093 [DOI] [PubMed] [Google Scholar]
- 22.Binkley MS, Trakul N, Jacobs LR, et al. Colorectal histology is associated with an increased risk of local failure in lung metastases treated with stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):1044-1052. doi: 10.1016/j.ijrobp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Loo BW Jr, Kavanagh BD, Meyer JL. Motion management and image guidance for thoracic tumor radiotherapy: clinical treatment programs Front Radiat Ther Oncol. 2011;43:271-291. doi: 10.1159/000322451 [DOI] [PubMed] [Google Scholar]
- 24.Yu AS, von Eyben R, Yamamoto T, et al. Anatomic optimization of lung tumor stereotactic ablative radiation therapy. Pract Radiat Oncol. 2015;5(6):e607-e613. doi: 10.1016/j.prro.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 25.Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non–small cell lung cancer. JAMA Oncol. 2018;4(9):1287-1288. doi: 10.1001/jamaoncol.2018.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. 2015;89(1):50-56. doi: 10.1016/j.lungcan.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 27.Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non–small-cell lung cancer: five-year mature results. J Thorac Oncol. 2015;10(6):960-964. doi: 10.1097/JTO.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 28.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291-298. doi: 10.1016/S0360-3016(99)00507-6 [DOI] [PubMed] [Google Scholar]
- 29.Dawson LA, Winter K, Knox J, et al. NRG/RTOG 1112: randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC). Int J Radiat Oncol Biol Phys . 2022;114(5):1057. doi: 10.1016/j.ijrobp.2022.09.002 [DOI] [Google Scholar]
- 30.Song CW, Glatstein E, Marks LB, et al. Biological principles of stereotactic body radiation therapy (SBRT) and stereotactic radiation surgery (SRS): indirect cell death. Int J Radiat Oncol Biol Phys. 2021;110(1):21-34. doi: 10.1016/j.ijrobp.2019.02.047 [DOI] [PubMed] [Google Scholar]
- 31.Singh AK, Gomez-Suescun JA, Stephans KL, et al. One versus three fractions of stereotactic body radiation therapy for peripheral stage I to II non–small cell lung cancer: a randomized, multi-institution, phase 2 trial. Int J Radiat Oncol Biol Phys. 2019;105(4):752-759. doi: 10.1016/j.ijrobp.2019.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woody NM, Stephans KL, Andrews M, et al. A Histologic basis for the efficacy of SBRT to the lung. J Thorac Oncol. 2017;12(3):510-519. doi: 10.1016/j.jtho.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellyer JA, Padda SK, Diehn M, Wakelee HA. Clinical implications of KEAP1-NFE2L2 mutations in NSCLC. J Thorac Oncol. 2021;16(3):395-403. doi: 10.1016/j.jtho.2020.11.015 [DOI] [PubMed] [Google Scholar]
- 34.Jeong Y, Hoang NT, Lovejoy A, et al. Role of KEAP1/NRF2 and tp53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 2017;7(1):86-101. doi: 10.1158/2159-8290.CD-16-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell JD, Alexandrov A, Kim J, et al. ; Cancer Genome Atlas Research Network . Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48(6):607-616. doi: 10.1038/ng.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binkley MS, Jeon Y-J, Nesselbush M, et al. KEAP1/NFE2L2 Mutations predict lung cancer radiation resistance that can be targeted by glutaminase inhibition. Cancer Discov. 2020;10(12):1826-1841. doi: 10.1158/2159-8290.CD-20-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Laba JM, Zayed S, Boldt RG, Palma DA, Louie AV. Safety and effectiveness of stereotactic ablative radiotherapy for ultra-central lung lesions: a systematic review. J Thorac Oncol. 2019;14(8):1332-1342. doi: 10.1016/j.jtho.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 38.Lindberg K, Grozman V, Karlsson K, et al. The HILUS-trial: a prospective Nordic multicenter phase 2 study of ultracentral lung tumors treated with stereotactic body radiotherapy. J Thorac Oncol. 2021;16(7):1200-1210. doi: 10.1016/j.jtho.2021.03.019 [DOI] [PubMed] [Google Scholar]
- 39.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non–small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13(8):802-809. doi: 10.1016/S1470-2045(12)70242-5 [DOI] [PubMed] [Google Scholar]
- 40.Spratt DE, Wu AJ, Adeseye V, et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non–small-cell lung cancer: implications for surveillance. Clin Lung Cancer. 2016;17(3):177-183.e2. doi: 10.1016/j.cllc.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee K, Le T, Hau E, et al. A systematic review into the radiologic features predicting local recurrence after stereotactic ablative body radiotherapy (SABR) in patients with non–small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2022;113(1):40-59. doi: 10.1016/j.ijrobp.2021.11.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Treatment Plan Data, Grouped by Assigned Dose.
eTable 2. Site of First Recurrence, Patient Level
eTable 3. Details of Cases of Equivocal Local Recurrence Reviewed by Panel
eTable 4. Local Recurrence by Patient Group (Kaplan-Meier Method)
eTable 5. Local Recurrence by Patient Group, First Enrollment Per Patient Only (Kaplan-Meier Method)
eTable 6. Treated Tumor Recurrence By Patient Group (Cumulative Incidence Method)
eTable 7. Treated Tumor Recurrence vs Competing Events by Tumor Size/Location
eTable 8. Distant Recurrence by Patient Group
eTable 9. Treated Tumor Recurrence by T Stage (Primary NSCLC Only, so Groups 1-2 Only)
eTable 10. Treated Tumor Recurrence by Histology (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 1. Treated Tumor Recurrence, per Tumor, Kaplan-Meier Plot
eFigure 2. Treated Tumor Recurrence by T stage, per Tumor (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 3. Treated Tumor Recurrence By Histology, per Tumor (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 4. Treated Tumor Recurrence by Primary Tumor Site, per Tumor (All Patients)
eFigure 5. Cumulative Incidence of Regional Recurrence, per Patient (Primary NSCLC Only, so Groups 1-2 Only)
eFigure 6. Cumulative Incidence of Distant Recurrence, per Patient
eFigure 7. Recurrence-Free Survival, per Patient
eFigure 8. Patient With Possible Grade 5 Toxicity
Data Sharing Statement


