Abstract
Mannitol salt agar was evaluated for detection of oxacillin resistance in 136 Staphylococcus aureus isolates. All mecA-positive isolates (n = 54) were correctly categorized as oxacillin resistant by the disk diffusion test (1-μg disk; zone diameter, <16 mm); the specificity was 97.6%. Agar screening (2 μg of oxacillin per ml) revealed a sensitivity of 98.1% and a specificity of 95.1%.
In the United States and Europe, nosocomial infections caused by methicillin-resistant Staphylococcus aureus (MRSA) remain a prominent problem (17, 33) resulting in the performance of many surveillance cultures for detection and monitoring of MRSA. Several body sites of MRSA index patients, contact patients, and nursing and medical staff members may require investigation, depending on the surveillance scheme in a hospital (9). Many methods to detect oxacillin resistance in S. aureus have been evaluated and are widely used in bacteriological laboratories, including the following: agar dilution on Mueller-Hinton agar with 6 μg of oxacillin per ml (26), disk diffusion (4 μg of oxacillin) on Mueller-Hinton agar (15), disk diffusion (1 μg of oxacillin) on Mueller-Hinton or Iso-Sensitest agar (10), MIC determination by broth dilution (18), oxacillin broth screening test (10), automatic systems such as the VITEK (11), or even flow cytometry (24). Mannitol salt agar (MSA) has been used since 1945 as a selective medium for the isolation of pathogenic staphylococci (3, 4) and was found to be valuable for use with specific specimens such as sputum from patients with cystic fibrosis (22). MSA was first investigated as a medium for susceptibility testing in 1985 (12). Its value as a screening medium for MRSA has been investigated in several studies (13, 16). It has been further evaluated as a primary isolation medium for recovery of MRSA when containing 6 μg of oxacillin per ml (28). Recently, it has been suggested that MSA might be a promising medium for use in the disk diffusion method with a 1-μg oxacillin disk or for agar screening, especially for surveillance cultures (10). Therefore, we evaluated MSA for use in disk diffusion and agar screen tests.
MATERIALS AND METHODS
Bacterial strains, genotyping, and identification.
One hundred fifty-one clinical isolates previously identified as S. aureus by agglutination tests were initially included in the study (71 MRSA isolates; 80 methicillin-susceptible S. aureus [MSSA] isolates). Five MRSA isolates originally came from Argentina, 8 were from Belgium, 24 were from Canada, 29 were from Germany, and 5 were from Switzerland; all 80 MSSA isolates were from Germany. All MRSA isolates were screened for clonal identity by pulsed-field gel electrophoresis (PFGE) (SmaI digest) prior to evaluation experiments (19). Patterns were compared by three investigators, and isolates were grouped in accordance with the criteria reported by Tenover et al. (25). Two isolates from Belgium and four isolates from Canada were excluded from further experiments because their PFGE patterns were found to be indistinguishable from those of other isolates. The remaining 65 MRSA isolates were found to be either distinct clones (n = 54) or epidemiologically related (n = 11). Identification of the species S. aureus was confirmed by detection of the coagulase gene by PCR (21) and by tube coagulation (rabbit plasma; bioMérieux, Nürtingen, Germany) by using the criteria of Sperber and Tatini (23). Eight of the remaining 145 isolates did not have a coagulase gene, and one of the coagulase-gene-positive isolates failed to show plasma coagulation; all nine of these isolates were excluded. This resulted in a total of 136 S. aureus isolates that were used for further experiments.
Disk diffusion test.
A sterile swab was dipped in an S. aureus suspension (McFarland standard 0.5) and plated onto MSA (CM 85; Oxoid, Basingstoke, England). Oxacillin disks (1 μg; Becton Dickinson, Heidelberg, Germany) were applied by using a sterile forceps. Agar plates were incubated at 36 ± 1°C for 24 h. The zone of inhibition was documented in millimeters (no inhibition zone was noted as 0 mm). An isolate was classified as resistant to oxacillin when the inhibition zone was less than 16 mm in diameter (10).
Agar screen test.
An aliquot of 10 μl of a 1:100 dilution of a bacterial suspension (McFarland standard 0.5) was placed on MSA with 2 μg of oxacillin per ml (18). All plates were incubated for 24 h at 36 ± 1°C and assessed for the presence of colonies. An isolate was regarded as resistant to oxacillin when growth of at least one typical S. aureus colony was detected at the inoculation site on the agar plate.
MIC of oxacillin (E-test).
The MICs (of oxacillin) were determined by using the E-test. A sterile swab was dipped into an inoculum suspension (McFarland standard 0.5). Excess fluid was removed by rotating and pressing the swab firmly against the inside wall of the test tube. The entire surface of a Mueller-Hinton agar plate (2% NaCl supplement) was swabbed three times by rotating the plate each time to ensure an even distribution of the inoculum (2). An E-test strip was placed aseptically onto the agar plate. After incubation at 36 ± 1°C for 18 to 24 h, the MIC was read at the point of intersection between the zone edge and the E-test strip.
PCR for detection of the mecA gene.
All S. aureus isolates were investigated for the presence of the mecA gene (PCR product, 533 bp) by using PCR with the mecA1 and mecA2 primers as described before (10).
Statistics.
Sensitivity and specificity rates were calculated for the disk diffusion and agar screen tests. To choose the best cutoff for the zone diameter of the disk diffusion test, sensitivity was plotted versus 1 − specificity in the form of a receiver operating characteristic (ROC) curve (1).
RESULTS
Of all 136 S. aureus isolates included in the study, 54 were found to be mecA positive and 82 were found to be mecA negative. The presence or absence of the mecA gene was regarded as the reference to detect oxacillin resistance (27).
All 54 mecA-positive isolates were correctly identified as oxacillin resistant by disk diffusion on MSA (sensitivity, 100%), and 80 of the 82 mecA-negative isolates were found to be oxacillin susceptible (specificity, 97.6%), resulting in a positive predictive value of 96.4% and a negative predictive value of 100% (Table 1). The agar screen test on MSA was found to be highly sensitive, too (98.1%), with a lower specificity (95.1%), resulting in a positive predictive value of 93.0% and a negative predictive value of 98.7% (Table 1).
TABLE 1.
Evaluation of the disk diffusion (1 μg of oxacillin) and agar screen (2 μg of oxacillin per ml) tests on MSA for detection of oxacillin resistance in S. aureus isolates (n = 136)
| Presence of the mecA gene | Total no. of isolates | No. of isolates
|
|||
|---|---|---|---|---|---|
| Disk diffusion on MSA with zone diam of:
|
Agar screening on MSA
|
||||
| <16 mm | ≥16 mm | Growth | No growth | ||
| mecA positive | 54 | 54 | 0 | 53 | 1 |
| mecA negative | 82 | 2 | 80 | 4 | 78 |
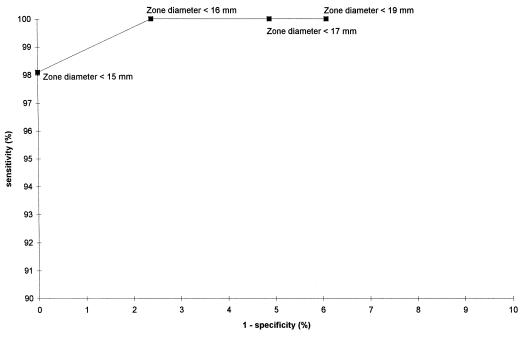
The inhibition zone diameters (in millimeters) relative to the number of isolates with the mecA gene present are shown in Table 2. A ROC curve was plotted (Fig. 1). A zone diameter of <15 mm results in a 100% specificity rate and a 98.1% sensitivity rate of detecting oxacillin resistance, whereas a zone diameter of <16 mm results in a 100% sensitivity rate and a 97.6% specificity rate.
TABLE 2.
Disk diffusion zone diameters (1-μg oxacillin disk) on MSA relative to the presence of the mecA gene in 136 S. aureus isolates
| Zone diam (mm) | No. of isolates
|
||
|---|---|---|---|
| mecA gene negative | mecA gene positive | Total | |
| 0 | 0 | 53 | 53 |
| 15 | 2 | 1 | 3 |
| 16 | 2 | 2 | |
| 18 | 1 | 1 | |
| 19 | 1 | 1 | |
| 20 | 4 | 4 | |
| 21 | 3 | 3 | |
| 22 | 9 | 9 | |
| 23 | 6 | 6 | |
| 24 | 13 | 13 | |
| 25 | 14 | 14 | |
| 26 | 11 | 11 | |
| 27 | 5 | 5 | |
| 28 | 4 | 4 | |
| 29 | 4 | 4 | |
| 30 | 1 | 1 | |
| 32 | 2 | 2 | |
| Total | 82 | 54 | 136 |
FIG. 1.
ROC curve based on specificity and sensitivity rates of the disk diffusion test on MSA (oxacillin, 1 μg) for S. aureus isolates positive for the mecA gene.
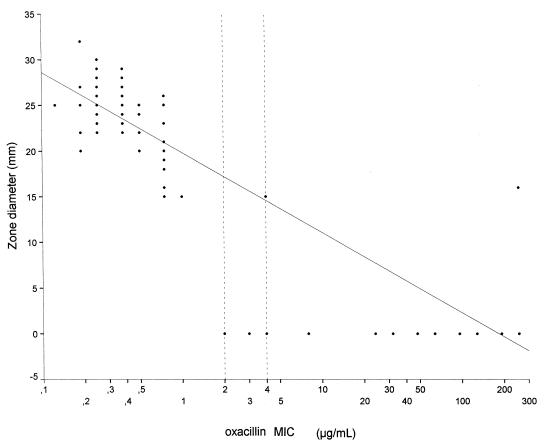
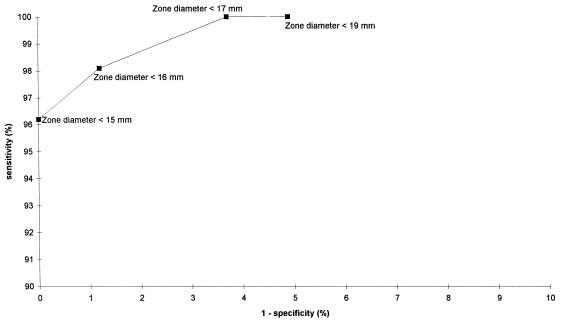
The zone diameters (in millimeters) relative to the MICs (of oxacillin) are demonstrated in Fig. 2 as a scattergram with a linear regression curve. The correlation coefficient was −0.802 (Pearson’s correlation coefficient, two-tailed). The breakpoint MICs (of oxacillin) for S. aureus are ≤2 μg/ml (susceptible) and ≥4 μg/ml (resistant) (18). For the resulting ROC curve, any isolate for which the MIC was ≤2 μg/ml was regarded as susceptible and the remaining isolates were regarded as resistant to oxacillin. Figure 3 shows that only with a zone diameter of <15 mm could oxacillin resistance be detected at a specificity rate of 100% and that zone diameters of ≤16 mm result in detection at a 100% sensitivity rate.
FIG. 2.
Scattergram with a linear regression curve resulting from plotting inhibition zone diameters for oxacillin resistance on MSA and the E-test oxacillin MICs. The National Committee for Clinical Laboratory Standards breakpoints for oxacillin resistance are shown as dotted lines.
FIG. 3.
ROC curve based on specificity and sensitivity rates of the disk diffusion test on MSA (oxacillin, 1 μg) for S. aureus isolates relative to MICs (oxacillin susceptible = MIC of ≤2 μg/ml; oxacillin resistant = MIC of ≥4 μg/ml); two isolates with MICs of 3 μg/ml were excluded from this analysis.
DISCUSSION
MSA was developed in 1945 as a selective medium for the isolation of pathogenic staphylococci (4). It is regarded as a valuable medium for the isolation of S. aureus from water, milk, the skin, respiratory tract secretions, and the nose (6, 20, 32), although subinhibitory concentrations of a beta-lactam compound have been reported to result in failure of S. aureus to grow on MSA (14). Since 1985, MSA has been studied with regards to its suitability as a medium for susceptibility testing (12).
In the present investigation, the disk diffusion test (1 μg of oxacilin) on MSA was found to be an excellent screening method to detect oxacillin resistance in S. aureus (sensitivity, 100%; specificity, 97.6%). Our data indicate that an S. aureus isolate with an inhibition zone diameter on MSA of <16 mm (oxacillin disk, 1 μg) should be reported as resistant. Four strains were mecA negative with zone diameters of ≤16 mm. It may be useful to consider isolates with a zone diameter of between 14 and 16 mm as intermediately resistant and to confirm the result by an alternate test (e.g., E-test). The agar screen test (2 μg of oxacillin per ml) on MSA was almost as sensitive (98.1%) and specific (95.1%) as the disk diffusion test. In another study, direct plating of swabs onto methicillin-containing MSA (4 μg/ml) revealed a lower detection rate of MRSA after 18 h of incubation (sensitivity, 66%) (5).
Lipovitellin-salt-mannitol agar has been evaluated for disk diffusion (1 μg of oxacillin) by using 97 MRSA (MIC breakpoint, ≤4 μg/ml) and 56 MSSA isolates. A zone diameter of <13 mm was interpreted as evidence of oxacillin resistance. The medium was found to have a sensitivity of 100% and a specificity of 70% in detecting MRSA isolates (31). In a previous report on 89 mecA-positive S. aureus isolates, the disk diffusion test on MSA was found to have a 100% sensitivity (zone diameter of <16 mm = resistant) (10). Oxacillin-MSA has been used as a screening medium in national and international studies on the frequency of MRSA (29, 30) and is recommended by the National Committee for Clinical Laboratory Standards (18).
The salt concentration in the medium is known to be of great importance for S. aureus. It has been reported that salt supplements of 2 or 4% to Iso-Sensitest agar significantly improve the sensitivity of disk diffusion testing with oxacillin (8, 10). MSA contains 7.5% NaCl. This salt concentration is unique in a medium for testing oxacillin susceptibility. It might be worthwhile to evaluate the use of Iso-Sensitest agar with an even larger salt supplement (e.g., 6 or 7.5%) as the medium for disk diffusion testing. Although Mueller-Hinton agar is still recommended and used, it has the disadvantage of a variable reproducibility of results (7).
In summary, our study shows that MSA is a suitable medium for disk diffusion and agar screen testing to detect oxacillin resistance in S. aureus.
ACKNOWLEDGMENTS
We thank Gabriele Rose and Petra Bähn for technical assistance in carrying out the PFGE and PCR. We thank C. Bantar (Buenos Aires, Argentina), G. Reybrouck (Leuven, Belgium), L. P. Jetté (Sainte-Anne-de-Bellevue, Canada), W. Witte (Werningerode, Germany), and F. H. Kayser (Zürich, Switzerland) for providing us a selection of their national MRSA isolates.
REFERENCES
- 1.Altman D G. Practical statistics for medical research. 1st ed. London, United Kingdom: Chapman & Hall; 1991. [Google Scholar]
- 2.Baker C N, Huang M B, Tenover F C. Optimizing testing of methicillin-resistant Staphylococcus species. Diagn Microbiol Infect Dis. 1994;19:167–170. doi: 10.1016/0732-8893(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 3.Blair E B, Emerson J S, Tull A H. A new medium, salt mannitol plasma agar, for the isolation of Staphylococcus aureus. Am J Clin Pathol. 1967;47:30–39. doi: 10.1093/ajcp/47.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Chapman G H. The significance of sodium chloride in studies of staphylococci. J Bacteriol. 1945;50:201–203. doi: 10.1128/JB.50.2.201-203.1945. [DOI] [PubMed] [Google Scholar]
- 5.Davies S, Zadik P M. Comparison of methods for the isolation of methicillin resistant Staphylococcus aureus. J Clin Pathol. 1997;50:257–258. doi: 10.1136/jcp.50.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilligan P H, Gage P A, Welch D F, Muszynski M J, Wait K R. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol. 1987;25:1258–1261. doi: 10.1128/jcm.25.7.1258-1261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindler J A, Warner N L. Effect of source of Mueller-Hinton agar on detection of oxacillin resistance in Staphylococcus aureus using a screening methodology. J Clin Microbiol. 1987;25:734–735. doi: 10.1128/jcm.25.4.734-735.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang M B, Gay T E, Baker C N, Banerjee S N, Tenover F C. Two percent sodium chloride is required for susceptibility testing of staphylococci with oxacillin when using agar-based dilution methods. J Clin Microbiol. 1993;31:2683–2688. doi: 10.1128/jcm.31.10.2683-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jernigan J A, Clemence M A, Stott G A, Titus M G, Alexander C H, Palumbo C M, Farr B M. Control of methicillin-resistant Staphylococcus aureus at a university hospital: one decade later. Infect Control Hosp Epidemiol. 1996;16:686–696. doi: 10.1086/647042. [DOI] [PubMed] [Google Scholar]
- 10.Kampf G, Weist K, Kegel M, Swidsinski S, Rüden H. Comparison of screening methods to identify methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1997;16:301–307. doi: 10.1007/BF01695635. [DOI] [PubMed] [Google Scholar]
- 11.Knapp C C, Ludwig M D, Washington J A. Evaluation of differential inoculum disk diffusion method and Vitek GPS-SA card for detection of oxacillin-resistant staphylococci. J Clin Microbiol. 1994;32:433–436. doi: 10.1128/jcm.32.2.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lally R T, Ederer M N, Woolfrey B F. Evaluation of mannitol salt agar with oxacillin as a screening medium for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;22:501–504. doi: 10.1128/jcm.22.4.501-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Zonby J G, Starzyk M J. Screening method for recovery of methicillin-resistant Staphylococcus aureus from primary plates. J Clin Microbiol. 1986;24:186–188. doi: 10.1128/jcm.24.2.186-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchese A, Saverino D, Debbia E A, Pesce A, Schito G C. Antistaphylococcal activity of cefdinir, a new oral third-generation cephalosporin, alone and in combination with other antibiotics, at supra- and sub-MIC levels. J Antimicrob Chemother. 1995;35:53–66. doi: 10.1093/jac/35.1.53. [DOI] [PubMed] [Google Scholar]
- 15.McDougal L K, Thornsberry C. New recommendations for disk diffusion antimicrobial susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1984;19:482–488. doi: 10.1128/jcm.19.4.482-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlino J, Gill R, Robertson G J. Application of lipovitellin-salt-mannitol agar for screening, isolation, and presumptive identification of Staphylococcus aureus in a teaching hospital. J Clin Microbiol. 1996;34:3012–3015. doi: 10.1128/jcm.34.12.3012-3015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michel M, Gutmann L. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: therapeutic realities and possibilities. Lancet. 1997;349:1901–1906. doi: 10.1016/s0140-6736(96)11192-2. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Pfaller M, Hollis R J, Sader H S. Pulsed-field gel electrophoresis of chromosomal DNA. In: Isenberg H D, editor. Clinical microbiology procedures handbook. Vol. 2. Washington, D.C: American Society for Microbiology; 1992. pp. 10.5.c.1–10.5.c.12. [Google Scholar]
- 20.Sabbour M M, el Zanfaly H T. Status of membrane-filter procedure in the examination of water and milk for Staphylococcus aureus. Zentbl Mikrobiol. 1987;142:379–386. [PubMed] [Google Scholar]
- 21.Schwarzkopf A, Karch H. Genetic variation in Staphylococcus aureus coagulase genes: potential and limits for use as epidemiologic marker. J Clin Microbiol. 1994;32:2407–2412. doi: 10.1128/jcm.32.10.2407-2412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparham P D, Lobban D I, Speller D C E. Isolation of Staphylococcus aureus from sputum in cystic fibrosis. J Clin Pathol. 1978;31:913–918. doi: 10.1136/jcp.31.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperber W H, Tatini S R. Interpretation of the tube coagulase test for identification of Staphylococcus aureus. Appl Microbiol. 1975;29:502–505. doi: 10.1128/am.29.4.502-505.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suller M T E, Stark J M, Lloyd D. A flow cytometric study of antibiotic-induced damage and evaluation as a rapid antibiotic susceptibility test for methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;40:77–83. doi: 10.1093/jac/40.1.77. [DOI] [PubMed] [Google Scholar]
- 25.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornsberry C, McDougal L K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983;18:1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokue Y, Shoji S, Satoh K, Watanabe A, Motomiya M. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:6–9. doi: 10.1128/aac.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Enk R A, Thompson K D. Use of a primary isolation medium for recovery of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1992;30:504–505. doi: 10.1128/jcm.30.2.504-505.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss A, Machka K, Lenz W, Milatovic D. Vorkommen, Häufigkeit und Resistenzverhalten von Methicillin-Oxacillin-resistenten Staphylococcus-aureus-Stämmen in Deutschland. Dtsch Med Wochenschr. 1992;117:1907–1912. doi: 10.1055/s-2008-1062528. [DOI] [PubMed] [Google Scholar]
- 30.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl V T, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]
- 31.Wanger A R, Moore D G, LaRocco M T. Latex agglutination for rapid identification of methicillin-resistant Staphylococcus aureus recovered from selective media. Eur J Clin Microbiol Infect Dis. 1991;10:564–567. doi: 10.1007/BF01967274. [DOI] [PubMed] [Google Scholar]
- 32.Webster J. Handwashing in a neonatal intensive care nursery: product acceptability and effectiveness of chlorhexidine gluconate 4% and triclosan 1% J Hosp Infect. 1992;21:137–141. doi: 10.1016/0195-6701(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 33.Witte W, Kresken M, Braulke C, Cuny C. Increasing incidence and widespread dissemination of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals in central Europe, with special reference to German hospitals. Clin Microbiol Infect. 1997;3:414–422. doi: 10.1111/j.1469-0691.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]