Abstract
While the cellular and molecular features of human inflammatory skin diseases are well-characterized, their tissue context and systemic impact remain poorly understood. We thus profiled human psoriasis (PsO) as a prototypic immune-mediated condition with a high predilection for extra-cutaneous involvement. Spatial transcriptomics (ST) analyses of 25 healthy, active lesion, and clinically uninvolved skin biopsies, and integration with public single-cell transcriptomics data revealed striking differences in immune microniches between healthy and inflamed skin. Tissue scale-cartography further identified core disease features across all active lesions, including the emergence of an inflamed suprabasal epidermal state and the presence of B lymphocytes in lesional skin. Importantly, both lesional and distal non-lesional samples stratified by skin disease severity, and not by presence of systemic disease. This segregation was driven by macrophage-, fibroblast- and lymphatic-enriched spatial regions with gene signatures associated with metabolic dysfunction. Taken together, these findings suggest that mild and severe forms of PsO have distinct molecular features and that severe PsO may profoundly alter the cellular and metabolic composition of distal unaffected skin sites. Additionally, our study provides a valuable resource for the research community to study spatial gene organization of healthy and inflamed human skin.
One Sentence Summary:
Spatial transcriptomics provides insight into the cellular, immune, and molecular landscape of psoriatic disease pathogenesis.
INTRODUCTION
Human skin constitutes a physical and immunological barrier to the surrounding environment. As such, this highly active immune organ has a strong predisposition to inflammatory diseases. Affecting between 2 to 3% of the population, psoriasis (PsO) is one of the most prevalent immune-mediated inflammatory diseases. PsO is clinically characterized by erythematous skin plaques, which are either restricted to a small skin area or cover a large portion of the body, and can vary in thickness (induration), scaliness (desquamation) and redness (erythema) (1). Disease severity, defined and measured by Psoriasis Area and Severity Index (PASI), differs considerably between patients. In addition, PsO often involves systemic complications such as metabolic syndrome, cardiovascular disease, depression, and synovioentheseal inflammation. For instance, up to 1 in 3 patients with PsO develop psoriatic arthritis (PsA) and up to 40% of PsO patients develop cardiometabolic comorbidities (2-5). Moreover, the risk of developing cardiovascular complications or PsA is known to increase with severity of skin disease (6-11). Navigating these complexities to define the mechanisms underlying disease severity and the progression from localized skin inflammation to systemic disease thus remains a major challenge (12).
Advances in single-cell transcriptomic technologies enabled the generation of the human cell atlas. Such precise cellular mapping of human tissues has transformed our understanding of healthy and inflamed skin (13-15). Profiling the cellular landscape of PsO and related conditions confirmed the clonal expansion of helper and cytotoxic T cell subsets and dysfunctional epithelial states in diseased skin when compared to healthy human cutaneous tissue (13-15). Despite providing a high cellular resolution, single cell RNA sequencing (scRNA-seq) requires enzymatic skin digestion and therefore does not preserve native tissue context. Consequently, 3D structural data and the distinct environments or “microniches” in which healthy and pathological cell-cell interactions occur are challenging to capture. Given the importance of cellular organization and interactomes in orchestrating health and disease, we postulated that agnostically mapping these features at the tissue-scale may reveal emergent characteristics of psoriatic disease severity and/or its systemic manifestations.
Here we employed spatial transcriptomics (ST), a method that positions tissue sections onto spatially barcoded arrays at 50-micron resolution to determine gene expression by cell type and histological location (16). In so doing, we defined cellular composition, localization, and interactomes of 25 healthy and matched psoriatic lesional (L) and non-lesional (NL) skin biopsies (table S1). In addition, we leveraged publicly-available scRNA-seq datasets to gain cellular resolution within in 50-micron spatial spot. Our whole tissue cross-sectional analyses revealed positional immune dynamics and regional pathological features that arise in the epithelium and upper dermis during inflammation, including the presence of B lymphocytes. Noticeably, unsupervised classification of PsO and PsA samples reveals stratification by disease severity or PASI score but not by systemic joint disease. This disease severity-dependent segregation was particularly evident in distal, non-lesional samples, and could be distinguished by dermal macrophage and fibroblast clusters, the lymphatic endothelium, and enrichment of pathways involved in metabolic dysregulation. Collectively, our high-resolution spatial analyses constitute a valuable resource for the scientific community that defines distinguishing cellular and structural skin tissue features of mild and severe PsO both at the site of inflammation and in clinically uninvolved distal skin.
RESULTS
Spatial Transcriptomics (ST) faithfully maps gene expression in healthy human skin
We developed a human skin specific protocol for 10X Genomics Visium platform and performed ST on 25 skin samples collected from 3 healthy controls and 11 patients with PsO/PsA (Fig. 1A, figs. S1, A to C, S14A and table S1). Healthy samples were obtained from three skin sites, either flank, forearm, or buttock. Unique molecular identifiers (UMIs) correlated with gene (feature) counts per spot and low-quality spots with gene counts below 200 were filtered out (fig. S2, A and B).
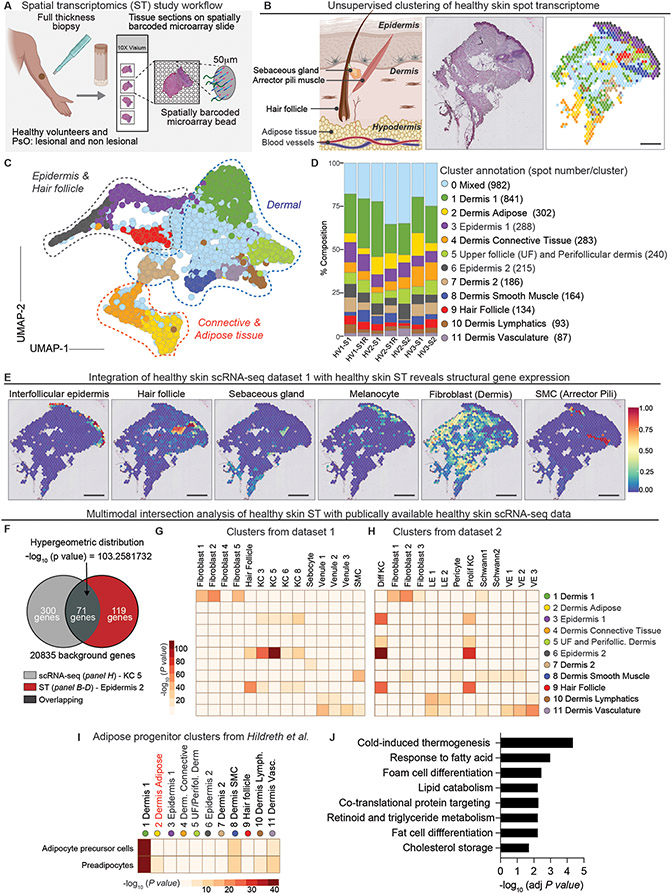
Figure 1. Spatial transcriptomics analysis of healthy human skin.
(A) Schematic of spatial transcriptomics study workflow. Table S1 contains metadata for each sample. (B) Schematic of skin, representative hematoxylin-eosin (H&E) image and corresponding ST plot (left-to-right). Scale bar = 440μm (C) UMAP visualization of 3,815 spots colored by cluster obtained from healthy skin samples (N=3, n=5). (D) Composition plots displaying relative abundance of each cluster by sample. Note up to two samples (labeled S) were collected from each Healthy Volunteer (HV). Replicate arrays are labeled “R” along the X axis. (E) Integration with a publicly-sourced single cell RNA-seq data set (dataset 1) with a representative ST spatial feature plot. See Figure S4 for UMAP of annotated cell type clusters. SMC=smooth muscle cell. Scale bar = 520μm (F) Multimodal intersection analysis (MIA) of overlap between data from datasets 1 and 2 and our ST-generated clusters. A sample hypergeometric distribution of keratinocyte cluster from dataset 1 and our epidermis cluster (cluster 6). MIA enrichment heatmaps of non-immune cell types in dataset 1 (G) and dataset 2 (H) and ST clusters from healthy skin. The X axis denotes the scRNA seq-identified cell types while the Y axis represents the ST-generated clusters. Differentiated keratinocytes (Diff KC) lymphatic endothelium (LE), proliferating keratinocytes (Prolif KC), vascular endothelium (VE), keratinocyte (KC). (I) MIA heatmap showing the enrichment of scRNA-seq-identified adipose- cell types from Hildreth et al. within pooled healthy skin ST clusters. (J) KEGG pathway analysis of the adipose cluster (cluster 2).
After Seurat anchor-based integration, we implemented graph-based clustering of the healthy skin spot transcriptome (3,815 spots). Dimensionality reduction, performed using uniform manifold approximation and projection (UMAP), revealed 12 distinct clusters (Fig. 1, B to D) (17). Cluster annotations based on differentially expressed marker genes had remarkable concordance with histopathologic tissue features (Fig. 1B and fig. S2C). Clusters grouped by the skin’s three primary histologically-defined layers (epidermis, dermis, and hypodermis) and captured discrete structures such as the hair follicle (Fig. 1, B to D). Harmony-based integration and graph-based clustering analysis yielded 11 distinct clusters and similarly delineated the distinct skin layers (fig. S3, A to C). Importantly, serial analysis of tissue sections (identified with ‘R’, X axis) yielded highly reproducible results and each of the 12 clusters were represented in all healthy samples when using both anchor-based and Harmony-based integration analyses (Fig. 1D and fig. S3C). We thus performed subsequent validations and analyses of healthy skin using anchor-based integration. Across all samples, epidermal and hair follicle clusters 3, 7, 9 had the highest number of UMIs followed by cluster 6 (upper follicle and perifollicular dermis) and dermis 2, highlighting regions that were the most cell dense and/or transcriptionally active (fig. S2D).
We made use of publicly available scRNA-seq data to gain cellular resolution within the ST spots and validate our analysis (fig. S4, A and B) (13, 14). Although these healthy skin cell atlases were generated from tissue discards (i.e., mammoplasty or abdominoplasty), integration of Hughes et al, herein called “dataset 1” with our ST plots revealed accurate prediction of the skin’s architectural elements, including the interfollicular epidermis, hair follicle, sebaceous gland, melanocyte, dermal fibroblasts and arrector pili muscle (Fig. 1E). To further confirm these findings, we used multimodal intersection analysis (MIA), a computational method that infers enrichment of specific cell type(s) in tissue regions based on the degree of overlap in upregulated differentially expressed (DE) / marker genes between every ST and scRNA-seq cluster using a hypergeometric test (18, 19) (Fig. 1F). Mixed cluster 0 marker genes did not meet the MIA cutoff and was thus excluded from MIA analysis. MIA maps summarizing enrichment analyses of scRNA-seq clusters from Reynolds et al. (ENA - ERP116319), herein called “dataset 2”, and dataset 1 with our ST-generated clusters showed significant agreement in cell annotations and regional distributions (Fig. 1, G and H). For instance, differentiated keratinocyte (Diff KC) clusters from dataset 2 were enriched in epidermal clusters 3 and 6 and in the hair follicle clusters 6 and 9, underscoring the ability of ST to properly contextualize cell types of interest and their localization.
Unexpectedly, dermal adipose cluster 2 had a notable lack of cell enrichment in the MIA analysis (Fig. 1, G and H). We thus turned to a third scRNA-seq data set from Hildreth et al., which characterized adipocyte precursor cells and preadipocytes to dermal cluster 1 (20). Even this adipose tissue data set did not show signal in dermal adipose cluster 2 in MIA analysis (Fig. 1I). Instead, adipocyte precursor cells and preadipocyte clusters were enriched in dermal cluster 1 adjacent to dermal adipose cluster 2. Moreover, cells occupying the area under cluster 2 spots (yellow spots) morphologically appeared to be mature adipocytes with large lipid droplets in their cytoplasm (Fig. 1B). This prompted us to consider that ST analyses may uniquely capture gene expression of mature adipocytes that succumb to the enzymatic cell extraction process used for scRNA-seq. Indeed, the dermal adipose cluster 2 expressed genes involved in fat cell differentiation, cholesterol storage, and lipid-associated processes (Fig. 1J). The unique adipose tissue signatures were absent from the other spatial clusters and adipose progenitors, which can be captured by scRNA-seq, occupied distinct niches from mature adipocytes (fig. S4C). Taken together, these data provide a high degree of confidence in ST analysis of human skin and reveal an added technological advantage of this method in capturing gene expression of mature adipocytes.
A perifollicular immune niche monitors healthy skin
We next sought to map the immune landscape of healthy human skin and determine the spatial organization of immune activity. Toward this end, we applied MIA to immune populations from healthy skin identified in datasets 1 and 2 to determine enrichment of these cells within our ST clusters (Fig. 2, A and B) (13, 14). In so doing, we uncovered significant patterns of immune activity around the upper follicle and perifollicular dermis (cluster 5) and within the hair follicle itself (cluster 9). Dendritic cells (DCs), innate lymphoid cells (ILC2), T helper (Th), T cytotoxic (Tc) cells, and myeloid cells were all strongly enriched in these areas. Of note, many immune populations were also detected in dermal lymphatic and vascular endothelial clusters 10 and 11, albeit to a lesser degree than in follicular regions (Fig. 2, A to C).
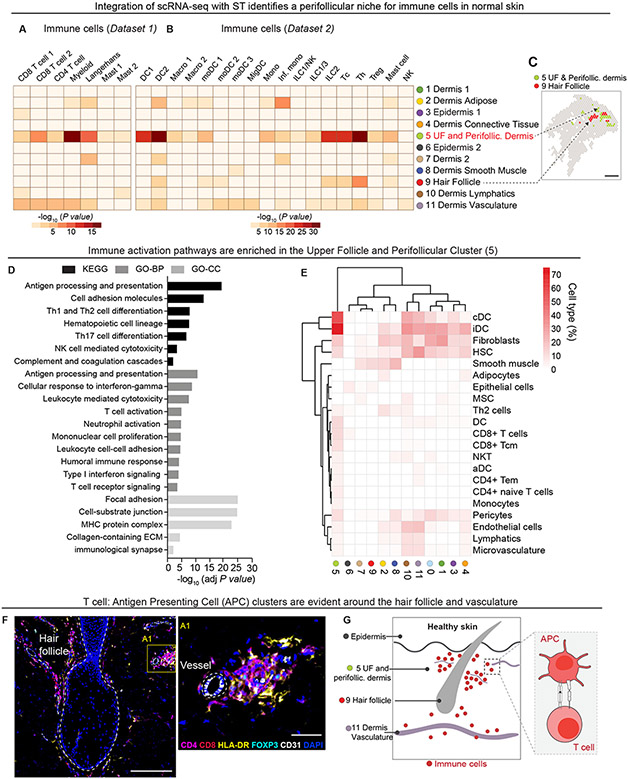
Figure 2. Immune cells are enriched in hair follicle and endothelial niches in healthy skin.
MIA enrichment heatmaps of immune cell types from integrated scRNA-seq dataset 1 (A) and dataset 2 (B). The X axis denotes the scRNA seq-identified immune cell types while the Y axis represents the ST-generated clusters. dendritic cell (DC), macrophage (macro), monocyte-derived dendritic cell (moDC), migratory dendritic cell (migDC), monocyte (mono), Inflammatory monocyte (inf. Mono), innate lymphoid cell (ILC), natural killer cell (NK cell), cytotoxic CD8+ T cell (Tc), CD4+ T helper cell (Th), regulatory T cell (Treg). (C) Representative spatial plot highlighting location of clusters 5 and 9 in healthy skin. (D) Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology – Biological Processes (GO-BP), GO-cellular component (GO-CC) pathway analyses of differentially expressed genes in upper follicle and perifollicular cluster 5. (E) xCell clustered heatmap of immune cells in cluster 5. Map is scaled by cell type fraction of total cells in a given cluster. conventional dendritic cell (cDC), immature dendritic cell (iDC), hematopoietic stem cell (HSC), mesenchymal stem cell (MSC), dendritic cell (DC), natural killer T cell (NKT), activated dendritic cell (aDC), effector memory T cell (Tem), lymphatic (ly), microvascular (mv). (F) Representative image illustrating the perifollicular and perivascular localization of CD4+ (magenta), CD8+ (red), HLA-DR+ (yellow), and FOXP3+ (cyan) immune cells clusters. DAPI nuclei (blue). Hair follicle and CD31+ (white) blood vessel demarcated by white dashed line. Scale bar = 200μm (left), Scale bar = 50μm (right) . (G) Schematic of immune cell activity in healthy skin around the hair follicle and vasculature.
Gene Ontology (GO) and KEGG pathways analyses of differentially expressed genes (average Log2FC > 0.25, FDR < 0.1) in the upper follicle and perifollicular dermis (cluster 5) relative to other ST clusters revealed enrichment of: 1) T cell activation pathways (antigen presentation, T cell receptor signaling, mononuclear cell proliferation), 2) cytokine signaling pathways, and 3) barrier function (focal adhesion and cell-substrate junction), (Fig. 2D and fig. S4C). To further validate these findings we employed xCell, a method to perform enrichment analysis of gene expression data curated from 64 human immune and stroma cell types (21), and found that hierarchical clustering distinguished the upper follicle and perifollicular (cluster 5) for gene signatures of antigen presenting populations (iDC and cDC) and T cells (CD8+/CD4+T cells and T central memory, cm) (Fig. 2E). To corroborate the cellular composition and location highlighted by these gene signatures, we visualized effector T cells (CD4+ or CD8+, Foxp3neg) and HLA-DR+ skin antigen presenting cells (APCs) using immunofluorescence (Fig. 2F). Large clusters of effector (Foxp3neg) T cells and APCs congregate around CD31+ blood vessels in close apposition of the hair follicle (Fig. 2G). Collectively, these data illustrate the DC-lymphocyte-mediated immune surveillance of the hair follicle and perivascular space in healthy skin.
Dysregulated epidermal-dermal interactomes in psoriatic lesions
We next performed ST on matched patient biopsies from plaque skin (lesional) and uninvolved skin from contralateral site (non-lesional). We once again compared Harmony and Seurat anchor-based integration methods followed by graph-based clustering and UMAP visualization of healthy and psoriatic lesional and non-lesional skin spot transcriptome to generate region-specific clusters (fig. S5 to 7). Clusters in both Harmony and anchor-integrated UMAPs were annotated based on differentially expressed genes and histological features (fig. S5 and 6). Cross examination of the two UMAPs indicated that Harmony integration delineated clusters with greater structural specificity (fig. S5). For example, UMAP visualization of Harmony-based integration discerned a specific pilosebaceous unit (cluster 8), which was grouped into the dermis cluster in Seurat anchor-based clustering (fig. S5C). Thus, we performed our downstream analyses using Harmony sample integration. Analysis of all healthy, lesional and non-lesional samples (16,424 total spots, average of 1,343 genes per spot) revealed 17 distinct clusters (Fig. 3, A to C) (22). Spatial spot clusters grouped by epidermal, dermis, pilosebaceous, and adipose regions and traced key histopathologic features of both lesional and non-lesional skin (Fig. 3, A to C).
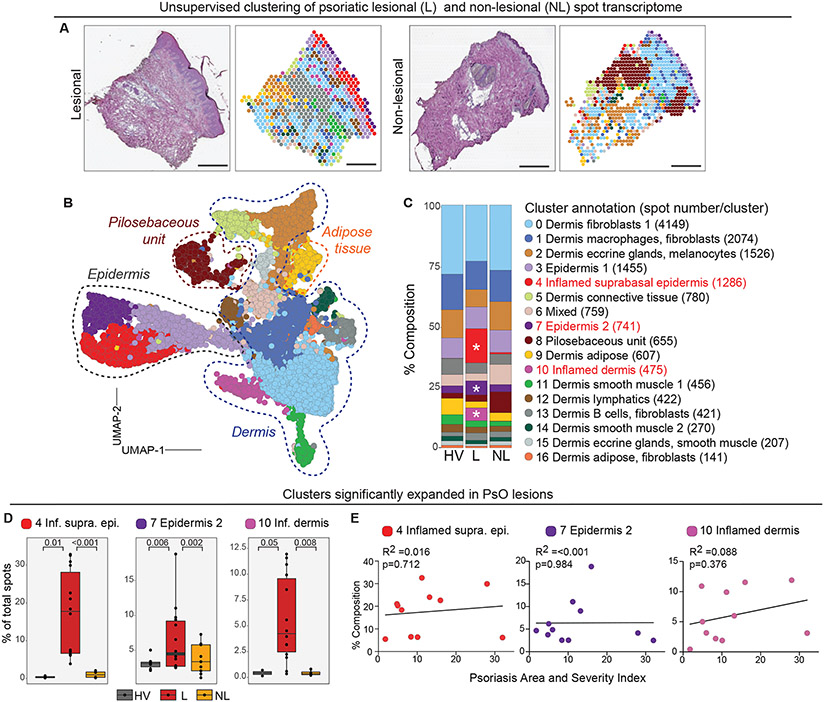
Figure 3. ST analyses of psoriatic (PsO) lesional and non lesional skin.
(A) Representative histological image (left) and corresponding ST plots following Harmony batch correction (right) of lesional PsO and non-lesional skin samples. Scale bar = 440μm (B) UMAP visualization of 16,424 spots obtained from combined psoriatic lesional (n=14) and non lesional (n=9), and healthy (n=5) skin samples. Dashed lines around clusters discern region specific clusters. (C) Composition plots indicating the relative abundance of each cluster in healthy volunteer (HV), lesional (L), non-lesional (NL) samples. Asterix denotes significantly expanded clusters (p≤0.05, see D for absolute p values) (D) Box plots depicting frequency of PsO-enriched clusters 4, 7, and 10 in different sample groups. Each black dot represents an individual biopsy sample. P values computed using Wilcoxon rank sum test. (E) Linear regression plots of clusters 4, 7, and 10 percent composition in PsO lesional samples (y-axis) and corresponding sample Psoriasis Area and Severity Index score (x-axis). Each dot represents an individual biopsy sample. n= number of skin sections analysed.
To identify spatial and molecular features specific to PsO skin, we examined aggregated healthy (5 samples), PsO lesional (14 samples), and when available, matched non-lesional (9 samples) cluster composition (table S1 and Fig. 3C). Comparing the frequency of cluster-specific spots across pooled samples revealed that epidermis 2 (cluster 7) was significantly expanded in lesional skin, and the emergence of inflamed suprabasal epidermal (cluster 4) and inflamed dermis (cluster 10) specifically in PsO lesional skin (Fig. 3, C and D). By contrast, the remaining clusters were evenly represented in all groups or showed a trend toward enrichment in healthy or non-lesional samples (fig. S8C). UMI and gene counts were also higher in inflamed skin sections (average of 7,382 UMIs) when compared to healthy (average of 2,244 UMIs) or non-lesional skin (average of 2,996 UMIs) indicative of higher transcriptional activity and/ or cell density (fig. S2A and S8A). Interestingly, suprabasal epidermis (cluster 4) specific to lesional skin had the highest average UMI counts per spot (30,153 UMIs), consistent with previous findings linking high inflammatory cytokine and antimicrobial peptide expression to this layer (fig. S8B) (23).
The psoriatic samples analyzed spanned the spectrum of mild (PASI 1.8) to severe (PASI 32) disease (table S1). We therefore examined if the composition of spatial clusters 4, 7, and 10, which were significantly expanded in PsO lesional skin, varied by disease severity. Linear regression analysis of the relative composition of each cluster within total sample (% composition) and PASI score did not reveal any meaningful associations, indicating that these pathologic features were common to PsO lesions irrespective of disease burden (Fig. 3D). Accordingly, the PsO-associated clusters were enriched for IL-17, IL-22, and STAT signaling pathways, all characteristic of this inflammatory skin disease (Fig. 4, A to C) (24, 25). In addition, metabolic pathways (sphingolipid metabolism, oxidative phosphorylation, and glycolysis), ribosome, cell-cell interactions (gap junction, desmosomes, focal adhesions), ECM interactions, and proliferative (RAS, AMPK signaling), and endocrine signaling (estrogen receptor, GnRH signaling) were all enhanced in PsO-specific clusters. Upregulation of genes involved in ichthyosis was also observed (Fig. 4C), supporting the previously-established shared pathogenic mechanisms of epidermal hyperdifferentiation and barrier dysfunction between the two conditions, in addition to their mutual dysregulation of the TH17/IL-23 axis (26).
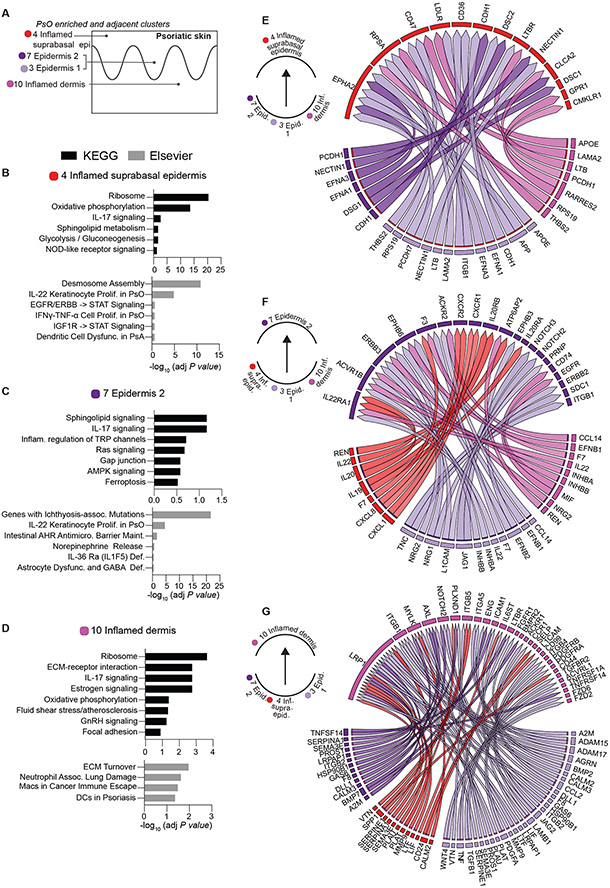
Figure 4. Pathways and ligand receptor interactions in psoriasis-enriched clusters.
(A) Schematic illustrating spatial locations of psoriasis-enriched (4,7,10) and adjacent cluster (3). (B-D) KEGG and Elsevier pathways analyses of clusters 4, 7, and 10, respectively. (E-G) Circle plots of NicheNet-predicted ligand-receptors interactions in clusters 4, 7, and 10 and ligands in cluster 3. Arrow point from putative ligand/sender to receptor/receiver clusters are denoted on the left of each circle plot. For total Niche-net output see data file S1.
To identify the cellular interactions that may fuel differentially-expanded lesional clusters, we turned to NicheNet (27), a computational method that predicts ligand-receptor pairs. Prioritization scores of interactions are reported based on both ligand-receptor expression patterns in designated sender–recipient clusters as well as the enrichment of their downstream signaling signatures in the recipient cluster (for total NicheNet output see data file S1). We examined the crosstalk between PsO- enriched clusters 4, 7, and 10 as well as their relationship with the adjacent dermal cluster 3 (Fig. 4, A to G). Consistent with the pathway analyses, the top 50 bona fide ligand-receptor interactions with the highest prioritization scores were enriched in inflammatory cytokine signaling (TNF/TNFRSF1A, IL-22/IL22RAI, IL20/IL20RB, IFNG/IFNGR, TGFB1/TGFBR2), chemotaxis (CCL14/ACKR2, CXCL8/CXCR2), lipid metabolism (APOE/LDLR, ADIPOQ/ ADIPOR1, LRPAP1/LRP1), cell adhesion and migration (ITGB2/ICAM1, DSG1/DSC2, LAMA2/RPSA, TNC/SDC1, L1CAM/ERBB3, THBS2/CD47), cell proliferation (GAS6/AXL, PDGFA/PDGFRB, EFNA1/EPHA2), and endocrine/hormonal processes (CGA/VIPR1, INHBA/ACVR1B) (Fig. 4 E to G, data file S2).
Dynamic re-organization of the immune milieu and fibroblasts in PsO lesional and non-lesional skin
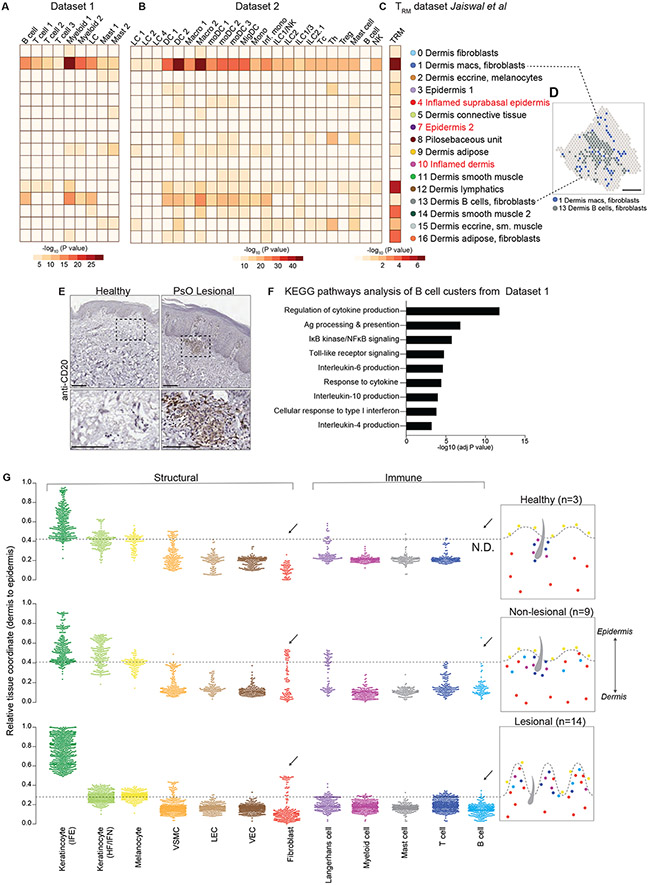
Integration of psoriatic skin ST specifically with psoriatic scRNA-seq samples from datasets 1 and 2 revealed the fidelity of ST in rendering histologically-compatible patterns of gene expression in diseased skin (Fig. S9, A to C). Additionally, we once again employed MIA and confirmed that key epidermal and dermal structural cells agreed with histological tissue architecture (fig. S9, D and E). We also used MIA to chart the spatial distribution of immune cells, including tissue-resident memory T cells (TRM) in psoriatic lesional and non-lesional ST clusters (Fig. 5, A to C) (28). In contrast to healthy skin, in which we observed an enrichment of immune activity around the hair follicle and endothelium, only a few subsets of immune cells (Th cells, mast cells and DCs) were mildly enriched in the pilosebaceous unit (cluster 8) (Fig. 5, A to C). Instead, MIA-based tissue mapping revealed the repositioning of immune cells to the papillary and upper reticular dermal clusters 1 and 13 (Fig. 5D). TRM signatures were also enriched in lymphatic cluster 12 (Fig. 5C). Accordingly, pathways analyses of these clusters reflected adaptive and innate immune activity (fig. S9F). In addition to Type 17 immune cells, neutrophils, and other inflammatory cells typically associated with psoriasis, the upper dermal clusters 1 and 13 were also enriched for B lymphocytes, which were notably absent from healthy skin. Accordingly, immunohistochemical staining with the B cell antigen CD20 confirmed the presence of B cell clusters in lesional skin (Fig. 5E). Pathways analysis on B cell clusters from dataset 1 revealed functional signatures of cytokine production and antigen presentation enriched in psoriatic skin, suggesting a potential role in pathology (Fig. 5F).
Figure 5. Psoriatic and healthy skin have distinct immune-fibro cellular neighborhoods.
MIA enrichment heatmaps of psoriatic skin immune cells in dataset 1 (A), dataset 2 (B), and skin T resident memory cell (TRM) signatures from Jaiswal et al (C) with ST-delineated clusters from figure 3. Langerhans cell (LF), dendritic cell (DC), macrophage (macro), monocyte-derived dendritic cell (moDC), migratory dendritic cell (migDC), monocyte (mono), inflammatory monocyte (inf.mono), innate lymphoid cell (ILC), natural killer cell (NK), cytotoxic CD8+ T cell (Tc), CD4+ T helper cell (Th), regulatory T cell (Treg), mast cell (mast), tissue-resident memory T cells (TRM). (D) Spatial plot highlighting location of immune-enriched clusters 1 and 13 in lesional skin. (E) Representative immunohistochemistry images of CD20+ cells PsO lesional skin and healthy skin. Scale bar = 100μm. (F) Significantly enriched pathways in B cells from sc-RNAseq dataset 1. (G) SpaceFold one-dimension projection (left) and summary schematic (right) of cell distribution from dataset 1 on aggregated ST healthy (N=3, n=5), lesional (N=11, n=14) and non lesional samples (N=9, n=9). Y-axis represents tissue position, starting with the lower dermis demarked as position 0 to suprabasal epidermis marked as position 1. Arrows highlight cell types whose relative coordinate is different between healthy and diseased samples. Dashed line represents epidermal-dermal junction, discerned by cell types in the basal epidermal layer (melanocytes and Langerhans cells). Interfollicular epidermis (IFE), Hair follicle and infundibulum (HF/IFN), Vascular Smooth Muscle Cell (VSMC), Lymphatic endothelial cells (LECs), Vascular endothelial cells (VEC). N= number of individuals biopsied, n= number of skin sections analyzed.
As a complementary strategy to MIA, we used deconvolution, integration, and projection methods, BayesPrism (29) and SpaceFold (30), to cartograph structural and immune cells from dataset 1 along the spatial dimensions of healthy, lesional, and non-lesional skin. SpaceFold uses non-linear dimensionality reduction to project tissue coordinates along a one-dimensional (1D) pseudo-space axis and thus, regardless of tissue thickness, each condition is scaled from 0-1 (lower dermis to suprabasal epidermis) axis (Fig. 5G). We used cells that are known to reside in the basal layer of the epidermis (melanocytes and Langerhans cells (LCs)) to define the basement membrane which separates the epidermis from the dermis, and marked by a dashed line on SpaceFold plots. SpaceFold projection recapitulated epidermal hyperplasia in lesional skin as noted by the compression of the dermis, and expansion of interfollicular epidermal (IFEs) keratinocytes along the 1D axis. We also observed a dynamic re-arrangement of many of the dermal structural elements. The median coordinate of lymphatic endothelial cells (LEC) and vascular endothelial cells (VEC) was closer to the basement membrane (denoted by a dashed line) in lesional tissue than in healthy or non-lesional skin. Perhaps most strikingly, a subset of papillary dermal spots was enriched for fibroblasts genes in both lesional and non-lesional PsO skin, denoted by presence of this signature above the basement membrane line (Fig. 5G). This tissue-scale computational cartography further highlighted the differences between non-lesional and healthy skin, indicating that even skin distal to clinically evident disease demonstrates cellular and molecular alterations.
Unsupervised clustering reveals stratification by psoriatic disease severity
Leveraging a priori knowledge of skin disease severity as measured by PASI score and concomitant systemic, extra-cutaneous disease [active skin psoriatic lesions and arthritis (PsA)], we sought to determine if unsupervised clustering algorithms could stratify the psoriatic and healthy skin samples into clinically-relevant transcriptomic endotype subsets. A PASI score of less than 12 was used to define mild psoriasis and a score of 12 or greater to define moderate-to-severe psoriasis (1, 31). Neither hierarchical clustering nor PCA of pseudobulked ST samples segregated patients based on presence or absence of PsA (Fig. 6A to C and fig. S10A); however, both lesional and non-lesional samples neatly separated by cutaneous disease severity. Noticeably, while non-lesional mild skin disease (PASI<12, herein called PASIlow) samples segregated with healthy skin, non-lesional moderate-to-severe skin disease (PASI ≥12, herein called PASIhigh) samples clustered with lesional groups (Fig. 6A and fig. S10A). Accordingly, we observed a gradient of transcriptomic variance on the first principal component ranging from lesional moderate-to-severe skin, to lesional mild skin, to non-lesional moderate-to-severe skin, and finally, to non-lesional mild skin that overlapped with healthy skin (Fig. 6C).
Figure 6. Psoriasis (PsO) and psoriatic arthritis (PsA) lesional and non-lesional samples stratify by cutaneous disease severity, not by presence or absence of joint disease.
(A) Heatmap with dendrogram showing hierarchical clustering of pseudo-bulked ST samples. Samples have been demarked by presence (PsA) or absence (PsO) of systemic disease and cutaneous disease severity (Psoriasis Area and Severity Index (PASI)). PCA plots of lesional (L) and non-lesional (NL) samples colored by presence or absence of arthritis (B) and by severity (PASI) of cutaneous disease (C). Each spot on the PCA plot represents an individual replicate.
Distinguishing features of mild and moderate-to-severe disease
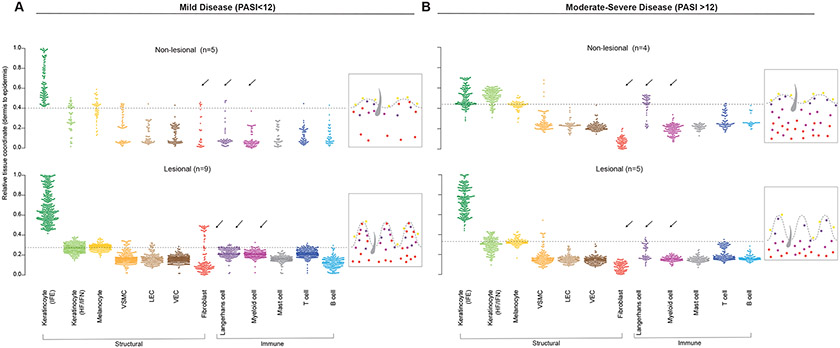
We then asked whether the spectrum of PsO disease severity could be defined by distinct gene signatures, both at the site of inflammation and in clinically-uninvolved skin. To test this, we performed BayesPrism and SpaceFold projection using dataset 1 on lesional and non-lesional samples grouped by mild or moderate-to-severe disease (Fig. 7, A and B). Localization of each cell type was projected across the dermal-epidermal tissue axis (0-1 pseudoscale) and using melanocytes as a cellular marker of the basal layer, we denoted the basement membrane separating the epidermis and dermis with a dashed line. Similar to the pooled lesional skin analyses (Fig. 5G), 1D SpaceFold projection revealed epidermal hyperplasia in lesional skin across the spectrum of disease severity. However, we uncovered differences in the regional enrichment of a number of different cell types between mild and moderate-to-severe psoriasis. Most notably, fibroblast median coordinates were relegated to the lower dermis in the PASIhigh group, but extended to the upper dermis in proximity of the demarked basement membrane (dashed line) in the PASIlow group. This striking difference in fibroblast localization was also noted in non-lesional PASIhigh vs. PASIlow groups. In addition to fibroblasts, lymphatic endothelial cells, vascular endothelial, and myeloid median coordinates were also observed in proximity of the demarked basement membrane (dashed line) in lesional PASIlow samples, but were much lower in the dermis of PASIlow non-lesional and all samples in PASIhigh group.
Figure 7. Disease severity stratifies by emergent cellular ecosystems in the upper dermis.
SpaceFold one dimensional projection of cell distribution based on cell types from dataset 1 on aggregated ST lesional and non lesional samples from mild (PASIlow) (A) and moderate-severe (PASIhigh) samples (B). Y axis represents tissue position, starting with the lower dermis demarked as position 0 to suprabasal epidermis marked as position 1. Arrows highlight cell types whose position is changed between healthy and diseased samples. Dashed line represents epidermal-dermal junction, discerned by cell types in the basal epidermal layer (melanocytes and Langerhans cells). Interfollicular epidermis (IFE), Hair follicle and infundibulum (HF/IFN),Vascular Smooth Muscle Cell (VSMC), Lymphatic endothelial cells (LECs), Vascular endothelial cells (VEC). n= number of skin sections analyzed.
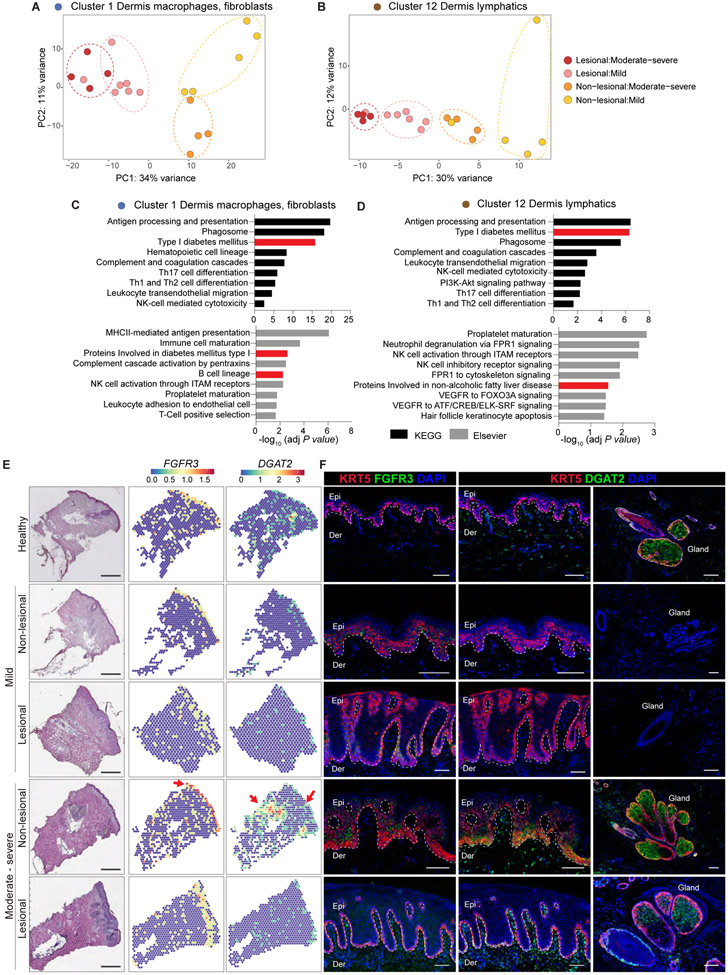
To validate the differences uncovered by SpaceFold-based tissue cartography, we performed unsupervised PCA on each of the 17 ST-generated clusters (Figs. 4, A to C, 8A, and B and fig. S10A). Consistent with the enrichment of fibroblasts, lymphatics, and myeloid cells in the upper dermis of PASIlow samples, separation of the phenotypic severity groups was most pronounced in the macrophage and fibroblast cluster (cluster 1), and in the dermal lymphatic cluster (cluster 12) (Fig. 8, A and B and fig. S11A). Of note, the differentially expanded clusters in lesional psoriatic skin (clusters 4, 7, and 10) did not aggregate by disease severity, suggesting that these clusters represented more generalizable features of disease (fig. S11A). KEGG and Elsevier-based pathway analysis of marker genes from clusters 1 and 12 revealed enrichment of key T cell and innate immune cell activation associated pathways (antigen processing, complement and coagulation cascades, leukocyte migration, T-helper cell differentiation, innate immune cell activation) and a notable enrichment of B cells (Fig. 8, C and D). Additionally, pathways associated with metabolic dysfunction were also enriched in these skin clusters (type 1 diabetes, proteins involved in nonalcoholic fatty liver disease, cholesterol metabolism, and lipolysis in adipocytes) (Fig. 8, C and D, and fig. S12, A and B).
Figure 8. Macrophage, fibroblast, and lymphatic endothelium-associated spatial clusters distinguish mild and moderate-severe disease endotypes.
Post pseudo-bulking PCA of lesional and non-lesional samples colored by disease severity in spatial clusters 1 (A) and 12 (B). KEGG and Elsevier Pathway analyses of clusters 1 (C) and 12 (D) genes. (E) Representative histological images and corresponding spatial gene expression plots of selected differentially expressed genes (∣log2(FC)∣ ≥ 1.5 and FDR <0.05) in healthy, lesional, and non-lesional samples. Red arrows point to areas of enriched gene expression. Fibroblast growth factor receptor 3 (FGFR3), diacylglycerol O-acyltransferase 2 (DGAT2). Scale bar = 470μm (F) Representative non-lesional moderate-severe skin exhibits robust expression of FGFR3 throughout the epidermis (green, left) and DGAT2 in the epidermis (green, center) and adnexal glands (green, right). Keratin (KRT) 5 (red), DAPI nuclei (blue). Scale bar = 100μm.
To gain insights into specific genes underlying the sample stratification, we performed pairwise differential gene expression between PASIhigh and PASIlow groups (log2(FC) ≥ 1.5 and FDR <0.05) in clusters 1 and 12. We visualized gene expression of top hits in healthy, lesional, and non-lesional samples that illustrated the patterns of stratification. (Fig. 8E and fig. S13). Two lipid metabolism genes (FADS2 and DGAT2) and FGFR3 were notably enriched in the PASIhigh non-lesional samples compared to all other groups, whereas FABP5 and SERPINB4 were strongly expressed in lesional samples from PASIhigh group. Remarkably, immunofluorescence imaging of DGAT2 and FGFR3 confirmed that these two factors were enriched in non-lesional PASIhigh skin, and particularly enriched in keratin 14-expressing epidermal compartment (Fig. 8F). Collectively, these data illustrate that distinct molecular features stratify mild and severe psoriasis and that clinically-uninvolved skin is altered in severe disease.
DISCUSSION
Our study highlights the ability of ST to concomitantly cartograph gene expression and define distinct, biologically-relevant, cellular “neighborhoods” in healthy skin. In so doing, we uncovered a distinct advantage of ST to capture gene expression of mature fat laden cells (mature adipocytes, sebocytes). This analysis also revealed that immune cells are enriched in the perifollicular and perivascular niches in healthy skin, and that this focal positioning is significantly altered in inflamed skin. PsO lesions have striking changes in immune cell spatial distribution, spanning from a predominantly perifollicular location in healthy skin to a superficial to mid-dermal localization in psoriatic skin. Thus, in addition to the compositional changes in immune populations reported in active PsO lesions, their positions may provide much needed insights into their regulation and function (13, 14, 32). For instance, we find that homeostatic effector T cells (Th/Tc) and Tregs cluster with antigen-presenting cells in the perifollicular region around the vasculature. The follicle is home to commensal microbes and a portal for pathogen and allergen entry as well as to richly express chemokines and immune activation and survival factors (33). Indeed, murine studies have revealed that homeostatic immune surveillance is dependent on follicle-dwelling commensals (34, 35). Understanding the specific commensal bacterial populations in healthy and diseased human skin and how they may influence lymphocyte function are avenues of active investigation.
Loss of cutaneous immune tolerance has long been thought to contribute to psoriasis initiation and perpetuation (36). Thus, in addition to the influx of inflammatory cells, disturbances in homeostatic immune surveillance and tolerogenic mechanisms around the follicular niche may also be key instigators of lesion formation. (37, 38). Consistent with this notion, global transcriptomics analysis revealed that mild PsO lesions were enriched in immune regulatory signatures when compared to severe disease (1). Clinically, psoriatic lesions on the scalp, the body’s most follicle-dense skin, are known to be more treatment refractory and have a higher likelihood of systemic involvement (39-42). Moreover, hair follicle dropout along with down-sizing and sebaceous gland atrophy are observed in both scalp and non-scalp psoriasis (43-45). Mechanisms underlying the strategic positioning and ratio of effector/regulatory T cells around the follicular epithelium that contribute to (or protect from) skin inflammation warrant further investigation.
Applying ST to active psoriatic lesions and biopsies from uninvolved, non-lesional skin allowed us to uncover distortions of cellular organization and cell-cell communication at the tissue-scale. In so doing, we illustrated the value of this platform for placing publicly available skin single-cell transcriptional atlases into their native tissue context. Our results identified two epidermal and one dermal spatial cluster as significantly enriched (and in some cases uniquely expressed) in lesional skin cross sections. These clusters expressed genes that were involved in inflammatory (e.g. Th17 activation) and epidermal proliferative and differentiation pathways, that are also seen during epidermal repair (19, 46). Indeed, PsO has long been thought of as a pathology of wound overhealing. PsO-enriched clusters were represented in all samples, and thus likely represent “core signatures” of disease, irrespective of severity or presence of comorbidities. Previous work reported a paradoxical increase in IL-17A expression in samples from participants with mild disease compared to severe disease (1). Nevertheless, IL-17 blockade has been clinically effective against both mild and severe disease, validating the established notion that mechanisms that drive the entire spectrum of disease severity converge along the Type 17 immune axis (1, 25).
The preponderance of CD20+ B cells in lesional skin as demonstrated by SpaceFold and confirmed by IHC is also noteworthy. Varying ratios of B cells have been found in both the peripheral blood and lesional skin of patients with psoriasis and correlate with presence of arthritis and cutaneous disease severity (49-51). More recently, depletion of IL-10-producing regulatory B cells (Bregs) has also been proposed as a trigger for activation of the IL-23/Th17 axis (52, 53). An enrichment of Breg progenitors and concomitant loss of mature IL-10-producing Bregs was observed in patients with psoriasis, compared to healthy individuals, and this imbalance was reversed by biologic therapy (54). Thus, the B cell population detected in our study may include both functionally-immature Breg progenitors and mature B cells with the capacity for antigen presentation and cytokine production.
We homed in on discrete location-specific changes that distinguish the mild and severe forms of disease. Using unsupervised transcriptome clustering, we uncovered that stratification occurs according to cutaneous clinical severity and not by synovioentheseal involvement, a distinction that was also reflected in the clinically-uninvolved, non-lesional samples. Dermal macrophages, fibroblasts, and lymphatic rich regions were all key distinguishing features of psoriasis severity. In particular, molecular cartography revealed a specific accumulation of fibroblasts in the upper dermis of mild psoriasis (Fig 7, A and B). Given the known role of heterogenous fibroblast subsets in both activating and suppressing the local immune response, understanding if and how upper-dermal fibroblasts are functionally contributing to or restraining disease may provide insights into mechanisms underlying psoriasis and related skin inflammatory conditions (47). Examination of distal uninvolved skin similarly revealed high interferon signatures in patients with cutaneous lupus (48). Whether other organs or tissues beyond the skin are also impacted by inflammatory skin disease while maintaining their normal function, or if signatures of skin inflammation are uniquely observed in distal skin, remains to be explored. It is conceivable that an accumulation of such inflammatory and metabolic conditioning in unaffected tissues could promote systemic co-morbidities over time (55).
Notably, DGAT2 and FGFR3 protiens were enriched in the distal skin of clinically uninvolved PASIhigh samples compared to all other groups. DGAT2, diacylglycerol O-acyltransferase 2, is an enzyme that catalyzes the synthesis of triglycerides. Dgat2-deficient animals display perinatal lethality due to defects in barrier function, underscoring the contribution of this enzyme in maintaining the epidermal lipid barrier (56). However, we find that this enzyme is strikingly enriched in PASIhigh non-lesional skin compared to both lesional and healthy skin, suggesting a metabolic rewiring of distal skin and potential enrichment in production of triglycerides. Indeed, murine studies of psoriatic-like inflammation have revealed altered cholesterol metabolism and entrapment in distal skin (57), prompting a broader assessment of other lipid alterations in uninvolved skin. If and how such alterations occur in human disease and whether they contribute to the development of systemic co-morbidities (e.g. cardiovascular disease) awaits further investigation.
FGFR3, a member of the FGFR family, is involved in various homeostatic processes including keratinocyte proliferation, differentiation, and apoptosis (58-60). Single nucleotide polymorphisms in the FGFR3 gene locus have been identified in seborrheic keratoses, epidermal nevi, and acanthosis nigricans (61-64), with established associations with insulin resistance and obesity (65, 66). While FGFR1/2 mutations have been implicated in the development of the psoriatic phenotype by inducing abnormalities in tight junction protein expression and distribution (67), the involvement of FGFR3 within this framework, if any, has not been clarified. It is possible that upregulation of FGFR3 in our non-lesional skin samples serves as a regulatory mechanism employed by the tissue to reduce keratinocyte proliferation and/or increased apoptosis that preclude development of lesions of psoriasis (60). Thus, upregulation of DGAT2 and FGFR3 may signal the development of systemic disease, portend an imminently lesional (“pre-lesional”) state and/or, paradoxically, protect against overt lesional development. Indeed, baseline gene expression in nonlesional skin was found to be a stronger predictor of anti-TNF therapy responses when compared to lesional skin signatures (68). Thus, the spatial profiling of non-lesional skin in relation to therapeutic responses may provide important insights into disease pathogenesis and potentially aid in patient stratification in the clinic when deciding upon various available agents.
Our study is limited by the currently available resolution of the spatial transcriptomics technology, which captures gene expression at 50-micron increments. Nevertheless, we integrated scRNA-seq data sets to gain higher cellular resolution and spatial context. As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states. Another key consideration is the depth of sequencing and the dropout rate of genes. We have therefore avoided “absence of evidence” arguments and only focused on positive enrichment of genes to draw conclusions. It is also possible that rare cell types or very lowly-expressed genes were not captured by our analysis. SpaceFold makes two assumptions about the tissue structure: the tissue should have stereotypical structures, and the cell type fraction of each spot is indicative of this cell type’s physical coordinate, as it relates to the originating spot. Thus, the coordinate of spots within the heterogeneous tissue regions may not correspond to the physical cell type location and hence is not directly interpretable. However, the relative coordinates between regions of different cell type distributions are still comparable with the use of tissue cellular landmarks (e.g., Langerhans cells and melanocytes to discern the basal epidermal layer and basement membrane) to draw conclusions. Finally, some spatial clusters (0, 1, 2, 3, 8, 15, and 16) trended toward enrichment in non-lesional skin when compared to lesional samples (fig. S8C); however, sample-to-sample variation observed in these clusters and the low sample size of analysis rendered these differences statistically insignificant.
Collectively, our findings underscore the value of spatially-resolved gene profiling in understanding emergent cellular ecosystems underlying health and disease. Thus, in addition to unearthing previously undescribed disease features, our data will serve as an invaluable resource for the research community.
MATERIALS AND METHODS
Study design
The goal of this study was to unbiasedly characterize and agnostically map the cellular niches and molecular pathways driving psoriatic disease pathology and severity. We thus obtained skin punch biopsy samples were from patients with skin-limited psoriasis (PsO) and psoriatic arthritis (PsA), and from healthy volunteers. We started by evaluating the spatial landscape of healthy skin and verified the utility of spatial transcriptomics (ST) in faithfully defining cellular microenvrionments of the skin using immunoflourence and by integrating publically avaible single cell data with our ST data. Next we analysed our pooled PsO lesonal and non lesional samples along with healthy skin to delinate disease associated changes in cellular ecosystems. We used SpaceFold-based tissue cartography to determine changes in the relative coordinates of cell types under different conditions. Finally, we performed unsupervised clustering of our samples to discern if and how presence of systemic disease or skin disease severity are captured in our ST data.
Skin sampling and cryopreservation
Four mm punch biopsies of skin from healthy controls (3 donors, 2 of whom provided 2 distinct skin site samples, n=5 biopsies) and paired lesional (11 donors, 9 of whom provided non-lesional skin samples, n=14 biopsies) and non-lesional (9 donors, n=9 biopsies) skin from patients with psoriatic disease were performed under protocols approved by the Institutional Review Board of NYU Langone Health (study IRB numbers S20-01167 and S12-00831). Duplicate runs on the spatial transcriptomics platform were performed on 2 samples from 2 unique healthy control donors and 3 samples from 3 unique lesional skin donors. Replicate samples are labeled with an “R”. Tissue samples were embedded as previously described (19). Briefly, within 30 minutes of biopsy, specimens were rinsed with phosphate buffered saline (PBS), embedded in optimal cutting temperature (OCT) medium (Tissue-Tek®), and snap frozen in liquid nitrogen-cooled isopentane. Samples were stored in −80°C prior to use in the spatial transcriptomics workflow.
Spatial transcriptomics (ST)
Optimization of conditions for human skin ST:
Serial sections were placed onto capture areas on the Visium Spatial Tissue Optimization slide (Cat# 1000193, 10X Genomics). Per the manufacturer’s protocol, the sections were fixed, stained, and subsequently permeabilized over different time points. Optimal permeabilization time, marked by a yellow dashed rectangle of 4-6 minutes and 10 μm tissue thickness, were selected based signal-to-noise ratio of fluorescent cDNA footprint in Visium optimization slides (fig. S1, B and C).
Sample Processing:
10 μm-thick cryosections were mounted onto the ST array slides (Cat# 1000184, 10X Genomics). Tissue sections were fixed in methanol at −20°C and then stained with hematoxylin and eosin. Brightfield images were taken on a Leica AT2 wide slide scanner at 20X resolution to be used downstream for gene mapping. The slides were inserted into cassettes that ensured leakproof wells for adding reagents. Tissues were permeabilized with Permeabilization Enzyme (Cat# 2000214, 10X Genomics) for 5 minutes at 37°C as established by the tissue optimization protocol. RT Master Mix (10X Genomics) was added to the permeabilized tissue sections and incubated for 45 minutes at 53°C. Spatially barcoded full-length cDNA from poly-adenylated mRNA were generated followed by second strand synthesis at 65°C for 15 minutes. The cDNA from each capture area was denatured and transferred to a corresponding tube for amplification and library construction. Samples were sequenced on Illumina NovaSeq 6000.
Sequence alignment and annotation:
Sequencing output and the histology images were processed using Space Ranger software (10x Genomics). The Space Ranger mkfastq function was used for sample demultiplexing and to convert spatial barcodes and reads into FASTQ format. Space Ranger count function was used to align reads and calculate counts based on the human reference genome (GRCh38, GENCODE v32 / Ensembl 98) and then align microscopic slide images and transcriptomes to generate barcode/UMI counts and feature spot matrices.
Data analysis:
A graphical representation of the data and the data analysis pipeline incorporating figure information alongside analysis performed can be seen in supplementary figure 14. To account for differences in tissue heterogeneity and individual heterogeneity, each technical and biological replicate or each capture area on the 4-sample 10X Visium slide was treated as an individual batch. The Space Ranger output was further processed using the Seurat package in R programming environment. Scatter plots were generated to ascertain data quality for each sample.
Quality Control and Sample Filtering:
Sequencing results were plotted on R2 plots to evaluate concordance between Unique Molecular Identifiers (UMIs) from library prep and detected features (genes) (fig. S2A and fig. S8A). In each sample, spots with low depth of coverage, less than 200 features/spot, were filtered out (fig. S2B).
Anchor-based integration (Figs. 1 and 2 and figs. S2, C and D, S7):
Healthy skin samples in figures 1 and 2 were batch corrected using anchor integration workflow as described in the Seurat package vignette. The full ST dataset consisting of psoriatic lesional, psoriatic non-lesional, and healthy skin (fig. S7) were also processed with anchor-based integration. Following quality control and sample filtering, ST samples were normalized independently using variance stabilizing transformation by running SCTransform function in Seurat package with default parameters as described in the vignette, setting parameter variable.features.n to 3000, i.e. the top 3000 most variable genes ranked by residual variance were used for downstream integration and cluster analysis. Top 20 PCs with resolution of 0.6 were used to identify spatial clusters by running the FindNeighbours and FindClusters functions. The clusters were manually annotated based on cluster-specific marker genes identified using the FindAllMarkers function with default parameters including statistical test set to Wilcox rank sum test, minimum cell percentage set to 0.25, and Log2Fold change cutoff set to 0.25. Heatmap plots were generated using DoHeatmap, showing top 8 marker genes per cluster (fig. S2, S7).
Harmony-based integration (Fig. 3 and figs. S3 and S5 and S6):
Healthy skin samples were batch-corrected using Harmony-based integration (fig S3). The full ST dataset consisting of psoriatic lesional, psoriatic non-lesional, and healthy skin (Fig. 3 and fig. S5) was also integrated with Harmony. Both groups of data were first merged into a single Seurat object using merge function and were normalized using the SCTransform function using default parameters, setting parameter variable.features.n to 3000, i.e. using the top 3000 most variable genes ranked by residual variance. PCA was performed on the normalized data as needed before running Harmony batch correction. Harmony batch correction was performed by running the RunHarmony Function from the Harmony package using default parameters with assay set to SCT counts and group variable set to sample ID column in the metadata. The downstream analysis of batch-corrected data was performed using Seurat and the top 40 dimensions from Harmony embeddings were used for UMAP-based dimensionality reduction and graph-based clustering with the resolution set to 0.35. The clusters were then manually annotated using cluster-specific marker genes after running the FindAllMarkers function using default parameters including a Wilcox rank sum statistical test, minimum cell percentage set to 25%, and Log2Fold change cutoff set to 0.25. Heatmaps were generated using DoHeatmap, showing top 10 marker genes per cluster (fig. S6).
Integration with publicly available single cell RNA seq data (Fig.1E, figs. S4, A and B, S9, A to C):
Healthy skin ST data was integrated with scRNA-seq data taken from scRNA dataset 1 (GEO: GSE150672), scRNA data set 2 (ENA - ERP116319), and Hildreth at el. (GEO: GSE155960 and GSE156110). For scRNA-seq data, we first selected and made a subset to only include cells from healthy skin samples (Fig. S4 – A, B). The first integration was performed using anchor-based integration in Seurat package to obtain spot-based enrichment of each reference cell type using prediction scores calculated for every spot. These values were then plotted using SpatialFeaturePlot function (Fig. 1E). The full skin ST data was integrated with scRNA-seq data taken from scRNA data set 1 (GEO: GSE150672) and scRNA data set 2 (ENA - ERP116319)). For disease-related samples, subseted cells scRNA-seq data only from the psoriatic skin disease group (fig. S9 – B, C) were used for integration. Integration was performed using the same methods as described for healthy skin data.
Multimodal Integration Analysis (MIA) (Figs. 1, F to I, 2, A and B, 5, A to C and fig. S9, D to E):
MIA uses a hypergeometric test to assess overlap in marker genes between each ST cluster and scRNA-seq cluster (18). The top 300 upregulated marker genes with adjusted p-value less than or equal to 0.05 and log-fold-change greater than 0.25 were used to calculate MIA enrichment scores. Pathway analysis was performed using the ClusterProfiler and EnrichR packages and results were filtered by false discovery rate (FDR)/p-adjusted value less than 0.1. P-adjusted values were computed using Benjamini-Hochberg method of correction for multiple hypothesis testing. For tissue resident memory T cells (TRM) data, we obtained relevant TRM genes from Jaiswal et al. (28). In the original study, the genes were provided in four lists which includes TRM exclusive, signature, upregulated, and highly expressed genes. The unique genes from these four lists were selected and then used in the hypergeometric test for MIA. Cluster 0 (mixed) in healthy ST samples and cluster 6 (mixed) in full ST skin data had no marker genes that passed the cutoff for MIA and hence were excluded from MIA (Fig.1, G to I, 2, A and B).
xCell (Fig. 2E):
xCell (21) R package was used to generate enrichment scores for reference immune and stromal cell types across clusters.
NicheNet Ligand-Receptor Analysis (Fig. 4, E to G and data files S1 and S2):
Ligand receptor (LR) analysis was performed using NicheNet (27) with epidermis clusters 4 and 7 and inflamed dermis cluster 10 from Harmony-integrated ST data set in Figure 3 as receptors/niches of interest . Clusters 3, 4, 7, and 10 were selected as ligands. NicheNet default parameters of LogFC 0.15 and ≤0.1 p adjusted values for receiver/receptor genes were used. This analysis yielded 117,360 total ligand-receptor pairs (LR) (data file S1). Ligand-receptor pairs were ranked based on prioritization scores, a weighed sum of the scaled scores of properties of interest such as differential expression scores, scaled expression, and expression fraction for ligand and receptor, plus prior knowledge quality of the LR interaction, and expression of downstream genes. “Bona fide” LR interactions (labeled “true” in data file S1) were marked by NicheNet based on their inclusion in validated databases. The top 50 bona-fide LR pairs for each ligand were ranked according to prioritization scores (data file S2) and further cross-referenced for biological relevance and known cognate ligand-receptor interactions with CellTalkDB, a comprehensive, manually-curated database that combines information from SoptSC, CellPhoneDB, and SingleCellSignalR, and other databases. The LR pairs validated by both NicheNet and CellTalkDB were selected for plotting in Figure 4.
BayesPrism and SpaceFold (Figs. 5D, 7, A and B):
BayesPrism-(29) based cell type deconvolution was performed on the psoriatic lesional skin, psoriatic non-lesional skin, and healthy skin ST samples independently. Visium ST data and single cell RNA-seq data from data set 1 (GEO: GSE150672) was split into healthy and psoriatic skin for respective analysis. The reference single cell data was filtered to remove all genes on the X and Y chromosomes, mitochondrial genes, and ribosomal (RB) protein-related genes. Lowly expressed genes which were expressed in less than 5 cells were also removed. The labels for cell types were taken from the processed scRNA meta-data table using the CellType column from Hughes et al. The labels for cell states were taken from the Specific_CellType column from the same source. Cell types with fewer than 16 cells in healthy and 20 cells from the psoriatic skin were removed to avoid unstable inference. To improve the signal-to-noise ratio for deconvolution, we searched for signature genes for each cell type by performing differential analysis between cell states from different cell types using pairwise one-sided t-test for log transformed normalized count using the findMarker function from the scran package (69). We then selected genes that were expressed at a significantly higher level in at least one cell state by requiring the maximum p value to be less than 0.05, and the minimum log2 fold change to be above 0.1. This generated a total of 6,031 genes for the psoriatic skin dataset and 5,874 genes for the healthy skin dataset. The cell type fraction matrix produced by BayesPrism for each ST spot was then used as the input for SpaceFold pipeline (29, 30) which uses PHATE (70), a non-linear dimensionality reduction method to reduce the cell-type fractions vector to a 1-dimensional (1D) pseudo-space axis for each spot. SpaceFold axis for spots containing the top 10 % quantile of each cell type was visualized using a beeswarm plot. To define location of the basement membrane within each condition while also accounting for differences in different tissue states (e.g., epidermal thickness), we use cell types that reside in the basal epidermis as cellular landmarks along the space fold axis. Specifically, the location with the highest density of melanocytes and Langerhans cells was demarcated with a dashed line to note proximity to the basement membrane on each beeswarm plot.
Pseudo-bulk analysis (Figs. 6, A to C, 8, A and B and fig. S11):
Pseudo-bulk analysis of each ST sample was performed separately. Technical pseudobulked replicates were then combined prior to downstream analysis to ensure that each sample represented 1 biological individual. Pseudo-bulk raw counts for sample-specific and cluster-specific comparisons were generated using the PseudobulkExpression function from Seurat. Downstream analysis was performed using DESeq2 package in R, where replicates were merged using collapseReplicates function. The counts matrix was then transformed to generated variance stabilized data (vsd) counts using vst function that provides a variance stabilizing transformation. A heatmap of sample distances with hierarchical clustering was generated following that after calculating a distance matrix using dist function on the vsd assay with the final visualization generated using pheatmap package in R (Fig. 6A). A dendrogram-based visualization of the hierarchical clustering (fig. S10A) was also generated in R. Principal component analysis was also performed using the variance stabilized counts to compare sample similarity and dissimilarity with plotPCA function. The analysis was split into multiple groups including: All samples (PsO + healthy), PsO only, and cluster-specific (Fig. 6 A, B and fig. S11A). For cluster specific PCA analysis (fig. S11), we set nsub parameter to 100 genes for the vst function
Multiplex Immunoflourence Staining
Four-micron paraffin-embedded sections were stained either with hematoxylin and eosin or with Akoya Biosciences® Opal™ multiplex automation kit reagents (Leica Cat #ARD1001EA) on a Leica BondRX® autostainer, according to manufacturers’ instructions. Briefly, all slides underwent sequential staining cycles with Leica Biosystems epitope retrieval 2 solution (ER2, pH9, Cat. AR9640), followed by primary and HRP-linked secondary Mouse+Rabbit polymer incubation (Akoya ARH1001EA) and tyramide signal amplification (TSA) with Akoya Opal fluorophores. Figure 2F panel antibodies: CD8, Clone C8/144B;CD4, Clone EPR6855; Foxp3 clone 236A/E7; HLA-DR, Clone CR3/43; CD31, Clone JC/70A. Figure 8F panel antibodies: FGFR3, Clone B-9; DGAR2, Polyclonal, CK5, Clone EP1601Y. Sections were counter-stained with DAPI and mounted with Prolong Gold Antifade Reagent (Invitrogen P36930). Semi-automated image acquisition was performed on a Vectra® Polaris multispectral imaging system. After whole slide scanning at 20X the tissue was manually outlined to select fields for spectral unmixing using InForm® version 2.4.11 software from Akoya Biosciences.
Statistical analysis
Differential gene expression analysis were performed using Wilcox rank sum test. MIA method uses a hypergeometric test to assess overlap in marker genes between each ST cluster and scRNA-seq cluster. The marker genes used in MIA method were filtered using adjusted p-value less than or equal to 0.05, computed using bonferroni correction. Pathway analysis results were filtered using false discovery rate (FDR)/p-adjusted value less than 0.1. P-adjusted values were computed using Benjamini-Hochberg method of correction for multiple hypothesis testing.
Supplementary Material
Acknowledgements:
We thank R. Bonneau, S. Maniatis, I. Yanai, A. Rao, R. Moncada, A. Daly and H. Phatnani, A. Hettinghouse, and Scher/Naik Lab members for their helpful discussions, advice, and/or critical reading of this manuscript. The following core facilities enabled our study: NYULMC High Performance Computing, Flow Cytometry, Genome Technology Center, Experimental Pathology Core, the Microscopy Laboratory, Center for Biospecimen Research and Development, and Applied Bioinformatics Laboratories.
Funding:
This work was supported by funds from the Cancer Center Support Grant P30CA016087 at the Laura and Isaac Perlmutter Cancer Center and Shared Instrumentation Grant S10 OD021747 (core facility subsidies), National Psoriasis Foundation Psoriatic Disease Research Fellowship (R.L.C.), National Psoriasis Foundation Early Career Research Grant (P.K.), National Psoriasis Foundation Diagnostic Challenge Grant (J.U.S.), Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Pilot Research, Grant in Rheumatology/Dermatology (R.L.C., D.Y.), Rheumatology Research Foundation Scientist Development Award (R.H.H.), Pew Stewart Scholar Award grant 00034119 (S.N.) , National Institutes of Health grant AR 069515 (J.U.S.; R.L.C. trainee), National Institutes of Health grant UL1TR001445 (R.L.C.), National Institutes of Health grant 1DP2AR079173-0 (S.N.), National Institutes of Health grant R01-AI168462 (S.N.), National Institutes of Health grant UC2AR081029 (J.U.S., S.N., J.G.), Translational Immunology Center (TrIC) at NYU Langone Health (S.N., J.U.S.) , New York Stem Cell Foundation Robertson Stem Cell Investigator award (S.N.), Packard Fellowship in Science and Engineering (S.N.), NYU Judith and Stewart Colton Center for Autoimmunity (J.U.S., S.N.), The Beatrice Snyder Foundation (J.U.S.), The Riley Family Foundation (J.U.S.)
Footnotes
Competing interests: S.N. is on the SAB of Seed Inc. and a consultant for BiomX and receives funding from Takeda Pharmaceuticals. A.L.N. served as a consultant for Janssen, UCB, AbbVie, BMS. J.C. receives funding from Regeneron, Sanofi, and Genentech. J.U.S. served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and BMS and receives research funding from Janssen and Pfizer. The remaining authors have no competing interests to disclose.
Data and materials availability: Genomic data is publicly available at GEO under the accession number GSE202011. Code used for data analysis of single cell and spatial RNA sequencing is deposited in Zenodo: https://zenodo.org/record/7813973. RDS files and other processed data is also deposited in Zenodo: https://zenodo.org/record/7562864. All other data needed to support the conclusions in this paper can be found in the paper or the Supplementary Materials.
References
- 1.Kim J et al. , The Spectrum of Mild to Severe Psoriasis Vulgaris Is Defined by a Common Activation of IL-17 Pathway Genes, but with Key Differences in Immune Regulatory Genes. Journal of Investigative Dermatology 136, 2173–2182 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Coates LC, FitzGerald O, Helliwell PS, Paul C, Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin Arthritis Rheum 46, 291–304 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Husni ME, Merola JF, Davin S, The psychosocial burden of psoriatic arthritis. Seminars in Arthritis and Rheumatism 47, 351–360 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK, Prevalence of the Metabolic Syndrome in Psoriasis: Results From the National Health and Nutrition Examination Survey, 2003-2006. Archives of Dermatology 147, 419–424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husted JA et al. , Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken) 63, 1729–1735 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Gelfand JM et al. , Risk of myocardial infarction in patients with psoriasis. Jama 296, 1735–1741 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Neimann AL et al. , Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55, 829–835 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Tey HL et al. , Risk factors associated with having psoriatic arthritis in patients with cutaneous psoriasis. J Dermatol 37, 426–430 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM et al. , Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 53, 573 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Haroon M, Kirby B, FitzGerald O, High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 72, 736–740 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Eder L et al. , The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis Rheumatol 68, 915–923 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Scher JU, Ogdie A, Merola JF, Ritchlin C, Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nature Reviews Rheumatology 15, 153–166 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Hughes TK et al. , Second-Strand Synthesis-Based Massively Parallel scRNA-Seq Reveals Cellular States and Molecular Features of Human Inflammatory Skin Pathologies. Immunity 53, 878–894.e877 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds G et al. , Developmental cell programs are co-opted in inflammatory skin disease. Science 371, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J et al. , Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8(+) T cells in autoimmunity and cancer. J Allergy Clin Immunol 147, 2370–2380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ståhl PL et al. , Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Stuart T et al. , Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e1821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada R et al. , Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat Biotechnol 38, 333–342 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Konieczny P et al. , Interleukin-17 governs hypoxic adaptation of injured epithelium. Science 377, eabg9302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildreth AD et al. , Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nature Immunology 22, 639–653 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aran D, Hu Z, Butte AJ, xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biology 18, 220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korsunsky I et al. , Fast, sensitive and accurate integration of single-cell data with Harmony. Nature Methods 16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston A et al. , IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol 140, 109–120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y, Fleming C, Yan J, New insights of T cells in the pathogenesis of psoriasis. Cellular & Molecular Immunology 9, 302–309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi X et al. , IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J Invest Dermatol 131, 2401–2408 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Paller AS et al. , An IL-17-dominant immune profile is shared across the major orphan forms of ichthyosis. J Allergy Clin Immunol 139, 152–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browaeys R, Saelens W, Saeys Y, NicheNet: modeling intercellular communication by linking ligands to target genes. Nature Methods 17, 159–162 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Jaiswal A et al. , An activation to memory differentiation trajectory of tumor-infiltrating lymphocytes informs metastatic melanoma outcomes. Cancer Cell 40, 524–544.e525 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu T, Wang Z, Pe’er D, Danko CG, Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nature Cancer 3, 505–517 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niec RE et al. , A lymphatic-stem cell interactome regulates intestinal stem cell activity. bioRxiv, 2022.2001.2029.478341 (2022). [Google Scholar]
- 31.Feldman SR, A quantitative definition of severe psoriasis for use in clinical trials. Journal of Dermatological Treatment 15, 27–29 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Li B et al. , Transcriptome Analysis of Psoriasis in a Large Case–Control Sample: RNA-Seq Provides Insights into Disease Mechanisms. Journal of Investigative Dermatology 134, 1828–1838 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naik S, One Size Does Not Fit All: Diversifying Immune Function in the Skin. The Journal of Immunology 208, 227–234 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik S et al. , Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naik S et al. , Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nussbaum L, Chen YL, Ogg GS, Role of regulatory T cells in psoriasis pathogenesis and treatment. British Journal of Dermatology 184, 14–24 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, Naik S, Nagao K, Choreographing Immunity in the Skin Epithelial Barrier. Immunity 50, 552–565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabashima K, Honda T, Ginhoux F, Egawa G, The immunological anatomy of the skin. Nat Rev Immunol 19, 19–30 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Wilson FC et al. , Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum 61, 233–239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlica L, Perić-Hajzler Z, Jovelić A, Sekler B, Damjanović M, Psoriatic arthritis: a retrospective study of 162 patients. Vojnosanit Pregl 62, 613–620 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Hjuler KF et al. , Localization of treatment-resistant areas in patients with psoriasis on biologics. Br J Dermatol 181, 332–337 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Larko O, Problem sites: scalp, palm and sole, and nail. Dermatol Clin 13, 771–777 (1995). [PubMed] [Google Scholar]
- 43.Headington JT et al. , A morphometric and histologic study of the scalp in psoriasis. Paradoxical sebaceous gland atrophy and decreased hair shaft diameters without alopecia. Arch Dermatol 125, 639–642 (1989). [PubMed] [Google Scholar]
- 44.Rittié L et al. , Sebaceous Gland Atrophy in Psoriasis: An Explanation for Psoriatic Alopecia? J Invest Dermatol 136, 1792–1800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George SMC, Taylor MR, Farrant PBJ, Psoriatic alopecia. Clinical and Experimental Dermatology 40, 717–721 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Guenin-Mace L, Konieczny P, Naik S, Immune-Epithelial Cross Talk in Regeneration and Repair. Annu Rev Immunol, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson S et al. , Fibroblasts as immune regulators in infection, inflammation and cancer. Nature Reviews Immunology 21, 704–717 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Billi AC et al. , Nonlesional lupus skin contributes to inflammatory education of myeloid cells and primes for cutaneous inflammation. Sci Transl Med 14, eabn2263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Ding Y, Yi X, Zheng J, CD19+ B cell subsets in the peripheral blood and skin lesions of psoriasis patients and their correlations with disease severity. Braz J Med Biol Res 49, e5374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoud F et al. , Elevated B-lymphocyte levels in lesional tissue of non-arthritic psoriasis. J Dermatol 26, 428–433 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Niu J et al. , Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol 29, 1791–1796 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Fetter T, Niebel D, Braegelmann C, Wenzel J, Skin-Associated B Cells in the Pathogenesis of Cutaneous Autoimmune Diseases-Implications for Therapeutic Approaches. Cells 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizumaki K, Horii M, Kano M, Komuro A, Matsushita T, Suppression of IL-23-mediated psoriasis-like inflammation by regulatory B cells. Sci Rep 11, 2106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi M et al. , IL-10-producing regulatory B cells are decreased in patients with psoriasis. J Dermatol Sci 81, 93–100 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Naik S, Fuchs E, Inflammatory memory and tissue adaptation in sickness and in health. Nature 607, 249–255 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone SJ et al. , Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 279, 11767–11776 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Huang LH et al. , Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis. Cell Metab 29, 475–487.e477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.L'Hôte CG, Knowles MA, Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res 304, 417–431 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Johnson DE, Williams LT, in Advances in Cancer Research, Vande Woude GF, Klein G, Eds. (Academic Press, 1992), vol. 60, pp. 1–41. [Google Scholar]
- 60.Hafner C et al. , FGFR3 mutation affects cell growth, apoptosis and attachment in keratinocytes. Exp Cell Res 316, 2008–2016 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Li L, Zhang S, Li H, Chou H, FGFR3 promotes the growth and malignancy of melanoma by influencing EMT and the phosphorylation of ERK, AKT, and EGFR. BMC Cancer 19, 963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hafner C et al. , FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Modern Pathology 20, 895–903 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Hernández S et al. , Fibroblast growth factor receptor 3 mutations in epidermal nevi and associated low grade bladder tumors. J Invest Dermatol 127, 1664–1666 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Berk DR, Spector EB, Bayliss SJ, Familial Acanthosis Nigricans Due to K650T FGFR3 Mutation. Archives of Dermatology 143, 1153–1156 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Sadeghian G, Ziaie H, Amini M, Ali Nilfroushzadeh M, Evaluation of insulin resistance in obese women with and without acanthosis nigricans. J Dermatol 36, 209–212 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Sinha S, Schwartz RA, Juvenile acanthosis nigricans. J Am Acad Dermatol 57, 502–508 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Yang J et al. , Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol 188, 935–952 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsoi LC et al. , Cytokine responses in nonlesional psoriatic skin as clinical predictor to anti-TNF agents. Journal of Allergy and Clinical Immunology 149, 640–649.e645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lun AT, McCarthy DJ, Marioni JC, A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res 5, 2122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moon KR et al. , Visualizing structure and transitions in high-dimensional biological data. Nature Biotechnology 37, 1482–1492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.