Abstract
Background
Omadacycline is an aminomethylcycline antibiotic in the tetracycline class that was approved by the US FDA in 2018 for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. It is available in both IV and oral formulations. Omadacycline has broad-spectrum in vitro activity and clinical efficacy against infections caused by Gram-positive and Gram-negative pathogens. Omadacycline is being evaluated in a 3 month placebo-controlled Phase 2 clinical trial of oral omadacycline versus placebo in adults with non-tuberculous mycobacteria (NTM) pulmonary disease caused by Mycobacterium abscessus (NCT04922554).
Objectives
To determine if omadacycline has intracellular antimicrobial activity against NTM, bacteria that can cause chronic lung disease, in an ex vivo model of intracellular infection.
Methods
Two strains of M. abscessus were used to infect THP-1 macrophages. Intracellular M. abscessus was then challenged with omadacycline and control antibiotics at multiples of the MIC over time to evaluate intracellular killing.
Results
At 16 × the MIC at 72 h, omadacycline treatment of intracellular NTM yielded a log10 reduction in cfu of 1.1 (91.74% reduction in cfu) and 1.6 (97.65% reduction in cfu) consistent with killing observed with tigecycline, whereas amikacin and clarithromycin at 16 × the MIC did not show any reduction in cfu against the intracellular M. abscessus.
Conclusions
Omadacycline displayed intracellular activity against M. abscessus within macrophages. The activity was similar to that of tigecycline; as expected, intracellular killing was not observed with clarithromycin and amikacin.
Introduction
Non-tuberculous mycobacteria (NTM) are inherently antibiotic resistant, grow slowly, and have a complex intracellular lifestyle in the host, often evading the antibacterial effects of the phagolysosome by preventing maturation of the phagosome through neutralization of pH and prevention of host antimicrobial production.1,2 NTM are opportunistic pathogens, have known reservoirs in water systems of medical facilities, and often cause infections through injections of contaminated substances or through medical device implants.3Mycobacterium abscessus, one species of NTM, causes respiratory infections in patients with cystic fibrosis (CF), AIDS, COPD and other diseases in immunocompromised patients.4,5 Although NTM typically are not transmitted person-to-person, M. abscessus has been documented to spread between patients with CF.6
Omadacycline is a semi-synthetic derivative of tetracycline with indications of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.7 Omadacycline has activity against both Gram-positive and Gram-negative organisms; not only does it have a tetracycline-unique ribosomal interaction, but it also retains activity against the vast majority of clinically relevant tetracycline-resistance mechanisms.8
In vitro, omadacycline displays activity against M. abscessus in broth microdilution and time–kill kinetics assays.9,10 Additionally, tigecycline, has shown activity against NTM in an intracellular killing assay targeting NTM in macrophages, whereas amikacin and clarithromycin did not show any intracellular activity.11 This study sought to determine whether omadacycline maintained antibacterial activity against intracellular M. abscessus in a differentiated human macrophage cell line, THP-1.
Methods
Test compounds
Omadacycline was provided by Paratek Pharmaceuticals (Lot No. CA20-0964). Micromyx provided the following comparators: tigecycline (USP; R09410), amikacin (Sigma; 058k0803) and clarithromycin (USP; G2I235), which were handled following CLSI guidelines.12 Testing ranges for omadacycline, tigecycline and clarithromycin were 0.03–32 mg/L, whereas for amikacin it was 0.06–64 mg/L.
Test organisms
The test organisms evaluated were M. abscessus ATCC 19977 and MMX 9450, an isolate collected from a patient sputum sample in Indiana, USA in 2017. NTM were grown on Middlebrook 7H11 selective agar (Hardy Diagnostics; 137898, 14213) for 3–5 days at 30°C. They were then subcultured onto Middlebrook 7H11 non-selective agar (Hardy Diagnostics; 498795, 501472) and incubated for approximately 5 days at 30°C prior to use in the MIC assay. All test organisms were identified by a Bruker MALDI Biotyper (Bruker Daltonics).
Broth microdilution MIC assay
MIC values were determined in duplicate using a broth microdilution procedure described by CLSI (M24, M62 and M100).12–14 The test medium used was CAMHB (BD; 1242967). All drug stocks evaluated were within CLSI published QC ranges against Staphylococcus aureus ATCC 29213 and Mycobacterium peregrinum ATCC 700686.
Cell culture and intracellular antibacterial activity assay
Medium, conditions, treatment and recovery for intracellular killing assays were based on previous studies.11,15 THP-1 cells (ATCC-TIB-202; 70043382) were grown at 37°C with 5% CO2. To differentiate into macrophages, 1 mL of cell suspension (approximately 5 × 105 cells/mL) was added per well in Roswell Park Memorial Institute culture medium (RPMI) with 10% FBS, 1% pen/strep and 200 nM phorbol 12-myristate 13-acetate (PMA; Sigma; MKCL1143) in a 24-well tissue culture plate containing sterile glass coverslips on the bottom of each well and incubated for 48 h. Following incubation, PMA medium was replaced with RPMI with 10% FBS without antibiotics and the cells were incubated for 3 days. Following incubation, the medium was removed from the wells with adherent macrophage, and 1 mL of RPMI with 2% FBS containing 5–7 × 105 cfu/mL of a log-phase bacterial suspension at a multiplicity of infection (MOI) of 1:1 was added to the wells. Plates were then incubated for 6 h at 37°C with 5% CO2.
Medium was removed from each well prior to washing twice in prewarmed Dulbecco’s phosphate-buffered saline (DPBS; Sigma; RNK9608). After washing, 1 mL of 200 µg/mL amikacin suspended in RPMI with 2% FBS was added to each well, then plates were incubated for an additional 2 h at 37°C with 5% CO2 to kill extracellular bacteria. The medium was removed prior to washing twice in prewarmed PBS. After washing, 1 mL of 0×, 0.5×, 1×, 4× and 16× the MIC of four antibiotics (omadacycline, tigecycline, amikacin and clarithromycin) suspended in RPMI with 2% FBS was added to the wells. At 0, 24, 48 and 72 h, coverslips were removed, blotted onto sterile cloth, and added to 50 mL conical tubes with 5 mL 1% (v/v) Triton X-100 (Sigma; 033K0605) in saline with 5 to 10 sterile 3 mm glass beads. Conical tubes were vortexed on high for 1 min to lyse the cells and release intracellular bacteria. Cell suspensions were serially diluted 1:10, plated on trypticase soy agar with 5% sheep blood, and agar plates were incubated at 35°C for approximately 72 h to allow for enumeration of viable bacteria.
Statistical analysis
Changes in cfu/mL were compared by Student’s unpaired two-tailed t-test for time–kill assays. P values ≤0.05 were considered significant. All statistical analyses were performed using Microsoft Excel (Microsoft 365).
Results
Broth microdilution testing
The MIC values for omadacycline, tigecycline, amikacin and clarithromycin were 0.12, 0.12, 8 and 2 mg/L against M. abscessus ATCC 19977, respectively, and 0.5, 0.25, 8 and 2 mg/L against M. abscessus MMX 9450, respectively (Table S1, available as Supplementary data at JAC-AMR Online). Both strains were susceptible to amikacin and clarithromycin; there are no interpretive criteria established for omadacycline and tigecycline.13 These data were used to determine the drug exposures at multiples of the MIC described below.
Intracellular activity of antimicrobials
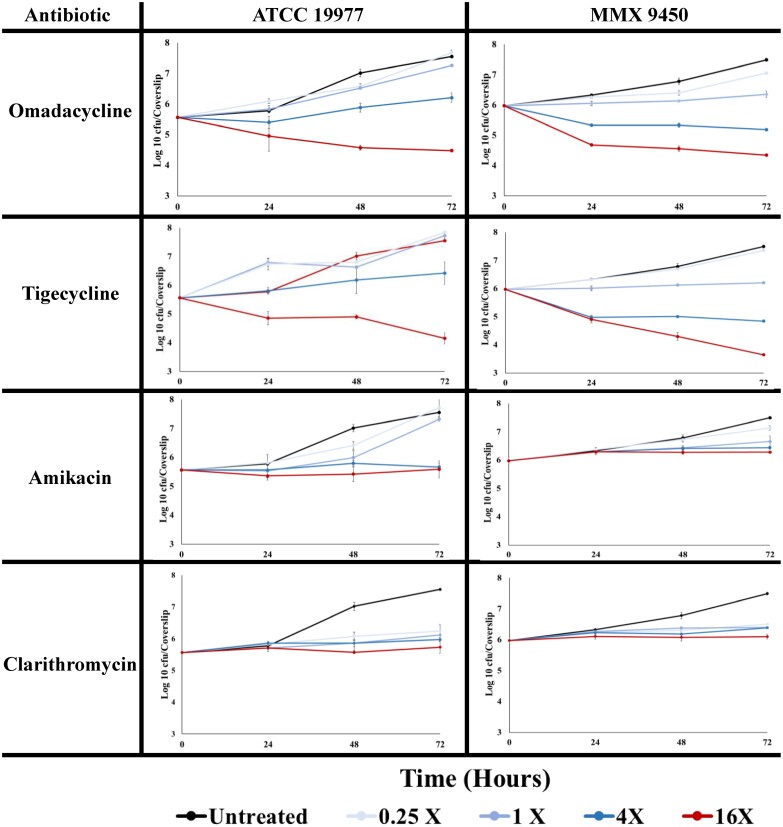
In this study, four antibiotics at four concentrations were evaluated against two M. abscessus strains in triplicate. The change in mean log10 cfu/coverslip and time–kill kinetics for omadacycline, tigecycline, amikacin and clarithromycin compared with untreated controls at 0 h against M. abscessus ATCC 19977 and MMX 9450 are shown in Table 1 and Figure 1, respectively. Omadacycline at 16 × MIC demonstrated intracellular killing activity at 48 and 72 h, with log reductions of 1.0 (P value <0.001) and 1.1 (P value <0.001), respectively, for ATCC 19977, and log reductions of 1.4 (P value = 0.003) and 1.6 (P value = 0.002), respectively, for MMX 9450. Tigecycline at 16 × MIC at 48 and 72 h had log reductions of 0.7 (P value <0.001) and 1.4 (P value <0.001), respectively, against ATCC 19977, and log reductions of 1.7 (P value = 0.003) and 2.3 (P value = 0.002) when testing against MMX 9450. At 72 h and 16 × the MIC value against ATCC 19977 and MMX 9450, the percent reduction by omadacycline was 91.74% and 97.65% against the two isolates, whereas tigecycline showed percent reduction of 96.12% and 99.53%. Amikacin and clarithromycin did not exhibit any intracellular effect on either strain infecting THP-l macrophages (Table 1 and Figure 1).
Table 1.
Change in cfu/coverslip of intracellular M. abscessus over time treated with omadacycline and comparators
| Organism | Multiple of the MIC | Δ log10 cfu/coverslip relative to untreated control at 0 h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Omadacycline | Tigecycline | Amikacin | Clarithromycin | ||||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| M. abscessus ATCC 19799 | 0.25× | 0.5 | 1.0 | 2.1 | 1.2 | 1.1 | 2.2 | 0.3 | 0.9 | 2.2 | 0.3 | 0.5 | 0.7 |
| 1× | 0.3 | 1.0 | 1.7 | 1.2 | 1.2 | 2.3 | 0.0 | 0.4 | 1.8 | 0.1 | 0.3 | 0.6 | |
| 4× | −0.2 | 0.3 | 0.6 | 0.2 | 0.6 | 0.9 | 0.0 | 0.2 | 0.1 | 0.3 | 0.3 | 0.4 | |
| 16× | −0.6 | −1.0 | −1.1 | −0.7 | −0.7 | −1.4 | −0.2 | −0.1 | 0.0 | 0.1 | 0.0 | 0.2 | |
| M. abscessus MMX 9450 | 0.25× | 0.3 | 0.4 | 1.1 | 0.3 | 0.7 | 1.4 | 0.4 | 0.8 | 1.2 | 0.3 | 0.3 | 0.5 |
| 1× | 0.1 | 0.2 | 0.4 | 0.0 | 0.2 | 0.2 | 0.3 | 0.5 | 0.7 | 0.3 | 0.4 | 0.4 | |
| 4× | −0.6 | −0.6 | −0.8 | −1.0 | −1.0 | −1.1 | 0.3 | 0.4 | 0.5 | 0.3 | 0.2 | 0.4 | |
| 16× | −1.3 | −1.4 | −1.6 | −1.1 | −1.7 | −2.3 | 0.3 | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | |
Bold text denotes ≥ 1-log killing. Mean Δlog10 determined by the change in cfu/mL versus the starting intracellular cfu (0 h timepoint) from sampling triplicate wells of each condition. The difference in mean log10 cfu/coverslip recovered for two intracellular NTM isolates treated with multiples of the MIC of omadacycline, tigecycline, amikacin and clarithromycin over time versus the starting intracellular cfu.
Figure 1.
Intracellular activity of antibiotics over time against two M. abscessus strains in THP-1 macrophages. Graphs show the mean log10 cfu/coverslip of intracellular M. abscessus recovered at each timepoint in each condition during the intracellular killing assay. Error bars represents the SD of three independent wells.
Discussion
There is a critical need for new antibiotics to treat diseases caused by M. abscessus, which is one of the most difficult-to-treat NTM species.9 Currently, few treatment options are available for M. abscessus MDR infections, and there is a lack of efficacy data against NTM in clinical trials, so new antibiotic therapeutics are urgently needed.5,16 However, most antibacterial studies for M. abscessus are conducted in in vitro models that do not account for the intracellular presence of M. abscessus. In this study, four antibiotics were evaluated in an ex vivo model of infection in a differentiated human macrophage cell line: omadacycline, tigecycline, amikacin and clarithromycin, for intracellular antimicrobial activity against two strains of M. abscessus.
Our data showed that both omadacycline (2 mg/L for ATCC 19977 and 8 mg/L for MMX 9450) and tigecycline (2 mg/L for ATCC 19977 and 4 mg/L for MMX 9450) at 16 × MIC have intracellular activity against each M. abscessus isolate tested, with the percent reduction for omadacycline ranging from 91.74% to 97.65% compared with tigecycline ranging from 96.12% to 99.53% at similar timepoints (Figure 1). The results support the findings of Nicklas et al.,17 who reported bactericidal activity of omadacycline against M. abscessus in a time–kill assay and in a mouse model of pulmonary infection. Conversely, amikacin and clarithromycin demonstrated no intracellular effect across the evaluated concentrations and timepoints. These results for amikacin and tigecycline are in agreement with the data of Molina-Torres et al.,11 who investigated the intracellular activity of amikacin, clarithromycin and tigecycline against M. abscessus in human macrophages.
In a Phase 1 pharmacokinetic study of healthy subjects administered the FDA-approved IV dose (100 mg twice on Day 1 followed by 100 mg once daily, the exposure of which matches the FDA-approved 300 mg oral dose), omadacycline was demonstrated to have a large volume of distribution and penetrated lung tissues, including epithelial lining fluid (ELF) and alveolar macrophages, with the observed steady-state concentration of omadacycline 25.79-fold higher in alveolar cells than in plasma, and 1.47-fold higher in ELF than in plasma.7,18 Based on the data presented here, the concentrations of omadacycline that demonstrated intracellular activity (2 and 8 mg/L, 16 × the MIC for each strain) are expected to be covered by the expected human alveolar cell concentration (∼11 ± 3.72 µg/mL at 24 h), suggesting that omadacycline would be efficacious against intracellular M. abscessus in lung tissues.18 Omadacycline may be better tolerated than tigecycline due to reduced nausea—2.4% versus 47.6%, respectively—and preferred over tigecycline as it is administered once daily and can be given intravenously or orally.7,18 Although no new antibiotics were added to the most recently published clinical practice guidelines for the treatment of NTM pulmonary disease in 2020, recent publications have recommended oral omadacycline as a preferred initial treatment for M. abscessus pulmonary infections.19,20 However, it remains to be seen if omadacycline treatment will lead to better outcomes compared with current regimens.20 Presently, a Phase 2 study evaluating oral omadacycline versus placebo in adults with NTM pulmonary disease caused by M. abscessus is underway (NCT04922554).
In conclusion, we found that omadacycline and tigecycline demonstrated similar intracellular activities against both M. abscessus isolates at 16 × the MIC, whereas amikacin and clarithromycin displayed no intracellular activity. Our results suggest that omadacycline is a potential new agent for the treatment of M. abscessus infection and further studies are warranted investigating the efficacy of this therapeutic in patients with NTM pulmonary disease.
Supplementary Material
Acknowledgements
We thank Olivia Walser for careful review of the manuscript.
Contributor Information
S Jahanbakhsh, Microbiologics Antibiotic and Microbiome Research Center, Kalamazoo, MI, USA.
J Howland, Microbiologics Antibiotic and Microbiome Research Center, Kalamazoo, MI, USA.
M O Ndayishimiye Uwineza, Microbiologics Antibiotic and Microbiome Research Center, Kalamazoo, MI, USA.
M T Thwaites, Microbiologics Antibiotic and Microbiome Research Center, Kalamazoo, MI, USA.
C M Pillar, Microbiologics Antibiotic and Microbiome Research Center, Kalamazoo, MI, USA.
A W Serio, Paratek Pharmaceuticals, Inc., King of Prussia, PA, USA.
D M Anastasiou, Paratek Pharmaceuticals, Inc., King of Prussia, PA, USA.
D A Hufnagel, Microbiologics Antibiotic and Microbiome Research Center, Kalamazoo, MI, USA.
Funding
This work was supported by Paratek Pharmaceuticals, Inc.
Transparency declarations
A.W.S. and D.M.A. are employees and shareholders of Paratek Pharmaceuticals, Inc. Microbiologics received financial support from Paratek Pharmaceuticals, Inc. in connection with the study and the development of this manuscript. S.J., J.H., M.O.N.U., M.T.T., D.A.H. and C.M.P. are employees of Microbiologics.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med 2013; 34: 110–23. 10.1055/s-0033-1333573 [DOI] [PubMed] [Google Scholar]
- 2. Shamaei M, Mirsaeidi M. Nontuberculous mycobacteria, macrophages, and host innate immune response. Infect Immun 2021; 89: e0081220. 10.1128/IAI.00812-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis 2001; 33: 1363–74. 10.1086/323126 [DOI] [PubMed] [Google Scholar]
- 4. Lee M-R, Sheng W-H, Hung C-Cet al. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 2015; 21: 1638–46. 10.3201/2109.141634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopeman RC, Harrison J, Desai Met al. Mycobacterium abscessus: environmental bacterium turned clinical nightmare. Microorganisms 2019; 7: 90. 10.3390/microorganisms7030090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryant JM, Grogono DM, Greaves Det al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013; 381: 1551–60. 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. FDA . NUZYRA (omadacycline). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209816_209817lbl.pdf
- 8. Karlowsky JA, Steenbergen J, Zhanel GG. Microbiology and preclinical review of omadacycline. Clin Infect Dis 2019; 69: S6–S15. 10.1093/cid/ciz395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bax HI, de Vogel CP, Mouton JWet al. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother 2019; 74: 2930–3. 10.1093/jac/dkz267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown-Elliott BA, Wallace RJ. In vitro susceptibility testing of omadacycline against nontuberculous mycobacteria. Antimicrob Agents Chemother 2021; 65: e01947-20. 10.1128/AAC.01947-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molina-Torres CA, Tamez-Peña L, Castro-Garza Jet al. Evaluation of the intracellular activity of drugs against Mycobacterium abscessus using a THP-1 macrophage model. J Microbiol Methods 2018; 148: 29–32. 10.1016/j.mimet.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 12. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Second Edition: M100. 2022. [Google Scholar]
- 13. CLSI . Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes—First Edition: M62. 2018. [Google Scholar]
- 14. CLSI . Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes—Third Edition: M24. 2018. [PubMed] [Google Scholar]
- 15. Cassidy SKB, Hagar JA, Kanneganti TDet al. Membrane damage during Listeria monocytogenes infection triggers a caspase-7 dependent cytoprotective response. PLoS Pathog 2012; 8: e1002628. 10.1371/journal.ppat.1002628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz M, Fisher S, Story-Roller Eet al. Activities of dual combinations of antibiotics against multidrug-resistant nontuberculous mycobacteria recovered from patients with cystic fibrosis. Microb Drug Resist 2018; 24: 1191–7. 10.1089/mdr.2017.0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicklas DA, Maggioncalda EC, Story-Roller Eet al. Potency of omadacycline against Mycobacteroides abscessus clinical isolates in vitro and in a mouse model of pulmonary infection. Antimicrob Agents Chemother 2022; 66: e0170421. 10.1128/AAC.01704-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gotfried MH, Horn K, Garrity-Ryan Let al. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother 2017; 61: e01135-17. 10.1128/AAC.01135-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daley CL, Iaccarino JM, Lange Cet al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020; 56: 2000535. 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Griffith DE, Daley CL. Treatment of Mycobacterium abscessus pulmonary disease. Chest 2022; 161: 64–75. 10.1016/j.chest.2021.07.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.