Abstract
Nuclear receptor 4A1 (NR4A1) plays an important role in endometriosis progression; levels of NR4A1 in endometriotic lesions are higher than in normal endometrium, and substituted bis-indole analogs (NR4A1) antagonists suppress endometriosis progression in mice with endometriosis. In addition, the flavonoids kaempferol and quercetin are natural products that directly bind NR4A1 and significantly repress the intrinsic NR4A1-dependent transcriptional activity in human endometriotic epithelial and stromal cells and Ishikawa endometrial cancer cells. NR4A1 knockdown and inhibition of NR4A1 by kaempferol and quercetin suppressed proliferation of human endometriotic epithelial cells and Ishikawa cells by inhibiting epidermal growth factor receptor/c-Myc/survivin-mediated growth-promoting and survival pathways, The mammalian target of rapamycin (mTOR) signaling and αSMA/CTGF/COL1A1/FN-mediated fibrosis signaling but increasing Thioredoxin domain Containing 5/SESN2-mediated oxidative/estrogen receptors stress signaling. In human endometriotic stromal cells, NR4A1 knockdown and inhibition of NR4A1 by kaempferol and quercetin primarily inhibited mTOR signaling by suppressing proliferation of human endometrial stromal cells. In addition, kaempferol and quercetin treatment also effectively suppressed the growth of endometriotic lesions in mice with endometriosis compared with the vehicle without any body weight changes. Therefore, kaempferol and quercetin are NR4A1 antagonists with potential as nutritional therapy for endometriosis.
Keywords: quercetin, kaempferol, endometriosis, NR4A1 antagonists
Endometriosis is a common but complex estrogen-dependent inflammatory disease that occurs in cells lining the uterus and are implanted at distal sites such as peritoneal surfaces, bowel, and ovaries. Over 5 500 000 women in the United States and 176 000 000 women worldwide suffer from symptoms of endometriosis, pelvic pain, and infertility (1-4). Once diagnosed, the staging of the disease (ie, stage I-IV) and its location are essential for determining the appropriate treatment regimen. Excision surgery and hormonal therapy (such as progestins, oral contraceptives, and gonadotropin-releasing hormone antagonists) to systemically deplete estrogen levels have been employed for treating patients with endometriosis (5-11). However, the current treatments for endometriosis do not effectively relieve endometriosis symptoms and causes adverse effects in other estrogen target tissues, such as bone and brain, because hormonal therapy causes postmenopausal symptoms in patients with endometriosis (3-7, 11). Therefore, the development of new nonhormonal therapies to improve the efficacy of endometriosis treatment without the accompanying adverse effects of current endometriosis treatment is highly desirable (3, 4).
Nuclear receptor 4A1 (NR4A1) is an orphan nuclear receptor that was identified as an immediate–early gene activated by multiple stressors (12). Moreover, NR4A1 and other NR4A members (NR4A2, Nurr1, NR4A3, Nor1) are overexpressed and play a role in inflammatory-related diseases, including cancer, T-cell exhaustion, fibrosis, and brain injury (13-23). NR4A1 regulates 1 or more of cancer cell proliferation, survival, cell cycle progression, migration, and invasion in various solid tumors (such as lung, melanoma, lymphoma, pancreatic, colon, cervical, ovarian, and gastric cancer), and NR4A1 is effectively inhibited by bis-indole derived compounds (CDIM) NR4A1 ligands that act as antagonists to suppress cancer progression (24-28). For instance, NR4A1 knockdown and CDIM/NR4A1 antagonists treatment effectively suppressed the growth of endometrial cancer cell lines (25). In addition, NR4A1 knockdown and CDIM/NR4A1 antagonist inhibited the growth of human endometriotic lesions isolated from patients with endometriosis by inhibiting NR4A1-regulated proliferation, The mammalian target of rapamycin (mTOR) signaling, and fibrosis in vitro (26). CDIM/NR4A1 antagonists also suppressed the growth of endometriotic lesions in mice with endometriosis, suggesting that NR4A1 plays an essential role in endometriosis progression and is a new therapeutic target for the nonhormonal treatment of endometriosis.
Flavonoids are phytochemicals that naturally exist in plants, fruits, vegetables, and leaves and these compounds have many medicinal benefits, such as anticancer, antioxidant, anti-inflammatory, antiviral, neuroprotective, and cardioprotective properties (29-36). Interestingly, the flavonoid kaempferol inhibits hepatic gluconeogenesis and induces GLUT4 expression and glucose uptake in muscle cells, like CDIMs and other NR4A1 ligands (37-39). Recent studies also showed that the flavonoid kaempferol and CDIMs regulate expression of the histone methyltransferase G9a gene, which was recently shown to be an NR4A1-dependent gene in Rh30 cells (27, 40). A recent study showed that the flavonoids quercetin and kaempferol bound NR4A1 and acted as receptor antagonists to inhibit NR4A1-dependent pro-oncogenic pathways/genes in rhabdomyosarcoma cells (41). Since NR4A1 plays a critical role in endometriosis, this study has investigated the potential applications of the flavonoids quercetin and kaempferol as nutraceuticals for treatment of endometriosis.
Materials and Methods
Mice
Luciferase-expressing FVB (Tg[CAG-luc, GFP]L2G85Chco) and FVB female mice were purchased from Jackson Laboratory and maintained in the designated animal care facility at Baylor College of Medicine according to the Institutional Animal Care and Use Committee (IACUC) guidelines for the care and use of laboratory animals. An IACUC-approved protocol was followed for all animal experiments in this study.
Primary Human Endometriotic Cells From Endometriosis Patients
Using our primary human endometrial stromal cells isolated from an ovarian endometrioma (26), we generated immortalized human endometriotic stromal cells using lentivirus expressing human telomerase reverse transcriptase (26, 42). In addition, the immortalized human endometriotic epithelial cells generated from ovarian endometrioma cells were employed (43). Primary normal endometrial stromal cells (NESCs) were isolated from the biopsy of the eutopic endometrium of normal women (NEM) in the proliferative phase (26). Ishikawa cells were purchased from Millipore Sigma (catalog number: 99040201). These cells were maintained in Dulbecco’s modified Eagle’s medium/F12 growth medium supplemented with 10% fetal bovine serum and 1× antibiotic/antimycotic solution (Sigma-Aldrich, St Louis, MO). All cells were incubated at 37 °C in CO2 incubator in an atmosphere of humidified 5% CO2% and 95% air. The short tandem repeat analysis (9) validated that these cells were not contaminated with another type of cells. In addition, the identity of human endometrial epithelial and stromal cells was validated by the expression of cytokeratin 18 and vimentin by Western blot analyses, respectively.
Reagents and Antibodies
The primary antibodies used were epidermal growth factor receptor (EGFR; RRID:AB_2246311), survivin (RRID:AB_2063948), mTOR (RRID:AB_330978), p-S6RP (RRID:AB_916156), S6RP (RRID:AB_331355), p-4E-BP1 (RRID:AB_330947), 4E-BP1 (RRID:AB_2097841) from Cell Signaling Technology (Danvers, MA, US); Sestrin 2 (RRID:AB_2716805), and p-mTOR (RRID:AB_10888105) from Abcam (Boston, MA); c-Myc (RRID:AB_627268) from Santacruz Biotechnology (Santacruz, CA, US); NR4A1 (RRID:AB_10797121) antibody was purchased from LSBio (Seattle, WA); β-actin (RRID:AB_476743) from Sigma Aldrich Corporation (Milwaukee, WI, US); Thioredoxin domain Containing 5 (TXNDC5) (RRID:AB_11170188), α-smooth muscle actin (α-SMA; RRID:AB_1240408), collagen type 1 α-1 (COL1A1; RRID:AB_10721155), corrective tissue growth factor (CTGF; RRID:AB_11169640), and fibronectin (FN; RRID:AB_1950298) from GeneTex, Inc. (Irvine, CA, US). Secondary antibodies for rabbit (7074), mouse (7076), and antirabbit Alexa Fluor (4412) were purchased from Cell Signaling Technology (Danvers, MA, US). Two siNR4A1 oligonucleotides used in this study were 5′-CAGUGGCUCUGACUACUAU-3′ (1) and 5′-GAGAGCUAUUCCAUGCCUA-3′ (2) and nontargeted scrambled small interfering RNAs (siRNA; iGL2) were used as a control from Sigma Aldrich Corporation (The Woodlands, TX, US). Quercetin and kaempferol were purchased from Indofine (Hillsborough, NJ).
Cell Proliferation Assay
Patient-derived endometriotic cells, immortalized human endometriotic stromal cells (IHESCs) and immortalized human endometriotic epithelial cells (IHEECs), human endometrial adenocarcinoma cells, Ishikawa cells, and normal cells from NEM were seeded into a 96-well plate. The cells were treated for 24 hours with either dimethyl sulfoxide (DMSO) or different concentrations of quercetin and kaempferol (25-100 µM or 25-150 µM). IHESCs and IHEECs were treated with 50 nM of 2 siNR4A1 oligonucleotides to downregulate NR4A1; 50 nM of nontarget siRNAs was employed as the control of siRNA. Afterward, the medium was removed, and the MTT solution diluted in phosphate-buffered saline (PBS) was added to cell cultures. After 3 hours of incubation, the medium was aspirated and washed with PBS. DMSO was added and incubated at 37 °C for 10 minutes, and absorbance was measured at 570 nM.
Western Blotting
IHESCs, IHEECs, and Ishikawa cells (2 × 105) were seeded and allowed to attach for 24 hours, and cells were then treated for 24 hours with either DMSO or different concentrations of quercetin and kaempferol. Cells were then lysed, and whole-cell lysates were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Proteins were transferred using polyvinylidene fluoride membrane by wet blotting followed by primary and secondary antibody incubation and detected using enhanced chemiluminescence reagent as previously described.
Immunofluorescence
IHEECs and Ishikawa cells were seeded in a Nunc chambered coverglass followed by various drug treatments. The cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at 37 °C. Cells were then blocked and incubated overnight with primary α-SMA antibody in the buffer (5% bovine serum albumin in PBS) at 4 °C, followed by Alexa Fluor–conjugated secondary antibody at a dilution of 1:250 for 2 hours at room temperature. Finally, cells were observed using a Zeiss confocal fluorescence microscope.
RNA Interference
IHESCs, Ishikawa cells, and IHEECs were seeded in 6-well plates and allowed to grow to 60% confluence (24 hours), and then transfections were performed with Lipofectamine 2000 according to the manufacturer's protocol. Both siNR4A1 oligonucleotides and nontargeted control siRNAs were used. Six hours after transfection, the medium was replaced with fresh medium and left for 72 hours; then the cells were harvested, and protein expression was determined. NR4A1 knockdown efficiency by NR4A1 siRNA was determined by Western blotting.
Determination of the Intrinsic Transcriptional Activity of NR4A1 Upon Flavonoids Exposure.
IHESCs, Ishikawa cells, and IHEECs were plated on 12-well plates in Dulbecco’s modified Eagle’s medium/F12 supplemented with 2.5% charcoal-stripped fetal bovine serum. Cells were allowed to attach and grow for 12 hours, and various amounts of DNA (ie, upstream activation sequence [UAS] x5-Luc [500 ng], GAL4-NR4A1 [50 ng]) were cotransfected into each well using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. After 6 hours of transfection, cells were treated with a plating medium containing either solvent (DMSO) or indicated concentrations of quercetin and kaempferol for 18 hours. Cells were then lysed using a freeze–thaw protocol, and cell extract was used for luciferase and b-gal assays. LumiCount (Packard) was used to quantify luciferase and β-gal activities. Luciferase activity values were normalized against corresponding β-gal activity values and protein concentrations determined by Bradford assay. GAL4-NR4A1 constructs contain full-length NR4A1 coding sequences and all the plasmids used in this study were previously described (24, 26, 41).
Computation-Based Molecular Modeling
Molecular modeling studies were conducted using Maestro (Schrödinger Release 2020-1, Schrödinger, LLC, New York, NY, 2020). The version of Maestro used for these studies is licensed to the Laboratory for Molecular Simulation, a Texas A&M University core user facility for molecular modeling, and is associated with the Texas A&M University High Performance Research Computing (HPRC) facility (College Station, TX, 77843). All Maestro-associated applications were accessed via the graphical user interface VNC interactive application through the HPRC Ada OnDemand portal. The crystal structure coordinates for human orphan nuclear receptor NR4A1 ligand binding domain (LBD) (38) were downloaded from the Protein Data Bank (https://www.rcsb.org; PDB ID 3V3Q). The human NR4A1 LBD crystal structure was prepared for ligand docking utilizing the Maestro Protein Preparation Wizard; restrained minimization of the protein structure was performed utilizing the OPLS3e force field. Each ligand (quercetin and kaempferol) 3-dimensional structure was prepared for docking utilizing the Maestro LigPrep, again using the OPLS3e force field. Maestro Glide (41, 44, 45) was utilized with the default settings to dock each prepared ligand to the prepared protein, predict the lowest energy ligand binding orientation, calculate the predicted binding energy in units of kcal/mol, and determine specific amino acid/ligand interactions.
Noninvasive Reporter Mouse Model of Endometriosis
To test drug effects on endometriosis, a new noninvasive reporter mouse model of endometriosis was generated by using luciferase reporter mice (FVB-Tg[CAG-Luc, GFP]L2G85Chco/J, Jackson Laboratory, catalog #: 008450). Under anesthesia, uterine horns were isolated from a female luciferase reporter mouse (8 weeks old) at the estrus stage. The isolated horns were opened longitudinally in a Petri dish. The endometrial tissues were scraped away from the myometrial layer using a scalpel to ensure that only endometrial cells without myometrial and perimetrical tissues were injected to mimic the menstrual dissemination of endometrial tissues. The scraped endometrial mass was suspended in PBS buffer, and 1 ×106 cells were injected intraperitoneally into 1 recipient ovariectomized syngeneic female mouse (8 weeks old, FVB) implanted with an estrogen pellet (3.6 mg, 60 days lease) to induce endometriosis (26).
Endometriosis Treatment With Kaempferol and Quercetin
Endometriosis was induced in mice with the noninvasive reporter mouse model of endometriosis method. After ectopic lesions were established in mice (third week after endometriosis induction), we randomly divided mice with endometriosis into 3 groups and then intraperitoneally injected mice with vehicle (5% DMSO and 10% 2-hydroxypropyl-β-cyclodextrin), 100 mg/kg of kaempferol, and 100 mg/kg of quercetin (once a day, for 14 days). After drug treatment, we determined the luciferase activity of the ectopic lesions treated with vehicle, kaempferol, and quercetin using the in vivo image system.
Quantifying Bioluminescence Data
Mice were anesthetized with a 1.5% isoflurane/air mixture using an Inhalation Anesthesia System (VetEquip). Next, d-Luciferin (ThermoFisher, catalog number: L2916) was intraperitoneally injected at 40 mg/kg mouse body weight. Ten minutes after the D-luciferin injection, the mice were imaged using an IVIS Imaging System (Xenogen) with continuous 1% to 2% isoflurane exposure. Imaging variables were maintained for comparative analysis. Grayscale-reflected and pseudocolorized images reflecting bioluminescence were superimposed and analyzed using Living Image software (Version 4.4, Xenogen). A region of interest (ROI) was manually selected over the relevant signal intensity regions. The area of the ROI was kept constant across experiments, and the intensity was recorded as total photon counts per second per cm2 within the ROI.
Statistical Analysis
All experiments have been repeated a minimum of 3 times. The data are expressed as the mean ± SE. A 1-way analysis of variance was used to determine statistical significance. P < .05 was considered to be statistically significant.
Results
Kaempferol and Quercetin Are NR4A1 Antagonists.
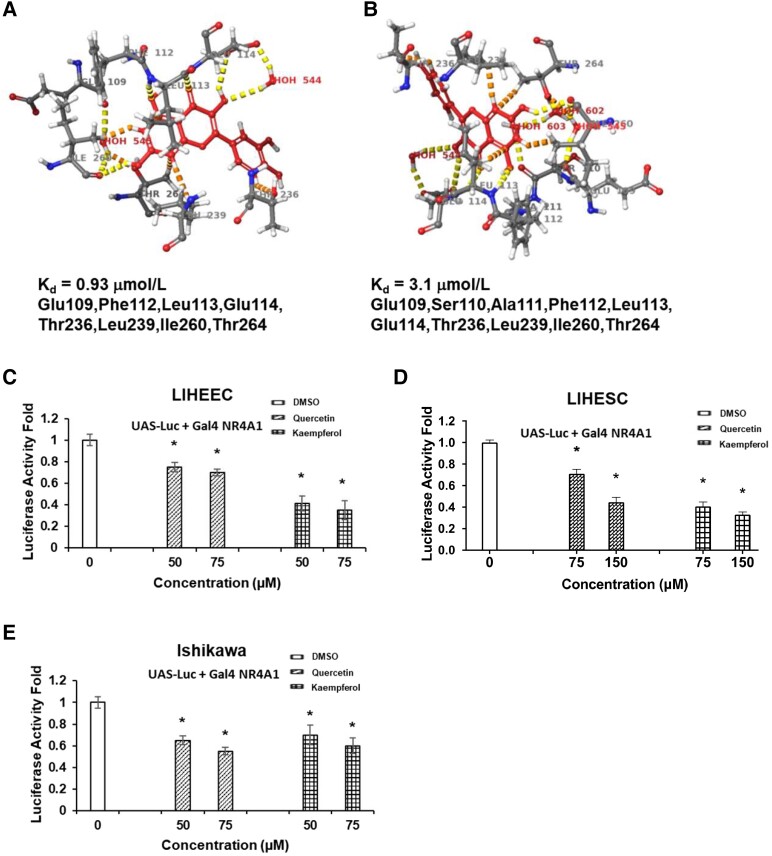
The 3-dimensional interactions of kaempferol and quercetin with the LBD of NR4A1 and analysis which measures quenching of intrinsic tryptophan fluorescence revealed that quercetin (Kd = 0.93 µmol/L) had a higher binding affinity to the LBD of NR4A1 than kaempferol (Kd = 3.1 µmol/L) (41). The Schrodinger/Maestro modeling showed that quercetin bound to positive amino acids (Glu109, Phe112, Leu113, Glu114, and Ile260) (a yellow dashed line) and negative amino acids (Thr236, Leu239, Ile260, and Thr264) (orange dashed lines) in LBD of NRA41 (Fig. 1A). Kaempferol also bound to positive amino acids (Glu109, Ser110, Ala111, Phe112, Leu113, Glu114, and Ile260) and negative amino acids (Thr236, Leu239, Ile260, and Thr264) in LBD of NR4A1 (Fig. 1B). Therefore, both quercetin and kaempferol have a similar binding orientation within the LBD binding pocket of NR4A1.
Figure 1.
Kaempferol and quercetin as NR4A1 ligands. Quercetin (A) and kaempferol (B) interactions with the LBD of NR4A1 using a modeling approach as described (42-44) and critical interacting amino acids are indicated. Effects of kaempferol and quercetin on luciferase activity were determined in IHEECs (C), IHESCs (D), and Ishikawa cells (E) transfected with a chimeric GAL4-NR4A1 construct and a UAS-luc reporter gene. Luciferase activity was determined as outlined in “Materials and Methods.” Results (C-E) are expressed as means ± SE for at least 3 determinations, and significant (P < .05) changes compared with DMSO (control) are indicated (*).
Next, we determined how the binding of quercetin and kaempferol changes NR4A1 activity. To determine the intrinsic transcriptional activity of NR4A1, the expression vector for GAL4 DNA binding domain fused to NR4A1 (Gal4 NR4A1) and the luciferase reporter plasmid containing 5 copies of Gal4 binding UAS were cotransfected into IHEECs, IHESCs, and Ishikawa cells. Compared with vehicle treatment, both quercetin and kaempferol treatment decreased the intrinsic transcriptional activity of NR4A1 in a dose-dependent manner in all tested endometrial cells (Figs. 1C-1E). Therefore, quercetin and kaempferol are natural flavonoids that directly bind to NR4A1 to decrease its transcriptional activity.
Quercetin and Kaempferol Suppressed the Growth of Human Endometriotic Cells but not Normal Endometrial Cells.
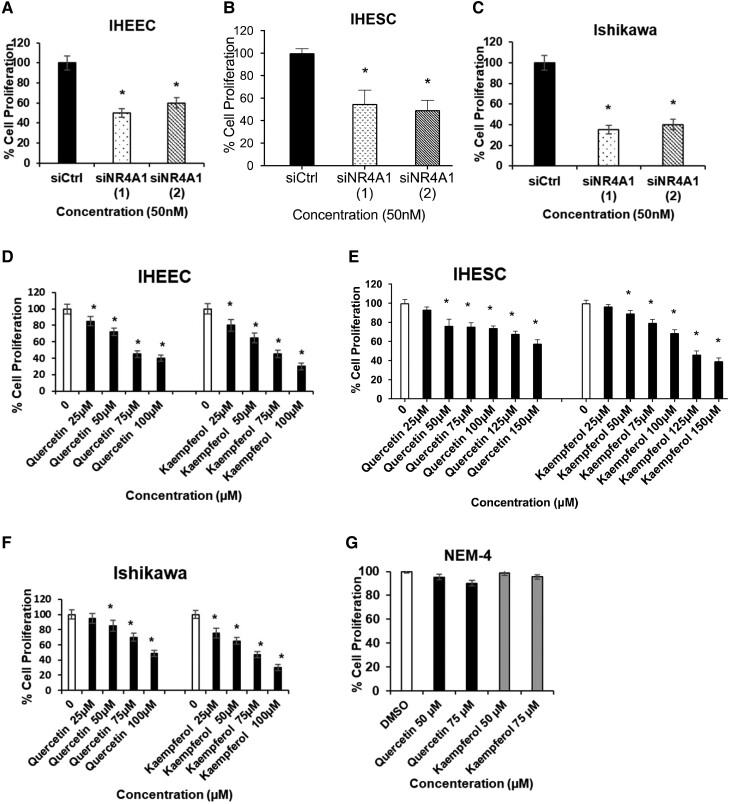
To define whether NR4A1 has an essential role in the progression of IHEECs, IHESCs, and Ishikawa cells, NR4A1 levels in these cells were decreased by transfection with 2 different siRNAs against NR4A1. Ishikawa endometrial cancer cells are routinely used in endometriosis studies and were included in this research. As the siRNA control, nontargeting siRNA (siCtrl) was employed. Compared with siCtrl, NR4A/siRNA effectively reduced NR4A1 protein levels in IHEECs, IHESCs, and Ishikawa cells (Fig. 2A) and then suppressed proliferation of IHEECs, IHESCs, and Ishikawa cells (Fig. 2B-2D). Therefore, NR4A1 is essential in human endometriotic epithelial and stromal cell proliferation. Next, we determined whether inhibition of NR4A1 activity by quercetin and kaempferol also suppressed growth of human endometriotic cells. Both quercetin and kaempferol suppressed proliferation of IHEECs and IHESCs in a dose-dependent manner (Fig. 2E and 2F). Furthermore, in addition to human endometriotic cells, quercetin and kaempferol treatment also suppressed the proliferation of Ishikawa cells (Fig. 2G). In contrast, the proliferation of NEM was not inhibited by quercetin and kaempferol (Fig. 2H). Therefore, quercetin and kaempferol specifically inhibited growth of human endometriotic cells from patients with endometriosis but not normal endometrial cells.
Figure 2.
Endometriotic cell growth inhibition by quercetin and kaempferol. (A) Knockdown efficiencies of NR4A1 in IHEECs, IHESCs, and Ishikawa cells determined by Western blots of whole cell lysates as outlined in “Materials and Methods.” (B-D) IHEECs (B), IHESCs (C), and Ishikawa cells (D) were transfected with 2 oligonucleotides targeting NR4A1, and levels of NR4A1 were determined with Western blot analysis. (E-G) The proliferation of IHEECs (E), IHESCs (F), and Ishikawa cells (G) transfected with siNR4A1 oligonucleotides and control siRNA (siCtrl) was determined by the MTT assays. (E-H) The proliferation of IHEECs (E), IHESCs (F), Ishikawa cells (G), and NEM-4 (H) cells treated with quercetin or kaempferol for 24 hours was determined by MTT assays. Results are expressed as means ± SE for at least 3 replicate determinations significant (P < .05) inhibition of cell growth is indicated (*).
Quercetin and Kaempferol Decrease the EGFR/c-Myc/Survivin Growth Axis in Human Endometriotic Cells.
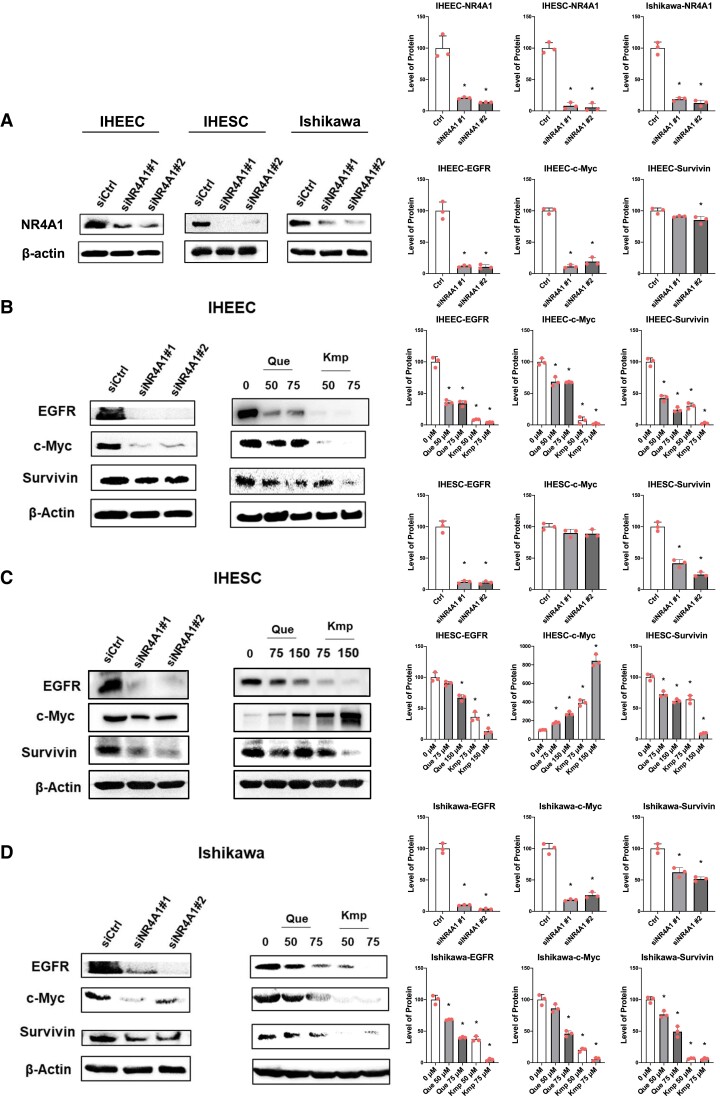
NR4A1 regulates the expression of growth-promoting and survival genes (EGFR, c-Myc, survivin) in endometriotic and endometrial cancer cells (25, 26). Thus, we determined whether quercetin and kaempferol also block the growth survival signaling in human endometriotic cells. The knockdown of NR4A1 decreased EGFR, c-Myc, and survivin levels in IHEECs compared with control siRNA (Fig. 3A). Also, quercetin and kaempferol treatment decreased levels of EGFR, c-Myc, and survivin in IHEECs compared with the vehicle. Therefore, quercetin and kaempferol treatment effectively suppressed the NR4A1-mediated EGFR/c-Myc/Survivin axis in IHEECS to inhibit the growth of these cells. Compared with IHEECs, the NR4A1 knockdown and quercetin, and kaempferol reduced levels of EGFR and survivin compared with their control (Fig. 3B). However, c-Myc levels were not reduced by NR4A1 knockdown in IHESCs and quercetin, and kaempferol treatment increased c-Myc in IHESCs compared with the vehicle (Fig. 3B). In human endometriotic stromal cells, therefore, NR4A1 did not regulate the c-MYC expression. The knockdown NR4A1 and quercetin and kaempferol treatment effectively suppressed levels of EGFR, c-Myc, and survivin in Ishikawa cells as also observed in IHEECs (Fig. 3C). Therefore, quercetin and kaempferol treatment suppress the NR4A1-mediated EFGR/c-Myc/survivin axis in human endometriotic epithelial but not in human endometriotic stromal cells.
Figure 3.
siNR4A1 knockdown, quercetin, and kaempferol decrease expression of growth-promoting and survival genes. (A-C) The levels of EGFR, c-Myc, and survivin in IHEECs (A), IHESCs (B), and Ishikawa cells (C) transfected with siNR4A1 oligonucleotides or treated with quercetin or kaempferol for 24 hours were analyzed by Western blotting. Results are expressed as means ± SE for at least 3 replicate determinations significant (P < .05) inhibition of cell growth is indicated (*).
Quercetin and Kaempferol Treatment Increased the Estrogen Receptors (ER) Oxidative/ER Stress by Reduction of TXNDC5 in Human Endometriotic Cells.
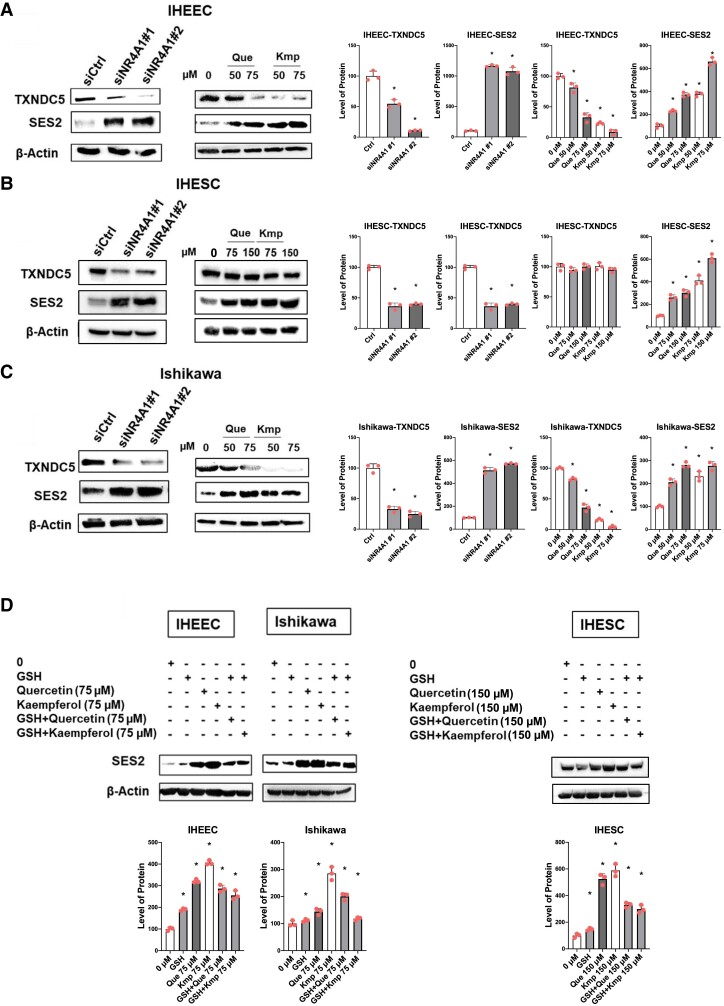
NR4A1 knockdown or treatment with NR4A1 antagonists induced oxidative/ER stress by decreasing TXNDC5 and elevating levels of the oxygen sensor protein setrin2 (SESN2) in Ishikawa cells (25). To validate whether quercetin and kaempferol treatment also induced similar responses in human endometriotic cells, expression levels of TXNDC5 and SESN2 were examined in IHEECs and IHESCs after treatment with these flavonoids. NR4A1 knockdown and quercetin and kaempferol treatment decreased levels of TXNDC5 and increased SESN2 in IHEECs compared with their control (Fig. 4A). NR4A1 knockdown also decreased TXNDC5 expression and increased SESN2 to enhance oxidative/ER stress (Fig. 4B). In contrast, kaempferol and quercetin treatment induced SESN2 without reducing TXNDC5 expression in IHESCs (Fig. 4B). Therefore, kaempferol and quercetin treatment induced the oxygen-sensing SENS2 in IHESCs independent of TXNDC5 suggesting roles for other NR4A1-regulated proreductant genes, and these are currently being investigated. NR4A1 knockdown, kaempferol and quercetin induced also decreased TXNDC5 and induced SESN2 levels in Ishikawa cells as observed for IHEECs (Fig. 4C). To determine whether induction of ROS associated with downregulation of TXNDC5 induced SESN2, changes in levels of SESN2 were determined in human endometriotic cells and Ishikawa cells in the presence or absence of 5 mM glutathione (GSH). GSH treatment alone did not affect SESN2 levels but significantly inhibited induction of SESN2 in IHEECs, IHESCs, and Ishikawa cells treated with quercetin and kaempferol alone (Fig. 4D).
Figure 4.
siNR4A1, quercetin, and kaempferol modulate oxidative/ER stress pathway genes in endometriotic cells. (A-C) Levels of TXNDC5, SESN2, β-actin in IHEECs (A), IHESCs (B), and Ishikawa cells (C) transfected with siNR4A1 oligonucleotides or treated with quercetin or kaempferol for 24 hours were determined by Western blot analysis. (D) Levels of SESN2 and β-actin in IHEECs, IHESCs, and Ishikawa cells treated with DMSO, GSH, quercetin, kaempferol, GSH plus quercetin, and GSH plus kaempferol for 24 hours were determined by Western blotting. Results are expressed as means ± SE for at least 3 replicate determinations significant (P < .05) inhibition of cell growth is indicated (*).
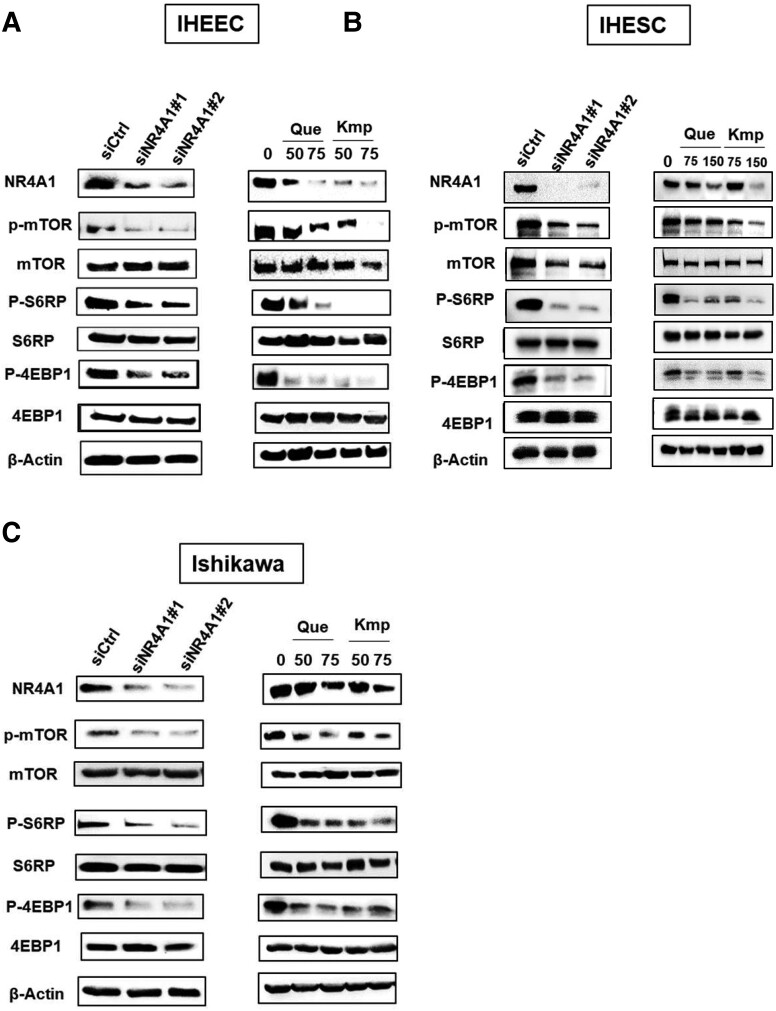
Quercetin and Kaempferol Inhibited mTOR Signaling in Human Endometriotic Cells.
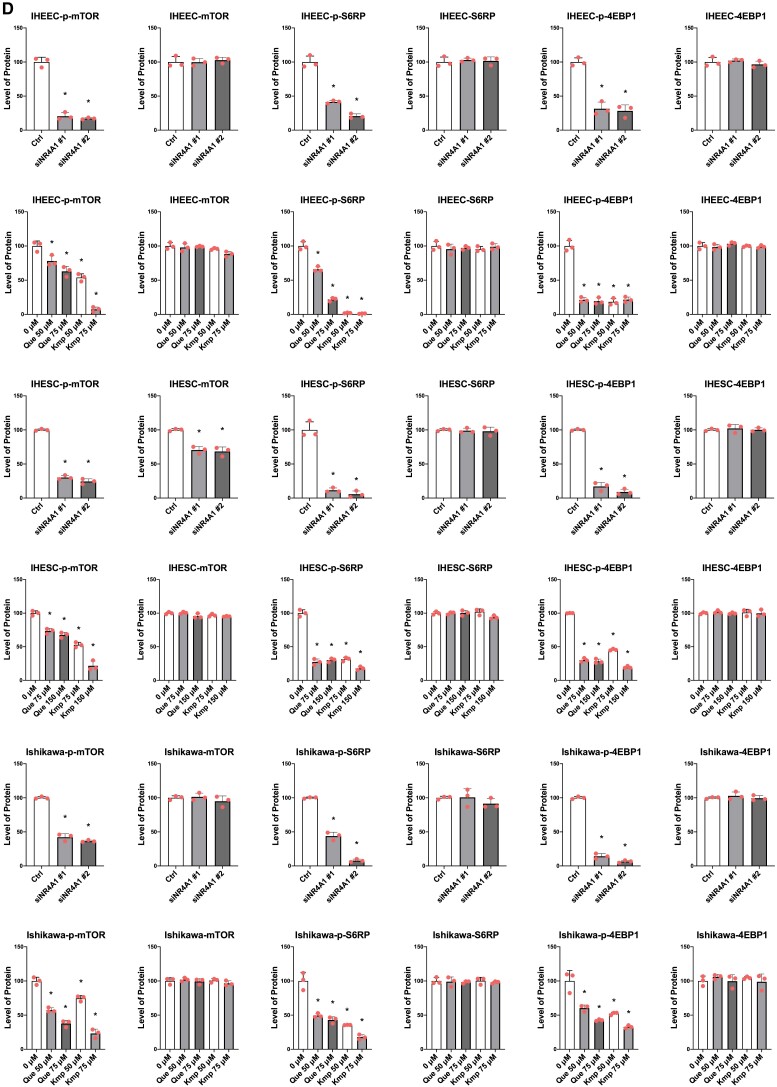
The induction of SESN2 inhibits mTOR signaling in various cancer cells (24, 25) and, therefore, we determined whether quercetin and kaempferol also inhibited SESN2-mediated mTOR signaling in human endometriotic cells. NR4A1 knockdown, quercetin, and kaempferol treatment significantly decreased phosphorylated (p)-mTOR, p-S6RP, and p-4EBP1 in both IHEECs and IHESCs compared with their control (Fig. 5A and 5B). NR4A1 knockdown and quercetin and kaempferol treatment also significantly inhibited mTOR signaling pathways by reducing p-mTOR, p-S6RP, and p-4EBP1 in Ishikawa cancer cells (Fig. 5C). The quantification of intensity of Western blotting bands are illustrated in Fig. 5D. Thus, quercetin and kaempferol effectively suppressed the mTOR signaling by increasing SESN2 in human endometriotic cells and this is consistent with their growth inhibitory effects (Fig. 2).
Figure 5.
siNR4A1, quercetin, and kaempferol inhibit mTOR signaling in human endometriotic cells. (A-C) Levels of mTOR marker proteins in IHEECs (A), IHESCs (B), and Ishikawa cells (C) transfected with siNR4A1 oligonucleotides or treated with kaempferol or quercetin for 24 hours were determined by Western blotting. (D) Quantitation of bands generated in Western blots (A-C) using β-actin as a loading control and compared with siCtrl band intensities. Results are expressed as means ± SE for at least 3 replicate determinations significant (P < .05) inhibition of cell growth is indicated (*).
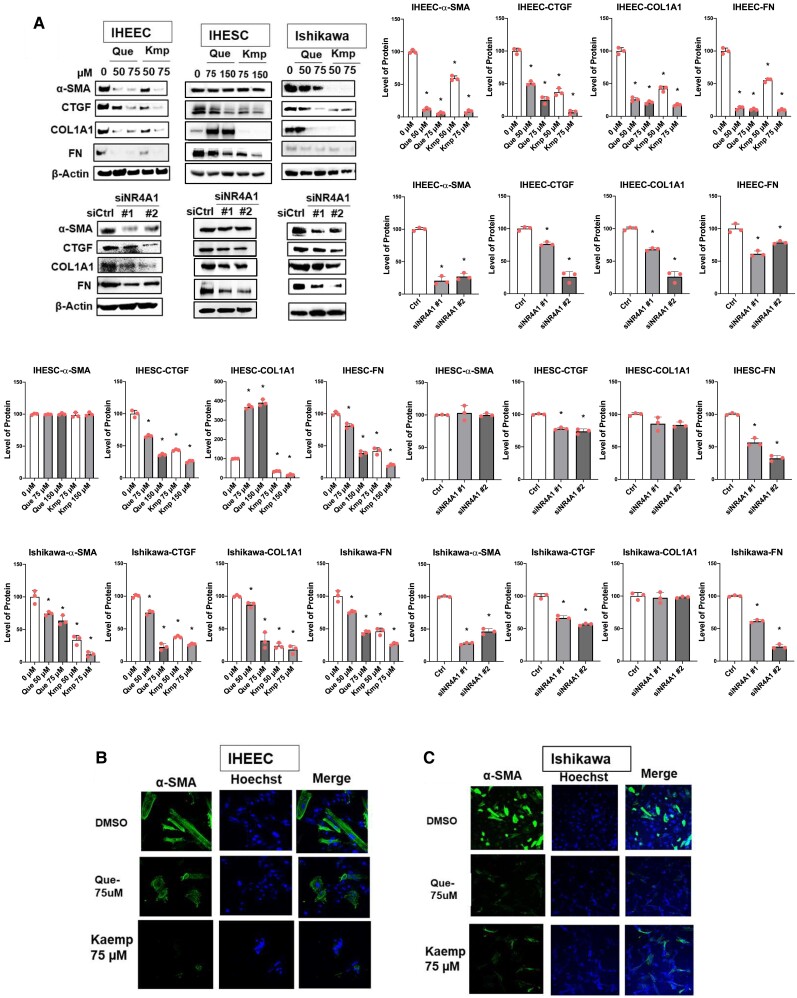
Quercetin and Kaempferol Inhibited Fibrosis Progression in Human Endometriotic Cells.
The fibrosis progression mediated by α-SMA and other profibrotic genes has a critical role in endometriosis, and NR4A1 regulates the expression of profibrotic genes in human endometriotic cells (26). The bis-indole-derived NR4A1 antagonist significantly reduced levels of α-SMA, FN, CTGF, and COL1A1 in human endometriotic cells (26). Therefore, we determined whether quercetin and kaempferol also suppressed fibrosis progression in human endometriotic cells. NR4A1 knockdown and quercetin and kaempferol treatment decreased expression of the fibrotic genes α-SMA, CTGF, COL1A1, and FN in IHEECs compared with their control (Fig. 6A). In contrast, NR4A1 silencing decreased the level of FN, but did not reduce levels of α-SMA, COL1A1, and CTGF in IHESCs compared with control siRNA (Fig. 6B). Treatment with quercetin and kaempferol also did not decrease α-SMA, but decreased CTGF and FN in IHESCs compared with the vehicle (Fig. 6B), whereas kaempferol but not quercetin decreased COL1A1 levels in IHESCs (Fig. 6B). Therefore, NR4A1 does not have an essential role in fibrosis progression in IHESCs. NR4A1 knockdown, quercetin, and kaempferol treatment reduced levels of profibrotic genes in Ishikawa cells compared with their control (Fig. 6C). Immunofluorescence analysis also validated the reduction of α-SMA in IHEECs and Ishikawa cells by quercetin and kaempferol compared with the vehicle (Fig. 6D and 6E).
Figure 6.
siNR4A1, quercetin and kaempferol inhibit fibrosis in human endometriotic cells. (A-C) Levels of fibrosis marker proteins in IHEECs (A), IHESCs (B), and Ishikawa cells (C) treated with quercetin or kaempferol or transfected with siRNAs were determined by Western blotting. (D-E). Levels of α-SMA in IHEECs (D) and Ishikawa cells (E) treated with 75 µM quercetin and kaempferol for 24 hours were determined by immunofluorescence. Hoechst-stained nucleus. Results are expressed as means ± SE for at least 3 replicate determinations significant (P < .05) inhibition of cell growth is indicated (*).
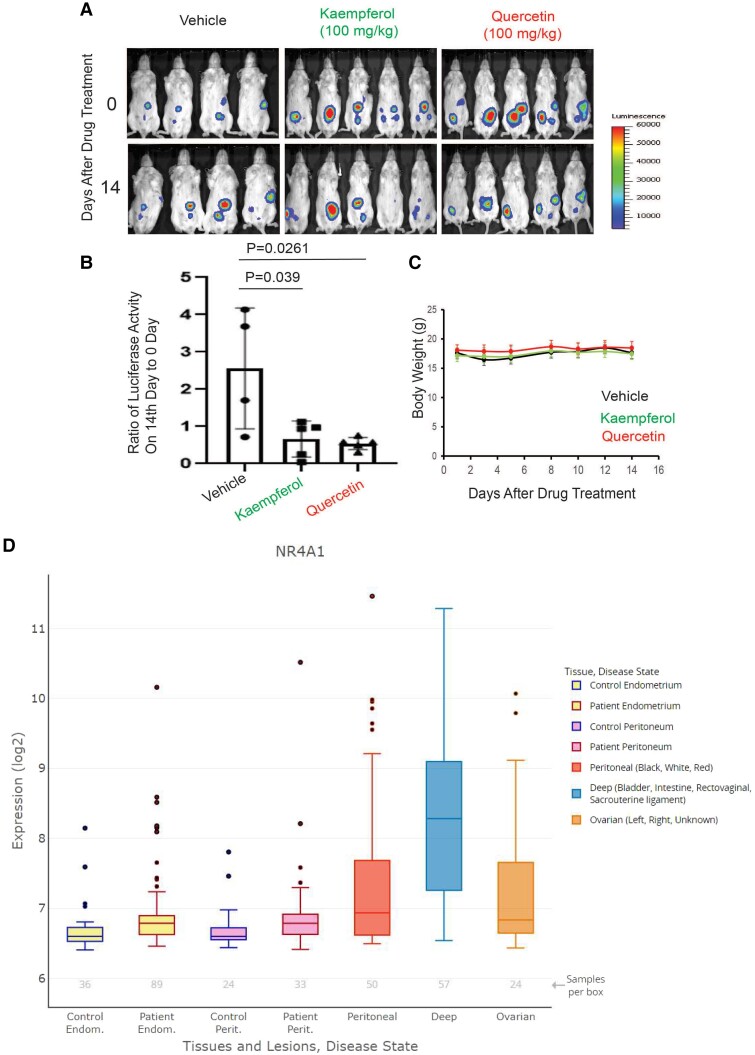
Quercetin and Kaempferol Reduced the Endometriosis Progression in Mice Without Toxicity.
To validate effects of quercetin and kaempferol on human endometriotic cells, we examined whether quercetin and kaempferol treatment suppress endometriosis progression in mice as described (26). The luciferase activity analysis revealed that endometriotic lesions were well established in mice with endometriosis (Fig. 7A, 0 days after drug treatment). In the vehicle-treated mice, luciferase activities were elevated compared with vehicle treatment (Fig. 7A and 7B). Therefore, endometriotic lesions were continuously grown in mice with endometriosis and treated with a vehicle. Compared with the vehicle, however, quercetin (100 mg/kg/d) and kaempferol (100 mg/kg/d) significantly decreased luciferase activities of ectopic lesions compared to the before drug treatment (Fig. 7A and 7B). Therefore, quercetin and kaempferol treatment effectively suppressed the growth of endometriotic lesions in mice with endometriosis. Even though quercetin and kaempferol treatment reduced the growth of endometriotic lesions, quercetin, and kaempferol treatment did not affect body weights compared with vehicle (Fig. 7C). Thus, flavonoids quercetin and kaempferol safely antagonized multiple NR4A1-dependent proendometriotic responses in both in vitro and in vivo assays. To delineate the differential expression levels of NR4A1 in patients with endometriosis, we utilized EndometDB, a specialized database that provides gene expression data from 115 patients and 53 controls (46). Our analysis using EndometDB revealed that peritoneal and deep infiltrating ectopic lesions and ovarian endometriomas exhibit significantly higher levels of NR4A1 compared to the control peritoneum (Fig. 7D). Therefore, elevated levels of NR4A1 are associated with ectopic lesions in patients with endometriosis.
Figure 7.
Quercetin and kaempferol treatment suppressed the growth of endometriotic lesions in mice with endometriosis. (A) Luciferase activity of ectopic lesions in mice with endometriosis treated with vehicle, quercetin, and kaempferol before drug treatment and 14th day after drug treatment. (B) Quantification of luciferase activity shown in A. (C) The body weight changing of mice treated with vehicle, quercetin, and kaempferol for 14 days. (D) aberrant levels of NR4A1 in endometriotic lesions compared with normal endometrium. The expression levels of NR4A1 in control endometrium, control peritoneum, endometrium, and peritoneum of patients with endometriosis, as well as peritoneal and deep infiltration ectopic lesions and ovarian endometriomas, were determined using EndometDB.
Discussion
NR4A1 and phosphorylated NR4A1 levels are elevated in patients with endometriosis, and NR4A1 is involved in cell division, inflammation, programmed cell death, and fibrosis in endometriosis progression (26, 47). Therefore, NR4A1 is considered a novel target for endometriosis treatment as a non-hormonal therapy to decrease the adverse effects of current hormonal therapies for endometriosis. In this context, bis-indole-derived compounds (CDIMs) have been identified as CDIM/NR4A1 antagonists effectively suppressed the growth of human endometrial cells derived from patients with endometriosis by inducing apoptosis and inhibiting fibrosis pathways and also suppressed the growth of endometriotic lesions in mice with endometriosis (25, 26). In addition to synthetic ligands, natural products have been applied for human disease treatment due to their beneficial therapeutic effects and relatively low toxicity (30, 38). Interestingly, flavonoids also work as NR4A1 ligands to modulate NR4A1-mediated cellular pathways. For instance, the flavonoids kaempferol and quercetin are NR4A1 ligands that inhibit rhabdomyosarcoma cell growth by suppressing NR4A1-regulated oncogenic genes and cellular pathways (41). Like CDIM/NR4A1 ligands, structurally diverse flavonoids such as quercetin, luteolin, myricetin, 3,6-dihydroxyflavone, chrysin, scutellarin, epigallocatechin-3-gallate, kaempferol, and flavonoid conjugates also suppressed endometriosis progression by inhibiting cell growth, migration, and invasion in vivo and in vitro (34-36, 48-55). These observations and our previous study (26) suggested that some flavonoids may also suppress endometriosis progression by inhibiting NR4A1-mediated cellular pathways.
How do flavonoids inhibit NR4A1? Like CDIMs, quercetin and kaempferol directly bind to NR4A1 with KD values of 0.93 and 3.1 µM, respectively, and inhibited the transcriptional activity of NR4A1 (41). The binding sites of quercetin and kaempferol in NR4A1 are similar to each other but not identical. For instance, kaempferol, but not quercetin, bound to Ser110, and Ala111 of NR4A1 with kaempferol and not quercetin. In contrast, interactions of quercetin and kaempferol were different from that previously reported by 1,1-bis(3′-indolyl)-1-(3,5-disubstitutedphenyl) methane analogs (56), suggesting that these NR4A1 ligands are selective receptor modulators, and this is consistent with their structure- and cell context–dependent differential effects on profibrotic genes (Fig. 6).
Quercetin and kaempferol are among the most ubiquitous polyphenols in fruit and vegetables. Kaempferol is safe for use, and quercetin supplements are added to the Food and Drug Administration's Generally Recognized as Safe list. Our mouse study also showed that quercetin and kaempferol treatment did not cause body weight loss, even though they suppressed the growth of endometriotic lesions (Fig. 7). In addition, quercetin and kaempferol have many other beneficial effects including lowering blood pressure and inflammation and potentially cardiovascular effects (29-36). Therefore, dietary fruit and vegetables containing quercetin and kaempferol (such as broccoli, kale, dill, and spinach) should benefit patients with endometriosis without any adverse effects compared with current hormonal therapy. Results of this study complement several previous reports linking dietary factors including quercetin and other flavonoids as beneficial agents in the treatment of endometriosis (57-59). For instance, network pharmacology studies show that some traditional Chinese medicine that have been used for treatment of endometriosis contain quercetin, which is predicted to be an important contributor to their effectiveness (60, 61). This is also supported by a comprehensive review of the pathophysiology of endometriosis which also identifies quercetin as a beneficial compound but concludes that this needs to be confirmed in “future clinical studies” (62).
Generally, flavonoids are extensively metabolized in the liver and circulate in the blood as sulfate, methyl, and glucuronide conjugates (63, 64). The 3 major plasma metabolites of quercetin were quercetin-3-sulfate, quercetin-30-sulfate, and quercetin-3-glucuronide. The major metabolite identified in plasma and urine was kaempferol-3-glucuronide (63). However, the role of metabolites of quercetin and kaempferol in NR4A1 function in endometriosis is not elucidated. Therefore, the bioactivity and metabolism of quercetin and kaempferol metabolites in body tissues must be investigated to further understand the mechanism of action on NR4A1 targeted suppression of endometriosis.
Flavonoid-mediated NR4A1 inhibition studies have focused on the role of NR4A1 in survival pathways (such as PI3K/AKT, mTOR, oxidative/ER stress, and fibrosis) for the growth of endometriotic lesions. In addition, NR4A1 also has an essential role in immune cell function (65). For instance, NR4A1 has a critical role in regulatory T cell differentiation, and regulatory T cells have a crucial role in endometriosis progression (66, 67). Thus, functional studies regarding the effects of flavonoid-targeted NR4A1 in immune cells will be needed to more fully understand the detailed molecular mechanism of how NR4A1 enhances endometriosis progression. Nevertheless, results of this study suggest that the nutraceutical applications of quercetin and kaempferol may be useful adjuncts for treating some symptoms of endometriosis.
Abbreviations
- CDIM
bis-indole derived compounds
- COL1A1
collagen type 1 α-1
- CTGF
corrective tissue growth factor
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- ER
estrogen receptors
- FN
fibronectin
- GSH
glutathione
- IHEEC
immortalized human endometriotic epithelial cell
- IHESC
immortalized human endometriotic stromal cell
- LBD
ligand binding domain
- mTOR
The mammalian target of rapamycin
- NEM
endometrium of normal women
- NESC
normal endometrial stromal cell
- NR4A1
nuclear receptor 4A1
- PBS
phosphate-buffered saline
- ROI
region of interest
- SESN2
sensor protein setrin2
- SMA
smooth muscle actin
- TXNDC5
Thioredoxin domain Containing 5
- UAS
upstream activation sequence
Contributor Information
Lei Zhang, Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, TX 77843, USA.
Kumaravel Mohankumar, Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, TX 77843, USA.
Gregory Martin, Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, TX 77843, USA.
Fuada Mariyam, Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, TX 77843, USA.
Yuri Park, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA.
Sang Jun Han, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA.
Stephen Safe, Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, TX 77843, USA.
Funding
This work was party supported by National Institute of Child Health and Human Development (NICHD) (R01 HD098059) to SH and the National Institute of Health ES029067 (SS) and the Syd Kyle Chair endowment (SS).
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235‐258. [DOI] [PubMed] [Google Scholar]
- 2. Buck Louis GM, Hediger ML, Peterson CM, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
- 4. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152(3):R63‐R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angioni S, Cofelice V, Pontis A, Tinelli R, Socolov R. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014;30(11):769‐773. [DOI] [PubMed] [Google Scholar]
- 6. Andres Mde P, Lopes LA, Baracat EC, Podgaec S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet. 2015;292(3):523‐529. [DOI] [PubMed] [Google Scholar]
- 7. Zito G, Luppi S, Giolo E, et al. Medical treatments for endometriosis-associated pelvic pain. Biomed Res Int. 2014;2014:191967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granese R, Perino A, Calagna G, et al. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial. Acta Obstet Gynecol Scand. 2015;94(6):637‐645. [DOI] [PubMed] [Google Scholar]
- 9. Strowitzki T, Faustmann T, Gerlinger C, Schumacher U, Ahlers C, Seitz C. Safety and tolerability of dienogest in endometriosis: pooled analysis from the European clinical study program. Int J Womens Health. 2015;7:393‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown J, Farquhar C. An overview of treatments for endometriosis. JAMA. 2015;313(3):296‐297. [DOI] [PubMed] [Google Scholar]
- 11. Practice Committee of the American Society for Reproductive Medicine . Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927‐935. [DOI] [PubMed] [Google Scholar]
- 12. Pearen MA, Muscat GEO. Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol. 2010;24(10):1891‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Safe S, Jin U-H, Hedrick E, Reeder A, Lee S-O. Minireview: role of orphan nuclear receptors in cancer and potential as drug targets. Mol Endocrinol. 2014;28(2):157‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SO, Abdelrahim M, Yoon K, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010;70(17):6824‐6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muscat GE, Eriksson NA, Byth K, et al. Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol. 2013;27(2):350‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SO, Andey T, Jin U-H, Kim K, Sachdeva M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265‐3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delgado E, Boisen MM, Laskey R, et al. High expression of orphan nuclear receptor NR4A1 in a subset of ovarian tumors with worse outcome. Gynecol Oncol. 2016;141(2):348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacey A, Rodrigues-Hoffman A, Safe S. PAX3-FOXO1A Expression in rhabdomyosarcoma is driven by the targetable nuclear receptor NR4A1. Cancer Res. 2017;77(3):732‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palumbo-Zerr K, Zerr P, Distler A, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21(2):150‐158. [DOI] [PubMed] [Google Scholar]
- 20. Dai Y, Sun Q, Zhang X, Hu Y, Zhou M, Shi J. Cyclosporin A ameliorates early brain injury after subarachnoid hemorrhage through inhibition of a Nur77 dependent apoptosis pathway. Brain Res. 2014;1556:67‐76. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, López-Moyado IF, Seo H, et al. NR4A Transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567(7749):530‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hibino S, Chikuma S, Kondo T, et al. Inhibition of Nr4a receptors enhances antitumor immunity by breaking treg-mediated immune tolerance. Cancer Res. 2018;78(11):3027‐3040. [DOI] [PubMed] [Google Scholar]
- 23. Ando M, Ito M, Srirat T, Kondo T, Yoshimura A. Memory T cell, exhaustion, and tumor immunity. Immunol Med. 2020;43(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 24. Lacey A, Hedrick E, Li X, et al. Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget. 2016;7(21):31257‐31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohankumar K, Li X, Sridharan S, Karki K, Safe S. Nuclear receptor 4A1 (NR4A1) antagonists induce ROS-dependent inhibition of mTOR signaling in endometrial cancer. Gynecol Oncol. 2019;154(1):218‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohankumar K, Li X, Sung N, Cho YJ, Han SJ, Safe S. Bis-Indole–Derived Nuclear Receptor 4A1 (NR4A1, Nur77) ligands as inhibitors of endometriosis. Endocrinology. 2020;161(4):bqaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shrestha R, Mohankumar K, Jin U-H, Martin G, Safe S. The histone methyltransferase gene G9A is regulated by Nuclear Receptor 4A1 (NR4A1) in alveolar rhabdomyosarcoma cells. Mol Cancer Res. 2021;20(3):612‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Safe S, Karki K. The paradoxical roles of orphan Nuclear Receptor 4A (NR4A) in cancer. Mol Cancer Res. 2021;19(2):180‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salaritabar A, Darvishi B, Hadjiakhoondi F, et al. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J Gastroenterol. 2017;23(28):5097‐5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abotaleb M, Samuel S, Varghese E, et al. Flavonoids in cancer and apoptosis. Cancers (Basel). 2019;11(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imran M, Salehi B, Sharifi-Rad J, et al. Kaempferol: A key emphasis to its anticancer potential. Molecules. 2019;24(12):2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kikuchi H, Yuan B, Hu X, Okazaki M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am J Cancer Res. 2019;9(8):1517‐1535. [PMC free article] [PubMed] [Google Scholar]
- 33. Abbaszadeh H, Keikhaei B, Mottaghi S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother Res. 2019;33(8):2002‐2014. [DOI] [PubMed] [Google Scholar]
- 34. Park S, Song G, Lim W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J Nutr Biochem. 2020;78:108328. [DOI] [PubMed] [Google Scholar]
- 35. Park S, Lim W, Bazer FW, Whang K-Y, Song G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. 2019;63:87‐100. [DOI] [PubMed] [Google Scholar]
- 36. Park S, Lim W, You S, Song G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J Nutr Biochem. 2019;67:161‐172. [DOI] [PubMed] [Google Scholar]
- 37. Kashyap B, Saikia K, Samanta SK, et al. Kaempferol 3-O-rutinoside from Antidesma acidum retz. Stimulates glucose uptake through SIRT1 induction followed by GLUT4 translocation in skeletal muscle L6 cells. J Ethnopharmacol. 2023;301:115788. [DOI] [PubMed] [Google Scholar]
- 38. Zhan YY, Chen Y, Zhang Q, et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol. 2012;8(11):897‐904. [DOI] [PubMed] [Google Scholar]
- 39. Mohankumar K, Lee J, Wu CS, Sun Y, Safe S. Bis-Indole-Derived NR4A1 ligands and metformin exhibit NR4A1-dependent glucose metabolism and uptake in C2C12 cells. Endocrinology. 2018;159(5):1950‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim TW, Lee SY, Kim M, Cheon C, Ko S-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9(9):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Halgren TA, Murphy RB, Friesner RA, et al. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47(7):1750‐1759. [DOI] [PubMed] [Google Scholar]
- 42. Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291‐2296. [DOI] [PubMed] [Google Scholar]
- 43. Bono Y, Kyo S, Takakura M, et al. Creation of immortalised epithelial cells from ovarian endometrioma. Br J Cancer. 2012;106(6):1205‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friesner RA, Murphy RB, Repasky MP, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J Med Chem. 2006;49(21):6177‐6196. [DOI] [PubMed] [Google Scholar]
- 45. Friesner RA, Banks JL, Murphy RB, et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739‐1749. [DOI] [PubMed] [Google Scholar]
- 46. Gabriel M, Fey V, Heinosalo T, et al. A relational database to identify differentially expressed genes in the endometrium and endometriosis lesions. Sci Data. 2020;7(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hedrick E, Safe S. Transforming growth factor β/NR4A1-inducible breast cancer cell migration and epithelial-to-mesenchymal transition is p38α (mitogen-activated protein kinase 14) dependent. Mol Cell Biol. 2017;37(18):e00306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang CC, Xu H, Man GCW, et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. 2013;16(1):59‐69. [DOI] [PubMed] [Google Scholar]
- 49. Yu M, Zhou Q. 3,6-dihydroxyflavone Suppresses the epithelial-mesenchymal transition, migration and invasion in endometrial stromal cells by inhibiting the notch signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):4009‐4017. [DOI] [PubMed] [Google Scholar]
- 50. Ilhan M, Ali Z, Khan IA, Taştan H, Küpeli Akkol E. The regression of endometriosis with glycosylated flavonoids isolated from Melilotus officinalis (L.) pall. In an endometriosis rat model. Taiwan J Obstet Gynecol. 2020;59(2):211‐219. [DOI] [PubMed] [Google Scholar]
- 51. Ding D, Cai X, Zheng H, Guo S-W, Liu X. Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis. Reprod Sci. 2019;26(11):1417‐1428. [DOI] [PubMed] [Google Scholar]
- 52. Ilhan M, Ali Z, Khan IA, Taştan H, Küpeli Akkol E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. And their effect on endometriosis rat model. J Ethnopharmacol. 2019;243:112100. [DOI] [PubMed] [Google Scholar]
- 53. Ryu S, Bazer FW, Lim W, Song G. Chrysin leads to cell death in endometriosis by regulation of endoplasmic reticulum stress and cytosolic calcium level. J Cell Physiol. 2019;234(3):2480‐2490. [DOI] [PubMed] [Google Scholar]
- 54. Toh MF, Mendonca E, Eddie SL, et al. Kaempferol exhibits progestogenic effects in ovariectomized rats. J Steroids Horm Sci. 2014;5(3):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsuzaki S, Darcha C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum Reprod. 2014;29(8):1677‐1687. [DOI] [PubMed] [Google Scholar]
- 56. Karki K, Mohankumar K, Schoeller A, Martin G, Shrestha R, Safe S. NR4A1 Ligands as potent inhibitors of breast cancer cell and tumor growth. Cancers (Basel). 2021;13(11):2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Signorile PG, Viceconte R, Baldi A. Novel dietary supplement association reduces symptoms in endometriosis patients. J Cell Physiol. 2018;233(8):5920‐5925. [DOI] [PubMed] [Google Scholar]
- 58. Markowska A, Antoszczak M, Markowska J, Huczyński A. The role of selected dietary factors in the development and course of endometriosis. Nutrients. 2023;15(12):2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yalcin Bahat P, Ayhan I, Üreyen Özdemir E, İnceboz Ü, Oral E. Dietary supplements for treatment of endometriosis: A review. Acta Biomed. 2022;93(1):e2022159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu YN, Hu X-J, Liu B, Shang Y-J, Xu W-T, Zhou H-F. Network pharmacology-based prediction of bioactive compounds and potential targets of wenjing decoction for treatment of endometriosis. Evid Based Complement Alternat Med. 2021;2021:4521843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu J, Xue X, He Z, Zhang J, Sun H. Using network pharmacology and molecular docking to explore the underlying anti-inflammatory mechanism of Wuyao-Danshen to treat endometriosis. Ann Transl Med. 2022;10(4):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hipolito-Reis M, Neto AC, Neves D. Impact of curcumin, quercetin, or resveratrol on the pathophysiology of endometriosis: A systematic review. Phytother Res. 2022;36(6):2416‐2433. [DOI] [PubMed] [Google Scholar]
- 63. Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298‐344. [DOI] [PubMed] [Google Scholar]
- 64. Ulusoy HG, Sanlier N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit Rev Food Sci Nutr. 2020;60(19):3290‐3303. [DOI] [PubMed] [Google Scholar]
- 65. Bending D, Zikherman J. Nr4a nuclear receptors: markers and modulators of antigen receptor signaling. Curr Opin Immunol. 2023;81:102285. [DOI] [PubMed] [Google Scholar]
- 66. Szukiewicz D. Epigenetic regulation and T-cell responses in endometriosis—something other than autoimmunity. Front Immunol. 2022;13:943839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xiao F, Liu X, Guo SW. Platelets and regulatory T cells may induce a type 2 immunity that is conducive to the progression and fibrogenesis of endometriosis. Front Immunol. 2020;11:610963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.