Abstract
Background
Pediatric brain tumor survivors (PBTS) are at risk of worse quality of life (QOL) due to the impact of neurotoxic treatments on the developing nervous system. Parenting factors such as protectiveness have been linked to worse QOL in childhood cancer survivors generally, but have yet to be explored for PBTS. We examined whether parenting behaviors moderated the association between neurotoxic treatment and QOL for PBTS.
Methods
PBTS (n = 40; ages 10–25) and their caregivers (n = 47) completed measures of parenting behaviors including warmth (support/connectedness) and psychological control (protectiveness) and QOL. We divided the sample into moderate/high and low neurotoxicity groups based on chart review using the Pediatric Neuro-Oncology Rating of Treatment Intensity and examined moderator effects.
Results
Survivor-reported primary caregiver warmth moderated the relationship between neurotoxicity and caregiver-reported QOL. Moderate/high neurotoxicity was associated with lower caregiver-reported QOL only when survivor-reported primary caregiver warmth was low, P = .02. Similar results were found for survivor-reported QOL. Caregiver-reported psychological control moderated the association between neurotoxicity and caregiver-reported QOL such that neurotoxicity only affected QOL at high levels of psychological control, P = .01.
Conclusions
Heightened associations between parenting and QOL in the context of neurotoxic treatments underscore the need to better support PBTS. Findings are consistent with research suggesting that family factors may be particularly important for children with other neurological insults. Limitations include cross-sectional design and a small/heterogeneous clinical sample with low ethnic/racial diversity. Prospective studies are needed to refine evidence-based screening and develop psychosocial intervention strategies to optimize QOL for PBTS and their families.
Keywords: caregiver, central nervous system tumor, quality of life
For the over 60 000 pediatric brain tumor survivors (PBTS) in the United States, lifesaving treatments may adversely impact the child’s developing central nervous system (CNS), resulting in chronic physical and neurocognitive challenges, often termed late effects of cancer treatment.1 Intellectual and academic deficits, relative to peers, may emerge over time2,3 and may have a long-term, cascading effect on the survivor’s quality of life (QOL).4 QOL is a multidimensional concept that encompasses physical, psychological, and social domains of functioning.5 One meta-analysis of 15 studies indicated that PBTS have worse global health-related QOL compared to both healthy controls and non-CNS cancer survivors.6 PBTS report difficulties with physical aspects of QOL including mobility, sleep, fatigue, and pain,7 poorer social adjustment,8 and have elevated risk for anxiety, depression, suicidal ideation, and post-traumatic stress symptoms.9–11
Prior research has largely focused on identifying demographic, treatment, and disease-related risk factors for poorer QOL. Demographic risk factors include younger age at diagnosis, female sex,12 lower income,13 public insurance,14 and identification as a racially or ethnically minoritized individual.15 Disease and treatment-related risk factors include cancer recurrence and receiving more intensive treatments such as cranial radiation or chemotherapy, which are known to be toxic to the developing nervous system (ie, “neurotoxicity”).16–18 As cancer treatments evolve to minimize neurotoxicity, additional studies are needed to link updated neurotoxicity criteria19 to QOL outcomes for PBTS.
More recent work has shifted to identifying modifiable protective and risk factors, such as family factors, with several systematic reviews underscoring that family functioning is associated with PBTS’ QOL.20,21 Longitudinal research suggests that initial disruptions in family functioning following a brain tumor diagnosis improve over time for most families.22 Existing work also indicates that more adaptive family functioning, such as greater family cohesion and less caregiver-child conflict, is consistently related to higher adaptive23 and social functioning for PBTS and caregiver-reported emotional functioning, behavioral outcomes, and QOL.24 Still, current evidence linking family functioning to PBTS QOL remains methodologically limited by case series, brief measures of global family functioning, and shared method variance, suggesting that further work to identify specific and modifiable family factors is needed.
Parenting behaviors constitute a modifiable family factor that has been associated with QOL for survivors of multiple cancer types, but not PBTS.25 Children with neurological conditions may be particularly sensitive to parenting behaviors, as highlighted in research from the traumatic brain injury literature,26 suggested by theoretical models in pediatric cancer,20 and identified in a recent paper examining parenting in school-aged pediatric cancer survivors.27 PBTS are also more likely than non-CNS pediatric cancer survivors to require ongoing social support throughout young adulthood, with literature showing they are less likely to live independently or be employed full time.28 Thus, the parenting role may be prolonged through young adulthood and may continue to be an important predictor of long-term QOL for PBTS.29 Among non-CNS cancer survivors, some studies have linked protective parenting to poorer adjustment and QOL.30,31 Caregivers have reported feeling inclined to protect pediatric childhood cancer survivors due to perceived vulnerability, with adolescent and young adult survivors often resisting these efforts in pursuit of greater autonomy.32 Therefore, psychological control, which is characterized by attempts to manage children’s thoughts and feelings or protect them from harm,33 may be a relevant parenting construct prevalent among caregivers of PBTS. In normative samples, psychological control is a risk factor for psychopathology.33 By contrast, parental focus on child accomplishments in qualitative interviews, as opposed to focus on vigilance and protection, has been associated with more favorable survivor-reported QOL for survivors of non-CNS cancer.34
To optimize psychosocial interventions for PBTS, we utilized a multi-informant design to: (1) identify risk and protective factors at a family systems level that contribute to PBTS QOL, and (2) examine how family systems factors moderate the relationship between neurotoxicity and QOL. We hypothesized that more adaptive family functioning and higher caregiver warmth would be protective factors associated with better QOL outcomes, particularly in the context of moderate/high neurotoxicity. Conversely, we hypothesized that caregiver psychological control would be a risk factor associated with worse QOL. Specifically, we predicted psychological control would exacerbate the impact of treatment neurotoxicity on QOL, such that higher psychological control would be associated with poorer QOL, particularly in the context of high neurotoxicity.
Method
Procedure
Following Institutional Review Board approval, the study team screened the institutional Brain Tumor Program Survivorship Clinic Registry for PBTS between the ages of 10 and 25 years. The age range was selected in order to focus our study on emerging adolescence through emerging adulthood, who could provide self-report of their functioning during a critical transitional developmental period.35 Data collection occurred in the summer and fall of 2018. The study team approached potentially eligible survivors during clinic appointments, by a mailed letter, email, and/or phone. The study team collaborated with the neuro-oncology clinical team to determine eligibility. After reviewing potential benefits and risks of participation and providing verbal or digital consent/assent (ie, assent for PBTS age ≤18 years), eligible PBTS and one of their caregivers completed standardized measures with paper/pencil or online via REDCap surveys. The team made multiple reminder calls to encourage participation when one member of the dyad participated. The study team also reviewed PBTS’ medical records to abstract information about diagnosis and treatment. Families received gift cards ($5 per participant) for participation.
Participants
Eligibility criteria based on previous research with PBTS36 included: (1) history of brain tumor diagnosed before age 18, (2) at least 5 years post-diagnosis, (3) at least one prior therapeutic brain tumor treatment completed 3 or more years ago, (4) current age between 10 and 25, and (5) ability to understand and complete surveys. Non-English-speaking families were excluded due to using measures validated only in English.
Measures
Demographic characteristics
Caregivers and survivors who were 18 years and over completed a self-reported demographic questionnaire (eg, race/ethnicity, age, and education level). Demographics for survivors under the age of 18 were obtained from caregiver reports. Addresses from the medical record were utilized to identify 2018 estimated tract median family income (hereafter: “family income”).
General family functioning
Survivors and caregivers completed the Family Assessment Device, General Functioning scale (FAD-GF), a 12-item, widely utilized and “well established” measure of family functioning.37,38 Respondents rated items on a 4-point scale Likert scale from “strongly agree” to “strongly disagree.” Scores range from 1 to 4, with higher scores reflecting more family dysfunction and scores ≥2 indicative of clinically significant family dysfunction.
Parenting behaviors
The revised Children’s Report of Parenting Behaviors Inventory (CRPBI) and the Parenting Behaviors Inventory (PBI)39 are measures of the dyadic caregiver-child relationship completed by PBTS (30 items, completed separately for the primary and secondary caregiver) and caregivers (30 items), respectively. Characteristics were rated on a 3-point Likert scale: “not like,” “somewhat like,” and “a lot like.” The warmth/acceptance scale (hereafter “warmth”) and psychological control/psychological autonomy scale (hereafter “psychological control”) were utilized, given their consistent associations with youth adjustment in meta-analyses.40 Sample items included: My caregiver is a person who “enjoys doing things with me” (warmth) and “would like to be able to tell me what to do all the time” (psychological control). The warmth and psychological control subscales have demonstrated fair to excellent internal consistency previously (αs:0.65–0.93)39,41 and in the current sample (αs = 0.71–0.92). Although designed for children and adolescents, prior studies have utilized the CRPBI with young adult populations as a retrospective measure of parenting behaviors in their lifetime,42 and this measure has been previously used in pediatric populations38 and in pediatric oncology.27 Scores range from 10 to 30 for each subscale, with higher scores reflecting more of the respective parenting behavior reported by the survivor.
Quality of life
Survivors and their caregivers completed the Patient Reported Outcomes Measurement Information System (PROMIS) scales as a measure of health-related QOL.43 These measures have been established as feasible and valid measures with a pediatric oncology survivorship population.43 Depending on the survivor’s age, either the PROMIS Pediatric/Parent Proxy Profile 25 v2.0 (ages 10–17) or the PROMIS-29 Profile v1.2 (ages 18–25) were completed as self and proxy reports. Both included 4-item short-form scales assessing anxiety, depressive symptoms, fatigue, pain interference, mobility, and peer relationships.
Participants responded about the past 7 days, rating each item on a 5-point Likert scale. The health measures scoring service was utilized to generate standardized scores (T-scores) for the appropriate age version. In order to compare the entire sample on QOL, the adult and pediatric versions of the PROMIS were linked by converting the pediatric T-scores to adult T-scores for analogous subscales based on previous work.44,45 Administering the age-appropriate versions of the PROMIS scales ensured that the items were developmentally tailored45 and the pediatric/adult versions have been highly correlated with one another in previous work (rs.84–0.95).45 Scores range from 20 to 80, with higher scores for the subscales indicating more of the construct being assessed. Clinical cutoffs for at least mild difficulties for the subscales of anxiety, depression, fatigue, and pain interference included T-scores ≥55, and for the subscales of mobility/physical functioning and social roles/peer relationships included scores ≤40.
Based on prior factor analyses,46 the PROMIS subscales were also scored and combined into three composites: physical QOL (physical function/mobility, pain, and fatigue), emotional QOL (anxiety and depression), and social QOL (peer relationships/ability to participate in social roles and activities). Scores ranged from 4 to 20. A global QOL composite was also created, ranging from 12 to 60. Higher domain level and global QOL indicate higher QOL.
Neurotoxicity of treatment
Two trained research assistants reviewed survivors’ electronic medical charts to abstract information about their cancer treatment and diagnosis, and rated treatment neurotoxicity using the Pediatric Neuro-Oncology Rating of Treatment Intensity (PNORTI), which is a tool intended for use by researchers to rate the intensity of treatment for pediatric brain tumor.19 A rating of “one” indicated treatment involved surgical resection only, focal radiation, and/or low-intensity chemotherapy. A rating of “two” indicated treatment with cranial radiation or craniospinal radiation with or without medium or lower chemotherapy. A rating of “three” indicated treatment involved high-intensity chemotherapy. A random subset of charts was double-coded (n = 12), which yielded excellent interrater reliability (100% agreement). Given that only a few children received a rating of 3 in this sample (n = 8), PNORTI ratings of 2 and 3 were combined to separate the sample into a low neurotoxicity group (PNORTI rating of 1, n = 32) and moderate/high neurotoxicity group (PNORTI rating of 2 or 3, n = 23).
Data Analysis Plan
The data were tested for violations of the assumptions of regression, including linearity, homoscedasticity, and an absence of multicollinearity. Descriptive statistics and bivariate correlations were conducted for primary outcome measures, with separate models for primary and secondary caregiver outcomes. Intraclass correlations to compare across survivor and caregiver reports were also calculated. Independent samples t-tests examined whether QOL, parenting, and family functioning differed across neurotoxicity levels. We decided not to correct for multiple comparisons, given that in small samples, there is a higher likelihood of Type II error over Type I error.47,48 We also report effect sizes (Cohen’s d) for further interpretability of the findings. For the interaction effects, separate models were run to examine cross-informant models (ie, survivor-reported family factors with caregiver-reported global QOL, n = 29 pairs) and secondary analyses were run to explore caregiver-reported models (n = 43) and survivor-reported models (n = 37). Based on previous literature,49,50 we anticipated medium to large effects. The sample size of at least 43 yielded at least 80% power to detect large effects, with up to 59 participants needed to detect medium effects. Thus, we were underpowered for the cross-informant and survivor-reported models. Moderation analyses were conducted with the PROCESS macro for SPSS with mean centering and bias-corrected bootstrapping with 10 000 iterations (See Figure 2),51 covarying for family income, which was significantly correlated with QOL (r = 0.35). A heteroskedasticity-consistent standard error estimator (HC3—Davidson– MacKinnon) was selected and utilized, given the evidence for this approach with small sample sizes.52
Figure 2.
Caregiver-reported psychological control as a moderator of neurotoxicity on caregiver-reported quality of life. Note. *P < .05, Controlling for family income; psych control = psychological control, low psychological control = 1 standard deviation below the mean, average psychological control = sample mean, high psychological control = 1 standard deviation above the mean.
Results
Sample Characteristics
Of 90 families who were potentially eligible and approached to participate, 55 completed measures (61.1%), while 8 eligible PBTS declined participation and 27 eligible PBTS were unable to be contacted. Participants did not differ from non-participants on PBTS sex, race/ethnicity, age, age at diagnosis, or years since last cancer treatment. A total of 40 survivors and 47 caregivers completed at least part of the study measures. Families in which both survivors and caregivers participated (n = 29) did not differ in demographic characteristics from those in which only 1 member participated, and thus all available data were retained and analyzed. Caregivers were primarily biological mothers (n = 39), with a few biological fathers (n = 3), grandparents (n = 2), and legal guardians (n = 3). Most of the PBTS responded in reference to their mother as the primary caregiver, but survivors were able to write in alternative primary caregivers, as appropriate (n = 3 identified an alternative, non-parent caregiver such as a grandparent on the parenting behavior questionnaire). Our sample of PBTS was primarily male (55%), White (85%), in the young adult age range (65%, ages 18–25), and diagnosed with an astrocytoma/glioma (60%). See Table 1 for a display of demographic characteristics.
Table 1.
Demographic and Treatment Characteristics of Participants
| Survivors (N = 55) |
Caregivers (n = 47) |
Adult Survivors Self-reported (n = 28) |
|
|---|---|---|---|
| Male, n (%) | 30 (55%) | - | - |
| Race/Ethnicity, n (%) | - | - | - |
| White, n (%) | 47 (85.5%) | 41 (87.2%) | - |
| Black/African American, n (%) | 4 (7.3%) | 3 (6.4%) | - |
| Native American/Alaskan/Hawaiian | 4 (7.3%) | 2 (4.3 %) | - |
| Hispanic, n (%) | 3 (5.5%) | 1 (2.1%) | - |
| Asian, n (%) | 2 (3.6%) | 0 (0%) | - |
| Age, years, M (SD) | 18.44 (4.12) | 46.84 (8.47) | - |
| Pre-teen (ages 10–12), n (%) | 7 (12.7%) | - | - |
| Teen (ages 13–17), n (%) | 12 (21.8%) | - | - |
| Young adult (ages 18–25), n (%) | 36 (65.5%) | - | - |
| Current living situation, n (%) | |||
| Living with caregiver/guardian | - | - | 20 (71.4%) |
| Living independently (alone/roommate) | - | - | 6 (21.4%) |
| Living with a significant other/partner | - | - | 2 (7.1%) |
| Relationship status, n (%) | - | - | - |
| Married | - | 34 (72.3%) | 1 (3.6%) |
| Widowed | - | 1 (2.1%) | - |
| Divorced | - | 7 (14.9%) | - |
| Separated | - | 1 (2.1%) | - |
| Never Married | - | 3 (6.4%) | - |
| Live with someone | - | 1 (2.1%) | - |
| Single, never married | - | - | 23 (82.1%) |
| In a committed relationship | - | - | 3 (10.7%) |
| Employment status, n (%) | - | ||
| Full time | - | 29 (61.7%) | 4 (14.3%) |
| Part-time | - | 6 (12.8%) | 11 (39.3%) |
| Homemaker | - | 7 (14.9%) | -- |
| Student | - | -- | 5 (17.9%) |
| Retired | - | 1 (2.1%) | -- |
| Disabled | - | 2 (4.3%) | 2 (7.1%) |
| Unemployed | - | 1 (2.1%) | 6 (21.4%) |
| Public assistance, n (%) | - | 26 (55.3%) | 8 (28.6%) |
| 2018 Census Tract Median Income, dollars, M (SD), range | 81.67 (27.79), 33–146 | - | - |
| Caregiver Highest Level of Education, n (%) | - | - | - |
| No high school diploma/GED | - | 1 (2.1%) | - |
| High school diploma/GED | - | 17 (36.2%) | 12 (42.9%) |
| At least 2 years of college | - | 10 (21.3%) | 3 (10.7%) |
| Bachelor’s degree | - | 7 (14.9%) | 9 (32.1%) |
| Graduate/professional degree | - | 11 (23.4%) | 4 (14.3%) |
| Time since diagnosis, years, M (SD),range | 12.00 (4.41), 5.28–22.42 | - | - |
| Age at diagnosis, years, M (SD), range | 6.44 (4.53), 0.33–17 | - | - |
| Time since treatment, years, M (SD), range | 10.63 (4.32), 3.76–20.43 | - | - |
| Diagnosis category, n (%) | - | - | - |
| Astrocytoma/glioma | 33 (60%) | - | - |
| Medulloblastoma | 9 (16.4%) | - | - |
| Germ cell tumor | 3 (5.5%) | - | - |
| Ependymoma | 2 (3.6%) | - | - |
| Atypical teratoid-rhabdoid tumor | 2 (3.6%) | - | - |
| Other | 6 (10.9%) | - | - |
| Radiation, n (%) | 26 (47.3%) | - | - |
| Relapse, n (%) | 17 (30.9%) | - | - |
| Neurosurgery, n (%) | 52 (94.5%) | - | - |
| Neurosurgery only, n (%) | 23 (41.8%) | - | - |
| Chemotherapy, n (%) | 27 (49.0%) | - | - |
| Chemotherapy only | 2 (3.6%) | - | - |
| Autologous bone marrow transplant | 7 (12.7%) | - | - |
| Neurofibromatosis type 1, n (%) | 2 (3.6%) | - | - |
| Ventricular- peritoneal shunt, n (%) | 18 (32.7%) | - | - |
| Hydrocephalus, n (%) | 24 (43.6%) | - | - |
Note. Participants could select multiple races/ethnicities, Adult survivors (n = 28) are included in the overall 55 participants.
Survivor versus Caregiver Report
The proportion of survivors with clinical elevations indicating at least mild difficulties on domains of QOL were between 21% and 45% for caregiver/proxy report (n = 47 caregivers) and between 33% and 55% for survivor report (n = 40 survivors), with the most common elevations in the areas of anxiety and mobility (See Table 2). Intraclass correlations indicated that caregivers and survivors reported similarly on most aspects of QOL and parenting, except for social QOL, and primary caregiver warmth (See Table 2).53 Independent samples t-tests by neurotoxicity indicated that there were no significant differences for parenting behaviors nor global QOL, except for family functioning, in which survivors in the low neurotoxicity group reported marginally higher levels of family dysfunction (M = 1.88, SD = 0.51) as compared to survivors in the moderate/high neurotoxicity group (M = 1.61, SD = 0.37), t(38) = 1.77, P = .08, d = 0.59 (See Supplementary Table 1). There were no age effects for QOL, main effects for neurotoxicity and global QOL, nor a main effect between parenting factors/family functioning and global QOL.
Table 2.
Descriptive Statistics for Primary Outcome Variables
| Survivor Report M (SD) |
Survivor Clinical Elevation (n, %) |
Caregiver Report M (SD) |
Caregiver Clinical Elevation (n, %) |
ICC | ICC Classification | |
|---|---|---|---|---|---|---|
| Anxiety (PROMIS) | - | 22 (55) | 53.50 (9.44) | 21 (45) | 0.78 | Good |
| Depression (PROMIS) | - | 13 (33) | 49.19 (9.88) | 16 (34) | 0.76 | Good |
| Physical function/mobility (PROMIS) | - | 20 (50) | 47.72 (9.90) | 17 (36) | 0.83 | Good |
| Fatigue (PROMIS) | - | 13 (33) | 49.06 (9.64) | 10 (21) | 0.73 | Moderate |
| Pain interference (PROMIS) | - | 20 (50) | 48.22 (7.47) | 11 (23) | 0.72 | Moderate |
| Social roles/ peer relationships | - | 20 (50) | 49.56 (10.15) | 15 (32) | 0.40 | Poor |
| Global QOL (PROMIS) | - | - | 48.84 (8.40) | - | 0.77 | Good |
| Primary caregiver warmth (CRPBI/PBI) | 26.97 (2.91) | - | 27.00 (2.92) | - | 0.11 | Poor |
| Primary caregiver psychological control (CRPBI/PBI) | 16.33 (4.47) | - | 13.17 (3.05) | - | 0.63 | Moderate |
| Secondary caregiver warmth/acceptance (CRPBI/PBI) | 25.40 (4.67) | - | - | - | - | - |
| Secondary caregiver psychological control (CRPBI/PBI) | 14.94 (4.93) | - | - | - | - | - |
| Family functioning (FAD-GF) | 1.77 (0.47) | - | 1.65 (0.38) | - | 0.57 | Moderate |
Notes. PROMIS, Patient Reported Outcomes Measurement System; CRPBI, Children’s Report of Parent Behavior Inventory; PBI, Parenting Behaviors Inventory; FAD, Family Assessment Device; PROMIS subscales are presented as T-scores (M = 50, SD = 10), with higher quality of life (QOL) indicative of higher of the construct presented; Global QOL is presented as the sum of raw scores (range 12–60), with higher scores indicative of higher QOL; Clinical elevation for the PROMIS includes mild, moderate or severe difficulties on T-score of PROMIS and FAD-GF scores ≥ 2; An ICC of below.50 indicates poor agreement; an ICC of 0.50–0.75 indicates moderate agreement; an ICC of 0.75–0.90 indicates good agreement; and an ICC of 0.90–1.00 indicates excellent agreement (Koo and Li, 2016).
Primary Analyses: Cross-Informant Moderation Models
Consistent with hypotheses, survivor-reported primary caregiver warmth moderated the relationship between neurotoxicity and caregiver-reported global QOL (See Figure 1; n = 29 survivor and caregiver pairs), b = 2.39 (SE = 0.88), t(4,24) = 2.70, P = .01. At low levels of survivor-reported primary caregiver warmth, neurotoxicity of treatment was significantly associated with global QOL, b = −9.31(SE = 3.84), t(1,24) = −2.43, P = .02. However, for average or high levels of survivor-reported primary caregiver warmth, neurotoxicity of treatment was not significantly associated with global QOL, b = −1.88(SE = 2.52), t(1,24) = −0.75, P = 0.46 and b = 5.56(SE = 3.63), t(1,24) = 1.53, P = .14, respectively. Contrary to hypotheses, PBTS-reported primary caregiver psychological control, secondary caregiver psychological control, secondary caregiver warmth, and family functioning did not moderate associations between treatment neurotoxicity and caregiver-reported global QOL (see Supplementary Table 2).
Figure 1.
Survivor-reported primary caregiver warmth as a moderator of neurotoxicity on caregiver-reported quality of life. Note: * indicates P < .05, Controlling for family income; low primary caregiver warmth = 1 standard deviation below the mean, average primary caregiver warmth = sample mean, high primary caregiver warmth = 1 standard deviation above the mean.
Secondary Analysis: Parallel Informant Moderation Models
Secondary analyses with the same informant indicated similar results. For analyses with caregiver-reported variables for the moderator and outcome variables (n = 43), caregiver-reported psychological control moderated the relationship between neurotoxicity and caregiver-reported global QOL, b = −1.95 (SE = 0.71), t(4,38) = −2.75, P = .009 (See Figure 2). At high levels of caregiver-reported psychological control, treatment neurotoxicity was significantly associated with global QOL, b = −8.73 (SE = 3.34), t(4,38) = −2.61, P = .01. However, for average or low levels of caregiver-reported psychological control, neurotoxicity of treatment was not significantly associated with global QOL, b = −3.00 (SE = 2.26), t(4,38) = −2.61, P = 0.19 and b = 2.72(SE = 2.77), t(4,38) = 0.98, P = .33, respectively. Caregiver-reported warmth and family functioning did not moderate the association of neurotoxicity with caregiver-reported global QOL (see Supplementary Table 3).
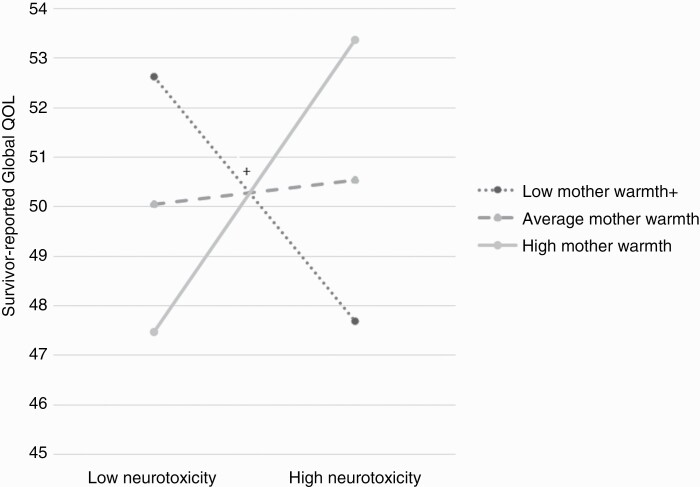
For survivor-reported models (n = 37), there was a significant interaction effect, wherein survivor-reported primary caregiver warmth moderated the relationship between neurotoxicity and survivor-reported global QOL, b = 2.09 (SE = 0.91), t(3,33) = 2.29, P = .03 (See Figure 3). At low survivor-reported primary caregiver warmth, neurotoxicity of treatment was marginally associated with survivor-reported global QOL, b = −4.93 (SE = 2.63), t(3,33) = −1.87, P = .07, such that higher neurotoxicity of treatment was related to lower survivor-reported global QOL. For average or high levels of survivor-reported primary caregiver warmth, neurotoxicity of treatment was not significantly associated with global QOL, b = 0.48 (SE = 2.59), t(3,33) = 1.40, P = .85 and b = 5.90 (SE = 4.20), t(3,33) = 1.40, P = .17, respectively. There were null findings for the interaction effects between neurotoxicity and survivor-reported family functioning and psychological control (See Supplementary Table 4).
Figure 3.
Survivor-reported primary caregiver warmth as a moderator of neurotoxicity on survivor-reported quality of life. Note: + indicates P < .10; Higher score indicates better quality of life; low warmth = 1 standard deviation below the mean, average warmth = sample mean, high warmth = 1 standard deviation above the mean.
Discussion
We examined associations between family risk and protective factors and QOL for PBTS. Consistent with previous findings,54 approximately half of PBTS were reported to demonstrate moderate to severe impairments in QOL on one or more domains, relative to typically developing peers. Our results indicated that the associations between parenting behaviors and QOL were amplified in the context of survivor neurological vulnerability. As hypothesized, we found that the association of treatment neurotoxicity with survivor QOL varied as a function of parenting behaviors. Specifically, treatment neurotoxicity was only associated with poorer QOL by both survivor and caregiver reports, in the context of low levels of survivor-reported caregiver warmth. There was also some preliminary indication in the caregiver-reported analyses that the association of neurotoxic treatments with QOL was amplified at high levels of parental protectiveness, suggesting that psychological control is associated with lower QOL among survivors with more neurotoxic treatments. Counter to hypotheses, we failed to find evidence that family functioning moderated outcomes. Taken together, our findings indicated that parenting factors and survivor functioning are more closely related following neurotoxic treatments, which highlights more thorough consideration of both survivor and family-level factors when caring for this vulnerable population.
Given the nature of the cross-sectional data, the significant interactions between parenting behavior and neurotoxicity with QOL suggest 2 different explanations. There may be a potential buffering/exacerbating effect of parenting behaviors in the context of higher neurotoxicity. It has been speculated in previous studies that the parenting role may be slightly prolonged for PBTS,32 and thus survivors in the higher neurotoxicity group may rely on their primary caregivers for more support for longer into adolescence and adulthood due to their elevated risk for neurocognitive difficulties and physical disabilities.55 Previous work has supported a negative association between overprotectiveness and QOL for non-CNS cancer survivors,31,34 but this is the first study to suggest that parenting (in our study, psychological control) was only related to QOL for PBTS with more neurotoxic treatment. Notably, comparing to previous studies of non-CNS tumors can be challenging, as parenting constructs such as overprotectiveness and vigilance may have different meanings in the context of CNS disease. For instance, PBTS have a higher likelihood of recurrence and the emergence of continued medical complexities as they age. Thus, these constructs likely require additional examination and understanding of mixed-method approaches with the PBTS population. Still, similar to other neurological populations such as traumatic brain injury,20,26 our findings may suggest that survivors with more CNS-involved treatments may be more sensitive to parenting and family factors.
An alternative explanation for our findings is that parents of PBTS with lower QOL in the context of higher neurotoxicity, may implement more psychological control and less warmth, in response to their child’s functioning. Specifically, we speculate that in the context of PBTS with lower QOL and higher neurotoxicity, caregivers may have more demands and higher caregiver burden,56 which may “spillover” into less capacity to implement positive parenting behaviors. For instance, caregivers may exert more control and protectiveness as way of supporting, scaffolding, and compensating for their child’s functioning,57,58 or may have less capacity for warmth in response to their child’s lower QOL. Although these relationships have been suggested in general child psychopathology and other types of cancer, these relationships have yet to be studied prospectively in PBTS and their caregivers. It is also plausible that these relationships could be reciprocal, such that lower QOL may lead to less adaptive parenting behaviors (ie, lower warmth, higher psychological control), which further exacerbates QOL over time. For instance, for survivors of non-CNS cancer, one past study suggested a vicious cycle, such that overprotectiveness and low family cohesiveness led to lower survivor QOL, which then contributed to more overprotectiveness and lower cohesiveness.59 Prospective data will be essential in further delineating the directionality of these relationships and implications for intervention.
The findings of this study should be interpreted considering several limitations. Firstly, this study was limited by a small sample, which limited statistical power to detect effects and increased the chance of Type II error. We also did not control for multiple comparisons, given the small sample size.47 There was also heterogeneity in the sample with a wide range of brain tumor diagnoses, treatment types, developmental stages (ages 10–25), and variability in time since diagnosis and other confounding life stressors. These factors may have increased variability in QOL and diluted environmental effects, especially for measures of general family functioning. For example, it is possible that parenting was less impactful on QOL for older PBTS who were further from treatment or that survivors who were younger at diagnosis had lower QOL; although our small sample size precluded detecting these relationships. Secondly, recruitment relied on a clinic-based convenience sample, of English-speaking participants, and a predominantly White composition. Therefore, we are unable to form conclusions about these relationships for racial and ethnic minoritized PBTS and linguistically diverse families. Thirdly, parenting behavior inventories are based on the dominant culture and nuclear family structures with 2-parent homes and are not normed or sensitive to other family constellations, and may have limited validity in ethnically, racially, and linguistically diverse samples.60 Fourthly, there are limitations to the measures selected in that we combined pediatric/adult proxy PROMIS profiles and used retrospective reports of parenting. Additionally, PBTS who have received neurotoxic treatments may have poorer self-insight and awareness of their own deficits, which is suggested by literature that PBTS often underestimate social difficulties as compared to peers.61 Furthermore, we were unable to obtain standardized assessments of cognitive functioning in this study, which could have provided more information about PBTS’ need for parental support and accommodation. Finally, some of our significant findings were based on single informant-reported analyses, which are limited by shared method variance.
Future work could extend the current study by implementing a prospective design with a larger, more racially/ethnically diverse sample of PBTS to further examine the influence of parenting and family factors and neurotoxicity on QOL. A longitudinal study design would facilitate the examination of potential reciprocal relationships between variables to better delineate directionality of relationships. It would also be interesting to examine the relationship between age at diagnosis, time since diagnosis, late effects, and protectiveness, as late effects emerge for PBTS and may exacerbate protective parenting behaviors over time. In addition, a resilience-oriented perspective could examine predictors of positive outcomes, including post-traumatic growth. Future work could include a mixed-method assessment of parenting, neurotoxicity, and QOL to better understand how these constructs interact. Measures that directly assess parental overprotectiveness, perceived vulnerability, and communication patterns may inform more specific targets of intervention. Future studies may consider observational-based measures of parenting behaviors, which can be used to assess dyadic interactions and relationship quality. For QOL, an assessment of QOL based on clinician rating or diagnostic interview may help to reduce shared method variance and any potential minimization of symptom burden. Finally, future studies should consider including an assessment of caregiver distress or caregiver’s own self-report of QOL, which is known to have a downstream influence on caregiver interactions and survivor functioning.62
Consistent with the Pediatric Psychosocial Preventative Health Model,63 the findings of the current study suggest that psychosocial teams might provide universal screening throughout survivorship to identify PBTS at greatest risk for poor QOL, particularly among those who received highly neurotoxic cancer treatments and those with family systems level risk factors. Notably, evidence-based screening tools such as the Psychosocial Assessment Tool64 and the Adolescent and Young Adult Psycho-Oncology Screening Tool65 assess global family functioning, but may not be sensitive to specific parenting factors (eg, caregiver warmth, psychological control). Findings from the current study also may have implications for the design of clinical interventions for PBTS, and suggest that targeting parenting behaviors for children with highly neurotoxic treatments, including increasing caregiver warmth, acceptance, and empathy66 may be a promising avenue for future work. Clinical interventions may involve aspects of evidence-based care known to facilitate warm interactions between caregivers and survivors, such as identifying opportunities for the family to enjoy quality time together and teaching active listening techniques.66 Interventions could also be adapted to foster support with other important relationships in the survivor’s life as appropriate, such as extended family, close friends, or romantic relationships. Future work should examine the efficacy of these strategies in the PBTS population, how they impact parenting behaviors and survivor QOL, and the optimal timing of intervention.
In conclusion, the results of this small-scale cross-sectional study indicated that parenting factors are related to QOL only in the context of high neurotoxicity for PBTS, such that low primary caregiver warmth and high psychological control were related to lower QOL only for PBTS who received highly neurotoxic treatments. Implications for future research and clinical practice may be further refined with additional findings from larger longitudinal studies. Survivorship teams should consider implementing evidence-based screening tools to identify PBTS and caregivers at risk for poorer QOL based on demographic, treatment, and parenting factors. Prospective studies extending this line of research could inform the development of psychosocial interventions for the families of these at-risk groups, with a possible intervention target to improve the caregiver-survivor relationship. Our work in conjunction with the existing literature suggests that prioritizing the screening and care of the entire family system may optimize the QOL for survivors of pediatric brain tumors.
Supplementary Material
Contributor Information
Emily L Moscato, Division of Pediatric Rehabilitation Medicine, Cincinnati Children’s Hospital Medical Center; Department of Psychology, University of Cincinnati, Cincinnati, Ohio, USA.
Allison P Fisher, Division of Pediatric Rehabilitation Medicine, Cincinnati Children’s Hospital Medical Center; Department of Psychology, University of Cincinnati, Cincinnati, Ohio, USA.
Natasha Pillay-Smiley, Cancer and Blood Diseases Institute, The Cure Starts Now Foundation Brain Tumor Center; Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Ralph Salloum, Division of Hematology and Oncology, Nationwide Children’s Hospital; Department of Pediatrics, The Ohio State University, Columbus, Ohio, USA.
Shari L Wade, Division of Pediatric Rehabilitation Medicine, Cincinnati Children’s Hospital Medical Center; Department of Psychology, University of Cincinnati, Cincinnati, Ohio, USA; Department of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Funding
University of Cincinnati’s University Research Council Grant (to E.M. and S.W.).
Conflict of Interest
None.
References
- 1. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Ruiter MA, Van Mourik R, Schouten-Van Meeteren AYN, et al. Neurocognitive consequences of a paediatric brain tumour and its treatment: A meta-analysis. Dev Med Child Neurol. 2013;55(5):408–417. [DOI] [PubMed] [Google Scholar]
- 3. Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–531. [DOI] [PubMed] [Google Scholar]
- 4. Duckworth J, Nayiager T, Pullenayegum E, et al. Health-related quality of life in long-term survivors of brain tumors in childhood and adolescence: a serial study spanning a decade. J Pediatr Hematol Oncol. 2015;37(5):362–367. [DOI] [PubMed] [Google Scholar]
- 5. CDC. Health Related Quality of Life Concepts. 2018. https://www.cdc.gov/hrqol/concept.htm. Accessed March 6, 2023.
- 6. Schulte F, Russell KB, Cullen P, et al. Systematic review and meta-analysis of health‐related quality of life in pediatric CNS tumor survivors. Pediatr Blood Cancer. 2017;64(8):e26442. [DOI] [PubMed] [Google Scholar]
- 7. Macartney G, VanDenKerkhof E, Harrison MB, et al. Symptom experience and quality of life in pediatric brain tumor survivors: A cross-sectional study. J Pain Symptom Manage. 2014;48(5):957–967. [DOI] [PubMed] [Google Scholar]
- 8. Hocking MC, McCurdy M, Turner E, et al. Social competence in pediatric brain tumor survivors: Application of a model from social neuroscience and developmental psychology. Pediatr Blood Cancer. 2015;62(3):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah SS, Dellarole A, Peterson EC, et al. Long-term psychiatric outcomes in pediatric brain tumor survivors. Childs Nerv Syst. 2015;31(5):653–663. [DOI] [PubMed] [Google Scholar]
- 10. Bruce M, Gumley D, Isham L, et al. Post-traumatic stress symptoms in childhood brain tumour survivors and their parents. Child Care Health Dev. 2011;37(2):244–251. [DOI] [PubMed] [Google Scholar]
- 11. Sharkey CM, Espeleta HC, Traino KA, et al. Psychological adjustment outcomes among pediatric brain tumor survivors: a meta-analysis. Pediatr Blood Cancer. 2020;67(10):e28644. [DOI] [PubMed] [Google Scholar]
- 12. Olsson IT, Perrin S, Lundgren J, et al. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51(4):515–521. [DOI] [PubMed] [Google Scholar]
- 13. Barakat LP, Li Y, Hobbie WL, et al. Health-related quality of life of adolescent and young adult survivors of childhood brain tumors. Psychooncology. 2015;24(7):804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deatrick JA, Barakat LP, Knafl GJ, et al. Patterns of family management for adolescent and young adult brain tumor survivors. J Fam Psychol. 2018;32(3):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meeske KA, Patel SK, Palmer SN, et al. Factors associated with health-related quality of life in pediatric cancer survivors. Pediatr Blood Cancer. 2007;49(3):298–305. [DOI] [PubMed] [Google Scholar]
- 16. Patel SK, Schulte F, Kelly NC, et al. Neurocognitive late effects in children with cancer. In: Abrams AN, Muriel AC, Wiener L, eds. Pediatric Psychosocial Oncology: Textbook for Multidisciplinary Care. Cham, Switzerland: Springer; 2016:157–174. [Google Scholar]
- 17. Rizzo D, Peruzzi L, Attinà G, et al. Neurocognitive outcomes in pediatric brain tumors survivors. Med One. 2017;2(4):e170015. [Google Scholar]
- 18. Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):34943494–349433500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hocking MC, Hobbie W, Fisher MJ.. Development of the Pediatric Neuro-Oncology Rating of Treatment Intensity (PNORTI). J Neurooncol. 2018;136(1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peterson CC, Drotar D.. Family impact of neurodevelopmental late effects in survivors of pediatric cancer: review of research, clinical evidence, and future directions. Clin Child Psychol Psychiatry. 2006;11(3):349–366. [DOI] [PubMed] [Google Scholar]
- 21. Moscato E, Patronick J, Wade SL.. Family functioning and adaptation following pediatric brain tumor: a systematic review. Pediatr Blood Cancer. 2022;69(2):e29470. [DOI] [PubMed] [Google Scholar]
- 22. Penn A, Lowis SP, Stevens MC, et al. Family, demographic and illness-related determinants of HRQL in children with brain tumours in the first year after diagnosis. Pediatr Blood Cancer. 2009;53(6):1092–1099. [DOI] [PubMed] [Google Scholar]
- 23. Adduci A, Jankovic M, Strazzer S, et al. Parent-child communication and psychological adjustment in children with a brain tumor. Pediatr Blood Cancer. 2012;59(2):290–294. [DOI] [PubMed] [Google Scholar]
- 24. Hocking MC, Quast LF, Brodsky C, et al. Caregiver perspectives on the social competence of pediatric brain tumor survivors. Support Care Cancer. 2017;25(12):3749–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orbuch TL, Parry C, Chesler M, et al. Parent-child relationships and quality of life: Resilience among childhood cancer survivors. Fam Relat. 2005;54(2):171–183. [Google Scholar]
- 26. Wade SL, Taylor HG, Drotar D, et al. Parent-adolescent interactions after traumatic brain injury: Their relationship to family adaptation and adolescent adjustment. J Head Trauma Rehabil. 2003;18(2):164–176. [DOI] [PubMed] [Google Scholar]
- 27. Winning AM, Howard Sharp K, Ferrante AC, et al. CNS-directed cancer treatment and child adjustment: Moderating effects of maternal parenting. J Pediatr Psychol. 2022;47(8):916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schulte F, Kunin-Batson AS, Olson-Bullis BA, et al. Social attainment in survivors of pediatric central nervous system tumors: a systematic review and meta-analysis from the Children’s Oncology Group. J Cancer Surviv. 2019;13:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodgate RL, Tailor K, Yanofsky R, et al. Childhood brain cancer and its psychosocial impact on survivors and their parents: A qualitative thematic synthesis. Eur J Oncol Nurs. 2016;20:140–149. [DOI] [PubMed] [Google Scholar]
- 30. Hullmann SE, Wolfe-Christensen C, Meyer WH, et al. The relationship between parental overprotection and health-related quality of life in pediatric cancer: The mediating role of perceived child vulnerability. Qual Life Res. 2010;19(9):1373–1380. [DOI] [PubMed] [Google Scholar]
- 31. Schepers SA, Okado Y, Russell K, et al. Adjustment in childhood cancer survivors, healthy peers, and their parents: The mediating role of the parent–child relationship. J Pediatr Psychol. 2019;44(2):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aukema EJ, Last BF, Schouten-Van Meeteren AYN, et al. Explorative study on the aftercare of pediatric brain tumor survivors: A parents’ perspective. Support Care Cancer. 2011;19(10):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gorostiaga A, Aliri J, Balluerka N, et al. Parenting styles and internalizing symptoms in adolescence: A systematic literature review. Int J Environ Res Public Health. 2019;16(17):31923192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eiser C, Eiser JR, Greco V.. Surviving childhood cancer: Quality of life and parental regulatory focus. Pers Soc Psychol Bull. 2004;30(2):123–133. [DOI] [PubMed] [Google Scholar]
- 35. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–228. [DOI] [PubMed] [Google Scholar]
- 36. Moscato EL, Miley AE, LeBlond AE, et al. Feasibility and acceptability of an online problem-solving therapy intervention for adolescent and young adult brain tumor survivors. Clin Pract Pediatr Psychol. 2019;7(1):68–78. [Google Scholar]
- 37. Epstein NB, Baldwin LM, Bishop DS.. The McMaster family assessment device. J Marital Fam Ther. 1983;9(2):171–180. [Google Scholar]
- 38. Alderfer MA, Fiese BH, Gold JI, et al. Evidence-based assessment in pediatric psychology: Family measures. J Pediatr Psychol. 2007;33(9):1046–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schludermann S, Schludermann E.. Questionnaire for children and youth (CRPBI-30). Unpublished manuscript. Winnipeg: University of Manitoba; 1988. [Google Scholar]
- 40. Yap MBH, Jorm AF.. Parental factors associated with childhood anxiety, depression, and internalizing problems: A systematic review and meta-analysis. J Affect Disord. 2015;175:424–440. [DOI] [PubMed] [Google Scholar]
- 41. Schludermann E, Schludermann S.. Replicability of factors in children’s report of parent behavior (CRPBI). J Psychol. 1970;76(2):239–249. [Google Scholar]
- 42. Cross HJ. College students’ memories of their parents: A factor analysis of the CRPBI. J Consult Clin Psychol. 1969;33(3):275–278. [Google Scholar]
- 43. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. [DOI] [PubMed] [Google Scholar]
- 44. Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007:S22–S31. [DOI] [PubMed] [Google Scholar]
- 45. Tulsky DS, Kisala PA, Boulton AJ, et al. Determining a transitional scoring link between PROMIS® pediatric and adult physical health measures. Qual Life Res. 2019;28(5):1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hays RD, Spritzer KL, Schalet BD, et al. PROMIS®-29 v2. 0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;43:46. [PubMed] [Google Scholar]
- 48. Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7):1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hocking MC, Hobbie WL, Deatrick JA, et al. Family functioning mediates the association between neurocognitive functioning and health-related quality of life in young adult survivors of childhood brain tumors. J Adolesc Young Adult Oncol. 2015;4(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quast LF, Phillips PC, Li Y, et al. A prospective study of family predictors of health-related quality of life in pediatric brain tumor survivors. Pediatr Blood Cancer. 2018;65(6):e26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications; 2017. [Google Scholar]
- 52. Hayes AF, Cai L.. Using heteroskedasticity-consistent standard error estimators in OLS regression: An introduction and software implementation. Behav Res Methods. 2007;39(4):709–722. [DOI] [PubMed] [Google Scholar]
- 53. Koo TK, Li MY.. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siembida EJ, Reeve BB, Zebrack BJ, et al. Measuring health-related quality of life in adolescent and young adult cancer survivors with the National Institutes of Health Patient-Reported Outcomes Measurement Information System: Comparing adolescent, emerging adult, and y. Psychooncology. 2021;30(3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anderson DM, Rennie KM, Ziegler RS, et al. Medical and neurocognitive late effects among survivors of childhood central nervous system tumors. Cancer. 2001;92(10):2709–2719. [DOI] [PubMed] [Google Scholar]
- 56. Salvador A, Crespo C, Martins AR, et al. Parents’ perceptions about their child’s illness in pediatric cancer: Links with caregiving burden and quality of life. J Child Fam Stud. 2015;24(4):1129–1140. [Google Scholar]
- 57. Scharf M, Goldner L.. “If you really love me, you will do/be…”: Parental psychological control and its implications for children’s adjustment. Dev Rev. 2018;49:16–30. [Google Scholar]
- 58. Sharkey CM, Clawson AH, Mullins LL, et al. The relationship of child executive functions to parenting capacities in childhood acute lymphoblastic leukemia survivors. Pediatr Blood Cancer. 2019;66(8):e27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang I-C, Brinkman TM, Mullins L, et al. Child symptoms, parent behaviors, and family strain in long-term survivors of childhood acute lymphoblastic leukemia. Psychooncology. 2018;27(8):2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barber BK, Stolz HE, Olsen JA.. Parental support, psychological control, and behavioral control: assessing relevance across time, culture, and method. Monogr Soc Res Child Dev. 2005;70(4):1–137. [DOI] [PubMed] [Google Scholar]
- 61. Salley CG, Gerhardt CA, Fairclough DL, et al. Social self-perception among pediatric brain tumor survivors compared with peers. J Dev Behav Pediatr. 2014;35(7):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bakula DM, Sharkey CM, Perez MN, et al. The relationship between parent distress and child quality of life in pediatric cancer: A meta-analysis. J Pediatr Nurs. 2020;50:14–19. [DOI] [PubMed] [Google Scholar]
- 63. Kazak AE. Pediatric Psychosocial Preventative Health Model (PPPHM): Research, practice, and collaboration in pediatric family systems medicine. Fam Syst Health. 2006;4(4):381. [Google Scholar]
- 64. Pai ALH, Patiño-Fernández AM, McSherry M, et al. The Psychosocial Assessment Tool (PAT20): Psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. J Pediatr Psychol. 2008;33(1):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patterson P, D’Agostino NM, Mcdonald FEJ, et al. ; International AYA Cancer Distress Screening Group. Screening for distress and needs: findings from a multinational validation of the Adolescent and Young Adult Psycho-Oncology Screening Tool with newly diagnosed patients. Psychooncology. 2021;30(11):1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moretti MM, Obsuth I, Craig SG, et al. An attachment-based intervention for parents of adolescents at risk: Mechanisms of change. Attach Hum Dev. 2015;17(2):119–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.